We find that in the absence of p38 activity, human cells form longer spindles on which mitotic checkpoint satisfaction is transiently delayed. However, the cells ultimately divide normally. We conclude that normal p38 activity is required for the timely attachment of kinetochores to the spindle, but not for the fidelity of mitosis.
Abstract
Although p38 activity is reported to be required as cells enter mitosis for proper spindle assembly and checkpoint function, its role during the division process remains controversial in lieu of direct data. We therefore conducted live cell studies to determine the effect on mitosis of inhibiting or depleting p38. We found that in the absence of p38 activity the duration of mitosis is prolonged by ∼40% in nontransformed human RPE-1, ∼80% in PtK2 (rat kangaroo), and ∼25% in mouse cells, and this prolongation leads to an elevated mitotic index. However, under this condition chromatid segregation and cytokinesis are normal. Using Mad2/YFP-expressing cells, we show the prolongation of mitosis in the absence of p38 activity is directly due to a delay in satisfying the mitotic checkpoint. Inhibiting p38 did not affect the rate of chromosome motion; however, it did lead to the formation of significantly (10%) longer metaphase spindles. From these data we conclude that normal p38 activity is required for the timely stable attachment of all kinetochores to spindle microtubules, but not for the fidelity of the mitotic process. We speculate that p38 activity promotes timely checkpoint satisfaction by indirectly influencing those motor proteins (e.g., Klp10, Klp67A) involved in regulating the dynamics of kinetochore microtubule ends.
INTRODUCTION
p38, a member of the mitogen-activated protein kinase (MAPK) family, mediates a major cell cycle checkpoint control pathway that guards entry into mitosis (i.e., the G2/M transition). This evolutionarily conserved serine/threonine kinase was discovered in the early 1990s as a key player in the cell cycle delay induced by sudden osmotic changes (Brewster et al., 1993; Han et al., 1994; Millar et al., 1995). It is now evident that p38 is rapidly activated by a variety of stresses including proinflammatory cytokines (Raingeaud et al., 1995), heat shock (Rouse et al., 1994), UV light (Bulavin et al., 2001), and microtubule (Matsusaka and Pines, 2004), topoisomerase II (Mikhailov et al., 2004) and ribosome (Bunyard et al., 2003) poisons. When activated in G2 p38 quickly causes a transient G2 delay or sometimes a permanent arrest (Bulavin et al., 2002; Mikhailov et al., 2005; Coulthard et al., 2009).
Four isoforms of p38 have been identified including p38α, β, γ, and δ. Although each shares ∼60% identity, p38γ and δ are specifically expressed in only a few tissues, whereas p38α and β are ubiquitous (Coulthard et al., 2009). Although the loss of p38β, γ, or δ, or both γ and δ, does not interfere with normal mouse development, disruption of p38α (i.e., p38 MAPK) results in embryonic death (Adams et al., 2000). Like all MAPKs, p38 has a TGY dual phosphorylation motif that is phosphorylated and activated by cellular stresses. The primary upstream activators of p38 are the MAPK kinases (MKKs) 3, 4 and 6 (Brancho et al., 2003; Zarubin and Han, 2005), whereas its primary downstream target is the MAPK–activated protein kinase MAPKAPK2 (MK2; Rouse et al., 1994; Ronkina et al., 2007).
A number of studies have reported that p38 activity is required for normal “mitotic progression,” “mitotic transit,” or mitosis (Diehl et al., 2000; Fan et al., 2005; Yang et al., 2005; Cha et al., 2007; Tang et al., 2008). Most, however, are based on indirect population studies that are equally consistent with the interpretation that p38 plays a critical role in progression through G2 and not mitosis. Regardless, it is clear from biochemical work on HeLa and NIH 3T3 cells (Takenaka et al., 1998; Fan et al., 2005; Tang et al., 2008), as well as immunocytochemical studies on developing nonstressed rat retina (Campos et al., 2002) that compared with G2, p38 activity increases as cells enter mitosis. It is similarly evident from indirect immunofluorescence (IMF) observations that activated (phosphorylated) p38 (P-p38) is distributed throughout the cytoplasm of mitotic cells (Fan et al., 2005), and also that it concentrates in the centrosomes during spindle assembly (Cha et al., 2007; Tang et al., 2008).
Although the centrosomal localization of P-p38 suggests it plays a direct role in mitosis, the data for this are conflicting. Early flow cytometric studies led Diehl et al. (2000) to conclude that the persistent activation of p38 arrests fetal mouse thymocytes in mitosis. However, more recent population studies on HeLa cells conclude just the opposite: that inhibiting or depleting p38 leads to defective spindles and an arrest in mitosis (Fan et al., 2005; Tang et al., 2008). In contrast to these conflicting results Takenaka et al., (1998) reported, based on H1 kinase levels in NIH 3T3 cells treated with p38 inhibitors, that inhibiting p38 activity abrogates the mitotic checkpoint in nocodazole-treated mouse cells (see also Yen and Yang, 2010). The major conclusion of these studies is that P38 activity is important for proper spindle formation and mitosis. However, this verdict is not consistent with other reports, based on cell sorting studies, that inhibiting p38 has no effect on cell cycle progression in HeLa (Deacon et al., 2003) or A431 cells (Boldt et al., 2002), nor does it abrogate a taxol-induced block in mitosis (Boldt et al., 2002). These latter findings are consistent with the report that when the placental defect in fetal mice lacking p38α is rescued, embryos lacking p38α develop to term and appear normal (Adams et al., 2000), a conclusion that implies that p38 activity is not critical for normal cell division in mice.
The confusion over the role of p38 in mitosis arises from a lack of direct data on the topic. We therefore conducted a series of studies on live human, mouse, and rat-kangaroo cells to determine the effects of inhibiting or depleting p38 on mitosis. Surprisingly these studies reveal that in the absence of functional p38 cells form longer but otherwise normal spindles on which satisfaction of the mitotic checkpoint is transiently delayed. Although this delay in mitosis does not compromise the fidelity of the mitotic process, it does lead to a higher mitotic index (MI) in cultures lacking p38 activity.
MATERIALS AND METHODS
Cell Culture and Reagents
A PtK2 cell line stably expressing yellow fluorescent protein (YFP)/Mad2 (Yang et al., 2009) was cultured in Ham's F12 (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS). Mouse embryonic fibroblasts (MEFs), hTERT-RPE-1 (RPE-1), nontransformed human RPE-1 stably expressing green fluorescent protein (GFP)/CENP-A, and RPE-1 stably expressing GFP/centrin, were maintained in DMEM supplemented with 10% FBS. Small molecule p38 inhibitors SB202190 and SB203580, and their nonfunctional analogue (SB202474; all from Calbiochem, La Jolla, CA) were diluted in DMSO and used at 30-μM. Stock cultures of all cells were maintained in a humidified incubator at 37°C in a 5% CO2 environment. Before experimentation, cells were seeded onto 22-mm2 coverslips and grown for at least 16 h in the same environment.
