Abstract
While most cigarette smokers endorse a desire to quit smoking, only 14–49% will achieve abstinence after 6 months or more of treatment. A greater understanding of the effects of smoking on brain function may result in improved pharmacological and behavioral interventions for this condition. Research groups have examined the effects of acute and chronic nicotine/cigarette exposure on brain activity using functional imaging; the purpose of this chapter is to synthesize findings from such studies and present a coherent model of brain function in smokers. Responses to acute administration of nicotine/smoking include reduced global brain activity; activation of the prefrontal cortex, thalamus, and visual system; activation of the thalamus and visual cortex during visual cognitive tasks; and increased dopamine (DA) concentration in the ventral striatum/nucleus accumbens. Responses to chronic nicotine/cigarette exposure include decreased monoamine oxidase (MAO) A and B activity in the basal ganglia and a reduction in α4β2 nicotinic acetylcholine receptor (nAChR) availability in the thalamus and putamen (accompanied by an overall upregulation of these receptors). These findings indicate that smoking enhances neurotransmission through cortico–basal ganglia–thalamic circuits by direct stimulation of nAChRs, indirect stimulation via DA release or MAO inhibition, or a combination of these and possibly other factors. Activation of this circuitry may be responsible for the effects of smoking seen in tobacco-dependent smokers, such as improvements in attentional performance, mood, anxiety, and irritability.
1 Introduction
Smoking remains a major health issue in USA and quitting smoking continues to be a challenge. In a recent survey, approximately 23% of Americans were found to smoke cigarettes (Balluz et al. 2004). While most smokers endorse a desire to quit (Fiore et al. 2000), very few will quit smoking without treatment, and only about 14–49% will achieve abstinence after 6 months or more of effective treatment (Holmes et al. 2004; Hughes et al. 1999; Hurt et al. 1997; Jorenby et al. 1999; Killen et al. 2000, 1999). Because cigarette smoking carries both considerable health risks (Bartal 2001; Mokdad et al. 2004) and high societal costs (Leistikow et al. 2000a, b), there is an urgent need for improved treatments for this condition. Functional brain imaging (in conjunction with other lines of research) holds great promise for elucidating both brain circuits and molecular targets that mediate the acute effects of cigarette smoking and the chronic effects of tobacco dependence. A greater understanding of brain function associated with smoking may result in improved pharmacological (and behavioral) interventions.
Many functional brain imaging studies of tobacco use and dependence have been performed, using four primary imaging modalities: (i) functional magnetic resonance imaging (fMRI), (ii) positron emission tomography (PET), (iii) single photon emission computed tomography (SPECT), and (iv) autoradiography. These imaging modalities have been used to determine relationships between brain function and the effects of acute and chronic cigarette smoking and of smoking-related behaviors. For this chapter, the MEDLINE database was searched using keywords for the four imaging techniques mentioned above, cross-referenced with the words “nicotine”, “cigarette”, and “tobacco.” Only data-driven functional imaging studies were included in this review, and reference lists within papers found on MEDLINE were also examined and relevant studies included here. In order to maintain focus in this chapter, functional imaging techniques that provide measures of blood flow and metabolism (which are closely related under normal conditions; Paulson 2002) are combined under the general heading of brain activity (including fMRI and certain types of SPECT, PET, and autoradiography studies). Also, in order to build a cohesive model of brain activity responses to acute and chronic smoking, nicotine and cigarette studies will be reviewed together while recognizing that cigarette smoke has many constituents other than nicotine (Baker et al. 2004; Fowles and Dybing 2003).
The purpose of this chapter is to synthesize findings from functional brain imaging studies of tobacco use and dependence, and present a coherent model of brain function in smokers. Acute brain responses to nicotine/smoking will be reviewed first, followed by chronic responses to nicotine/smoking, and concluding with a discussion of these imaging findings in the context of neuroanatomical work and the clinical effects of smoking in tobacco-dependent subjects.
2 Brain Function Responses to Acute Nicotine Administration and Cigarette Smoking
2.1 Brain Activity Responses to Nicotine/Cigarette Administration
Many functional brain imaging studies have been performed examining the effects of administration of nicotine or cigarette smoking compared with a placebo or control state (Table 1). Though a wide range of brain regions have been reported to have altered activity in response to nicotine or cigarette smoking, several global and regional findings have been replicated, leading to general conclusions about the acute effects of nicotine or smoking on brain activity.
Table 1.
Functional brain imaging studies of nicotine or cigarette administration
| Authors | Subjects | Method | Intervention | Results |
|---|---|---|---|---|
| Animal studies | ||||
| London et al. (1988a, b) | Rats | 2-Deoxy-D-[1–14C]glucose autoradiography | SC nic (0.1–1.75 mg kg−1) | ↑ Nicotine rich regions, including thal, cereb, visual system, others |
| Marenco et al. (2000) | Rats – chronically nic exposed vs. nic naive | 2-Deoxy-D-[1–14C] glucose autoradiography | SC nic (0.4 mg kg−1) vs. saline | ↑ Thal, superior colliculus in chronically exposed; ↑ thal, superior colliculus, medial habenula, and dorsal lateral geniculate in nic naive |
| Human studies | ||||
| Rourke et al. (1997) | 8 Smokers; 8former smokers; 17 nonsmokers |
Iodine-123 iodoamphetamine (IMP) SPECT |
Smokers smoked the morning of the scan; other groups did not | ↓ Cortical uptake of IMP (a measure of blood flow) in current smokers compared to other groups |
| Stein et al. (1998) | 16 Smokers | fMRI | IV nic (0.75–2.25 mg/70 kg wt) vs. placebo | ↑ R NAc and bilateral amyg, cingulate, frontal lobes, thal, others |
| Domino et al. (2000a) | 18 Smokers | 15O-PET | Nic nasal spray vs. pepper spray | ↑ Thal, pons, visual cortex, cereb |
| Domino et al. (2000b) | 11 Smokers | FDG-PET | Nic nasal spray vs. pepper spray | Small ↓ global; ↑ L IFG, L PC, R thal, visual cortex; ↓ normalized L ins and R inf occ ctx |
| Zubieta et al. (2001) | 18 Smokers | 15O-PET | Nic nasal spray vs. pepper spray | ↑ Anterior thal; ↓ L ant temp and R amyg |
| Rose et al. (2003) | 34 Smokers | 15O-PET | Cigarette vs. no nic control conditions | ↑ L frontal factor (incl. prefrontal and ACC), ↓ L amyg rCBF |
| Yamamoto et al. (2003) | 10 Smokers | 99mTc-ECD SPECT | Cigarette vs. abstinence | ↓ Global blood flow |
| Stapleton et al. (2003a) | 4 Smokers; two nonsmokers | 2 FDG-PETs (fully quantified) | IV nic (1.5 mg) vs. placebo | ↓ Global and most regions studied |
| Zubieta et al. (2005) | 19 smokers | 15O-PET | Nicotine containing vs. denicotinized cigarettes | ↓ Global blood flow |
| Staley et al. (2006) | 16 Smokers; 16 nonsmokers | 5 IA-SPECT | Recent abstinence | ↑ Striatum, parietal cortex, frontal cortex, anterior cingulated, temporal cortex, occipital cortex, cerebellum |
All regional changes represent normalized activity, unless otherwise stated. SC subcutaneous, nic nicotine, thal thalamus, cereb cerebellum, SPECT single photon emission computed tomography, fMRI functional magnetic resonance imaging, IV intravenous, R right, L left, NAc nucleus accumbens, amyg amygdala, FDG 18F-fluorodeoxyglucose, PET positron emission tomography, IFG inferior frontal gyrus, PC posterior cingulate, ins insula, inf occ ctx inferior occipital cortex, ant anterior, temp temporal lobe, ACC anterior cingulate cortex
One common finding is that nicotine administration (Domino et al. 2000b; Stapleton et al. 2003b) or cigarette smoking (Yamamoto et al. 2003) results in decreased global brain activity. Similarly, smokers who smoke ad lib prior to SPECT scanning (including the morning of the scan) have decreased global brain activity compared to former smokers and nonsmokers (Rourke et al. 1997). These findings are generally supported by studies using transcranial Doppler ultrasound or the Xe 133 inhalation method to measure responses to smoking, with some (Cruickshank et al. 1989; Kubota et al. 1983, 1987; Rogers et al. 1983), but not all (Kodaira et al. 1993; Terborg et al. 2002), studies showing diminished cerebral blood flow.
A large (n = 86), recent study (Fallon et al. 2004) further characterized this decreased global activity with nicotine administration. 18F-fluorodeoxyglucose (FDG) PET was performed while smokers and exsmokers performed the Bushman aggression task (designed to elicit an aggressive state) and wearing either a 0, 3.5-, or 21-mg nicotine patch. Smokers who were rated high on the personality trait hostility had widespread cerebral metabolic decreases while wearing the 21-mg patch and performing the aggression task. Low-hostility smokers did not have these changes during PET, suggesting that personality profile may determine which smokers have global metabolic decreases in response to nicotine.
In studies examining regional activity responses to nicotine or smoking, the most common findings are relative increases in activity in the prefrontal cortex (including the dorsolateral prefrontal cortex, and inferior frontal, medial frontal, and orbitofrontal gyri) (Domino et al. 2000b; Rose et al. 2003; Stein et al. 1998), thalamus (Domino et al. 2000a, b; London et al. 1988a, b; Stein et al. 1998; Zubieta et al. 2001), and visual system (Domino et al. 2000a, b; London et al. 1988a, b). Additionally, a Xe 133 inhalation study reported increases in frontal lobe and thalamic blood flow in smokers who smoked a cigarette (Nakamura et al. 2000). The human studies here examined cigarette smokers, while the animal studies here used non-dependent rats, with strong concordance of findings between these sets of studies. Functional brain imaging studies of nicotine or cigarette administration to human nonsmokers have not yet been reported, and would be important for a more complete understanding of the effects of tobacco on brain activity. While this group of studies demonstrates specific regional activation with nicotine or smoking, they also imply activation of cortico–basal ganglia–thalamic brain circuits (Alexander et al. 1990) that mediate the subjective effects of smoking (see Sect. 4). Zubieta et al. (2005) have conducted a 15O-PET study in 19 smokers using nicotine and denicotinized cigarettes, who were abstinent of smoking for 12 h before PET. In this study, increases in the regional cerebral blood flow (rCBF) in visual cortex and cerebellum, and reductions in rCBF in the anterior cingulate, the right hippocampus, and ventral striatum were found. Cigarette craving in chronic smokers also was correlated with rCBF in the right hippocampus, which is a region involved in associating environmental cues with drugs, and in the left dorsal anterior cingulate, an area implicated in drug craving and relapse to drug-seeking behavior.
Since regional activity was normalized to whole brain activity in at least some of these studies, and whole brain activity has been found to decrease with nicotine or cigarette administration, the regional findings presented here may represent either increased regional activity or, possibly, less of a decrease in regional activity than in other brain areas. Regional decreases in activity are generally not seen with nicotine or cigarette administration, though at least two studies found relatively decreased activity in the amygdala, left (Rose et al. 2003) and right (Zubieta et al. 2001)).
2.2 Effect of Nicotine on Brain Activation During Cognitive Tasks
There is evidence that nicotine administration improves performance on tasks that require vigilant attention in nicotine-dependent smokers (Newhouse et al. 2004). Nicotine administration also has been reported to improve reaction time, regardless of smoking status (Ernst et al. 2001a). Consistent with these findings are studies that demonstrate that acute abstinence from smoking (within 12 h) results in slowed response times (Bell et al. 1999; Gross et al. 1993; Thompson et al. 2002).
In examining brain mediation of the cognitive effects of smoking, several groups have performed functional imaging studies in subjects performing cognitive tasks during administration of nicotine (compared to a control condition) (Table 2). For most of these studies, subjects performed a cognitive task that involved visual recognition and working memory, such as the n-back task. Results of these studies have been somewhat mixed, showing both decreased (Ernst et al. 2001b; Ghatan et al. 1998) and increased (Jacobsen et al. 2004; Kumari et al. 2003) anterior cingulate cortex (ACC) activation in response to nicotine administration while performing the task. Brain activation responses to nicotine during cognitive tasks have been more consistent in other brain areas such as the thalamus (Jacobsen et al. 2004; Lawrence et al. 2002) and visual cortex (Ghatan et al. 1998; Lawrence et al. 2002), while nicotine had no effect on the visual cortex during photic stimulation (Jacobsen et al. 2002). This last finding indicates that nicotine activates the visual cortex only during demanding visual tasks, rather than on simple stimulation.
