Abstract
Penumbra is the viable tissue around the irreversibly damaged ischemic core. The purpose of acute stroke treatment is to salvage penumbral tissue and to improve brain function. However, the majority of acute stroke patients who have treatable penumbra are left untreated. Therefore, developing an effective non-recanalizational therapeutics, such as neuroprotective agents, has significant clinical applications. Part I of this serial review on “targeting penumbra” puts special emphases on penumbral pathophysiology and the development of therapeutic strategies. Bioenergetic intervention by massive metabolic suppression and direct energy delivery would be a promising future direction. An effective drug delivery system for this purpose should be able to penetrate BBB and achieve high local tissue drug levels while non-ischemic region being largely unaffected. Selective drug delivery to ischemic stroke penumbra is feasible and deserves intensive research.
Keywords: stroke, cerebral ischemia, neuroprotection, penumbra, treatment, energy state, cerebral energy metabolism
Introduction
Each year, approximately 795 000 people experience a new or recurrent stroke. On average, every 40 seconds, someone in the United States has a stroke. Overall stroke prevalence during 2003 to 2006 is around 2.9%. Of all strokes, 87% are ischemic. (Lloyd-Jones et al.) Due to stroke's high incidence and prevalence rates and the lack of effective treatment, stroke remains one of the major diseases causing most mortality and disability. Stroke is the third leading cause of death, behind diseases of the heart and cancer, and is a leading cause of serious, long-term disability in the United States. Although treatments for ischemic stroke have been rigorously investigated for two decades, up to now there is only one FDA-approved pharmacological treatment for ischemic stroke, the intravenous thrombolytic treatment using recombinant tissue plasminogen activator (r-tPA).(Jahan and Vinuela 2009), which can only be available to a very limited number of patients (Kleindorfer et al. 2004).
Acute stroke causes an irreversibly damaged ischemic core and salvageable surrounding tissue. “Penumbra” is the term used for the reversibly injured brain tissue around ischemic core; which is the pharmacological target for acute ischemic stroke treatment (Astrup et al. 1981a). The goal to treat ischemic stroke is to salvage the penumbra as much and early as possible. It has been reported that roughly half of all acute ischemic patients show penumbra on MRI (Rivers et al. 2006) and are potentially treatable. However, only 8% of all ischemic stroke patients eligible for treatment with recombinant tissue plasminogen activator (r-tPA) (Kleindorfer et al. 2004). Effective pharmacological treatment with or without recanalization could be used for the majority of stroke patients, having invaluable clinical significance. The development of neuroprotective treatment for ischemic stroke is obstructed by the blood-brain barrier and reduced blood supply to ischemic brain tissue, facing repeated translational failure in recent 20 years. Drug delivery to brain tissue, especially the ischemic brain tissue has long been the technical bottleneck limiting acute stroke treatments. A breakthrough in this area will possibly bring in numerous related applications. The technology to be developed in this field may also be extended to other fields, such as traumatic brain injury, brain tumor, and CNS inflammatory diseases. This review summarizes advances for ischemic stroke penumbra, and puts special emphases on strategy development from a metabolic point of view for effective drug delivery to ischemic penumbra.
Penumbra and infarct expansion: the “time is brain” concept
In animal studies, the dynamic changes of penumbra area and infarct expansion can be better illustrated based on the data obtained from experimental strokes, in which the timing of occlusion and reperfusion was precisely controlled. After middle cerebral artery (MCA) occlusion, the infarct evolves rapidly in the first few hours, supporting the interventional concept that “time is brain” (Saver 2006). For an example, in a 300 g rat, 2-h MCA occlusion (MCAO) produces a big infarct of 400-450 mm3 that is close to the infarct caused by 24-h permanent MCAO (Greco et al. 2007; Masada et al. 2001). Ninety minute transient MCAO results in a smaller infarct about 250–380 mm3 (Eschenfelder et al. 2008; Liu et al. 2006) whilst 60-min MCAO only produces approximately 170 mm3 infarct (Han et al. 2008). Therefore, in a 300g rat, at 1-h post-MCA occlusion approximately 170 mm3 brain tissue has already been irreversibly injured. At this moment the occlusion has caused approximately 230 mm3 tissue in danger. Roughly 140 mm3 of this 230 mm3 in-danger brain tissue will die in 30 min, and the left 90 mm3 will die in 60 min. If we assume the specific gravity of rat brain is 1.0 mg/mm3, the average speed of infarct expansion for a 300g rat is approximately 3.3 mg/min after MCA occlusion.
Imaging penumbra
For identifying the salvageable brain tissue in acute stroke, the direct method is to image penumbra. In acute ischemic stroke, the viability and size of penumbra change dynamically (Kuge et al. 2001; Shimosegawa et al. 2005) in response to regional cerebral blood flow, pathophysiological environment and treatment. Penumbra can be imaged using different technologies, such as MRI, CT (Kumar et al.), PET, and SPECT (Meerwaldt et al. 2009). For targeting penumbra in stroke patients, imaging penumbra is necessary for monitoring treatment response as well as for patient screening. The “mismatch” of perfusion-weighted and diffusion-weighted images (PWI-DWI mismatch) is the most commonly used method for imaging penumbra and may serve for this purpose (Ebinger et al. 2009; Rivers et al. 2006). The diffusion-weighted image may represent reversibly injured tissue in the early hours after stroke (Muller et al. 1995; Sakoh et al. 2001) whereas the perfusion-weighted image may include area of benign oligemia (Sobesky et al. 2005). The mismatched tissue represents “tissue-at-risk”, not “tissue-doomed-to die”; therefore it does not identify lesion growth by itself (Rivers et al. 2006). (For infarct expansion see the following paragraph.) Penumbra may resolve spontaneously (Koga et al. 2005), either by merging with the ischemic core, or becoming normal tissue. When recanalizational therapy started early enough, the mismatched tissue, the penumbra, may be salvaged, which has been observed using both CT (Murphy et al. 2006) and MRI (Olivot et al. 2008) methods.
