Abstract
The retinas of teleost fish have long been of interest to developmental neurobiologists for their persistent plasticity during growth, life history changes, and response to injury. Because the vertebrate retina is a highly conserved tissue, the study of persistent plasticity in teleosts has provided insights into mechanisms for postembryonic retinal neurogenesis in mammals. In addition, in the past 10 years there has been an explosion in the use of teleost fish—zebrafish (Danio rerio) in particular—to understand the mechanisms of embryonic retinal neurogenesis in a model vertebrate with genetic resources. This review summarizes the key features of teleost retinal neurogenesis that make it a productive and interesting experimental system, and focuses on the contributions to our knowledge of retinal neurogenesis that uniquely required or significantly benefited from the use of a fish model system.
Keywords: Teleost, Retina, Neurogenesis, Regeneration, Zebrafish, Development
I. Introduction: The Fish Retina as a Model System for Retinal Neurogenesis
The multilayered vertebrate retina, like other parts of the vertebrate central nervous system (CNS), develops from a relatively homogeneous, single-layered sheet of neuroepithelial cells. The process of vertebrate retinal neurogenesis has served as a model for understanding CNS neurogenesis in general and has provided important insights into the basis for some human visual disorders, as well as directions to pursue for regenerative therapies. Several animal model systems have been important in the historical and recent advances in the field of retinal neurogenesis, including those of mouse, chick, and Xenopus. The retinas of fish—particularly, though not exclusively, those of zebrafish (Danio rerio)—have offered a unique set of insights into vertebrate retinal neurogenesis in part due to the distinctive features and advantages of the teleost fish retina. These distinctive features will be briefly summarized in this introductory section, and will serve as the detailed focus of this review.
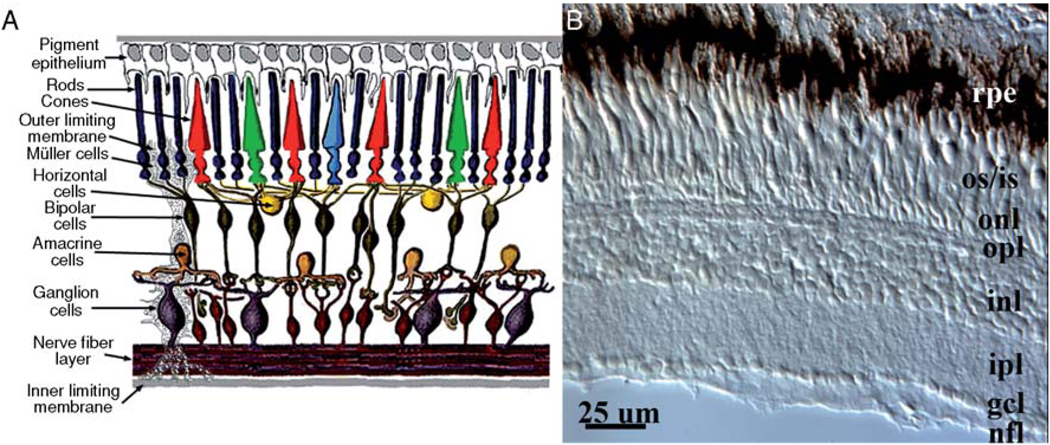
The retina is a highly conserved structure in vertebrates, having almost identical anatomical and physiological characteristics in multiple taxa. All vertebrates have a three-layered retina consisting of a photoreceptor layer containing rod and cone photoreceptors, an inner nuclear layer containing the cell bodies of processing neurons, and a ganglion cell layer containing the cell bodies of ganglion cells, the projection neurons of the retina (Fig. 1). Rod photoreceptors in all vertebrates are exquisitely light sensitive but do not discriminate spectral information, while the cone photoreceptors are less sensitive and in most vertebrates come in several types with distinct spectral sensitivities. The vertebrate retina processes information primarily to detect and enhance luminance and color contrast, and the output of the retina projects to a number of conserved visual relay and processing centers. This degree of evolutionary conservation supports the use of the teleost fish retina as a model to understand retinal biology.
FIG. 1. Histology of the vertebrate retina.
(A) Diagram illustrating the cell types of the vertebrate retina. (Reproduced with permission from WebVision http://webvision.med.utah.edu/.) (B) Radial cryosection of zebrafish retina, showing retinal pigmented epithelium (rpe), outer and inner segments of photoreceptors (os/is), outer nuclear layer (onl) containing photoreceptor nuclei, outer plexiform layer (opl), inner nuclear layer (inl), inner plexiform layer (ipl), ganglion cell layer (gcl), and nerve fiber layer (nfl).
The retinas of fish are also particularly suited as developmental models for the following reasons (Easter and Malicki, 2002; Hitchcock et al., 2004). The first is largely one of practicality: fish develop externally, and relatively rapidly, and most fish used for experimental purposes have high fecundity. The embryonic period of retinal neurogenesis in fish is therefore highly accessible and easy to manipulate experimentally, and large numbers of embryos can be obtained, contributing to statistical power in interpreting results. For the zebrafish, there are additional advantages such as embryonic transparency and the existence of a wide array of genetic tools. A second advantage to the teleost fish retina as a model system is that in these species, retinal neurogenesis persists beyond the embryonic period. Fish display indeterminate growth, becoming larger and larger with age, and continuously generate new tissue of all types, including nervous system tissue. Persistent neurogenesis in the retina permits the study of developmental processes in a mature organism, providing additional opportunities for experimental manipulation. A third advantage is that the retinas of teleost fish respond to damage by replacing lost retinal neurons. This regenerative response is in contrast to the gliotic response of the damaged mammalian retina. An understanding of retinal regeneration in fish should lead to strategies for restoring retinal function in human visual disorders. A final advantage to the use of teleost fish is that fish have a truly duplex retina, containing large numbers of both rod and cone photoreceptors, and rod neurogenesis and cone neurogenesis are temporally and spatially distinct. Because the loss of cone photoreceptors in human retinal disorders is responsible for the most debilitating loss of vision, knowledge of cone photoreceptor cell biology, aging, and development will be the key to adequate treatment of these disorders. This knowledge will be dependent more upon animal models with color vision, such as fish, than on those with limited or no capacity for true color vision, such as rodents.
The use of several teleost fish species has contributed to our understanding of retinal neurogenesis, but some fish models deserve special mention. The goldfish (Carrasius auratus) has long been a favorite of retinal physiologists and anatomists, and work on goldfish retina provided the first important insights into mechanisms for persistent neurogenesis and whole-tissue retinal regeneration. The cichlids, threespine sticklebacks, and blind cavefish are notable as models for understanding visual system evolution and color vision-mediated behaviors. Fish with unique life history traits, such as those involving migration (i.e., salmonids) or metamorphosis (i.e., the winter flounder), have provided opportunities for understanding plasticity of the visual system as a naturally adaptive mechanism. The medaka has offered advantages for understanding developmental biology and developmental genetics of retinal neurogenesis. The real star, however, is now the zebrafish, which has emerged as the outstanding model for improving our understanding of developmental genetics of retinal neurogenesis. Superb genomic resources and the growing collaborative network of zebrafish biologists have made Danio rerio an attractive model to develop for related experimental questions.
The remainder of this review will summarize and discuss the historic and recent findings that have shaped our current knowledge of retinal neurogenesis. The focus will be on the unique contributions to this body of knowledge that required or significantly benefited from the use of a fish model system.
II. Neurogenesis in the Embryonic Fish Retina
A. Spatiotemporal Patterns
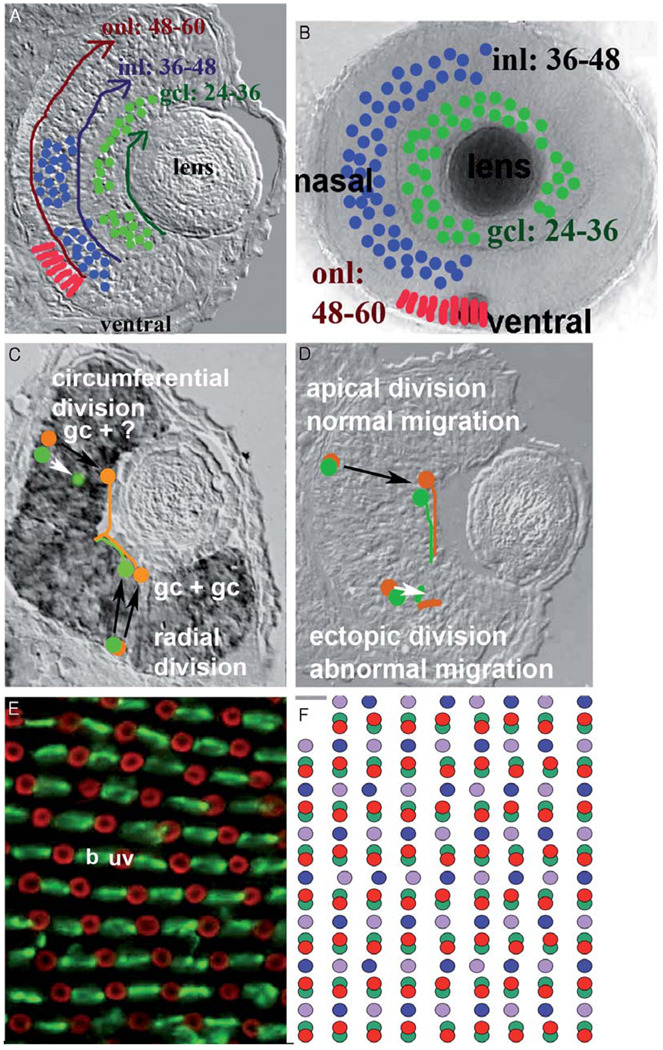
Embryonic retinal neurogenesis in all vertebrates begins with an apparently uniform population of neuroepithelial cells. From this uniform population, seven major cell classes are generated in the correct ratios for retinal function. Very little cell death takes place in the embryonic, wild-type zebrafish retina (Cole and Ross, 2001). Also in all vertebrates, this process occurs in a stereotyped spatiotemporal pattern. The pattern of retinal neurogenesis in teleost fishes is particularly striking; it has provided hints as to the mechanisms underlying the process, and its predictability has served as an assay for the identification of factors that regulate neurogenesis and cell differentiation. This spatiotemporal pattern has several components: cell-specific timing of cell cycle exit, tissue polarity and neuronal migration, fan-shaped neurogenic waves, and the generation of precise two-dimensional cell patterns (Fig. 2).
FIG. 2. Spatiotemporal patterns of neurogenesis in the fish retina.
(A) Radial retinal cryosection, and (B) whole mounted eye of zebrafish embryo, depicting sequential, fan-shaped waves of cell production, with cells of the ganglion cell layer (gcl; green profiles) generated from 24–36 hpf, those of the inner nuclear layer (inl; blue profiles) generated from 36–48 hpf, and those of the outer nuclear layer (onl; red profiles) generated from 48–60 hpf (Hu and Easter, 1999). (C) Orientation of terminal mitosis predicts cell fate. Circumferential divisions (within the plane of the image) of identified progenitors lead to asymmetric fates, while radial divisions (perpendicular to the plane of the image) of identified progenitors lead to symmetric fates; i.e., two ganglion cells (gc) (Poggi et al., 2005). (Figure modified with permission from Stenkamp et al., 2002, Fig. 3J.) (D) Ectopic sites of terminal mitosis are associated with abnormal migration and ectopic differentiation (Pujic and Malicki, 2001). (E) Whole mounted zebrafish retina hybridized with a combination of cRNA probe corresponding to blue cone opsin (b; visualized in green) and UV cone opsin (uv; visualized in red) (F) Illustration of the cone mosaic of the zebrafish retina. Red profiles correspond to red-sensitive cones; green profiles to green-sensitive cones, etc. (Raymond et al., 1993).
1. Cell-Specific Timing of Cell Cycle Exit
The cell classes of the vertebrate retina are generated sequentially, and in the zebrafish, this sequence generates cells layer by layer, from outside to inside relative to the apical surface of the retina (Hu and Easter, 1999). Ganglion cells are the first to be generated, and undergo their final mitotic divisions from 24 to 36 h postfertilization (hpf), as measured by incorporation of the S-phase marker bromodeoxyuridine (BrdU). Cells of the inner nuclear layer are generated next, born from 36 to 48 hpf. Cone photorecept or cells are born later, between 48 and 60 hpf. These precise 12 h intervals of neurogenesis suggest little or no overlap in the production of each retinal layer, and offer opportunities to identify developmental mechanisms distinct for each neurogenic period (Fig. 2A, B). The genesis of rod photoreceptors in the teleost retin a is delayed and protracted relative to that of cone photoreceptors, and rods are generated from a defined neural lineage (Raymond, 1985). Rod neurogenesis will be considered in further detail in Section III.B.
In the zebrafish, a series of cell-specific waves of apoptotic cell death has been documented to take place subsequent to the waves of cell birth (Biehlmaier et al., 2001). These waves of cell death follow the same sequence, with an initial wave of cell death in the ganglion cell layer followed by cell death in the inner and then outer nuclear layer. However, the relative timing of each event does not precisely mirror the relative timing of the waves of neurogenesis; the wave of apoptosis in the ganglion cell layer peaks at 3 days postfertilization (3 dpf), followed immediately by a lesser wave of cell death in the inner nuclear layer at 4 dpf and a delayed wave of cell death in the outer nuclear layer peaking at 7 dpf. In contrast to mammals, however, the total amount of cell death in the embryonic and larval zebrafish retina is very low.
A recent study using video time-lapse photography to follow the fates of dividing progenitors in zebrafish has revealed yet another intriguing aspect of vertebrate retinal neurogenesis (Poggi et al., 2005). As progenitor cells progress through M-phase at the apical surface of the retina, the plane of cleavage may assume a circumferential or radial orientation with respect to the retinal hemisphere. During the period of ganglion cell neurogenesis, circumferential divisions are likely to generate asymmetric or different fates, such as a ganglion cell and some other cell type. However, radial divisions tend to generate symmetric fates—both daughters became ganglion cells (Fig. 2C). It remains to be seen whether this principle will apply to the generation of other retinal cell types and to retinal neurogenesis in other vertebrates.
2. Tissue Polarity and Neuronal Migration
As each layer of the vertebrate retina is generated, the newly born neurons migrate from the apical surface to their final positions relative to other neurons. The importance of tissue polarity during this process was recognized through the identification of lamination mutants in zebrafish (Malicki et al., 1996). In these mutants, although all major retinal cell classes are generated, retinal cells migrate abnormally and the consequence is a retina with profound patterning defects and no sign of correct lamination. For example, photoreceptors in these mutants can be found near the lens, and ganglion cells can be found near the retinal pigmented epithelium. In all of the lamination mutants, these patterning defects are preceded by abnormalities in position of mitotic figures during retinal proliferation (Fig. 2D). In a wild-type retina, nuclei of proliferative progenitor cells migrate as they progress through the cell cycle, with S-phase taking place at the vitreal surface and M-phase at the apical surface. Mitotic figures of lamination mutants are instead located ectopically, at various radial positions. In many cases, the mutated genes have been identified and their protein products are known to play a role in the establishment and maintenance of cell polarity. For example, the glass onion (glo) locus corresponds to cadherin-2 (cad2), encoding a Ca2+-dependent cell adhesion molecule (Malicki et al., 2003), and the nagie oko (nok) locus corresponds to a membrane-associated guanylate cyclase (MAGUK) scaffolding factor (Wei and Malicki, 2002). These data indicate that cell polarity during retinal neurogenesis is a tissue characteristic essential for the laminar patterning of the retina. The mechanisms behind these defects are still under study. The abnormal position of M-phase progenitors may expose new neurons to an inappropriate environment for directing cell migration and/or differentiation; alternatively, the absence of polarity cues may itself lead to abnormal neuronal migration and/or differentiation.
3. Fan-Shaped Neurogenic Waves
Within each layer of the developing zebrafish retina, neurogenesis takes place asynchronously. For example, in the ganglion cell layer, a small number of cells located in the ventronasal quadrant of the retina, adjacent to the choroid fissure, exit the cell cycle prior to any other retinal cell (Hu and Easter, 1999). This precocious patch of ganglion cells is next joined by newly born ganglion cells in nasal, and then dorsal, and finally temporal retina (Fig. 2A, B). This wave of ganglion cell neurogenesis has been compared to the opening of a fan and is distinct from the centrifugal neurogenic gradient in the retinas of other vertebrates. Fan gradients of neurogenesis have been described for the other layers of the teleost retina, with the first-born cells of each layer residing in a ventral patch near the choroid fissure, followed by the sequential recruitment of cells in nasal, dorsal, and then temporal retina (Raymond et al., 1995; Schmitt and Dowling, 1996; Stenkamp et al., 1996). Rod neurogenesis in the zebrafish follows a similar pattern, with an early-forming ventronasal patch of differentiating rods, but it displays a more generalized ventral-to-dorsal developmental gradient, rather than an obvious fan-shaped pattern.
Why do fish display the fan gradient rather than the central-to-peripheral gradient of other vertebrates? The fan gradient of teleosts is succeeded in the larval phase by the addition of new retina at the periphery of the embryonic retina and persists with new retina continuously generated in a central-to-peripheral pattern, reminiscent of the embryonic pattern of other vertebrates (McCabe et al., 1999). It is possible that only the teleost model offers the level of resolution needed to reveal the early fan gradient. A more interesting explanation that may provide mechanistic insights is that the fan gradient reflects an earlier, proximal-to-distal patterning gradient laid down when the optic primordium was a solid, paddle-shaped mass (Li et al., 2000). This hypothesis will be discussed further in Section II.D. Regardless of the reasons for the fan gradient, this stereotyped, asynchronous pattern has been compared to the similarly asynchronous, posterior-to-anterior neurogenic wave of the Drosophila retina, and as such, has inspired a search for neurogenic mechanisms similar to those known for Drosophila (Raymond and Barthel, 2004; Stenkamp and Cameron, 2002). The fan gradient has also served as an important experimental tool in pursuing these and other mechanisms behind the neurogenic waves.
4. Generation of Precise Cell Patterns
The adult teleost fish retina, from an anatomical standpoint, is a structure of exquisite geometric precision (Fig. 2E, F). Each cell class not only occupies a defined laminar position, but also it is distributed within each retinal layer in a two-dimensional array that can be described by statistical pattern analysis methods as nonrandom, or regular. In the ganglion cell layer, and in the inner nuclear layer, the regular patterns of each cell class are independent of the patterns of other cell classes (Cameron and Carney, 2004). A combination of modeling and empirical work in the goldfish retina demonstrated that the two-dimensional patterns of inner retinal neurons arise as a consequence of information from like-cell types, and that cell death and cell migration are not involved (Tyler et al., 2005). In the photoreceptor layers of teleost fish, the four spectral types of cone photoreceptors are arranged in a geometric lattice, referred to as the cone mosaic (Raymond et al., 1993). The positions of each cone type can be predicted based on the positions of any other cone type, and these relationships can be described by statistical pattern analysis methods as interdependent (Stenkamp et al., 2001). The most frequently described cone mosaic of teleost fish is termed the “square mosaic” in which single cones—generally blue or ultraviolet (UV) sensitive—occupy the center and corners of the mosaic, respectively, and double cones sensitive to longer wavelengths— generally green or red sensitive—occupy the sides of the square. The zebrafish is somewhat exceptional in that it displays a “row mosaic” of its cone photo-receptors. In the zebrafish, rows of blue and UV-sensitive cones alternate with rows of green and red-sensitive double cones. The retinas of other vertebrates also show independent, regular mosaics of inner retinal neurons (Rockhill et al., 2000). However, the patterns of cone photoreceptors in nonteleost vertebrates typically show a less geometric pattern of regularity than those of teleosts, and mosaics of each cone type are not anatomically dependent upon those of any remaining cone type (Wikler and Rakic, 1994).
