Abstract
ADAMTS metalloprotease family member ADAMTS9 maps to 3p14.2 and shows significant associations with the aerodigestive tract cancers esophageal squamous cell carcinoma (ESCC) and nasopharyngeal carcinoma (NPC). However, the functional impact of ADAMTS9 on cancer development has not been explored. In this study, we evaluated hypothesized anti-angiogenic and tumor suppressive functions of ADAMTS9 in ESCC and NPC, in stringent tumorigenicity and matrigel plug angiogenesis assays. ADAMTS9 activation suppressed tumor formation in nude mice. Conversely, knockdown of ADAMTS9 resulted in clones reverting to the tumorigenic phenotype of the parental cells. In vivo angiogenesis assays revealed a reduction in microvessel numbers in gel plugs injected with tumor-suppressive cell transfectants. Similarly, conditioned media from cell transfectants dramatically reduced the tube-forming capacity of human umbilical vein endothelial cells (HUVECs). These activities were associated with a reduction in expression levels of the pro-angiogenic factors MMP9 and VEGFA, which were consistently reduced in ADAMTS9 transfectants derived from both cancers. Taken together, our results indicate that ADAMTS9 contributes an important function in the tumor microenvironment that acts to inhibit angiogenesis and tumor growth in both ESCC and NPC.
Keywords: ADAMTS9, tumor suppression, angiogenesis, esophageal carcinoma, nasopharyngeal carcinoma
Introduction
The A Disintegrin-like and Metalloproteinase (reprolysin type) with Thrombospondin Type 1 Motifs (ADAMTS) family consists of 19 secreted proteases having a well-defined domain structure. These enzymes consist of a pro-metalloproteinase domain and a characteristic ancillary domain containing one or more thrombospondin type 1 motifs (1). Through analysis of mutant mice and human genetic disorders, ADAMTS roles in skin pigmentation, organogenesis, limb development, connective tissue assembly, and fertility were demonstrated (2). Moreover, altered expression of some ADAMTS genes has been shown in various cancers and arthritis (1, 2). Three ADAMTS proteases (ADAMTS1, ADAMTS8, and ADAMTS9), were previously shown to have anti-angiogenic activity. ADAMTS1 and ADAMTS8 inhibited VEGF-induced angiogenesis as assayed by the chick chorioallantoic membrane assay, suppressed FGF-induced vascularization in the cornea pocket assay, and inhibited endothelial cell proliferation in vitro (3). ADAMTS9 was recently demonstrated to be a constitutive product of microvascular endothelial cells in both embryonic and adult mice and to act as a cell-autonomous angiogenesis inhibitor (4).
The ability of a tumor to progress from a non-angiogenic to angiogenic phenotype is critical to cancer progression and is termed the “angiogenic switch” (5). Expansion of a tumor mass beyond its initial microscopic size is dependent on the recruitment of its own vascular supply, by angiogenesis and/or blood vessel cooption (6–8). Failure of a tumor to recruit new microvascular endothelial cells or to reorganize the existing surrounding vasculature results in growth-limited, non-angiogenic tumors (9). Although related matrix metalloproteases, ADAM and ADAMTS proteases, have been implicated in tumor progression and angiogenesis, the specific role of ADAMTS9 in tumor angiogenesis is less clearly defined. Our previous functional genomic studies show that ADAMTS9 is associated with tumor suppression in two aerodigestive tract cancers, namely esophageal squamous cell carcinoma (ESCC) and nasopharyngeal carcinoma (NPC). Down-regulation of ADAMTS9 expression was commonly observed in tumor tissues and cell lines of both cancers. Promoter hypermethylation contributes to ADAMTS9 gene silencing in both ESCC and NPC (10, 11). Importantly, previous studies indicate that ADAMTS9 protein expression in NPC is significantly associated with lymph node metastases (11). The role of this protein in cancer development remains unclear. In the present study, we investigated the in vivo and in vitro functional roles of ADAMTS9 in angiogenesis and ESCC and NPC tumorigenesis. Anti-angiogenic and tumor suppressive activities of ADAMTS9 were studied by stringent in vivo tumorigenicity and matrigel plug angiogenesis assays. The effects of conditioned media from ADAMTS9 stable transfectants were assessed in in vitro tube formation ability assays using human umbilical vein endothelial cells (HUVECs) to better understand its role in this important process.
Materials and methods
Cell lines and culture conditions
The ESCC cell line KYSE30 obtained from DSMZ (Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH, Braunschweig, Germany) (12) and immortalized esophageal epithelial cell line NE1 were cultured as previously described (10). Stable ESCC ADAMTS9 transfectants (EC-AD clones) and pCR3.1 vector-alone control (EC-V clone) were cultured in medium containing 400 µg/ml neomycin. The recipient NPC HONE1 cell line and the previously established HONE1/chromosome 3 microcell hybrid (MCH) cell line MCH8.12 were used for the ADAMTS9 knockdown analysis. MCH8.12 contains an extra truncated chromosome 3 (deleted at 3p24) transferred by microcell-mediated chromosome transfer (MMCT) to the recipient HONE1 cell; it exhibits a prolonged latency period before tumor formation. HONE1 and MCH8.12 were maintained as previously described (13). The stable ADAMTS9 knockdown clones were maintained in culture medium containing 500 µg/ml neomycin and 5 µg/ml blasticidin. The immortalized nasopharyngeal epithelial cell line NP460 was cultured as described (14). Construction of a pETE-Bsd responsive vector and a HONE1 cell line, HONE1–2, producing the tetracycline transactivator tTA, was described in Protopopov et al. (15). Stable NPC transfectants with ADAMTS9 transgene (NPC-AD clones) or with pETE-Bsd vector-alone (NPC-V clone) were maintained in culture medium containing 500 µg/ml neomycin and 5 µg/ml blasticidin. Human umbilical vein endothelial cells (HUVEC) (Lonza, Walkersville, MD) were cultured as previously described (16). All cultures were regularly monitored for mycoplasma contamination and were uniformly negative.
Reverse transcription-PCR and real-time quantitative RT-PCR analyses
Semi-quantitative and quantitative PCR were performed as previously reported (10, 11). The real-time quantitative PCRs were performed using ADAMTS9 and GAPDH Taqman probes or the SYBR Green PCR master mix in a StepOnePlus Real-Time PCR System (Applied Biosystems, Foster City, CA). The primers used for semi-quantitative PCR are listed in Supplementary Table 1. All PCR assays were performed in triplicate in two independent experiments. For the analysis of mRNA stability of MMP9 and VEGFA, the transcription inhibitor, actinomycin D (Sigma-Aldrich, St. Louis, MO, 5µg/ml) (17), was added to the ADAMTS9 stable transfectants.