Penumbra in stroke patients: the majority of potentially treatable patients are not treated
The use of imaging modalities detecting the existence of penumbra in stroke patients brought in new lights in patient management. Theoretically, all patients having penumbra zone should be treated. However, the number of patients treated by recanalizational intervention is only a small portion of all acute stroke patients who have a salvageable penumbra. When further looking into the subtypes according to the Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification (Adams et al. 1993), the existence of penumbral tissue significantly correlates to stroke subtypes. The majority (about 94%)of intracranial large artery atherosclerotic (IC-LAA) stroke patients had perfusion-diffusion mismatch, whereas in cargioembolic strokes the penumbra existed in 35.7% patients (Boomer et al. 2009). Although the initial penumbral volume is similar among large-vessel stroke, cardioembolic stroke and cryptogenic embolic stroke, the mean perfusion defect in IC-LAA stroke was less severe than in other groups. This finding may indicate that the penumbral tissue in intracranial large artery atherosclerotic stroke may be more responsive to acute treatment. When an infarct involves white matter, it is associated with a relatively greater penumbral zone than in gray matter because white matter is more resistant to cerebral ischemia (Arakawa et al. 2006; Bristow et al. 2005; Koga et al. 2005) possibly due to the difference in constituent cell population and NMDA receptor dexpression‥ Lacunar infarction is caused by occlusion of perforating artery, which is end-artery without collateral circulation; and its occlusion is thought not to result in a penumbral zone. Because of the small volume of lacunar infarcts, the finding of a perfusion-diffusion mismatch in lacunar stroke is affected by MRI technical issue. Studies using a 1.5-T scanner (Gerraty et al. 2002; Ohashi et al. 2005), or CT perfusion imaging and CT angiography (Vergoni et al. 2000), found no PWI abnormality in patients with a final diagnosis of lacunar infarct. In a most recent study of lacunar infarcts using a 3-T scanner that provide a higher spatial resolution, only 68.2% patients was found having abnormal PWI at the site of the diffusion-weighted imaging lesion (Poppe et al. 2009).
The fate of penumbra: role of energy state
While cerebral blood flow determines both the metabolic process (Hata et al. 2000; Hossmann 1994) and the fate of ischemic tissue (Bardutzky et al. 2007; Murphy et al. 2006; Ohashi et al. 2005), energy state of an ischemic cell determines the pathway (Eguchi et al. 1997; Nicotera and Leist 1997; Nicotera et al. 1998) (Leist et al. 1997; Lieberthal et al. 1998) and the destination (Galeffi et al. 2000) (Wang et al. 2000) of a cell to die or to survive. For detailed discussion please refer to our previous publication (Liu and Levine 2008) and figure 1 and figure 2. Cerebral ischemia causes a disturbance of energy metabolism. In global ischemia, brain ATP levels decrease to approximately 60% of baseline in one minute (Winn et al. 1979). In focal cerebral ischemia, the ischemic core is depleted with ATP whilst the penumbra has decreased ATP level, see figure 3. Theoretically, intervention that maintains cell energy state may provide robust neuroprotection. Such examples can be found in some classic neuroprotectants (Warner et al. 1996). Bioenergetic intervention could be equally important and effective as recanalizational intervention for acute stroke treatment.
Figure 1.

Infarct expansion and treatment strategies. During the first few hours after middle cerebral occlusion of a 300 gram rat, the infarct expands quickly at an average speed of 3.3 mg brain tissue per minute assuming the specific gravity of rat brain is 1.0 mg/mm3. Neuroprotective treatment should be able to penetrate the blood-brain barrier and reach penumbral zone. Such treatment should be made available to most acute stroke patients who have salvageable penumbral tissue.
Figure 2.
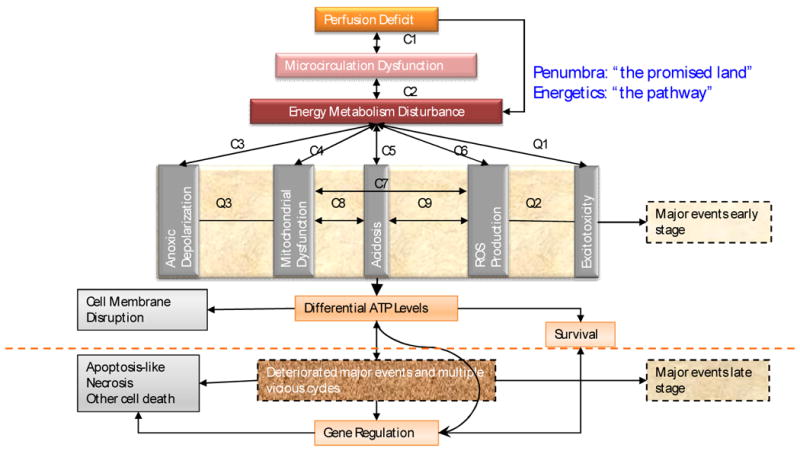
From pathophysiology to therapeutic strategy. Salvaging penumbra is the goal for acute stroke treatment. Neuroprotection for acute ischemic stroke should target the upper stream event that determines the fate of ischemic penumbra. Bioenergetic intervention could be the therapeutic modality equivalent to recanalizational therapies at metabolic levels because the disturbance of energy metabolism after acute brain ischemia differentiates the ischemic cascades. C1-C9: pathological cycles between major events that are supported by literature; Q1-Q3: suspected pathological cycles between major events that need more literature support.
Figure 3.