One of the more remarkable aspects of teleost retinal mosaics is that they are established at the time each neuronal class is initially generated (Branchek and Bremiller, 1984; Fadool, 2003; Prabhudesai et al., 2005). This has been interpreted to mean that little or no tangential cell movement takes place after terminal mitosis. An additional interpretation is that cell fate may be dependent upon cell position during neurogenesis. Mechanisms that regulate cell fate based upon cell position are well described for Drosophila retinal neurogenesis, and so this interpretation has fueled the search for conserved mechanisms in vertebrates (Raymond and Barthel, 2004; Stenkamp and Cameron, 2002). The precise cone mosaic of the zebrafish has served as an assay for potential roles of signaling factors in regulating cone cell fate, since any factor that influences cone fate will theoretically disrupt the cone mosaic (Bernardos et al., 2005; Prabhudesai et al., 2005). The findings from these studies will be discussed in detail in Section II.C. As a final note, although the rod photoreceptor pattern in teleost retina has long been viewed as nonrandom, a recent study using a transgenic reporter line has revealed that rods, too, are arranged with geometric regularity that can be appreciated at the level of the rod inner segment (Fadool, 2003).
B. Intrinsic Factors
A longstanding, hypothesis-based debate that has driven the field of vertebrate retinal neurogenesis is the question of relative contributions of cell lineage versus cell environment in the regulation of retinal cell fate. Cell lineage tracing experiments demonstrated that retinal progenitor cells remain multipotent until their final mitotic division, a finding consistent with the cell environment providing the necessary information for cell fate decisions (Turner et al., 1990). Furthermore, manipulation of the cell environment in cell culture experiments confirmed the persistent plasticity of retinal progenitor cells (Adler and Hatlee, 1989; Reh and Kljavin, 1989; Watanabe and Raff, 1990). However, these and similar studies also revealed that not all progenitor cells are alike. Over developmental time, the cell fate potential of retinal progenitor cells changes: early and late retinal progenitors generate different sets of retinal cell types even when placed in the same environment. Although this does not necessarily defeat the cell environment hypothesis, it does indicate that factors intrinsic to retinal progenitor cells are also important in the determination of retinal cell fate. The current model suggests that retinal progenitor cells pass through a series of “competence states” over developmental time, with each state biasing progenitors toward a limited number of fates (Livesey and Cepko, 2001). These states can be defined by factors—predominantly transcription factors—intrinsic to the progenitor cells, which may allow for responsiveness to environmental cues. Extrinsic factors remain essential for regulating final fate choice and for promoting cell differentiation.
The model outlined above was derived from information from nonteleost vertebrate models, primarily the chick and mouse. However, recent studies using zebrafish have added some important details regarding function and regulation of specific intrinsic and extrinsic factors, as well as a testing ground for the degree of conservation of the current model. As teleost fish have contributed to information from other animal models, further principles have emerged or have been solidified. These include the conservation of molecular mechanisms for retinal neurogenesis among metazoans, and the efficiency with which evolution has utilized the same molecular factors at multiple developmental times and locations. The key transcription factors belong predominantly to two major classes: the homeobox-containing class and those containing a basic helix-loop-helix (bHLH) motif. In general, homeobox-containing genes are initially involved in specification of the optic primordium and regulation of cell proliferation, and later are involved in cell-specific differentiation (Dyer, 2003). In contrast, there is evidence that the bHLH transcription factors, together with other transcription factors, help define the “competence states” of progenitor cells described above (Vetter and Brown, 2001). In the remainder of this section, selected specific transcription factors of these and other classes will be discussed, with an emphasis on recent functional information derived from teleost fish models. Table I summarizes these factors and their known roles.
TABLE 1.
Intrinsic Factors Regulating Retinal Neurogenesis in Teleost Fish
| Eye morphogenesis | Retinal progenitor cell cycle control |
Retinal cell determination and differentiation |
||
|---|---|---|---|---|
| Homeobox | Pax6 |
Macdonald et al., 1995; Nornes et al., 1998 |
Hitchcock et al., 1996; Otteson et al., 2001 | |
| Pax2 | Macdonald et al., 1995, 1997 | |||
| Vax | Take-uchi et al., 2003 | |||
| Chx/vsx |
Barabino et al., 1997; Passini et al., 1998 |
Passini et al., 1998 | ||
| Crx | Shen and Raymond, 2004 | Y. Liu et al., 2001; Shen and Raymond, 2004 | ||
| Rx |
Chuang and Raymond, 2001; Loosli et al, 2003; Rojas-Munoz et al., 2005 |
Chuang et al., 1999 | ||
| bHLH | Ath5 | Poggi et al., 2005 |
Kay et al, 2001, 2005; Masai et al, 2000; Poggi et al., 2005; Stenkamp and Frey, 2003a |
|
| NeuroD |
Hitchcock and Kakuk-Atkins, 2004; Korzh et al., 1998 |
|||
| Nuclear | RAR/RXR | Tallafuss et al., 2006 | ||
| hormone receptor |
PNR | Chen et al., 2005 | ||
| Other | Brn3 | DeCarvalho et al.,2004 | ||
| Chromatin remodeling |
brgl | Gregg et al., 2003; Link et al., 2000 | ||
| add | Yamaguchi et al., 2005 |
1. Homeodomain-Containing Genes
The pax6 genes (pax6.1 and pax6.2) in zebrafish (Nornes et al., 1998) encode transcription factors containing two DNA-binding domains: a paired domain and a homeodomain. Involvement of pax6 homologs in eye development has been demonstrated or indirectly supported for nearly all metazoans that have visual structures. In Drosophila, pax6 (eyeless) is required for eye development; in the absence of this gene, eyes do not form (Quiring et al., 1994). Also in Drosophila, ectopic expression of eyeless is sufficient to orchestrate the formation of ectopic eyes (Gehring, 1996). In vertebrates, pax6 genes are expressed in eye primordia (Macdonald et al., 1995), and this expression persists through the early stages of retinal neurogenesis when pax6 is downregulated in all retinal cell types except ganglion cells and amacrine cells (Hitchcock et al., 1996). Overexpression of pax6 in vertebrates results in the formation of ectopic eye tissue (though not whole eyes [Chow et al., 1999]), and disruption of pax6 expression results in eye abnormalities (Lauderdale et al., 2000). In the zebrafish, a transition zone defined by the proximal limit of pax6 expression defines tissues of the future eye (retina and retinal pigmented epithelium) as distinct from the optic stalk and more proximal structures, which express a related factor, pax2 (Macdonald et al., 1995). The position of this transition zone can be manipulated experimentally in zebrafish by changing the expression of embryonic midline signals such as those of the hedgehog family of signaling proteins. In the absence of the midline signal (mutant zebrafish lacking prechordal plate mesoderm), pax6 expression is not limited at the anterior midline, and the fish embryo develops a single, cyclopic eye. When hedgehog genes are overexpressed during early embryonic development, expression of pax6 becomes more spatially restricted and the result is small eyes and enlarged optic stalks (Macdonald et al., 1995). The role of pax6 in differentiated and mature ganglion and amacrine cells is not known.
The retinal homeobox (rx; also known as rax) genes are homeodomain-containing genes that do not have a paired domain (Mathers et al., 1997). Like pax6, a functional rx gene is required for the formation of vertebrate eyes, and overexpression of rx genes results in ectopic retinal tissue. The zebrafish genome has three rx genes, the most of any vertebrate. Two of these, rx1 and rx2, are highly conserved relative to each other and to those of other vertebrates, while rx3 has a slightly divergent homeodomain. The rx1 and rx2 genes are expressed in anterior neuroepithelium and later become restricted to retinal progenitor cells (Chuang et al., 1999). As neurogenesis proceeds, rx1 and rx2 are downregulated but then are reexpressed in photo-receptors and some inner nuclear layer cells. In contrast, rx3 is initially expressed in anterior neuroepithelium, but then becomes restricted to the developing hypothalamus and pineal organ, and appears in the retina in only a few cells in the inner nuclear layer. These distinct expression patterns are consistent with the hypothesis that a duplication of the rx gene in the teleost lineage was followed by selective pressures to retain this gene for functions distinct from those of the concurrently evolving parent gene. Interestingly, although overexpression of rx1 and rx2 generates ectopic retinal tissue (Chuang and Raymond, 2001), rx3 is the only one of the three that has been shown to be required for eye development in the zebrafish and medaka; mutants for rx3 do not have eyes (Loosli et al., 2001, 2003). The examination of hypomorphic rx3 mutants in zebrafish has also revealed that this rx gene is essential for specification of the retinal pigmented epithelium (RPE [Rojas-Munoz et al., 2005]). It is possible that subfunctionalization may have taken place for an alternative activity of rx. For example, only rx1 and rx2 are expressed in photoreceptors in the zebrafish, and in vitro (cell-free) studies have demonstrated that the rx protein can regulate photoreceptor-specific genes. Also in zebrafish, rx1 and rx2 appear to be cone specific (Chuang et al., 1999). However, our laboratory has preliminary evidence that at least rx1 is expressed in the progenitor lineage that gives rise to rods, as well as in rods themselves; this will be discussed further in Section III.B. For an outstanding review of rx gene structure and function, readers are directed to Bailey et al. (2004).
The cone–rod homeobox (crx) is another homeodomain-containing transcription factor. The mouse Crx protein was identified as a key regulator of expression of photoreceptor-specific genes in a heterologous expression system (Furukawa et al., 1997). Mutations in human crx lead to a rod–cone dystrophy that results in blindness. In mammals, the crx gene is expressed predominantly by developing and mature photoreceptors, while in zebrafish, crx expression is also detectable in the outer half of the inner nuclear layer and earlier in development, in retinal progenitors (Shen and Raymond, 2004). In fact, crx expression anticipates neurogenesis as it is initially detectable in a small ventral patch of progenitor cells at 24 hpf. Treatment of zebrafish embryos with crx antisense morpholino oligonucleotides effectively blocks translation of Crx protein and generates several intriguing morphant phenotypes. Morphants show a distal invasion of pax2 expression, suggesting a role for crx in regionalization of the optic primordia. Retinas of morphants also display delayed withdrawal from the cell cycle and delayed retinal cell differentiation (Shen and Raymond, 2004). The crx gene in teleosts therefore has functions important for retinal neurogenesis that precede its role in regulating photoreceptor differentiation.
2. bHLH Motif-Containing Genes
The bHLH genes involved in retinal neurogenesis can be described as proneural genes—those that promote the adoption of a neuronal fate. Two major categories of vertebrate proneural genes are the ash group and the ath group. The ash genes are homologs of the Drosophila achaete-scute complex genes, while the ath genes are homologs of the Drosophila atonal gene, which is required for the neurogenesis of sensory neurons. Studies of gene function in several vertebrate models suggest that specific proneural genes, or combinations of proneural genes with other transcription factors, are required for certain cell fates (Vetter and Brown, 2001). For example, ganglion cells may require ath5, bipolar cells ash1, and photoreceptors and amacrine cells NeuroD. The teleost fish model has offered the most insights into the functions of ath5 and NeuroD and these will be the focus of the rest of this section.
Zebrafish ath5 was identified based on its very close similarity to the Drosophila atonal gene. Atonal expression is required for photoreceptor neurogenesis in the Drosophila eye imaginal disc (Jarman et al., 1994). In zebrafish, the ath5 gene is expressed prior to the production of retinal neurons, in a fan-shaped gradient that anticipates the fan-shaped gradients of retinal neurogenesis (Masai et al., 2000). The initial site of ath5 expression is in the ventronasal quadrant, immediately adjacent to the optic stalk and the pax2/pax6 expression boundary. This boundary has therefore been suggested as the site of a signaling activity that may initiate retinal neurogenesis and will be discussed further in Section II.D. The zebrafish ath5 mutant lakritz does not have ganglion cells (Kay et al., 2001). Instead, supernumerary amacrine and bipolar neurons and Müller glia are found ectopically in a reduced ganglion cell layer. In other animal models, ath5 has been shown to regulate the expression of ganglion cell-specific genes, including the transcription factor Brn3b, and is considered essential for the production of ganglion cells (W. Liu et al., 2001). In a recent study that takes full advantage of experimental resources in the zebrafish model, Poggi et al. (2005) used time-lapse video photography to follow the fates of progenitor cells that express a transgenic ath5:GFP reporter gene. This reporter gene faithfully recapitulates the onset of native ath5 expression but persists due to stability of the green fluorescent protein (GFP) reporter protein. GFP+ cells were seen to divide only once at the apical surface of the developing retina, producing one daughter that migrates and differentiates as a ganglion cell, and a second daughter whose fate remains a mystery. The role of ath5 for ganglion cell determination is now better defined, but ath5 may have functions beyond this role. When the experiment is performed using a lakritz (ath5-null) genetic background, some GFP+ cells divided more than once, and sometimes both daughter cells acquired a ganglion cell fate. Therefore, while progenitor cell lineage in the form of expression of the intrinsic factor ath5 is confirmed as a contributing determinant of cell fate, extrinsic factors in the retinal cell environment also play a key role in regulating this process.
Another bHLH transcription factor involved in retinal neurogenesis in teleosts is NeuroD. NeuroD is an atonal homolog that was originally ascribed the function of directing a neuronal cell differentiation program (Kageyama et al., 1997). In the retina, there is evidence that the function of NeuroD is more specific. Most of this evidence comes from nonteleost animal model systems, where both gain- and loss-of-function approaches have demonstrated that NeuroD is involved in the determination of photoreceptors and amacrine cells (Morrow et al., 1999; Yan and Wang, 1998). In the zebrafish embryo, NeuroD expression is first detectable in ventronasal retina at 31 hpf and later is found in the inner and outer retinal layers (P. F. Hitchcock, personal communication). In teleosts, NeuroD is also expressed in the rod photoreceptor lineage (Hitchcock and Kakuk-Atkins, 2004); this subject will be covered in more detail in Section III.B of this review.
3. Nuclear Hormone Receptors
An additional class of transcription factors that deserves mention in this review, due primarily to its importance in regulating photoreceptor development, is the nuclear hormone receptor family. Genes in this family are activated by small molecule hydrophobic ligands and form homodimers and heterodimers that then interact with specific response elements in regulatory regions of target genes (Applebury et al., 2000; Bugge et al., 1992). This family includes genes encoding the retinoid receptors (RARs and RXRs), thyroid hormone receptors (TRs), and a number of “orphan” receptors for which ligands have not been identified. In other vertebrates, most notably the mouse, there is evidence that nuclear hormone receptors are involved in determining photoreceptor identity. In the wild-type mouse, most cone photoreceptors express some combination of middle and short wavelength-sensitive visual pigments, M- and S-opsin (Applebury et al., 2000; Shupe et al., 2005), although higher levels of M-opsin expression are found in dorsal retina, and a fraction of cones in dorsal retina does not express S-opsin. In mice that are null for the gene encoding TRβ, M-opsin expression is eliminated (Ng et al., 2001), and in mice null for the gene encoding RXRγ, the M-opsin gradient is eliminated (Roberts et al., 2005), consistent with possible regulation of M-opsin through TRβ/RXRγ heterodimers.
Rod versus cone identity in mammals is regulated by an orphan nuclear hormone receptor family member, NR2E3 (also known as PNR [Chen et al., 2005]). PNR transcriptionally activates rod-specific genes, and in mice and humans lacking PNR activity, S-opsin expression is enhanced and this expression takes place in morphologically hybrid photoreceptors (Corbo and Cepko, 2005). Surprisingly little information is available from the teleost fish models regarding the role of nuclear hormone receptors, with the exception of the recent identification of the zebrafish PNR gene and its transient expression in cones, followed by persistent expression in rods (Chen et al., 2005). The expression of zebrafish RXRγ has been localized to the retinal photoreceptor layer (Tallafuss et al., 2006). It is likely, then, that cone development in teleost fish may also involve the activity of RXRγ. Despite the paucity of information regarding expression and function of nuclear hormone receptor genes in teleosts, the embryonic zebrafish has been an outstanding model for understanding the activities of one of the known ligands, retinoic acid (RA). These activities, as well as those of other signaling factors, will be discussed in Section II.C.
4. Other Intrinsic Factors: Chromatin Remodeling
An unexpected new category of intrinsic factors that regulates retinal neurogenesis has recently emerged from studies of zebrafish developmental mutants. These intrinsic factors do not themselves regulate transcription but instead alter chromatin structure through modification of histone position and/or chemistry. These alterations in turn influence gene transcription. The two examples discussed here, young (yng) and ascending and descending (add), were independently identified through forward genetic screens for mutations deficient in retinal cell differentiation (Gregg et al., 2003; Yamaguchi et al., 2005). Yng mutants display arrested differentiation of retinal cells: the major retinal cell types are generated and early markers of cell differentiation are expressed, but cells fail to develop morphological specializations and do not express later markers (Link et al., 2000). The yng gene corresponds to the brahma-related gene 1 (brg1), which encodes a subunit of a chromatin remodeling complex (Gregg et al., 2003). Interestingly, the yng mutation acts non-cell-autonomously, in that mosaic embryos containing both yng−/− and yng+/+ cells have a wild-type phenotype. Therefore, the brg1 gene may regulate genes involved in generating cell–cell signals. Data demonstrating disruption of the mitogen-activated protein kinase (MAPK) intracellular signaling pathway in yng embryos suggest that these putative cell–cell signals influence the MAPK pathway. The add mutants similarly show failed retinal cell differentiation, but in these embryos, no differentiation takes place at all, and retinal cells continue to proliferate (Yamaguchi et al., 2005). The add gene likely corresponds to the gene encoding histone deacetylase 1, an enzyme that removes acetyl groups from histones, resulting in chromatin compaction. In add mutants, retinal progenitor cells do not exit the cell cycle, and this function is cell autonomous. The genes that add regulates likely correspond to those involved in cell cycle regulation. In both examples, yng and add, the mutant phenotype is retina specific, indicating that chromatin-related factors that influence gene transcription can be highly tissue specific in function. It is likely that additional examples of these factors will be uncovered and will contribute to a better understanding of the mechanisms driving retinal neurogenesis.
C. Extrinsic Factors
Several cell–environmental factors are now firmly established as having important functions in regulating retinal neurogenesis. These include insulin-like growth factors (IGFs), fibroblast growth factors (FGFs), hedgehog signaling proteins (Hh), Wnts, RA, thyroid hormone (TH), and cell surface proteins such as Notch and the cadherins. Some of these factors play several independent roles in retinal neurogenesis, and some are known to have distinct functions in different animal models. A comprehensive review would be considerably beyond the scope of this article, and so instead this section will focus on the contributions to our understanding of cell–cell signaling factors in retinal development that are based upon teleost fish model systems. Table II summarizes these factors and their known roles.
TABLE II.