Table 2.
Functional brain imaging studies of nicotine or cigarette administration during cognitive tasks/stimulation
| Authors | Subjects | Method/task | Intervention | Effect of nicotine during task |
|---|---|---|---|---|
| Ghatan et al. (1998) | 12 Smokers; 6 nonsmokers |
15O-butanol PET/computerized maze |
IV nic infusion versus abstinence | ↓ ACC and cerebellum; ↑ occ ctx |
| Ernst et al. (2001b) | 11 Smokers; 11 former smokers | 15O-PET/2-back | Two pieces of 2-mg nic gum vs. placebo gum | ↓ ACC and PFC activation in smokers |
| Jacobsen et al. (2002) | 9 Smokers | fMRI/photic stimulation | IV nic 10 mcg kg−1 vs. saline | No effect on visual cortex |
| Lawrence et al. (2002) | 15 Smokers | fMRI/rapid visual information-processing | 21-mg nic vs. placebo patch | ↑ Parietal and occipital ctx,, thal, caudate |
| Kumari et al. (2003) | 11 Nonsmoking men | fMRI/n-back | SC nic (1 mg) vs. saline | ↑ ACC, superior frontal ctx, superior parietal ctx |
| Jacobsen et al. (2004) | 13 Schizophrenic smokers; 13 smokers | fMRI/n-back | 28- or 35-mg nic vs. placebo patch | ↑ ACC and bilateral thal activation (schizophrenic, nonschizophrenic) |
PET positron emission tomography, IV intravenous, nic nicotine, ACC anterior cingulate cortex, occ ctx occipital cortex, PFC prefrontal cortex, thal thalamus, fMRI functional magnetic resonance imaging
2.3 Brain Dopamine Responses to Nicotine and Smoking
A common pathway for the positive reinforcement associated with most, if not all, addictive drugs is the brain dopamine (DA) reward pathway (Koob 1992; Leshner and Koob 1999). Laboratory animal studies demonstrate that DA release in the ventral striatum (VST)/nucleus accumbens (NAc) underlies the reinforcing properties of nicotine (Koob 1992; Leshner and Koob 1999). Microdialysis (Damsma et al. 1989; Di Chiara and Imperato 1988; Pontieri et al. 1996; Sziraki et al. 2001) and lesion (Corrigall et al. 1992) studies in rats indicate that nicotine-induced DA release is strongest in this region, and is more robust than the DA release found in associated structures receiving dopaminergic input, such as the dorsal striatum (Di Chiara and Imperato 1988). These studies generally used nicotine dosages that simulated human cigarette smoking. Acute exposure to cigarette smoke and nicotine has been found to upregulate dopamine transporter mRNA in the ventral tegmental area (VTA) and substantia nigra (Li et al. 2004), and chronic exposure to cigarette smoke, more so than chronic nicotine alone, has also been found to upregulate D1 and D2 receptor mRNA in the VST (Bahk et al. 2002). Additionally, many in vitro studies of the VST have reported DA release in response to nicotine administration (Connelly and Littleton 1983; Marien et al. 1983; Rowell et al. 1987; Sakurai et al. 1982; Westfall et al. 1983).
Functional brain imaging studies of the DA system (Table 3) corroborate and expand upon these laboratory findings. Striatal DA release in response to a nicotine or cigarette challenge has been demonstrated repeatedly in both nonhuman primates and humans (Brody et al. 2004b, 2006; Dewey et al. 1999; Marenco et al. 2004; Tsukada et al. 2002), with most of these studies using PET and the radiotracer 11C-raclopride (a specific D2/D3 DA receptor binder) to demonstrate DA release through radiotracer displacement. These studies have reported a wide range of DA concentration change. In two studies that examined the question directly (Marenco et al. 2004; Tsukada et al. 2002), nicotine was found to result in less radiotracer displacement than amphetamine, while it has also been reported that nicotine-induced DA release is comparable in magnitude to that induced by other addictive drugs (Pontieri et al. 1996). Also, an association between 11C-raclopride displacement and the hedonic effects of smoking (defined as elation and euphoria) has been demonstrated (Barrett et al. 2004), though this study did not find an overall difference between the smoking and nonsmoking conditions. Thus, while most studies do provide evidence for nicotine/smoking-induced DA release, there are disparities between studies in the extent of human smoking-induced DA release, leaving this issue currently unresolved. Disparities between these studies may be due to differences in methodology (e.g., nicotine administration vs. cigarette smoking) and/or technical complexities in performing such studies. (As an aside, effects of smoking on dopamine projections to the prefrontal cortex (Goldman-Rakic et al. 1989) have not yet been reported with functional brain imaging.)
Table 3.
Functional imaging studies of the effects of nicotine or cigarette smoking on the dopamine (DA) system
| Authors | Subjects | Method | Intervention | Results/conclusions |
|---|---|---|---|---|
| Dewey et al. (1999) | 16 Baboons | 11C-Raclopride PET (double bolus) | IV nic (0.3 mg) | ↓ DV tracer (indicating ↑ DA concentration) in NAc |
| Dagher et al. (2001) | 11 Smokers; 18 nonsmokers | 11C-SCH 23390 PET | ↓ BP in smokers (indicating ↓ D1 receptor density) in ventral striatum | |
| Tsukada et al. (2002) | 4 Macada mulatto monkeys | 11C-Raclopride PET (B/I) | IV nic (B/I) | Slight ↓ BP (indicating ↑ DA concentration) in anesthetized, but not conscious monkeys, in dorsal striatum |
| Salokangas et al. (2000) | 9 Smokers; 10 nonsmokers | 18F-DOPA PET | ↑ Uptake (indicating ↑ DA activity) in cd and Put of smokers | |
| Krause et al. (2002) | 11 Smokers w/ADHD; 11 nonsmokers w/ADHD | [99mTc]TRODAT SPECT | ↓ DAT (striatal) in smokers | |
| Staley et al. (2001) | 21 Smokers; 21 nonsmokers | [123I] β-CIT SPECT | No overall binding difference between smokers and nonsmokers; ↑ brainstem 5-HT transporters in male smokers | |
| Marenco et al. (2004) | 5 Rhesus monkeys | 11C-Raclopride PET (double bolus and B/I) | IV nic (0.01–0.06 mg kg−1) | ↓ BP (indicating ↑ DA concentration) in basal ganglia with nic administration |
| Brody et al. (2004b) | 20 Smokers | 11C-Raclopride PET (B/I) | Single cigarette vs. no smoking | ↓ BP (indicating ↑ DA concentration) in smoking, but not no smoking, condition in L ventral cd and put |
| Barrett et al. (2004) | 10 Smokers | 11C-Raclopride PET (double bolus) | Smoking every 12 min vs. no smoking | ↓ BP correlated with hedonic response to smoking in cd and posterior put |
PET positron emission tomography, IV intravenous, nic nicotine, DV volume of distribution, DA dopamine, BP binding potential, B/I bolus-plus-infusion, cd caudate, put putamen, SPECT single photon emission computed tomography, DAT dopamine transporter, ADHD attention deficit hyperactivity disorder, β-CIT 2 β-carbomethoxy-3 β-(4-iodophenyl)-tropane, 5-HT serotonin
Nicotine-induced DA release in the NAc has been reported to be mediated by stimulation of nicotinic acetylcholine receptors (nAChRs) on cells of the VTA that project to the NAc rather than by nicotinic receptors within the NAc itself (Nisell et al. 1994). Lesioning of mesolimbic VTA neurons projecting to the NAc leads to decreased nicotine self-administration (Corrigall et al. 1992; Lanca et al. 2000). Additionally, the effects of nicotine on the dopaminergic system appear to be modulated by glutamatergic and GABAergic neurons (Picciotto and Corrigall 2002), with nicotine stimulation of gluatamatergic tracts from the prefrontal cortex to the VTA leading to increased DA neuron firing (Kenny and Markou 2001) and GABA agonism leading to a dampening of DA neuron responses (Cousins et al. 2002). Recent work indicates that nicotine administration causes prolonged depression of GABAergic firing, leading to relatively large excitatory (glutamatergic) input into the mesolimbic DA system and increased DA neuron firing (Mansvelder et al. 2002).
Other functional imaging studies of the DA system have reported decreased D1 receptor density (Dagher et al. 2001), increased 18F-DOPA uptake (a marker for increased DA turnover) (Salokangas et al. 2000), and both decreased (Krause et al. 2002) and no alterations (Staley et al. 2001) in dopamine transporter binding in smokers.
To summarize these studies of the DA system, there is extensive evidence that nicotine administration and smoking result in activation of the brain DA mesolimbic pathway, resulting in increased DA release and turnover in the VST/NAc. Because dopaminergic input to the NAc modulates neurotransmission through cortico–basal ganglia–thalamic circuitry (Haber and Fudge 1997), smoking-induced increases in DA concentration may explain some of the clinical effects of smoking, as discussed in Sect. 4.
2.4 Functional Imaging of Nicotinic Acetylcholine Receptors (nAChRs)
Because stimulation of nAChRs is intimately linked with the effects of smoking, a longstanding and still developing area of research is the labeling of nAChRs using functional brain imaging. Nicotinic acetylcholine receptors are ligand-gated ion channels consisting of α and β subunits (Court et al. 2000; Hogg et al. 2003). Many nAChRs have been identified, with the heteromeric α4β2 being the most common subtype in the brain and the homomeric α7 being the next most common. Postmortem (Benwell et al. 1988; Breese et al. 1997) and laboratory (Yates et al. 1995) studies demonstrate that smokers have widespread upregulation of nAChRs, likely related to desensitization of these receptors from nicotine exposure. Many animal studies also demonstrate upregulation of nAChRs in response to chronic nicotine administration (e.g., Pauly et al. 1996; Shoaib et al. 1997; Zhang et al. 2002). Thus, nAChRs are a natural target for tracer development in the pursuit of a greater understanding of tobacco dependence and other illnesses with abnormal nAChR levels.
Animal research demonstrates that nicotine binds to nAChRs in the brain to mediate a variety of behavioral states (Lukas 1998), such as heightened arousal and improved reaction time and psychomotor function (Paterson and Nordberg 2000). Nicotine administration also produces reward through DA release in the NAc, at least in part through stimulation of nAChRs in the VTA (Blaha et al. 1996; Corrigall et al. 1994; Nisell et al. 1994; Yeomans and Baptista 1997; Yoshida et al. 1993). Nicotinic acetylcholine receptors are widespread throughout the brain, with a rank order distribution of nAChR density being thalamus > basal ganglia > cerebral cortex > hippocampus > cerebellum (Broussolle et al. 1989; Cimino et al. 1992; Clarke et al. 1984; Davila-Garcia et al. 1999, 1997; London et al. 1985, 1995; Pabreza et al. 1991; Pauly et al. 1989; Perry and Kellar 1995; Valette et al. 1998; Villemagne et al. 1997).
Radiotracers for the nAChR have been developed in recent years, with labeled A-85380 (3-(2(S)-azetidinylmethoxy pyridine) (Koren et al. 1998) compounds having the most widespread use. Radiolabeling of A-85380 was a major advance in imaging nAChRs, because administration of radiolabeled nicotine (used for previous imaging studies) resulted in high nonspecific binding and short drug–receptor interaction times (Sihver et al. 2000). 2-[18F]F-A-85380 or simply 2-FA and related compounds (Chefer et al. 1999; Horti et al. 1998; Koren et al. 1998) are being used for PET imaging, and 5-[123/125I]iodo-A85380 is being used for SPECT imaging (Chefer et al. 1998; Horti et al. 1999; Mukhin et al. 2000) of α4β2 nAChRs.
