Abstract
The nature of the ligand is an important aspect of controlling structure and reactivity in coordination chemistry. In connection with our study of heme/copper/oxygen reactivity relevant to cytochrome c oxidase O2-reduction chemistry, we compare the molecular and electronic structure of two high-spin heme-peroxo-copper [FeIII-O22--CuII]+ complexes containing N4-tetradentate (1) or N3-tridentate (2) copper ligands. Combining previously reported and new resonance Raman and EXAFS data coupled to DFT calculations we report a geometric structure and more complete electronic description of the high-spin heme-peroxo-copper complexes 1 and 2, which establish μ-(O22-) side-on to the FeIII and end-on to CuII (μ-η2:η1) binding for the complex 1 but side-on/side-on (μ-η2:η2) μ-peroxo coordination for the complex 2. We also compare and summarize the differences and similarities of these two complexes in their reactivity toward CO, PPh3, acid and phenols. The comparison of a new X-ray structure of μ-oxo complex 2a with the previously reported 1a X-ray structure, two thermal decomposition products respectively of 2 and 1, reveals a considerable difference in the Fe-O-Cu angle between the two μ-oxo complexes (∠Fe-O-Cu = 178.2° in 1a, ∠Fe-O-Cu = 149.5° in 2a). The reaction of 2 with one equivalent of exogenous N-donor axial base leads to the formation of a distinctive low-temperature stable, low-spin heme-O2-Cu complex (2b), but under the same conditions the addition of an axial base to 1 leads to the dissociation of the heme-peroxo-Cu assembly and the release of O2. 2b reacts with phenols performing hydrogen-atom (e– + H+) abstraction resulting in O-O bond cleavage and the formation of high-valent ferryl [FeIV=O] complex (2c). The nature of 2c was confirmed by comparison of its spectroscopic features and reactivity with those of an independently prepared ferryl complex. The phenoxyl radical generated by the hydrogen-atom abstraction was either 1) directly detected by EPR spectroscopy using phenols that produce stable radicals or 2) indirectly by detection of the coupling product of two phenoxyl radicals.
Introduction
In the last few years, we have been engaged in heme/copper/O2 reactivity studies using synthetic systems, with the goal being to provide fundamental information of possible relevance to biological heme-copper oxidases (HCO's) which include cytochrome c oxidase (CcO). HCO's are the terminal enzymes of the respiratory chain which catalyze the reduction of molecular oxygen to water, a process that requires four electrons and four protons,
The reaction is coupled to the pumping of four additional protons across the mitochondrial or bacterial membrane which ultimately is utilized to drive ATP synthetase activity and thus generate ATP which is stored and used in subsequent metabolic functions.1-6
In the heme-copper active-site mediated O2-reduction by HCO's, dioxygen reacts with a fully reduced FeII···CuI binuclear center (Scheme 1) initially forming a detectable transient, the FeIII−(O2–)···CuI complex A. A exhibits UV-vis and resonance Raman (rR) spectroscopic properties very similar to those known for oxy-hemoglobin or myoglobin. A DFT calculated structure of A supports the FeIII(O2–)···CuI formulation with the Cu ion positioned very close to the superoxo O-atom, Cu···O = 2.12 Å.7 The next transformation seems optimized to prevent leakage of deleterious intermediates such as hydroxyl radical or hydrogen peroxide, wherein rapid overall four-electron reductive O–O cleavage is completed, giving PM, in which a closeby coupled Tyr (Scheme 1) or nearby Trp8 residue efficiently provides a needed electron. A peroxo-bridged FeIII-(O22–)-CuII transient has been discussed as possibly forming from A prior to PM formation.9-12 Also discussed and calculated is an electron-transfer from CuBI in A which is accompanied by H+ uptake to give a μ-hydroperoxo transient, Fea3III-O-O(H)-CuBII; the protonation event in this scenario would trigger electron transfer (from the heme a3 and the Tyr244) yielding a cleaved O—O product ferryl moiety (state PM, Scheme 1).10,13-15
Scheme 1.
Synthetic modeling presents a chemically interesting if not necessary approach to study metalloprotein active site structure (electronic and connectivity) and reactivity and its relationship with the inherent fundamental chemistry associated with the metal ion and its ligand environment.16-18 Of particular interest is the A to PM conversion, as O-O cleavage chemistry is of broad fundamental and practical importance in the utilization of molecular oxygen such as in biological “O2-activation” occurring in heme, non-heme iron and copper oxidases or oxygenases. The use of O2 (from air) in practical (chemical/industrial) organic oxidations, or energy production in fuel cell technologies also involves O-O cleavage. The reverse process, O-O bond formation, occcurs in photosynthesis and is of great contemporary interest and importance as related to worldwide energy concerns. This timely forum highlights the importance of understanding the elementary kinetic steps required to reductively cleave the O—O bond, and the many ways researchers are tackling such issues.
In this article we will highlight our own studies in the synthetic modeling of heme-copper oxidases, also providing new insights obtained on heme-Cu/O2 adduct structure, bonding and reactivity. In our earlier studies, we have employed a systematic approach varying the design of heme/Cu complexes.6 The approach entails synthetic modulation of copper ligand type, denticity, arrangement of donors, heme axial ‘base’ ligand and availability of protons or reducing equivalents. The overarching question is how such factors influence heme-copper/O2 derived structures, along with the resulting complex Fe-O2(H)-bonding and electronic structure (i.e., spin state, level of reduction of dioxygen, etc.). Such factors have been shown or are expected to dictate the absence or presence of further reactivity, and the nature of such reactivity in the context of O-O cleavage chemistry. With similar emphases, in part, the research groups of Collman and coworkers5,19 and Naruta and coworkers20,21 have been very active and have made major contributions. Collman has also presented a longtime program in biomimetic electrode surface electrocatalysis of the four-electron O2-reduction to water.5,22,23 Despite the many achievements and advancements from all groups, there remains a considerable lack of detail concerning structure (i.e., of heme-Cu/O2 derived adducts) and therefore we do not have firm correlations between structure/bonding and ensuing reactivity. The latter involves protonation and electron-transfer steps, for which a deeper understanding of the thermodynamics and timing or order is required.
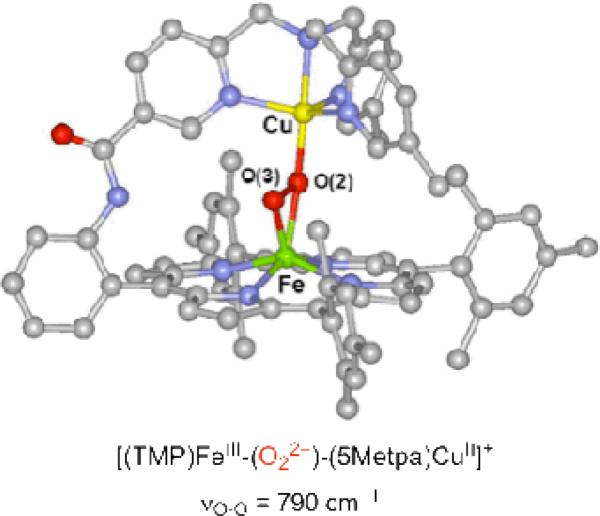
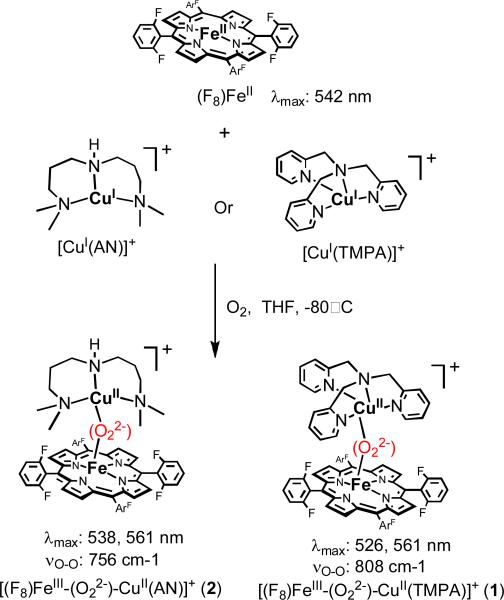
In our initial attempts to mimic the O2 reduction chemistry of CcO, we utilized TMPA-CuI complexes, where TMPA (tris(2-pyridylmethylamine)) is a tetradentate N4 ligand. We observed that a low-temperature stable hemeFeIII-peroxo-CuII complex [(F8)FeIII-(O22-)-CuII(TMPA)]+ (1, Chart 1) could be generated from the O2 reaction with an equimolar mixture of (F8)FeII and [CuI(TMPA)]+ (see discussion below), were F8 = tetrakis(2,6-difluorophenyl)porphyrinate2–24 Structural investigation of the ensuing complex using resonance Raman (rR) and X-ray absorption (XANES and EXAFS) spectroscopies coupled to DFT calculations indicated that the peroxide unit is bound side-on to the high-spin iron(III) (overall hexa-coordinate with the iron out of the porphyrinate plane) and end-on to the copper(II) (overall penta-coordinate), thus an FeIII-(μ-η2:η1-peroxo)-CuII coordination mode.25,26 An intraperoxide stretching band was observed in the rR spectrum at 808 cm-1.24,25 The plausibility of such a coordination mode was affirmed by Naruta and co-workers, through crystallographic characterization of such a complex possessing a closely related ligand framework.27 There, νO-O was determined to be 790 cm-1 (Figure 1).
Chart 1.
Figure 1.
X-ray structure of [(TMP)FeIII-(O22-)-(5Metpa)CuII]+ synthesized and characterized by Naruta and coworkers.
We also established the same μ-η2:η1 coordination mode in three other peroxo complexes containing tetradenate copper ligands (Chart 1). In [(6L)FeIII-(O22-)-CuII]+ (3), the tetradentate TMPA ligand is covalently appended to the periphery of a 2,6-difluorophenyl-substituted porphyrin and νO-O = 788 cm-1.28, 29 In [(F8)FeIII-(O22–)-CuII(LN4OH)]+ (5a) and [(F8)FeIII-(O22–)-CuII(LN4OMe)]+ (5b) the copper ligand is a modified version of TMPA were one pyridine is replaced by protected (5b) or non-protected (5a) imidazole-phenol moiety to mimic the cross-linked histidine-tyrosine in CcO active site. The O-O stretching frequencies in 5a and 5b are 813 cm-1 and 815 cm-1, respectively.6,30 Extensive previous (and ongoing) CuI/O2 chemical investigations have shown that even subtle differences in ligand/denticity can dramatically change the nature of the copper–dioxygen adduct and its reactivity toward substrates.31,32 Tetradentate ligands, such as TMPA, induce formation of end-on (μ-1,2) peroxo dicopper(II) structures (Chart 2), whereas tridentate ligands generate side-on (μ-η2:η2) peroxo dicopper(II) species.33 The former possess relatively average νO–O values, typically above 800 cm–1 (Chart 2). However, the latter side-on complexes have reduced (< 760 cm–1) νO–O values (Chart 2), ascribed to back bonding from copper to the peroxo antibonding σ* orbital, which considerably weakens the O–O bond.34-36
Chart 2.
To compare and contrast the chemistry with that for heme-Cu system possessing tetradentate copper ligands, we expanded our study to heme-Cu systems containing tridentate copper ligands. Within that context, we reported the synthesis and the characterization of three high-spin heme-peroxo-Cu complexes with tridentate copper ligands (Chart 1): 1) [(F8)FeIII-(O22–)-CuII(AN)]+ (2) (AN = bis(3-dimethylaminopropyl)amine),37 2) [(F8)FeIII-(O22–)-CuII(LMe2N)]+ (6) (LMe2N = N,N-bis[2-(2-pyridyl)ethyl]methylamine),38 and 3) [(2L)FeIII-(O22–)-CuII]+ (4) (2L = tethered prophryn-N,N-bis[2-(2-pyridyl)ethyl]methylamine).39,40 In concordance with expectations from copper-dioxygen homodimer chemistry (Chart 2), the three complexes present O-O stretching frequencies below 760 cm-1 thereby suggesting a side-on (μ-η2:η2) mode of coordination for the peroxo bridge. Thus, the heme-peroxo-copper complexes depicted in Chart 1 were tentatively assigned as such.40 In this paper, this point is addressed in detail, following new rR and EXAFS spectroscopic studies supported by DFT calculations.
Further, in this report we have selected [(F8)FeIII-(O22-)-CuII(TMPA)]+ (1) and [(F8)FeIII-(O22-)-CuII(AN)]+ (2) containing tetra- and tridentate copper ligands, respectively, to compare and contrast molecular structure, electronic-structure/bonding and resultant reactivity, combining some previously published information, along with new data. The overall goal is to contribute a better understanding of the relationship between the denticity of the copper ligand to the structure and reactivity of high-spin heme-peroxo-Cu complexes. We also describe the formation and initial characterization of a new related low-spin heme-peroxo-Cu complex and compare the high-spin and low-spin complexes in their reactivity toward phenols as hydrogen-atom (H+ + e–) donors.
Results and Discussion
Generation of High-Spin Heme-Peroxo-Cu Complexes
As mentioned, [(F8)FeIII-(O22-)-CuII(TMPA)]+ (1) has been structurally characterized, through a variety of spectroscopic studies, computational chemistry and comparison to the Naruta complex. These possess a μ-η2:η1-peroxo ligation. A major goal of the present work is to elucidate or confirm the μ-η2:η2- side-on peroxo structure previously proposed for 4 (and thus 6) employing [(F8)FeIII-(O22-)-CuII(AN)]+ (2) for such studies; the latter possesses synthetically more accessible precursor components, and has been found to be more stable and amenable to spectroscopic interrogation.