RNA Interference and Immunoblotting
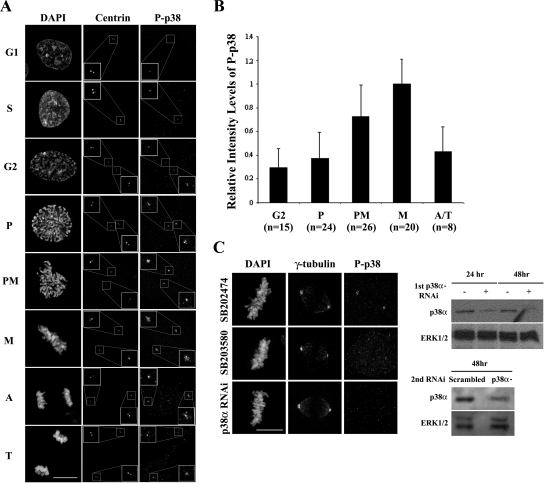
Two different synthetic double-stranded small interfering RNAs (siRNAs) were used to deplete endogenous p38α. One, based on information provided by Tang et al. (2008), was custom-made by Dharmacon (Lafayette, CO) to knock down a sequence of p38 (5′-AACTGCGGTTACTTAAACATA-3′) that we provided. The other ON-TARGET plus SMART pool sequence was provided by Dharmacon (Catalogue no. L-00351200-00). We also used a nonsense control (ON-TARGET plus NonTargeting Pool; Dharmacon D-001810-10). Targeted duplexes were transfected at 100 nM with oligofectamine (Invitrogen) according to the manufacturer's instructions. In some instances RPE-1 cells were harvested after a 24–48-h treatment with siRNAs or the nonsense control and lysed with 1× sample buffer (62.5 mM Tris, pH 6.8, 2% SDS, 50 mM DTT, 10% glycerol, 0.01% bromophenol blue) for subsequent immunoblotting. The primary antibody used was anti-p38 (no.9212, Cell Signaling, Beverly, MA).
IMF and Quantification
For IMF microscopy, coverslip cultures were washed with PBS, fixed with cold 100% methanol (−20°C), and permeabilized with 0.5% Triton X-100 in PBS as previously detailed (Lee and Song, 2007). The following primary antibodies were used: mouse anti-γ-tubulin (clone GTU-88, Sigma, St. Louis, MO) and rabbit anti-phospho-p38 (no. 9211, Cell Signaling). FITC-conjugated anti-mouse (Sigma) and rabbit Alexa Fluor 568 (Invitrogen) were used as the secondary antibodies. Image stacks were acquired and deconvolved on a Delta Vision System (Applied Precision, Issaquah, WA) centered on an Olympus IX70 microscope (Melville, NY) and equipped with a CM350 Photometrics camera (Huntington Beach, CA). Phospho-p38 (P-p38) intensity was determined using the method of Salmon and colleagues (Hoffman et al., 2001). In brief, immunofluorescence image stacks were acquired using 300-nm steps on the Delta Vision System, and the acquisition settings were the same for all samples. The best in-focus images were selected, and the fluorescence intensity of P-p38 was measured at the centrosomes in each of the mitotic stages. For Figures 1 and 2 the average value from individual centrosomes in untreated control metaphase (Figure 1) or prometaphase (Figure 2) cells was designated as 1, and the values for the other mitotic stages or treatment conditions were normalized to this number. Integrated intensities were measured by ImageJ (http://rsb.info.nih.gov/ij/; National Institutes of Health, Bethesda, MD), and the results were processed by Microsoft Excel software (Redmond, WA).
Figure 1.
Active p38 (P-p38) is associated with interphase centrosomes and its activity at this location increases during mitosis. (A) Distribution of P-p38 on centrosomes during the cell cycle. (B) Average fluorescence intensity histogram, relative to that of metaphase (M) cells, of P-p38 staining on G2, prophase (P), prometaphase (PM), and anaphase/telophase (A/T) centrosomes. n = the number of cells examined. (C) Active p38 is not seen on centrosomes of cells treated with a p38 inhibitor (SB203580) or after depleting p38 for 48 h with RNAi. See text for details. Bars, 10 μm.
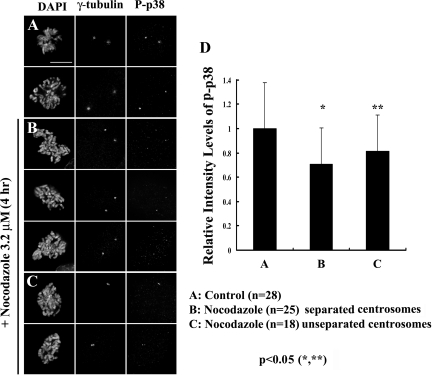
Figure 2.
P-p38 staining associated with individual centrosomes in prometaphase cells. When microtubule assembly is inhibited with nocodazole (B and C), centrosomes in mitotic cells concentrate significantly less P-p38 than in untreated control prometaphase cells (A). (D) Fluorescence intensity was significantly reduced compared with individual centrosomes in nontreated prometaphase cells. n = number of cells. See text for details. Bar, 10 μm.
To determine the relative frequencies of the mitotic stages, we treated growing RPE-1 coverslip cultures with small molecule inhibitors of p38 or its nonfunctional analogue, and we also depleted p38 by RNA interference (RNAi). After 16 h in drugs or 48 h after transfection with RNAi, we fixed the cultures and stained them with DAPI. The population of mitotic cells within each was then scored for the number in prometaphase, metaphase, or anaphase. This experiment was conducted twice on two different days, and because the results of the two experiments were the same, the data were pooled to generate the histograms in Figure 4, A and B.
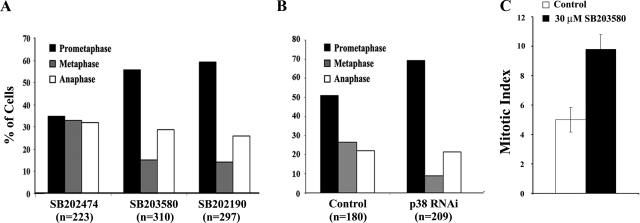
Figure 4.
Inhibiting p38 in growing RPE-1 cultures with small molecule inhibitors (A), or depleting it with RNAi (B), leads to an accumulation of prometaphase cells and a concurrent increase in the mitotic index or percentage of mitotic cells (C). n = number of mitotic cells in each data set. See text for details.
For mitotic index studies (see Figure 4C) we inhibited p38 in growing RPE-1 coverslip cultures with SB203580. Six hours later two untreated and two drug-treated coverslips were fixed and stained with DAPI. The number of prometaphase, metaphase, and anaphase/telophase cells within a random population of 500 cells per coverslip was then determined. The next day the experiment was repeated, and the results of the two separate experiments were pooled to obtain the total number of mitotic figures per 2000 cells for each condition.
To determine the length of the metaphase spindle (see Figure 6) coverslip cultures of RPE-1 cells at 37°C were treated with or without 30-μM SB203580 for 6 h before fixation and immunostaining for γ-tubulin (antibody clone GTU-88, Sigma) and chromosomes (DAPI). For this study we fixed two control and three drug-treated coverslips, and on these coverslips we located a total of 105 control and 116 drug-treated true metaphase cells. Each cell was photographed as a 3D image stack on the Delta Vision System, which was then deconvolved, and the distance between the centrosomes in the maximum intensity projection was calculated by two different individuals on different days, using the measuring feature in ImageJ.
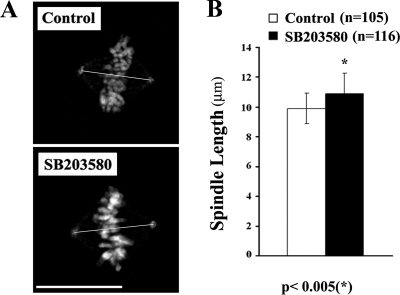
Figure 6.