Studies of nonhuman primates and humans have examined distributions of nAChRs with these new radiotracers, and found regional densities of these receptors similar to those in the animal work cited above (Chefer et al. 2003, 1999; Fujita et al. 2002, 2003; Kimes et al. 2003; Valette et al. 1999). Two recent studies on baboons examined effects of nicotine or tobacco smoke on nAChR availability. In a 2-FA PET study (Valette et al. 2003), IV nicotine (0.6 mg), inhalation of tobacco smoke from one cigarette (0.9 mg nicotine), and IV nornicotine were all found to reduce the volume of distribution of the tracer by roughly 30–60% in the thalamus and putamen at 80 min, and this reduction of 2-FA binding was relatively long lived (up to 6 h). Similarly, a 50% reduction in nAChR availability was found with IV nicotine administration to baboons using an epibatidine analog and PET scanning (Ding et al. 2000). Taken together, these studies demonstrate that radiotracers for nAChRs can be administered safely to measure nAChR densities, and that nicotine and smoking substantially decrease α4β2 nAChR availability.
In a recent study (Brody et al. 2006), human cigarette smokers were studied using 2-FA and PET scanning. In this study, only one to two puffs of a cigarette resulted in 50% occupancy of brain α4β2 nAChRs, and this occupancy lasted for at least 3.1 h after smoking. Smoking a full cigarette resulted in 88% occupancy, and was accompanied by a reduction in cigarette craving. Binding of nicotine to α4β2 nAChR causes desensitization of these receptors, and this 2-FA PET study indicated that smoking may lead to withdrawal alleviation by maintaining nAChRs in the desensitized state.
[123 I]5-IA or simply 5-I-A is a SPECT radioligand that binds to β2nAChRs. In a recent study, Staley et al. (2006) hypothesized that an abnormally high number of β2nAChRs in early abstinence may be responsible for continued tobacco usage. In this study, 16 smokers and 16 nonsmokers underwent 5-I-A SPECT scanning. Smokers were imaged in the abstinent phase, 7 days after their last cigarette. Each group consisted of seven men and nine women who were matched for age. Women smokers and nonsmokers were also matched by phase of menstrual cycle. Smokers quit cigarettes with brief behavioral counseling, and no medication was used for smoking cessation. In this study, recently abstinent smokers were found to have significantly higher 5-I-A uptake in the striatum, parietal cortex, frontal cortex, anterior cingulate, temporal cortex, occipital cortex, and cerebellum, which suggests that smoking upregulates the number of β2nAChRs.
2.5 Glutamatergic (and Other) Effects of Nicotine/Cigarette Smoking
Recent autoradiography studies of rodents have examined the effects of nicotine/smoking in other neurotransmitter systems that may be activated by nAChR stimulation. For example, in response to nicotine, glutamate release has been demonstrated in the prelimbic prefrontal cortex (Gioanni et al. 1999), and glutamate and aspartate release have been demonstrated in the VTA (Schilstrom et al. 2000). The finding of nAChR-induced glutamate release in the prefrontal cortex has also been demonstrated by measuring spontaneous excitatory postsynaptic currents (Lambe et al. 2003). Importantly, one of these studies (Gioanni et al. 1999) also demonstrated that nicotine administration facilitates thalamo-cortical neurotransmission through stimulation of nAChRs on glutamatergic neurons.
3 Brain Function Responses to Chronic Nicotine Administration and Cigarette Smoking
3.1 Functional Brain Imaging of Cigarette Craving
As for brain imaging studies of chronic tobacco/nicotine dependence, cigarette smokers experience craving for cigarettes (urge to smoke) within minutes after the last cigarette, and the intensity of craving rises over the next 3–6 h (Jarvik et al. 2000; Schuh and Stitzer 1995). Cigarette-related cues have been shown to reliably enhance craving during this period, compared to neutral cues (Carter and Tiffany 1999).
Two studies used a cigarette versus neutral cue paradigm paired with functional imaging to evaluate brain mediation of cigarette craving. In one study (Due et al. 2002), six smokers and six nonsmokers underwent event-related fMRI when presented with smoking-related images (color photographs) compared with neutral images, for 4 s each. For the smoker group, craving increased during the testing session and exposure to smoking-related images resulted in activation of mesolimbic (right posterior amygdala, posterior hippocampus, VTA, and medial thalamus) and visuospatial cortical attention (bilateral prefrontal and parietal cortex and right fusiform gyrus) circuitry, whereas the nonsmoker group did not have these changes. In the second study (Brody et al. 2002), 20 smokers and 20 nonsmokers underwent two FDG–PET sessions. For one PET session, subjects held a cigarette and watched a cigarette-related video, while for the other, subjects held a pen and watched a nature video (randomized order) during the 30-min uptake period of FDG. When presented with smoking-related (compared to neutral) cues, smokers had higher regional metabolism in bilateral (ACC), left orbitofrontal cortex (OFC), and left anterior temporal lobe. Change in craving scores was also positively correlated with change in metabolism in the OFC, dorsolateral prefrontal cortex, and anterior insula bilaterally.
Taken together, these studies of cigarette craving indicate that immediate responses to visual smoking-related cues (fMRI study) activate the brain reward system, limbic regions, and the visual processing system, while longer exposure to cues (FDG–PET study) leads to activation of the ACC, which mediates anxiety, alertness, and arousal (Chua et al. 1999; Critchley et al. 2001; Kimbrell et al. 1999; Naito et al. 2000; Rauch et al. 1999) and the OFC, which functions in part as a secondary processing center for sensory information (Rolls et al. 1998; Rolls and Baylis 1994).
In a related preliminary study, 17 smokers underwent the same FDG–PET craving versus neutral cue protocol as in the second study of craving listed above (Brody et al. 2002) after treatment with a standard course of bupropion HCl (tapered up to 150 mg orally twice a day for a mean of 5.6 weeks). This group of treated subjects had a significant reduction in smoking levels from pre- to post-treatment (mean 27.1 down to 3.7 cigarettes per day). These treated smokers also had reduced cigarette cue-induced craving and diminished ACC activation when presented with cigarette-related cues, compared to untreated smokers (Brody et al. 2004a). This diminished ACC activation was due to elevated baseline-normalized ACC activity in treated smokers, giving an indication that bupropion treatment of smokers increases resting ACC metabolism.
A more recent study examined (Brody et al. 2007) brain activation during resistance of the urge to smoke when smokers were presented with cigarette-related cues. In this study, activation was found in the cigarette cue resist condition compared with the cigarette cue crave condition in the left dorsal ACC, posterior cingulate cortex (PCC), and precuneus. Other findings of this study include lower magnetic resonance signal for the cigarette cue resist in the cuneus bilaterally, left lateral occipital gyrus, and right postcentral gyrus. These activations and deactivations were stronger when the cigarette cue resist condition was compared with the neutral cue condition. The urge to smoke scale (craving) score had positive correlations with MR signal in the medial aspect of superior frontal gyrus, supramarginal gyrus, precuneus, inferior frontal gyrus/anterior insula, bilateral corpus callosum, left precentral gyrus, putamen, and middle frontal gyrus, and right lingual gyrus extending to the fusiform gyrus. Negative correlations were found for the cuneus, left occipital gyrus, anterior temporal lobe, postcentral gyrus, insula, and right angular gyrus. This study concludes that active suppression of craving during cigarette cue exposure is associated with activation of limbic and related brain regions and deactivation of primary sensory and motor cortices.
3.2 Functional Brain Imaging of Cigarette Withdrawal
Abstinence-induced changes have also been studied (McClernon et al. 2005) in 13 dependent smokers using event-related fMRI. FMRI images were taken after usual smoking and following overnight abstinence. Self-reported craving measures were also conducted before, during, and after scanning. Results revealed larger hemodynamic responses to smoking compared to control cues in ventral anterior cingulate gyrus and superior frontal gyrus. Results show that brain responses to smoking cues, while relatively stable at the group level following short-term abstinence, may be modulated by individual differences in craving in response to abstinence, particularly in regions subserving attention and motivation.
Rose et al. (2007) also studied smokers (n = 15) with functional brain imaging following treatment for nicotine dependence. In this study, subjects were given nicotine patches and denicotinized cigarettes. PET scans were obtained at baseline, after 2 weeks of nicotine patch and denicotinized cigarettes, and 2 weeks after patients returned back to smoking. Craving of cigarettes was lower at the second session compared to the other two. After 2 weeks’ exposure to nicotine patches and denicotinized cigarettes, the authors found decreased brain metabolic activity in the right hemisphere anterior cingulate cortex.
Brain activity changes (measured with fMRI) during cigarette withdrawal were recently reported for nicotine-dependent rats (Shoaib et al. 2004). In this study, subcutaneous mecamylamine (1 mg kg−1), a nicotine receptor antagonist, was administered to precipitate withdrawal during scanning, and this state was compared to a control state after subcutaneous saline administration. After subcutaneous mecamy-lamine, nicotine-dependent rats had bilateral increases in NAc activity compared to the control state.
3.3 Monoamine Oxidase (MAO) Function in Smokers
Fowler and colleagues have performed a series of important studies demonstrating decreases in MAO A and B activity in cigarette smokers using the PET tracers [11C]clorgyline (Fowler et al. 1996b) and [11C]L-deprenyl-D2 (Fowler et al. 1996a, 1998b), respectively. When compared to former smokers and nonsmokers, average reductions for current smokers are 30 and 40% for MAO A and B (Fowler et al. 2003a). These reductions were the result of chronic smoking behavior rather than a single administration of intravenous nicotine (Fowler et al. 1998a) or smoking a single cigarette (Fowler et al. 1999, 2000, 2005), and are less than those seen with antidepressant MAO inhibitors (Fowler et al. 1994, 1996b). MAO A levels were found to be reduced up to 50% in peripheral organs (heart, lungs, and kidneys) in smokers when compared to nonsmokers. Additionally, a human postmortem study of chronic smokers demonstrated a modest reduction in MAO A binding that did not reach statistical significance (Klimek et al. 2001). Peripheral MAO B is also reduced in cigarette smokers (Fowler et al. 2003b).
MAO participates in the catabolism of dopamine, norepinephrine, and serotonin (Berlin and Anthenelli 2001; Fowler et al. 2003a), and it has been postulated that some of the clinical effects of smoking are due to MAO inhibition, leading to decreases in monoamine breakdown with a subsequent increase in monoamine availability (Berlin and Anthenelli 2001). Thus, smoking may enhance DA availability and the rewarding properties of smoking both through DA release (as described above) and MAO inhibition. Smoking may also alter mood and anxiety through MAO inhibition effects on norepinephrine and serotonin availability and turnover. Comprehensive reviews of the role of MAO in tobacco dependence have recently been published (Berlin and Anthenelli 2001; Fowler et al. 2003a).
4 Discussion: Functional Neuroanatomy of Tobacco Use and Dependence
Both acute and chronic effects of nicotine/cigarette exposure have been elucidated with functional brain imaging. Replicated responses to acute administration of nicotine/smoking include a reduction in global brain activity (perhaps most prominently in smokers with high levels of hostility as a personality trait); activation of the prefrontal cortex, thalamus, and visual system; activation of the thalamus and visual cortex (and possibly ACC) during visual cognitive tasks; and increased DA concentration in the ventral striatum/NAc. Replicated responses to chronic nicotine/cigarette exposure include decreased MAO A and B activity and a substantial reduction in α4β2 nAChR availability in the thalamus and putamen (accompanied by an overall upregulation of these receptors).
This group of findings demonstrates a number of ways in which smoking might enhance neurotransmission through cortico–basal ganglia–thalamic circuits (Alexander et al. 1990), in addition to demonstrating direct effects of chronic nicotine exposure on nAChR availability (Fig. 1). Given that the thalamus (Groenewegen et al. 1999; Herrero et al. 2002; Sommer 2003) and ventral striatum/NAc (Groenewegen et al. 1999; Herrero et al. 2002) function as relay centers for information and for paralimbic and motor processing in the brain, the net effect of smoking may be to enhance neurotransmission along cortico–basal ganglia–thalamic loops originating in the paralimbic cortex. Neurotransmission through these circuits may be stimulated directly by the interconnected (Sherman 2001; Sillito and Jones 2002) nAChR-rich thalamus and visual systems, and/or indirectly through effects on MAO inhibition and DA release in the ventral striatum/NAc, as well as through nicotine stimulation of excitatory glutamatergic input to the dopaminergic system (Mansvelder et al. 2002). In the thalamus, for example, nicotine has direct agonist action on excitatory thalamocortical projection neurons and local circuit neurons, although nicotine also stimulates GABAergic interneurons, so that the relationship between nicotine stimulation and thalamocortical stimulation may be complex (Clarke 2004). There is mixed evidence as to whether or not nicotine stimulates corticothalamic neurons (Clarke 2004).
