Abstract
In response to light, the retinal pigment epithelium (RPE) generates a series of slow potentials that can be recorded as the c-wave, fast oscillation (FO), and light peak (LP) of the electroretinogram (ERG). As these potentials can be related to specific cellular events, they provide information about RPE function and how that may be altered by disease or experimental manipulation. In the present study we describe a noninvasive means for recording the light-evoked responses of the mouse RPE and use this to define the stimulus–response properties of the major components in three inbred strains of mice (BALBc/ByJ, C57BL/6J, and 129/SvJ) and two mouse mutants that reduce activity in the rod pathway. All of the major ERG components generated by the RPE are readily measured in the mouse. In albino strains (BALBc/ByJ and 129/SvJ) the intensity–response functions for the c-wave, FO, and LP are shifted toward lower intensities in comparison to those for C57BL/6J mice. Each of these components was markedly reduced in mice lacking transducin in which rod phototransduction is interrupted, indicating that they reflect primarily rod photoreceptor activity. All components were observed in no b-wave (nob) mutant mice, indicating that inner retinal activity does not make a major contribution to these potentials. Further studies of mutant mice will allow us to define the functional consequences of gene manipulation on RPE function and to evaluate specific hypotheses regarding the generation of ERG components.
INTRODUCTION
The electroretinogram (ERG) has been used to measure retinal function for more than a century (Armington 1974) and has found particular application in patient diagnosis and clinical research (Fishman et al. 2001). During the last decade, the ERG has been widely used to understand the functional consequences of retinal gene manipulation (reviewed in Peachey and Ball 2003). These applications have spurred the development of stimulation, recording, and analysis protocols that allow the functional properties of different retinal cell types to be evaluated in normal and mutant mice.
Following the relatively rapid a- and b-waves, which reflect primarily the activity of photoreceptors and bipolar cells, respectively (Robson et al. 2003; Robson and Frishman 1995), the ERG is known to include a series of slow potentials that are generated in response to neural activity by nonneuronal elements of the retina (Steinberg et al. 1985). The positive polarity c-wave occurs within several seconds following a light stimulus and represents the sum of two potentials that are generated in response to the light-induced decline in subretinal [K+]. A positive potential, generated by hyperpolarization of the apical membrane of the retinal pigment epithelium (RPE) (Oakley and Green 1976; Steinberg et al. 1970, 1980) is offset somewhat by slow PIII, a negative polarity signal that is generated by the Müller cells (Kofuji et al. 2000; Witkovsky et al. 1975). In reptiles and higher vertebrates, the c-wave is followed by the fast oscillation (FO), which has a negative polarity that reaches a minimum level within the first 2 min after a flash (Griff and Steinberg 1984; Kikiwada 1968; Linsenmeier and Steinberg 1984). The FO is generated in part by the recovery of slow PIII and the c-wave from their peaks as [K+] increases in the IPM. The major factor underlying the FO, however, is a hyperpolarization of the basal membrane of the RPE in response to the initial decline in subretinal [K+], which is transmitted relatively slowly through the RPE cells (Griff and Steinberg 1984; Linsenmeier and Steinberg 1984). The light peak (LP) follows the c-wave and FO, with a positive polarity that reaches a peak only several minutes after the FO (Linsenmeier and Steinberg 1982). The LP is known to reflect a depolarization of the basal membrane of the RPE, although the “light-peak substance” that initiates this response component has not been identified.