Western blot analysis
Western blot analysis of ADAMTS9 was performed as previously reported (18). The ADAMTS9 propeptide domain targeting antibody (Abcam, Cambridge, UK) and Ab-1 (Calbiochem, Darmstadt, Germany) were used as primary antibodies for the detection of ADAMTS9 and α-tubulin, respectively.
Stable transfection of ADAMTS9
To generate stable clones, which express wild type ADAMTS9 in ESCC and NPC cell lines, KYSE30 and HONE1–2 cells were transfected with pCR3.1-ADAMTS9 and pETE-Bsd-ADAMTS9, respectively, as previously reported (11, 18).
Knockdown of ADAMTS9 in MCH8.12 cells
The ADAMTS9 knockdown was achieved by using the BLOCK-iT™ Pol II miR RNAi Expression Vector Kit (Invitrogen, Carlsbad, CA) and the sequences of the pair of the shRNA oligonucleotides are 5'- TGCTGTCACCAGCCAGGTTAATCCTTGTTTTGGCCACTGACTGACAAGGATTACTGGCTGGTGA −3' and 5'- CCTGTCACCAGCCAGTAATCCTTGTCAGTCAGTGGCCAAAACAAGGATTAACCTGGCTGGTGAC-3', which target at nucleotide position, 770–780, of the human ADAMTS9 cDNA (NM_182920). In brief, the pcDNA6.2GM-shRNA770 plasmid with the ADAMTS9 shRNA oligonucleotide or the vector-alone pcDNA6.2-GW/EmGFP-miR (pcDNA6.2GM) plasmid was stably transfected into the recipient cell line, MCH8.12, which strongly expresses ADAMTS9 (11).
Tumorigenicity assay and tumor segregant (TS) analysis
The cell lines were injected subcutaneously into three 6 to 8 week old female athymic Balb/c Nu/Nu mice. Subcutaneous injection and preparation of tumor segregants (TSs) were performed as previously described (10, 19). In brief, 5 × 106 and 1 × 107 cells were injected into both flanks of three nude mice (six sites) for each ESCC and NPC cell line, respectively. The tumor sizes were measured weekly. Tumors arising from non-suppressing ADAMTS9 transfectants were subsequently excised and reconstituted into tissue culture. These are the TS cell lines utilized for further analysis. For inhibition of the tetracycline-inducible expression of ADAMTS9 in NPC transfectant cell lines in vivo, 200 µg/ml dox was added to the drinking water of mice one week before injection; water containing dox was changed twice a week.
HUVEC tube formation assay
The conditioned media were collected by incubating the ADAMTS9 and the vector-alone ESCC and NPC transfected cells with Dulbecco’s Modified Eagle Medium (DMEM) without serum for 24 hrs. For the NPC ADAMTS9 and vector-alone transfectants, the conditioned media ± 0.2 µg/ml dox were obtained. A total of 4 × 104 HUVEC cells were seeded into each well coated with 50 µl matrigel (BD Biosciences, San Jose, CA) and incubated with 100 µl conditioned media from vector-alone and ADAMTS9 transfectants plus 1% FBS. The cells were then incubated for 5 hrs to allow formation of tube-like structures (16). The images at 100X magnification were captured using an inverted microscope (Nikon Instruments Inc., Melville, NY). Total tube length was measured and compared for three different viewing fields by the SPOT software (Diagnostic Instruments, Sterling Heights, MI). The primary ADAMTS9 targeting antibody (Abcam, Cambridge, UK) was used as a neutralizing antibody for blocking the effects of the extracellular ADAMTS9 protein in the conditioned media. Another irrelevant rabbit polyclonal antibody was used as a negative control immunoglobulin.
In vivo matrigel plug angiogenesis assay
A total of 5 × 106 ESCC cells or 1 × 107 NPC cells in 50 µl DMEM mixed with 250 µl ice-cold matrigel (BD Biosciences) were subcutaneously injected into the nude mice. Each cell line was injected into one site for five nude mice. The matrigel containing the cell suspension polymerized after injection and formed a plug impregnated with tumor cells. The gel plugs were removed after 7 days, fixed with formalin, and embedded in paraffin. Histological sections were stained with hematoxylin and eosin (H&E) and endothelial cell marker anti-CD34 monoclonal antibody (Santa Cruz, Santa Cruz, CA). The slides were incubated with the anti-CD34 antibody (1:40 dilution) for immunohistochemistry as previously described (18). The CD34-positive staining of vascular endothelial cells was analyzed by ImageScope v10 software (Aperio, Vista, CA).
Human angiogenesis antibody array
Conditioned media were obtained as previously described. Proteins in the conditioned media were hybridized with a human angiogenesis antibody array dotted with 43 human angiogenesis-related antibodies (RayBiotech, Norcross, GA). The assay was performed as described in the manufacturer’s manual.
Gelatin zymography
The MMP9 protein expression was measured by gelatin zymography and was performed as previously described (20). In brief, conditioned medium was mixed with loading buffer without β-mercaptoethanol, and loaded onto a 10% SDS-PAGE gel with 0.1% gelatin. After the samples were fractionated, the gel was washed twice with 2.5% Triton X-100, and then incubated at 37°C with reaction buffer (50mM Tris-Cl pH7.5, 5mM CaCl2, and 0.02% NaN3) overnight. The gel was stained with 0.1% Coomassie brilliant blue R-250 (Sigma-Aldrich, St. Louis, MO). The MMP9 activity was visualized as a clear band on a blue background with a size of 92 kDa. The assay was performed in three independent experiments. Quantitation of the MMP band was performed by using the Quantity One Gel Documentation System (Biorad, Hercules, CA).
Human VEGF immunoassay
The VEGFA in the conditioned media secreted by various ESCC and NPC cell lines was detected by the Quantikine Human VEGF Immunoassay system (R&D Systems, Minneapolis, MN). The assay was performed according to the manufacturer’s instructions. The absorbance was detected by the Labsystems Multiskan MS Plate Reader (Thermo Fisher Scientific Inc, Waltham, MA). The assay was performed in three independent experiments.
Statistical analysis
Statistical analysis was performed using SPSS11.0 statistics calculation software (SPSS Inc., Chicago, IL). Comparisons between ADAMTS9 and vector-alone transfectants in all experiments were performed by Student’s t test. A p-value of <0.05 was considered as significant.
