Transition metal-catalyzed allylic substitution reactions (ASR) have been widely applied in organic synthesis.1 Many classes of carbon-centered nucleophiles can be employed, including active methylene compounds,2 enolates,3 enamines4 and organometallic reagents.5 Oppolzer has also demonstrated that olefins may be used as nucleophile equivalents in catalytic intramolecular ASR.6 However, catalytic intermolecular allylic substitution of simple alkenes has not been extensively investigated.7 Such a transformation would enable the construction of 1,4-dienes (“skipped” dienes) prevalent in natural compounds.8 Moreover, many simple alkenes, e.g., alpha olefins, are inexpensive feedstock chemicals and, as compared to enolates and other organometallic reagents, are generally easier to synthesize and compatible with a greater range of reaction conditions. Herein we report the first examples of catalytic intermolecular allylic substitution of unactivated, simple alkenes. Catalyst loadings as low as 2.5 mol% Ni afford the desired product in high yield in both gram-scale and smaller scale coupling reactions.
In the course of investigating nickel-catalyzed carbon-carbon bond-forming reactions in which alkenes serve as nucleophiles,9,10 we observed the ASR of ethylene (1 atm) by cinnamyl methyl ether (1a). Catalyzed by Ni(cod)2 and P(o-anisyl)3 in the presence of triethylsilyl trifluoromethanesulfonate (Et3SiOTf) and triethylamine at room temperature, this reaction afforded linear 1,4-diene 2a in 91% yield (Table 1, entry 1), along with a small amount of conjugated 1,3-diene 3a (5%), yet no detectable 1,4-diene isomers of 2a. Ethylene also undergoes substitution with a wide range of allylating reagents, including electrophiles bearing classically poor leaving groups, such as, alkyl ethers, Me3Si ethers, and even allylic alcohols (entries 1–4). Cinnamyl derivatives with classical leaving groups (acetate, chloride, and methyl carbonate (1b), entries 5–7) also performed well in this transformation. In the case of 1b, a catalyst loading of 2.5 mol% Ni provided a 91% yield of 2a (entry 7).
Table 1.
Scope of Leaving Group in Ni-Catalyzed ASR
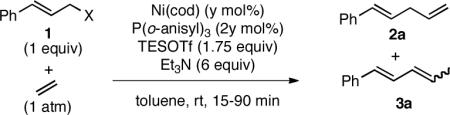
| Entry | X | y | yield of 2aa | yield of 3aa,b |
|---|---|---|---|---|
| 1 | OMe (1a) | 10 | 91 | 5 |
| 2 | OEt | 10 | 85 | 4 |
| 3c | OTMS | 10 | 75 | trace |
| 4d | OH | 20 | 56 | <5 |
| 5 | OAc | 10 | 86 | 10 |
| 6 | Cl | 10 | 63 | 20 |
| 7e | OCO2Me (1b) | 2.5 | 91 | 7 |
Determined by 1H NMR.
For 3a byproduct E:Z = approx 3:1 in all cases.
Me3SiOTf (1.75 equiv) used in place of Et3SiOTf, 4h.
Me3SiOTf (3 equiv) used instead of Et3SiOTf.
10 mol% P(o-anisyl)3 used, 3 h.
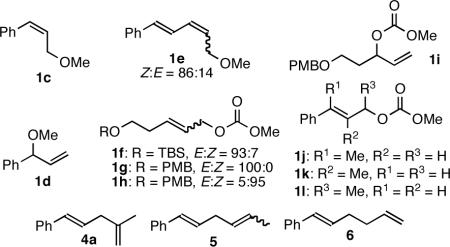
An examination of the scope of the ethylene ASR revealed that both Z-cinnamyl methyl ether (1c) and the corresponding branched isomer 1d provided linear product 2a in good yield with complete E selectivity (Table 2, entries 2–3). A broad range of allylic alcohol derivatives functioned well (entries 4–8), with a small amount of branched product observed with substrates bearing alkyl substituents (entries 5–8). Generally, substituents at any position of the allyl carbonate were tolerated (entries 9–11).