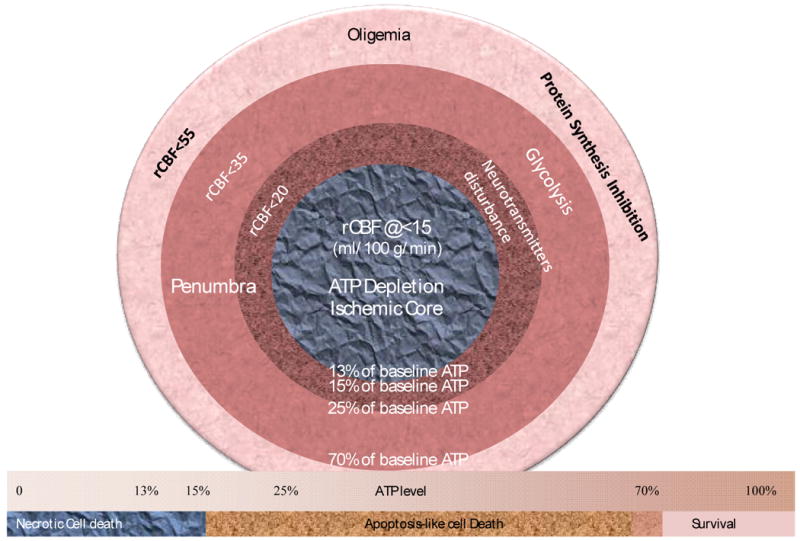
Correlation between energy thresholds and blood flow thresholds. The form and pathway of cell death closely are closely associated with energy state levels. Blood flow reduction causes specific metabolic disturbances at certain blood flow thresholds. The ischemic core has depleted ATP level whilst the penumbra has gradient reduction of ATP level between normal or oligemic tissue and ischemic core.
Potential of neuroprotection: view from metabolic suppression
Neuroprotection can be achieved through metabolic suppression that decreases energy demand, therefore, maintains energy state. The human brain is metabolically highly active, and the majority of its metabolism is for functional purposes and can be suppressed. The human brain constitutes only about 2% of the body weight, yet the energy-consuming processes that ensure proper brain function account for approximately 25% of total body glucose utilization. The average ATP concentration of normal rat brain tissue is between 2.38 to 2.75 nmole/mg wet weight (Hsu et al. 1991; Plaschke et al. 1998; Winn et al. 1979). The main energy-consuming process of the brain is the maintenance of ionic gradients across the plasma membrane and function-related activities (Ames 2000). About 87% of total energy consumed reflects function-related activities (Magistretti 2002), which could be suppressed to decrease energy consumption. Metabolic suppression happens naturally in hibernating animals without causing tissue injury. Hibernation and torpid state can reduce basal metabolic rate to 1-5% of resting normothermic metabolic rate below ischemic threshold for causing irreversible injury (Geiser 2004). Decreasing energy demand by metabolic suppression is the classic method for achieving neuroprotection. Metabolic rate could be drastically reduced by hypothermia (Astrup et al. 1981b; Berger et al. 1998; Mori et al. 1998), anesthetics and sedatives (Astrup et al. 1981b; Warner et al. 1996); but hypothermia-related (Jian et al. 2003; Schwab et al. 2001) and drug-related systemic complications (Coupey 1997) have limited their use in acute strokes. Recent advances in CNS drug delivery system may provide a solution for these problems.
Direct energy delivery
ATP molecules are negatively charged and cannot freely pass membrane barriers entering intracellular space (Gordon 1986). Because extracellular ATP are rapidly degraded by ectonucleotidases (Winn et al. 1979), investigators have tried using nanoliposome-entrapped ATP to deliver energy to ischemic tissue. Nanoliposome-encapsulated ATP(Arakawa et al. 1998) has shown protective effects in intestinal injury from hemorrhagic shock (Zakaria el et al. 2005), forebrain ischemia (Laham et al. 1988; Puisieux et al. 1994), myocardial ischemia.(Verma et al. 2005a; Verma et al. 2006; Verma et al. 2005b), and skin wound healing (Chiang et al. 2007). ATP blood levels can be increased drastically after the administration of ATP-loaded nanoliposomes; a similar administration of carboxyfluorescein-loaded nanoliposomes showed that nanoliposomes can reach the ischemic cerebral parenchyma in rats (Chapat et al. 1991).
Direct energy delivery for brain ischemia
ATP molecules are highly recycled in living cells. It is not practical and neither necessary to provide the total consumption amount of exogenous ATP because injured cells still have, although limited, ability to regenerate ATP. Because ATP is released into, and degraded in, extracellular space, theoretically, it could also be beneficial for ischemic cells if such loss of intracellular ATP can be replenished through exogenous resources by targeted intracellular ATP delivery. Administration of liposomal ATP has been shown to be promising in a forebrain ischemia model.(Puisieux et al. 1994)
The liposomal ATP solution for in vivo experiments could reach a high concentration about 12 mg/ml (21.8 μmole/ml). (Verma et al. 2005a) With a bolus injection of serum stable pH-sensitive liposomes, 50%, 24%, and 15% of injected dose could remain in the blood at 1-h, 10-h, and 24-h post-injection, respectively (Slepushkin et al. 1997). Considering the regional cerebral blood flow (rCBF) in the inner penumbra being approximately 15 ml/100g/min (0.00015 ml/mg/min), (Murphy et al. 2006; Ohashi et al. 2005) therefore, an injection of 1 ml such ATP-loaded liposomes (12 mg/ml) into a 300g rat could deliver ATP to the inner boundary of penumbra with a speed of 0.079 nmole/mg/min (21.8*0.5/21*0.00015*1000) at 1-h post-injection, assuming the total blood volume being 21 ml. At this delivery speed, it will only need about 30-min (2.38/0.079) to replenish the total ATP base pool (2.38 nmole/mg wet weight) in the inner penumbra through the residue blood flow.
