Abstract
Background - Aims: Chronic hepatitis C (CHC) can cause a series of neuropsychiatric symptoms, whereas the currently approved treatment for this disease often induces similar symptoms as well. The aim of the present study was to compare Greek CHC patients' health-related quality of life (HRQoL) with that of healthy controls, to identify any possible relationships between HRQoL and demographic and laboratory parameters and to study the fluctuation of HRQoL during therapy and follow-up.
Patients and Methods: Ninty nine patients with CHC and 91 healthy controls were enrolled in the study. ALT, viral load, HCV genotype, fibrosis stage by liver biopsy and BMI, were determined at baseline. All patients completed the SF-36 quality of life questionnaire, which was self-administered, before treatment. They were treated with pegylated interferon α2-a or α-2b and ribavirin for 24 or 48 weeks and evaluated in the middle of therapy, at the end and six months after treatment cessation. SF-36 questionnaire was also completed in each evaluation.
Results: Patients' HRQoL was found to be below that of healthy controls in all SF-36 scales before treatment. There was a significant negative association between history of drug abuse and general health and a positive association between age and mental health. Multivariate analysis revealed that history of drug abuse seemed to play a significant role in bodily pain and general health of patients, as well as age did in vitality and mental health. The course of patients' HRQoL showed that in the middle of treatment values in all SF-36 scales were below those of baseline and they returned to pretreatment levels at the end of therapy. However, at the end of the six month follow-up period, an improvement in almost all scales compared to baseline was noted.
Conclusion: Our results showed that a) Greek CHC patients' HRQoL was worse than that of healthy individuals and fluctuated significantly during treatment b) A history of drug abuse and age can independently affect HRQoL c) During treatment values of HRQoL are worsened possibly due to interferon-a treatment and d) In the long-term treatment results in improvement of HRQoL.
Keywords: Health-related quality of life, chronic hepatitis C, pegylated interferon, sustained virological response
Hepatitis C virus (HCV) infection is currently a major public health problem worldwide with a prevalence ranging from 0.5% to about 20% in highly endemic countries1. Although HCV infection has been thought to be an asymptomatic disease, it is now understood that it can significantly compromise patients' health-related quality of life (HRQoL)2–4. Furthermore the currently approved treatment for chronic hepatitis C (CHC) with pegylated interferon and ribavirin can cause a worsening of patients' general health during therapy, followed by a marked improvement in those individuals achieving sustained virological response (SVR)5.
Multiple studies worldwide have shown that chronic hepatitis C is associated with a considerable impairment of HRQoL as compared with healthy controls4,6–10. Nonetheless, different results have also been reported implying possible interaction of ethnicity11. However, no relevant study was conducted in Greek patients with chronic hepatitis C.
The aim of this study was to compare Greek CHC patients' HRQoL before therapy with that of healthy subjects and to study HRQoL fluctuation during treatment and 24 weeks post-treatment. Moreover we explored possible relationships with patients' demographics and laboratory parameters.
Methods
Patients
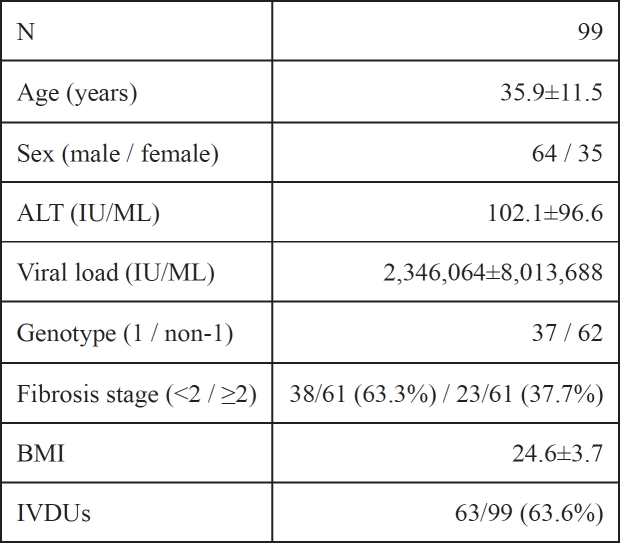
Ninety-nine patients (64 males, mean age 35.9±11.5) with chronic hepatitis C and 91 healthy subjects (63 males, mean age of 37.1±9.4) were enrolled in the study. Demographic and laboratory data before treatment are summarized in Table 1. All patients were evaluated in the Liver Unit of the 2nd Internal Medicine Dpt, Aristotle University, at Hippokratio Hospital of Thessaloniki, Greece. Patients were antiHCV / HCV RNA (+) and naive for antiviral therapy. A history of other chronic diseases or liver transplantation before entry was considered as an exclusion criterion. Patients with a decreased Greek language perception or other ethnic origin were excluded. A similar number of healthy controls with paired demographic characteristics were also enrolled in the study.
