Abstract
Objective
Several lines of evidence suggest that antipsychotic drug efficacy is mediated by dopamine D2 receptor blockade. Therefore, it seems plausible that variation in the DRD2 gene is associated with clinical response to antipsychotic drug treatment. We conducted the first meta-analysis to examine the relationship between DRD2 polymorphisms and antipsychotic drug response.
Method
Medline search (12/31/2008) yielded 18 prospective studies examining DRD2 variation and antipsychotic response in schizophrenia patients, of which 10 independent studies met criteria for inclusion. Clinical response to antipsychotic treatment was defined as a 50% reduction of either BPRS or PANSS total score at approximately 8 weeks follow-up. Odds ratio (OR) was the primary effect size measure and was computed for each polymorphism in each study. Sufficient data were available for two DRD2 polymorphisms, -141C Ins/Del and Taq1A.
Results
Six studies reported results on the -141C Ins/Del polymorphism (n=698). The Del allele carrier was significantly associated with poorer antipsychotic drug response, compared to the Ins/Ins genotype, OR=.65, p=.03. Eight studies assessed the Taq1A polymorphism and antipsychotic response (n=748). There was no significant difference in response rate in A1 carrier vs. A2/A2 genotype or A2 carrier vs. A1/A1 genotype.
Conclusion
DRD2 genetic variation is associated with clinical response to antipsychotic drug treatment. This data may provide proof-of-principle for pharmacogenetic studies in schizophrenia.
Introduction
Schizophrenia is a chronic and debilitating disorder, for which antipsychotic drugs are the treatment of choice(1). However, many patients with schizophrenia discontinue or switch antipsychotic drug regimens due to lack of efficacy and/or treatment-emergent side effects, and a large proportion of patients remain symptomatic despite treatment(2-4). The factors that influence the variation in response to antipsychotic drug treatment have not been well-elucidated, rendering it difficult to develop effective treatment strategies tailored to individual patients.
Pharmacogenetics research focuses on the identification of genetic variants that predict who may optimally benefit from antipsychotic treatment(5). Variants in genes that code for neurotransmitter receptors have been the primary targets, including multiple loci in the dopamine and serotonin receptor systems. However, there remain surprisingly few studies on the relationship between the most obvious candidate gene, DRD2, and antipsychotic drug response. Several lines of evidence suggest that the D2 receptor plays a critical role in antipsychotic drug action. Earlier studies showed that antipsychotic clinical potency was highly correlated with the binding affinity to a particular type of dopamine receptor(6), which was later found to be the D2 receptor(7, 8). Recent functional imaging studies suggest that binding to the D2 receptor by antipsychotic agents may be “necessary and sufficient” for antipsychotic efficacy(9). Finally, all known antipsychotic drugs bind to the D2 receptor, and drugs that have targeted non-D2 receptors without at least some element of D2 blockade have failed to treat schizophrenia effectively(8, 10).
Some of the earliest studies of DRD2 single nucleotide polymorphisms (SNPs, specifically, the -141C Ins/Del and Taq1A variants) revealed promising associations with antipsychotic efficacy(11-13). However, the subsequent literature has been marked by mixed results and small sample sizes, complicating the evaluation of such associations. A potentially useful methodology to overcome this limitation is by use of meta-analytic techniques that incorporate results from multiple studies in an unbiased fashion. In the present study, we conducted the first pharmacogenetics meta-analysis to examine the association between variation in the DRD2 gene and antipsychotic drug response.
As described in detail below, relevant studies in the literature have utilized a variety of designs, trial durations, symptom measures, and response criteria. Consequently, we developed a systematic and consistent methodology to harmonize the reported phenotypes, contacting the original investigators when necessary to re-evaluate raw data. Additionally, while multiple DRD2 SNPs have been studied, including Taq1B(14, 15), Taq1D(15, 16), T939C(14), S311C(17), and C957T(16, 18), most of these SNPs were reported in only one or two studies, with the exception of the Taq1A and -141C Ins/Del variants. Finally, studies to date have included patients across all phases of the illness, ranging from first-episode schizophrenia patients with no or limited prior exposure to antipsychotic drugs to clozapine-treated patients with poor prior antipsychotic drug responses and lengthy prior medication histories. As antipsychotic drug exposure has been demonstrated to alter the expression of multiple CNS receptors(19), including the dopamine D2 receptor, this factor may introduce additional variance into studies of genetic sources of variability. Therefore, we conducted exploratory analyses to investigate whether studies examining first episode cohorts yielded stronger results than those comprised primarily of chronically ill subjects.
Methods
Literature Search
To identify studies eligible for this meta-analysis, we searched Medline for all publications available up to 12/31/2008 that examined the association between the DRD2 gene and antipsychotic drug response. The following key words were used in the literature search: DRD2, polymorphism, antipsychotic, clinical response, gene, and schizophrenia. We also used the reference lists from identified papers and recent literature review articles to identify additional relevant studies. Furthermore, to find unpublished studies, we also searched meeting abstracts that were likely to contain relevant studies. Each paper included in the meta-analysis meets the following criteria: 1) reported the association between DRD2 polymorphisms and clinical antipsychotic drug response; 2) the majority of patients met DSM-IV criteria for a diagnosis of schizophrenic or schizoaffective disorder, and diagnoses were confirmed with a standardized structured clinical interview; 3) drug response was assessed with a standardized rating scale, such as the Brief Psychiatric Rating Scale (BPRS), the Positive and Negative Syndrome Scale (PANSS), or the Clinical Global Impression scale (CGI), at baseline and follow-up; and 4) the follow-up period was no longer than three months. We selected this duration of follow-up because our major goal was to assess acute antipsychotic treatment response, and response rates in treatment trials longer than three months may reflect other factors related to non-compliance(20), relapse prevention, illness course, and psychosocial variables(21), which may confound the genotype-drug response relationships.
Selection of candidate polymorphisms
DRD2 (see Figure 1) contains a number of SNPs with differing frequencies amongst populations. Several DRD2 polymorphisms have been studied in association with antipsychotic drug response(22). In order to conduct a robust meta-analysis, we selected polymorphisms that were reported on in at least three studies. Two polymorphisms fit this criterion: the -141C Ins/Del and Taq1A polymorphisms. Minor allele frequency for the Taq1A ranges from 20% in Caucasians to 44% in other ethnic groups. Minot allele frequency for the -141C Ins/Del ranges from about 10% in Japanese and Caucasians to more than 50% in Africans.