As previously described, [(F8)FeIII-(O22-)-CuII(TMPA)]+ (1) and [(F8)FeIII-(O22-)-CuII(AN)]+ (2) can be cleanly generated by bubbling O2 at low temperature through an equimolar solution of (F8)FeII and [CuI(AN)](B(C6F5)4) or [CuI(TMPA)](B(C6F5)4,6,37 Scheme 2. With tridentate chelating ligands for the copper ion, complexes 2, 4 and 6 possess the same UV-Vis features in the Q-band region, but which are clearly distinguishable from those of 1, 3 and 5 with their tetradentate copper ligands.39 In the former case a 2nd prominent absorption occurs, e.g. at 538 nm for 2 (Scheme 2). Low-temperature 1H NMR characterization of the μ-peroxo products 1 and 2 show a downfield-shifted pyrrole resonance at ~ 95 ppm which has been confirmed by 2H NMR studies using the pyrrole-deuterated (d8-F8)FeII analogue) in the synthesis This finding exclude the possibility that the product observed is (i) the η2-peroxo compound [(F8)FeIII]2−(O22-) (~ 17.5 ppm, −80° C), (ii) an iron–superoxide complex, that is, (F8)FeIII−(O2-) (~ 8.9 ppm, −80° C), (iii) the ferryl species (F8)FeIV=O (~ 3.5 ppm, −80° C) or (iv) (F8)FeIII−OH (~ 135 ppm, −80° C).41 In fact, the 95 ppm pyrrole resonance is in the range expected for a high-spin FeIII (d5, S = 5/2) system which is strongly antiferromagnetically coupled to a CuII ion (d9, S = 1/2), giving an overall S = 2 spin system (confirmed in 1 by solution magnetic moment measurement μeff = 5.1 μB at −40° C). Further, in the case of [(F8)FeIII-(O22-)-CuII(TMPA)]+ (1) MALDI-TOF-MS reveal the presence of a dioxygen adduct with a stoichiometry of (F8)Fe: CuII(TMPA):O2 = 1:1:1 ratio and solution mass spectral data give the correct O2-drived moiety (for which m/z increases by four when using 18O2).26
Scheme 2.
Coordination Geometry and Electronic Structural Comparisons of 1 and 2
Cu EXAFS
The k3-weighted Cu K-edge EXAFS spectroscopic data and Fourier transforms (FT) of 2 are shown in Figure 2. The EXAFS fit parameters are given in Table 1. The first-shell EXAFS data were fit best with five Cu-O/N contributions at 2.00 Å.42 The theoretical phase and amplitude parameters were calculated from a Cu-N path. Using a Cu-O path the best first-shell fit resulted in a coordination number of four. This difference in the first-shell coordination is within the estimated 20-25% error. Split first-shell fits with a combination of one to four short Cu-O/N with one to four longer Cu-N/O were also performed. These fits gave higher error with the split in distance between the two paths less than the resolution of the data (0.14 Å). The paths corresponding to the FT peak in the R = 2–2.8 Å range was fit using single scattering (SS) and multiple scattering (MS) contributions from the AN alkyl backbone. The FT peak in the R = 2.8–3.8 Å range was fit with a Cu···Fe SS contribution at 3.62 Å with its corresponding MS Cu-O-Fe vector refined to 3.75 Å when both distances and σ2 values were allowed to float independently for these paths. Linking the σ2 values for these paths resulted in a fit with Cu···Fe distance of 3.63 Å and Cu-O-Fe MS distance of 3.76 Å with a σ2 value of 627×10-5 Å2. The Cu···Fe vector distance varied between fits to data of four different samples and with slightly different splines giving an average Cu···Fe distance of 3.63 ± 0.04 Å.
Figure 2.
Cu K-edge EXAFS data to k = 12.8 Å-1 (inset) and non-phase-shift-corrected Fourier transform of the EXAFS data for 2 in solution. Phase shift in the first shell is ~0.4 Å. Data ( ); fit (
); fit ( ).
).
Table 1.
EXAFS least-squares fitting results for 2.
| Cu Coord./Path | R(Å)a | σ2(Å2)b | ΔE0(eV) | Fc | Fe Coord./Path | R(Å)a | σ2(Å2)b | ΔE0(eV) | Fc |
|---|---|---|---|---|---|---|---|---|---|
| 5 Cu-N/O | 2.00 | 963 | 1 Fe-O/N | 1.94 | 257 | ||||
| 6 Cu-C | 2.92 | 701 | 5 Fe-N/O | 2.09 | 397 | ||||
| 16 Cu-N-C | 3.22 | 311 | -9.61 | 0.07 | 8 Fe-C | 3.10 | 368 | ||
| 1 Cu-Fe | 3.62 | 653 | 16 Fe-N-Cd | 3.32 | 368 | ||||
| 2 Cu-O-Fe | 3.75 | 809 | 4 Fe-C | 3.49 | 864 | ||||
| 1 Fe-Cu | 3.64 | 370 | -2.09 | 0.23 | |||||
| 2 Fe-O-Cud | 3.71 | 370 | |||||||
| 5 Fe-C | 3.81 | 461 | |||||||
| 12 Fe-N-C | 4.38 | 513 | |||||||
| 16 Fe-C-C | 4.91 | 377 | |||||||
| 8 Fe-C-C | 4.98 | 291 |
The estimated standard deviations in R for each fit is ±0.02 Å.
The σ2 values are multiplied by 105.
The error, F, is given by Σ[(χobsd – χcalcd)2k6]/Σ[(χobsd)2k6].
The σ2 for the multiple scattering path is linked to that of the corresponding single scattering path. The errors in ΔE0 values are ±0.22 eV for Cu and ±0.27 eV for Fe. The estimated errors in σ2 are 5-30%. The error in coordination number is 20-25% and that in the identity of the scatterer Z is ±1.
Fe EXAFS
The k3-weighted Fe K-edge EXAFS spectroscopic data and FT of 2 are shown in Figure 3. The first backscattering shell was fit best using one Fe-O/N contribution at 1.94 Å and five Fe-N/O contributions at 2.09 Å. The second-shell FT peak in the R = 2–3 Å range was fit using the porphyrin pyrrole α carbon SS and MS contributions, along with the SS contribution from the meso carbons. The Fe···Cu contribution was fit at 3.64 Å with its corresponding MS Fe-O-Cu vector refined to 3.71 Å when the two σ2 values were linked to each other. Floating the σ2 values for these paths resulted in very similar fit parameters. The Fe···Cu vector was not required to get a good fit to the EXAFS data. This is because the SS and MS contributions from the porphyrin pyrrole β carbons interfere with the signal from the Fe···Cu interaction making the metal-metal vector hard to determine. The reliable distance of the Fe···Cu interaction was thus obtained from Cu EXAFS. The intensity in the R = 3.5–5 Å FT range was fit with SS and MS components from the pyrrole β and γ carbon atoms.
Figure 3.
Fe K-edge EXAFS data to k = 16 Å-1 (inset) and non-phase-shift-corrected Fourier transform of [(F8)FeIII-O2-CuII(AN)](ClO4) in solution form. Phase shift in the first shell is ~0.4 Å. Data ( ); fit (
); fit ( ).
).
Comparison of EXAFS between 1 and 2
The Cu K-edge EXAFS and FT of frozen solutions of 1 and 2 are compared in Figure S6. Both species have a first-shell coordination of 5 Cu-N/O, which in the case of 1 can be split to obtain a short Cu-O/N of 1.87 Å. The two EXAFS patterns between k = 4–12 Å-1 are significantly different and account for the contrasting copper ligands and Cu···Fe vectors in the two species. The slight phase shift to lower k in 1 is consistent with a lenghthened Cu···Fe vector in 1 of 3.72 Å compared to 3.63 Å in 2. The higher EXAFS intensity in 1 is reflected in the higher FT intensity in which a major difference is observed from R = 3–4 Å. The signal in this region originates from the Cu···Fe interaction, which in 1 is much more intense because the Cu-O-Fe MS is stronger than that in 2. This difference arises from the more linear Cu-O-Fe angle of 150° in 1 compared to 134° in 2. Both angles have been calculated from EXAFS parameters.43 The deconvoluted sine waves from the SS and MS of Cu-Fe and Cu-O-Fe, respectively, are shown in Figure S8. A Cu-O-Fe angle of 150° is not enough to produce such an intense MS wave in 1. Some of the intensity in the R = 3–4 Å region is a result of SS and MS contribution from the light atoms of TMPA. The remainder of the difference between R = 2.5–5 Å is due to the different SS and MS contributions from the pyridine versus the alkylamine side-chains.
The Fe K-edge EXAFS and FT of the solution data of 1 and 2 are compared in Figure S7. Both have a split first-shell with a short 1Fe-O/N at 1.92 Å and 1.94 Å, respectively, and 5Fe-N/O at 2.09 Å. The small changes in the first-shell FT are possibly from the different interference of the two sine waves in each system. The EXAFS beat patterns are very similar in both species. The higher intensity in 1 is again from the stronger Fe-O-Cu MS. This higher intensity translates into the FT peak at 3.5 Å in 1 that is missing in 2. Both 1 and 2 have a very similar coordination environment around Fe as seen from the Fe EXAFS. The Fe K-edge and pre-edge (Figure S9) lend further support to the Fe being in a very similar coordination environment. The pre-edge peak intensity at ~7113.5 eV is a result of a 1s→3d transition that is dipole forbidden but quadrupole allowed. This peak gains intensity from 4p mixing into the 3d ground state. Distortion away from centrosymmetry increases 4p-3d mixing as the Fe is pulled out of the heme plane. The similar pre-edge peak intensity in the two systems suggests that the peroxo binding to Fe is similar in both species, i.e. side-on, as a consequence of similar Fe coordination.
Resonance Raman Spectroscopy
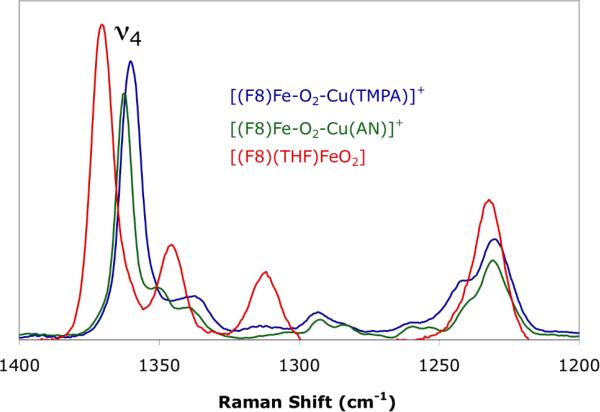
The O—O stretching frequencies of both 1 and 2 have been previously reported as 810 and 756 cm-1 respectively,24,37 indicating the peroxo character of the bound dioxygen moiety. However, the significant difference between these two energies was hypothesized as due to a distinct core geometry, namely a μ:η2:η2 for 2 relative to the μ-η2:η1 found in 1. To evaluate this possibility and an alternative η1 coordination mode at the Fe, rR spectroscopy was performed to assess the displacement of the iron out of the heme pocket. In particular, the energy of the oxidation and spin state sensitive marker band ν4 was recorded. From the spectra in Figure 4, the ν4 band of 2 is observed at 1362 cm-1 which compares well to that of 1 (1360 cm-1). A bona fide end-on dioxygen bound heme, [(F8)(THF)Fe(O2)]24,26,38,44 with the iron in the porphryn plane was measured as a control, and exhibits a ν4 of 1370 cm-1.
Figure 4.
Resonance Raman spectra with 413 nm excitation of 1 (blue), 2 (green), [(F8)(THF)FeO2] (red).
Geometric Structures
Spin-unrestricted DFT calculations were performed using the BP86 functional on [(F8)Fe-O2-Cu(TMPA)]+ (1) and [(F8)Fe-O2-Cu(AN)]+ (2). The optimized structures are shown in Figure 5 and selected bond angles and distances are reported in Table 2. An optimized structure of a truncated model lacking phenyl rings of the heme of 1 was previously reported,25,26 however, the full structure of 1 was reoptimized herein to facilitate structural comparisons between 1 and 2. The metrical parameters of the μ:η2:η1 Fe—O2—Cu core in the present structure of 1 agrees well with the previously reported metrical parameters of the truncated model, and thus indicates that the heme phenyl substituents exhibit only a minor effect on the core. The core structure of 1 is likewise very analogous to that of the crystallographically characterized tethered complex of Naruta and co-workers,27 {[(TMP)Fe]-O2-[Cu(5MeTPA)]}+. Both molecules display a degree of ruffling of the porphyrin macrocycles, a feature not observed in the previous calculations of 1, presumably due to their lack of phenyl substituents. The ruffling causes the four coordinating nitrogen atoms to deviate slightly from planarity. Thus, the displacement of the Fe out of the heme pseudo-plane (0.56 Å in the case of 2) is reported as an average displacement.
Figure 5.
Unrestricted DFT (BS, ST = 2) optimized molecular structures of [(F8)Fe-(O22-)-Cu(TMPA)]+ (1) (right) and [(F8)Fe-(O22-)-Cu(AN)]+ (2) (left). Hydrogen atoms omitted for clarity.
Table 2.
