Abstract
Introduction
Adipose derived stem cells (ADSCs) are a somatic stem cell population contained in fat tissue that possess the ability for self-renewal, differentiation into one or more phenotypes and functional regeneration of damaged tissue, which will benefit the recovery of erectile function by using a stem cell based therapy.
Aim
To review available evidence concerning adipose derived stem cell availability, differentiation into functional cells, and the potential of these cells for the treatment of erectile dysfunction (ED).
Methods
We examined the current data associated with the definition and characterization of adipose derived stem cells, including the differentiation of these cells and the initial effects of adipose derived stem cell therapy in a rat model of erectile dysfunction.
Main Outcome Measures
There is strong evidence supporting the concept that ADSCs are a potential stem cell therapy source for treatment of erectile dysfunction.
Results
The adipose derived stem cells are paravascularly localized in the adipose tissue. Under specific induction medium conditions, these cells differentiated into neuron-like cells, smooth muscle cells and endothelium in vitro. The insulin-like growth factor/insulin-like growth factor receptor (IGF/IGFR) pathway participates in neuronal differentiation while the fibroblast growth factor 2 (FGF2) pathway is involved in endothelium differentiation. In addition, the internal ribosomal entry sites (IRES) regulated gene translation is related to these types of differentiation. In a preliminary in-vivo experiment, the adipose derived stem cells functionally recovered the damaged erectile function. Therefore, the underlying mechanism needs be further examined.
Conclusion
The adipose derived stem cells are a potential source of stem cells for treatment of erectile dysfunction, which highlights the possibility of an effective clinical therapy for ED in the near future.
Keywords: Erectile Dysfunction, Stem Cells, Adipose Derived Stem Cell, Differentiation, FGF2
Introduction
Stem cells (SC) are endowed with the capacity to self-renew and differentiate into various cell types, depending on the stimuli or signals that they receive. While embryonic stem cells (ESC) have greater differentiation potential than adult stem cells (ASC), the former will most likely take years to reach clinical application due to ethical concerns and governmental restrictions associated with its use. Since firstly reported in 1998[1], the human stem cell researches have attracted thousands of scientists worldwide. Actually, the wide range of uses for treatment of multitude of human diseases is the major argument in favor of human stem cell research [2,3].
Adipose-derived stromal cells (ADSCs) [4] have previously been shown to possess stem cell properties such as transdifferentiation and self-renewal. They are a somatic stem cell population contained in fat tissue and posses the properties of self-renewal, differentiation into one or more phenotypes and functional reproduction of damaged tissue [5,6]. ADSCs share the same properties of all stem cells, such as the ability to divide and renew themselves for long periods, and differentiate into specialized cells. Right now, the sources of adipose derived stem cells include three types of adipose tissues, including brown adipose tissue (BAT), white adipose tissue (WAT), and bone marrow adipose tissue.
ADSCs are virtually identical to Bone marrow stem cells (BMSC) in differentiation and therapeutic potential and are much easier and safer to obtain in large quantities. ADSCs therefore appear to be a better choice for future clinical application within the stem cell field[7]. Meanwhile, the ADSCs have many advantages compared to BMSCs, including the ability to isolate many more stem cells using a minimally invasive surgical procedure for extraction of the adipose tissue. Although the isolation and characterization of ADSCs have been well documented, the in-situ localization of ADSCs in BAT / WAT is poorly understood. By applying an extensive scan of the stem cell markers with immunohistology, it was shown that CD34+ cells, the well described progenitor/stem cell in adipose tissue, were clearly localized perivascularly [8]. Also, the isolated and purified ADSCs have successfully differentiated into neuron-like cells, smooth muscle cells, and endothelium in vitro [9–11].
In recent years, there has been much interest surrounding adipose derived stem cells, but the ADSCs field of research has actually been around for a long time. In 1964 [12], Rodbell developed the method of isolation of the stromal vascular fraction (SVF) from adipose tissue. In 1978, Bjontorp [13] found that periadipocytes have the ability for differentiation, which is defined as one of the properties of stem cells. It wasn’t until 2001 that Zuk [4] first purified and named the adipose derived stem cells. Since their official inception, the adipose derived stem cells have been extensively studied from basic science to clinical research. In 2004, a German group successfully transplanted adipose derived stem cells to repair the cranium of a 7-year-old girl [14]. In 2005, an experiment for the treatment of incontinence using the adipose derived stem cells was also implemented [11]. The ADSCs have also been shown to improve erectile function in neurogenic and diabetic animal models [15].
Compared to other kinds of adult stem cells, such as bone marrow-derived mesenchymal stem cells, ADSCs possess a clear advantage due to easy and repeatable access to subcutaneous adipose tissue and the simple isolation procedure. Nevertheless, limitations on the purification of ADSCs and the lack of knowledge regarding their molecular characterization are the major hindrances associated with the use of ADSCs.
Characterization and Localization of adipose derived stem cell in situ of the tissue
Despite the importance of ADSCs and many publications on their characterization, the molecular characterization of ADSCs is not well established. By employing flow cytometry [16], histology [8] and other methods [17,18], several candidate cellular markers and genes have been screened (Table). Meanwhile, the cellular origin of ADSCs within adipose tissue remains unknown. Recently, Yamamoto et al [19] used immunofluorescence (IF) staining of mouse adipose tissue to identify cells expressing CD90, CD105, Sca-1, and/or p75NTR. The results showed widespread distribution of each of these markers, suggesting that they are not specific for ADSCs. In another recent study by Zannettino et al [20] attempted to identify ADSCs in human adipose tissue by employing IF staining for cellular markers 1A6.12, 1B5, STRO-1, CD146, and 3G5. While these markers were detected in two large blood vessels of unknown identity, their location in the adipose tissue cannot be inferred due to the lack of adipocytes or any other landmarks in the neighborhood of these two blood vessels. Furthermore, the study did not examine the small vessels (arterioles, venules, or capillaries) in adipose tissue, although the authors did acknowledge that mesenchymal stem cells (MSC), such as ADSCs, likely reside in specialized niches within the micro-vascular networks.