Inhibiting p38 activity leads to the formation of longer metaphase spindles. (A) Maximum intensity projections, from a deconvolved IMF Z-series, of typical control and p38-inhibited metaphase cells showing the aligned chromosomes (white), the two centrosomes stained for γ-tubulin (white dots) and a line between the centrosomes. (B) Histogram of metaphase spindle length for control and p38-inhibited cells. n = number of cells. See text for details. Bar, 10 μm.
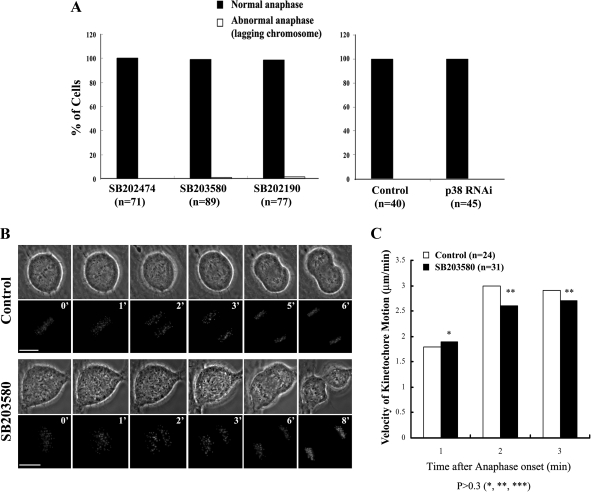
For our studies on lagging chromosomes we treated growing RPE-1 coverslip cultures with either a p38 inhibitor or its nonfunctional analogue, or we depleted p38 by RNAi. We then fixed a single control and experimental coverslip 16 h later, or 48 h after transfection, and stained each with DAPI. These were then examined to determine the percentage of anaphase cells on each coverslip that contained one or more lagging chromosome. We then repeated this experiment two more times over a 2-d period, and the data were pooled to generate the histograms in Figure 7A.
Figure 7.
p38 activity is not required during mitosis for the fidelity of the process. (A) Histograms depicting the incidence of lagging chromosomes in anaphase cells after inhibiting or depleting p38. Notice that there is no significant difference between controls and p38-inhibited or -depleted cells. (B) Frames from a time-lapse sequence of untreated and p38-inhibited CENP-A/GFP RPE-1 cells proceeding through anaphase. Note the absence of lagging chromosomes. (C) Histograms of average kinetochore velocities during anaphase in control (□) and p38 inhibited (■) cells. n = number of cells.
Live-Cell Microscopy
For live-cell imaging, coverslip cultures were assembled into Rose chambers containing phenol-free L-15 medium (Invitrogen) with 10% FBS (Khodjakov and Rieder, 2006). For studies on the duration of mitosis time-lapse images were captured every minute for 2–24 h at 37°C with either a 10× 0.3 NA or a 20× 0.5 NA Plan Fluor objective lens mounted on a Nikon Eclipse TE2000-U microscope (Melville, NY) equipped with shuttered Micromax (Roper Scientific, Tucson, AZ) or Orca ER (Hamamatsu Photonics, Bridgewater, NJ) cameras. High-magnification studies used a 100× 1.4 NA Plan Apo phase-contrast objective lens mounted on a Nikon Eclipse TE2000-U microscope equipped with a shuttered Micromax camera. Time-lapse image sequences were compiled with ImageJ. For studies on kinetochore-associated CENP-A/GFP (Figure 7, B and C) or Mad2/YFP (see Figure 5) we used a 100× 1.4 NA Plan Apo objective lens mounted on a Nikon model TE 2000-U microscope equipped with a shuttered Micromax (Roper Scientific) camera. Z-series image stacks were collected in phase-contrast and GFP or YFP epifluorescence at 0.5-μm steps throughout the depth of the cell. A narrow band-pass excitation filter was used to minimize photobleaching. Maximum intensity projections of GFP or YFP data stacks were generated with ImageJ.
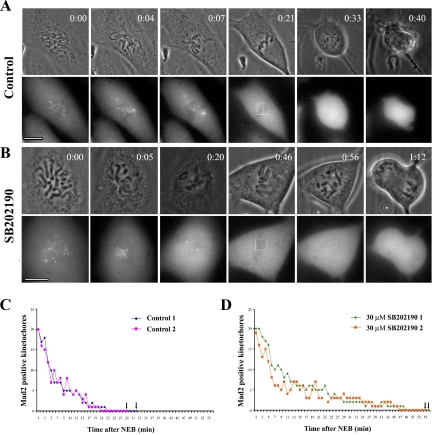
Figure 5.
Inhibiting p38 delays satisfaction of the mitotic checkpoint. Control (A) and p38-inhibited (B) YFP/Mad2-expressing PtK2 cells were followed at 37°C from nuclear envelope breakdown (NEB) through anaphase by near simultaneous phase-contrast (top frame) and 4D fluorescence (bottom frame) microscopy. These records were then analyzed to determine the rate at which Mad2-positive kinetochores disappeared (C and D) and also when the last Mad2-positive kinetochore (boxed in fourth frame in the A and B fluorescent images) disappeared relative to NEB and anaphase onset. Note that compared with untreated control cells (C) the time required for all kinetochores to form a stable attachment to the spindle is substantially longer in cells lacking p38 activity (D). Black arrows on the abscissa in C and D indicate points of anaphase onset. See text for details and also Table 2. Bars, 10 μm.
Kinetochore Tracking
The velocity of kinetochore motion in GFP/CENP-A RPE-1 cells (see Figure 7C) was tracked in maximal-intensity projection time-lapse Z series using the manual tracking feature in ImageJ.
RESULTS
Centrosome-associated p38 Activity Is Enhanced in a Microtubule-dependent Manner as Cells Enter Mitosis
Although active P-p38 is concentrated in centrosomes in mitotic HeLa cells (Cha et al., 2007; Tang et al., 2008), when this occurs and whether it is dependent on microtubules (MTs) has not yet been determine. We therefore fixed growing cultures of RPE-1 cells after treatment with 30 μM SB203580 or SB202190 (potent and selective inhibitors of p38α and β; Jiang et al., 1996; Davies et al., 2000) or after depletion of p38 by RNAi. We then stained them for IMF analyses using antibodies against either nonactivated p38 or P-p38. Subsequent observations on control cultures revealed that nonactivated and activated p38 were distributed throughout the cells in a weakly punctuate pattern (data not shown). However, unlike the nonactivated form, P-p38 was a conspicuous component of the single centrosome in G1 cells containing two centrioles (centrin-positive dots), and it remained associated with this centrosome during S phase (centriole replication; Figure 1A). During G2 P-p38 was seen associated with both centrosomes of the replicated diplosome, although more of it usually appeared on one than the other. Then, near the time of nuclear envelope breakdown (NEB) its activity (i.e., P-p38 fluorescence intensity) became significantly enhanced on both centrosomes (Figure 1A). Centrosome-associated P-p38 remained enhanced throughout spindle assembly (prometaphase), peaking at metaphase, after which its activity progressively decreased during anaphase and telophase (Figure 1, A and B). Because P-p38 was not seen on interphase (data not shown) or on mitotic centrosomes in cultures depleted of p38 by RNAi, or after treatment with p38 inhibitors (Figure 1C), we conclude that it is a component of interphase centrosomes and that its activity in this location increases as cells enter mitosis.