In a forebrain ischemia model, it has been observed that when being entrapped into nanoliposomes and administered intracarotidally, ATP greatly increased the number of ischemic episodes that can be tolerated before brain electrical silence and death appeared (Laham et al. 1988; Puisieux et al. 1994) because of improvement in energy metabolism. Direct energy delivery remains an attractive treatment for ischemic stroke, yet it still needs extensive research before its successful translation to clinic settings. Efforts need to be put on aspects such as giving synergistic adjunctive treatments, improving the bioavailability of ATP-loaded nanoliposomes, and minimizing the interaction of exogenous ATP purinergic receptors (Boucsein et al. 2003; Chen et al. 2007; Siow et al. 2005). A non-selective P2 receptor antagonist, such as suramin (Kharlamov et al. 2002; Millart et al. 2009), can be used for minimizing these compounding effects. Suramin can be encapsulated into liposomes (Chang and Flanagan 1994; Chang and Flanagan 1995).
Delivery of a metabolic suppressor
Nanoliposomes have been used as a carrier for CNS drug delivery and can be tissue selective. Selective delivery of a metabolic suppressor to a specific brain region makes it possible to reach a desired regional drug concentration with minimized drug-related systemic adverse effects (CNS depression, hypotension, etc.), therefore, having its application in acute stroke treatments. Some local anesthetics and sedatives have been reported of their liposomal formulation for topical application and controlled release, such as lidocaine (Fransson et al. 2002), benzocaine (Avila and Martinez 2003), diazepam (Fatouros and Antimisiaris 2002; Sznitowska et al. 2000). Because the amphiphilic drug diazepam, which binds to the same GABAA receptor as pentobarbital does, can be used in liposomal formulation, the more water-soluble pentobarbital will theoretically be better encapsulated in nanoliposomes and be bioactive.
Penumbral drug delivery strategy
Conventional drug delivery methods cause unwanted drug exposure to other tissue or brain regions, leading to severe side effects and toxicity, especially when high dose is being used for reaching therapeutic drug levels in ischemic tissue. For examples, the classic metabolic suppressor pentobarbital could reduce metabolic rate by 56%, (Warner et al. 1996) having a proven neuroprotective effect; but it cannot be used with a sufficient dose to achieve the desired maximal metabolic suppression because of its drug-related respiratory suppression. Neuroprotection by providing exogenous energy has also been facing problems of adverse effects and low bioavailability. With the advancement of CNS drug delivery, those problems can be tackled through innovative approaches (see following paragraph).
Brain ischemia causes a serial of pathological changes that affect drug delivery. In the ischemic local region there is limited blood supply while the blood-brain barrier and the shrunk extracellular space further limit drug access to ischemic brain tissue. However, there are also some pathological changes that may be utilized for facilitating drug delivery to local ischemic tissue. For example, brain ischemia causes a metabolic shift towards anaerobic glycolysis, resulting in a lower intracellular pH value in the ischemic brain tissue. Targeting at this property of ischemic brain tissue, liposomal nanocarrier may be optimized to release their cargos under acidic condition (Collins et al. 1989) similar to the intracellular environment of ischemic brain tissue(pH<6.75) (Anderson et al. 1999). Another example, ischemia induced molecular structure changes can also be used for selective drug delivery to ischemic brain tissue. A most recently discovered special peptide has showed the homing ability to ischemic brain tissue (Hong et al. 2008). Therefore, the strategy for drug delivery to ischemic brain tissue should be to overcome the disadvantages and to utilize the advantages of ischemia induced pathological changes for achieving maximal bioavailability. And the neuroprotective strategy is to deliver a treatment that has the largest protection potential using the most efficient drug delivery system.
Summary
It is of great clinical significance to develop a neuroprotective treatment that can be made available to most acute stroke patients. Bioenergetic intervention by massive metabolic suppression and direct energy delivery would be a promising future direction. An effective drug delivery system for this purpose should be able to penetrate BBB and achieve high local tissue drug levels while non-ischemic region being largely unaffected. Selective drug delivery to ischemic stroke penumbra is feasible and deserves intensive research. See Figure 1.
Acknowledgments
This work was supported by NIH grant 5T32NS051147-02 and NS 21076-24. The author appreciates and acknowledges Dr. Levine and Dr. Winn at Mount Sinai School of Medicine for his contribution on revising this paper.
References