Figure 1.
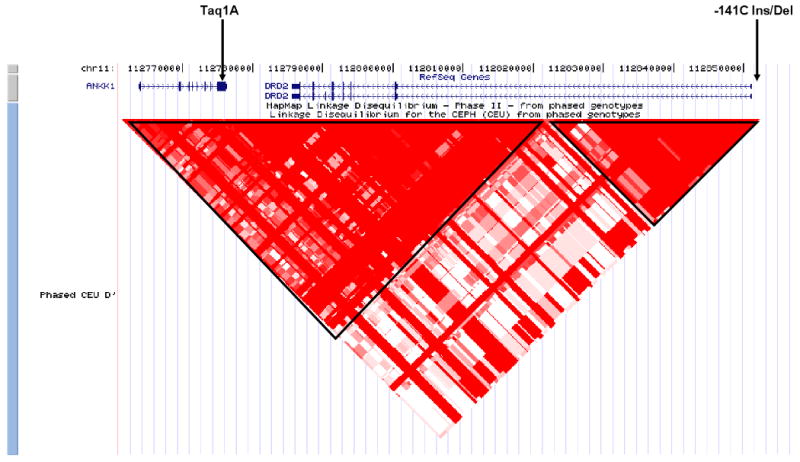
Location of the Taq1A and -141C Ins/Del polymorphisms in the context of ANKK1 and DRD2 at chromosome 11q22. Red triangles represent areas of high linkage equilibrium (D′).
-141C Ins/Del (rs1799732)
This polymorphism represents a deletion (versus insertion) of cytosine at position -141, located in the 5′ promoter region of DRD2. In vitro data by Arinami and colleagues(23) showed that cell lines transfected with the Del allele were less active in a luciferase reporter assay than cell lines transfected with the Ins allele. In vivo data with PET imaging (22) have also suggested that this polymorphism may influence D2 receptor density in the striatum of healthy volunteers unexposed to antipsychotic drug treatment. For the purposes of this meta-analysis, we pooled the Del/Del and Ins/Del genotype groups into one group (Del carrier) because of the low frequency of the Del/Del genotype in the general population and then tested for association between this group versus the Ins/Ins genotype group.
Taq1A (rs1800497)
This SNP involves a C >T substitution, located about 10kb downstream of DRD2. The A1 allele is associated with reduced DRD2 gene expression(24, 25). Recently, the Taq1A SNP was found to be part of the kinase gene “ankyrin repeat and kinase domain containing 1” (ANKK1) (26, 27). This SNP has been studied in association with substance abuse, alcohol dependence, eating disorder, and smoking cessation. Given the lack of unequivocal data for Taq1A genotype pooling, we tested both dominant and recessive hypotheses: A1/A1 versus A1/A2 + A2/A2, and A2/A2 versus A1/A2 + A1/A1.
Definition of clinical response
Clinical response to antipsychotic drug treatment was defined as a 50% reduction of either BPRS or PANSS total score from baseline to follow-up. Studies have shown that a 50% reduction of BPRS total score is approximately equivalent to a 50% reduction in PANSS total score, which equates to a rating of 1 or 2 on the CGI-Improvement scale(28). To be consistent across studies, we chose to define clinical response at the 8-week follow-up (or closest time point thereto), because this was the most common follow-up time point available. If a study did not use 50% reduction as the definition of clinical response, effort was made to contact the authors to obtain additional data. If data with 50% reduction was not obtainable, the original definition of clinical response reported in the paper was used in the meta-analysis.
Odds ratio (OR) was the primary effect size measure and was computed for each polymorphism in each study. If a study did not report the categorical outcomes of responders vs. non-responders, we requested data from the authors. If categorical data were not available, the study was not included in the meta-analysis.
Statistical analysis
Data were entered into and analyzed by the Cochrane Collaboration review manager software (RevMan version 5). Heterogeneity between the studies was assessed by the χ2 test. Individual OR and associated 95% confidence intervals (CI) were calculated, and pooled to compute the mean effect size by the Mantel-Haenszel method(29). A fixed-effect model was used in all analyses(30), a similar approach used in other pharmacogenetic meta-analyses(30, 31). Separate meta-analysis was conducted for each SNP and for each genotype. Publication bias was assessed with the funnel plot, the “Trim and Fill” method(32), and Egger's test(33), which was conducted using the “metatrim” and “metabias” macro procedures in the Stata program version 7.
Results
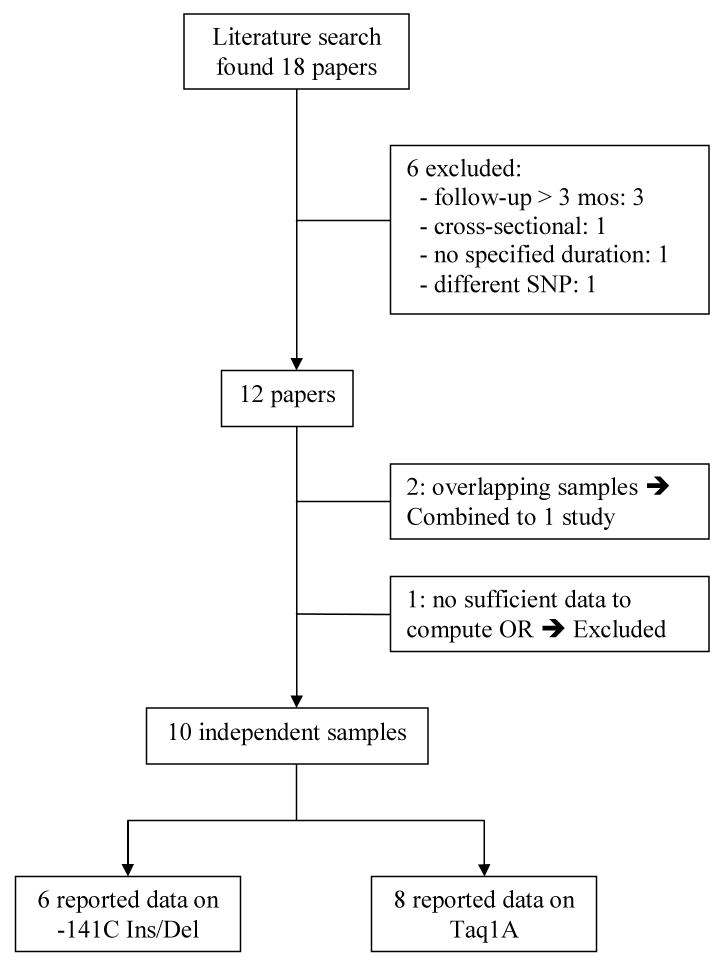
To conduct the meta-analysis on the relationship between DRD2 variation and antipsychotic drug response, we searched the literature with a cut-off date of 12/31/2008, which yielded 18 published papers. Six papers were not included in the meta-analysis: three studies only reported long-term outcomes greater than three months from baseline to follow-up(15, 16, 34); one study(35) was cross-sectional; one paper did not state duration of follow-up, and nor did it include the BPRS, PANSS, or CGI(36); and one study contained no data on the Taq1A or -141C Ins/Del polymorphisms(17). Two papers(37, 38) published by the same research group contained overlapping data, and therefore were assessed as one study subsequent to the authors providing the additional data needed to compute categorical outcome of clinical response. Finally, we were unable to obtain sufficient information to calculate response rate from an additional study(39), therefore these data were not included in the meta-analysis.