The RPE is critically involved in many activities required for normal retinal function, including the flow of nutrients and waste products between the photoreceptors and the choroidal circulation, the visual cycle, and the phagocytosis of shed outer segment disks (reviewed in Bok 1993; Marmorstein 2001). In addition, mutations in RPE genes have been found to underlie a wide range of hereditary retinal diseases, such as retinitis pigmentosa (Maw et al. 1997), Lebers congenital amaurosis (Gu et al. 1997; Marlhens et al. 1997), Malattia Leventinese and Doyne honeycomb retinal dystrophy (Marmorstein et al. 2002), Sorsby’s fundus dystrophy (Weber et al. 1994), congenital hypotrichosis (Sprecher et al. 2001), and Best vitelliform macular dystrophy (Marmorstein et al. 2000; Petrukhin et al. 1998). This information motivates the development of mouse mutants for these retinal disorders and for other RPE-specific genes. Although the ERG provides a means to better understand the functional consequences of gene manipulation, only Kikiwada (1968) has reported that RPE-derived components may be recorded from mice. Recently, we reported a new method to record these responses from the rat (Peachey et al. 2002). In the present study, we report a modification of this noninvasive procedure for recording light-evoked activity of mouse RPE and use this to determine the stimulus–response properties of three wild-type (WT) mouse strains that are widely used in vision research. We also describe results obtained from mutant mice with well-defined defects of the rod pathway that allow us to begin to evaluate specific issues regarding the generation of the components that comprise the mouse dc-ERG.
METHODS
Animals and anesthesia
This study examined two strains of WT mice that are frequently used in vision research (C57BL/6J and BALBc/ByJ) and a third strain of WT mouse that is widely used in generating knockout mutants (129/SvJ). WT mice were obtained from The Jackson Laboratory (Bar Harbor, ME). The nob (no b-wave) mouse is a spontaneous mutant that was originally identified by the lack of an ERG b-wave (Pardue et al. 1998). The nob defect is an 85-bp deletion in the nyctalopin gene (Gregg et al. 2003b), and the mice tested here were derived from a breeding colony in which nob is maintained on a C57BL/6J background. As nob is an X-linked trait, only male mice were used (i.e., nob/Y or +/Y). Transducin (Tr) mutant mice were obtained from a cross between the original Tr-null (Tr−/−) background strain (Calvert et al. 2000) and C57BL/6J mice. These mice, all Tr+/− heterozygotes, were crossed with Tr−/− mice to generate the mice studied here (i.e., Tr+/− or Tr−/−). In all cases, recordings were made between 5 and 16 wk of age. ERG recordings were used to identify nob and Tr mutant mice (cf. Calvert et al. 2000; Candille et al. 1999).
After overnight dark adaptation, mice were anesthetized with ketamine (80 mg/kg) and xylazine (16 mg/kg). This dose is usually effective for ≥25 min, and the duration of each experimental session was chosen to not require supplemental anesthetic and disturbing the recording preparation. Eye drops were used to anesthetize the cornea (1% proparacaine HCl) and to dilate the pupil (1% mydriacyl, 2.5% phenylephrine HCl, and 1% cyclopentolate HCl). Mice were placed on a temperature-regulated heating pad throughout the recording session. All procedures involving animals were approved by the local institutional animal care and use committee and were in accordance with the Institute for Laboratory Animal Research Guide for Care and Use of Laboratory Animals.
Recording and stimulation
Two stimulation and recording systems and protocols were used in this study. To measure ERG components generated by the RPE, we used a variation of a technique developed for the rat (Peachey et al. 2002). In brief, responses were recorded from the corneal surface of the left eye using an unpulled 1-mm-diam glass capillary tube with filament (BF100–50-10, Sutter Instruments; Novato, CA) that was filled with HBSS to make contact with a Ag/AgCl wire electrode with an attached connector. A similar electrode placed in contact with the right eye served as a reference lead. Both electrodes were shielded in a black tube, and a baffle constructed from black electrical tape was used to shield the right eye from light stimulation. Responses were differentially amplified (DP-301, Warner Instruments, Hamden, CT; dc-100 Hz; gain = 1000×) digitized at 20 Hz and stored using LabScribe Data Recording Software (iWorx, Dover, NH). After these initial setup procedures were finished, the stability of the recording was monitored for several minutes prior to stimulus presentation. Under these conditions, mice did not usually develop reversible cataracts, probably because the corneal surface was moistened by the saline solution used to fill the capillary tube (Ridder et al. 2002).
