Abstract
We have recently shown that the gene encoding the truncated form of protein tyrosine phosphatase receptor-type O (PTPROt) expressed predominantly in hematopoietic cells, is epigenetically silenced in human primary chronic lymphocytic leukemia (B-CLL). To determine whether increased phosphorylation of the PTPROt substrates following PTPROt suppression alters signal transduction pathway(s) that impart a growth advantage to the leukemic lymphocytes, it is critical to discern the key substrates of PTPROt. Here, we used substrate-trapping assay to identify two novel substrates of PTPROt, the tyrosine kinases Lyn and ZAP70. Both Lyn and ZAP70 were dephosphorylated by wild type PTPROt, but not by its catalytic site (CS) mutant. A critical phosphorylation site in Lyn, Y397, essential for its activity was dephosphorylated by PTPROt. Consequently, the activity of Lyn kinase was compromised when co-expressed with PTPROt-WT compared to vector control or upon co-expression with PTPROt-CS. Ectopic expression of PTPROt in Raji cells reduced phosphorylation of Lyn in the absence of any change in its protein levels. These results have revealed the physiological importance of PTPROt in regulating B-cell receptor signaling at Lyn kinase. Further, ectopic expression of PTPROt also sensitized the cells to the VEGF-R inhibitor Pazopanib.
Keywords: Lyn, tyrosine phosphatase, PTPROt, ZAP70, CLL
Protein phosphorylation and dephosphorylation is a dynamic process that is intricately regulated by protein kinases and phosphatases, respectively, to maintain normal cellular physiology. These posttranslational modifications of proteins play a key role in signal transduction pathways in executing the function of extracellular or membrane associated signals (see [Ostman and Bohmer, 2001] for a review). Although protein phosphatases modulate the intensity and duration of the signal transmitted by the cascade of kinases, they do not appear to occupy a prominent spot in most signaling pathways. This could, in part, be due to the limited number of known protein phosphatase substrates.
B-cell receptor (BCR) signaling has been widely studied in normal B-cell development as well as in lymphoid malignancies. The central initiating events in this pathway consist of phosphorylation of Src family kinase, immunoreceptor tyrosine-based activation motifs (ITAMs) and SYK tyrosine kinase. These primary activations then transmit their message via diverse signaling molecules in the MAPK cascade, Akt, PKC and other pathways involving phosphorylation of either serine/threonine or tyrosine residues of proteins. It is, therefore, evident that serine/threonine and/or tyrosine phosphatases and kinases, especially those that may be specifically expressed in B-cells, would be an integral part of the BCR signaling.
Our laboratory has been studying the role of protein tyrosine phosphatases in tumor suppression and in apoptosis [Motiwala and Jacob, 2006; Motiwala et al., 2004]. The truncated variant of the protein tyrosine phosphatase receptor-type O (PTPROt) is expressed in B-lymphoid organs and cells of macrophage/monocyte lineage. It is developmentally regulated in B-lymphocytes and induces cell cycle arrest, suggesting a role in B-cell development and growth suppression [Aguiar et al., 1999]. We have established that the full length isoform of PTPRO (PTPRO-FL) is a candidate tumor suppressor in a rat model of folate-methyl deficient diet induced hepatocarcinogenesis using genome wide screening of hypermethylated genes [Motiwala et al., 2003]. Subsequently, our detailed functional studies on PTPRO-FL and PTPROt were the first to prove the growth suppressive role of this tyrosine phosphatase in solid [Motiwala et al., 2004] and liquid tumors [Motiwala et al., 2009], respectively. Epigenetic silencing of PTPRO in these tumors has raised the possibility that re-expression of this protein tyrosine phosphatase could form a novel molecular basis for cancer therapy (for reviews see [Jacob and Motiwala, 2005; Motiwala and Jacob, 2006]). Recently, we have also demonstrated extensive methylation of the CpG island in the gene encoding PTPROt in primary B-cell chronic lymphocytic leukemia (B-CLL) relative to normal B-lymphocytes [Motiwala et al., 2007]. As observed in liver and lung tumors [Motiwala et al., 2003; Motiwala et al., 2004], this methylation inversely correlated with PTPROt expression. Further, the suppressed gene could be re-activated upon treatment with the DNA hypomethylating drug decitabine (5-aza-deoxycytidine). It is conceivable that silencing of PTPROt at an early stage of leukemogenesis can lead to increased accumulation of its p-tyr substrates, some of which can trigger oncogenic signals.
A recent study has shown that Syk, a tyrosine kinase, is a substrate of PTPROt in B-cells, and that PTPROt expression suppresses BCR-triggered SYK tyrosine phosphorylation and downstream signaling events [Chen et al., 2006]. Here, we demonstrate that Lyn kinase that functions upstream of Syk kinase in BCR signaling, is a substrate of this protein tyrosine phosphatase. Further, ZAP70, also a tyrosine kinase analogous to Syk and involved in T-cell receptor signaling, is a substrate of PTPROt. Although ZAP70 is normally expressed in T-cells it is aberrantly expressed in B-CLL and is an indicator of poor prognosis [Durig et al., 2003; Rosenwald et al., 2001; Wiestner et al., 2003]. This study, therefore, shows that utilizing its multilayer control, PTPROt can maintain a tight regulation of BCR signaling in normal cells. Additionally, we show here that inactivation of Lyn kinase by PTPROt facilitates the action of VEGF-R inhibitor Pazopanib emphasizing the significance of multi-kinase targeting for anti-cancer treatment.
MATERIALS AND METHODS
Reagents
The antibodies used in this study were obtained from the following sources: anti-pY cocktail (4G10 from Millipore, pY20 and pY99 from Santa Cruz Biotechnology), anti-GST (GE Healthcare), anti-HA (Covance), anti-ZAP70 (Millipore), anti-Src (detects Lyn), anti-Syk (Santa Cruz Biotechnology), anti-pY(416)Src [detects pY(397)Lyn] and anti-pY(507)Lyn (Cell Signaling Techology), anti-Flag M2 (Sigma), anti-GAPDH (Chemicon), anti-Ku70 (NeoMarkers) and anti-myc (Covance).
Generation of GST-tagged substrate-trapping mutants of PTPROt
Three rounds of PCR were used to introduce the aspartic acid to alanine (D→A) mutation by site directed mutagenesis essentially as described before [Aiyar et al., 1996]. Briefly, WT and CS mutants of PTPU2L (kind gift of Dr. Hiroyuki Seimiya, [Seimiya et al., 1995]) were used as templates with the following primers: pair 1; hPTP-DA-Bgl-F1 (5′-GCAGATCTTCCACTGAATCGA-3′) and hPTP-DA-R1 (5′-TGTGGGCACACCATGcgCAGGCCATGCAGT-3′), pair 2; hPTP-DA-F2 (5′-ACTGCATGGCCTGcgCATGGTGTGCCCACA-3′) and hPTP-DA-Apa-R 2 ( 5′-AGAATAGGGCCCACTAAAACAATCTGG-3′). The mutagenic bases are in lower case and restriction sites are underlined. The mutated fragment carrying the Bgl II and Apa I restriction sites was used to replace the Bgl II/Apa I fragment in the PTPU2L construct. The three different templates (PTPU2L-WT, CS, DA) were then used to amplify PTPROt using the primers PTPt-F-Bam-GST (5′-CAGGATCCCAATGGTTACAGAGATGAATCC-3′) and PTP-R (5′-CCGGTACCCACATCATGTCCTCTCTGC-3′) for cloning into the Bam HI and SmaI sites of pGEX-5X-1 (GE Healthcare).