In sum, ten independent studies (total sample size: 889) met criteria for inclusion in the meta-analysis. Figure 2 summarizes the literature search process. The clinical characteristics of each study are summarized in Table 1. Six studies reported outcomes conditioned on the -141C Ins/Del SNP (N = 687) and eight reported outcomes on the Taq1A SNP (N = 748). Six of the 10 studies reported continuous outcomes, but the authors generously provided the additional data needed to compute ORs.
Figure 2.
Flow chart of literature search.
Table 1.
Characteristics of studies investigating the association between DRD2 polymorphisms and antipsychotic drug response in schizophrenic patients.
| Author/ year |
N | Country | Ethnicity | Setting | Diagnosis | Study Design |
Patient Type |
Meds |
DRD2 SNP |
Treatment Length |
Outcome Measure |
Follow-up Period |
Definition of Response |
Mean Change at Follow-up |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Xing, 2007(14) | 125 | China | Chinese | not reported | SCZ | RCT | Chronic | Risperidone | -141C Ins/Del; Taq1A | 8 weeks | BPRS | 8 weeks | 40% reduction | not reported |
| Schafer, 2001(11) | 57 | Germany | Caucasian | Inpatient | Mostly psychotic disorders |
RCT | Acute | Haloperidol | Taq1A | 4 weeks | PANAS | 4 weeks | 50% reduction | not reported |
| Wu, 2005(46) | 135 | China | Chinese | Inpatient | SCZ | RCT | Mostly 1st eptsode | Chlorpromazine | -141C Ins/Del; Taq1A | 8 weeks | BPRS | 8 weeks | 50% reduction | not reported |
| Yamanouchi, 2003(37) and Ikeda, 2008(38) | 166 | Japan | Japanese | not reported | SCZ SAD | Open-label | 120 were 1st episode | Risperidone | -141C Ins/Del; Taq1A | 8 weeks | PANSS | 8 weeks | 50% reduction | 23.1% |
| Lencz, 2006(43) | 61 | USA | Mixed Caucasian & AA | Inpatient | SCZ | RCT | 1st episode | Risperidone Olanzapine | -141C Ins/Del | 16 weeks | CGI-I | 8 weeks | 1 or 2 | N/A |
| Malhotra, 1999(12) | 72 | USA | Mixed Causian & AA | Inpatient | SCZ | RCT | Treatment refractory | Clozapine | -141C Ins/Del | 10 weeks | BPRS | 10 weeks | 20% reduction | not reported |
| Suzuki, 2000(13) | 25 | Japan | Japanese | Inpatient | SCZ | RCT | Acute | Nemonapride | Taq1 A | 3 weeks | BPRS | 3 weeks | 50% reduction | 65.9% |
| Suzuki, 2001(47) | 30 | Japan | Japanese | Inpatient | SCZ | RCT | Chronic | Bromperidol | Taq1 A | 3 weeks | BPRS | 3 weeks | 50% reduction | 56.8% |
| Kwon 2008(48) | 90 | South Korea | Korean | Inpatient | SCZ | RCT | Acute | Aripiprazole | Taq1A | 26 weeks | PANSS | 8 weeks | 50% reduction | 30.0% |
| Shen 2008(18) | 128 | Taiwan | Chinese | Inpatient | SCZ | Open-label | Chronic | Aripiprazole | -141C Ins/Del; Taq1A | 4 weeks | PANSS | 4 weeks | 50% reduction | 24.2% |
Note: SCZ = Schizophrenia; BPRS = Brief Psychiatric Rating Scale; PANSS = Positive and Negative Syndrome Scale; CGI = Clinical Global Impression scale; AA = African American.
-141C Ins/Del polymorphism and antipsychotic drug response
Six studies that met inclusion criteria reported results on the -141C Ins/Del polymorphism, with a total sample size of 687 patients. Figure 3 presents ORs for the individual studies and the pooled analyses in different genotype groups. There was a significant difference in response rate between the Del carrier vs. Ins/Ins genotypes (pooled OR = .65, 95% CI = .43 ∼ .97, p = .03), indicating that Del carriers tend to have less favorable antipsychotic drug responses than patients with the Ins/Ins genotype. The heterogeneity χ2 test was not significant, χ2 = 9.23, df = 5, p = .10, I2 = 46%. To deal with potentially undetected heterogeneity across samples, a sensitivity analysis was conducted excluding two studies with the largest and smallest effect size(12, 14). Another reason to exclude these two studies was that they may be different from other studies because the 50% reduction of BPRS or PANSS was not used to define clinical response. For the sensitivity analysis, I2 changed from 46% to 10%, and the χ2 test for the heterogeneity analysis dropped from 9.23 to 3.33, which was non-significant and the p-value changed from .10 to .34. The pooled OR became .60, 95% CI = .38∼.97, and p = .04.
Figure 3.