White light stimuli were derived from an optical channel using a Leica microscope illuminator as the light source and delivered to the test eye with a 1-cm-diam fiber optic bundle. The unattenuated stimulus luminance was 4.4 log cd/m2. For the mouse eye, this luminance corresponds to 6.8 log photoisomerizations per rod/s, based on the assumption that 1 photopic cd/m2 equals 1.4 scotopic cd/m2 for the tungsten halogen light source (Wyszecki and Stiles 1982) and that 1 scotopic cd/m2 is equivalent to 100 photoisomerizations per rod/s (Hetling and Pepperberg 1999). Neutral density filters (Oriel Instruments, Stratford, CT) placed in the light path reduced stimulus luminance. Luminance calibrations were made with a LS-110 photometer (Minolta, Ramsey, NJ) focused on the output of the fiber optic bundle. A Uniblitz shutter system was used to control stimulus duration at 7 min. Each mouse was tested only once on a given day, using only a single stimulus condition. Intensity–response functions were developed from recordings made in different recording sessions that were separated by at least 2 days.
To record conventional ERGs, a second stimulation and recording protocol was used that has been developed for mouse ERG recording (Peachey and Ball 2003). The ERG was recorded using a stainless-steel electrode that made contact with the corneal surface through a thin layer of 0.7% methylcellulose. Needle electrodes placed in the cheek and the tail served as reference and ground leads, respectively. Under these conditions, mice typically develop reversible cataracts. Responses were differentially amplified (0.3–1500 Hz), averaged, and stored using a UTAS E-3000 signal averaging system (LKC Technologies, Gaithersburg, MD). After overnight dark adaptation, ERGs were recorded to flash stimuli presented in an LKC ganzfeld. Stimulus flashes ranging from −3.6 to 2.1 log cd s/m2 were presented to the dark-adapted eye. In some cases, cone ERGs were obtained to strobe flashes (1.4 log cd s/m2) superimposed on a steady adapting field after a 7-min adaptation period (Peachey et al. 1993).
RESULTS
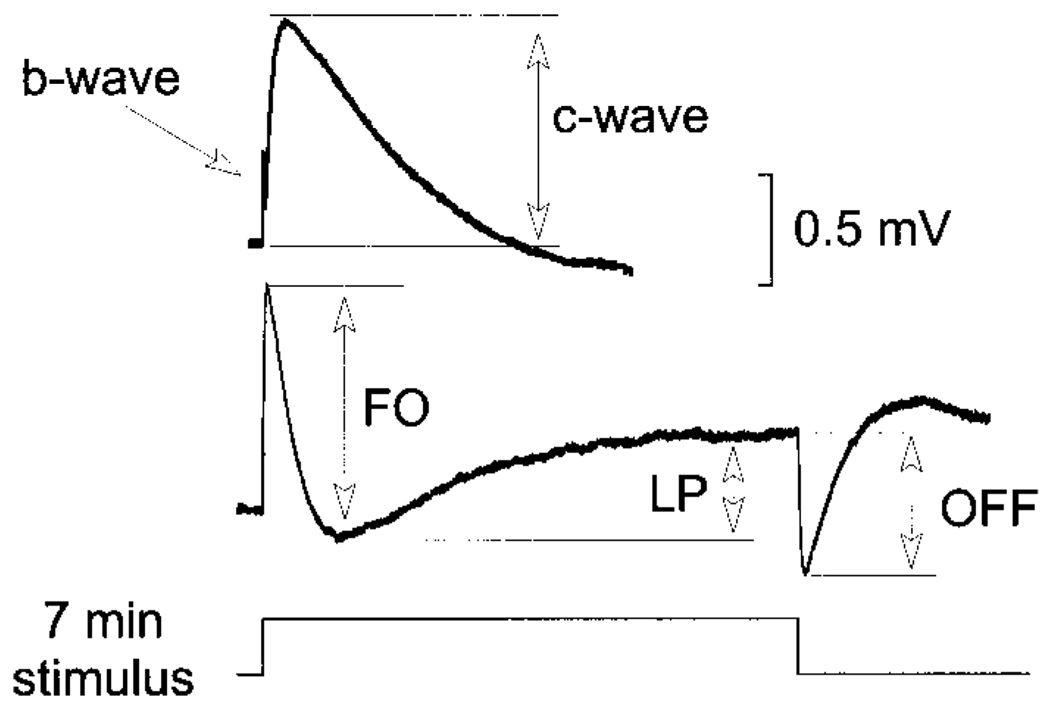
Figure 1 presents a representative response recorded from a C57BL/6J mouse in response to a 7-min duration stimulus flash, indicated by the lower trace. The lower waveform shows a complete recording, while the upper waveform presents only the initial part of the response on a 5× expanded time scale. In this expanded format, the initial portion of the response can be seen to include a positive polarity b-wave, which is followed by a second positive wave, the c-wave, that peaks several seconds following flash onset. The lower waveform shows that the b- and c-waves are followed by a negative polarity FO and then a positive polarity LP. At flash offset, the off-response is negative in polarity, although this feature is intensity dependent (see following text). In agreement with Kikiwada (1968), the mouse ERG includes all of the major dc-ERG components seen in other vertebrates.
