Abstract
It is widely believed that discovery of specific, sensitive and reliable tumor biomarkers can improve the treatment of cancer. The goal of this study was to develop a novel fractionation protocol targeting hydrophobic proteins as possible cancer cell membrane biomarkers. Hydrophobic proteins of breast cancer tissues and cell lines were enriched by polymeric reverse phase columns. The retained proteins were eluted and digested for peptide identification by nano-liquid chromatography with tandem mass spectrometry using a hybrid linear ion-trap Orbitrap.
Hundreds of proteins were identified from each of these three specimens: tumors, normal breast tissue, and breast cancer cell lines. Many of the identified proteins defined key cellular functions. Protein profiles of cancer and normal tissues from the same patient were systematically examined and compared. Stem cell markers were overexpressed in triple negative breast cancer (TNBC) compared with non-TNBC samples. Because breast cancer stem cells are known to be resistant to radiation and chemotherapy, and can be the source of metastasis frequently seen in patients with TNBC, our study may provide evidence of molecules promoting the aggressiveness of TNBC.
The initial results obtained using a combination of hydrophobic fractionation and nano-LC mass spectrometry analysis of these proteins appear promising in the discovery of potential cancer biomarkers. When sufficiently refined, this approach may prove useful for early detection and better treatment of breast cancer.
Keywords: Hydrophobic fractionation, Cancer biomarker, Mass spectrometry, Triple negative breast cancer
Introduction
Breast cancer is the most common cancer in women, the leading cause of death among young women age 15–54, and the second most common cause of cancer death in American women (Jemal et al., 2008). Approximately 15% of all invasive breast cancers are triple negative breast cancers (TNBC), with negative expression of estrogen receptors (ER), progesterone receptors (PR), and human epidermal growth factor receptor Her2/neu (Cleator et al., 2007; Kang et al., 2008). Recent gene analysis studies suggest that TNBC arises from basal cells of the mammary epithelium (Nielsen et al., 2004; Harris et al., 2007). TNBCs are more frequently seen in African-American women, young women and women with the BRCA1 mutations (Kang et al., 2008; Carey et al., 2006; Bauer et al., 2007). They are not only among the most aggressive breast tumors but also the only subtype of breast cancer without targeted therapy. Efforts to identify new targets that contribute to the unique biology of these tumors are urgently needed to develop better treatment for this neglected patient cohort.
Proteomics has been employed recently to identify new disease related biomarkers for cancer diagnosis and development of targeted treatment (He et al., 2007; Shau et al., 2003; He et al., 2009; Whelan et al., 2009). Since tumor tissues and tumor cell lines are rich in cancer related proteins, they were selected to study the hydrophobic sub-proteome of human breast cancer.
A common strategy used in proteomics research is to enrich a target set of proteins in order to identify the lower abundance peptides that specify relevant cellular functions. Many fractionation methods have been explored including isolation/enrichment of the membrane sub-proteome, such as membrane glycoproteins (Whelan et al., 2009), that may be a key site for tumor targeting. It is estimated that approximately 30–35% of all open reading frames of sequenced human genomes encode polytopic transmembrane proteins (Hirokawa et al., 1998; Hopkins et al., 2007). Despite their critical biological significance, membrane proteins remain underrepresented in proteomic studies due to poor water solubility, making separation and mass analysis difficult (Speers et al., 2007; Whitelegge et al., 2006). In this study, we analyzed and report the hydrophobic sub-proteome of breast cancer using an enrichment method of normal cell hydrophobic proteins as first described by (Whitelegge et al., 1998; Whitelegge et al., 1999; Whitelegge et al., 2004; Whitelegge, 2005a; Whitelegge, 2005b). These studies demonstrated that not only cell membrane proteins with a variety of functions, but also sub-cellular organelle membrane proteins and acylated non-membrane proteins were found in the hydrophobic sub-proteome. Therefore, we focused our search of cancer biomarkers on a class of proteins possessing hydrophobicity.
Hydrophobicity is a common feature of many cellular proteins especially those residing within, or associated with bilayer membranes. Since membrane proteins play critical roles within cells and endow cancer cells with many of their unique properties, a strategy that enriches this class of proteins may prove useful. While integral membrane proteins can be predicted from their primary sequences, association of other globular proteins with membranes can be challenging if not impossible to predict. Choice of hydrophobicity as a property for enrichment is novel and offers the chance of finding ‘biomarker events’ that result in gain or loss of membrane association. The hydrophobic sub-proteome of breast cancer was analyzed by combining LTQOrbitrap mass spectrometry with several computational methods to identify a cohort of moderate abundance proteins including several candidate biomarkers related to malignancy.
Material and Methods
Cell culture
Human breast cancer cell lines MDAMB231, MDAMB468, MCF7 and T47D were obtained from American Tissue Type Culture Collection (ATCC, Rockville, MD). Cells were maintained in Dulbecco’s minimal essential medium (InVitrogen, Carlsbad, CA) or RPMI 1640 (Invitrogen) with 10% heat-inactivated fetal calf serum, 100,000 units/L penicillin, and 100 mg/L streptomycin, at 37°C in 5% CO2.
This panel of cell lines was chosen based on ER, PR and Her2 status, which are surface marker used clinically to select optimal adjuvant therapy for breast cancer patients. All cell lines were tested and authenticated for the ER, PR and Her2 status by western-blot (Supplementary Data). MDAMB231 and MDAMB468 are TNBC while MCF7 and T47D are hormone receptor positive but Her2 negative breast cancer.
Human samples
Breast specimens were collected prospectively from participants of IRB approved clinical studies. Breast tissue was collected immediately after needle biopsy or surgical removal. The collected specimen was delivered on ice to the Tissue Bank where it was divided into three parts: one part placed in OCT embedding medium for frozen tissue specimens (Tissue-Tek®) and two parts directly frozen in liquid nitrogen. All specimens were stored frozen at −80°C.
Paired breast cancer and normal breast tissue were collected from two patients with stage III disease, Case A and Case B. Case A was a TNBC, while Case B was hormone receptor positive and Her2 negative, a non-TNBC breast cancer.
Protein extraction of cell lysates and tissue lysates
Confluent monolayer of cultured breast cancer cells was extracted in lysing buffer (150 mM NaCl, 50 mM Tris-Cl, 2 mM EDTA, 1 mM sodium orthovanadate, containing 1% Triton X-100) for 10 min. The cell lysate was centrifuged at 12,000 g for 10 min to collect the supernatant. Protein concentrations were measured by Bradford assays.
Breast tumors and normal tissues with fat trimmed were rinsed in cold PBS and homogenized in the same lysing buffer. The homogenizer was immersed in slushy ice during 30 slow passes. The homogenate of each specimen was centrifuged at 12,000 g for 10 min at 4°C to remove debris. All supernatants were collected at 4°C, and frozen in liquid nitrogen for storage at −80°C after protein concentration was determined.