Table 1.
Molecular phenotype of adipose derived stem cells
| ADSC positive cellular markers and gene | ADSC negative cellular markers and gene |
|---|---|
| CD9 | CD11b |
| CD10 | CD14 |
| CD13 | CD19 |
| CD29 | CD31 |
| CD34 | CD45 |
| CD44 | CD79a |
| CD49 | CD80 |
| CD54 | CD117 |
| CD55 | CD133 |
| CD59 | CD144 |
| CD73 | HLA-DR |
| CD90 | CD105 |
| MyD88 | PDGFR-β/CD140 |
| CD106 | HLA-II |
| CD146 | Lin |
| CD166 | ASMA |
| HLA-I | Wnt5a |
| STRO-1 | β-cat |
| Sca1 | |
| SSEA1 | |
| OCT3/4 | |
| P75NTR | |
| Telomerase |
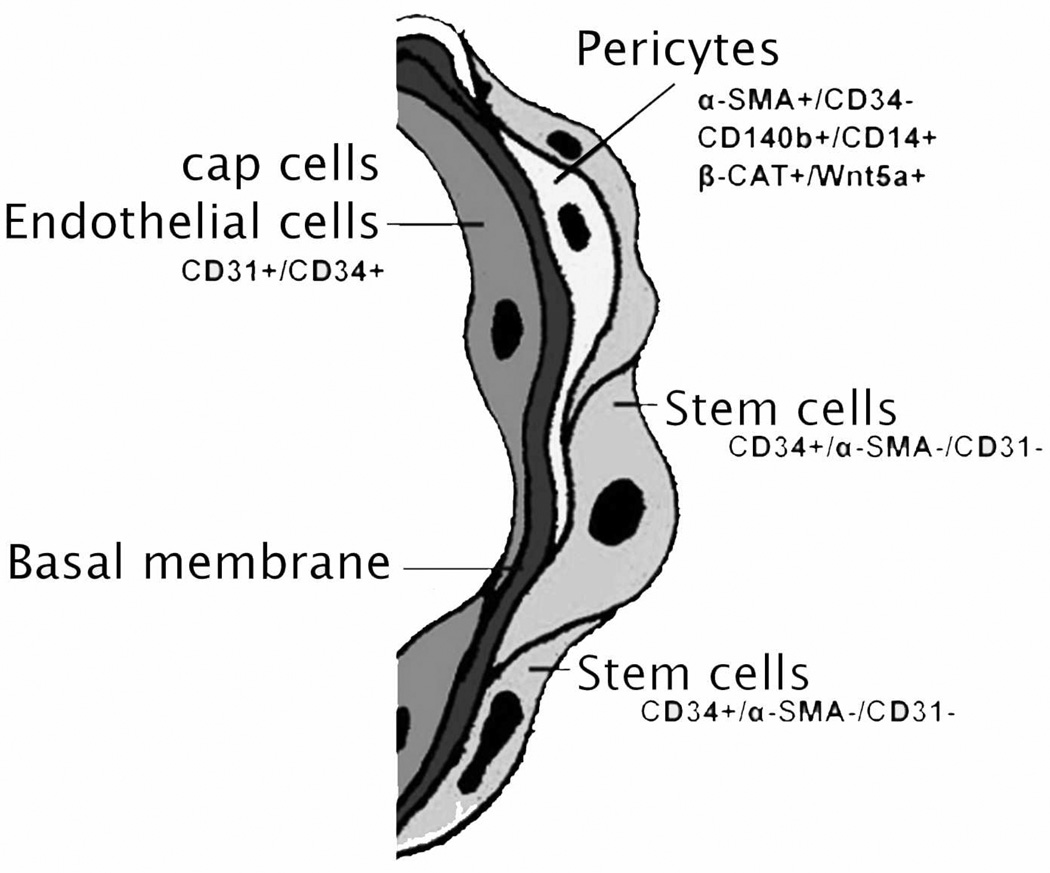
Actually, several lines of evidence suggest that ADSCs are vascular precursor cells. Many studies have shown that SVF contains progenitor cells that are able to differentiate into endothelial cells and participate in blood vessel formation. Also, a recent study demonstrated that SVF cells expressing both pericyte and mesenchymal markers reside in a periendothelial location and stabilize endothelial networks [21]. Another recent study showed that ADSCs transplanted into ischemic cortex preferentially migrate toward micro-vessels where they differentiate into vascular smooth muscle cells [2]. By extensive histology, flow cytometric assay, an updated results show that smooth muscle actin (SMA) and CD31 localized within smooth muscle and endothelial cells, respectively, in all blood vessels examined. CD34 localized to both the intima (endothelium) and adventitia, neither of which expressed SMA. The niche marker Wnt5a was confined exclusively to the vascular wall, within mural smooth muscle cells. Surprisingly, the widely accepted mesenchymal stem cell marker STRO-1 was expressed exclusively in the endothelium of capillaries and arterioles but not in the endothelium of arteries. The embryonic stem cell marker SSEA1 localized to a pericytic location in capillaries and in certain smooth muscle cells of arterioles. Cells expressing the embryonic stem cell markers telomerase and OCT4 were rare and observed only in capillaries. Tang et al also identified the progenitor cells in white adipose tissue within the adipose vasculature [18]. Notably, Matthew S. Rodeheffer reported a similar result by employing a variety of approaches [17]. Based on these findings and evidence gathered from the existing literature, it has been proposed that ADSCs are vascular precursor (stem) cells at various stages of differentiation [8]. These studies serve to solidify the idea that adipose derived stem cells are in fact derived from the adipose vasculature where the stem cells reside in a microenvironment that has been hypothetically named as the adipose derived stem cell niche (Fig 1).