Extrinsic Factors Regulating Retinal Neurogenesis in Teleost Fish
| Eye morphogenesis | Retinal progenitor cell cycle control | Retinal cell determination and differentiation | |
|---|---|---|---|
| IGFs |
Boucher and Hitchcock, 1998a; Mack and Fernald, 1993; Otteson et al., 2002 |
||
| FGFs |
Heisenberg et al., 1999; Picker and Brand, 2005 |
Mack and Fernald, 1993; Martinez-Morales et al., 2005 |
|
| Wnts |
Masai et al., 2005; Yamaguchi et al., 2005 |
||
| Hh |
Macdonald et al., 1995; Stenkamp and Frey, 2003a; Take-uchi et al., 2003 |
Masai et al., 2005; Shkumatava and Neumann, 2005; Stenkamp et al., 2002 |
Masai et al., 2005; Neumann and Nuesslein-Volhard, 2000; Shkumatava et al., 2004; Stenkamp and Frey, 2003a; Stenkamp et al., 2000 |
| RA |
Hyatt et al., 1992, 1996b; Marsh-Armstrong et al., 1994 |
Biehlmaier et al., 2005; Hyatt et al., 1996a; Prabhudesai et al., 2005 |
|
| Notch | Yamaguchi et al., 2005 | Bernardos et al., 2005; Scheer et al., 2001 | |
| Cadherins | Babb et al., 2005; Malicki et al., 2003 |
1. IGFs and wnts
Although these extracellular signaling factors are not highly evolutionarily related to each other, they are both primarily involved in the regulation of retinal progenitor proliferation. IGFs interact with cell-surface receptors of the tyrosine kinase receptor family and have mitogenic effects (Romano, 2003). The most information regarding the function of IGFs in the retina has come from the study of persistent neurogenesis in the mature retinas of goldfish and cichlids, and will be discussed in more detail in Section III.A.
The wnt signaling pathway has only recently emerged as a regulator of the cell cycle in the embryonic teleost retina. The canonical wnt signaling pathway involves the intracellular activity of B-catenin and a cyclin D1 target (Wang and Wynshaw-Boris, 2004). Suppression of wnt signaling in the zebrafish results in failure of retinal progenitors to incorporate BrdU, suggesting a requirement for this signaling pathway in promoting passage through the cell cycle (Masai et al., 2005). The introduction of an expression construct encoding B-catenin under a regulable promoter resulted in hyperproliferation in the retina and inhibition of cell cycle exit. Interestingly, the intracellular wnt signaling pathway is suppressed when Hdac1 (add) is active (Yamaguchi et al., 2005). Therefore, some component of the wnt pathway may be a target gene for this chromatin-modifying enzyme. In addition, now that tools are available for manipulation of the wnt pathway in zebrafish, other functions of wnt signaling during retinal neurogenesis may be uncovered.
2. Fibroblast Growth Factors
Two distinct functions for FGF signaling have recently been demonstrated using the zebrafish model: an early influence on nasal-temporal patterning of the retina (Picker and Brand, 2005) and a later role in initiating the production of retinal neurons (Martinez-Morales et al., 2005). The FGFs interact with tyrosine kinase receptors and engage the activity of the ras-MAPK pathway (Thisse and Thisse, 2005); there are at least 25 FGFs in the zebrafish genome (Katoh and Katoh, 2005). FGF8 and FGF3 are expressed in the developing telencephalon, the optic stalk, and newly generated ganglion cells, and all of these are potentially signaling centers that influence retinal neurogenesis (Picker and Brand, 2005). The identification of these signaling functions was facilitated by the use of the acerebellar (ace/fgf8) mutant, the lia (fgf3) mutant, and the FGF signal transduction inhibitor SU5402.
The ace mutant displays misprojections of retinal ganglion cells to the optic tectum, with the nasal–temporal axes of the retina failing to map appropriately (inversely) onto the anterior–posterior axes of the tectum (Picker and Brand, 2005). Clearly FGF8 signaling is in some way involved in retinotectal mapping, but the timing of this requirement came as somewhat of a surprise. Only when FGF signaling was inhibited between the 5- and 10-somite stage, corresponding to the onset of eye morphogenesis, was the axon trajectory phenotype replicated. The main site of fgf8 expression at that developmental time is the telencephalon, suggesting this tissue as the source of the nasal-temporal patterning signal. In the absence of the signal, specific nasal or temporal markers, such as ephrin a4b, are misexpressed, resulting in inappropriate ganglion cell identities. The importance of early midline signaling centers for later retinal neurogenesis and differentiation has arisen as a significant theme in the pursuit of mechanisms regulating these processes. A broader discussion of other early developmental signaling events involved in regulating retinal neurogenesis will be provided in Section II.D.
The second documented role for FGF signaling in retinal neurogenesis in teleost fish is in initiating the wave of ganglion cell differentiation. The source of the FGF signal in this case is thought to be the distal optic stalk, which abuts the developing retina precisely at the region where neurogenesis is initiated (Martinez-Morales et al., 2005). Exposure of embryos to the FGF signaling inhibitor SU5402, just prior to the generation of the first ganglion cells, prevents the expression of ath5. And while the retina of the ace mutant progresses through retinal neurogenesis in a relatively normal fashion, the retina of a double mutant, ace/lia, does not, and very closely phenocopies the pharmacological reduction of FGF signaling. Correspondingly, implantation of an FGF-soaked bead promotes ganglion cell production, as well as expression of phosphorylated ERK (dp-ERK), consistent with the effects of FGF being mediated through the ras/MAPK pathway. These data collectively demonstrate the importance of FGF8 and FGF3 signaling for initiating, and perhaps propagating, the wave of ganglion cell differentiation. One aspect of this regulatory activity is that another extracellular signaling molecule, Hh, is expressed by ganglion cells and may be downstream of FGF. This signaling pathway is discussed next.
3. Hedgehog
The hedgehog family of signaling proteins has recently received tremendous attention in the visual system development literature, with evidence for multiple key roles, from several tissue sources, at a number of developmental times. The secreted Hh protein interacts with a cell surface receptor complex consisting of two proteins—patched (ptc), and smoothened (smo). Hh signaling results in a change in activity of the Gli transcription factors, and the signal transduction pathway can be antagonized by activated protein kinase A (PKA) (Cohen, 2003). The search for Hh genes and their developmental functions in the vertebrate eye was inspired by the known role of Hh signaling in propagating photoreceptor neurogenesis in the Drosophila eye imaginal disk (Dominguez, 1999). This role is conserved in zebrafish, but with an intriguing twist: multiple retinal cell types express Hh genes, and the differentiation of multiple retinal cell types is regulated by Hh signaling. For example, two zebrafish hh genes, sonic hedgehog (shh) and tiggy-winkle hedgehog (twhh), are expressed in newly generated ganglion cells, and Hh signaling is required for the progression of ganglion cell differentiation (Neumann and Nuesslein-Volhard, 2000). Shh and twhh are also expressed in the RPE (Stenkamp et al., 2000) and in amacrine cells (Shkumatava et al., 2004), and the Hh signal from these sources is essential for the progression of photoreceptor differentiation. Demonstration of expression of the receptor complex has been difficult (Shkumatava et al., 2004), although our laboratory has detected expression of ptc2 within zebrafish embryo eyes, with tentative localization to retinal progenitor cells (Stenkamp et al., 2000).
If Hh signaling is reduced, such as in the sonic-you (syu) shh deletion mutant, the progression of ganglion cell differentiation is slowed or arrested (Neumann and Nuesslein-Volhard, 2000). In addition, cell proliferation is reduced (Stenkamp et al., 2002). A recent study provided more mechanistic evidence for this dual effect: the Hh signal is secreted not only from newly generated ganglion cells, but also slightly earlier, from ath5+ cells (only some of which will differentiate as ganglion cells; see Section II.B). Hh signaling from ath5+ cells likely promotes cell cycle exit of retinal progenitor cells, while Hh signaling from ganglion cells promotes the maturation of subsequent ganglion cells (Masai et al., 2005; Shkumatava and Neumann, 2005). This “sequential induction” process results in propagation of Hh expression as well, reminiscent of the wave of Hh signaling that takes place during retinal neurogenesis in Drosophila (Dominguez, 1999). These insights were gained largely through the use of loss-of-function strategies: specific zebrafish mutants and pharmacological agents that antagonize Hh signaling. Of great interest is that treatment of zebrafish embryos with the teratogenic alkaloid cyclopamine, which selectively interferes with Hh signaling, arrests ganglion cell differentiation, but only slightly reduces the progression of ath5 expression and neurogenesis in general (Kay et al., 2005; Stenkamp and Frey, 2003a). These data indicate that the progression of ath5, and perhaps cell cycle exit itself, may also be regulated by additional factors, although Hh signaling is an absolute requirement for ganglion cell differentiation. Treatment of zebrafish embryos with forskolin, an activator of PKA, completely arrests retinal neurogenesis (although this treatment must take place prior to progression of the neurogenic wave) (Masai et al., 2005). Therefore, the regulation of cell cycle exit very likely involves multiple signals, such as wnts, Hh, and perhaps FGFs, converging onto several interacting signal transduction pathways, such as those involving PKA and/or ras/MAPK. It is also very likely that the zebrafish model, with the potential for supporting future signal interaction experiments, will allow the elucidation of these complex signaling mechanisms.
Reduced Hh signaling, such as in the syu mutant, or as accomplished through the use of antisense gene knockdown strategies or cyclopamine treatment, also results in significant attenuation of photoreceptor differentiation (Stenkamp and Frey, 2003a; Stenkamp et al., 2000, 2002). The knock -down strategies applied were specifically targeted to the time when shh and twhh expression spreads throughout the RPE, leading to the conclusion that the Hh signal needed for photoreceptor differentiation likely originates in RPE cells. In support of this conclusion is that the spatiotemporal expression pattern of shh and twhh expression in the RPE anticipates the subsequent pattern of photoreceptor differentiation. In addition, there is evidence that the Hh protein is secreted from the apical surface of the RPE, toward the developing photoreceptor layer (Stenkamp et al., 2000). However, there is also evidence that the Hh signal arising from amacrine cells may be the key signal for promoting photoreceptor differentiation. The evidence for this conclusion comes from a highly creative experiment (Shkumatava et al., 2004). Mosaic embryos were generated, consisting of a combination of syu−/− cells and wild-type shh:GFP cells. These mosaic embryos were examined to determine whether failure of photoreceptor differentiation was associated with nearby syu−/− RPE or in nearby syu−/− amacrine cells, which could be recognized by the absence of the GFP transgene. The retinal regions with normal photoreceptor differentiation were predominantly radially contiguous with regions of wild-type shh:GFP amacrine cells and not wild-type shh:GFP RPE. The amacrine cell Hh signal, rather than the RPE Hh signal, may therefore act to promote photoreceptor differentiation, although there are several alternative interpretations that are consistent with these and prior data. For example, both signals may be required for photoreceptor differentiation, or total Hh signaling must exceed a threshold to promote differentiation. These hypotheses would be difficult to test using the mosaic embryo strategy, because the twhh gene is expressed in the syu−/− amacrine and RPE cells. A further complication of this approach is that globally reduced Hh signaling during the time of photoreceptor differentiation is also associated with a high rate of retinal cell death (Stenkamp et al., 2002). Therefore, the reported failure of photoreceptor differentiation (and inner retinal neuron differentiation) observed (Shkumatava et al., 2004) may actually be due to loss of retinal cells rather than a failure to differentiate. We hope to explore these issues as well as many others through the use of transgenic zebrafish that express shh under the control of a heat shock promoter. Preliminary experiments using mosaic expression have demonstrated that precocious Hh signaling in RPE is associated with precocious photoreceptor differentiation, while ectopic Hh signaling from the lens does not promote photoreceptor differentiation in adjacent cells (Stenkamp and Frey, 2003b).
4. Retinoic Acid
RA is a metabolite of vitamin A and has multiple developmental roles. RA interacts with nuclear receptors RARs and RXRs, which form heterodimers and act as transcription factors. Through the use of gain- and loss-of-function experiments in zebrafish, functions for RA have been uncovered for both eye morphogenesis and photoreceptor differentiation. For example, the application of exogenous RA at early developmental stages can result in what appears to be a duplicated retina (Hyatt et al., 1992), while pharmacological reduction of RA synthesis can prevent closure of the choroid fissure, essentially eliminating the ventral retina (Marsh-Armstrong et al., 1994). There are both dorsal and ventral sources of RA in the developing zebrafish eye (Marsh-Armstrong et al., 1994), although primarily the ventral source activates RA signaling in transgenic zebrafish expressing eYFP under the control of a series of retinoic acid response elements (Perz-Edwards et al., 2001; Prabhudesai et al., 2005).
In addition to a role for RA in regulating eye morphogenesis, several in vitro experiments in nonteleost vertebrate model systems have revealed that RA promotes photoreceptor differentiation, photoreceptor survival, and the generation of photoreceptors at the expense of other retinal cell types (Kelley et al., 1994; Soderpalm et al., 2000; Stenkamp et al., 1993). However, in vivo experiments in zebrafish have not supported a role for RA in regulating photoreceptor cell fate (Hyatt et al., 1996a; Prabhudesai et al., 2005). The zebrafish model oVers a particular advantage in that eVects of RA treatment can be evaluated for five different photoreceptor cell types. Exogenous RA promotes differentiation of rods and red-sensitive cones, while inhibiting differentiation of blue- and UV-sensitive cones and having a negligible influence on the differentiation of green-sensitive cones. Although this observation is consistent withRA effecting changes in cell fate, a creative experiment determined that this is not the case (Prabhudesai et al., 2005). If RA did actually manipulate photoreceptor cell fate—for example, changing photoreceptors fated to become UV-sensitive cones to ones becoming red-sensitive cones or rods—then the precise photoreceptor mosaic would be disrupted. Several theoretical experiments provided convincing evidence that statistical pattern analysis methods could reliably detect this predicted outcome of photoreceptor fate change. However, similar analysis of empirically generated data sets showed that the photoreceptor mosaics of RA-treated embryos were not statistically different from those of control embryos. Exogenous RA is therefore selectively changing the rate of photoreceptor differentiation in specified photoreceptors, rather than changing photoreceptor fate. Our laboratory is currently pursuing the cell-specific role of endogenous RA by performing signaling knockdown experiments. It is worth noting here that in most RA experiments, as well as in the Hh knockdown experiments described previously, photoreceptor differentiation was examined almost exclusively through evaluation of expression of specific opsin genes. An emerging principle of photoreceptor differentiation is that specific features of this process (morphological differentiation, opsin expression, expression of other photoreceptor-specific genes) may be independently regulated by a number of distinct signaling factors (Bradford et al., 2005). This principle was uncovered in in vitro studies of chick photoreceptor cells but now can be tested in vivo in the zebrafish model. For example, do Hh and RA regulate only opsins or an independently targeted collection of photoreceptor-specific genes?
5. Cell Surface Signals
Signaling mechanisms mediated by cell–cell contact are also important in regulating retinal neurogenesis. Several of these mechanisms have been identified through the generation of retinal lamination mutants, as discussed in Section II.A. An additional cell surface signaling mechanism, the Notch-Delta interaction, has been explored through selective gain- and loss-of-function methods in the zebrafish. Notch proteins are transmembrane receptors that interact with Delta/Serrate/Lag-2 transmembrane ligands on adjacent cells. Activation of Notch results in cleavage of its intracellular domain, which can then function as a transcription factor. Typically in the nervous system, activated Notch signaling favors the maintenance of a proliferating progenitor cell phenotype, or glial differentiation, at the expense of neuronal differentiation (Livesey and Cepko, 2001). Consistent with this, in zebrafish embryos, global expression of a constitutively activated Notch1a inhibits the differentiation of retinal neurons while promoting the differentiation of Müller glia (Scheer et al., 2001). The unusual consequences for a developing retina under these conditions also include considerable cell death. Experiments that block Notch signaling have taken advantage of the mind bomb (mib) mutant, in which both Notch- and Delta-mediated signal transduction is greatly reduced. The mib embryo therefore does not represent the reciprocal experiment to Notch gain of function, but instead offers a rather intriguing retinal phenotype (Bernardos et al., 2005). The differentiation of Müller glia is prevented, as would be predicted. Retinal neurons are generated, but in reduced numbers and with a slight degree of laminar disorganization. This phenotype is replicated by the use of a γ-secretase inhibitor, a pharmacological agent that also prevents both Notch- and Delta-mediated signal transduction. In the presence of this drug, the developing retina also fails to properly pattern in the tangential plane, with profound consequences for the cone mosaic. Although the disruption of the cone mosaic has not been quantified through pattern analysis, the appearance of the mosaic is enormously convincing that this is the case (Bernardos et al., 2005). This stunning result has provided the only evidence to date that a specific cell surface signaling interaction is required for cone patterning in teleosts. The current approach to identify additional signals and unravel the molecular mechanisms of cone mosaic formation involves zebrafish mutagenesis and screening for mutants with abnormal cone patterns (Allison et al., 2005).
D. Early Signaling Events Required for Later Neurogenesis
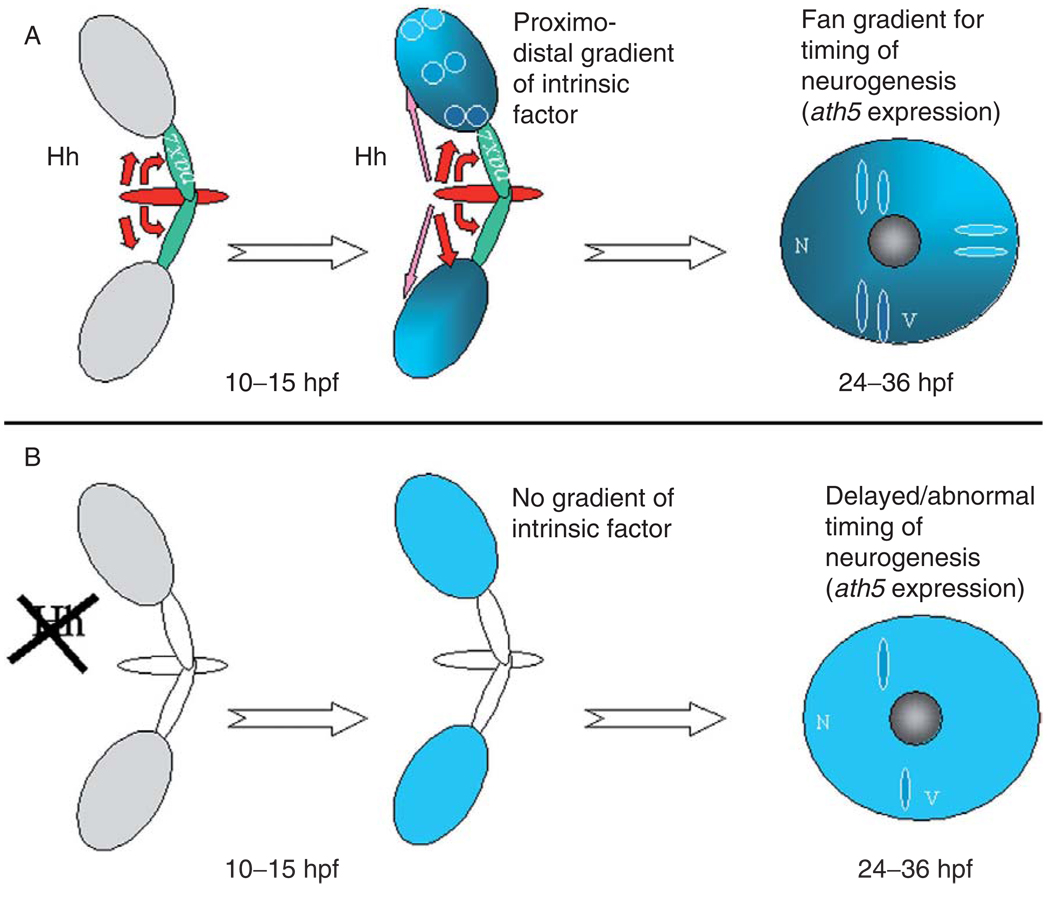
The zebrafish model for retinal neurogenesis has recently led the field in a somewhat unexpected direction—backward in developmental time. A series of studies taking full advantage of specific mutants, pharmacological agents, and transplant and mosaic experiments have established that patterning signals arising from the embryonic midline at approximately the time of neurulation are required for the later initiation and progression of retinal neurogenesis. The first of these studies (Masai et al., 2000) examined zebrafish mutants with defects in development migration of the prechordal plate—axial mesodermal tissue anterior to the notochord. All of these mutants are cyclopic because the midline signals needed during late gastrulation for separation of the developing eye fields are missing. In some cases the cyclopic eyes form laminated retinas, but in mutants in which the prechordal plate defect persisted through the time of neurulation, the single eye fails to initiate retinal neurogenesis. It was concluded that a midline signal must be required for retinal neurogenesis, and because the signaling event takes place far earlier than its target event, it was further surmised that signaling must be indirect. Because midline signals are also needed for optic stalk development, and because the distal optic stalk abuts the region where retinal neurogenesis is initiated, the optic stalk was pursued as the tissue that relayed the midline signal. The results of transplant experiments were consistent with this idea.
