Abstract
Alzheimer's disease (AD) is the leading cause of dementia affecting over 25 million people worldwide. Classical studies focused on the description and characterization of the pathological hallmarks found in AD patients including the neurofibrillary tangles and the amyloid plaques. Current strategies focus on the etiology of these hallmarks and the different mechanisms contributing to neurodegeneration. Among them, recent studies reveal the close interplay between the immunological and the neurodegenerative processes. This article examines the implications of the alpha7 nicotinic acetylcholine receptor (alpha7nAChR) as a critical link between inflammation and neurodegeneration in AD. Alpha7nAChRs are not only expressed in neurons but also in Glia cells where they can modulate the immunological responses contributing to AD. Successful therapeutic strategies against AD should consider the connections between inflammation and neurodegeneration. Among them, alpha7nAChR may represent a pharmacological target to control these two mechanisms during the pathogenesis of neurodegenerative and behavioral disorders.
Keywords: Inflammation, Neurodegeneration, Alzheimer's disease, Microglia, Beta amyloid, Tau, Nicotinic receptors, Alpha7nicotinic receptors, Acetylcholine
1. Introduction
Alzheimer's disease (AD) is the leading cause of dementia worldwide and the seventh leading cause of death in 2004 killing over 65,000 patients in the USA. AD is the third most costly disease with an annual national cost of $100 billion and an average lifetime cost of care of nearly $175,000 per patient (Smith, 1998). Over 5 million Americans are currently afflicted with AD, and the number of individuals over 65 with AD is estimated to reach 16 millions in USA by 2050 (http://www.alz.org/national/documents/Report_2007FactsAndFigures.pdf). AD is a neurodegenerative disorder characterized by widespread cognitive impairments that begin with episodic memory declines. As the neurodegenerative disorder progresses, AD is characterized by a progressive cognitive impairment that extends to the domains of language (aphasia), skilled movements (apraxia), recognition (agnosia), and executive functions (such as decision-making and planning).
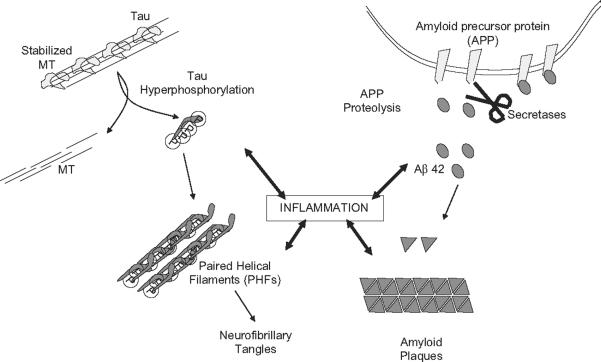
The typical hallmarks of AD include the neurofibrillary tangles and amyloid plaques that represent the two characteristic pathological processes of the disease: hyperphosphorylation of tau and misfolding of amyloid beta (Fig. 1). AD is considered a tauopathy due to abnormal aggregation of the tau protein in the brain (Rubio et al., 2006; Tolnay and Probst, 1999). Tau is a microtubule-associated protein that stabilizes the microtubules and contributes to the cellular transport of vesicles along the neurites. Like most microtubule-associated proteins, the binding of tau to microtubules is regulated by phosphorylation. Hyperphosphorylation of tau in AD patients favors its dissociation from the microtubules and its accumulation in paired helical filaments (PHFs) (Kosik et al., 1986; Wolozin et al., 1986). PHFs aggregate into detrimental clusters inside nerve cell bodies known as neurofibrillary tangles (NFTs) and dystrophic neurites associated with amyloid plaques. In addition to this process, the massive dissociation of tau from microtubules interferes with the axonal transport, contributing to the loss of synapses and neuronal degeneration correlated with the characteristic cognitive impairments of AD (DeKosky and Scheff, 1990; Terry et al., 1991). AD is also associated with the accumulation of abnormal folded amyloid beta1–42 (Aβ42) in the extracellular senile plaques. Aβ42 results from the abnormal proteolysis of the transmembrane amyloid beta precursor protein (APP) by the β- and γ-secretases (Wilson et al., 1999). The causative role of the amyloid in AD is based on its increased production and its early deposition in brain plaques of individuals with familial AD caused by mutations in APP, presenilin 1 (PS1) and PS2 genes (Selkoe, 1998). However, the extent of amyloid beta accumulation does not correlate well with pathogenesis (Slow et al., 2006) and there are a significant number of individuals with amyloid plaques who do not suffer dementia. Actually, soluble amyloid beta correlates better with the cognitive decline of AD patients than the insoluble, fibril deposits (Cleary et al., 2005). In APP transgenic models, neurological deficits precede the deposition of significant amounts of Aβ42, suggesting that the pathophysiology of AD may occur prior to amyloid deposition (Westerman et al., 2002).
Fig. 1.
Characteristic pathological mechanisms and hallmarks found in AD patients. The typical pathological hallmarks of AD include the neurofibrillary tangles and amyloid plaques that represent the two characteristic biochemical processes of the disease: hyperphosphorylation of tau and misfolding of amyloid beta. Hyperphosphorylation of tau in AD patients favors its dissociation from the microtubules and its accumulation in paired helical filaments (PHFs). PHFs aggregate into detrimental clusters inside nerve cell bodies known as neurofibrillary tangles and as dystrophic neurites associated with amyloid plaques. The massive dissociation of tau from microtubules also interferes with the axonal transport and contributes to the loss of synapses and neuronal degeneration correlated with the cognitive impairments of the AD patients. AD is also associated with the accumulation of abnormal folded amyloid beta1–42 (Aβ42) in the amyloid plaques. More comprehensive strategies are currently directed to determine the etiology of AD and the potential contribution of inflammation to both the pathogenesis and prognosis of AD.
The development of successful therapeutic strategies for AD is limited by our understanding of the pathological processes and the contribution of immunologic mechanisms to the pathogenesis. This article analyzes the contribution of inflammatory processes to neurodegeneration and whether anti-inflammatory strategies may provide a beneficial effect against AD. A classical example is that nicotine was used to compensate the loss of cholinergic neuron observed in the frontal cortex of AD patients, and administration of nicotine to AD patients reduced anxiety and improved cognitive performance in clinical trials (Levin et al., 2006). However, nicotine does not only target cholinergic receptors in neurons but it also controls the immune responses in glia cells. Thus, it may not be surprising that the alpha7 nicotinic acetylcholine receptor (alpha7nAChR), the receptor proposed for treatment of AD, is the same receptor that appears to mediate the anti-inflammatory potential of nicotine in glia cells. Here, we discuss the implications of alpha7nAChR as a critical link between inflammation and neurodegeneration, and its potential pharmacological implications for the treatment of AD.
2. Cholinergic implications in neurodegenerative disorders: from acetylcholine to nicotinic receptors
Cognitive impairments in AD patients are mainly characterized by the loss of cholinergic neurons, which are the most affected in the disease. In the central nervous system (CNS), acetylcholine (ACh) has a variety of effects as a neuromodulator involved in synaptic plasticity and stability. Acetylcholine enhances the amplitude of synaptic potentials following long-term potentiation (LTP) in many regions of the brain but mainly in the neocortex, CA1, dentate gyrus and piriform cortex. Acetylcholine receptors (AChRs) decrease the conductance of voltage-gated and Ca2+-dependent currents. There are two types of acetylcholine receptors, muscarinic (mAChRs) and nicotinic receptors (nAChRs). Neuronal mAChRs are metabotropic receptors stimulated by acetylcholine and muscarine but blocked by atropine. They belong to the G-protein coupled family of receptors, and they also activate other ionic channels via a second messenger cascade. For instance mAChR can activate M1, M3, M5 via phospholipase C or inhibit M2, M4 via adenylate cyclase. The nicotinic receptors (nAChRs) are cation-selective, ligand-gated ion pentameric channels composed out of selected alpha and/or beta subunits. To date, 12 neuronal subunits have been described including nine alpha (α2–α10) and three beta (β2–β4) subunits. The alpha subunits contain two adjacent cysteine residues for the binding of acetylcholine, whereas the beta subunits lack them (Alkondon and Albuquerque, 1993; Lukas et al., 1999). The combination of these subunits defines the function and affinity of the receptor for specific ligands (Sudweeks and Yakel, 2000). Nicotinic receptors are stimulated by acetylcholine and nicotine, but blocked by curare, α-conotoxin, α-bungarotoxin or mecamylamine (Itier and Bertrand, 2001). Both muscarinic and nicotinic acetylcholine receptors are not specific of the central system as they also appear in the peripheral nervous system.
Among the nAChRs, the alpha7nAChR has major clinical and pharmacological implications for AD. The alpha7nAChR is a homomeric pentameric ligand-gate ion channel with five acetylcholine binding sites (Drisdel and Green, 2000). Originally described as a sodium channel, the alpha7 subunit presents a high conductance for calcium (Berg and Conroy, 2002), low sensitivity to acetylcholine (Clarke, 1992) and a high affinity for α-bungarotoxin (Marks and Collins, 1982; Rangwala et al., 1997). This receptor also appears to trigger alternative signal pathways in neuronal and non-neuronal cells. In neuronal cells activation of the alpha7nAChR causes an increase in intracellular Ca2+ directly through voltage activated channels (activation of ERK1/2 in a Ca2+ and PKA dependent manner) (Dajas-Bailador et al., 2002b) and indirectly from intracellular sources following nicotinic ryanodine receptor channels activation (Dajas-Bailador et al., 2002a). In astrocytes, the alpha7nAChRs appears to modulate Ca2+ release from intracellular stores (Sharma and Vijayaraghavan, 2001). In neurons, nicotinic-induced increase in intracellular Ca2+ modulates glutamate and GABA activity (Maggi et al., 2001; Radcliffe and Dani, 1998) and regulates CREB, the transcription factor involved in the formation of the LTP (Lynch, 2004; Silva et al., 1998) via glutamatergic transmission (Hu et al., 2002).
Alpha7nAChRs are widely distributed in brain. Radioligand binding of [I125] α-bungarotoxin shows a high to moderate even distribution of the alpha7nAChR in the CA1 region of the hippocampus and entorhinal cortex (Court et al., 2000). In the prefrontal and the motor cortex, the alpha7nAChR localizes in the pyramidal neurons of the layers II/III, V and VI (Wevers et al., 1994). Alpha7-mRNA has high and intermediate expression in the nucleus reticularis and lateral/medial geniculate bodies as opposed to the low expression in the thalamus (Breese et al., 1997; Court et al., 2000; Rubboli et al., 1994; Spurden et al., 1997). Basal ganglia has high expression of alpha7nAChR in substantia nigra, intermediate in caudate and putamen, and a lower expression in striatum (Court et al., 2000). Cerebellum exhibits strong expression of alpha7nAChR in selectively Purkinge cells and shows higher density in the molecular than in the granular layer (Court et al., 2000).
Decline in nicotinic receptors, particularly the alpha7nAChR in the frontal cortex, is associated with aging (Utsugisawa et al., 1999). Radioligand binding of [I125] α-bungarotoxin to samples from patients ages 20–100 years old (Court et al., 1997) and 65–80 years old (Nordberg and Winblad, 1986) show significant reductions in the entorhinal cortex and the thalamus. No changes were observed in hippocampus (ages 20–100) or putamen (ages 22–80) (Court et al., 1997; Utsugisawa et al., 1999). Decline in nAChRs, including the alpha7nAChR, is also associated with AD. These deficits appear early in the disease and correlate with the progressive loss of cognitive abilities (Nordberg, 1994, 2001; Whitehouse and Kalaria, 1995). In AD patients, the alpha7nAChRs protein levels are reduced in the cortex and hippocampus (Burghaus et al., 2000; Guan et al., 2000; Martin-Ruiz et al., 1999; Nordberg, 2001; Wevers et al., 2000). Although the protein loss is evident in AD, reductions in gene expression at the transcriptional level are less clear. The 36% reduction in alpha7nAChR-protein levels in the hippocampus of patients with AD (Guan et al., 2000) contrast with the 65% increase in alpha7nAChR-mRNA expression reported (Hellstrom-Lindahl et al., 1999). Apparent contradictory results were also found in the frontal cortex of AD patients showing no differences in [I125] α-bungarotoxin binding (Davies and Feisullin, 1981; Sugaya et al., 1990), or a significant reduction of the alpha7nAChR-protein expression levels (Engidawork et al., 2001). These apparent contradictions may suggest a compensatory mechanism at the transcriptional level in both the cerebral cortex and the hippocampus (Nordberg, 2001). In conclusion, one of the major features in AD is the decline of nAChRs in disease-relevant brain regions such as the cerebral cortex and the hippocampus. This loss may be explained by the loss of cholinergic cells, which contribute to the cognitive dysfunction.