Fig 1.
A hypothetical scheme of the adipose derived stem cells niche consists of four components: cap cells-endothelial cells, basal membrane, supporting cells-pericytes, and stem cells.
The isolation and differentiation of adipose derived stem cells in vitro
In recent years, the adipose derived stem cells as a new source of adult stem cells has been extensively explored [14,22,23]. The adipose derived stem cells can be developed with a series of steps including isolation, sorting, culture, differentiation and application. Firstly, the adipose tissue need be digested with collagenase type IA, sorted by a cell strainer and specific stem cell markers. These cells can be cultured in regular culture medium and can be induced into different cell types using induction medium. After described steps, the cells can be used for many different purposes. Using flow cytometric sorting, these adipose derived stem cells have been shown to share many of the same cellular markers with the Bone marrow stem cells, such as CD9, CD10, CD13, CD29, CD117, CD34, CD90, CD105 and STRO-1 (Table ).
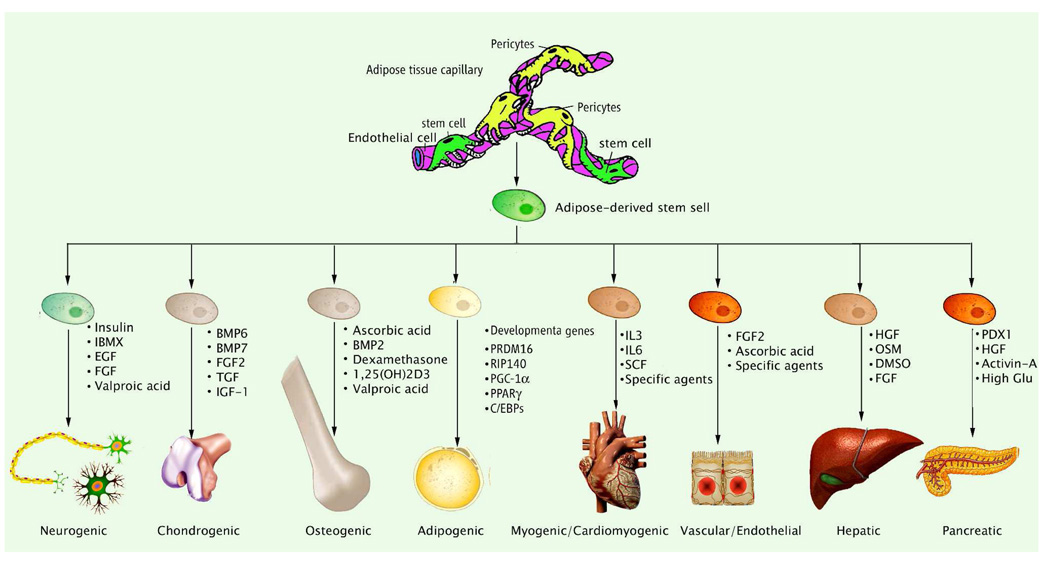
The ADSCs display multipotency by retaining the ability to differentiate into cell types of different lineages, including neurogenic, chondrogenic, ostogenic, adipogenic, myogenic, cardiomyogenic, vascular/endothelial, hepatic, and pancreatic differentiation (Fig. 2). In the current review, we mainly focus on neurogenic differentiation, smooth muscle differentiation, and endothelial differentiation, which encompass the dominant differentiation pathways for functional cells located within the penile erectile tissue.
Fig 2.
Schematic for the mutilineage differentiation of adipose derived stem cells and associated factors.
Neuron differentiation
It has been shown that ADSCs can be induced by isobutylmethylxanthine (IBMX) to differentiate into neuron-like cells in vitro [5,10,24]. This IBMX-induced neuronal differentiation was mediated by the IGF-I signaling pathway. The significance of these studies is that ADSCs has the potential to treat degenerative neurological diseases including neurogenic ED, which frequently occurs in patients who have undergone pelvic floor surgeries or radiation.
Due to the fact that future clinical applications will likely to use these adult stem cells in an autologous fashion, ADSCs could be induced to differentiate into neuron-like cells which expressed neuronal markers S100, nestin, and NF70. Isobutylmethylxanthine (IBMX), indomethacin (INDO), and insulin were the active ingredients in a previously established neural induction medium (NIM). It was also found that IBMX alone was as effective as NIM in the induction of morphological changes as well as neuronal marker expression. The cellular signaling pathways involved in this neuron differentiation were studied by treating ADSCs with IBMX in the presence or in the absence of each of eight specific inhibitors of different signaling pathways (JAK/STAT, PKA, PI3K, MEK, Wnt/Frizzled, ERK/MAPK, TGF-b, and insulin growth factor [IGF]- I). PPP, a specific inhibitor of IGF-I signaling, was the only inhibitor that showed significant inhibition of IBMX-induced ADSCs neuronal differentiation, as determined by changes in cell morphology in the initial screening. Further examination by immunofluorescence staining showed that the neuronal marker, β-III-tubulin, was highly induced in IBMX-treated ADSCs, and the induction was significantly suppressed by PPP. Western blotting followed by densitometry showed that PPP suppressed IBMX-induced β-III-tubulin expression by 43%, 88%, and 84% when used to treat the cells for 1, 3, and 24 hr, respectively. Treatment of ADSCs with IBMX also led to the phosphorylation of IGF-I receptor at tyrosine 1136 (Y1136), as determined by immunofluorescence staining with an antibody that reacts specifically with Y1136. This effect was also abrogated by PPP. Thus, the IBMX-induced neuron-like differentiation of ADSCs is mediated by IGF signaling through the phosphorylation of IGF-IR at Y1136 [25].