The identity of the midline signal was uncovered in our own laboratory (Stenkamp and Frey, 2003a). Our examination of retinal gene expression in the syu mutant exposed an interesting retinal phenotype: in half of the mutants, the retinas had failed to initiate neurogenesis (Stenkamp et al., 2002), similar to the situation in some of the prechordal plate mutants (Masai et al., 2000). Therefore, we next pursued Hh as the signal arising from prechordal plate required for retinal neurogenesis. A series of temporally selective Hh signaling knockdown experiments confirmed that retinal neurogenesis was prevented only if Hh signaling was inhibited during the time of neurulation, indicating that Hh from the midline, and not from retinal sources, was the key signal. We also examined zebrafish embryos with mutations in the smoothened gene (smu), and found that in some cases, retinal neurogenesis was blocked, and also in many cases, optic stalks were not present, consistent with a role for optic stalks in mediating the effects of the midline Hh signal. However, the optic stalk phenotype could be uncoupled from the retinal neurogenesis phenotype in that some smu mutants display normal neurogenesis despite the lack of optic stalks. A midline Hh signal is therefore important for both optic stalk development and for the initiation of retinal neurogenesis, but these roles are independent (Stenkamp and Frey, 2003a).
The studies of Kay and colleagues (2005) took these findings one step further, and their results also challenge the “sequential induction” model for propagation of ganglion cell neurogenesis described in Section II.C. They performed another series of temporally selective Hh knockdown experiments over the time of neurulation and over the time of initial retinal neurogenesis. In these experiments, again only the early knockdown could result in blocked or delayed retinal neurogenesis. Of great interest, the timing of the spread of retinal neurogenesis may also be dependent upon this early signal rather than upon signals derived from adjacent cells. Retinal progenitor cells transplanted from nasal to temporal retina expressed ath5 at the developmental time appropriate for their site of origin rather than the host site. Furthermore, the lakritz mutant, lacking functional ath5 and therefore lacking ganglion cells and the ganglion cell-derived Hh signal, progresses otherwise normally through retinal neurogenesis. These data suggest that the early Hh signal, from the embryonic midline, patterns the optic primordium such that retinal progenitor cells retain an intrinsic memory of position relative to this signal, and this intrinsic information directs the timing of cell cycle exit during retinal neurogenesis (Fig. 3). In this model, the hypothetical intermediate signal (from optic stalk or other tissue) would not necessarily be required (Kay et al., 2005). Also in this model, signals within the retina, such as Hh and FGF, would not be required for propagation of neurogenesis. One possible resolution to data that are in apparent conflict may involve the activity of PKA as a mediator of the proposed intrinsic information, and it is this intracellular signaling activity that is manipulated experimentally as neurogenesis proceeds.
FIG. 3. Midline hedgehog signaling is required to establish cell -intrinsic timing of retinal neurogenesis.
(A) At the time of neurulation (10–15 hpf), the Hh signal from the prechordal plate (red) (Masai et al., 2000; Stenkamp and Frey, 2003a) regulates pax2 expression in optic stalk and separates the eye fields ( Macdonald et al., 1995). Evidence suggests that Hh signaling also establishes a proximal–distal gradient of an unknown intrinsic factor (Kay et al., 2005) that predicts the fan gradient (Hu and Easter, 1999; Li et al., 2000) of neurogenesis, as revealed by the spatiotemporal pattern of ath5 expression ( Kay et al., 2005; Stenkamp and Frey, 2003a). (B) If Hh signaling is blocked by cyclopamine treatment at the time of neurulation ( Kay et al., 2005; Stenkamp and Frey, 2003a), pax2 expression may be disrupted (Stenkamp and Frey, 2003a), and neurogenesis is either blocked or proceeds with inappropriate timing ( Kay et al., 2005; Stenkamp and Frey, 2003a). N, nasal; V, ventral.
The significance of these findings should not be underestimated, as they lend insight into the possible etiology of microphthalmia as part of the human syndrome of holoprosencephaly. Holoprosencephaly results from impaired separation of the embryonic forebrain along the midline, and at its most extreme can include complete cyclopia and severe craniofacial abnormalities (Hahn and Pinter, 2002). Milder cases of holoprosencephaly can instead include microphthalmia, and this has always presented somewhat of a puzzle as to why the same defective gene (generally shh) or teratogenic insult can produce either cyclopia or microphthalmia. We suggest that the midline Hh signal, at the time of neurulation, is not only separating the developing eye fields but is orchestrating (in advance) retinal neurogenesis. Defective midline signaling therefore has later consequences for eye size and function. Altered midline hedgehog signaling is also implicated as an evolutionary mechanism leading to eye degeneration in the blind cavefish, Astyanax mexicanus (Yamamoto et al., 2004). However, in this case midline signaling is expanded, leading to excess activity of genes regulated by Hh signaling. Ultimately the ocular lens undergoes degeneration and this arrests growth and development of other ocular structures, including the retina.
III. Persistent Retinal Neurogenesis in the Fish Retina
In mammals, a complete complement of retinal neurons and glia is generated early in the animal’s life history, and neurogenesis does not persist thereafter. In teleost fish and amphibians, the neurons and glia generated embryonically represent a very small fraction of the total number present in adults, and retinal neurogenesis is continuous throughout the animal’s lifespan (Johns and Easter, 1977). Teleost fish display indeterminate growth, becoming larger with age, while mammals reach a relatively stable adult size early in life. Therefore fish have ongoing needs to generate new nervous system tissue and to match the rate of neuron production to that of growth of other tissues (Raymond et al., 1983). Persistent retinal neurogenesis must also be regulated and coordinated coincident with ongoing retinal function.
In fish, the retina increases in size throughout adulthood by three mechanisms (Johns, 1977). The first mechanism is one of balloon-like expansion of existing retina and enlargement of existing retinal cells. The second mechanism is the addition of new retinal tissue at the retinal periphery, generated by a circumferential germinal zone (CGZ). The third mechanism is the insertion of additional rod photoreceptors into the existing sheet of photoreceptors in the outer nuclear layer. The latter two mechanisms, which involve the generation of new neurons, have offered unique insights into retinal neurogenesis in general, and these will be the focus of this section of the review. Readers are also directed to other excellent recent reviews (Hitchcock et al., 2004; Otteson and Hitchcock, 2003).
A. The Circumferential Germinal Zone
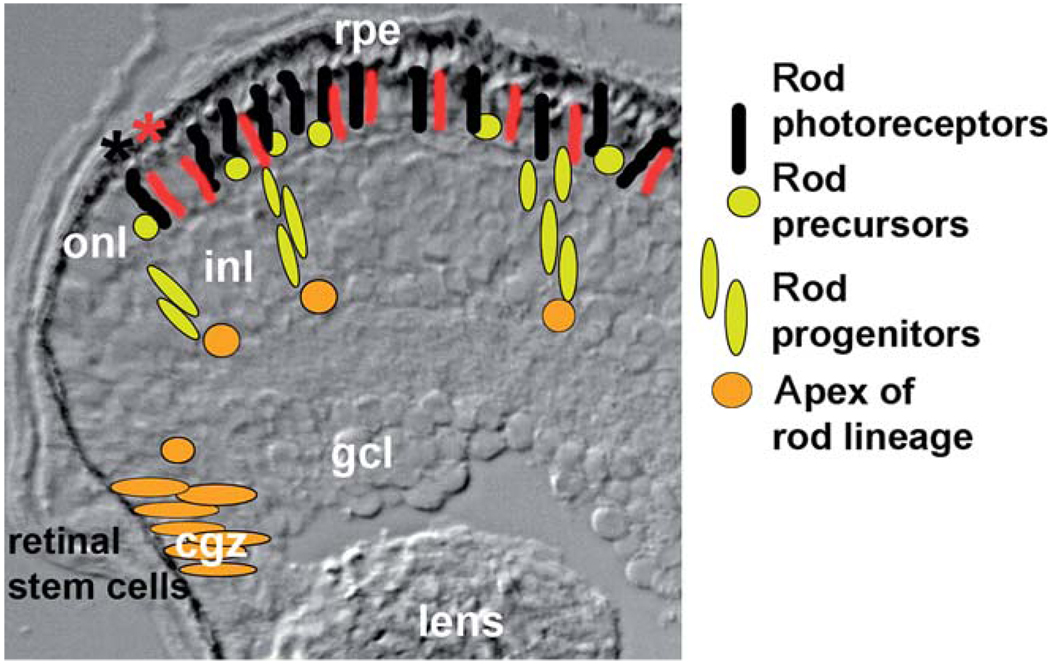
The circumferential germinal zone is found at the ciliary margin where the retina meets the iris epithelium, and it consists of retinal progenitor cells and correspondingly has a neuroepithelial appearance (Fig. 4). This region has been considered a remnant of the embryonic retina (Johns, 1977), but recent genetic discoveries argue that the CGZ has features that distinguish it from the embryonic retinal neuroepithelium (Wehman et al., 2005). Nevertheless, the CGZ behaves as an embryonic neuroepithelium, continuously generating new retinal neurons of all cell classes (except rod photoreceptors, as discussed in Section III.B), which are appositionally added to the existing retina. The fish retina therefore grows in concentric annuli, such that the developmentally oldest cells are located centrally, and the developmentally youngest cells are located peripherally (Johns, 1977).
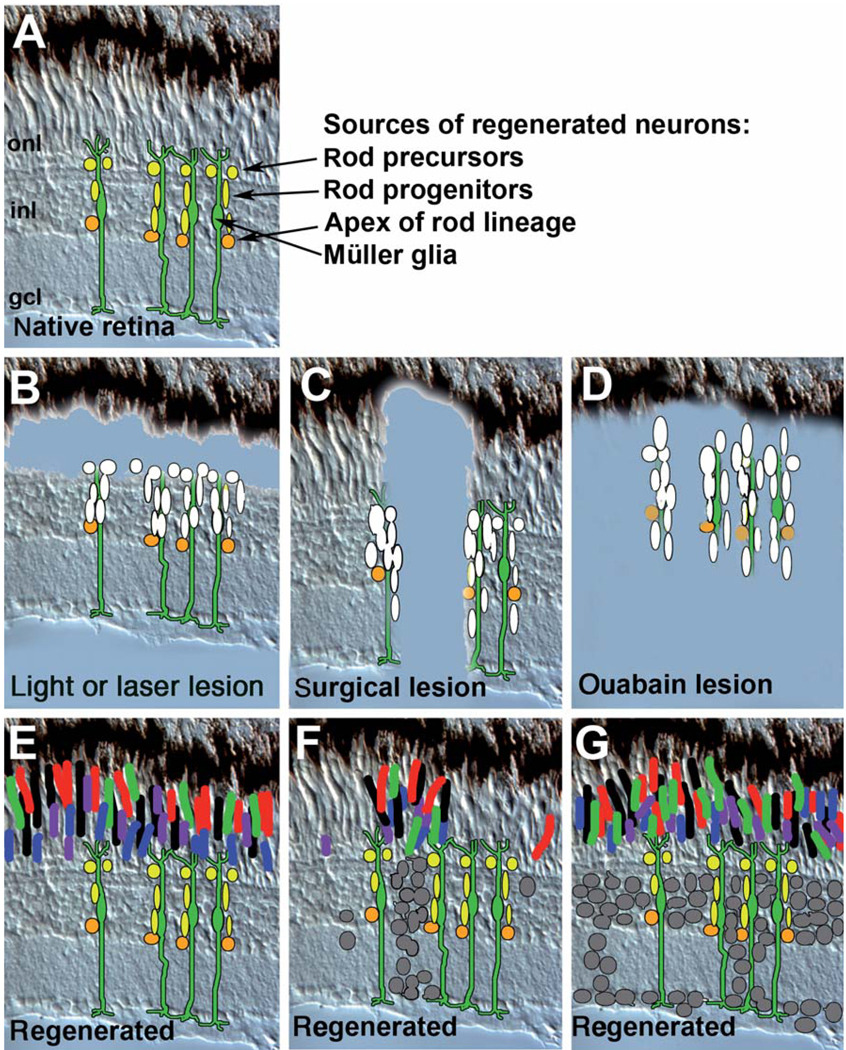
FIG. 4.
Retinal neurogenesis and the rod lineage in teleost fish (see also Otteson and Hitchcock, 2003). Radial cryosection of larval zebrafish retina; only dorsal retina is shown. Orange profiles represent retinal stem cells of the circumferential germinal zone (cgz) or pax6+ cells of the inner nuclear layer (inl) residing at the apex of the rod lineage. Yellow profiles represent NeuroD+ proliferative progenitor cells of the rod lineage (Hitchcock and Kakuk-Atkins, 2004); those residing in the outer nuclear layer (onl) are referred to as rod precursors (Raymond and Rivlin, 1987). Dark stripes represent rod photoreceptors; red stripes represent red cone photoreceptors (the remaining photoreceptor types are not depicted in this illustration to prevent clutter). Dark asterisk (*) shows the location of the “youngest” rod photoreceptor (closest to the cgz) with respect to the “youngest” cone photoreceptor (red asterisk) (Stenkamp et al., 1997; Wan and Stenkamp, 2000). Note the region of onl containing new cone photoreceptors that have not yet differentiated. This region has been referred to as the circumferential larval zone (Otteson and Hitchcock, 2003).
1. Developmental Timelines
The developmental timelines contained within a growing fish or amphibian retina have been exploited as a means to investigate the sequence of developmental events in retinal neurogenesis. For example, the most vigorous proliferative activity occurs in cells located nearest the iris epithelium, as demonstrated by incorporation of S-phase markers [3H]thymidine and BrdU, and by the expression of proliferating cell nuclear antigen (PCNA) and other cell cycle markers (Johns, 1977; Negishi et al., 1990; Stenkamp et al., 1997). Genes involved in regulating retinal neurogenesis, such as rx and pax6, are also expressed in the CGZ (Chuang et al., 1999; Hitchcock et al., 1996). Comparative expression patterns have been described for a number of these genes in the CGZ of the growing Xenopus retina, where the temporal sequence of expression—implied by differential distance from the iris epithelium—recapitulates the sequence of gene expression during embryonic retinal neurogenesis (Perron et al., 1998). Although this comparative expression experiment has not been performed using a teleost fish model, many of the same genes have been evaluated independently and are found in the CGZ.
The combined use of an S-phase marker (BrdU) and a cell-specific marker in growing fish retina has revealed comparative time courses of photoreceptor differentiation (Stenkamp et al., 1997; Wan and Stenkamp, 2000). In adult goldfish, the time between cell birth and expression of short-wavelength-sensitive cone opsins (such as blue and UV) is substantially greater than the time between cell birth and expression of the long-wavelength-sensitive cone opsins (such as red and green). This has been interpreted to mean that red- and green-sensitive cones differentiate more quickly than blue- and UV-sensitive cones. Of additional interest is that although rod photoreceptor genesis is delayed, as the rod lineage includes additional rounds of cell division, rods differentiate the most rapidly of all photoreceptor types. The consequence is that rod photoreceptors and red-sensitive cone photoreceptors begin to express opsin at approximately the same distance from the iris epithelium (Fig. 4). The periphery of the growing fish retina has therefore revealed that developing cone photoreceptors appear to “wait” for an encounter with developing rods prior to expressing a specific visual pigment. This prompted our laboratory group to test the hypothesis that developing rods play a role in regulating cone differentiation or formation of the cone mosaic by selectively killing a cohort of rod progenitors with 5-fluorouracil. In these experiments, cone differentiation and the cone mosaic were unaffected, indicating that developing rods do not play a role in regulating cone differentiation (Wan and Stenkamp, 2000). Any functional significance of this tight coordination of rod and cone differentiation therefore currently remains a mystery.
2. Regulation and Genetics of CGZ Neurogenesis
The rate of proliferation of cells of the CGZ, and by inference, the rate of production of new retinal neurons, appear to be linked to body growth in teleost fish through the regulatory activity of the growth hormone (GH)—insulin-like growth factor-I (IGF-I) axis. Receptors for GH and for IGF-I are present in the growing goldfish retina (Boucher and Hitchcock, 1998a), and injection of recombinant GH upregulates expression of IGF-I in retina (Otteson et al., 2002). Both GH and IGF-I promote proliferation in the CGZ of goldfish (Boucher and Hitchcock, 1998b) and of rod precursor cells in cichlids (Mack and Fernald, 1993). These findings are consistent with the roles of GH/IGF-I in regulating body size in fish: systemic GH regulates synthesis of IGF-I in target tissues, and IGF-I is a local regulator of growth for these target tissues.
Forward genetic screens in zebrafish have identified several mutations that selectively affect the CGZ but do not interfere with embryonic retinal neurogenesis (Fadool et al., 1997; Wehman et al., 2005). The existence of these mutations means that although in many ways neurogenesis in the CGZ does indeed recapitulate embryonic retinal neurogenesis, the two processes must utilize some distinct genetic pathways. Several mutants were identified in independent screens that have highly reduced CGZs (Fadool et al., 1997; Wehman et al., 2005); in some of these, the RPE was expanded, suggesting the existence of genetic pathways that regulate the RPE versus retinal stem cell phenotype. In addition, several mutants have recently been identified that have expanded CGZs relative to wild-type age-matched zebrafish larvae, and in these mutants the CGZ fails to actually generate new neurons, suggesting the existence of genetic pathways that selectively regulate postembryonic retinal neurogenesis (Wehman et al., 2005). Of great interest, none of the genes identified in the latter screen could be matched to candidate genes known to be involved in retinal neurogenesis or growth-regulating signaling pathways. These mutations therefore represent a novel genetic resource for enhancing our understanding of persistent retinal neurogenesis in the CGZ.
The existence of the CGZ in fish and frogs has inspired the search for cells with similar developmental potential in birds and mammals at the junction of retina and iris epithelium. Although similar cell populations do exist, they are reduced in size and highly reduced in proliferative and neurogenerative potential in vivo (Fischer and Reh, 2000; Moshiri and Reh, 2004; Tropepe et al., 2000). However, cells obtained from the ciliary margin of mammals may be coaxed to perform as retinal progenitors in vitro (Tropepe et al., 2000). The identification of genetic and other regulatory pathways required for retinal stem cells to function in teleost fish will be of tremendous importance in furthering these studies toward a therapeutic goal.
B. The Rod Photoreceptor Lineage
In addition to the production of new retinal tissue at the margin, the teleost retina grows throughout the lifetime of the animal by expansion of the existing tissue and by the continuous insertion of new rod photoreceptors (Johns and Fernald, 1981; Raymond and Rivlin, 1987). The density of rod photoreceptors therefore remains somewhat constant as the animal grows (Johns, 1982), while the density of all other cell types—including cone photoreceptors—decreases.