Fig. 1.
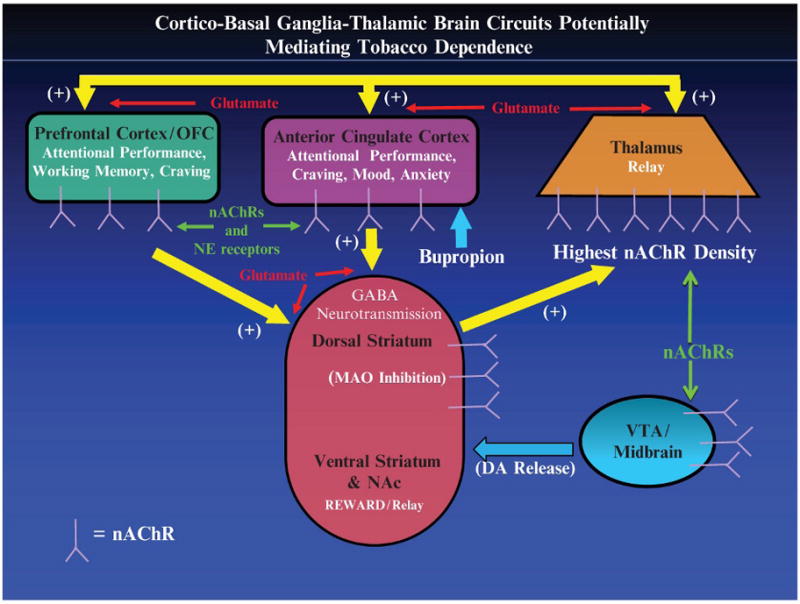
Representation of the cortico–basal ganglia–thalamic brain circuitry that may mediate the effects of nicotine/smoking on attentional control, craving, mood, and anxiety. Potential targets for nicotine/smoking to enhance attention (and improve craving, mood, and anxiety) include (1) direct stimulation of nicotinic acetylcholine receptors (nAChRs) in cortex, (2) stimulation of the nAChR-rich thalamus and basal ganglia, (3) activation of dopaminergic mesolimbic reward pathways originating in the VTA and projecting to the striatum, and (4) monoamine oxidase (MAO) inhibition in the basal ganglia. NAc nucleus accumbens; VTA ventral tegmental area
Enhancement of neurotransmission through prefrontal and paralimbic cortico–basal ganglia–thalamic circuits may account for the most commonly reported cognitive effect of cigarette smoking, namely, improved attentional performance (Newhouse et al. 2004), and also related effects, such as improvements in reaction times (Hatsukami et al. 1989; Pritchard et al. 1992; Shiffman et al. 1995), arousal (Parrott and Kaye 1999), motivation (Powell et al. 2002), and sustained attention (Rusted et al. 2000). Prefrontal (including both dorsolateral and ventrolateral) (Duncan and Owen 2000; Rees and Lavie 2001; Smith and Jonides 1999) and ACC (Carter et al. 1999; Duncan and Owen 2000; Peterson et al. 1999; Smith and Jonides 1999) cortices are reported to activate during attentional control tasks (especially visuospatial tasks) (Pessoa et al. 2003). Cigarette smoking may enhance attentional control through direct stimulation of nAChRs within these structures or perhaps through subcortical stimulation of nAChRs in the thalamus and via DA release and/or MAO inhibition in the basal ganglia.
In addition to improvement in attention, smoking improves withdrawal symptoms, such as depressed mood, anxiety, and irritability in tobacco-dependent smokers (Cohen et al. 1991; Parrott 2003), and all these effects depend (at least in part) on the expectations of the smoker (Perkins et al. 2003). Though nicotine administration generally results in increased activity along prefrontal and paralimbic brain circuits, it is interesting that both increased and decreased ACC activation during cognitive task performance has been reported (see Sect. 2.2). ACC activity has been associated with anxiety and mood, with increased activity being associated with greater anxiety (Chua et al. 1999; Kimbrell et al. 1999) and decreased activity being associated with depressed mood (Drevets et al. 1997). This combination of findings suggests a potential interaction between expectation of the effects of smoking (e.g., mood improvement, anxiety reduction, or decreased irritability) and direction of ACC activity change during cognitively demanding tasks. Perhaps smokers who expect to and do have anxiety alleviation from smoking have deactivation or decreased activation of the ACC while performing cognitive tasks, whereas those who expect to and do experience mood improvement from smoking have increased activation of the ACC.
In addition to these primary effects of nicotine and smoking, other functional imaging studies reviewed here focus on smoking-related states, such as cue-induced cigarette craving. Such studies are part of a large body of literature examining cue-induced craving for addictive drugs. Studies specific for cigarette cues/craving reveal that exposure to visual cigarette cues immediately activates mesolimbic (VTA, amygdala, and hippocampus) and visuospatial cortical attention areas of the brain, and acutely (over a 30-min period) activate paralimbic regions (ACC and OFC), and that this cue-induced activation may be diminished by a course of bupropion treatment. These results are similar to those of functional imaging studies for drugs other than tobacco (Goldstein and Volkow 2002; Miller and Goldsmith 2001), and it has been posited that at least some of the activations seen with cigarette-related cues (cortical attention areas and OFC) are associated with an expectation of smoking in the nontreatment-seeking subjects who participated in these studies (Wilson et al. 2004).
5 Future Directions
New radioligands are in development for nAChRs. Currently, 2-FA, 6-FA, and 5-I-A radiotracers are available, which have affinity to bind to the α4β2 nAChR subtype. Other radiotracers are in development for this subtype, but there is need for radioligands for imaging of other subtypes of nicotinic receptors, including the α7 subtype, which is abundant in humans. Future research is likely to focus on radioligands for imaging α4β2 nAChR in the thalamus with faster kinetics than 2-FA, 6-FA, and 5-I-A. Radiolabeled antagonists for imaging of α4β2 nAChR may prove very beneficial for greater understanding of receptor binding and ultimately in development of pharmacological agents to help with quitting smoking (Pomper et al. 2005; Horti et al. 2006).
New treatments are being discovered for smoking cessation, and the Food and Drug Administration has recently approved varenicline, which is a partial nAChR agonist and antagonist. The agonist effect is caused by binding to nicotinic receptors and stimulating receptor-mediated activity. The antagonist effect occurs when varenicline blocks the ability of nicotine to activate nicotinic receptors. Imaging studies with varenicline may tell us more about nicotine dependence and the role of the α4β2 nicotine receptor.
References
- Alexander GE, Crutcher MD, DeLong MR. Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Prog Brain Res. 1990;85:119–146. [PubMed] [Google Scholar]
- Bahk JY, Li SP, Park MS, Kim MO. Dopamine D-1 and D-2 receptor mRNA up-regulation in the caudate-putamen and nucleus accumbens of rat brains by smoking. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:1095–1104. doi: 10.1016/s0278-5846(02)00243-9. [DOI] [PubMed] [Google Scholar]
- Baker RR, Massey ED, Smith G. An overview of the effects of tobacco ingredients on smoke chemistry and toxicity. Food Chem Toxicol. 2004;42(Suppl):S53–S83. doi: 10.1016/j.fct.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Balluz L, Ahluwalia IB, Murphy W, Mokdad A, Giles W, Harris VB. Surveillance for certain health behaviors among selected local areas–United States, Behavioral Risk Factor Surveillance System, 2002. MMWR Surveill Summ. 2004;53:1–100. [PubMed] [Google Scholar]
- Barrett SP, Boileau I, Okker J, Pihl RO, Dagher A. The hedonic response to cigarette smoking is proportional to dopamine release in the human striatum as measured by positron emission tomography and [11C]raclopride. Synapse. 2004;54:65–71. doi: 10.1002/syn.20066. [DOI] [PubMed] [Google Scholar]
- Bartal M. Health effects of tobacco use and exposure. Monaldi Arch Chest Dis. 2001;56:545–554. [PubMed] [Google Scholar]
- Bell SL, Taylor RC, Singleton EG, Henningfield JE, Heishman SJ. Smoking after nicotine deprivation enhances cognitive performance and decreases tobacco craving in drug abusers. Nicotine Tob Res. 1999;1:45–52. doi: 10.1080/14622299050011141. [DOI] [PubMed] [Google Scholar]
- Benwell ME, Balfour DJK, Anderson JM. Evidence that tobacco smoking increases the density of (−)-[3H]nicotine binding sites in human brain. J Neurochem. 1988;50:1243–1247. doi: 10.1111/j.1471-4159.1988.tb10600.x. [DOI] [PubMed] [Google Scholar]
- Berlin I, Anthenelli RM. Monoamine oxidases and tobacco smoking. Int J Neuropsychopharmacol. 2001;4:33–42. doi: 10.1017/S1461145701002188. [DOI] [PubMed] [Google Scholar]
- Blaha CD, Allen LF, Das S, Inglis WL, Latimer MP, Vincent SR, Winn P. Modulation of dopamine efflux in the nucleus accumbens after cholinergic stimulation of the ventral tegmental area in intact, pedunculopontine tegmental nucleus-lesioned, and laterodorsal tegmental nucleus-lesioned rats. J Neurosci. 1996;16:714–722. doi: 10.1523/JNEUROSCI.16-02-00714.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese CR, Marks MJ, Logel J, Adams CE, Sullivan B, Collins AC, Leonard S. Effect of smoking history on [3H]nicotine binding in human postmortem brain. J Pharmacol Exp Ther. 1997;282:7–13. [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, London ED, Childress AR, Bota RG, Ho ML, Lee GS, Saxena S, Baxter LR, Madsen D, Jarvik ME. Brain metabolic changes during cigarette craving. Arch Gen Psychiatry. 2002;59:1162–1172. doi: 10.1001/archpsyc.59.12.1162. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Lee G, Smith E, Sadeghi M, Saxena S, Jarvik ME, London ED. Attenuation of cue-induced cigarette craving and anterior cingulate cortex activation in bupropion-treated smokers: a preliminary study. Psych Res Neuroimaging. 2004a;130:269–281. doi: 10.1016/j.pscychresns.2003.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, Olmstead RE, London ED, Farahi J, Meyer JH, Grossman P, Lee GS, Huang J, Hahn EL, Mandelkern MA. Smoking-induced ventral striatum dopamine release. Am J Psychiatry. 2004b;161:1211–1218. doi: 10.1176/appi.ajp.161.7.1211. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, London ED, Olmstead RE, Farahi J, Scheibal D, Jou J, Allen V, Tiongson E, Chefer SI, Koren AO, Mukhin AG. Cigarette smoking saturates brain alpha 4 beta 2 nicotinic acetylcholine receptors. Arch Gen Psychiatry. 2006;63:907–915. doi: 10.1001/archpsyc.63.8.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Olmstead RE, Jou J, Tiongson E, Allen V, Scheibal D, London ED, Monterosso JR, Tiffany ST, Korb A, Gan JJ, Cohen MS. Neural substrates of resisting craving during cigarette cue exposure. Biol Psychiatry. 2007;62:642–651. doi: 10.1016/j.biopsych.2006.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broussolle EP, Wong D, Fanelli RJ, London ED. In vivo specific binding of [3H]-nicotine in the mouse brain. Life Sci. 1989;44:1123–1132. doi: 10.1016/0024-3205(89)90340-8. [DOI] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94:327–340. [PubMed] [Google Scholar]
- Carter CS, Botvinick MM, Cohen JD. The contribution of the anterior cingulate cortex to executive processes in cognition. Rev Neurosci. 1999;10:49–57. doi: 10.1515/revneuro.1999.10.1.49. [DOI] [PubMed] [Google Scholar]