3. Alpha7nAChR and information processing
CHRNA7 knock out mice have cognitive dysfunction, including attention and working memory deficiencies (Fernandes et al., 2006; Hoyle et al., 2006; Young et al., 2007). Likewise, there is general consensus that the alpha7nAChR plays an important role in information processing in humans. In a series of studies Freedman and colleagues demonstrated that variants in the CHRNA7 gene influence susceptibility to schizophrenia and that the alpha7nAChR is involved in attentional gating as measured by the P50 ERP paradigm (Freedman et al., 2006; Martin et al., 2004). In this paradigm abnormalities in the alpha7nAChR system result in failures to reduce amplitude to the second auditory stimulus of a pair, a finding which suggests a basic defect in the filtering of novel from non-novel events. Furthermore, a specific alpha7nAChR agonist (GTS21) improved cognition in schizophrenia, including attention, working memory, speed and total score of a brief cognitive screening instrument (Olincy et al., 2006). P50 inhibition also improved. More detailed information on this area is available in several recent reviews (Levin, 2002; Martin et al., 2004; Potter et al., 2006).
4. Epidemiological implications of nicotine in neurodegenerative disorders
Epidemiological studies show that nicotine decreases the risk for Parkinson's disease (PD) and AD (Fratiglioni and Wang, 2000). This negative association is in agreement with postmortem studies showing diminutions of amyloid plaque deposits in former smokers with AD (Hellstrom-Lindahl et al., 2004b). Nicotine treatment appears to interfere with the formation of the amyloid plaques in vitro and in vivo (Hellstrom-Lindahl et al., 2004a; Ono et al., 2002; Utsuki et al., 2002) and to reduce the accumulation of insoluble Aβ42 peptides (Nordberg et al., 2002) through a mechanism mediated by alpha7nAChRs as shown with mecamylamine that blocks the increase of sAPP produced by treating SH-SY5Y neuroblastoma cells with nicotine (Hellstrom-Lindahl et al., 2004a). Several clinical trials indicate that chronic administration of nicotine in individuals with age-associated memory impairments and AD improves cognition in attention but not in memory (Snaedal et al., 1996; White and Levin, 1999, 2004; Wilson et al., 1995). Similar studies indicate that ABT418, a nicotinic alpha4beta2-agonist, improves memory and learning skills in AD patients (Potter et al., 1999). Nicotine also improves cognition in patients with psychiatric disorders such as schizophrenia where the loss of alpha7nAChRs may contribute to neurocognitive and sensory gating deficits (Adler et al., 1998; Depatie et al., 2002; Harris et al., 2004; Myers et al., 2004; Smith et al., 2002, 2006). Smoking improved auditory sensory gating in schizophrenic patients (Adler et al., 1993) and these effects appear to be mediated by the alpha7nAChR as shown by using alpha-bungarotoxin and tubocurarine in rat hippocampus (Adler et al., 1998; Luntz-Leybman et al., 1992). The effects of smoking in memory remain controversial due to studies with negative (Depatie et al., 2002; Harris et al., 2004) and positive results (Myers et al., 2004). In conclusion, for both schizophrenia and AD, nicotine improves performance in attention through the alpha7nAChRs, while memory improvements seem to be driven through the alpha4beta2-nAChRs.
Nicotine seems to protect against the development of AD and PD through anti-inflammatory mechanisms. Both AD and PD are characterized by local inflammatory responses sustained by activated microglial cells. Nicotine induces anti-inflammatory mechanisms that diminish local inflammatory responses (Hellstrom-Lindahl et al., 2004a; Streit, 2002; Wang et al., 2000a, b). Among other, nicotine abrogates the production of TNF in culture of microglia through a mechanism dependent on ERK and p38 MAPK (Shytle et al., 2004; Suzuki et al., 2006). Experimental models of AD indicate that alpha7nAChR is the central core of the nicotine-mediated neuroprotection. Nicotine decreases accumulation of beta-amyloid in the cortex and hippocampus of APP (V717I) transgenic mice. These studies also indicate that nicotine prevents the activation of the NF-κB and c-Myc pathways by inhibiting the ERK and p38 MAPK kinases via alpha7nAChRs (Liu et al., 2007). This mechanism of the alpha7nAChR function was confirmed by using RNA interference in the experiments of nicotine-mediated neuroprotection. However, nicotine also has other beneficial effects on AD that are not mediated through the alpha7nAChRs. For instance, nicotine appears to protect the neuron cell against the Aβ42 toxicity by scavenging ROS and NO free radicals (Liu et al., 2003; Liu and Zhao, 2004), chelating copper and iron (Bridge et al., 2004; Zhang et al., 2006) and protecting antioxidants in cells (Linert et al., 1999; Liu et al., 2003).
5. Inflammation in neurodegenerative disorders
Activated microglia increase in aging brain and are associated with degenerative disorders such as AD, Parkinson and amyotrophic lateral sclerosis (ALS). Microglia exert neuroprotective functions by secreting growth factors or diffusible anti-inflammatory mediators, which help to resolve inflammation and restore tissue homeostasis (Klegeris and McGeer, 2002; Streit, 2002). However, microglia can also be neurotoxic by producing free radicals, inflammatory cytokines and other toxic factors. Recent studies indicate that neurons and astrocytes can regulate innate immune responses in microglia via both alpha7nAChRs and purinergic P2X7 receptors (Shytle et al., 2004; Suzuki et al., 2006). Primary cultures of both resting and actívate microglia and astrocytes show choline acetyltransferase (ChAT) activity and synthesize acetylcholine (De Rosa et al., 2005) suggesting that this neurotransmitter act as a local immune regulator and contribute to the regulation of microglial functions.
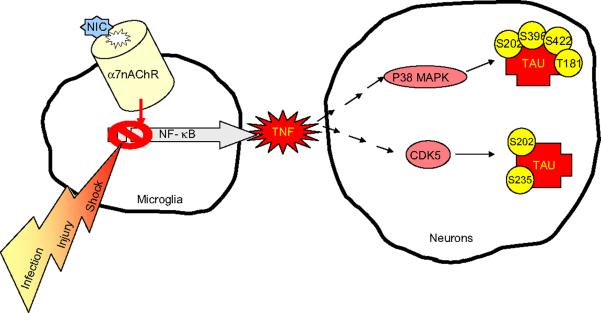
Stimulation of purinergic P2X7 receptors on microglia by neuronal released ATP induces small TNF production, which protects neurons (Zhang et al., 2007). On the other hand, LPS-stimulated microglia causes massive TNF production leading to inflammation (Suzuki et al., 2006). Acetylcholine and nicotine inhibit the production of TNF in LPS-stimulated mouse microglial cultures (De Rosa et al., 2005; Shytle et al., 2004). This anti-inflammatory potential of acetylcholine and nicotine is based on the inhibition of the NF-κB pathway through a specific `nicotinic anti-inflammatory pathway' dependent on the alpha7nAChR (Wang et al., 2004). The inhibition of TNF production in LPS-stimulated microglial cultures is also associated with a reduction in the activation of ERK and p38MAPK (Shytle et al., 2004; Suzuki et al., 2006) (Fig. 2). P38MAPK expression appears restricted to neurons and glial cells containing hyperphosphorylated tau, as well as dystrophic neurites of senile plaques in AD (Pei et al., 2001). P38MAPK phosphorylates tau in vitro at the threonine 181 and serines 202, 396 and 422 with different efficiencies for particular sites (Buee-Scherrer and Goedert, 2002; Ferrer et al., 2005; Goedert et al., 1997) (Fig. 3). Thus, inhibition of p38MAPK prevents tau phosphorylation (Fig. 2). Nicotine also acts as an anti-inflammatory agent on microglia by increasing the expression of COX-2 and the synthesis of prostaglandin PGE2, known to down-regulate microglial activation and expression of proinflammatory genes including TNF (De Rosa et al., 2005; Suzuki et al., 2006). However, nicotine has no effect on the release of IL-1β or IL-10, but it down-regulates nitric oxide (NO) production (Liu et al., 2007). The effect of nicotine on both, LPS-induced TNF production and PGE2 release, is counteracted by alpha-bungarotoxin, the specific antagonist of the alpha7nAChRs.
Fig. 2.
Effect of nicotine-alpha7nAChRs interaction on tau phosphorylation. The nicotine-alpha7nAChRs interaction inhibits the production of TNF in LPS-stimulated mouse microglial cultures through inhibition of the NF-κB pathway. This inhibition of TNF is associated with a reduction in phosphorylation of ERK and p38 MAPK. P38 MAPK can phosphorylates tau in neurons and glia. A reduction of p38 MAPK may prevent tau phosphorylation at S202, S296, S422 and T181. Activated microglia colocalize with amyloid plaques and facilitate amyloid clearance but can also phosphorylate tau through release of proinflammatory cytokines (including TNF), chemokines and other inflammatory components. Activation of the cdk5/p25 complex has also been proposed as underlying mechanism in the phosphorylation of tau at S202 and S235.
Fig. 3.
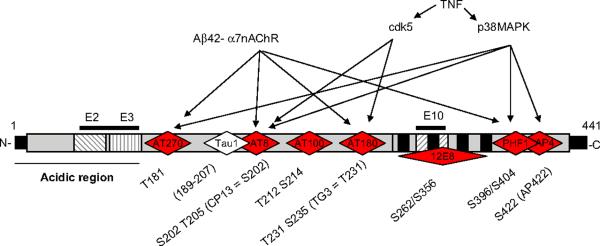
Schematic diagram showing the phospho tau epitopes affected by the Abeta42-alpha7nAChR interaction and the kinases activated by TNF in the longest isoform of tau. Tau isoforms are generated by splicing in or out exons 2, 3 and 10 (E2, E3 and E10). Red rhomboids are the different antibodies at specific phospho-tau epitopes. Abeta42 can phosphorylate tau at specific sites (T181, S202, T231 and S396/404) and TNF can activate kinases cdk5 and p38MAPK that phosphorylate tau at S202 and S235 (cdk5) and T181, S202, S396 and S422 (p30MAPK).
Activated microglia in the brain colocalize with amyloid plaques (Akiyama et al., 2000) and facilitate amyloid clearance by a phagocytic response (Frautschy et al., 1992; Rogers et al., 2002). Activated microglia can also exacerbate tau pathology (Kitazawa et al., 2005; Li et al., 2003; Yoshiyama et al., 2007) through a mechanism dependent on the cdk5/p25 (Kitazawa et al., 2005) (Fig. 2). Brain microglia increase p25, a calpain-induced cleaved fragment of p35 that activates cdk5 (Humbert et al., 2000; Lee and Tsai, 2003). Cyclin-dependent kinase-5 (cdk5) then hyper-phosphorylates tau at the serines 202 and 235, representing the disease-associated phospho-epitopes recognizes by the antibodies AT8 and TG3 respectively (Noble et al., 2003; Sengupta et al., 1997). P35 localizes in the cell membranes meanwhile p25 localizes in the cytoplasm and nucleus (Lee and Tsai, 2003; Weishaupt et al., 2003) becoming a neurotoxic fragment implicated in neuronal apoptosis (Lee et al., 2000). Over-expression of p25 has been confirmed in the brain of AD patients (Patrick et al., 1999). In this sense, little is known about the regulation of cdk5 by nicotinic receptors. Recent studies indicate that nicotine inhibits cdk5 phosphorylation of DARPP-32 (dopamine- and cAMP-regulated phosphoprotein of 32 kDa) at threonine 75 neostriatal slices through a mechanism possibly mediated by alpha4beta2nAChRs (Hamada et al., 2005).