Table 2.
Ni-Catalyzed Allylic Substitution of H2C=CH2a

| Entry | Substrate | Product | Yieldb | l:bc | E:Zd |
|---|---|---|---|---|---|
| 1 | 1a |
|
75% | >99:1 | >99:1 |
| 2 | 1c | 2a | 83% | >99:1 | >99:1 |
| 3 | 1d | 2a | 74% | >99:1 | >99:1 |
| 4 | 1e |
|
84% | >99:1 | 83:17 |
| 5 | 1f |
|
97% | 95:5 | 94:6 |
| 6 | 1g |
|
73% | 98:2 | 92:8 |
| 7 | 1h | 2d | 82% | 98:2 | 92:8 |
| 8 | 1i | 2d | 76% | 98:2 | 92:8 |
| 9 | 1j |
|
57% | >99:1 | 94:6 |
| 10 | 1k |
|
81% | >99:1 | 88:12 |
| 11 | 1l |
|
71%e | >99:1 | >99:1 |
Isolated yield.
Linear:branched product.
Ratio of geometric isomers of linear products.
Approx. 15% yield of 1-phenylbutadiene also obtained; see Supporting Information.
The scope with respect to the alkene was also investigated (Table 3). The ASR of propylene by 1b under the same conditions as were used for ethylene (Table 2) afforded a mixture of 4a, 5, and 6 in an unselective manner (52:20:28). The use of PCy2Ph in place of P(o-anisyl)3 dramatically improved the selectivity for the 1,1-disubstituted product (4a, Table 3, entry 1, 77% yield, >98% selectivity), but higher boiling (i.e., not a gas at STP) monosubstituted alkenes (alpha olefins) such as 1-octene gave the corresponding product in low yield (approx 20%) under the same conditions. A solution to this problem was ultimately found by changing three reaction parameters: Increasing the initial substrate concentration, using a combination of PCy2Ph and P(OPh)3, and mixing 1b with the nickel complex prior to addition of alkene. Under these conditions, many alpha olefins gave the coupling products 4 in good yield and with excellent selectivity, including the more sterically demanding vinylcyclohexane (Table 3, entries 2–6).11 The opposite regioselectivity was observed in the case of styrene, with 7 being a sole coupling product (entry 7). It is worthy of note that in the case of all aliphatic olefins, C-C bond formation occurs at the more substituted position of the alkene.
Table 3.
Ni-Catalyzed Allylic Substitution of Alkenesa
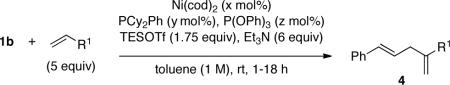
| entry | x | y | z | R1 (product) | yield (%)a |
|---|---|---|---|---|---|
| 1b | 10 | 20 | 0 | Me (4a) | 77 (71)c |
| 2 | 10 | 10 | 10 | n-hexyl (4b) | 79 |
| 3 | 20 | 20 | 20 | CH2OTES (4c) | 73 |
| 4 | 10 | 10 | 10 | (CH2)2OTBS (4d) | 83d |
| 5 | 20 | 20 | 20 | CH2CHMe2 (4e) | 87 |
| 6e | 20 | 40 | 0 | cyclohexyl (4f) | 64f |
| 7 | 20 | 40 | 0 |
|
25 |
Isolated yield; E/Z selectivity >98:2 in all cases.
Propylene pressure 1 atm (balloon); toluene (0.2 M).
5 mol% Ni(cod)2, 10 mol% PCy2Ph.
Yield of free alcohol after treatment with 1N HCl.
Et3SiOTf added over 4 h.
Yield includes trace amounts of regioisomers (total < 8%).
Although more detailed studies are required, we propose the mechanism delineated in Figure 1, in which methyl carbonate 1b is used as a representative substrate.12 The Ni(0) complex reacts with 1b without the assistance of Et3SiOTf,13 affording allyl nickel complex 8. The methoxy group is removed upon reaction with Et3SiOTf, generating cationic allylnickel complex 9, poised for olefin coordination. Migratory insertion (giving 10) orients the alkene substituent R away from the Ni, and (PhO)3P-facilitated10d β-H elimination and reductive elimination provide the 1,4-diene product and regenerate the catalyst.
