Abstract
Rough titanium (Ti) surface microarchitecture and high surface energy have been shown to increase osteoblast differentiation, and this response occurs through signaling via the α2β1 integrin. However, clinical success of implanted materials is dependent not only upon osseointegration but also on neovascularization in the peri-implant bone. Here we tested the hypothesis that Ti surface microtopography and energy interact via α2β1 signaling to regulate the expression of angiogenic growth factors. Primary human osteoblasts (HOB), MG63 cells and MG63 cells silenced for α2 integrin were cultured on Ti disks with different surface microtopographies and energies. Secreted levels of vascular endothelial growth factor-A (VEGF-A), basic fibroblast growth factor (FGF-2), epidermal growth factor (EGF), and angiopoietin-1 (Ang-1) were measured. VEGF-A increased 170% and 250% in MG63 cultures, and 178% and 435% in HOB cultures on SLA and modSLA substrates, respectively. In MG63 cultures, FGF-2 levels increased 20 and 40-fold while EGF increased 4 and 6-fold on SLA and modSLA surfaces. These factors were undetectable in HOB cultures. Ang-1 levels were unchanged on all surfaces. Media from modSLA MG63 cultures induced more rapid differentiation of endothelial cells and this effect was inhibited by anti-VEGF-A antibodies. Treatment of MG63 cells with 1α,25(OH)2D3 enhanced levels of VEGF-A on SLA and modSLA. Silencing the α2 integrin subunit increased VEGF-A levels and decreased FGF-2 levels. These results show that Ti surface microtopography and energy modulate secretion of angiogenic growth factors by osteoblasts and that this regulation is mediated at least partially via α2β1 integrin signaling.
Keywords: Titanium, microstructure, surface energy, osteoblast, angiogenesis, VEGF
Introduction
Biomaterial surface properties play a significant role in determining host cellular responses to implant materials used in tissue engineering and regenerative medicine applications. Modifications to surface microarchitecture, chemistry, or energy can alter cell adhesion, proliferation, and gene expression [1-3]. By designing materials to present specific surface properties, there is the potential to control cell responses to achieve the desired application.
Titanium (Ti) is a widely used biomaterial in the orthopaedic and dental industries because of the biocompatibility and resistance to wear of the Ti oxide layer that forms on its surface. In vitro studies have shown that modifications to Ti surface microtopography affect the attachment and differentiation of osteoblasts, including MG63 and MC3T3-E1 cell lines, as well as fetal rat calvarial cells and normal human osteoblasts [4]. MG63 cells cultured on Ti surfaces with microrough topographies that resemble osteoclast resorption pits display a more differentiated phenotype than cells grown on smooth Ti substrates, characterized by decreased alkaline phosphatase specific activity and higher levels of osteocalcin [5]. The combination of microstructure and high surface energy further enhances osteoblast differentiation on Ti surfaces [6]. In vivo, microstructured implant surfaces support greater bone-to-implant contact than smooth surfaces do, resulting in greater removal torque strength [7-9].
Osteoblasts interact with their substrate via integrin binding to extracellular matrix proteins [10]. On tissue culture polystyrene (TCPS) surfaces, osteoblasts primarily express α5β1 integrins [11]. However, when grown on Ti substrates, expression of α2 and β1 integrin subunits is increased [12], suggesting that the surface roughness dependent differentiation of osteoblasts may be mediated specifically through α2β1 signaling. Knockdown of either the α2 or β1 integrin subunits in MG63 cells blocks surface roughness dependent differentiation of those cells [13,14], supporting this hypothesis.
The overall success of biomaterial implants in orthopaedic and dental applications however, is not only dependent on achieving the desired cellular response at the host tissue/implant interface but also by the integration of the implant into the surrounding host tissue. Angiogenesis, the sprouting of new capillary blood vessels from the pre-existing vasculature, is a critical process during embryonic development and in several physiological conditions, including the formation of new bone and bone fracture healing [15,16], as well as during bone regeneration and osseointegration of implanted materials [17]. This suggests that materials that support peri-implant bone formation may support angiogenesis as well as osteogenesis.
The formation of blood vessels in vivo is a complex process and involves the coordination of multiple growth factors and events. Among the many identified growth factors that serve to initiate and control angiogenesis are vascular endothelial growth factor-A (VEGF-A) [18], basic fibroblast growth factor (FGF-2) [19], epidermal growth factor (EGF) [20], and angiopoietin-1 (Ang-1) [21]. Both VEGF-A and FGF-2 are two of the growth factors necessary for initiating angiogenesis and both are chemotactic for endothelial cells [22]. VEGF-A is produced by multiple cell types, including osteoblasts [23] and hypertrophic chondrocytes [24], and affects vascular permeability in vivo [25]. The interaction of VEGF with its receptors Flt-1 and Flk-1/KDR is one of the first signal transduction pathways activated during angiogenesis in endothelial cells [26]. FGF-2 is a heparin-binding polypeptide that induces proliferation, migration, and protease production in cultured endothelial cells and promotes neovascularization in vivo [27]. EGF has also been implicated in angiogenesis by stimulating the proliferation of endothelial cells through its interaction with the tyrosine kinase EGF receptor [28]. EGF treatment of prostate cancer cells increases VEGF mRNA expression suggesting that EGF may also exert its effect by stimulating VEGF production [29]. Ang-1, a member of the angiopoietin family of signaling molecules, binds to its cognate receptor tyrosine kinase Tie1 present on the surface of endothelial cells, inducing signaling events that serve to control later stages of blood vessel formation, such as the stabilization of the endothelial sprout and its interaction with pericytes [30].
Recent studies suggest that osteoblasts may play a role in directly stimulating endothelial cells. Osteoblasts produce VEGF-A [31] and FGF-2 [32], and levels of these angiogenic factors are regulated by factors that stimulate osteogenesis in vivo, including 1,25 dihydroxyvitamin D3 [1,25(OH)2D3] [33], 17β-estradiol [34], and bone morphogenetic protein-2 (BMP-2) [35]. Others have noted that neovascularization is increased in peri-implant bone when microstructured Ti implants are used [36].
Mesenchymal stem cells (MSCs) that have been induced to become osteoblasts produce greater levels of angiogenic factors than unstimulated MSCs [37]. This suggests that this is a function of mature secretory cells and those factors that enhance osteoblast differentiation may also enhance their ability to promote angiogenesis. While it has been established that Ti surface properties influence osteoblast maturation and differentiation and enhance osseointegration in vivo, the potential role that surface properties may have in enhancing angiogenesis surrounding the implant surface through the secretion of angiogenic stimulators by osteoblasts has not been investigated.