Various types of anti-inflammatory agents have been assessed in clinical trials for AD. Meta-analysis of studies of AD in relation to the use of NSAIDs have found no beneficial effect of NSAIDs in preventing cognitive decline (de Craen et al., 2005). Prednisone, a glucocorticoid that inhibits the induction of COX-2, was also ineffective in improving cognition in AD (Aisen et al., 2000). Likewise, randomized clinical trials with COX-2 inhibitors (e.g. Rofecoxib, Naproxen) have failed to improve cognition in patients with mild to moderate AD (Aisen et al., 2003; Reines et al., 2004). Certain NSAIDs, including ibuprofen, have differential mechanisms of action on the inflammatory response and deposition of beta-amyloid. Ibuprofen possesses preferential Abeta-42 lowering activity not related to the inhibition of cyclooxygenases, but altering gamma-secretase activity (Leuchtenberger et al., 2006; Weggen et al., 2001). Indomethacin, also shown to lower Abeta-42, stabilizes cognitive decline in clinical trials of AD patients, perhaps independently of its Cox-2 inhibitory properties (Rogers et al., 1993; Weggen et al., 2001). Negative studies may have resulted from use of NSAIDs relatively late in the disease process or imprecise targeting of the inflammation response.
6. Alpha7nAChR neurotoxicity and neuroprotection
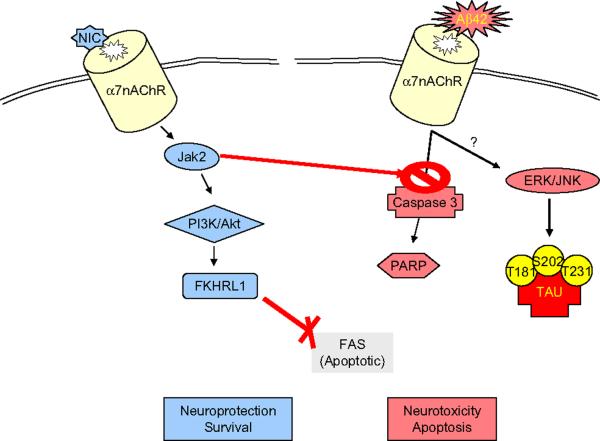
Alpha7nAChR mediates the toxicity of the beta-amyloid 42 (Aβ42). Many studies indicate the direct binding of Aβ42 to the alpha7nAChR as a triggering factor of neuronal cell death and Alzheimer's pathology. The binding of Aβ42 to the alpha7nAChRs on the neuronal surfaces leads to internalization of the alpha7nAChR-Aβ42 complex and its accumulation within the lysosomal compartment (D'Andrea et al., 2001; Wang et al., 2000a, b). This alpha7nAChR-Aβ42 interaction inhibits acetylcholine release and calcium flux, and contributes to cell death suggesting that this interaction may be a key event in the pathogenesis of AD (Fig. 4). The most vulnerable neurons to neurodegeneration appear to be those that express the alpha7nAChR. Aβ42 first accumulates in these neurons producing cell death prior to plaque formation (D'Andrea et al., 2001; Gyure et al., 2001; Shie et al., 2003; Wirths et al., 2001). This mechanism (mediated via ERK1/2 signaling) links the amyloid and the cholinergic system modulating synaptic plasticity and cognitive performance (Lynch, 2004; Silva et al., 1998). These results suggest that the alpha7nAChR could be a promising therapeutic target for treatment of AD. The Aβ42–alpha7nAChR interaction may induce tau phosphorylation at the Serine 202, Threonines 181 and 231 via ERKs and c-Jun N-terminal kinase (JNK-1) (Wang et al., 2003b) (Fig. 4). Alpha7nAChR gene delivery into mouse hippocampal neurons indicates that overexpression of alpha7nAChR prompted tau phosphorylation at both serine 202 and threonine 205 as recognized by AT8 (a monoclonal antibody that recognizes tau protein phosphorylated at these sites) (Ren et al., 2007) (Fig. 3). These studies depict the alpha7nAChR connecting the two classical pathological landmarks of AD beta-amyloid and hyperphosphorylation of tau, and support this receptor as a potential pharmacological target to inhibit tau phosphorylation in AD.
Fig. 4.
Schematic of the nicotinic-alpha7nAChR mediated activation of Jak2 neuroprotective pathway. The Abeta42-alpha7nAChR interaction activates the apoptotic enzyme caspase 3 and produces cleavage of the DNA-repairing enzyme poly-(ADP-ribose) polymerase causing eventually neurotoxicity and cell death. This cascade is inhibited by nicotinic activation of the JAK2-PI3K-Akt signaling pathway, promoting neuroprotection and cell survival. Activation of the anti-apoptotic kinase Akt involves phosphorylation of the forkhead transcription factor (FKHRL1), blocking eventually the expression of the apoptotic FAS protein. The Abeta42-alpha7nAChR interaction also produces increases in intracellular Ca2+ with activation of mitogen-activated kinase proteins ERK and JNK inducing tau hyperphosphorylation.
Alpha7nAChR-agonists trigger activation of tyrosine kinase Janus 2 (JAK2) and phosphorylation of Akt via activation of phosphatidylinositol-3-kinase (PI3K) (Kihara et al., 2001; Shaw et al., 2002) (Fig. 4). This mechanism seems to play a role in the neuroprotective properties of the alpha7nAChR versus amyloid neurotoxicity. In the neuronal cell line PC12, nicotine competes with Aβ42 for the binding to alpha7nAChR (in a “dominant” way), and prevents the Aβ42-induction of caspase 3 and apoptosis. The latter seems to be the result of nicotinic activation of the JAK2-PI3K-Akt signalling pathway, rather than blockade of Aβ42 binding to the alpha7nAChR (Shaw et al., 2002). This effect appears to be mediated by alpha7nAChR because the protection is blocked by alpha-bungarotoxin and is mimicked by the alpha7nAChR-agonist TC-1698 (Marrero et al., 2004; Shaw et al., 2002, 2003). Treatment with nicotine for ten days in the APPsw mice model (transgenic mice overproducing mutant amyloid β protein precursor, βAPP) reduced insoluble amyloid Aβ1–40 and Aβ1–42 peptides by 80% in the brain cortex of 9 month-old mice (Hellstrom-Lindahl et al., 2004a). This effect is mediated, at least in part, by the alpha7nAChRs as shown by using mecamylamine (Hellstrom-Lindahl et al., 2004a). Together, these findings support the notion that JAK2 mediates the alpha7nAChR-induced neuroprotection against Aβ42. Hence, these studies strengthen the potential of alpha7nAChR as a pharmacological target for neuroprotection in AD by preventing neuronal apoptosis.
7. Alpha7nAChR-agonists and allosteric modulators in neurodegenerative disorders
Alpha7nAChR-agonists were developed for the treatment of disorders with a cognitive component such as AD and schizophrenia despite early concerns that the rapid desensitization of this receptor would limit their therapeutical potential. One of the most characteristic alpha7nAChR-agonist is GTS21. GTS21 (3-[(2,4-dimethoxy) benzylidene]-anabaseine), a partial alpha7nAChR-agonist, enhances attention, working memory and episodic memory measured in healthy humans (Kitagawa et al., 2003). This compound was well tolerated at doses of up 450 mg/day, doses higher than those allowed by nicotine, and no significant side effects were observed. Unlike nicotine, GTS21 has no effect on locomotor activity in mice or on dopamine turnover in experimental rats indicating that it is less toxic than nicotine (Kitagawa et al., 2003; Meyer et al., 1998; Ulloa, 2005). A recent proof-of-concept trial indicate that GTS21 improve cognition and P50 sensory gating (a positive component of the auditory evoked potential peaking around 50 ms post-stimulus, which provides a measure of sensory motor gating) in schizophrenic patients (Martin et al., 2004; Olincy et al., 2006). Although the cognitive improvements were primarily in attention, further studies are needed to clarify if the improvements apply to other cognitive functions (e.g. immediate and delayed memory functions). Based in these initial results achieved with GTS-21, clinical trials in participants between 50 and 80 years old with AD were developed by Athenagen Biopharmaceuticals to evaluate both safety and cognitive improvements in these patients though results are not yet published. In vitro, GTS-21 can protect neurons against damage induced by amyloid peptides, this is in agreement with previous studies suggesting that alpha7nAChRs can have a neuroprotective potential. GTS21 have three major adversities for clinical use: (1) GTS21 is not specific for alpha7nAChR and it affects other receptors including alpha4beta2-nAChRs (Gerzanich et al., 1995; Meyer et al., 1998; Stokes et al., 2004) and 5-HT3A (Machu et al., 2001), (2) GTS21 has high affinity for the rodent receptor but it presents low affinity for the human alpha7nAChR (Gerzanich et al., 1995; Meyer et al., 1998; Stokes et al., 2004), and (3) GTS21 appears to have a limited brain penetration (Kem et al., 2004). These limitations appear to be overcome by a second generation of alpha7nAChR-agonists including 4OHGTS (3-(4-hydroxy, 2-methoxybenzylidene) anabaseine) (Meyer et al., 1998; Uteshev et al., 2003), compound that was developed to increase its affinity for human alpha7nAChR (Gerzanich et al., 1995). SSR180711 (1, 4-Diazabicyclo [3.2.2] nonane-4-carboxylic acid, 4-bromophenyl ester), was specifically design as a selective alpha7nAChR-agonist, which rapidly penetrates into the brain and displays high affinity for human alpha7nAChRs. Microdialysis studies show that administration of this compound increases acetylcholine release, glutamatergic neurotransmission and LTP in rat hippocampus in a dose-dependent manner (Biton et al., 2007). Thus, this new generation of alpha7nAChR holds high expectancies to improve cognitive deficits.
MEM 3454 (Memory Pharmaceuticals/Roche) is a novel partial agonist of the alpha7nAChR with 5-HT3 receptor antagonist properties. MEM 3454 was developed as a potential therapy for AD and schizophrenia and it is currently in phase-II clinical trial. Results from phase I showed significant improvements of memory and concentration in healthy subjects after 13 days of daily 15 mgrs (Callahan et al., 2006), though the exact paradigm used to assess its effects were somewhat unveil. Quinuclidines are also partial alpha7nAChR-agonists (e.g., PNU-282987, Pfizer; PNU-282987 ([N-[(3R)-1-Azabicyclo [2.2.2] oct-3-yl]-4-chlorobenzamide hydrochloride])) that improve cognitive functions in experimental animals models (Bodnar et al., 2005; Leonik et al., 2007). A novel set of azabicyclic aryl amides to improve the properties of PNU-282987 were recently identified as selective alpha7nAChR-agonist with a potential for treating cognitive deficits (Walker et al., 2006). Among them, compound 14, N-[(3R)-1-Azabicyclo [2.2.2]oct-3-yl] furo [2,3-c] pyridine-5-carboxamide (14, PHA-543,613), presents improved oral bioavailability, brain penetration, efficacy improving auditory sensory gating and novel object recognition (Wishka et al., 2006). ABBF (Bayer group), N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl]-7-[2-(methoxy)phenyl]-1-benzofuran-2-carboxamide, is also a novel agonist with higher affinity for the alpha7 receptor (no agonist activity at other nAChRs subtypes) and a better binding selectivity over the 5-HT3 receptors, that improves working and recognition memory in rodents (Boess et al., 2007).