1. Spatiotemporal Patterns
The proximal source of new rods is the population of proliferating cells in the outer nuclear layer (ONL). These cells, called rod precursors, were identified through the application of [3H]thymidine and have since been confirmed through other means (Johns, 1982; Knight and Raymond, 1990; Mack and Fernald, 1997). The proliferative and neurogenerative activities of rod precursors have been studied in goldfish, cichlids, salmonids, and zebrafish. In cichlids a peak of proliferative activity in the ONL near the middle of the dark period of a light–dark cycle has been documented (Chiu et al., 1995). Furthermore, following terminal mitosis, the cell bodies of nascent rods move from an apical (scleral) position in the ONL to a more distal (vitreal) position (Mack and Fernald, 1995). In adult goldfish, the time between terminal mitosis and rod opsin expression is 3 days (Knight and Raymond, 1990; Stenkamp et al., 1997), while in cichlids this process is slightly faster—2 days (Henderson and Fernald, 2004). This rate of differentiation is rapid compared to the 6–12 days required for new cone photo-receptors to express specific markers (Stenkamp et al., 1997; Wan and Stenkamp, 2000) (Fig. 4). In general, more proliferative activity within the ONL can be found near the retinal margin, in the “younger” part of the retina, than in the central “older” part of the retina (Johns, 1982). In the zebrafish, a teleost that reaches a somewhat static size, the proliferative activity within the ONL slows considerably with age (Marcus et al., 1999), suggesting that the rate of rod production may be yoked to animal growth, as is the case with the rate of CGZ neurogenesis. Consistent with this idea, treatment of retinal slice cultures with IGF-I stimulates rod precursor proliferation (Zygar et al., 2005).
Where do the rod precursors come from? An elegant study in larval goldfish demonstrated that the rod precursors arise from a population of progenitor cells sequestered in the inner nuclear layer (INL) (Johns, 1982). In this study, [3H]thymidine labeling revealed elongated, mitotically active clusters of cells that appeared to migrate along the processes of Müller glia from the ONL to the INL. More recent experiments have confirmed the existence of this neurogenic cell population in teleost retinas of rainbow trout (Julian et al., 1998), goldfish (Otteson et al., 2001), and zebrafish (Stenkamp, 2004). The proliferative cells in the INL divide slowly, and so had not been consistently seen following intraocular injection of BrdU. However, they are revealed when fish are immersed for up to several days in dilute BrdU as a means of systemic administration. The spatiotemporal pattern of the rod photoreceptor lineage has now been well characterized, and appears to consist of three cell types that can be defined based upon position and nuclear morphology (Otteson et al., 2001). The first has a spherical nucleus and is stationary within the INL and associated with Müller glia. This cell type represents the apex of the rod lineage—a stem cell that can replace itself, as well as generate other cell types. These stem cells of the rod lineage are seeded into the INL from the CGZ, providing each “generation” of retinal tissue with a source of continuous rod neurogenesis (Fig. 4). The second cell type also found in the INL has a spindle-shaped fusiform nucleus and migrates toward the ONL along Müller glia processes. The two types of proliferative inner nuclear cells have been referred to as PINCs (Julian et al., 1998). The third cell type of the rod lineage is the rod precursor, which is located in the ONL and has an ovoid nucleus.
2. Developmental Genetics
The capacity to identify the rod lineage with BrdU has permitted an analysis of gene expression within this lineage, through colabeling experiments on retinal tissue derived from BrdU-exposed fish. For example, the cells at the apex of the rod lineage express the transcription factor pax6 (Otteson et al.,2001). However, fusiform progenitor cells and rod precursors are pax6 negative, and instead express the proneural gene NeuroD (Hitchcock and Kakuk-Atkins, 2004). We have recently confirmed that these expression patterns hold true at the earliest establishment of a rod lineage in the zebrafish embryo. In addition, the homeodomain-containing transcription factors crx and rx1 are also expressed in the rod lineage (R.A. Frey et al., unpublished observations). The separation of rod and cone neurogenesis in the developing teleost retina will permit continued analysis of comparative developmental genetics, and a more thorough understanding of rod neurogenesis, which would not be possible in other animal models.
C. Life History-Related Retinal Plasticity
The capacity for ongoing neurogenesis in a growing, postembryonic animal is in some cases accompanied by adjustments in retinal neurogenesis as the visual requirements of the animal change. These adjustments can include changes in the types of cells generated, and/or selective cell death, or phenotype changes within a differentiated cell. Each case represents a remarkable opportunity to study mechanisms for retinal plasticity that take place as a natural occurrence, rather than from a pathological insult.
1. Metamorphosis in Flounder
Flatfish offer dramatic examples of changes to the visual system that can occur in a metamorphic teleost. The metamorphic winter flounder grows considerably in size, changes position of one eye such that eyes are no longer bilaterally symmetrical, and adopts a benthic marine lifestyle. As its eyes grow (and as one of them migrates), the complement of photoreceptor types within each retina expands (Evans et al., 1993). Premetamorphic flounder have only one type of visual pigment, a green-sensitive pigment expressed in cone photoreceptors. Postmetamorphic flounder have two additional cone pigments, red- and blue-sensitive pigments selectively expressed in specific cone photoreceptors, and a rhodopsin pigment expressed in rods. In addition, the green-sensitive pigment is expressed in a cone photoreceptor type that is different in morphology from the green-sensitive cones of premetamorphic flounder (Mader and Cameron, 2004). Metamorphosis in Atlantic halibut is accompanied by similar changes, although some red- and blue-sensitive photoreceptors are present in the premetamorphic retina (Helvik et al., 2001). Because the eye grows several thousandfold during metamorphosis, the new features of the postmetamorphic retina are likely laid down by mechanisms of persistent neurogenesis; new retina generated during metamorphosis has a different complement of photoreceptors and the necessary circuitry than the remnant of premetamorphic retina. The organism-wide metamorphosis process is regulated by changes in circulating thyroid hormone levels (TH) (Inui and Miwa, 1985), therefore, increasing levels of TH may be involved in changing the complement of photoreceptors generated in a growing retina as metamorphosis progresses. Indeed, recent data suggest that in postmetamorphic flounder rendered hypothyroid through a pharmacological treatment, newly generated retina contains only the cone photoreceptor type found in premetamorphic retina (Mader and Cameron, 2006).
2. Smoltification in Salmonids
Anadromous salmonids undergo embryonic development and initial post-embryonic growth in freshwater, and then migrate to saltwater where they continue to grow prior to becoming reproductively mature and returning to spawn in their natal streams. Behavioral and physiological measurements of visual function in salmonids have shown that the adaptive changes for the saltwater environment (the parr-to-smolt transition, or smoltification) are accompanied by the loss of UV-sensitive vision (Browman and Hawryshyn, 1992). The cone mosaics of parr and smolt-stage fish are correspondingly distinct: parr-stage fish have cone photoreceptors residing in the “corner” position of the square mosaic, while smolt-stage fish have far fewer cones in this position (Bowmaker and Kunz, 1987). These findings collectively suggest that smolts have lost UV-sensitive corner cones from the cone mosaic.
The mechanisms behind this retinal plasticity have been under study for some time, in a number of different salmonids. Documented mechanisms include apoptosis of UV-sensitive cones in existing retina (Kunz et al., 1994), production of new retina at the CGZ that does not contain UV-sensitive cones (Flamarique, 2001), and production of new retina at the CGZ in which UV-sensitive cones are present only transiently (Allison et al., 2003). There is also some evidence that as some salmonids reach reproductive maturity, UV-sensitive vision is reestablished, and UV-sensitive cones reappear in the retina (Beaudet et al., 1997). Two nuclear receptor-based signaling systems have been implicated as regulating these changes: thyroid hormone (Browman and Hawryshyn, 1992) and retinoic acid (Browman and Hawryshyn, 1994), suggesting that the mechanisms for the control of retinal plasticity accompanying changes in life history are similar to those regulating photoreceptor differentiation in a developing embryo. Recently, an additional mechanism for visual system plasticity has been proposed: a switch in expression of opsin genes from the UV-sensitive opsin to a blue-sensitive opsin in individual cone photoreceptors (Cheng and Novales Flamarique, 2004). Remarkably, this process takes place prior to smoltification, leaving a reduced number of UV-sensitive cones in the retina. The continued study of teleosts with life history-related changes in visual function will likely reveal a vast repertoire of mechanisms for regulating these changes.
IV. Injury-Induced Neurogenesis in the Fish Retina
In contrast to the situation in mammals, when the retina of a teleost fish is injured, neurogenic mechanisms are engaged that replace the lost retinal cells. This regenerative capacity of the teleost retina has been known since the experiments of Lombardo (1968, 1972). In these experiments, a portion of the retina was surgically removed. The edge of the resulting lesion then became populated with proliferating cells, and this blastema advanced inward toward the lesion’s center, leaving regenerated retina in its wake. Since the time of these experiments, a variety of alternative approaches to retinal damage have been employed to study the regenerative response. In addition to describing the process and patterns of the regenerative phenomenon, attention has also focused on the anatomical and physiological attributes of the regenerated tissue, its capacity for function, the cellular source of new retina, and the developmental genetics of the regenerative process. Traditional models, such as goldfish, are giving way to the zebrafish to take advantage of genetic resources. Regeneration of teleost fish retina has served as a powerful model system for understanding the capacities as well as the limitations of injury-induced neurogenesis and has inspired the search for similar capacities in other model systems, including mammals.
A. Injury Models and Spatiotemporal Patterns of Regeneration
1. Chemical Injury
A technically straightforward means of damaging retinal tissue and initiating a regenerative response is the use of a cellular toxin. The Na+/K+-ATPase poison ouabain has been used by many investigators since it reliably destroys retinal neurons, but allows the cellular source of regenerated retina to survive and proliferate (Maier and Wolburg, 1979; Raymond et al., 1988). New retina is actually generated from two distinct sources: the CGZ more vigorously generates new retina at the ciliary margin, and scattered “neurogenic clusters” of proliferating cells fill in the more central region of the destroyed retina (Fig. 5A, D, and G). The former is not considered true regeneration, although it likely contributes to functional recovery.
FIG. 5. Retinal regeneration: the process and putative stem cells.
(A) Retinal cryosection of undamaged zebrafish retina, with superimposed depiction of probable sources of new retina following a lesion: Müller glia (green profiles) (Wu et al., 2001; Yurco and Cameron, 2005) and cells of the rod lineage (orange and yellow profiles) (Raymond et al., 1988; Wu et al., 2001). (B, C, D) The same cryosection, but images have been altered to represent (B) loss of the photoreceptor layer following a laser lesion or light damage, (C) surgical removal of a piece of retina, or (D) complete loss of neurons following treatment with ouabain. In each case, cell proliferation is increased, as depicted by the positions of the white profiles, and Müller glia reenter the cell cycle (Wu et al., 2001; Yurco and Cameron, 2005) (although this has not been documented for the ouabain lesion). For surgical lesions, proliferation also increases at sites not immediately adjacent to the lesion (Cameron, 2000). (E, F, G) Images of a cryosection have been altered to represent a retina that has (E) regenerated photoreceptors lost to light damage or a laser lesion, (F) regenerated following a surgical lesion, or (G) a retina that has regenerated following a ouabain lesion. New photoreceptors are depicted by the appropriately colored stripes, and new cells in other retinal layers are depicted by gray profiles. In each case, the regenerated cone mosaic is disorganized (Stenkamp and Cameron, 2002; Stenkamp et al., 2001; Vihtelic and Hyde, 2000). In retina regenerated following surgical or chemical lesions, cells are found ectopically, in the inner plexiform layer (Hitchcock et al., 1992; Raymond et al., 1988). Onl, outer nuclear layer; inl, inner nuclear layer; gcl, ganglion cell layer.
The ouabain model has been useful for the analysis of anatomical features of the regenerated versus native retina. These analyses have revealed that while regenerated retina contains a full complement of retinal cell types predominantly localized to the correct retinal layers, some errant cells are misplaced within plexiform layers, primarily the inner plexiform layer (Raymond et al., 1988). In addition, regenerated retina is not well organized in the tangential plane (Stenkamp et al., 2001). The topographical arrangements of inner and outer nuclear layer neurons are disrupted in regenerated retina, and measures of pattern analysis confirm that these arrangements are not statistically regular. The cone mosaic is most strikingly disordered, but the degree of disorder varies according to whether the new retina was generated at the CGZ following ouabain treatment, or was generated by the scattered neurogenic clusters. Truly regenerated retina shows a cone pattern that can be statistically characterized as “clumped,” perhaps reflecting the pattern of the regenerative mechanism—clumps of neurogenic cells. In contrast, new retina arising from an accelerated neurogenesis process at the CGZ shows a cone pattern that can be statistically characterized as “random,” a curious finding given that this same CGZ had generated statistically regular cone patterns prior to the injury (Stenkamp et al., 2001). These data are consistent with the hypothesis that retinal patterning in the tangential plane requires instructive information from a template of preexisting retinal cells. The study of retinal regeneration in teleosts can therefore lead to insights into neurogenesis in general, as well as a better understanding of this favorable response to retinal injury (Stenkamp and Cameron, 2002).
The ouabain model, in comparison to other chemical lesioning methods, has also provided insight into the mechanisms behind the regenerative response. A series of experiments was designed to evaluate the capacity of the teleost retina to launch a full regenerative response following the administration of cell-selective toxins. For example, intraocular injections of smaller concentrations of ouabain that fail to destroy the entire retina also fail to engage a regenerative response (Raymond et al., 1988). Similarly, intraocular injections of 6-hydroxydopamine (6-HODA) result in a regenerative response only when the concentration of 6-HODA is sufficient to kill over 30% of retinal neurons in the INLs and ONLs (Braisted and Raymond, 1992). When lesser concentrations are used, 6-HODA selectively destroys only dopaminergic amacrine cells, and the retina does not respond in a regenerative manner. It appears that the ONL must be damaged to initiate regeneration. Specifically, cone photoreceptors must die; tunicamycin treatment, which selectively kills rod photoreceptors, results only in continued generation of rods, but no other cell types are generated (Braisted and Raymond, 1993). These findings, along with other evidence to be discussed below, led to the preliminary conclusion that the rod precursor, which resides in the ONL and therefore in a position to “detect” cone damage, may be the stem cell source of the regenerated cells.
2. Surgical Injury
The original methods of Lombardo have been modified and applied to several teleosts, including goldfish (Hitchcock et al., 1992), sunfish (Cameron and Easter, 1995), and zebrafish (Cameron, 2000). Generally, rather small portions (1–2 mm2) of adult retina are removed, using a transscleral approach, with the flap of sclera sutured back into place in the case of larger lesions. Following the injury, mitotically active cells can be found within the outer and inner nuclear layers of retina surrounding the wound, forming radial clusters reminiscent of the neurogenic clusters of the ouabain lesion (Fig. 5A, C, and F). Recently, an upregulation of proliferative activity has been documented at greater distances from the lesion, suggesting that the factors regulating proliferation/regeneration in response to damage are likely diVusible, or there exists a mechanism for the propagation of this information (Yurco and Cameron, 2005). The blastema surrounding the wound generates new retinal neurons and glia that eventually populate the lesion from the outside in, much as the CGZ generates new retinal tissue from the edge of the retina outward.
The anatomical features of the regenerated retina have also been studied following this injury paradigm. As with the ouabain model, neurons in regenerated retina show reduced spatial order, with a tendency toward a random distribution, and the cone mosaic is not properly patterned (Cameron and Carney, 2000; Stenkamp and Cameron, 2002). Laminar organization to the regenerated retina is also imprecise, with “laminar fusions” common (Hitchcock et al., 1992). However, new neurons in regenerated retina become physiologically integrated with existing, undamaged retina, as evidenced by the presence of function gap junctions among amacrine cells (Hitchcock, 1997).
3. Focal Injuries and Light Damage
In the pursuit of more precise methods for generating cell-selective damage in the teleost retina, several innovative lesioning techniques have been developed. These include the use of constant light conditions to destroy the photoreceptor layer of albino zebrafish (Vihtelic and Hyde, 2000), and the use of an argon laser focused through the cornea to heat and kill a small patch of photoreceptors (Braisted et al., 1994). Currently several alternatives are under development, such as focal application of hot or cold probes to the sclera, in order to destroy the underlying photoceptors and RPE. The goals of these studies are in part to generate models for human retinal degenerative diseases in which photoreceptors die, but also to observe more carefully the proliferative activities of the inner nuclear layer in the interests of identifying the stem cell source of regenerated retina.
The laser lesioning technique has permitted a targeted and spatially restricted evaluation of cellular activities over the time course of regenerative photoreceptor replacement (Fig. 5A, B, and E). The technique involves the use of an ophthalmic argon laser focused through the cornea, upon the photoreceptor layer. The result is heat damage and photoreceptor cell death in a 100-µm-diameter region. This damage triggers proliferation and replacement of rod and cone photoreceptors. Interestingly, when laser lesions are accompanied by intraocular injections of cell-selective concentrations of 6-HODA, dopaminergic neurons are still not replaced (Braisted et al., 1994). Therefore, cone regeneration requires outer nuclear damage, but replacement of inner retinal neurons requires damage to both inner and outer retinal layers. The mechanisms regulating retinal cell replacement in teleosts are therefore complex and not solely dependent upon signals present in the ONL.
More recent laser lesioning experiments have cast additional doubt on the notion that the signals and cells involved in regeneration reside in the ONL (Wu et al., 2001). Following a lesion, the majority of proliferating cells are localized to the INL, forming a neurogenic cluster at the lesion site similar to the clusters described for chemical lesions and the blastema described for surgical lesions. Therefore, although cone and rod photoreceptors are selectively being replaced, the neurogenic source of these regenerated cells more likely resides within the INL, or at least is not exclusively found in the ONL. These and other experiments aimed at identifying the stem cell source of regenerated retinal neurons will be discussed further in the next section.
Light damage to the photoreceptor layer of a zebrafish is most effectively accomplished using the albino (alb) strain of zebrafish (Vihtelic and Hyde, 2000). Seven days of constant light (preceded by 7 days of constant dark) results in apoptotic photoreceptor cell death very soon after lighting conditions are changed—within 24 h. Within 3 days the retina responds to damage by an increase in proliferation in the INL, as measured by BrdU incorporation. During a recovery period consisting of a standard photoperiod, these proliferating cells generate new rod and cone photoreceptors. The recovered retina displays normal lamination, but the cone mosaic is disordered, as in other regenerative models. This finding suggests that cone pattern information does not reside within the adjacent layer of the retina. The light damage studies in general also provide further support for the hypothesis that damage to the photoreceptor layer alone is sufficient to result in a regenerative response. However, these studies, like the laser lesioning experiments, demonstrate that increased mitotic activity spanned the thickness of the retina and therefore involves proliferative cells in the INL (Vihtelic and Hyde, 2000).
B. Stem Cells and Developmental Genetics of Regeneration
Two key issues regarding retinal regeneration in teleosts have received recent attention: the first is the identity of the stem cells that generate new retinal neurons following injury; the second is the degree to which the regenerative process recapitulates embryonic retinal neurogenesis.
1. Stem Cells
For the purposes of this discussion, a stem cell is considered capable of both self-renewal and of generating all cell types within the retina. This latter capacity may be condition dependent, such as following injury. Several cell types within the mature teleost retina have been considered candidates as the stem cells from which new retina is generated following injury. These include cells of the CGZ, rod precursors, other cells of the rod lineage such as PINCs, and Müller glia (Fig. 5A). The CGZ most certainly contains stem cells that meet the criteria above, although they continuously generate new retinal tissue and their activities are not dependent upon injury. Under conditions of injury, neurogenesis at the CGZ is accelerated, but has been shown not to contribute to retinal cell replacement in regions beyond the retinal periphery (i.e., cells derived from the CGZ do not migrate within the retina to replace cells at a distance) (Raymond et al., 1988; Stenkamp et al., 2001).