Membrane preparation
By traditional centrifugation method
Samples were centrifuged at 1,500 g for 5 min at 4°C to remove large debris, and then centrifuged at 100,000 g for 1 hour to remove nucleocytosolic fractions from crude membrane fractions. Membrane fractions were solubilized in 40 mM Tris-HCL pH 8.3, 6 M guanidine HCL, 0.2% RapiGest, 5 mM DTT. Insoluble debris was removed by centrifugation and the supernatant was diluted with 1 M guanidine HCl.
By hydrophobic liquid chromatography
Briefly, the protein samples were centrifuged at 8,000 g for 10 min at 4°C before injection of supernatants onto an HPLC reverse-phase chromatography using a polymeric stationary phase (2.1 × 150 mm, 5 μm, 300 Å, PLRP/S, Polymer Labs, Part#PL1912-3501). The system was first equilibrated for 30 minutes at 95% Buffer A and 5% Buffer B (A, 0.1% TFA in water; B, 0.1% TFA in freshly prepared acetonitrile/isopropanol, 50/50, v/v) before injection and starting a linear gradient of increasing organic concentration (18). The column was eluted with a compound linear gradient from 5% Buffer B at 3 min to 90% Buffer B at 75 minutes. A280 nm measurement was recorded for assessment of chromatographic performance. Proteins not eluted during the acetonitrile/isopropanol gradient were displaced by injection of formic acid (88%, 100 μl) and elution with Buffer C (chloroform/methanol/1% aqueous formic acid, 4/4/1). Fractions were collected, dried in a vacuum concentrator and stored at −20°C.
By using disposable spin cartridge as an alternative to hydrophobic column
Our experience in working with HPLC showed that pressure build-up in HPLC columns was a limiting factor in enriching hydrophobic proteins. An alternative method was successfully developed by us to overcome this problem. Beads from the Polymer Lab (Part# PL1412-2101) were packed into a single-use disposable spin cartridge with the same volumn as the conventional hydrophobic column. Our unpublished data comparing the same cell lysate profiled by the commercial hydrophobic column and a disposable spin cartridge prepared in our laboratory showed more than 95% of proteins detected by either methods were identical. The disposable spin column is low-cost, has a zero sample-sample cross contamination, does not have pressure build-up, and has therefore become our choice for hydrophobic fractionation. Briefly, each cartridge was activated with two sequential methanol rinses, followed by washing with three sequential rinses of water/acetonitrile/TFA (95/5/0.1, all by vol.). Specimens of 1 mg cell lysates were loaded into the cartridges. These cartridges were spun for 1 min. at 110 g. Hydrophobic proteins were retained, while soluble proteins, salts, and polar solutes like DNA were eluted with the liquid and discarded. Non-retained components were removed by five sequential barrel washes using the following solvents:
water/acetonitrile/isopropanol/TFA (90/05/05/0.1, all by vol.);
water/acetonitrile/isopropanol/TFA (70/15/15/0.1, all by vol.);
water/acetonitrile/isopropanol/TFA (50/25/25/0.1, all by vol.);
water/acetonitrile/isopropanol/TFA (30/35/35/0.1, all by vol.);
water/acetonitrile/isopropanol/TFA (10/45/45/0.1, all by vol.).
The retained hydrophobic proteins were eluted with 1 mL 88% formic acid followed by 2 mL chloroform/methanol/H2O (4/4/1, v/v, freshly prepared daily). Samples were collected and dried in a vacuum concentrator and stored at −20°C.
Reduction, alkylation and trypsinization of proteins for LC/MS/MS
The dried samples were dissolved in freshly prepared guanidine HCl (6 M, 20 μl) containing 10 mM DTT and 0.2% RapiGest (Waters Corp. MA), vortexed, and incubated at 37°C for 1 hour. Additional guanidine HCL (6 M, 2 μl) containing 300 mM iodoacetamide was added, mixed, and incubated at 37°C for 1 hour. The sample was mixed with the trypsin (sequencing grade, Promega) solution containing 1.6 ml 0.5 M ammonium bicarbonate and incubated at 37°C for 4 hours. Reverse phase C18 cartridges (AccuBond II ODSC18) were used and the manufacturers protocol was followed to remove salt from the samples.
LC-MS/MS analysis and peptide data analysis
LC-MS/MS and data analysis were modified from Whelan et al. (2009). Briefly, samples were redissolved in Buffer D (H2O/acetonitrile/formic acid, 98.9/1/0.1, typically 50 μL), separated by nanospray LC (Eskigent Technologies, Inc. Dublin, CA), and analyzed using online tandem mass spectrometry (LTQ Orbitrap, Thermo Fisher). Aliquots were injected (10 μL) onto a reverse phase column (New Objective C18, 15 cm, 75 μM diameter, 5 μm particle size equilibrated in Buffer D) and eluted (300 nL/min) with an increasing concentration of Buffer E (acetonitrile/water/formic acid, 98.9/1/0.1; min 0/5, 10/10, 112/40, 130/60, 135/90, 140/90). The effluent from the column was passed directly into an integrated nanospray emitter tip connected to the LTQ Orbitrap mass spectrometer. Eluted peptides were analyzed by MS and datadependent MS/MS acquisition (collision-induced dissociation (CID)), previously optimized for samples, selecting the 7 most abundant precursor ions for MS/MS with a dynamic exclusion duration of 15.0 s.
Biowork software searchers were conducted using a human trypsin cleavage indexed peptide database, with variable modifications of carboxyamidomethylation (57.02146) and methionine oxidation (15.99492). Scaffold data analysis (Proteome Software, Inc. Version 2.2.03) was conducted using Bioworks search file results with a high stringency filter with a 99% minimum protein ID probability, a minimum number of 2 unique peptides for each protein identified, and with a minimum peptide ID probability of 95% except when stated otherwise. Scaffold uses X! Tandem (Craig et al., 2003) ProteinProphet computer algorithms (Nesvizhskii et al., 2003) and PeptideProphet (Keller et al., 2002) to verify peptide identifications derived from MS/MS sequencing results. Scaffold is also used to quantitate spectral counts by normalizing MS/MS data between samples. Each sample analyzed was a combination of 3 replicate experiments and was normalized by averaging spectral counts for all samples, multiplying spectral counts in each sample by the average and then dividing by each samples sum.
Results
Hydrophobic column chromatography combined with the LTQ-Orbitrap MS/MS analysis detected hundreds of proteins from whole cell lysates
The reverse-phase chromatography system effectively enriched hydrophobic proteins of cultured cells and human tissues. Combined with the LTQ-Orbitrap, hundreds of proteins were identified in each sample by high stringency search engines. The significant number of proteins identified provided a meaningful analysis of disease-related biomarkers. Table 1 lists the total number of proteins identified by the LTQ-Orbitrap in the hydrophobic fractions of 8 samples.