- Adams HP, Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE., 3rd Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- Ames A., 3rd CNS energy metabolism as related to function. Brain Res Brain Res Rev. 2000;34:42–68. doi: 10.1016/s0165-0173(00)00038-2. [DOI] [PubMed] [Google Scholar]
- Anderson RE, Tan WK, Meyer FB. Brain acidosis, cerebral blood flow, capillary bed density, and mitochondrial function in the ischemic penumbra. J Stroke Cerebrovasc Dis. 1999;8:368–379. doi: 10.1016/s1052-3057(99)80044-5. [DOI] [PubMed] [Google Scholar]
- Arakawa A, Ishiguro S, Ohki K, Tamai M. Preparation of liposome-encapsulating adenosine triphosphate. Tohoku J Exp Med. 1998;184:39–47. doi: 10.1620/tjem.184.39. [DOI] [PubMed] [Google Scholar]
- Arakawa S, Wright PM, Koga M, Phan TG, Reutens DC, Lim I, Gunawan MR, Ma H, Perera N, Ly J, Zavala J, Fitt G, Donnan GA. Ischemic thresholds for gray and white matter: a diffusion and perfusion magnetic resonance study. Stroke. 2006;37:1211–1216. doi: 10.1161/01.STR.0000217258.63925.6b. [DOI] [PubMed] [Google Scholar]
- Astrup J, Siesjo BK, Symon L. Thresholds in cerebral ischemia - the ischemic penumbra. Stroke. 1981a;12:723–725. doi: 10.1161/01.str.12.6.723. [DOI] [PubMed] [Google Scholar]
- Astrup J, Sorensen PM, Sorensen HR. Inhibition of cerebral oxygen and glucose consumption in the dog by hypothermia, pentobarbital, and lidocaine. Anesthesiology. 1981b;55:263–268. doi: 10.1097/00000542-198109000-00013. [DOI] [PubMed] [Google Scholar]
- Avila CM, Martinez F. Thermodynamics of partitioning of benzocaine in some organic solvent/buffer and liposome systems. Chem Pharm Bull (Tokyo) 2003;51:237–240. doi: 10.1248/cpb.51.237. [DOI] [PubMed] [Google Scholar]
- Bardutzky J, Shen Q, Henninger N, Schwab S, Duong TQ, Fisher M. Characterizing tissue fate after transient cerebral ischemia of varying duration using quantitative diffusion and perfusion imaging. Stroke. 2007;38:1336–1344. doi: 10.1161/01.STR.0000259636.26950.3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger R, Jensen A, Hossmann KA, Paschen W. Effect of mild hypothermia during and after transient in vitro ischemia on metabolic disturbances in hippocampal slices at different stages of development. Brain Res Dev Brain Res. 1998;105:67–77. [PubMed] [Google Scholar]
- Boomer JA, Qualls MM, Inerowicz HD, Haynes RH, Patri VS, Kim JM, Thompson DH. Cytoplasmic delivery of liposomal contents mediated by an acid-labile cholesterol-vinyl ether-PEG conjugate. Bioconjug Chem. 2009;20:47–59. doi: 10.1021/bc800239b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucsein C, Zacharias R, Farber K, Pavlovic S, Hanisch UK, Kettenmann H. Purinergic receptors on microglial cells: functional expression in acute brain slices and modulation of microglial activation in vitro. Eur J Neurosci. 2003;17:2267–2276. doi: 10.1046/j.1460-9568.2003.02663.x. [DOI] [PubMed] [Google Scholar]
- Bristow MS, Simon JE, Brown RA, Eliasziw M, Hill MD, Coutts SB, Frayne R, Demchuk AM, Mitchell JR. MR perfusion and diffusion in acute ischemic stroke: human gray and white matter have different thresholds for infarction. J Cereb Blood Flow Metab. 2005;25:1280–1287. doi: 10.1038/sj.jcbfm.9600135. [DOI] [PubMed] [Google Scholar]
- Chang HC, Flanagan DR. Liposomal entrapment of suramin. J Pharm Sci. 1994;83:1043–1046. doi: 10.1002/jps.2600830723. [DOI] [PubMed] [Google Scholar]
- Chang HC, Flanagan DR. Liposomal entrapment of suramin(II): interaction of suramin with phospholipids of various chain lengths. J Pharm Sci. 1995;84:1078–1082. doi: 10.1002/jps.2600840909. [DOI] [PubMed] [Google Scholar]
- Chapat S, Frey V, Claperon N, Bouchaud C, Puisieux F, Couvreur P, Rossignol P, Delattre J. Efficiency of liposomal ATP in cerebral ischemia: bioavailability features. Brain Res Bull. 1991;26:339–342. doi: 10.1016/0361-9230(91)90004-4. [DOI] [PubMed] [Google Scholar]
- Chen HH, Schock SC, Xu J, Safarpour F, Thompson CS, Stewart AF. Extracellular ATP-dependent upregulation of the transcription cofactor LMO4 promotes neuron survival from hypoxia. Exp Cell Res. 2007;313:3106–3116. doi: 10.1016/j.yexcr.2007.04.026. [DOI] [PubMed] [Google Scholar]
- Chiang B, Essick E, Ehringer W, Murphree S, Hauck MA, Li M, Chien S. Enhancing skin wound healing by direct delivery of intracellular adenosine triphosphate. Am J Surg. 2007;193:213–218. doi: 10.1016/j.amjsurg.2006.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins D, Maxfield F, Huang L. Immunoliposomes with different acid sensitivities as probes for the cellular endocytic pathway. Biochim Biophys Acta. 1989;987:47–55. doi: 10.1016/0005-2736(89)90453-7. [DOI] [PubMed] [Google Scholar]
- Coupey SM. Barbiturates. Pediatr Rev. 1997;18:260–264. doi: 10.1542/pir.18-8-260. quiz 265. [DOI] [PubMed] [Google Scholar]
- Ebinger M, De Silva DA, Christensen S, Parsons MW, Markus R, Donnan GA, Davis SM. Imaging the penumbra - strategies to detect tissue at risk after ischemic stroke. J Clin Neurosci. 2009;16:178–187. doi: 10.1016/j.jocn.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Eguchi Y, Shimizu S, Tsujimoto Y. Intracellular ATP levels determine cell death fate by apoptosis or necrosis. Cancer Res. 1997;57:1835–1840. [PubMed] [Google Scholar]
