Abstract
Microvascular barrier dysfunction is implicated in the initiation and progression of inflammation, posttraumatic complications, sepsis, ischaemia–reperfusion injury, atherosclerosis, and diabetes. Under physiological conditions, a precise equilibrium between endothelial cell–cell adhesion and actin–myosin-based centripetal tension tightly controls the semi-permeability of microvascular barriers. Myosin light chain kinase (MLCK) plays an important role in maintaining the equilibrium by phosphorylating myosin light chain (MLC), thereby inducing actomyosin contractility and weakening endothelial cell–cell adhesion. MLCK is activated by numerous physiological factors and inflammatory or angiogenic mediators, causing vascular hyperpermeability. In this review, we discuss experimental evidence supporting the crucial role of MLCK in the hyperpermeability response to key cell signalling events during inflammation. At the cellular level, in vitro studies of cultured endothelial monolayers treated with MLCK inhibitors or transfected with specific inhibiting peptides have demonstrated that induction of endothelial MLCK activity is necessary for hyperpermeability. Ex vivo studies of live microvessels, enabled by development of the isolated, perfused venule method, support the importance of MLCK in endothelial permeability regulation in an environment that more closely resembles in vivo tissues. Finally, the role of MLCK in vascular hyperpermeability has been confirmed with in vivo studies of animal disease models and the use of transgenic MLCK210 knockout mice. These approaches provide a more complete view of the role of MLCK in vascular barrier dysfunction.
Keywords: MLCK, MLC, Contractile cytoskeleton, Endothelial barrier function, Microvascular permeability
1. Introduction
Myosin light chain kinases (MLCK) are a family of soluble protein kinases that function principally to phosphorylate the 20 kDa regulatory myosin light chain (MLC-2) and thereby induce ATPase driven actin–myosin contraction.1,2 In most cells, MLCK is a transducer for signalling MLC phosphorylation in response to Ca2+ binding to MLCK-associated calmodulin. MLCK-mediated MLC phosphorylation and actomyosin contractility is important in muscle contraction, cell migration, and endo/exocytic processes, and is recognized for its central role in signalling endothelial cell–cell adhesion and barrier function. In this review, we discuss the molecular physiology of MLCK, and the biochemical basis for actin–myosin contraction in the context of vascular endothelial permeability. We use an experimental approach that incorporates molecular information from cultured endothelial cell monolayers into physiological responses in intact microvessels, ex vivo and in vivo, providing a more complete understanding of the control of endothelial permeability by MLCK in pathophysiological conditions.
2. MLCK structure and molecular physiology
MLCK contains a C2 immunoglobin domain that binds to unphosphorylated MLC, a catalytic site for kinase activity,3 and a calmodulin-binding regulatory domain that functions as an autoinhibitory domain to suppress constitutive activity in the absence of calmodulin.4,5 By way of binding to calmodulin, Ca2+ is an essential regulator of MLCK activity. While the structures required for MLCK activity and binding to MLC are conserved across species and tissue types,6 there are differences in the components required for regulation of MLCK variants in different cell types. For example, in smooth muscle cells, Ca2+ binding to calmodulin is sufficient to activate MLCK and induce actin–myosin contraction. In other cell types, Ca2+ binding to calmodulin is necessary for MLCK activity, but alone is not sufficient to elicit an actin–myosin contractile response.7 Differences in regulation are pronounced in muscle vs. non-muscle MLCK.
2.1. Muscle MLCK isoforms
There are three types of muscle MLCK: skeletal (skMLCK), cardiac (cMLCK), and smooth muscle (smMLCK), products of genes mylk2, mylk3, and mylk1, respectively.1,8,9 MLCK is activated in response to Ca2+ released from intracellular stores. Ca2+ binding to calmodulin induces MLCK to phosphorylate MLC on serine 19, which increases the actomyosin contractile response. In skeletal or cardiac myocytes, Ca2+-binding troponin triggers actomyosin contraction, and MLCK activation increases contractile strength.1,10 In smooth muscle, smMLCK induces actin–myosin contraction. The smMLCK isoform differs markedly in structure from skMLCK or cMLCK, having greater similarity to non-muscle MLCK (discussed below).11 In contrast to skMLCK or cMLCK, smMLCK bears an additional C-terminal insert, and a long N-terminus that contains a fibronectin domain, two immunoglobin domains, and numerous putative phosphorylation sites.1,6,10 The smMLCK has 1147 amino acids 12 and is also called MLCK108 based on its predicted molecular weight of 108 kDa. MLCK108 shows apparent weights ranging 125–155 kDa,4 compared with 77–103 kDa for skMLCK.13
2.2. Non-muscle MLCK isoforms
Earlier work by Garcia's group has identified and cloned a single gene of human non-muscle MLCK (nmMLCK) that encodes four high molecular weight MLCK isoforms (MLCK1-4).6 Non-muscle MLCK differs from smMLCK mainly in that nmMLCK contains an additional stretch of 922 amino acids spliced into the N-terminus, with multiple sites for protein–protein interactions including p60Src-mediated tyrosine phosphorylation.14,15 All nmMLCK isoforms are splice variants derived from the mylk1 gene on human chromosome 3 (3qcen-q21).9,16,17 MLCK1, previously known as endothelial MLCK (eMLCK), was originally cloned from human endothelial cells,7 and believed to have a predominantly endothelial tissue distribution. Recent evidence shows that MLCK1 is also expressed in other tissues including gut epithelium, and more recently was observed in neutrophils. MLCK isoforms 1 and 2 are the most highly expressed in endothelium.6 MLCK1 is the highest molecular weight MLCK variant with 1914 amino acids (predicted 210 kDa), also known as MLCK210.6 MLCK2 is identical to MLCK1, but lacks a stretch of 69-amino acid containing two tyrosine residues necessary for phosphorylation and activation of MLCK1 by p60Src.6,14 MLCK 3a and 3b are identical to MLCK1 and 2, respectively, but bearing an additional distal deletion (exon 30) corresponding to a stretch of 51 amino acids. The complete sequence information for MLCK4 is not yet known, but has similar tissue distribution to other nmMLCK isoforms: human lung, liver, brain, and kidney tissues, as well as endothelial cells. The remainder of this review is dedicated to the role of MLCK in regulating permeability of vascular endothelial barriers.
3. Endothelial barriers
The microvascular endothelium consists of a layer of closely apposed endothelial cells, forming a semi-permeable barrier between blood and tissue to control exchange of fluids, electrolytes, and proteins.18 The integrity of this barrier is crucial in maintaining circulatory homeostasis and physiological organ function. Pathological aberration of endothelial barrier function leads to microvascular hyperpermeability and plasma extravagation, resulting in tissue oedema and organ dysfunction.18–21 The problem is associated with inflammatory disease, traumatic or thermal injury, diabetes mellitus, myocardial infarction, and tumorigenesis.21–24 Although transcellular transport of albumin does occur, it is now clear that leakage of fluid and macromolecules across the endothelial barrier during the aforementioned disease processes occurs in a largely paracellular fashion via cell–cell junctions.25–27
The cell–cell junctional structures of the vascular endothelium include tight junctions and adherens junctions. Tight junctions are detected primarily in blood-brain, blood-retinal, or blood-testis barrier microvasculature.26,28 Tight junctions are zipper-like structures formed of homophilic interactions between occludin, claudins, and junction adhesion molecule (JAM) A. In vivo, tight junctions exhibit high transendothelial electrical resistance (TER)29,30 and are exceptionally impermeant to the passage of solutes.31 Adherens junctions are found in nearly all vascular beds, especially in the peripheral microvasculature.25,26,32 Adherens junctions are mainly composed of homophilic interactions of the junctional adhesion protein VE-cadherin, as well as JAM A, B, and C.
Intracellularly, tight junction proteins are connected to actin filaments via zona occludens-1/2 (ZO-1/2); adherens junction proteins are connected via catenins (α, β, γ, and p120).32–35 By way of these connections, endothelial cytoskeletal contractile forces strongly influence cell–cell junctions and thus paracellular permeability. In addition, the vascular endothelium is anchored to extracellular matrix through focal adhesions, mediated by transmembrane integrins and actin-linking proteins, e.g. focal adhesion kinase, talin, paxillin, and vinculin.35,36 Cell–cell and cell–matrix adhesion structures act coordinately with cytoskeleton proteins to maintain the integrity of the endothelial barrier and a low basal permeability.33
4. Actomyosin contractile machinery
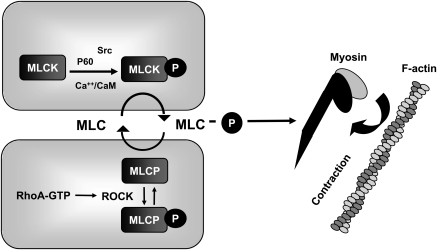
The contractile machinery of cells is driven by a mechanochemical interaction between actin and myosin.37 In vascular endothelial cells, the actin–myosin interaction is regulated by the phosphorylation status of MLC, and activation of MLCK is a key step in the development of actomyosin-based contractile forces.38,39 Upon activation by Ca2+/calmodulin or by tyrosine kinase-mediated phosphorylation at Tyr-464 and Tyr-471, MLCK phosphorylates MLC at Ser-19 and subsequently at Thr-18, resulting in a change in the myosin tertiary structure favouring contractile movement against actin (Figure 1).40–42 Opposing MLCK, myosin light chain phosphatase (MLCP) dephosphorylates MLC, decreasing tension and relaxing the cytoskeleton.43 Therefore, optimal control of the contractile status arises from the balance between MLCK and MLCP activity. RhoA, a member of Rho family small GTPases, plays a critical role in regulating MLCP activity. Once activated in a GTP-bound form, RhoA can activate its downstream Rho kinase (ROCK) that subsequently phosphorylates and inhibits MLCP, resulting in increased MLC phosphorylation and actomyosin contraction (Figure 1).32,44,45 There is also evidence that ROCK directly phosphorylates MLC in vitro,46 or increases endothelial permeability by inducing VE-cadherin phosphorylation, though the relative importance of these events is unclear.46,47 In many cases, endothelial paracellular permeability is controlled by MLCK-dependent processes; however, endothelial hyperpermeability can also occur through MLCK-independent mechanisms.48,49 This review is focused on control of MLCK activity and actomyosin contractility in endothelial hyperpermeability, which is central to the pathophysiology of vascular barrier dysfunction.