During the G2/M transition the interphase MT complex is replaced by two robust radial arrays of dynamic “astral” MTs nucleated by each of the separating centrosomes. During this time many noncentrosomal proteins become concentrated in and around each centrosome due to the centripetal transport properties of their associated astral MT arrays. Thus the question arises as to whether P-p38 accumulates in centrosomes during spindle assembly because it is a bona fide centrosomal component or if its accumulation depends on the presence of MTs. To answer this question, we first determined the intensity of P-p38 staining on each of the two centrosomes (centriole pairs) in untreated early-to-midprometaphase cells (Figure 2, A and D). Next we treated RPE-1 cultures with 3.2 μM nocodazole to prevent spindle MT formation, before fixing and staining them 4 h later with the phospho-p38 antibody. Within these cultures we then measured the intensity of P-p38 staining associated with individual centrosomes in prometaphase cells containing separated centrosomes (Figure 2B) and also the intensity of P-p38 staining associated with the single replicated diplosome in those cells containing unseparated centrosomes (Figure 2C). For this study we restricted our analyses to those prometaphase cells in which the degree of chromosome condensation was similar to that of untreated prometaphase cells. We found that although some P-p38 remained associated with each of the two individual centrosomes in prometaphase cells lacking MTs, its activity (fluorescence intensity) was significantly reduced compared with individual centrosomes in nontreated prometaphase cells (Figure 2D). In fact, the combined P-p38 fluorescence intensity of the entire diplosome in nocodazole-treated cells containing unseparated centrosomes was still significantly less than that associated with individual centrosomes in untreated cells (Figure 2D). From these data we conclude that spindle MT assembly stimulates the accumulation of additional P-p38 at centrosomes, i.e., that the sudden increase in centrosome MT nucleation at the onset of mitosis leads to the recruitment of additional P-p38 to the centrosomes.
Normal p38 Activity Is Required for Timely Progression through Mitosis
We next asked what role if any p38 plays in mitosis. To answer this question we used low- (10–20×) magnification phase-contrast light microscopy to followed fields of RPE-1 and MEFs at one frame per minute as they entered mitosis over a 6–12-h period, in the presence of small molecule inhibitors of p38. From these records we determined the duration of mitosis defined in this study as that period between NEB and the first signs of telophase (cytokinesis/membrane blebbing). We found (Table 1) that the duration of mitosis was prolonged in both small molecule p38 inhibitors (SB203580 and SB202190), but not in their nonfunctional control (SB 202474), by ∼40% in RPE-1 and to a lesser extent (∼25%) in mouse embryo fibroblasts (MEFs). Relative to a scrambled control, mitosis was also prolonged by 20–30% in RPE-1 cells in which p38 was knocked down (but not out) by each of two different synthetic double strand siRNAs. From these novel data we conclude that normal p38 activity is required at some point in the cell cycle for timely progression through mitosis.
Table 1.
Duration of mitosis
| Cell type | Treatment | DM (min)a |
|---|---|---|
| RPE-1 | None (control) | 20.4 ± 4 (n = 160) |
| 30 μM SB202474b | 19.9 ± 4.1 (59) | |
| 30 μM SB203580 | 28.3 ± 12.6 (98) | |
| 30 μM SB202190 | 28.1 ± 9.3 (95) | |
| Scrambled control | 23.4 ± 4.2 (165) | |
| p38 RNAi-1 | 32.4 ± 9.5 (61) | |
| p38 RNAi-2 | 28.7 ± 8.6 (173) | |
| Mouse embryonic fibroblast | None (control) | 25 ± 7 (72) |
| 30 μM SB202474 | 25.5 ± 6 (42) | |
| 30 μM SB203580 | 31.7 ± 9.6 (63) |
aDuration of mitosis (DM) is defined as NEB to initial signs of cytokinesis and/or telophase membrane blebbing. Values are mean ± SD, with number of cells in parentheses.
bNonfunctional p38 inhibitor analogue.
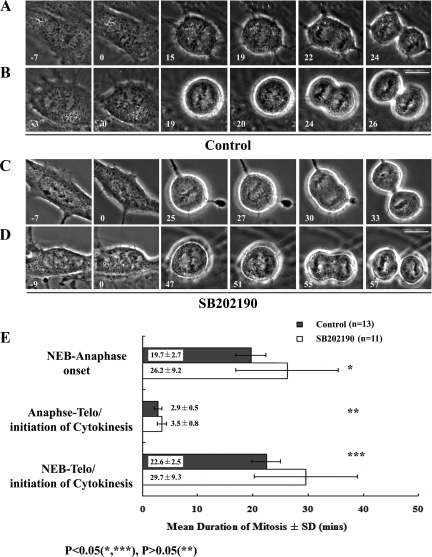
What stage(s) of mitosis are prolonged when p38 is inhibited? To answer this question we followed individual RPE-1 cells at higher magnification (100×; 1 frame/min) as they entered mitosis in the presence (Figure 3, A and B) or absence (Figure 3, C and D) of functional p38. An analysis of these records (Figure 3E) revealed that inhibiting p38 prolongs the period from NEB to anaphase onset and not anaphase onset to cytokinesis. As will be discussed later, a more dramatic prolongation of this period (∼80%) was seen when p38 was inhibited in rat kangaroo (PtK2) cells (Table 2). From these data we conclude that inhibiting p38 selectively prolongs the period in mitosis between NEB and anaphase onset, which is commonly referred to as spindle assembly.
Figure 3.
Normal P-p38 activity is required for timely progress through prometaphase/metaphase but not anaphase/telophase. High-magnification phase-contrast time-lapse images of mitosis in untreated control RPE-1 cells (A and B) and cells treated with SB202190 (C and D) reveal that inhibiting p38 prolongs the period between nuclear envelope breakdown and anaphase onset (E), and not between anaphase onset to telophase/cytokinesis. See text for details. n = number of cells. Bars, 10 μm.
Table 2.
Duration of the various mitotic phases in Mad2/YFP PtK2 cells
| Control (n = 20) | SB202474 (n = 20) | SB203580 (n = 25) | SB202190 (n = 20) | |
|---|---|---|---|---|
| NEB-initiation of anaphase | 34.9 ± 8.7 | 36.7 ± 7.2 | 64.7 ± 19.8 | 63.5 ± 15.6 |
| NEB-Mad2/YFP disappearance on last kinetochore | 24 ± 7.5 | 26.3 ± 7.1 | 50.4 ± 21.5 | 52.2 ± 15.7 |
| Mad2/YFP disappearance last kinetochore- initiation of anaphase | 10.9 ± 5.3 | 10.5 ± 2.1 | 14.3 ± 8.6 | 11.4 ± 2.5 |
Values are mean ± SD, expressed in minutes; n = number of cells.
Is the prolongation of spindle assembly in p38 inhibitors due to a requirement for p38 activity during mitosis, or at some earlier point(s) in the cell cycle? To address this issue, we conducted another study to determine the duration of spindle assembly (NEB to anaphase onset) in RPE-1 cells that underwent NEB within the first 90 min of drug treatment. Such cells were in late G2 at the time p38 was inhibited. The results of this study revealed that when p38 was inhibited during the G2/M transition by SB202190, spindle assembly required 25.9 ± 6.3 min (n = 75) compared with 19.7 ± 4.2 min (n = 73) in untreated controls, an increase of ∼30%. From these data we conclude that p38 activity is required at the time cells enter mitosis for timely progression through the division process.