- Eschenfelder CC, Krug R, Yusofi AF, Meyne JK, Herdegen T, Koch A, Zhao Y, Carl UM, Deuschl G. Neuroprotection by oxygen in acute transient focal cerebral ischemia is dose dependent and shows superiority of hyperbaric oxygenation. Cerebrovasc Dis. 2008;25:193–201. doi: 10.1159/000113856. [DOI] [PubMed] [Google Scholar]
- Fatouros DG, Antimisiaris SG. Effect of amphiphilic drugs on the stability and zeta-potential of their liposome formulations: a study with prednisolone, diazepam, and griseofulvin. J Colloid Interface Sci. 2002;251:271–277. doi: 10.1006/jcis.2002.8432. [DOI] [PubMed] [Google Scholar]
- Fransson BA, Peck KE, Smith JK, Anthony JA, Mealey KL. Transdermal absorption of a liposome-encapsulated formulation of lidocaine following topical administration in cats. Am J Vet Res. 2002;63:1309–1312. doi: 10.2460/ajvr.2002.63.1309. [DOI] [PubMed] [Google Scholar]
- Galeffi F, Sinnar S, Schwartz-Bloom RD. Diazepam promotes ATP recovery and prevents cytochrome c release in hippocampal slices after in vitro ischemia. J Neurochem. 2000;75:1242–1249. doi: 10.1046/j.1471-4159.2000.0751242.x. [DOI] [PubMed] [Google Scholar]
- Geiser F. Metabolic rate and body temperature reduction during hibernation and daily torpor. Annu Rev Physiol. 2004;66:239–274. doi: 10.1146/annurev.physiol.66.032102.115105. [DOI] [PubMed] [Google Scholar]
- Gerraty RP, Parsons MW, Barber PA, Darby DG, Desmond PM, Tress BM, Davis SM. Examining the lacunar hypothesis with diffusion and perfusion magnetic resonance imaging. Stroke. 2002;33:2019–2024. doi: 10.1161/01.str.0000020841.74704.5b. [DOI] [PubMed] [Google Scholar]
- Gordon JL. Extracellular ATP: effects, sources and fate. Biochem J. 1986;233:309–319. doi: 10.1042/bj2330309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco R, Amantea D, Blandini F, Nappi G, Bagetta G, Corasaniti MT, Tassorelli C. Neuroprotective effect of nitroglycerin in a rodent model of ischemic stroke: evaluation of Bcl-2 expression. Int Rev Neurobiol. 2007;82:423–435. doi: 10.1016/S0074-7742(07)82024-1. [DOI] [PubMed] [Google Scholar]
- Han JL, Kollmar R, Tobyas B, Schwab S. Inhibited glutamate release by granulocyte-colony stimulating factor after experimental stroke. Neurosci Lett. 2008;432:167–169. doi: 10.1016/j.neulet.2007.07.056. [DOI] [PubMed] [Google Scholar]
- Hata R, Maeda K, Hermann D, Mies G, Hossmann KA. Evolution of brain infarction after transient focal cerebral ischemia in mice. J Cereb Blood Flow Metab. 2000;20:937–946. doi: 10.1097/00004647-200006000-00006. [DOI] [PubMed] [Google Scholar]
- Hong HY, Choi JS, Kim YJ, Lee HY, Kwak W, Yoo J, Lee JT, Kwon TH, Kim IS, Han HS, Lee BH. Detection of apoptosis in a rat model of focal cerebral ischemia using a homing peptide selected from in vivo phage display. J Control Release. 2008;131:167–172. doi: 10.1016/j.jconrel.2008.07.020. [DOI] [PubMed] [Google Scholar]
- Hossmann KA. Viability thresholds and the penumbra of focal ischemia. Ann Neurol. 1994;36:557–565. doi: 10.1002/ana.410360404. [DOI] [PubMed] [Google Scholar]
- Hsu SS, Meno JR, Zhou JG, Gordon EL, Winn HR. Influence of hyperglycemia on cerebral adenosine production during ischemia and reperfusion. Am J Physiol. 1991;261:H398–403. doi: 10.1152/ajpheart.1991.261.2.H398. [DOI] [PubMed] [Google Scholar]
- Jahan R, Vinuela F. Treatment of acute ischemic stroke: intravenous and endovascular therapies. Expert Rev Cardiovasc Ther. 2009;7:375–387. doi: 10.1586/erc.09.13. [DOI] [PubMed] [Google Scholar]
- Jian S, Yongming Q, Zhihua C, Yan C. Feasibility and safety of moderate hypothermia after acute ischemic stroke. Int J Dev Neurosci. 2003;21:353–356. doi: 10.1016/s0736-5748(03)00070-4. [DOI] [PubMed] [Google Scholar]
- Kharlamov A, Jones SC, Kim DK. Suramin reduces infarct volume in a model of focal brain ischemia in rats. Exp Brain Res. 2002;147:353–359. doi: 10.1007/s00221-002-1251-1. [DOI] [PubMed] [Google Scholar]
- Kleindorfer D, Kissela B, Schneider A, Woo D, Khoury J, Miller R, Alwell K, Gebel J, Szaflarski J, Pancioli A, Jauch E, Moomaw C, Shukla R, Broderick JP. Eligibility for recombinant tissue plasminogen activator in acute ischemic stroke: a population-based study. Stroke. 2004;35:e27–29. doi: 10.1161/01.STR.0000109767.11426.17. [DOI] [PubMed] [Google Scholar]
- Koga M, Reutens DC, Wright P, Phan T, Markus R, Pedreira B, Fitt G, Lim I, Donnan GA. The existence and evolution of diffusion-perfusion mismatched tissue in white and gray matter after acute stroke. Stroke. 2005;36:2132–2137. doi: 10.1161/01.STR.0000181066.23213.8f. [DOI] [PubMed] [Google Scholar]
- Kuge Y, Yokota C, Tagaya M, Hasegawa Y, Nishimura A, Kito G, Tamaki N, Hashimoto N, Yamaguchi T, Minematsu K. Serial changes in cerebral blood flow and flow-metabolism uncoupling in primates with acute thromboembolic stroke. J Cereb Blood Flow Metab. 2001;21:202–210. doi: 10.1097/00004647-200103000-00003. [DOI] [PubMed] [Google Scholar]
- Kumar G, Goyal MK, Sahota PK, Jain R. Penumbra, the basis of neuroimaging in acute stroke treatment: current evidence. J Neurol Sci. 288:13–24. doi: 10.1016/j.jns.2009.09.027. [DOI] [PubMed] [Google Scholar]