Figure 1.
Control of actin–myosin contraction in endothelium. Increased MLC phosphorylation in response to MLCK activation by Ca2+-calmodulin binding and src kinase activity, or to inhibition of MLCP by ROCK activation downstream of RhoA, increases MLC ATPase-driven force generation relative to actin.
5. MLCK in cultured endothelial cells
Endothelial cell monolayers are powerful tools for investigating molecular mechanisms that control cell junction structure and permeability. Many studies employ endothelial cells of macrovascular or arterial origin, such as human umbilical vein endothelial cells (HUVECs) or aortic endothelial cells. These are useful models for studying cell morphology or signalling; however, permeability responses in these cell lines may not resemble the microvascular exchange process in vivo, because these cells are derived from non-exchange vessels. Even cultured cells of microvascular origin do not retain all physiological barrier properties present in the microvasculature. Adamson et al. have pointed out that FITC-albumin leakage in intact microvessels occurs at less than 5% of endothelial cells,49 therefore cultured endothelial cells (especially clonal populations) may not retain the complete phenotype observed in intact microvessels. Despite this limitation, however, we have gained much information about the molecular mechanisms underlying the MLCK-dependent permeability response by studying cultured cells.
Our laboratory and others have shown that the phosphorylation status of MLC is critical in the barrier response to histamine, thrombin, oxygen radicals, and activated neutrophils.20,40,50–53 Also, the pattern of hyperpermeability response varies depending on agonists. Histamine exposure increases MLC phosphorylation and actomyosin contraction, manifest as rapid and transient (5 min) hyperpermeability in HUVEC monolayers.53–55 Similar effects are seen in endothelial cells from carotid artery and aorta, with hyperpermeability beginning within 10 min and lasting for up to 2 h.56 Thrombin also induces MLC phosphorylation, cellular contraction, and intercellular gap formation; however, in HUVEC monolayers, the hyperpermeability response to thrombin is sustained (up to 5.5 h) relative to the transient effect of histamine.55,57 This indicates either that different stimuli activate different signalling processes, or that different resolution mechanisms are involved. A pattern of response similar to that of thrombin is seen with the serine/threonine phosphatase inhibitor calyculin-A, suggesting that sustained hyperpermeability may be due to the inability to dephosphorylate MLC.40,57 Conversely, inhibition of serine/threonine MLC phosphorylation reduces the hyperpermeability elicited by these agonists.40,50,51,53
The necessity of activated MLCK in MLC phosphorylation and endothelial permeability was confirmed using a protein transference technique.58,59 Briefly, proteins or engineered peptides can be transfected directly into endothelial cells using the polyamine transfection reagent transIT, without apparent toxicity, producing protein transfection efficiencies of up to 90%.59,60 Furthermore, transIT transfection of endothelial cells with a protein kinase C (PKC)-specific inhibiting peptide dramatically reduced intracellular PKC activity to the same extent as application of the PKC inhibitor to cell lysates in vitro, illustrating the effectiveness of this method for perturbing specific elements of cell signalling in live cells. To determine that activated MLCK is sufficient to elicit endothelial hyperpermeability, we introduced purified, constitutively active MLCK protein into coronary venular endothelial cells (CVEC) as a polyamine-conjugated complex.58 Transfected MLCK significantly increased the phosphorylation level of MLC (∼60%), and was accompanied by an increase in transendothelial flux of albumin across the CVEC monolayer. Similar to the effect of calyculin-A on MLC, MLCK transfection exclusively induced the diphosphorylated form of MLC. This is significant in that Thr-18/Ser-19 diphosphorylation of MLC generates higher myosin ATPase activity than does monophosphorylation,61 and also speaks to the complexity of signalling to MLC. Fluorescent microscopy studies further revealed that increased MLCK activity led to widespread intracellular gap formation in the monolayer, loss of peripheral catenin, and contractile cytoarchitecture.58 All of these MLCK-mediated effects on MLC phosphorylation, endothelial cell morphology, and barrier function are abrogated by inhibition of MLCK. Thus, accumulating evidence indicates that abnormally activated MLCK is a major determinant in microvascular barrier dysfunction in response to many signalling mediators and in a variety of pathophysiological processes.
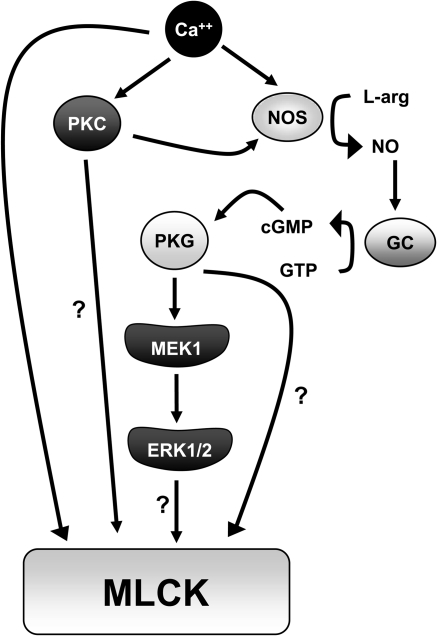
PKC activation and NO production are important for the MLCK-dependent vascular endothelial hyperpermeability responses to thrombin, histamine, and vascular endothelial growth factor (VEGF).62–65 There is significant controversy over the effects of nitric oxide (NO) on endothelial hyperpermeability, as some groups have reported barrier enhancing effects of NO and others have reported hyperpermeability-inducing effects of NO.66 The reasons for these differences are not clear, however may be due to heterogenous responses to different experimental conditions, differential expression of nitric oxide synthase (NOS) isoforms, or to cell type-specific effects of NO on a multitude of cellular processes including impaired Ca++ homeostasis, or interaction with reactive oxygen species.67–70 In general, activation of endothelial nitric oxide synthase (eNOS) and NO production induce vascular endothelial permeability through activation of guanylate cyclase (GC), production of cyclic guanosine 3′,5′-cyclic monophosphate (cGMP), and activation of protein kinase G (PKG) (Figure 2).20,21,60,71 In addition, the hyperpermeability response to these agents or to NO donors is prevented by MLCK inhibitors, indicating that NO-cGMP signalling lies upstream of MLCK activation. Also, NO may activate MLCK by elevating intracellular calcium levels.70 In HUVEC cells, treatment with VEGF causes activation of NO, PKG and subsequent activation of Raf-1, mitogen-activated protein kinases, and extracellular regulated kinase (ERK1/2).72,73 This suggests that NO- and PKG-dependent activation of MLCK is signalled through MAP/ERK (MEK) kinases (Figure 2). PKC is also an important signalling molecule in endothelial hyperpermeability responses. Treatment of bovine pulmonary artery endothelial monolayers with the phorbol ester 12-phorbol,13-myristate acetate (PMA) causes a dose-dependent increase in PKC activation accompanied by increased transendothelial albumin flux.63 In HUVEC monolayers, PKC activation worked synergistically with elevated intracellular Ca2+ to increase MLC phosphorylation and hyperpermeability,40 demonstrating that MLCK-dependent and -independent signalling pathways contribute to MLC phosphorylation and hyperpermeability. Furthermore, both pathways may be modulated by PKC.
Figure 2.
MLCK activation in endothelial hyperpermeability. Endothelial MLCK is activated in response to multiple cell signalling events including elevated intracellular Ca++, protein kinase C (PKC) activation, and signalling through nitric oxide synthase (NOS)- dependent production of NO, and guanylate cyclase (GC)-dependent production of cGMP. Activation of cGMP-dependent protein kinase (PKG) activates MLCK through activation of MEK1 and ERK1/2. Possible direct activation by PKC, PKG, or ERK1/2 is represented by question marks.
