Abstract
Astrocytes respond to environmental cues and play a multifaceted role in the response to trauma in the central nervous system. As the most prevalent contributors to the glial scar, astrocytes are targeted as barriers to regeneration. However, there is also strong evidence that astrocytes are vital for neuroprotection and metabolic support after injury. In addition, consistent with their role during development, astrocytes may be capable of supporting the growth of injured axons. Therefore, we hypothesized that with appropriate stimulation, the reparative functions of endogenous astrocytes could be harnessed to promote axon growth and recovery after spinal cord injury. Transforming growth factor-α (TGF-α) is a mitogenic growth factor that is active on astrocytes and is poised to contribute to such a strategy. Recombinant TGF-α was administered intrathecally to adult C57BL/6 mice for two weeks following a moderate mid-thoracic spinal cord contusion. By three weeks post-injury, TGF-α infusion had not affected locomotor recovery, but promoted extensive axon growth and altered the composition of the lesion site. The center of the lesion in the treated mice contained greater numbers of new cells and increased astrocyte invasion. Despite the expression of inhibitory proteoglycans, there was a marked increase in axons expressing neurofilament and GAP-43 immunoreactivity, and the new axons were closely associated with increased laminin expression within and beyond the astrocyte matrix. The results demonstrate that astrocytes are dynamic players in the response to spinal cord injury, and the growth supportive role of these cells can be enhanced by TGF-α infusion.
Keywords: spinal cord injury, astrocyte, extracellular matrix, transforming growth factor, regeneration, sprouting, CSPG, neurocan
Introduction
Astrocytes play multifaceted roles in the developing and mature central nervous system, and contribute to repair after central nervous system (CNS) injury. During development, radial glial cells, precursors to astrocytes, are produced alongside neurons and are essential for the support of neuronal migration and axon guidance (Rakic, 1978; Vaccarino et al., 2007). In the mature intact CNS, astrocytes regulate synaptic activity (Norenberg, 1979; Anderson and Swanson, 2000; Schousboe et al., 2004; Tanaka, 2007), modulate the extracellular ionic environment (Walz et al., 1984; Walz, 2000), control volume homeostasis (Sykova et al., 1992), and maintain the characteristics of the blood-brain barrier (Vise et al., 1975; Haseloff et al., 2005; Abbott et al., 2006). After injury, mature astrocytes respond rapidly to local environmental cues by undergoing hypertrophy, proliferating, and migrating to the edge of the lesion site (Buffo et al., 2008). One consequence of these responses is the production of the glial scar, a physical and chemical barrier to regeneration (Luizzi and Lasek, 1987; Rudge and Silver, 1990; Fitch and Silver, 1997).
Within the local environment of a CNS injury, the pro-inflammatory and growth inhibitory effects of reactive astrocytes are offset by the essential role of the astrocyte response in neuroprotection and the potential for astrocytes to support axon growth. Astrocytes contribute to restoring the extracellular ionic environment (Sykova et al., 1992), sequestering extracellular glutamate (Rothstein et al., 1996), and producing neurotrophic factors (Lee et al., 1998; Krenz and Weaver, 2000; Ikeda et al., 2001; do Carmo Cunha et al., 2007) after injury. Although reactive astrocytes produce chondroitin sulfate proteoglycans (CSPGs) (Zuo et al., 1998; Morgenstern et al., 2002; Fitch and Silver, 2008), molecules that are inhibitory to axon growth, they also produce adhesive extracellular matrix (ECM) molecules, such as laminin, that provide a substrate supportive to growth (Frisen et al., 1995; Costa et al., 2002). Thus, the astrocyte response to injury is necessary for successful homeostasis and tissue repair. Indeed, if astrocyte proliferation (Faulkner et al., 2004) or migration (Okada et al., 2006) is disrupted after spinal cord injury, wound healing and recovery is diminished. Understanding the balance between the protective and inhibitory functions of astrocytes is important in order to devise strategies that will prompt beneficial astrocytic repair following injury.
TGF-α is an endogenous, mitogenic ligand that can promote changes in astrocytes and other cells via activation of the epidermal growth factor receptor (EGFR) (Lee et al., 1995; Junier, 2000). TGF-α administration increases glial proliferation and survival both in vitro (Sharif et al., 2006b) and in vivo (Fallon et al., 2000). In vitro, TGF-α alters astrocyte phenotype by increasing expression of the radial glial markers BLBP and RC2 and inducing an immature, bipolar astrocytic morphology (Zhou et al., 2001; Sharif et al., 2006a). In vivo, TGF-α overexpression induces astrocyte hypertrophy (Rabchevsky et al., 1998) and neuroprotection (Boillee et al., 2001), and administration of exogenous TGF-α induces the migration of neural and glial progenitors from the subventricular zone (Fallon et al., 2000). Induction of the ErbB2 EGFR subunit, which upregulates TGF-α and EGFR (Xie et al., 1999), also promotes a neural supportive radial glial phenotype in the adult cerebral cortex (Ghashghaei et al., 2007). These bipolar and radial glial phenotypes are supportive to neuronal migration in the developing brain (Vaccarino et al., 2007) and axonal growth in the developing spinal cord (Joosten and Gribnau, 1989), suggesting that this pattern of protein expression and morphology is a growth-supportive phenotype.
Based on these findings, we hypothesized that administration of TGF-α to the injured spinal cord would alter the glial response to injury and create a glial environment that would support axonal growth. Following a two-week infusion of human recombinant TGF-α, we detected a striking astrocyte-rich matrix that extended into the lesion site of the TGF-α-treated mice. The treated injury site included an enhanced axonal plexus that extended throughout and beyond the edges of the glial border into the center of the lesion. The increased axonal growth in the lesion core prevailed despite production of the inhibitory CSPG neurocan by surrounding astrocytes. The axon-rich matrix was associated with increased laminin immunoreactivity throughout the lesion site. Thus, administration of exogenous TGF-α alters the evolution of the local environment after spinal cord injury, resulting in the production of an extracellular matrix that is permissive to both astrocyte invasion and axonal growth.
Materials and Methods
Subjects
Adult female C57BL/6 mice 10 weeks of age weighing 17 to 20 g (Jackson Laboratories, Bar Harbor, ME) were housed in barrier cages in a temperature and humidity controlled room with ad libidum access to food and water. After 1 week of acclimation, all mice were evaluated for normal locomotor function using the Basso Mouse Scale (BMS, Basso et al., 2006, see below). All animal experimentation procedures were performed according to approved protocols and in accordance with the NIH Guide to the Care and Use of Laboratory Animals.
Subdural Catheter Surgeries
Animals were anesthetized with a ketamine (Vedco, St. Joseph, MO) (80 mg/kg) and xylazine (Ben Venue Laboratories, Bedford, OH) (10 mg/kg) cocktail via intraperitoneal injection. After a thoracic level 13 (T13) laminectomy, the dura was cut with microscissors and a vehicle-filled cannula constructed from Intramedic Polyethylene Tubing (PE-50, Clay Adams, Parsippany, NJ) was inserted 0.7 cm in the rostral direction so that the tip reached T10. The end of the tubing was sealed and the catheter secured to the L1 paraspinous muscles and lateral cutaneous truncii muscles using silk sutures and a drop of cyanoacrylate adhesive. Skin openings were then closed with vicryl, the mice were given 2 cc of saline, and were allowed to recover in a warmed cage.
Groups
At 1 day post catheter surgery, mice were evaluated for locomotor function and were randomly assigned to 4 groups after ensuring a balance of equal numbers of mice with normal locomotion (BMS=9) and mice with mild trunk instability (BMS=7.5–8) in each group. The following groups were assigned: vehicle + spinal cord injury (SCI), TGF-α + SCI, vehicle + laminectomy, TGF-α + laminectomy. Prior studies have shown that the catheter placement in mice results in a local inflammatory response and a transient behavioral impairment, but has no long-term effects on behavioral recovery compared with animals with no catheter (Mire et al., 2008).
Spinal Cord Injury
Spinal cord injury or laminectomy sham surgeries were performed 7 days after subdural catheter surgeries to ensure sealing of the dura and full recovery. Mice were reanesthetized with ketamine/xylazine and a T9 laminectomy was performed under aseptic conditions. The OSU ESCID device was used to administer a moderate contusion injury (0.5 mm displacement) as described previously (Jakeman et al., 2000). One mouse was eliminated from further study due to an inadequate impact as revealed by real-time force measurements. After impact or laminectomy alone, the overlying muscles were sutured with 4-0 silk sutures. The sealed tip of the catheter was then cut and a primed Alzet mini-pump (see below) was attached and secured to the trunk muscle fascia with silk sutures and a drop of cyanoacrylate. Skin openings were closed with vicryl sutures and the mice were allowed to recover as described above. Surgeons were blinded to the pump contents. At 14 days after injury, mice were anesthetized with isofluorane, the Alzet pumps were removed, and the ends of the catheters were cauterized. Adequate pump function was assessed by determining residual volume. All pumps contained < 50 µl of solution at the time of pump removal. One animal had a pump that was disconnected from the catheter and was removed from further analysis. Two injured mice with vehicle treatment and two mice with TGF-α treatment were perfused at the time of pump removal to evaluate the distribution of the EGFR during the infusion period. All remaining mice survived 21 days. Injury groups had 5–6 animals per group, and laminectomy groups had 3 animals per group.
Post Operative Care
Bladders were expressed twice daily for the duration of the experiment. Throughout the experiment, mice were given peanut butter and sweetened cereal once daily to reduce weight loss after injury. To prevent urinary infection, subcutaneous injections of Baytril (Bayer Health Care, Shawnee Mission, KS) (5 ml/kg) and 0.9% saline were administered for 5 days after injury. If needed, mice were individually housed to control chewing of sutures resulting in damage to the catheter.
Mini-Osmotic Pumps and Treatment
Alzet Mini-Osmotic Pumps (Alzet, Cupertino, CA, Model 2002) were used for drug or vehicle administration. This model delivers fluid at a rate of 0.5 µl/hour for a duration of 14 days. Pumps were filled with either vehicle (1% normal mouse serum (Sigma Aldrich, St. Louis, MO) in sterile phosphate buffered saline (PBS)) or recombinant human TGF-α (R&D Systems, Minneapolis, MN), 50 µg in 200 µl PBS with 1% normal mouse serum. The dose was chosen based upon evidence of in vivo efficacy following intraventricular infusion using similar methods (Fallon et al., 2000). Pumps were weighed before and after injections to insure proper filling. The pumps were primed in a water bath at 37°C for 24 hours prior to insertion.
BrdU Injections
To determine the numbers and distribution of cells that proliferated during the first week after injury, intraperitoneal injections of bromodeoxyuridine (BrdU, Roche, Basel, Switzerland) (25 mg/kg in saline) were given once daily for the first seven days post-injury. Identical injections were given to animals in all injury and laminectomy groups.
Behavioral Testing
The Basso Mouse Scale (BMS) was used to assess locomotor function at 1, 3, 7, 14 and 21 days after injury or laminectomy (Basso et al., 2006). Animals were allowed to ambulate freely for 4 minutes per session in a molded plastic circular field with a nonskid floor and a clear plastic wall. They were assigned a BMS score and BMS subscore by 2 trained observers who were blind to surgery and treatment groups.
Tissue Preparation
All mice were anesthetized at the indicated survival time with a lethal dose of ketamine (120 mg/kg) and xylazine (15 mg/kg), and then transcardially perfused with a 0.1 M PBS solution and then with 4% paraformaldehyde in 0.1 M PBS. Brains and spinal cords were post-fixed in 4% paraformaldehyde for 2 hours at 4°C, then placed in 0.2 M phosphate buffer (PB) for 24 hours at 4°C. Tissues were then submerged in 30% sucrose for 2–5 days for cryoprotection. Spinal cords were cut and frozen into 0.8 cm blocks centered on the laminectomy site and serial transverse sections were sliced with a cryostat to a thickness of 10 µm in 10 sets of sections spaced 100 µm apart.
Immunohistochemistry
One set of sections was stained with eriocryome cyanine (EC, Sigma Aldrich, St. Louis, MO) to determine the distribution of residual myelin and to define the epicenter of the injury, defined as the section with the least amount of white matter sparing (WMS) (Basso et al., 1996). Two mice were removed from the study after EC analysis showed that the contusion injuries were at the incorrect thoracic level. This resulted in 4–5 animals per injury group and 3 animals per laminectomy group. The remaining sections were processed for subsequent immunohistochemistry.
For BrdU immunohistochemistry, sections were pretreated in 2N HCl in dH2O for 25 minutes at 37°C. After blocking of endogenous peroxidases, tissues were incubated in blocking solution for 20 minutes. Sections were then incubated for two hours at room temperature with rat anti-BrdU (proliferating cells, 1:100, AbD Serotec, Oxford, UK). The slides were incubated with biotinylated rabbit anti-rat (1:200, Vector Laboratories, Burlingame, CA), and then incubated in Avidin-Biotin Complex (ABC Elite, Vector Laboratories). 3, 3’ diaminobenzidine (DAB; Vector Laboratories) substrate was added in the presence of H2O2 and then slides were dehydrated and coverslipped. An additional set of sections was sequentially stained with rat anti-BrdU and rabbit anti-glial fibrillary acidic protein to identify distribution of proliferating astrocytes. After DAB, the sections were treated with blocking solution for one hour and incubated with rabbit anti-GFAP (astrocytes, 1:5000 dilution, Dako, Carpinteria, CA). Sections were then incubated with biotinylated goat anti-rabbit (1:1000, Vector Laboratories), followed by ABC Elite and H2O2 with SG substrate (Vector Laboratories).