In this study, we examined the production of the pro-angiogenic growth factors VEGF-A, FGF-2, EGF and Ang-1 by MG63 human osteoblast-like cells and normal human osteoblasts cultured on Ti surfaces with varying microtopographies and surface energies. In addition, we investigated whether surface-dependent production of those factors is sensitive to systemic regulation by treating the cells with 1α,25(OH)2D3. We verified that factors produced by the cells were angiogenic by assessing endothelial cell differentiation in response to the conditioned media from MG63 cell cultures from the different Ti substrates. The specific contribution of VEGF-A was determined by treating the endothelial cell cultures with conditioned media in the presence of a neutralization antibody to VEGF-A. Finally, we examined whether the production of angiogenic growth factors is modulated through specific integrin adhesion receptor signaling by silencing of the α2 integrin subunit.
Materials & Methods
Ti Surfaces
Ti disks were prepared from 1 mm thick sheets of grade 2 unalloyed commercially pure Ti punched into 15mm diameter disks and supplied by Institut Straumann AG (Basel, Switzerland). The production and characterization of smooth pretreatment (PT), sand blasted and acid etched (SLA), and modified SLA (modSLA) surfaces have been described previously [6]. PT surfaces were degreased by washing Ti disks in acetone and processed in a 2% ammonium fluoride/2% hydrofluoric acid/10% nitric acid solution. SLA surfaces were made by coarse grit-blasting of the PT surfaces with 0.25-0.50 mm corundum grit followed by acid etching. modSLA surfaces were made using the same procedure as SLA surfaces under nitrogen rinsing to prevent exposure to air and were then stored under aqueous conditions under nitrogen to retain high surface free energy. The PT surface has an overall average roughness (Ra) of less than 0.7 μm. SLA and modSLA surfaces have a complex microtopography with craters varying from 30 to 100 μm in diameter overlaid with pits from 1 to 3 μm in diameter. The acid etch creates sharp edges approximately 700 nm in height, resulting in an overall Ra of approximately 4 μm. PT, SLA and modSLA Ti disks all have a TiO2 surface layer, with the PT and SLA surfaces being hydrophobic due to the adsorption of atmospheric hydrocarbons while the modSLA surface is hydrophilic. Advancing contact angles were used to calculate the hydrophilicity of the surfaces as PT (95.8°), SLA (139.80°), and modSLA (∼0°). Surface free energy for PT, SLA, and modSLA surfaces was calculated according to Zisman (critical surface tension), Equation of State (EOS), and Geometric Mean approaches and is described in detail elsewhere [38].
Cell Culture
Human MG63 osteoblast like cells were cultured in 24-well tissue culture plates on tissue culture treated polystyrene (TCPS, used as a control for all studies), PT, SLA, and modSLA surfaces using Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin. Cells were seeded at an initial density of 10,000 cells/cm2 and media were exchanged 24 hours after seeding and every 48 hours thereafter. When the cells were confluent on TCPS, media from all cultures were collected and examined for VEGF-A, FGF-2, EGF, Ang-1 and osteocalcin levels. Additionally, conditioned media from MG63 cell cultures were used to assess differentiation of human aortic endothelial cells as described below.
Primary human osteoblasts (HOB cells) were isolated from the mandible of a 50 year old male donor by use of an explant culture. Briefly, isolated bone chips were cleaned of all soft and connective tissues and cut into approximately 1.5 mm fragments. The bone fragments were then cultured in DMEM containing 10% fetal bovine serum and 1% penicillin/streptomycin for two weeks to allow cells to migrate out of the tissue. The migrated osteoblasts were then collected and cultured in DMEM containing 10% FBS and 1% penicillin/streptomycin. Fourth passage human osteoblasts were used for experiments.
To assess the role of α2β1 signaling, we used an MG63 cell line that was stably silenced for α2. This cell line was generated by transfection with α2 integrin shRNA using a P-suppressor-neo vector system and shown to have a 70% reduction in α2 protein.[13] The α2-silenced MG63 cells were maintained in media containing geneticin (G418; Invitrogen, Carlsbad, CA) at a concentration of 600 μg/mL.
Effect of 1,25(OH)2D3
Confluent MG63 cell cultures were treated with 10-8M or 10-9M 1α,25(OH)2D3 or vehicle for 24 hours prior to harvest.
Cell Number
Cell number was determined for all cell types at time of harvest. At confluence or after 24 hour treatment with 1α,25(OH)2D3, cells were released from TCPS and Ti surfaces using two sequential incubations with 0.25% trypsin for 10 minutes at 37°C to ensure that no cells remained on the rough Ti surfaces and counted using an automated cell counter (Z1 Particle counter, Beckman Coulter, Fullerton, CA).
Alkaline Phosphatase Specific Activity
Alkaline phosphatase specific activity (orthophosphoric monoester phosphohydrolase, alkaline; E.C. 3.1.3.1) was measured in the cell lysates as a marker of osteoblastic differentiation. Enzyme activity was determined using a colorimetric assay measuring the release of p-nitrophenol from p-nitrophenylphosphate at 37°C. Samples were read on a plate reader at 415nm [39].
Osteocalcin
Osteocalcin levels in the conditioned medium of MG63 cells and human osteoblasts grown on Ti surfaces were determined as a marker of osteoblast maturation using a commercially available radioimmunoassay (Biomedical Technologies, Inc., Stoughton, MA) following the manufacturer's protocol.
VEGF-A, FGF-2, EGF, Ang-1
The levels of the angiogenic growth factors VEGF-A, FGF-2, EGF, and Ang-1 were determined in the conditioned medium using commercially available sandwich ELISA assays (Duoset ELISA Development Systems, R&D Systems, Minneapolis, MN) following the manufacturer's protocols.
Endothelial Cell Differentiation
To determine if the conditioned media were angiogenic, we examined their ability to support endothelial tube formation. Human aortic endothelial cells (HAEC) were purchased from Lonza (Walkersville, MD) and grown in 75cm2 tissue culture flasks using endothelial cell basal medium (EGM-2, Lonza). At confluence, cells were passaged and used for the endothelial tube formation assay. Endothelial cell differentiation was assessed using two separate assays; a Matrigel tube formation assay (Millipore, St. Charles, MO), and a fibrin gel assay (Millipore).
Matrigel Tube Formation Assay
Briefly, 50 μL ECMatrix™ was added to each well of a 96-well tissue culture plate and allowed to solidify for 1 hour at 37°C. HAECs were then seeded at a density of 1 × 104 cells/well using a mixture of 100μL conditioned medium from MG63 cells cultured on TCPS, PT, SLA, and modSLA surfaces and 50μL EGM-2 for growth maintenance. Cells were cultured for 24 hours, and at 4, 8, 12, and 24 hours after seeding, pictures were taken for morphometric analysis to determine the total endothelial tube length and total number of branch points.
To assess the specific role of VEGF-A, endothelial cell differentiation in the presence of a competitive VEGF-A blocking antibody was determined. Goat anti-human VEGF-A neutralization antibody was purchased from R&D Systems (Minneapolis, MN) and was added to the culture medium at a concentration of 5 μg/mL.
