Abstract
Melanopsin is the photopigment of intrinsically photosensitive retinal ganglion cells (ipRGCs). Melanopsin immunoreactivity reveals two dendritic plexuses within the inner plexiform layer (IPL), and morphologically heterogeneous retinal ganglion cells. Using enhanced immunohistochemistry, we provide a fuller description of murine cell types expressing melanopsin, their contribution to the plexuses of melanopsin dendrites, and mosaics formed by each type. M1 cells, corresponding to the originally described ganglion-cell photoreceptors, occupy the ganglion cell or inner nuclear layers. Their large, sparsely branched arbors (mean diameter 275 µm) monostratify at the outer limit of the OFF sublayer. M2 cells also have large, monostratified dendritic arbors (mean diameter 310 µm), but ramify in the inner third of the IPL, within the ON sublayer. There are approximately 900 M1 cells and 800 M2 cells per retina; each type comprises roughly 1–2% of all ganglion cells. The cell bodies of M1 cells are slightly smaller than those of M2 cells (mean diameters: 13 µm for M1, 15 µm for M2). Dendritic field overlap is extensive within each type (coverage factors ~3.8 for M1 and 2.5 for M2 cells). Rare bistratified cells deploy terminal dendrites within both melanopsin-immunoreactive plexuses. Because these are too sparsely distributed to permit complete retinal tiling, they lack a key feature of true ganglion cell types and may be anomalous hybrids of the M1 and M2 types. Finally, we observed weak melanopsin immunoreactivity in other ganglion cells, mostly with large somata, that may constitute one or more additional types of melanopsin-expressing cells.
Keywords: intrinsically photosensitive, ipRGC, coverage, dendrites, types
INTRODUCTION
A small minority of mammalian retinal ganglion cells expresses the photopigment melanopsin (Provencio et al., 2000; Provencio et al., 2002). These cells are capable of autonomous phototransduction and are thus true photoreceptors (Berson et al., 2002; Do et al., 2009; Graham et al., 2008; Schmidt and Kofuji, 2009; Schmidt et al., 2008; Viney et al., 2007; Warren et al., 2003). These intrinsically photosensitive retinal ganglion cells (ipRGCs) project directly to a subset of subcortical visual nuclei of the brain and have been implicated in circadian, pupillary, and hormonal responses to light (Baver et al., 2008; Dacey et al., 2005; Gooley et al., 2003; Hannibal et al., 2004; Hattar et al., 2006; Hattar et al., 2002; Morin et al., 2003; Viney et al., 2007).
The retinal ganglion cells exhibiting either melanopsin expression or intrinsic photosensitivity were initially described as belonging to a single, distinctive morphological type (termed M1 or Type 1 in rodents). This type has a relatively small cell body and a large, sparsely branched dendritic arbor narrowly monostratified at the outer margin of the inner plexiform layer (IPL), within the OFF sublayer. These cells are invariably intensely melanopsin immunoreactive. In rodents, they have been shown to project to the hypothalamic suprachiasmatic nucleus, among other targets (Baver et al., 2008; Berson et al., 2002; Hattar et al., 2006; Hattar et al., 2002; Morin et al., 2003; Sollars et al., 2003; Viney et al., 2007) and to be intrinsically photosensitive (Berson et al., 2002; Do et al., 2009; Fu et al., 2005; Hartwick et al., 2007; Hattar et al., 2002; Lucas et al., 2003; Schmidt et al., 2008; Warren et al., 2003). A probable homolog has been described in the retinas of primates, including humans (Dacey et al., 2005; Gamlin et al., 2007; Hannibal and Fahrenkrug, 2004; Jusuf et al., 2007; Rollag et al., 2003).
However, various observations point to the existence of one or more additional types of melanopsin-expressing ganglion cells. In primates and rodents, a second plexus of melanopsin-immunoreactive processes marks the inner third of the IPL (within the ON sublayer and juxtaposed to the ganglion cell layer) (Baver et al., 2008; Dacey et al., 2005; Hattar et al., 2006; Jusuf et al., 2007; Provencio et al., 2002; Schmidt and Kofuji, 2009; Schmidt et al., 2008; Viney et al., 2007). Dendrites within this plexus have been traced to a second population of RGCs (Schmidt et al., 2008; Viney et al., 2007), termed M2 or Type 2 in rodents. M2 cells have larger somata and dendritic fields than M1 cells (Schmidt and Kofuji, 2009). In addition, their arbors are more highly branched than the M1 cells, and labeling of adjacent M1 and M2 cells suggests an extensive overlap of dendritic fields (Schmidt and Kofuji, 2009). Like the outer-stratifying M1 type, M2 cells are intrinsically photosensitive (Dacey et al., 2005; Schmidt et al., 2008). Recent evidence suggests that these cells may have distinct patterns of projection to visual centers of the brain (Baver et al., 2008). Finally, there are also scattered reports of ipRGCs and/or melanopsin-expressing cells with bistratified dendritic arbors (Pu, 1999; Schmidt and Kofuji, 2009; Schmidt et al., 2008; Viney et al., 2007; Warren et al., 2003) see also (Pu, 1999)).
The mouse has become an indispensable tool to study this relatively new photoreceptive system. In addition to the standard knock-out and knock-in models previously described (Hattar et al., 2002; Panda et al., 2002; Ruby et al., 2002), mice expressing Cre recombinase under the control of the melanopsin gene promoter are now available (Hatori et al., 2008), providing investigators with the ability to express a large array of reporters, enzymes, and receptors specifically within these new photoreceptor cells. A deeper understanding of the cellular elements and the dendritic organization of this system will be critical for the interpretation of future experiments. To this end, in this report we contrast the dendritic architecture, soma sizes, and mosaics of the M1 and M2 cells in the adult mouse retina. We demonstrate that both types of cells form highly overlapping mosaics. By contrast, we show that melanopsin-immunoreactive cells with bistratified dendritic arbors are too sparsely distributed to tile the retina and are presumably not a true type. Finally, we provide preliminary evidence that there may be one or more additional ganglion cell types which express melanopsin at very low levels.
MATERIALS and METHODS
Animals
All data in this study were obtained from adult male C57BL/6J mice (Jackson Labs, Bar Harbor, ME). All procedures were consistent with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Institutional Animal Care and Use Committees of the University of Virginia and Brown University. Animals were housed in individual cages and maintained under 12 hours light:12 hours dark cycle. Mice were killed by carbon dioxide asphyxiation and immediate decapitation.
Antibodies
The primary polyclonal antiserum (UF006; Advanced Targeting Systems, San Diego, CA) was raised in rabbit against a synthetic peptide consisting of the 15 N-terminal amino acids of mouse melanopsin (Genbank accession NP_038915) conjugated to keyhole limpet hemocyanin. Specificity of this antiserum has been confirmed in control studies showing a dose-dependent loss of immunoreactivity by preadsorption with the immunogen and by the lack of immunoreactivity in the retinas of melanopsin-null mice (Panda et al., 2002). We confirmed the lack of immunoreactivity in melanopsin knockout mice when using the more sensitive immunoperoxidase method described below. The secondary antibody was either Cy3-conjugated donkey anti-rabbit IgG (Jackson ImmunoResearch Laboratories, West Grove, PA), for immunofluorescence, or biotinylated goat anti-rabbit IgG (Vector Laboratories, Burlingame, CA), for immunoperoxidase labeling.
Immunohistochemistry
Immunofluorescent labeling of retinal sections
Eyes were removed immediately after death and placed into cold aerated Ames medium (Sigma-Aldrich, St. Louis, MO). The cornea was removed and the eye fixed at 4°C for 24 h in 4% paraformaldehyde (Electron Microscopy Sciences, Ft. Washington, PA) freshly prepared in phosphate-buffered saline (PBS; pH 7.4). The lenses were subsequently removed, eyecups cryoprotected in 30% sucrose in PBS (4°C for 24 h), and embedded in OCT medium (Sakura Finetek, Torrance, CA). Embedded eyecups were sectioned by cryostat at 16–20 µm thickness and thaw-mounted onto gelatin-coated slides. The mounted tissue was washed 3 times (10 min, 4°C) in Tris-buffered saline (TBS, pH 7.4; Quality Biological, Gaithersburg, MD) and blocked for 30 min at 4°C in 1.5% normal goat serum (Vector Laboratories, Burlingame, CA) in TBS. After three washes in TBS (10 min, 4°C), the tissue was incubated for 24 hr at 4°C in unpurified primary antiserum (UF006) diluted 1:2500 in “incubating buffer” consisting of TBS containing 1% bovine serum albumin, 0.25% carrageenan lambda, and 0.3% Triton-X-100 (Sigma-Aldrich, St. Louis, MO). Tissue was washed three times in TBS (10 min, 22°C) and then incubated in the Cy3-conjugated secondary antibody diluted 1:500 in incubating buffer (1 hour, 22°C). Again, sections were washed three times in TBS (10 min, 22°C) and excess buffer was removed by blotting the slide edges on absorbent paper towels. Sections were mounted in DAPI-containing Vectashield (Vector Laboratories, Burlingame, CA), coverslipped, and sealed with clear fingernail polish.
Immunoperoxidase labeling of retinal flat mounts
Immediately after eye removal, the retina was gently dissected from the eyecup, mounted on filter paper, and fixed for two hours in 4% buffered paraformaldehyde, as above. To enhance penetration, the retina was cryoprotected in 30% sucrose in PBS for several hours, twice briefly frozen over crushed dry ice and thawed, and finally immersed overnight in 0.5% dimethylsulfoxide (DMSO) and 2.0% Triton-X-100. Endogenous peroxidase activity was suppressed by immersion in 0.1% hydrogen peroxide in PBS (45 min, 22°C). After a blocking step (6% goat serum, 2% Triton-X-100, 0.5% DMSO in TBS; 1 hour, 22°C), tissue was incubated for 3 days in the unpurified primary antiserum (UF006) diluted 1:2500 in “incubating buffer” consisting of TBS containing 6% goat serum, 2% Triton-X-100, and 0.5% DMSO. After three TBS washes (10 min, 22°C), flat mounts were incubated for three hours in the goat anti-rabbit biotinylated secondary IgG antibody diluted 1:200 in an incubation buffer that matched that used for the primary antiserum. Thereafter, the avidin-biotin-peroxidase reaction (Elite ABC kit, Vector Laboratories) was carried out according to the protocol supplied by the vendor, using diaminobenzidine as the chromogen. After a final three washes in TBS (10 min, 22°C), the flat-mounts were removed from the filter paper, transferred onto to glass microscope slides, mounted in glycerol, coverslipped, and sealed with fingernail polish.