Table 1.
Total number of protein in hydrophobic fraction of 2mg tissue lysates identified by LTQ-Orbitrap.
| Sample (2mg) | Tumor Type | Proteins Identified |
|---|---|---|
| Case A normal tissue | 172 | |
| Case A cancer tissue | TNBC | 227 |
| Case B normal tissue | 146 | |
| Case B cancer tissue | Non-TNBC | 147 |
| MDAMB231 cells | TNBC | 392 |
| MDAMB468 cells | TNBC | 206 |
| MCF7 cells | Non-TNBC | 346 |
| T47D cells | Non-TNBC | 290 |
More proteins were identified in the hydrophobic sub-proteomes than in the whole cell lysates
Several highly abundant proteins such as structural proteins (e.g. tubulin and actinin) in whole cell lysates overwhelm the mass spectrometry system and mask the lower abundance proteins from being detected. Hydrophobic fractionation removed many of these high abundant proteins and efficiently enriched a unique sub-proteome. When this hydrophobic sub-proteome was compared to the whole cell lysate of the same cell line, not only were there more proteins identified but there were also more cancer-related proteins consistently found in each sample (Table 2).
Table 2.
Comparison of proteins identified in the hydrophobic fraction and the whole cell lysate of three breast cancer cell lines by LTQ-Orbitrapmass spectrometry.
| Cell lines studied | Unique proteins in hydrophobic fraction | Shared proteins by the two preparations | Unique proteins in whole cell lysate |
|---|---|---|---|
| MDAMB231 cells | 251 | 141 | 4 |
| MCF7 cells | 230 | 116 | 6 |
| T47D cells | 170 | 120 | 13 |
Hydrophobic fractionation allowed for the identification of more membrane proteins than by conventional centrifugation methods
Most proteins enriched by the hydrophobic fraction were membrane origin. The hydrophobic proteins were therefore directly compared to membrane proteins prepared by the conventional ultra-centrifugation method. There are many proteins only detected in the hydrophobic fractions but not in the conventional membrane preparation. Several examples of these proteins and their protein accession numbers, functions and cellular/sub-cellular location of these novel membrane proteins were summarized in Table 3.
Table 3.
Unique MCF7 membrane proteins enriched by hydrophobic chromatography that are not found by conventional cell membrane preparation.
| Identified Proteins | Accession Number | Cellular Function | Membrane Location |
|---|---|---|---|
| G-protein coupled receptor 123 | gi|143811399 | G-protein coupled receptor protein signaling pathway | Cell membrane; Multi-pass membrane protein |
| Integrin beta-1 (Fibronectin receptor subunit β) (CD29 antigen) | gi|124963 (+1) | Homophilic cell adhesion, stem cell marker | Sarcolemma |
| Nesprin-1 (Nuclear envelope spectrin repeat protein 1) | gi|29839561 | Anchoring protein involved in the maintenance of nuclear organization and structural integrity. | Nucleus outer membrane |
| Phosphatidylinositol 3,4,5-trisphosphate-dependent Rac exchanger 1 protein (PRex1) | gi|148886999 | Actin filament polymerization | Plasma membrane |
| Long-chain-fatty-acid--CoA ligase 6 (LACS 6) | gi|146322303 | Acyl-CoA metabolism | Plasma membrane |
| Prolow-density lipoprotein receptor-related protein 1 precursor (LRP) | gi|1708865 | Cell proliferation | Integral to plasma membrane |
| Thioredoxin reductase 2 | gi|34925455 | Maintains thioredoxin in a reduced state. Implicated in the defenses against oxidative stress. May play a role in redox-regulated cell signaling. | Mitochondrion |
| Elongation factor Tu | gi|1706611 | Promotes the GTP-dependent binding of aminoacyl-tRNA to the Asite of ribosomes during protein biosynthesis. | Mitochondrion |
| Polycystin-1 (Autosomal dominant polycystic kidney disease protein 1) | gi|45645177 | Calcium-independent cellmatrix adhesion | Integral to plasma membrane |
| ATP-binding cassette subfamily A member 12 | gi|51315963 | Cell homeostasis | Integral to membrane |
| Desmocollin-1 | gi|2493422 | Cell adhesion and cell junction | Gap junction |
| Long-chain-fatty-acid--CoA ligase 6 | gi|146322303 | Fatty acid metabolism | Mitochondrion outer membrane |
Some membrane proteins were also found in both preparation. For example, Caprin-1 (gi|2498733) and transferrin receptor protein 1 (TfR1, gi|108935939), both integral to plasma membrane, were found in MCF7 cells by both methods. However, the proteins in the conventional cell membrane preparation were not all included in the findings made by the hydrophobic method. There were proteins unique to the conventional cell membrane preparation, although most of them were not membrane origin. For example, in MCF7 cells, only three trans-membrane proteins, voltage-dependent anion channel 1 (gi|4507879), solute carrier family-3 member-2 isoform-f (gi|61744483) and N-acetylglucosamine-specific receptor-1 (gi|627551), were identified exclusively in the conventional cell membrane preparation.
Comparing the two methods of membrane protein enrichment, our data suggests that the hydrophobic fractionation is superior because significantly more proteins were found by the method. In the same cell line, 346 proteins were detected by the hydrophobic method while only 145 proteins were found in the conventional membrane preparation.
Many shared and unique proteins were detected in breast cancer and normal breast tissue of the same individual
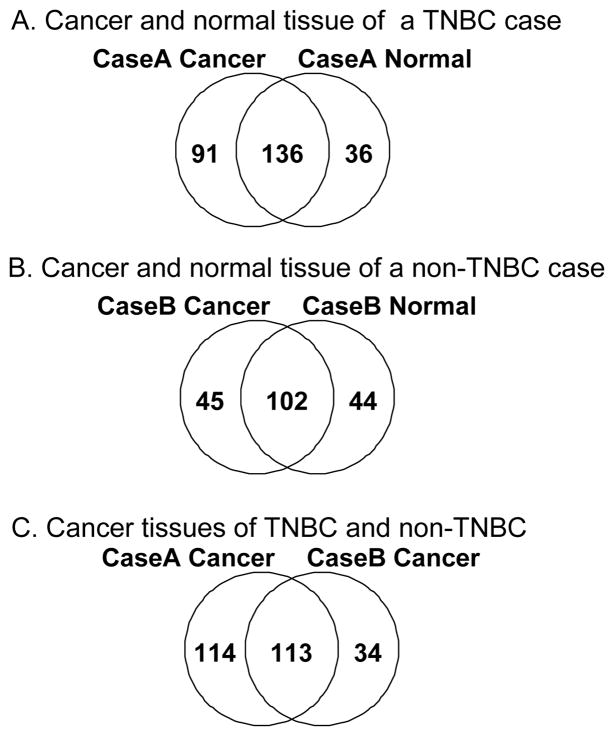
We compared hydrophobic proteins between paired breast cancer and normal breast tissue of the same individual (Figure 1A and 1B). As expected, we found many proteins shared by tumor and normal tissues of the same individual. We also found a significant number of unique proteins in either cancer or normal tissues. When confirmed, some proteins unique to cancer may represent valuable biomarkers for cancer diagnosis or targeted therapy (Hopkins et al., 2002; Russ et al., 2005).