Figure 1.
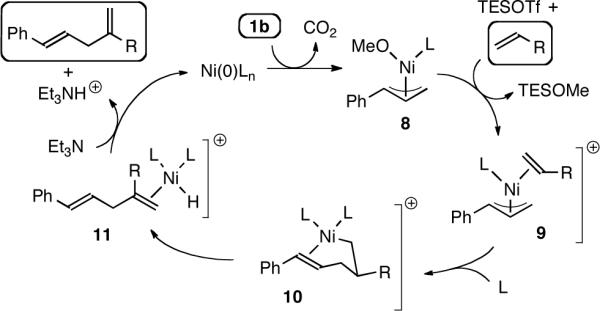
Proposed Mechanism of Ni-Catalyzed Allylic Substitution of Olefins (L = organophosphine; triflate (TfO−) omitted for clarity).
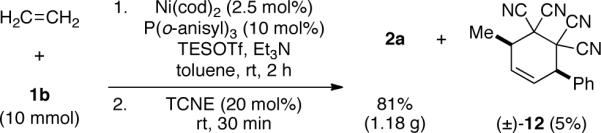
As a demonstration of the scalability of this transformation, the ASR of ethylene was conducted on 10-mmol scale. Filtration of the reaction mixture through a pad of silica gel and treatment with tetracyanoethylene (TCNE) cleanly and completely removed the major 1,3-diene byproduct (E-3a) via [4+2] cycloaddition (giving 12). The desired coupling product (2a) was not affected by TCNE treatment and was isolated in 81% yield (1.18 g) in >98% purity.
In summary, we report the first examples of catalytic allylic substitution of simple alkenes. This method accommodates a wide range of allylic alcohol derivatives and non-activated terminal alkenes, such as ethylene and propylene, affording synthetically valuable 1,4-dienes. High selectivity for substitution at the 2-position of alpha olefins is generally observed, favoring 1,4-diene products with a 1,1-disubstituted alkene. Further investigation of the reaction mechanism and the development of a mediator-free (i.e., without Et3SiOTf) process are underway.
Supplementary Material
Scheme 1.
Gram-Scale Allylic Substitution Reaction of Ethylene
Acknowledgment
Support for this work was provided by the NIGMS (GM-63755). We are grateful to Dr. Li Li for mass spectrometric data. R. M. thanks JSPS Postdoctoral Fellowships for Research Abroad for financial support.
Footnotes
Supporting Information Available: Experimental procedures and data for all new compounds (PDF). This material is available free of charge via the Internet at http://pubs.acs.org.
References
- (1).Trost BM, Lee CB. Chapter 8E. In: Ojima I, editor. Catalytic Asymmetric Synthesis II. Wiley-VCH; New York: 2000. pp. 593–650. [Google Scholar]
- (2).Trost BM. Tetrahedron. 1977;33:2615. [Google Scholar]
- (3).Braun M, Meier T, Laicher F, Meletis P, Fiden M. Adv. Synth. Catal. 2008;350:303. [Google Scholar]
- (4).Zhao X, Liu D, Xie F, Zhang W. Tetrahedron. 2009;65:512. [Google Scholar]
- (5).(a) Yasui H, Mizutani K, Yorimitsu H, Oshima K. Tetrahedron. 2006;62:1410. [Google Scholar]; (b) Son S, Fu GC. J. Am. Chem. Soc. 2008;130:2756. doi: 10.1021/ja800103z. [DOI] [PubMed] [Google Scholar]; (c) Porcel S, López-Carrillo V, García-Yebra C, Echavarren AM. Angew. Chem. Int. Ed. 2008;47:1883. doi: 10.1002/anie.200704500. [DOI] [PubMed] [Google Scholar]; (d) Nishikata T, Lipshutz BH. J. Am. Chem. Soc. 2009;131:12103. doi: 10.1021/ja905082c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Oppolzer W. Angew. Chem. Int. Ed. 1989;28:38. [Google Scholar]
- (7).Catalytic intermolecular ASRs of conjugated alkenes leading to 1,4-dienes have been reported: Tsukada N, Sato T, Inoue Y. Chem. Commun. 2001:237. doi: 10.1039/b307705e.. Tsukada N, Sato T, Inoue Y. Chem. Commun. 2003:2404. doi: 10.1039/b307705e..