Selected DFT optimized bond distances (Å) and angles (°) of models [(F8)Fe-(O22-)-Cu(TMPA)]+ (1) and [(F8)Fe-(O22-)-Cu(AN)]+ (2).
| DFT optimized coordinates of 1 |
DFT optimized coordinates of 2 |
|
|---|---|---|
| Cu—O (Å) | 1.945, 2.764 | 2.094, 2.001 |
| O—O (Å) | 1.433 | 1.463 |
| Fe—O (Å) | 2.066, 1.889 | 2.060, 1.902 |
| Fe···Cu (Å) | 3.994 | 3.727 |
| Cu—O—O (°) | 109.4 | 72.5, 65.7 |
| Fe—O—O (°) | 75.7, 62.4 | 74.2, 62.7 |
| Fe—O—O—Cu (°) | 172.7 | 166.1 |
In contrast to the μ-η2:η1 structure of 1, the geometry optimized core structure of 2 displays the peroxo moiety spanning the metals in a μ:η2:η2 configuration, where the peroxo ligand has gone from η1 to η2 on Cu in going from 1 to 2. This is the first example of such coordination in heme-peroxo-Cu chemistry. The most notable metrical parameters predicted by DFT of 2 and listed in Table 2 are: 1) an O—O bond length of 1.463 Å, 2) an intermetallic Fe···Cu distance of 3.727 Å, 3) slightly inequivalent Cu—O bond lengths of 2.094 and 2.001 Å (compared to the η1 structure of 1, where a Cu-O bond length of 1.945 Å is calculated), 4) slightly inequivalent Fe—O bond lengths of 2.060 and 1.902 Å, 5) a slight butterfly of the peroxo core characterized by an Fe—O—O—Cu dihedral angle of 166.1°, and 6) displacement of the Fe out of the pseudo-plane of the porphyrn by 0.53 Å. In general, the structural parameters predicted by theory are in good agreement with those measured by Fe and Cu K-edge EXAFS. In particular, DFT predicts an intermetallic distance (3.727 Å) in good agreement with the Fe···Cu vector observed from Cu and Fe EXAFS (3.63 Å). Furthermore, the predicted O—O bond length (1.463 Å) is well within the classical peroxo manifold. Analytical frequency calculations were performed on 1 and 2, yielding νO—O energies of 889 and 821 cm-1 respectively, which parallel the observed ordering and difference of 810 and 756 cm-1 derived experimentally in 1 compared to 2. Thus, the optimized core is well described by the calculations. Alternative coordination modes were assessed to ensure that a global minimum had been obtained. Specifically, a μ:η2:η1 core geometry was found to be a local minimum on the HS potential energy surface. However, this coordination mode lies higher in energy then the μ:η2:η2 (by ~ 65 kcal/mol), and deviates from the experimentally observed Fe···Cu distance by 0.5 Å (the predicted distance for this coordination mode is ~ 4.2 Å).
Electronic Structure
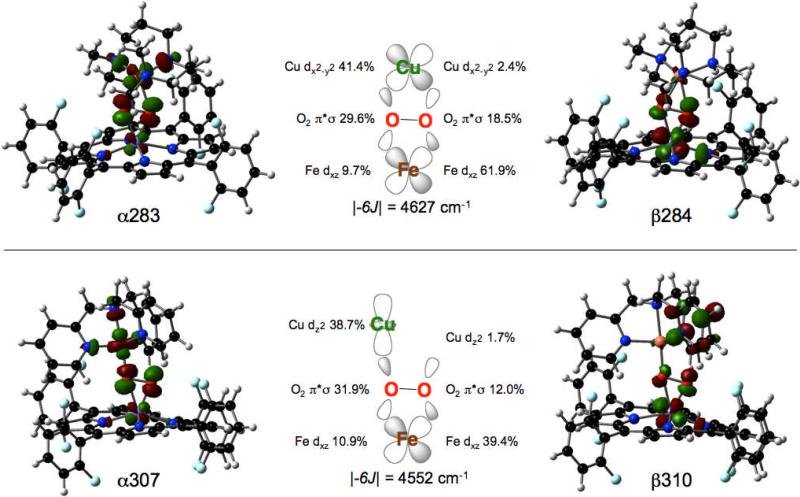
In agreement with the experimentally determined spin of the complexes, both molecules are well described by broken-symmetry wavefunctions, yielding ST = 2 ground state electronic structures, in which the FeIII and CuII are anti-ferromagnetically coupled. In both molecules, one α-hole is localized on the Cu whereas five β-holes are localized on the Fe (Figures 4 and 5),45 The magnitude of the coupling in 1 and 2 between the ST = 2 and ST = 3 states, |-6J|, calculated using Equation 1, is 4552 and 4627 cm-1 respectively (using the Hamiltonian -2JS1•S2), and is in general agreement with the experimentally observed strong antiferromagnetic coupling. However, the nature of the ligands on copper, specifically the denticity, engenders distinct character to the singly occupied, and thus net bonding, orbital on the Cu. In the case of 1, given the propensity of TMPA for 5-coordinate trigonal bypyramidal molecular geometry with O22- bound in a end-on fashion, the singly occupied orbital is of 3dz2 character. In contrast, with the less conformationally demanding AN ligand which allows for square pyramidal geometry and bi-dentate O22-, the singly occupied Cu d orbital of 2 is of 3dx2-y2 character. As depicted graphically in Figure 6, the superexchange pathways in 1 and 2 are via the sigma bonding framework. Specifically, the coupling involves the 3dxz orbital of the Fe (z ∞ to the heme plane and x ∥ to the O—O vector) with the singly occupied orbital of the Cu, 3dz2 of 3dx2-y2 respectively.
Figure 6.
Isosurface plots (0.05 isovalue) and schematic diagrams of the dominant superexchange pathways of 2 (top) and 1 (bottom).
In both molecules, the Fe-O22- bonding is characterized by two interactions (Figures S5 and S6). The in-plane σ interaction is between the Fe 3dxz and the O22- π*σ orbitals and the out-of-plane δ interaction is between the Fe dxy and the O22- π*v orbitals. The orbital basis for the interactions are similar in 1 and 2, but do reflect the differences in peroxo-copper bonding (Tables 3 and 4). In the case of 1, the spin down σ bonding contributions are 12.0 % and 39.4 % for O22- and Fe (Figure 4, Table 3, orbital β310) and the δ bonding contributions are 15.9 % and 76.7 % for O22- and Fe (Figure 4, Table 3, orbital β303). In 2, the spin down σ bonding contributions are 18.5 % and 61.9 % for O22- and Fe (Figure 5, Table 4, orbital β284) and the δ bonding contributions are 13.4 and 80.5 % for O22- and Fe (Figure 5, Table 4, orbital β279). Given that the Fe-porphyrin fragments in 1 and 2 are identical, any differences in bonding must be a result of modulation of the Cu fragment-peroxo bonding. As can be observed from the unoccupied spin-up Cu-O22- anti-bonding molecular orbital of 1, the Cu 3dz2 (38.7%) interacts strongly with the in-plane O2 π*σ (31.9%), which does contain some contribution from the Fe (10.9%) and ligands (Figure 4, Table 3, orbital α307). The unfilled spin-up Cu-O22- anti-bonding orbital of 2 indicates σ-overlap of the 3dx2-y2 (41.4%) with the in-plane O2 π*σ (29.6%), with some Fe (9.7%) and ligand contributions (Figure 5, Table 4, orbital α283). In both molecules, the out-of-plane O2 π*v is largely non-bonding with the otherwise filled Cu d manifold.
Table 3.
Mulliken population analysis of molecular orbitals from a spin-unrestricted BP86 calculation of 1 in the BS (ST = 2) state.
| orbital | level | occ | E(eV) | Cu | O2 | Fe | rest |
|---|---|---|---|---|---|---|---|
| α307 | Cu – O2π*σ | 0 | -5.417 | 38.7 | 31.9 | 10.9 | 18.5 |
| β313 | Fe x2-y2 | 0 | -3.661 | 0.1 | 2.46 | 67.5 | 30.0 |
| β310 | Fe xz | 0 | -4.108 | 1.7 | 12.0 | 39.4 | 47.0 |
| β305 | Fe z2 | 0 | -4.825 | 2.3 | 6.2 | 70.4 | 21.1 |
| β304 | Fe yz | 0 | -5.126 | 0.0 | 9.2 | 66.2 | 24.7 |
| β303 | Fe xy - O2π*ν | 0 | -5.482 | 1.2 | 15.9 | 76.7 | 6.2 |
| | |||||||
| spin density | -0.36 | 0.20, 0.02 | 3.76 | ||||
Table 4.
Mulliken population analysis of molecular orbitals from a spin-unrestricted BP86 calculation of 2 in the BS (S = 2) state.
| orbital | level | occ | E(eV) | Cu | O2 | Fe | rest |
|---|---|---|---|---|---|---|---|
| α283 | Cu – O2π*σ | 0 | -5.653 | 41.4 | 29.6 | 9.7 | 19.3 |
| β285 | Fe x2-y2 | 0 | -4.021 | 0.3 | 2.5 | 68.3 | 28.9 |
| b284 | Fe xz | 0 | -4.508 | 2.4 | 18.5 | 61.9 | 17.2 |
| β281 | Fe z2 | 0 | -5.121 | 1.2 | 5.9 | 75.6 | 17.3 |
| β280 | Fe yz | 0 | -5.511 | 0.6 | 5.6 | 72.6 | 21.2 |
| β279 | Fe xy - O2π*ν | 0 | -5.834 | 0.5 | 13.4 | 80.5 | 5.7 |
| | |||||||
| spin density | -0.56 | 0.10, -0.19 | 0.85 | ||||
Comparison to the bonding observed in μ-η2:η2 and μ-η1:η1 Cu2O2 homo-dimers is instructive.46 In the side-on peroxo complex, [{Cu(Tp)}2-(O2)], which displays a relatively low νO—O of ~760 cm-1, the unoccupied Cu d – O2 π*σ orbital was calculated to be composed of 68 % Cu and 22 % O2. In contrast, in the end-on complex [{Cu(TMPA)}2-(O2)]2+, which displays a νO—O of 830 cm-1 , the unoccupied Cu d – O2 π*σ orbital consists of 43 % Cu and 9 % O2. Thus, the low intraperoxide stretch observed in the side-on case was not a result of differences in the σ bonding framework (i.e. in the side-on complex [{Cu(Tp)}2-(O2)], the peroxo is a better π*σ donor which removes e- density from an anti-bonding orbital, thus, the νO—O should be greater), but rather backbonding into the O22- σ* (O22-σ* mixing into occupied, predominantly Cu d orbitals).47 On this basis, the ~2.3 % calculated difference in Cu-O2 covalency in the σ framework of 1 and 2 does not sufficiently explain the ~50 cm-1 downward shift in the intraperoxide stretching energy observed experimentally between 1 and 2 (2 having more π*σ character) and reproduced by DFT calculations. The more covalent (as defined by the mixing of the filled peroxo π*σ orbital into the unfilled Cu d orbital) σ interaction in fact would argue for a higher νO—O in 2, inconsistent with experiment. Differences in mechanical coupling as a rationale to account for the lower intraperoxide stretch were also considered, but determined to be negligible by systematically increasing the mass of the Cu bound ligand atoms. Surveying the occupied valence orbitals of 1 and 2 for O22- σ* character indicates ~ 4x greater σ* character in the occupied valence orbitals of 2 compared to those of 1. The σ* in 2 acts as a π-acceptor, which weakens the O—O bond, leading to a low νO—O stretching energy. Due to the fact that the σ* orbital is strongly antibonding, a few percent increase of the mixing of the σ* character into an occupied metal based orbital is enough to greatly impact the observed νO—O. Thus, as for in the Cu2O2 dimers, the low observed νO—O in 2 is a result of σ* backbonding in the μ-η2:η2 core structure.
| Eq. 1 |
Thermal Decomposition of 1 and 2
The thermal decomposition of high-spin heme-peroxo-Cu complexes 1 and 2 yields the corresponding μ-oxo compound [(F8)FeIII-(O2-)-CuII(TMPA)]+ (1a) and [(F8)FeIII-(O2-)-CuII(AN)]+ (2a) with distinct UV-vis bathochromic shifts in their Soret and Q bands (1: λmax = 412, 526 nm;48 1a: λmax = 433, 553 nm49,50 and 2: λmax = 418, 538, 561 nm; 2a: λmax = 440, 557 nm). The μ-oxo complexes also have distinct 1H-NMR features at –80° C with a pyrrole resonance signal at ~105 ppm. Separate measurements29 indicate that ~0.5 equiv. of dioxygen is released during the μ-peroxo to μ-oxo conversion, indicating this process involves a disproportionation reaction, 2 [FeIII-(O22-)-CuII]+ → 2 [FeIII-(O2-)-CuII]+ + O2. Insights into the mechanism(s) for such reactions have not been sought or obtained, but similar kinds of processes are known and studied for related non-heme diiron species.51,52
X-ray Structure of μ-oxo complex [(F8)FeIII-(O2-)-CuII(AN)]+ (2a)
The comparison between a new X-ray structure of [(F8)FeIII-(O2-)-CuII(AN)]+ (2a), presented here (also see Supporting Information), and the previously described X-ray structure of [(F8)FeIII)-(O2-)-CuII(TMPA)]+ (1a)49 (Figure 7), reveals that in both cases the metal-oxygen bond distances are all similar and characteristically short.53,54 The Fe-O bond length in 1a [1.740 Å] and 2a [1.747 Å] are similar to those of μ-oxo-diiron(III)-(porphyrinates),55 and the short Cu-O bond distances [1.856 Å in 1a and 1.815 Å in 2a] are not far off from values observed for bis-μ-oxodicopper(III) complexes (Cu-O = 1.8 Å).33,56 To further compare, a high-spin (heme)FeIII-hydroxide Fe-O bond lengths is 1.86 Å,57 while for example CuII-hydroxide Cu-O(H) bond lengths in complexes with the CuII-O(H)-FeIII (1.89-1.95 Å)50,58 or CuII2(OH–)2 / CuII(OH–) core (1.9 – 2.0 Å) are greater.59-67
Figure 7.