- Chefer SI, Horti AG, Lee K, Koren A, Jones DW, Gorey J, Links JM, Mukhin AG, Weinberger DR, London ED. In vivo imaging of brain nicotinic receptors with 5-[123I]iodo-A-85380 using single photon emission computed tomography. Life Sci. 1998;63:PL355–PL360. doi: 10.1016/s0024-3205(98)00514-1. [DOI] [PubMed] [Google Scholar]
- Chefer SI, Horti AG, Koren AO, Gündrisch D, Links JM, Kurian V, Dannals RF, Mukhin AG, London ED. 2-[18F]F-A-83580: a PET radioligand for α4β2 nicotinic acetylcholine receptors. Neuroreport. 1999;10:2715–2721. doi: 10.1097/00001756-199909090-00005. [DOI] [PubMed] [Google Scholar]
- Chefer SI, London ED, Koren AO, Pavlova OA, Kurian V, Kimes AS, Horti AG, Mukhin AG. Graphical analysis of 2-[F-18]FA binding to nicotinic acetylcholine receptors in rhesus monkey brain. Synapse. 2003;48:25–34. doi: 10.1002/syn.10180. [DOI] [PubMed] [Google Scholar]
- Chua P, Krams M, Toni I, Passingham R, Dolan R. A functional anatomy of anticipatory anxiety. Neuroimage. 1999;9:563–571. doi: 10.1006/nimg.1999.0407. [DOI] [PubMed] [Google Scholar]
- Cimino M, Marini P, Fornasari D, Cattabeni F, Clementi F. Distribution of nicotinic receptors in cynomolgus monkey brain and ganglia: localization of alpha 3 subunit mRNA, alpha-bungarotoxin and nicotine binding sites. Neuroscience. 1992;51:77–86. doi: 10.1016/0306-4522(92)90472-e. [DOI] [PubMed] [Google Scholar]
- Clarke PBS. Nicotinic modulation of thalamocortical neurotransmission. Acetylcholine in the cerebral cortex. Prog Brain Res. 2004;145:253–260. doi: 10.1016/S0079-6123(03)45017-6. [DOI] [PubMed] [Google Scholar]
- Clarke PBS, Pert C, Pert A. Autoradiographic distribution of nicotine receptors in rat brain. Brain Res. 1984;323:390–395. doi: 10.1016/0006-8993(84)90320-2. [DOI] [PubMed] [Google Scholar]
- Cohen C, Pickworth WB, Henningfield JE. Cigarette smoking and addiction. Clin Chest Med. 1991;12:701–710. [PubMed] [Google Scholar]
- Connelly MS, Littleton JM. Lack of stereoselectivity in ability of nicotine to release dopamine from rat synaptosomal preparations. J Neurochem. 1983;41:1297–1302. doi: 10.1111/j.1471-4159.1983.tb00824.x. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Franklin KB, Coen KM, Clarke PB. The mesolimbic dopaminergic system is implicated in the reinforcing effects of nicotine. Psychopharmacology. 1992;107:285–289. doi: 10.1007/BF02245149. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM, Adamson KL. Self-administered nicotine activates the mesolimbic dopamine system through the ventral tegmental area. Brain Res. 1994;653:278–284. doi: 10.1016/0006-8993(94)90401-4. [DOI] [PubMed] [Google Scholar]
- Court JA, Martin-Ruiz C, Graham A, Perry E. Nicotinic receptors in human brain: topography and pathology. J Chem Neuroanat. 2000;20:281–298. doi: 10.1016/s0891-0618(00)00110-1. [DOI] [PubMed] [Google Scholar]
- Cousins MS, Roberts DC, de Wit H. GABA(B) receptor agonists for the treatment of drug addiction: a review of recent findings. Drug Alcohol Depend. 2002;65:209–220. doi: 10.1016/s0376-8716(01)00163-6. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Dolan RJ. Neural activity in the human brain relating to uncertainty and arousal during anticipation. Neuroimage. 2001;13:S392. doi: 10.1016/s0896-6273(01)00225-2. [DOI] [PubMed] [Google Scholar]
- Cruickshank JM, Neildwyer G, Dorrance DE, Hayes Y, Patel S. Acute effects of smoking on blood-pressure and cerebral blood-flow. J Hum Hypertension. 1989;3:443–449. [PubMed] [Google Scholar]
- Dagher A, Bleicher C, Aston JAD, Gunn RN, Clarke PBS, Cumming P. Reduced dopamine D1 receptor binding in the ventral striatum of cigarette smokers. Synapse. 2001;42:48–53. doi: 10.1002/syn.1098. [DOI] [PubMed] [Google Scholar]
- Damsma G, Day J, Fibiger HC. Lack of tolerance to nicotine-induced dopamine release in the nucleus accumbens. Eur J Pharmacol. 1989;168:363–368. doi: 10.1016/0014-2999(89)90798-x. [DOI] [PubMed] [Google Scholar]
- Dávila-García MI, Musachio J, Perry D, Xiao Y, Horti A, London E, Dannals RF, Kellar K. [125I]IPH, an epibatidine analog, binds with high affinity to neuronal nicotinic cholinergic receptors. J Pharmacol Exp Ther. 1997;282:445–451. [PubMed] [Google Scholar]
- Davila-Garcia MI, Houghtling RA, Qasba SS, Kellar KJ. Nicotinic receptor binding sites in rat primary neuronal cells in culture: characterization and their regulation by chronic nicotine. Mol Brain Res. 1999;66:14–23. doi: 10.1016/s0169-328x(98)00344-1. [DOI] [PubMed] [Google Scholar]
- Dewey SL, Brodie JD, Gerasimov M, Horan B, Gardner EL, Ashby CRJ. A pharmacologic strategy for the treatment of nicotine addiction. Synapse. 1999;31:76–86. doi: 10.1002/(SICI)1098-2396(199901)31:1<76::AID-SYN10>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding YS, Volkow ND, Logan J, Garza V, Pappas N, King P, Fowler JS. Occupancy of brain nicotinic acetylcholine receptors by nicotine doses equivalent to those obtained when smoking a cigarette. Synapse. 2000;35:234–237. doi: 10.1002/(SICI)1098-2396(20000301)35:3<234::AID-SYN9>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Domino EF, Minoshima S, Guthrie S, Ohl L, Ni L, Koeppe RA, Zubieta JK. Nicotine effects on regional cerebral blood flow in awake, resting tobacco smokers. Synapse. 2000a;38:313–321. doi: 10.1002/1098-2396(20001201)38:3<313::AID-SYN10>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Domino EF, Minoshima S, Guthrie SK, Ohl L, Ni L, Koeppe RA, Cross DJ, Zubieta J. Effects of nicotine on regional cerebral glucose metabolism in awake resting tobacco smokers. Neuroscience. 2000b;101:277–282. doi: 10.1016/s0306-4522(00)00357-2. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Simpson JR, Jr, Todd RD, Reich T, Vannier M, Raichle ME. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- Due DL, Huettel SA, Hall WG, Rubin DC. Activation in mesolimbic and visuospatial neural circuits elicited by smoking cues: evidence from functional magnetic resonance imaging. Am J Psychiatry. 2002;159:954–960. doi: 10.1176/appi.ajp.159.6.954. [DOI] [PubMed] [Google Scholar]
- Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000;23:475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- Ernst M, Heishman SJ, Spurgeon L, London ED. Smoking history and nicotine effects on cognitive performance. Neuropsychopharmacology. 2001a;25:313–319. doi: 10.1016/S0893-133X(01)00257-3. [DOI] [PubMed] [Google Scholar]
- Ernst M, Matochik JA, Heishman SJ, Van Horn JD, Jons PH, Henningfield JE, London ED. Effect of nicotine on brain activation during performance of a working memory task. Proc Natl Acad Sci USA. 2001b;98:4728–4733. doi: 10.1073/pnas.061369098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon JH, Keator DB, Mbogori J, Turner J, Potkin SG. Hostility differentiates the brain metabolic effects of nicotine. Brain Res Cogn Brain Res. 2004;18:142–148. doi: 10.1016/j.cogbrainres.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Fiore MC, Bailey WC, Cohen SJ, Dorfman SF, Goldstein MG, Gritz ER, Heyman RB, Jaen CR, Kottke TE, Lando HA, Mecklenburg RE, Mullen PD, Nett LM, Robinson L, Stitzer ML, Tommasello AC, Villejo L, Wewers ME. Clinical Practice Guideline. U.S. Department of Health and Human Services. Public Health Service; Rockville, MD: 2000. Treating tobacco use and dependence. [Google Scholar]
- Fowler JS, Volkow ND, Logan J, Wang GJ, MacGregor RR, Schyler D, Wolf AP, Pappas N, Alexoff D, Shea C. Slow recovery of human brain MAO B after L-deprenyl (Selege-line) withdrawal. Synapse. 1994;18:86–93. doi: 10.1002/syn.890180203. [DOI] [PubMed] [Google Scholar]
- Fowler JS, Volkow ND, Wang GJ, Pappas N, Logan J, MacGregor R, Alexoff D, Shea C, Schlyer D, Wolf AP, Warner D, Zezulkova I, Cilento R. Inhibition of monoamine oxidase B in the brains of smokers. Nature. 1996a;379:733–736. doi: 10.1038/379733a0. [DOI] [PubMed] [Google Scholar]
- Fowler JS, Volkow ND, Wang GJ, Pappas N, Logan J, Shea C, Alexoff D, MacGregor RR, Schlyer DJ, Zezulkova I, Wolf AP. Brain monoamine oxidase A inhibition in cigarette smokers. Proc Natl Acad Sci USA. 1996b;93:14065–14069. doi: 10.1073/pnas.93.24.14065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler JS, Volkow ND, Logan J, Pappas N, King P, MacGregor R, Shea C, Garza V, Gatley SJ. An acute dose of nicotine does not inhibit MAO B in baboon brain in vivo. Life Sci. 1998a;63:L19–L23. doi: 10.1016/s0024-3205(98)00251-3. [DOI] [PubMed] [Google Scholar]
- Fowler JS, Volkow ND, Wang GJ, Pappas N, Logan J, MacGregor R, Alexoff D, Wolf AP, Warner D, Cilento R, Zezulkova I. Neuropharmacological actions of cigarette smoke: brain monoamine oxidase B (MAO B) inhibition. J Addict Dis. 1998b;17:23–34. doi: 10.1300/J069v17n01_03. [DOI] [PubMed] [Google Scholar]
- Fowler JS, Wang GJ, Volkow ND, Franceschi D, Logan J, Pappas N, Shea C, MacGregor RR, Garza V. Smoking a single cigarette does not produce a measurable reduction in brain MAO B in non-smokers. Nicotine Tob Res. 1999;1:325–329. doi: 10.1080/14622299050011451. [DOI] [PubMed] [Google Scholar]
- Fowler JS, Wang GJ, Volkow ND, Franceschi D, Logan J, Pappas N, Shea C, MacGregor RR, Garza V. Maintenance of brain monoamine oxidase B inhibition in smokers after overnight cigarette abstinence. Am J Psychiatry. 2000;157:1864–1866. doi: 10.1176/appi.ajp.157.11.1864. [DOI] [PubMed] [Google Scholar]
- Fowler JS, Logan J, Wang GJ, Volkow ND. Monoamine oxidase and cigarette smoking. Neurotoxicology. 2003a;24:75–82. doi: 10.1016/s0161-813x(02)00109-2. [DOI] [PubMed] [Google Scholar]
- Fowler JS, Logan J, Wang GJ, Volkow ND, Telang F, Zhu W, Franceschi D, Pappas N, Ferrieri R, Shea C, Garza V, Xu YW, Schlyer D, Gatley SJ, Ding YS, Alexoff D, Warner D, Netusil N, Carter P, Jayne M, King P, Vaska P. Low monoamine oxidase B in peripheral organs in smokers. Proc Natl Acad Sci USA. 2003b;100:11600–11605. doi: 10.1073/pnas.1833106100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler JS, Logan J, Wang GJ, Volkow ND, Telang F, Zhu W, Franceschi D, Shea C, Garza V, Xu Y, Ding YS, Alexoff D, Warner D, Netusil N, Carter P, Jayne M, King P, Vaska P. Comparison of monoamine oxidase a in peripheral organs in nonsmokers and smokers. J Nucl Med. 2005;46:1414–1420. [PubMed] [Google Scholar]