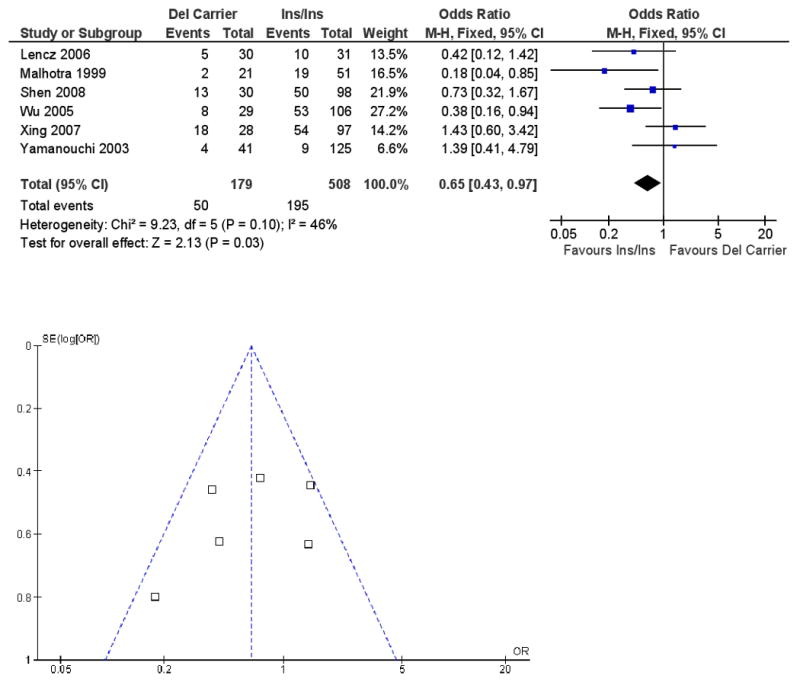
Meta-analysis of the association between the -141C Ins/Del polymorphism (Del carrier vs. Ins/Ins) and antipsychotic drug response: Forrest plot and funnel plot.
Note: M-H: Mantel-Haenszel method.
As a post hoc analysis, we restricted our analysis to the studies comprised of patients with first-episode schizophrenia (N = 316). The pooled OR was .53 for Del carrier vs. Ins/Ins (95% CI = .28 ∼ .99, p = .05), demonstrating poorer clinical response in Del carriers. In contrast, for the studies that did not include first-episode schizophrenic patients (N = 371), we obtained a pooled OR of .75 (95% CI = .44 ∼ 1.27, p = .28). (Please see supplemental data Figure 1.)
Funnel plot is presented with Figure 3 and did not indicate evidence of publication bias. Duval and Tweedie's “trim and fill” analysis showed that it was not necessary to trim any existing study and fill any additional “unpublished” study. In addition, Egger's test did not show evidence of publication bias (B = .79, 95% CI = -3.05 ∼ 4.63, p = .60).
Taq1A polymorphism and antipsychotic drug response
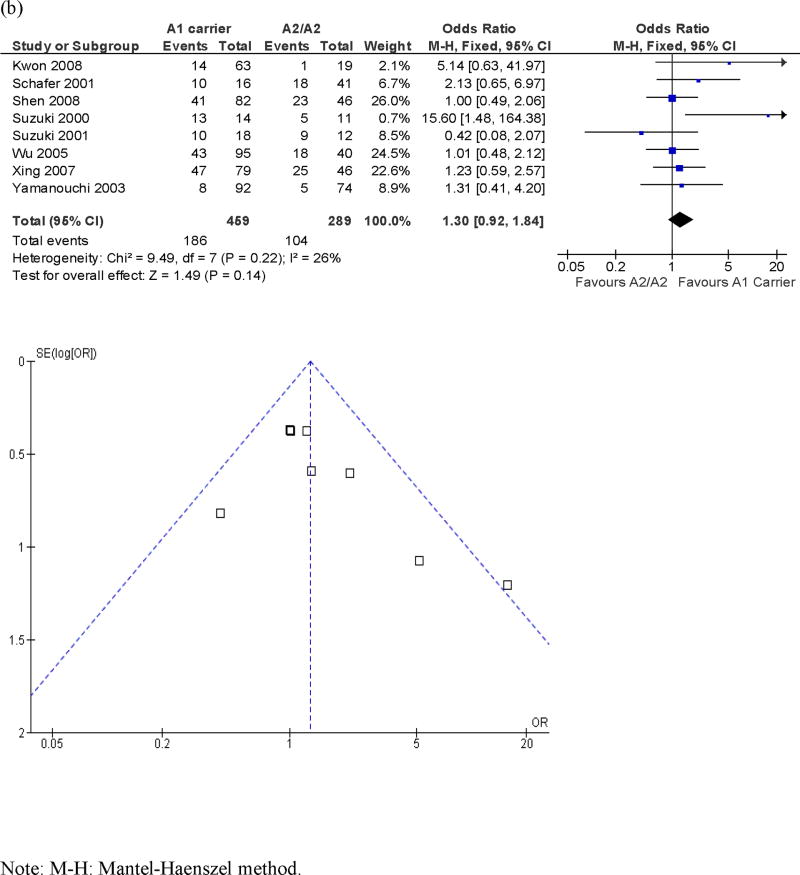
Eight studies assessed the Taq1A polymorphism and antipsychotic response, with a total sample size of 748 patients. Figure 4 presents ORs for the individual studies and the pooled analyses in different genotype groups. There was no significant difference in response rate in A1/A1 vs. A2 carrier or A1 carrier vs. A2/A2 genotype (pooled ORs = 1.39 and 1.30, p's = .13 and .14, respectively). There was no significant heterogeneity across studies in the two comparisons, χ2 = 10.40 and 9.49, p's = .11 and .22, I2 = 42% and 26%, respectively.
Figure 4.