Microscopy and analysis
Immunofluorescence images were captured on a Zeiss Axiophot epifluorescence microscope equipped with an ORCA camera (Hamamatsu). Immunoperoxidase images were obtained on a Nikon E600 microscope equipped with Spot RT Slider camera. Image files were pseudocolored and enhanced globally for brightness and contrast using Photoshop 6.0 (Adobe Systems, San Jose, CA). Final figures were composed in Photoshop, Canvas X (Deneba Software, Miami, FL) or PowerPoint (Microsoft Corporation, Redmond, WA).
To reconstruct the dendritic profiles of individual melanopsin-expressing ganglion cells, we analyzed in detail a quadrant of retina that had been optimally stained by the immunoperoxidase method (see Figs. 2A and 2E). We assembled a comprehensive high-resolution library of digital photomicrographs in which every melanopsin immunoreactive element was in sharp focus in at least one image. With the optic axis positioned at one margin of the retinal sample, we used a 20× objective lens (numerical aperture: 0.75) to capture in a series of approximately 30 images incremented in focal depth by 1 µm and ranging from the optic fiber layer to the outermost immunopositive dendrites. The optic axis was then offset laterally and the process repeated. Altogether, we captured 17 such “z-stacks”, each covering an area of 435 × 580 µm. Each stack slightly overlapped those adjacent to it so that individual processes could be followed continuously from one stack to the next. These stacks were assembled in Photoshop, with one image per layer. Individual somadendritic profiles were traced in a process similar to that used to make a camera lucida drawing. Using the Photoshop pencil tool, we traced each cell’s profile on a separate, overlying transparent layer, displaying or hiding underlying layers as needed to visualize each process as it coursed in depth. Each cell’s profile was drawn in a unique color on a separate layer, so that cells could be displayed individually or as a mosaic.
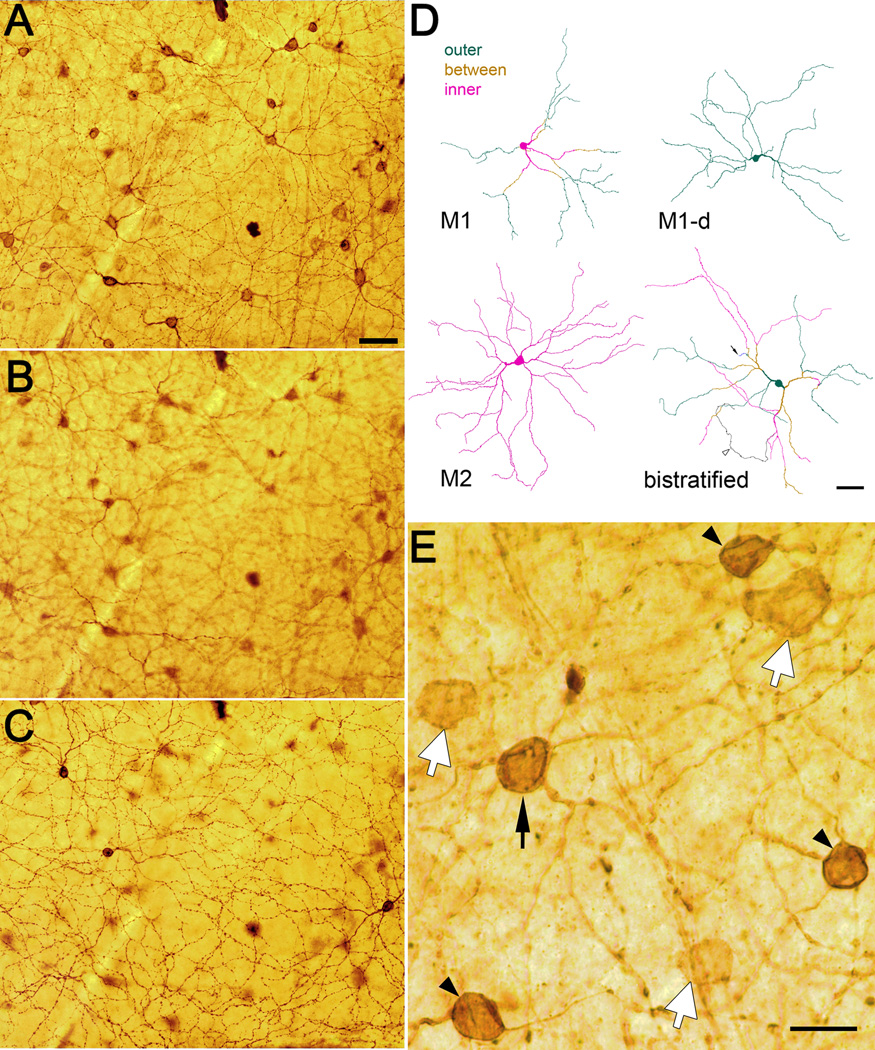
Figure 2.
Melanopsin immunostaining in retinal wholemounts and the cell types it reveals. A–C: Three planes of focus through a mouse wholemount retina showing melanopsin-like immunoreactivity as revealed by immunoperoxidase labeling. Focal plane in A lies in the middle of the inner (M2) plexus of immunoreactive dendrites. Conventionally placed immunoreactive cell bodies are also in sharp focus. Focal plane in B lies between the two plexuses of immunoreactive dendrites. Immunoreactive processes in sharp focus are rare, and consist almost entirely of the proximal dendrites of conventionally placed M1 cells ascending to the outer IPL. Focal plane in C is in the middle of the outer (M1) plexus of immunoreactive dendrites. Somata of three displaced M1 cells are also in sharp focus. Scale bar in A (50 µm) applies to A–C. D: Representative examples of the dendritic architecture of each of four varieties of melanopsin immunopositive ganglion cells as reconstructed from the immunoperoxidase-stained retina shown in A–C. Processes and somata are color coded to distinguish those in or near the inner (M2) plexus (magenta) from those in or near the outer (M1) plexus (green). Processes in the middle of the IPL, between the two plexuses, are shown in gold. The M1 cell has a conventionally placed cell body and terminal dendrites in the outer dendritic plexus, but proximal dendrites must course through the inner plexus. The displaced M1 cell (“M1-d”) has a soma in the inner nuclear layer and all processes within the outer plexus. The M2 cell has a conventionally placed soma and dendrites restricted to the inner plexus. The bistratified cell illustrated has a displaced soma (though others are conventionally placed) and terminal dendrites in both the inner and outer plexuses. An unusual feature of this bistratified cell is the presence of one short process ascending from the IPL through the INL to terminate near the outer plexiform layer (arrow), a feature described previously for melanopsin expressing dendrites in rat retina (Hattar et al., 2002). The arrowhead marks the axon (black), which arose from a dendrite. Scale bar: 50 µm. E: Three types of melanopsin-immunoreactive ganglion cell bodies in the mouse retina. Several large, weakly melanopsin immunopositive cells (white arrows) are shown in relation to three neighboring M1 cells (black arrowheads) and a single M2 cell (black arrow). The photomicrograph is drawn from the same retinal wholemount illustrated in Fig. 2; focal plane is within the ganglion cell layer. Scale bar: 20 µm.
We used the same three-dimensional digital photomontage for several other purposes. We assessed the basic stratification pattern of every immunolabeled cell in the retinal piece (many more than it was possible to fully trace) to develop the map of cell types shown in Fig. 3C. We traced the outlines of each labeled cell body and the minimal convex polygon enclosing the dendritic field of each traced somadendritic profile. These outlines were exported to ImageJ (http://rsbweb.nih.gov/ij/) which we used to measure the areas of somatic and dendritic profiles. Soma and dendritic field sizes were expressed as equivalent diameters, that is, the diameter of a circle of equal area. To estimate the level of stratification of M2 dendrites within the IPL, we identified, at many retinal locations, the plane of best focus for the innermost and outermost processes of the M2 dendritic plexus. These depths were then expressed as a percentage of the full thickness of the IPL, with 100% depth corresponding to the ganglion cell layer, taken as the depth of best focus of ganglion cell somata, and 0% corresponding to the outer border of the IPL, inferred from the plane of best focus of the outermost dendrites of the M1 plexus.
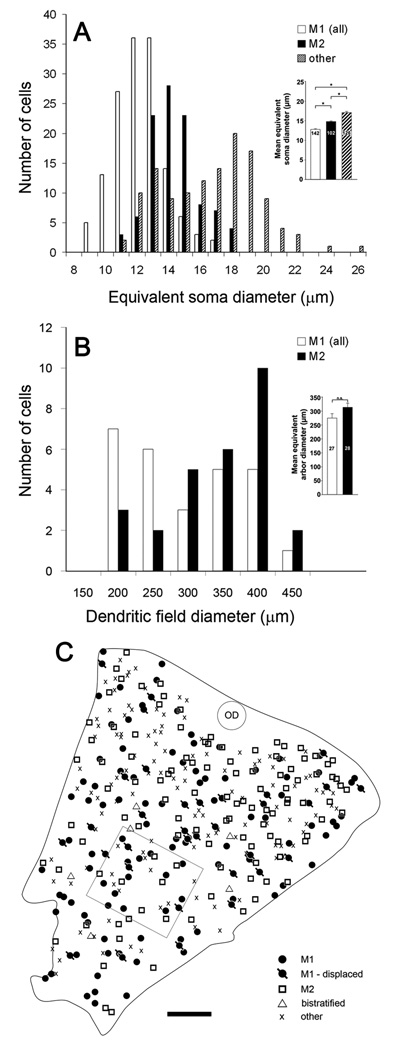
Figure 3.
Somatic and dendritic-field sizes of several types of melanopsin immunopositive ganglion cells and their spatial distribution in the retina. A: Histogram of equivalent soma diameter of M1 cells (combining conventionally placed and displaced varieties), M2 cells, and the very weakly immunopositive cell bodies without appreciable dendritic staining (“other”). Bistratified cells are not included. Inset : Soma sizes (mean ± SEM) of M1, M2, and “other” cells. Asterisks indicate statistically significant differences (p<0.05). B: Distribution of dendritic field diameters of M1 cells, including displaced M1 cells (white bars;) and M2 cells (black bars). Inset: equivalent dendritic-field diameters (mean ± SEM) of M1 and M2 cells. Asterisks indicate statistically significant difference (p<0.01). C: Map of the distribution of melanopsin immunoreactive cells within a piece of retinal wholemount tissue (rectangular outline indicates location of zone shown in Fig. 2A–C). Different symbols represent different types of melanopsin cells, as determined for each cell from its dendritic stratification and somatic location (ganglion cell layer or inner nuclear layer). The “other” category refers to weakly immunopositive cell bodies without appreciable dendritic staining. OD : optic disk. Scale bar: 250 µm.