Expression and purification of GST-tagged PTPROt
The pGEX-5X-1 plasmid carrying PTPROt (WT, CS, DA) was transformed into M15 bacteria. An overnight culture was induced with 1 mM IPTG for 4 hrs. The bacterial pellet was suspended in bead binding buffer (50 mM potassium phosphate pH 7.4, 150 mM KCl, 1 mM MgCl2 and protease inhibitors) followed by sonication (6 X 60 pulses). Glycerol and Triton X-100 were added to a final concentration of 10% and 1%, respectively. The extract was rocked at room temperature for 30 mins prior to centrifugation at 16,000 rpm for 45 mins at 4°C. The clear extract was used to purify GST-tagged PTPROt using glutathione Sepharose 4B beads (GE Healthcare) as described before [Datta et al., 2005].
Phosphatase activity using pNPP hydrolysis
For each assay, 10 μl enzyme (alkaline phosphatase or PTPROt) was added to 70 μl assay buffer (25 mM HEPES pH 7.4, 50 mM NaCl, 5 mM DTT, 2.5 mM EDTA, 250 μg/ml BSA) and incubated for 15 min at 37°C. The pNPP substrate, 120 μl of 1.5 mg/ml stock, was then added followed by incubation at 37°C for 15 mins. The reaction was stopped by addition of 20 μl 13% K2HPO4 and the absorbance measured at 405 nm. A standard curve of absorbance with increasing concentration of alkaline phosphatase (0.25 to 1.0 units) was plotted to determine the relative activity of equal amounts of PTPROt (WT and mutants).
In vitro substrate-trapping assay
The assay was performed essentially as described [Blanchetot et al., 2005]. Briefly, whole cell extract of B-cell line WaC3CD5 treated with 750 μM pervanadate was prepared in lysis buffer B (20 mM Tris pH 7.5; 100 mM NaCl, 1% Triton X-100, 10% glycerol, 5 mM iodoacetic acid, 1 mM sodium orthovanadate and protease inhibitors). Following 30 min incubation on ice the iodoacetic acid and orthovanadate were neutralized with 10 mM DTT and 1 mM EDTA. After 15 mins on ice the extract was centrifuged at 14,000 rpm for 15 mins to remove any insoluble debris. The protein (6 mg) was then incubated for 90 mins at 4°C with either GST alone or GST-tagged PTPROt (WT, CS, DA) bound to GSH sepharose beads. Following washing with lysis buffer A (20 mM Tris pH 7.5, 100 mM NaCl, 1% Triton X-100, 10% glycerol, 1 mM EDTA, 5 mM iodoacetic acid and protease inhibitors) the beads were suspended in 2X SDS-PAGE loading buffer and proteins were separated on 8% SDS-PAGE. The proteins were transferred to nitrocellulose membrane for Western blot analysis with antibodies indicated in the results.
Cloning HA-tagged Lyn, HA-tagged ZAP70 and constitutively active Lck
The coding region of human Lyn kinase was PCR amplified from bone marrow cDNA using the primers hLyn-Bam-F (5′-ATGGATCCTACGAGCGGGAAATATGG-3′) and hLyn-XbaI-R (5′-GCTCTAGACTAAGGCTGCTGCTGGTA-3′). The restriction sites are underlined. The PCR products for both isoforms (p53 and p56) were cloned into the BamHI/XbaI sites of pcDNA-HA to introduce a HA-tag at the N-terminal end of Lyn kinase. HA-tagged ZAP70 was a kind gift from Dr. Arthur Weiss [Chan et al., 1992]. The expression vector for constitutively active Lck (pTEJ8-Lckmyc505F) designated LckY505F in this paper was a generous gift from Dr. Ugo D’Oro [D’Oro et al., 1996].
In vitro Lyn kinase assay
H293T cells were co-transfected with HA-tagged Lyn and Flag(Vector)/Flag-tagged PTPROt using Lipofectamine 2000. Forty-eight hour post-transfection, cell free extract was prepared in IPK lysis buffer [50 mM Tris pH 7.5, 1 mM EDTA, 1 mM EGTA, 0.5 mM sodium orthovanadate, 10 mM sodium β-glycerophosphate, 50mM sodium fluoride, 5 mM sodium pyrophosphate, 1% triton X-100, 0.1mM PMSF and protease inhibitor cocktail (Sigma)]. Pre-equilibrated recombinant protein G-agarose was incubated with anti-HA and anti-Flag antibodies in the IPK lysis buffer with constant rocking at 4°C for 1h followed by thorough washing with IPK cell lysis buffer. Equal amount of anti-HA and anti-Flag antibody bound protein G-agarose beads were then incubated with whole cell extracts (~500μg protein) containing HA-Lyn and Flag-PTPROt/Flag (vector) (in triplicate) for 3h on a rotator at 4°C to immunoprecipitate both Lyn and PTPROt proteins. The protein G-agarose/enzyme immunocomplexes were then washed thoroughly with IPK lysis buffer followed by one wash with Tris Assay Dilution Buffer (TADB; 50 mM Tris pH 7.2, 0.1 mM EGTA, 15 mM DTT, protease inhibitors and 1 mg/ml BSA). The beads were then incubated in 50μl TADB containing 250 μM Src kinase peptide, 15 mM MgCl2 and ATP mixture [(100μM unlabeled ATP and 1 μCi γ-32P ATP; 3000Ci/mmol (Perkin Elmer)] for 10 min at 30°C in a shaking incubator. Reaction mixture without any Lyn kinase (protein G-agarose beads) was used as control. After incubation, the immunocomplex was pelleted by centrifugation and the supernatant fraction containing the phosphorylated peptide was transferred onto phosphocellulose paper (p81). Air-dried papers were then washed thoroughly with 1% phosphoric acid(five times for 5 min each) followed by once with acetone. The dried p81 papers were then transferred into a vial containing scintillation fluid and read in a scintillation counter. The radioactivity (CPM) of the enzyme samples was compared to that of control samples that contained no enzyme (background).
In vitro dephosphorylation of ZAP70
HA-tagged ZAP70 (along with constitutively active Lck) was transfected into H293T cells using Lipofectamine 2000 as per manufacturer’s recommendations. Prior to harvest at 48 hrs the cells were treated with 100 μM pervanadate to enhance phosphorylation of the over-expressed substrate. Whole cell extract was prepared inlysis buffer for IP (50 mM Tris pH 7.5, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 1 mM sodium orthovanadate, 1 mM NaF and protease inhibitors). After 30 mins incubation on ice the extract was centrifuged at maximum speed to remove any insoluble debris. The buffer components were then adjusted to be optimal for the phosphatase assay (10 mM Tris, 30 mM NaCl, 0.2 mM EDTA, 1.875 mM DTT, 0.2% Triton X-100 and 125 μg/ml BSA). Equal aliquots of this extract were incubated with either GST alone or GST-PTPROt (WT or CS) at 37°C for 45 mins. At the end of the reaction the glutathione bead bound GST or GST-PTPROt was removed by centrifugation. The buffer constituents were again optimized for immunoprecipitation (150 mM NaCl, 0.5% Triton X-100, 50 mM Tris pH 7.5) and HA-tagged ZAP70 was immunoprecipitated using anti-HA antibody. The immunoprecipitated proteins were then separated on SDS-PAGE and immunoblotted with anti-pY cocktail followed by washing and re-probing with anti-HA.
Generation of Raji cells stably expressing PTPROt
Raji cells were maintained in RPMI1640 supplemented with 10% FBS, 2 mM glutamine and penicillin/streptomycin. Retroviral infection with virus generated by transfecting Phoenix cells with pRetro-On (vector) (Takara Bioscience) or PTPROt-WT cloned in pRetro-On (WT) was essentially as described [Motiwala et al., 2007]. Cells expressing PTPROt were selected with 0.75 μg/ml puromycin and individual clones were isolated by limited dilution.
Western blot analysis
Whole cell extracts were prepared in cell lysis buffer (50 mM Tris pH 8.1, 10 mM EDTA and 1% SDS) followed by sonication and centrifugation at 14,000 rpm for 20 mins at 4°C. Proteins were separated on 10% SDS-PAGE, transferred to nitrocellulose membrane and immunoblotted with the appropriate antibodies as per manufacturer’s recommendations.