FIG. 1.
Representative dc-electroretinogram (ERG) recorded from a C57BL/6J mouse in response to a 7-min stimulus flash. The arrows indicate the manner in which the fast oscillation (FO), light peak (LP), and off-response are measured. The upper record presents the initial portion of the response on an expanded (×5) time scale, where the early components can be seen more clearly. The arrows indicate the manner in which the c-wave was measured. Amplitude calibration indicates 0.5 mV.
Comparison of inbred mouse strains
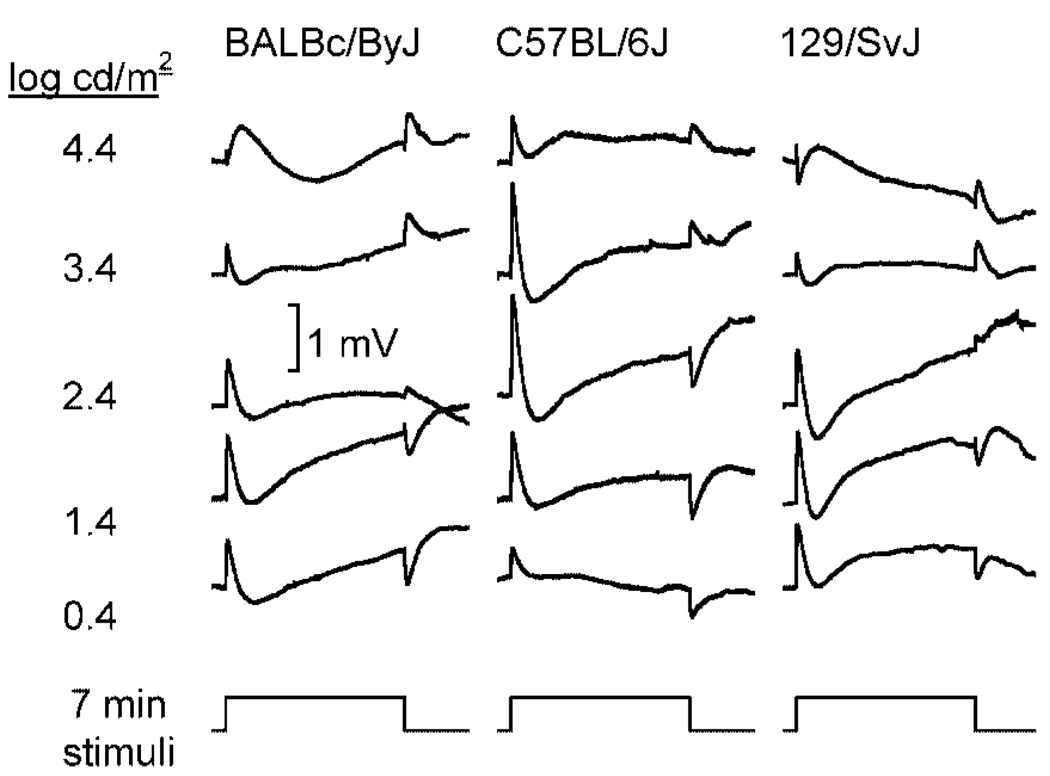
To define the basic characteristics of the ERG components generated by the RPE, responses were recorded over a 5-log unit range of intensity from three different strains of WT mice. Figure 2 presents responses obtained from BALBc/ByJ (left), C57BL/6J (middle), and 129/SvJ (right) mice to five different stimulus intensities. Each waveform represents the grand average of the responses obtained from 5 to 12 individual animals. These responses have not been corrected for dc drift. Instead, we found that the drift present in our recordings tended to cancel out in this grand average format. Throughout most of the intensity range examined, it was possible to identify the major response components generated by the RPE. The largest amplitudes were, however, obtained from stimuli that fell in the middle of our intensity range, and responses declined at the highest flash intensities. The decrease in response amplitude that was observed at higher flash intensities cannot reflect light adaptation from prior stimuli, as mice were tested only once on a given day using a single stimulus condition. In comparison to the other components, the polarity of the off-response was intensity dependent. At low flash intensities, the off-response was negative in all three strains of mice tested. As flash intensity increased, the response decreased somewhat, and then reversed to a positive polarity. This reversal was observed in all three strains examined, although it occurred at lower stimulus intensities in albino BALBc/ByJ and 129/SvJ mice than in pigmented C57BL/6J mice.
FIG. 2.