To generate the images in Fig. 2A–C, we manipulated the z-stacks to correct for the imperfect flatness of the retina. Using the outer margin of the M1 plexus and the inner margin of the M2 plexus as benchmarks, we generated a contour map encoding the displacement in the z-dimension of every point in the retinal piece relative to a reference region of excellent flatness. We then used this contour map to correct the distortion by selecting the relevant region within the image and transposing it, in every image of the stack, either up or down the stack by the appropriate number of images. These corrected images were used only to illustrate the inner and outer melanopsin plexuses as they would appear in a perfectly flat piece of tissue (Fig. 2A–C). All reconstructions of dendritic profiles and analyses of dendritic stratification were done on the uncorrected z-stacks, with the depth of any process being inferred by reference to the local position within the stack of the inner and outer plexuses. Dendritic profiles shown in Fig 4 were subjected to Sholl analysis to compare their dendritic arborizations quantitatively. We used the ImageJ Sholl analysis plug-in courtesy of the Ghosh Lab (http://www-biology.ucsd.edu/labs/ghosh/software/) with the following settings: starting radius, 10 µm; ending radius, 400 µm; radius step size, 20 µm; radius span, 3 µm; and span type, median.
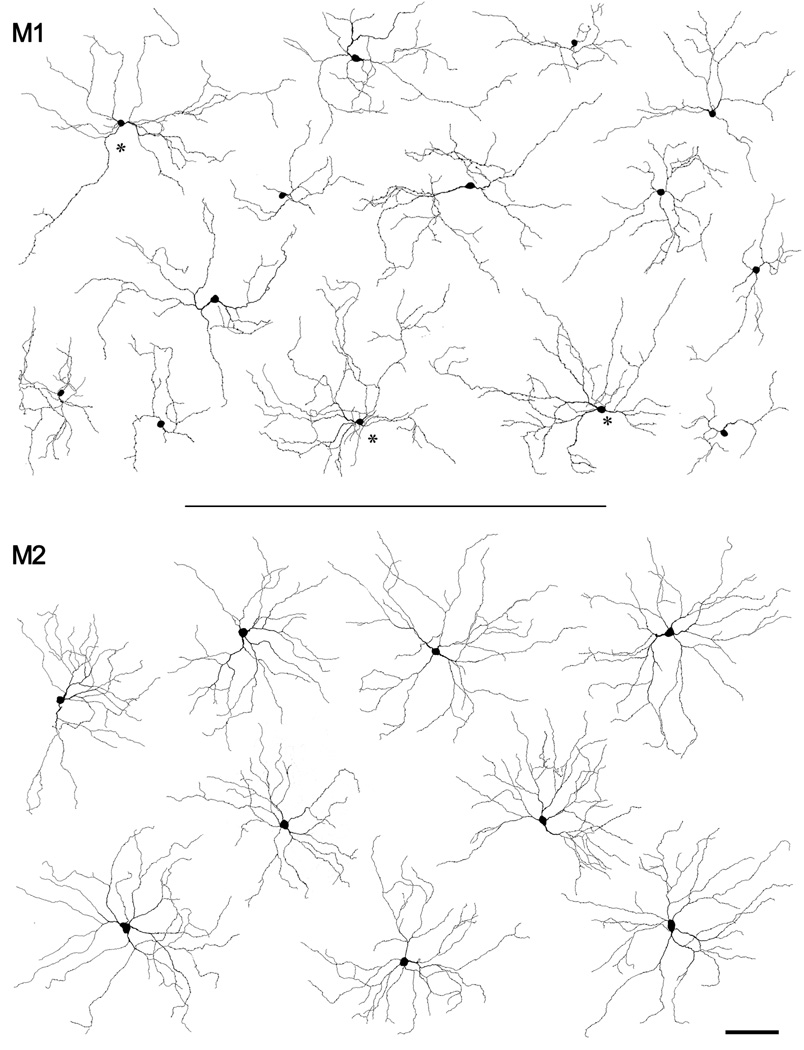
Figure 4.
Examples of the somadendritic profiles of melanopsin immunopositive ganglion cells of the M1 and M2 types. Top: Tracings of fourteen M1 cells. The three cells marked with asterisks had somas displaced to the inner nuclear layer; the rest were conventionally placed. Bottom: Drawings of nine cells of the M2 type. All cells were found in or near the region illustrated in Fig. 2A–C. Scale bar: 50 µm.
RESULTS
Pattern of anti-melanopsin immunoreactivity
Fig. 1 illustrates the appearance of melanopsin immunofluorescence in vertical sections of the mouse retina. Immunofluorescent cell bodies appeared at low density in the ganglion cell layer and at even lower density within the innermost cell row of the inner nuclear layer. Two plexuses of immunofluorescent dendrites were also evident within the IPL. One of these occupied the outer margin of the IPL, abutting the inner boundary of the INL. This corresponds to the outer part of the OFF sublamina of the IPL. The other plexus occupied roughly the inner third of the IPL, within inner part of the ON sublayer. Processes of the outer plexus were more narrowly stratified in depth than those of the inner plexus and were consistently more boldly stained than those in the inner plexus, giving them a coarser appearance. In some material, the inner plexus was faint and barely detectable.
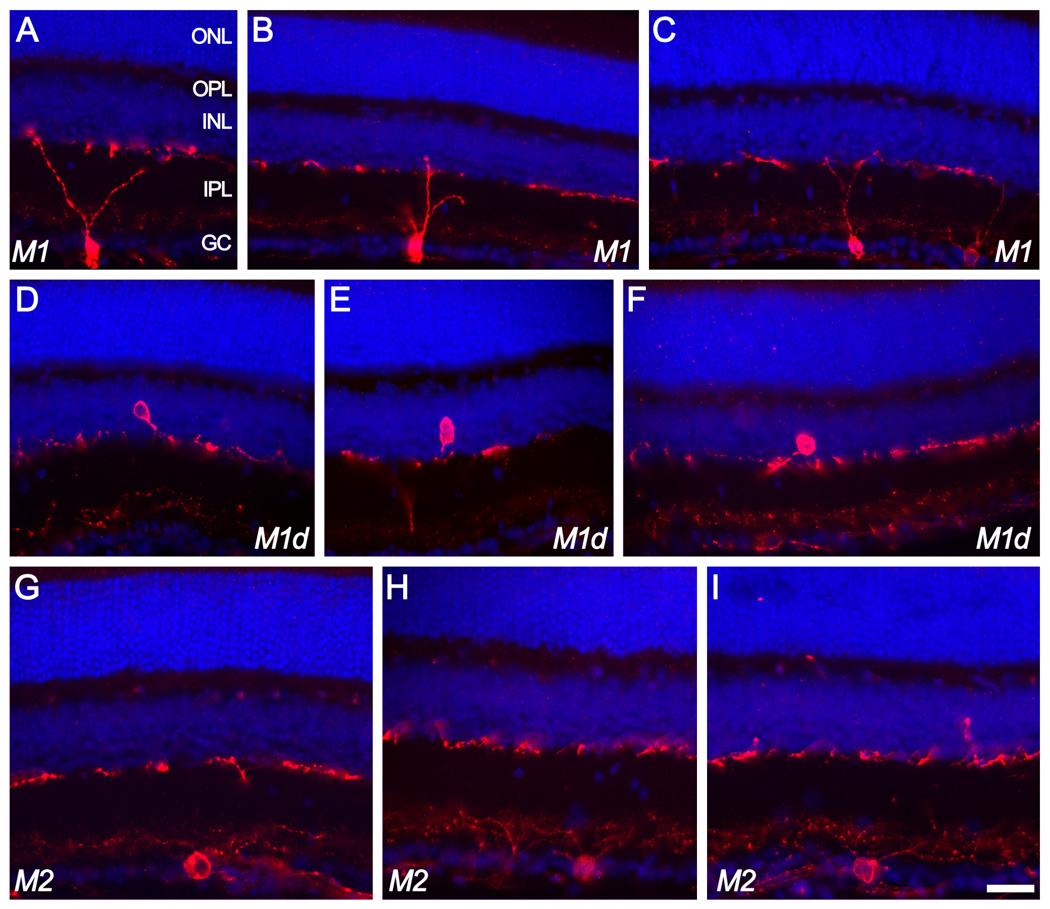
Figure 1.
Ganglion cells of the M1 and M2 types as revealed in vertical sections of the mouse retina immunostained for melanopsin (red). A nuclear counterstain (DAPI; blue) reveals cellular laminae. Top two rows of images (A–F) illustrate melanopsin-immunoreactive cells of the M1 type, characterized by dendritic arborizations in the outer melanopsin immunoreactive plexus lying at the boundary between inner plexiform and inner nuclear layers. Some of these M1 cells were conventionally placed with somata in the ganglion cell layer (top row; A–C) while others (“M1d”) had somata displaced to the inner nuclear layer (middle row; D–F). The fainter inner (M2) plexus is barely detectable in some of these images. M2 cells (bottom row; G–I) had conventionally placed somata and laterally spreading dendrites entering the inner (M2) plexus of immunopositive dendrites. Scale bar: 30 µm. ONL=outer nuclear layer; OPL=outer plexiform layer; INL=inner nuclear layer; IPL=inner plexiform layer; GC=ganglion cell layer.
Even in vertical sections, many cells retained enough of their proximal dendritic arbor to provide at least a rough impression of their level of dendritic stratification. Such cells appeared to contribute processes mainly to one of the two melanopsin immunopositive plexuses. Examples are shown in Fig. 1, with cells contributing to the outer plexus (including those with somata displaced to the INL) labeled “M1”, and those arborizing in the inner plexus labeled “M2”.