Table 1. Baseline characteristics of patients.
Data are presented as the meanstandard deviation. ALT: alanine aminotransferase, BMI: body mass index, IVDUs: intravenous drug users
Former intravenous drug users (IVDUs) participating in the study, were defined as past users of illicit drugs, who were abstinent from use for a minimum period of three months.
The study was carried out in accordance with the Declaration of Helsinki. The study was approved by the ethics committee of the Hospital and all patients signed an informed consent.
Laboratory evaluation
Values for alanine aminotransferase (ALT) levels and complete blood count were assessed by standard techniques. HCV-RNA measurements were performed by the Combas Amplicor® HCV (ROCHE Diagnostics) and HCV genotyping by INNOLIPA (Bayer Diagnostics). Determination of fibrosis stage on liver biopsy specimens was performed according to modified Knodell classification system12.
Treatment
Patients were treated according to current guidelines with pegylated interferon α2-a (180g per week) or α- 2b (1.5g/kg per week) subcutaneously, and ribavirin (800 mg per day for patients with genotype 2 or 3 and 1000-1200 mg for those with genotype 1 or 4 according to their body weight) orally13–15. Treatment duration was 24 and 48 weeks for genotypes 2 or 3 and 1 or 4 respectively.
Health-related quality of life
The Greek version of SF-36 questionnaire (SF-36™ Health Survey) was completed by all patients during their evaluations before treatment, in the middle of therapy, at the end of treatment and six months after treatment cessation. Laboratory results of the previous visit were only released at the time of questionnaire completion in order to eliminate any possible bias. Moreover SF-36 was selfadministered.
SF-36 is a widely used questionnaire, already validated in Greek population16. It consists of 36 questions measuring eight concepts: physical function (PF), role physical (RP), bodily pain (BP), general health (GH), vitality (VT), social function (SF), role emotional (RE), mental health (MH).
The scoring of the SF-36 questionnaire in our study was conducted upon a 0-100 scale, with higher scores reflecting better health status.
Statistics
Comparison of values between patients and healthy subjects was analyzed using the student t test. Correlations among HRQoL components and demographic and laboratory characteristics was performed with the Pearson and Spearman test and also with multiple linear regression analysis. Values across different time-points were analyzed by one-way ANOVA method using multiple and pair post hoc comparisons according to Bonferroni and Tukey.
Results
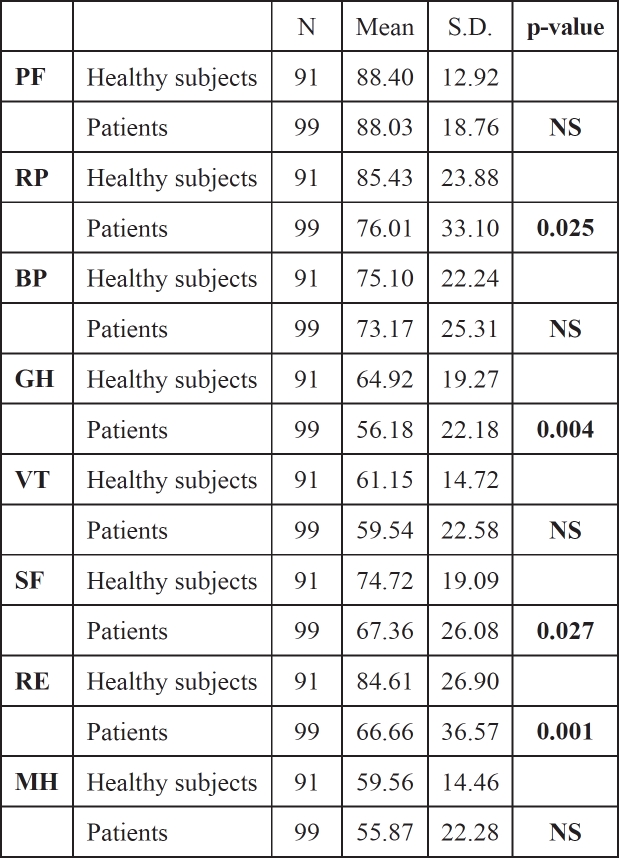
1. Differences in quality of life between patients and healthy subjects before treatment
At baseline patients' quality of life was found to be below that of healthy controls in all SF-36 scales (Table 2). Significant differences were detected for physiological rate (RP, p: 0.025), general health (GH, p: 0.004), social function (SF, p: 0.027) and emotional rate (RE, p: 0.001). The mean scores of patients remained lower than healthy controls in the majority of SF-36 scales after adjusting for the IVDU status (data not shown).