Smooth muscle cells differentiation
The smooth muscle is a major component of erectile tissues and essential for the normal erectile function. The use of adipose derived stem cells for cell-based tissue engineering and regeneration strategies represents a promising alternative for the treatment of erectile dysfunction. For such strategies to succeed, a reliable source of smooth muscle precursor cells must be identified. In 2006 [11], Rodríguez LV et al checked the capacity of adipose-derived stem cells to differentiate into phenotypic and functional smooth muscle cells in vitro. The cells were cultured in smooth muscle differentiation medium for the differentiation. Smooth muscle differentiation of ADSCs induced genetic expression of all smooth muscle markers and further confirmed by increased protein expression of smooth muscle cell-specific alpha actin (ASMA), calponin, SM22, myosin heavy chain (MHC), and smoothelin. Clonal studies of adipose derived multipotent cells demonstrated differentiation of these cells into smooth muscle cells in addition to trilineage differentiation capacity. Importantly, smooth muscle-differentiated cells, but not their precursors, exhibit the functional ability to contract and relax in direct response to pharmacologic agents. From their result, it has been demonstrated that the adipose-derived cells have the potential to differentiate into functional smooth muscle cells and thus, adipose tissue can be a useful source of cells for treatment of injured tissues where smooth muscle plays an important role.
Furthermore, Lee WC et al [26] explored the regulation of smooth muscle cell (SMC) marker α-smooth muscle actin (α-SMA) during the differentiation of ADSCs to smooth muscle cell. The ADSCs differentiated toward the SMC lineage under the exogenous biochemical stimulation. The immunofluorescence staining and Western blot analysis detected protein expression of the early SMC marker α-SMA in both control and experiment groups. Expression of α-SMA in ADSCs significantly increased when treated with transforming growth factor-β1, while α-SMA expression only slightly increased in the presence of retinoic acid (RA), β-mercaptoethanol and ascorbic acid. Treatment with platelet-derived growth factor-BB, RA and dibutyryl-cyclic adenosine monophosphate decreased the expression of α-SMA significantly.
More interestingly, in 2008, Andersen DC [27] found a non-haematopoietic "side population" of CD45(−) cells within ADSCs possessing the ability to differentiate into smooth muscle cells. Simultaneous qRT-PCR of 64 genes revealed that the freshly isolated CD45(−) was highly enriched for cells expressing genes related to stem cells, the Notch pathway, and early vascular precursors. Notably, the expression of smooth muscle actin, C-met and CD34 together with Angpt2, Flk1, VE-cadherin, and CD31 suggested a phenotypic resemblance to pericytes. It has been observed in vitro myogenic specification of CD45(−) cells when co-cultured with myoblasts. Furthermore, immediate intramuscular engraftment of non-cultured CD45(−) cells gave rise to myofibres and cells lining blood vessels.
Endothelium differentiation
Endothelial dysfunction is a prominent feature of a wide variety of diseases including ED, and therapeutic angiogenesis is one of the strategies that have been considered for the restoration of endothelial function. Earlier efforts of this therapeutic strategy largely focused on injection of proangiogenic proteins or vectors expressing such proteins. These efforts were fruitful in pre-clinical studies but encountered obstacles in clinical trials. Growth factor therapy for ED has also been successfully demonstrated in animal models but has not reached clinical trials [28]. On the other hand, recent studies have shifted to cell-based therapies, such as using progenitor and stem cells [29–31].
It has been previously shown that ADSCs were localized within the vasculature of adipose tissue [8] and displayed multipotency, as witnessed by the ability of ADSCs to differentiate into endothelial cells. It has been hypothesized that ADSCs could be used to restore the endothelial function in vasculogenic ED. As a first step, we demonstrated that indeed ADSCs differentiated into endothelial cells in the penile sinusoids. We then sought to identify the mechanism underlying ADSCs endothelial differentiation. Using cell cultures we first tested whether ADSCs could differentiate into endothelial cells when grown in Endothelial Growth Medium 2 (EGM2), a commonly used endothelial growth medium. We observed that switching ADSCs from DMEM to EGM2 for approximately 7 days was sufficient to induce expression of endothelial markers, LDL-uptake, and endothelial tube formation. We also observed that this endothelial differentiation was reversible because switching ADSCs back to DMEM resulted in the disappearance of all of the above-mentioned endothelial characteristics. Upon returning to EGM2, the endothelial characteristic reappeared, albeit at reduced levels. Thus, EGM2 did contain factors necessary and sufficient to induce ADSCs endothelial differentiation. Subsequent experiments led to the identification of FGF2 and vitamin C as the only two plausible factors.
Mechanism of the differentiation of adipose derived stem cells
A central question in stem cell research is how stem cells maintain the ability to self-renew and replicate while producing differentiated daughter cells, in addition to which signaling events control stem cell proliferation and differentiation [1,3,7,32,33]. Meanwhile, much research effort has been implemented in manipulating stem cells into desired cell types for use in clinical application. Studies have revealed that stem cells use evolutionarily conserved molecular pathways and machinery for their differentiation and self-renewal. Genes that participate in these events are regulated at different levels, such as transcription, translation, and protein modification.