Endothelial hyperpermeability may also mediated by MLCK-dependent activation of stores operated Ca++ (SOC) channels.74,75 In pulmonary artery or brain capillary endothelial cells, MLCK activates stores-operated membrane transient receptor potential (TRPC) Ca++ channels, thereby increasing junctional permeability.76,77 Application of the MLCK inhibitor ML-9 prevents activation of TRPC and vascular leakage.76,78 This suggests that activation of TRPC channels depends upon MLCK activity, and that MLCK-dependent endothelial hyperpermeability depends upon Ca++ entry following MLC phosphorylation. On the other hand, activation of MLCK and subsequent MLC phosphorylation is also dependent upon intracellular Ca++.79
The neutrophil is an important inducer of endothelial hyperpermeability.80,81 Although the signalling events involved in neutrophil-endothelial cell interaction have been extensively studied, the molecular mechanisms by which neutrophils cause microvascular leakage have not been fully established. Conventional theories in this area emphasize neutrophil migration-mediated mechanical disruption of endothelium, which is dependent on proteases released from neutrophils 82–84 during transendothelial migration via paracellular and/or transcellular routes.85 Fluorescence microscopy images of transcellular diapedesis indicate that transendothelial pore formation only occurs at surfaces in close contact with invading leucocytes, suggesting that leucocyte transcellular migration is not accompanied by serum leakage.86,87 In addition, convincing ultrastructural evidence by Lewis and Granger88 demonstrates paracellular neutrophil transmigration across microvessel endothelium in the absence of serum protein extravasation. Therefore, neutrophil transmigration may not necessarily cause hyperpermeability.
Neutrophil adhesion to vascular endothelial cells (CVEC or HUVEC) induces tyrosine phosphorylation of adherens junction proteins, and increases cytosolic Ca2+-dependent opening of intercellular junctions, and endothelial hyperpermeability.89,90 Because Ca2+-calmodulin activates MLCK,10,33 we investigated MLCK-mediated MLC phosphorylation and the contractile cytoskeleton in microvascular endothelial cells in response to activated neutrophils. In CVEC monolayers, exposure to C5a-activated neutrophils induced MLCK-dependent transendothelial albumin flux.91 Activated neutrophils induced a concentration- and time-dependent phosphorylation of MLC at Thr-18/Ser-19 shown on urea gels, which was significantly abrogated by pre-treatment with ML-7 or by transference of an MLCK-inhibiting peptide.91–93 Further, exposure of endothelial cells to activated neutrophils resulted in increased contractile stress fibre formation and intercellular gaps as shown by immunocytochemical staining. Both the neutrophil-induced MLC phosphorylation and stress fibre formation are substantially attenuated through inhibition of MLCK with ML-7.91 This suggests that inhibiting MLCK-mediated MLC phosphorylation improves vascular barrier function during inflammatory injury. We have seen that increased isometric force is generated in confluent CVEC monolayers in the presence of activated neutrophils that closely parallels decreased TER, and that both effects are abrogated by ROCK inhibitors.93 We also found that activated neutrophils potentiate the hyperpermeability effect caused by MLCK transference.91 Therefore, mechanisms in addition to MLCK activation may be involved in neutrophil-induced microvascular barrier dysfunction, including RhoA-ROCK-mediated MLCP inhibition (Figure 2).18,35
6. MLCK in isolated, intact microvessels
In order to interrogate the molecular mechanisms of endothelial permeability in exchange microvessels under physiologically relevant conditions, we developed an isolated and perfused venule model.94 Briefly, this model entails dissection of postcapillary venules 20–50 μm in diameter from living tissues. The vessel is cannulated with a pipette-in-pipette system and is perfused with a physiological salt solution containing fluorescently labelled albumin under a selected perfusion pressure and flow rate. The apparent permeability coefficient of albumin (Pa) is determined based on the ratio of albumin flux to its transmural concentration difference.95 This approach enables quantitative assessment of the permeability of intact microvascular endothelium in its native environment, where physical forces and chemical conditions are tightly controlled, and extrinsic confounding factors are eliminated.94 Other applications include direct observation and real-time quantification of neutrophil–endothelium interactions. This model has advantages over cultured endothelial cells in that microvessels isolated from living tissues behave with permeability characteristics that more closely resemble in vivo systems. On the other hand, in vivo studies do not provide specific information needed to understand the molecular bases for physiological phenomena. The isolated microvessel preparation is better suited to address mechanistic questions about vascular endothelium-specific processes because there is limited interference from other cell types or systemic factors as would occur in vivo.
Using the isolated microvessel technique, we have examined the effects of MLCK activity on endothelial barrier function in porcine coronary venules as well as microvessels from rodent skeletal muscle and mesentery.51 An interesting finding is that the MLCK inhibitor ML-7 significantly reduced basal permeability to FITC-albumin. The inhibitory effect of ML-7 was dose-dependent and persisted in the presence of this inhibitor. In contrast, treatment with calyculin-A increased MLC phosphorylation and significantly increased the basal vascular permeability.51,58 We postulate that MLCK-mediated actomyosin activity plays a role in maintaining basal barrier function in intact microvascular endothelium.
We have previously shown that NO production and cGMP mediate shear stress- and agonist-induced hyperpermeability responses.94,96,97 In isolated venules, increasing intraluminal flow velocity induces hyperpermeability, which is abrogated by the NOS inhibitor NG-monomethyl-l-arginine (L-NMMA) or mimicked by the NO precursor l-arginine.94 In a similar fashion, histamine increases coronary venular permeability through a phospholipase C (PLC)-NOS-cGMP signalling cascade (Figure 2).96 VEGF also increases microvascular permeability via increased NO synthesis and subsequent PKG activation.97 VEGF binding to its membrane receptor KDR initiates PLC-mediated cytosolic Ca2+ elevation and PKC activation, activating eNOS and inducing venular hyperpermeability.62 In as much as the cytoskeleton is a ubiquitous structural end point for intracellular signalling events, this indicates the possibility that MLCK activity is downstream of the NO-cGMP cascade. Using isolated microvessels, we have demonstrated that activated MLCK is a critical mediator of NO- or cGMP-induced microvascular hyperpermeability.51 Venule hyperpermeability in response to the NO donor sodium nitroprusside (SNP), or the PKG activator 8-bromoguanosine 3′,5′-cyclic monophosphate (8Br-cGMP) is substantially attenuated by MLCK inhibition. It is not clear how PKG activates MLCK, however, in isolated coronary venules, VEGF-, histamine-, SNP-, or 8Br-cGMP-induced hyperpermeability are attenuated by treatment with U0126 or PD98059, indicating signalling through MEK1 and ERK1/2.98
To study the MLCK-dependent mechanism in PKC-induced endothelial dysfunction, which is known to occur in the early stages of diabetes,99 we treated coronary venules with the PKC activator PMA.51 PMA-induced hyperpermeability was considerably reduced by ML-7 in a dose-dependent manner, reinforcing the notion that activated MLCK is a common downstream effector in executing the hyperpermeability effects of many signalling mediators.
As indicated above, neutrophil binding to endothelium causes elevated intracellular Ca2+, and hence Ca2+-calmodulin in vitro.89,90 Because Ca2+-calmodulin increases MLCK activity,10,33 we investigated the microvascular endothelial responses to activated neutrophils focusing on MLCK-mediated MLC phosphorylation and contractile cytoskeleton. In isolated coronary venules, we found that perfusion of microvessels with C5a-activated neutrophils induced a time- and concentration-dependent increase in albumin permeability.91 Inhibition of MLC phosphorylation by treatment with ML-7 significantly attenuated neutrophil-induced hyperpermeability. Based on the endothelial cell transfection technique, we have further developed the technique with an enhanced capacity to transfect large molecules or proteins into intact microvessels.100 We demonstrated inhibition of neutrophil-induced hyperpermeability in microvessels transfected with either an MLCK-inhibiting peptide or dominant negative (purified, inactivated by proteolysis) MLCK.91 Taken together, these results confirm the importance of MLCK signalling in neutrophil-mediated hyperpermeability at the microvascular level.
7. MLCK and hyperpermeability in vivo
In vivo studies are regarded as the most realistic representations of actual biological conditions. Models used to study microvascular permeability include intravital microscopy in mesenteric tissues that are semi-transparent, displaying well-defined vessels.95,101 Using such preparations, it is possible to spread the tissue across the microscope visual field, to measure fluorescent tracer flux out of the vessels, while the tissue remains connected to a live anaesthetized animal. The hamster cheek pouch is another commonly used model, where the time-dependent tracer distribution in the intravascular vs. extravascular space is monitored and tracer flux measured as an indicator of permeability.102 In hamster cheek pouch vessels, agonists that elevate endothelial intracellular NO/cGMP and increase paracellular permeability include platelet activating factor (PAF),103 ADP,104 and bradykinin. By inhibiting eNOS or its downstream signalling, microvascular permeability to water and macromolecules is significantly reduced. Therefore, NO production and cGMP are important for inducing hyperpermeability in vivo, as we have shown in isolated microvessels and in cultured cells.