ERGs recorded to 7-min duration stimulus flashes from BALBc/ByJ mice (left column of waveforms), C57BL/6J mice (middle column of waveforms), and from 129/SvJ mice (right column of waveforms). Each record indicates the grand average of responses obtained from 5 to 12 individual mice. Each row of waveforms corresponds to a different stimulus intensity. Stimulus presentation is represented by the lower trace. Amplitude calibration indicates 1 mV.
As indicated in Fig. 1, we measured the peak-to-trough amplitude of each ERG component. The amplitude of the c-wave was measured from the prestimulus baseline to the peak of the c-wave. The FO was measured from the c-wave peak to the trough of the FO. The amplitude of the LP was measured from the FO trough to the asymptote of the LP. Finally, the amplitude of the off-response was measured from the LP value just prior to flash offset to the peak of the initial component that, depending on stimulus intensity, was either positive or negative in polarity. Figure 3 presents intensity– response functions for each component. The c-wave (Fig. 3A) increased with flash intensity to a maximum, and then declined with increasing stimulus intensity. The maximal response, as well as the subsequent decline, occurred approximately 1–2 log units lower for albino than for pigmented mice. In addition, the maximum amplitude of the c-wave was higher for C57BL/6J mice than for the two albino strains examined. The FO (Fig. 3B) and LP (Fig. 3C) displayed similar strain differences, where the response maximum occurred at a lower stimulus intensity for albino than for pigmented mice. Although the maximum FO was obtained for C57BL/6J mice, LPs of 129/SvJ were somewhat larger in amplitude than for the other two strains examined. Although there were no major differences in amplitude for the off-response (Fig. 3D), the stimulus intensity at which this response component reversed polarity was also lower for albino than for pigmented mice.
FIG. 3.
Intensity–response functions for the amplitude of the major ERG components generated by the RPE in response to light. The different panels plot the amplitude of the c-wave (A), fast oscillation (B), light peak (C), or off-response (D) as a function of flash intensity for adult mice (○, BALBc/ByJ; ●, C57BL/6J; ◊, 129/SvJ). Data points indicate the average (±SE) of 5 to 12 responses obtained from different mice.