Initiation of translation of most eukaryotic mRNAs commences with 5’ end–dependent recruitment of 40S ribosomal subunits to the mRNA. The 40S subunit carrying the initiator methionine-tRNA and certain eukaryotic initiation factors (eIFs) are thought to scan the mRNA in a 5’ to 3’ direction until an appropriate start codon is encountered, at which stage a 60S subunit joins to form an 80S ribosome that can decode the RNA into protein. A subset of mRNAs contain internal ribosomal entry sites (IRES), usually in the 5’ UTR, enable end-independent initiation to occur and has been recruited as a major translational format under certain stimuli (i.e. stress) which is an initial step in activation of stem cells for differentiation [34,35].
Recently, it has been demonstrated that translational regulation of fibroblast growth factor 2 (FGF2) plays a critical role in maintaining self-renewal and differentiation of ADMSC [36–39]. The human FGF2 mRNA is one of the most striking systems of translational regulation. Its highly structured 5′ leader region contains 5 initiation codons; an AUG coding for an 18 KDa isoform and 4 noncanonical CUGs, coding for high-molecular-weight isoforms (HMW) of 22, 22.5, 24 and 34 KDa. It was shown previously that translation initiation (except for the 34 kDa FGF2 isoform) could be driven by IRES located in the mRNA 5′ untranslated region, allowing translation to occur by a non-classical cap-independent mechanism [36,40]. These different FGF2 isoforms have different localizations and functions. The AUG-initiated 18 kDa isoform is cytoplasmic, and it is secreted and acts by the classical para- or autocrine way involving membrane receptor recognition. In contrast, the CUG initiated FGF-2 isoforms are nuclear and have an intracrine action that involves interaction with nuclear partners. FGF2 IRES activity is regulated in a developmental and tissue-specific manner. It was also noted that FGF2 is the key molecule for maintenance of stemness in ADSCs [41]. In this regard, FGF2 appears to be of similar importance for hADSCs self-renewal as leukemia inhibitory factor (LIF) is for mouse ESCs.
Application of stem cells/adipose derived stem cells
Stem cell-based therapy for erectile dysfunction
In 2003 Deng et al showed that bone marrow stem cells (BMSC) transduced with eNOS were able to improve the erectile function of aged rats [42]. In 2004 our research showed that ESC transfected with brain-derived neurotrophic factor (BDNF) could restore the erectile function of rats whose cavernous nerves were experimentally damaged [31]. Bivalacqua et al [29,30] showed that BMSC alone or transduced with eNOS were able to reverse age-associated ED. Song et al [43] showed that magnetic resonance could be used to non-invasively evaluate human BMSC in corpus cavernous of rats and rabbits. Also, Song et al showed that immortalized human BMSC (by v-myc transfection) transplanted into rat corpus cavernosum could differentiate into endothelial and smooth muscle cells.
Also, the skeletal muscle-derived stem cells (MDSCs) have been converted into smooth muscle cells (SMCs) both in vitro and in vivo, and were shown to recover erectile function in the aged rats [44]. Although these results suggest exogenous stem cell implantation and/or endogenous stem cell modulation might be a viable therapeutic approach for age-related ED, the application of MDSCs obtained from muscle is limited due to the source and number of this kind of stem cell.
Song et al investigated the feasibility of applying neural crest stem cells (NCSCs) to repair injury in the penile cavernosum and found that these stem cells differentiated into either endothelial cells or smooth muscle cells, as shown by their expression of cell type-specific markers. However, the function of these differentiated cells had not been explored in their experiment [45].
Application of adipose derived stem cells
Garcia et al isolated human ADSCs from a liposuction procedure, and administered according to a strict protocol that involves infusion of the cells into the target lesion along with fibrin glue in a human phase IIb clinical study [46]. The clinical results satisfied the effectiveness to induce healing in a complex of perianal fistulas, though the underling mechanism needs to be further explored.
Also, human ADSCs have been successfully applied to reverse urethral incontinence in an animal model in 2005 [47]. The ADSCs were also isolated from human lipoaspirate, labeled with a fluorescent tag and suspended in Hanks' balanced salt solution. The multiple animal models, including 8 Rnu athymic rats and 6 SCID mice underwent laparotomy and injection of ADSCs into the bladder and urethra. The ADSCs remained viable for up to 12 weeks in the lower urinary tract and differentiated into smooth muscle cells, which implies that ADSCs may provide a feasible and cost-effective cell source for urinary tract reconstruction. The major defect of this study is the lack of functional studies on those animals.
Adipose derived stem cells for erectile dysfunction
In our laboratory, a pilot experiment has been conducted [15]. Thirty 3-month old Sprague-Dawley male rats were randomly divided into three groups, including the sham, control and ADSCs injection groups. Following bilateral cavernous nerve crush injury in the control and ADSCs injection groups, the autologous adult ADSCs were isolated from the rats. The ADSCs were cultured in vitro and then transplanted back into the rat penis in the ADSCs injection group, while the sham and control group were injected with PBS into the penis. Five weeks later, the erectile function of each group was checked by measuring the intracavernosal pressure (ICP), and the fate of transplanted ADSCs was assayed by immunochemical histology. The results indicated that the mean increase in ICP for animals in the sham vs. untreated crush injury groups was 106.2 ±14.1cmH2O versus 18.0 ±8.0 cmH2O, respectively (p<0.05). Rats treated with ADSCs after crush injury significantly demonstrated greater ICP responses relative to untreated controls (p<0.05) with ICP values at 85.1 ±7.5cmH2O. There was evidence that some stem cells labeled with BrdU appeared to differentiate into endothelial cells and smooth muscle cells within the erectile tissue.