To reconstruct more fully the stratification patterns of individual cells, we turned to immunoperoxidase preparations, which yielded dendritic labeling of higher sensitivity and contrast than achievable by immunofluorescence. An additional advantage was that the mosaic could be studied at length without fading. Fig. 2A–C shows the immunoperoxidase labeling in one region of a wholemounted retina as viewed at three focal planes: in the middle of the inner (M2) plexus (Fig. 2A), between the two plexuses (2B), and in the middle of the outer (M1) plexus (2C). Processes of M2 as well as of M1 cells appeared to be well labeled all the way to their tips, and nearly all could be traced back with confidence to their somatic origin. This allowed us to reconstruct both the somadendritic profile of individual immunopositive cells and the mosaic of cells arborizing in each sublayer. These reconstructions are subject to some minor errors. Wherever a pair of very fine terminal processes of similar caliber cofasciculated for short stretches or crossed one another obliquely within the same focal plane, it was difficult to trace the continuity with certainty. However, such instances were rare, and because they occurred in the far periphery of the dendritic fields, erroneous assignments of such terminal processes had little impact on the size or general form of reconstructed dendritic profiles.
An overview of melanopsin-expressing ganglion cell types
We fully reconstructed 61 immunopositive cells and partially reconstructed 19 others. These cells could be readily sorted into four distinct morphological patterns. Fig. 2D shows one representative somatodendritic profile of each of these forms as reconstructed from the wholemount, color-coded to illustrate the depth of individual processes.
M1 cells of the ganglion-cell layer
The great majority of immunostained dendrites in the outer melanopsin plexus could be traced to cells with somata conventionally placed in the ganglion cell layer. The terminal dendrites of these RGCs stratified exclusively in the outer plexus. We use the designation “M1” to refer to these cells. They match the morphology of melanopsin-positive ipRGCs in mice (Lucas et al., 2003) and rats (Berson et al., 2002; Warren et al., 2003) that are retrolabeled from suprachiasmatic nucleus and the Type I (or Type 1) melanopsin-expressing cells in mice (Schmidt et al., 2008; Viney et al., 2007). Because the cell bodies of M1 cells reside in the RGC layer, their dendrites pass through the ON sublamina of the IPL, but these are a small minority of the processes in the inner plexus and are relatively easy to distinguish from the other processes in that plexus due to their intense immunostaining, coarse caliber, and their typically steep and direct outward trajectory toward the outer plexus (see top three panels of Fig. 1).
Displaced M1 cells
Nearly all processes in the outer melanopsin plexus not derived from conventional M1 cells could be traced back to melanopsin positive ganglion-cell somata displaced to the INL. These are certainly ganglion cells rather than amacrine cells because axons can be traced from them through the IPL to the optic fiber layer (data not shown). These cells closely resembled conventional M1 cells in their dendritic caliber and branching pattern. Their terminal dendrites also narrowly costratified with those of conventional M1 cells, though their proximal dendrites immediately joined the outer melanopsin plexus, whereas conventional M1 cells did so only after ascending through the full thickness of the IPL. For this reason, and because they do not tile the retina (see below), we consider them as a variant of the M1 type and therefore refer to them as “displaced M1 cells”. With the exception of a very few bistratified cells (see below), every melanopsin-positive cell body within the INL was a displaced M1 cell.
M2 cells
These monostratified cells have somata within the ganglion cell layer and dendritic arbors restricted to the inner plexus of melanopsin-positive dendrites, within the ON sublayer of the IPL. They match the morphology of melanopsin-positive ganglion cells in mice previously shown to be intrinsically photosensitive and variously termed M2, Type 2 or Type II cells (Schmidt and Kofuji, 2009; Schmidt et al., 2008; Viney et al., 2007). The dendrites of M2 cells account for virtually all immunopositive processes within the inner melanopsin-immunopositive plexus. The few remaining processes comprised either the thicker caliber, proximal dendrites of conventionally placed M1 cells coursing toward the outer melanopsin plexus or dendrites of bistratified cells (see below).
Bistratified cells
We encountered a small number of immunopositive cells that extended dendrites into both melanopsin immunopositive plexuses. Somata of these cells could be either displaced to the INL, as in the example shown in Fig. 2D, or located conventionally in the ganglion cell layer. While some bistratified cells, such as that in Fig. 2D, have roughly equal numbers of terminal dendrites in the two plexuses, many of these cells stratify primarily in the inner IPL within the plexus of M2 dendrites. Bistratified melanopsin immunoreactive cells have been termed “Type 3” cells by Viney et al. (2007) and “Type III“ by Schmidt et al. (2008) though evidence presented below indicates that they may not constitute a true type.
Other cells
In optimally stained preparations, an additional group of very weakly immunopositive somata could be discerned within the RGC layer. Fig. 2E shows three such cells (white arrows) along with one M2 cell (black arrow) and several M1 cells (black arrowheads). The faintly stained cells are presumably ganglion cells because nearly all of them are too large to be displaced amacrine cells (see below). Staining was so faint that only the proximal 50–100 µm of dendritic arbor could be discerned, so little can be said about the dendritic architecture of these cells. The initial trajectory of these dendrites does suggest stratification at least partly in the ON sublayer. It is unclear whether these cells comprise a single type.
Soma size
Fig. 3A shows the distribution of soma sizes among M1, M2 and the weakly stained “other” melanopsin RGCs. Conventionally placed and displaced M1 cells differed little in size (conventional, 13.0 µm ± 1.4 µm; displaced, 12.1 µm ± 1.7 µm; mean ± s.d.) and have been pooled in this histogram. On average, M1 cells had the smallest somata among the melanopsin immunopositive cells, M2 cells were of intermediate size, and the “other” weakly immunoreactive melanopsin cells were largest (see Fig. 3A inset; M1: 12.8±1.6 µm mean± SD, n =142; M2: 14.8±1.5, n=102; “other”: 17.1±;2.9, n=126) While the mean equivalent soma diameters were statistically distinguishable, there was considerable overlap among the soma diameter distributions of these classes (Fig. 3A), so that soma size alone is a morphological parameter of limited utility for discriminating among these types. Bistratified cells are not included in this histogram, but in our very small sample of such cells, they were comparable in soma diameter to M1 and M2 cells (mean = 14.5 µm; range: 12.7 – 16.9 µm; n = 6). Both M1 and M2 somatic profiles were roughly circular. Mean form factor (i.e., the ratio of least to greatest diameter) was 0.81 ± 0.11 for M1 cells and 0.77 ± 0.12 for M2 cells (n = 320 for each type).
Overall numbers, spatial density and distribution of types
To characterize the spatial distribution of these cell types, we determined the identity of every melanopsin positive cell in an optimally stained retinal sample from which the images of Fig. 2A–C were obtained. Fig. 3C plots the location and type of each cell within this tissue sample. To estimate the spatial density and total numbers of M1, M2 and “other” melanopsin cells, we counted total numbers of each type within a retinal area of 1.9 mm2, corresponding to nearly the entire retinal region illustrated in Fig. 3C, but excluding its margins and the area around the optic disk where staining appeared incomplete or was otherwise difficult to analyze. There were 120 M1 cells in this region (86 conventionally placed, and 34 displaced), for an estimated average spatial density of 63 cells/mm2. There were 112 M2 cells, giving a density of 59 cells/mm2, while the weakly stained “other” melanopsin-positive cells numbered 115, for an estimated density of 61 cells/mm2. Thus, M1, M2 and “other” melanopsin cells were present at essentially equivalent spatial density. These density values, when multiplied by an estimated total retinal area of 14 mm2 (Lyubarsky and Pugh, 1996; Williams and Goldowitz, 1992), yield rough estimates of the total numbers of each type within a retina: 890 M1 cells of which 640 have somata in the RGC layer and 250 have displaced cell bodies, 830 M2 cells, and 850 “other” melanopsin cells. These estimates are based on the assumption that these cell types do not exhibit substantial topographic variations in density, which is broadly confirmed and further validated by earlier estimates of the abundance of M1 and M2 cells (Baver et al., 2008; Hattar et al., 2006; Hattar et al., 2002). Regarding bistratified cells, only a rough approximation can be made of their prevalence. Their bistratified arbors were typically not obvious from casual inspection, so we restrict our consideration to a subregion of the retinal piece illustrated in Fig. 2E, within which we reconstructed all of the M1 and M2 cells completely enough to distinguish them from bistratified cells. Within this zone we counted 6 bistratified cells, as compared with 41 M1 and 33 M2 cells. By this analysis, bistratified cells account for less than 10% of the overall population of melanopsin immunoreactive cells, an estimate that drops even further if the weakly stained “other”’ ganglion cells are included.
The M1 mosaic
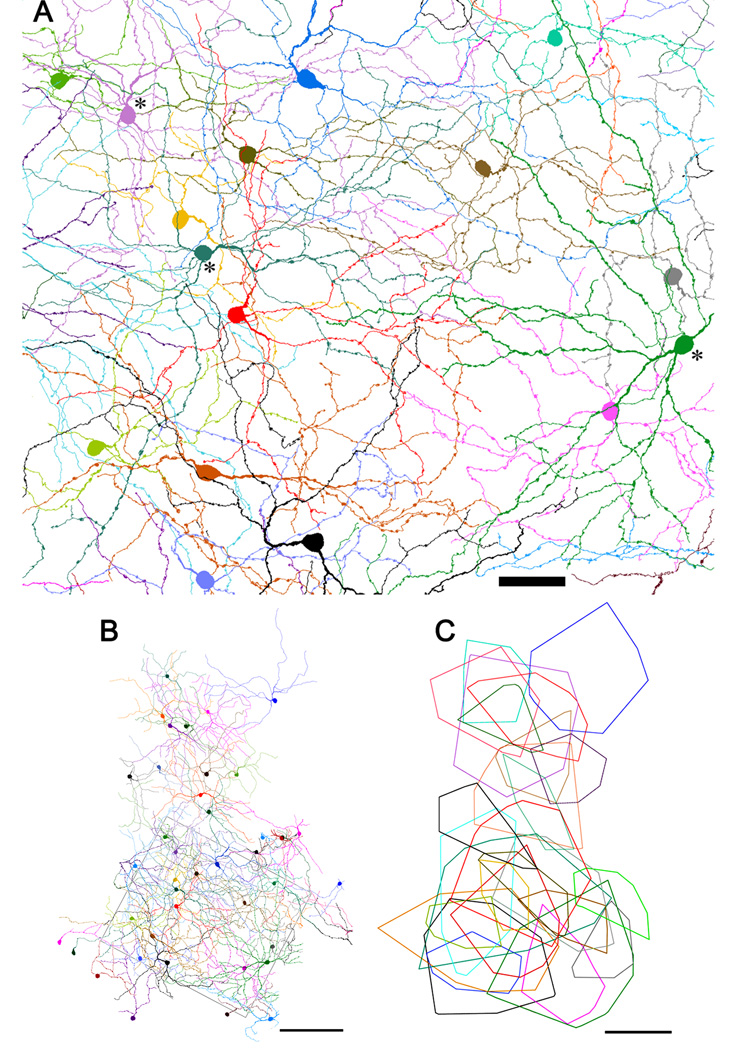
Fig. 4A shows tracings of fourteen M1 cells drawn from within or near the sample zone depicted in Fig. 2A–C. Three of these, indicated by asterisks, had displaced somata while the rest were conventionally placed. Their large, sparsely branched dendritic arbors had a disorganized appearance with some tortuous processes, highly variable distances between branch points, much denser branching in some sectors of the field than in others, and some mutual overlap among dendrites.