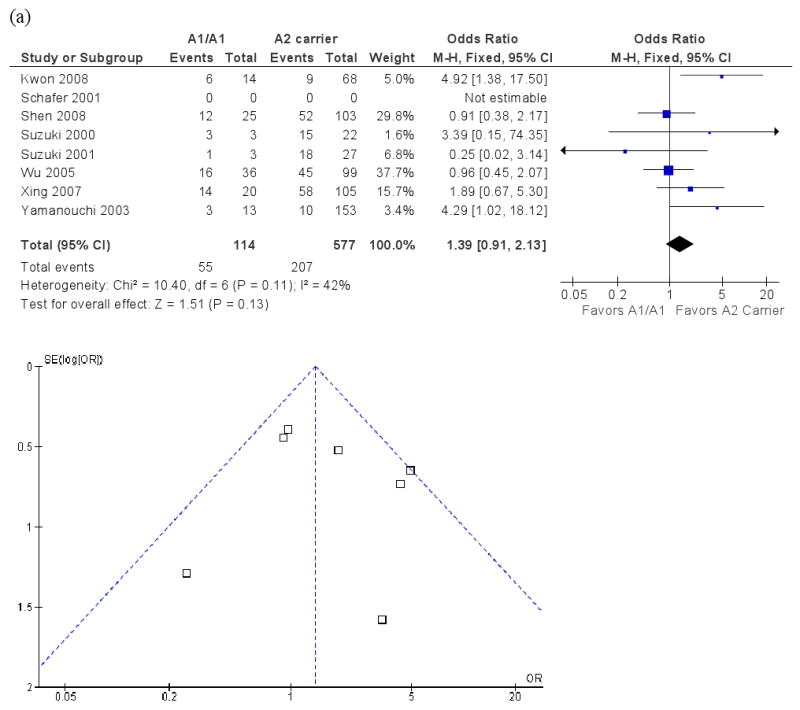
Meta-analysis of the association between the Taq1A polymorphism and antipsychotic drug response: Forrest plot and funnel plot. (a) A1/A1 vs. A2 carrier; (b) A1 carrier vs. A2/A2.
Note: M-H: Mantel-Haenszel method.
Funnel plots are presented in Figure 4. Duval and Tweedie's “trim and fill” analysis showed that it was necessary to “fill” an additional “unpublished” study for both the A1/A1 vs A2 carrier comparison and the A1 carrier vs A2/A2 comparison. The pooled OR's for the two comparisons with an additional “filled” study became 1.24 and 1.17, p's = .30 and .39, respectively. In contrast, Egger's test did not show evidence of publication bias for either comparison (B's = -.07 and -.60, p's = .93 and .26, respectively). In summary, the evidence regarding publication bias for the Taq1A polymorphism was inconsistent. Even if we were able to eliminate publication bias, it seemed that the association between the Taq1A polymorphism and antipsychotic drug response was still not significant.
Discussion
In order to assess the relationship between DRD2 genetic variation and antipsychotic drug response, we conducted the first meta-analysis of two commonly studied DRD2 SNPs: -141C Ins/Del and Taq1A, and clinical response to antipsychotic drug treatment. The primary result is that the -141C Ins/Del polymorphism significantly influences antipsychotic drug response (n = 687 patients), whereas we were not able to detect a relationship between clinical response and the Taq1A variant.
These data are consistent with prior research indicating an important role for the dopamine D2 receptor in antipsychotic drug response. Antipsychotic clinical potency is highly correlated with the binding affinity to the dopamine D2 receptor(6-8), D2 receptor occupancy by antipsychotic agents has been demonstrated to occur with all antipsychotic agents(9), and drugs targeting other receptor sites without dopamine D2 blockade have not yet been successfully developed as antipsychotics(8). To our knowledge, this is the first meta-analysis in pharmacogenetics to demonstrate the importance of DRD2 genetic variation in antipsychotic drug response.
Of note, we observed a significant genotype-phenotype relationship in patients with first-episode schizophrenia. This may be due to the limited or lack of prior exposure to antipsychotic drugs in these patients. Differential amounts of prior drug exposure, as commonly observed in chronically ill samples, could result in considerable variation in levels of dopamine receptor up-regulation(40, 41) and potentially mask subtle genetic effects on dopamine receptor availability(42) that could mediate antipsychotic response. However, other factors including greater drug response rates in first-episode patients should be considered, as well as the limitation that first-episode studies are less common than the studies that include chronic patients.
There was no significant association between the Taq1A genotype and antipsychotic drug response in the meta-analysis of eight studies and over 700 patients. Although the Taq1A polymorphism has been found to be associated with drug response in several studies(11, 18, 38), it is not clear how it is related to DRD2, and it is actually located in a non-coding region of the DRD2 locus. In contrast, we did find a significant association between the -141C Ins/Del polymorphism and antipsychotic drug response. This may be due to the fact that this SNP is located in the 5′ promoter region of DRD2, where it may influence modulation of transcriptional activities(23) and D2 receptor density(42). Interestingly, another DRD2 SNP, A-241G, which is also located in the promoter region of DRD2, has also been associated with antipsychotic drug response(43).
Although sample size limitations do not provide us with an opportunity to conduct drug-specific analysis, it is not unexpected that DRD2 variation might influence clinical response to all antipsychotics. First, all antipsychotic drugs bind potently to the D2 receptors. Second, there are few data to suggest that any one antipsychotic has markedly improved efficacy over another, and similar response rates suggest phenotypic overlap and provide the rationale for grouping of individual drug responses for analysis. Third, and perhaps most importantly, each of these drugs was specifically developed because of the common mechanism of action of antagonism of dopamine D2 receptors, and therefore a common effect of DRD2 variation across the antipsychotic drugs seems highly plausible. Nevertheless, the development of drugs with antipsychotic efficacy that do not act at the D2 receptor will be needed to empirically assess this question.
There are several limitations of the study. First, odds ratio was used as the effect size measure in the meta-analysis. Because this requires dichotomizing a continuous measure of either BPRS or PANSS, this may diminish statistical power, and may explain why some studies reported significant findings of an association between DRD2 and antipsychotic drug response, whereas the OR's were not individually significant in the meta-analysis. Therefore, meta-analysis of OR may lack some sensitivity to detect small effect sizes. This is consistent with an exploratory sensitivity analysis using a random effect model (see supplemental data Figure 2), which produced a less robust p value than the fixed effect model. Nevertheless, categorical response, instead of incremental differences in scores on the BPRS or PANSS, may be more meaningful from a clinical perspective. To clarify the clinical relevance of DRD2 genetic variations it may be necessary to use an even more clinically meaningful outcome measure, such as days to discharge following acute treatment or assessments of functional disability.