After analyzing our results, several possible mechanisms underlying the treatment were proposed. First, the cell-based mechanism indicated that ADSCs differentiated into local functional cells, including smooth muscle cells and endothelium. Second, the growth factor-based mechanism indicated that the growth factors secreted from the ADSCs underwent one of the following situations: (1) Recruit local stem cells and activity to differentiation; (2) attract the systemic stem cells to the penis by homing effect; (3) IGF-1 promote local functional cell growth; (4) the neurotrophic effect to promote nerve regeneration, which need to be confirmed by further studies.
Perspectives.
The adipose-derived stem cells are regarded as candidates for the treatment of erectile dysfunction due to many factors. These stem cells can easily be obtained in large quantities under local anesthesia, they possess the potential to undergo long-term proliferation, self-renewal and multipotent differentiation, and they can serve as a vehicle for release of neurotrophins, such as nerve growth factor, to repair damages due to diabetes or pelvic surgery.
In addition to using adipose derived stem cells for treatment of erectile dysfunction, there are several other kinds of andrology disorders that can benefit from stem cell therapy, including the reconstruction of penile tissue, enlargement of the penis, and strengthening of the vaginal muscle. There is also the potential application for use of adipose derived stem cells in steroid hormone therapy.
Reference
- 1.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 2.Halvorsen YC, Wilkison WO, Gimble JM. Adipose-derived stromal cells--their utility and potential in bone formation. Int J Obes Relat Metab Disord. 2000;24 Suppl 4:S41–S44. doi: 10.1038/sj.ijo.0801503. [DOI] [PubMed] [Google Scholar]
- 3.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 4.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 5.Kokai LE, Rubin JP, Marra KG. The potential of adipose-derived adult stem cells as a source of neuronal progenitor cells. Plast Reconstr Surg. 2005;116:1453–1460. doi: 10.1097/01.prs.0000182570.62814.e3. [DOI] [PubMed] [Google Scholar]
- 6.Kurita M, Matsumoto D, Shigeura T, Sato K, Gonda K, Harii K, Yoshimura K. Influences of centrifugation on cells and tissues in liposuction aspirates: Optimized centrifugation for lipotransfer and cell isolation. Plast Reconstr Surg. 2008;121:1033–1041. doi: 10.1097/01.prs.0000299384.53131.87. [DOI] [PubMed] [Google Scholar]
- 7.Yoshimura K, Sato K, Aoi N, Kurita M, Inoue K, Suga H, Eto H, Kato H, Hirohi T, Harii K. Cell-assisted lipotransfer for facial lipoatrophy: Efficacy of clinical use of adipose-derived stem cells. Dermatol Surg. 2008 doi: 10.1111/j.1524-4725.2008.34256.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 8.Lin G, Garcia M, Ning H, Banie L, Gio YL, Lue TF, Lin CS. Defining stem and progenitor cells within adipose tissue. Stem Cells Dev. 2008 doi: 10.1089/scd.2008.0117. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kurita M, Aiba-Kojima E, Shigeura T, Matsumoto D, Suga H, Inoue K, Eto H, Kato H, Aoi N, Yoshimura K. Differential effects of three preparations of human serum on expansion of various types of human cells. Plast Reconstr Surg. 2008;122:438–448. doi: 10.1097/PRS.0b013e31817d618d. [DOI] [PubMed] [Google Scholar]
- 10.Ning H, Lin G, Lue TF, Lin CS. Neuron-like differentiation of adipose tissue-derived stromal cells and vascular smooth muscle cells. Differentiation. 2006;74:510–518. doi: 10.1111/j.1432-0436.2006.00081.x. [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez LV, Alfonso Z, Zhang R, Leung J, Wu B, Ignarro LJ. Clonogenic multipotent stem cells in human adipose tissue differentiate into functional smooth muscle cells. Proc Natl Acad Sci U S A. 2006;103:12167–12172. doi: 10.1073/pnas.0604850103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodbell M. Metabolism of isolated fat cells. I. Effects of hormones on glucose metabolism and lipolysis. J Biol Chem. 1964;239:375–380. [PubMed] [Google Scholar]
- 13.Björntorp PKM, Pertoft H, Pettersson P, Sjöström L, Smith U. Isolation and characterization of cells from rat adipose tissue developing into adipocytes. J Lipid Res. 1978;19:316–324. [PubMed] [Google Scholar]
- 14.Lendeckel S, Jodicke A, Christophis P, Heidinger K, Wolff J, Fraser JK, Hedrick MH, Berthold L, Howaldt HP. Autologous stem cells (adipose) and fibrin glue used to treat widespread traumatic calvarial defects: Case report. J Craniomaxillofac Surg. 2004;32:370–373. doi: 10.1016/j.jcms.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 15.Bella AJ, Lin G, Phonsombat S, Lin CS, Garcia M, Brant WO, Lue TF. Non-cell line induced autologous adult adipose tissue derived stem cells enhance recovery of erectile function in the rat following bilateral cavernous nerve crush injury; Sexual Medicine Society of North America Meeting; 2008. 68 pp. [Google Scholar]