Our in vivo studies have focused on rodent models of full-thickness burns covering 25–40% total body surface area. Severe burns are a common form of trauma that often induces a systemic inflammatory response affecting multiple organs.23,105–107 The reaction is initiated by overproduction of inflammatory mediators, many of which target the microvasculature leading to impaired blood-tissue perfusion and exchange. As a cardinal component of systemic inflammation, microvascular leak occurs not only at the local wound, but also in distal tissues, especially in the splanchnic microvessels.108,109 Plasma fluid loss and accumulation in tissues result in hypovolemic shock, pulmonary oedema, abdominal compartment syndrome, and generalized tissue malperfusion that ultimately lead to multiple organ failure.109,110 Our previous in vivo studies show that plasma extravasation in the splanchnic microvasculature is significantly increased following burns.111 Consistent with the in vivo observation, our experiments with endothelial monolayers and isolated microvessels show that endothelial permeability is increased by circulating factors released during burn injury.18,112 However, clinical studies have shown that targeting specific inflammatory pathways has limited efficacy in treating burn oedema. Likewise, we have seen that pharmacological inhibition of signalling molecules generally considered to lie upstream of the hyperpermeability response, such as Src and PKC, has negligible inhibitory effects on burn-induced microvascular leakage.111 This is not surprising considering the wide spectrum of extracellular inflammatory mediators and intracellular signalling intermediaries that cause endothelial hyperpermeability,113–118 notwithstanding that there is crosstalk between parallel signalling pathways. These events can compensate for each other such that selective inhibition of individual pathways may not be sufficient to block the massive, collective detrimental response. We believe that a better therapeutic strategy for treatment of burn oedema is one that specifically targets common terminal effectors of these signalling pathways. Our studies show that treatment with ML-7 blocks MLC phosphorylation and significantly attenuates burn-induced venular hyperpermeability in a dose-dependent manner.111 This finding supports our hypothesis that MLCK is a common endpoint effector for multiple signalling pathways triggered by circulating inflammatory factors that induce endothelial hyperpermeability in trauma.
The construction of MLCK-210 knock-out mice119 has enabled in vivo testing of the hypothesis that nmMLCK kinase activity is necessary for microvascular barrier response to stress or injury. The lungs are frequently involved in trauma- or sepsis-induced inflammation,18,120 and we have observed that pulmonary microvessels are particularly susceptible to hyperpermeability in response to inflammatory mediators. Studies of human populations show altered susceptibility to acute lung injury or acute respiratory distress syndrome associated with single nucleotide polymorphisms in the mylk1 gene, in the regions specifically coding nmMLCK.121,122 This suggests that lung hyperpermeability during trauma or sepsis is specifically mediated by nmMLCK isoforms. In addition, MLCK210 knockout animals have lowered susceptibility to septic injury, especially in the lung tissue.119 Therefore, we investigated the specific role of nmMLCK in microvascular hyperpermeability during severe burns using MLCK210 knockout mice.123 When compared with wild-type mice that show substantially increased albumin transflux and hydraulic conductivity (Lp) after severe burns, microvascular hyperpermeability was significantly attenuated in MLCK210 knockouts and accompanied by improved survival.123 This study provides strong in vivo evidence that nmMLCK mediates microvascular barrier dysfunction in response to systemic inflammation during severe traumatic injury. Further studies of endothelial-specific overexpression of MLCK2 in transgenic mice shows enhanced serum protein leakage into lung tissue during sepsis or injury, suggesting that individual nmMLCK isoforms mediate endothelial hyperpermeability in vivo.124
8. Summary
Microvascular endothelial barriers face diverse challenges in the form of physical forces, chemical factors, and circulating cells, and are critical for maintaining fluid/electrolyte homeostasis and physiological organ function. As the initiator and consequence of many diseases and disorders associated with microvascular inflammation, disruption of endothelial integrity is a critical problem that is difficult to correct clinically. The research efforts of several laboratories spanning decades has revealed that endothelial barrier dysfunction is generated by an imbalance between interendothelial adhesive forces and actomyosin-based centripetal tension. MLCK is a central regulator of actomyosin-based contractile cytoskeleton for a multitude of inflammatory cell signalling pathways. In this review, we provided experimental evidence ranging from in vitro to ex vivo and in vivo that supports the concept that MLCK is a common mediator for microvascular endothelial barrier dysfunction induced by several signalling molecules (e.g. NO/GMP and PKC), activated neutrophils, and severe thermal injury. Notably, MLCK-mediated MLC phosphorylation does not account for the action of all inflammatory agonists. There is evidence that MLCK-dependent actomyosin contractile mechanisms do not contribute significantly to PAF- or bradykinin-induced hyperpermeability in rat venular microvessels.49 Additionally, evidence suggests that the role of actomyosin cytoskeletal contraction in controlling endothelial permeability in vivo is different from endothelial cell monolayers in vitro. For example, in cultured endothelial cells, inhibition of the ROCK/MLC cascade exhibits a strong protective effect on the endothelial barrier during lethal toxin-induced inhibition of Rac1 activity, an effect believed to stabilize the endothelial barrier at the level of VE-cadherin.125 In the same study, antagonism of actomyosin contractility did not prevent toxin-induced hyperpermeability. Hence the mechanisms that mediate endothelial hyperpermeability may be more complex for intact vessels in vivo than for cell monolayers in vitro. Given the central role of nmMLCK in mediating microvascular leakage associated with the many inflammatory conditions described above, targeting nmMLCK as a therapeutic intervention may prove an effective alternative for treating these problems. Thus far, clinical use of non-specific MLCK inhibitors such as ML-7 and ML-9 has not been possible, due to systemic complications and non-specific effects on other kinases. Optimally, more specific small molecule or peptide inhibitors of nmMLCK isoforms can be designed that selectively target endothelial tissues and prevent oedema. More recent evidence regarding the protective effects of sphingosine-1-phosphate (S1P) indicate that activation of the receptor SIPR1 or of downstream tyrosine kinases may offer other approaches to therapeutic inhibition of endothelial nmMLCK activity, and suppression of vascular hyperpermeability.126,127
Conflict of interest: none declared.
Funding
This work is supported by the National Institutes of Health grants HL61507, HL70752, HL73324, HL84542, and HL96640.
References
- 1.Takashima S. Phosphorylation of myosin regulatory light chain by myosin light chain kinase, and muscle contraction. Circ J. 2009;73:208–213. doi: 10.1253/circj.cj-08-1041. doi:10.1253/circj.CJ-08-1041. [DOI] [PubMed] [Google Scholar]
- 2.Harrington WF, Rodgers ME. Myosin. Annu Rev Biochem. 1984;53:35–73. doi: 10.1146/annurev.bi.53.070184.000343. doi:10.1146/annurev.bi.53.070184.000343. [DOI] [PubMed] [Google Scholar]
- 3.Guerriero V, Jr, Russo MA, Olson NJ, Putkey JA, Means AR. Domain organization of chicken gizzard myosin light chain kinase deduced from a cloned cDNA. Biochemistry. 1986;25:8372–8381. doi: 10.1021/bi00374a007. doi:10.1021/bi00374a007. [DOI] [PubMed] [Google Scholar]