Susceptibility of Raji clones to VEGF-R inhibitor Pazopanib
Raji clones were seeded at 0.2E6/ml and treated with either DMSO (vehicle control) or Pazopanib (5 or 10 μM). Viable cell count by trypan blue exclusion was determined at 96 h and the data represented as percent viability of Pazopanib treated cells relative to DMSO treated cells.
RESULTS
In vitro substrate trapping assay identifies ZAP70 and Lyn as substrates of PTPROt in leukemia cells
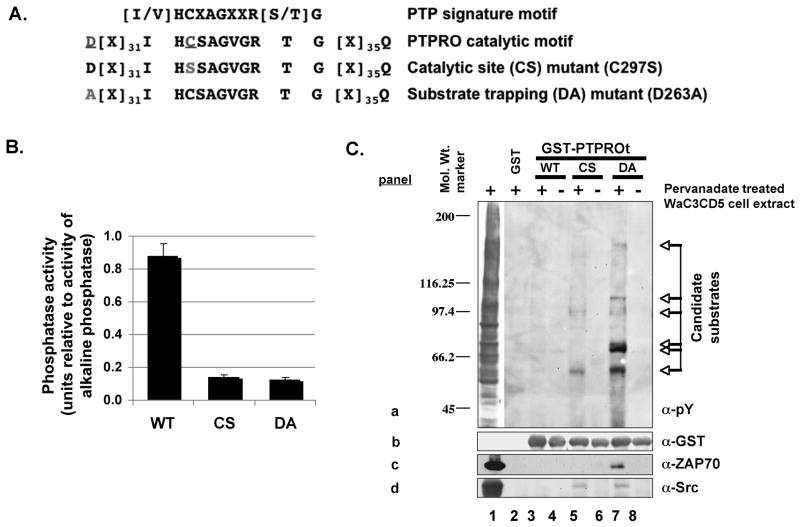
The truncated isoform of PTPRO (PTPROt) that is predominantly expressed in cells of lymphoid and myeloid origin is a transmembrane protein with a short extracellular domain of only 7 amino acids and a single catalytic domain in its intracellular region. To identify the lymphoid specific substrates of PTPROt that catalyzes very rapid dephosphorylation reactions, we generated substrate-trapping mutants as originally developed to identify PTP substrates [Flint et al., 1997]. The DA mutant was generated by mutating the conserved asp 34 aa upstream of the catalytic site cys to ala using site directed mutagenesis (Fig. 1A). The conserved asp residue is part of a WPD loop that closes around the phosphotyrosine chain of the substrate. Its mutation to ala was found to decrease the kcat while maintaining the km of the enzyme-substrate reaction [Tiganis and Bennett, 2007].
Fig. 1. Substrate-trapping assay identified several candidate pY-polypeptides as PTPROt substrate.
(A) Schematic representation of the catalytic site and substrate-trapping mutants of PTPROt used in this study. (B) Activity of PTPROt (WT and mutants) measured using pNPP as substrate. Equal amounts of GST-tagged PTPROt purified using GSH-sepharose was assayed for activity in parallel with increasing concentration of alkaline phosphatase. A plot of units of alkaline phosphatase v/s optical density of product p-nitrophenol was used to compute the activity of PTPROt. (C) Whole cell extract of pervanadate treated B-cell line WaC3CD5 was used to bind bacterially expressed and purified GST-tagged PTPROt (WT, CS, DA). The pulled-down proteins separated by SDS-PAGE were immonoblotted with anti-pY cocktail (4G10, pY20, pY99) (panel a), anti-GST (panel b), anti-ZAP70 (panel c) or anti-Src (panel d). The positions of molecular weight standards (in kDa) are indicated on the left and candidate substrates are denoted by arrows on the right.
Next, the WT and mutants (DA and CS) of PTPROt were expressed as GST-fusion protein and purified using glutathione (GSH) beads. Among these only the WT protein was catalytically active with p-nitrophenyl phosphate (pNPP) as substrate (Fig. 1B). To identify substrates typical of PTPROt expressing B-cells, the GST-fusion proteins bound to glutathione beads were allowed to bind potential tyrosyl phosphorylated (pY) substrates from extracts of leukemia cells WaC3CD5 of B-cell origin, pretreated with pervanadate (a potent inhibitor of tyrosine phosphatases). The p-tyr polypeptides that formed complex with WT and mutant GST-PTPROt were identified by immunoblot analysis with anti-phosphotyrosine (pY) antibodies (Fig. 1C, panel a). The inability of GST alone or PTPROt-WT to pull down any p-tyr proteins (Fig. 1C, panel a, lanes 2–4) demonstrates specificity of the enzyme-substrate interaction. While both mutants (CS and DA) were able to trap potential substrates, the DA mutant was most efficient (Fig. 1C, panel a, compare lanes 5 and 7). The absence of candidate polypeptides pulled-down with PTPROt-WT suggests that these proteins are substrates rather than interacting proteins. Alternatively, it is possible that incubation of the extract with catalytically active phosphatase (PTPROt-WT) resulted in dephosphorylation of the proteins. Reprobing the blot with anti-GST antibody showed comparable amount of GST-PTPROt used for each pull-down assay (Fig. 1C, panel b). These results revealed at least six potential p-tyr substrates of PTPROt in WaC3CD5 cells ranging in molecular size from 55 kDa to 150 kDa.
We then predicted the identity of the p-tyr polypeptides pulled down by the DA mutant of PTPROt (Fig. 1C) based on their size and expression in B-cells. Two candidates of ~55 kDa and 70 kDa were of particular interest, as their molecular sizes were identical to those of two important protein tyrosine kinases Lyn and ZAP70, respectively. Lyn, a Src family protein kinase, is known to be overexpressed and constitutively activated in B-CLL [Contri et al., 2005] whereas ZAP70, another tyrosine kinase, is frequently induced/activated in CLL [Chen et al., 2005; Gobessi et al., 2007; Rosenwald et al., 2001]. Western blot analysis showed that ZAP70 (Fig. 1C, panel c) and Lyn (Fig. 1C, panel d, detected with anti-Src) were indeed pulled down either by DA alone or both CS as well as DA mutants, respectively. This observation was consistent with the potential of these tyrosine kinases as being substrates of PTPROt. It should be noted that these substrates (irrespective of their phosphorylation state) were not detected in the PTPROt-WT pull-down lane (Fig. 1C, lane 3) suggesting that their interaction with the WT phosphatase could be transient. Since Syk kinase has been shown to be a substrate of PTPROt, the blot was also probed with anti-Syk antibody. As expected Syk was specifically associated with the substrate-trapping mutant of PTPROt (data not shown).