Our conclusions from live cells predict that inhibiting p38 activity in growing cultures will lead to an enrichment of prometaphase and/or metaphase cells. To test this, we treated RPE-1 cultures with SB202190 or SB203580 or their nonfunctional analogue (SB202474). After 16 h these cultures were fixed, and the population of mitotic cells within each was scored for the number in prometaphase, metaphase, or anaphase. The results of this study (Figure 4A) revealed that inhibiting p38 leads to a significant increase in prometaphase cells and a decrease in metaphase cells. Similar results were obtained after depleting p38 in RPE-1 by RNAi (Figure 4B). From these data we conclude, as predicted from our live cell studies, that inhibiting p38 in growing cultures leads to an accumulation of prometaphase cells.
Our finding that inhibiting p38 prolongs spindle assembly in RPE-1 by 30–40% implies that under this condition the MI, i.e., the percentage of cells in mitosis at any given time in a growing cell culture, should also increase by 30–40%. However, we found (Figure 4C) that although the MI of control cultures was ∼5%, the MI of cultures treated for 6 h with a p38 inhibitor was ∼10 or ∼100% greater than in controls and 35–40% greater than predicted. We interpret this to mean that inhibiting p38 activity not only leads to an increase in the mitotic index by delaying progression through mitosis but also, as recently reported by Fornace and colleagues (Cha et al., 2007), that it accelerates the passage of G2 cells into M.
The Prolongation of Mitosis in Response to Inhibiting p38 Is Due to a Delay in Satisfying the Mitotic Checkpoint And Not to a Retardation of APC/C Activity or Proteasome Function
Indirect assays (e.g., based on histone H3 phosphorylation or Cdc2 activity) have led several groups to conclude that P-p38 is required during mitosis for proper mitotic checkpoint function (e.g., Takenaka et al., 1998; Campos et al., 2002; Fan et al., 2005; Yen and Yang, 2010). To determine if this is so, we prevented MT assembly in RPE-1 cells with 3.2 μM nocodazole and then followed them through mitosis in the presence or absence of a p38 inhibitor (SB203580). Under this condition we found that nocodazole-treated RPE-1 cells averaged 1229 ± 508 (n = 104) min in mitosis before slipping into the next G1 as 4N cells, whereas those treated with both nocodazole and a p38 inhibitor averaged 1637 ± 594 (n = 154) min in mitosis. These results are similar to those reported for rat kangaroo (PtK1) cells (Mikhailov et al., 2004), and they clearly reveal that p38 activity is not required for a functional mitotic checkpoint.
An obvious reason why prometaphase cells accumulate in cultures treated with p38 inhibitors is because P-p38 activity is required during mitosis for timely satisfaction of the mitotic checkpoint, i.e., for the timely stable attachment of all kinetochores to the spindle. To evaluate this we used 4D light microscopy to follow PtK2 cells expressing YFP/Mad2 (Yang et al., 2009) as they entered and proceeded through mitosis in the presence or absence of p38 inhibitors (e.g., Figure 5). As previously noted (see Table 2) inhibiting p38 with SB203580 prolongs mitosis in PtK2 YFP/Mad2 cells by ∼80%. We then analyzed these time-lapse video records to determine if the duration between disappearance of the last Mad2-positive kinetochore and if chromatid disjunction was the same or different after inhibiting p38. We found that in the absence of p38 activity the disappearance of Mad2 from the last kinetochore occurred ∼23 min later than in controls (cf., Figure 5, C and D; Table 2); however, once it occurred anaphase was initiated on time (∼10–15 min later; Table 2). That is, the delay between satisfaction of the checkpoint (last kinetochore stably attached) and anaphase onset in controls and p38-inhibited cells was the same. From these direct data we conclude that normal p38 activity is required during mitosis for timely satisfaction of the mitotic checkpoint. Furthermore, our observation that inhibiting p38 does not prolong the period between checkpoint satisfaction and anaphase onset (i.e., metaphase) implies that p38 activity is not required during mitosis for normal APC/C or proteasome function.
P-p38 Activity Is Required during Mitosis for Normal Spindle Length
Why is P-p38 required for timely satisfaction of the mitotic checkpoint? From the literature it is clear that checkpoint satisfaction is delayed whenever the normal dynamic behavior of kinetochore-associated MT ends are perturbed, as occurs in low concentrations of MT poisons or when the activity of those spindle-associated molecules that control MT tip dynamics are disrupted. In addition to delaying checkpoint satisfaction, these treatments commonly induce length changes in the spindle (Goshima et al., 2005; Brito and Rieder, 2008) by modifying the length of kinetochore fiber MTs (Goshima et al., 2005; Dumont and Mitchison, 2009). We therefore asked if inhibiting p38 in RPE-1 leads to the formation of shorter or longer spindles relative to controls. To answer this, we fixed growing RPE-1 cultures, with or without pretreatment with a p38 inhibitor, and then measured the distance between the centrosomes (γ-tubulin spots) in those cells in which all of the chromosomes were aligned on the spindle equator (i.e., true metaphase cells). We found (Figure 6) that the metaphase spindles in nontreated control cultures averaged 9.9 ± 1.1 μm and that they were significantly shorter than those in cultures treated with p38 inhibitors, which averaged 10.9 ± 1.4 μm. Thus, inhibiting p38 leads to the formation of normal-looking metaphase spindles that are ∼10% longer than in control cells.
P-p38 Activity Is Not Required during Mitosis for the Fidelity of the Process
At this point we can conclude that inhibiting p38 during mitosis leads to the formation of slightly longer spindles on which satisfaction of the mitotic checkpoint is transiently delayed. We next sought to determine whether normal p38 activity is required for the fidelity of chromosome segregation. To answer this, we treated growing RPE-1 cultures with a p38 inhibitor or its nonfunctional analogue. We then fixed and stained them with DAPI 16 h later and examined each to determine the number of anaphase cells that contained one or more lagging chromosome. We found that inhibiting p38, or knocking it down by RNAi, did not significantly increase the incidence of lagging chromosomes over untreated control cells (Figure 7A). This result is consistent with our live-cell analyses of RPE-1 cells expressing GFP-CENP-A, which revealed that other than prolonging prometaphase, inhibiting p38 had no obvious effect on the duration or rate of anaphase chromosome motion or the fidelity of kinetochore segregation (Figure 7, B and C).
DISCUSSION
The accumulation of active p38 on centrosomes during mitosis, combined with cell cycle timing data based on fixed cell populations stained for phospho-histone H3 and cyclin B, has led to the conclusion that centrosome-associated P-p38 plays an important functional role in regulating entry into (Cha et al., 2007) and progression through (Tang et al., 2008) mitosis. We find, however, that the enhanced accumulation of P-p38 on centrosomes during mitosis depends largely on the presence of centrosomal MTs, i.e., that additional P-p38 is recruited to centrosomes during mitosis via their MT arrays. This readily explains why the activity of p38 at centrosomes increases and decreases in accordance with mitotic progression, because the amount accumulated at the centrosome depends on the density of MTs, which changes during mitosis. This novel finding, combined with reports that the sudden increase in centrosomal MT nucleation at the G2/M transition is due to cyclin B/CDK1 activation (Ohta et al., 1993; Gabrielli et al., 1996), implies that centrosome-associated P-p38 has no essential role in initiating the events that lead to NEB or spindle assembly. Indeed, our studies reveal that other than inducing a transient delay in satisfying the mitotic checkpoint, preventing the accumulation of P-p38 at centrosomes with drugs or via RNAi depletion neither delays entry into mitosis nor induces the formation of abnormal spindles.