Second, variation in the antipsychotic drugs administered in these studies limited the possibility of examining the association of DRD2 with any specific drug. In the studies included in the meta-analysis, multiple antipsychotic drugs were utilized, including typical agents such as chlorpromazine and haloperidol, and atypical drugs such as clozapine, risperidone, olanzapine, and aripiprazole. While all of these drugs act on the D2 receptor, they exhibit different affinity profiles for many of the candidate receptors(44), making direct comparisons more complex. For example, non-D2 receptors such as D3, D4, and 5-HT2A may also be important in antipsychotic drug action(44), as well as new mechanisms of action such as mGluR2 and mGluR3 stimulation(45). Additionally, it should be noted that studies have included patients from several different ethnic groups, with an over-representation of Asians (e.g., Chinese, Korean, and Japanese) and under-representation of individuals of African descent. As allele frequencies may vary considerably between ethnic groups, careful consideration of the potential impact of population genetics on genotypic and phenotypic distribution is warranted, but the limited samples currently available have hampered this effort. Finally, the relatively small number of studies included in the meta-analysis makes it difficult to conduct any meaningful moderator analyses.
Due to the heterogeneity of medication used, duration of illness in different samples, and racial groups, it is possible that we have under-estimated the effect size of the gene–drug response association. Furthermore, none of these studies formally accounted for medication non-compliance, which is prevalent among patients with schizophrenia. Put simply, when a patient does not take the prescribed antipsychotic drug, the measured effect size of gene-drug response association is assessed as zero, whereas the true effect of genotype on the phenotype is perhaps larger. Nevertheless, despite the potential under-estimation of effect size produced by these uncontrolled factors, we were still able to detect a significant association between -141C Ins/Del and antipsychotic drug response in the meta-analysis. Data on -141C Ins/Del from larger studies such as the CATIE trial and industry efforts will be informative to further establishing the role of this SNP in antipsychotic drug response.
In summary, our meta-analysis indicates that DRD2 genetic variation is significantly associated with antipsychotic drug response. SNPs in the DRD2 promoter region, such as -141C Ins/Del, may be particularly important in predicting clinical response to antipsychotic drug treatment. Studies with larger cohorts examined with prospective designs may be needed to fully understand the nature of this relationship.
Supplementary Material
Figure 1. Meta-analysis of the association between -141C Ins/Del polymorphism (Del carrier vs. Ins/Ins) and antipsychotic drug response in studies of first-episode schizophrenia patients.
Note: M-H: Mantel-Haenszel method.
Figure 2. Meta-analysis of the association between -141C Ins/Del polymorphism (Del carrier vs. Ins/Ins) and antipsychotic drug response: A random effect model.
Note: M-H: Mantel-Haenszel method.
Acknowledgments
The study was partially supported by NIMH grants 1P30MH074543 (PI: J. Kane, MD), 1P50MH080173 (PI: J. Kane, MD), 1R01MH79800-01 to Dr. Malhotra, and K01MH65580 to Dr. Lencz. We would like to thank the investigators who provided additional data from their studies to make it possible to compute odds ratios for the meta-analysis.
Footnotes
Disclosures of Conflict of Interest: Dr. Malhotra is a consultant/advisor to Eli Lilly, Janssen, Wyeth, Vanda, and a member of the Speakers Bureau for BMS. Dr. Lencz is a consultant to Eli Lilly.
References
- 1.Kane JM. Pharmacologic treatment of schizophrenia. Biol Psychiatry. 1999;46(10):1396–408. doi: 10.1016/s0006-3223(99)00059-1. [DOI] [PubMed] [Google Scholar]
- 2.Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, Keefe RS, Davis SM, Davis CE, Lebowitz BD, Severe J, Hsiao JK. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209–23. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- 3.Jones PB, Barnes TR, Davies L, Dunn G, Lloyd H, Hayhurst KP, Murray RM, Markwick A, Lewis SW. Randomized controlled trial of the effect on Quality of Life of second- vs first-generation antipsychotic drugs in schizophrenia: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study (CUtLASS 1) Arch Gen Psychiatry. 2006;63(10):1079–87. doi: 10.1001/archpsyc.63.10.1079. [DOI] [PubMed] [Google Scholar]
- 4.Kahn RS, Fleischhacker WW, Boter H, Davidson M, Vergouwe Y, Keet IP, Gheorghe MD, Rybakowski JK, Galderisi S, Libiger J, Hummer M, Dollfus S, Lopez-Ibor JJ, Hranov LG, Gaebel W, Peuskens J, Lindefors N, Riecher-Rossler A, Grobbee DE. Effectiveness of antipsychotic drugs in first-episode schizophrenia and schizophreniform disorder: an open randomised clinical trial. Lancet. 2008;371(9618):1085–97. doi: 10.1016/S0140-6736(08)60486-9. [DOI] [PubMed] [Google Scholar]
- 5.Malhotra AK, Murphy GM, Jr, Kennedy JL. Pharmacogenetics of psychotropic drug response. Am J Psychiatry. 2004;161(5):780–96. doi: 10.1176/appi.ajp.161.5.780. [DOI] [PubMed] [Google Scholar]
- 6.Creese I, Burt DR, Snyder SH. Dopamine receptor binding predicts clinical and pharmacological potencies of antischizophrenic drugs. Science. 1976;192(4238):481–3. doi: 10.1126/science.3854. [DOI] [PubMed] [Google Scholar]
- 7.Snyder SH. Dopamine receptors, neuroleptics, and schizophrenia. Am J Psychiatry. 1981;138(4):460–4. doi: 10.1176/ajp.138.4.460. [DOI] [PubMed] [Google Scholar]