Figure 1. Comparison of the hydrophobic proteins identified from cancer breast tissue and normal breast tissue of the same patient (A and B) as well as cancer breast tissues of two different types of breast cancer (C).
1A and 1B: While a number of hydrophobic proteins were found to be shared between cancer and normal breast tissues derived from the same individual, unique proteins were also found to be associated with either cancer or normal breast specimen. The pool of the unique proteins may include disease related biomarkers, and may potentially be used as therapy targets. 1C: Comparison between the hydrophobic sub-proteome of a TNBC (Case A) and a non-TNBC (Case B) cancer specimen showed the difference between two cancers.
A comparison of the hydrophobic sub-proteome of breast cancer tissues of two different types of breast cancer revealed many differences (Figure 1C). While some of the differences may be related to the types of breast cancer, the majority of unique proteins of each case may simply reflect differences of the two different individuals.
Protein overlaps seen between cancer specimens and cell lines
Cell lines traditionally have been used to study cancer biology, and to build pre-clinical data guiding therapeutic strategies. Our study shows that a great number of proteins are shared by tumor tissues and cancer cell lines, although there are more similarities between cell lines (both TNBC and non-TNBC) than the similarities between tissue and cell lines of the same type of breast cancer (Figure 2A and 2B). Some of the differences observed between cancer tissues and cell lines, may reflect cellular changes resulting from long term in vitro culture as well as the presence of in vivo stromal components found only in tumor tissue. Because of the significant differences seen between the proteins of the two sources, findings from cell line studies need to be interpreted with caution for clinical appreciation.
Figure 2. Comparison of hydrophobic sub-proteome of cancer tissue and two breast cancer cell lines of the same receptor type.
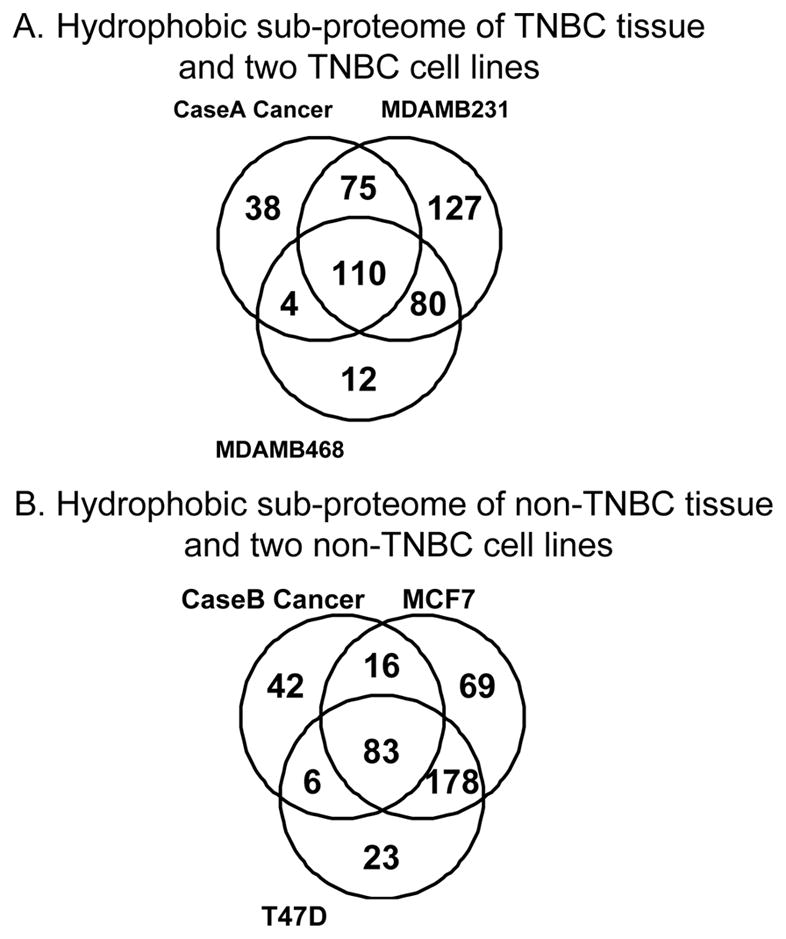
A: Comparison of a triple negative breast cancer (TNBC) tissue vs. cell lines—MDAMB231 and MDAMB468. B: Comparison of a non-triple negative breast cancer tissue vs. cell lines—MCF7 and T47D cells. All three are ER+/PR+/Her2-.
Differentially expressed proteins by TNBC when compared with non-TNBC tissues and cell lines
Using semi-quantitative analysis of TNBCs vs. non-TNBCs performed by Scaffold 2.2.03 software, we found that numerous proteins were down-regulated (≤0.5 fold), or up-regulated (≥2.0 fold). When comparing the two cancer cases, we found 59 proteins were up-regulated in the TNBC, 42 proteins were down-regulated, and 47 proteins were similar in the two specimens. Comparison between cell lines of MDAMB231 and MCF7 showed that 139 proteins were upregulated in the TNBC cell line, 178 proteins were down-regulated, and 57 proteins were similar in both cell lines.
From a pool of up-regulated proteins in TNBC biopsy samples, a list of 20 proteins with more than a 2 fold increase in the TNBC tumor (Table 4A) and cell line (Table 4B) was reported. Several stem cell markers, such as 4-trimethylaminobutyraldehyde dehydrogenase (ALDH), CD44, integrin alpha-6 (CD49F), integrin beta-1 precursor (fibronectin receptor subunit beta, and CD29) were found expressed in the TNBC samples and their presence were confirmed by flow cytometry analysis (Supplementary Data). While the overlap in protein expression between TNBC cancer tissues and TNBC cell lines were limited, however there was a remarkable correlation of significantly up-regulated hydrophobic proteins between the two. Our data suggest that these proteins may include bona fide markers associated with TNBC.
Table 4A.