Fibrin Gel Assay
Endothelial cell differentiation was further assessed with the use of a fibrin gel assay. 30 μL of fibrinogen solution and 20 μL of thrombin solution were added to each well of a 96-well plate and the mixture was allowed to polymerize for 30 minutes at 37°C. HAECs were plated in 100 μL of EGM-2 at a density of 5 × 103 cells/well and cultured at 37°C for 24h. At 24h, media were removed and a second layer of fibrin was added on top of cells by again mixing 30 μL of fibrinogen and 20μL of thrombin. The mixture was allowed to polymerize for 5 minutes before 100 μL of conditioned media from MG63 cell cultures on Ti substrates was added. At 12, 24, 36, and 48h after the addition of conditioned media, images were taken for morphometric analysis to determine total endothelial tube length.
Statistical Analysis
The data presented here are from one of two separate sets of experiments. Both sets of experiments yielded comparable observations. For any given experiment, each data point represents the mean ± standard error of six individual cultures. Data were analyzed by ANOVA and when statistical differences were detected, Student's t-test for multiple comparisons using Bonferroni's modification was used. p-values < 0.05 were considered significant.
Results
MG63 and HOB Cell Response
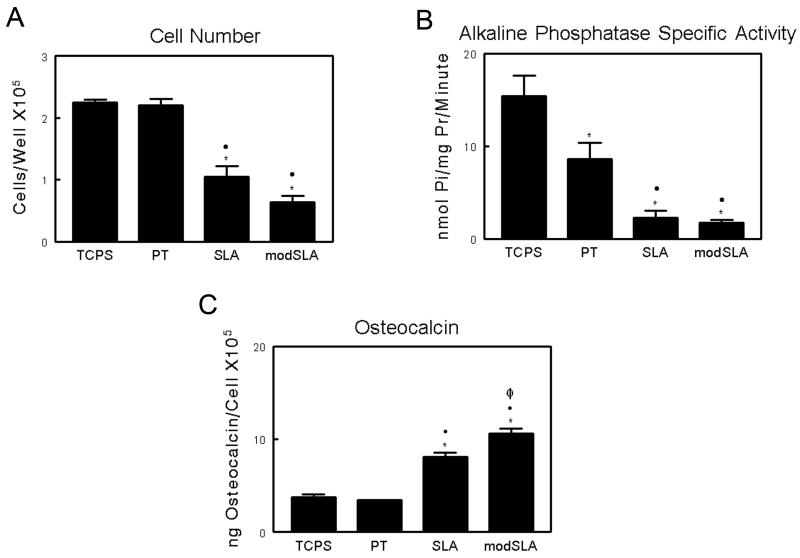
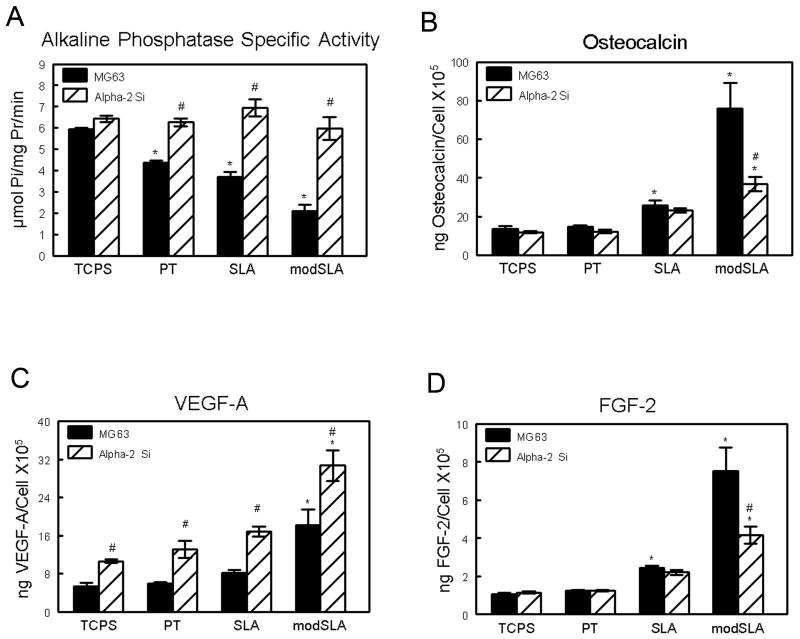
As noted previously [6], MG63 cell differentiation was increased on the SLA and modSLA substrates compared to cells on TCPS and PT, validating the model. Total cell number was comparable on TCPS and smooth PT surfaces, whereas cells cultured on microrough SLA Ti surfaces demonstrated a significant decrease (p<0.05) in cell number when compared to TCPS and smooth PT Ti surfaces (Figure 1A). The addition of high surface energy on modSLA Ti surfaces did not result in any further decrease in overall cell number. MG63 cells exhibited a more differentiated phenotype when grown on Ti substrates compared to TCPS. Alkaline phosphatase specific activity on all three Ti substrates was significantly reduced (Figure 1B). Osteocalcin, a late marker of osteoblast differentiation was found to be similar on TCPS and smooth PT substrates, and was increased 212% in MG63 cell cultures on SLA (Figure 1C). The combination of high surface energy and microstructure on modSLA surfaces resulted in a further increase in osteocalcin levels compared to SLA.
Figure 1.
Response of MG63 cells cultured on tissue culture polystyrene and Ti surfaces: (A) cell number, (B) alkaline phosphatase specific activity in the cell lysate, and (C) osteocalcin levels in the conditioned medium. Cells were cultured on control (TCPS), PT, SLA, and modSLA Ti surfaces. Values presented are mean ± SEM of six independent cultures. The data presented are from one of two separate experiments with comparable results. Data were analyzed using ANOVA and statistical significance between groups was determined using Bonferroni's modification of Student's t-test. *p< 0.05 vs. TCPS; ·p<0.05 vs. PT; Φp<0.05 vs. SLA.