Both Lyn and ZAP70 are dephosphorylated by PTPROt
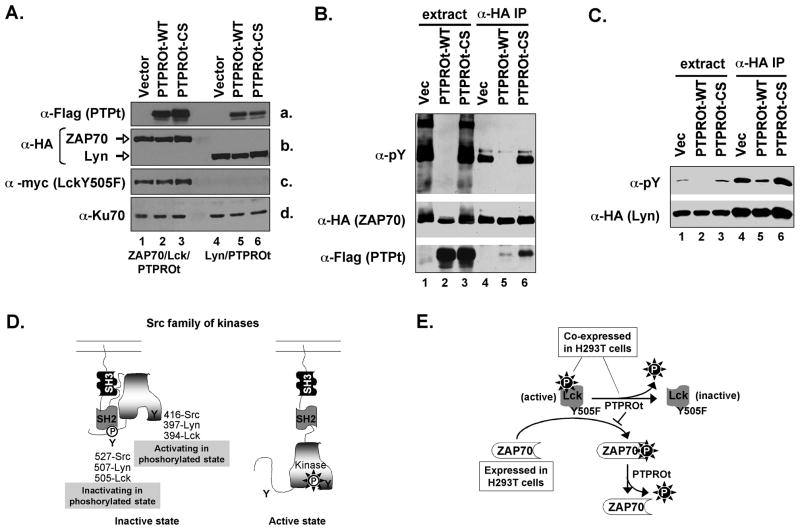
To confirm that Lyn and ZAP70 are indeed substrates of PTPROt, we investigated whether these proteins can be dephosphorylated by PTPROt in vivo. To address this issue, HA-tagged substrate (Lyn or ZAP70) along with Flag-tagged PTPROt was transiently expressed in H293T cells. These cells were used because of higher efficiency of transfection and overexpression of transgenes. Further, such an approach is routinely used to study the in vivo relationship between a tyrosine phosphatase and its candidate substrate [Palka et al., 2003; Wu et al., 2006]. Phosphorylation of ZAP70 in T-cells (where it is expressed under physiological conditions) requires activity of the Src kinase Lck [Chan et al., 1992; Palacios and Weiss, 2004]. To phosphorylate ZAP70 efficiently in H293T cells it was, therefore, necessary to co-express a constitutively active mutant of Lck (myc tagged LckY505F) with ZAP70 [Tiganis and Bennett, 2007]. Immunoblotting of whole cell extracts from transfected cells confirmed the ectopic expression of PTPROt (Fig. 2A, panel a, detected with anti-Flag), ZAP70 and Lyn (Fig. 2A, panel b, detected with anti-HA), LckY505F (Fig. 2A, panel c, detected with anti-myc). The substrates (ZAP70 and Lyn) were immunoprecipitated using anti-HA antibody. Immunoblot analysis of the extract and the immunoprecipitated substrate with anti-pY antibodies showed that Lyn or ZAP70 co-expressed with PTPROt-WT were in a dephosphorylated state compared to that co-expressed with PTPROt-CS or vector alone (Fig. 2B,C; top panel, compare lane 5 to lanes 4 and 6). Although Fig. 2C represents data for p53Lyn, similar observations were made for p56Lyn (data not shown). It is noteworthy that the constitutively active Lck failed to phosphorylate any cellular proteins when co-expressed with PTPROt-WT (Fig. 2B, top panel, compare lane 2 to lanes 1 and 3). This observation suggests that Lck, which belongs to the same family of Src kinases as Lyn kinase, is also a substrate of PTPROt.
Fig. 2. PTPROt dephosphorylates its substrates in vivo.
(A) Whole cell extracts from H293 cells transfected with ZAP70/LckY505F/PTPROt (lanes 1–3) or Lyn/PTPROt (lanes 4–6) were separated on SDS-PAGE and probed with anti-Flag (for PTPROt, panel a), anti-HA (for ZAP70/Lyn, panel b), anti-myc (for LckY505F, panel c) and Ku70 (for normalization, panel d). HA-tagged ZAP70 (B) or HA-tagged Lyn (C) co-expressed with Flag-tagged PTPROt (WT or CS) in H293T cells was immunopercipitated with anti-HA antibody. The extracts as well as the immunoprecipitated proteins were separated on SDS-PAGE and immunoblotted either with anti-pY cocktail, anti-HA or anti-Flag as indicated in the figure. Each experiment was repeated three times with similar results. (D) Schematic representation of the inactive and active states of Src kinase. In its inactive form Src kinases adopt a closed conformation resulting from phosphorylation of the inactivating tyrosine (Y505), which creates a binding motif for the SH2 domain, as well as the intramolecular interaction between the SH3 domain and the linker region between the SH2 and the kinase domain. Phosphorylation of the activating tyrosine (Y394) in the kinase domain results in an open and active conformation. It is noteworthy that phosphorylation of Y394 is inhibited when Y505 is phosphorylated. Mutation of Y505 (Y505F), therefore, prevents its phosphorylation thereby promoting phosphorylation of Y394 resulting in a constitutively active kinase. The positions of the activating and inactivating tyrosines of Src, Lyn and Lck are indicated. (E) Schematic representation of the inactivation of Lck(Y505F) by PTPROt when co-expressed in H293T cells. This inactivation prevented the phosphorylation of ZAP70.
Although dephosphosrylation of Lyn by PTPROt was evident (Fig. 2C), the inability of its T-cell counterpart Lck to phosphorylate co-expressed ZAP70 in the presence of PTPROt-WT prompted us to investigate the specific regulation of this Src kinase by PTPROt. It is known that the activities of Lyn/Lck are differentially regulated by phosphorylation at Y394 and Y505 (with respect to Lck). While phosphorylation of Y394 is necessary for Lck activity, phosphorylation at Y505 exhibits a negative effect on its activity [Abraham and Veillette, 1990; Thomas and Brugge, 1997; Veillette et al., 1992] (see Fig. 2D for schematic representation). Therefore, it was critical to determine which p-tyr is dephosphorylated by PTPROt. The constitutively active Y505F mutant prevents its phosphorylation at Y505 and subsequent inactivation [Amrein and Sefton, 1988; Marth et al., 1988; Veillette et al., 1992]. We entertained the possibility that PTPROt dephosphorylates the activating tyrosine at position 394 thereby inhibiting Lck function (see Fig. 2E for schematic representation).
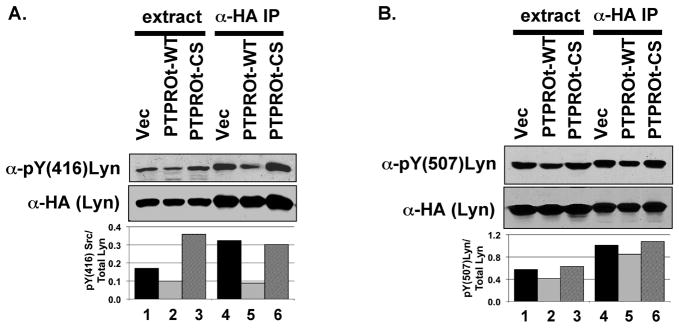
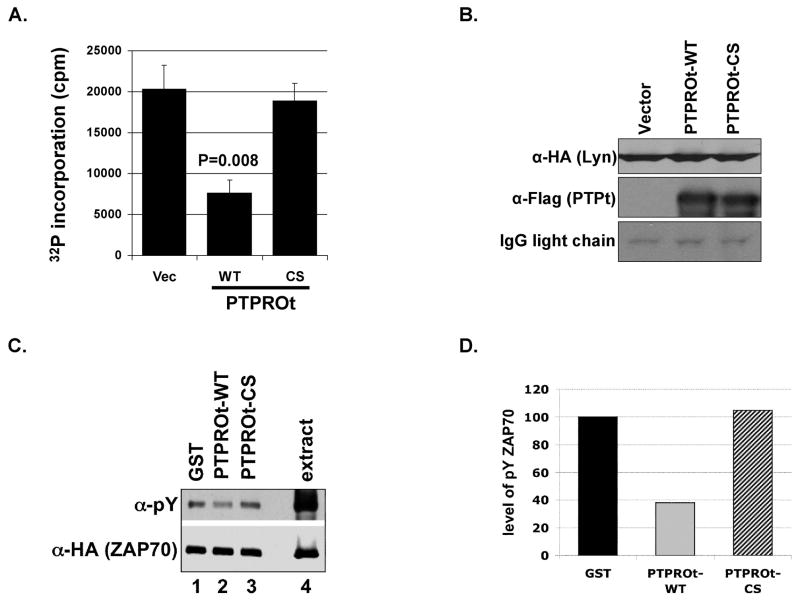
To confirm that PTPROt indeed dephosphorylates the activating tyrosine on these Src family kinases, we used the Lyn/PTPROt co-expression system (as in Fig. 2C) since Lck and Lyn belonging to the same family of Src kinases are similarly regulated. Further, our primary interest was in studying Lyn as a B-cell specific PTPROt substrate. Lyn immunoprecipitated from H293T cells over-expressing both HA-Lyn and Flag-PTPROt as well as cell extracts were separated on SDS-PAGE in duplicate. The blots were probed with antibody specific for phosphorylated forms of the activating Y394 [anti-pY(416)Src, which cross reacts with its analog pY(394)Lyn] and inactivating Y507 [anti-pY(507)Lyn] (see Fig. 3A,B). The data revealed dramatic hypophosphorylation of the activating tyrosine (pY416) compared to the inactivating tyrosine (pY507) by PTPROt-WT (Fig. 3A,B; compare lane 2 to lanes 1&3 and lane 5 to lanes 4&6, respectively). Quantification of the pY signal normalized to total Lyn showed ~60% reduction in phosphorylation of activating tyrosine (Fig. 3A) but only ~20% decrease in phosphorylation of the inactivating tyrosine (Fig. 3B) by PTPROt-WT compared to that in vector-transfected or PTPROt-CS mutant cell extracts. This data affirms the fact that the constitutively active LckY505F cannot phosphorylate ZAP70 in the presence of PTPROt because of its dephosphorylation and inactivation by the phosphatase (see Fig. 2E). The lack of efficient phosphorylation at Y416 in the presence of PTPROt-WT suggests that activity of lyn kinase could be compromised by PTPROt. To test this possibility we performed in vitro kinase assay on HA-Lyn co-expressed with vector alone or PTPROt (WT and CS). For this purpose, Lyn and PTPROt were immunoprecipitated using anti-HA and anti-Flag antibodies, respectively. PTPROt was co-immunoprecipitated for the kinase assay to prevent autophosphorylation and activation of Lyn during the assay in absence of PTPROt. The activity of immunoprecipitated Lyn was measured by 32P incorporation into synthetic substrate peptide. The data demonstrated significant (p=0.008) reduction in Lyn activity when co-expressed with PTPROt-WT (63% inhibition) compared to its activity when co-expressed with vector alone or PTPROt-CS (Fig. 4A). The immunoprecipitated proteins when separated on SDS-PAGE followed by immunoblotting with anti-HA and anti-Flag antibody demonstrated equal pull-down of Lyn-HA and PTPROt-Flag, respectively (Fig. 4B).