Early experiments utilizing the cytotoxin ouabain suggested rod precursors as the stem cell origin of regenerated retina. Because rod precursors survived the ouabain insult, and because their proliferative activity increases following injury, the rod precursor was proposed as the stem cell source of regenerated retina (Raymond et al., 1988). In addition, damage to the ONL was needed to initiate a regenerative response, and the distribution of neurogenic clusters resembled the distribution of the scattered rod precursors. Rod precursors therefore seemed the most logical candidates as the stem cell source of regenerated retina. However, these experiments, and this preliminary conjecture, predated the more complete characterization of the rod lineage.
Identification of the rod lineage in the INL (i.e., PINCs) stimulated interest in these cells as candidates for those that generate new retinal neurons of all cell types following injury. Their slow rate of proliferation was more consistent with a stem cell identity than was the rapid rate of rod precursor proliferation. In addition, the INL shows robust proliferative activity following multiple types of retinal injury. After a laser lesion, for example, BrdU is incorporated into cells in the INL, and they or their progeny migrate to the ONL and differentiate as rods or cones (Wu et al., 2001). A clear demonstration that the PINCs are indeed the source of regenerated retina is lacking, however. Indirect evidence of stem cell identity is suggested by the expression of pax6 by cells residing at the apex of the rod lineage, as expression of this marker is typical of multipotent retinal progenitor cells (Otteson and Hitchcock, 2003).
The Müller glial cell type has also received attention recently as a potential stem cell for retinal regeneration. In laser lesion experiments, Müller glia were observed to reenter the cell cycle, suggesting that they participate in the regenerative process (Wu et al., 2001). This proliferative activity has also been observed following a surgical lesion, and in this case, the nuclei of Müller glia were seen to migrate in a manner reminiscent of interkinetic nuclear migration seen in embryonic neural progenitor cells (Yurco and Cameron, 2005). Finally, in the zebrafish, proliferating Müller glia have been shown to generate neurons, as well as additional glia, following injury (Fausett and Goldman, 2006). This was demonstrated through cell lineage tracing methods with transgenic zebrafish in which the transgene is expressed in a subset of Müller glia. There is also evidence from a different model system, the chemically lesioned posthatch chicken retina, that Müller glia can divide and generate retinal neurons (Fischer and Reh, 2001). Interestingly, it is not yet clear whether multiple cell types within the teleost retina—those of the rod lineage as well as Müller glia—may act as stem cells following injury.
2. Developmental Genetics of Regeneration
Does regeneration recapitulate development? From an anatomical and histological perspective, this appears to be the case. There is also evidence that the genetic cascades described and tested for roles during embryonic retinal neurogenesis are reengaged during the regenerative process. Early in the regeneration process, within days of the lesion, cells of the neurogenic clusters express genes typical of retinal progenitors, including pax6 (Hitchcock et al., 1996), vsx-1 (Levine et al., 1994), Notch-3 (Sullivan et al., 1997), and n-cadherin (Liu et al., 2002). Preliminary studies from our laboratory have also verified transient expression of ath5 following a ouabain lesion, similar to the transient expression of ath5 during embryonic retinal neurogenesis (Spritzer et al., 2005). We have also noted delayed expression of the ganglion cell marker neurolin (zn-8), a cell-surface molecule involved in axon outgrowth and pathfinding. The expression of this marker is transient, as it is downregulated to the low levels seen in adult, undamaged retina, several weeks following the lesion. Collectively these studies support the hypothesis that regeneration recapitulates development, suggesting that information derived from the study of embryonic retinal neurogenesis can be applied to the study and potential application of the regenerative process. This hypothesis is also consistent with a wealth of anatomical studies aimed at comparative description of regenerated versus native retina. For example, all retinal cell types are present in regenerated zebrafish retina (Cameron and Carney, 2000), the full and appropriate complement of opsins and visual pigments is reestablished (Cameron et al., 1997; Mader and Cameron, 2004), though incorrectly patterned (Stenkamp et al., 2001), and regenerated ganglion cells display normal dendritic arbors (Cameron et al., 1999).
A more comprehensive evaluation of gene expression during retinal regeneration has recently been achieved through the use of zebrafish gene chip microarrays (Cameron et al., 2005). Gene expression data were collected from adult zebrafish brain, adult undamaged retina, and from adult retina 2, 3, 5, and 14 days following a surgical lesion. Genes upregulated in lesioned retina at early time points include those involved in wound healing, such as matrix metallo-proteinase 9 and chemokine CXC motif receptor 4b. In addition, genes related to cell and tissue growth were also significantly upregulated. These included α-tubulin, c-fos, progranulin, growth-associated protein 43 (GAP43), and cadherin 2. At 14 days following the lesion a number of genes related to cell cycle regulation were upregulated including protein regulator of cytokinesis 1, proliferation associated protein 100, deoxycytidine kinase, class I γ-tubulin, activating transcription factor 3, cyclin B1, and tumor suppressor p53-binding protein. At this time point genes related to cell differentiation also showed an increase in expression, such as engrailed 2b, zic2, and smad7. Interestingly, similar datasets are not yet available cataloguing gene expression over an embryonic developmental time frame. We are currently conducting gene profiling experiments using the embryonic zebrafish eye, in part to further test the hypothesis that regeneration recapitulates development.
C. Functional Recovery
Does regenerated retina actually work? Are correct synaptic connections established, including functional connections to the brain? Functional recovery has been among the most challenging issues to address in the teleost system, but a number of independent studies collectively indicate that at least some degree of visual function can be regained following retinal injury. The anatomy of regenerated retina, while not perfect, is nevertheless laminated and contains all of the cell types found in uninjured retina (Hitchcock et al., 1992; Raymond et al., 1988). In the surgical model, regenerated neurons become functionally integrated with neurons in nearby undamaged retina via gap junctions (Hitchcock, 1997), and analysis of regenerated retina by electron microscopy reveals the presence of chemical synapses (Hitchcock and Cirenza, 1994). Functional activity of these synapses has been demonstrated through the recording of electroretinograms (ERGs) several months after a ouabain lesion in goldfish (Mensinger and Powers, 1999). These ERGs, however, did not entirely recapitulate the ERGs of undamaged goldfish retina in that the photopic b-wave did not return to predamage levels. As this feature may indicate function of bipolar cells mediating cone photoreceptor input, the finding suggests defects in specific aspects of visual function. It has been suggested that these defects may be in part related to a defective cone pattern in regenerated retina (Stenkamp and Cameron, 2002).
It has also been challenging to test the hypothesis that following retinal regeneration, visual information is relayed to the brain in a functional manner. Ongoing studies from this laboratory have documented growth in diameter of the optic nerve head from 20 to 200 days following ouabain-mediated retinal destruction in the zebrafish (Sherpa and Stenkamp, unpublished observations). These data suggest that the axons of regenerated ganglion cells reach (and pass through) the optic nerve head, but they do so asynchronously. In the goldfish, recovery of functional connections of the optic tract to the tectum has been established through the use of metabolic labeling (Melzer and Powers, 2001).In these experiments, fish were subjected to visual stimuli coincident with systemic administration of [3H]deoxyglucose. This method documented both the loss of metabolic activity in tecta lacking input from a (ouabain-treated) retina, as well as recovery of this activity 80–120 days after the treatment. Finally, an incredibly simple behavioral assay based upon the dorsal light reflex has been applied to verify both unilateral loss of vision following intraocular injection of ouabain, and recovery of visual function as a consequence of retinal regeneration. Fish orient themselves in part according to the perceived direction of downwelling light; in the absence of visual information from one eye, the fish tilts its body such that the injured eye faces upward. In goldfish, a normal dorsal light reflex is regained approximately 200 days after an initial cytotoxic injury, indicating recovery of a visually mediated behavior (Mensinger and Powers, 1999). Collectively these studies provide evidence that retina regenerated after chemical injury not only develops in a manner that supports visual function, but also forms active long-distance connections with targets in the brain that mediate behavior. The teleost fish model is arguably the best established example of whole-tissue nervous system functional regeneration in vertebrates, lending considerable optimism for application of similar regenerative strategies in human disease.
V. Conclusions
The teleost fish retina has been an overwhelmingly productive resource for developmental neurobiologists with interests in retinal neurogenesis. The emergence of a developmentally tractable and genetic model, the zebrafish, has catalyzed research in embryonic retinal neurogenesis, uncovering mechanisms and activities that would not be appreciated in other models. These include the discovery of novel genes involved in retinal neurogenesis through the use of forward genetic screens, and the discovery of key spatiotemporal patterns visible only in a transparent, externally developing animal. In the future, these technical advantages, combined with high-throughput technologies for describing and quantifying gene expression, will reveal the tissue- and cell-specific gene networks coordinating these patterning activities.
The phenomenology and mechanisms underlying persistent neurogenesis and regeneration, while initially regarded as quirks of the teleosts, are now considered powerful systems for understanding the roles of retinal stem cells in a mature, functioning retina. These findings have inspired the search for cells with retinal stem cell properties in other vertebrates. Since such cells have been shown to exist, at least to a limited extent, the challenge now is to uncover the extrinsic and/or intrinsic factors that permit functional recovery in teleosts or those that block regenerative processes in mammals. Curiously, little attention has been paid to the reality that any regenerative therapy for human retinal disorders will need to take place amid underlying pathology. New models for retinal disease are currently being generated and identified in zebrafish, and these will oVer a testing ground for understanding the capacities and limitations of the regenerative process under such conditions.
Acknowledgments
Dr. Brian Link (Medical College of Wisconsin), Dr. David Cameron (SUNY Upstate Medical University), and members of the Stenkamp laboratory (Dr. Craig Stevens and Ms. Ruth Frey) provided critical evaluations of the manuscript. Dr. Cameron also kindly provided data prior to publication. Work in this laboratory is supported by National Institutes of Health Grant EY012146, a Grant-in-Aid from the Glaucoma Foundation, a Macular Degeneration Program grant from the American Health Assistance Foundation, and additional graduate and undergraduate student support through NIH Grant P20 RR016454 from the INBRE Program of the NIH/NCRR, Fight For Sight summer research fellowships, NSF 0243885 REU Site: Summer Computational Neuroscience & Technology Research Experience for Undergraduates, and undergraduate research awards from the Department of Biological Sciences.
References
- Adler R, Hatlee M. Plasticity and differentiation of embryonic retinal cells after terminal mitosis. Science. 1989;243(4889):391–393. doi: 10.1126/science.2911751. [DOI] [PubMed] [Google Scholar]
- Allison WT, Dann SG, Helvik JV, Bradley C, Moyer HD, Hawryshyn CW. Ontogeny of ultraviolet-sensitive cones in the retina of rainbow trout (Oncorhynchus mykiss) J. Comp. Neurol. 2003;461(3):294–306. doi: 10.1002/cne.10682. [DOI] [PubMed] [Google Scholar]
- Allison WT, Barthel LK, Yan B, Chi C, Ross JL, Yang X, Kuang C, Goldman DJ, Kawamura S, Raymond PA. Genetic analysis of the cone photoreceptor mosaic in zebrafish; Ft. Lauderdale, FL. Association for Research in Vision and Ophthalmology Annual Meeting; May 1–5, 2005.2005. [Google Scholar]
- Applebury ML, Antoch MP, Baxter LC, Chun LL, Falk JD, Farhangfar F, Kage K, Krzystolik MG, Lyass LA, Robbins JT. The murine cone photoreceptor: A single cone type expresses both S and M opsins with retinal spatial patterning. Neuron. 2000;27(3):513–523. doi: 10.1016/s0896-6273(00)00062-3. [DOI] [PubMed] [Google Scholar]
- Babb SG, Kotradi SM, Shah B, Chiappini-Williamson C, Bell LN, Schmeiser G, Chen E, Liu Q, Marrs JA. Zebrafish R-cadherin (Cdh4) controls visual system development and differentiation. Dev. Dyn. 2005;233(3):930–945. doi: 10.1002/dvdy.20431. [DOI] [PubMed] [Google Scholar]
- Bailey TJ, El-Hodiri H, Zhang L, Shah R, Mathers PH, Jamrich M. Regulation of vertebrate eye development by Rx genes. Int. J. Dev. Biol. 2004;48(8–9):761–770. doi: 10.1387/ijdb.041878tb. [DOI] [PubMed] [Google Scholar]
- Barabino SM, Spada F, Cotelli F, Boncinelli E. Inactivation of the zebrafish homologue of Chx10 by antisense oligonucleotides causes eye malformations similar to the ocular retardation phenotype. Mech. Dev. 1997;63(2):133–143. doi: 10.1016/s0925-4773(97)00036-1. [DOI] [PubMed] [Google Scholar]
- Beaudet L, Novales Flamarique I, Hawryshyn CW. Cone photoreceptor topography in the retina of sexually mature Pacific salmonid fishes. J. Comp. Neurol. 1997;383(1):49–59. [PubMed] [Google Scholar]
- Bernardos RL, Lentz SI, Wolfe MS, Raymond PA. Notch-Delta signaling is required for spatial patterning and Müller glia differentiation in the zebrafish retina. Dev. Biol. 2005;278(2):381–395. doi: 10.1016/j.ydbio.2004.11.018. [DOI] [PubMed] [Google Scholar]
- Biehlmaier O, Neuhauss SC, Kohler K. Onset and time course of apoptosis in the developing zebrafish retina. Cell Tissue Res. 2001;306(2):199–207. doi: 10.1007/s004410100447. [DOI] [PubMed] [Google Scholar]
- Biehlmaier O, Lampert JM, von Lintig J, Kohler K. Photoreceptor morphology is severely affected in the beta,beta-carotene-15, 15’-oxygenase (bcox) zebrafish morphant. Eur. J. Neurosci. 2005;21(1):59–68. doi: 10.1111/j.1460-9568.2004.03830.x. [DOI] [PubMed] [Google Scholar]
- Boucher SE, Hitchcock PF. Insulin-like growth factor-I binds in the inner plexiform layer and circumferential germinal zone in the retina of the goldfish. J. Comp. Neurol. 1998a;394(3):395–401. doi: 10.1002/(sici)1096-9861(19980511)394:3<395::aid-cne10>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Boucher SE, Hitchcock PF. Insulin-related growth factors stimulate proliferation of retinal progenitors in the goldfish. J. Comp. Neurol. 1998b;394(3):386–394. [PubMed] [Google Scholar]
- Bowmaker JK, Kunz YW. Ultraviolet receptors, tetrachromatic colour vision and retinal mosaics in the brown trout (Salmo trutta): Age-dependent changes. Vision Res. 1987;27(12):2101–2108. doi: 10.1016/0042-6989(87)90124-6. [DOI] [PubMed] [Google Scholar]
- Bradford RL, Wang C, Zack DJ, Adler R. Roles of cell-intrinsic and microenvironmental factors in photoreceptor cell differentiation. Dev. Biol. 2005;286(1):31–45. doi: 10.1016/j.ydbio.2005.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braisted JE, Raymond PA. Regeneration of dopaminergic neurons in goldfish retina. Development. 1992;114(4):913–919. doi: 10.1242/dev.114.4.913. [DOI] [PubMed] [Google Scholar]
- Braisted JE, Raymond PA. Continued search for the cellular signals that regulate regeneration of dopaminergic neurons in goldfish retina. Brain Res. Dev. Brain Res. 1993;76(2):221–232. doi: 10.1016/0165-3806(93)90210-2. [DOI] [PubMed] [Google Scholar]
- Braisted JE, Essman TF, Raymond PA. Selective regeneration of photoreceptors in goldfish retina. Development. 1994;120(9):2409–2419. doi: 10.1242/dev.120.9.2409. [DOI] [PubMed] [Google Scholar]
- Branchek T, Bremiller R. The development of photoreceptors in the zebrafish, Brachydanio rerio. I. Structure. J. Comp. Neurol. 1984;224(1):107–115. doi: 10.1002/cne.902240109. [DOI] [PubMed] [Google Scholar]
- Browman HI, Hawryshyn CW. Thyroxine induces a precocial loss of ultraviolet photosensitivity in rainbow trout (Oncorhynchus mykiss, Teleostei) Vision Res. 1992;32(12):2303–2312. doi: 10.1016/0042-6989(92)90094-y. [DOI] [PubMed] [Google Scholar]
- Browman H, Hawryshyn C. Retinoic acid modulates retinal development in the juveniles of a teleost fish. J. Exp. Biol. 1994;193(1):191–207. doi: 10.1242/jeb.193.1.191. [DOI] [PubMed] [Google Scholar]
- Bugge TH, Pohl J, Lonnoy O, Stunnenberg HG. RXR alpha, a promiscuous partner of retinoic acid and thyroid hormone receptors. EMBO J. 1992;11(4):1409–1418. doi: 10.1002/j.1460-2075.1992.tb05186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron DA. Cellular proliferation and neurogenesis in the injured retina of adult zebrafish. Vis. Neurosci. 2000;17(5):789–797. doi: 10.1017/s0952523800175121. [DOI] [PubMed] [Google Scholar]