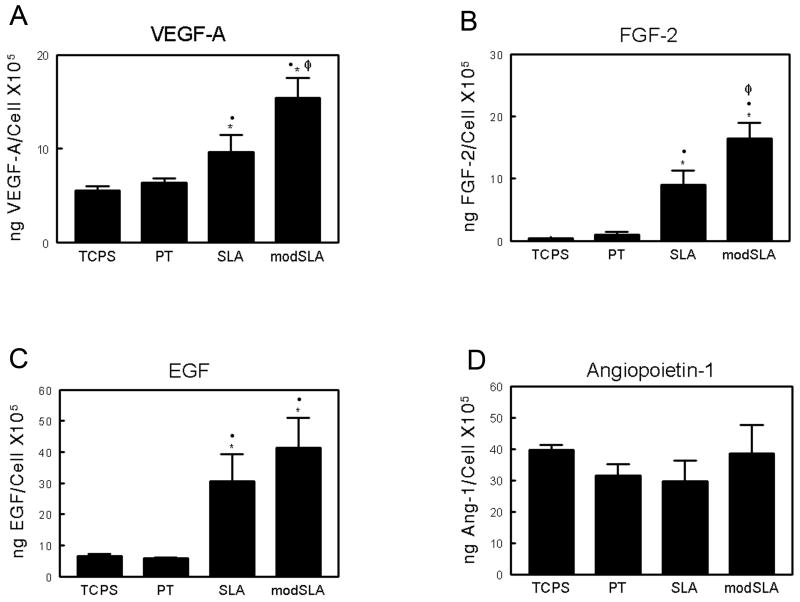
Secretion of angiogenic factors by MG63 cells was differentially regulated as a function of substrate morphology and surface energy. Secreted levels of VEGF-A on TCPS and PT substrates were comparable (Figure 2A). Levels of secreted VEGF-A increased nearly 2-fold on SLA while MG63 cell cultures produced almost 3-fold higher levels of VEGF-A on modSLA. Secretion of FGF-2 and EGF exhibited similar substrate-dependent effects. FGF-2 levels were similar on both TCPS and PT substrates; secreted levels observed on SLA were approximately 10-fold higher than TCPS levels; and the combination of a hydrophilic surface with the rough surface microstructure observed on modSLA resulted in a further enhancement of FGF-2 production (Figure 2B). EGF production on SLA and modSLA was significantly higher than on either TCPS or PT (Figure 2C). In contrast to secreted levels of VEGF-A, FGF-2, and EGF, the levels of Ang-1 were comparable on all surfaces examined (Figure 2D).
Figure 2.
Production of angiogenic growth factors by MG63 cells on tissue culture polystyrene and Ti surfaces: (A) VEGF-A, (B) FGF-2, (C) EGF, and (D) Ang-1 levels were determined in the conditioned medium by ELISA. Cells were cultured on control (TCPS), PT, SLA, and modSLA Ti surfaces. Values presented are mean ± SEM of six independent cultures. The data presented are from one of two separate experiments with comparable results. Data were analyzed using ANOVA and statistical significance between groups was determined using Bonferroni's modification of Student's t-test. *p< 0.05, vs. TCPS; ·p<0.05, vs. PT; Φp<0.05, vs. SLA.
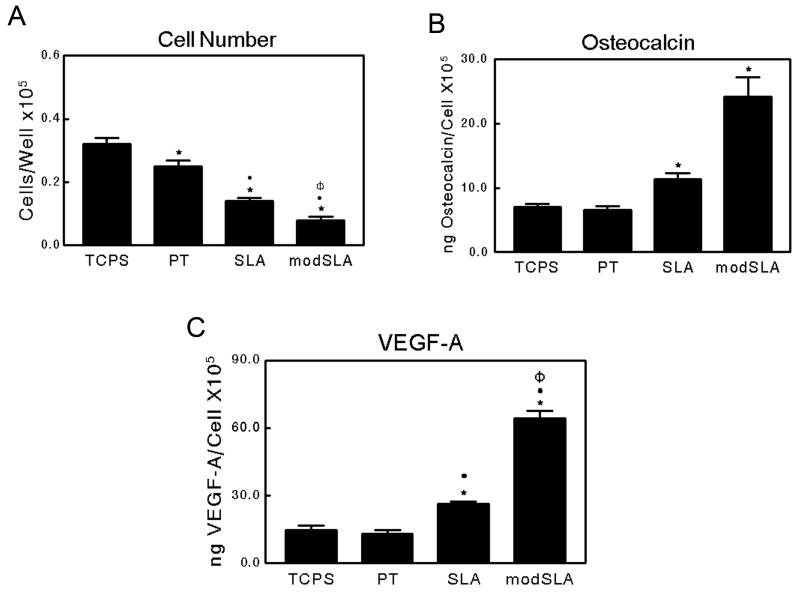
HOB cells cultured on TCPS as well as on all three Ti surfaces exhibited similar responses to those observed in MG63 cell cultures. Total cell number on TCPS was significantly higher than that observed on all three Ti substrates (Figure 3A). Further, a rough microstructure reduced total cell number compared to smooth pre-treated Ti surfaces. High surface energy further reduced total cell number on modSLA surfaces. Osteocalcin production by HOB cells on TCPS and PT substrates was comparable, while osteocalcin levels increased approximately 1.6 fold and 3.5 fold on SLA and modSLA surfaces, respectively (Figure 3B). Similarly, VEGF-A levels were comparable on TCPS and PT. Secreted VEGF-A levels were doubled on SLA substrates, while cultures on modSLA surfaces showed increased levels over SLA (Figure 3C). Levels of FGF-2 and EGF in the conditioned media of cells grown on all substrates were undetectable by ELISA (data not shown).
Figure 3.
Characterization of normal human osteoblasts cultured on TCPS, PT, SLA, and modSLA Ti surfaces: (A) cell number, (B) osteocalcin, and (C) VEGF-A levels in the conditioned medium. Values presented are mean ± SEM of six independent cultures. The data presented are from one of two separate experiments with comparable results. Data were analyzed using ANOVA and statistical significance between groups was determined using Bonferroni's modification of Student's t-test. *p< 0.05 vs. TCPS; ·p<0.05 vs. PT; Φp<0.05 vs. SLA.
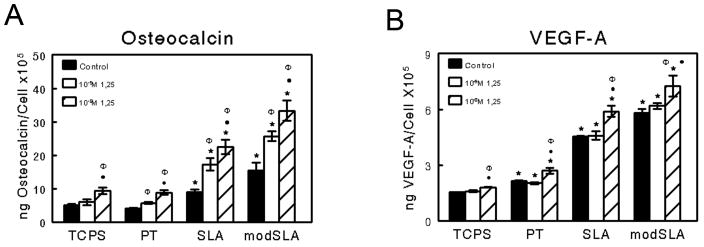
Treatment of MG63 cells with the vitamin D metabolite 1α,25(OH)2D3 caused a dose dependent increase in both osteocalcin (Figure 4A) and VEGF-A (Figure 4B) on all surfaces in addition to the increase observed in response to Ti surface microstructure. The effects of surface roughness and 1α,25(OH)2D3 were synergistic for both osteocalcin and VEGF-A. No effect of 1α,25(OH)2D3 on FGF-2 and EGF was observed regardless of substrate (data not shown).
Figure 4.