In addition to characteristic nicotinic agonists, the nAChR can be activated by a novel class of drugs, called allosteric enhancers because they activate these receptors without binding to the acetylcholine-binding site (Akk and Steinbach, 2005; Pereira et al., 2002). The most characteristic examples are physostigmine and galantamine (Reminyl; Janssen Pharmaceutica, Titusville, NJ); both belong to a class of acetylcholinesterase inhibitors approved for symptomatic treatment of schizophrenia and AD. These drugs presumably act by raising and prolonging the profile of acetylcholine via an inhibitory effect on the esterase. However, these drugs can also bind directly to nAChRs and modulate its activation. Galantamine produces a brief, voltage-dependent channel block, consistent with a simple, linear open channel blocking mechanism (Cooper et al., 1996). Galantamine does not interfere with the binding of nicotinic agonist such as acetylcholine, carbachol, choline or [I125]-alpha-bungarotoxin (Akk and Steinbach, 2005). These acetylcholinesterase inhibitors have no significant effect on either the amplitude or kinetics of alpha7nAChRs activated by ACh, but they slowed the rate of (Fayuk and Yakel, 2004) recovery from desensitization through an indirect mechanism; responses activated with either choline or carbachol seem unaffected. The existence of multiple classes of binding sites is well established for other ligand-gated ion channels. For example, the GABAA receptor can be activated by several classes of drugs that bind to no overlapping regions of the receptor: while GABA and muscimol interact with the characteristic ligand-binding site, barbiturates and steroids bind different domains (Ueno et al., 1997). Further studies are needed to identify the precise binding site of these potential allosteric enhancers to nAChRs, determine its pharmacological interest and contribution to the therapeutic effect of these compounds in AD. Future studies are needed to determine whether the cognitive potential of these agonists is based on their binding to neuronal receptors or whether their anti-inflammatory potential may contribute to their therapeutic potential in neurological disorders.
More selective allosteric enhancers for nAChRs are just starting to emerge. PNU-120596 is the first selective allosteric potentiator reported for the alpha7nAChR, with no effects on alpha4beta2, alpha3beta4 or alpha9beta10-nAChRs (Hurst et al., 2005), Compounds 2087101, 2087133 and 1078733 have been recently described as selectively potentiate alpha7, alpha2beta4, alpha4beta2 and alpha4beta4, but not alpha3- or alpha1-containing nAChRs or other ion channels (Broad et al., 2006). These compounds should be useful in establishing if nAChR potentiators can improve desensitization, have better tolerance, while producing comparable cognitive enhancement to the nAChR agonists. In addition, their selectivity potential for central nAChRs, but not ganglionic or neuromuscular nAChRs, may help to understand its clinical efficacy.
8. Concluding remarks and future directions
Recent studies suggest that cholinergic attrition associated with AD may actually link inflammation and neurodegeneration. Although acetylcholine is historically considered to be a neurotransmitter, it can also function as an immune cytokine and might represent a common ancestral mediator in cellular biology. Acetylcholine can be synthesized by both nervous and immune cells and serve as link for the neural-immune coordination (Ulloa, 2005). Deficiencies in the cholinergic system can explain cognitive impairments as well as a chronic inflammatory environment contributing to neurodegeneration. Chronic microglial activation contributes to neurodegenerative events such as plaque formation, dystrophic neurite growth and tau hyperphosphorylation. A recent study in a P301S tauopathy mouse model describes synaptic loss and microglial activation preceding tangle formation (Yoshiyama et al., 2007). Thus, microglia driven neuropathological responses constitute itself a pathogenic factor in neurodegenerative diseases such as AD. Among the cholinergic receptors, alpha7nAChR stimulation appears to be required for the anti-inflammatory potential of cholinergic agonists in immune cells including microglia. Giving this connection, it may not be surprising that nicotine and the alpha7nAChR have been extensively involved to the pathogenesis of AD. These studies suggest that the beneficial effects of nicotine in neurological disorders may be, at least in part, mediated by its anti-inflammatory role in microglia cells. Nicotine may impinge directly in both mechanisms. It can target neurons and improve cognitive performance, but it may also attenuate inflammatory responses in glial cells. This dual mechanism can explain some discrepancies found in the literature. Nicotine may prevent tau hyperphosphorylation in vivo, but not in neuronal cultures of cell lines. These discrepancies can be explained by the effects of nicotine in glial cells and warrant future studies in co-culture of glial and neuronal cells. Future studies are also needed to determine whether the cognitive potential of nicotinic agonists is based on its neuronal or anti-inflammatory potential.
Experimental evidence links the alpha7nAChR with inflammatory processes (Wang et al., 2003a). The alpha7nAChR expression is increased in astrocytes from hippocampus and entorhinal cortex of AD patients when compared with age-matched controls (Graham et al., 2002, 2003; Teaktong et al., 2003). It may represent a compensatory mechanism to restrain TNF production in astrocytes during AD. If so, alpha7nAChRs-agonists may provide a pharmacological strategy to restrain the production of inflammatory cytokines like TNF, but also to selectively modulate the NF-κB pathway in immune cells (Huston et al., 2006). This pathway contributes to inflammatory responses in immune cells but it also has a neuroprotective potential in neurons. Little is known about the contribution of this pathway to AD, but this hypothesis is in agreement with studies in apoE4 mice indicating that the NF-κB pathway plays a critical role in neuroinflammation associated with AD (Ophir et al., 2005). Future studies are needed to determine whether alpha7nAChRs can induce a differential regulation of NF-κB in different cell lines.
Acknowledgements
LU is supported by the faculty program of the Department of Surgery of the University of Medicine of New Jersey, and grants from the the American Heart Association (AHA06352230N) and the US Army Medical Research Command (USAMRMC#05308004).
References
- Adler LE, Hoffer LD, Wiser A, Freedman R. Normalization of auditory physiology by cigarette smoking in schizophrenic patients. American Journal of Psychiatry. 1993;150:1856–1861. doi: 10.1176/ajp.150.12.1856. [DOI] [PubMed] [Google Scholar]
- Adler LE, Olincy A, Waldo M, Harris JG, Griffith J, Stevens K, Flach K, Nagamoto H, Bickford P, Leonard S, Freedman R. Schizophrenia, sensory gating, and nicotinic receptors. Schizophrenia Bulletin. 1998;24:189–202. doi: 10.1093/oxfordjournals.schbul.a033320. [DOI] [PubMed] [Google Scholar]
- Aisen PS, Davis KL, Berg JD, Schafer K, Campbell K, Thomas RG, Weiner MF, Farlow MR, Sano M, Grundman M, Thal LJ. A randomized controlled trial of prednisone in Alzheimer's disease. Alzheimer's Disease Cooperative Study [see comment] Neurology. 2000;54:588–593. doi: 10.1212/wnl.54.3.588. [DOI] [PubMed] [Google Scholar]
- Aisen PS, Schafer KA, Grundman M, Pfeiffer E, Sano M, Davis KL, Farlow MR, Jin S, Thomas RG, Thal LJ, Study., A.S.D.C. Effects of rofecoxib or naproxen vs placebo on Alzheimer disease progression: a randomized controlled trial. Journal of the American Medical Association. 2003;289:2819–2826. doi: 10.1001/jama.289.21.2819. [DOI] [PubMed] [Google Scholar]
- Akiyama H, Arai T, Kondo H, Tanno E, Haga C, Ikeda K. Cell mediators of inflammation in the Alzheimer disease brain. Alzheimer Disease and Associated Disorders. 2000;14(Suppl. 1):S47–S53. doi: 10.1097/00002093-200000001-00008. [DOI] [PubMed] [Google Scholar]
- Akk G, Steinbach JH. Galantamine activates muscle-type nicotinic acetylcholine receptors without binding to the acetylcholine-binding site. Journal of Neuroscience. 2005;25:1992–2001. doi: 10.1523/JNEUROSCI.4985-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkondon M, Albuquerque EX. Diversity of nicotinic acetylcholine receptors in rat hippocampal neurons. I. Pharmacological and functional evidence for distinct structural subtypes. Journal of Pharmacology and Experimental Therapeutics. 1993;265:1455–1473. [PubMed] [Google Scholar]
- Berg DK, Conroy WG. Nicotinic alpha 7 receptors: synaptic options and downstream signaling in neurons. Journal of Neurobiology. 2002;53:512–523. doi: 10.1002/neu.10116. [DOI] [PubMed] [Google Scholar]
- Biton B, Bergis OE, Galli F, Nedelec A, Lochead AW, Jegham S, Godet D, Lanneau C, Santamaria R, Chesney F, Leonardon J, Granger P, Debono MW, Bohme GA, Sgard F, Besnard F, Graham D, Coste A, Oblin A, Curet O, Vige X, Voltz C, Rouquier L, Souilhac J, Santucci V, Gueudet C, Francon D, Steinberg R, Griebel G, Oury-Donat F, George P, Avenet P, Scatton B. SSR180711, a novel selective alpha7 nicotinic receptor partial agonist: (1) binding and functional profile. Neuropsychopharmacology. 2007;32:1–16. doi: 10.1038/sj.npp.1301189. [DOI] [PubMed] [Google Scholar]
- Bodnar AL, Cortes-Burgos LA, Cook KK, Dinh DM, Groppi VE, Hajos M, Higdon NR, Hoffmann WE, Hurst RS, Myers JK, Rogers BN, Wall TM, Wolfe ML, Wong E. Discovery and structure-activity relationship of quinuclidine benzamides as agonists of alpha7 nicotinic acetylcholine receptors. Journal of Medicinal Chemistry. 2005;48:905–908. doi: 10.1021/jm049363q. [DOI] [PubMed] [Google Scholar]
- Boess FG, De Vry J, Erb C, Flessner T, Hendrix M, Luithle J, Methfessel C, Riedl B, Schnizler K, van der Staay FJ, van Kampen M, Wiese WB, Koenig G. The novel alpha7 nicotinic acetylcholine receptor agonist N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl]-7-[2-(methoxy)phenyl]-1-benzofuran-2-carboxamide improves working and recognition memory in rodents. Journal of Pharmacology and Experimental Therapeutics. 2007;321:716–725. doi: 10.1124/jpet.106.118976. [DOI] [PubMed] [Google Scholar]