Immunofluorescence
To examine the distribution of axons and cells within the lesion site, equally spaced sets of sections were incubated with selected antibodies and detected using immunofluorescence. Sections were incubated in blocking solution and then overlaid with primary antibodies (rabbit anti-GFAP, astrocytes, 1:2000, Dako; chicken anti-NF, axons, 1:500, Aves Labs, Inc., Tigard, OR; sheep anti-EGFR, epidermal growth factor receptor, 1:1000, Abcam, Cambridge, MA; rabbit anti-p75, Schwann cells, 1:200 dilution, Promega, Madison, WI; rat anti-laminin B2, laminin, 1:2000 dilution, Millipore, Billerica, MA; rabbit anti-GAP-43, growing axons, 1:4000 dilution, Millipore; rat anti-CD68, macrophages, 1:100, AbD Serotec; rabbit anti-neurocan, neurocan, 1:10000; gift of Dr. Richard Margolis Lab, New York University Medical Center; rabbit anti-calcitonin related peptide (CGRP), peptidergic axons, 1:8000, Sigma; goat anti-5-HT, serotonergic axons, 1:5000, ImmunoStar, Hudson, WI; rabbit anti-collagen IV, collagen IV, 1:400; Millipore) which were diluted in the blocking solution. The sections were then incubated in the appropriate Alexafluor (Invitrogen, Carlsbad, CA) secondary fluorescent antibodies (goat anti rabbit 488; goat anti-chicken 568; goat anti-chicken 647; donkey anti-goat 546; donkey anti-sheep 546; goat anti-rat 546). The nuclear marker Draq5 (1:000 dilution, Biostatus Limited, Leicestershire, UK) was added to the secondary antibody dilution of selected sections. Sections were then rinsed with PBS and coverslipped.
Quantitative Analysis
White Matter Sparing
White matter sparing was analyzed in tissue stained for myelin with EC. The area of normal intensity of blue (myelin) staining on sections obtained from the lesion epicenter was determined using the MCID image analysis software. Proportional white matter sparing was calculated as white matter area/total cross sectional area (Basso et al., 1996; Ma et al., 2001).
BrdU+ Cell Counting
The number of BrdU+ cells per tissue section was counted using the MCID 4.0 image analysis software (Imaging Research, Inc., St. Catherines, Ontario, Canada). Stained cells were defined at low magnification using a density threshold measure and criteria set to reflect the size of an average BrdU stained nucleus, which was confirmed by high power magnification. In addition, the number of BrdU+ cells in selected areas of the spinal cord (lesion center, dorsal column, dorsal horns, and spared white matter) at lesion epicenters was manually counted using a Zeiss Axioplan microscope with a 40x objective (2500 µm2 area).
Quantification of NF, GFAP, and Laminin Immunofluorescence
Images of NF, GFAP, and laminin immunofluorescence were captured using the MCID program and a Zeiss Axioplan microscope with a 10x objective. A sample box with an area of 290,000 µm2 was placed at the center of the section to measure the area of immunoreactivity in the lesion center. The MCID analysis program was used to measure the area of fluorescent immunoreactivity in the reference area. The proportional area (stained area/290,000 µm2) was then computed. For GFAP and NF immunoreactivity, the volume across the length of the lesion was determined (V = Σ[(stain area mm2 per section) ×1 × 10−6 mm2 between sections)]).
Confocal Microscopy
The Zeiss Z10 Confocal Microscope at The Ohio State University Confocal Microscopy Imaging Facility was used to capture dual and triple stained immunofluorescent images and identify colocalization.
Statistical Analysis
GraphPad Prizm 4.0 was used for statistical analysis. Repeated measure two-way ANOVAs were used to analyze BrdU+ cell counts, GFAP and NF immunoreactivity, and BMS scores. Bonferroni corrected post-tests were used for post-hoc analyses in two-way ANOVAs. Data extrapolation was performed in cases with missing values due to folded sections. Student’s t tests were used for analysis of GFAP and NF immunoreactivity volume, laminin immunoreactivity, and spared white matter Significance was set at p < 0.05. For all figures, error bars represent standard error of the mean (SEM).
Results
TGF-α infusion increases the number of newborn cells in the lesion center and dorsa horn after contusion injury
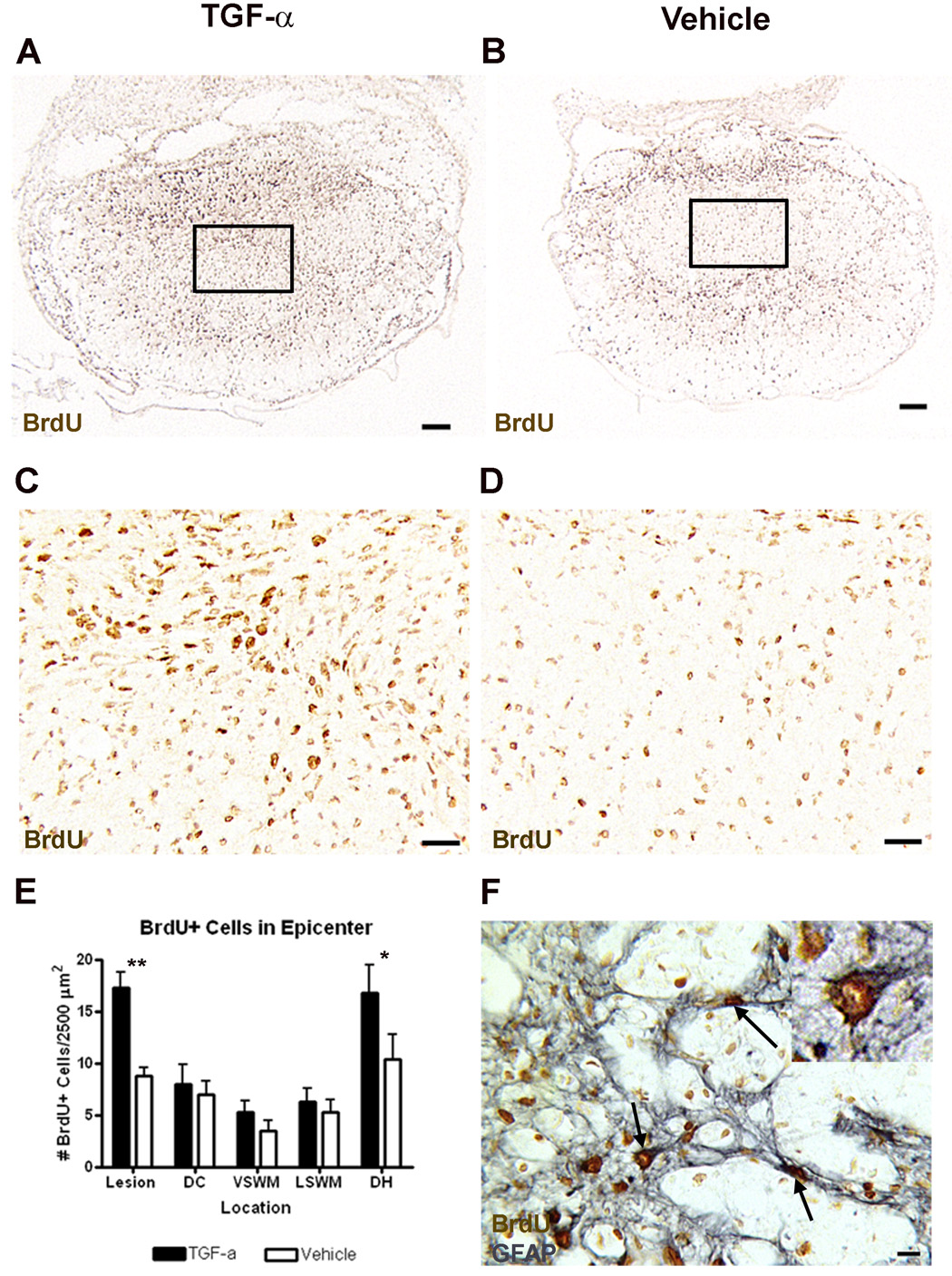
TGF-α can stimulate proliferation of a variety of cell types. To determine the effects of TGF-α infusion on cell proliferation after SCI, BrdU was administered daily for the first week after laminectomy or contusion injury. At 3 weeks after laminectomy, there were very few BrdU+ nuclei in TGF-α- or vehicle-infused animals and there was no effect of TGF-α on the total number of BrdU+ nuclei or BrdU+ nuclei in gray or white matter regions (not shown). However, after SCI, BrdU+ nuclei were prevalent throughout the spinal cord in all specimens (Fig. 1). Although there was no effect of TGF-α on the total number of BrdU+ nuclei across the entire epicenter section (TGF-α = 1552 ± 145 vs. Vehicle = 1342 ± 118), there were differences in the regional density of BrdU+ nuclei, reflecting an effect on the distribution of newborn cells. There were significantly more BrdU+ cells in the lesion center (Bonferroni post-hoc p<0.01) and the dorsal horns (Bonferroni post-hoc p<0.05) in TGF-α-treated than in vehicle-treated mice. Many of the BrdU+ cells in these regions were GFAP+, indicating that at least some of the cells that proliferated after injury were astrocytes (Fig. 1F).
Figure 1.
TGF-α-infused mice have increased BrdU+ cells in the lesion center and dorsal horn compared to vehicle-infused mice, and some of these cells are astrocytes. Low (A–B) and high (C–D) power images of BrdU immunoreactivity at the lesion epicenter of TGF-α (A,C) and vehicle (B,D) infused animals. Boxes in (A) and (B) indicate area shown in (C) and (D), respectively. (E) Quantification of the distribution of BrdU+ cells at the lesion epicenter. Lesion = Lesion Core, DC = Dorsal Column, VSWM = Ventral Spared White Matter, LSWM = Lateral Spared White Matter, DH = Dorsal Horn. *p<0.05, **p<0.01. (F) High power image of BrdU and GFAP immunoreactivity caudal to the lesion epicenter, showing that some BrdU+ cells in the lesion center are astrocytes. Arrow = cell double-labeled with GFAP and BrdU. Scale = 100 µm (A–B), 20 µm (C–D), 10 µm (F).