- 4.Blue EK, Goeckeler ZM, Jin Y, Hou L, Dixon SA, Herring BP, et al. 220- and 130-kDa MLCKs have distinct tissue distributions and intracellular localization patterns. Am J Physiol Cell Physiol. 2002;282:C451–C460. doi: 10.1152/ajpcell.00333.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ito M, Guerriero V, Jr, Chen XM, Hartshorne DJ. Definition of the inhibitory domain of smooth muscle myosin light chain kinase by site-directed mutagenesis. Biochemistry. 1991;30:3498–3503. doi: 10.1021/bi00228a021. doi:10.1021/bi00228a021. [DOI] [PubMed] [Google Scholar]
- 6.Lazar V, Garcia JG. A single human myosin light chain kinase gene (MLCK; MYLK) Genomics. 1999;57:256–267. doi: 10.1006/geno.1999.5774. doi:10.1006/geno.1999.5774. [DOI] [PubMed] [Google Scholar]
- 7.Garcia JG, Lazar V, Gilbert-McClain LI, Gallagher PJ, Verin AD. Myosin light chain kinase in endothelium: molecular cloning and regulation. Am J Respir Cell Mol Biol. 1997;16:489–494. doi: 10.1165/ajrcmb.16.5.9160829. [DOI] [PubMed] [Google Scholar]
- 8.Chan JY, Takeda M, Briggs LE, Graham ML, Lu JT, Horikoshi N, et al. Identification of cardiac-specific myosin light chain kinase. Circ Res. 2008;102:571–580. doi: 10.1161/CIRCRESAHA.107.161687. doi:10.1161/CIRCRESAHA.107.161687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watterson DM, Schavocky JP, Guo L, Weiss C, Chlenski A, Shirinsky VP, et al. Analysis of the kinase-related protein gene found at human chromosome 3q21 in a multi-gene cluster: organization, expression, alternative splicing, and polymorphic marker. J Cell Biochem. 1999;75:481–491. doi:10.1002/(SICI)1097-4644(19991201)75:3<481::AID-JCB12>3.0.CO;2-5. [PubMed] [Google Scholar]
- 10.Kamm KE, Stull JT. Dedicated myosin light chain kinases with diverse cellular functions. J Biol Chem. 2001;276:4527–4530. doi: 10.1074/jbc.R000028200. doi:10.1074/jbc.R000028200. [DOI] [PubMed] [Google Scholar]
- 11.Shoemaker MO, Lau W, Shattuck RL, Kwiatkowski AP, Matrisian PE, Guerra-Santos L, et al. Use of DNA sequence and mutant analyses and antisense oligodeoxynucleotides to examine the molecular basis of nonmuscle myosin light chain kinase autoinhibition, calmodulin recognition, and activity. J Cell Biol. 1990;111:1107–1125. doi: 10.1083/jcb.111.3.1107. doi:10.1083/jcb.111.3.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gallagher PJ, Herring BP, Griffin SA, Stull JT. Molecular characterization of a mammalian smooth muscle myosin light chain kinase. J Biol Chem. 1991;266:23936–23944. [PMC free article] [PubMed] [Google Scholar]
- 13.Takio K, Blumenthal DK, Walsh KA, Titani K, Krebs EG. Amino acid sequence of rabbit skeletal muscle myosin light chain kinase. Biochemistry. 1986;25:8049–8057. doi: 10.1021/bi00372a038. doi:10.1021/bi00372a038. [DOI] [PubMed] [Google Scholar]
- 14.Birukov KG, Csortos C, Marzilli L, Dudek S, Ma SF, Bresnick AR, et al. Differential regulation of alternatively spliced endothelial cell myosin light chain kinase isoforms by p60(Src) J Biol Chem. 2001;276:8567–8573. doi: 10.1074/jbc.M005270200. doi:10.1074/jbc.M005270200. [DOI] [PubMed] [Google Scholar]
- 15.Garcia JG, Siflinger-Birnboim A, Bizios R, Del Vecchio PJ, Fenton JW, 2nd, Malik AB. Thrombin-induced increase in albumin permeability across the endothelium. J Cell Physiol. 1986;128:96–104. doi: 10.1002/jcp.1041280115. doi:10.1002/jcp.1041280115. [DOI] [PubMed] [Google Scholar]
- 16.Herring BP, El-Mounayri O, Gallagher PJ, Yin F, Zhou J. Regulation of myosin light chain kinase and telokin expression in smooth muscle tissues. Am J Physiol Cell Physiol. 2006;291:C817–C827. doi: 10.1152/ajpcell.00198.2006. doi:10.1152/ajpcell.00198.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Potier MC, Chelot E, Pekarsky Y, Gardiner K, Rossier J, Turnell WG. The human myosin light chain kinase (MLCK) from hippocampus: cloning, sequencing, expression, and localization to 3qcen-q21. Genomics. 1995;29:562–570. doi: 10.1006/geno.1995.9965. doi:10.1006/geno.1995.9965. [DOI] [PubMed] [Google Scholar]
- 18.Kumar P, Shen Q, Pivetti CD, Lee ES, Wu MH, Yuan SY. Molecular mechanisms of endothelial hyperpermeability: implications in inflammation. Expert Rev Mol Med. 2009;11:e19. doi: 10.1017/S1462399409001112. doi:10.1017/S1462399409001112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Nieuw Amerongen GP, van Hinsbergh VW. Targets for pharmacological intervention of endothelial hyperpermeability and barrier function. Vascul Pharmacol. 2002;39:257–272. doi: 10.1016/s1537-1891(03)00014-4. doi:10.1016/S1537-1891(03)00014-4. [DOI] [PubMed] [Google Scholar]
- 20.Yuan SY. Protein kinase signaling in the modulation of microvascular permeability. Vascul Pharmacol. 2002;39:213–223. doi: 10.1016/s1537-1891(03)00010-7. doi:10.1016/S1537-1891(03)00010-7. [DOI] [PubMed] [Google Scholar]
- 21.Yuan SY, Breslin JW, Perrin R, Gaudreault N, Guo M, Kargozaran H, et al. Microvascular permeability in diabetes and insulin resistance. Microcirculation. 2007;14:363–373. doi: 10.1080/10739680701283091. doi:10.1080/10739680701283091. [DOI] [PubMed] [Google Scholar]
- 22.Hashizume H, Baluk P, Morikawa S, McLean JW, Thurston G, Roberge S, et al. Openings between defective endothelial cells explain tumor vessel leakiness. Am J Pathol. 2000;156:1363–1380. doi: 10.1016/S0002-9440(10)65006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lund T, Onarheim H, Reed RK. Pathogenesis of edema formation in burn injuries. World J Surg. 1992;16:2–9. doi: 10.1007/BF02067107. doi:10.1007/BF02067107. [DOI] [PubMed] [Google Scholar]
- 24.Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. doi:10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 25.Komarova Y, Malik AB. Regulation of endothelial permeability via paracellular and transcellular transport pathways. Annu Rev Physiol. 2010;72:463–493. doi: 10.1146/annurev-physiol-021909-135833. doi:10.1146/annurev-physiol-021909-135833. [DOI] [PubMed] [Google Scholar]
- 26.Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev. 2006;86:279–367. doi: 10.1152/physrev.00012.2005. doi:10.1152/physrev.00012.2005. [DOI] [PubMed] [Google Scholar]
- 27.Vogel SM, Minshall RD, Pilipovic M, Tiruppathi C, Malik AB. Albumin uptake and transcytosis in endothelial cells in vivo induced by albumin-binding protein. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1512–L1522. doi: 10.1152/ajplung.2001.281.6.L1512. [DOI] [PubMed] [Google Scholar]
- 28.Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4. doi:10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- 29.Crone C, Olesen SP. Electrical resistance of brain microvascular endothelium. Brain Res. 1982;241:49–55. doi: 10.1016/0006-8993(82)91227-6. doi:10.1016/0006-8993(82)91227-6. [DOI] [PubMed] [Google Scholar]
- 30.Smith QR, Rapoport SI. Cerebrovascular permeability coefficients to sodium, potassium, and chloride. J Neurochem. 1986;46:1732–1742. doi: 10.1111/j.1471-4159.1986.tb08491.x. [DOI] [PubMed] [Google Scholar]
- 31.Huber JD, Egleton RD, Davis TP. Molecular physiology and pathophysiology of tight junctions in the blood-brain barrier. Trends Neurosci. 2001;24:719–725. doi: 10.1016/s0166-2236(00)02004-x. doi:10.1016/S0166-2236(00)02004-X. [DOI] [PubMed] [Google Scholar]
- 32.Vandenbroucke E, Mehta D, Minshall R, Malik AB. Regulation of endothelial junctional permeability. Ann N Y Acad Sci. 2008;1123:134–145. doi: 10.1196/annals.1420.016. doi:10.1196/annals.1420.016. [DOI] [PubMed] [Google Scholar]
- 33.Dudek SM, Garcia JG. Cytoskeletal regulation of pulmonary vascular permeability. J Appl Physiol. 2001;91:1487–1500. doi: 10.1152/jappl.2001.91.4.1487. [DOI] [PubMed] [Google Scholar]
- 34.Prasain N, Stevens T. The actin cytoskeleton in endothelial cell phenotypes. Microvasc Res. 2009;77:53–63. doi: 10.1016/j.mvr.2008.09.012. doi:10.1016/j.mvr.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shen Q, Wu MH, Yuan SY. Endothelial contractile cytoskeleton and microvascular permeability. Cell Health Cytoskeleton. 2009;1:43–50. doi: 10.2147/chc.s5118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu MH. Endothelial focal adhesions and barrier function. J Physiol. 2005;569:359–366. doi: 10.1113/jphysiol.2005.096537. doi:10.1113/jphysiol.2005.096537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schnittler HJ, Wilke A, Gress T, Suttorp N, Drenckhahn D. Role of actin and myosin in the control of paracellular permeability in pig, rat and human vascular endothelium. J Physiol. 1990;431:379–401. doi: 10.1113/jphysiol.1990.sp018335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cai S, Pestic-Dragovich L, O'Donnell ME, Wang N, Ingber D, Elson E, et al. Regulation of cytoskeletal mechanics and cell growth by myosin light chain phosphorylation. Am J Physiol. 1998;275:C1349–C1356. doi: 10.1152/ajpcell.1998.275.5.C1349. [DOI] [PubMed] [Google Scholar]