We also find, as others have, that inhibiting p38 in growing cultures leads to an elevated MI. In some studies this increased MI has been attributed to a stimulation of cell proliferation (Engel et al., 2006). In others it fostered the conclusion that inhibiting p38 leads to the formation of abnormal spindles and a mitotic arrest (e.g., Takenaka et al., 1998; Fan et al., 2005; Tang et al., 2008). However, our live cell studies reveal that when p38 is inhibited, or knocked down by RNAi, normal prometaphase cells accumulate in cultures, and these can easily be mistaken for abnormal spindles by those not well versed in mitosis (see Khodjakov and Rieder, 2009). Furthermore, we found that this accumulation of mitotic cells is due to a transient delay, and not a failure, in satisfying the mitotic checkpoint. The duration of this delay varied depending on the organism. In RPE-1 cells the delay was 6–10 min, which increased the total duration of mitosis from ∼20 to ∼30 min at 37°C. By contrast in rat kangaroo (PtK2) cells it was ∼30 min, which doubled the duration of mitosis. We conclude that it is this delay, combined with an acceleration of G2 progression (Cha et al., 2007), that produces the increased MI seen in cell cultures treated with p38 inhibitors.
The only phenotypic change we detected during mitosis in response to inhibiting p38 was that it leads to the formation of slightly (∼10%) longer spindles. In spite of this and the delay in checkpoint satisfaction, cells lacking active p38 invariably completed a normal mitosis, i.e., the ensuing stages of chromosome segregation and cytokinesis occurred on schedule and without apparent defects. From these observations we conclude that p38 activity during mitosis (or at any other stage of the cell cycle) is not essential for a normal division, a conclusion that is consistent with reports that when the placental defect is rescued, mice lacking p38 (or the MK2 and MK3 downstream targets) develop to term and are normal in appearance (Adams et al., 2000; Hegen et al., 2006; Ronkina et al., 2007). Here it is noteworthy that in the absence of p38 activity the mitotic checkpoint functions exactly as it should: it delays chromatid separation until all of the kinetochores are stably attached to the spindle. As a result it prevents chromosome segregation errors under conditions in which, if the checkpoint were not present, a high incidence of aneuploidy would be expected.
Our finding that p38 activity is not required for the fidelity of mitosis, even though it is required for normal spindle length and timely checkpoint satisfaction, implies that during evolution this kinase has come to have a nonessential influence on spindle assembly. Our unpublished preliminary data suggest that knocking down MK2 by RNAi, which is the major downstream target of p38, also leads to a delay in satisfying the mitotic checkpoint. This suggests that p38 indirectly influences the duration of mitosis via its action on its downstream substrates. One possibility is that p38 activity has a minor indirect influence on the behavior of those MT depolymerases that regulate kinetochore MT dynamics and spindle length. This includes Klp10A (kinesin 13), which, like P-p38, becomes concentrated during mitosis in the centrosomes and spindle poles where it controls the behavior of kinetochore-microtubule minus ends (Rogers et al., 2004). It also includes KLP67 (kinesin 8), which, unlike Klp10A, concentrates in kinetochores during spindle assembly where it regulates the behavior of kinetochore microtubule plus ends (Savoian et al., 2004). Minor changes in the activity of these kinesin-like proteins, especially Klp10A, will lead to changes in the dynamics of kinetochore MTs, which in turn could produce both longer spindles and a delay in mitotic checkpoint satisfaction. This scenario draws support from recent reports linking p38 activity to microtubule dynamics during interphase, e.g., via the phosphorylation of microtubule-associated proteins like MAP4 and Op18 (Hu et al., 2010) or Tau (Feijoo et al., 2005). Alternatively, P-p38 and its primary MK2 target normally form a tight physiological complex (Ben-Levy et al., 1998; White et al., 2007), and within the centrosome P-p38/MK2 complexes colocalize with and are reported to phosphorylate the polo-like kinase 1 (Tang et al., 2008). In turn, polo-like kinase 1 may play a role in regulating the microtubule depolymerases discussed above, or it may influence kinetochore MT stability via phosphorylation of the BubR1 kinetochore protein (Elowe et al., 2007).
Chromosomal instability (CIN) is a condition in which cells exhibit a high rate of single chromosome missegregation, and it is a hallmark of aggressive malignancies. There is recent evidence that CIN is due to an enhanced stability of kinetochore MTs, which make it more difficult to correct erroneous kinetochore attachments (which are not seen by the mitotic checkpoint) before anaphase onset (Bakhoum et al., 2009). Because we found that inhibiting p38 significantly delays anaphase onset, possibly via its influence on kinetochore MT stability, we conducted a preliminary study to determine if we could reduce the chromosome segregation errors in CIN cells by inhibiting p38. Unfortunately, at least for U2OS, we found that there was no significant difference before or after inhibiting p38 in the number of cells exhibiting lagging chromosomes during anaphase (∼30–35% under both conditions; not shown).
Finally, although we found that p38 activity is not essential during the G2/M transition and mitosis for normal chromosome distribution or cytokinesis, it is increasingly clear that its activity during this period is an important determinant of cell fate. In this regard p38 is a key component of a quality control feedback pathway that functions during mitosis to detect perturbations in spindle assembly which does not, by itself, prevent the cell from completing division, i.e., from satisfying the mitotic checkpoint. These disturbances range in severity from a lack of centrosomes (Mikule et al., 2007) to minor prolongations of the process by nanomolar concentrations of spindle poisons (Y. Uetake and G. Sluder, unpublished data). During mitosis these problems are subsequently converted, via p38, into a robust G1 arrest in the daughter cells produced from the division, often in a p53-dependent manner by, e.g., blocking the expression of cyclin D (Ellinger-Ziegelbauer et al., 1999). Similarly, the MT-stabilizing drug taxol is effective against a variety of solid tumors, and its efficacy requires that cells enter mitosis in its presence (Lee et al., 2004; Sudo et al., 2004) where they are delayed for a variable period, depending on the concentration (Yang et al., 2009). At least in some cancer cell lines the death induced by taxol during or after mitosis is mediated by p38 (Bacus et al., 2001; Deacon et al., 2003). Clearly, although p38 activity during mitosis only subtly influences the process of cell division, its activity at this time plays a critical role in defining the subsequent fate of the daughter cells.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Drs. Alexey Khodjakov, Chris O'Connell, and Jadranka Loncarek (Wadsworth Center) for sharing some of their RPE-1 cell lines and for discussions related to this project. We also thank Susan Nowogrodzki for editorial assistance and Dr. Greenfield Sluder for allowing us to cite his unpublished results. This work was supported by a National Institutes of Health, National Institute of General Medical Sciences Grant 40198 to C.L.R.
Abbreviations used:
- IMF
indirect immunofluorescence
- NEB
nuclear envelope breakdown
- MT
microtubule
- P-p38
activated (phosphorylated) p38.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E10-02-0125) on May 12, 2010.