- 8.Kapur S, Mamo D. Half a century of antipsychotics and still a central role for dopamine D2 receptors. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27(7):1081–90. doi: 10.1016/j.pnpbp.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Kapur S, Seeman P. Does fast dissociation from the dopamine d(2) receptor explain the action of atypical antipsychotics?: A new hypothesis. Am J Psychiatry. 2001;158(3):360–9. doi: 10.1176/appi.ajp.158.3.360. [DOI] [PubMed] [Google Scholar]
- 10.Truffinet P, Tamminga CA, Fabre LF, Meltzer HY, Riviere ME, Papillon-Downey C. Placebo-controlled study of the D4/5-HT2A antagonist fananserin in the treatment of schizophrenia. Am J Psychiatry. 1999;156(3):419–25. doi: 10.1176/ajp.156.3.419. [DOI] [PubMed] [Google Scholar]
- 11.Schafer M, Rujescu D, Giegling I, Guntermann A, Erfurth A, Bondy B, Moller HJ. Association of short-term response to haloperidol treatment with a polymorphism in the dopamine D(2) receptor gene. Am J Psychiatry. 2001;158(5):802–4. doi: 10.1176/appi.ajp.158.5.802. [DOI] [PubMed] [Google Scholar]
- 12.Malhotra AK, Buchanan RW, Kim S. Allelic variation in the promotor region of the dopamine D2 receptor gene and clozapine response. Schizophr Res. 1999;36:92–93. [Google Scholar]
- 13.Suzuki A, Mihara K, Kondo T, Tanaka O, Nagashima U, Otani K, Kaneko S. The relationship between dopamine D2 receptor polymorphism at the Taq1 A locus and therapeutic response to nemonapride, a selective dopamine antagonist, in schizophrenic patients. Pharmacogenetics. 2000;10(4):335–41. doi: 10.1097/00008571-200006000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Xing Q, Qian X, Li H, Wong S, Wu S, Feng G, Duan S, Xu M, Gao R, Qin W, Gao J, Meng J, He L. The relationship between the therapeutic response to risperidone and the dopamine D2 receptor polymorphism in Chinese schizophrenia patients. Int J Neuropsychopharmacol. 2007;10(5):631–7. doi: 10.1017/S146114570600719X. [DOI] [PubMed] [Google Scholar]
- 15.Vijayan NN, Bhaskaran S, Koshy LV, Natarajan C, Srinivas L, Nair CM, Allencherry PM, Banerjee M. Association of dopamine receptor polymorphisms with schizophrenia and antipsychotic response in a South Indian population. Behav Brain Funct. 2007;3:34. doi: 10.1186/1744-9081-3-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hwang R, Shinkai T, De Luca V, Muller DJ, Ni X, Macciardi F, Potkin S, Lieberman JA, Meltzer HY, Kennedy JL. Association study of 12 polymorphisms spanning the dopamine D(2) receptor gene and clozapine treatment response in two treatment refractory/intolerant populations. Psychopharmacology (Berl) 2005;181(1):179–87. doi: 10.1007/s00213-005-2223-5. [DOI] [PubMed] [Google Scholar]
- 17.Lane HY, Lee CC, Chang YC, Lu CT, Huang CH, Chang WH. Effects of dopamine D2 receptor Ser311Cys polymorphism and clinical factors on risperidone efficacy for positive and negative symptoms and social function. Int J Neuropsychopharmacol. 2004;7(4):461–70. doi: 10.1017/S1461145704004389. [DOI] [PubMed] [Google Scholar]
- 18.Shen YC, Chen SF, Chen CH, Lin CC, Chen SJ, Chen YJ, Luu SU. Effects of DRD2/ANKK1 gene variations and clinical factors on aripiprazole efficacy in schizophrenic patients. J Psychiatr Res. 2008 doi: 10.1016/j.jpsychires.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 19.Strange PG. Antipsychotic drugs: importance of dopamine receptors for mechanisms of therapeutic actions and side effects. Pharmacol Rev. 2001;53(1):119–33. [PubMed] [Google Scholar]
- 20.Robinson D, Woerner MG, Alvir JM, Bilder R, Goldman R, Geisler S, Koreen A, Sheitman B, Chakos M, Mayerhoff D, Lieberman JA. Predictors of relapse following response from a first episode of schizophrenia or schizoaffective disorder. Arch Gen Psychiatry. 1999;56(3):241–7. doi: 10.1001/archpsyc.56.3.241. [DOI] [PubMed] [Google Scholar]
- 21.Schooler NR. Relapse prevention and recovery in the treatment of schizophrenia. J Clin Psychiatry. 2006;67 5:19–23. [PubMed] [Google Scholar]
- 22.Arranz MJ, de Leon J. Pharmacogenetics and pharmacogenomics of schizophrenia: a review of last decade of research. Mol Psychiatry. 2007;12(8):707–47. doi: 10.1038/sj.mp.4002009. [DOI] [PubMed] [Google Scholar]
- 23.Arinami T, Gao M, Hamaguchi H, Toru M. A functional polymorphism in the promoter region of the dopamine D2 receptor gene is associated with schizophrenia. Hum Mol Genet. 1997;6(4):577–82. doi: 10.1093/hmg/6.4.577. [DOI] [PubMed] [Google Scholar]
- 24.Ritchie T, Noble EP. Association of seven polymorphisms of the D2 dopamine receptor gene with brain receptor-binding characteristics. Neurochem Res. 2003;28(1):73–82. doi: 10.1023/a:1021648128758. [DOI] [PubMed] [Google Scholar]
- 25.Pohjalainen T, Rinne JO, Nagren K, Lehikoinen P, Anttila K, Syvalahti EK, Hietala J. The A1 allele of the human D2 dopamine receptor gene predicts low D2 receptor availability in healthy volunteers. Mol Psychiatry. 1998;3(3):256–60. doi: 10.1038/sj.mp.4000350. [DOI] [PubMed] [Google Scholar]
- 26.Neville MJ, Johnstone EC, Walton RT. Identification and characterization of ANKK1: a novel kinase gene closely linked to DRD2 on chromosome band 11q23.1. Hum Mutat. 2004;23(6):540–5. doi: 10.1002/humu.20039. [DOI] [PubMed] [Google Scholar]
- 27.Dubertret C, Gouya L, Hanoun N, Deybach JC, Ades J, Hamon M, Gorwood P. The 3′ region of the DRD2 gene is involved in genetic susceptibility to schizophrenia. Schizophr Res. 2004;67(1):75–85. doi: 10.1016/s0920-9964(03)00220-2. [DOI] [PubMed] [Google Scholar]
- 28.Leucht S, Kane JM, Etschel E, Kissling W, Hamann J, Engel RR. Linking the PANSS, BPRS, and CGI: clinical implications. Neuropsychopharmacology. 2006;31(10):2318–25. doi: 10.1038/sj.npp.1301147. [DOI] [PubMed] [Google Scholar]
- 29.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. Journal of the National Cancer Institute. 1959;22:719–748. [PubMed] [Google Scholar]