2. Correlation between HRQoL scores and patients' demographics and laboratory findings
We evaluated possible correlations between patients' scores in SF-36 scales at baseline and the following parameters: age, gender, ALT levels, HCV RNA level, HCV genotype, fibrosis stage, BMI and history of drug abuse. A significant negative association between history of drug abuse and GH (r =-0.339, p=0.001) and a positive association between age and mental health (MH, r: 0.337, p: 0.001) were found. According to these findings IVDUs had worse GH than non-IVDUs, whereas older patients had better MH. The multiple linear regression analysis showed that a history of drug abuse seemed to play a significant role in patients' bodily pain (BP, p: 0.03, R: 0.351) and GH (p: 0.002, R: 0.462), as well as age did in vitality (VT, p: 0.036, R: 0.26) and MH (p: 0.004, R: 0.351) (Table 3).
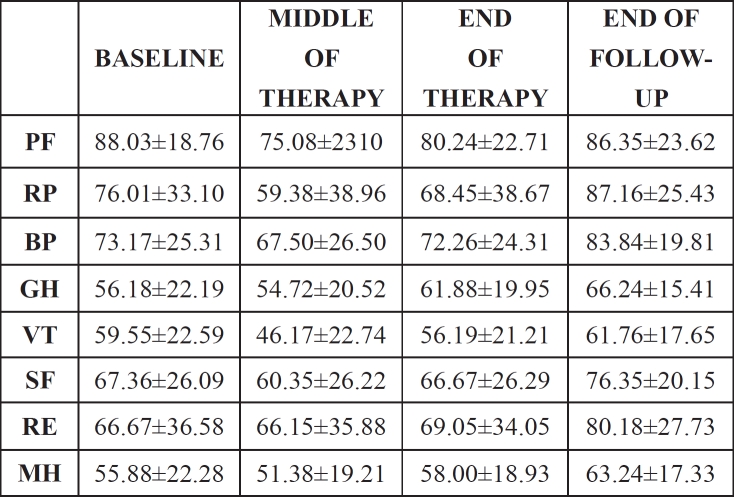
Table 3. SF-36 scores at different time-points of therapy.
Data are presented as the meanstandard deviation. S.D.= standard deviation, PF= physical function, RP= role physical, BP= bodily pain, GH= general health, VT= vitality, SF= social function, RE= role emotional, MH= mental health
3. SF-36 scores during therapy
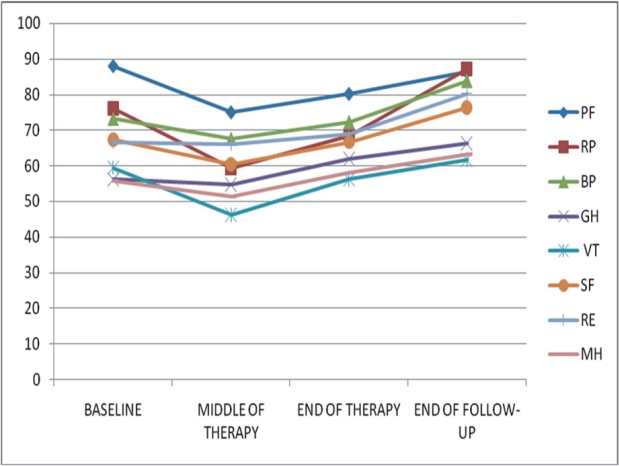
The evaluation of the course of patients' HRQoL during therapy and follow-up showed significant differences (p<0.05) between the four time points in the following scales: PF, RP, BP, GH, VT, SF, MH. Mean HRQoL values are summarized in Table 3. In the middle of therapy values in all SF-36 scales were below that of baseline. Scores returned to pre-treatment level in the end of therapy and showed an improvement in almost all scales compared to baseline at the end of the six month follow-up period (Figure 1).
Figure 1. SF-36 scores in different time-points of therapy. S.D.= standard deviation, PF= physical function, RP= role physical, BP= bodily pain, GH= general health, VT= vitality, SF= social function, RE= role emotional, MH= mental health.