TGF-α alters GFAP and CD68 distribution and morphology independent of white matter sparing
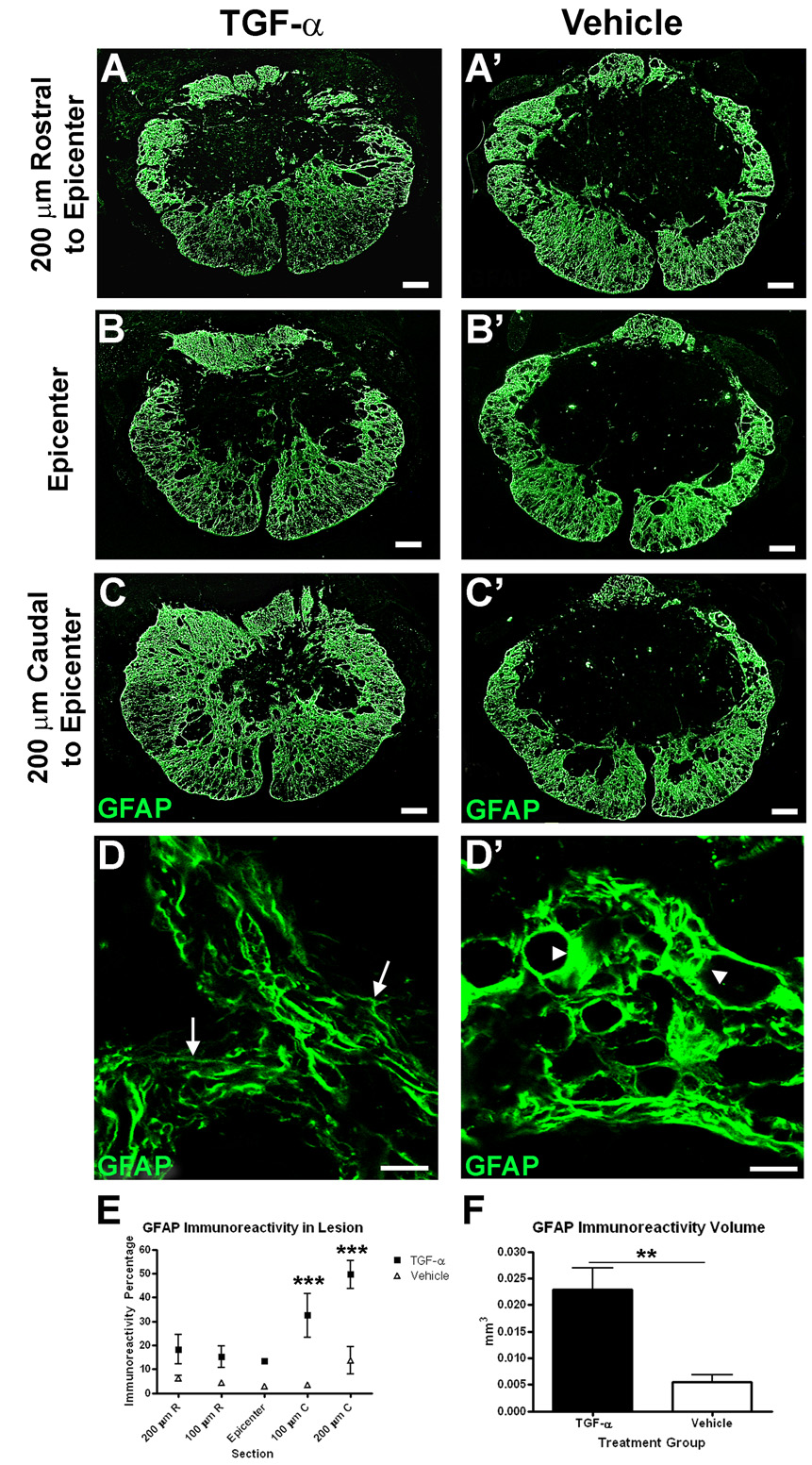
To test the hypothesis that TGF-α infusion would alter the astrocyte response to injury, sections through the lesion were labeled with an antibody raised against GFAP and examined by fluorescence microscopy. The distribution of GFAP immunoreactivity in vehicle-treated animals was similar to that previously described, with GFAP reactivity increased along the residual rim of the lesion site and little to no GFAP within the lesion core (Fig. 2). In contrast, GFAP immunoreactivity in TGF-α-infused mice extended further into the center of the lesion. The percentage of GFAP immunoreactivity in the lesion core was increased throughout the length of the lesion in TGF-α-infused mice compared to vehicle-infused controls (Two-way ANOVA treatment effect p<0.01) (Fig. 2E). This effect was especially prominent at 100–200 µm caudal to the epicenter, corresponding to the site of the tip of the infusion catheter (Bonferroni post-hoc p<0.001 at 100 µm caudal, p<0.001 at 200 µm caudal). Furthermore, the total volume of GFAP-immunoreactivity in the lesion was greater in TGF-α-treated animals compared to controls (TGF-α = 0.022 ± 0.004 mm3 vs. Vehicle = 0.005 ± 0.001 mm3) (Fig. 2F). TGF-α can also alter astrocyte morphology both in vitro (Sharif et al., 2006a) and in vivo (Rabchevsky et al., 1998). In laminectomy mice, there was no obvious effect of TGF-α infusion on cells with GFAP+ immunoreactivity (not shown). After injury, however, GFAP+ cells and processes at the lesion edge of TGF-α-treated specimens were thinner and oriented toward the lesion center. In similar regions from vehicle treated animals, astrocytes had thicker processes surrounding vessels and cysts and arranged in parallel with the perimeter of the lesion core (Fig. 2D). These differences suggest that TGF-α may modify the migration or morphological characteristics of reactive astrocytes at the site of injury.
Figure 2.
TGF-α alters GFAP distribution and morphology. (A–C) Images of GFAP immunoreactivity throughout the length of the lesion in TGF-α- (A–C) and vehicle- (A’–C’) infused mice. (D) High power magnification images of GFAP immunoreactivity along the lesion border of TGF-α- (D) and vehicle- (D’) infused mice. Arrows in (D) show lacy, elongated astrocytic process in contrast to arrowheads in (D’) showing hypertrophied GFAP+ processes. (E–F) Quantification of percentage (E) and volume (F) of GFAP immunoreactivity throughout the length of the lesion. Scale = 100 µm (A–C), 10 µm (D). **p<0.01, ***p<0.001.
To determine if TGF-α infusion also altered the local inflammatory response, additional sections were stained with antibodies directed toward the CD68 marker found on macrophages and microglia. Sections double-labeled with GFAP and CD68 revealed subtle differences in macrophage distribution between treatment groups that were complementary to the increased GFAP distribution (Supplemental Fig. 1). In both groups of mice, small phagocytic profiles were found in the peripheral rim while the center was occupied by dense CD68 immunoreactivity. In the TGF-α-treated subjects, the CD68+ clusters were smaller and the borders less pronounced. Notably, the relationship between the macrophage clusters and the astrocytes was conserved in that the macrophages were primarily restricted to areas that were free of GFAP immunoreactivity.
TGF-α has neuroprotective effects in some models. To determine if the effects of TGF-α on GFAP distribution reflected a protective action on overall tissue sparing at the epicenter, a series of sections through the lesion were stained with EC and evaluated with conventional measures (Supplemental Fig. 2). There was no effect of treatment on the total cross sectional area (TGF-α = 0.81 ± 0.06 mm2 vs. Vehicle = 0.83 ± 0.03 mm2) (not shown) or percentage of white matter sparing at the epicenter (TGF-α = 27.6 ± 3.6% vs. Vehicle = 27.3 ± 1.5%). Consistent with past studies showing a tight correlation between white matter sparing and behavioral recovery, no significant differences in BMS scores were observed (TGF-α = 4.4 ± 1.3 vs. Vehicle = 4.6 ± 1.2).
TGF-α infusion increases axons within the lesion
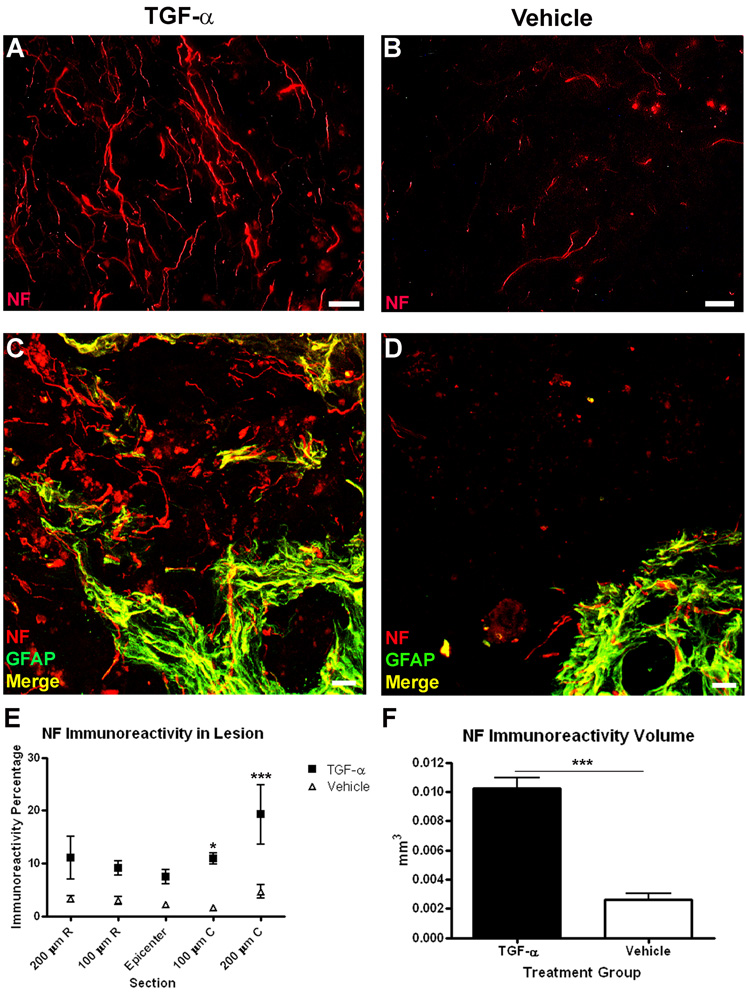
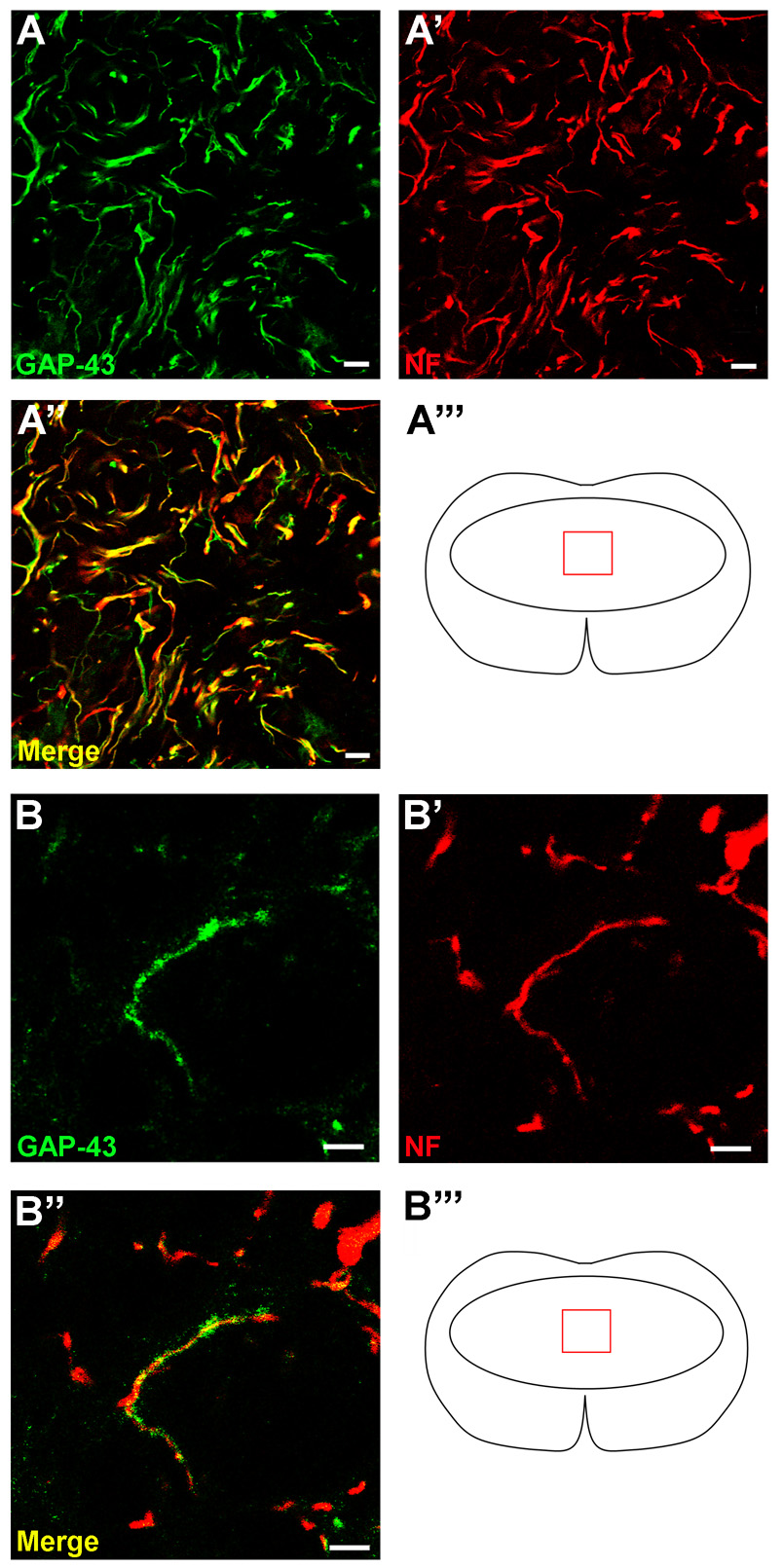
Examination of sections labeled with an antibody raised against the heavy-chain neurofilament protein (NF, 200 kD) revealed many axons within the center of the lesion in TGF-α treated specimens (Fig. 3). In contrast, in vehicle-infused mice, NF+ profiles were rarely found within the lesion core. Co-labeling with NF and GFAP antibodies illustrated differences in the distribution of NF profiles. TGF-α-treated specimens contained NF axons both within and beyond the GFAP+ border of the lesion, while in the vehicle-infused mice, the NF-immunoreactivity mirrored that of the GFAP+ immunoreactivity, in that very few axonal profiles extended past the glial border into the lesion center (Fig. 3C,D). As a measure of the density of axons within the lesion, the percent area of NF immunoreactivity was calculated at 100 µm intervals through the lesion center. NF+ profile area percentage (Two-way ANOVA treatment effect p<0.0001) and total volume (TGF-α = 0.010 ± 0.001 mm3 vs. Vehicle = 0.002 ± 0.000 mm3) measures were greater throughout the lesion core in the TGF-α-infused mice compared to controls (Fig. 3E,F). Similar to patterns of GFAP immunoreactivity, the increases in NF distribution were most robust caudal to the injury epicenter (Bonferroni post-hoc p<0.05 at 100 µm caudal, p<0.001 at 200 µm caudal). To differentiate between the contribution of spared and growing axons in the lesion core of TGF-α-infused mice, one set of sections was stained with both NF and growth-associated protein 43 (GAP-43), which is preferentially found in sprouting and regenerating axons (Skene and Willard, 1981) (Fig. 4). In TGF-α-infused mice, virtually all NF+ axons within the lesion were double-labeled with GAP-43, suggestive of regenerating, rather than spared axons. Double-labeled profiles were evident both along the glial border and in the core of the lesion.