- 30.Munafo MR, Flint J. Meta-analysis of genetic association studies. Trends Genet. 2004;20(9):439–44. doi: 10.1016/j.tig.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 31.Serretti A, Kato M, De Ronchi D, Kinoshita T. Meta-analysis of serotonin transporter gene promoter polymorphism (5-HTTLPR) association with selective serotonin reuptake inhibitor efficacy in depressed patients. Mol Psychiatry. 2007;12(3):247–57. doi: 10.1038/sj.mp.4001926. [DOI] [PubMed] [Google Scholar]
- 32.Duval SJ, Tweedie RL. A non-parametric “trim and fill” method of assessing publication bias in meta-analysis. Journal of the American Statistical Association. 2000;95:89–98. [Google Scholar]
- 33.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hwang R, Shinkai T, Deluca V, Macciardi F, Potkin S, Meltzer HY, Kennedy JL. Dopamine D2 receptor gene variants and quantitative measures of positive and negative symptom response following clozapine treatment. Eur Neuropsychopharmacol. 2006;16(4):248–59. doi: 10.1016/j.euroneuro.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 35.Alenius M, Wadelius M, Dahl ML, Hartvig P, Lindstrom L, Hammarlund-Udenaes M. Gene polymorphism influencing treatment response in psychotic patients in a naturalistic setting. J Psychiatr Res. 2008;42(11):884–93. doi: 10.1016/j.jpsychires.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 36.Arranz MJ, Li T, Munro J, Liu X, Murray R, Collier DA, Kerwin RW. Lack of association between a polymorphism in the promoter region of the dopamine-2 receptor gene and clozapine response. Pharmacogenetics. 1998;8(6):481–4. doi: 10.1097/00008571-199812000-00004. [DOI] [PubMed] [Google Scholar]
- 37.Yamanouchi Y, Iwata N, Suzuki T, Kitajima T, Ikeda M, Ozaki N. Effect of DRD2, 5-HT2A, and COMT genes on antipsychotic response to risperidone. Pharmacogenomics J. 2003;3(6):356–61. doi: 10.1038/sj.tpj.6500211. [DOI] [PubMed] [Google Scholar]
- 38.Ikeda M, Yamanouchi Y, Kinoshita Y, Kitajima T, Yoshimura R, Hashimoto S, O'Donovan MC, Nakamura J, Ozaki N, Iwata N. Variants of dopamine and serotonin candidate genes as predictors of response to risperidone treatment in first-episode schizophrenia. Pharmacogenomics. 2008;9(10):1437–43. doi: 10.2217/14622416.9.10.1437. [DOI] [PubMed] [Google Scholar]
- 39.Dahmen N, Muller MJ, Germeyer S, Rujescu D, Anghelescu I, Hiemke C, Wetzel H. Genetic polymorphisms of the dopamine D2 and D3 receptor and neuroleptic drug effects in schizophrenic patients. Schizophr Res. 2001;49(1-2):223–5. doi: 10.1016/s0920-9964(99)00147-4. [DOI] [PubMed] [Google Scholar]
- 40.Lidow MS, Goldman-Rakic PS. Differential regulation of D2 and D4 dopamine receptor mRNAs in the primate cerebral cortex vs. neostriatum: effects of chronic treatment with typical and atypical antipsychotic drugs. J Pharmacol Exp Ther. 1997;283(2):939–46. [PubMed] [Google Scholar]
- 41.Silvestri S, Seeman MV, Negrete JC, Houle S, Shammi CM, Remington GJ, Kapur S, Zipursky RB, Wilson AA, Christensen BK, Seeman P. Increased dopamine D2 receptor binding after long-term treatment with antipsychotics in humans: a clinical PET study. Psychopharmacology (Berl) 2000;152(2):174–80. doi: 10.1007/s002130000532. [DOI] [PubMed] [Google Scholar]
- 42.Jonsson EG, Nothen MM, Grunhage F, Farde L, Nakashima Y, Propping P, Sedvall GC. Polymorphisms in the dopamine D2 receptor gene and their relationships to striatal dopamine receptor density of healthy volunteers. Mol Psychiatry. 1999;4(3):290–6. doi: 10.1038/sj.mp.4000532. [DOI] [PubMed] [Google Scholar]
- 43.Lencz T, Robinson DG, Xu K, Ekholm J, Sevy S, Gunduz-Bruce H, Woerner MG, Kane JM, Goldman D, Malhotra AK. DRD2 promoter region variation as a predictor of sustained response to antipsychotic medication in first-episode schizophrenia patients. Am J Psychiatry. 2006;163(3):529–31. doi: 10.1176/appi.ajp.163.3.529. [DOI] [PubMed] [Google Scholar]
- 44.Gardner DM, Baldessarini RJ, Waraich P. Modern antipsychotic drugs: a critical overview. Cmaj. 2005;172(13):1703–11. doi: 10.1503/cmaj.1041064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ghose S, Gleason KA, Potts BW, Lewis-Amezcua K, Tamminga CA. Differential expression of metabotropic glutamate receptors 2 and 3 in schizophrenia: a mechanism for antipsychotic drug action? Am J Psychiatry. 2009;166(7):812–20. doi: 10.1176/appi.ajp.2009.08091445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu S, Xing Q, Gao R, Li X, Gu N, Feng G, He L. Response to chlorpromazine treatment may be associated with polymorphisms of the DRD2 gene in Chinese schizophrenic patients. Neurosci Lett. 2005;376(1):1–4. doi: 10.1016/j.neulet.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 47.Suzuki A, Kondo T, Mihara K, Yasui-Furukori N, Otani K, Furukori H, Kaneko S, Inoue Y. Association between TaqI A dopamine D2 receptor polymorphism and therapeutic response to bromperidol: a preliminary report. Eur Arch Psychiatry Clin Neurosci. 2001;251(2):57–9. doi: 10.1007/s004060170053. [DOI] [PubMed] [Google Scholar]
- 48.Kwon JS, Kim E, Kang DH, Choi JS, Yu KS, Jang IJ, Shin SG, Aplus Study G. Taq1A polymorphism in the dopamine D2 receptor gene as a predictor of clinical response to aripiprazole. Eur Neuropsychopharmacol. 2008;18(12):897–907. doi: 10.1016/j.euroneuro.2008.07.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure 1. Meta-analysis of the association between -141C Ins/Del polymorphism (Del carrier vs. Ins/Ins) and antipsychotic drug response in studies of first-episode schizophrenia patients.
Note: M-H: Mantel-Haenszel method.
Figure 2. Meta-analysis of the association between -141C Ins/Del polymorphism (Del carrier vs. Ins/Ins) and antipsychotic drug response: A random effect model.
Note: M-H: Mantel-Haenszel method.