REFERENCES
- Adams R. H., Poras A., Alonso G., Jones M., Vintersten K., Panelli S., Valladares A., Perez L., Klein R., Nebreda A. R. Essential role of p38α MAP kinase in placental but not embryonic cardiovascular development. Mol. Cell. 2000;6:109–116. [PubMed] [Google Scholar]
- Bacus S. S., Gudkov A. V., Lowe M., Lyass L., Yung Y., Komarov A. P., Keyomarsi K., Yarden Y., Seger R. Taxol-induced apoptosis depends on MAP kinase pathways (ERK and p38) and is independent of p53. Oncogene. 2001;20:147–155. doi: 10.1038/sj.onc.1204062. [DOI] [PubMed] [Google Scholar]
- Bakhoum S. F., Genovese G., Compton D. A. Deviant kinetochore microtubule dynamics underlie chromosomal instability. Curr. Biol. 2009;19:1937–1942. doi: 10.1016/j.cub.2009.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Levy R., Hooper S., Wilson R., Patterson H. F., Marshall C. J. Nuclear export of the stress-activated protein kinase p38 mediated by its substrate MAPKAP kinase-2. Curr. Biol. 1998;8:1049–1057. doi: 10.1016/s0960-9822(98)70442-7. [DOI] [PubMed] [Google Scholar]
- Boldt S., Weidle U. H., Kolch W. The role of MAPK pathways in the action of chemotherapeutic drugs. Carcinogenesis. 2002;23:1831–1838. doi: 10.1093/carcin/23.11.1831. [DOI] [PubMed] [Google Scholar]
- Brancho D., Tanaka N., Jaeschke A., Ventura J.-J., Kelkar N., Tanaka Y., Kyuuma M., Takeshita T., Flavell R. A., Davis R. J. Mechanism of p38 MAP kinase activation in vivo. Genes Dev. 2003;17:1969–1978. doi: 10.1101/gad.1107303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewster J. L., de Valoir T., Dwyer N. D., Winter E., Gustin M. C. An osmosensing signal transduction pathway in yeast. Science. 1993;259:1760–1763. doi: 10.1126/science.7681220. [DOI] [PubMed] [Google Scholar]
- Brito D., Rieder C. L. The ability to survive mitosis in the presence of microtubule poisons differs significantly between human nontransformed (RPE-1) and cancer (U2OS, HeLa) cells. Cell Motil. Cytoskelet. 2008;66:437–447. doi: 10.1002/cm.20316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulavin D. V., Amundson S. A., Fornace A. J. p38 and Chk1 kinases: different conductors for the G2/M checkpoint symphony. Cur. Opin. Genet. Dev. 2002;12:92–97. doi: 10.1016/s0959-437x(01)00270-2. [DOI] [PubMed] [Google Scholar]
- Bulavin D. V., Higashimoto Y., Popoff I. J., Gaarde W. A., Basrur V., Potapova O., Appella E., Fornace A. J. Initiation of a G2/M checkpoint after ultraviolet radiation requires p38 kinase. Nature. 2001;411:102–107. doi: 10.1038/35075107. [DOI] [PubMed] [Google Scholar]
- Bunyard P., Handley M., Pollara G., Rutault K., Wood I., Chaudry M., Alderman C., Foreman J., Katz D. R., Chain B. M. Ribotoxic stress activates p38 and JNK kinases and modulates the antigen-presenting activity of dendritic cells. Mol. Immunol. 2003;39:815–827. doi: 10.1016/s0161-5890(02)00262-6. [DOI] [PubMed] [Google Scholar]
- Campos C.B.L., Bedard P. A., Linden R. Activation of p38 mitogen-activated protein kinase during normal mitosis in the developing retina. Neuroscience. 2002;112:583–591. doi: 10.1016/s0306-4522(02)00096-9. [DOI] [PubMed] [Google Scholar]
- Cha H., Wang X., Li H., Fornace A. J. A functional role for p38 MAPK in modulating mitotic transit in the absence of stress. J. Biol. Chem. 2007;282:22984–22992. doi: 10.1074/jbc.M700735200. [DOI] [PubMed] [Google Scholar]
- Coulthard L. R., White D. E., Jones D. L., McDermott M. F., Burchill S. A. p38MAPK: stress responses from molecular mechanisms to therapeutics. Trends Mol. Med. 2009;15:369–379. doi: 10.1016/j.molmed.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies S. P., Reddy H., Caivano M., Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem. J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon K., Mistry P., Chernoff J., Blank J. L., Patel R. p38 mitogen-activated protein kinase mediates cell death and p21-activated kinase mediates cell survival during chemotherapeutic drug-induced mitotic arrest. Mol. Biol. Cell. 2003;14:2071–2087. doi: 10.1091/mbc.E02-10-0653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl N. L., Enslen H., Fortner K. A., Merritt C., Stetson N., Charland C., Flavell R. A., Davis R. J., Rincon M. Activation of the p38 mitogen-activated protein kinase pathway arrests cell cycle progression and differentiation of immature thymocytes in vitro. J. Exp. Med. 2000;191:321–334. doi: 10.1084/jem.191.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont S., Mitchison T. J. Compression regulates mitotic spindle length by a mechanochemical switch at the poles. Curr. Biol. 2009;19:1086–1095. doi: 10.1016/j.cub.2009.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellinger-Ziegelbauer H., Kelly K., Siebenlist U. Cell cycle arrest and reversion of Ras-induced transformation by a conditionally activated form of mitogen-activated protein kinase kinase kinase 3. Mol. Biol. Cell. 1999;19:3857–3868. doi: 10.1128/mcb.19.5.3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elowe S., Hummer S., Li X., Nigg E. A. Tension-sensitive Plk1 phosphorylation on BubR1 regulates the stability of kinetochore microtubule interactions. Genes Dev. 2007;21:2205–2219. doi: 10.1101/gad.436007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel F. B., Hsieh P.C.H., Lee R. T., Keating M. T. FGF1/p38 MAP kinase inhibitor therapy induces cardiomyocyte mitosis, reduces scarring, and rescues function after myocardial infarction. Proc. Natl. Acad. Sci. USA. 2006;103:15546–15551. doi: 10.1073/pnas.0607382103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan L., Yang X., Du J., Marshall M., Blanchard K., Ye X. A novel role of p38α MAPK in mitotic progression independent of its kinase activity. Cell Cycle. 2005;4:1616–1624. doi: 10.4161/cc.4.11.2125. [DOI] [PubMed] [Google Scholar]
- Feijoo C., Campbell D. G., Jakes R., Goedert M., Cuenda A. Evidence that phosphorylation of the microtubule-associated protein Tau by SAPK4/p38γ at Thr50 promotes microtubule assembly. J. Cell Sci. 2005;118:397–408. doi: 10.1242/jcs.01655. [DOI] [PubMed] [Google Scholar]
- Gabrielli B. G., DeSouza C. P., Tonks I. D., Clark J. M., Hayward N. K., Ellem K. A. Cytoplasmic accumulation of cdc25B phosphatase in mitosis triggers centrosomal microtubule nucleation in HeLa cells. J. Cell Sci. 1996;109:1081–1093. doi: 10.1242/jcs.109.5.1081. [DOI] [PubMed] [Google Scholar]
- Goshima G., Wollman R., Stuurman N., Scholey J. M., Vale R. D. Length control of the metaphase spindle. Curr. Biol. 2005;15:1979–1988. doi: 10.1016/j.cub.2005.09.054. [DOI] [PubMed] [Google Scholar]