Figure 3.
TGF-α infusion increases axons in the lesion core. NF immunoreactivity in the lesion core at the lesion epicenter of TGF-α- (A) and vehicle- (B) infused mice. (C–D) Confocal images of GFAP and NF immunoreactivity, showing NF+ axons extending past the glial border in TGF-α- (C) infused mice, but not vehicle- (D) infused mice. (E–F) Quantification of percentage (E) and volume (F) of GFAP immunoreactivity throughout the length of the lesion. Scale = 20 µm. *p<0.05, ***p<0.001.
Figure 4.
The majority of axons in the lesion core and on the glial border are new axons or collaterals. (A) GAP-43 and NF immunofluorescence in the lesion core of a TGF-α- infused subject. (B) High power magnification image of GAP-43 and, NF immunoreactivity showing one axon double-labeled in the lesion core. Scale = 10 µm (A), 5 µm (B).
Axons within the lesion grow alongside astrocyte or Schwann cell processes
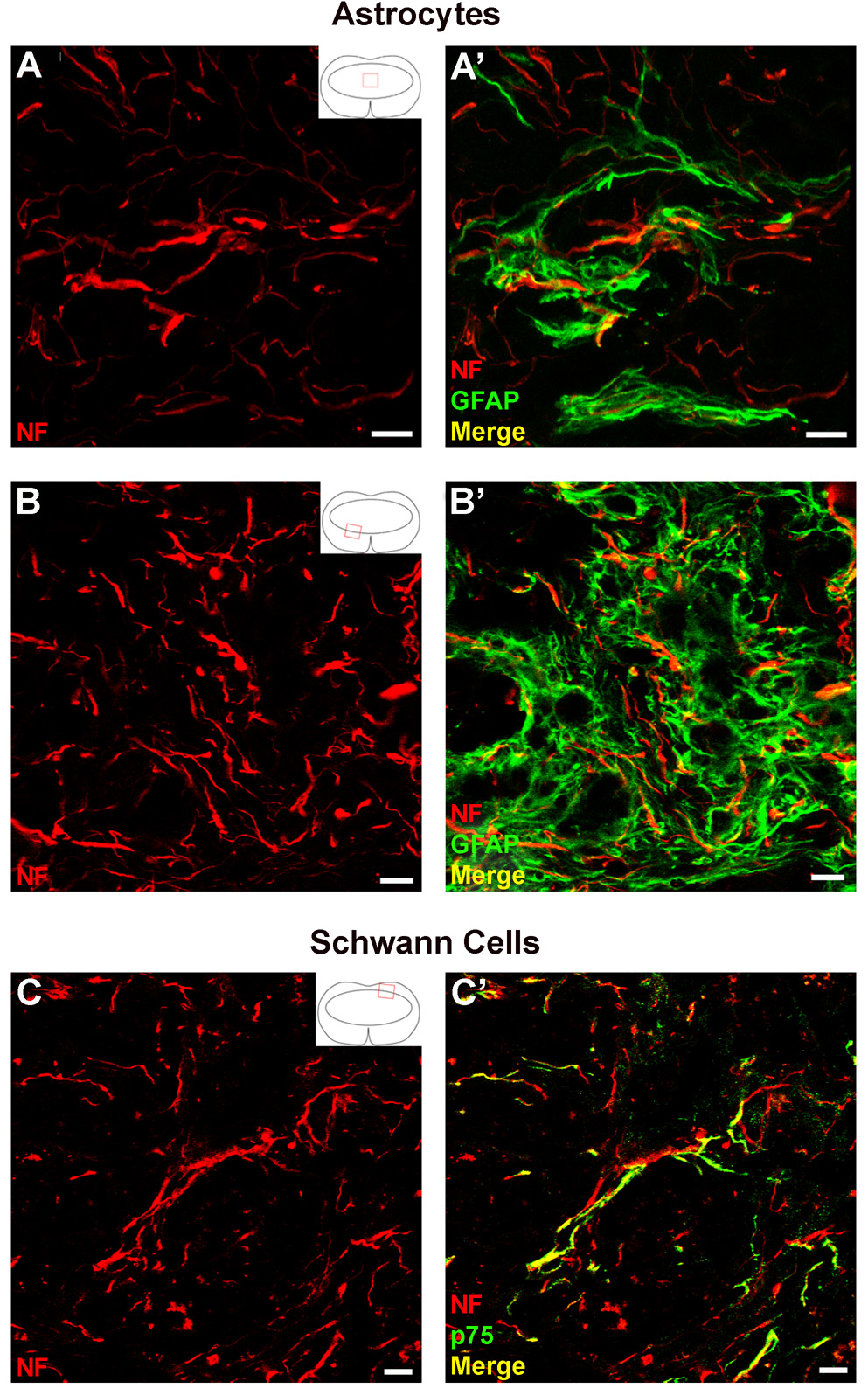
Recent examples of intervention strategies have shown that neurotrophic factors or cell transplants can induce axonal growth after spinal cord injury by enhancing Schwann cell invasion (Blesch and Tuszynski, 2007; Hill et al., 2007). Double immunofluorescence of NF and either GFAP or p75 (to identify Schwann cells) was performed and confocal microscopy was used to determine if astrocytes or Schwann cells were associated with the axons in the lesion (Fig. 5). Within the lesion core and along the glial border, many axons were in close contact with GFAP+ processes (Fig. 5A,B). In the dorsal root entry zone area, where Schwann cells commonly infiltrate the lesion, most axons were associated with p75+ processes (Fig. 5C). Thus, both astrocytes and Schwann cells are candidates for support of growing axons in the TGF-α-infused mice.
Figure 5.
Axons within the lesion associate with either astrocytes or Schwann cells. (A–B) High power magnification images of NF+ axons associated with GFAP+ processes in the lesion core (A, Z-stack projection) and glial border (B). (C) High power magnification image of NF+ axons growing alongside p75+ processes in the dorsal root entry zone. Scale = 10 µm.
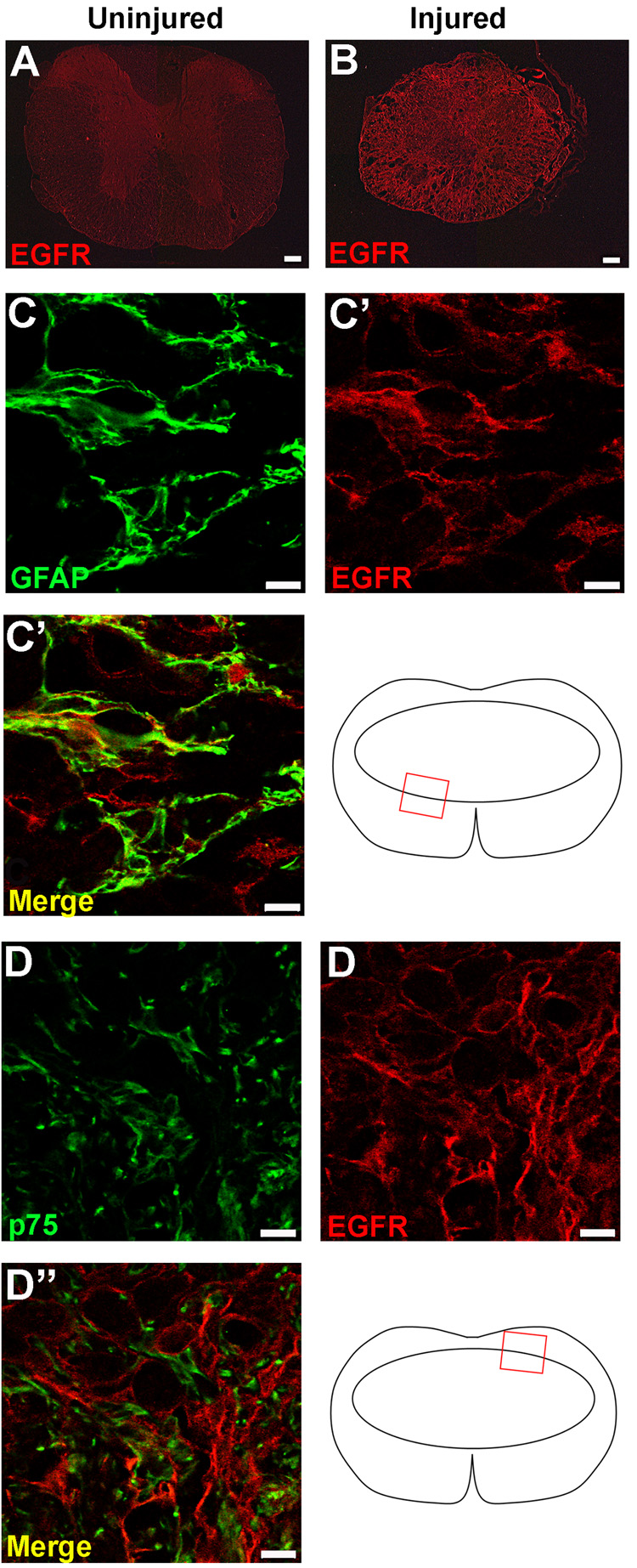
The EGFR is located on astrocytes, but not Schwann cells, in the lesion epicenter
Because axons were associated with both astrocytes and Schwann cells in the lesion site, sections from animals perfused two weeks after injury (during the infusion period) were immunolabeled with antibodies against EGFR and either GFAP or p75 to determine the likely cellular targets of TGF-α. There was very little EGFR immunoreactivity in uninjured animals treated with either vehicle or TGF-α (Fig. 6A). EGFR immunoreactivity increased markedly after SCI in both treatment groups, with no obvious differences in the intensity or distribution of receptor expression between TGF-α- and vehicle-treated mice (Fig. 6B). The most prominent EGFR immunoreactivity was co-localized with GFAP+ cells in both groups (Fig. 6C). In contrast, although EGFR immunoreactivity was located in proximity to p75+ Schwann cells in the dorsal quadrant of the spinal cord lesion in both groups, no colocalization was evident with these cells (Fig. 6D). Axons in the lesion did not express EGFR, but EGFR expression was found on fibroblast-like mesenchymal cells along the dorsal surface and dorsal regions of the lesion site and modest expression of the EGFR was found in macrophage-like profiles within the lesion core. Thus, TGF-α can act directly on astrocytes, macrophages or mesenchymal cells after injury, while any effects on Schwann cells or axons are likely to be indirect.
Figure 6.
The EGFR is upregulated after injury and is located on astrocytes, but not Schwann cells. EGFR immunoreactivity in uninjured (A) and injured (B) mice two weeks following injury. The EGFR colocalizes with GFAP+ astrocytes in on the glial border (C), but is not located on Schwann cells infiltrating the lesion from the dorsal root entry zone (D). Scale = 100 µm (A–B), 10 µm (C–D).