Core structure depiction of μ-oxo complexes [(F8)Fe-(O2-)-Cu(TMPA)]+ (1a) and [(F8)Fe-(O2-)-Cu(AN)]+ (2a). An ORTEP diagram of the cationic portion of 2a along with other X-ray crystallographic data are given in the Supporting Information.
Most interestingly, there is a large difference in the bond angle between the two structures. Complex 1a, as most other (porphyrinate)FeIII-O-CuII complexes with tetradentate copper ligands,4,6 have near-linear core structures, but the Fe-O-Cu bridge in 2a is severely bent to an angle of 149.5° as observed in the other complex [(F8)FeIII-O-CuII(LMe2N)]+ (6) with tridentate copper ligand.38 We note that a somewhat related μ-hydroxo complex, [(OEP)Fe-(OH)-Cu(Me5dien)(OClO3)](C1O4) (OEP = octaethylporphyrinate2–); Me5dien = tris(2-(N,N-dimethylamino)-ethyl)amine), possesses a bent FeIII-(OH-)-CuII core (),58 as would be expected for a protonated oxo group changing its hybridzation from sp (μ-oxo) to sp2 (μ-hydroxo). For the μ-oxo complexes [(F8)Fe-(O2-)-Cu(TMPA)]+ (1a) and [(F8)Fe-(O2-)-Cu(AN)]+ (2a), the differences in observed structures once again illustrate how the nature of the copper chelate (tri- versus tetradentate) influences the structure of heme-copper/dioxygen adducts. Such structural (Chart 2) and reactivity effects are already well known in copper-dioxygen chemistry.31,32,68
Reactivity of high-spin heme–peroxo–copper complexes 1 and 2
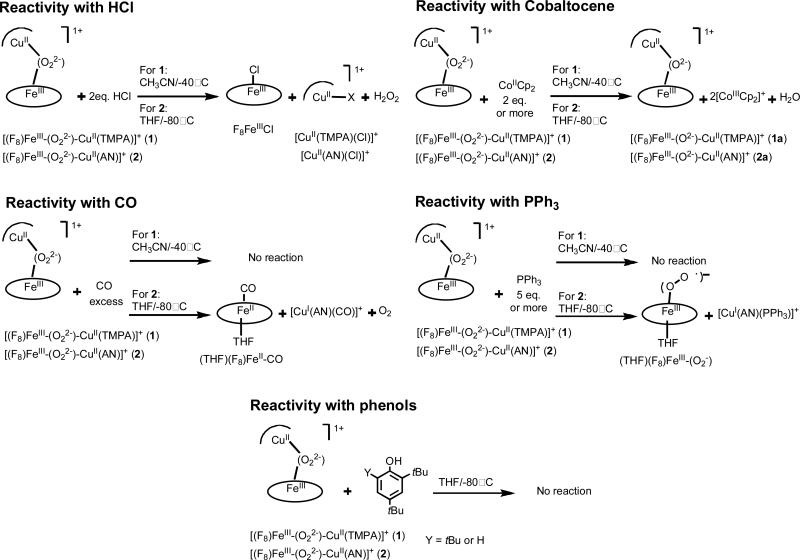
We have previously37 described the reactivity of [(F8)Fe-(O22-)Cu(TMPA)]+ (1) and [(F8)Fe-(O22-)-Cu(AN)]+ (2), along with peroxo complexes 3 and 4 (Chart 1), toward different reagents, HCl, Co(Cp)2, CO, PPh3 and tBu-substituted phenols. As an important goal of the current report is to contrast reactivity when the spin-state of the heme-iron is changed (vide infra), we summarize here major aspects of the reactivity observed for the HS complexes 1 and 2 (Scheme 3).
Scheme 3.
Hydrochloric acid was added to 1 and 2, in order to study the possibility of forming and studying protonated dioxygen species (e.g. hydroperoxo complexes).9,10,12 Protonation and formation of such species are likely important in triggering HCO enzymatic O-O reductive cleavage events. When peroxo complexes 1 and 2 were exposed to one or two equivalents of HCl, neither low-temperature UV-vis nor 1H NMR spectroscopic investigations showed any evidence for a hydroperoxo intermediate, i.e., formation of a new absorption feature was not found in the reaction. Further, electron paramagnetic resonance (EPR) spectroscopic and ESI-MS analyses of the reactions products revealed the formation of F8FeIII-Cl and CuII-Cl. H2O2 was released providing complementary evidence that O2 adducts 1 and 2 have a peroxidic nature.37
Peroxo complexes 1 and 2 possess dioxygen in its two-electron reduced state. Thus, to complete the reductive cleavage of dioxygen to the level of water, two more electrons are needed. The strong outer-sphere reductant cobaltocene (CoCp2) was, thus, used to provide these reducing equivalents.29,37,48 Reaction of two equivalents of CoCp2 does effect O-O cleavage and the formation of the corresponding previously structurally characterized μ-oxo-bridged compounds [(F8)FeIII-(O2-)-CuII(TMPA)]+ (1a) and [(F8)FeIII-(O2-)-CuII(AN)]+ (2a).37 This result differs from an earlier work of Collman and coworkers where they showed that the addition of CoCp2 to heme–dioxygen-copper complexes containing appended imidazole axial base for iron lead to fully reduced FeII/CuI and the reduction of O2 to H2O2.69-72
1 and 2 were also exposed to carbon monoxide and PPh3,37 reagents known to either displace the peroxide or to be subject to oxidation giving CO2 (in the case of CO (in CcO))73 or O=PPh3 in the case of triphenylphosphine. Compound 2 reacted with excess CO(g) to form the CO-adduct (THF)(F8)FeII-CO and copper monoxide adduct [CuI(AN)(CO)]+ while also releasing dioxygen. Surprisingly, 1 does not react with CO. 2 reacts with PPh3 to displace the peroxo ligand from copper, but not from iron, thus involving a formally partial reduction reaction. The products are the iron–superoxo species (solvent)(F8)FeIII−(O2•–) and the reduced CuI–phosphine adduct [CuI(AN)(PPh3)]+. Interrogation of the product solutions using 31P-NMR spectroscopy showed only the presence of PPh3 (and no O=PPh3). Unexpectedly, complex 1 displays no reaction toward PPh3 under these conditions. In summary, there are clear differences in FeIII−(O22-)−CuII reactions with CO or PPh3 that reflect variations in the detailed nature of the peroxo structure and reactivity induced by the nature of the copper ligand (tri- vs tetradentate).
The examination of the addition of phenols to heme–Cu/O2 adducts was inspired by reactivity observed for the “mixed-valent” CcO enzyme form, wherein an active-site tyrosine, found crystallographically to be covalently tethered to a copper bound histidine, is thought to act as a net hydrogen atom donor (see the Introduction and further discussion below).4 When either 2,4-tBu2-phenol or 2,4,6-tBu3-phenol, i.e. good hydrogen atom (H+ + e–) donors, was added to low-temperature solutions of 1 or 2, no reaction occured (Scheme 3). This is an important finding, especially when contrasted with the chemistry of a low-spin analog (vide infra).
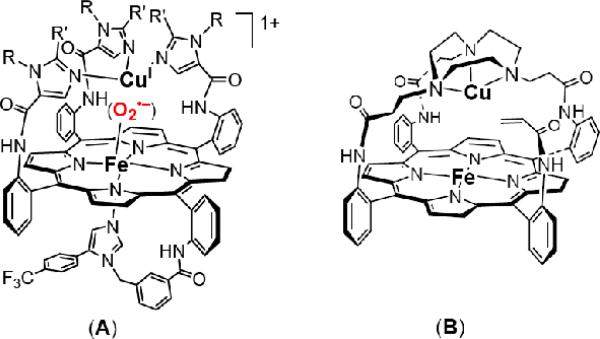
Collman and co-workers74 recently reported that a phenol as hydrogen-atom source can be added to a heme–superoxo–copper complex, (i.e. a low-spin FeIII(O2•–)···CuI moiety) resulting in reductive O—O cleavage and formation of a phenoxyl radical (A, Chart 3). This heme–Cu complex possesses an internal (i.e., appended) imidazole axial base for iron. A closely related complex possessing an internal phenol group (i.e., with one of the R-groups, Chart 3 (A), is a phenol) can electrocatalytically reduce dioxygen to water.22 In that system, electronic structural and spin-state issues have not been addressed beyond the superoxo complex and possible intermediates after O-O cleavage. In systems we have studied, (F8)FeIII(O2-)···CuI species are transient (but detectable) intermediates (Figure 8)39 in the reduction of O2 from the FeII···CuI to FeIII-(O22–)-CuII((ligand) complex stage, the latter being stable at –80° C and amenable to study, i.e., those complexes in Chart 1.
Chart 3.
Figure 8.
UV-vis detction of the transient [(2L)FeIII-(O2-)...CuI(NCEt)]+ preceding the formation of [(2L)FeIII-(O22-)-CuII(NCEt)]+ by oxygenation of [(2L)FeII···CuI(NCEt)]+ at -90° C in EtCN/CH2Cl2 6%.
It is notable that for non-heme iron FeIII−OOR complexes, low-spin rather than high-spin species are amenable to reductive O−O cleavage chemistry as a consequence of weakened peroxide O−O bonds and strengthened Fe–O bonds.75,76 From all the information available, as discussed thus far in this report, it was clear one should investigate, compare and contrast the chemistry of high-spin FeIII−(O22-)−CuII complexes with low-spin analogues, if we could generate the latter. This was accomplished, as described below.
Generation of a low-spin heme-peroxo-copper Complex
The low-spin heme−O2−copper complex which we formulate as [DCHIm(F8)FeIII-(O22-)-CuII(AN)]+ (2b) was generated by the addition of one equivalent of 1,5-dicyclohexylimidazole (DCHIm) to a solution of high-spin [(F8)FeIII-(O22-)-CuII(AN)]+ (2) in THF at -80° C (Scheme 4). Collman's research group reported the first example of a well-characterized discrete dioxygen adduct in a heme/Cu assembly using a sophisticated superstructured “capped” porphyrin with appended triazacyclononane as ligand for copper (B, Chart 3).69,71 When a solution of [(α3TACNαAcr)FeIICuI]+ is mixed with large excess (500 equiv.) of DCHIm and subsequently exposed to dioxygen, rapid and irreversible formation of a low-spin adduct [(DCHIm)(α3TACNαAcr)FeIII−(O22-)-CuII]+ occurs.
Scheme 4.
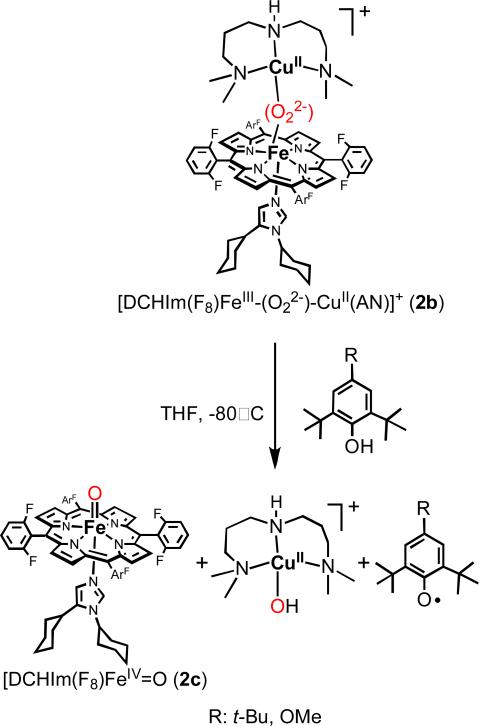
With our system, the UV-vis monitoring show that only one equivalent of DCHIm is required for the complete transformation of high-spin species 2 (418, 538, 561 nm) to low-spin species [(DCHIm(F8)FeIII-(O22-)-CuII(AN)]+ (2b) (421, 537 nm) (Figure 9). A 2H-NMR study of 2b formation was performed using pyrrole-deuterated version of F8 (Figure 10). After the generation of the high-spin peroxo (2) (pyrrole resonance at ~ 95 ppm), the addition of 1 equiv. of DCHIm is accompanied by the shift of the pyrrole signal to the diamagnetic region (δpyrrole ~ 8.3 ppm). This behavior suggests that in 2b, the presence of antiferromagnetic coupling between the low-spin (S = 1/2) six-coordinate Fe(III) and the d9 Cu(II) (also S = ½) centers occurs through the peroxo bridge. The latter's peroxidic nature is not yet unequivocally determined.77 However, evidence that that [DCHIm(F8)FeIII-(O22-)-CuII(AN)]+ (2b) possesses a peroxidic intact O-O bond is suggested by its reactivity with hydrochloric acid to release hydrogen peroxide in good yield (see Experimental Section). Alternative formulations of the new species 2b as either 1) a mixture of (F8)FeIII-(O22-)-FeIII(F8) (δpyrrole 15-19 ppm},26 and [(AN)CuII-(O22-)-CuII(AN)]+ (formed by disproportionation of 2b) or 2) a O—O cleaved78 product give ferryl complex [(DCHIm)(F8)FeIV=O] (2c) (δpyrrole 4-5 ppm), can be ruled out based on its δpyrrole ~ 8.3 ppm assignment and UV-vis spectroscopic peak positions.41 The formation of an analogous complex to 2b was also observed using 4-(dimethylamino)pyridine (DMAP) instead of DCHIm as the exogenous axial ligand.