- Breese CR, Adams C, Logel J, Drebing C, Rollins Y, Barnhart M, Sullivan B, Demasters BK, Freedman R, Leonard S. Comparison of the regional expression of nicotinic acetylcholine receptor alpha7 mRNA and [125I]-alpha-bungarotoxin binding in human postmortem brain. Journal of Comparative Neurology. 1997;387:385–398. doi: 10.1002/(sici)1096-9861(19971027)387:3<385::aid-cne5>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Bridge MH, Williams E, Lyons ME, Tipton KF, Linert W. Electrochemical investigation into the redox activity of Fe(II)/Fe(III) in the presence of nicotine and possible relations to neurodegenerative diseases. Biochimica et Biophysica Acta. 2004;1690:77–84. doi: 10.1016/j.bbadis.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Broad LM, Zwart R, Pearson KH, Lee M, Wallace L, McPhie GI, Emkey R, Hollinshead SP, Dell CP, Baker SR, Sher E. Identification and pharmacological profile of a new class of selective nicotinic acetylcholine receptor potentiators. Journal of Pharmacology and Experimental Therapeutics. 2006;318:1108–1117. doi: 10.1124/jpet.106.104505. [DOI] [PubMed] [Google Scholar]
- Buee-Scherrer V, Goedert M. Phosphorylation of microtubule-associated protein tau by stress-activated protein kinases in intact cells. FEBS Letters. 2002;515:151–154. doi: 10.1016/s0014-5793(02)02460-2. [DOI] [PubMed] [Google Scholar]
- Burghaus L, Schutz U, Krempel U, de Vos RA, Jansen Steur EN, Wevers A, Lindstrom J, Schroder H. Quantitative assessment of nicotinic acetylcholine receptor proteins in the cerebral cortex of Alzheimer patients. Brain Research. Molecular Brain Research. 2000;76:385–388. doi: 10.1016/s0169-328x(00)00031-0. [DOI] [PubMed] [Google Scholar]
- Callahan PM, Wang S, Xie W, Dragan S, Sun S, Michael T, Herbert B, Rowe W, Tehim A, Lowe D, Barret JE. Pharmacological characterization of MEM 3454, a novel nicotinic alpha7 receptor partial agonist: theurapeutic potential for the cognitive deficits associated with Alzheimer's disease and schizophrenia. Neuropsychopharmacology. 2006;31:198. [Google Scholar]
- Clarke PB. The fall and rise of neuronal alpha-bungarotoxin binding proteins. Trends in Pharmacological Sciences. 1992;13:407–413. doi: 10.1016/0165-6147(92)90125-p. [DOI] [PubMed] [Google Scholar]
- Cleary JP, Walsh DM, Hofmeister JJ, Shankar GM, Kuskowski MA, Selkoe DJ, Ashe KH. Natural oligomers of the amyloid-beta protein specifically disrupt cognitive function. Nature Neuroscience. 2005;8:79–84. doi: 10.1038/nn1372. [DOI] [PubMed] [Google Scholar]
- Cooper JC, Gutbrod O, Witzemann V, Methfessel C. Pharmacology of the nicotinic acetylcholine receptor from fetal rat muscle expressed in Xenopus oocytes. European Journal of Pharmacology. 1996;309:287–298. doi: 10.1016/0014-2999(96)00294-4. [DOI] [PubMed] [Google Scholar]
- Court JA, Lloyd S, Johnson M, Griffiths M, Birdsall NJ, Piggott MA, Oakley AE, Ince PG, Perry EK, Perry RH. Nicotinic and muscarinic cholinergic receptor binding in the human hippocampal formation during development and aging. Developmental Brain Research. 1997;101:93–105. doi: 10.1016/s0165-3806(97)00052-7. [DOI] [PubMed] [Google Scholar]
- Court JA, Martin-Ruiz C, Graham A, Perry E. Nicotinic receptors in human brain: topography and pathology. Journal of Chemical Neuroanatomy. 2000;20:281–298. doi: 10.1016/s0891-0618(00)00110-1. [DOI] [PubMed] [Google Scholar]
- Dajas-Bailador FA, Mogg AJ, Wonnacott S. Intracellular Ca2+ signals evoked by stimulation of nicotinic acetylcholine receptors in SH-SY5Y cells: contribution of voltage-operated Ca2+ channels and Ca2+ stores. Journal of Neurochemistry. 2002a;81:606–614. doi: 10.1046/j.1471-4159.2002.00846.x. [DOI] [PubMed] [Google Scholar]
- Dajas-Bailador FA, Soliakov L, Wonnacott S. Nicotine activates the extracellular signal-regulated kinase 1/2 via the alpha7 nicotinic acetylcholine receptor and protein kinase A, in SH-SY5Y cells and hippocampal neurones. Journal of Neurochemistry. 2002b;80:520–530. doi: 10.1046/j.0022-3042.2001.00725.x. [DOI] [PubMed] [Google Scholar]
- Davies P, Feisullin S. Postmortem stability of alpha-bungarotoxin binding sites in mouse and human brain. Brain Research. 1981;216:449–454. doi: 10.1016/0006-8993(81)90148-7. [DOI] [PubMed] [Google Scholar]
- de Craen AJ, Gussekloo J, Vrijsen B, Westendorp RG. Meta-analysis of nonsteroidal antiinflammatory drug use and risk of dementia. American Journal of Epidemiology. 2005;161:114–120. doi: 10.1093/aje/kwi029. [DOI] [PubMed] [Google Scholar]
- De Rosa MJ, Esandi Mdel C, Garelli A, Rayes D, Bouzat C. Relationship between alpha 7nAChR and apoptosis in human lymphocytes. Journal of Neuroimmunology. 2005;160:154–161. doi: 10.1016/j.jneuroim.2004.11.010. [DOI] [PubMed] [Google Scholar]
- DeKosky ST, Scheff SW. Synapse loss in frontal cortex biopsies in Alzheimer's disease: correlation with cognitive severity. Annals of Neurology. 1990;27:457–464. doi: 10.1002/ana.410270502. [DOI] [PubMed] [Google Scholar]
- Depatie L, O'Driscoll GA, Holahan AL, Atkinson V, Thavundayil JX, Kin NN, Lal S. Nicotine and behavioral markers of risk for schizophrenia: a double-blind, placebo-controlled, cross-over study. Neuropsychopharmacology. 2002;27:1056–1070. doi: 10.1016/S0893-133X(02)00372-X. [DOI] [PubMed] [Google Scholar]
- Drisdel RC, Green WN. Neuronal alpha-bungarotoxin receptors are alpha7 subunit homomers. Journal of Neuroscience. 2000;20:133–139. doi: 10.1523/JNEUROSCI.20-01-00133.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Andrea MR, Nagele RG, Wang HY, Peterson PA, Lee DH. Evidence that neurones accumulating amyloid can undergo lysis to form amyloid plaques in Alzheimer's disease. Histopathology. 2001;38:120–134. doi: 10.1046/j.1365-2559.2001.01082.x. [DOI] [PubMed] [Google Scholar]
- Engidawork E, Gulesserian T, Balic N, Cairns N, Lubec G. Changes in nicotinic acetylcholine receptor subunits expression in brain of patients with Down syndrome and Alzheimer's disease. Journal of Neural Transmission Supplementum. 2001:211–222. doi: 10.1007/978-3-7091-6262-0_17. [DOI] [PubMed] [Google Scholar]
- Fayuk D, Yakel JL. Regulation of nicotinic acetylcholine receptor channel function by acetylcholinesterase inhibitors in rat hippocampal CA1 interneurons. Molecular Pharmacology. 2004;66:658–666. doi: 10.1124/mol.104.000042. [DOI] [PubMed] [Google Scholar]
- Fernandes C, Hoyle E, Dempster E, Schalkwyk LC, Collier DA. Performance deficit of alpha7 nicotinic receptor knockout mice in a delayed matching-to-place task suggests a mild impairment of working/episodic-like memory. Genes, Brain, and Behavior. 2006;5:433–440. doi: 10.1111/j.1601-183X.2005.00176.x. [DOI] [PubMed] [Google Scholar]
- Ferrer I, Barrachina M, Puig B, Martinez de Lagran M, Marti E, Avila J, Dierssen M. Constitutive Dyrk1A is abnormally expressed in Alzheimer disease, Down syndrome, Pick disease, and related transgenic models. Neurobiology of Disease. 2005;20:392–400. doi: 10.1016/j.nbd.2005.03.020. [DOI] [PubMed] [Google Scholar]
- Fratiglioni L, Wang HX. Smoking and Parkinson's and Alzheimer's disease: review of the epidemiological studies. Behavioural Brain Research. 2000;113:117–120. doi: 10.1016/s0166-4328(00)00206-0. [DOI] [PubMed] [Google Scholar]
- Frautschy SA, Cole GM, Baird A. Phagocytosis and deposition of vascular beta-amyloid in rat brains injected with Alzheimer beta-amyloid. American Journal of Pathology. 1992;140:1389–1399. [PMC free article] [PubMed] [Google Scholar]
- Freedman R, Leonard S, Waldo M, Gault J, Olincy A, Adler LE. Characterization of allelic variants at chromosome 15q14 in schizophrenia. Genes, Brain, and Behavior. 2006;5(Suppl. 1):14–22. doi: 10.1111/j.1601-183X.2006.00190.x. [DOI] [PubMed] [Google Scholar]
- Gerzanich V, Peng X, Wang F, Wells G, Anand R, Fletcher S, Lindstrom J. Comparative pharmacology of epibatidine: a potent agonist for neuronal nicotinic acetylcholine receptors. Molecular Pharmacology. 1995;48:774–782. [PubMed] [Google Scholar]
- Goedert M, Hasegawa M, Jakes R, Lawler S, Cuenda A, Cohen P. Phosphorylation of microtubule-associated protein tau by stress-activated protein kinases. FEBS Letters. 1997;409:57–62. doi: 10.1016/s0014-5793(97)00483-3. [DOI] [PubMed] [Google Scholar]
- Graham AJ, Martin-Ruiz CM, Teaktong T, Ray MA, Court JA. Human brain nicotinic receptors, their distribution and participation in neuropsychiatric disorders. Current Drug Targets—CNS and Neurological Disorders. 2002;1:387–397. doi: 10.2174/1568007023339283. [DOI] [PubMed] [Google Scholar]
- Graham AJ, Ray MA, Perry EK, Jaros E, Perry RH, Volsen SG, Bose S, Evans N, Lindstrom J, Court JA. Differential nicotinic acetylcholine receptor subunit expression in the human hippocampus. Journal of Chemical Neuroanatomy. 2003;25:97–113. doi: 10.1016/s0891-0618(02)00100-x. [DOI] [PubMed] [Google Scholar]
- Guan ZZ, Zhang X, Ravid R, Nordberg A. Decreased protein levels of nicotinic receptor subunits in the hippocampus and temporal cortex of patients with Alzheimer's disease. Journal of Neurochemistry. 2000;74:237–243. doi: 10.1046/j.1471-4159.2000.0740237.x. [DOI] [PubMed] [Google Scholar]
- Gyure KA, Durham R, Stewart WF, Smialek JE, Troncoso JC. Intraneuronal abeta-amyloid precedes development of amyloid plaques in Down syndrome. Archives of Pathology and Laboratory Medicine. 2001;125:489–492. doi: 10.5858/2001-125-0489-IAAPDO. [DOI] [PubMed] [Google Scholar]
- Hamada M, Hendrick JP, Ryan GR, Kuroiwa M, Higashi H, Tanaka M, Nairn AC, Greengard P, Nishi A. Nicotine regulates DARPP-32 (dopamine- and cAMP-regulated phosphoprotein of 32 kDa) phosphorylation at multiple sites in neostriatal neurons. Journal of Pharmacology and Experimental Therapeutics. 2005;315:872–878. doi: 10.1124/jpet.105.090852. [DOI] [PubMed] [Google Scholar]
- Harris JG, Kongs S, Allensworth D, Martin L, Tregellas J, Sullivan B, Zerbe G, Freedman R. Effects of nicotine on cognitive deficits in schizophrenia. Neuropsychopharmacology. 2004;29:1378–1385. doi: 10.1038/sj.npp.1300450. [DOI] [PubMed] [Google Scholar]