Effect of 1α,25 (OH)2D3 treatment on MG63 cell differentiation. MG63 cells were cultured on TCPS, PT, SLA, and modSLA Ti surfaces. At confluence, cultures were treated with 10-9M or 10-8M 1α,25 (OH)2D3 for 24 hours. (A) Osteocalcin and (B) VEGF-A levels in the conditioned medium were determined. Values are mean ± SEM of six independent cultures. The data presented are from one of two experiments with comparable results. Data were analyzed using ANOVA and statistical significance between groups was determined using Bonferroni's modification of Student's t-test. *p< 0.05 vs. TCPS; ·p<0.05 vs. PT; Φp<0.05 vs. SLA.
Endothelial Cell Differentiation
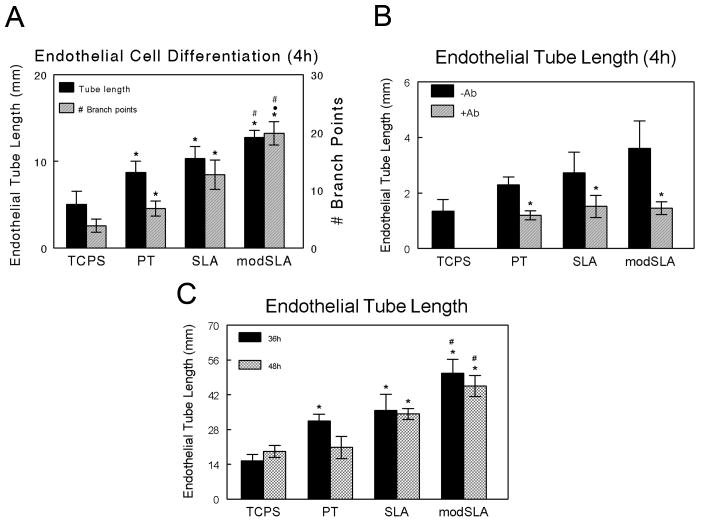
Conditioned media from MG63 cell cultures affected endothelial tube formation in a Matrigel® tube formation assay in a substrate dependent manner. Conditioned media from all three Ti substrates caused a greater degree of endothelial cell differentiation than conditioned media from cells grown on TCPS based on total endothelial tube length (Figure 6A). Moreover, surface energy had a further stimulating effect. Using the number of branch points (the number of points where endothelial networks split into two or more tube like structures) as another marker of endothelial cell differentiation, we found that at 4h of culture, the number of branch points induced by conditioned media from all Ti substrates was significantly higher than those observed in the presence of TCPS media. Media from cultures grown on SLA increased the number of endothelial branch points observed versus media from PT substrates (SLA vs. PT), and media from cultures on the high surface energy substrate caused a further increase in endothelial branch points (modSLA vs. SLA).
Figure 6.
Endothelial cell differentiation. Endothelial tube formation was assessed using a Matrigel® tube formation assay with conditioned medium from MG63 cells cultured on TCPS, PT, SLA, and modSLA surfaces. (A) Total endothelial tube length and total number of branch points at 4h. Values are mean ± SEM of six independent cultures. Data were analyzed using ANOVA and statistical significance between groups was determined using Bonferroni's modification of Student's t-test. *p< 0.05 vs. TCPS; ·p<0.05 vs. PT; #p<0.05 vs. SLA. (B) Addition of VEGF-A neutralization antibody inhibits endothelial cell differentiation in response to MG63 conditioned media. Endothelial tube formation was assessed using conditioned medium from MG63 cells cultured on TCPS, PT, SLA, and modSLA surfaces in the presence of a VEGF-A neutralization antibody. Total endothelial tube length is presented for cultures with and without the addition of neutralization antibody. *p<0.05 vs. no antibody. (C) Endothelial cell differentiation was further assessed using a fibrin gel assay to determine total endothelial tube length within a fibrin gel matrix. *p< 0.05 vs. TCPS.
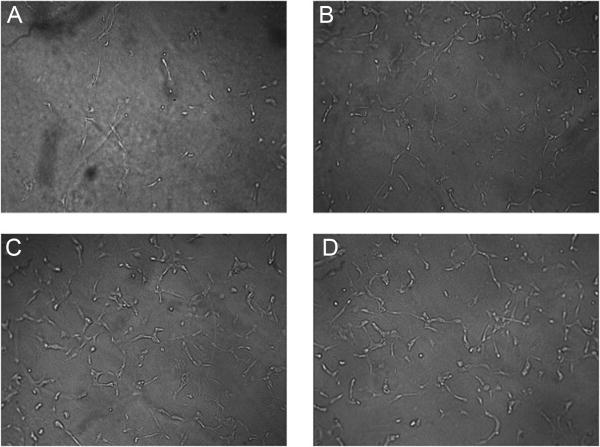
The increase in endothelial cell differentiation seen at earlier time points in response to conditioned media from cultures grown on SLA and modSLA surfaces was due in part to an increase in the levels of VEGF-A. Addition of a VEGF-A neutralization antibody to conditioned media from these cultures reduced endothelial cell differentiation to levels observed using conditioned media from TCPS cultures in the absence of antibody (Figure 6B). Results from the fibrin gel assay further demonstrate that there were increased levels of pro-angiogenic growth factors in the conditioned media of MG63 cell cultures on Ti substrates. Similar to the Matrigel® assay, endothelial cells cultured in media from all three Ti substrates exhibited a greater degree of differentiation as determined by tubular network length after 36h of cultured within a fibrin gel network (Figure 5 and Figure 6C). There were no significant differences in total tube length between the different Ti substrates however.
Figure 5.
Endothelial cell differentiation. Representative images of endothelial cells cultured on a fibrin gel matrix with conditioned media from (A) TCPS, (B) PT, (C) SLA, and (D) modSLA cultures of MG63 cells.
Integrin α2 Knockdown
VEGF-A and FGF-2 levels in the conditioned media were regulated by α2β1 signaling. When grown on Ti substrates, the α2 silenced cells exhibited alkaline phosphatase specific activity comparable to levels seen in MG63 cells grown on TCPS (Figure 7A). However, α2-silencing had no effect on enzyme activity in TCPS cultures. Similarly, α2-silencing blocked the surface dependent increases in osteocalcin production but had no effect on osteocalcin in cultures grown on TCPS (Figure 7B). VEGF-A levels were increased in the α2 silenced cells by 100% on all surfaces, including TCPS (Figure 7C). α2 silencing had no effect on FGF-2 produced in response to microstructure, but it reduced FGF-2 levels to those seen in cultures of wild type MG63 on modSLA (Figure 7D).
Figure 7.
Integrin α2 signaling regulates pro-angiogenic growth factor production. Stably silenced α2 cells and MG63 cells were cultured on TCPS, PT, SLA, and modSLA substrates. Total cell number (A), osteocalcin (B), VEGF (C), and FGF-2 (D) levels were determined in the conditioned media. Values are mean ± SEM of six independent cultures. Data were analyzed using ANOVA and Student's t-test. *p<0.05 vs. MG63 wild-type on TCPS; #p<0.05 vs. MG63 wild-type.