Figure 9.
UV-visible spectra monitoring the generation of the low-spin peroxo complex [DCHIm(F8)FeIII-(O22-)-CuII(AN)]+ (2b) (λmax= 421, 537 nm) from the high-spin peroxo complex [(F8)FeIII-(O22-)-CuII(AN)]+ (2) (λmax= 418, 538, 561 nm) in THF at -80° C.
Figure 10.
2H NMR spectra of the generation of [(F8)FeIII-(O22-)-CuII(AN)]+ (2) and [DCHIm(F8)FeIII-(O22-)-CuII(AN)]+ (2b) successively from oxygenation of a 1:1 mixture of [CuI(AN)]+ and the pyrrole-deuterated version of [(F8)FeII] (Acetone-d6 at – 80° C).
By contrast, the addition of an axial base such as DCHIm or DMAP to a solution of [(F8)FeIII-(O22-)-CuII(TMPA)]+ (1) effects the release of O2 and the formation of a mixture of [CuI(TMPA)]+ and six-coordinate low-spin compound (F8)FeII–(Base)2 (Scheme 4). The reaction was followed by UV-vis spectroscopy as shown in the Supporting Information (Figure S2): the peroxo compound 1 exhibits Q band at 558 nm with a shoulder at 535 nm (CH3CN, – 40° C) and upon addition of 1 to 5 equiv. of DMAP a new spectrum with λmax = 527 nm is formed assigned to (F8)FeII(DMAP)2. To support this assignment a control experiment was conducted in which the addition of DMAP to a solution of the mixture of (F8)FeII/[CuI(TMPA)]+ yielded the same spectrum because of the formation of (F8)FeII(DMAP)2. The evolution of 0.9 equiv O2 was determined by exposing evolved gases to an alkaline pyrogallol test solution;79 within experimental error, this finding is consistent with the quantitative oxidation of the peroxide moiety to molecular oxygen.
Reactivity of low-spin peroxo [DCHIm(F8)FeIII-(O22-)-CuII(AN)]+ complex (2b) toward phenols
To study the reactivity of the low-spin peroxo [DCHIm(F8)FeIII-(O22-)-CuII(AN)]+ (2b) complex toward phenols, 2,4-tBu2-phenol was employed first as substrate. The rationale for selection of this phenol was if hydrogen atom abstraction (H+ + e-) occured, the phenol would couple to form 3,3’,5,5’tetra-tert-butyl-2,2’-dihydroxylbiphenyl which can be detected and quantified as stable product.80-84 We have shown above (Scheme 3) that the high-spin peroxo complex 2 doesn't react with phenols. The addition of 1 equiv. of 2,4-tBu2-phenol to a solution of low-spin peroxo complex 2b in THF at -80° C leads to the formation of the coupled phenol product (3,3’,5,5’tetra-tert-butyl-2,2’-dihydroxylbiphenyl) (Scheme 5). GC-MS analyses of the reaction mixture at room temperature revealed that the yield of the reaction is greater than 65%. Further spectroscopic investigations show that the oxidative coupling of the phenol is accompanied by the reductive cleavage of the peroxo bridge and the formation of CuII-hydroxide complex ([CuII(AN)(OH)]+) and ferryl heme [(DCHIm)(F8)FeIV=O] (2c) (Scheme 5). The evidence for the presence of [CuII(AN)(OH)]+ comes from observation of a characteristic CuII-OH EPR signal of the frozen reaction mixture (A∥ = 127 × 10-4 cm-1, A∞ = 35 × 10-4 cm-1, g∥ = 2.24 and g∞ = 2.06) (Figure S3) and from ESI-MS spectrometry at room temperature (calculated m/z = 267.1 for C10H26CuN3O; found, m/z = 267.5). The EPR spectrum of the reaction mixture after warming up to room temperature presents the same signal for CuII-OH in addition to a characteristic signal for the well known stable compound (F8)FeIII-OH (g = 5.8), which derives from thermal decay of [(DCHIm)(F8)FeIV=O] (2c).41 The UV-vis monitoring of the O-O cleavage reaction shows a shift of the 2b Q band at 535 nm to a new band at 540 nm (Supporting Information, Figure S1). This band was also observed for an authentic [DCHIm(F8)FeIV=O] generated by the reaction of [(F8)FeIII]2-(O22-) with DCHIm in noncoordinating solvent.41,85 The ferryl nature of the product solution and presence of 2c was also established by its reactivity toward different substrates: (a) the low-temperature addition of one equivalent of [CuI(AN)]+ leads to the formation of [(F8)FeIII-(O2-)-CuII(AN)]+ (2a) (90% yield calculated by UV-vis spectroscopy) (Scheme 5); the same redox reaction is also observed with the authentic ferryl-oxo complex, as determined in a separate ‘control’ experiment, (b) the addition of triphenylphosphine to the reaction solution led to triphenylphosphine oxide, also a criterion used to demonstrate the presence of a ferryl (FeIV=O) species.86
Scheme 5.
To confirm the electron and proton transfer from the phenol to 2b and the formation of the phenoxyl radical prior to the phenol coupling, we used 2,4,6-tri-tert-butylphenol and the 2,6-di-tert-buyl-4-methoxyphenol (Scheme 6), two phenols known to form relatively stable and detectable phenoxyl radicals.87-89 The reaction of EPR-silent 2b with those phenols in THF at -80° C leads to the formation of phenoxyl radical which displays an intense and characteristic EPR signal at 2.006 in the case of 2,4,6-tri-tert-butylphenol and at 2.008 in the case of 2,6-di-tert-buyl-4-methoxyphenol (Figure 11).
Scheme 6.
Figure 11.
a) EPR spectra recorded at 77 K of generated phenoxyl radicals by the reaction of [DCHIm(F8)FeIII-(O22-)-CuII(AN)]+ (2b) and 2,4,6-tri-tert-butylphenol (A) or 2,4-di-tert-butyl-4-methoxylphenol (B) at -80° C in THF. As determined in separate experiments, at equal concentrations of a phenoxyl radical with a copper(II) complex, the radical g ~ 2 signal peak intensity is vastly greater than the peak intensities for the g ~ 2.0-to-2.3 signals resultant from the copper(II) ion due to the differences in peak width, which is why the latter is essentially not observable. Also, within the resolution of the EPR experiments conducted, the g-values are indistinguishable.90,91 b) UV-vis monitoring of the reaction of 2b with 2,4-di-tert-butyl-phenol at -80° C.
Summary & Perspectives
Our investigation into the nature of heme-peroxo-copper complexes has revealed that the denticity (tri- vs. tetradentate) of the copper ligand can have major influence on the molecular structure and the electronic-structure/bonding in such species. Using rR and EXAFS spectroscopic analyses coupled to DFT calculations, we have previously established a μ-(O22-) side-on to the FeIII ion and end-on to CuII (i.e., μ-η2:η1-peroxo) center binding in the complex with tetradentate copper ligand [(F8)FeIII-(O22-)-CuII(TMPA)]+ (1). However, in new investigations described here, side-on/side-on (μ-η2:η2) μ-peroxo coordination for the complex with tridentate copper ligand is established for [(F8)FeIII-(O22-)-CuII(AN)]+ (2). Insights into the electronic-structure/bonding of 2 and comparisons to 1 are described. A structural difference, for tridentate versus tetradentate copper ligand, was also directly observed by comparison of the X-ray structures of the μ-oxo complexes [(F8)FeIII-(O2--CuII(TMPA)]+ (1a) and newly reported species [(F8)FeIII-(O2--CuII(AN)] (2a), these obtained respectively by thermal decomposition of 1 and 2.
These electronic and structural differences between the two dioxygen adducts result in a difference in their reactivity toward substrates such as CO or PPh3. More interestingly, the difference between the two high-spin heme-peroxo complexes 1 and 2 is the ability of the latter with tridentate copper ligand to form a new low temperature stable low-spin complex [DCHIm(F8)FeIII-(O22-)-CuII(AN)]+ (2b) in the presence of an axial base, tentatively assigned as a peroxo complex. Under the same conditions, complex 1 (tetradentate copper ligand) reacts with DCHIm only to break apart the heme-peroxo-copper assembly, essentially reversing its formation and releasing O2.
In this study, we have also shown that in contrast to the behavior of the high-spin complexes 1 and 2, the new low-spin complex 2b reacts with phenols performing O-O bond cleavage which is accompanied by formation of the high valent ferryl heme species [DCHIm(F8)FeIV=O] (2c).77 This reaction occurs as a net hydrogen atom abstraction (H+ + e-) from the phenol with generation of a phenoxyl radical.
Given the new reactivity demonstrated by the low-spin complex, considerable future efforts are needed to determine the geometric and electronic structure of the low-spin system in comparison to the structure of high-spin system determined here. Such comparisons can contribute to a better understanding of the differenential reactivity observed dependent on the spin state in addition to different peroxo bridging modes. It is intruiging to consider if a bridging peroxo species could be identified as a fleeting intermediate in cytochrome c oxidase, would its spin state also be of central importance? Based on chemical precedent, and given the proximal histidine present in heme-copper oxidase enzymes, one would surmise a low-spin state was at work. Nonetheless, previous studies have suggested heme a3 proximal histidine dissociation from iron, a mechanism which is known to occur in the evolutionarily related heme/non-heme diiron NO reductases, suggestive of a high-spin oxidant.
However, in addition to the spin state contribution to the O—O bond cleavage in heme-copper oxidases, proton and electron transport must also be considered. The order in which protons and electrons enter catalysis may shed light on the nature of the active oxidant. Our interest is especially piqued by the speculation of a discreet hydroperoxo species (either Cu or Fe bound, or both), given the generality of such a potential reaction. As such we have embarked on studies, some of which are alluded to herein, to test the possibility of such species and their respective reactivity for insight into the mechanism of heme-copper oxidases.
Within the context of 1) the nature of the active oxidant and 2) the role of protons and electrons in driving a particular reaction coordinate, the issue of the active site tyrosine remains intruiging to us from a mechanistic standpoint. While it is generally accepted that a tyrosine radical forms at some point during the reaction, it remains an open question as to when its proton and electron enter the catalytic cycle and by what means. Specifically, 1) does the active site oxidant do direct H-atom abstraction from the tyrosine, or does the reaction occur stepwise, and 2) do the transfers occur before or after the O—O bond is cleaved. Stepwise mechanisms would indicate the importance of the Tyr-His crosslink to form an adequate superexchange pathway for electron transfer and would significantly enhance the functional role of copper in mediating the electron transfer. Finally, how would such a crosslink be formed in an active site? Presumably one would invoke some sort of oxidative coupling involving reduced oxygen species in the proenzyme before the first turnover (the cofactor biogenesis reaction), but would that process require both copper and iron? Hopefully, our foray into small molecule analogs as probes of these potential mechanistic scenarios continues to shed light on these critical questions, given the enormous scope of the processes involved and applicability to problems ranging from disease to energy conversion.
Experimental Section
Materials and Methods
All reagents and solvents were purchased from commercial sources and were of reagent quality unless otherwise noted. Tetrahydrofuran (THF) was distilled from sodium/benzophenone under argon, and acetonitrile (CH3CN) was purified over and activated alumina column. Preparation and handling of air-sensitive compounds were performed under MBraun Labmaster 130 inert atmosphere (<1 ppm O2, <1 ppm H2O) glove box filled with nitrogen. Deoxygenation of solvents was effected either by repeated freeze/pump/thaw cycles of by bubbling with argon for 30-45 min.
Low-temperature UV-vis Low-temperature UV-vis spectra were recorded on a Hewlett-Packard Model 8453A diode array spectrometer with HP Chemstation software; the instrument was equipped with a variable-temperature Dewar and cuvette. 1H and 2H NMR spectra were measured on a Varian XL-400 NMR instrument and Bruker 400 MHz spectrometer at ambient or a low temperatures. All spectra were recorded in 5 mm o.d. NMR tubes, and chemical shifts δ (ppm) were referenced either to an internal standard (Me4Si) or to residual solvent peaks. Electron paramagnetic resonance (EPR) spectra were obtained in frozen solutions with 4 mm o.d. quartz tubes in a Bruker EMX spectrometer operating at X-band using microwave frequencies around 9.5 GHz, with sample temperature maintained at 77 K. EPR spectra were referenced to 2,2-diphenyl-1-picrylhydrazyl (g = 2.0036). Elemental analyses were performed by Desert Analytics (Tuscon, AZ). The coupled phenol was analyzed by gas chromatography-mass spectrometry (GC-MA) on a Shimadzu GC17A/QP5050A instrument equipped with a non-polar column (DB-5 ms). Helium was used as the carrier gas at a flow rate of 27 cm s-1. Injections were made in split mode using an initial column temperature of 100° C. The temperature was raised by 20° C per min to 300° C with an initial and final hold time of 3 min and 2 min.