- Hellstrom-Lindahl E, Mousavi M, Zhang X, Ravid R, Nordberg A. Regional distribution of nicotinic receptor subunit mRNAs in human brain: comparison between Alzheimer and normal brain. Brain Research. Molecular Brain Research. 1999;66:94–103. doi: 10.1016/s0169-328x(99)00030-3. [DOI] [PubMed] [Google Scholar]
- Hellstrom-Lindahl E, Court J, Keverne J, Svedberg M, Lee M, Marutle A, Thomas A, Perry E, Bednar I, Nordberg A. Nicotine reduces A beta in the brain and cerebral vessels of APPsw mice. European Journal of Neuroscience. 2004a;19:2703–2710. doi: 10.1111/j.0953-816X.2004.03377.x. [DOI] [PubMed] [Google Scholar]
- Hellstrom-Lindahl E, Mousavi M, Ravid R, Nordberg A. Reduced levels of Abeta 40 and Abeta 42 in brains of smoking controls and Alzheimer's patients. Neurobiology of Disease. 2004b;15:351–360. doi: 10.1016/j.nbd.2003.11.024. [DOI] [PubMed] [Google Scholar]
- Hoyle E, Genn RF, Fernandes C, Stolerman IP. Impaired performance of alpha7 nicotinic receptor knockout mice in the five-choice serial reaction time task. Psychopharmacology. 2006;189:211–223. doi: 10.1007/s00213-006-0549-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M, Liu QS, Chang KT, Berg DK. Nicotinic regulation of CREB activation in hippocampal neurons by glutamatergic and nonglutamatergic pathways. Molecular and Cellular Neurosciences. 2002;21:616–625. doi: 10.1006/mcne.2002.1202. [DOI] [PubMed] [Google Scholar]
- Humbert S, Dhavan R, Tsai L. p39 activates cdk5 in neurons, and is associated with the actin cytoskeleton. Journal of Cell Science. 2000;113:975–983. doi: 10.1242/jcs.113.6.975. [DOI] [PubMed] [Google Scholar]
- Hurst RS, Hajos M, Raggenbass M, Wall TM, Higdon NR, Lawson JA, Rutherford-Root KL, Berkenpas MB, Hoffmann WE, Piotrowski DW, Groppi VE, Allaman G, Ogier R, Bertrand S, Bertrand D, Arneric SP. A novel positive allosteric modulator of the alpha7 neuronal nicotinic acetylcholine receptor: in vitro and in vivo characterization. Journal of Neuroscience. 2005;25:4396–4405. doi: 10.1523/JNEUROSCI.5269-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huston JM, Ochani M, Rosas-Ballina M, Liao H, Ochani K, Pavlov VA, Gallowitsch-Puerta M, Ashok M, Czura CJ, Foxwell B, Tracey KJ, Ulloa L. Splenectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis. Journal of Experimental Medicine. 2006;203:1623–1628. doi: 10.1084/jem.20052362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itier V, Bertrand D. Neuronal nicotinic receptors: from protein structure to function. FEBS Letters. 2001;504:118–125. doi: 10.1016/s0014-5793(01)02702-8. [DOI] [PubMed] [Google Scholar]
- Kem WR, Mahnir VM, Prokai L, Papke RL, Cao X, LeFrancois S, Wildeboer K, Prokai-Tatrai K, Porter-Papke J, Soti F. Hydroxy metabolites of the Alzheimer's drug candidate 3-[(2,4-dimethoxy)benzylidene]-anabaseine dihydrochloride (GTS-21): their molecular properties, interactions with brain nicotinic receptors, and brain penetration. Molecular Pharmacology. 2004;65:56–67. doi: 10.1124/mol.65.1.56. [DOI] [PubMed] [Google Scholar]
- Kihara T, Shimohama S, Sawada H, Honda K, Nakamizo T, Shibasaki H, Kume T, Akaike A. Alpha 7 nicotinic receptor transduces signals to phosphatidylinositol 3-kinase to block A beta-amyloid-induced neurotoxicity. Journal of Biological Chemistry. 2001;276:13541–13546. doi: 10.1074/jbc.M008035200. [DOI] [PubMed] [Google Scholar]
- Kitagawa H, Takenouchi T, Azuma R, Wesnes KA, Kramer WG, Clody DE, Burnett AL. Safety, pharmacokinetics, and effects on cognitive function of multiple doses of GTS-21 in healthy, male volunteers. Neuropsychopharmacology. 2003;28:542–551. doi: 10.1038/sj.npp.1300028. [DOI] [PubMed] [Google Scholar]
- Kitazawa M, Oddo S, Yamasaki TR, Green KN, LaFerla FM. Lipopolysaccharide-induced inflammation exacerbates tau pathology by a cyclin-dependent kinase 5-mediated pathway in a transgenic model of Alzheimer's disease. Journal of Neuroscience. 2005;25:8843–8853. doi: 10.1523/JNEUROSCI.2868-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klegeris A, McGeer PL. Cyclooxygenase and 5-lipoxygenase inhibitors protect against mononuclear phagocyte neurotoxicity. Neurobiology of Aging. 2002;23:787–794. doi: 10.1016/s0197-4580(02)00021-0. [DOI] [PubMed] [Google Scholar]
- Kosik KS, Joachim CL, Selkoe DJ. Microtubule-associated protein tau (tau) is a major antigenic component of paired helical filaments in Alzheimer disease. Proceedings of the National Academy of Sciences of the United States of America. 1986;83:4044–4048. doi: 10.1073/pnas.83.11.4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MS, Tsai LH. Cdk5: one of the links between senile plaques and neurofibrillary tangles? Journal of Alzheimer's Disease. 2003;5:127–137. doi: 10.3233/jad-2003-5207. [DOI] [PubMed] [Google Scholar]
- Lee MS, Kwon YT, Li M, Peng J, Friedlander RM, Tsai LH. Neurotoxicity induces cleavage of p35 to p25 by calpain. Nature. 2000;405:360–364. doi: 10.1038/35012636. [DOI] [PubMed] [Google Scholar]
- Leonik FM, Papke RL, NA H. Quinuclidines as selective agonists for alpha-7 nicotinic acetylcholine receptors. Bioorganic & Medicinal Chemistry Letters. 2007;17:1520–1522. doi: 10.1016/j.bmcl.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Leuchtenberger S, Beher D, Weggen S. Selective modulation of Abeta42 production in Alzheimer's disease: non-steroidal anti-inflammatory drugs and beyond. Current Pharmaceutical Design. 2006;12:4337–4355. doi: 10.2174/138161206778793029. [DOI] [PubMed] [Google Scholar]
- Levin ED. Nicotinic receptor subtypes and cognitive function. Journal of Neurobiology. 2002;53:633–640. doi: 10.1002/neu.10151. [DOI] [PubMed] [Google Scholar]
- Levin ED, McClernon FJ, Rezvani AH. Nicotinic effects on cognitive function: behavioral characterization, pharmacological specification, and anatomic localization. Psychopharmacology. 2006;184:523–539. doi: 10.1007/s00213-005-0164-7. [DOI] [PubMed] [Google Scholar]
- Li Y, Liu L, Barger SW, Griffin WS. Interleukin-1 mediates pathological effects of microglia on tau phosphorylation and on synaptophysin synthesis in cortical neurons through a p38-MAPK pathway. Journal of Neuroscience. 2003;23:1605–1611. doi: 10.1523/JNEUROSCI.23-05-01605.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linert W, Bridge MH, Huber M, Bjugstad KB, Grossman S, Arendash GW. In vitro and in vivo studies investigating possible antioxidant actions of nicotine: relevance to Parkinson's and Alzheimer's diseases. Biochimica et Biophysica Acta. 1999;1454:143–152. doi: 10.1016/s0925-4439(99)00029-0. [DOI] [PubMed] [Google Scholar]
- Liu Q, Zhao B. Nicotine attenuates beta-amyloid peptide-induced neurotoxicity, free radical and calcium accumulation in hippocampal neuronal cultures. British Journal of Pharmacology. 2004;141:746–754. doi: 10.1038/sj.bjp.0705653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Tao Y, Zhao B. ESR study on scavenging effect of nicotine on free radicals. Applied Magnetic Resonance. 2003;24:105–112. [Google Scholar]
- Liu Q, Zhang J, Zhu H, Qin C, Chen Q, Zhao B. Dissecting the signaling pathway of nicotine-mediated neuroprotection in a mouse Alzheimer disease model. FASEB Journal. 2007;21:61–73. doi: 10.1096/fj.06-5841com. [DOI] [PubMed] [Google Scholar]
- Lukas RJ, Changeux JP, Le Novere N, Albuquerque EX, Balfour DJ, Berg DK, Bertrand D, Chiappinelli VA, Clarke PB, Collins AC, Dani JA, Grady SR, Kellar KJ, Lindstrom JM, Marks MJ, Quik M, Taylor PW, Wonnacott S. International Union of Pharmacology. XX. Current status of the nomenclature for nicotinic acetylcholine receptors and their subunits. Pharmacological Reviews. 1999;51:397–401. [PubMed] [Google Scholar]
- Luntz-Leybman V, Bickford PC, Freedman R. Cholinergic gating of response to auditory stimuli in rat hippocampus. Brain Research. 1992;587:130–136. doi: 10.1016/0006-8993(92)91437-j. [DOI] [PubMed] [Google Scholar]
- Lynch MA. Long-term potentiation and memory. Physiological Reviews. 2004;84:87–136. doi: 10.1152/physrev.00014.2003. [DOI] [PubMed] [Google Scholar]
- Machu TK, Hamilton ME, Frye TF, Shanklin CL, Harris MC, Sun H, Tenner TE, Jr., Soti FS, Kem WR. Benzylidene analogs of anabaseine display partial agonist and antagonist properties at the mouse 5-hydroxytryptamine(3A) receptor. Journal of Pharmacology and Experimental Therapeutics. 2001;299:1112–1119. [PubMed] [Google Scholar]
- Maggi L, Sher E, Cherubini E. Regulation of GABA release by nicotinic acetylcholine receptors in the neonatal rat hippocampus. Journal of Physiology. 2001;536:89–100. doi: 10.1111/j.1469-7793.2001.00089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks MJ, Collins AC. Characterization of nicotine binding in mouse brain and comparison with the binding of alpha-bungarotoxin and quinuclidinyl benzilate. Molecular Pharmacology. 1982;22:554–564. [PubMed] [Google Scholar]
- Marrero MB, Papke RL, Bhatti BS, Shaw S, Bencherif M. The neuroprotective effect of 2-(3-pyridyl)-1-azabicyclo[3.2.2]nonane (TC-1698), a novel alpha7 ligand, is prevented through angiotensin II activation of a tyrosine phosphatase. Journal of Pharmacology and Experimental Therapeutics. 2004;309:16–27. doi: 10.1124/jpet.103.061655. [DOI] [PubMed] [Google Scholar]
- Martin LF, Kem WR, Freedman R. Alpha-7 nicotinic receptor agonists: potential new candidates for the treatment of schizophrenia. Psychopharmacology. 2004;174:54–64. doi: 10.1007/s00213-003-1750-1. [DOI] [PubMed] [Google Scholar]
- Martin-Ruiz CM, Court JA, Molnar E, Lee M, Gotti C, Mamalaki A, Tsouloufis T, Tzartos S, Ballard C, Perry RH, Perry EK. Alpha4 but not alpha3 and alpha7 nicotinic acetylcholine receptor subunits are lost from the temporal cortex in Alzheimer's disease. Journal of Neurochemistry. 1999;73:1635–1640. doi: 10.1046/j.1471-4159.1999.0731635.x. [DOI] [PubMed] [Google Scholar]
- Meyer EM, Kuryatov A, Gerzanich V, Lindstrom J, Papke RL. Analysis of 3-(4-hydroxy, 2-methoxybenzylidene)anabaseine selectivity and activity at human and rat alpha-7 nicotinic receptors. Journal of Pharmacology and Experimental Therapeutics. 1998;287:918–925. [PubMed] [Google Scholar]
- Myers CS, Robles O, Kakoyannis AN, Sherr JD, Avila MT, Blaxton TA, Thaker GK. Nicotine improves delayed recognition in schizophrenic patients. Psychopharmacology. 2004;174:334–340. doi: 10.1007/s00213-003-1764-8. [DOI] [PubMed] [Google Scholar]