- Fowles J, Dybing E. Application of toxicological risk assessment principles to the chemical constituents of cigarette smoke. Tob Control. 2003;12:424–430. doi: 10.1136/tc.12.4.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita M, Seibyl JP, Vaupel DB, Tamagnan G, Early M, Zoghbi SS, Baldwin RM, Horti AG, Koren AO, Mukhin AG, Khan S, Bozkurt A, Kimes AS, London ED, Innis RB. Whole-body biodistribution, radiation absorbed dose, and brain SPET imaging with [123I]5-I-A-85380 in healthy human subjects. Eur J Nucl Med Mol Imaging. 2002;29:183–190. doi: 10.1007/s00259-001-0695-z. [DOI] [PubMed] [Google Scholar]
- Fujita M, Ichise M, van Dyck CH, Zoghbi SS, Tamagnan G, Mukhin AG, Bozkurt A, Seneca N, Tipre D, DeNucci CC, Iida H, Vaupel DB, Horti AG, Koren AO, Kimes AS, London ED, Seibyl JP, Baldwin RM, Innis RB. Quantification of nicotinic acetylcholine receptors in human brain using [I-123]5-I-A-85380 SPET. Eur J Nucl Med Mol Imaging. 2003;30:1620–1629. doi: 10.1007/s00259-003-1320-0. [DOI] [PubMed] [Google Scholar]
- Ghatan PH, Ingvar M, Eriksson L, Stone-Elander S, Serrander M, Ekberg K, Wahren J. Cerebral effects of nicotine during cognition in smokers and non-smokers. Psychopharmacology. 1998;136:179–189. doi: 10.1007/s002130050554. [DOI] [PubMed] [Google Scholar]
- Gioanni Y, Rougeot C, Clarke PB, Lepouse C, Thierry AM, Vidal C. Nicotinic receptors in the rat prefrontal cortex: increase in glutamate release and facilitation of mediodorsal thalamo-cortical transmission. Eur J Neurosci. 1999;11:18–30. doi: 10.1046/j.1460-9568.1999.00403.x. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Leranth C, Williams SM, Mons N, Geffard M. Dopamine synaptic complex with pyramidal neurons in primate cerebral cortex. Proc Natl Acad Sci USA. 1989;86:9015–9019. doi: 10.1073/pnas.86.22.9015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewegen HJ, Galisde Graaf Y, Smeets WJAJ. Integration and segregation of limbic cortico-striatal loops at the thalamic level: an experimental tracing study in rats. J Chem Neuroanat. 1999;16:167–185. doi: 10.1016/s0891-0618(99)00009-5. [DOI] [PubMed] [Google Scholar]
- Gross TM, Jarvik ME, Rosenblatt MR. Nicotine abstinence produces content-specific Stroop interference. Psychopharmacology. 1993;110:333–336. doi: 10.1007/BF02251289. [DOI] [PubMed] [Google Scholar]
- Haber SN, Fudge JL. The primate substantia nigra and VTA: integrative circuitry and function. Crit Rev Neurobiol. 1997;11:323–342. doi: 10.1615/critrevneurobiol.v11.i4.40. [DOI] [PubMed] [Google Scholar]
- Hatsukami D, Fletcher L, Morgan S, Keenan R, Amble P. The effects of varying cigarette deprivation duration on cognitive and performance tasks. J Subst Abuse. 1989;1:407–416. [PubMed] [Google Scholar]
- Herrero MT, Barcia C, Navarro JM. Functional anatomy of thalamus and basal ganglia. Childs Nervous Syst. 2002;18:386–404. doi: 10.1007/s00381-002-0604-1. [DOI] [PubMed] [Google Scholar]
- Hogg RC, Raggenbass M, Bertrand D. Nicotinic acetylcholine receptors: from structure to brain function. Rev Physiol Biochem Pharmacol. 2003;147:1–46. doi: 10.1007/s10254-003-0005-1. [DOI] [PubMed] [Google Scholar]
- Holmes S, Zwar N, Jimenez-Ruiz CA, Ryan PJ, Browning D, Bergmann L, Johnston JA. Bupropion as an aid to smoking cessation: a review of real-life effectiveness. Int J Clin Pract. 2004;58:285–291. doi: 10.1111/j.1368-5031.2004.00153.x. [DOI] [PubMed] [Google Scholar]
- Horti AG, Scheffel U, Koren AO, Ravert HT, Mathews WB, Musachio JL, Finley PA, London ED, Dannals RF. 2-[F-18]fluoro-A-85380, an in vivo tracer for the nicotinic acetylcholine receptors. Nucl Med Biol. 1998;25:599–603. doi: 10.1016/s0969-8051(98)00031-6. [DOI] [PubMed] [Google Scholar]
- Horti AG, Koren AO, Lee KS, Mukhin AG, Vaupel DB, Kimes AS, Stratton M, London ED. Radiosynthesis and preliminary evaluation of 5-[123/125I]iodo-3-(2(S)-azetidinylmethoxy)pyridine: a radioligand for nicotinic acetylcholine receptors. Nucl Med Biol. 1999;26:175–182. doi: 10.1016/s0969-8051(98)00086-9. [DOI] [PubMed] [Google Scholar]
- Horti AG, Villemagne VL. The quest for Eldorado: development of radioligands for in vivo imaging of nicotinic acetylcholine receptors in human brain. Curr Pharm Des. 2006;12:3877–3900. doi: 10.2174/138161206778559605. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Lesmes GR, Hatsukami DK, Richmond RL, Lichtenstein E, Jorenby DE, Broughton JO, Fortmann SP, Leischow SJ, McKenna JP, et al. Are higher doses of nicotine replacement more effective for smoking cessation? Nic Tobacco Res. 1999;1:169–174. doi: 10.1080/14622299050011281. [DOI] [PubMed] [Google Scholar]
- Hurt RD, Sachs DP, Glover ED, Offord KP, Johnston JA, Dale LC, Khayrallah MA, Schroeder DR, Glover PN, Sullivan CR, Croghan IT, Sullivan PM. A comparison of sustained-release bupropion and placebo for smoking cessation. NEJM. 1997;337:1195–1202. doi: 10.1056/NEJM199710233371703. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Gore JC, Skudlarski P, Lacadie CM, Jatlow P, Krystal JH. Impact of intravenous nicotine on BOLD signal response to photic stimulation. Magn Reson Imaging. 2002;20:141–145. doi: 10.1016/s0730-725x(02)00494-0. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, D’Souza DC, Mencl WE, Pugh KR, Skudlarski P, Krystal JH. Nicotine effects on brain function and functional connectivity in schizophrenia. Biol Psychiatry. 2004;55:850–858. doi: 10.1016/j.biopsych.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Jarvik ME, Madsen DC, Olmstead RE, Iwamoto-Schaap PN, Elins JL, Benowitz NL. Nicotine blood levels and subjective craving for cigarettes. Pharmacol Biochem Behav. 2000;66:553–558. doi: 10.1016/s0091-3057(00)00261-6. [DOI] [PubMed] [Google Scholar]
- Jorenby DE, Leischow SJ, Nides MA, Rennard SI, Johnston JA, Hughes AR, Smith SS, Muramoto ML, Daughton DM, Doan K, Fiore MC, Baker TB. A controlled trial of sustained-release bupropion, a nicotine patch, or both for smoking cessation. NEJM. 1999;340:685–691. doi: 10.1056/NEJM199903043400903. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Markou A. Neurobiology of the nicotine withdrawal syndrome. Pharmacol Biochem Behav. 2001;70:531–549. doi: 10.1016/s0091-3057(01)00651-7. [DOI] [PubMed] [Google Scholar]
- Killen JD, Fortmann SP, Davis L, Strausberg L, Varady A. Do heavy smokers benefit from higher dose nicotine patch therapy? Exp Clin Psychopharm. 1999;7:226–233. doi: 10.1037//1064-1297.7.3.226. [DOI] [PubMed] [Google Scholar]
- Killen JD, Fortmann SP, Schatzberg AF, Hayward C, Sussman L, Rothman M, Strausberg L, Varady A. Nicotine patch and paroxetine for smoking cessation. J Consult Clin Psych. 2000;68:883–889. [PubMed] [Google Scholar]
- Kimbrell TA, George MS, Parekh PI, Ketter TA, Podell DM, Danielson AL, Repella JD, Benson BE, Willis MW, Herscovitch P, Post RM. Regional brain activity during transient self-induced anxiety and anger in healthy adults. Biol Psychiatry. 1999;46:454–465. doi: 10.1016/s0006-3223(99)00103-1. [DOI] [PubMed] [Google Scholar]
- Kimes AS, Horti AG, London ED, Chefer SI, Contoreggi C, Ernst M, Friello P, Koren AO, Kurian V, Matochik JA, Pavlova O, Vaupel DB, Mukhin AG. 2-[18F]F-A-85380: PET imaging of brain nicotinic acetylcholine receptors and whole body distribution in humans. FASEB J. 2003;17:1331–1333. doi: 10.1096/fj.02-0492fje. [DOI] [PubMed] [Google Scholar]
- Klimek V, Zhu MY, Dilley G, Konick L, Overholser JC, Meltzer HY, May WL, Stockmeier CA, Ordway GA. Effects of long-term cigarette smoking on the human locus coeruleus. Arch Gen Psychiatry. 2001;58:821–827. doi: 10.1001/archpsyc.58.9.821. [DOI] [PubMed] [Google Scholar]
- Kodaira K, Fujishiro K, Wada T, Maie K, Satoi T, Tsukiyama E, Fukumoto T, Uchida T, Yamazaki S, Okamura T. A study on cerebral nicotine receptor distribution, blood flow, oxygen consumption, and other metabolic activities–a study on the effects of smoking on carotid and cerebral artery blood flow. Yakubutsu Seishin Kodo. 1993;13:157–165. [PubMed] [Google Scholar]
- Koob GF. Drugs of abuse: anatomy, pharmacology and function of reward pathways. Trends Pharm Sci. 1992;13:177–184. doi: 10.1016/0165-6147(92)90060-j. [DOI] [PubMed] [Google Scholar]
- Koren AO, Horti AG, Mukhin AG, Gundisch D, Kimes AS, Dannals RF, London ED. 2-, 5-, and 6-halo-3-(2(S)-azetidinylmethoxy)pyridines: synthesis, affinity for nicotinic acetylcholine receptors, and molecular modeling. J Med Chem. 1998;41:3690–3698. doi: 10.1021/jm980170a. [DOI] [PubMed] [Google Scholar]
- Krause KH, Dresel SH, Krause J, Kung HF, Tatsch K, Ackenheil M. Stimulant-like action of nicotine on striatal dopamine transporter in the brain of adults with attention deficit hyper-activity disorder. Int J Neuropsychopharmacol. 2002;5:111–113. doi: 10.1017/S1461145702002821. [DOI] [PubMed] [Google Scholar]
- Kubota K, Yamaguchi T, Abe Y, Fujiwara T, Hatazawa J, Matsuzawa T. Effects of smoking on regional cerebral blood-flow in neurologically normal subjects. Stroke. 1983;14:720–724. doi: 10.1161/01.str.14.5.720. [DOI] [PubMed] [Google Scholar]
- Kubota K, Yamaguchi T, Fujiwara T, Matsuzawa T. Effects of smoking on regional cerebral blood-flow in cerebral vascular-disease patients and normal subjects. Tohoku J Exp Med. 1987;151:261–268. doi: 10.1620/tjem.151.261. [DOI] [PubMed] [Google Scholar]
- Kumari V, Gray JA, Ffytche DH, Mitterschiffthaler MT, Das M, Zachariah E, Vythelingum GN, Williams SCR, Simmons A, Sharma T. Cognitive effects of nicotine in humans: an fMRI study. Neuroimage. 2003;19:1002–1013. doi: 10.1016/s1053-8119(03)00110-1. [DOI] [PubMed] [Google Scholar]
- Lambe EK, Picciotto MR, Aghajanian GK. Nicotine induces glutamate release from thalamocortical terminals in prefrontal cortex. Neuropsychopharmacology. 2003;28:216–225. doi: 10.1038/sj.npp.1300032. [DOI] [PubMed] [Google Scholar]
- Lanca AJ, Adamson KL, Coen KM, Chow BL, Corrigall WA. The pedunculopontine tegmental nucleus and the role of cholinergic neurons in nicotine self-administration in the rat: a correlative neuroanatomical and behavioral study. Neuroscience. 2000;96:735–742. doi: 10.1016/s0306-4522(99)00607-7. [DOI] [PubMed] [Google Scholar]