XAS Data Acquisition
The Cu and Fe K-edge X-ray absorption spectra of [(F8)FeIII-O2-CuII(AN)](ClO4) in solution form were measured at the Stanford Synchrotron Radiation Lightsource (SSRL) on the focused 16-pole, 2.0-T wiggler beam line 9-3 under storage ring parameters of 3 GeV and 80-100 mA. A Rh-coated premonochromator flat bent mirror was used for harmonic rejection and vertical collimation, while a bent cylindrical Rh-coated post-monochromator mirror was used for focusing. A Si(220) double crystal monochromator was used for energy selection. ~150 μL of the solution samples were loaded into 2 mm Delrin XAS cells with a 38 μm Kapton window. The samples were immediately frozen and stored under liquid nitrogen. The samples were maintained at a constant temperature of ~10 K during data collection using an Oxford Instruments CF 1208 continuous-flow liquid helium cryostat. A Canberra solid-state Ge 30-element array detector was used to collect Kα fluorescence data. Data reported here are up to k = 12.8 Å-1 for Cu in order to avoid interference from the Zn K-edge, and up to k = 16 Å-1 for Fe. Internal energy calibration was performed by simultaneous measurement of the absorption of the corresponding metal foil placed between two ionization chambers located after the sample. The first inflection point of the foil spectrum was assigned to 8980.3 eV for Cu and 7111.2 eV for Fe. To reduce photoreduction, data were collected on four distinct and physically separate spots, two spots for Cu and two spots for Fe. A total of 12 and 19 scans were measured at the Cu and Fe K-edge respectively with no significant change from photoreduction observed.
XAS Data Analysis
The energy-calibrated averaged data were processed by fitting a second-order polynomial to the pre-edge region and subtracting this from the entire spectrum as a background. A three-region spline of orders 2, 3, and 3 was used to model the smoothly decaying post-edge region. The data were normalized by scaling the spline function to an edge jump of 1.0 at 9000 eV for Cu and 7130 for Fe. This background subtraction and normalization was done using PySpline.92 The least-squares fitting program OPT in EXAFSPAK93 was used to fit the data. Initial ab initio theoretical phase and amplitude functions were generated in FEFF 7.094 using crystallographic parameters of the oxo complex [(F8)Fe-O-Cu (AN)]+ as the starting model. The final model used was based on the DFT optimized structure. Atomic coordinates were further adjusted as necessary as fits were improved. During the fitting process, the bond distance (R), and the mean-square thermal and static deviation in R (σ2) were varied for all components. The threshold energy (ΔE0) was also allowed to vary for each fit but was constrained to the same value for all components in a given fit. Coordination numbers (N) were systematically varied to provide the best chemically viable agreement to the EXAFS data and Fourier transform but was fixed within a given fit.
Resonance Raman
Samples were prepared as previously described as 1mM THF solutions in NMR tubes. Samples were excited at 413 nm using a Coherent I90C-K Kr+ ion laser while the sample was immersed in a liquid nitrogen cooled (77 K) EPR finger dewar (Wilmad). Power was 2 mW at the sample. Data were recorded while rotating the sample to minimize photodecomposition. The spectra were recorded using a Spex 1877 CP triple monochromator with a 1200 groves/mm holographic spectrograph grating, and an Andor Newton CCD cooled to -80° C. Spectra were calibrated to toluene.
Computational methods
Density functional calculations were performed using Gaussian03.95 Models of 1 and 2 and were constructed based loosely on crystallographically derived parameters of the monometallic molecular fragments. Calculations were performed with the BP86 functional within the unrestricted formalism (B3LYP incorrectly predicts the ground state as HS (S = 3), and a previous assessment of functional dependence for the truncated [(P)Fe-O-Cu(AN)]+ complex supports the choice of the BP86 functional). A triple-ζ basis set with polarization (6-311G*) was used on the Cu, Fe, and O2. A split-valence basis (6-31G) was used on all remaining atoms (F, C, H), supplemented by polarization functions in the case of N (6-31g*). Density fitting was employed and models were optimized to default convergence criteria (unless otherwise noted) on ultrafine integration grids. Models were first subjected to a coarse optimization on the high-spin (ST = 3) surface, followed by a single point calculation to obtain a spin-polarized broken-symmetry solution. The models were then reoptimized on the BS (ST = 2) surface to yield final atomic coordinates. Analytical frequency calculations were performed to ensure stationary points on the models’ respective potential energy surfaces had been reached. No imaginary frequencies were obtained. Mulliken population analysis was performed with QMForge.96
Generation of [(F8)Fe-(O2-)-Cu(AN)]+ BArF- (2a)
In a 100 mL Schlenk flask equipped with a stir bar were placed, in the drybox, 210 mg (0.2 mmol) of (F8)FeII and 186 mg (0.2 mmol, 1.0 equiv.) of [(AN)CuI](BArF), to which was added air-free freshly distilled THF (20 mL). The reaction mixture was cooled to -80° C (dry ice-acetone bath) and stirred for 30 min. The solution was subject to O2 purging and finally allowed to warm slowly at room temperature and then layered with 50 mL of deoxygenated heptane. After 12 h, the solution was filtered and the black microcrystalline solid dried in vacuo (245 mg, 70% yield). Anal. Calcd. (found): C, 53.28 (53.22); H, 2.58 (2.59); N, 5.58 (5.63). 1ååH NMR (CD2Cl2): δ (s, 8H, pyrrole), 9.8-9.14 (d, 8H, m-phenyl), 7.67-7.51 (m, 16H, 8H BArF + p-phenyl); UV-visible (CH2Cl2): 440, 557 nm.
Generation and Characterization of [(DCHIm)(F8)FeIII-(O22-)-CuII(AN)]+ (2b)
(a) UV-vis Spectroscopy
[(F8)FeIII-(O22-)-CuII(AN)]+ (2) was synthesized as previously reported.37 To 4 mL of 0.36 mM THF solution of dioxygen adduct (2), 1.0 equiv. (100 μL of 14.4 mM, 0.30 mg) of DCHIm was added to generate low spin [(DCHIm)(F8)FeIII-(O22-)-CuII(AN)]+ which was characterized by UV-vis spectra [λmax (ε, mM-1cm-1) = 421 (soret: 146.5), 537 (8.2) nm]. Upon warming up the solution leads to the formation of hydroxo complex [(F8)FeIII-OH] with λmax (ε, mM-1cm-1) at 412 (156.3) and 572 nm (9.3).
(b) 2H NMR Spectroscopy
In the glovebox, 1:1 mixture of (d8-F8)FeII.H2O (3.0 mg, 3.5 mM) and [CuI(AN)]+ (3.32 mg, 3.5mM) was taken in 5 mm NMR tube and dissolved in 0.5 mL of THF. To the cold THF solution (at – 80° C, dry ice – acetone) of (d8-F8)FeII.H2O and [CuI(AN)]+, 2 mL of O2 was bubbled and the NMR was recorded at -80° C. A peak at 96 ppm was observed as reported previously, corresponds to the chemical shift of pyrrole deuterium atoms of [(d8F8)FeIII-(O22-)-CuII(AN)]+. To the same NMR tube 1.5 equiv. (1.2 mg of DCHIm in 0.05 mL of THF) of DCHIm was added, new peak at upfield 8.31 ppm was observed.
UV-vis spectroscopy monitoring of the reaction of [(F8)FeIII-(O22-)-CuII(TMPA)]+ (1) with axial ligand bases
In a modified low-temperature cuvette assembly equipped with a Schlenk-type sidearm, a solution of (1) was generated in CH3CN at – 40° C as previously described.37 A stock solution of equimolar amounts of (F8)FeII•H2O (13 mg) and [CuI(TMPA)(CH3CN)](ClO4) (8 mg) in 6 g deoxygenated CH3CN is prepared in glovebox and 0.3 g of stock solution is diluted to 10 g and transferred to the UV-vis cuvette. Generation of the peroxo adduct (λmax = 558, 535 (sh) nm) is accomplished by bubbling excess dioxygen to the solution at -40° C. Removal of excess O2 is performed by application of 3-5 vacuum/Ar cycles. Then 1, 3 and 5 equiv of 4-(dimethylamino)pyridine (DMAP) (50, 150 and 250 μl respectively of DMAP stock solution prepared with 19.5 mg DMAP in 10 ml CH3CN) were added and the respective spectra recorded at – 40° C (see Figure S2). The new Q-band spectral feature (λmax = 527 nm) is assigned to the formation of (F8)FeII(DMAP)2. A control experiment by addition of DMAP to a solution of the mixture of (F8)FeII/[CuI(TMPA)(CH3CN]+ yielded the same spectrum. The experiment was repeated in the non-polar solvent CH2Cl2 (instead of CH3CN) and also using 1,5-(dicyclohexyl)imidazole (DCHIm) as axial ligand base (instead of DMAP) in CH3CN, and the results were essentially the same in both cases.
Reaction of [(F8)FeIII-(O22-)-CuII(TMPA)]+ (1) with DMAP – Determination of dioxygen evolution
Dioxygen evolution was quantitatively determined using an alkaline pyrogallol solution (1,2,3-trihydroxybenzene), following a methodology used frequently in our laboratory.29,37 In the glovebox, equimolar amounts of (F8)FeII•H2O (40 mg) and [CuI(TMPA)(CH3CN)](ClO4) (24 mg) were dissolved in 10 ml of deoxygenated CH3CN, transferred to a 50 ml Schlenk flask with a stir-bar and capped with a rubber septum. The flask was taken out of the glovebox, the solution was cooled down to –40° C (adding dry ice to acetone bath, temperature followed by thermocouple), and then O2 was gently bubbled into the solution using a syringe with long needle. To ensure complete formation of the peroxo compound, the solution was allowed to stand in the cold bath for 30 minutes. Excess/free O2 is removed via 5 freeze-pump-thaw cycles. In parallel with the reaction, 4.0 g of pyrogallol were dissolved in 25 ml of deoxygenated 50% KOH solution in a specially designed 100 ml Schlenk flask possessing a 2 mm path length cuvette; its UV-vis spectrum was recorded. Excess DMAP (10 equiv.: 1 ml solution prepared with 293 mg DMAP in 5 ml CH3CN) was added to the peroxo adduct solution thus allowing O2 release. The O2 was then moved from the reaction flask to the pyrogallol flask by passing slowly argon through the headspace of the reaction flask thus moving the O2 through a cannula to the pyrogallol flask. The O2 was then bubbled directly into the pyrogallol solution whose color changed from faint beige to dark brown after some minutes. After 20-30 minutes, the pyrogallol solution ceased becoming darker and its UV-vis spectrum was recorded. The amount of O2 released was determined using a calibration curve where absorbance (400 nm) = (0.0716 × mL O2) + 0.025.29 Based on the stoichiometry of the reaction where 1 equiv. of peroxo compound decomposes to give 1 equiv. of O2, two experiments yielded 90 and 91% O2 detection.
Reaction of [(F8)FeIII-(O22-)-CuII(TMPA)]+ (1) with DMAP – Characterization of the product (F8)FeII(DMAP)2
In the same experiment described above, (F8)FeII(DMAP)2 precipitated spontaneously and was isolated by filtration under argon, washed, vacuum-dried for 2 hours and finally stored in glove box. Yield was 70 %. The purple solid was characterized by elemental analysis consistent with a formulation as monohydrate solid, (F8)FeII(DMAP)2.H2O, Desert Analytics, C58H42N8F8FeO found/calc: %C 64.5/64.8; %H 3.6/3.9; %N 10.1/10.4; UV-vis (CH3CN, 293 K) λmax = 418.5 nm (Soret), 527 nm (Q-band); 1H-NMR showing a typical spectrum of low-spin iron(II) porphyrin, 400 MHz, CD2Cl2, 300 K, δ (ppm) = 7.6 (pyrrole-H); 7.2 (phenyl-H); 1.5 (H2O); MALDI-TOF mass spectrum shows a peak characteristic of the monobase (F8)FeII(DMAP) (probably the other base is lost by effect of the laser): [M – DMAP – H2O] m/z = 935, [M – 2 DMAP – H2O] m/z = 812.
UV-vis spectroscopy monitoring of the reaction of [DCHIm(F8)FeIII-(O22-)-CuII(AN)]+ (2b) with 2,4-di-tert-butylphenol
In a UV-vis cuvette assembly, a solution of [(DCHIm)(F8)FeIII-(O22-)-CuII(AN)]+ (0.36 mM) was generated in THF at –80° C as described above; 1.0 equiv of 2,4-di-tert-butylphenol (0.29 mg, 14.4 mM) was then added. UV-vis absorption at λmax (ε, mM-1cm-1) = 419 (Soret: 133.8), 541 (8.5) nm] corresponds to high-valent oxyferryl [(DCHIm)(F8)FeIV=O] (spectra are given in Supporting Information, Figure S1).