By tracing all the dendrites from individual M1 somata through the z-stack, we were able to reconstruct a virtually complete mosaic of these cells. Fig. 5A shows such a reconstruction for the sample zone shown in Fig. 2A–C, and an expanded reconstruction including the sample zone (rectangle) is shown in Fig. 5B. It is clear from these images that the dendritic fields of neighboring M1 cells overlapped extensively. This is shown more clearly in Fig. 5C, in which a minimal convex polygon has been fitted around the dendritic profile of each fully reconstructed M1 cell in Fig. 5B. Dendritic fields were among the largest of any mouse RGCs, with an equivalent diameter of 275 ± 82 µm (mean ± s.d., n = 27). The accuracy of these measurements is limited by the possible assignment of very fine distal branches to the wrong M1 cell, as noted above. Such errors would be expected to have minimal effect on the estimated dendritic field size, because virtually all dendrites within the outer plexus were assigned to one cell or another. Accordingly, any inflation of one cell’s dendritic field from erroneously assigning it a given dendritic branch would in general be counterbalanced by a corresponding shrinkage in the apparent field size of the cell to which the incorrectly assigned dendrite actually belonged. In other words, the main impact of such errors would be to increase the variability of field size, rather than to alter mean size.
Figure 5.
The mosaic of M1 cells. A: Reconstruction of the M1 cell mosaic within the retinal region illustrated in Figs. 2A–C. Three displaced cells are included, indicated by asterisks near cell bodies. The processes of bistratified cells within the outer plexus have been excluded. Scale bar: 50 µm B: Reconstruction of the same mosaic over a larger area, shown at reduced magnification. Rectangular outline marks the region illustrated in panel A and Figs. 2A–C. Not all cells outside the rectangle have been fully reconstructed. Scale bar: 200 µm. C: Dendritic field areas of fully reconstructed M1 cells from panel B represented as minimal convex polygons fully enclosing the dendritic profile. Scale bar: 200 µm.
Image magnification of the adult mouse eye is 34 µm/deg (Schmucker and Schaeffel, 2004). Thus, the mean dendritic field of individual M1 cells covers a region of visual space about 8 degrees in diameter. A standard index of the degree of dendritic overlap among neighboring cells of the same type is the coverage factor, the average number of dendritic fields of that type overlapping any retinal point. This is calculated as the product of dendritic field area and the local spatial density of cell of the same type. For M1 cells, we obtained a coverage factor of 3.8, based on an average density of 63 cells/mm2 (see above) and the mean field diameter of 275 µm (field area of 0.059 mm2).
It is generally assumed that every retinal point falls within the dendritic field of at least one cell of any true ganglion cell type (Wässle, 2004). Thus, the coverage factor of a true type should exceed unity. To address the question of whether displaced M1 cells constitute a distinct cell type, we deleted conventionally placed M1 cells from the mosaic in Fig. 5B so that only displaced M1 cells were shown (Fig. 6). There was substantial mutual overlap among the dendritic fields of these cells in many locations, but there were also clearly gaps in coverage. Note, for example, the dendrite-free patch within the rectangular sample zone in Fig. 6. The failure of displaced M1 cells to tile the retina suggests that they do not constitute a distinct cell type but are simply members of a broader M1 type that happen to exhibit an atypical somatic location.
Figure 6.
Displaced M1 cells do not tile the retina. Reconstruction of the mosaic form by displaced M1 cells. The mosaic is identical to that in Fig. 2C except that conventionally placed M1 cells have been deleted, leaving only displaced M1 cells. Scale bar: 100 µm.
The M2 mosaic
In the best stained preparations with clearly resolvable immunopositive dendrites, nearly all processes in the inner plexus could be traced back to their cells of origin. The vast majority of these arose from M2 cells. The only exception to this were the proximal dendrites of M1 cells with somata in the ganglion-cell layer and a very few inner processes of bistratified cells. The M1 proximal dendrites were easily distinguished from M2 dendrites because they were of greater caliber, generally more intensely stained, and passed fairly directly to the outer plexus, following vertical or steeply oblique trajectories through the inner M2 plexus (Fig. 1). A few M1 cells had dendrites running for several hundred micrometers within the M2 plexus before ascending, but such instances were very rare. Through-focus analysis placed the outer border of the M2 plexus at 67% ± 0.06% depth and its inner border at 83% ± 0.03% depth (mean ± s.d., n=10 in both cases).
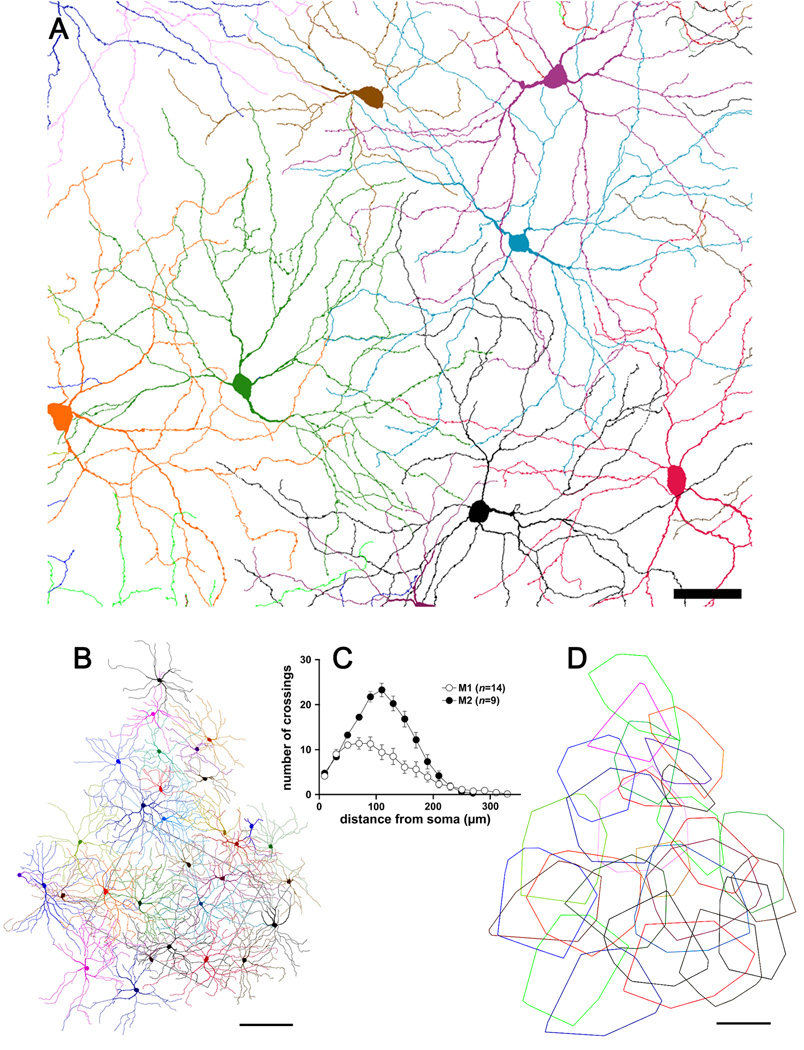
Tracing every immunopositive dendrite in and near the sample zone of Fig. 2A–C back to its somatic origin allowed us to characterize the morphology of large numbers of M2 cells and the mosaic they form. Fig. 7A shows tracings of nine M2 cells drawn from within or near the sample zone depicted in Fig. 2A–C. All had cell bodies within the ganglion cell layer. In fact, we have never observed an example of a displaced M2 cell, although two displaced bistratified melanopsin cells deployed the great majority of their dendritic arbor with the M2 dendritic plexus and these may represent aberrant M2 cells (see below). The dendritic fields of M2 cells were large, like those of M1 cells, but their profiles were subtly different. In particular, they appeared more orderly, with straighter, more radiate and more regularly branching dendrites, and a more uniform density of dendritic branching within the dendritic field. Fig. 7C compares the complexity of the dendritic arbors of M1 and M2 cells (Fig. 4) by Sholl analysis (Sholl, 1953). Arbors of M2 cells were more highly branched than those of M1 cells, particularly within 100 µm of the soma, where dendrites of M2 cells bifurcated approximately twice as frequently as those of M1 cells.
Figure 7.
The mosaic of M2 cells. A: Reconstruction of the M2 cell mosaic within the retinal region illustrated in Figs. 2A–C. The processes of bistratified cells within the inner plexus have been excluded. Scale bar: 50 µm. B: Reconstruction of the same mosaic over a larger area, shown at reduced magnification. Rectangular outline marks the region illustrated in panel A and Figs. 2A–C and Fig. 5A. C: Sholl analysis of dendritic branching pattern of M2 cells in comparison to that in M1 cells. Mean number of dendritic crossings ± SEM. D: Dendritic field areas of fully reconstructed M2 cells from panel B represented as minimal convex polygons fully enclosing the dendritic profile. Scale bar: 200 µm.