- Laham A, Claperon N, Durussel JJ, Fattal E, Delattre J, Puisieux F, Couvreur P, Rossignol P. Intracarotidal administration of liposomally-entrapped ATP: improved efficiency against experimental brain ischemia. Pharmacol Res Commun. 1988;20:699–705. doi: 10.1016/s0031-6989(88)80117-6. [DOI] [PubMed] [Google Scholar]
- Leist M, Single B, Castoldi AF, Kuhnle S, Nicotera P. Intracellular adenosine triphosphate (ATP) concentration: a switch in the decision between apoptosis and necrosis. J Exp Med. 1997;185:1481–1486. doi: 10.1084/jem.185.8.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberthal W, Menza SA, Levine JS. Graded ATP depletion can cause necrosis or apoptosis of cultured mouse proximal tubular cells. Am J Physiol. 1998;274:F315–327. doi: 10.1152/ajprenal.1998.274.2.F315. [DOI] [PubMed] [Google Scholar]
- Liu S, Levine SR. The continued promise of neuroprotection for acute stroke treatment. Journal of Experimental Stroke & Translational Medicine. 2008;1:1–8. doi: 10.6030/1939-067x-1.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Liu W, Ding W, Miyake M, Rosenberg GA, Liu KJ. Electron paramagnetic resonance-guided normobaric hyperoxia treatment protects the brain by maintaining penumbral oxygenation in a rat model of transient focal cerebral ischemia. J Cereb Blood Flow Metab. 2006;26:1274–1284. doi: 10.1038/sj.jcbfm.9600277. [DOI] [PubMed] [Google Scholar]
- Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Stafford R, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Circulation. Vol. 121. Heart disease and stroke statistics--2010 update: a report from the american heart association; pp. e46–e215. [DOI] [PubMed] [Google Scholar]
- Magistretti P. Brain Energy Metabolism. In: Squire L, Roberts J, Spitzer N, et al., editors. Fundamental Neuroscience. Elsevier Science & Technology Books; 2002. pp. 339–360. [Google Scholar]
- Masada T, Hua Y, Xi G, Ennis SR, Keep RF. Attenuation of ischemic brain edema and cerebrovascular injury after ischemic preconditioning in the rat. J Cereb Blood Flow Metab. 2001;21:22–33. doi: 10.1097/00004647-200101000-00004. [DOI] [PubMed] [Google Scholar]
- Meerwaldt R, Slart RH, van Dam GM, Luijckx GJ, Tio RA, Zeebregts CJ. PET/SPECT imaging: From carotid vulnerability to brain viability. Eur J Radiol. 2009 doi: 10.1016/j.ejrad.2009.01.034. [DOI] [PubMed] [Google Scholar]
- Millart H, Alouane L, Oszust F, Chevallier S, Robinet A. Involvement of P2Y receptors in pyridoxal-5′-phosphate-induced cardiac preconditioning. Fundam Clin Pharmacol. 2009;23:279–292. doi: 10.1111/j.1472-8206.2009.00677.x. [DOI] [PubMed] [Google Scholar]
- Mori K, Maeda M, Miyazaki M, Iwase H. Effects of mild (33 degrees C) and moderate (29 degrees C) hypothermia on cerebral blood flow and metabolism, lactate, and extracellular glutamate in experimental head injury. Neurol Res. 1998;20:719–726. doi: 10.1080/01616412.1998.11740590. [DOI] [PubMed] [Google Scholar]
- Muller TB, Haraldseth O, Jones RA, Sebastiani G, Godtliebsen F, Lindboe CF, Unsgard G. Combined perfusion and diffusion-weighted magnetic resonance imaging in a rat model of reversible middle cerebral artery occlusion. Stroke. 1995;26:451–457. doi: 10.1161/01.str.26.3.451. discussion 457-458. [DOI] [PubMed] [Google Scholar]
- Murphy BD, Fox AJ, Lee DH, Sahlas DJ, Black SE, Hogan MJ, Coutts SB, Demchuk AM, Goyal M, Aviv RI, Symons S, Gulka IB, Beletsky V, Pelz D, Hachinski V, Chan R, Lee TY. Identification of penumbra and infarct in acute ischemic stroke using computed tomography perfusion-derived blood flow and blood volume measurements. Stroke. 2006;37:1771–1777. doi: 10.1161/01.STR.0000227243.96808.53. [DOI] [PubMed] [Google Scholar]
- Nicotera P, Leist M. Energy supply and the shape of death in neurons and lymphoid cells. Cell Death Differ. 1997;4:435–442. doi: 10.1038/sj.cdd.4400265. [DOI] [PubMed] [Google Scholar]
- Nicotera P, Leist M, Ferrando-May E. Intracellular ATP, a switch in the decision between apoptosis and necrosis. Toxicol Lett. 1998;102-103:139–142. doi: 10.1016/s0378-4274(98)00298-7. [DOI] [PubMed] [Google Scholar]
- Ohashi M, Tsuji A, Kaneko M, Matsuda M. Threshold of regional cerebral blood flow for infarction in patients with acute cerebral ischemia. J Neuroradiol. 2005;32:337–341. doi: 10.1016/s0150-9861(05)83165-7. [DOI] [PubMed] [Google Scholar]
- Olivot JM, Mlynash M, Thijs VN, Kemp S, Lansberg MG, Wechsler L, Schlaug G, Bammer R, Marks MP, Albers GW. Relationships between infarct growth, clinical outcome, and early recanalization in diffusion and perfusion imaging for understanding stroke evolution (DEFUSE) Stroke. 2008;39:2257–2263. doi: 10.1161/STROKEAHA.107.511535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaschke K, Bardenheuer HJ, Weigand MA, Martin E, Hoyer S. Increased ATP production during long-term brain ischemia in rats in the presence of propentofylline. Eur J Pharmacol. 1998;349:33–40. doi: 10.1016/s0014-2999(98)00172-1. [DOI] [PubMed] [Google Scholar]