Reactivity of [DCHIm(F8)FeIII-(O22-)-CuII(AN)]+ (2b) in presence of HCl and quantitive determination of H2O2
Equimolar amounts of (F8)FeII·H2O (20 mg) and [CuI(AN)(CH3CN)]ClO4 (9.8 mg) were dissolved in 10 mL of CH3CN (conc = 3 mM) in the glovebox. The solution was brought out of the glovebox and immersed in a cold bath at − 40° C, and dioxygen was then bubbled through the solution to generate the high-spin peroxo compound 2a. Excess O2 was removed by several vacuum/Ar cycles; then 1.0 equiv. of 1,5-dicyclohexylimidazole (DCHIm) (5.6 mg in 0.1 mL of CH3CN) was added to form the low-spin peroxo complex 2b. 10 equiv. of HCl (0.24 mL of 1.0 M diethyl ether solution of hydrogen chloride, Aldrich) was added, and the dark red solution turned to dark green. The solution was removed from the cold bath, followed by addition of 20 mL of distilled water (final volume = 30.3 mL). A suspension is formed because of the insolubility of (F8)FeIII−Cl in the solvent mixture. H2O2 was determined spectrophotometrically (at 408 nm) upon addition of 1 mL of titanyl reagent (titanium(IV) oxysulfate in sulfuric acid, Riedel de Haën) to a 10 mL suspension, followed by centrifugation. A calibration curve was made in the same solvent mixture of the reaction (CH3CN/Ether/H2O). The yield of H2O2 was determined to be 70% on the basis of two experiments.
UV-vis spectroscopy monitoring of the reaction of [(DCHIm)(F8)FeIV=O] (1c) with [CuI(AN)][B(C6F5)4]
To the UV-vis cuvette containing THF solution of [(DCHIm)(F8)FeIV=O] (prepared by reaction of (2b) with 2,4-di-tert-butylphenol. as discussed above, 1.0 equiv. of [CuI(AN)]+ (1.33 mg in 0.1 mL in THF) at – 80° C. UV-vis absorption at λmax 440, 553 nm corresponds to [(F8)FeIII-(O2-)-CuII(AN)]+.
EPR spectroscopy Identification of [CuII(AN)(OH)]+ as product of the reaction of [(DCHIm)(F8)FeIII-(O22-)-CuII(AN)]+ (2b) with 2,4-di-tert-butyl phenol
In the glove box, 1.6 mg of (F8)FeII.H2O and 1.8 mg of [CuI(AN)]+ is dissolved in 0.4 mL of deoxygenated acetone in a EPR tube. Outside the glovebox, the solution was cooled to -80° C (acetone/dry ice bath) and bubbled with dioxygen to generate the peroxo compound (2b). 1 equiv. of DCHIm (50 μL of 40 mM acetone solution of DCHIm) was added to the reaction mixture followed by addition of 50 μL of 40 mM acetone solution of 2,4-di-tert-butylphenol (1 equiv.). Then, the EPR spectrum was recorded at 77 K. [EPR parameters A∥ = 127.33 × 10-4 cm-1, A⊥ = 35 × 10-4 cm-1, g∥ = 2.24 and g⊥ = 2.06]. The solution was warmed up to room temperature, and again an EPR spectrum was recorded at 77 K. The thermally decomposed products [(F8)FeIII–OH] (g = 5.8) and [CuII(AN)(OH)]+ were formed (spectra are given Supporting Information, Figure S3).
Identification and quantification of coupled phenol (3,3’,5,5’-tetra-tert-butyl-(1,1’-biphenyl)-2,2’-diol) by GC-MS
In the glove box, 16.6 mg of (F8)FeII.H2O and 18.5 mg of [CuI(AN)]+ was dissolved in 2 mL of THF in a Schlenk flask. Outsied the glovebox, the solution was cooled to -80 °C and bubbled with dioxygen to generate the peroxo adduct. The excess dioxygen was removed by employing vacuum and Argon bubbling through the solution for 2 cycles. To the solution, 4.6 mg of DCHIm in 0.2 mL of THF (1 equiv.) was added, followed by addition 1 equiv. of 2,4-di-tert-butylphenol (4.6 mg of phenol in 0.4 mL of THF). Then the solution was stirred for 2 hrs and warmed up to RT. The volume of the solution was reduced to 0.5 mL and the metal complexes are precipitated by the addition of 10 mL of pentane, and separated by filtration. The filterate was carefully collected and pentane was removed by vacuum, the organic residue was redissolved in 4 mL of CH3CN. The coupled phenol generated in this reaction was quantitatively determined by GC-MS. Yield of 3,3’,5,5’-tetra-tert-butyl-(1,1’-biphenyl)-2,2’-diol is 68.5 % (GC-MS trace is given in Supporting Information, Figure S4).
Identification of 2,4,6-tri-tert-butylphenoxy radical and 2,4-di-tert-butyl-4-methoxylphenoxy radical by EPR spectroscopy
This reaction was carried out in a manner similar to that described above except that either 2,4,6-tri-tert-butylphenol or 2,4-di-tert-butyl-4-methoxylphenol was used. Then, the EPR spectrum was recorded at 77 K, g value for 2,4,6-tri-tert-butylphenoxy radical and 2,4-di-tert-butyl-4-methoxylphenoxy radical are 2.0056 and 2.0077 respectively.
Supplementary Material
Figure 4.
Isosurface plots (isovalue 0.05 a.u.) of unfilled Cu and Fe d orbitals of 1 from a spin-unrestricted BP86 calculation in the BS (ST = 2) state.
Figure 5.
Isosurface plots (isovalue 0.05 a.u.) of unfilled Cu and Fe d orbitals of 2 from a spin-unrestricted BP86 calculation in the BS (ST = 2) state.
Acknowledgements
These studies were supported in part by the NIH (DK031450 E.I.S.; RR-001208 K.O.H.. Computational resources used in these studies were provided in part by the Bioinformatics Center of the University of Arkansas for Medical Sciences (NIH P20 RR-16460 from the IDeA Networks of Biomedical Research Excellence (INBRE) Program of the National Center for Research Resources) and in part by the National Science Foundation through TeraGrid resources provided by the NCSA (TG-CHE080054N). K.D.K. acknowledges support from the NIH (GM60353) and WCU-Program R31-2008-000-10010-0. The XAS data were measured at SSRL, the operations of which is funded by the Department of Energy, Office of Basic Energy Sciences. The SSRL Structural Molecular Biology program is supported by the National Institutes of Health, National Center for Research Resources, Biomedical Technology Program and by the Department of Energy, Office of Biological and Environmental Research. This publication was made possible by Grant Number 5 P41 RR001209 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
Footnotes
Supporting Information Available. UV-visible spectra of the reaction of (2b) with 1 equiv. of 2,4-di-tertbutylphenol (Figure S1), UV-vis spectra of the reaction of [(F8)FeIII-(O22-)-CuII(TMPA)]+ (1) with DMAP (Figure S2), EPR spectra of (2b) reaction with 1 equiv. of 2,4-di-tert-butylphenol (Figure S3), GC-MS trace of the oxidative coupling of 2,4-di-tert-butylphenol in presence of (2b) (Figure S4), ORTEP diagram (Figure S5) and crystal data and structure refinement for [(F8)FeIII-(O2-)-CuII(AN)]+ (2a) X-ray structure (Table S1), XAS spectra and computational data.
References
- 1.Ferguson-Miller S, Babcock GT. Chem. Rev. 1996;96:2889–2907. doi: 10.1021/cr950051s. [DOI] [PubMed] [Google Scholar]
- 2.Iwata S, Ostermeier C, Ludwig B, Michel H. Nature. 1995;376:660–669. doi: 10.1038/376660a0. [DOI] [PubMed] [Google Scholar]
- 3.Yoshikawa S, Shinzawa-Itoh K, Nakashima R, Yaono R, Yamashita E, Inoue N, Yao M, Jei-Fei M, Libeu CP, Mizushima T, Yamaguchi H, Tomizaki T, Tsukihara T. Science. 1998;280:1723–1729. doi: 10.1126/science.280.5370.1723. [DOI] [PubMed] [Google Scholar]
- 4.Kim E, Chufán EE, Kamaraj K, Karlin KD. Chem. Rev. 2004;104:1077–1133. doi: 10.1021/cr0206162. [DOI] [PubMed] [Google Scholar]
- 5.Collman JP, Boulatov R, Sunderland CJ, Fu L. Chem. Rev. 2004;104:561–588. doi: 10.1021/cr0206059. [DOI] [PubMed] [Google Scholar]
- 6.Chufán EE, Puiu SC, Karlin KD. Acc. Chem. Res. 2007;40:563–572. doi: 10.1021/ar700031t. [DOI] [PubMed] [Google Scholar]
- 7.Blomberg LM, Blomberg MRA, Siegbahn PEM. J. Inorg. Biochem. 2005;99:949–958. doi: 10.1016/j.jinorgbio.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 8.de Vries S. Biochimica et Biophysica Acta (BBA) - Bioenergetics. 2008;1777:925–928. doi: 10.1016/j.bbabio.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 9.Proshlyakov DA, Pressler MA, Babcock GT. Proc. Natl. Acad. Sci. USA. 1998;95:8020–8025. doi: 10.1073/pnas.95.14.8020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blomberg MRA, Siegbahn PEM, Wikström M. Inorg, Chem. 2003;42:5231–5243. doi: 10.1021/ic034060s. [DOI] [PubMed] [Google Scholar]
- 11.Ogura T, Kitagawa T. Biochim. Biophys. Acta. 2004;1655:290–297. doi: 10.1016/j.bbabio.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 12.Blomberg MRA, Siegbahn PEM. Biochim. Biophys. Acta. 2006;1757:969–980. doi: 10.1016/j.bbabio.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 13.Himo F, Siegbahn PEM. Chem. Rev. 2003;103:2421–2456. doi: 10.1021/cr020436s. [DOI] [PubMed] [Google Scholar]
- 14.Huynh MHV, Meyer TJ. Chem. Rev. 2007;107:5004–5064. doi: 10.1021/cr0500030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoshioka Y, Satoh H, Mitani M. J. Inorg. Biochem. 2007;101:1410–1427. doi: 10.1016/j.jinorgbio.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 16.Karlin KD. Science. 1993;261:701–708. doi: 10.1126/science.7688141. [DOI] [PubMed] [Google Scholar]
- 17.Holm RH, Solomon EI, editors. Biomimetic Inorganic Chemistry. Chem. Rev. 2004;104(2) doi: 10.1021/cr0206364. [DOI] [PubMed] [Google Scholar]
- 18.Lee Y, Karlin KD. Highlights of Copper Protein Active-Site Structure/Reactivity and Synthetic Model Studies. In: Metzler-Nolte N, Kraatz H-B, editors. Concepts and Models in Bioinorganic Chemistry. Wiley-VCH; New York: 2006. pp. 363–395. [Google Scholar]
- 19.Collman JP, Decréau RA. Chem. Commun. 2008:5065–5076. doi: 10.1039/b808070b. [DOI] [PubMed] [Google Scholar]
- 20.Liu JG, Naruta Y, Tani F. Angew. Chem. Int. Ed. 2005;44:1836–1840. doi: 10.1002/anie.200462582. [DOI] [PubMed] [Google Scholar]
- 21.Liu J-G, Naruta Y, Tani F. Chem. Eur. J. 2007;13:6365–6378. doi: 10.1002/chem.200601884. [DOI] [PubMed] [Google Scholar]
- 22.Collman JP, Devaraj NK, Decreau RA, Yang Y, Yan Y-L, Ebina W, Eberspacher TA, Chidsey CED. Science. 2007;315:1565–1568. doi: 10.1126/science.1135844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bröring M. Angew. Chem. Int. Ed. 2007;46:6222–6224. doi: 10.1002/anie.200702254. [DOI] [PubMed] [Google Scholar]
- 24.Ghiladi RA, Hatwell KR, Karlin KD, Huang H.-w., Moënne-Loccoz P, Krebs C, Huynh BH, Marzilli LA, Cotter RJ, Kaderli S, Zuberbühler AD. J. Am. Chem. Soc. 2001;123:6183–6184. doi: 10.1021/ja010602y. [DOI] [PubMed] [Google Scholar]
- 25.del Rio D, Sarangi R, Chufán EE, Karlin KD, Hedman B, Hodgson KO, Solomon EI. J. Am. Chem. Soc. 2005;127:11969–11978. doi: 10.1021/ja043374r. [DOI] [PubMed] [Google Scholar]
- 26.Ghiladi RA, Chufan EE, del Rio D, Solomon EI, Krebs C, Huynh BH, Huang H. w., Moenne-Loccoz P, Kaderli S, Honecker M, Zuberbuhler AD, Marzilli L, Cotter RJ, Karlin KD. Inorg. Chem. 2007;46:3889–3902. doi: 10.1021/ic061726k. [DOI] [PubMed] [Google Scholar]
- 27.Chishiro T, Shimazaki Y, Tani F, Tachi Y, Naruta Y, Karasawa S, Hayami S, Maeda Y. Angew. Chem. Int. Ed. 2003;42:2788–2791. doi: 10.1002/anie.200351415. [DOI] [PubMed] [Google Scholar]
- 28.Ghiladi RA, Ju TD, Lee D-H, Moënne-Loccoz P, Kaderli S, Neuhold Y-M, Zuberbühler AD, Woods AS, Cotter RJ, Karlin KD. J. Am. Chem. Soc. 1999;121:9885–9886. [Google Scholar]
- 29.Ghiladi RA, Huang HW, Moënne-Loccoz P, Stasser J, Blackburn NJ, Woods AS, Cotter RJ, Incarvito CD, Rheingold AL, Karlin KD. J. Biol. Inorg. Chem. 2005;10:63–77. doi: 10.1007/s00775-004-0609-1. [DOI] [PubMed] [Google Scholar]
- 30.Kim E, Kamaraj K, Galliker B, Rubie ND, Moënne-Loccoz P, Kaderli S, Zuberbühler AD, Karlin KD. Inorg. Chem. 2005;44:1238–1247. doi: 10.1021/ic048907b. [DOI] [PubMed] [Google Scholar]
- 31.Hatcher LQ, Karlin KD. Adv. Inorg. Chem. 2006;58:131–184. [Google Scholar]
- 32.Hatcher LQ, Karlin KD. J. Biol. Inorg. Chem. 2004;9:669–683. doi: 10.1007/s00775-004-0578-4. [DOI] [PubMed] [Google Scholar]
- 33.Mirica LM, Ottenwaelder X, Stack TDP. Chem. Rev. 2004;104:1013–1045. doi: 10.1021/cr020632z. [DOI] [PubMed] [Google Scholar]
- 34.Ross PK, Solomon EI. J. Am. Chem. Soc. 1991;113:3246–3259. [Google Scholar]
- 35.Ross PK, Solomon EI. J. Am. Chem. Soc. 1990;112:5871–5872. [Google Scholar]
- 36.Solomon EI, Tuczek F, Root DE, Brown CA. Chem. Rev. 1994;94:827–856. [Google Scholar]
- 37.Chufan EE, Mondal B, Gandhi T, Kim E, Rubie ND, Moenne-Loccoz P, Karlin KD. Inorg. Chem. 2007;46:6382–6394. doi: 10.1021/ic700363k. [DOI] [PubMed] [Google Scholar]
- 38.Kim E, Helton ME, Wasser IM, Karlin KD, Lu S, Huang H.-w., Moënne-Loccoz P, Incarvito CD, Rheingold AL, Honecker M, Kaderli S, Zuberbühler AD. Proc. Natl. Acad. Sci. USA. 2003;100:3623–3628. doi: 10.1073/pnas.0737180100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim E, Helton ME, Lu S, Moënne-Loccoz P, Incarvito CD, Rheingold AL, Kaderli S, Zuberbühler AD, Karlin KD. Inorg, Chem. 2005;44:7014–7029. doi: 10.1021/ic050446m. [DOI] [PubMed] [Google Scholar]
- 40.Kim E, Shearer J, Lu S, Moënne-Loccoz P, Helton ME, Kaderli S, Zuberbühler AD, Karlin KD. J. Am. Chem. Soc. 2004;126:12716–12717. doi: 10.1021/ja045941g. [DOI] [PubMed] [Google Scholar]
- 41.Ghiladi RA, Kretzer RM, Guzei I, Rheingold AL, Neuhold Y-M, Hatwell KR, Zuberbühler AD, Karlin KD. Inorg. Chem. 2001;40:5754–5767. doi: 10.1021/ic0105866. [DOI] [PubMed] [Google Scholar]
- 42.The EXAFS envelopes obtained from a single scatterer (SS) with atomic number Z and Z±1 at the same distance from the absorber are indistinguishable. Ths, Cu-O/N refers to a single scattering contribution from either O or N.