Results
Activation of ADAMTS9 expression suppresses tumor formation in vivo
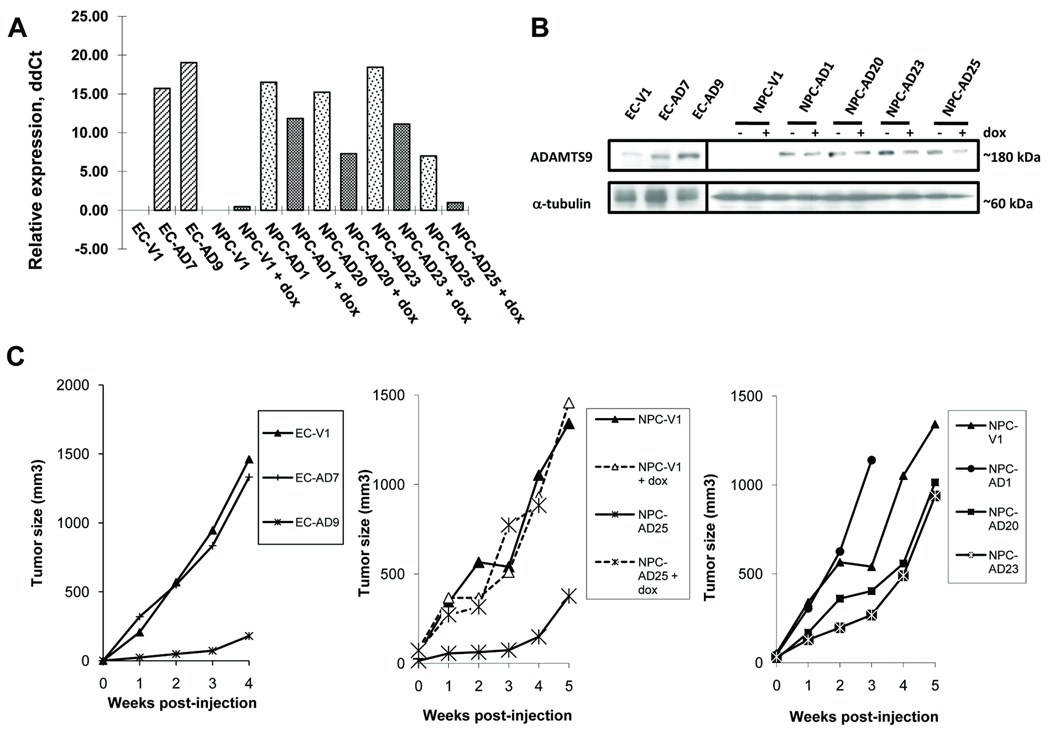
ADAMTS9 expression is down-regulated in the cell lines KYSE30 and HONE1 derived from ESCC and NPC, respectively (10, 11); therefore, they were chosen for the stable ADAMTS9 transfection and the subsequent functional analyses. As shown in Figs. 1A and B, the gene and protein expression of ADAMTS9 was induced in both ESCC ADAMTS9 transfectants, EC-AD7 and -AD9, which were analyzed by quantitative real-time PCR and Western blot analyses, respectively. ADAMTS9 expression in four NPC transfectants, NPC-AD1, -AD20, -AD23, and -AD25 was induced in the absence of dox (Figs. 1A and B). Dox treatment inactivates the tTA transcriptional activator, resulting in reduction of transgene and protein expression. Reduction of ADAMTS9 transcript and protein levels were observed following dox treatment in the HONE1–2 stable clones (Figs. 1A and B). High gene and protein expression levels were detected in NPC-AD1, -AD20, -AD23, and -AD25 cell lines (−dox); ADAMTS9 levels only dropped to those observed in the vector-alone control with NPC-AD25 (+dox) (Figs. 1A and B).
Fig. 1.
(A) Real-time quantitative RT-PCR analysis of ADAMTS9 in ESCC and NPC ADAMTS9 stable clones. Relative ddCt of each clone was compared to its respective vector-alone control. (B) Western blot analysis of the ADAMTS9 protein in stable clones. α-tubulin was used as an internal control. (C) Tumor growth kinetics of ESCC and NPC ADAMTS9 and vector-alone transfectants. Each data point represents an average tumor volume at six sites inoculated for each cell line.
The recipient KYSE30 and HONE1 cells are highly tumorigenic and formed palpable tumors of 150 mm3 one week after injection in all six injection sites. When ADAMTS9 was expressed in EC-AD9 and NPC-AD25 (−dox), prolonged latency periods for tumor formation of up to four to six weeks were observed, which was significantly longer than the one to two weeks latency period observed with vector-alone clones (Fig. 1C and Table 1). When the ADAMTS9 expression was reduced in NPC-AD25 (+dox), the average tumor size was 883 mm3, whereas when transcription was switched on, the average size was reduced to 149 mm3 four weeks after injection. The difference was statistically significant (p value = 0.02). These results suggest that ADAMTS9 expression by tumor potently suppresses tumor formation in both ESCC and NPC in vivo.
Table 1.
Tumorigenicity assays of ADAMTS9 transfectants and ADAMTS9-knockdown MCHs
| Cell line | Identification | Dox | Tumor formation (# tumors/# sites) |
Latency period (wk) for tumor volume of 150 mm3 |
p valuea/b/c/d |
|---|---|---|---|---|---|
| EC-V1 | KYSE30× pCR3.1 | − | 6/6 | 1–2 | |
| EC-AD7 | KYSE30× pCR3.1-ADAMTS9 | − | 6/6 | 1 | 0.71a |
| EC-AD9 | KYSE30× pCR3.1-ADAMTS9 | − | 3/6 | 4–6 | 0.002a |
| NPC-V1 | HONE1-2× pETE-Bsd | − | 6/6 | 1–2 | - |
| + | 6/6 | 1–2 | 0.4b | ||
| NPC-AD1 | HONE1-2× pETE-Bsd-ADAMTS9 | − | 6/6 | 1–2 | 0.14a |
| NPC-AD20 | HONE1-2× pETE-Bsd-ADAMTS9 | − | 4/6 | 1–4 | 0.35a |
| NPC-AD23 | HONE1-2× pETE-Bsd-ADAMTS9 | − | 5/6 | 1–4 | 0.23a |
| NPC-AD25 | HONE1-2× pETE-Bsd-ADAMTS9 | − | 5/6 | 4–6 | 0.01a |
| + | 5/6 | 1–4 | 0.45a, 0.02b | ||
| HONE1 | Parental NPC cell line | − | 6/6 | 1–3 | - |
| 8.12-pcDNA-V1 | 8.12 × pcDNA6.2GM | − | 2/6 | NA | 0.004c |
| 8.12-A9-shRNA770-N59 | 8.12 × ADAMTS9-shRNA770 | − | 4/6 | 4–7 | 0.01c, 0.02d |
| 8.12-A9-shRNA770-N60 | 8.12 × ADAMTS9-shRNA770 | − | 5/6 | 4–6 | 0.003c, 0.016d |
p value obtained by comparison with EC-V1/NPC-V1 (−dox).
p value obtained by comparison between tumor sizes ±dox.
p value obtained by comparison with HONE1.
p value obtained by comparison with pcDNA6.2GM.