- Cameron DA, Carney LH. Cell mosaic patterns in the native and regenerated inner retina of zebrafish: Implications for retinal assembly. J. Comp. Neurol. 2000;416(3):356–367. [PubMed] [Google Scholar]
- Cameron DA, Carney LH. Cellular patterns in the inner retina of adult zebrafish: Quantitative analyses and a computational model of their formation. J. Comp. Neurol. 2004;471(1):11–25. doi: 10.1002/cne.11040. [DOI] [PubMed] [Google Scholar]
- Cameron DA, Easter SS., Jr Cone photoreceptor regeneration in adult fish retina: Phenotypic determination and mosaic pattern formation. J. Neurosci. 1995;15(3 Pt. 2):2255–2271. doi: 10.1523/JNEUROSCI.15-03-02255.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron DA, Cornwall MC, MacNichol EF., Jr Visual pigment assignments in regenerated retina. J. Neurosci. 1997;17(3):917–923. doi: 10.1523/JNEUROSCI.17-03-00917.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron DA, Vafai H, White JA. Analysis of dendritic arbors of native and regenerated ganglion cells in the goldfish retina. Vis. Neurosci. 1999;16(2):253–261. doi: 10.1017/s0952523899162060. [DOI] [PubMed] [Google Scholar]
- Cameron DA, Gentile KL, Middleton FA, Yurco P. Gene expression profiles of intact and regenerating zebrafish retina. Mol. Vis. 2005;11:775–791. [PubMed] [Google Scholar]
- Chen J, Rattner A, Nathans J. The rod photoreceptor-specific nuclear receptor Nr2e3 represses transcription of multiple cone-specific genes. J. Neurosci. 2005;25(1):118–129. doi: 10.1523/JNEUROSCI.3571-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CL, Novales Flamarique I. Opsin expression: New mechanism for modulating colour vision. Nature. 2004;428(6980):279–280. doi: 10.1038/428279a. [DOI] [PubMed] [Google Scholar]
- Chiu JF, Mack AF, Fernald RD. Daily rhythm of cell proliferation in the teleost retina. Brain Res. 1995;673(1):119–125. doi: 10.1016/0006-8993(94)01411-a. [DOI] [PubMed] [Google Scholar]
- Chow RL, Altmann CR, Lang RA, Hemmati-Brivanlou A. Pax6 induces ectopic eyes in a vertebrate. Development. 1999;126(19):4213–4222. doi: 10.1242/dev.126.19.4213. [DOI] [PubMed] [Google Scholar]
- Chuang JC, Raymond PA. Zebrafish genes rx1 and rx2 help define the region of forebrain that gives rise to retina. Dev. Biol. 2001;231(1):13–30. doi: 10.1006/dbio.2000.0125. [DOI] [PubMed] [Google Scholar]
- Chuang JC, Mathers PH, Raymond PA. Expression of three Rx homeobox genes in embryonic and adult zebrafish. Mech. Dev. 1999;84(1–2):195–198. doi: 10.1016/s0925-4773(99)00077-5. [DOI] [PubMed] [Google Scholar]
- Cohen MM., Jr The hedgehog signaling network. Am. J. Med. Genet. A. 2003;123(1):5–28. doi: 10.1002/ajmg.a.20495. [DOI] [PubMed] [Google Scholar]
- Cole LK, Ross LS. Apoptosis in the developing zebrafish embryo. Dev. Biol. 2001;240(1):123–142. doi: 10.1006/dbio.2001.0432. [DOI] [PubMed] [Google Scholar]
- Corbo JC, Cepko CL. A hybrid photoreceptor expressing both rod and cone genes in a mouse model of enhanced S-cone syndrome. PLoS Genet. 2005;1(2):e11. doi: 10.1371/journal.pgen.0010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCarvalho AC, Cappendijk SL, Fadool JM. Developmental expression of the POU domain transcription factor Brn-3b (Pou4f2) in the lateral line and visual system of zebrafish. Dev. Dyn. 2004;229(4):869–876. doi: 10.1002/dvdy.10475. [DOI] [PubMed] [Google Scholar]
- Dominguez M. Dual role for hedgehog in the regulation of the proneural gene atonal during ommatidia development. Development. 1999;126(11):2345–2353. doi: 10.1242/dev.126.11.2345. [DOI] [PubMed] [Google Scholar]
- Dyer MA. Regulation of proliferation, cell fate specification and differentiation by the homeodomain proteins Prox1, Six3, and Chx10 in the developing retina. Cell Cycle. 2003;2(4):350–357. [PubMed] [Google Scholar]
- Easter SS, Jr, Malicki JJ. The zebrafish eye: Developmental and genetic analysis. Results Probl. Cell Differ. 2002;40:346–370. doi: 10.1007/978-3-540-46041-1_17. [DOI] [PubMed] [Google Scholar]
- Evans BI, Harosi FI, Fernald RD. Photoreceptor spectral absorbance in larval and adult winter flounder (Pseudopleuronectes americanus) Vis. Neurosci. 1993;10(6):1065–1071. doi: 10.1017/s0952523800010178. [DOI] [PubMed] [Google Scholar]
- Fadool JM. Development of a rod photoreceptor mosaic revealed in transgenic zebrafish. Dev. Biol. 2003;258(2):277–290. doi: 10.1016/s0012-1606(03)00125-8. [DOI] [PubMed] [Google Scholar]
- Fadool JM, Brockerhoff SE, Hyatt GA, Dowling JE. Mutations affecting eye morphology in the developing zebrafish (Danio rerio) Dev. Genet. 1997;20(3):288–295. doi: 10.1002/(SICI)1520-6408(1997)20:3<288::AID-DVG11>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Fausett BV, Goldman D. A role for alpha1 tubulin-expressing Muller glia in regeneration of the injured zebrafish retina. J. Neurosci. 2006;26(23):6303–6313. doi: 10.1523/JNEUROSCI.0332-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer AJ, Reh TA. Identification of a proliferating marginal zone of retinal progenitors in postnatal chickens. Dev. Biol. 2000;220(2):197–210. doi: 10.1006/dbio.2000.9640. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Reh TA. Muller glia are a potential source of neural regeneration in the postnatal chicken retina. Nat. Neurosci. 2001;4(3):247–252. doi: 10.1038/85090. [DOI] [PubMed] [Google Scholar]
- Flamarique IN. Gradual and partial loss of corner cone-occupied area in the retina of rainbow trout. Vision Res. 2001;41(24):3073–3082. doi: 10.1016/s0042-6989(01)00201-2. [DOI] [PubMed] [Google Scholar]
- Furukawa T, Morrow EM, Cepko CL. Crx, a novel otx-like homeobox gene, shows photoreceptor-specific expression and regulates photoreceptor differentiation. Cell. 1997;91(4):531–541. doi: 10.1016/s0092-8674(00)80439-0. [DOI] [PubMed] [Google Scholar]
- Gehring WJ. The master control gene for morphogenesis and evolution of the eye. Genes Cells. 1996;1(1):11–15. doi: 10.1046/j.1365-2443.1996.11011.x. [DOI] [PubMed] [Google Scholar]
- Gregg RG, Willer GB, Fadool JM, Dowling JE, Link BA. Positional cloning of the young mutation identifies an essential role for the Brahma chromatin remodeling complex in mediating retinal cell differentiation. Proc. Natl. Acad. Sci. USA. 2003;100(11):6535–6540. doi: 10.1073/pnas.0631813100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn JS, Pinter JD. Holoprosencephaly: Genetic, neuroradiological, and clinical advances. Semin. Pediatr. Neurol. 2002;9(4):309–319. doi: 10.1053/spen.2002.32507. [DOI] [PubMed] [Google Scholar]
- Heisenberg CP, Brennan C, Wilson SW. Zebrafish aussicht mutant embryos exhibit widespread overexpression of ace (fgf8) and coincident defects in CNS development. Development. 1999;126(10):2129–2140. doi: 10.1242/dev.126.10.2129. [DOI] [PubMed] [Google Scholar]
- Helvik JV, Drivenes O, Harboe T, Seo HC. Topography of different photoreceptor cell types in the larval retina of Atlantic halibut (Hippoglossus hippoglossus) J. Exp. Biol. 2001;204(Pt. 14):2553–2559. doi: 10.1242/jeb.204.14.2553. [DOI] [PubMed] [Google Scholar]
- Henderson RG, Fernald RD. Timing and location of rhodopsin expression in newly born rod photoreceptors in the adult teleost retina. Brain Res. Dev. Brain Res. 2004;151(1–2):193–197. doi: 10.1016/j.devbrainres.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Hitchcock PF. Tracer coupling among regenerated amacrine cells in the retina of the goldfish. Vis. Neurosci. 1997;14(3):463–472. doi: 10.1017/s095252380001213x. [DOI] [PubMed] [Google Scholar]
- Hitchcock PF, Cirenza P. Synaptic organization of regenerated retina in the goldfish. J. Comp. Neurol. 1994;343(4):609–616. doi: 10.1002/cne.903430410. [DOI] [PubMed] [Google Scholar]
- Hitchcock P, Kakuk-Atkins L. The basic helix-loop-helix transcription factor neuroD is expressed in the rod lineage of the teleost retina. J. Comp. Neurol. 2004;477(1):108–117. doi: 10.1002/cne.20244. [DOI] [PubMed] [Google Scholar]
- Hitchcock PF, Lindsey Myhr KJ, Easter SS, Jr, Mangione-Smith R, Jones DD. Local regeneration in the retina of the goldfish. J. Neurobiol. 1992;23(2):187–203. doi: 10.1002/neu.480230209. [DOI] [PubMed] [Google Scholar]
- Hitchcock PF, Macdonald RE, VanDeRyt JT, Wilson SW. Antibodies against Pax6 immunostain amacrine and ganglion cells and neuronal progenitors, but not rod precursors, in the normal and regenerating retina of the goldfish. J. Neurobiol. 1996;29(3):399–413. doi: 10.1002/(SICI)1097-4695(199603)29:3<399::AID-NEU10>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Hitchcock P, Ochocinska M, Sieh A, Otteson D. Persistent and injury-induced neurogenesis in the vertebrate retina. Prog. Retin. Eye Res. 2004;23(2):183–194. doi: 10.1016/j.preteyeres.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Hu M, Easter SS. Retinal neurogenesis: The formation of the initial central patch of postmitotic cells. Dev. Biol. 1999;207(2):309–321. doi: 10.1006/dbio.1998.9031. [DOI] [PubMed] [Google Scholar]
- Hyatt GA, Schmitt EA, Marsh-Armstrong NR, Dowling JE. Retinoic acid-induced duplication of the zebrafish retina. Proc. Natl. Acad. Sci. USA. 1992;89(17):8293–8297. doi: 10.1073/pnas.89.17.8293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyatt GA, Schmitt EA, Fadool JM, Dowling JE. Retinoic acid alters photoreceptor development in vivo. Proc. Natl. Acad. Sci. USA. 1996a;93(23):13298–13303. doi: 10.1073/pnas.93.23.13298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyatt GA, Schmitt EA, Marsh-Armstrong N, McCaffery P, Drager UC, Dowling JE. Retinoic acid establishes ventral retinal characteristics. Development. 1996b;122(1):195–204. doi: 10.1242/dev.122.1.195. [DOI] [PubMed] [Google Scholar]
- Inui Y, Miwa S. Thyroid hormone induces metamorphosis of flounder larvae. Gen. Comp. Endocrinol. 1985;60(3):450–454. doi: 10.1016/0016-6480(85)90080-2. [DOI] [PubMed] [Google Scholar]
- Jarman AP, Grell EH, Ackerman L, Jan LY, Jan YN. Atonal is the proneural gene for Drosophila photoreceptors. Nature. 1994;369(6479):398–400. doi: 10.1038/369398a0. [DOI] [PubMed] [Google Scholar]
- Johns PR. Growth of the adult goldfish eye. III. Source of the new retinal cells. J. Comp. Neurol. 1977;176(3):343–357. doi: 10.1002/cne.901760304. [DOI] [PubMed] [Google Scholar]
- Johns PR. Formation of photoreceptors in larval and adult goldfish. J. Neurosci. 1982;2(2):178–198. doi: 10.1523/JNEUROSCI.02-02-00178.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns PR, Easter SS., Jr Growth of the adult goldfish eye. II. Increase in retinal cell number. J. Comp. Neurol. 1977;176(3):331–341. doi: 10.1002/cne.901760303. [DOI] [PubMed] [Google Scholar]
- Johns PR, Fernald RD. Genesis of rods in teleost fish retina. Nature. 1981;293(5828):141–142. doi: 10.1038/293141a0. [DOI] [PubMed] [Google Scholar]
- Julian D, Ennis K, Korenbrot JI. Birth and fate of proliferative cells in the inner nuclear layer of the mature fish retina. J. Comp. Neurol. 1998;394(3):271–282. [PubMed] [Google Scholar]
- Kageyama R, Ishibashi M, Takebayashi K, Tomita K. bHLH transcription factors and mammalian neuronal differentiation. Int. J. Biochem. Cell Biol. 1997;29(12):1389–1399. doi: 10.1016/s1357-2725(97)89968-2. [DOI] [PubMed] [Google Scholar]
- Katoh Y, Katoh M. Comparative genomics on FGF7, FGF10, FGF22 orthologs, and identification of fgf25. Int. J. Mol. Med. 2005;16(4):767–770. [PubMed] [Google Scholar]
- Kay JN, Finger-Baier KC, Roeser T, Staub W, Baier H. Retinal ganglion cell genesis requires lakritz, a Zebrafish atonal homolog. Neuron. 2001;30(3):725–736. doi: 10.1016/s0896-6273(01)00312-9. [DOI] [PubMed] [Google Scholar]
- Kay JN, Link BA, Baier H. Staggered cell-intrinsic timing of ath5 expression underlies the wave of ganglion cell neurogenesis in the zebrafish retina. Development. 2005;132(11):2573–2585. doi: 10.1242/dev.01831. [DOI] [PubMed] [Google Scholar]
- Kelley MW, Turner JK, Reh TA. Retinoic acid promotes differentiation of photoreceptors in vitro. Development. 1994;120(8):2091–2102. doi: 10.1242/dev.120.8.2091. [DOI] [PubMed] [Google Scholar]
- Knight JK, Raymond PA. Time course of opsin expression in developing rod photoreceptors. Development. 1990;110(4):1115–1120. doi: 10.1242/dev.110.4.1115. [DOI] [PubMed] [Google Scholar]
- Korzh V, Sleptsova I, Liao J, He J, Gong Z. Expression of zebrafish bHLH genes ngn1 and nrd defines distinct stages of neural differentiation. Dev. Dyn. 1998;213(1):92–104. doi: 10.1002/(SICI)1097-0177(199809)213:1<92::AID-AJA9>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Kunz YW, Wildenburg G, Goodrich L, Callaghan E. The fate of ultraviolet receptors in the retina of the Atlantic salmon (Salmo salar) Vision Res. 1994;34(11):1375–1383. doi: 10.1016/0042-6989(94)90136-8. [DOI] [PubMed] [Google Scholar]
- Lauderdale JD, Wilensky JS, Oliver ER, Walton DS, Glaser T. 3’ deletions cause aniridia by preventing PAX6 gene expression. Proc. Natl. Acad. Sci. USA. 2000;97(25):13755–13759. doi: 10.1073/pnas.240398797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine EM, Hitchcock PF, Glasgow E, Schechter N. Restricted expression of a new paired-class homeobox gene in normal and regenerating adult goldfish retina. J. Comp. Neurol. 1994;348(4):596–606. doi: 10.1002/cne.903480409. [DOI] [PubMed] [Google Scholar]
- Li Z, Joseph NM, Easter SS., Jr The morphogenesis of the zebrafish eye, including a fate map of the optic vesicle. Dev. Dyn. 2000;218(1):175–188. doi: 10.1002/(SICI)1097-0177(200005)218:1<175::AID-DVDY15>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Link BA, Fadool JM, Malicki J, Dowling JE. The zebrafish young mutation acts non-cell-autonomously to uncouple differentiation from specification for all retinal cells. Development. 2000;127(10):2177–2188. doi: 10.1242/dev.127.10.2177. [DOI] [PubMed] [Google Scholar]
- Liu Q, Londraville RL, Azodi E, Babb SG, Chiappini-Williamson C, Marrs JA, Raymond PA. Up-regulation of cadherin-2 and cadherin-4 in regenerating visual structures of adult zebrafish. Exp. Neurol. 2002;177(2):396–406. doi: 10.1006/exnr.2002.8008. [DOI] [PubMed] [Google Scholar]
- Liu W, Mo Z, Xiang M. The Ath5 proneural genes function upstream of Brn3 POU domain transcription factor genes to promote retinal ganglion cell development. Proc. Natl. Acad. Sci. USA. 2001;98(4):1649–1654. doi: 10.1073/pnas.98.4.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Shen Y, Rest JS, Raymond PA, Zack DJ. Isolation and characterization of a zebrafish homologue of the cone rod homeobox gene. Invest. Ophthalmol. Vis. Sci. 2001;42(2):481–487. [PubMed] [Google Scholar]
- Livesey FJ, Cepko CL. Vertebrate neural cell-fate determination: Lessons from the retina. Nat. Rev. Neurosci. 2001;2(2):109–118. doi: 10.1038/35053522. [DOI] [PubMed] [Google Scholar]
- Lombardo R. The regeneration of the retina in the adult teleost. Accad. Lincei-Rendtconti Sci. Fisic. Matemat. Nat. 1968;45:631–635. [Google Scholar]
- Lombardo R. Course and localization of mitoses during the regeneration of the retina of an adult teleost. Accad. Lincei-Rendtconti Sci. Fisic. Matemat. Nat. 1972;53:323–327. [Google Scholar]
- Loosli F, Winkler S, Burgtorf C, Wurmbach E, Ansorge W, Henrich T, Grabher C, Arendt D, Carl M, Krone A, Grzebisz E, Wittbrodt J. Medaka eyeless is the key factor linking retinal determination and eye growth. Development. 2001;128(20):4035–4044. doi: 10.1242/dev.128.20.4035. [DOI] [PubMed] [Google Scholar]
- Loosli F, Staub W, Finger-Baier KC, Ober EA, Verkade H, Wittbrodt J, Baier H. Loss of eyes in zebrafish caused by mutation of chokh/rx3. EMBO Rep. 2003;4(9):894–899. doi: 10.1038/sj.embor.embor919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald R, Barth KA, Xu Q, Holder N, Mikkola I, Wilson SW. Midline signalling is required for Pax gene regulation and patterning of the eyes. Development. 1995;121(10):3267–3278. doi: 10.1242/dev.121.10.3267. [DOI] [PubMed] [Google Scholar]
- Macdonald R, Scholes J, Strahle U, Brennan C, Holder N, Brand M, Wilson SW. The Pax protein Noi is required for commissural axon pathway formation in the rostral forebrain. Development. 1997;124(12):2397–2408. doi: 10.1242/dev.124.12.2397. [DOI] [PubMed] [Google Scholar]
- Mack AF, Fernald RD. Regulation of cell division and rod differentiation in the teleost retina. Brain Res. Dev. Brain Res. 1993;76(2):183–187. doi: 10.1016/0165-3806(93)90206-p. [DOI] [PubMed] [Google Scholar]
- Mack AF, Fernald RD. New rods move before differentiating in adult teleost retina. Dev. Biol. 1995;170(1):136–141. doi: 10.1006/dbio.1995.1202. [DOI] [PubMed] [Google Scholar]
- Mack AF, Fernald RD. Cell movement and cell cycle dynamics in the retina of the adult teleost Haplochromis burtoni. J. Comp. Neurol. 1997;388(3):435–443. [PubMed] [Google Scholar]
- Mader MM, Cameron DA. Photoreceptor differentiation during retinal development, growth, and regeneration in a metamorphic vertebrate. J. Neurosci. 2004;24(50):11463–11472. doi: 10.1523/JNEUROSCI.3343-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mader MM, Cameron DA. Effects of induced systemic hypothyroidism upon the retina: Regulation of thyroid horomone receptor alpha and photoreceptor production. Mol. Vis. 2006;12:915–930. [PubMed] [Google Scholar]
- Maier W, Wolburg H. Regeneration of the goldfish retina after exposure to different doses of ouabain. Cell Tissue Res. 1979;202(1):99–118. doi: 10.1007/BF00239223. [DOI] [PubMed] [Google Scholar]
- Malicki J, Neuhauss SC, Schier AF, Solnica-Krezel L, Stemple DL, Stainier DY, Abdelilah S, Zwartkruis F, Rangini Z, Driever W. Mutations affecting development of the zebrafish retina. Development. 1996;123:263–273. doi: 10.1242/dev.123.1.263. [DOI] [PubMed] [Google Scholar]
- Malicki J, Jo H, Pujic Z. Zebrafish N-cadherin, encoded by the glass onion locus, plays an essential role in retinal patterning. Dev. Biol. 2003;259(1):95–108. doi: 10.1016/s0012-1606(03)00181-7. [DOI] [PubMed] [Google Scholar]
- Marcus RC, Delaney CL, Easter SS., Jr Neurogenesis in the visual system of embryonic and adult zebrafish (Danio rerio). off. Vis. Neurosci. 1999;16(3):417–424. doi: 10.1017/s095252389916303x. [DOI] [PubMed] [Google Scholar]