- Lawrence NS, Ross TJ, Stein EA. Cognitive mechanisms of nicotine on visual attention. Neuron. 2002;36:539–548. doi: 10.1016/s0896-6273(02)01004-8. [DOI] [PubMed] [Google Scholar]
- Leistikow BN, Martin DC, Milano CE. Estimates of smoking-attributable deaths at ages 15–54, motherless or fatherless youths, and resulting Social Security costs in the United States in 1994. Prev Med. 2000a;30:353–360. doi: 10.1006/pmed.2000.0657. [DOI] [PubMed] [Google Scholar]
- Leistikow BN, Martin DC, Milano CE. Fire injuries, disasters, and costs from cigarettes and cigarette lights: a global overview. Prev Med. 2000b;31:91–99. doi: 10.1006/pmed.2000.0680. [DOI] [PubMed] [Google Scholar]
- Leshner AI, Koob GF. Drugs of abuse and the brain. Proc Assoc Am Phys. 1999;111:99–108. doi: 10.1046/j.1525-1381.1999.09218.x. [DOI] [PubMed] [Google Scholar]
- Li SP, Kim KY, Kim JH, Kim JH, Park MS, Bahk JY, Kim MO. Chronic nicotine and smoking treatment increases dopamine transporter mRNA expression in the rat midbrain. Neurosci Lett. 2004;363:29–32. doi: 10.1016/j.neulet.2004.03.053. [DOI] [PubMed] [Google Scholar]
- London ED, Waller SB, Wamsley JK. Autoradiographic localization of [3H] nicotine binding sites in the rat brain. Neurosci Lett. 1985;53:179–184. doi: 10.1016/0304-3940(85)90182-x. [DOI] [PubMed] [Google Scholar]
- London ED, Connolly RJ, Szikszay M, Wamsley JK, Dam M. Effects of nicotine on local cerebral glucose-utilization in the rat. J Neurosci. 1988a;8:3920–3928. doi: 10.1523/JNEUROSCI.08-10-03920.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London ED, Dam M, Fanelli RJ. Nicotine enhances cerebral glucose utilization in central components of the rat visual system. Brain Res Bull. 1988b;20:381–385. doi: 10.1016/0361-9230(88)90067-6. [DOI] [PubMed] [Google Scholar]
- London ED, Scheffel U, Kimes AS, Kellar KJ. In vivo labeling of nicotinic acetylcholine receptors in brain with [3H]epibatidine. Eur J Pharmacol. 1995;278:R1–R2. doi: 10.1016/0014-2999(95)00178-n. [DOI] [PubMed] [Google Scholar]
- Lukas RJ. Neuronal nicotinic acetylcholine receptors. In: Barrantes FJ, editor. The nicotinic acetylcholine receptor: current views and future trends. R.G. Landes; Georgetown: 1998. pp. 145–173. [Google Scholar]
- Mansvelder HD, Keath JR, McGehee DS. Synaptic mechanisms underlie nicotine-induced excitability of brain reward areas. Neuron. 2002;33:905–919. doi: 10.1016/s0896-6273(02)00625-6. [DOI] [PubMed] [Google Scholar]
- Marenco T, Bernstein S, Cumming P, Clarke PBS. Effects of nicotine and chlorisondamine on cerebral glucose utilization in immobilized and freely-moving rats. Br J Pharmacol. 2000;129:147–155. doi: 10.1038/sj.bjp.0703005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marenco S, Carson RE, Berman KF, Herscovitch P, Weinberger DR. Nicotine-induced dopamine release in primates measured with [C-11]raclopride PET. Neuropsychopharmacology. 2004;29:259–268. doi: 10.1038/sj.npp.1300287. [DOI] [PubMed] [Google Scholar]
- Marien M, Brien J, Jhamandas K. Regional release of [3H]dopamine from rat brain in vitro: effects of opioids on release induced by potassium, nicotine, and L-glutamic acid. Can J Physiol Pharmacol. 1983;61:43–60. doi: 10.1139/y83-005. [DOI] [PubMed] [Google Scholar]
- McClernon FJ, Huettel SA, Rose JE. Abstinence-induced changes in self-report craving correlate with event-related fMRI responses to smoking cues. Neuropsychopharmacology. 2005;301:940–1947. doi: 10.1038/sj.npp.1300780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller NS, Goldsmith RJ. Craving for alcohol and drugs in animals and humans: biology and behavior. J Addict Dis. 2001;20:87–104. doi: 10.1300/J069v20n03_08. [DOI] [PubMed] [Google Scholar]
- Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA. 2004;291:1238–1245. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- Mukhin AG, Gundisch D, Horti AG, Koren AO, Tamagnan G, Kimes AS, Chambers J, Vaupel DB, King SL, Picciotto MR, Innis RB, London ED. 5-Iodo-A-85380, an alpha 4 beta 2 subtype-selective ligand for nicotinic acetylcholine receptors. Mol Pharmacol. 2000;57:642–649. doi: 10.1124/mol.57.3.642. [DOI] [PubMed] [Google Scholar]
- Naito E, Kinomura S, Geyer S, Kawashima R, Roland PE, Zilles K. Fast reaction to different sensory modalities activates common fields in the motor areas, but the anterior cingulate cortex is involved in the speed of reaction. J Neurophysiol. 2000;83:1701–1709. doi: 10.1152/jn.2000.83.3.1701. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Tanaka A, Nomoto Y, Ueno Y, Nakayama Y. Activation of fronto-limbic system in the human brain by cigarette smoking: evaluated by a CBF measurement. Keio J Med. 2000;49(Suppl 1):A122–A124. [PubMed] [Google Scholar]
- Newhouse PA, Potter A, Singh A. Effects of nicotinic stimulation on cognitive performance. Curr Opin Pharmacol. 2004;4:36–46. doi: 10.1016/j.coph.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Nisell M, Nomikos GG, Svensson TH. Systemic nicotine-induced dopamine release in the rat nucleus accumbens is regulated by nicotinic receptors in the ventral tegmental area. Synapse. 1994;16:36–44. doi: 10.1002/syn.890160105. [DOI] [PubMed] [Google Scholar]
- Pabreza LA, Dhawan S, Kellar KJ. [3H]Cytisine binding to nicotinic cholinergic receptors in brain. Mol Pharmacol. 1991;39:9–12. [PubMed] [Google Scholar]
- Parrott AC. Cigarette-derived nicotine is not a medicine. World J Biol Psychiatry. 2003;4:49–55. doi: 10.3109/15622970309167951. [DOI] [PubMed] [Google Scholar]
- Parrott AC, Kaye FJ. Daily uplifts, hassles, stresses and cognitive failures: in cigarette smokers, abstaining smokers, and non-smokers. Behav Pharmacol. 1999;10:639–646. doi: 10.1097/00008877-199911000-00010. [DOI] [PubMed] [Google Scholar]
- Paterson D, Nordberg A. Neuronal nicotinic receptors in the human brain. Prog Neurobiol. 2000;61:75–111. doi: 10.1016/s0301-0082(99)00045-3. [DOI] [PubMed] [Google Scholar]
- Paulson OB. Blood-brain barrier, brain metabolism and cerebral blood flow. Eur Neuropsychopharmacol. 2002;12:495–501. doi: 10.1016/s0924-977x(02)00098-6. [DOI] [PubMed] [Google Scholar]
- Pauly JR, Stitzel JA, Marks MJ, Collins AC. An autoradiographic analysis of cholinergic receptors in mouse brain. Brain Res Bull. 1989;22:453–459. doi: 10.1016/0361-9230(89)90072-5. [DOI] [PubMed] [Google Scholar]
- Pauly JR, Marks MJ, Robinson SF, van de Kamp JL, Collins AC. Chronic nicotine and mecamylamine treatment increase brain nicotinic receptor binding without changing alpha 4 or beta 2 mRNA levels. J Pharmacol Exp Ther. 1996;278:361–369. [PubMed] [Google Scholar]
- Perkins K, Sayette M, Conklin C, Caggiula A. Placebo effects of tobacco smoking and other nicotine intake. Nicotine Tob Res. 2003;5:695–709. doi: 10.1080/1462220031000158636. [DOI] [PubMed] [Google Scholar]
- Perry DC, Kellar KJ. [3H]Epibatidine labels nicotinic receptors in rat brain: an autoradiographic study. J Pharmacol Exp Ther. 1995;285:1030–1034. [PubMed] [Google Scholar]
- Pessoa L, Kastner S, Ungerleider LG. Neuroimaging studies of attention: from modulation of sensory processing to top-down control. J Neurosci. 2003;23:3990–3998. doi: 10.1523/JNEUROSCI.23-10-03990.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson BS, Skudlarski P, Gatenby JC, Zhang HP, Anderson AW, Gore JC. An fMRI study of Stroop word-color interference: evidence for cingulate subregions subserving multiple distributed attentional systems. Biol Psychiatry. 1999;45:1237–1258. doi: 10.1016/s0006-3223(99)00056-6. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Corrigall WA. Neuronal systems underlying behaviors related to nicotine addiction: neural circuits and molecular genetics. J Neurosci. 2002;22:3338–3341. doi: 10.1523/JNEUROSCI.22-09-03338.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomper MG, Phillips E, Fan H, McCarthy DJ, Keith RA, Gordon JC, Scheffel U, Dannals RF, Musachio JL. Synthesis and biodistribution of radiolabeled alpha 7 nicotinic acetylcholine receptor ligands. J Nucl Med. 2005;46:326–334. [PubMed] [Google Scholar]
- Pontieri FE, Tanda G, Orzi F, Di Chiara G. Effects of nicotine on the nucleus accumbens and similarity to those of addictive drugs. Nature. 1996;382:255–257. doi: 10.1038/382255a0. [DOI] [PubMed] [Google Scholar]
- Powell J, Dawkins L, Davis RE. Smoking, reward responsiveness, and response inhibition: tests of an incentive motivational model. Biol Psychiatry. 2002;51:151–163. doi: 10.1016/s0006-3223(01)01208-2. [DOI] [PubMed] [Google Scholar]
- Pritchard WS, Robinson JH, Guy TD. Enhancement of continuous performance task reaction-time by smoking in nondeprived smokers. Psychopharmacology. 1992;108:437–442. doi: 10.1007/BF02247417. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Dougherty DD, Alpert NM, Orr SP, Lasko M, Macklin ML, Fischman AJ, Pitman RK. Neural activation during sexual and competitive arousal in healthy men. Psychiatry Res Neuroimaging. 1999;91:1–10. doi: 10.1016/s0925-4927(99)00020-7. [DOI] [PubMed] [Google Scholar]
- Rees G, Lavie N. What can functional imaging reveal about the role of attention in visual awareness? Neuropsychologia. 2001;39:1343–1353. doi: 10.1016/s0028-3932(01)00122-1. [DOI] [PubMed] [Google Scholar]
- Rogers RL, Meyer JS, Shaw TG, Mortel KF, Hardenberg JP, Zaid RR. Cigarette-smoking decreases cerebral blood-flow suggesting increased risk for stroke. JAMA. 1983;250:2796–2800. [PubMed] [Google Scholar]
- Rolls ET, Baylis LL. Gustatory, olfactory, and visual convergence within the primate orbitofrontal cortex. J Neurosci. 1994;14:5437–5452. doi: 10.1523/JNEUROSCI.14-09-05437.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET, Critchley HD, Browning A, Hernadi I. The neurophysiology of taste and olfaction in primates, and umami flavor. Ann N Y Acad Sci. 1998;855:426–437. doi: 10.1111/j.1749-6632.1998.tb10602.x. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM, Westman EC, Mathew RJ, London ED, Hawk TC, Turkington TG, Coleman RE. PET studies of the influences of nicotine on neural systems in cigarette smokers. Am J Psychiatry. 2003;160:323–333. doi: 10.1176/appi.ajp.160.2.323. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM, Salley AN, Bates JE, Coleman RE, Hawk TC. Regional brain activity correlates of nicotine dependence. Neuropsychopharmacology. 2007;32:2441–2452. doi: 10.1038/sj.npp.1301379. [DOI] [PubMed] [Google Scholar]