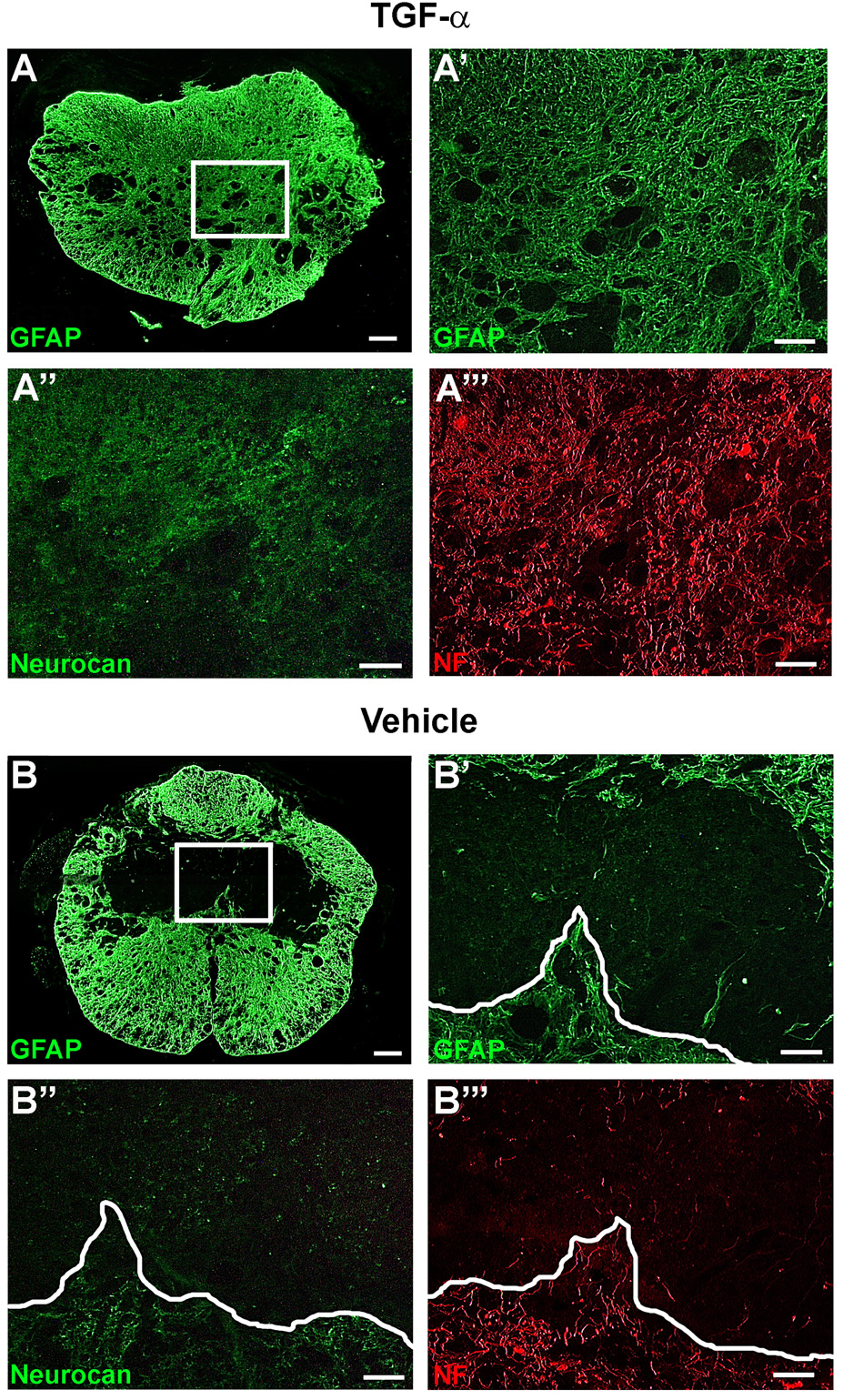
TGF-α increases neurocan expression, but does not restrict axon growth
In vitro studies have shown that TGF-α administration to astrocytes increases production of inhibitory chondroitin sulfate proteoglycans (CSPGs) (Smith and Strunz, 2005). To determine if the increased astrocyte distribution in lesion core was accompanied by increased CSPG expression, sections were labeled with an antibody to the inhibitory CSPG core protein neurocan. In both TGF-α- and vehicle-infused mice, neurocan immunoreactivity was closely associated with astrocytes along the glial border (Fig. 7). In TGF-α-infused mice, neurocan immunoreactivity was increased, as staining extended into the lesion core with a similar distribution pattern as GFAP, and axons were found both within and beyond regions of neurocan staining (Fig. 7A). In contrast, in vehicle-infused mice, neurocan was restricted to the edge of the lesion, and axons did not extend past the glial border and corresponding region of neurocan expression (Fig. 7B).
Figure 7.
TGF-α increases neurocan within the lesion, but does not inhibit axon growth. (A and B) Low power GFAP immunoreactivity caudal to the epicenter. Box = area shown in (A’-A’’’) and (B’-B’’’). High power magnification of GFAP (A’,B’), neurocan (A’’,B’’), and NF (A’’’,B’’’) immunoreactivity in the lesion core. White line dictates glial border in vehicle-infused mice (B’-B’’’). Scale = 100 µm (A–B), 20 µm (A’-A’’’, B’-B’’’).
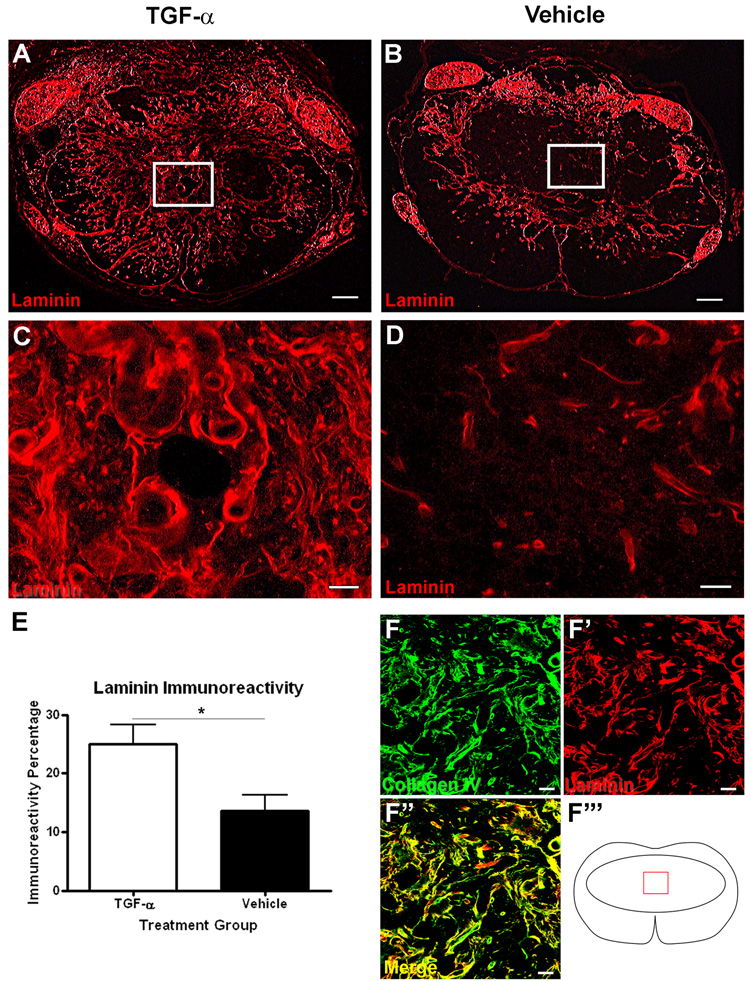
TGF-α administration increases laminin in the lesion core
In addition to CSPGs, astrocytes also produce ECM molecules supportive to axon growth, such as laminin (Frisen et al., 1995). Therefore, we examined the lesion site to determine if astrocytes in TGF-α-treated mice within the lesion were producing increased laminin in addition to CSPGs (Fig. 8). In vehicle-infused mice, laminin immunoreactivity was restricted to the lesion border and areas surrounding blood vessels (Fig. 8B,D). In TGF-α-infused mice, laminin staining was found throughout the lesion core (Fig. A,C). The percentage of laminin immunoreactivity in the lesion core of TGF-α-infused mice was greater than that found in vehicle-infused mice (TGF-α = 25.0 ± 3.2% vs. Vehicle = 13.6 ± 2.7%) (Fig. 8E). In addition to laminin, astrocytes and Schwann cells also produce Collagen IV, which forms a major component of the basal lamina. Sections were double-labeled with antibodies to laminin and collagen IV and examined using confocal microscopy. Nearly all laminin profiles within the lesion colocalized with collagen IV, showing that the laminin produced as a result of TGF-α infusion was primarily associated with basal lamina as opposed to cells (Fig. 8F).
Figure 8.
TGF-α treatment increases laminin immunoreactivity in the lesion core. Low (A–B) and high (C–D) power images of laminin immunoreactivity at the lesion epicenter of TGF-α- (A,C) and vehicle- (B,D) infused mice. (E) Quantification of the proportional area of laminin immunoreactivity at the lesion epicenter. (F) High power confocal image of collagen IV and laminin in the lesion epicenter of TGF-α-treated mice. Scale = 100 µm (A–B), 20 μm (C–D,F). *p<0.05.
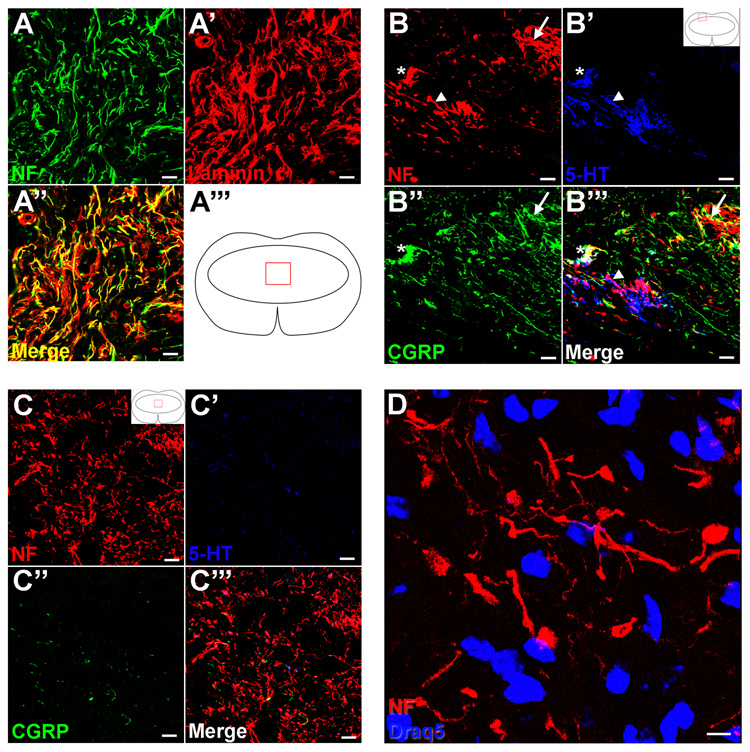
Axons within the lesion core grow alongside laminin and are CGRP and 5-HT negative
Based on the dense immunoreactivity of both NF and laminin within the lesion core, we hypothesized that axons in this area were located directly alongside the laminin profiles. To address this question, sections were stained with antibodies against NF and laminin, and confocal microscopy revealed that almost all NF+ axons were directly aligned with laminin (Figure 9A). To further explore the possible origin of these axons, sections were triple-labeled with antibodies to NF, calcitonin-related gene peptide (CGRP) to identify small diameter peptidergic Aδ and C fibers, and 5-HT to identify descending serotonergic axons (Fig. 9B,C). Along the dorsal and rostral lesion borders, there were axons in both TGF-α- and vehicle-treated mice that expressed CGRP or 5-HT, and small clusters of terminals where all three markers were localized. However, the NF+ axons found throughout the lesion core of TGF-α-infused mice were negative for both CGRP and 5-HT (Fig. 9C). To ensure that the NF antibody used was not detecting neuronal cell bodies, additional sections were double-labeled with NF and Draq5, a nuclear counterstain (Fig. 9D). The frequent rounded NF profiles were negative for the nuclear marker, suggesting that these were enlarged axonal profiles as opposed to cell bodies.
Figure 9.
Axons comingle with laminin and are CGRP and 5-HT negative. (A) High power confocal image of NF and laminin in the lesion epicenter of a TGF-α-infused subject. (B,C) High power confocal image of NF, 5-HT, and CGRP in the dorsal root entry zone (B) and the lesion core (C). Arrows in (B) show axons double-labeled with CGRP, arrowheads in (B) show axons double-labeled with 5-HT, and asterisks in (B) show axons that label with all three markers. (D) Confocal z-stack projection of NF and the nuclear marker Draq5 in the lesion core of a TGF-α-treated mouse. Scale = 20 µm (A–C), 5 µm (D).
Discussion
The astrocytes of the glial scar comprise a major impediment to recovery after SCI by forming a physical and chemical barrier that inhibits axon growth into and beyond the lesion edge. A common strategy to improve axonal growth and regeneration is to try to reduce the glial and astrocytic response to injury while maintaining the supportive functions of these cells. However, through an alternative approach, by stimulating astrocytes and other cells to divide, migrate into the lesion and modify the ECM composition, TGF-α infusion altered both the physical and chemical components of the glial scar, thus ameliorating the barrier to axonal growth. Administration of TGF-α modified the physical hurdle of the scar by transforming the astrocyte border from a dense network of tightly woven and thickened processes to a more open meshwork of elongated and thin processes that extended deep into the lesion. In addition, the infusion altered the balance of extracellular mediators of axonal growth by enhancing the production of laminin throughout the lesion site. By using TGF-α to manipulate and stimulate the endogenous cellular response to injury, a pro-reparative astrocytic infiltration zone was created that was able to support increased axonal growth at the site of a spinal contusion injury.
Cellular proliferation and increased astrocyte migration alter the composition of the lesion site
Cellular proliferation is minimal in the intact spinal cord, with small numbers of endogenous dividing cells located primarily in the periphery (Horner et al., 2000). SCI initiates a large increase in cell proliferation that peaks within the first week, with new cells derived from surrounding gray and white matter regions (McTigue et al., 2001; Zai and Wrathall, 2005; Horky et al., 2006). In the absence of treatment, the center of the lesion becomes occupied by cells of macrophage/microglial origin, while the majority of remaining newborn cells accumulates abruptly at the edge of the lesion site, forming a glial border enriched in microglia, glial progenitors and mature glial cells (Yang et al., 2006; Lytle and Wrathall, 2007; Tripathi and McTigue 2007). Despite its well-known role as a cellular mitogen, TGF-α administration after SCI did not significantly affect total numbers of newborn cells at the epicenter of the lesion site at three weeks post-injury, but it increased the numbers of newborn cells found in the dorsal quadrants and deep within the center of the lesion site. Co-labeling of BrdU and GFAP demonstrated that many of the cells in these regions were astrocytes. There was no evidence of acute neuroprotection, as measures of white matter sparing did not differ between groups. Instead, morphological evidence of elongated and delicate processes along the ventral and dorsal borders and the increased overall distribution of GFAP immunoreactivity throughout the lesion site support the interpretation that TGF-α stimulated the proliferation and migration of astrocytes toward the lesion center. This effect is consistent with the effects of TGF-α on astrocyte morphology and migration in vitro (Zhou et al., 2001; Sharif et al., 2006a) and resulted in a significant change in the cellular composition of the SCI site.