- 16.Schaffler A, Buchler C. Concise review: Adipose tissue-derived stromal cells--basic and clinical implications for novel cell-based therapies. Stem Cells. 2007;25:818–827. doi: 10.1634/stemcells.2006-0589. [DOI] [PubMed] [Google Scholar]
- 17.Rodeheffer MS, Birsoy K, Friedman JM. Identification of white adipocyte progenitor cells in vivo. Cell. 2008;135:240–249. doi: 10.1016/j.cell.2008.09.036. [DOI] [PubMed] [Google Scholar]
- 18.Tang W, Zeve D, Suh JM, Bosnakovski D, Kyba M, Hammer RE, Tallquist MD, Graff JM. White fat progenitor cells reside in the adipose vasculature. Science. 2008;322:583–586. doi: 10.1126/science.1156232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamamoto N, Akamatsu H, Hasegawa S, Yamada T, Nakata S, Ohkuma M, Miyachi E, Marunouchi T, Matsunaga K. Isolation of multipotent stem cells from mouse adipose tissue. J Dermatol Sci. 2007;48:43–52. doi: 10.1016/j.jdermsci.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 20.Zannettino AC, Paton S, Arthur A, Khor F, Itescu S, Gimble JM, Gronthos S. Multipotential human adipose-derived stromal stem cells exhibit a perivascular phenotype in vitro and in vivo. J Cell Physiol. 2008;214:413–421. doi: 10.1002/jcp.21210. [DOI] [PubMed] [Google Scholar]
- 21.Stashower M, Smith K, Williams J, Skelton H. Stromal progenitor cells present within liposuction and reduction abdominoplasty fat for autologous transfer to aged skin. Dermatol Surg. 1999;25:945–949. doi: 10.1046/j.1524-4725.1999.99098.x. [DOI] [PubMed] [Google Scholar]
- 22.Clavijo-Alvarez JA, Rubin JP, Bennett J, Nguyen VT, Dudas J, Underwood C, Marra KG. A novel perfluoroelastomer seeded with adipose-derived stem cells for soft-tissue repair. Plast Reconstr Surg. 2006;118:1132–1142. doi: 10.1097/01.prs.0000221037.34883.0a. [DOI] [PubMed] [Google Scholar]
- 23.Dudas JR, Marra KG, Cooper GM, Penascino VM, Mooney MP, Jiang S, Rubin JP, Losee JE. The osteogenic potential of adipose-derived stem cells for the repair of rabbit calvarial defects. Ann Plast Surg. 2006;56:543–548. doi: 10.1097/01.sap.0000210629.17727.bd. [DOI] [PubMed] [Google Scholar]
- 24.Lee WC, Maul TM, Vorp DA, Rubin JP, Marra KG. Effects of uniaxial cyclic strain on adipose-derived stem cell morphology, proliferation, and differentiation. Biomech Model Mechanobiol. 2007;6:265–273. doi: 10.1007/s10237-006-0053-y. [DOI] [PubMed] [Google Scholar]
- 25.Ning H, Lin G, Fandel T, Banie L, Lue TF, Lin CS. Insulin growth factor signaling mediates neuron-like differentiation of adipose tissue-derived stem cells. Differentiation. 2008;76:488–494. doi: 10.1111/j.1432-0436.2007.00240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee WC, Rubin JP, Marra KG. Regulation of alpha-smooth muscle actin protein expression in adipose-derived stem cells. Cells Tissues Organs. 2006;183:80–86. doi: 10.1159/000095512. [DOI] [PubMed] [Google Scholar]
- 27.Andersen DC, Schroder HD, Jensen CH. Non-cultured adipose-derived CD45- side population cells are enriched for progenitors that give rise to myofibres in vivo. Exp Cell Res. 2008;314:2951–2964. doi: 10.1016/j.yexcr.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 28.Fandel TM, Bella AJ, Tantiwongse K, Garcia M, Nunes L, Thuroff JW, Tanagho EA, Pohl J, Lue TF. The effect of intracavernosal growth differentiation factor-5 therapy in a rat model of cavernosal nerve injury. BJU Int. 2006;98:632–636. doi: 10.1111/j.1464-410X.2006.06375.x. [DOI] [PubMed] [Google Scholar]
- 29.Bivalacqua TJ, Deng W, Champion HC, Hellstrom WJ, Kadowitz PJ. Gene therapy techniques for the delivery of endothelial nitric oxide synthase to the corpora cavernosa for erectile dysfunction. Methods Mol Biol. 2004;279:173–185. doi: 10.1385/1-59259-807-2:173. [DOI] [PubMed] [Google Scholar]
- 30.Bivalacqua TJ, Deng W, Kendirci M, Usta MF, Robinson C, Taylor BK, Murthy SN, Champion HC, Hellstrom WJ, Kadowitz PJ. Mesenchymal stem cells alone or ex vivo gene modified with endothelial nitric oxide synthase reverse age-associated erectile dysfunction. Am J Physiol Heart Circ Physiol. 2007;292:H1278–H1290. doi: 10.1152/ajpheart.00685.2006. [DOI] [PubMed] [Google Scholar]
- 31.Bochinski D, Lin GT, Nunes L, Carrion R, Rahman N, Lin CS, Lue TF. The effect of neural embryonic stem cell therapy in a rat model of cavernosal nerve injury. BJU Int. 2004;94:904–909. doi: 10.1111/j.1464-410X.2003.05057.x. [DOI] [PubMed] [Google Scholar]