We also evaluated the kinetics of the ERG components generated by the RPE. The implicit times of the c-wave and FO were measured from flash onset to the peak of the c-wave or the FO trough, respectively. The kinetics of the FO and LP were analyzed by fitting a single exponential function to derive a time constant (τ) for the isolated FO (i.e., from the peak of the c-wave to the FO trough) or the isolated LP (i.e., from the FO trough to the LP asymptote) (cf. Peachey et al. 2002). As stimulus intensity increased, the implicit time of the c-wave (Fig. 4A) increased to a relatively stable level and then declined at the highest stimulus levels. In comparison, the implicit time of the FO remained relatively stable through the lower portion of the stimulus intensity range used here and then declined at the highest stimulus intensities; the magnitude of this decline was greatest for the two albino strains. For the FO, values of τ (Fig. 4C) were relatively stable at low stimulus intensities and then declined with increasing stimulus intensity. Although there was good agreement in τ values between strains through the lower portion of the intensity range, in albino mice the values of FO τ were markedly lower at the highest stimulus intensity. Values of τ derived for the LP were consistently lower for albino mouse strains, indicating that the kinetics of this ERG component were decreased steadily with increasing stimulus intensity. This reduction was most pronounced at the highest intensity, consistent with the waveform changes observed in albino, but not pigmented strains at the highest stimulus intensities.
FIG. 4.
Intensity–response functions for the timing of the major ERG components generated by the RPE in response to light. The different panels plot the implicit time of the c-wave (A) or fast oscillation (B), as well as the values of τ fit to the FO (C) or LP (D) as a function of flash intensity for adult mice (○, BALBc/ByJ; ●, C57BL/6J; ◊, 129/SvJ). Data points indicate the average (±SE) of 5 to 12 responses obtained from different mice.
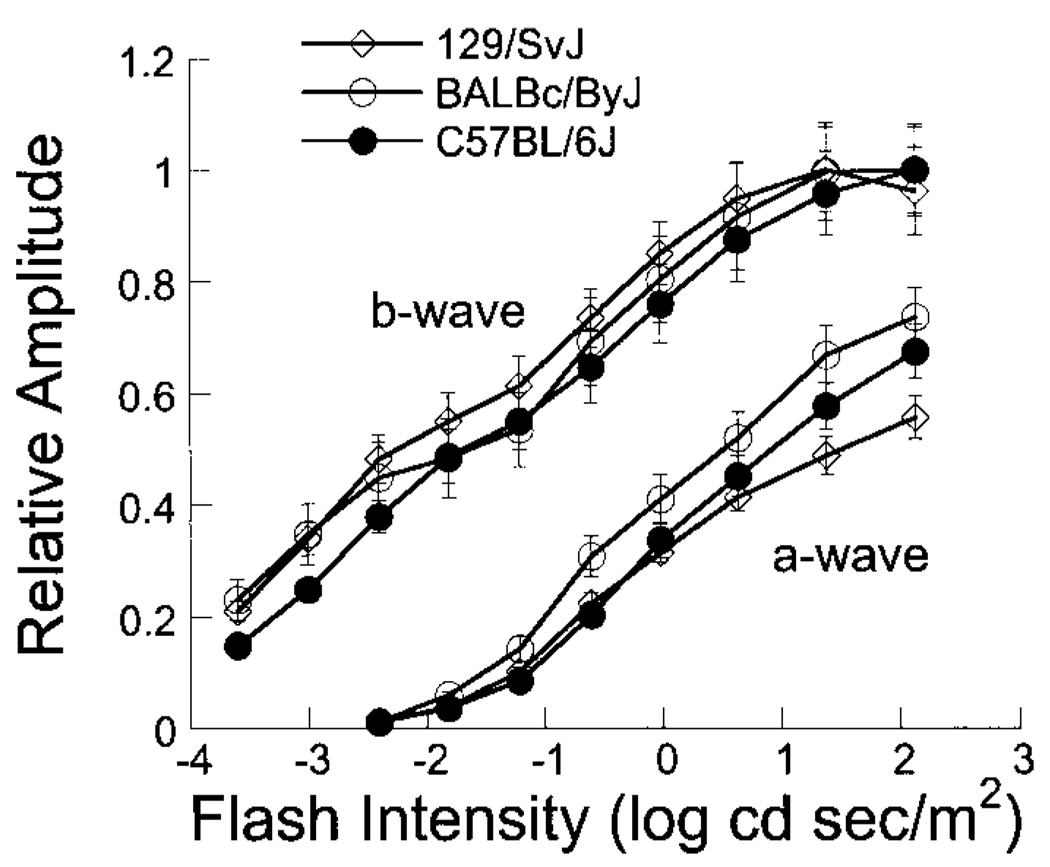
For both strains of albino mice tested, the intensity–response functions for RPE-derived ERG components were shifted to the left along the intensity axis in comparison to those for pigmented C57BL/6J mice. To examine whether this shift reflects a difference in the photoreceptor response, we recorded dark-adapted intensity–response functions for the ERG a- and b-waves. Figure 5 plots response functions for the ERG a- and b-waves. To better compare the relative position of these functions along the intensity axis, the data set corresponding to each mouse strain was normalized by the average maximum b-wave for that strain (129/SvJ: 1316.6 µV; BALBc/ByJ: 887 µV; and C57BL/6J: 1134.2 µV). In general, the response functions of albino mice were shifted toward lower intensities, as noted also by