NA, not applicable
Loss of expression of ADAMTS9 in tumors and a tumor segregant derived from tumorigenic transfectants is associated with tumorigenicity
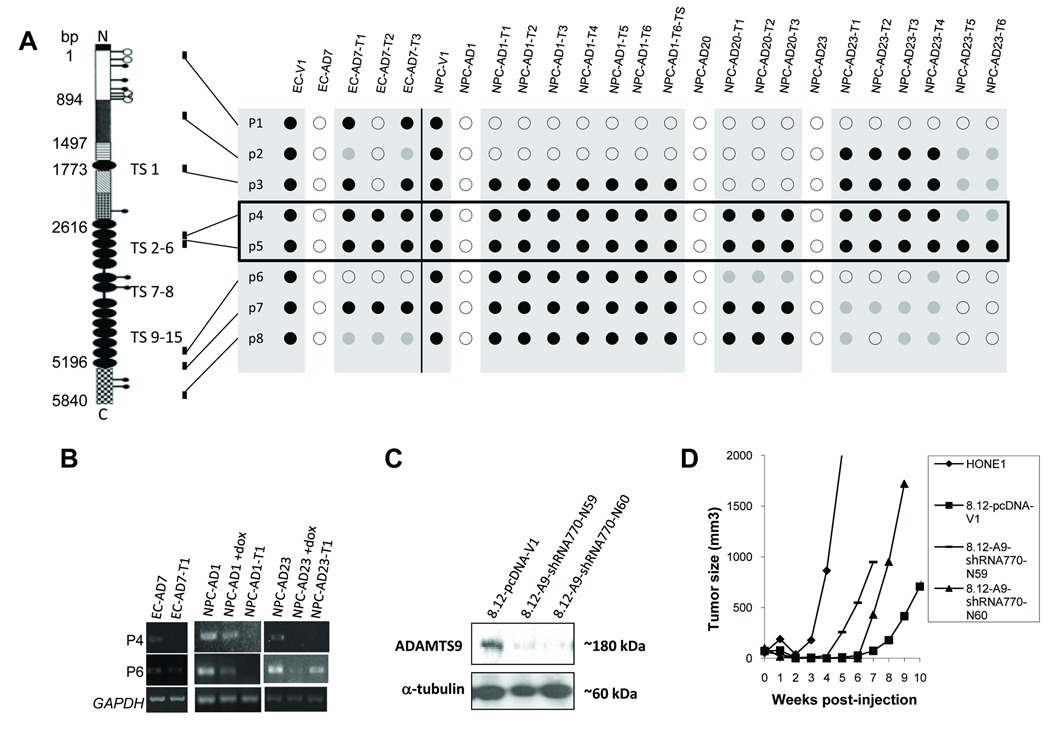
The in vivo tumorigenicity results showed that there is no significant tumor suppression for the other ADAMTS9 stable transfectants, EC-AD7 and the NPC-AD1, -AD20, and -AD23 (−dox), when compared with the vector-alone transfectants, EC-V1 and NPC-V1 (−dox) (Fig. 1C and Table 1). To check the status of ADAMTS9 following tumor formation in these tumorigenic ADAMTS9 clones, total RNA was isolated from excised tumors (EC-AD7-T1-T3, NPC-AD1-T1-T6, NPC-AD20-T1-T3, NPC-AD23-T1-T6) and cells from one tumor (NPC-AD1-T6-TS) were reconstituted in culture medium. Eight primer pairs spanning the ADAMTS9 transcript were used to analyze the regional loss of ADAMTS9 gene expression in those tumors and the TSs by RT-PCR of the 7335 bp ADAMTS9 transcript. In general, the NPC-AD1 series of tumors and its TSs showed an extensive deletion in the ADAMTS9 mRNA. The other tumors derived from the EC-AD7 and NPC-AD20 and -AD23 showed smaller discrete deletions. RT-PCR analysis using the primers p4 and p5 consistently demonstrated the loss of ADAMTS9 transcript in all tumors and the tumor segregant; a common minimum region of deletion was observed in the third and fourth thrombospondin type I repeats of ADAMTS9 (Fig. 2A). Representative RT-PCR results with and without loss of ADAMTS9 transcript regions in the selected tumors are shown in Fig. 2B. We conclude that functional inactivation of ADAMTS9 during tumorigenesis in nude mice contributes to the lack of tumor suppression of EC-AD7, NPC-AD1, -AD20, and -AD23.
Fig. 2.
(A) RT-PCR analysis of ESCC and NPC tumorigenic ADAMTS9 stable clone-derived tumors and tumor segregants with eight primer pairs covering the entire ADAMTS9 transcript. Relative localizations of the primer pairs are indicated. ○, positive expression; ●, no expression;  , reduced expression. (B) Representative RT-PCR results of the ADAMTS9 transcript regions P4 and P6 in selected tumors derived from the stable ADAMTS9 clones. GADPH served as a loading control. (C) Western blot analysis of the ADAMTS9 knockdown transfectants in two NPC clones and the vector-alone control. (D) Tumor growth kinetics of the two ADAMTS9 knockdown and vector-alone clones and HONE1 cells.
, reduced expression. (B) Representative RT-PCR results of the ADAMTS9 transcript regions P4 and P6 in selected tumors derived from the stable ADAMTS9 clones. GADPH served as a loading control. (C) Western blot analysis of the ADAMTS9 knockdown transfectants in two NPC clones and the vector-alone control. (D) Tumor growth kinetics of the two ADAMTS9 knockdown and vector-alone clones and HONE1 cells.
Tumorigenicity restored by ADAMTS9-knockdown in a microcell hybrid cell line
In order to further confirm the tumor suppressive effect of ADAMTS9, ADAMTS9 knockdown in the non-tumorigenic HONE1/chromosome 3 MCH8.12, which expresses high levels of ADAMTS9 mRNA (11), was performed. Stable ADAMTS9 knockdown transfectants were obtained by transfecting pcDNA6.2GM-shRNA770 into MCH8.12. Reduction of ADAMTS9 protein expression was observed in the stable clones, 8.12-A9-shRNA770-N59 and -N60 (Fig. 2C), when compared with the vector-alone 8.12-pcDNA-V1. The specificity of the shRNA oligonucleotide was tested, RT-PCR results show that the gene expression of ADAMTS1 and ADAMTS8 was not affected in the stable ADAMTS9 knockdown transfectants 8.12-A9-shRNA770-N59 and -N60 (data not shown). Tumorigenicity was suppressed in the vector-alone clone 8.12-pcDNA-V1 and tumors were observed at only two sites of injection for up to 8 weeks after inoculation (Fig. 2D and Table 1). Tumors appeared with both ADAMTS9-knockdown clones in at least four injection sites, which showed similar growth kinetics. The differences were statistically significant when compared with the vector-alone controls (p values = 0.01 and 0.003, respectively). When compared with the tumor growth rate of HONE1 cells, the two ADAMTS9-knockdown MCHs were significantly slower (p values = 0.02 and 0.016, respectively) (Fig. 2D and Table 1).