Discussion
Angiogenesis is an essential process for the clinically successful integration of orthopaedic and dental implants. Here we demonstrate that the surface properties of biomaterials affect cellular response with regard to the production of pro-angiogenic growth factors and show that these factors stimulate endothelial cell differentiation. These observations suggest that microstructured, high energy surfaces induce angiogenesis during osseointegration. Moreover, we show that α2β1 signaling plays an important role in mediating the effects of the surface properties on production of these local mediators of angiogenesis.
Our results show that in addition to producing increased levels of VEGF-A on SLA and modSLA [40], MG63 cells also produce increased FGF-2 levels on these substrates. Part of the increase is due to microstructure and part is due to surface energy. MG63 cells produce elevated levels of EGF as well, but this effect is due primarily to substrate microstructure since no further increase was seen on modSLA. Not all angiogenic factors are regulated in this manner, however, either as a function of surface chemistry or surface microarchitecture. Levels of angiopoeitin-1 were comparable in the conditioned media of all cultures examined.
The human osteoblasts used in the present study exhibited marked increases in VEGF-A when grown on SLA and further increases when grown on modSLA, but unlike the MG63 cells they did not exhibit substrate dependent changes in FGF-2 or EGF. We examined cells from only a single human donor. Numerous studies demonstrate the variability among donors as a function of sex, age, and donor site [41,42]. In addition, it is likely that failure to observe such changes was because these factors were below the limits of detection of the assay kits we used. Our results support the hypothesis that production of angiogenic factors is related to the state of osteogenic maturation of the osteoblasts. MG63 cells and normal human osteoblasts exhibited a more differentiated phenotype on SLA and modSLA than on TCPS and PT, with reduced alkaline phosphatase and increased osteocalcin typical of secretory cells. These cells also exhibited increased production of angiogenic factors, suggesting that this is linked to the acquisition of a more differentiated phenotype.
The systemic osteotropic hormone 1α,25(OH)2D3 is a key regulator of bone metabolism [33]. Treatment of late-stage osteoblast cultures with 1α,25(OH)2D3 upregulates expression of both alkaline phosphatase and type I collagen, markers of osteoblast differentiation [43]. Previous results from our laboratory have shown that treatment of MG63 cells cultures with 1α,25(OH)2D3 enhances cell response to Ti surface microstructure [44]. Our results here support this data and further show that production of VEGF-A is regulated in the same manner.
Substrates that presented a microrough surface topography and high surface energy enhanced the synthesis of several pro-angiogenic growth factors, which subsequently resulted in an enhancement of the differentiation of human aortic endothelial cells in vitro. This supports the hypothesis that more mature osteoblasts produce the angiogenic factors needed to recruit a vascular supply. The addition of a VEGF-A neutralization antibody to endothelial cell cultures inhibited this increase in endothelial cell differentiation, indicating that the increased levels of VEGF-A produced by MG63 cells on SLA and modSLA substrates contributes to endothelial cell differentiation.
The mechanism by which Ti surface microstructure and energy regulate the production of pro-angiogenic growth factors by osteoblasts is unclear. We have previously shown that osteoblasts cultured on SLA and modSLA substrates increase expression of the adhesion receptor α2 integrin subunit [12,13]. These cells have a more differentiated phenotype displaying increased levels of osteocalcin and the local factor prostaglandin E2. Knockdown of the α2 integrin subunit in these cells resulted in the loss of differentiation in response to Ti surface microstructure. We found here that knockdown of the α2 subunit differentially regulated the production of VEGF-A and FGF-2, with the secreted levels of VEGF-A increasing in knockdown cells and levels of FGF-2 decreasing, suggesting that signaling events through this adhesion receptor may regulate the production of pro-angiogenic growth factors to induce endothelial cell migration and subsequent capillary formation from the surrounding vasculature during healing. Further examination of the effect of conditioned media from knockdown cell cultures is necessary to determine if the increase in VEGF expression influences endothelial cell differentiation. Also, it is possible that signaling events through other integrin adhesion receptors may modulate the production of angiogenic growth factors by osteoblasts. In addition to the α2β1 integrin, osteoblasts express several other integrin subunits including α3, α4, α5, α6, αv, and β3 [13,45].
In a clinical setting, establishment of a vasculature preceding or concomitant with bone formation allows for not only the delivery of oxygen, systemic hormones, and nutrients to the injury site but also the migration of mesenchymal stem cells. In the absence of neovascularization, the implant may be surrounded by a fibrous capsule, resulting in implant loosening and ultimately failure, demonstrating the importance of the initial reaction of the first cells to come in contact with an implanted material.
Conclusions
We show here that substrate microstructure and surface energy regulate the production of angiogenic growth factors by osteoblasts and that this regulation occurs at least partially through signaling of the α2 integrin subunit. The increase in endothelial cell differentiation observed in response to conditioned media from SLA and modSLA cultures further demonstrate that Ti substrate features control osseointegration by enhancing angiogenesis at the material/tissue interface.
Acknowledgments
The authors thank Institut Straumann AG (Basel, Switzerland) for their generous gift of Ti culture disks for this study. This research was supported by grants from the NIH, the ITI Foundation (Basel, Switzerland), and Children's Healthcare of Atlanta (Atlanta, GA).
Funding Sources: NIH US PHS AR052102, Cell and Tissue Engineering (CTEng) NIH Biotechnology Training Grant (TG GM08433), ITI Foundation, Children's Healthcare of Atlanta
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Andrew L. Raines, Email: andrew.raines@bme.gatech.edu.
Rene Olivares-Navarrete, Email: rene.olivares-navarrete@bme.gatech.edu.
Marco Wieland, Email: wielandmar@yahoo.com.
David L. Cochran, Email: Cochran@uthscsa.edu.
Zvi Schwartz, Email: zvi.schwartz@bme.gatech.edu.