- 43.Cu-O-Fe = cos-1{[(Cu-O)2+(Fe-O)2-(Cu-Fe)2]/2(Cu-O)(Fe-O)}
- 44.Wasser IM, Huang HW, Moenne-Loccoz P, Karlin KD. J. Am. Chem. Soc. 2005;127:3310–3320. doi: 10.1021/ja0458773. [DOI] [PubMed] [Google Scholar]
- 45.We use the “holes” here to reflect uncompensated occupied valence orbitals; the Cu(II) has negative spin density and the Fe(III) has positive spin density,
- 46.Chen P, Fujisawa K, Helton ME, Karlin KD, Solomon EI. J. Am Chem. Soc. 2003;125:6394–6408. doi: 10.1021/ja0214678. [DOI] [PubMed] [Google Scholar]
- 47.Henson MJ, Mahadevan V, Stack TDP, Solomon EI. Inorg. Chem. 2001;40:5068–5069. doi: 10.1021/ic015539s. [DOI] [PubMed] [Google Scholar]
- 48.Chufán EE, Karlin KD. J. Am Chem. Soc. 2003;125:16160–16161. doi: 10.1021/ja036632d. [DOI] [PubMed] [Google Scholar]
- 49.Karlin KD, Nanthakumar A, Fox S, Murthy NN, Ravi N, Huynh BH, Orosz RD, Day EP. J. Am. Chem. Soc. 1994;116:4753–4763. [Google Scholar]
- 50.Fox S, Nanthakumar A, Wikström M, Karlin KD, Blackburn NJ. J. Am. Chem. Soc. 1996;118:24–34. [Google Scholar]
- 51.Du Bois J, Mizoguchi TJ, Lippard SJ. Coord. Chem. Rev. 2000;200-202:443–485. [Google Scholar]
- 52.Feig a. L., Becker M, Schindler S, van Eldik F, Lippard SJ. Inorg. Chem. 2003;42:3704–3704. doi: 10.1021/ic951242g. [DOI] [PubMed] [Google Scholar]
- 53.Scheidt WR, Reed CA. Chem. Rev. 1981;81:543–555. [Google Scholar]
- 54.Obias HV, van Strijdonck GPF, Lee D-H, Ralle M, Blackburn NJ, Karlin KD. J. Am. Chem. Soc. 1998;120:9696–9697. [Google Scholar]
- 55.Kurtz DM., Jr. Chem. Rev. 1990;90:585–606. [Google Scholar]
- 56.Que L, Jr., Tolman WB. Angew. Chem. Int. Ed. 2002;41:1114–1137. doi: 10.1002/1521-3773(20020402)41:7<1114::aid-anie1114>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 57.Yeh C-Y, Chang CJ, Nocera DG. J. Am. Chem. Soc. 2001;123:1513–1514. doi: 10.1021/ja003245k. [DOI] [PubMed] [Google Scholar]
- 58.Scott MJ, Zhang HH, Lee SC, Hedman B, Hodgson KO, Holm RH. J. Am. Chem. Soc. 1995;117:568–569. [Google Scholar]
- 59.Lee SC, Holm RH. Inorg. Chem. 1993;32:4745–4753. [Google Scholar]
- 60.Koval IA, van der Schilden K, Schuitema AM, Gamez P, Belle C, Pierre JL, Luken M, Krebs B, Roubeau O, Reedijk J. Inorg. Chem. 2005;44:4372–4382. doi: 10.1021/ic0501770. [DOI] [PubMed] [Google Scholar]
- 61.Mahapatra S, Halfen JA, Wilkinson EC, Pan G, Wang X, Young J,VG, Cramer CJ, Que J,L, Tolman WB. J. Am. Chem. Soc. 1996;118:11555–11574. [Google Scholar]
- 62.Berreau LM, Mahapatra S, Halfen JA, Young VG, Tolman WB. Inorg. Chem. 1996;35:6339–&. [Google Scholar]
- 63.Obias HV, Lin Y, Murthy NN, Pidcock E, Solomon EI, Ralle M, Blackburn NJ, Neuhold Y-M, Zuberbühler AD, Karlin KD. J. Am. Chem. Soc. 1998;120:12960–12961. [Google Scholar]
- 64.Shearer J, Zhang CX, Zakharov LN, Rheingold AL, Karlin KD. J. Am. Chem. Soc. 2005;127:5469–5483. doi: 10.1021/ja045191a. [DOI] [PubMed] [Google Scholar]
- 65.Izzet G, Akdas H, Hucher N, Giorgi M, Prange T, Reinaud O. Inorg. Chem. 2006;45:1069–1077. doi: 10.1021/ic051221e. [DOI] [PubMed] [Google Scholar]
- 66.Hong S, Hill LMR, Gupta AK, Naab BD, Gilroy JB, Hicks RG, Cramer CJ, Tolman WB. Inorg. Chem. 2009;48:4514–4523. doi: 10.1021/ic9002466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Contaldi S, Nicola CD, Garau F, Karabach YY, Martins LMDRS, Monari M, Pandolfo L, Pettinari C, Pombeiro AJL. Dalton Trans. 2009:4928–4941. doi: 10.1039/b823370e. [DOI] [PubMed] [Google Scholar]
- 68.Itoh S, Tachi Y. Dalton Trans. 2006:4531–4538. doi: 10.1039/b607964d. [DOI] [PubMed] [Google Scholar]
- 69.Collman JP, Herrmann PC, Boitrel B, Zhang X, Eberspacher TA, Fu L, Wang J, Rousseau DL, Williams ER. J. Am. Chem. Soc. 1994;116:9783–9784. [Google Scholar]
- 70.Collman JP, Fu L, Herrmann PC, Zhang X. Science. 1997;275:949–951. doi: 10.1126/science.275.5302.949. [DOI] [PubMed] [Google Scholar]
- 71.Collman JP. Inorg. Chem. 1997;36:5145–5155. [Google Scholar]
- 72.Collman JP, Fu L, Herrmann PC, Wang Z, Rapta M, Bröring M, Schwenninger R, Boitrel B. Angew. Chem. Int. Ed. 1998;37:3397–3400. doi: 10.1002/(SICI)1521-3773(19981231)37:24<3397::AID-ANIE3397>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 73.Kim Y, Shinzawa-Itoh K, Yoshikawa S, Kitagawa T. J. Am. Chem. Soc. 2001;123:757–758. doi: 10.1021/ja0027867. [DOI] [PubMed] [Google Scholar]
- 74.Collman JP, Decréau RA, Sunderland CJ. Chem. Commun. 2006:3894–3896. doi: 10.1039/b607277a. [DOI] [PubMed] [Google Scholar]
- 75.Lehnert N, Ho RYN, Que L, Jr., Solomon EI. J. Am Chem. Soc. 2001;123:12802–12816. doi: 10.1021/ja011450+. [DOI] [PubMed] [Google Scholar]
- 76.Lehnert N, Ho RYN, Que L, Jr., Solomon EI. J. Am. Chem. Soc. 2001;123:8271–8290. doi: 10.1021/ja010165n. [DOI] [PubMed] [Google Scholar]
- 77.Experiments to assign the structure of 2b are in progress.
- 78.Balch AL. Inorg. Chim. Acta. 1992;198-200:297–307. [Google Scholar]
- 79.Park GY, Deepalatha S, Puiu SC, Lee D-H, Mondal B, Narducci Sarjeant AA, del Rio D, Pau MYM, Solomon EI, Karlin KD. J. Biol. Inorg. Chem. 2009;14:1301–1311. doi: 10.1007/s00775-009-0575-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Paul PP, Tyeklár Z, Jacobson RR, Karlin KD. J. Am. Chem. Soc. 1991;113:5322–5332. [Google Scholar]
- 81.Lucchese B, Humphreys KJ, Lee D-H, Incarvito CD, Sommer RD, Rheingold AL, Karlin KD. Inorg. Chem. 2004;43:5987–5998. doi: 10.1021/ic0497477. [DOI] [PubMed] [Google Scholar]
- 82.Halfen JA, Young VG, Jr., Tolman WB. Inorg. Chem. 1998;37:2102–2103. doi: 10.1021/ic971216d. [DOI] [PubMed] [Google Scholar]
- 83.Mahadevan V, DuBois JL, Hedman B, Hodgson KO, Stack TDP. J. Am. Chem. Soc. 1999;121:5583–5584. [Google Scholar]
- 84.Kushioka K. J. Org. Chem. 1983;48:4948–4950. [Google Scholar]
- 85.Chin DH, Lamar GN, Balch AL. J. Am. Chem. Soc. 1980;102:5945–5947. [Google Scholar]
- 86.Collman JP, Decreau RA, Yan Y, Yoon J, Solomon EI. J. Am. Chem. Soc. 2007;129:5794–5795. doi: 10.1021/ja0690969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Manner VW, Markle TF, Freudenthal JH, Roth JP, Mayer JM. Chem. Commun. 2008:256–258. doi: 10.1039/b712872j. [DOI] [PubMed] [Google Scholar]
- 88.Hicks RG. Org. Biomol. Chem. 2007;5:1321–1338. doi: 10.1039/b617142g. [DOI] [PubMed] [Google Scholar]
- 89.Altwicker ER. Chem. Rev. 1967;67:475. [Google Scholar]
- 90.Nakanishi I, Fukuhara K, Shimada T, Ohkubo K, Iizuka Y, Inami K, Mochizuki M, Urano S, Itoh S, Miyata N, Fukuzumi S. J Chem Soc Perk T 2. 2002:1520–1524. [Google Scholar]
- 91.Yamaji T, Saiful ISM, Baba M, Yamauchi S, Yamauchi J. J. Phys. Chem. A. 2007;111:4612–4619. doi: 10.1021/jp071263j. [DOI] [PubMed] [Google Scholar]
- 92.Tenderholt A, Hedman B, Hodgson KO. PySpline. Stanford Synchrotron Radiation Laboratory; Stanford, CA: 2006. [Google Scholar]
- 93.George GN. EXAFSPAK. Stanford Synchrotron Radiation Laboratory; Stanford, CA: 2000. [Google Scholar]
- 94.Rehr JJ, Albers RC. Rev Mod Phys. 2000;72:621–654. [Google Scholar]
- 95.Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA, Jr., Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA. Gaussian 03, Revision C.02. Gaussian, Inc.; Wallingford CT: 2004. [Google Scholar]
- 96.Tenderholt Adam L. QMForge, Version 2.1. Stanford University; Stanford, CA, USA: [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.