- 32.Allera-Moreau C, Delluc-Clavieres A, Castano C, Van den Berghe L, Golzio M, Moreau M, Teissie J, Arnal JF, Prats AC. Long term expression of bicistronic vector driven by the FGF-1 IRES in mouse muscle. BMC Biotechnol. 2007;7:74. doi: 10.1186/1472-6750-7-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gonda K, Shigeura T, Sato T, Matsumoto D, Suga H, Inoue K, Aoi N, Kato H, Sato K, Murase S, Koshima I, Yoshimura K. Preserved proliferative capacity and multipotency of human adipose-derived stem cells after long-term cryopreservation. Plast Reconstr Surg. 2008;121:401–410. doi: 10.1097/01.prs.0000298322.70032.bc. [DOI] [PubMed] [Google Scholar]
- 34.Audigier S, Guiramand J, Prado-Lourenco L, Conte C, Gonzalez-Herrera IG, Cohen-Solal C, Recasens M, Prats AC. Potent activation of FGF-2 IRES-dependent mechanism of translation during brain development. RNA. 2008;14:1852–1864. doi: 10.1261/rna.790608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prats AC, Prats H. Translational control of gene expression. Role of IRESs and consequences for cell transformation and angiogenesis. Prog Nucleic Acid Res Mol Biol. 2002;72:367–413. doi: 10.1016/s0079-6603(02)72075-8. [DOI] [PubMed] [Google Scholar]
- 36.Gonzalez-Herrera IG, Prado-Lourenco L, Pileur F, Conte C, Morin A, Cabon F, Prats H, Vagner S, Bayard F, Audigier S, Prats AC. Testosterone regulates FGF-2 expression during testis maturation by an IRES-dependent translational mechanism. Faseb J. 2006;20:476–478. doi: 10.1096/fj.04-3314fje. [DOI] [PubMed] [Google Scholar]
- 37.Kakudo N, Shimotsuma A, Kusumoto K. Fibroblast growth factor-2 stimulates adipogenic differentiation of human adipose-derived stem cells. Biochem Biophys Res Commun. 2007;359:239–244. doi: 10.1016/j.bbrc.2007.05.070. [DOI] [PubMed] [Google Scholar]
- 38.Rider DA, Dombrowski C, Sawyer AA, Ng GH, Leong D, Hutmacher DW, Nurcombe V, Cool SM. Autocrine fibroblast growth factor 2 increases the multipotentiality of human adipose-derived mesenchymal stem cells. Stem Cells. 2008;26:1598–1608. doi: 10.1634/stemcells.2007-0480. [DOI] [PubMed] [Google Scholar]
- 39.Suga H, Eto H, Shigeura T, Inoue K, Aoi N, Kato H, Nishimura S, Manabe I, Gonda K, Yoshimura K. Ifats series. FGF-2-induced HGF secretion by adipose-derived stromal cells inhibits post-injury fibrogenesis through a JNK-dependent mechanism. Stem Cells. 2008 doi: 10.1634/stemcells.2008-0261. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 40.Gonzalez-Herrera IG, Prado-Lourenco L, Teshima-Kondo S, Kondo K, Cabon F, Arnal JF, Bayard F, Prats AC. IRES-dependent regulation of FGF-2 mRNA translation in pathophysiological conditions in the mouse. Biochem Soc Trans. 2006;34:17–21. doi: 10.1042/BST20060017. [DOI] [PubMed] [Google Scholar]
- 41.Zaragosi LE, Ailhaud G, Dani C. Autocrine fibroblast growth factor 2 signaling is critical for self-renewal of human multipotent adipose-derived stem cells. Stem Cells. 2006;24:2412–2419. doi: 10.1634/stemcells.2006-0006. [DOI] [PubMed] [Google Scholar]
- 42.Deng W, Bivalacqua TJ, Chattergoon NN, Hyman AL, Jeter JR, Jr, Kadowitz PJ. Adenoviral gene transfer of eNOS: High-level expression in ex vivo expanded marrow stromal cells. Am J Physiol Cell Physiol. 2003;285:C1322–C1329. doi: 10.1152/ajpcell.00141.2003. [DOI] [PubMed] [Google Scholar]
- 43.Song YS, Lee HJ, Park IH, Kim WK, Ku JH, Kim SU. Potential differentiation of human mesenchymal stem cell transplanted in rat corpus cavernosum toward endothelial or smooth muscle cells. Int J Impot Res. 2007;19:378–385. doi: 10.1038/sj.ijir.3901539. [DOI] [PubMed] [Google Scholar]
- 44.Nolazco G, Kovanecz I, Vernet D, Gelfand RA, Tsao J, Ferrini MG, Magee T, Rajfer J, Gonzalez-Cadavid NF. Effect of muscle-derived stem cells on the restoration of corpora cavernosa smooth muscle and erectile function in the aged rat. BJU Int. 2008;101:1156–1164. doi: 10.1111/j.1464-410X.2008.07507.x. [DOI] [PubMed] [Google Scholar]
- 45.Song YS, Lee HJ, Park IH, Lim IS, Ku JH, Kim SU. Human neural crest stem cells transplanted in rat penile corpus cavernosum to repair erectile dysfunction. BJU Int. 2008;102:220–224. doi: 10.1111/j.1464-410X.2008.07469.x. discussion 224. [DOI] [PubMed] [Google Scholar]
- 46.Garcia-Olmo D, Garcia-Arranz M, Herreros D. Expanded adipose-derived stem cells for the treatment of complex perianal fistula including crohn's disease. Expert Opin Biol Ther. 2008;8:1417–1423. doi: 10.1517/14712598.8.9.1417. [DOI] [PubMed] [Google Scholar]
- 47.Jack GS, Almeida FG, Zhang R, Alfonso ZC, Zuk PA, Rodriguez LV. Processed lipoaspirate cells for tissue engineering of the lower urinary tract: Implications for the treatment of stress urinary incontinence and bladder reconstruction. J Urol. 2005;174:2041–2045. doi: 10.1097/01.ju.0000176489.96993.84. [DOI] [PubMed] [Google Scholar]