Fig. 3. Differential hypophosphorylation of activating ad inactivating tyrosines of Lyn kinase by PTPROt.
Lyn-HA was immunoprecipitated from whole cell extracts of H293T cells co-expressing HA-tagged Lyn and Flag-tagged PTPROt. The extracts along with the immunoprecipitated complex were separated on SDS-PAGE (in duplicate) followed by immunoblotting either with anti-pY(416)Src (A) or anti-pY(507)Lyn (B). The same blot was reprobed with anti-HA to measure total Lyn. Quantification of phosphorylation of the tyrosine residues of Lyn normalized to Lyn-HA is provided in the respective panels. The experiment was repeated three times with similar observation. One representative data was quantified for illustration.
Fig. 4. Inactivation of Lyn and dephosphorylation of ZAP70 by PTPROt.
(A) Activity of Lyn kinase immunoprecipitated from whole cell extracts of H293T cells co-expressing HA-tagged Lyn and Flag-tagged PTPROt was measured using a peptide substrate. The activity is represented as a measure of 32P incorporation into the peptide following incubation of immunoprecipitated Lyn with peptide and 32P-gammaATP (in cpm). The experiment was repeated twice with triplicate measurements. (B) The co-immunoprecipitated Lyn-HA and PTPROt (WT, CS) used for kinase assay were separated on SDS-PAGE followed by Western blot analysis with anti-HA (for Lyn, top panel) and anti-Flag (for PTPROt, middle panel). (C) Whole cell extract from pervanadate treated H293T cells over-expressing HA-tagged ZAP70 was incubated with either GST alone or GST-tagged PTPROt (WT or CS) under conditions optimal for phosphatase reaction. Following the reaction, ZAP70 was immunoprecipated with anti-HA antibody. The immunoprecipitated proteins were separated on SDS-PAGE and immunoblotted with anti-pY cocktail and anti-HA antibody. (D) Quantification of ZAP70 phosphorylation normalized to total ZAP70 upon incubation with GST alone or GST-PTPROt (WT or CS). Scanned images were quantified using KODAK imaging software. The experiment was repeated three times with similar observation. One representative data was quantified for illustration.
Although these results established Lyn/Lck as a substrate for PTPROt it was not certain that ZAP70 was also a substrate of this phosphatase in vivo. In the absence of evidence showing direct in vivo dephosporylation of ZAP70 by PTPROt, we explored the potential in vivo interaction between these proteins. For this purpose, the immunoprecipitated substrate (ZAP70-HA) was immunoblotted with anti-Flag antibody to detect any Flag-tagged PTPROt that co-immunoprecipitated with ZAP70. The results showed that ZAP70-HA was able to pull-down PTPROt-CS confirming their in vivo interaction (Fig. 2B, bottom panel, lane 6). In this experiment PTPROt-WT served as a negative control. Minimal interaction of ZAP70 (not efficiently phosphorylated due to inactivation of LckY505F by PTPROt-WT) with PTPROt-WT (Fig. 2B, bottom panel, lane 5) reiterates the fact that the enzyme-substrate interaction is transient and is contingent upon phosphorylation of the substrate. To confirm that ZAP-70 can be dephosphorylated by PTPROt we performed in vitro dephosphorylation assay. For this purpose, HA-tagged ZAP70 was co-expressed with LckY505F in H293T cells and the cells were treated with pervanadate prior to harvest to preserve protein phosphorylation. The extract from these cells was then used as a source of the phosphorylated substrate for GST-PTPROt in an in vitro phosphatase assay. GST alone was used as a negative control. Following incubation with the respective proteins under conditions optimal for phosphatase reaction, ZAP70 was immunoprecipitated with anti-HA antibody. The immunoprecipitated proteins were then assessed for phosphotyrosine and ZAP70-HA profiles. The results demonstrate reduced phosphorylation of ZAP70 when incubated with PTPROt-WT compared to GST alone or PTPROt-CS (Fig. 4C, top panel, compare lane 2 to 1 and 3). Normalization of phosphorylated ZAP70 with total ZAP70 (Fig. 4C, bottom panel) indicates approximately 60% dephosphorylation by GST-PTPROt relative to GST alone or PTPROt-CS (Fig. 4D).
Ectopic expression of PTPROt in Raji B-cells reduces Src phosphorylation
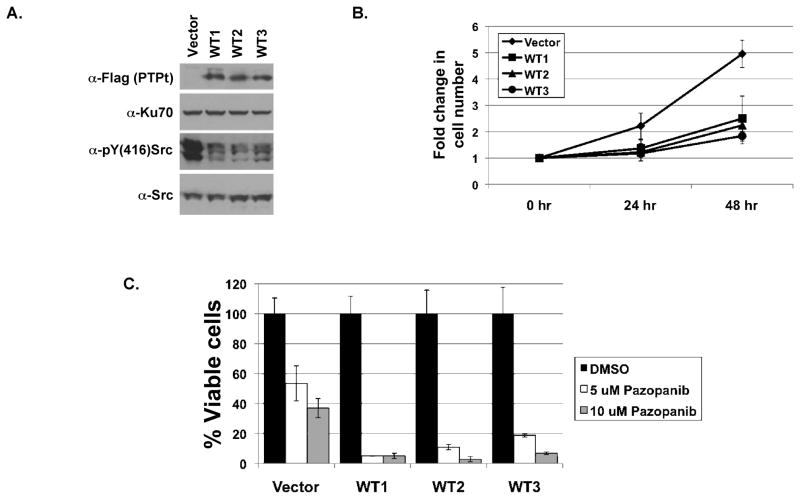
To evaluate the physiological effect of PTPROt, we ectopically expressed it as a flag-tagged protein in the lymphoblastoid cell line Raji. Several puromycin resistant clones were isolated by limited dilution following one week of puromycin selection. PTPROt expression in each clone was assessed by Western blot analysis with anti-Flag M2 antibody. Three representative clones expressing PTPROt were then used for further studies (Fig. 5A, top panel). Immunoblotting with anti-pY(416)Src confirmed that phosphorylation of endogenous Lyn at its activating tyrosine was lower in cells expressing PTPROt-WT compared to the vector control cells (Fig. 5A, third panel). This observation confirms that PTPROt is able to dephosphorylate the activating tyrosine on Lyn kinase in B-cells. Consistent with the demonstrated tumor suppressor characteristics of PTPROt, growth of these clones was much slower than the vector transfected cells (Fig. 5B). Cell growth was inhibited 49.5%, 54.7% and 62.8% at 48 hrs in PTPROt expressing clones 1, 2 and 3, respectively.