Kashani et al. (2003). The magnitude of this shift does not, however, account completely for the strain differences noted in Fig. 3, indicating that the RPE of albino and pigmented strains may respond differently to subretinal ionic changes. In addition, any difference in retinal sensitivity does not account for the strain differences noted in overall amplitude for the c-wave (Fig. 3A), FO (Fig. 3B), or LP (Fig. 3C), since the functions cannot be brought into close alignment by simply shifting them along the intensity axis.
FIG. 5.
Intensity–response functions for amplitude of the ERG a- and b-waves. Data points indicate the average (±SE) result obtained from 6 different mice of each strain (○, BALBc/ByJ; ●, C57BL/6J; ◊, 129/SvJ).
Inner retinal contribution
The possibility that activity of the inner retina produces some of the driving force for RPE-derived components has been examined on a single occasion. Gallemore and co-workers (1988) examined responses while the retina was perfused with agents that suppress postreceptoral synaptic transmission. Although no component was pharmacologically abolished, the various agents examined (Co2+, Mg2+, 2-amino-4-phosphonobutyric acid, and cis-2, 3-piperidine-dicarboxylic acid) induced distinct but reproducible changes. As a result, while these results indicate that inner retinal activity is not required for the generation of any specific component, they do not rule out the possibility of some inner retinal contribution. Moreover, these studies were carried out using an isolated chicken retina preparation, and it is not clear that these results will apply directly to the mouse. To examine the possibility that inner retinal activity may evoke RPE-derived components, we have made recordings in nob mutant nice. As shown in Fig. 6A, nob mice lack the ERG b-wave, but retain an a-wave with normal kinetics and amplitude (Pardue et al. 1998). Although nob mice have normal retinal histology (Pardue et al. 1998) and a normal distribution of many proteins in the outer plexiform layer (Ball et al. 2003), light-evoked inner retinal activity is substantially diminished in these animals, as evidenced by the lack of an ERG b-wave (Pardue et al. 1998), an overall decrease in visual sensitivity (Gregg et al. 2003b), and in responses of depolarizing bipolar cells to glutamate (Gregg et al. 2003a). As a consequence, the nob mouse provides a model with which to examine the possibility that ionic changes generated by inner retinal activity might contribute to any of the components generated by the RPE.
FIG. 6.
ERGs of nob mutant mice. (A) ERGs recorded from a representative nob mouse (right) and a control littermate (left) in response to a strobe flash presented to the dark-adapted eye. (B) ERGs recorded from 7-min duration stimulus flashes from nob mice. Each record indicates the grand average of responses obtained from ≥5 individual mice.