Selected over-expressed proteins (≥2 fold) in a triple negative breast cancer (TNBC, Case A) tumor when compared with a hormone-receptor-positive-Her2-negative (Non-TNBC, Case B) tumor specimen.
| Identified Proteins | Accession # | Cellular Function |
|---|---|---|
| 4-trimethylaminobutyraldehyde dehydrogenase (ALDH) | gi|62511242 | Catalyzes the irreversible oxidation of aldehydes to the corresponding acids in an NAD-dependent reaction. Stem cell marker |
| Annexin VI isoform 1 | gi|71773329 | Mediates the intracellular calcium Signal |
| ATP synthase, beta subunit precursor | gi|32189394 | ATP synthesis |
| Dipeptidyl peptidase III | gi|18491024 | Protease that is a member of the S9B family. Increased activity of this protein is associated with endometrial and ovarian cancers. |
| Elongation factor Tu, mitochondrial precursor (EF-Tu) | gi|1706611 | Promotes the GTP-dependent binding of aminoacyl-tRNA to the A-site of ribosomes during protein synthesis. |
| Enolase 1 | gi|4503571 | Myc promoter-binding protein |
| Eukaryotic translation elongation factor 2 | gi|4503483 | Essential factor for protein synthesis |
| Heat shock 70kDa protein 5 | gi|16507237 | Cellular stress response |
| Heat shock 70kDa protein 8 isoform 1 | gi|5729877 | Cellular stress response |
| L-plastin | gi|4504965 | Actin-binding proteins expressed only in hemopoietic cell lineages in benigh tissue, but in malignant human cells of non-hemopoietic origin suggesting that its expression is induced accompanying tumorigenesis in solid tissues. |
| Macrophage Migration Inhibitory Factor (Mif) | gi|13399777 | Inhibits macrophage migration and causes adherence |
| Mutated melanoma-associated antigen 1 | gi|152032608 | Melanoma-associated antigen |
| Proteasome (prosome, macropain) subunit, alpha type 6 | gi|8394076 | Central enzyme of nonlysosomal protein degradation in both the cytosol and nucleus |
| Proteasome subunit, α5 | gi|7106387 | Protein degradation |
| Protein disulfide isomerase-associated 3 precursor | gi|21361657 | Endoplasmic reticulum protein interacting with lectin chaperones calreticulin and calnexin to modulate folding of newly synthesized glycoproteins |
| Protein disulfide isomerase-associated 4 | gi|4758304 | Catalyzing the formation of disulfide bonds of newly synthesized polypeptides in the endoplasmic reticulum |
| Pyruvate kinase 3 isoform 1 | gi|33286418 (+2) | Catalyzes the rate-limiting step of glycolysis |
| Thioredoxin peroxidase | gi|5453549 | Antioxidant enzyme belongs to the peroxiredoxin family. Reduce hydrogen peroxide and alkyl hydroperoxides to water and alcohol. Regulate the activation of the transcription factor NF-kappaB |
| Tumor rejection antigen (gp96) 1 | gi|4507677 | Cellular stress response |
| Vesicle amine transport protein 1 | gi|18379349 | An abundant integral membrane protein involved in vesicular transport |
Table 4B.
Selected over-expressed proteins (≥ 2 Fold) in MDAMB231 cells when compared with MCF7 cells.
| Identified Proteins | Accession # | Cellular Function |
|---|---|---|
| Adenylyl cyclase-associated protein 1 | gi|116241280 | Establishment and/or polarity maintenance of cell |
| Annexin 5 | gi|4502107 | Mediates the intracellular calcium signal |
| Annexin A2 | gi|4757756 | Mediates the intracellular calcium signal |
| Annexin I | gi|4502101 | Mediates the intracellular calcium signal |
| CD44 antigen precursor | gi|2507241 | Cell-cell adhesion. Stem cell marker |
| Enolase 1 | gi|4503571 | Myc promoter-binding protein |
| Eukaryotic translation elongation factor 1 alpha 1 | gi|4503471 | Essential factor for protein synthesis |
| Eukaryotic translation initiation factor 3 subunit D | gi|4503523 | Protein biosynthesis |
| Eukaryotic translation initiation factor 3, subunit 10 theta | gi|4503509 | Protein biosynthesis |
| Heat shock 10kDa protein 1 | gi|4504523 | Cellular stress response |
| Integrin alpha-6 (CD49F) | gi|12644170 | A receptor for laminin in epithelial cells, stem cell marker |
| Integrin beta-1 precursor (Fibronectin receptor subunit beta, CD29) | gi|124963 | Homophilic cell adhesion, cellular defense response, stem cell marker |
| Keratin, type I cytoskeletal 10 | gi|147744568 | Response to oxidative stress |
| Polymerase I and transcript release factor (PTRF) | gi|42734430 | Augments ribosomal gene transcription |
| Proteasome (prosome, macropain) 26S subunit, ATPase 1 | gi|6679501 | Protein degradation |
| Proteasome 26S ATPase subunit 2 | gi|4506209 | Protein degradation |
| proteasome alpha 1 subunit isoform 2 | gi|4506179 | Protein degradation |
| proteasome alpha 7 subunit | gi|4506189 (+1) | Protein degradation |
| Ras related v-ral simian leukemia viral oncogene homolog A | gi|33946329 (+1) | Belongs to the small GTPase superfamily, Ras family of proteins. GTP-binding proteins mediate the transmembrane signaling initiated by the occupancy of certain cell surface receptors |
| Ribosome-binding protein 1 (Ribosome receptor protein) | gi|23822112 | Protein biosynthesis |
| Ubiquitin-activating enzyme E1 | gi|23510338 | Protein degradation |
Effect of high stringency vs. low stringency filters on the protein identification
In order to ensure confidence in the proteins reported, we chose a high stringency filter (Minimum protein 99%; Minimum # peptide 2; Minimum peptide 95%) in most of our results. However, the double-edged sword of the high stringency filter on one hand increases the confidence in reporting the protein identified, but it also misses low abundant proteins that could be clinically more important such as HER2, PR, and ER. We therefore compared the results using both high and low stringency in two normal tissues. Forty-eight unique proteins were identified in Case A normal breast tissue with high stringency filter, in contrast to 237 proteins identified by a low stringency filter (Minimum protein. 90%; Minimum # peptide, 1; Minimum peptide, 90%). Similarly the unique proteins in Case B normal breast tissue increased from 22 to 159 when low stringency filter was used. The proteins shared between Case A and Case B were 124 and 190 identified by the high and low stringency filters respectively. Our study showed by lowering the stringency filter to accept 1 peptide that the number of unique proteins identified was 5 times greater than reported with the high stringency filter.
Our finding suggested that data from both high and low stringency filters should be explored in proteomics research. Many of the proteins, especially those with critical biological functions, such as membrane receptors or kinases, are expressed at much lower levels than structure proteins or metabolism-related enzymes. To avoid either missing potential biomarkers or getting false positive results, all findings, especially those found by the low stringency filters should be carefully validated by other experimental methods such as immunological assays.