- Noble W, Olm V, Takata K, Casey E, Mary O, Meyerson J, Gaynor K, LaFrancois J, Wang L, Kondo T, Davies P, Burns M, Veeranna, Nixon R, Dickson D, Matsuoka Y, Ahlijanian M, Lau LF, Duff K. Cdk5 is a key factor in tau aggregation and tangle formation in vivo. Neuron. 2003;38:555–565. doi: 10.1016/s0896-6273(03)00259-9. [DOI] [PubMed] [Google Scholar]
- Nordberg A. Human nicotinic receptors—their role in aging and dementia. Neurochemistry International. 1994;25:93–97. doi: 10.1016/0197-0186(94)90059-0. [DOI] [PubMed] [Google Scholar]
- Nordberg A. Nicotinic receptor abnormalities of Alzheimer's disease: therapeutic implications. Biological Psychiatry. 2001;49:200–210. doi: 10.1016/s0006-3223(00)01125-2. [DOI] [PubMed] [Google Scholar]
- Nordberg A, Winblad B. Reduced number of [3H]nicotine and [3H]acetylcholine binding sites in the frontal cortex of Alzheimer brains. Neuroscience Letters. 1986;72:115–119. doi: 10.1016/0304-3940(86)90629-4. [DOI] [PubMed] [Google Scholar]
- Nordberg A, Hellstrom-Lindahl E, Lee M, Johnson M, Mousavi M, Hall R, Perry E, Bednar I, Court J. Chronic nicotine treatment reduces beta-amyloidosis in the brain of a mouse model of Alzheimer's disease (APPsw) Journal of Neurochemistry. 2002;81:655–658. doi: 10.1046/j.1471-4159.2002.00874.x. [DOI] [PubMed] [Google Scholar]
- Olincy A, Harris JG, Johnson LL, Pender V, Kongs S, Allensworth D, Ellis J, Zerbe GO, Leonard S, Stevens KE, Stevens JO, Martin L, Adler LE, Soti F, Kem WR, Freedman R. Proof-of-concept trial of an alpha7 nicotinic agonist in schizophrenia. Archives of General Psychiatry. 2006;63:630–638. doi: 10.1001/archpsyc.63.6.630. [DOI] [PubMed] [Google Scholar]
- Ono K, Hasegawa K, Yamada M, Naiki H. Nicotine breaks down preformed Alzheimer's beta-amyloid fibrils in vitro. Biological Psychiatry. 2002;52:880–886. doi: 10.1016/s0006-3223(02)01417-8. [DOI] [PubMed] [Google Scholar]
- Ophir G, Amariglio N, Jacob-Hirsch J, Elkon R, Rechavi G, Michaelson DM. Apolipoprotein E4 enhances brain inflammation by modulation of the NF-kappaB signaling cascade. Neurobiology of Disease. 2005;20:709–718. doi: 10.1016/j.nbd.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Patrick GN, Zukerberg L, Nikolic M, de la Monte S, Dikkes P, Tsai LH. Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration [see comment] Nature. 1999;402:615–622. doi: 10.1038/45159. [DOI] [PubMed] [Google Scholar]
- Pei JJ, Braak E, Braak H, Grundke-Iqbal I, Iqbal K, Winblad B, RF C. Localization of active forms of C-jun kinase (JNK) and p38 kinase in Alzheimer's disease brains at different stages of neurofibrillary degeneration. Journal of Alzheimer's Disease. 2001;3:41–48. doi: 10.3233/jad-2001-3107. [DOI] [PubMed] [Google Scholar]
- Pereira EF, Hilmas C, Santos MD, Alkondon M, Maelicke A, Albuquerque EX. Unconventional ligands and modulators of nicotinic receptors. Journal of Neurobiology. 2002;53:479–500. doi: 10.1002/neu.10146. [DOI] [PubMed] [Google Scholar]
- Potter A, Corwin J, Lang J, Piasecki M, Lenox R, Newhouse PA. Acute effects of the selective cholinergic channel activator (nicotinic agonist) ABT-418 in Alzheimer's disease. Psychopharmacology. 1999;142:334–342. doi: 10.1007/s002130050897. [DOI] [PubMed] [Google Scholar]
- Potter D, Summerfelt A, Gold J, Buchanan RW. Review of clinical correlates of P50 sensory gating abnormalities in patients with schizophrenia. Schizophrenia Bulletin. 2006;32:692–700. doi: 10.1093/schbul/sbj050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radcliffe KA, Dani JA. Nicotinic stimulation produces multiple forms of increased glutamatergic synaptic transmission. Journal of Neuroscience. 1998;18:7075–7083. doi: 10.1523/JNEUROSCI.18-18-07075.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangwala F, Drisdel RC, Rakhilin S, Ko E, Atluri P, Harkins AB, Fox AP, Salman SS, Green WN. Neuronal alpha-bungarotoxin receptors differ structurally from other nicotinic acetylcholine receptors. Journal of Neuroscience. 1997;17:8201–8212. doi: 10.1523/JNEUROSCI.17-21-08201.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reines SA, Block GA, Morris JC, Liu G, Nessly ML, Lines CR, Norman BA, Baranak CC, Rofecoxib Protocol 091 Study, G. Rofecoxib: no effect on Alzheimer's disease in a 1-year, randomized, blinded, controlled study. Neurology. 2004;62:66–71. doi: 10.1212/wnl.62.1.66. [DOI] [PubMed] [Google Scholar]
- Ren K, Thinschmidt J, Liu J, Ai L, Papke RL, King MA, Hughes JA, Meyer EM. Alpha7 Nicotinic receptor gene delivery into mouse hippocampal neurons leads to functional receptor expression, improved spatial memory-related performance, and tau hyperphosphorylation. Neuroscience. 2007;145:314–322. doi: 10.1016/j.neuroscience.2006.11.023. [DOI] [PubMed] [Google Scholar]
- Rogers J, Kirby LC, Hempelman SR, Berry DL, McGeer PL, Kaszniak AW, Zalinski J, Cofield M, Mansukhani L, Willson P. Clinical trial of indomethacin in Alzheimer's disease. Neurology. 1993;43:1609–1611. doi: 10.1212/wnl.43.8.1609. [DOI] [PubMed] [Google Scholar]
- Rogers J, Strohmeyer R, Kovelowski CJ, Li R. Microglia and inflammatory mechanisms in the clearance of amyloid beta peptide. Glia. 2002;40:260–269. doi: 10.1002/glia.10153. [DOI] [PubMed] [Google Scholar]
- Rubboli F, Court JA, Sala C, Morris C, Chini B, Perry E, Clementi F. Distribution of nicotinic receptors in the human hippocampus and thalamus. European Journal of Neuroscience. 1994;6:1596–1604. doi: 10.1111/j.1460-9568.1994.tb00550.x. [DOI] [PubMed] [Google Scholar]
- Rubio A, Perez M, Avila J. Acetylcholine receptors and tau phosphorylation. Current Molecular Medicine. 2006;6:423–428. doi: 10.2174/156652406777435444. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. The cell biology of beta-amyloid precursor protein and presenilin in Alzheimer's disease. Trends in Cell Biology. 1998;8:447–453. doi: 10.1016/s0962-8924(98)01363-4. [DOI] [PubMed] [Google Scholar]
- Sengupta A, Wu Q, Grundke-Iqbal I, Iqbal K, Singh TJ. Potentiation of GSK-3-catalyzed Alzheimer-like phosphorylation of human tau by cdk5. Molecular and Cellular Biochemistry. 1997;167:99–105. doi: 10.1023/a:1006883924775. [DOI] [PubMed] [Google Scholar]
- Sharma G, Vijayaraghavan S. Nicotinic cholinergic signaling in hippocampal astrocytes involves calcium-induced calcium release from intracellular stores [see comment] Proceedings of the National Academy of Sciences of the United States of America. 2001;98:4148–4153. doi: 10.1073/pnas.071540198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw S, Bencherif M, Marrero MB. Janus kinase 2, an early target of alpha 7 nicotinic acetylcholine receptor-mediated neuroprotection against Abeta-(1–42) amyloid. Journal of Biological Chemistry. 2002;277:44920–44924. doi: 10.1074/jbc.M204610200. [DOI] [PubMed] [Google Scholar]
- Shaw S, Bencherif M, Marrero MB. Angiotensin II blocks nicotine-mediated neuroprotection against beta-amyloid (1–42) via activation of the tyrosine phosphatase SHP-1. Journal of Neuroscience. 2003;23:11224–11228. doi: 10.1523/JNEUROSCI.23-35-11224.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shie FS, LeBoeuf RC, Jin LW. Early intraneuronal Abeta deposition in the hippocampus of APP transgenic mice. Neuroreport. 2003;14:123–129. doi: 10.1097/01.wnr.0000051151.87269.7d. [erratum appears in Neuroreport. 2004 Aug 26;15(12):1993 Note: LeBoeur, Renee C [corrected to LeBoeuf, Renee C]] [DOI] [PubMed] [Google Scholar]
- Shytle RD, Mori T, Townsend K, Vendrame M, Sun N, Zeng J, Ehrhart J, Silver AA, Sanberg PR, Tan J. Cholinergic modulation of microglial activation by alpha 7 nicotinic receptors. Journal of Neurochemistry. 2004;89:337–343. doi: 10.1046/j.1471-4159.2004.02347.x. [DOI] [PubMed] [Google Scholar]
- Silva AJ, Kogan JH, Frankland PW, Kida S. CREB and memory. Annual Review of Neuroscience. 1998;21:127–148. doi: 10.1146/annurev.neuro.21.1.127. [DOI] [PubMed] [Google Scholar]
- Slow EJ, Graham RK, Hayden MR. To be or not to be toxic: aggregations in Huntington and Alzheimer disease. Trends in Genetics. 2006;22:408–411. doi: 10.1016/j.tig.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Smith MA. Alzheimer disease. International Review of Neurobiology. 1998;42:1–54. doi: 10.1016/s0074-7742(08)60607-8. [DOI] [PubMed] [Google Scholar]
- Smith RC, Singh A, Infante M, Khandat A, Kloos A. Effects of cigarette smoking and nicotine nasal spray on psychiatric symptoms and cognition in schizophrenia. Neuropsychopharmacology. 2002;27:479–497. doi: 10.1016/S0893-133X(02)00324-X. [DOI] [PubMed] [Google Scholar]
- Smith RC, Warner-Cohen J, Matute M, Butler E, Kelly E, Vaidhyanathaswamy S, Khan A. Effects of nicotine nasal spray on cognitive function in schizophrenia. Neuropsychopharmacology. 2006;31:637–643. doi: 10.1038/sj.npp.1300881. [DOI] [PubMed] [Google Scholar]
- Snaedal J, Johannesson T, Jonsson JE, Gylfadottir G. The effects of nicotine in dermal plaster on cognitive functions in patients with Alzheimer's disease. Dementia. 1996;7:47–52. doi: 10.1159/000106852. [DOI] [PubMed] [Google Scholar]
- Spurden DP, Court JA, Lloyd S, Oakley A, Perry R, Pearson C, Pullen RG, Perry EK. Nicotinic receptor distribution in the human thalamus: autoradiographical localization of [3H]nicotine and [125I] alpha-bungarotoxin binding. Journal of Chemical Neuroanatomy. 1997;13:105–113. doi: 10.1016/s0891-0618(97)00038-0. [DOI] [PubMed] [Google Scholar]
- Stokes C, Papke JK, Horenstein NA, Kem WR, McCormack TJ, Papke RL. The structural basis for GTS-21 selectivity between human and rat nicotinic alpha7 receptors. Molecular Pharmacology. 2004;66:14–24. doi: 10.1124/mol.66.1.14. [DOI] [PubMed] [Google Scholar]
- Streit WJ. Microglia as neuroprotective, immunocompetent cells of the CNS. Glia. 2002;40:133–139. doi: 10.1002/glia.10154. [DOI] [PubMed] [Google Scholar]