References
- 1.Hench LL, Polak JM. Third-generation biomedical materials. Science. 2002;295:1014–7. doi: 10.1126/science.1067404. [DOI] [PubMed] [Google Scholar]
- 2.Jell G, Stevens MM. Gene activation by bioactive glasses. J Mater Sci Mater Med. 2006;17:997–1002. doi: 10.1007/s10856-006-0435-9. [DOI] [PubMed] [Google Scholar]
- 3.Kriparamanan R, Aswath P, Zhou A, Tang L, Nguyen KT. Nanotopography: cellular responses to nanostructured materials. J Nanosci Nanotechnol. 2006;6:1905–19. doi: 10.1166/jnn.2006.330. [DOI] [PubMed] [Google Scholar]
- 4.Goto T, Yoshinari M, Kobayashi S, Tanaka T. The initial attachment and subsequent behavior of osteoblastic cells and oral epithelial cells on titanium. Biomed Mater Eng. 2004;14:537–44. [PubMed] [Google Scholar]
- 5.Boyan BD, Schwartz Z, Lohmann CH, Sylvia VL, Cochran DL, Dean DD, Puzas JE. Pretreatment of bone with osteoclasts affects phenotypic expression of osteoblast-like cells. J Orthop Res. 2003;21:638–47. doi: 10.1016/S0736-0266(02)00261-9. [DOI] [PubMed] [Google Scholar]
- 6.Zhao G, Schwartz Z, Wieland M, Rupp F, Geis-Gerstorfer J, Cochran DL, Boyan BD. High surface energy enhances cell response to titanium substrate microstructure. J Biomed Mater Res A. 2005;74:49–58. doi: 10.1002/jbm.a.30320. [DOI] [PubMed] [Google Scholar]
- 7.Buser D, Schenk RK, Steinemann S, Fiorellini JP, Fox CH, Stich H. Influence of surface characteristics on bone integration of titanium implants. A histomorphometric study in miniature pigs. J Biomed Mater Res. 1991;25:889–902. doi: 10.1002/jbm.820250708. [DOI] [PubMed] [Google Scholar]
- 8.Gotfredsen K, Wennerberg A, Johansson C, Skovgaard LT, Hjorting-Hansen E. Anchorage of TiO2-blasted, HA-coated, and machined implants: an experimental study with rabbits. J Biomed Mater Res. 1995;29:1223–31. doi: 10.1002/jbm.820291009. [DOI] [PubMed] [Google Scholar]
- 9.Cochran DL, Schenk RK, Lussi A, Higginbottom FL, Buser D. Bone response to unloaded and loaded titanium implants with a sandblasted and acid-etched surface: a histometric study in the canine mandible. J Biomed Mater Res. 1998;40:1–11. doi: 10.1002/(sici)1097-4636(199804)40:1<1::aid-jbm1>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 10.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–87. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 11.Gronowicz G, McCarthy MB. Response of human osteoblasts to implant materials: integrin-mediated adhesion. J Orthop Res. 1996;14:878–87. doi: 10.1002/jor.1100140606. [DOI] [PubMed] [Google Scholar]
- 12.Raz P, Lohmann CH, Turner J, Wang L, Poythress N, Blanchard C, Boyan BD, Schwartz Z. 1alpha,25(OH)2D3 regulation of integrin expression is substrate dependent. J Biomed Mater Res A. 2004;71:217–25. doi: 10.1002/jbm.a.30134. [DOI] [PubMed] [Google Scholar]
- 13.Olivares-Navarrete R, Raz P, Zhao G, Chen J, Wieland M, Cochran DL, Chaudhri RA, Ornoy A, Boyan BD, Schwartz Z. Integrin alpha2beta1 plays a critical role in osteoblast response to micron-scale surface structure and surface energy of titanium substrates. Proc Natl Acad Sci U S A. 2008;105:15767–72. doi: 10.1073/pnas.0805420105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang L, Zhao G, Olivares-Navarrete R, Bell BF, Wieland M, Cochran DL, Schwartz Z, Boyan BD. Integrin beta1 silencing in osteoblasts alters substrate-dependent responses to 1,25-dihydroxy vitamin D3. Biomaterials. 2006;27:3716–25. doi: 10.1016/j.biomaterials.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 15.Duvall CL, Taylor WR, Weiss D, Wojtowicz AM, Guldberg RE. Impaired angiogenesis, early callus formation, and late stage remodeling in fracture healing of osteopontin-deficient mice. J Bone Miner Res. 2007;22:286–97. doi: 10.1359/jbmr.061103. [DOI] [PubMed] [Google Scholar]
- 16.Geris L, Gerisch A, Sloten JV, Weiner R, Oosterwyck HV. Angiogenesis in bone fracture healing: a bioregulatory model. J Theor Biol. 2008;251:137–58. doi: 10.1016/j.jtbi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 17.Abshagen K, Schrodi I, Gerber T, Vollmar B. In vivo analysis of biocompatibility and vascularization of the synthetic bone grafting substitute NanoBone(R) J Biomed Mater Res A. 2008 doi: 10.1002/jbm.a.32237. [DOI] [PubMed] [Google Scholar]
- 18.Connolly DT, Heuvelman DM, Nelson R, Olander JV, Eppley BL, Delfino JJ, Siegel NR, Leimgruber RM, Feder J. Tumor vascular permeability factor stimulates endothelial cell growth and angiogenesis. J Clin Invest. 1989;84:1470–8. doi: 10.1172/JCI114322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schweigerer L, Neufeld G, Friedman J, Abraham JA, Fiddes JC, Gospodarowicz D. Capillary endothelial cells express basic fibroblast growth factor, a mitogen that promotes their own growth. Nature. 1987;325:257–9. doi: 10.1038/325257a0. [DOI] [PubMed] [Google Scholar]
- 20.Mehta VB, Besner GE. HB-EGF promotes angiogenesis in endothelial cells via PI3-kinase and MAPK signaling pathways. Growth Factors. 2007;25:253–63. doi: 10.1080/08977190701773070. [DOI] [PubMed] [Google Scholar]
- 21.Koblizek TI, Weiss C, Yancopoulos GD, Deutsch U, Risau W. Angiopoietin-1 induces sprouting angiogenesis in vitro. Curr Biol. 1998;8:529–32. doi: 10.1016/s0960-9822(98)70205-2. [DOI] [PubMed] [Google Scholar]
- 22.Deckers MM, Karperien M, van der Bent C, Yamashita T, Papapoulos SE, Lowik CW. Expression of vascular endothelial growth factors and their receptors during osteoblast differentiation. Endocrinology. 2000;141:1667–74. doi: 10.1210/endo.141.5.7458. [DOI] [PubMed] [Google Scholar]
- 23.Clarkin CE, Emery RJ, Pitsillides AA, Wheeler-Jones CP. Evaluation of VEGF-mediated signaling in primary human cells reveals a paracrine action for VEGF in osteoblast-mediated crosstalk to endothelial cells. J Cell Physiol. 2008;214:537–44. doi: 10.1002/jcp.21234. [DOI] [PubMed] [Google Scholar]
- 24.Carlevaro MF, Cermelli S, Cancedda R, Descalzi Cancedda F. Vascular endothelial growth factor (VEGF) in cartilage neovascularization and chondrocyte differentiation: auto-paracrine role during endochondral bone formation. J Cell Sci. 2000;113:59–69. doi: 10.1242/jcs.113.1.59. [DOI] [PubMed] [Google Scholar]