- Poppe AY, Coutts SB, Kosior J, Hill MD, O'Reilly CM, Demchuk AM. Normal Magnetic Resonance Perfusion-Weighted Imaging in Lacunar Infarcts Predicts a Low Risk of Early Deterioration. Cerebrovasc Dis. 2009;28:151–156. doi: 10.1159/000225908. [DOI] [PubMed] [Google Scholar]
- Puisieux F, Fattal E, Lahiani M, Auger J, Jouannet P, Couvreur P, Delattre J. Liposomes, an interesting tool to deliver a bioenergetic substrate (ATP). in vitro and in vivo studies. J Drug Target. 1994;2:443–448. doi: 10.3109/10611869408996820. [DOI] [PubMed] [Google Scholar]
- Rivers CS, Wardlaw JM, Armitage PA, Bastin ME, Carpenter TK, Cvoro V, Hand PJ, Dennis MS. Do acute diffusion- and perfusion-weighted MRI lesions identify final infarct volume in ischemic stroke? Stroke. 2006;37:98–104. doi: 10.1161/01.STR.0000195197.66606.bb. [DOI] [PubMed] [Google Scholar]
- Sakoh M, Ostergaard L, Gjedde A, Rohl L, Vestergaard-Poulsen P, Smith DF, Le Bihan D, Sakaki S, Gyldensted C. Prediction of tissue survival after middle cerebral artery occlusion based on changes in the apparent diffusion of water. J Neurosurg. 2001;95:450–458. doi: 10.3171/jns.2001.95.3.0450. [DOI] [PubMed] [Google Scholar]
- Saver JL. Time is brain--quantified. Stroke. 2006;37:263–266. doi: 10.1161/01.STR.0000196957.55928.ab. [DOI] [PubMed] [Google Scholar]
- Schwab S, Georgiadis D, Berrouschot J, Schellinger PD, Graffagnino C, Mayer SA. Feasibility and safety of moderate hypothermia after massive hemispheric infarction. Stroke. 2001;32:2033–2035. doi: 10.1161/hs0901.095394. [DOI] [PubMed] [Google Scholar]
- Shimosegawa E, Hatazawa J, Ibaraki M, Toyoshima H, Suzuki A. Metabolic penumbra of acute brain infarction: a correlation with infarct growth. Ann Neurol. 2005;57:495–504. doi: 10.1002/ana.20427. [DOI] [PubMed] [Google Scholar]
- Siow NL, Xie HQ, Choi RC, Tsim KW. ATP induces the post-synaptic gene expression in neuron-neuron synapses: Transcriptional regulation of AChE catalytic subunit. Chem Biol Interact. 2005;157-158:423–426. doi: 10.1016/j.cbi.2005.10.088. [DOI] [PubMed] [Google Scholar]
- Slepushkin VA, Simoes S, Dazin P, Newman MS, Guo LS, Pedroso de Lima MC, Duzgunes N. Sterically stabilized pH-sensitive liposomes. Intracellular delivery of aqueous contents and prolonged circulation in vivo. J Biol Chem. 1997;272:2382–2388. doi: 10.1074/jbc.272.4.2382. [DOI] [PubMed] [Google Scholar]
- Sobesky J, Zaro Weber O, Lehnhardt FG, Hesselmann V, Neveling M, Jacobs A, Heiss WD. Does the mismatch match the penumbra? Magnetic resonance imaging and positron emission tomography in early ischemic stroke. Stroke. 2005;36:980–985. doi: 10.1161/01.STR.0000160751.79241.a3. [DOI] [PubMed] [Google Scholar]
- Sznitowska M, Janicki S, Gajewska M, Kulik M. Investigation of diazepam lipospheres based on Witepsol and lecithin intended for oral or rectal delivery. Acta Pol Pharm. 2000;57:61–64. [PubMed] [Google Scholar]
- Vergoni AV, Ottani A, Botticelli AR, Zaffe D, Guano L, Loche A, Genedani S, Gessa GL, Bertolini A. Neuroprotective effect of gamma-hydroxybutyrate in transient global cerebral ischemia in the rat. Eur J Pharmacol. 2000;397:75–84. doi: 10.1016/s0014-2999(00)00246-6. [DOI] [PubMed] [Google Scholar]
- Verma DD, Hartner WC, Levchenko TS, Bernstein EA, Torchilin VP. ATP-loaded liposomes effectively protect the myocardium in rabbits with an acute experimental myocardial infarction. Pharm Res. 2005a;22:2115–2120. doi: 10.1007/s11095-005-8354-x. [DOI] [PubMed] [Google Scholar]
- Verma DD, Levchenko TS, Bernstein EA, Mongayt D, Torchilin VP. ATP-loaded immunoliposomes specific for cardiac myosin provide improved protection of the mechanical functions of myocardium from global ischemia in an isolated rat heart model. J Drug Target. 2006;14:273–280. doi: 10.1080/10611860600763103. [DOI] [PubMed] [Google Scholar]
- Verma DD, Levchenko TS, Bernstein EA, Torchilin VP. ATP-loaded liposomes effectively protect mechanical functions of the myocardium from global ischemia in an isolated rat heart model. J Control Release. 2005b;108:460–471. doi: 10.1016/j.jconrel.2005.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Chambers G, Cottrell JE, Kass IS. Differential fall in ATP accounts for effects of temperature on hypoxic damage in rat hippocampal slices. J Neurophysiol. 2000;83:3462–3472. doi: 10.1152/jn.2000.83.6.3462. [DOI] [PubMed] [Google Scholar]
- Warner DS, Takaoka S, Wu B, Ludwig PS, Pearlstein RD, Brinkhous AD, Dexter F. Electroencephalographic burst suppression is not required to elicit maximal neuroprotection from pentobarbital in a rat model of focal cerebral ischemia. Anesthesiology. 1996;84:1475–1484. doi: 10.1097/00000542-199606000-00024. [DOI] [PubMed] [Google Scholar]
- Winn HR, Rubio R, Berne RM. Brain adenosine production in the rat during 60 seconds of ischemia. Circ Res. 1979;45:486–492. doi: 10.1161/01.res.45.4.486. [DOI] [PubMed] [Google Scholar]
- Zakaria el R, Ehringer WD, Tsakadze N, Li N, Garrison RN. Direct energy delivery improves tissue perfusion after resuscitated shock. Surgery. 2005;138:195–203. doi: 10.1016/j.surg.2005.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]