- 39.Chrzanowska-Wodnicka M, Burridge K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J Cell Biol. 1996;133:1403–1415. doi: 10.1083/jcb.133.6.1403. doi:10.1083/jcb.133.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garcia JG, Davis HW, Patterson CE. Regulation of endothelial cell gap formation and barrier dysfunction: role of myosin light chain phosphorylation. J Cell Physiol. 1995;163:510–522. doi: 10.1002/jcp.1041630311. doi:10.1002/jcp.1041630311. [DOI] [PubMed] [Google Scholar]
- 41.Goeckeler ZM, Wysolmerski RB. Myosin light chain kinase-regulated endothelial cell contraction: the relationship between isometric tension, actin polymerization, and myosin phosphorylation. J Cell Biol. 1995;130:613–627. doi: 10.1083/jcb.130.3.613. doi:10.1083/jcb.130.3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Verin AD, Gilbert-McClain LI, Patterson CE, Garcia JG. Biochemical regulation of the nonmuscle myosin light chain kinase isoform in bovine endothelium. Am J Respir Cell Mol Biol. 1998;19:767–776. doi: 10.1165/ajrcmb.19.5.3126. [DOI] [PubMed] [Google Scholar]
- 43.Verin AD, Patterson CE, Day MA, Garcia JG. Regulation of endothelial cell gap formation and barrier function by myosin-associated phosphatase activities. Am J Physiol. 1995;269:L99–L108. doi: 10.1152/ajplung.1995.269.1.L99. [DOI] [PubMed] [Google Scholar]
- 44.Essler M, Amano M, Kruse HJ, Kaibuchi K, Weber PC, Aepfelbacher M. Thrombin inactivates myosin light chain phosphatase via Rho and its target Rho kinase in human endothelial cells. J Biol Chem. 1998;273:21867–21874. doi: 10.1074/jbc.273.34.21867. doi:10.1074/jbc.273.34.21867. [DOI] [PubMed] [Google Scholar]
- 45.Totsukawa G, Yamakita Y, Yamashiro S, Hartshorne DJ, Sasaki Y, Matsumura F. Distinct roles of ROCK (Rho-kinase) and MLCK in spatial regulation of MLC phosphorylation for assembly of stress fibers and focal adhesions in 3T3 fibroblasts. J Cell Biol. 2000;150:797–806. doi: 10.1083/jcb.150.4.797. doi:10.1083/jcb.150.4.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, et al. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) J Biol Chem. 1996;271:20246–20249. doi: 10.1074/jbc.271.34.20246. doi:10.1074/jbc.271.34.20246. [DOI] [PubMed] [Google Scholar]
- 47.Sanchez T, Skoura A, Wu MT, Casserly B, Harrington EO, Hla T. Induction of vascular permeability by the sphingosine-1-phosphate receptor-2 (S1P2R) and its downstream effectors ROCK and PTEN. Arterioscler Thromb Vasc Biol. 2007;27:1312–1318. doi: 10.1161/ATVBAHA.107.143735. doi:10.1161/ATVBAHA.107.143735. [DOI] [PubMed] [Google Scholar]
- 48.Adamson RH, Ly JC, Sarai RK, Lenz JF, Altangerel A, Drenckhahn D, et al. Epac/Rap1 pathway regulates microvascular hyperpermeability induced by PAF in rat mesentery. Am J Physiol Heart Circ Physiol. 2008;294:H1188–1196. doi: 10.1152/ajpheart.00937.2007. doi:10.1152/ajpheart.00937.2007. [DOI] [PubMed] [Google Scholar]
- 49.Adamson RH, Zeng M, Adamson GN, Lenz JF, Curry FE. PAF- and bradykinin-induced hyperpermeability of rat venules is independent of actin-myosin contraction. Am J Physiol Heart Circ Physiol. 2003;285:H406–H417. doi: 10.1152/ajpheart.00021.2003. [DOI] [PubMed] [Google Scholar]
- 50.Sheldon R, Moy A, Lindsley K, Shasby S, Shasby DM. Role of myosin light-chain phosphorylation in endothelial cell retraction. Am J Physiol. 1993;265:L606–612. doi: 10.1152/ajplung.1993.265.6.L606. [DOI] [PubMed] [Google Scholar]
- 51.Yuan Y, Huang Q, Wu HM. Myosin light chain phosphorylation: modulation of basal and agonist-stimulated venular permeability. Am J Physiol. 1997;272:H1437–1443. doi: 10.1152/ajpheart.1997.272.3.H1437. [DOI] [PubMed] [Google Scholar]
- 52.Hixenbaugh EA, Goeckeler ZM, Papaiya NN, Wysolmerski RB, Silverstein SC, Huang AJ. Stimulated neutrophils induce myosin light chain phosphorylation and isometric tension in endothelial cells. Am J Physiol. 1997;273:H981–H988. doi: 10.1152/ajpheart.1997.273.2.H981. [DOI] [PubMed] [Google Scholar]
- 53.Moy AB, Shasby SS, Scott BD, Shasby DM. The effect of histamine and cyclic adenosine monophosphate on myosin light chain phosphorylation in human umbilical vein endothelial cells. J Clin Invest. 1993;92:1198–1206. doi: 10.1172/JCI116690. doi:10.1172/JCI116690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van Hinsbergh VW, van Nieuw Amerongen GP. Intracellular signalling involved in modulating human endothelial barrier function. J Anat. 2002;200:549–560. doi: 10.1046/j.1469-7580.2002.00060.x. doi:10.1046/j.1469-7580.2002.00060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van Nieuw Amerongen GP, Draijer R, Vermeer MA, van Hinsbergh VW. Transient and prolonged increase in endothelial permeability induced by histamine and thrombin: role of protein kinases, calcium, and RhoA. Circ Res. 1998;83:1115–1123. doi: 10.1161/01.res.83.11.1115. [DOI] [PubMed] [Google Scholar]
- 56.Langeler EG, Snelting-Havinga I, van Hinsbergh VW. Passage of low density lipoproteins through monolayers of human arterial endothelial cells. Effects of vasoactive substances in an in vitro model. Arteriosclerosis. 1989;9:550–559. doi: 10.1161/01.atv.9.4.550. [DOI] [PubMed] [Google Scholar]
- 57.Shasby DM, Stevens T, Ries D, Moy AB, Kamath JM, Kamath AM, et al. Thrombin inhibits myosin light chain dephosphorylation in endothelial cells. Am J Physiol. 1997;272:L311–L319. doi: 10.1152/ajplung.1997.272.2.L311. [DOI] [PubMed] [Google Scholar]
- 58.Tinsley JH, De Lanerolle P, Wilson E, Ma W, Yuan SY. Myosin light chain kinase transference induces myosin light chain activation and endothelial hyperpermeability. Am J Physiol Cell Physiol. 2000;279:C1285–C1289. doi: 10.1152/ajpcell.2000.279.4.C1285. [DOI] [PubMed] [Google Scholar]
- 59.Tinsley JH, Hawker J, Yuan Y. Efficient protein transfection of cultured coronary venular endothelial cells. Am J Physiol. 1998;275:H1873–H1878. doi: 10.1152/ajpheart.1998.275.5.H1873. [DOI] [PubMed] [Google Scholar]
- 60.Yuan SY. Signal transduction pathways in enhanced microvascular permeability. Microcirculation. 2000;7:395–403. doi:10.1038/sj.mn.7300123. [PubMed] [Google Scholar]
- 61.Ikebe M, Koretz J, Hartshorne DJ. Effects of phosphorylation of light chain residues threonine 18 and serine 19 on the properties and conformation of smooth muscle myosin. J Biol Chem. 1988;263:6432–6437. [PubMed] [Google Scholar]
- 62.Wu HM, Yuan Y, Zawieja DC, Tinsley J, Granger HJ. Role of phospholipase C, protein kinase C, and calcium in VEGF-induced venular hyperpermeability. Am J Physiol. 1999;276:H535–H542. doi: 10.1152/ajpheart.1999.276.2.H535. [DOI] [PubMed] [Google Scholar]
- 63.Lynch JJ, Ferro TJ, Blumenstock FA, Brockenauer AM, Malik AB. Increased endothelial albumin permeability mediated by protein kinase C activation. J Clin Invest. 1990;85:1991–1998. doi: 10.1172/JCI114663. doi:10.1172/JCI114663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Johnson A, Hocking DC, Ferro TJ. Mechanisms of pulmonary edema induced by a diacylglycerol second messenger. Am J Physiol. 1990;258:H85–H91. doi: 10.1152/ajpheart.1990.258.1.H85. doi: [DOI] [PubMed] [Google Scholar]
- 65.Lum H, Malik AB. Regulation of vascular endothelial barrier function. Am J Physiol. 1994;267:L223–L241. doi: 10.1152/ajplung.1994.267.3.L223. [DOI] [PubMed] [Google Scholar]
- 66.Granger DN, Kubes P. Nitric oxide as antiinflammatory agent. Methods Enzymol. 1996;269:434–442. doi: 10.1016/s0076-6879(96)69044-2. doi:10.1016/S0076-6879(96)69044-2. [DOI] [PubMed] [Google Scholar]