- 25.Collins PD, Connolly DT, Williams TJ. Characterization of the increase in vascular permeability induced by vascular permeability factor in vivo. Br J Pharmacol. 1993;109:195–9. doi: 10.1111/j.1476-5381.1993.tb13553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stoelcker B, Echtenacher B, Weich HA, Sztajer H, Hicklin DJ, Mannel DN. VEGF/Flk-1 interaction, a requirement for malignant ascites recurrence. J Interferon Cytokine Res. 2000;20:511–7. doi: 10.1089/10799900050023933. [DOI] [PubMed] [Google Scholar]
- 27.Baffour R, Berman J, Garb JL, Rhee SW, Kaufman J, Friedmann P. Enhanced angiogenesis and growth of collaterals by in vivo administration of recombinant basic fibroblast growth factor in a rabbit model of acute lower limb ischemia: dose-response effect of basic fibroblast growth factor. J Vasc Surg. 1992;16:181–91. [PubMed] [Google Scholar]
- 28.Wakui S. Epidermal growth factor receptor at endothelial cell and pericyte interdigitation in human granulation tissue. Microvasc Res. 1992;44:255–62. doi: 10.1016/0026-2862(92)90085-4. [DOI] [PubMed] [Google Scholar]
- 29.Ravindranath N, Wion D, Brachet P, Djakiew D. Epidermal growth factor modulates the expression of vascular endothelial growth factor in the human prostate. J Androl. 2001;22:432–43. [PubMed] [Google Scholar]
- 30.Cai J, Kehoe O, Smith GM, Hykin P, Boulton ME. The angiopoietin/Tie-2 system regulates pericyte survival and recruitment in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2008;49:2163–71. doi: 10.1167/iovs.07-1206. [DOI] [PubMed] [Google Scholar]
- 31.Wang DS, Miura M, Demura H, Sato K. Anabolic effects of 1,25-dihydroxyvitamin D3 on osteoblasts are enhanced by vascular endothelial growth factor produced by osteoblasts and by growth factors produced by endothelial cells. Endocrinology. 1997;138:2953–62. doi: 10.1210/endo.138.7.5275. [DOI] [PubMed] [Google Scholar]
- 32.Hurley MM, Abreu C, Gronowicz G, Kawaguchi H, Lorenzo J. Expression and regulation of basic fibroblast growth factor mRNA levels in mouse osteoblastic MC3T3-E1 cells. J Biol Chem. 1994;269:9392–6. [PubMed] [Google Scholar]
- 33.St-Arnaud R. The direct role of vitamin D on bone homeostasis. Arch Biochem Biophys. 2008;473:225–30. doi: 10.1016/j.abb.2008.03.038. [DOI] [PubMed] [Google Scholar]
- 34.McMillan J, Kinney RC, Ranly DM, Fatehi-Sedeh S, Schwartz Z, Boyan BD. Osteoinductivity of demineralized bone matrix in immunocompromised mice and rats is decreased by ovariectomy and restored by estrogen replacement. Bone. 2007;40:111–21. doi: 10.1016/j.bone.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 35.Cole BJ, Bostrom MP, Pritchard TL, Sumner DR, Tomin E, Lane JM, Weiland AJ. Use of bone morphogenetic protein 2 on ectopic porous coated implants in the rat. Clin Orthop Relat Res. 1997:219–28. [PubMed] [Google Scholar]
- 36.Schwartz Z, Raz P, Zhao G, Barak Y, Tauber M, Yao H, Boyan BD. Effect of micrometer-scale roughness of the surface of Ti6Al4V pedicle screws in vitro and in vivo. J Bone Joint Surg Am. 2008;90:2485–98. doi: 10.2106/JBJS.G.00499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mayer H, Bertram H, Lindenmaier W, Korff T, Weber H, Weich H. Vascular endothelial growth factor (VEGF-A) expression in human mesenchymal stem cells: autocrine and paracrine role on osteoblastic and endothelial differentiation. J Cell Biochem. 2005;95:827–39. doi: 10.1002/jcb.20462. [DOI] [PubMed] [Google Scholar]
- 38.Rupp F, Scheideler L, Olshanska N, de Wild M, Wieland M, Geis-Gerstorfer J. Enhancing surface free energy and hydrophilicity through chemical modification of microstructured titanium implant surfaces. J Biomed Mater Res A. 2006;76:323–34. doi: 10.1002/jbm.a.30518. [DOI] [PubMed] [Google Scholar]
- 39.Martin JY, Schwartz Z, Hummert TW, Schraub DM, Simpson J, Lankford J, Jr, Dean DD, Cochran DL, Boyan BD. Effect of titanium surface roughness on proliferation, differentiation, and protein synthesis of human osteoblast-like cells (MG63) J Biomed Mater Res. 1995;29:389–401. doi: 10.1002/jbm.820290314. [DOI] [PubMed] [Google Scholar]
- 40.Rausch-fan X, Qu Z, Wieland M, Matejka M, Schedle A. Differentiation and cytokine synthesis of human alveolar osteoblasts compared to osteoblast-like cells (MG63) in response to titanium surfaces. Dent Mater. 2008;24:102–10. doi: 10.1016/j.dental.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 41.Siddappa R, Licht R, van Blitterswijk C, de Boer J. Donor variation and loss of multipotency during in vitro expansion of human mesenchymal stem cells for bone tissue engineering. J Orthop Res. 2007;25:1029–41. doi: 10.1002/jor.20402. [DOI] [PubMed] [Google Scholar]
- 42.Katopodi T, Tew SR, Clegg PD, Hardingham TE. The influence of donor and hypoxic conditions on the assembly of cartilage matrix by osteoarthritic human articular chondrocytes on Hyalograft matrices. Biomaterials. 2009;30:535–40. doi: 10.1016/j.biomaterials.2008.09.064. [DOI] [PubMed] [Google Scholar]
- 43.Owen TA, Aronow MS, Barone LM, Bettencourt B, Stein GS, Lian JB. Pleiotropic effects of vitamin D on osteoblast gene expression are related to the proliferative and differentiated state of the bone cell phenotype: dependency upon basal levels of gene expression, duration of exposure, and bone matrix competency in normal rat osteoblast cultures. Endocrinology. 1991;128:1496–504. doi: 10.1210/endo-128-3-1496. [DOI] [PubMed] [Google Scholar]
- 44.Boyan BD, Lossdorfer S, Wang L, Zhao G, Lohmann CH, Cochran DL, Schwartz Z. Osteoblasts generate an osteogenic microenvironment when grown on surfaces with rough microtopographies. Eur Cell Mater. 2003;6:22–7. doi: 10.22203/ecm.v006a03. [DOI] [PubMed] [Google Scholar]
- 45.Siebers MC, ter Brugge PJ, Walboomers XF, Jansen JA. Integrins as linker proteins between osteoblasts and bone replacing materials. A critical review. Biomaterials. 2005;26:137–46. doi: 10.1016/j.biomaterials.2004.02.021. [DOI] [PubMed] [Google Scholar]