From the reconstructions of the M2 mosaics (Figs. 7A and 7B), it is apparent that the somata of this cell type were fairly regularly spaced and that the dendritic fields completely covered the retina. The region reconstructed in Fig. 7A is that same as that for the M1 mosaic reconstruction in Fig. 5A and shown in the photomicrographs of Fig. 2A–C. The M2 mosaic (Fig. 7A) appears more orderly than that formed by M1 cells (Fig. 5A), with straighter dendrites, more regular spacing of cells and a more uniform density of dendritic branches. The dimensions and mutual overlap among M2 dendritic fields is shown more clearly in Fig. 7D, in which each field is mapped as a convex polygon, as in the M1 plot in Fig. 5C. Dendritic fields were very large, with equivalent diameters averaging 314 ± 76 µm (mean ± s.d., n = 28). This was about 40 µm larger than the mean diameter of M1 dendritic fields by (see above), but the distributions of field size for the two types overlapped extensively (Fig. 3B) and were not statistically different (Fig. 3B inset). The image magnification of the adult mouse eye (34 µm/degree) (Schmucker and Schaeffel, 2004) implies that, on average, the dendritic arbor of an M2 cell encompasses a region of visual space 9 degrees in diameter. Coverage factor for M2 cells was 4.6, calculated from a spatial density of 59 cells/mm2 and a dendritic field diameter of 314 µm (field area of 0.077 mm2).
Bistratified cells
Within the thoroughly analyzed retinal region covered by the reconstructed mosaics in Figs. 5A and 7A, we encountered six cells that deployed terminal dendrites within both the inner and outer melanopsin immunoreactive plexuses. In the inner plexus, the terminal dendrites of these cells were easily distinguished from the proximal dendrites of conventionally placed M1 cells because they were of finer caliber and ended within the inner plexus, rather than ascending to terminate in the outer plexus.
Three of these six bistratified melanopsin cells had somata displaced to the inner nuclear layer. These cells gave rise to one or more primary dendrites that descended through the outer plexus to the middle of the IPL, whereupon daughter branches either descended further to terminate in the inner plexus or ascended once more to terminate in the outer plexus. The bistratified cell illustrated in Fig. 2D, which adhered to this pattern, had roughly equal numbers of terminal dendrites in the two plexuses, but the other two displaced bistratified cells had a preponderance of their terminal dendrites in the inner plexus. The remaining three bistratified cells had somata placed conventionally, within the ganglion cell layer. All three of them arborized predominantly in the inner plexus, but gave rise to one or a few processes that ascended to terminate in the outer plexus. A few of the bistratified cells had one or two dendrites running for extended distances at an intermediate depth between the inner and outer plexuses, but virtually every dendritic tip lay in one melanopsin immunoreactive plexus or the other.
Stratification aside, the dendritic branching patterns of the bistratified cells were similar to those of M1 and M2 cells, and their dendritic fields similarly large. However, their spatial density was so low that they fell far short of tiling the retina. Because such tiling is generally considered to be a prerequisite for status as a true ganglion cell type (Wässle, 2004), we currently consider these bistratified cells an anomalous transitional form between two otherwise distinct types of melanopsin expressing cells, the M1 and M2 cells, with slightly greater similarity to M2 cells in terms of stratification and soma size.
DISCUSSION
The present findings clarify a number of outstanding issues regarding the types of murine ganglion cells expressing melanopsin and the mosaics formed by these types. They describe more fully the two main types, one stratifying in the outer IPL (M1 cells) and the other in the inner IPL (M2 cells). They suggest that displaced melanopsin RGCs are not a distinct type, but are instead mainly members of the M1 mosaic. Further, the data confirm the existence of bistratified melanopsin expressing cells, but suggest that these are too rare to tile the retina or constitute a true cell type; a few of these have displaced somata. They also suggest that there may be one or more “other” cells, some with very large cell bodies, that express melanopsin at low levels. These may represent additional morphologically and functionally distinct ipRGC types.
M1 cells
We have taken advantage of the complete staining of M1 cells by a melanopsin antiserum to provide a fuller characterization of the architecture of these cells and the mosaic they form. Our data indicate that displaced melanopsin immunoreactive neurons are ganglion cells, because they send axons into the optic fiber layer. Excluding the very rare displaced bistratified cells, all of these cells closely resemble conventionally placed M1 cells in terms of their dendritic branching pattern, dendritic field size, depth of stratification and soma size. The failure of the displaced cells to tile the retina uniformly further argues against their constituting a distinct ganglion cell type. For all of these reasons, we consider displaced monostratified melanopsin cells to be members of the M1 mosaic. A similar conclusion was reached by Jusuf et al. (2007) in the marmoset retina, in which melanopsin-positive ganglion cells stratify in either the innermost or outermost level of the IPL (Jusuf et al., 2007). Outer-stratifying (M1-like) cells in the marmoset could have somata in either the ganglion cell layer or the inner nuclear layer, although in contrast with our data in the mouse, the displaced population outnumbered the conventionally placed population.
Our estimate of the total number of M1 cells (about 900 per retina, including displaced cells) is in good agreement with prior estimates from Hattar et al.(2006) and Baver et al. (2008) of about 800 cells per retina. Gonzalez-Menendez et al. (2009) obtained much lower estimates, perhaps because they used mice with a different genetic background (C3H). The large, sparsely branched dendritic fields we reconstructed for M1 cells are consistent with earlier descriptions of these cells in the mouse (Coombs et al., 2006; Lucas et al., 2003; Provencio et al., 2002; Schmidt and Kofuji, 2009; Schmidt et al., 2008; Viney et al., 2007). Our estimates of M1 dendritic field size (~275 µm diam) are in line with those of Schmidt et al., 2008, although they apparently grouped M1, M2 and bistratified cells in their analysis. Slightly larger M1 dendritic fields were reported by other groups (Coombs et al., 2006: ~300–400 µm diameter; Viney et al., 2007: 410µm; their Fig. S1), but disparate labeling methods or other technical differences may account for this discrepancy.
The overlap among M1 dendritic fields is remarkably high. Coverage factor, the product of field size and local density, is nearly 4, and Fig. 5C confirms that most retinal points lie within the dendritic arbors of at least three M1 cells. Surprisingly, though Viney et al. (2007) obtained a larger estimate of mean M1 dendritic field size than we did, their estimate of M1 dendritic coverage (2.5) was substantially lower than ours. This is probably attributable to an underestimate in their study of the spatial density of M1 cells. They estimated the mean nearest neighbor distance between M1 somas at 250 µm, more than two and a half times greater than that they reported for M2 cells. Though we have not directly calculated this metric of spatial density, casual inspection of Fig. 3C suggests that M1 cells are far more tightly spaced than measured by Viney and colleagues and at least as tightly spaced as M2 cells. Our estimate of coverage is much higher than reported for other types of ganglion cells (Balasubramanian and Sterling, 2009). It should be noted, however, that these other estimates have been obtained in species other than the mouse, so it remains unclear whether such extensive coverage is unusual among mouse ganglion cells. In this vein, it is of interest that in the marmoset retina, outer-stratifying (M1-like) melanopsin cells apparently tile the retina with minimal dendritic overlap (Jusuf et al., 2007).
M2 cells
Information on the inner plexus of melanopsin immunoreactive dendrites has until now come mainly from dye filling of single melanopsin immunopositive cells (Schmidt and Kofuji, 2009; Schmidt et al., 2008; Viney et al., 2007). Here, we have been able to resolve the origin of these dendrites in three varieties of cells. A minor component derives from conventionally placed M1 cells, which send coarse primary dendrites through this plexus en route to the outer IPL. Nearly all of these ascend so steeply that their contribution to the inner plexus is negligible. Bistratified cells also contribute a few dendrites to the inner plexus, as discussed further below. All of the remaining branches can be traced back to a population of large-field, monostratified ganglion cells of the ganglion-cell layer. We call these M2 cells in keeping with several earlier reports (Baver et al., 2008; Gonzalez-Menendez et al., 2009; Hattar et al., 2006; Schmidt and Kofuji, 2009); others have referred to them as “Type 2” melanopsin cells (Schmidt et al., 2008; Viney et al., 2007).
M2 cells have been described morphologically not only in recent studies of melanopsin-expressing ganglion cells (Viney et al., 2007; Schmidt et al., 2008; Schmidt and Kofuji, 2009) but apparently also in several comprehensive surveys of murine ganglion cells (Badea and Nathans, 2004; Coombs et al., 2006; Kong et al., 2005; Sun et al., 2002), as summarized in Table 1. Specifically, our M2 cells resemble the RGC3 cell of Sun et al. (2002), the Cluster 8 ganglion cells of Kong et al. (2005), the M6-on cells of Coombs et al. (2006) and some cells of Cluster 9 of Badea et al. (2004; their Fig. 17.E.a). All of these cell types are characterized by somas of intermediate size and dendritic arbors that are large, radiate, relatively sparsely branched and monostratified in the inner (ON) sublamina of the IPL. The correspondence is not complete (note for example the smaller mean field size of Badea et al.’s cluster 9 cells). Further study will be required to determine if these discrepancies are attributable to technical differences among these studies or because M2 cells represent only a subset of the ganglion cells included within these cell types.
Table 1.
Possible equivalence of M2 cells to other mouse RGC types
| Study | Type | IPL depth % |
Field diam. (mean; µm) |
Soma diam. (µm) |
|---|---|---|---|---|
| this paper | M2 | 67–83 | 314 | 14.8 |
| Schmidt & Kofuji, 2009 | M2 | ____ | 423 | 21.8 |
| Viney et al., 2007 | Type 2 | 70 | 230 | ____ |
| Sun et al. 2002 | RGC3 | 68 | 296 | 15 |
| Badea & Nathans 2004 | Cluster 9 | ~75–80 | 200 | ____ |
| Kong et al. 2005 | Cluster 8 | 80 | 300 | ____ |
| Coombs et al. 2006 | M6-on | 92–97 | 300–415 | 14–17 |
Many mammalian ganglion cell types can be grouped into paramorphic pairs, types more closely related to one another than to any other type and differing from one another mainly by their level of stratification in the IPL. One member of the pair stratifies in the inner sublayer of the IPL, and has an ON center receptive field, while the other terminates in the outer sublayer and has an OFF center receptive field. Examples include the ON and OFF midget (P) and parasol (M) types in primate retina and the ON and OFF alpha (Y) and beta (X) types in cats. It is natural to ask, therefore, whether M2 cells might be considered a paramorphic pair of the M1 cells. The subtle differences in the somadendritic silhouette (soma size, branching structure, dendritic field size) might be offered as evidence against this idea, but similar differences have been noted between ON and OFF alpha cells, so this is hardly determinative. There are at least two stronger reasons to question such a characterization. First, available evidence suggests that these two cell types may have distinct patterns of central projection (Baver et al., 2008; J. Ecker, O.Dumitrescu, K. Wong, D. Berson and S. Hattar, unpublished observations; F. Dunn, M. Takao and D. Berson, unpublished observations), whereas this is not generally true for known paramorphic pairs. Second, these two types are apparently both ON center, rather than having the complementary ON and OFF center receptive organization expected of paramorphic pairs. The intrinsic photoresponse is depolarizing (ON) for both types (Berson et al., 2002; Schmidt and Kofuji, 2009; Schmidt et al., 2008; Warren et al., 2003). For M1 cells, the synaptically mediated drive from rods and cones is also of the ON type (Perez-Leon et al., 2006; Wong et al., 2007), paradoxically so since their dendrites arborize in the OFF sublayer. M2 cells also seem virtually certain to receive a synaptically mediated ON response, although corroboration on this point is currently indirect; all ipRGCs tested exhibited ON synaptic inputs in both Wong et al. (2007) and Schmidt et al. (2008), and though some of these recordings were presumably obtained from M2 cells, this was not directly confirmed in either study. A dominant ON synaptic input to M2 cells would hardly be surprising given their dendritic stratification in the ON sublayer of the IPL and direct evidence that M2-like inner-stratifying cells in macaque monkeys receive such an input (Dacey et al., 2005).