Fig. 5. Ectopic expression of PTPROt in Raji cells leads to dephosphorylation of Lyn kinase.
(A) Whole cell extract of Raji cells (vector control and expressing Flag-tagged PTPROt) were separated by SDS-PAGE and immunoblotted with anti-Flag (first panel), Ku-70 (second panel), anti-pY(416)Src (third panel) and anti-Src antibodies. (B) Raji cells seeded at 0.2E6/ml were counted every 24 hours using hemocytometer. Growth of cells relative to 0 hour is plotted. The experiment was repeated three times with duplicate counts. (C) Raji cells seeded at 0.2E6/ml were treated with DMSO (solvent control), 5 μM Pazopanib or 10 μM Pazopanib. Viable cells were counted by trypan blue exclusion using hemocytometer. Percent viable cells relative to the corresponding DMSO treated cells is plotted. The experiment was repeated three times with duplicate counts.
Next, we investigated whether PTPROt expression and hence inactivation of Lyn would render these cells more sensitive to drugs against other targets important in CLL therapy. CLL B-cells are known to spontaneously secrete vascular endothelial growth factor (VEGF) [Kay et al., 2002] that is linked to apoptosis resistance in addition to angiogenesis [Lee et al., 2004]. Further, VEGF receptors (VEGF-R1 and VEGF-R2) are constitutively activated in CLL [Lee et al., 2004]. Since Raji cells also secrete VEGF and express VEGF-R1 and VEGF-R2 [Wang et al., 2004], we treated Raji cells expressing PTPROt with the VEGF-R inhibitor Pazopanib, anorally available small molecule inhibitor of all three VEGF receptors. As anticipated, treatment of the PTPROt expressing cells with Pazopanib resulted in increased susceptibility of these cells to the drug compared to the vector control cells (Fig. 5C). Notably, survival of Pazopanib treated PTPROt expressing cells was dramatically inhibited compared to DMSO (vehicle) treated PTPROt expressing cells.
DISCUSSION
Although the functional characteristics of most tyrosine phosphatases are known, their mode of action remains to be elucidated due to lack of knowledge about their upstream and downstream partners. The epithelial full-length (PTPRO-FL) and the hematopoietic specific truncated (PTPROt) isoforms of the protein tyrosine phosphatase receptor-type O have a proven role in inhibiting cell proliferation and facilitating apoptosis [Aguiar et al., 1999; Chen et al., 2006; Motiwala et al., 2004; Motiwala et al., 2009]. Our recent observation on methylation-mediated suppression of PTPROt in B-CLL and the effect of PTPROt on fludarabine-induced growth inhibition [Motiwala et al., 2007] prompted us to identify the substrates of this isoform in CLL. Using the B-cell line WaC3CD5 in substrate-trapping assay we were able to identify two substrates, ZAP70 and Lyn kinase among the few candidate phosphoproteins.
The potential roles of PTPROt substrates ZAP70 and Lyn in inhibiting apoptosis in CLL merit discussion. ZAP70, a tyrosine kinase, is an integral part of T-cell receptor signaling (TCR). Although it is normally expressed only in T-cells its aberrant expression in B-CLL cells has prognostic value [Chen et al., 2002; Crespo et al., 2003; Wiestner et al., 2003]. ZAP70-positive CLL cells are also hyper-responsive to BCR stimulation compared to the ZAP70 negative cells [Chen et al., 2002]. Thus, although the expression of immunoglobin (Ig) receptors is restricted on CLL cells, ZAP70 facilitates the amplification of growth and/or survival signals [Gobessi et al., 2007]. The expression of PTPROt, therefore, appears to be critical for limiting the stimulatory action of ZAP70 in B-CLL cells. Although a recent study demonstrated that ZAP70 lacking functional kinase activity can still function as an adaptor molecule to enhance B-cell receptor signaling in B-CLL [Chen et al., 2008], we cannot rule out the role of specific tyrosine phosphorylations in this process that can be regulated by PTPROt.
Lyn kinase, a member of the Src family of kinases, is one of the initial kinases activated upon ligation of B-cell receptor complex [Niiro and Clark, 2002]. The expression and activity of Lyn are aberrantly upregulated in B-CLL. While activation of Lyn in normal B-lymphocytes is dependent upon receptor ligation, it is constitutively active in the resting B-CLL cells. Further, this anomalous activity of Lyn kinase has been linked to the defective apoptosis that is characteristic of B-CLL cells [Contri et al., 2005]. It is possible that loss of PTPROt, which plays an important role in negative regulation of Lyn activity, could play a key role in the abnormal activation of Lyn kinase and subsequent resistance to apoptosis in CLL cells. Consistent with our finding of Lyn as a PTPROt substrate and ~20% loss of its phosphorylation at the inactivating tyrosine, PTP-oc/PTPROt has been shown to interact with Src kinase and dephosphorylate its inactivating tyrosine (Y527) in osteoclast cells [Amoui et al., 2007; Amoui et al., 2004]. However, osteoclasts from transgenic mice expressing rabbit PTP-oc specifically in cells of osteoclast lineage show increased c-Src activity compared to osteoclasts from WT littermates [Sheng et al., 2009]. It is noteworthy that this report also shows increased phosphorylation of paxillin that has been identified as a substrate of PTPphi/PTPROt in macrophage cells [Pixley et al., 2001]. This paradox could be due to cell-type specific function of PTPROt. In this context, it is noteworthy that the phosphatase CD45 can either positively or negatively regulate the activity of Src family kinases [Hermiston et al., 2009]. These differential effects could occur in hematopoietic cells of different lineages or in cells of the same lineage at different developmental stages [Hermiston et al., 2009]. The demonstration that PTPROt that is suppressed in CLL can negatively regulate Lyn raises the possibility that loss of PTPROt could be one of the mechanisms of constitutive activation of Lyn in CLL. In this context, it will be interesting to study whether there is an inverse relationship between Lyn activity and PTPROt expression in primary CLL samples. Nevertheless, this finding provides an alternative mechanism of regulation of antigen receptor signaling and has significant implications for treatment of leukemia/lymphoma.
It is evident from this and a previous study [Chen et al., 2006] that PTPROt maintains tight regulation of lymphocyte receptor signaling by dephosphorylating the Src kinase Lyn/Lck as well as Syk/ZAP70. Inhibitors against these aberrantly active kinases have been in clinical development. With increasing understanding of abnormally active signaling pathways in cancer and the development of small molecule inhibitors against specific kinases and those inhibiting multiple targets, it would be advantageous to target multiple signaling molecules to treat cancer [Faivre et al., 2006]. Indeed, in this study we observed that inhibition of Lyn kinase by PTPROt facilitated the action of the orally available VEGF-R inhibitor Pazopanib on Raji cells. This synergism between Lyn kinase inhibition (by PTPROt) and VEGF-R inhibition (by Pazopanib) indicates the potential for co-targeting these kinases. Currently, the most common VEGF inhibitor Bevacizumab is in Phase II trials in combination with commonly used CLL therapeutics like cyclophosphamide and rituximab. In the absence of tools to introduce functional PTPROt into CLL cells, it would be worthwhile to test small molecule inhibitors against Lyn kinase. In this context, it is important to note that Dasatinib, the common c-abl/Src kinase inhibitor, is in Phase I/II clinical trials for use in CLL either alone or in combination with routinely used chemotherapies (e.g. rituximab).