Discussion
Success of the human genome project has led to an increased understanding of cancer at the molecular level (Lander et al., 2001; Venter et al., 2001). Elucidation of the human genome identified approximately 23,000 genes that encode for 100,000 to 150,000 different transcripts (transcriptome). The functional products, the human proteome, are much more complex with a 10-fold increase in number. Traditional antibody based and target directed analysis is limited to known proteins and is not able to detect, compare and identify hundreds of unknown proteins simultaneously. Proteomics techniques, such as mass spectrometry (MS) coupled with powerful bioinformatic tools, now allow high through-put discovery of novel proteins, and are evolving rapidly to meet the formidable challenge of protein diversity in biomarker research.
Complexity of cancer proteome far exceeds the dynamic range of any single analytical method or instrument, and precludes the identification of most low abundance proteins. In this study, we focused on the hydrophobic sub-proteome to enrich these key low abundance proteins for enhanced biomarker detection. The rationale for choosing hydrophobicity of proteins to enrich cancer biomarkers is based on recent observations reported by Whitelegge et al. (2004) that many integral membrane proteins elute with low efficiency from polymeric reverse-phase columns (PLRP/S) but hydrophobic proteins retained on the column can be recovered by a formic acid for elution.
In this study, we have demonstrated that hydrophobic fractionation enhances detection of novel membrane proteins by mass spectrometry compared to conventional membrane preparations or whole cell lysate. Hydrophobic columns do not bind highly abundant cytosolic or soluble proteins, such as pyruvate kinase, structural components tubulin and actinin, or serum albumin present in tissue specimens, which allowed us to detect the less abundant proteins of interest. Several proteins usually considered to be non-hydrophobic were found to the hydrophobic matrix. This may be due to: 1). Protein-protein complex formation between a lipophilic membrane protein and cytosolic protein bound tightly that elute with the hydrophobic proteins; 2). Fatty acylation of these proteins or denatured protein exposing a hydrophobic core.
In this study, we have identified a rich source of hydrophobic proteins from selected human tumors and cell lines. Many of these proteins have known important cellular functions, including heat shock proteins (Soo et al., 2008), translation elongation factors (White-Gilbertson et al., 2008), EGFR (Charpidou et al., 2008), cytokeratin (Diaz et al., 2007), CD44 (Ginestier et al., 2007), cadherin (Wang et al., 2008), mitochondrial aldehyde dehydrogenase (Croker et al., 2008), endothelial cell growth factors (Mohammed et al., 2007; Relf et al., 1997), mucin (Rubinstein et al., 2009), and annexin (Imai et al., 2008).
When breast tumors, adjacent normal tissues and cell lines of TNBC and non-TNBC origins were compared, protein expression of the two groups were assigned into 3 categories: upregulated, unchanged, and down-regulated. Since up-regulated proteins are probably more useful as cancer biomarkers and drug targets, we focused our report on these proteins.
The unregulated proteins in TNBC were classified into 7 categories according to their cellular activities: 1). Metabolism related proteins, such as ATP synthase, glutathione transferase, mitochondrial aldehyde dehydrogenase, pyruvate kinase, glucosidase and fatty acid synthase. 2) Growth factors, such as endothelial cell growth factor, which plays an important role in angiogenesis. 3). Protein degradation pathways, such as proteosome subunits, ubiquitin conjugation factors, and ubiquitin-activating enzymes. 4) Transcription and translation regulatory proteins, such as DNA helicase, calreticulin, enolase, eukaryotic translation elongation factors, nucleolin, polymerase I, and transcript release factor, ribosome-binding proteins, DNA topoisomerase, and RNA polymerase. 5) Membrane channel or channel related proteins, such as annexin, voltage-dependent P/Q-type calcium channel subunits. 6) Cell-cell adhesion, which is important in the micro-environment of cancer cells, potentially helps cell migration and cancer metastasis, such as cadherin and CD44. 7) Cellular stress response, heat shock proteins, keratin, and tumor rejection antigen. TNBC specific up-regulation in the 7 functionalities was seen both in cell lines and human cancers, which may help account for the aggressiveness of TNBC. These invasive cancer cells are likely equipped with mechanisms capable of responding to cellular stress, such as hypoxia and nutritional depletion caused by their propensity to out-grow the existing vascular supply.
Annexin related proteins also were highly over-expressed in TNBC, especially in the cell lines with a greater than 100-fold increase. Our findings are in accord with previous reports of annexin over-expression correlating with the aggressiveness of cancer. Annexin A3 has been found to be significantly up-regulated in invasive lung adenocarcinomas with lymph node metastasis compared to those without lymph node metastasis (Liu et al., 2009). Similarly, annexin is significantly elevated in lymphatic metastasis of mouse hepatocarcinoma (Liu et al., 2008). Our previous study also showed that altered expression of annexin A1 is correlated with breast cancer development and progression (Shen et al., 2005; Shen et al., 2006). Together, these findings provide strong evidence that Annexin family proteins are likely to contribute to the aggressive phenotype and metastatic potential of cancer cells.
Our study also identified over expression of several important stem-cell markers in TNBC cancer specimens and TNBC cell lines compared with non-TNBC samples. For example, CD44 (gi|2507241), a stem cell marker, was found oeverexpressed in both MDAMB231 cells and MDAMB468 cells. CD44, is from a family of transmembrane p-glycoproteins, which are adhesion molecules binding to the extracellular matrix containing hyaluronic acid, collagen, fibronectin, laminin, and FGF-2 (Günthert, 1993). CD44 has been shown to contribute to both the metastatic potential in pancreatic cancer (Wielenga et al., 1993; Günthert et al., 1991) and drug resistance (Li et al., 2008). Other stem cell markers, such as integrin α6 (gi|12644170, also known as CD49F), and integrin beta-1 precursor (gi|124963, also called CD29), were also found to be over-expressed in MDAMB-231 cells. CD49F is highly-expressed by the basal layer of proliferating skin epithelial cells and by breast cancer stem cells. It regulates cell adhesion to the extracellular matrix and is involved in cancer cell migration, invasion, pathologic angiogenesis and tumor cell survival (Mercurio et al., 2001; Nikolopoulous et al., 2004). Another stem-cell marker, aldehyde dehydrogenase (ALDH, gi|62511242) was found in Case A—a TNBC tumor. ALDH1 is a detoxifying enzyme responsible for the oxidation of intracellular aldehydes and may play a role in early differentiation of stem cells through oxidizing retinol to retinoic acid. High levels of ALDH1 activity also have been found in other human stem cells of hematopoietic and neural origin. Because breast cancer stem cells have been implicated in radiation and chemotherapy resistance, as well as increasing the potential of metastasis, these findings may explain treatment failure as well as metastasis that are frequently seen in TNBC patients. The development of an effective therapeutic strategy for this disease may depend on finding a new way to target the stem cell population.
Although still preliminary, cancer proteomic discoveries have shown real promise in improving the understanding of tumor biology. Our study provides evidence that it is possible to identify hundreds of relevant proteins in a selected sub-proteome using only mg of cancer tissue. However, detection of very low abundance proteins remains to be a challenge. Further improvements in protein separation methods coupled with mass spectrometry to isolate different types of proteins and proteins with post-translational modifications may allow deeper profiling of the low abundance proteins in the near future.