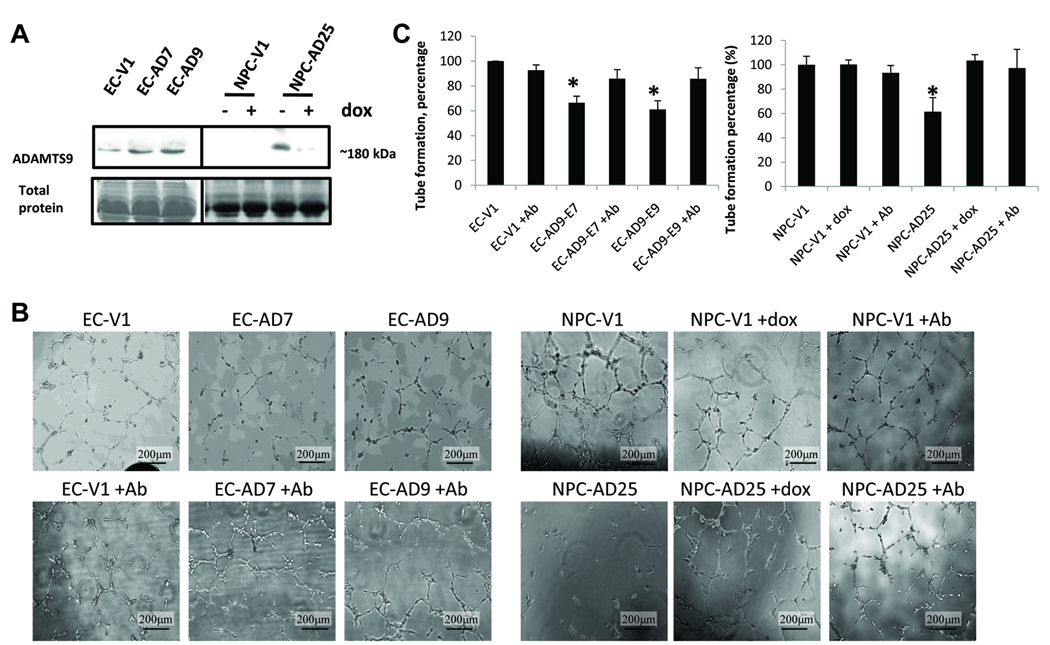
ADAMTS9 reduces tube formation by HUVEC in vitro
The “angiogenic switch” is critical for solid tumor formation (5) and ADAMTS9 was shown to suppress tumor formation in ESCC and NPC in the current study. This study examines the anti-angiogenic property of ADAMTS9 and how it can contribute to its tumor suppressive function. The HUVEC tube formation assay was used to test the effect of ADAMTS9 on angiogenesis in vitro. ADAMTS9 is a secreted protein associated with the cell surface, but has also been shown to be present in the conditioned medium of expressing cells (4). Concentrated conditioned media collected from both ESCC and NPC ADAMTS9 transfectants were analyzed by Western blotting to show the presence of secreted ADAMTS9 proteins compared to vector-alone transfectants, EC-V1 and NPC-V1 (Fig. 3A). The secreted ADAMTS9 protein expression was significantly reduced in NPC-AD25 (+dox) (Fig. 3A). The conditioned media from EC-AD7 and -AD9 and NPC-AD25 (−dox) inhibited formation of tube-like structures of HUVEC cells, as compared to their respective vector-alone controls (Fig. 3B). The tube-forming ability of HUVEC cells was significantly decreased to 66.4%, 61.1%, and 61.5% after incubation with EC-AD7 and -AD9 and NPC-AD25 (−dox) cell conditioned media, respectively, as compared with the vector-alone controls (Fig. 3C). The inhibitory effect on tube formation was restored to control levels in NPC-AD25 (+dox), when transgene expression is repressed (Figs. 3B and C). In order to further validate the specific inhibitory effect of the secreted ADAMTS9 protein in the conditioned media, ADAMTS9 neutralizing antibody was added together with the conditioned medium to block its activities; reduction in the secreted ADAMTS9 activities subsequently decreased tube-forming ability of the three ESCC/NPC ADAMTS9 transfectants (Figs. 3B and C). The ADAMTS9 neutralizing antibody has no significant effect on the tube formation in the vector-alone clones (Figs. 3B and C). An irrelevant negative control antibody has no significant effect on the tube formation in the ADAMTS9 transfectants (data not shown).
Fig. 3.
(A) Western blot analysis of conditioned medium from the ADAMTS9 and vector-alone stable clones. Total protein staining by Coomassie blue staining was used to indicate equal loading. (B) Representative results of the HUVEC tube formation assay of the ADAMTS9 and vector-alone transfectants and after treatment with the ADAMTS9 neutralizing antibody. (C) The percentage of tube formation ability of the ADAMTS9 clones with or without the ADAMTS9 neutralizing antibody, as compared to their corresponding vector-alone controls. “*” designates a statistically significant difference from the vector-alone clone (p < 0.05).
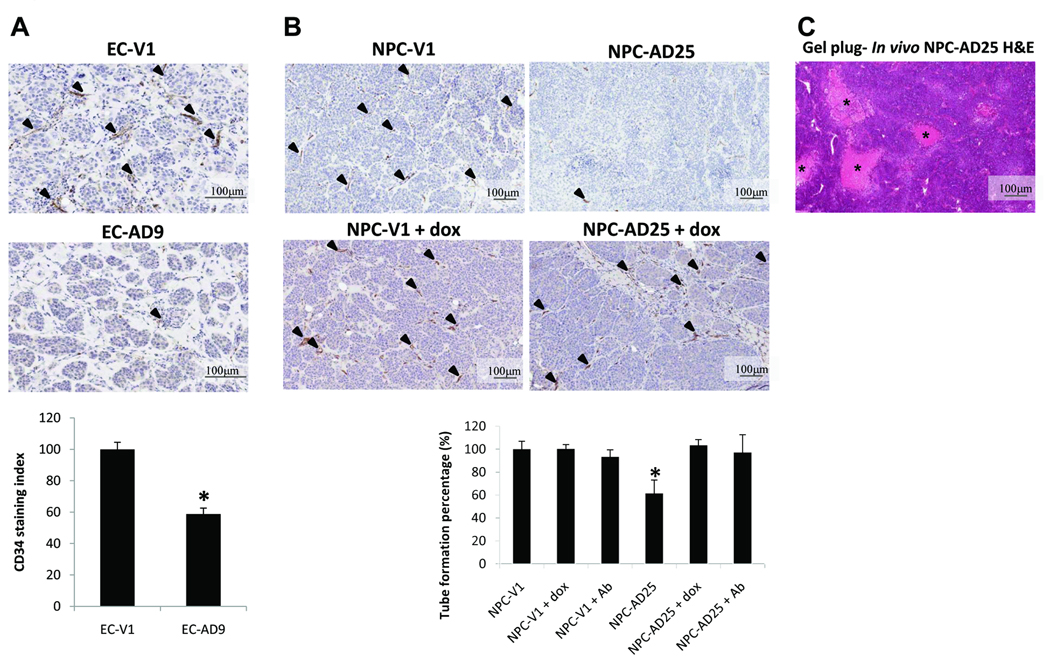
ADAMTS9 inhibits in vivo angiogenesis
The anti-angiogenic activity of ADAMTS9 in vivo was assessed in mice with the matrigel plug assay. The tumor-suppressive ESCC and NPC transfectants, EC-AD9 and NPC-AD25, were utilized, as the ADAMTS9 transgenes of these clones are stable in the in vivo conditions. The anti-CD34 antibody microvessel-stained gel plugs were analyzed; representative results are shown in Figs. 4A and B, upper panels. The numbers of microvessels of both ESCC and NPC clones, EC-AD9 and NPC-AD25 (−dox), were substantially reduced to 58.8% and 46.9%, respectively, of the vector-alone controls (Figs. 4A and B, lower panels). Repression of the transgene in NPC-AD25 (+dox) restored the angiogenesis levels observed in the gel plug to that of the vector-alone control (Fig. 4B). In addition, cell necrosis was frequently observed in central regions of tumor nodules in the gel plug of NPC-AD25 (−dox) (Fig. 4C). This is possibly connected with poorer vasculature of the tumor cells in these plugs.