In summary, this study has elucidated the significance of PTPROt expression in B-cells, loss of which in B-CLL could result in constitutively active Lyn. Further, it provides a rationale to test the efficacy of Lyn kinase inhibitors like Dasatinib in therapeutic regimens against CLL, specifically in combination with Bevacizumab or other VEGF-R inhibitors like Pazopanib.
Acknowledgments
We thank Dr. Arthur Weiss (University of California, San Francisco) for the HA-tagged ZAP70 expression vector pcDNA3 ZAP70 (HA-C), Dr. Hiroyuki Seimiya (Japanese Foundation for Cancer Research, Tokyo) for the WT and CS mutants of PTPU2L (PTPRO) and Dr. Ugo D’Oro (Novartis Vaccines and Diagnostics S.r.l.) for the expression vector for constitutively active Lck (pTEJ8-Lckmyc505F). We also thank Drs. Kalpana Ghoshal and Sarmila Majumder for valuable discussions and comments.
This study was supported, in part, by grants CA101956 and CA86978 from the National Cancer Institute.
References
- Abraham N, Veillette A. Activation of p56lck through mutation of a regulatory carboxy-terminal tyrosine residue requires intact sites of autophosphorylation and myristylation. Mol Cell Biol. 1990;10:5197–206. doi: 10.1128/mcb.10.10.5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguiar RC, Yakushijin Y, Kharbanda S, Tiwari S, Freeman GJ, Shipp MA. PTPROt: an alternatively spliced and developmentally regulated B-lymphoid phosphatase that promotes G0/G1 arrest. Blood. 1999;94:2403–13. [PubMed] [Google Scholar]
- Aiyar A, Xiang Y, Leis J. Site-directed mutagenesis using overlap extension PCR. Methods Mol Biol. 1996;57:177–91. doi: 10.1385/0-89603-332-5:177. [DOI] [PubMed] [Google Scholar]
- Amoui M, Sheng MH, Chen ST, Baylink DJ, Lau KH. A transmembrane osteoclastic protein-tyrosine phosphatase regulates osteoclast activity in part by promoting osteoclast survival through c-Src-dependent activation of NFkappaB and JNK2. Arch Biochem Biophys. 2007;463:47–59. doi: 10.1016/j.abb.2007.02.025. [DOI] [PubMed] [Google Scholar]
- Amoui M, Suhr SM, Baylink DJ, Lau KH. An osteoclastic protein-tyrosine phosphatase may play a role in differentiation and activity of human monocytic U-937 cell-derived, osteoclast-like cells. Am J Physiol Cell Physiol. 2004;287:C874–84. doi: 10.1152/ajpcell.00294.2003. [DOI] [PubMed] [Google Scholar]
- Amrein KE, Sefton BM. Mutation of a site of tyrosine phosphorylation in the lymphocyte-specific tyrosine protein kinase, p56lck, reveals its oncogenic potential in fibroblasts. Proc Natl Acad Sci U S A. 1988;85:4247–51. doi: 10.1073/pnas.85.12.4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchetot C, Chagnon M, Dube N, Halle M, Tremblay ML. Substrate-trapping techniques in the identification of cellular PTP targets. Methods. 2005;35:44–53. doi: 10.1016/j.ymeth.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Chan AC, Iwashima M, Turck CW, Weiss A. ZAP-70: a 70 kd protein-tyrosine kinase that associates with the TCR zeta chain. Cell. 1992;71:649–62. doi: 10.1016/0092-8674(92)90598-7. [DOI] [PubMed] [Google Scholar]
- Chen L, Apgar J, Huynh L, Dicker F, Giago-McGahan T, Rassenti L, Weiss A, Kipps TJ. ZAP-70 directly enhances IgM signaling in chronic lymphocytic leukemia. Blood. 2005;105:2036–41. doi: 10.1182/blood-2004-05-1715. [DOI] [PubMed] [Google Scholar]
- Chen L, Huynh L, Apgar J, Tang L, Rassenti L, Weiss A, Kipps TJ. ZAP-70 enhances IgM signaling independent of its kinase activity in chronic lymphocytic leukemia. Blood. 2008;111:2685–92. doi: 10.1182/blood-2006-12-062265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Juszczynski P, Takeyama K, Aguiar RC, Shipp MA. Protein tyrosine phosphatase receptor-type O truncated (PTPROt) regulates SYK phosphorylation, proximal B-cell-receptor signaling, and cellular proliferation. Blood. 2006;108:3428–33. doi: 10.1182/blood-2006-03-013821. [DOI] [PubMed] [Google Scholar]
- Chen L, Widhopf G, Huynh L, Rassenti L, Rai KR, Weiss A, Kipps TJ. Expression of ZAP-70 is associated with increased B-cell receptor signaling in chronic lymphocytic leukemia. Blood. 2002;100:4609–14. doi: 10.1182/blood-2002-06-1683. [DOI] [PubMed] [Google Scholar]
- Contri A, Brunati AM, Trentin L, Cabrelle A, Miorin M, Cesaro L, Pinna LA, Zambello R, Semenzato G, Donella-Deana A. Chronic lymphocytic leukemia B cells contain anomalous Lyn tyrosine kinase, a putative contribution to defective apoptosis. J Clin Invest. 2005;115:369–78. doi: 10.1172/JCI22094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo M, Bosch F, Villamor N, Bellosillo B, Colomer D, Rozman M, Marce S, Lopez-Guillermo A, Campo E, Montserrat E. ZAP-70 expression as a surrogate for immunoglobulin-variable-region mutations in chronic lymphocytic leukemia. N Engl J Med. 2003;348:1764–75. doi: 10.1056/NEJMoa023143. [DOI] [PubMed] [Google Scholar]
- D’Oro U, Sakaguchi K, Appella E, Ashwell JD. Mutational analysis of Lck in CD45-negative T cells: dominant role of tyrosine 394 phosphorylation in kinase activity. Mol Cell Biol. 1996;16:4996–5003. doi: 10.1128/mcb.16.9.4996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta J, Majumder S, Bai S, Ghoshal K, Kutay H, Smith DS, Crabb JW, Jacob ST. Physical and functional interaction of DNA methyltransferase 3A with Mbd3 and Brg1 in mouse lymphosarcoma cells. Cancer Res. 2005;65:10891–900. doi: 10.1158/0008-5472.CAN-05-1455. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Durig J, Nuckel H, Cremer M, Fuhrer A, Halfmeyer K, Fandrey J, Moroy T, Klein-Hitpass L, Duhrsen U. ZAP-70 expression is a prognostic factor in chronic lymphocytic leukemia. Leukemia. 2003;17:2426–34. doi: 10.1038/sj.leu.2403147. [DOI] [PubMed] [Google Scholar]
- Faivre S, Djelloul S, Raymond E. New paradigms in anticancer therapy: targeting multiple signaling pathways with kinase inhibitors. Semin Oncol. 2006;33:407–20. doi: 10.1053/j.seminoncol.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Flint AJ, Tiganis T, Barford D, Tonks NK. Development of “substrate-trapping” mutants to identify physiological substrates of protein tyrosine phosphatases. Proc Natl Acad Sci U S A. 1997;94:1680–5. doi: 10.1073/pnas.94.5.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobessi S, Laurenti L, Longo PG, Sica S, Leone G, Efremov DG. ZAP-70 enhances B-cell-receptor signaling despite absent or inefficient tyrosine kinase activation in chronic lymphocytic leukemia and lymphoma B cells. Blood. 2007;109:2032–9. doi: 10.1182/blood-2006-03-011759. [DOI] [PubMed] [Google Scholar]