- 67.Boueiz A, Hassoun PM. Regulation of endothelial barrier function by reactive oxygen and nitrogen species. Microvasc Res. 2009;77:26–34. doi: 10.1016/j.mvr.2008.10.005. doi:10.1016/j.mvr.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 68.Davidson SM, Duchen MR. Endothelial mitochondria: contributing to vascular function and disease. Circ Res. 2007;100:1128–1141. doi: 10.1161/01.RES.0000261970.18328.1d. doi:10.1161/01.RES.0000261970.18328.1d. [DOI] [PubMed] [Google Scholar]
- 69.Qian J, Zhang Q, Church JE, Stepp DW, Rudic RD, Fulton DJ. Role of local production of endothelium-derived nitric oxide on cGMP signaling and S-nitrosylation. Am J Physiol Heart Circ Physiol. 2010;298:H112–H118. doi: 10.1152/ajpheart.00614.2009. doi:10.1152/ajpheart.00614.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tran QK, Watanabe H. Calcium signalling in the endothelium. Handb Exp Pharmacol. 2006:145–187. doi: 10.1007/3-540-32967-6_5. doi:10.1007/3-540-32967-6_5. [DOI] [PubMed] [Google Scholar]
- 71.Yuan SY. New insights into eNOS signaling in microvascular permeability. Am J Physiol Heart Circ Physiol. 2006;291:H1029–H1031. doi: 10.1152/ajpheart.00509.2006. doi:10.1152/ajpheart.00509.2006. [DOI] [PubMed] [Google Scholar]
- 72.Hood J, Granger HJ. Protein kinase G mediates vascular endothelial growth factor-induced Raf-1 activation and proliferation in human endothelial cells. J Biol Chem. 1998;273:23504–23508. doi: 10.1074/jbc.273.36.23504. doi:10.1074/jbc.273.36.23504. [DOI] [PubMed] [Google Scholar]
- 73.Parenti A, Morbidelli L, Cui XL, Douglas JG, Hood JD, Granger HJ, et al. Nitric oxide is an upstream signal of vascular endothelial growth factor-induced extracellular signal-regulated kinase1/2 activation in postcapillary endothelium. J Biol Chem. 1998;273:4220–4226. doi: 10.1074/jbc.273.7.4220. doi:10.1074/jbc.273.7.4220. [DOI] [PubMed] [Google Scholar]
- 74.Tiruppathi C, Ahmmed GU, Vogel SM, Malik AB. Ca2+ signaling, TRP channels, and endothelial permeability. Microcirculation. 2006;13:693–708. doi: 10.1080/10739680600930347. doi:10.1080/10739680600930347. [DOI] [PubMed] [Google Scholar]
- 75.Cioffi DL, Barry C, Stevens T. Store-operated calcium entry channels in pulmonary endothelium: the emerging story of TRPCS and Orai1. Adv Exp Med Biol. 2010;661:137–154. doi: 10.1007/978-1-60761-500-2_9. doi:10.1007/978-1-60761-500-2_9. [DOI] [PubMed] [Google Scholar]
- 76.Hicks K, O'Neil RG, Dubinsky WS, Brown RC. Trpc-mediated actin-myosin contraction is critical for BBB disruption following hypoxic stress. Am J Physiol Cell Physiol. 2010 doi: 10.1152/ajpcell.00458.2009. [Epub ahead of print] doi:10.1152/ajpcell.00458.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Norwood N, Moore TM, Dean DA, Bhattacharjee R, Li M, Stevens T. Store-operated calcium entry and increased endothelial cell permeability. Am J Physiol Lung Cell Mol Physiol. 2000;279:L815–L824. doi: 10.1152/ajplung.2000.279.5.L815. [DOI] [PubMed] [Google Scholar]
- 78.Luh C, Kuhlmann CR, Ackermann B, Timaru-Kast R, Luhmann HJ, Behl C, et al. Inhibition of myosin light chain kinase reduces brain edema formation after traumatic brain injury. J Neurochem. 2010;112:1015–1025. doi: 10.1111/j.1471-4159.2009.06514.x. doi:10.1111/j.1471-4159.2009.06514.x. [DOI] [PubMed] [Google Scholar]
- 79.Kuhlmann CR, Tamaki R, Gamerdinger M, Lessmann V, Behl C, Kempski OS, et al. Inhibition of the myosin light chain kinase prevents hypoxia-induced blood-brain barrier disruption. J Neurochem. 2007;102:501–507. doi: 10.1111/j.1471-4159.2007.04506.x. doi:10.1111/j.1471-4159.2007.04506.x. [DOI] [PubMed] [Google Scholar]
- 80.Rodrigues SF, Granger DN. Role of blood cells in ischemia-reperfusion induced endothelial barrier failure. Cardiovasc Res. 2010 doi: 10.1093/cvr/cvq090. [Epub ahead of print] doi:10.1093/cvr%2Fcvq090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.DiStasi MR, Ley K. Opening the flood-gates: how neutrophil-endothelial interactions regulate permeability. Trends Immunol. 2009;30:547–556. doi: 10.1016/j.it.2009.07.012. doi:10.1016/j.it.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Carman CV, Springer TA. Trans-cellular migration: cell-cell contacts get intimate. Curr Opin Cell Biol. 2008;20:533–540. doi: 10.1016/j.ceb.2008.05.007. doi:10.1016/j.ceb.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lewis RE, Granger HJ. Neutrophil-dependent mediation of microvascular permeability. Fed Proc. 1986;45:109–113. [PubMed] [Google Scholar]
- 84.Huber AR, Weiss SJ. Disruption of the subendothelial basement membrane during neutrophil diapedesis in an in vitro construct of a blood vessel wall. J Clin Invest. 1989;83:1122–1136. doi: 10.1172/JCI113992. doi:10.1172/JCI113992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Carman CV. Mechanisms for transcellular diapedesis: probing and pathfinding by ‘invadosome-like protrusions. J Cell Sci. 2009;122:3025–3035. doi: 10.1242/jcs.047522. doi:10.1242/jcs.047522. [DOI] [PubMed] [Google Scholar]
- 86.Carman CV, Sage PT, Sciuto TE, de la Fuente MA, Geha RS, Ochs HD, et al. Transcellular diapedesis is initiated by invasive podosomes. Immunity. 2007;26:784–797. doi: 10.1016/j.immuni.2007.04.015. doi:10.1016/j.immuni.2007.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Carman CV, Springer TA. A transmigratory cup in leukocyte diapedesis both through individual vascular endothelial cells and between them. J Cell Biol. 2004;167:377–388. doi: 10.1083/jcb.200404129. doi:10.1083/jcb.200404129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lewis RE, Granger HJ. Diapedesis and the permeability of venous microvessels to protein macromolecules: the impact of leukotriene B4 (LTB4) Microvasc Res. 1988;35:27–47. doi: 10.1016/0026-2862(88)90048-9. doi:10.1016/0026-2862(88)90048-9. [DOI] [PubMed] [Google Scholar]
- 89.Huang AJ, Manning JE, Bandak TM, Ratau MC, Hanser KR, Silverstein SC. Endothelial cell cytosolic free calcium regulates neutrophil migration across monolayers of endothelial cells. J Cell Biol. 1993;120:1371–1380. doi: 10.1083/jcb.120.6.1371. doi:10.1083/jcb.120.6.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tinsley JH, Wu MH, Ma W, Taulman AC, Yuan SY. Activated neutrophils induce hyperpermeability and phosphorylation of adherens junction proteins in coronary venular endothelial cells. J Biol Chem. 1999;274:24930–24934. doi: 10.1074/jbc.274.35.24930. doi:10.1074/jbc.274.35.24930. [DOI] [PubMed] [Google Scholar]
- 91.Yuan SY, Wu MH, Ustinova EE, Guo M, Tinsley JH, De Lanerolle P, et al. Myosin light chain phosphorylation in neutrophil-stimulated coronary microvascular leakage. Circ Res. 2002;90:1214–1221. doi: 10.1161/01.res.0000020402.73609.f1. doi:10.1161/01.RES.0000020402.73609.F1. [DOI] [PubMed] [Google Scholar]
- 92.Breslin JW, Yuan SY. Involvement of RhoA and Rho kinase in neutrophil-stimulated endothelial hyperpermeability. Am J Physiol Heart Circ Physiol. 2004;286:H1057–1062. doi: 10.1152/ajpheart.00841.2003. doi:10.1152/ajpheart.00841.2003. [DOI] [PubMed] [Google Scholar]
- 93.Breslin JW, Sun H, Xu W, Rodarte C, Moy AB, Wu MH, et al. Involvement of ROCK-mediated endothelial tension development in neutrophil-stimulated microvascular leakage. Am J Physiol Heart Circ Physiol. 2006;290:H741–H750. doi: 10.1152/ajpheart.00238.2005. doi:10.1152/ajpheart.00238.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yuan Y, Granger HJ, Zawieja DC, Chilian WM. Flow modulates coronary venular permeability by a nitric oxide-related mechanism. Am J Physiol. 1992;263:H641–H646. doi: 10.1152/ajpheart.1992.263.2.H641. [DOI] [PubMed] [Google Scholar]
- 95.Huxley VH, Curry FE, Adamson RH. Quantitative fluorescence microscopy on single capillaries: alpha-lactalbumin transport. Am J Physiol. 1987;252:H188–H197. doi: 10.1152/ajpheart.1987.252.1.H188. [DOI] [PubMed] [Google Scholar]
- 96.Yuan Y, Granger HJ, Zawieja DC, DeFily DV, Chilian WM. Histamine increases venular permeability via a phospholipase C-NO synthase-guanylate cyclase cascade. Am J Physiol. 1993;264:H1734–H1739. doi: 10.1152/ajpheart.1993.264.5.H1734. [DOI] [PubMed] [Google Scholar]
- 97.Wu HM, Huang Q, Yuan Y, Granger HJ. VEGF induces NO-dependent hyperpermeability in coronary venules. Am J Physiol. 1996;271:H2735–H2739. doi: 10.1152/ajpheart.1996.271.6.H2735. [DOI] [PubMed] [Google Scholar]