Fig. 4.
Representative results from matrigel plug assay in ESCC (A) and NPC (B). The endothelial cells were stained with anti-CD34 antibody, as indicated by arrows. CD34 staining index of the ADAMTS9 transfectants as compared with their corresponding vector-alone controls. “*” designates a statistically significant difference from the vector-alone clone (p < 0.05). (C) Representative image of cell necrosis (acellular areas indicated by “*”) observed in center region of tumor nodules formed by the NPC ADAMTS9 transfectant NPC-AD25 clone (−dox).
Down-regulation of Matrix Metallopeptidase9 (MMP9) and Vascular Endothelial Growth Factor A (VEGFA) gene and protein expression in the media derived from ADAMTS9 stable transfectants
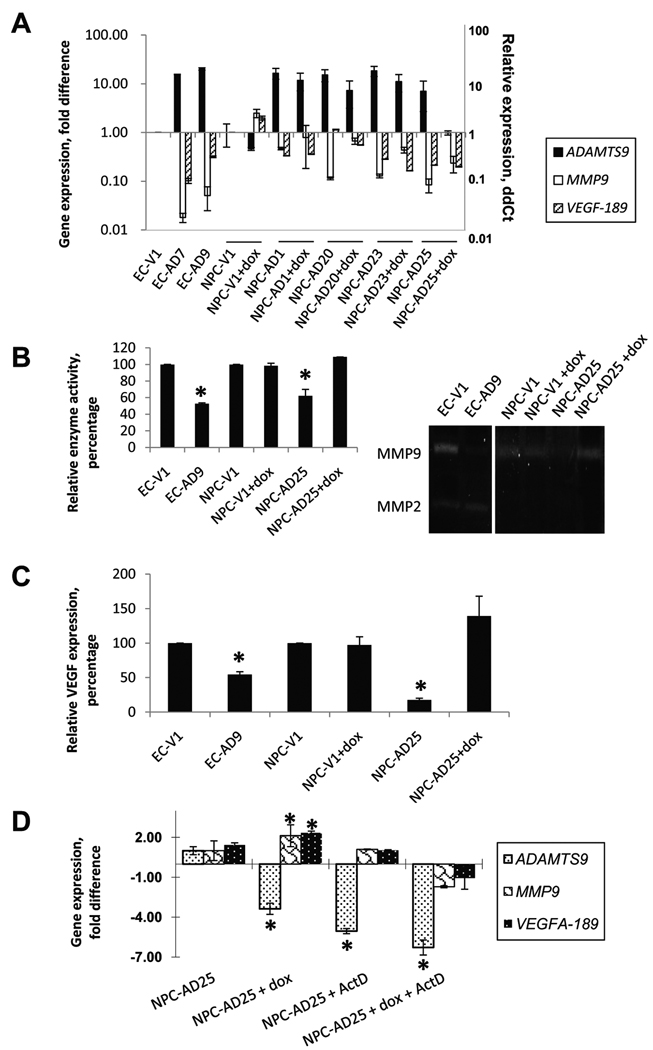
We used an antibody array containing several angiogenesis-related antibody probes to study the proteins associated with the anti-angiogenic activities of ADAMTS9. Conditioned media from the ESCC ADAMTS9 and vector-alone transfectants were collected and hybridized to this antibody array to determine differential expression levels of the angiogenesis-related proteins. Down-regulated expression of VEGFA, MMP9, IL-8, GM-CSF, and TGF-β1 was observed in ADAMTS9 transfectants (data not shown). Real-time quantitative PCR was used to confirm their expression in both ESCC and NPC transfectants, EC-AD7 and -AD9 and NPC-AD1, -AD20, -AD23, and -AD25. MMP9 gene expression was consistently decreased in all ADAMTS9-expressing ESCC and NPC clones (Fig. 5A). After transgene repression in NPC clones, MMP9 gene expression was higher in all four NPC transfectants (+dox), as compared to the clones expressing ADAMTS9 (−dox). We further used gelatin zymography to detect and quantify the MMP9 protein in the conditioned media. Strong MMP9 signals were observed in the vector-alone EC-V1 and NPC-V1 (Fig. 5B). When ADAMTS9 was over-expressed in EC-AD9 and NPC-AD25 (−dox), the MMP9 signals were significantly reduced. The MMP9 activity was restored, when the ADAMTS9 expression was suppressed in NPC-AD25 (+dox).
Fig. 5.
(A) Real-time quantitative PCR analysis of MMP9 and VEGFA-189 in ESCC and NPC ADAMTS9 transfectants. Fold-change of the relative gene expression of MMP9 and VEGFA-189 of each clone was compared to their corresponding vector-alone transfectants. Relative ddCt of the ADAMTS9 gene expression of each clone was compared to their corresponding vector-alone transfectants. (B) Zymography of conditioned media from ESCC and NPC ADAMTS9 stable transfectants. The average MMP9 activities were calculated from three independent experiments. Fold-change of the MMP9 activities of each clone was compared to the vector-alone controls. Representative zymography results are shown on the right hand side. The MMP9 and MMP2 are indicated. (C) Quantitative analysis of human VEGFA in conditioned media by ELISA. The relative VEGFA expression in conditioned medium from ADAMTS9 stable transfectants was compared to their vector-alone controls. “*” designates a statistically significant difference from the vector-alone clone (p < 0.05). (D) Real-time quantitative PCR analysis of MMP9 and VEGFA-189 in NPC-AD25 (±dox) after actinomycin D treatment. Fold-change of the relative gene expression of MMP9 and VEGFA-189 in each of the treated cells was compared to the untreated cells.