- Han J., Lee J.-D., Bibbs L., Ulevitch R. J. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994;265:808–811. doi: 10.1126/science.7914033. [DOI] [PubMed] [Google Scholar]
- Hegen M., Gaestele M., Nickerson-Nutter C. L., Lin L.-L., Telliez J.-B. MAPKAP kinase-2-deficient mice are resistant to collagen-induced arthritis. J. Immunol. 2006;177:1913–1917. doi: 10.4049/jimmunol.177.3.1913. [DOI] [PubMed] [Google Scholar]
- Hoffman D. B., Pearson C. G., Yen T. J., Howell B. J., Salmon E. D. Microtubule dependent changes in the assembly of microtubule motor proteins and mitotic spindle checkpoint proteins at kinetochores. Mol. Biol. Cell. 2001;12:1995–2009. doi: 10.1091/mbc.12.7.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J. Y., Chu Z. G., Han J., Dang Y. M., Yan H., Zhang Q., Liang G. P., Huang Y. S. The p38/MAPK pathway regulates microtubule polymerization through phosphorylation of MAP4 and Op18 in hypoxic cells. Cell Mol. Life Sci. 2010;67:321–333. doi: 10.1007/s00018-009-0187-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y., Chen C., Li Z., Guo W., Gegner J. A., Lin S., Han J. Characterization of the structure and function of a new mitogen-activated protein kinase (p38β) J. Biol. Chem. 1996;271:17920–17926. doi: 10.1074/jbc.271.30.17920. [DOI] [PubMed] [Google Scholar]
- Khodjakov A., Rieder C. L. Imaging the division process in living tissue culture cells. Methods. 2006;38:2–16. doi: 10.1016/j.ymeth.2005.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodjakov A., Rieder C. L. The nature of cell cycle checkpoints: facts and fallacies. J. Biol. 2009;8:88.1–88.5. doi: 10.1186/jbiol195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E. A., Keutmann M. K., Dowling M. L., Harris E., Chan G., Kao G. D. Inactivation of the mitotic checkpoint as a determinant of the efficacy of microtubule-targeted drugs in killing human cancer cells. Mol. Cancer Ther. 2004;3:661–669. [PubMed] [Google Scholar]
- Lee K., Song K. Actin dysfunction activates ERK1/2 and delays entry into mitosis in mammalian cells. Cell Cycle. 2007;6:1487–1495. [PubMed] [Google Scholar]
- Matsusaka T., Pines J. Chfr acts with the p38 stress kinase to block entry to mitosis in mammalian cells. J. Cell Biol. 2004;166:507–516. doi: 10.1083/jcb.200401139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikhailov A., Shinohara M., Rieder C. L. Topoisomerase II and histone deacetylase inhibitors delay the G2/M transition by triggering the p38 MAPK pathway. J. Cell Biol. 2004;166:517–526. doi: 10.1083/jcb.200405167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikhailov A., Shinohara M., Rieder C. L. The p38-mediated stress-activated checkpoint. Cell Cycle. 2005;4:57–62. doi: 10.4161/cc.4.1.1357. [DOI] [PubMed] [Google Scholar]
- Mikule K., Delavel B., Kaldis P., Jurczyk A., Hergert P., Doxsey S. Loss of centrosome integrity induces p38–p53-p21 G1-S arrest. Nat. Cell Biol. 2007;9:160–170. doi: 10.1038/ncb1529. [DOI] [PubMed] [Google Scholar]
- Millar J. B., Buck V., Wilkinson M. G. Pyp1 and Pyp2 PPTases dephosphorylate an osmosensing MAK kinase controlling cell size at division in fission yeast. Genes Dev. 1995;9:2117–2130. doi: 10.1101/gad.9.17.2117. [DOI] [PubMed] [Google Scholar]
- Ohta K., Shina N., Okumura E., Hisanaga S., Kishimoto T., Endo S., Gotoh Y., Nishida E., Sakai H. Microtubule nucleating activity of centrosomes in cell free extracts from Xenopus eggs: involvement of phosphorylation and accumulation of pericentriolar material. J. Cell Sci. 1993;104:125–137. doi: 10.1242/jcs.104.1.125. [DOI] [PubMed] [Google Scholar]
- Raingeaud J., Gupta S., Rogers J. S., Dickens M., Hans J., Ulevitch R. J., Davis R. J. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J. Biol. Chem. 1995;270:7420–7426. doi: 10.1074/jbc.270.13.7420. [DOI] [PubMed] [Google Scholar]
- Rogers G. C., Rogers S. L., Schwimmer T. A., Ems-McClung S. C., Walczak C. E., Vale R. D., Scholey J. M., Sharp D. J. Two mitotic kinesins cooperate to drive sister chromatid separation during anaphase. Nature. 2004;427:364–370. doi: 10.1038/nature02256. [DOI] [PubMed] [Google Scholar]
- Ronkina N., et al. The mitogen-activated protein kinase (MAPK)-activated protein kinases MK2 and MK3 cooperate in stimulation of tumor necrosis factor biosynthesis and stabilization of p38 MAPK. Mol. Cell. Biol. 2007;27:170–181. doi: 10.1128/MCB.01456-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouse J., Cohen P., Trigon S., Morange M., Alonso-Llamazares A., Zamanillo D., Hunt T., Nebreda A. R. A novel kinase cascade triggered by stress and heat shock that stimulates MAPKAP kinase-2 and phosphorylation of the small heat shock proteins. Cell. 1994;78:1027–1037. doi: 10.1016/0092-8674(94)90277-1. [DOI] [PubMed] [Google Scholar]
- Savoian M. S., Gatt M. K., Riparbelli M. G., Callaini G., Glover D. M. Drosophila Klp67A is required for proper chromosome congression and segregation during meiosis 1. J. Cell Sci. 2004;117:3669–3677. doi: 10.1242/jcs.01213. [DOI] [PubMed] [Google Scholar]
- Sudo T., Nitta M., Saya H., Ueno N. T. Dependence of paclitaxel sensitivity on a functional spindle assembly checkpoint. Cancer Res. 2004;64:2502–2508. doi: 10.1158/0008-5472.can-03-2013. [DOI] [PubMed] [Google Scholar]
- Takenaka K., Moriguchi T., Nishida E. Activation of the protein kinase p38 in the spindle assembly checkpoint and mitotic arrest. Science. 1998;280:599–602. doi: 10.1126/science.280.5363.599. [DOI] [PubMed] [Google Scholar]
- Tang J., Yang X., Liu X. Phosphorylation of Plk1 at Ser326 regulates its functions during mitotic progression. Oncogene. 2008;27:6635–6645. doi: 10.1038/onc.2008.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White A., Pargellis C. A., Studts J. M., Werneburg B. G., Farmer B. T. Molecular basis of MAPK-activated protein kinase 2, 38 assembly. Proc. Natl. Acad. Sci. USA. 2007;104:6353–6358. doi: 10.1073/pnas.0701679104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Du J., Marshall M., Blanchard K., Ye X. A novel role of p38α MAPK in mitotic progression independent of its kinase activity. Cell Cycle. 2005;4:e61–e69. doi: 10.4161/cc.4.11.2125. [DOI] [PubMed] [Google Scholar]
- Yang Z., Kenny A. E., Brito D. A., Rieder C. L. Cells satisfy the mitotic checkpoint in Taxol, and do so faster in concentrations that stabilize syntelic attachments. J. Cell Biol. 2009;186:675–684. doi: 10.1083/jcb.200906150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen A. H., Yang J. L. Cdc20 proteolysis requires p38 MAPK signaling and Cdh1-independent APC/C ubiquitination during spindle assembly checkpoint activation by cadmium. J. Cell Physiol. 2010;223:327–334. doi: 10.1002/jcp.22038. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.