Our study demonstrates that hydrophobic fractionation is an effective method to enrich an important class of tumor biomarkers and provides new evidence that LC/MS/MS can identify and quantify differences in cancer-related protein expression. When sufficiently refined, these powerful new technologies may pave the way for earlier detection and better treatment of breast cancer.
Supplementary Material
Acknowledgments
This work was supported in part by the California Breast Cancer Research Program (6JB-0013), the Department of Defense (DAMD17-01-1-0179), the National Institute of Health (1RO1CA93736), the Gonda Foundation, the EIF-Women Cancer Research Fund and Friends of the Breast Program at UCLA.
Abbreviations
- ER
Estrogen Receptors
- PR
Progesterone Receptors
- TNBC
Triple Negative Breast Cancer
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triplenegative phenotype: a populationbased study from the California cancer Registry. Cancer. 2007;109:1721–1728. doi: 10.1002/cncr.22618. [DOI] [PubMed] [Google Scholar]
- 2.Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. Jama. 2006;295:2492–2502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 3.Charpidou A, Blatza D, Anagnostou E, Syrigos KN. EGFR mutations in non-small cell lung cancer—clinical implications. In Vivo. 2008;22:529–536. [PubMed] [Google Scholar]
- 4.Cleator S, Heller W, Coombes RC. Triple-negative breast cancer: therapeutic options. Lancet Oncol. 2007;8:235–244. doi: 10.1016/S1470-2045(07)70074-8. [DOI] [PubMed] [Google Scholar]
- 5.Craig R, Beavis RC. A method for reducing the time required to match protein sequences with tandem mass spectra. Rapid Commun Mass Spectrom. 2003;17:2310–2316. doi: 10.1002/rcm.1198. [DOI] [PubMed] [Google Scholar]
- 6.Croker AK, Goodale D, Chu J, Postenka C, Hedley BD, et al. High aldehyde dehydrogenase and expression of cancer stem cell markers selects for breast cancer cells with enhanced malignant and metastatic ability. J Cell Mol Med. 2008 doi: 10.1111/j.1582-4934.2008.00455.x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diaz LK, Cryns VL, Symmans WF, Sneige N. Triple negative breast carcinoma and the basal phenotype: from expression profiling to clinical practice. Adv Anat Pathol. 2007;14:419–430. doi: 10.1097/PAP.0b013e3181594733. [DOI] [PubMed] [Google Scholar]
- 8.Ginestier C, Korkaya H, Dontu G, Birnbaum D, Wicha MS, et al. The cancer stem cell: the breast cancer driver. Med Sci. 2007;23:1133–1139. doi: 10.1051/medsci/200723121133. [DOI] [PubMed] [Google Scholar]
- 9.Günthert U, Hofmann M, Reber R, Rudy W, Reber S, et al. A new variant of glycoprotein CD44 confers metabolic potential to rat carcinoma cells. Cell. 1991;65:13–24. doi: 10.1016/0092-8674(91)90403-l. [DOI] [PubMed] [Google Scholar]
- 10.Günthert U. CD44: a multitude of isoforms with diverse functions. Curr Top Microbiol Immunol. 1993;184:47–63. doi: 10.1007/978-3-642-78253-4_4. [DOI] [PubMed] [Google Scholar]
- 11.Harris LN, You F, Schnitt SJ, Witkiewicz A, Lu X, et al. Predictors of resistance to preoperative trastuzumab and vinorelbine for HER2-positive early breast cancer. Clin Cancer Res. 2007;13:1198–1207. doi: 10.1158/1078-0432.CCR-06-1304. [DOI] [PubMed] [Google Scholar]
- 12.He J, Gornbein J, Shen D, Lu M, Rovai LE, et al. Detection of breast cancer biomarkers in nipple aspirate fluid by SELDI-TOF and their identification by combined liquid chromatography-tandem mass spectrometry. Int J Oncol. 2007;30:145–154. [PubMed] [Google Scholar]
- 13.He J, Shen D, Chung DU, Saxton RE, Whitelegge JP, et al. Tumor proteomic profiling predicts the susceptibility of breast cancer to chemotherapy. Int J Oncol. 2009;35:683–692. doi: 10.3892/ijo_00000380. [DOI] [PubMed] [Google Scholar]
- 14.Hirokawa T, Boon-Chieng S, Mitaku S. SOSUI: classification and secondary structure prediction system for membrane proteins. Bioinformatics. 1998;14:378–379. doi: 10.1093/bioinformatics/14.4.378. [DOI] [PubMed] [Google Scholar]
- 15.Hopkins AL, Groom CR. The druggable genome. Nat Rev Drug Discov. 2002;1:727–730. doi: 10.1038/nrd892. [DOI] [PubMed] [Google Scholar]
- 16.Imai K, Ichibangase T, Saitoh R, Hoshikawa Y. A proteomics study on human breast cancer cell lines by fluorogenic derivatization-liquid chromatography/tandem mass spectrometry. Biomed Chromatogr. 2008;22:1304–1314. doi: 10.1002/bmc.1102. [DOI] [PubMed] [Google Scholar]
- 17.Jemal A, Siegel R, Ward E, Hao Y, Xu J, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 18.Kang SP, Martel M, Harris LN. Triple negative breast cancer: current understanding of biology and treatment options. Curr Opin Obstet Gynecol. 2008;20:40–46. doi: 10.1097/GCO.0b013e3282f40de9. [DOI] [PubMed] [Google Scholar]
- 19.Keller A, Nesvizhskii AI, Kolker E, Aebersold R. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal Chem. 2002;74:5383–5392. doi: 10.1021/ac025747h. [DOI] [PubMed] [Google Scholar]
- 20.Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, et al. The international human genome sequencing consortium: initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- 21.Li X, Lewis MT, Huang J, Gutierrez C, Osborne CK, et al. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Clin Oncol. 2008;100:672–679. doi: 10.1093/jnci/djn123. [DOI] [PubMed] [Google Scholar]
- 22.Liu S, Sun MZ, Tang JW, Wang Z, Sun C, et al. High-performance liquid chromatography/nanoelectrospray ionization tandem mass spectrometry, two-dimensional difference in-gel electrophoresis and gene microarray identification of lymphatic metastasis-associated biomarkers. Rapid Commun Mass Spectrom. 2008;22:3172–3178. doi: 10.1002/rcm.3725. [DOI] [PubMed] [Google Scholar]
- 23.Liu Y, Xiao Z, Li M, Chen YH, Li C, et al. Quantitative proteome analysis reveals annexin A3 as a novel biomarker in lung adenocarcinoma. J Pathol. 2009;106:570–579. doi: 10.1002/path.2429. [DOI] [PubMed] [Google Scholar]