On the other hand, the real-time PCR results show that VEGFA-189 gene expression decreased in the two ESCC clones (EC-AD7 and -AD9) and three out of four NPC clones (NPC-AD1, -AD23, and -AD25) (Fig. 5A). The other angiogenesis-related genes, IL-8, TGF-β1, and GM-CSF, were less consistently down-regulated in the ESCC and NPC clones (data not shown). In order to quantitate the VEGFA protein expression, we used ELISA to detect the VEGFA secreted by the ADAMTS9 over-expressed transfectants in the conditioned media. Figure 5C shows that the VEGFA protein expression was reduced to 55% and 17% in EC-AD9 and NPC-AD25 (−dox), as compared with their vector-alone controls, respectively. The VEGFA protein expression was restored in NPC-AD25 (+dox), when the ADAMTS9 gene expression was switched off.
In order to investigate the regulatory mechanism of MMP9 and VEGF by ADAMTS9, NPC-AD25 was treated with actinomycin D for 24 hr to determine whether transcription or mRNA stability of MMP9 and VEGFA would be affected. Figure 5D shows that the MMP9 and VEGFA-189 transcripts were still stable after the treatment with actinomycin D. The increased expression of MMP9 and VEGFA-189 after addition of dox, was reduced to less than basal levels, when the cell line was treated with actinomycin D. Hence, it is likely that the transcription of both MMP9 and VEGFA-189 and not their stability was regulated by ADAMTS9.
Discussion
Functional analyses of ADAMTS9 strongly support its important role in vivo and in vitro. Using the inducible and constitutive gene expression systems, in vivo and in vitro angiogenesis and tumorigenicity assays clearly show that the over-expression of ADAMTS9 is sufficient to induce potent suppression of tumor formation and angiogenesis in both ESCC and NPC. Importantly, by knockdown of ADAMTS9 expression in a non-tumorigenic MCH, we show that the ADAMTS9-knockdown clones revert to the tumorigenic phenotype of the parental cells. Gene and protein expression analyses of ADAMTS9 transfectants revealed its reduced expression is associated with the transcriptional regulation of the pro-angiogenic factors, MMP9 and VEGFA, in both ESCC and NPC. Hence the data strongly suggest that ADAMTS9 plays a critical role in the “angiogenic switch” and transforms both ESCC and NPC cell lines from a pro-angiogenic to a non-angiogenic phenotype. Inhibition of tumor angiogenesis is a common mechanism for tumor suppression in both tumor types. Based on the deletion patterns of the ADAMTS9 transcript in tumors and a tumor segregant derived from the tumorigenic transfectants, it is tempting to speculate that the tumor-suppressive activity of ADAMTS9 in ESCC and NPC is associated with the thrombospondin (TSP) domains in the C-terminal region of the gene.
Our findings strongly suggest that the anti-angiogenic activities of ADAMTS9 play a critical role of tumor suppression in both ESCC and NPC. These findings are consistent with recent findings in which the ADAMTS9 protein expressed by the microvascular endothelial cells was demonstrated to be anti-angiogenic in both the Adamts9+/− mice and the siRNA knockdown of cultured human microvascular endothelial cells. In contrast, unlike ADAMTS1, which exhibits its anti-angiogenic effects by cleavage of TSPs and sequestration of VEGFA-165, ADAMTS9 neither cleaves TSP-1 and TSP-2, nor binds VEGFA-165 (4). A key conclusion of those studies was the cell-autonomous effect of ADAMTS9 in endothelial cells. The present studies highlight a non-cell autonomous mechanism by which of ADAMTS9 produced by tumor cells has an effect on angiogenesis; thus, ADAMTS9 may act on endothelial cells via a dual mechanism.
For the tumorigenic ADAMTS9 transfectants, EC-AD7 and NPC-AD1, -AD20, and AD-23 (−dox), tumors formed one week after injection. After inoculation of these clones in nude mice, both reduction and loss of the region encoding the third and the fourth thrombospondin type I repeats were observed by RT-PCR analysis of mRNA in all tumors and the representative tumor segregant derived from tumorigenic clones. This kind of functional inactivation due to elimination of the transgene over-expression was observed in our previous studies of the tumor suppressor gene (TSG) Cell Adhesion Molecule 1 (CADM1, formerly called TSLC1) and THY1 in NPC (19, 21). The in vitro angiogenesis assay results show that EC-AD7 could significantly suppress tube formation of HUVEC cells, as the ADAMTS9 transgene is stable and remains activated in the in vitro conditions.
The present functional studies were also performed using the stable NPC MCH8.12 ADAMTS9-knockdown clones. Both clones showing reduced ADAMTS9 expression clearly reverted back to their tumorigenic phenotype. However, since the tumor growth kinetics was still lower than that of the original recipient HONE1 cells, it is possible that other growth inhibitory gene(s) besides ADAMTS9 might be still present on chromosome 3. Previous studies indicate that PTPRG and RASSFIA and BLU, at the nearby 3p14–21 and 3p21.3 regions, respectively, are identified as TSGs in NPC (22–25).
Except for the initial association of the microvascular endothelial cells expressing ADAMTS9 with angiogenesis (4), its anti-angiogenic activities in human cancer cells have yet to be reported. The results of these studies, thus, provide clear evidence for the importance of ADAMTS9 in angiogenesis and tumor development in two important malignancies, ESCC and NPC. Our results suggest that ADAMTS9 inhibition of angiogenesis in both cancers is associated with reduction of the gene expression levels of the pro-angiogenic factors, MMP9 and VEGFA. In future studies it will be important to define the precise relationship between ADAMTS9 and these two key regulators of angiogenesis.
Supplementary Material
Acknowledgments
Grant support: The study was financed by the Research Grants Council of the Hong Kong Special Administrative Region, People’s Republic of China: Grant numbers HKU6617/08M and HKU6415/06M to MLL; the NIH award AR49930 to SSA; and the Swedish Cancer Society, the Swedish Research Council, the Swedish Institute, Cancer Research Institute in New York/Concern Foundation in Los Angeles and Karolinska Institute to ERZ.