- Hermiston ML, Zikherman J, Zhu JW. CD45, CD148, and Lyp/Pep: critical phosphatases regulating Src family kinase signaling networks in immune cells. Immunol Rev. 2009;228:288–311. doi: 10.1111/j.1600-065X.2008.00752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob ST, Motiwala T. Epigenetic regulation of protein tyrosine phosphatases: potential molecular targets for cancer therapy. Cancer Gene Ther. 2005;12:665–72. doi: 10.1038/sj.cgt.7700828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay NE, Bone ND, Tschumper RC, Howell KH, Geyer SM, Dewald GW, Hanson CA, Jelinek DF. B-CLL cells are capable of synthesis and secretion of both pro- and anti-angiogenic molecules. Leukemia. 2002;16:911–9. doi: 10.1038/sj.leu.2402467. [DOI] [PubMed] [Google Scholar]
- Lee YK, Bone ND, Strege AK, Shanafelt TD, Jelinek DF, Kay NE. VEGF receptor phosphorylation status and apoptosis is modulated by a green tea component, epigallocatechin-3-gallate (EGCG), in B-cell chronic lymphocytic leukemia. Blood. 2004;104:788–94. doi: 10.1182/blood-2003-08-2763. [DOI] [PubMed] [Google Scholar]
- Marth JD, Cooper JA, King CS, Ziegler SF, Tinker DA, Overell RW, Krebs EG, Perlmutter RM. Neoplastic transformation induced by an activated lymphocyte-specific protein tyrosine kinase (pp56lck) Mol Cell Biol. 1988;8:540–50. doi: 10.1128/mcb.8.2.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motiwala T, Ghoshal K, Das A, Majumder S, Weichenhan D, Wu YZ, Holman K, James SJ, Jacob ST, Plass C. Suppression of the protein tyrosine phosphatase receptor type O gene (PTPRO) by methylation in hepatocellular carcinomas. Oncogene. 2003;22:6319–31. doi: 10.1038/sj.onc.1206750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motiwala T, Jacob ST. Role of protein tyrosine phosphatases in cancer. Prog Nucleic Acid Res Mol Biol. 2006;81:297–329. doi: 10.1016/S0079-6603(06)81008-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motiwala T, Kutay H, Ghoshal K, Bai S, Seimiya H, Tsuruo T, Suster S, Morrison C, Jacob ST. Protein tyrosine phosphatase receptor-type O (PTPRO) exhibits characteristics of a candidate tumor suppressor in human lung cancer. Proc Natl Acad Sci U S A. 2004;101:13844–9. doi: 10.1073/pnas.0405451101. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Motiwala T, Majumder S, Ghoshal K, Kutay H, Datta J, Roy S, Lucas DM, Jacob ST. PTPROt inactivates the oncogenic fusion protein BCR/ABL and suppresses transformation of K562 cells. J Biol Chem. 2009;284:455–64. doi: 10.1074/jbc.M802840200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Motiwala T, Majumder S, Kutay H, Smith DS, Neuberg DS, Lucas DM, Byrd JC, Grever M, Jacob ST. Methylation and silencing of protein tyrosine phosphatase receptor type O in chronic lymphocytic leukemia. Clin Cancer Res. 2007;13:3174–81. doi: 10.1158/1078-0432.CCR-06-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niiro H, Clark EA. Regulation of B-cell fate by antigen-receptor signals. Nat Rev Immunol. 2002;2:945–56. doi: 10.1038/nri955. [DOI] [PubMed] [Google Scholar]
- Ostman A, Bohmer FD. Regulation of receptor tyrosine kinase signaling by protein tyrosine phosphatases. Trends Cell Biol. 2001;11:258–66. doi: 10.1016/s0962-8924(01)01990-0. [DOI] [PubMed] [Google Scholar]
- Palacios EH, Weiss A. Function of the Src-family kinases, Lck and Fyn, in T-cell development and activation. Oncogene. 2004;23:7990–8000. doi: 10.1038/sj.onc.1208074. [DOI] [PubMed] [Google Scholar]
- Palka HL, Park M, Tonks NK. Hepatocyte growth factor receptor tyrosine kinase met is a substrate of the receptor protein-tyrosine phosphatase DEP-1. J Biol Chem. 2003;278:5728–35. doi: 10.1074/jbc.M210656200. [DOI] [PubMed] [Google Scholar]
- Pixley FJ, Lee PS, Condeelis JS, Stanley ER. Protein tyrosine phosphatase phi regulates paxillin tyrosine phosphorylation and mediates colony-stimulating factor 1-induced morphological changes in macrophages. Mol Cell Biol. 2001;21:1795–809. doi: 10.1128/MCB.21.5.1795-1809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenwald A, Alizadeh AA, Widhopf G, Simon R, Davis RE, Yu X, Yang L, Pickeral OK, Rassenti LZ, Powell J, Botstein D, Byrd JC, Grever MR, Cheson BD, Chiorazzi N, Wilson WH, Kipps TJ, Brown PO, Staudt LM. Relation of gene expression phenotype to immunoglobulin mutation genotype in B cell chronic lymphocytic leukemia. J Exp Med. 2001;194:1639–47. doi: 10.1084/jem.194.11.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seimiya H, Sawabe T, Inazawa J, Tsuruo T. Cloning, expression and chromosomal localization of a novel gene for protein tyrosine phosphatase (PTP-U2) induced by various differentiation-inducing agents. Oncogene. 1995;10:1731–8. [PubMed] [Google Scholar]
- Sheng MH, Amoui M, Stiffel V, Srivastava AK, Wergedal JE, Lau KH. Targeted transgenic expression of an osteoclastic transmembrane protein-tyrosine phosphatase in cells of osteoclastic lineage increases bone resorption and bone loss in male young adult mice. J Biol Chem. 2009;284:11531–45. doi: 10.1074/jbc.M808324200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- Tiganis T, Bennett AM. Protein tyrosine phosphatase function: the substrate perspective. Biochem J. 2007;402:1–15. doi: 10.1042/BJ20061548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veillette A, Caron L, Fournel M, Pawson T. Regulation of the enzymatic function of the lymphocyte-specific tyrosine protein kinase p56lck by the non-catalytic SH2 and SH3 domains. Oncogene. 1992;7:971–80. [PubMed] [Google Scholar]
- Wang ES, Teruya-Feldstein J, Wu Y, Zhu Z, Hicklin DJ, Moore MA. Targeting autocrine and paracrine VEGF receptor pathways inhibits human lymphoma xenografts in vivo. Blood. 2004;104:2893–902. doi: 10.1182/blood-2004-01-0226. [DOI] [PubMed] [Google Scholar]
- Wiestner A, Rosenwald A, Barry TS, Wright G, Davis RE, Henrickson SE, Zhao H, Ibbotson RE, Orchard JA, Davis Z, Stetler-Stevenson M, Raffeld M, Arthur DC, Marti GE, Wilson WH, Hamblin TJ, Oscier DG, Staudt LM. ZAP-70 expression identifies a chronic lymphocytic leukemia subtype with unmutated immunoglobulin genes, inferior clinical outcome, and distinct gene expression profile. Blood. 2003;101:4944–51. doi: 10.1182/blood-2002-10-3306. [DOI] [PubMed] [Google Scholar]
- Wu J, Katrekar A, Honigberg LA, Smith AM, Conn MT, Tang J, Jeffery D, Mortara K, Sampang J, Williams SR, Buggy J, Clark JM. Identification of substrates of human protein-tyrosine phosphatase PTPN22. J Biol Chem. 2006;281:11002–10. doi: 10.1074/jbc.M600498200. [DOI] [PubMed] [Google Scholar]