- 98.Wu MH, Yuan SY, Granger HJ. The protein kinase MEK1/2 mediate vascular endothelial growth factor- and histamine-induced hyperpermeability in porcine coronary venules. J Physiol. 2005;563:95–104. doi: 10.1113/jphysiol.2004.076075. doi:10.1113/jphysiol.2004.076075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yuan SY, Ustinova EE, Wu MH, Tinsley JH, Xu W, Korompai FL, et al. Protein kinase C activation contributes to microvascular barrier dysfunction in the heart at early stages of diabetes. Circ Res. 2000;87:412–417. doi: 10.1161/01.res.87.5.412. [DOI] [PubMed] [Google Scholar]
- 100.Tinsley JH, Zawieja DC, Wu MH, Ustinova EE, Xu W, Yuan SY. Protein transfection of intact microvessels specifically modulates vasoreactivity and permeability. J Vasc Res. 2001;38:444–452. doi: 10.1159/000051077. doi:10.1159/000051077. [DOI] [PubMed] [Google Scholar]
- 101.Witte S. Microphotometric techniques in intravital microcirculatory studies. J Microsc. 1979;116:373–384. doi: 10.1111/j.1365-2818.1979.tb00222.x. [DOI] [PubMed] [Google Scholar]
- 102.Bekker AY, Ritter AB, Duran WN. Analysis of microvascular permeability to macromolecules by video-image digital processing. Microvasc Res. 1989;38:200–216. doi: 10.1016/0026-2862(89)90028-9. doi:10.1016/0026-2862(89)90028-9. [DOI] [PubMed] [Google Scholar]
- 103.Ramirez MM, Quardt SM, Kim D, Oshiro H, Minnicozzi M, Duran WN. Platelet activating factor modulates microvascular permeability through nitric oxide synthesis. Microvasc Res. 1995;50:223–234. doi: 10.1006/mvre.1995.1055. doi:10.1006/mvre.1995.1055. [DOI] [PubMed] [Google Scholar]
- 104.Mayhan WG. Role of nitric oxide in modulating permeability of hamster cheek pouch in response to adenosine 5(-diphosphate and bradykinin. Inflammation. 1992;16:295–305. doi: 10.1007/BF00917622. doi:10.1007/BF00917622. [DOI] [PubMed] [Google Scholar]
- 105.Arturson G. Forty years in burns research-the postburn inflammatory response. Burns. 2000;26:599–604. doi: 10.1016/s0305-4179(00)00069-3. doi:10.1016/S0305-4179(00)00069-3. [DOI] [PubMed] [Google Scholar]
- 106.Cioffi WG. What's new in burns and metabolism. J Am Coll Surg. 2001;192:241–254. doi: 10.1016/s1072-7515(00)00795-x. doi:10.1016/S1072-7515(00)00795-X. [DOI] [PubMed] [Google Scholar]
- 107.Gibran NS, Heimbach DM. Current status of burn wound pathophysiology. Clin Plast Surg. 2000;27:11–22. [PubMed] [Google Scholar]
- 108.Brouhard BH, Carvajal HF, Linares HA. Burn edema and protein leakage in the rat. I. Relationship to time of injury. Microvasc Res. 1978;15:221–228. doi: 10.1016/0026-2862(78)90020-1. doi:10.1016/0026-2862%2878%2990020-1. [DOI] [PubMed] [Google Scholar]
- 109.Demling RH. The burn edema process: current concepts. J Burn Care Rehabil. 2005;26:207–227. [PubMed] [Google Scholar]
- 110.Deitch EA. Multiple organ failure. Pathophysiology and potential future therapy. Ann Surg. 1992;216:117–134. doi: 10.1097/00000658-199208000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Huang Q, Xu W, Ustinova E, Wu M, Childs E, Hunter F, et al. Myosin light chain kinase-dependent microvascular hyperpermeability in thermal injury. Shock. 2003;20:363–368. doi: 10.1097/01.shk.0000079425.0000.db. doi:10.1097/01.shk.0000079425.0000.db. [DOI] [PubMed] [Google Scholar]
- 112.Tinsley JH, Teasdale NR, Yuan SY. Myosin light chain phosphorylation and pulmonary endothelial cell hyperpermeability in burns. Am J Physiol Lung Cell Mol Physiol. 2004;286:L841–L847. doi: 10.1152/ajplung.00341.2003. doi:10.1152/ajplung.00341.2003. [DOI] [PubMed] [Google Scholar]
- 113.Mileski WJ, Burkhart D, Hunt JL, Kagan RJ, Saffle JR, Herndon DN, et al. Clinical effects of inhibiting leukocyte adhesion with monoclonal antibody to intercellular adhesion molecule-1 (enlimomab) in the treatment of partial-thickness burn injury. J Trauma. 2003;54:950–958. doi: 10.1097/01.TA.0000030626.84680.11. doi:10.1097/01.TA.0000030626.84680.11. [DOI] [PubMed] [Google Scholar]
- 114.Piccolo MT, Wang Y, Verbrugge S, Warner RL, Sannomiya P, Piccolo NS, et al. Role of chemotactic factors in neutrophil activation after thermal injury in rats. Inflammation. 1999;23:371–385. doi: 10.1023/a:1020213717336. [DOI] [PubMed] [Google Scholar]
- 115.Shimizu S, Tanaka H, Sakaki S, Yukioka T, Matsuda H, Shimazaki S. Burn depth affects dermal interstitial fluid pressure, free radical production, and serum histamine levels in rats. J Trauma. 2002;52:683–687. doi: 10.1097/00005373-200204000-00011. doi:10.1097/00005373-200204000-00011. [DOI] [PubMed] [Google Scholar]
- 116.Gibran NS, Heimbach DM. Mediators in thermal injury. Semin Nephrol. 1993;13:344–358. [PubMed] [Google Scholar]
- 117.Kowal-Vern A, Walenga JM, Sharp-Pucci M, Hoppensteadt D, Gamelli RL. Postburn edema and related changes in interleukin-2, leukocytes, platelet activation, endothelin-1, and C1 esterase inhibitor. J Burn Care Rehabil. 1997;18:99–103. doi: 10.1097/00004630-199703000-00002. doi:10.1097/00004630-199703000-00002. [DOI] [PubMed] [Google Scholar]
- 118.Kurose I, Wolf R, Miyasaka M, Anderson DC, Granger DN. Microvascular dysfunction induced by nonsteroidal anti-inflammatory drugs: role of leukocytes. Am J Physiol. 1996;270:G363–G369. doi: 10.1152/ajpgi.1996.270.2.G363. [DOI] [PubMed] [Google Scholar]
- 119.Wainwright MS, Rossi J, Schavocky J, Crawford S, Steinhorn D, Velentza AV, et al. Protein kinase involved in lung injury susceptibility: evidence from enzyme isoform genetic knockout and in vivo inhibitor treatment. Proc Natl Acad Sci USA. 2003;100:6233–6238. doi: 10.1073/pnas.1031595100. doi:10.1073/pnas.1031595100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bhatia M, Moochhala S. Role of inflammatory mediators in the pathophysiology of acute respiratory distress syndrome. J Pathol. 2004;202:145–156. doi: 10.1002/path.1491. doi:10.1002/path.1491. [DOI] [PubMed] [Google Scholar]
- 121.Christie JD, Ma SF, Aplenc R, Li M, Lanken PN, Shah CV, et al. Variation in the myosin light chain kinase gene is associated with development of acute lung injury after major trauma. Crit Care Med. 2008;36:2794–2800. doi: 10.1097/ccm.0b013e318186b843. doi:10.1097/CCM.0b013e318186b843. [DOI] [PubMed] [Google Scholar]
- 122.Gao L, Grant A, Halder I, Brower R, Sevransky J, Maloney JP, et al. Novel polymorphisms in the myosin light chain kinase gene confer risk for acute lung injury. Am J Respir Cell Mol Biol. 2006;34:487–495. doi: 10.1165/rcmb.2005-0404OC. doi:10.1165/rcmb.2005-0404OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Reynoso R, Perrin RM, Breslin JW, Daines DA, Watson KD, Watterson DM, et al. A role for long chain myosin light chain kinase (MLCK-210) in microvascular hyperpermeability during severe burns. Shock. 2007;28:589–595. doi: 10.1097/SHK.0b013e31804d415f. doi:10.1097/SHK.0b013e31804d415f. [DOI] [PubMed] [Google Scholar]
- 124.Moitra J, Evenoski C, Sammani S, Wadgaonkar R, Turner JR, Ma SF, et al. A transgenic mouse with vascular endothelial over-expression of the non-muscle myosin light chain kinase-2 isoform is susceptible to inflammatory lung injury: role of sexual dimorphism and age. Transl Res. 2008;151:141–153. doi: 10.1016/j.trsl.2007.12.008. doi:10.1016/j.trsl.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Waschke J, Drenckhahn D, Adamson RH, Curry FE. Role of adhesion and contraction in Rac 1-regulated endothelial barrier function in vivo and in vitro. Am J Physiol Heart Circ Physiol. 2004;287:H704–H711. doi: 10.1152/ajpheart.01076.2003. doi:10.1152/ajpheart.01076.2003. [DOI] [PubMed] [Google Scholar]
- 126.Dudek SM, Jacobson JR, Chiang ET, Birukov KG, Wang P, Zhan X, et al. Pulmonary endothelial cell barrier enhancement by sphingosine 1-phosphate: roles for cortactin and myosin light chain kinase. J Biol Chem. 2004;279:24692–24700. doi: 10.1074/jbc.M313969200. doi:10.1074/jbc.M313969200. [DOI] [PubMed] [Google Scholar]
- 127.Zhao J, Singleton PA, Brown ME, Dudek SM, Garcia JG. Phosphotyrosine protein dynamics in cell membrane rafts of sphingosine-1-phosphate-stimulated human endothelium: role in barrier enhancement. Cell Signal. 2009;21:1945–1960. doi: 10.1016/j.cellsig.2009.09.002. doi:10.1016/j.cellsig.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]