References
- 1.Porter S, Clark IM, Kevorkian L, Edwards DR. The ADAMTS metalloproteinases. Biochem J. 2005;386:15–27. doi: 10.1042/BJ20040424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Apte SS. A disintegrin-like and metalloprotease (reprolysin-type) with thrombospondin type 1 motif (ADAMTS) superfamily: functions and mechanisms. J Biol Chem. 2009;284:31493–31497. doi: 10.1074/jbc.R109.052340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vazquez F, Hastings G, Ortega MA, et al. METH-1, a human ortholog of ADAMTS-1, and METH-2 are members of a new family of proteins with angio-inhibitory activity. J Biol Chem. 1999;274:23349–23357. doi: 10.1074/jbc.274.33.23349. [DOI] [PubMed] [Google Scholar]
- 4.Koo BH, David M, Coe DM, et al. ADAMTS9 is a cell-autonomously acting, anti-angiogenic metalloprotease expressed by microvascular endothelial cells. The American Journal of Pathology. 2009 doi: 10.2353/ajpath.2010.090655. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Folkman J, Watson K, Ingber D, Hanahan D. Induction of angiogenesis during the transition from hyperplasia to neoplasia. Nature. 1989;339:58–61. doi: 10.1038/339058a0. [DOI] [PubMed] [Google Scholar]
- 6.Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1990;82:4–6. doi: 10.1093/jnci/82.1.4. [DOI] [PubMed] [Google Scholar]
- 7.Holmgren L, O'Reilly MS, Folkman J. Dormancy of micrometastases: balanced proliferation and apoptosis in the presence of angiogenesis suppression. Nat Med. 1995;1:149–153. doi: 10.1038/nm0295-149. [DOI] [PubMed] [Google Scholar]
- 8.Pezzella F, Pastorino U, Tagliabue E, et al. Non-small-cell lung carcinoma tumor growth without morphological evidence of neo-angiogenesis. Am J Pathol. 1997;151:1417–1423. [PMC free article] [PubMed] [Google Scholar]
- 9.Naumov GN, Akslen LA, Folkman J. Role of angiogenesis in human tumor dormancy: animal models of the angiogenic switch. Cell Cycle. 2006;5:1779–1787. doi: 10.4161/cc.5.16.3018. [DOI] [PubMed] [Google Scholar]
- 10.Lo PH, Leung AC, Kwok CY, et al. Identification of a tumor suppressive critical region mapping to 3p14.2 in esophageal squamous cell carcinoma and studies of a candidate tumor suppressor gene, ADAMTS9. Oncogene. 2007;26:148–157. doi: 10.1038/sj.onc.1209767. [DOI] [PubMed] [Google Scholar]
- 11.Lung HL, Lo PH, Xie D, et al. Characterization of a novel epigenetically-silenced, growth-suppressive gene, ADAMTS9, and its association with lymph node metastases in nasopharyngeal carcinoma. Int J Cancer. 2008;123:401–408. doi: 10.1002/ijc.23528. [DOI] [PubMed] [Google Scholar]
- 12.Shimada Y, Imamura M, Wagata T, Yamaguchi N, Tobe T. Characterization of 21 newly established esophageal cancer cell lines. Cancer. 1992;69:277–284. doi: 10.1002/1097-0142(19920115)69:2<277::aid-cncr2820690202>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 13.Cheng Y, Poulos NE, Lung ML, et al. Functional evidence for a nasopharyngeal carcinoma tumor suppressor gene that maps at chromosome 3p21.3. Proc Natl Acad Sci U S A. 1998;95:3042–3047. doi: 10.1073/pnas.95.6.3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li HM, Man C, Jin Y, et al. Molecular and cytogenetic changes involved in the immortalization of nasopharyngeal epithelial cells by telomerase. Int J Cancer. 2006;119:1567–1576. doi: 10.1002/ijc.22032. [DOI] [PubMed] [Google Scholar]
- 15.Protopopov AI, Li J, Winberg G, et al. Human cell lines engineered for tetracycline-regulated expression of tumor suppressor candidate genes from a frequently affected chromosomal region, 3p21. J Gene Med. 2002;4:397–406. doi: 10.1002/jgm.283. [DOI] [PubMed] [Google Scholar]
- 16.Kong D, Li Y, Wang Z, Banerjee S, Sarkar FH. Inhibition of angiogenesis and invasion by 3,3'-diindolylmethane is mediated by the nuclear factor-kappaB downstream target genes MMP-9 and uPA that regulated bioavailability of vascular endothelial growth factor in prostate cancer. Cancer Res. 2007;67:3310–3319. doi: 10.1158/0008-5472.CAN-06-4277. [DOI] [PubMed] [Google Scholar]
- 17.Chen LC, Liu HP, Li HP, et al. Thymidine phosphorylase mRNA stability and protein levels are increased through ERK-mediated cytoplasmic accumulation of hnRNP K in nasopharyngeal carcinoma cells. Oncogene. 2009;28:1904–1915. doi: 10.1038/onc.2009.55. [DOI] [PubMed] [Google Scholar]
- 18.Lung HL, Bangarusamy DK, Xie D, et al. THY1 is a candidate tumour suppressor gene with decreased expression in metastatic nasopharyngeal carcinoma. Oncogene. 2005;24:6525–6532. doi: 10.1038/sj.onc.1208812. [DOI] [PubMed] [Google Scholar]
- 19.Lung HL, Leung Cheung AK, Xie D, et al. TSLC1 is a tumor suppressor gene associated with metastasis in nasopharyngeal carcinoma. Cancer Res. 2006;66:9385–9392. doi: 10.1158/0008-5472.CAN-06-0590. [DOI] [PubMed] [Google Scholar]
- 20.MacDougall JR, Bani MR, Lin Y, Rak J, Kerbel RS. The 92-kDa gelatinase B is expressed by advanced stage melanoma cells: suppression by somatic cell hybridization with early stage melanoma cells. Cancer Res. 1995;55:4174–4181. [PubMed] [Google Scholar]
- 21.Lung HL, Cheung AKL, Cheng Y, et al. Functional characterization of THY1 as a tumor suppressor gene with anti-invasive activity in nasopharyngeal carcinoma. Int J Cancer. 2009 doi: 10.1002/ijc.25047. In press. [DOI] [PubMed] [Google Scholar]
- 22.Cheung AK, Lung HL, Hung SC, et al. Functional analysis of a cell cycle-associated, tumor-suppressive gene, protein tyrosine phosphatase receptor type G, in nasopharyngeal carcinoma. Cancer Res. 2008;68:8137–8145. doi: 10.1158/0008-5472.CAN-08-0904. [DOI] [PubMed] [Google Scholar]
- 23.Yau WL, Lung HL, Zabarovsky ER, et al. Functional studies of the chromosome 3p21.3 candidate tumor suppressor gene BLU/ZMYND10 in nasopharyngeal carcinoma. Int J Cancer. 2006;119:2821–2826. doi: 10.1002/ijc.22232. [DOI] [PubMed] [Google Scholar]
- 24.Lo PH, Xie D, Chan KC, et al. Reduced expression of RASSF1A in esophageal and nasopharyngeal carcinomas significantly correlates with tumor stage. Cancer Lett. 2007;257:199–205. doi: 10.1016/j.canlet.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 25.Lo KW, Kwong J, Hui AB, et al. High frequency of promoter hypermethylation of RASSF1A in nasopharyngeal carcinoma. Cancer Res. 2001;61:3877–3881. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.