M2-like melanopsin expressing ganglion cells may be a conserved feature of mammalian retinal organization. Such cells have certainly been seen in human and non-human primates (Dacey et al., 2005; Jusuf et al., 2007). Paralleling our results in mice, M2-like cells in humans and macaque monkeys appear to have slightly larger somata and dendritic fields than M1-like cells (Dacey et al., 2005; Jusuf et al., 2007), although this seems not to be true in marmosets (Jusuf et al., 2007). Studies in a variety of other mammals have made no mention of M2-like melanopsin immunoreactive cells nor of a second, inner plexus of melanopsin-immunopositive dendrites, but these negative results should not be considered decisive. Even in the mouse, the M2 cells and their dendritic plexus are more difficult to visualize immunohistochemically than those of M1 cells. This is apparent from the weaker staining of M2 than M1 cells when using the N-terminal antiserum we employed here (UF006), and by the fact that detectable staining is lost in the M2 plexus before that in the M1 plexus when the antibody’s efficacy is compromised by subsaturating preadsorption with the immunizing peptide (Panda et al., 2003). Similarly, Baver et al. (2008) found that a C-terminal antibody, which is generally less sensitive than the N-terminal antibody used here, failed to stain the M2 cells, while effectively staining M1 cells. Finally, in a genetically modified mice in which reporter proteins are driven by the melanopsin promoter, M1 cells are more strongly labeled by the reporters (Baver et al., 2008; Hattar et al., 2006; Schmidt and Kofuji, 2009). Thus, the failure to detect M2-like cells in other species may simply reflect their lower level of expression of melanopsin protein as compared with M1-like cells. Indeed, a suggestion of an inner (M2-like) plexus of melanopsin immunoreactive dendrites is discernable in one published image from rat retina (Fahrenkrug et al., 2004) (their Fig. 2) and we have confirmed this using a commercially available melanopsin antibody (ABR PA1-781; F. Pucci and D. Berson, unpublished observations). It will be of interest to reexamine this question with more sensitive antibodies and immunohistochemical protocols or by other techniques.
As noted above, there is little doubt that, in mice, the concentration of melanopsin protein in the plasma membrane is substantially lower in M2 cells than in M1 cells. To the extent that photopigment density limits the sensitivity of the ipRGCs (Do et al., 2009), one would predict consistently lower thresholds for M1 than M2 cells, and this has recently been confirmed (Schmidt and Kofuji, 2009). This distinction may account for heterogeneity in the sensitivity of intrinsic light responses recorded in mouse ipRGCs by Tu et al. (2005). In a multielectrode array survey of intrinsic light-evoked spiking, they identified two populations of cells distinguishable, in part, by a 0.5–1 log unit difference in sensitivity (Tu et al., 2005). A reasonable hypothesis is that M1 cells correspond to the more sensitive Type III cells reported by Tu and colleagues, while the M2 cells correspond to the less sensitive Type II cells. The multielectrode technique does not permit morphological identification of the recorded cells, so explicit linkage of the functional types with the M1 and M2 cells remains to be resolved by other approaches. The distinctions between M1 and M2 cells may also underlie the heterogeneity among ipRGCs studied in calcium imaging experiments (Sekaran et al., 2003).
Bistratified cells
A number of earlier reports have reported bistratified cells among the ganglion cells that innervate the suprachiasmatic nucleus, are intrinsically photosensitive, and/or express melanopsin (Schmidt et al., 2008; Viney et al., 2007; Warren et al., 2003). We confirm the presence of bistratified cells among the melanopsin-expressing cells of the mouse retina, but find that they are far less common than observed in two recent reports. Viney et al. (2007) reported that bistratified cells (their Type 3 cells) comprised more than 20% of all melanopsin expressing cells, while Schmidt et al. (2008) reported an even higher incidence (26%), outnumbering even their Type I cells (i.e., M1 cells). We find that fewer than 10% of melanopsin immunopositive ganglion cells are truly bistratified, in the sense that their terminal dendrites occupy both the inner and outer melanopsin plexuses. It is unclear from the brief descriptions in the other two papers whether such a stringent criterion was applied. The only bistratified cell illustrated by Schmidt et al. (their Fig. 7C) would almost certainly meet this criterion, but it is not clear that the one illustrated by Viney et al. would do so (their Fig. 1F, bottom). It seems likely, therefore, that the differing estimates of the incidence of bistratified cells among these studies is a function of differing criteria and, in particular, whether conventionally placed M1 cells with proximal dendrites running for longer than usual within the ON sublayer are included within this group. Alternatively, methodological differences among the studies may have led to oversampling of one or more types. In this sense, the approach we have used, which permits exhaustive characterization of all immunopositive cells in a patch of retina, is probably less subject to sampling bias than the approaches used in the earlier studies. It is of interest in this context that in the marmoset, no truly bistratified cells were observed, although both the M1-like and M2-like types were occasionally seen to send a process to the ‘wrong’ plexus (Jusuf et al., 2007).
A key issue is whether bistratified cells are abundant enough to tile the retina, widely considered a core criterion for a bona fide ganglion cell type.. By our analysis in the mouse retina they are not. The expectation that a ganglion cell type should tile the retina emerged from studies of spatial sampling by ganglion cells implicated in mechanisms of pattern vision (Peichl, 1991). Extending this criterion to ganglion cells acting as irradiance detectors for non-image-forming visual networks can certainly be challenged on theoretical grounds because sparse and incomplete sampling might suffice to support such functions. However, the empirical evidence suggests otherwise. M1 and M2 cells each fully cover the retina with their dendritic fields, despite their well established roles in irradiance detection and non-image-forming functions. Of course, bistratified cells (or, for that matter, displaced M1 cells) may prove to be different from other melanopsin ganglion cells in functional properties or patterns of projection. If so, such specializations could promote their acceptance as distinct types and undermine the view that tiling is a universal feature of ganglion-cell types. At present, however, because almost nothing is known about the functional behavior or outputs of these cells, we believe it is premature to assign them a unique taxonomic status.
Other cells
We detected very weak melanopsin immunostaining in an additional population ganglion cells. We do not believe that these cells are simply weakly staining M1 or M2 cells for two reasons. First, their mean equivalent soma diameter is statistically distinct from that of M1 or M2 cells and many are larger than any M1 or M2 cell (Fig 3A). Furthermore, the dendritic staining in these cells was virtually nonexistent in our material, whereas in M1 and M2 cells it was strong enough to fully reconstruct dendritic profiles. These patterns of dendritic staining intensity were qualitatively distinct and readily discriminated.
These “other” cells may correspond to some of the cells that have been shown to express GFP under the control of the melanopsin promoter but to lack detectable melanopsin-like immunofluorescence (Hatori et al., 2008; J. Ecker, O. Dumitrescu, K. Wong, D. Berson and S. Hattar, unpublished observations).
Some large ON alpha-like cells in mice and rats are capable of generating weak intrinsic light responses under synaptic blockade (O. Dumitrescu and D. Berson, unpublished observations). Thus, even if displaced M1 cells and bistratified melanopsin cells are discounted as true types, there may be at least three types of melanopsin expressing ipRGCs in mice: M1 cells, M2 cells, and these large alpha-like cells.
It is likely that these morphologically distinct melanopsin-expressing retinal ganglion cells support functionally distinct roles. M1 and M2 cells differ physiologically (Schmidt and Kofuji, 2009) and are likely to project to different targets in the brain (Hattar et al., 2006; Baver et al., 2008). Progress on these issues may come from double labeling studies combining retrograde axon tracing and immunohistochemistry or genetic models with selective labeling of single subtypes of melanopsin ganglion cells.
Supplementary Material
Acknowledgments
Supported by National Institutes of Health grants EY12793 and EY17137 (to DB) and NS052112 (to IP), and by FAPESP and CNpq (Brazil) grants 06/03381-3 and 473658/2008-9 (to AMC).