In this study, we have used a conservative approach to define the size of the SCI lesion site based on white matter sparing. Our lab and others have traditionally used white matter sparing to define the lesion due to its tight correlation with locomotor recovery scores. However, if the lesion site is defined more rigidly as the core of the injury site devoid of GFAP staining and occupied only by inflammatory cells, then the effect of the infusion was to reduce the extent of the lesion core, providing a first step toward repair of the injury site. Importantly, these findings raise the question of whether astrocyte migration into the lesion is beneficial or detrimental to tissue integrity and potential recovery. In a recent study addressing that question, transgenic mice with perturbations of the cytoskeletal regulator Stat3 revealed that blocking migration of nestin-expressing cells toward the lesion exacerbated the extent of inflammation and injury. Conversely, enhancing Stat3 activation by a deficient expression of the negative regulator Socs3 resulted in increased astrocyte migration at the lesion edge and reduced inflammation and tissue loss (Okada et al., 2006). The widespread functions of Stat3, which is a critical downstream mediator of motility in nearly all cells and tissues (Hirano et al., 2000) make it an unlikely candidate for effective direct pharmacological approaches. However, in an ideal setting, a carefully timed and locally directed administration of a gliotrophin such as TGF-α might stimulate a more complete reinvasion of a lesion site with CNS derived glia, and promote growth of central axons across the full length of the lesion.
A modified ECM mediates enhanced axon growth
In addition to changing astrocyte distribution within the lesion, TGF-α administration promoted the growth of GAP-43 positive axons within and beyond the astrocyte border. Many of the axons in the ventral and lateral regions of the lesion were directly associated with fine astrocyte processes, while axons in the dorsal-most regions were often associated with p75+ Schwann cell profiles that migrated into the lesion site from the periphery. Given the multipotent roles of TGF-α on cell function, two approaches were taken to determine possible mechanisms for the increased axonal growth seen following TGF-α infusion. We first examined the distribution of the EGF receptor after injury and then identified selected ECM components that corresponded to the distribution of the new axon profiles.
TGF-α exerts its biological actions through activation of the EGFR, which is found on many different cell types. Consistent with past research (Erschbamer et al., 2007), we found that the EGFR was expressed at low levels in the intact spinal cord, and strongly upregulated after injury where it was located primarily on astrocytes, but not Schwann cells, at the time of infusion. EGFR immunoreactivity was also found in macrophage profiles in the lesion center and was associated with mesenchymal-like cells surrounding the spinal cord and within the lesion. Previous work has shown that the EGFR is located on dorsal root ganglion, cerebellar, and retinal neurons and their axons (Koprivica et al., 2005). However, the effects of EGFR activation on axons is still unclear. While EGFR phosphorylation is required for the inhibition of neurite growth on some inhibitory substrates (Koprivica et al., 2005), other data demonstrate that EGFR activation can facilitate neuron survival and neurite outgrowth (Santa-Olalla and Covarrubias, 1995; Boillee et al., 2001). In the present study, EGFR immunoreactivity was not present on the axonal profiles within the lesion, similar to results shown after optic nerve injury in rats (Liu et al., 2006). Based on these observations, we conclude that the primary effects of TGF-α infusion in this study were not a result of a direct action on axonal elongation, but instead due to indirect actions mediated by astrocytes, mesenchymal cells and/or macrophages.
Changes in the morphology and migration of astrocytes in the treated group and the close alignment of axons with astrocyte profiles are consistent with a primary role of these cells in the increased axonal growth in the lesion site. However, these findings appear paradoxical considering the well-known inhibitory nature of activated astrocytes on axon growth in vitro (Rudge and Silver, 1990; McKeon et al., 1991) and in vivo (Reier et al., 1983; Davies et al., 1999; Fitch and Silver, 2008). To reconcile these facts, it is important to consider that astrocytes comprise a vastly heterogeneous population that is capable of tremendous plasticity in response to local cues (DiProspero et al., 1997; Escartin et al., 2007; Gris et al., 2007). Notably, the ability of astrocytes to support axon growth is directly related to the composition of the extracellular matrix produced by these cells, and CSPGs are the major inhibitory molecules produced by astrocytes after CNS injury (Bovolenta et al., 1993; Canning et al., 1996; Properzi et al., 2005). Indeed, in vitro research has demonstrated that TGF-α and EGF administered to astrocytes in culture increases production of neurocan (Asher et al., 2000) and phosphacan (Dobbertin et al., 2003; Smith and Strunz, 2005), two important CSPGs associated with inhibition of axon growth. We looked at the expression of the CSPG, neurocan, and in agreement with the literature, we found that the astrocytes in treated and untreated groups were associated with neurocan immunoreactivity; in the TGF-α-treated mice, neurocan extended further into the lesion along the processes of the increased astrocyte milieu. However, astrocytes are also primary producers of laminin in development and after injury (Leisi and Kaupilla, 2001), so we examined the distribution of laminin in these mice and found that the TGF-α-treated mice also had a greater distribution of laminin throughout the lesion site. Laminin expression throughout lesion was colocalized with axons and with collagen IV, suggesting that activated cells contribute to the formation of basal lamina structures throughout the lesion, which provided a permissive substrate for axonal elongation. Several lines of evidence indicate that the key determinant of axonal growth after injury is a balance of permissive and inhibitory ECM molecules (Costa et al., 2002; Jones et al., 2003; Zhou et al., 2006). In particular, the ability of axons to extend neurites on laminin is inhibited by increasing CSPG composition, while the inhibitory influence of CSPGs can be overcome by the presence of laminin (McKeon et al., 1995; Costa et al., 2002; Snow et al., 2002). Thus, the increased axonal growth within the astrocyte matrix in TGF-α-infused mice may be attributed to the robust increase and change in laminin distribution in the lesion, which likely tipped the balance of the ECM in favor of the growth supportive rather than inhibitory substances.
While astrocytes were the intended target of TGF-α infusion, we also saw increased axonal growth in more dorsal regions of the lesion, where astrocytes were not present. Based on the distribution of EGFR immunoreactivity, we propose that mesenchymal cells or fibroblasts also respond to TGF-α and migrate into the lesion site. The distribution of collagen IV and laminin immunoreactivity supports the conclusion that cells in the dorsal region of the injury site also produce the basal lamina components that support axon growth. Therefore, based on the distribution of EGFR immunoreactivity and laminin and collagen deposition, we propose that astrocytes and mesenchymal cells directly activated by TGF-α proliferate and migrate into the lesion, providing a laminin-rich substrate that facilitates additional migration of Schwann cells and promotes axonal growth after spinal cord injury.
An additional hypothesis can be put forth based on the observation of EGFR immunoreactivity on macrophages after injury. Activated macrophages can enhance axonal growth through production of stimulatory factors, including oncomodulin and neurotrophic factors (Rapalino et al., 1998; Yin et al., 2006). Peripheral nerve models have shown that EGFR activation can enhance macrophage survival and thus improve phagocytosis leading to improved axonal regeneration (Hermann et al., 2005). However, macrophages in the injured spinal cord also produce toxic agents and pro-inflammatory cytokines that can compromise neuronal and glial survival, such as iNOS (Satake et al., 2000) and tumor necrosis factor (Pineau and Lacroix, 2007). In the present study, TGF-α administration resulted in subtle changes in the distribution of activated macrophages, so it is unclear if or how these cells contributed to the attraction and growth of axons into the center of the lesion.
TGF-α administration did not alter functional recovery
Although we demonstrated a significant increase in axonal growth into the lesion after TGF-α administration, improved functional recovery was not observed (Supplemental Fig. 2). This lack of behavioral effect is consistent with the lack of differences in white matter sparing at the lesion site, and suggests that the new axon growth does not result in sufficient new connections that lead to functional recovery (Macias et al., 2006). Similar to vehicle-treated mice, TGF-α-treated subjects exhibited CGRP and 5-HT staining in the dorsal area of the spinal cord at the epicenter and rostral to the epicenter. However, neither of these markers colocalized with the axons in the lesion core of TGF-α-infused mice, showing that these were of neither serotonergic or primary afferent peptidergic origin. Based on findings by Inman et al. (2003) that many of the axons found in the dorsal areas of the injured mouse spinal cord are sensory axons that originate from the dorsal root ganglia, we hypothesize that axons entering from dorsal regions and grew alongside Schwann cells or mesenchymal cells are large diameter primary sensory axons that do not innervate appropriate targets to contribute to functional motor recovery. However, the population of axons that are associated with astrocytes penetrating the ventral and lateral aspects of the lesion core may be of different origin and, if directed appropriately, may have potential to enhance locomotor function after injury.
Another possible reason for the lack of improved locomotion in the treated animals could be that modest improvements in recovery are counteracted by the striking meningeal proliferation observed in TGF-α-infused mice due to intrathecal administration of this mitogenic growth factor. This response led to increased compression in the lower thoracic spinal cord of the treated animals, which may have countered positive effects on functional recovery. This is consistent with other studies showing that robust meningeal proliferation in response to EGF and FGF-2 infusion by intrathecal administration leads to proliferation-related spinal cord compression and motor deficits (Parr and Tator, 2007). These effects of intrathecal infusion may be mitigated by direct intraspinal application of the growth factor or inhibitors into the surrounding parenchyma.
The effects of TGF-α infusion on axonal growth in this model are seemingly contrary to recent results showing that inhibition of EGFR activation can facilitate locomotor and sensory recovery following contusion injury in rats (Erschbaumer et al., 2007). Intrathecal administration of the irreversible EGFR inhibitor PD168393 had striking neuroprotective effects on white matter sparing and evidence of increased fiber sprouting, corresponding to functional improvements as early as 4 days post-injury. However, neither the effects of the treatment on astrocytes nor axon growth within the lesion were investigated. Since the structure of TGF-α and EGF differ and some of their biological actions are different (Ebner and Derynck, 1991; Santa-Olalla and Covarrubias, 1995; Junier, 2000), it is also possible that irreversible inhibition of the EGFR does not directly block the effects elicited by endogenous TGF-α or exogenous administration of this factor. Additional studies are needed to clearly distinguish the roles of TGF-α and EGF on axon growth and functional recovery after SCI.
Conclusions
The development of a glial scar at the edge of an injury to the CNS is a significant and well-established barrier to axonal growth and an impediment to maximizing functional recovery. However, it is clear that the principle cells that contribute to the scar are heterogeneous in structure and function, highly influenced by local cues, and exhibit plasticity in their phenotype and protein synthesis capacity. This study demonstrates the first step in the investigation of a unique and paradoxical approach to altering the glial response to injury. Using TGF-α as a representative of a class of mitogenic and gliotrophic factors, we propose that manipulating the glial response to injury by activating or stimulating astrocyte activity may one day be harnessed as part of a treatment strategy for spinal cord injury. The greatest advantage of this approach is that it preserves the supportive and survival effects of these cells that are highly evolved to protect and support neurons throughout life. Similar to most preclinical treatments, further combinatorial approaches need to be explored in order to fully exploit the growth-promoting effects of TGF-α. TGF-α administration coupled with controlled orientation of the extracellular matrix composition and/or directed axon growth to target cells may prove to result in the greatest functional recovery following SCI. The primary outcome of this work is a clear demonstration that TGF-α infusion altered astrocyte activation and produced a local environment that was conducive, rather than inhibitory, to axonal growth. This novel finding suggests that strategies to manipulate the astroglial scar may well include enhancing the proliferation and activation of this multipotent glial cell type.
Supplementary Material
TGF-α alters the distribution of macrophages, but not their relationship with astrocytes, after SCI. (A–B) CD68 immunoreactivity at the lesion epicenter of TGF-α- (A) and vehicle- (B) treated mice. (C–D) Corresponding GFAP immunoreactivity in TGF-α- (C) and vehicle- (D) treated mice. (E–H) High power confocal images of TGF-α- (E and G) and vehicle- (F and H) treated animals in the ventral spared white matter (E–F) and lesion center (G–H). Scale = 100 µm (A–D), 20 µm (E–H).
TGF-α treatment does not affect white matter sparing or functional recovery after SCI. (A–B) EC staining of TGF-α- (A) and vehicle- (B) treated mice. (C) Quantification of EC staining, showing no difference in white matter sparing between treatment groups. (D) BMS scores in all groups throughout the length of the study. No treatment differences were exhibited in injury or laminectomy groups. Scale = 100 µm.