- Rourke SB, Dupont RM, Grant I, Lehr PP, Lamoureux G, Halpern S, Yeung DW. Reduction in cortical IMP-SPET tracer uptake with recent cigarette consumption in a young group of healthy males. San Diego HIV Neurobehavioral Research Center. Eur J Nucl Med. 1997;24:422–427. doi: 10.1007/BF00881815. [DOI] [PubMed] [Google Scholar]
- Rowell PP, Carr LA, Garner AC. Stimulation of [3H]dopamine release by nicotine in rat nucleus accumbens. J Neurochem. 1987;49:1449–1454. doi: 10.1111/j.1471-4159.1987.tb01013.x. [DOI] [PubMed] [Google Scholar]
- Rusted JM, Caulfield D, King L, Goode A. Moving out of the laboratory: does nicotine improve everyday attention? Behav Pharmacol. 2000;11:621–629. doi: 10.1097/00008877-200011000-00009. [DOI] [PubMed] [Google Scholar]
- Ryan RE, Ross SA, Drago J, Loiacono RE. Dose-related neuroprotective effects of chronic nicotine in 6-hydroxydopamine treated rats, and loss of neuroprotection in alpha 4 nicotinic receptor subunit knockout mice. Br J Pharmacol. 2001;132:1650–1656. doi: 10.1038/sj.bjp.0703989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai Y, Takano Y, Kohjimoto Y, Honda K, Kamiya HO. Enhancement of [3H]dopamine release and its [3H]metabolites in rat striatum by nicotinic drugs. Brain Res. 1982;242:99–106. doi: 10.1016/0006-8993(82)90499-1. [DOI] [PubMed] [Google Scholar]
- Salokangas RK, Vilkman H, Ilonen T, Taiminen T, Bergman J, Haaparanta M, Solin O, Alanen A, Syvalahti E, Hietala J. High levels of dopamine activity in the basal ganglia of cigarette smokers. Am J Psychiatry. 2000;157:632–634. doi: 10.1176/appi.ajp.157.4.632. [DOI] [PubMed] [Google Scholar]
- Schilstrom B, Fagerquist MV, Zhang X, Hertel P, Panagis G, Nomikos GG, Svensson TH. Putative role of presynaptic alpha7* nicotinic receptors in nicotine stimulated increases of extracellular levels of glutamate and aspartate in the ventral tegmental area. Synapse. 2000;38:375–383. doi: 10.1002/1098-2396(20001215)38:4<375::AID-SYN2>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Schuh KJ, Stitzer ML. Desire to smoke during spaced smoking intervals. Psychopharmacology. 1995;120:289–295. doi: 10.1007/BF02311176. [DOI] [PubMed] [Google Scholar]
- Sherman SM. Thalamic relay functions. Prog Brain Res. 2001;134:51–69. doi: 10.1016/s0079-6123(01)34005-0. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paty JA, Gnys M, Elash C, Kassel JD. Nicotine withdrawal in chippers and regular smokers – subjective and cognitive effects. Health Psychol. 1995;14:301–309. doi: 10.1037//0278-6133.14.4.301. [DOI] [PubMed] [Google Scholar]
- Shoaib M, Schindler CW, Goldberg SR, Pauly JR. Behavioural and biochemical adaptations to nicotine in rats: influence of MK801, an NMDA receptor antagonist. Psychopharmacology. 1997;134:121–130. doi: 10.1007/s002130050433. [DOI] [PubMed] [Google Scholar]
- Shoaib M, Lowe AS, Williams SCR. Imaging localised dynamic changes in the nucleus accumbens following nicotine withdrawal in rats. Neuroimage. 2004;22:847–854. doi: 10.1016/j.neuroimage.2004.01.026. [DOI] [PubMed] [Google Scholar]
- Sihver W, Langstrom B, Nordberg A. Ligands for in vivo imaging of nicotinic receptor subtypes in Alzheimer brain. Acta Neurol Scand. 2000;102:27–33. doi: 10.1034/j.1600-0404.2000.00304.x. [DOI] [PubMed] [Google Scholar]
- Sillito AM, Jones HE. Corticothalamic interactions in the transfer of visual information. Philos Trans R Soc Lond Ser B Biol Sci. 2002;357:1739–1752. doi: 10.1098/rstb.2002.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EE, Jonides J. Neuroscience – Storage and executive processes in the frontal lobes. Science. 1999;283:1657–1661. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- Sommer MA. The role of the thalamus in motor control. Curr Opin Neurobiol. 2003;13:663–670. doi: 10.1016/j.conb.2003.10.014. [DOI] [PubMed] [Google Scholar]
- Staley JK, Krishnan-Sarin S, Zoghbi S, Tamagnan G, Fujita M, Seibyl JP, Maciejewski PK, O’Malley S, Innis RB. Sex differences in [123I]beta-CIT SPECT measures of dopamine and serotonin transporter availability in healthy smokers and nonsmokers. Synapse. 2001;41:275–284. doi: 10.1002/syn.1084. [DOI] [PubMed] [Google Scholar]
- Staley JK, Krishnan-Sarin S, Cosgrove KP, Krantzler E, Frohlich E, Perry E, Dubin JA, Estok K, Brenner E, Baldwin RM, Tamagnan GD, Seibyl JP, Jatlow P, Picciotto MR, London ED, O’Malley S, van Dyck CH. Human tobacco smokers in early abstinence have higher levels of beta2* nicotinic acetylcholine receptors than nonsmokers. J Neurosci. 2006;34:8707–8714. doi: 10.1523/JNEUROSCI.0546-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton JM, Gilson SF, Wong DF, Villemagne VL, Dannals RF, Grayson RF, Henningfield JE, London ED. Intravenous nicotine reduces cerebral glucose metabolism: a preliminary study. Neuropsychopharmacology. 2003a;28:765–772. doi: 10.1038/sj.npp.1300106. [DOI] [PubMed] [Google Scholar]
- Stapleton JM, Gilson SF, Wong DF, Villemagne VL, Dannals RF, Grayson RF, Henningfield JE, London ED. Intravenous nicotine reduces cerebral glucose metabolism: a preliminary study. Neuropsychopharmacology. 2003b;28:765–772. doi: 10.1038/sj.npp.1300106. [DOI] [PubMed] [Google Scholar]
- Stein E, Pankiewicz J, Harsch HH, Cho JK, Fuller SA, Hoffmann RG, Hawkins M, Rao S, Bandettini PA, Bloom AS. Nicotine-induced limbic cortical activation in the human brain: a functional MRI study. Am J Psychiatry. 1998;155:1009–1015. doi: 10.1176/ajp.155.8.1009. [DOI] [PubMed] [Google Scholar]
- Sziraki I, Lipovac MN, Hashim A, Sershen H, Allen D, Cooper T, Czobor P, Lajtha A. Differences in nicotine-induced dopamine release and nicotine pharmacokinetics between Lewis and Fischer 344 rats. Neurochem Res. 2001;26:609–617. doi: 10.1023/a:1010979018217. [DOI] [PubMed] [Google Scholar]
- Terborg C, Birkner T, Schack B, Witte OW. Acute effects of cigarette smoking on cerebral oxygenation and hemodynamics: a combined study with near-infrared spectroscopy and transcranial Doppler sonography. J Neurol Sci. 2002;205:71–75. doi: 10.1016/s0022-510x(02)00311-8. [DOI] [PubMed] [Google Scholar]
- Thompson JC, Wilby G, Stough C. The effects of transdermal nicotine on inspection time. Hum Psychopharmacol. 2002;17:157–161. doi: 10.1002/hup.377. [DOI] [PubMed] [Google Scholar]
- Tsukada H, Miyasato K, Kakiuchi T, Nishiyama S, Harada N, Domino EF. Comparative effects of methamphetamine and nicotine on the striatal [C-11]raclopride binding in unanesthetized monkeys. Synapse. 2002;45:207–212. doi: 10.1002/syn.10102. [DOI] [PubMed] [Google Scholar]
- Valette H, Bottlaender M, Dolle F, Guenther I, Coulon C, Hinnen F, Fuseau C, Ottaviani M, Crouzel C. Characterization of the nicotinic ligand 2-[F-18]fluoro-3-[2(S)-2-azetidinylmethoxy]pyridine in vivo. Life Sci. 1998;64:L93–L97. doi: 10.1016/s0024-3205(98)00573-6. [DOI] [PubMed] [Google Scholar]
- Valette H, Bottlaender M, Dolle F, Guenther I, Fuseau C, Coulon C, Ottaviani M, Crouzel C. Imaging central nicotinic acetylcholine receptors in baboons with [F-18]fluoro-A-85380. J Nucl Med. 1999;40:1374–1380. [PubMed] [Google Scholar]
- Valette H, Bottlaender M, Dolle F, Coulon C, Ottaviani M, Syrota A. Long-lasting occupancy of central nicotinic acetylcholine receptors after smoking: a PET study in monkeys. J Neurochem. 2003;84:105–111. doi: 10.1046/j.1471-4159.2003.01502.x. [DOI] [PubMed] [Google Scholar]
- Villemagne V, Horti A, Scheffel U, Ravert H, Finley P, Clough DJ, London E, Wagner H, Dannals RF. Imaging nicotinic acetylcholine receptors with fluorine-18-FPH, an epibatidine analog. J Nucl Med. 1997;38:1737–1741. [PubMed] [Google Scholar]
- Westfall TC, Grant H, Perry H. Release of dopamine and 5-hydroxytryptamine from rat striatal slices following activation of nicotinic cholinergic receptors. Gen Pharmacol. 1983;14:321–325. doi: 10.1016/0306-3623(83)90037-x. [DOI] [PubMed] [Google Scholar]
- Wilson SJ, Sayette MA, Fiez JA. Prefrontal responses to drug cues: a neurocognitive analysis. Nat Neurosci. 2004;7:211–214. doi: 10.1038/nn1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Nishiyama Y, Monden T, Satoh K, Ohkawa M. A study of the acute effect of smoking on cerebral blood flow using 99mTc-ECD SPET. Eur J Nucl Med Mol Imaging. 2003;30:612–614. doi: 10.1007/s00259-003-1119-z. [DOI] [PubMed] [Google Scholar]
- Yates SL, Bencherif M, Fluhler EN, Lippiello PM. Up-regulation of nicotinic acetylcholine receptors following chronic exposure of rats to mainstream cigarette smoke or alpha 4 beta 2 receptors to nicotine. Biochem Pharmacol. 1995;50:2001–2008. doi: 10.1016/0006-2952(95)02100-0. [DOI] [PubMed] [Google Scholar]
- Yeomans J, Baptista M. Both nicotinic and muscarinic receptors in ventral tegmental area contribute to brain-stimulation. Pharmacol Biochem Behav. 1997;57:915–921. doi: 10.1016/s0091-3057(96)00467-4. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Yokoo H, Tanaka T, Mizoguchi K, Emoto H, Ishii H, Tanaka M. Facilitatory modulation of mesolimbic dopamine neuronal-activity by a mu-opioid agonist and nicotine as examined with in-vivo microdialysis. Brain Res. 1993;624:277–280. doi: 10.1016/0006-8993(93)90087-4. [DOI] [PubMed] [Google Scholar]
- Zhang X, Tian JY, Svensson AL, Gong ZH, Meyerson B, Nordberg A. Chronic treatments with tacrine and (−)-nicotine induce different changes of nicotinic and muscarinic acetylcholine receptors in the brain of aged rat. J Neural Transm. 2002;109:377–392. doi: 10.1007/s007020200030. [DOI] [PubMed] [Google Scholar]
- Zubieta J, Lombardi U, Minoshima S, Guthrie S, Ni L, Ohl LE, Koeppe RA, Domino EF. Regional cerebral blood flow effects of nicotine in overnight abstinent smokers. Biol Psychiatry. 2001;49:906–913. doi: 10.1016/s0006-3223(00)01070-2. [DOI] [PubMed] [Google Scholar]
- Zubieta JK, Heitzeg MM, Xu Y, Koeppe RA, Ni L, Guthrie S, Domino EF. Regional cerebral blood flow responses to smoking in tobacco smokers after overnight abstinence. Am J Psychiatry. 2005;162:567–577. doi: 10.1176/appi.ajp.162.3.567. [DOI] [PubMed] [Google Scholar]