Discussion
The monitoring of health-related quality of life (HRQoL) in patients with chronic hepatitis C has recently become an important component in many clinical trials. Nonetheless, clinical use is still limited. Moreover, few studies have investigated the effect of ethnicity in HRQoL. Notably there is no other longitudinal study conducted in HCV infected Greek population. In our study we found that the HRQoL in Greek naive chronic hepatitis C patients was lower than that of healthy individuals. Former intravenous drug users had a worse level of HRQoL than non-IVDUs. We documented that HRQoL decreased during treatment, whereas it returned to pre-treatment levels at the end of therapy and furthermore improved six months after treatment cessation.
Multiple studies over the last decade have shown that chronic hepatitis C is associated with a considerable impairment of HRQoL as compared with healthy controls4,6–10. The majority of studies were performed in developed countries with similar socioeconomic status. However, a study performed in rural Egypt did not confirm these results11. A preliminary study in Greek population showed that naive CHC patients have worse HRQoL than controls in agreement with most of the worldwide studies17. Another Greek study focusing on interferon induced depression showed that gender and HCV-related factors are important determinants of depression development18. In our study, patients' quality of life at baseline was found to be inferior to healthy controls in all SF-36 scales showing no difference to most of the studies published.
Various baseline factors have been speculated to play an important role in HRQoL. A recent meta-analysis revealed that gender and BMI are the main determinants of HRQoL, although this relationship was weaker when the history of depression was included in the analysis9. Moreover, Teuber et al documented that advanced liver fibrosis is associated with reduced physical HRQoL and that female sex and older age are significant predictors of physical HRQoL, as well19. Past use of illicit drugs has been shown to lead to a worsening of HRQoL7. However, these findings are not homogeneous in the different studies, possibly due to the varying abstinence duration8. Further studies in the IVDU population stratified according the presence or not of HCV infection could help elucidate the role of illicit drug in HRQoL.
In our study, IVDUs had worse GH than non-IVDUs, possibly due to the fact that these patients usually encounter multiple socioeconomic problems which may interfere with their disease perception. However, older patients had better MH. The latter finding was surprising and more difficult to explain. It is likely that younger patients have lower levels of mental health, because they face their disease through a more pessimistic view, possibly related to a disease induced lack of hope for their future life.
Side effects of interferon and ribavirin have been shown to affect HRQoL during treatment1,2. It is also noteworthy that HRQoL returns to pretreatment levels at the end of treatment and even reaches higher levels in case of an SVR1,2,4,5,20. Our patients experienced this precise, previously reported variation. The high SVR rate in our cohort of patients (83.7%) did not allow a statistically powerful analysis between responders and non-responders, although the mean scores in non-responders were lower than responders in 7/8 SF-36 scales.
In conclusion, our findings confirm that Greek patients' quality of life shows a fluctuation during therapy and follow-up, similar to that previously reported. The history of drug abuse and age can independently affect HRQoL. Monitoring of HRQoL should be implemented in every-day clinical practice as a useful tool to recognize patients that could need additional psychological support.
Table 2. SF-36 scores for healthy subjects and patients before treatment.
S.D.= standard deviation, PF= physical function, RP= role physical, BP= bodily pain, GH= general health, VT= vitality, SF= social function, RE= role emotional, MH= mental health.