Acknowledgements
The authors gratefully acknowledge the assistance of Patricia Walters with surgeries and animal care. We thank Megan Detloff and Dr. Dana McTigue for critical review of the manuscript. This work was funded by R01 NS043246 and P30-NS045748.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- Anderson CM, Swanson RA. Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia. 2000;32:1–14. [PubMed] [Google Scholar]
- Asher RA, Morgenstern DA, Fidler PS, Adcock KH, Oohira A, Braistead JE, Levine JM, Margolis RU, Rogers JH, Fawcett JW. Neurocan is upregulated in injured brain and in cytokine-treated astrocytes. J. Neurosci. 2000;20:2427–2438. doi: 10.1523/JNEUROSCI.20-07-02427.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp. Neurol. 1996;139:244–256. doi: 10.1006/exnr.1996.0098. [DOI] [PubMed] [Google Scholar]
- Basso DM, Fisher LC, Anderson AJ, Jakeman LB, McTigue DM, Popovich PG. Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J. Neurotrauma. 2006;23:635–659. doi: 10.1089/neu.2006.23.635. [DOI] [PubMed] [Google Scholar]
- Blesch A, Tuszynski MH. Transient growth factor delivery sustains regenerated axons after spinal cord injury. J. Neurosci. 2007;27:10535–10545. doi: 10.1523/JNEUROSCI.1903-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boillee S, Cadusseau J, Coulpier M, Grannec G, Junier MP. Transforming growth factor alpha: a promoter of motoneuron survival of potential biological relevance. J. Neurosci. 2001;21:7079–7088. doi: 10.1523/JNEUROSCI.21-18-07079.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovolenta P, Wandosell F, Nieto-Sampedro M. Characterization of a neurite outgrowth inhibitor expressed after CNS injury. Eur. J. Neurosci. 1993;5(5):454–465. doi: 10.1111/j.1460-9568.1993.tb00512.x. [DOI] [PubMed] [Google Scholar]
- Buffo A, Rite I, Tripathi P, Lepier A, Colak D, Horn AP, Mori T, Gotz M. Origin and progeny of reactive gliosis: A source of multipotent cells in the injured brain. Proc. Natl. Acad. Sci. U.S.A. 2008;105:3581–3586. doi: 10.1073/pnas.0709002105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canning DR, Höke A, Malemud CJ, Silver J. Potent inhibitor of neurite outgrowth that predominates in the extracellular matrix of reactive astrocytes. Int. J. Dev. Neurosci. 1996;14(3):153–175. doi: 10.1016/0736-5748(96)00004-4. [DOI] [PubMed] [Google Scholar]
- do Carmo Cunha CJ, de Freitas Azevedo LB, de Luca BA, de Andrade MS, Gomide VC, Chadi G. Responses of reactive astrocytes containing S100beta protein and fibroblast growth factor-2 in the border and in the adjacent preserved tissue after a contusion injury of the spinal cord in rats: implications for wound repair and neuroregeneration. Wound Repair Regen. 2007;15:134–146. doi: 10.1111/j.1524-475X.2006.00194.x. [DOI] [PubMed] [Google Scholar]
- Costa S, Planchenault T, Charriere-Bertrand C, Mouchel Y, Fages C, Juliano S, Lefrancois T, Barlovatz-Meimon G, Tardy M. Astroglial permissivity for neuritic outgrowth in neuron-astrocyte cocultures depends on regulation of laminin bioavailability. Glia. 2002;37:105–113. doi: 10.1002/glia.10015. [DOI] [PubMed] [Google Scholar]
- Davies SJ, Goucher DR, Doller C, Silver J. Robust regeneration of adult sensory axons in degenerating white matter of the adult rat spinal cord. J. Neurosci. 1999;19(14):5810–5822. doi: 10.1523/JNEUROSCI.19-14-05810.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiProspero NA, Meiners S, Geller HM. Inflammatory cytokines interact to modulate extracellular matrix and astrocytic support of neurite outgrowth. Exp. Neurol. 1997;148(2):628–639. doi: 10.1006/exnr.1997.6700. [DOI] [PubMed] [Google Scholar]
- Dobbertin A, Rhodes KE, Garwood J, Properzi F, Heck N, Rogers JH, Fawcett JW, Faissner A. Regulation of RPTPbeta/phosphacan expression and glycosaminoglycan epitopes in injured brain and cytokine-treated glia. Mol. Cell Neurosci. 2003;24:951–971. doi: 10.1016/s1044-7431(03)00257-4. [DOI] [PubMed] [Google Scholar]
- Ebner R, Derynck R. Epidermal growth factor and transforming growth factor-alpha: differential intracellular routing and processing of ligand-receptor complexes. Cell Regul. 1991;2:599–612. doi: 10.1091/mbc.2.8.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erschbamer M, Pernold K, Olson L. Inhibiting epidermal growth factor receptor improves structural, locomotor, sensory, and bladder recovery from experimental spinal cord injury. J. Neurosci. 2007;27:6428–6435. doi: 10.1523/JNEUROSCI.1037-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escartin C, Pierre K, Colin A, Brouillet E, Delzescaux T, Guillermier M, Dhenain M, Déglon N, Hantraye P, Pellerin L, Bonvento G. Activation of astrocytes by CNTF induces metabolic plasticity and increases resistance to metabolic insults. J. Neurosci. 2007;27(27):7094–7104. doi: 10.1523/JNEUROSCI.0174-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon J, Reid S, Kinyamu R, Opole I, Opole R, Baratta J, Korc M, Endo TL, Duong A, Nguyen G, Karkehabadhi M, Twardzik D, Patel S, Loughlin S. In vivo induction of massive proliferation, directed migration, and differentiation of neural cells in the adult mammalian brain. Proc. Natl. Acad. Sci. U.S.A. 2000;97:14686–14691. doi: 10.1073/pnas.97.26.14686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner JR, Herrman JE, Woo MJ, Tansey KE, Doan NB, Sofroniew MV. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J. Neurosci. 2004;24:2143–2155. doi: 10.1523/JNEUROSCI.3547-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch MT, Silver J. Glial cell extracellular matrix: boundaries for axon growth in development and regeneration. Cell Tissue Res. 1997;290:379–384. doi: 10.1007/s004410050944. [DOI] [PubMed] [Google Scholar]
- Fitch MT, Silver J. CNS injury, glial scars, and inflammation: Inhibitory extracellular matrices and regeneration failure. Exp. Neurol. 2008;209:294–301. doi: 10.1016/j.expneurol.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisen J, Haegerstrand A, Risling M, Fried K, Johansson CB, Hammarberg H, Elde R, Hokfelt T, Cullheim S. Spinal axons in central nervous system scar tissue are closely related to laminin-immunoreactive astrocytes. Neuroscience. 1995;65:293–304. doi: 10.1016/0306-4522(94)00467-j. [DOI] [PubMed] [Google Scholar]
- Ghashghaei HT, Weimer JM, Schmid RS, Yokota Y, McCarthy KD, Popko B, Anton ES. Reinduction of ErbB2 in astrocytes promotes radial glial progenitor identity in adult cerebral cortex. Genes Dev. 2007;21:3258–3271. doi: 10.1101/gad.1580407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gris P, Tighe A, Levin D, Sharma R, Brown A. Transcriptional regulation of scar gene expression in primary astrocytes. Glia. 2007;55(11):1145–1155. doi: 10.1002/glia.20537. [DOI] [PubMed] [Google Scholar]
- Haseloff RF, Blasig IE, Bauer HC, Bauer H. In search of the astrocytic factor(s) modulating blood-brain barrier functions in brain capillary endothelial cells in vitro. Cell Mol. Neurobiol. 2005;25:25–39. doi: 10.1007/s10571-004-1375-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann PM, Nicol JJ, Nagle GT, Bulloch AG, Wildering WC. Epidermal growth factor-dependent enhancement of axonal regeneration in the pond snail Lymnaea stagnalis: role of phagocyte survival. J. Comp Neurol. 2005;492:383–400. doi: 10.1002/cne.20732. [DOI] [PubMed] [Google Scholar]
- Hill CE, Hurtado A, Blits B, Bahr BA, Wood PM, Bartlett BM, Oudega M. Early necrosis and apoptosis of Schwann cells transplanted into the injured rat spinal cord. Eur. J. Neurosci. 2007;26:1433–1445. doi: 10.1111/j.1460-9568.2007.05771.x. [DOI] [PubMed] [Google Scholar]
- Hirano T, Ishihara K, Hibi M. Roles of STAT3 in mediating the cell growth, differentiation and survival signals relayed through the IL-6 family of cytokine receptors. Oncogene. 2000;19(21):2548–2556. doi: 10.1038/sj.onc.1203551. [DOI] [PubMed] [Google Scholar]
- Horky LL, Galimi F, Gage FH, Horner PJ. Fate of endogenous stem/progenitor cells following spinal cord injury. J. Comp. Neurol. 2006;498:525–538. doi: 10.1002/cne.21065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner PJ, Power AE, Kempermann G, Kuhn HG, Palmer TD, Winkler J, Thal LJ, Gage FH. Proliferation and differentiation of progenitor cells throughout the intact spinal cord. J. Neurosci. 2000;20(6):2218–2228. doi: 10.1523/JNEUROSCI.20-06-02218.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda O, Murakami M, Ino H, Yamazaki M, Nemoto T, Koda M, Nakayama C, Moriya H. Acute up-regulation of brain-derived neurotrophic factor expression resulting from experimentally induced injury in the rat spinal cord. Acta Neuropathol. 2001;102:239–245. doi: 10.1007/s004010000357. [DOI] [PubMed] [Google Scholar]
- Inman DM, Steward O. Ascending sensory, but not other long-tract axons, regenerate into the connective tissue matrix that forms at the site of a spinal cord injury in mice. J. Comp. Neurol. 2003;462:431–449. doi: 10.1002/cne.10768. [DOI] [PubMed] [Google Scholar]
- Jakeman LB, Guan Z, Wei P, Ponnappan R, Dzwonczyk R, Popovich PG, Stokes BT. Traumatic spinal cord injury produced by controlled contusion in mouse. J. Neurotrauma. 2000;17:299–319. doi: 10.1089/neu.2000.17.299. [DOI] [PubMed] [Google Scholar]
- Jones LL, Sajed D, Tuszynski MH. Axonal regeneration through regions of chondroitin sulfate proteoglycan deposition after spinal cord injury: a balance of permissiveness and inhibition. J. Neurosci. 2003;23:9276–9288. doi: 10.1523/JNEUROSCI.23-28-09276.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joosten EA, Gribnau AA. Astrocytes and guidance of outgrowing corticospinal tract axons in the rat. An immunocytochemical study using anti-vimentin and anti-glial fibrillary acidic protein. Neuroscience. 1989;31:439–452. doi: 10.1016/0306-4522(89)90386-2. [DOI] [PubMed] [Google Scholar]
- Junier M. What role(s) for TGF-a in the central nervous system? Progress in Neurobiology. 2000;62:443–473. doi: 10.1016/s0301-0082(00)00017-4. [DOI] [PubMed] [Google Scholar]
- Koprivica V, Cho KS, Park JB, Yiu G, Atwal J, Gore B, Kim JA, Lin E, Tessier-Lavigne M, Chen DF, He Z. EGFR activation mediates inhibition of axon regeneration by myelin and chondroitin sulfate proteoglycans. Science. 2005;310:106–110. doi: 10.1126/science.1115462. [DOI] [PubMed] [Google Scholar]
- Krenz NR, Weaver LC. Nerve growth factor in glia and inflammatory cells of the injured rat spinal cord. J. Neurochem. 2000;74:730–739. doi: 10.1046/j.1471-4159.2000.740730.x. [DOI] [PubMed] [Google Scholar]
- Liesi P, Kauppila T. Induction of type IV collagen and other basement-membrane-associated proteins after spinal cord injury of the adult rat may participate in the formation of the glial scar. Exp. Neurol. 2002;173:31–45. doi: 10.1006/exnr.2001.7800. [DOI] [PubMed] [Google Scholar]
- Lee DC, Fenton SE, Berkowitz EA, Hissong MA. Transforming growth factor alpha: expression, regulation, and biological activities. Pharmacol. Rev. 1995;47(1):51–85. [PubMed] [Google Scholar]
- Lee MY, Kim CJ, Shin SL, Moon SH, Chun MH. Increased ciliary neurotrophic factor expression in reactive astrocytes following spinal cord injury in the rat. Neurosci. Lett. 1998;255:79–82. doi: 10.1016/s0304-3940(98)00710-1. [DOI] [PubMed] [Google Scholar]