- (8).There are far fewer ways to make 1,4-dienes with good regio- and stereocontrol than there are to make 1,3- and 1,5-dienes: Trost BM, Probst GD, Schoop A. J. Am. Chem. Soc. 1998;120:9228.. Wilson SR, Zucker PA. J. Org. Chem. 1988;53:4682.. Hilt G, du Mesnil F-X, Lüers S. Angew. Chem. Int. Ed. 2001;40:387.. Moreau B, Wu JY, Ritter T. Org. Lett. 2009;11:337. doi: 10.1021/ol802524r.. Morten CJ, Jamison TF. Tetrahedron. 2009;65:6648. doi: 10.1016/j.tet.2009.05.074..
- (9).(a) Ogoshi S, Oka M.-a., Kurosawa H. J. Am. Chem. Soc. 2004;126:11802. doi: 10.1021/ja0460716. [DOI] [PubMed] [Google Scholar]; (b) Ogoshi S, Haba T, Ohashi M. J. Am. Chem. Soc. 2009;131:10350. doi: 10.1021/ja903510u. [DOI] [PubMed] [Google Scholar]
- (10).(a) Ng S-S, Jamison TF. J. Am. Chem. Soc. 2005;127:14194. doi: 10.1021/ja055363j. [DOI] [PubMed] [Google Scholar]; (b) Ho C-Y, Ng S-S, Jamison TF. J. Am. Chem. Soc. 2006;128:5362. doi: 10.1021/ja061471+. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Schleicher KD, Jamison TF. Org. Lett. 2007;9:875. doi: 10.1021/ol063111x. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Ho C-Y, Jamison TF. Angew. Chem. Int. Ed. 2007;46:782. doi: 10.1002/anie.200603907. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Ho C-Y, Ohmiya H, Jamison TF. Angew. Chem. Int. Ed. 2008;47:1893. doi: 10.1002/anie.200705163. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Ng S-S, Ho C-Y, Schleicher KD, Jamison TF. Pure Appl. Chem. 2008;80:929. doi: 10.1351/pac200880050929. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Ho C-Y, Schleicher KD, Chan C-W, Jamison TF. Synlett. 2009:2565. doi: 10.1055/s-0029-1217747. [DOI] [PMC free article] [PubMed] [Google Scholar]; Reviews:
- (11).Methyl acrylate did not undergo allylation with 1b.
- (12).The mechanism of intramolecular ASR of olefins has been thoroughly investigated. Gómez-Bengoa E, Cuerva JM, Echavarren AM, Martorell G. Angew. Chem. Int. Ed. Engl. 1997;36:767.. Cárdenas DJ, Alcamí M, Cossío F, Méndez M, Echavarren AM. Chem. Eur. J. 2003;9:96. doi: 10.1002/chem.200390035.. A similar olefin insertion step has also been studied in reactions involving cationic metal hydrides. Mecking S, Keim W. Organometallics. 1996;15:2650.. DiRenzo GM, White PS, Brookhart M. J. Am. Chem. Soc. 1996;118:6225.. Joseph J, RajanBabu TV, Jemmis ED. Organometallics. 2009;28:3552. doi: 10.1021/om900045p..
- (13).NMR experiments revealed that, in contrast to cinnamyl methyl carbonate, cinnamyl methyl ether does not undergo oxidative addition to Ni(0) in the absence of Et3SiOTf. Yamamoto T, Ishizu J, Yamamoto A. J. Am. Chem. Soc. 1981;103:6863..
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.