- Sudweeks SN, Yakel JL. Functional and molecular characterization of neuronal nicotinic ACh receptors in rat CA1 hippocampal neurons. Journal of Physiology. 2000;527(Part 3):515–528. doi: 10.1111/j.1469-7793.2000.00515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugaya K, Giacobini E, Chiappinelli VA. Nicotinic acetylcholine receptor subtypes in human frontal cortex: changes in Alzheimer's disease. Journal of Neuroscience Research. 1990;27:349–359. doi: 10.1002/jnr.490270314. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Hide I, Matsubara A, Hama C, Harada K, Miyano K, Andra M, Matsubayashi H, Sakai N, Kohsaka S, Inoue K, Nakata Y. Microglial alpha7 nicotinic acetylcholine receptors drive a phospholipase C/IP3 pathway and modulate the cell activation toward a neuroprotective role. Journal of Neuroscience Research. 2006;83:1461–1470. doi: 10.1002/jnr.20850. [DOI] [PubMed] [Google Scholar]
- Teaktong T, Graham A, Court J, Perry R, Jaros E, Johnson M, Hall R, Perry E. Alzheimer's disease is associated with a selective increase in alpha7 nicotinic acetylcholine receptor immunoreactivity in astrocytes. Glia. 2003;41:207–211. doi: 10.1002/glia.10132. [DOI] [PubMed] [Google Scholar]
- Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, Hansen LA, Katzman R. Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Annals of Neurology. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- Tolnay M, Probst A. REVIEW: tau protein pathology in Alzheimer's disease and related disorders. Neuropathology and Applied Neurobiology. 1999;25:171–187. doi: 10.1046/j.1365-2990.1999.00182.x. [DOI] [PubMed] [Google Scholar]
- Ueno S, Bracamontes J, Zorumski C, Weiss DS, Steinbach JH. Bicuculline and gabazine are allosteric inhibitors of channel opening of the GABAA receptor. Journal of Neuroscience. 1997;17:625–634. doi: 10.1523/JNEUROSCI.17-02-00625.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulloa L. The vagus nerve and the nicotinic anti-inflammatory pathway. Nature Reviews Drug Discovery. 2005;4:673–684. doi: 10.1038/nrd1797. [DOI] [PubMed] [Google Scholar]
- Uteshev VV, Meyer EM, Papke RL. Regulation of neuronal function by choline and 4OH-GTS-21 through alpha 7 nicotinic receptors. Journal of Neurophysiology. 2003;89:1797–1806. doi: 10.1152/jn.00943.2002. [DOI] [PubMed] [Google Scholar]
- Utsugisawa K, Nagane Y, Tohgi H, Yoshimura M, Ohba H, Genda Y. Changes with aging and ischemia in nicotinic acetylcholine receptor subunit alpha7 mRNA expression in postmortem human frontal cortex and putamen. Neuroscience Letters. 1999;270:145–148. doi: 10.1016/s0304-3940(99)00473-5. [DOI] [PubMed] [Google Scholar]
- Utsuki T, Shoaib M, Holloway HW, Ingram DK, Wallace WC, Haroutunian V, Sambamurti K, Lahiri DK, Greig NH. Nicotine lowers the secretion of the Alzheimer's amyloid beta-protein precursor that contains amyloid beta-peptide in rat. Journal of Alzheimer's Disease. 2002;4:405–415. doi: 10.3233/jad-2002-4507. [DOI] [PubMed] [Google Scholar]
- Walker DP, Wishka DG, Piotrowski DW, Jia S, Reitz SC, Yates KM, Myers JK, Vetman TN, Margolis BJ, Jacobsen EJ, Acker BA, Groppi VE, Wolfe ML, Thornburgh BA, Tinholt PM, Cortes-Burgos LA, Walters RR, Hester MR, Seest EP, Dolak LA, Han F, Olson BA, Fitzgerald L, Staton BA, Raub TJ, Hajos M, Hoffmann WE, Li KS, Higdon NR, Wall TM, Hurst RS, Wong EH, Rogers BN. Design, synthesis, structure-activity relationship, and in vivo activity of azabicyclic aryl amides as alpha7 nicotinic acetylcholine receptor agonists. Bioorganic and Medicinal Chemistry. 2006;14:8219–8248. doi: 10.1016/j.bmc.2006.09.019. [DOI] [PubMed] [Google Scholar]
- Wang HY, Lee DH, D'Andrea MR, Peterson PA, Shank RP, Reitz AB. Beta-Amyloid(1–42) binds to alpha7 nicotinic acetylcholine receptor with high affinity. Implications for Alzheimer's disease pathology. Journal of Biological Chemistry. 2000a;275:5626–5632. doi: 10.1074/jbc.275.8.5626. [DOI] [PubMed] [Google Scholar]
- Wang HY, Lee DH, Davis CB, Shank RP. Amyloid peptide Abeta(1–42) binds selectively and with picomolar affinity to alpha7 nicotinic acetylcholine receptors. Journal of Neurochemistry. 2000b;75:1155–1161. doi: 10.1046/j.1471-4159.2000.0751155.x. [DOI] [PubMed] [Google Scholar]
- Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Wang H, Yang H, Ulloa L, Al-Abed Y, Czura CJ, Tracey KJ. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation [see comment] Nature. 2003a;421:384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- Wang HY, Li W, Benedetti NJ, Lee DH. Alpha 7 nicotinic acetylcholine receptors mediate beta-amyloid peptide-induced tau protein phosphorylation. Journal of Biological Chemistry. 2003b;278:31547–31553. doi: 10.1074/jbc.M212532200. [DOI] [PubMed] [Google Scholar]
- Wang H, Liao HY, Ochani M, Justiniani M, Lin X, Yang L, Al-Abed Y, Wang H, Metz C, Miller EJ, Tracey KJ, Ulloa L. Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis [see comment] Nature Medicine. 2004;10:1216–1221. doi: 10.1038/nm1124. [DOI] [PubMed] [Google Scholar]
- Weggen S, Eriksen JL, Das P, Sagi SA, Wang R, Pietrzik CU, Findlay KA, Smith TE, Murphy MP, Bulter T, Kang DE, Marquez-Sterling N, Golde TE, Koo EH. A subset of NSAIDs lower amyloidogenic Abeta42 independently of cyclooxygenase activity [see comment] Nature. 2001;414:212–216. doi: 10.1038/35102591. [DOI] [PubMed] [Google Scholar]
- Weishaupt JH, Kussmaul L, Grotsch P, Heckel A, Rohde G, Romig H, Bahr M, Gillardon F. Inhibition of CDK5 is protective in necrotic and apoptotic paradigms of neuronal cell death and prevents mitochondrial dysfunction. Molecular and Cellular Neurosciences. 2003;24:489–502. doi: 10.1016/s1044-7431(03)00221-5. [DOI] [PubMed] [Google Scholar]
- Westerman MA, Cooper-Blacketer D, Mariash A, Kotilinek L, Kawarabayashi T, Younkin LH, Carlson GA, Younkin SG, Ashe KH. The relationship between Abeta and memory in the Tg2576 mouse model of Alzheimer's disease. Journal of Neuroscience. 2002;22:1858–1867. doi: 10.1523/JNEUROSCI.22-05-01858.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wevers A, Jeske A, Lobron C, Birtsch C, Heinemann S, Maelicke A, Schroder R, Schroder H. Cellular distribution of nicotinic acetylcholine receptor subunit mRNAs in the human cerebral cortex as revealed by non-isotopic in situ hybridization. Brain Research. Molecular Brain Research. 1994;25:122–128. doi: 10.1016/0169-328x(94)90286-0. [DOI] [PubMed] [Google Scholar]
- Wevers A, Burghaus L, Moser N, Witter B, Steinlein OK, Schutz U, Achnitz B, Krempel U, Nowacki S, Pilz K, Stoodt J, Lindstrom J, De Vos RA, Jansen Steur EN, Schroder H. Expression of nicotinic acetylcholine receptors in Alzheimer's disease: postmortem investigations and experimental approaches. Behavioural Brain Research. 2000;113:207–215. doi: 10.1016/s0166-4328(00)00215-1. [DOI] [PubMed] [Google Scholar]
- White HK, Levin ED. Four-week nicotine skin patch treatment effects on cognitive performance in Alzheimer's disease. Psychopharmacology. 1999;143:158–165. doi: 10.1007/s002130050931. [DOI] [PubMed] [Google Scholar]
- White HK, Levin ED. Chronic transdermal nicotine patch treatment effects on cognitive performance in age-associated memory impairment. Psychopharmacology. 2004;171:465–471. doi: 10.1007/s00213-003-1614-8. [DOI] [PubMed] [Google Scholar]
- Whitehouse PJ, Kalaria RN. Nicotinic receptors and neurodegenerative dementing diseases: basic research and clinical implications. Alzheimer Disease and Associated Disorders. 1995;9(Suppl 2):3–5. doi: 10.1097/00002093-199501002-00002. [DOI] [PubMed] [Google Scholar]
- Wilson AL, Langley LK, Monley J, Bauer T, Rottunda S, McFalls E, Kovera C, McCarten JR. Nicotine patches in Alzheimer's disease: pilot study on learning, memory, and safety. Pharmacology, Biochemistry and Behavior. 1995;51:509–514. doi: 10.1016/0091-3057(95)00043-v. [DOI] [PubMed] [Google Scholar]
- Wilson CA, Doms RW, Lee VM. Intracellular APP processing and A beta production in Alzheimer disease. Journal of Neuropathology and Experimental Neurology. 1999;58:787–794. doi: 10.1097/00005072-199908000-00001. [DOI] [PubMed] [Google Scholar]
- Wirths O, Multhaup G, Czech C, Blanchard V, Moussaoui S, Tremp G, Pradier L, Beyreuther K, Bayer TA. Intraneuronal Abeta accumulation precedes plaque formation in beta-amyloid precursor protein and presenilin-1 double-transgenic mice. Neuroscience Letters. 2001;306:116–120. doi: 10.1016/s0304-3940(01)01876-6. [DOI] [PubMed] [Google Scholar]
- Wishka DG, Walker DP, Yates KM, Reitz SC, Jia S, Myers JK, Olson KL, Jacobsen EJ, Wolfe ML, Groppi VE, Hanchar AJ, Thornburgh BA, Cortes-Burgos LA, Wong EH, Staton BA, Raub TJ, Higdon NR, Wall TM, Hurst RS, Walters RR, Hoffmann WE, Hajos M, Franklin S, Carey G, Gold LH, Cook KK, Sands SB, Zhao SX, Soglia JR, Kalgutkar AS, Arneric SP, Rogers BN. Discovery of N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl]furo[2,3-c]pyridine-5-carboxamide, an agonist of the alpha7 nicotinic acetylcholine receptor, for the potential treatment of cognitive deficits in schizophrenia: synthesis and structure–activity relationship. Journal of Medicinal Chemistry. 2006;49:4425–4436. doi: 10.1021/jm0602413. [DOI] [PubMed] [Google Scholar]
- Wolozin BL, Pruchnicki A, Dickson DW, Davies P. A neuronal antigen in the brains of Alzheimer patients. Science. 1986;232:648–650. doi: 10.1126/science.3083509. [DOI] [PubMed] [Google Scholar]
- Yoshiyama Y, Higuchi M, Zhang B, Huang SM, Iwata N, Saido TC, Maeda J, Suhara T, Trojanowski JQ, VM L. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron. 2007;53:337–351. doi: 10.1016/j.neuron.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Young JW, Crawford N, Kelly JS, Kerr LE, Marston HM, Spratt C, Finlayson K, Sharkey J. Impaired attention is central to the cognitive deficits observed in alpha 7 deficient mice. European Neuropsychopharmacology. 2007;17:145–155. doi: 10.1016/j.euroneuro.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Zhang J, Liu Q, Chen Q, Liu NQ, Li FL, Lu ZB, Qin C, Zhu H, Huang YY, He W, Zhao BL. Nicotine attenuates beta-amyloid-induced neurotoxicity by regulating metal homeostasis. FASEB Journal. 2006;20:1212–1214. doi: 10.1096/fj.05-5214fje. [DOI] [PubMed] [Google Scholar]
- Zhang X, Chen Y, Wang CLYH. Neuronal somatic ATP release triggers neuron-satellite glial cell communication in dorsal root ganglia. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:9864–9869. doi: 10.1073/pnas.0611048104. [DOI] [PMC free article] [PubMed] [Google Scholar]