- Liu B, Chen H, Johns TG, Neufeld AH. Epidermal growth factor receptor activation: an upstream signal for transition of quiescent astrocytes into reactive astrocytes after neural injury. J. Neurosci. 2006;26:7532–7540. doi: 10.1523/JNEUROSCI.1004-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luizzi FJ, Lasek RJ. Astrocytes block axonal regeneration in mammals by activating the physiological stop pathway. Science. 1987;237:642–645. doi: 10.1126/science.3603044. [DOI] [PubMed] [Google Scholar]
- Lytle JM, Wrathall JR. Glial cell loss, proliferation and replacement in the contused murine spinal cord. Eur. J. Neurosci. 2007;25:1711–1724. doi: 10.1111/j.1460-9568.2007.05390.x. [DOI] [PubMed] [Google Scholar]
- Ma M, Basso DM, Walters P, Stokes BT, Jakeman LB. Behavioral and histological outcome following graded contusion injury in C57Bl/6 mice. Exp. Neurol. 2001;169:239–254. doi: 10.1006/exnr.2001.7679. [DOI] [PubMed] [Google Scholar]
- Macias MY, Syring MB, Pizzi MA, Crowe MJ, Alexanian AR, Kurpad SN. Pain with no gain: allodynia following neural stem cell transplantation in spinal cord injury. Exp. Neurol. 2006;201:335–348. doi: 10.1016/j.expneurol.2006.04.035. [DOI] [PubMed] [Google Scholar]
- McKeon RJ, Höke A, Silver J. Injury-induced proteoglycans inhibit the potential for laminin-mediated axon growth on astrocytic scars. Exp. Neurol. 1995;136(1):32–43. doi: 10.1006/exnr.1995.1081. [DOI] [PubMed] [Google Scholar]
- McKeon RJ, Schreiber RC, Rudge JS, Silver J. Reduction of neurite outgrowth in a model of glial scarring following CNS injury is correlated with the expression of inhibitory molecules on reactive astrocytes. J. Neurosci. 1991;11(11):3398–3411. doi: 10.1523/JNEUROSCI.11-11-03398.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTigue DM, Wei P, Stokes BT. Proliferation of NG2-positive cells and altered oligodendrocyte numbers in the contused rat spinal cord. J. Neurosci. 2001;21(10):3392–3400. doi: 10.1523/JNEUROSCI.21-10-03392.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mire E, Thomasset N, Jakeman LB, Rougon G. Modulating Sema3A signal with a L1 mimetic peptide is not sufficient to promote motor recovery and axon regeneration after spinal cord injury. Mol. Cell Neurosci. 2008;37:222–235. doi: 10.1016/j.mcn.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgenstern DA, Asher RA, Fawcett JW. Chondroitin sulphate proteoglycans in the CNS injury response. Prog. Brain Res. 2002;137:313–332. doi: 10.1016/s0079-6123(02)37024-9. [DOI] [PubMed] [Google Scholar]
- Norenberg MD. Distribution of glutamine synthetase in the rat central nervous system. J. Histochem. Cytochem. 1979;27:756–762. doi: 10.1177/27.3.39099. [DOI] [PubMed] [Google Scholar]
- Okada S, Nakamura M, Katoh H, Miyao T, Shimazaki T, Ishii K, Yamane J, Yoshimura A, Iwamoto Y, Toyama Y, Okano H. Conditional ablation of Stat3 or Socs3 discloses a dual role for reactive astrocytes after spinal cord injury. Nat. Med. 2006;12:829–834. doi: 10.1038/nm1425. [DOI] [PubMed] [Google Scholar]
- Parr AM, Tator CH. Intrathecal epidermal growth factor and fibroblast growth factor-2 exacerbate meningeal proliferative lesions associated with intrathecal catheters. Neurosurgery. 2007;60:926–933. doi: 10.1227/01.NEU.0000255441.59612.98. [DOI] [PubMed] [Google Scholar]
- Pineau I, Lacroix S. Proinflammatory cytokine synthesis in the injured mouse spinal cord: multiphasic expression pattern and identification of the cell types involved. J. Comp. Neurol. 2007;500:267–285. doi: 10.1002/cne.21149. [DOI] [PubMed] [Google Scholar]
- Properzi F, Carulli D, Asher RA, Muir E, Camargo LM, van Kuppevelt TH, ten Dam GB, Furukawa Y, Mikami T, Sugahara K, Toida T, Geller HM, Fawcett JW. Chondroitin 6-sulphate synthesis is up-regulated in injured CNS, induced by injury-related cytokines and enhanced in axon-growth inhibitory glia. Eur. J. Neurosci. 2005;21(2):378–390. doi: 10.1111/j.1460-9568.2005.03876.x. [DOI] [PubMed] [Google Scholar]
- Rabchevsky AG, Weinitz JM, Coulpier M, Fages C, Tinel M, Junier MP. A role for transforming growth factor alpha as an inducer of astrogliosis. J. Neurosci. 1998;18:10541–10552. doi: 10.1523/JNEUROSCI.18-24-10541.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. Neuronal migration and contact guidance in the primate telencephalon. Postgrad. Med.J. 1978;54 Suppl 1:25–40. [PubMed] [Google Scholar]
- Rapalino O, Lazarov-Spiegler O, Agranov E, Velan GJ, Yoles E, Fraidakis M, Solomon A, Gepstein R, Katz A, Belkin M, Hadani M, Schwartz M. Implantation of stimulated homologous macrophages results in partial recovery of paraplegic rats. Nat. Med. 1998;4:814–821. doi: 10.1038/nm0798-814. [DOI] [PubMed] [Google Scholar]
- Reier PJ, Stensaas LJ, Guth L. The astrocytic scar as an impediment to regeneration in the central nervous system. In: Kao CC, Bunge RP, Reier PJ, editors. Spinal Cord Reconstruction. New York: Raven Press; 1983. pp. 163–193. [Google Scholar]
- Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW, Kanai Y, Hediger MA, Wang Y, Schielke JP, Welty DF. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16:675–686. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- Rudge JS, Silver J. Inhibition of neurite outgrowth on astroglial scars in vitro. J. Neurosci. 1990;10:3594–3603. doi: 10.1523/JNEUROSCI.10-11-03594.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santa-Olalla J, Covarrubias L. Epidermal growth factor (EGF), transforming growth factor-alpha (TGF-alpha), and basic fibroblast growth factor (bFGF) differentially influence neural precursor cells of mouse embryonic mesencephalon. J Neurosci. Res. 1995;42:172–183. doi: 10.1002/jnr.490420204. [DOI] [PubMed] [Google Scholar]
- Satake K, Matsuyama Y, Kamiya M, Kawakami H, Iwata H, Adachi K, Kiuchi K. Nitric oxide via macrophage iNOS induces apoptosis following traumatic spinal cord injury. Brain Res. Mol. Brain Res. 2000;85:114–122. doi: 10.1016/s0169-328x(00)00253-9. [DOI] [PubMed] [Google Scholar]
- Schousboe A, Sarup A, Bak LK, Waagepetersen HS, Larsson OM. Role of astrocytic transport processes in glutamatergic and GABAergic neurotransmission. Neurochem. Int. 2004;45:521–527. doi: 10.1016/j.neuint.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Sharif A, Legendre P, Allet C, Romao L, Studler JM, Chneiweiss H, Junier MP. Transforming growth factor alpha promotes sequential conversion of mature astrocytes into neural progenitors and stem cells. Oncogene. 2006a:1–12. doi: 10.1038/sj.onc.1210071. [DOI] [PubMed] [Google Scholar]
- Sharif A, Prevot V, Renault-Mihara F, Allet C, Studler JM, Canton B, Chneiweiss H, Junier MP. Transforming growth factor alpha acts as a gliatrophin for mouse and human astrocytes. Oncogene. 2006b;25:4076–4085. doi: 10.1038/sj.onc.1209443. [DOI] [PubMed] [Google Scholar]
- Skene JH, Willard M. Axonally transporter proteins associated with axon growth in rabbit central and peripheral nervous systems. J. Cell Biol. 1981;89:96–103. doi: 10.1083/jcb.89.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GM, Strunz C. Growth factor and cytokine regulation of chondroitin sulfate proteoglycans by astrocytes. Glia. 2005;52:209–218. doi: 10.1002/glia.20236. [DOI] [PubMed] [Google Scholar]
- Snow DM, Smith JD, Gurwell JA. Binding characteristics of chondroitin sulfate proteoglycans and laminin-1, and correlative neurite outgrowth behaviors in a standard tissue culture choice assay. J. Neurobiol. 2002;51:285–301. doi: 10.1002/neu.10060. [DOI] [PubMed] [Google Scholar]
- Sykova E, Svoboda J, Simonova Z, Jendelova P. Role of astrocytes in ionic and volume homeostasis in spinal cord during development and injury. Prog. Brain Res. 1992;94:47–56. doi: 10.1016/s0079-6123(08)61738-0. [DOI] [PubMed] [Google Scholar]
- Tanaka K. Role of glutamate transporters in astrocytes. Brain Nerve. 2007;59:677–688. [PubMed] [Google Scholar]
- Tripathi R, McTigue DM. Prominent oligodendrocyte genesis along the border of spinal contusion lesions. Glia. 2007;55:698–711. doi: 10.1002/glia.20491. [DOI] [PubMed] [Google Scholar]
- Vaccarino FM, Fagel DM, Ganat Y, Maragnoli ME, Ment LR, Ohkubo Y, Schwartz ML, Silbereis J, Smith KM. Astroglial cells in development, regeneration, and repair. Neuroscientist. 2007;13:173–185. doi: 10.1177/1073858406298336. [DOI] [PubMed] [Google Scholar]
- Vise WM, Liss L, Yashon D, Hunt WE. Astrocytic processes: A route between vessels and neurons following blood-brain barrier injury. J. Neuropathol. Exp. Neurol. 1975;34:324–334. doi: 10.1097/00005072-197507000-00002. [DOI] [PubMed] [Google Scholar]
- Walz W. Role of astrocytes in the clearance of excess extracellular potassium. Neurochem. Int. 2000;36:291–300. doi: 10.1016/s0197-0186(99)00137-0. [DOI] [PubMed] [Google Scholar]
- Walz W, Wuttke W, Hertz L. Astrocytes in primary cultures: membrane potential characteristics reveal exclusive potassium conductance and potassium accumulator properties. Brain Res. 1984;292:367–374. doi: 10.1016/0006-8993(84)90772-8. [DOI] [PubMed] [Google Scholar]
- Xie W, Chow LT, Paterson AJ, Chin E, Kudlow JE. Conditional expression of the ErbB2 oncogene elicits reversible hyperplasia in stratified epithelia and up-regulation of TGFalpha expression in transgenic mice. Oncogene. 1999;18:3593–3607. doi: 10.1038/sj.onc.1202673. [DOI] [PubMed] [Google Scholar]
- Yang H, Lu P, McKay HM, Bernot T, Keirstead H, Steward O, Gage FH, Edgerton VR, Tuszynski MH. Endogenous neurogenesis replaces oligodendrocytes and astrocytes after primate spinal cord injury. J. Neurosci. 2006;26(8):2157–2166. doi: 10.1523/JNEUROSCI.4070-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Henzl MT, Lorber B, Nakazawa T, Thomas TT, Jiang F, Langer R, Benowitz LI. Oncomodulin is a macrophage-derived signal for axon regeneration in retinal ganglion cells. Nat. Neurosci. 2006;9:843–852. doi: 10.1038/nn1701. [DOI] [PubMed] [Google Scholar]
- Zai LJ, Wrathall JR. Cell proliferation and replacement following contusive spinal cord injury. Glia. 2005;50:247–257. doi: 10.1002/glia.20176. [DOI] [PubMed] [Google Scholar]
- Zhou FQ, Walzer M, Wu YH, Zhou J, Dedhar S, Snider WD. Neurotrophins support regenerative axon assembly over CSPGs by an ECM-integrin- independent mechanism. J. Cell Sci. 2006;119:2787–2796. doi: 10.1242/jcs.03016. [DOI] [PubMed] [Google Scholar]
- Zhou R, Wu X, Skalli O. TGF-alpha induces a stationary, radial-glia like phenotype in cultured astrocytes. Brain Res. Bull. 2001;56:37–42. doi: 10.1016/s0361-9230(01)00591-3. [DOI] [PubMed] [Google Scholar]
- Zuo J, Neubauer D, Dyess K, Ferguson TA, Muir D. Degradation of chondroitin sulfate proteoglycan enhances the neurite-promoting potential of spinal cord tissue. Exp. Neurol. 1998;154:654–662. doi: 10.1006/exnr.1998.6951. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TGF-α alters the distribution of macrophages, but not their relationship with astrocytes, after SCI. (A–B) CD68 immunoreactivity at the lesion epicenter of TGF-α- (A) and vehicle- (B) treated mice. (C–D) Corresponding GFAP immunoreactivity in TGF-α- (C) and vehicle- (D) treated mice. (E–H) High power confocal images of TGF-α- (E and G) and vehicle- (F and H) treated animals in the ventral spared white matter (E–F) and lesion center (G–H). Scale = 100 µm (A–D), 20 µm (E–H).
TGF-α treatment does not affect white matter sparing or functional recovery after SCI. (A–B) EC staining of TGF-α- (A) and vehicle- (B) treated mice. (C) Quantification of EC staining, showing no difference in white matter sparing between treatment groups. (D) BMS scores in all groups throughout the length of the study. No treatment differences were exhibited in injury or laminectomy groups. Scale = 100 µm.