- 24.Mercurio AM, Rabinovitz I. Towards a mechanistic understanding of tumor invasion – lessons from the α6 β4 integrin. Semin Cancer Biol. 2001;11:129–141. doi: 10.1006/scbi.2000.0364. [DOI] [PubMed] [Google Scholar]
- 25.Mohammed RA, Green A, El-Shikh S, Paish EC, Ellis IO, Martin SG. Prognostic significance of vascular endothelial cell growth factors -A, -C and -D in breast cancer and their relationship with angio- and lymphangiogenesis. Br J Cancer. 2007;96:1092–1100. doi: 10.1038/sj.bjc.6603678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nesvizhskii AI, Keller A, Kolker E, Aebersold R. A statistical model for identifying proteins by tandem mass spectrometry. Anal Chem. 2003;75:4646–4658. doi: 10.1021/ac0341261. [DOI] [PubMed] [Google Scholar]
- 27.Nielsen TO, Hsu FD, Jensen K, Cheang M, Karaca G, et al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res. 2004;10:5367–5374. doi: 10.1158/1078-0432.CCR-04-0220. [DOI] [PubMed] [Google Scholar]
- 28.Nikolopoulous SN, Blaikie P, Yoshioka T, Guo W, Giancotti FG. Integrin β4 signaling promotes tumor angiogenesis. Cancer Cell. 2004;6:471–483. doi: 10.1016/j.ccr.2004.09.029. [DOI] [PubMed] [Google Scholar]
- 29.Relf M, LeJeune S, Scott PA, Fox S, Smith K, et al. Expression of the angiogenic factors vascular endothelial cell growth factor, acidic and basic fibroblast growth factor, tumor growth factor beta-1, platelet-derived endothelial cell growth factor, placenta growth factor, and pleiotrophin in human primary breast cancer and its relation to angiogenesis. Cancer Res. 1997;57:963–969. [PubMed] [Google Scholar]
- 30.Rubinstein DB, Karmely M, Pichinuk E, Ziv R, Benhar I, et al. The MUC1 oncoprotein as a functional target: immunotoxin binding to alpha/beta junction mediates cell killing. Int J Cancer. 2009;124:46–54. doi: 10.1002/ijc.23910. [DOI] [PubMed] [Google Scholar]
- 31.Russ AP, Lampel S. The druggable genome: An update. Drug Discov Today. 2005;10:1607–1610. doi: 10.1016/S1359-6446(05)03666-4. [DOI] [PubMed] [Google Scholar]
- 32.Shau H, Chandler GS, Whitelegge JP, Gornbein JA, Faull KF, et al. Proteomic profiling of cancer biomarkers. Brief Funct Genomic Proteomic. 2003;2:147–158. doi: 10.1093/bfgp/2.2.147. [DOI] [PubMed] [Google Scholar]
- 33.Shen D, Chang HR, Chen Z, He J, Lonsberry V, et al. Loss of annexin A1 expression in human breast cancer detected by multiple high-throughput analyses. Biochem Biophys Res Commun. 2005;326:218–227. doi: 10.1016/j.bbrc.2004.10.214. [DOI] [PubMed] [Google Scholar]
- 34.Shen D, Nooraie F, Elshimali Y, Lonsberry V, He J, et al. Decreased expression of annexin A1 is correlated with breast cancer development and progression as determined by a tissue microarray analysis. Hum Pathol. 2006;37:1583–1591. doi: 10.1016/j.humpath.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 35.Soo ET, Yip GW, Lwin ZM, Kumar SD, Bay BH. Heat shock proteins as novel therapeutic targets in cancer. In Vivo. 2008;22:311–315. [PubMed] [Google Scholar]
- 36.Speers AE, Wu CC. Proteomics of integral membrane proteins –theory and application. Chem Rev. 2007;107:3687–3714. doi: 10.1021/cr068286z. [DOI] [PubMed] [Google Scholar]
- 37.Venter JC, Adams MD, Myers EW, Li PW, Mural RJ, et al. The sequence of the human genome. Science. 2001;291:1304–1351. doi: 10.1126/science.1058040. [DOI] [PubMed] [Google Scholar]
- 38.Wang GY, Lu CQ, Zhang RM, Hu XH, Luo ZW. The E-cadherin gene polymorphism 160C->A and cancer risk: A HuGE review and meta-analysis of 26 case-control studies. Am J Epidemiol. 2008;167:7–14. doi: 10.1093/aje/kwm264. [DOI] [PubMed] [Google Scholar]
- 39.Whelan SA, Lu M, He J, Yan W, Saxton RE, et al. Mass spectrometry (LCMS/MS) site-mapping of N-glycosylated membrane proteins for breast cancer biomarkers. J Proteome Res. 2009;8:4151–4160. doi: 10.1021/pr900322g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.White-Gilbertson S, Rubinchik S, Voelkel-Johnson C. Transformation, translation and TRAIL: an unexpected intersection. Cytokine Growth Factor Rev. 2008;19:167–172. doi: 10.1016/j.cytogfr.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Whitelegge JP, Gunderson CB, Faulk KF. Electrospray-ionization mass spectrometry of intact intrinsic membrane proteins. Protein Sci. 1998;7:1423–1430. doi: 10.1002/pro.5560070619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Whitelegge JP, Halgand F, Souda P, Zabrouskov V. Top-down mass spectrometry of integral membrane proteins. Expert Rev Proteomics. 2006;3:585–596. doi: 10.1586/14789450.3.6.585. [DOI] [PubMed] [Google Scholar]
- 43.Whitelegge JP, Katz J, Pihakari K, Hale R, Aguilera R, et al. Subtle modification of isotope ratio proteomics (SMIRP); a new strategy for expression proteomics. Phytochemistry. 2004;65:1507–1515. doi: 10.1016/j.phytochem.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 44.Whitelegge JP, Le Coutre J, Lee JC, Engel CK, Privé GG, et al. Towards the bilayer proteome electrospray ionization mass spectrometry of large intact membrane proteins. Proc Natl Acad Sci. 1999;96:10695–10698. doi: 10.1073/pnas.96.19.10695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Whitelegge JP. Mass spectrometry for high throughput quantitative proteomics in plant research: lessons from thylakoid membranes. Plant Physiol Biochem. 2005a;42:919–927. doi: 10.1016/j.plaphy.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 46.Whitelegge JP. Tandem Mass Spectrometry of Integral Membrane Proteins for Top-Down Proteomics. Trends Anal Chem. 2005b;24:576–582. [Google Scholar]
- 47.Wielenga VJ, Heider KH, Offerhaus GJ, Adolf GR, van den Berg FM, et al. Expression of GD44 variant proteins in human colorectal cancer is related to tumor progression. Cancer Res. 1993;53:4754–4756. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.