LITERATURE CITED
- Badea TC, Nathans J. Quantitative analysis of neuronal morphologies in the mouse retina visualized by using a genetically directed reporter. J Comp Neurol. 2004;480(4):331–351. doi: 10.1002/cne.20304. [DOI] [PubMed] [Google Scholar]
- Balasubramanian V, Sterling P. Receptive fields and functional architecture in the retina. J Physiol. 2009;587(Pt 12):2753–2767. doi: 10.1113/jphysiol.2009.170704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baver SB, Pickard GE, Sollars PJ. Two types of melanopsin retinal ganglion cell differentially innervate the hypothalamic suprachiasmatic nucleus and the olivary pretectal nucleus. Eur J Neurosci. 2008;27(7):1763–1770. doi: 10.1111/j.1460-9568.2008.06149.x. [DOI] [PubMed] [Google Scholar]
- Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295(5557):1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- Coombs J, van der List D, Wang GY, Chalupa LM. Morphological properties of mouse retinal ganglion cells. Neuroscience. 2006;140(1):123–136. doi: 10.1016/j.neuroscience.2006.02.079. [DOI] [PubMed] [Google Scholar]
- Dacey DM, Liao HW, Peterson BB, Robinson FR, Smith VC, Pokorny J, Yau KW, Gamlin PD. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature. 2005;433(7027):749–754. doi: 10.1038/nature03387. [DOI] [PubMed] [Google Scholar]
- Do MT, Kang SH, Xue T, Zhong H, Liao HW, Bergles DE, Yau KW. Photon capture and signalling by melanopsin retinal ganglion cells. Nature. 2009;457(7227):281–287. doi: 10.1038/nature07682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahrenkrug J, Nielsen HS, Hannibal J. Expression of melanopsin during development of the rat retina. Neuroreport. 2004;15(5):781–784. doi: 10.1097/00001756-200404090-00008. [DOI] [PubMed] [Google Scholar]
- Fu Y, Zhong H, Wang MH, Luo DG, Liao HW, Maeda H, Hattar S, Frishman LJ, Yau KW. Intrinsically photosensitive retinal ganglion cells detect light with a vitamin A-based photopigment, melanopsin. Proc Natl Acad Sci U S A. 2005;102(29):10339–10344. doi: 10.1073/pnas.0501866102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamlin PD, McDougal DH, Pokorny J, Smith VC, Yau KW, Dacey DM. Human and macaque pupil responses driven by melanopsin-containing retinal ganglion cells. Vision Res. 2007;47(7):946–954. doi: 10.1016/j.visres.2006.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Menendez I, Contreras F, Cernuda-Cernuda R, Garcia-Fernandez JM. Daily rhythm of melanopsin-expressing cells in the mouse retina. Front Cell Neurosci. 2009;3:3. doi: 10.3389/neuro.03.003.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooley JJ, Lu J, Fischer D, Saper CB. A broad role for melanopsin in nonvisual photoreception. J Neurosci. 2003;23(18):7093–7106. doi: 10.1523/JNEUROSCI.23-18-07093.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham DM, Wong KY, Shapiro P, Frederick C, Pattabiraman K, Berson DM. Melanopsin ganglion cells use a membrane-associated rhabdomeric phototransduction cascade. J Neurophysiol. 2008;99(5):2522–2532. doi: 10.1152/jn.01066.2007. [DOI] [PubMed] [Google Scholar]
- Hannibal J, Fahrenkrug J. Melanopsin containing retinal ganglion cells are light responsive from birth. Neuroreport. 2004;15(15):2317–2320. doi: 10.1097/00001756-200410250-00003. [DOI] [PubMed] [Google Scholar]
- Hannibal J, Hindersson P, Ostergaard J, Georg B, Heegaard S, Larsen PJ, Fahrenkrug J. Melanopsin is expressed in PACAP-containing retinal ganglion cells of the human retinohypothalamic tract. Invest Ophthalmol Vis Sci. 2004;45(11):4202–4209. doi: 10.1167/iovs.04-0313. [DOI] [PubMed] [Google Scholar]
- Hartwick AT, Bramley JR, Yu J, Stevens KT, Allen CN, Baldridge WH, Sollars PJ, Pickard GE. Light-evoked calcium responses of isolated melanopsin-expressing retinal ganglion cells. J Neurosci. 2007;27(49):13468–13480. doi: 10.1523/JNEUROSCI.3626-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatori M, Le H, Vollmers C, Keding SR, Tanaka N, Buch T, Waisman A, Schmedt C, Jegla T, Panda S. Inducible ablation of melanopsin-expressing retinal ganglion cells reveals their central role in non-image forming visual responses. PLoS ONE. 2008;3(6):e2451. doi: 10.1371/journal.pone.0002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattar S, Kumar M, Park A, Tong P, Tung J, Yau KW, Berson DM. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J Comp Neurol. 2006;497(3):326–349. doi: 10.1002/cne.20970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science. 2002;295(5557):1065–1070. doi: 10.1126/science.1069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jusuf PR, Lee SC, Hannibal J, Grunert U. Characterization and synaptic connectivity of melanopsin-containing ganglion cells in the primate retina. Eur J Neurosci. 2007;26(10):2906–2921. doi: 10.1111/j.1460-9568.2007.05924.x. [DOI] [PubMed] [Google Scholar]
- Kong JH, Fish DR, Rockhill RL, Masland RH. Diversity of ganglion cells in the mouse retina: unsupervised morphological classification and its limits. J Comp Neurol. 2005;489(3):293–310. doi: 10.1002/cne.20631. [DOI] [PubMed] [Google Scholar]
- Lucas RJ, Hattar S, Takao M, Berson DM, Foster RG, Yau KW. Diminished pupillary light reflex at high irradiances in melanopsin-knockout mice. Science. 2003;299(5604):245–247. doi: 10.1126/science.1077293. [DOI] [PubMed] [Google Scholar]
- Lyubarsky AL, Pugh EN., Jr Recovery phase of the murine rod photoresponse reconstructed from electroretinographic recordings. J Neurosci. 1996;16(2):563–571. doi: 10.1523/JNEUROSCI.16-02-00563.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin LP, Blanchard JH, Provencio I. Retinal ganglion cell projections to the hamster suprachiasmatic nucleus, intergeniculate leaflet, and visual midbrain: bifurcation and melanopsin immunoreactivity. J Comp Neurol. 2003;465(3):401–416. doi: 10.1002/cne.10881. [DOI] [PubMed] [Google Scholar]
- Panda S, Provencio I, Tu DC, Pires SS, Rollag MD, Castrucci AM, Pletcher MT, Sato TK, Wiltshire T, Andahazy M, Kay SA, Van Gelder RN, Hogenesch JB. Melanopsin is required for non-image-forming photic responses in blind mice. Science. 2003;301(5632):525–527. doi: 10.1126/science.1086179. [DOI] [PubMed] [Google Scholar]
- Panda S, Sato TK, Castrucci AM, Rollag MD, DeGrip WJ, Hogenesch JB, Provencio I, Kay SA. Melanopsin (Opn4) requirement for normal light-induced circadian phase shifting. Science. 2002;298(5601):2213–2216. doi: 10.1126/science.1076848. [DOI] [PubMed] [Google Scholar]
- Peichl L. Alpha ganglion cells in mammalian retinae: common properties, species differences, and some comments on other ganglion cells. Vis Neurosci. 1991;7(1–2):155–169. doi: 10.1017/s0952523800011020. [DOI] [PubMed] [Google Scholar]
- Provencio I, Rodriguez IR, Jiang G, Hayes WP, Moreira EF, Rollag MD. A novel human opsin in the inner retina. J Neurosci. 2000;20(2):600–605. doi: 10.1523/JNEUROSCI.20-02-00600.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provencio I, Rollag MD, Castrucci AM. Photoreceptive net in the mammalian retina. This mesh of cells may explain how some blind mice can still tell day from night. Nature. 2002;415(6871):493. doi: 10.1038/415493a. [DOI] [PubMed] [Google Scholar]
- Pu M. Dendritic morphology of cat retinal ganglion cells projecting to suprachiasmatic nucleus. J Comp Neurol. 1999;414(2):267–274. [PubMed] [Google Scholar]
- Rollag MD, Berson DM, Provencio I. Melanopsin, ganglion-cell photoreceptors, and mammalian photoentrainment. J Biol Rhythms. 2003;18(3):227–234. doi: 10.1177/0748730403018003005. [DOI] [PubMed] [Google Scholar]
- Ruby NF, Brennan TJ, Xie X, Cao V, Franken P, Heller HC, O'Hara BF. Role of melanopsin in circadian responses to light. Science. 2002;298(5601):2211–2213. doi: 10.1126/science.1076701. [DOI] [PubMed] [Google Scholar]
- Schmidt TM, Kofuji P. Functional and morphological differences among intrinsically photosensitive retinal ganglion cells. J Neurosci. 2009;29(2):476–482. doi: 10.1523/JNEUROSCI.4117-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt TM, Taniguchi K, Kofuji P. Intrinsic and extrinsic light responses in melanopsin-expressing ganglion cells during mouse development. J Neurophysiol. 2008;100(1):371–384. doi: 10.1152/jn.00062.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmucker C, Schaeffel F. A paraxial schematic eye model for the growing C57BL/6 mouse. Vision Res. 2004;44(16):1857–1867. doi: 10.1016/j.visres.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Sekaran S, Foster RG, Lucas RJ, Hankins MW. Calcium imaging reveals a network of intrinsically light-sensitive inner-retinal neurons. Curr Biol. 2003;13(15):1290–1298. doi: 10.1016/s0960-9822(03)00510-4. [DOI] [PubMed] [Google Scholar]
- Sholl DA. Dendritic organization in the neurons of the visual and motor cortices of the cat. J Anat. 1953;87(4):387–406. [PMC free article] [PubMed] [Google Scholar]
- Sollars PJ, Smeraski CA, Kaufman JD, Ogilvie MD, Provencio I, Pickard GE. Melanopsin and non-melanopsin expressing retinal ganglion cells innervate the hypothalamic suprachiasmatic nucleus. Vis Neurosci. 2003;20(6):601–610. doi: 10.1017/s0952523803206027. [DOI] [PubMed] [Google Scholar]
- Sun W, Li N, He S. Large-scale morphological survey of mouse retinal ganglion cells. J Comp Neurol. 2002;451(2):115–126. doi: 10.1002/cne.10323. [DOI] [PubMed] [Google Scholar]
- Tu DC, Zhang D, Demas J, Slutsky EB, Provencio I, Holy TE, Van Gelder RN. Physiologic diversity and development of intrinsically photosensitive retinal ganglion cells. Neuron. 2005;48(6):987–999. doi: 10.1016/j.neuron.2005.09.031. [DOI] [PubMed] [Google Scholar]
- Viney TJ, Balint K, Hillier D, Siegert S, Boldogkoi Z, Enquist LW, Meister M, Cepko CL, Roska B. Local retinal circuits of melanopsin-containing ganglion cells identified by transsynaptic viral tracing. Curr Biol. 2007;17(11):981–988. doi: 10.1016/j.cub.2007.04.058. [DOI] [PubMed] [Google Scholar]
- Warren EJ, Allen CN, Brown RL, Robinson DW. Intrinsic light responses of retinal ganglion cells projecting to the circadian system. Eur J Neurosci. 2003;17(9):1727–1735. doi: 10.1046/j.1460-9568.2003.02594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wässle H. Parallel processing in the mammalian retina. Nat Rev Neurosci. 2004;5(10):747–757. doi: 10.1038/nrn1497. [DOI] [PubMed] [Google Scholar]
- Williams RW, Goldowitz D. Structure of clonal and polyclonal cell arrays in chimeric mouse retina. Proc Natl Acad Sci U S A. 1992;89(4):1184–1188. doi: 10.1073/pnas.89.4.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.