References
- 1.Perz ZF, Farrington LA, Pecoraro C, Hutin YJF, Armstrong GL. 42nd Annual Meeting of the Infectious Diseases Society of America. Boston, MA, USA: 2004. Estimated global prevalence of hepatitis C virus infection. [Google Scholar]
- 2.Bonkovsky HL, Wooley JM Consensus Interferon Study Group. Reduction of health-related quality of life in chronic hepatitis C and improvement with interferon therapy. Hepatology. 1999;29:264–270. doi: 10.1002/hep.510290124. [DOI] [PubMed] [Google Scholar]
- 3.Ware J, Jr, Bayliss M, Mannocchia M, Davis GL International Hepatitis Interventional Therapy Group. Health-related quality of life in chronic hepatitis C: Impact of disease and treatment response. Hepatology. 1999;30:550–555. doi: 10.1002/hep.510300203. [DOI] [PubMed] [Google Scholar]
- 4.McHutchison JG, Ware JEJr, Bayliss MS, Pianko S, Albrecht JK, Cort S, et al. The effects of interferon alpha-2b in combination with ribavirin on health related quality of life and work productivity. J Hepatol. 2001;34:140–147. doi: 10.1016/s0168-8278(00)00026-x. [DOI] [PubMed] [Google Scholar]
- 5.Bernstein D, Kleinman L, Barker CM, Revicki DA, Green J. Relationship of health-related quality of life to treatment adherence and sustained response in chronic hepatitis C patients. Hepatology. 2002;35:704–708. doi: 10.1053/jhep.2002.31311. [DOI] [PubMed] [Google Scholar]
- 6.Spiegel B, Younossi Z, Hays R, Hays RD, Revicki D, Robins S, et al. Impact of hepatitis C on health related quality of life: a systematic review and quantitative assessment. Hepatology. 2005;41:790–800. doi: 10.1002/hep.20659. [DOI] [PubMed] [Google Scholar]
- 7.Foster G, Goldin R, Thomas H. Chronic hepatitis C virus infection causes a significant reduction in quality of life in the absence of cirrhosis. Hepatology. 1998;27:209–212. doi: 10.1002/hep.510270132. [DOI] [PubMed] [Google Scholar]
- 8.Hussain K, Fontana R, Moyer C, Su GL, Sneed-Pee N, Lok ASF. Comorbid illness is an important determinant of health-related quality of life in patients with chronic hepatitis C. Am J Gastroenterol. 2001;96:2737–2744. doi: 10.1111/j.1572-0241.2001.04133.x. [DOI] [PubMed] [Google Scholar]
- 9.Dan A, Martin L, Crone C, Ong JP, Farmer DW, Wise Th, et al. Depression, anemia and health-related quality of life in chronic hepatitis C. J Hepatol. 2006;44:491–498. doi: 10.1016/j.jhep.2005.11.046. [DOI] [PubMed] [Google Scholar]
- 10.Dalgard O, Egeland A, Skaug K, Villimas K, Steen T. Healthrelated quality of life in active injecting drug users with and without chronic hepatitis C virus infection. Hepatology. 2004;39:74–80. doi: 10.1002/hep.20014. [DOI] [PubMed] [Google Scholar]
- 11.Schwarzinger M, Dewedar S, Rekacewicz C, Elaziz KMA, Fontanet A, Carrat F, et al. Chronic hepatitis C virus infection: does it really impact health-related quality of life? A study in rural Egypt. Hepatology. 2004;40:1434–1441. doi: 10.1002/hep.20468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brunt E. Grading and staging the histopathological lesions of chronic hepatitis: The Knodell Histology Activity Index and beyond. Hepatology. 2000;31:241–246. doi: 10.1002/hep.510310136. [DOI] [PubMed] [Google Scholar]
- 13.Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, et al. Peginterferon α2b plus ribavirin compared with interferon 2b plus ribavirin for initial treatment of chronic hepatitis C: a randomized trial. Lancet. 2001;358:958–965. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 14.Fried MW, Shiffmann ML, Reddy R, Smith C, Marinos G, Goncales F, et al. Peginterferon α2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 15.Hadziyannis SJ, Sette H, Jr, Morgan TR, Balan V, Diago M, Marcellin P, et al. Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann Intern Med. 2004;140:346–355. doi: 10.7326/0003-4819-140-5-200403020-00010. [DOI] [PubMed] [Google Scholar]
- 16.Pappa E, Kontodimopoulos N, Niakas D. Validating and norming of the Greek SF-36 Health Survey. Qual Life Res. 2005;14:1433–1438. doi: 10.1007/s11136-004-6014-y. [DOI] [PubMed] [Google Scholar]
- 17.Yfantopoulos G, Pierrakos G, Zanakis B. Quality of life in patients with hepatitis C infection. Arch Hellen Med. 2001;18:288–296. [Google Scholar]
- 18.Koskinas J, Merkouraki P, Manesis E, Hadziyiannis S. Assessment of depression in patients with chronic hepatitis: effect of interferon treatment. Dig Dis. 2002;20:284–288. doi: 10.1159/000067682. [DOI] [PubMed] [Google Scholar]
- 19.Teuber G, Sch?fer A, Rimpel J, Paul K, Keicher Ch, Scheurlen M, et al. Deterioration of health-related quality of life and fatigue in patients with chronic hepatitis C: Association with demographic factors, inflammatory activity, and degree of fibrosis. J Hepatol. 2008;49:923–929. doi: 10.1016/j.jhep.2008.07.025. [DOI] [PubMed] [Google Scholar]
- 20.Neary MP, Cort S, Bayliss MS, Ware JE, Jr, et al. Sustained virologic response is associated with improved health-related quality of life in relapsed chronic hepatitis C patients. Semin Liver Dis. 1999;19(Suppl 1):77–85. [PubMed] [Google Scholar]