- Marsh-Armstrong N, McCaffery P, Gilbert W, Dowling JE, Drager UC. Retinoic acid is necessary for development of the ventral retina in zebrafish. Proc. Natl. Acad. Sci. USA. 1994;91(15):7286–7290. doi: 10.1073/pnas.91.15.7286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Morales JR, Del Bene F, Nica G, Hammerschmidt M, Bovolenta P, Wittbrodt J. Differentiation of the vertebrate retina is coordinated by an FGF signaling center. Dev. Cell. 2005;8(4):565–574. doi: 10.1016/j.devcel.2005.01.022. [DOI] [PubMed] [Google Scholar]
- Masai I, Stemple DL, Okamoto H, Wilson SW. Midline signals regulate retinal neurogenesis in zebrafish. Neuron. 2000;27(2):251–263. doi: 10.1016/s0896-6273(00)00034-9. [DOI] [PubMed] [Google Scholar]
- Masai I, Yamaguchi M, Tonou-Fujimori N, Komori A, Okamoto H. The hedgehog-PKA pathway regulates two distinct steps of the differentiation of retinal ganglion cells: The cell-cycle exit of retinoblasts and their neuronal maturation. Development. 2005;132(7):1539–1553. doi: 10.1242/dev.01714. [DOI] [PubMed] [Google Scholar]
- Mathers PH, Grinberg A, Mahon KA, Jamrich M. The Rx homeobox gene is essential for vertebrate eye development. Nature. 1997;387(6633):603–607. doi: 10.1038/42475. [DOI] [PubMed] [Google Scholar]
- McCabe KL, Gunther EC, Reh TA. The development of the pattern of retinal ganglion cells in the chick retina: Mechanisms that control differentiation. Development. 1999;126(24):5713–5724. doi: 10.1242/dev.126.24.5713. [DOI] [PubMed] [Google Scholar]
- Melzer P, Powers MK. Metabolic activity in optic tectum during regeneration of retina in adult goldfish. Vis. Neurosci. 2001;18(4):599–604. doi: 10.1017/s0952523801184099. [DOI] [PubMed] [Google Scholar]
- Mensinger AF, Powers MK. Visual function in regenerating teleost retina following cytotoxic lesioning. Vis. Neurosci. 1999;16(2):241–251. doi: 10.1017/s0952523899162059. [DOI] [PubMed] [Google Scholar]
- Morrow EM, Furukawa T, Lee JE, Cepko CL. NeuroD regulates multiple functions in the developing neural retina in rodent. Development. 1999;126(1):23–36. doi: 10.1242/dev.126.1.23. [DOI] [PubMed] [Google Scholar]
- Moshiri A, Reh TA. Persistent progenitors at the retinal margin of ptc+/− mice. J. Neurosci. 2004;24(1):229–237. doi: 10.1523/JNEUROSCI.2980-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negishi K, Stell WK, Takasaki Y. Early histogenesis of the teleostean retina: Studies using a novel immunochemical marker, proliferating cell nuclear antigen (PCNA/cyclin) Brain Res. Dev. Brain Res. 1990;55(1):121–125. doi: 10.1016/0165-3806(90)90112-c. [DOI] [PubMed] [Google Scholar]
- Neumann CJ, Nuesslein-Volhard C. Patterning of the zebrafish retina by a wave of sonic hedgehog activity. Science. 2000;289(5487):2137–2139. doi: 10.1126/science.289.5487.2137. [DOI] [PubMed] [Google Scholar]
- Ng L, Hurley JB, Dierks B, Srinivas M, Salto C, Vennstrom B, Reh TA, Forrest D. A thyroid hormone receptor that is required for the development of green cone photoreceptors. Nat. Genet. 2001;27(1):94–98. doi: 10.1038/83829. [DOI] [PubMed] [Google Scholar]
- Nornes S, Clarkson M, Mikkola I, Pedersen M, Bardsley A, Martinez JP, Krauss S, Johansen T. Zebrafish contains two pax6 genes involved in eye development. Mech. Dev. 1998;77(2):185–196. doi: 10.1016/s0925-4773(98)00156-7. [DOI] [PubMed] [Google Scholar]
- Otteson DC, Hitchcock PF. Stem cells in the teleost retina: Persistent neurogenesis and injury-induced regeneration. Vision Res. 2003;43(8):927–936. doi: 10.1016/s0042-6989(02)00400-5. [DOI] [PubMed] [Google Scholar]
- Otteson DC, D’Costa AR, Hitchcock PF. Putative stem cells and the lineage of rod photoreceptors in the mature retina of the goldfish. Dev. Biol. 2001;232(1):62–76. doi: 10.1006/dbio.2001.0163. [DOI] [PubMed] [Google Scholar]
- Otteson DC, Cirenza PF, Hitchcock PF. Persistent neurogenesis in the teleost retina: Evidence for regulation by the growth-hormone/insulin-like growth factor-I axis. Mech. Dev. 2002;117(1–2):137–149. doi: 10.1016/s0925-4773(02)00188-0. [DOI] [PubMed] [Google Scholar]
- Passini MA, Raymond PA, Schechter N. Vsx-2, a gene encoding a paired-type homeodomain, is expressed in the retina, hindbrain, and spinal cord during goldfish embryogenesis. Brain Res. Dev. Brain Res. 1998;109(2):129–135. doi: 10.1016/s0165-3806(98)00069-8. [DOI] [PubMed] [Google Scholar]
- Perron M, Kanekar S, Vetter ML, Harris WA. The genetic sequence of retinal development in the ciliary margin of the Xenopus eye. Dev. Biol. 1998;199(2):185–200. doi: 10.1006/dbio.1998.8939. [DOI] [PubMed] [Google Scholar]
- Perz-Edwards A, Hardison NL, Linney E. Retinoic acid-mediated gene expression in transgenic reporter zebrafish. Dev. Biol. 2001;229(1):89–101. doi: 10.1006/dbio.2000.9979. [DOI] [PubMed] [Google Scholar]
- Picker A, Brand M. Fgf signals from a novel signaling center determine axial patterning of the prospective neural retina. Development. 2005;132(22):4951–4962. doi: 10.1242/dev.02071. [DOI] [PubMed] [Google Scholar]
- Poggi L, Vitorino M, Masai I, Harris WA. Influences on neural lineage and mode of division in the zebrafish retina in vivo. J. Cell Biol. 2005;171(6):991–999. doi: 10.1083/jcb.200509098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhudesai SN, Cameron DA, Stenkamp DL. Targeted effects of retinoic acid signaling upon photoreceptor development in zebrafish. Dev. Biol. 2005;287(1):157–167. doi: 10.1016/j.ydbio.2005.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujic Z, Malicki J. Mutation of the zebrafish glass onion locus causes early cell-nonautonomous loss of neuroepithelial integrity followed by severe neuronal patterning defects in the retina. Dev. Biol. 2001;234(2):454–469. doi: 10.1006/dbio.2001.0251. [DOI] [PubMed] [Google Scholar]
- Quiring R, Walldorf U, Kloter U, Gehring WJ. Homology of the eyeless gene of Drosophila to the Small eye gene in mice and Aniridia in humans. Science. 1994;265(5173):785–789. doi: 10.1126/science.7914031. [DOI] [PubMed] [Google Scholar]
- Raymond PA. Cytodifferentiation of photoreceptors in larval goldfish: Delayed maturation of rods. J. Comp. Neurol. 1985;236(1):90–105. doi: 10.1002/cne.902360108. [DOI] [PubMed] [Google Scholar]
- Raymond PA, Barthel LK. A moving wave patterns the cone photoreceptor mosaic array in the zebrafish retina. Int. J. Dev. Biol. 2004;48(8–9):935–945. doi: 10.1387/ijdb.041873pr. [DOI] [PubMed] [Google Scholar]
- Raymond PA, Rivlin PK. Germinal cells in the goldfish retina that produce rod photoreceptors. Dev. Biol. 1987;122(1):120–138. doi: 10.1016/0012-1606(87)90338-1. [DOI] [PubMed] [Google Scholar]
- Raymond PA, Easter SS, Jr, Burnham JA, Powers MK. Postembryonic growth of the optic tectum in goldfish. II. Modulation of cell proliferation by retinal fiber input. J. Neurosci. 1983;3(5):1092–1099. doi: 10.1523/JNEUROSCI.03-05-01092.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond PA, Reifler MJ, Rivlin PK. Regeneration of goldfish retina: Rod precursors are a likely source of regenerated cells. J. Neurobiol. 1988;19(5):431–463. doi: 10.1002/neu.480190504. [DOI] [PubMed] [Google Scholar]
- Raymond PA, Barthel LK, Rounsifer ME, Sullivan SA, Knight JK. Expression of rod and cone visual pigments in goldfish and zebrafish: A rhodopsin-like gene is expressed in cones. Neuron. 1993;10(6):1161–1174. doi: 10.1016/0896-6273(93)90064-x. [DOI] [PubMed] [Google Scholar]
- Raymond PA, Barthel LK, Curran GA. Developmental patterning of rod and cone photoreceptors in embryonic zebrafish. J. Comp. Neurol. 1995;359(4):537–550. doi: 10.1002/cne.903590403. [DOI] [PubMed] [Google Scholar]
- Reh TA, Kljavin IJ. Age of differentiation determines rat retinal germinal cell phenotype: Induction of differentiation by dissociation. J. Neurosci. 1989;9(12):4179–4189. doi: 10.1523/JNEUROSCI.09-12-04179.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts MR, Hendrickson A, McGuire CR, Reh TA. Retinoid X receptor (gamma) is necessary to establish the S-opsin gradient in cone photoreceptors of the developing mouse retina. Invest. Ophthalmol. Vis. Sci. 2005;46(8):2897–2904. doi: 10.1167/iovs.05-0093. [DOI] [PubMed] [Google Scholar]
- Rockhill RL, Euler T, Masland RH. Spatial order within but not between types of retinal neurons. Proc. Natl. Acad. Sci. USA. 2000;97(5):2303–2307. doi: 10.1073/pnas.030413497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas-Munoz A, Dahm R, Nusslein-Volhard C. chokh/rx3 specifies the retinal pigment epithelium fate independently of eye morphogenesis. Dev. Biol. 2005;288(2):348–362. doi: 10.1016/j.ydbio.2005.08.046. [DOI] [PubMed] [Google Scholar]
- Romano G. The complex biology of the receptor for the insulin-like growth factor-1. Drug News Perspect. 2003;16(8):525–531. doi: 10.1358/dnp.2003.16.8.829351. [DOI] [PubMed] [Google Scholar]
- Scheer N, Groth A, Hans S, Campos-Ortega JA. An instructive function for Notch in promoting gliogenesis in the zebrafish retina. Development. 2001;128(7):1099–1107. doi: 10.1242/dev.128.7.1099. [DOI] [PubMed] [Google Scholar]
- Schmitt EA, Dowling JE. Comparison of topographical patterns of ganglion and photoreceptor cell differentiation in the retina of the zebrafish, Danio rerio. J. Comp. Neurol. 1996;371(2):222–234. doi: 10.1002/(SICI)1096-9861(19960722)371:2<222::AID-CNE3>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Shen YC, Raymond PA. Zebrafish cone-rod (crx) homeobox gene promotes retinogenesis. Dev. Biol. 2004;269(1):237–251. doi: 10.1016/j.ydbio.2004.01.037. [DOI] [PubMed] [Google Scholar]
- Shkumatava A, Neumann CJ. Shh directs cell-cycle exit by activating p57Kip2 in the zebrafish retina. EMBO Rep. 2005;6(6):563–569. doi: 10.1038/sj.embor.7400416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shkumatava A, Fischer S, Muller F, Strahle U, Neumann CJ. Sonic hedgehog, secreted by amacrine cells, acts as a short-range signal to direct differentiation and lamination in the zebrafish retina. Development. 2004;131(16):3849–3858. doi: 10.1242/dev.01247. [DOI] [PubMed] [Google Scholar]
- Shupe JM, Kristan DM, Austad SN, Stenkamp DL. The eye of the laboratory mouse remains anatomically adapted for natural conditions. Brain Behav. Evol. 2005;67(1):39–52. doi: 10.1159/000088857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderpalm AK, Fox DA, Karlsson JO, van Veen T. Retinoic acid produces rod photoreceptor selective apoptosis in developing mammalian retina. Invest. Ophthalmol. Vis. Sci. 2000;41(3):937–947. [PubMed] [Google Scholar]
- Spritzer SD, Mallory DE, Sand J, Stenkamp DL. Preliminary analysis of the developmental genetics of ganglion cell regeneration in zebrafish; Ft. Lauderdale, FL. Association for Research in Vision and Ophthalmology Annual Meeting; May 1–5, 2005.2005. [Google Scholar]
- Stenkamp DL, Cameron DA. Cellular pattern formation in the retina: Retinal regeneration as a model system. Mol. Vis. 2002;8:280–293. [PubMed] [Google Scholar]
- Stenkamp DL, Frey RA. Extraretinal and retinal hedgehog signaling sequentially regulate retinal differentiation in zebrafish. Dev. Biol. 2003a;258(2):349–363. doi: 10.1016/s0012-1606(03)00121-0. [DOI] [PubMed] [Google Scholar]
- Stenkamp DL, Frey RA. Temporally-selective induction of hedgehog signaling promotes photoreceptor differentiation in embryonic zebrafish; Ft. Lauderdale, FL. Association for Research in Vision and Ophthalmology Annual Meeting; May 4–8, 2003.2003b. [Google Scholar]
- Stenkamp DL, Wardwell SL. Developmental genetics of the rod photoreceptor lineage in the zebrafish retina; Ft. Lauderdale, FL. Association for Research in Vision and Ophthalmology Annual Meeting; April 25–29, 2005.2004. [Google Scholar]
- Stenkamp DL, Gregory JK, Adler R. Retinoid effects in purified cultures of chick embryo retina neurons and photoreceptors. Invest. Ophthalmol. Vis. Sci. 1993;34(8):2425–2436. [PubMed] [Google Scholar]
- Stenkamp DL, Hisatomi O, Barthel LK, Tokunaga F, Raymond PA. Temporal expression of rod and cone opsins in embryonic goldfish retina predicts the spatial organization of the cone mosaic. Invest. Ophthalmol. Vis. Sci. 1996;37(2):363–376. [PubMed] [Google Scholar]
- Stenkamp DL, Barthel LK, Raymond PA. Spatiotemporal coordination of rod and cone photoreceptor differentiation in goldfish retina. J. Comp. Neurol. 1997;382(2):272–284. doi: 10.1002/(sici)1096-9861(19970602)382:2<272::aid-cne10>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Stenkamp DL, Frey RA, Prabhudesai SN, Raymond PA. Function for hedgehog genes in zebrafish retinal development. Dev. Biol. 2000;220(2):238–252. doi: 10.1006/dbio.2000.9629. [DOI] [PubMed] [Google Scholar]
- Stenkamp DL, Powers MK, Carney LH, Cameron DA. Evidence for two distinct mechanisms of neurogenesis and cellular pattern formation in regenerated goldfish retinas. J. Comp. Neurol. 2001;431(4):363–381. doi: 10.1002/1096-9861(20010319)431:4<363::aid-cne1076>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Stenkamp DL, Frey RA, Mallory DE, Shupe EE. Embryonic retinal gene expression in sonic-you mutant zebrafish. Dev. Dyn. 2002;225(3):344–350. doi: 10.1002/dvdy.10165. [DOI] [PubMed] [Google Scholar]
- Sullivan SA, Barthel LK, Largent BL, Raymond PA. A goldfish Notch-3 homologue is expressed in neurogenic regions of embryonic, adult, and regenerating brain and retina. Dev. Genet. 1997;20(3):208–223. doi: 10.1002/(SICI)1520-6408(1997)20:3<208::AID-DVG4>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Take-uchi M, Clarke JD, Wilson SW. Hedgehog signalling maintains the optic stalk-retinal interface through the regulation of Vax gene activity. Development. 2003;130(5):955–968. doi: 10.1242/dev.00305. [DOI] [PubMed] [Google Scholar]
- Tallafuss A, Hale LA, Yan YL, Dudley L, Eisen JS, Postlethwait JH. Characterization of retinoid-X receptor genes rxra, rxrba, rxrbb and rxrg during zebrafish development. Gene Expr. Patterns. 2006;6(5):556–565. doi: 10.1016/j.modgep.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Thisse B, Thisse C. Functions and regulations of fibroblast growth factor signaling during embryonic development. Dev. Biol. 2005;287(2):390–402. doi: 10.1016/j.ydbio.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Tropepe V, Coles BL, Chiasson BJ, Horsford DJ, Elia AJ, McInnes RR, van der Kooy D. Retinal stem cells in the adult mammalian eye. Science. 2000;287(5460):2032–2036. doi: 10.1126/science.287.5460.2032. [DOI] [PubMed] [Google Scholar]
- Turner DL, Snyder EY, Cepko CL. Lineage-independent determination of cell type in the embryonic mouse retina. Neuron. 1990;4(6):833–845. doi: 10.1016/0896-6273(90)90136-4. [DOI] [PubMed] [Google Scholar]
- Tyler MJ, Carney LH, Cameron DA. Control of cellular pattern formation in the vertebrate inner retina by homotypic regulation of cell-fate decisions. J. Neurosci. 2005;25(18):4565–4576. doi: 10.1523/JNEUROSCI.0588-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter ML, Brown NL. The role of basic helix-loop-helix genes in vertebrate retinogenesis. Semin. Cell Dev. Biol. 2001;12(6):491–498. doi: 10.1006/scdb.2001.0273. [DOI] [PubMed] [Google Scholar]
- Vihtelic TS, Hyde DR. Light-induced rod and cone cell death and regeneration in the adult albino zebrafish (Danio rerio) retina. J. Neurobiol. 2000;44(3):289–307. doi: 10.1002/1097-4695(20000905)44:3<289::aid-neu1>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Wan J, Stenkamp DL. Cone mosaic development in the goldfish retina is independent of rod neurogenesis and differentiation. J. Comp. Neurol. 2000;423(2):227–242. [PubMed] [Google Scholar]
- Wang J, Wynshaw-Boris A. The canonical Wnt pathway in early mammalian embryogenesis and stem cell maintenance/differentiation. Curr. Opin. Genet. Dev. 2004;14(5):533–539. doi: 10.1016/j.gde.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Raff MC. Rod photoreceptor development in vitro: Intrinsic properties of proliferating neuroepithelial cells change as development proceeds in the rat retina. Neuron. 1990;4(3):461–467. doi: 10.1016/0896-6273(90)90058-n. [DOI] [PubMed] [Google Scholar]
- Wehman AM, Staub W, Meyers JR, Raymond PA, Baier H. Genetic dissection of the zebrafish retinal stem-cell compartment. Dev. Biol. 2005;281(1):53–65. doi: 10.1016/j.ydbio.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Wei X, Malicki J. nagie oko, encoding a MAGUK-family protein, is essential for cellular patterning of the retina. Nat. Genet. 2002;31(2):150–157. doi: 10.1038/ng883. [DOI] [PubMed] [Google Scholar]
- Wikler KC, Rakic P. An array of early differentiating cones precedes the emergence of the photoreceptor mosaic in the fetal monkey retina. Proc. Natl. Acad. Sci. USA. 1994;91(14):6534–6538. doi: 10.1073/pnas.91.14.6534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu DM, Schneiderman T, Burgett J, Gokhale P, Barthel L, Raymond PA. Cones regenerate from retinal stem cells sequestered in the inner nuclear layer of adult goldfish retina. Invest. Ophthalmol. Vis. Sci. 2001;42(9):2115–2124. [PubMed] [Google Scholar]
- Yamaguchi M, Tonou-Fujimori N, Komori A, Maeda R, Nojima Y, Li H, Okamoto H, Masai I. Histone deacetylase 1 regulates retinal neurogenesis in zebrafish by suppressing Wnt and Notch signaling pathways. Development. 2005;132(13):3027–3043. doi: 10.1242/dev.01881. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Stock DW, Jeffery WR. Hedgehog signalling controls eye degeneration in blind cavefish. Nature. 2004;431(7010):844–847. doi: 10.1038/nature02864. [DOI] [PubMed] [Google Scholar]
- Yan RT, Wang SZ. NeuroD induces photoreceptor cell overproduction in vivo and de novo generation in vitro. J. Neurobiol. 1998;36(4):485–496. [PMC free article] [PubMed] [Google Scholar]
- Yurco P, Cameron DA. Responses of Müller glia to retinal injury in adult zebrafish. Vision Res. 2005;45(8):991–1002. doi: 10.1016/j.visres.2004.10.022. [DOI] [PubMed] [Google Scholar]
- Zygar CA, Colbert S, Yang D, Fernald RD. IGF-1 produced by cone photoreceptors regulates rod progenitor proliferation in the teleost retina. Brain Res. Dev. Brain Res. 2005;154(1):91–100. doi: 10.1016/j.devbrainres.2004.10.009. [DOI] [PubMed] [Google Scholar]