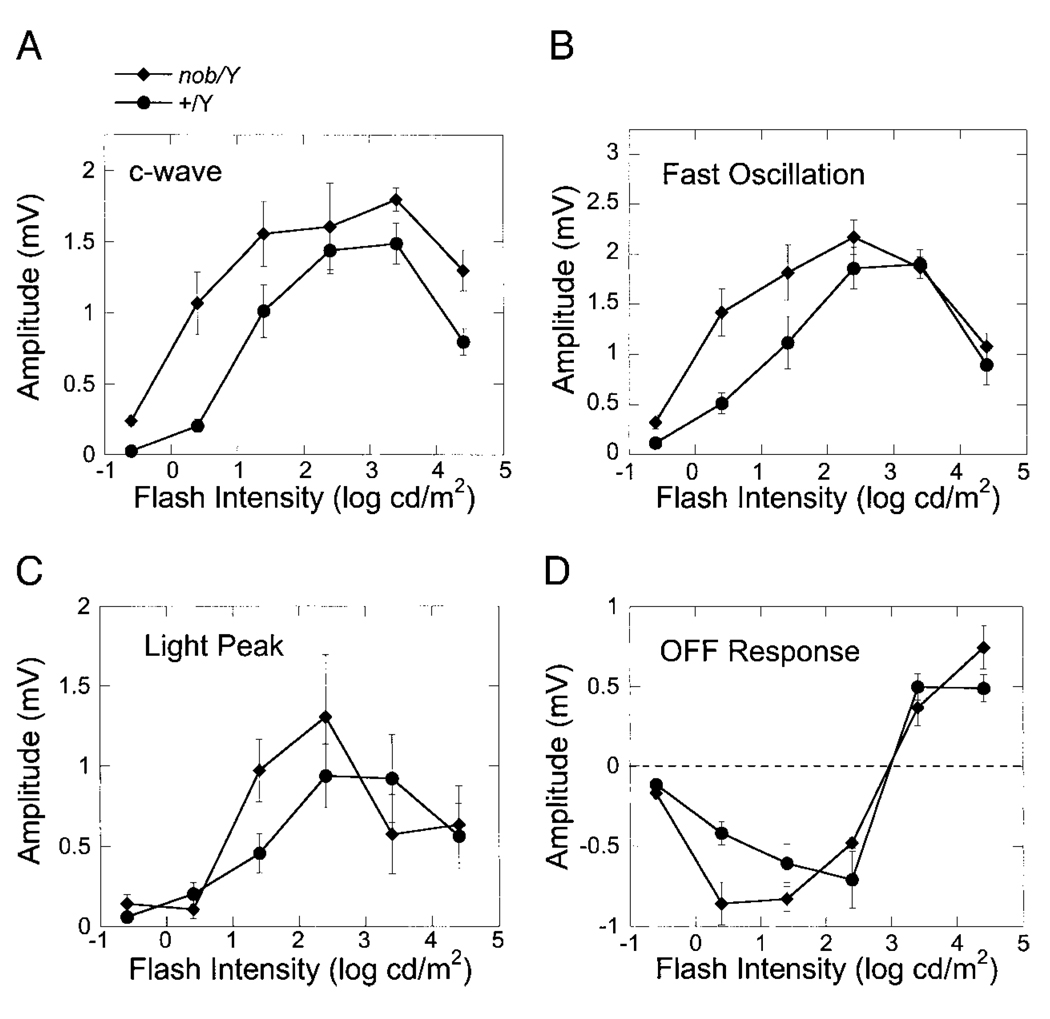
Figure 6B presents a series of responses obtained from nob mice. It is clear that these signals contain each of the ERG components generated by the RPE, indicating that inner retinal activity is not required to generate the mouse c-wave, FO, or LP. Like WT mice, the off-response shows an intensity-dependent polarity inversion. Peak-to-trough measures from individual nob mouse recordings are summarized in Fig. 7 for each of the major ERG components. The intensity dependence of the c-wave (Fig. 7A) was similar in nob and WT mice, although the overall amplitude was consistently larger in nob mutant mice. At lower intensities, the amplitude of the FO was also larger in nob mice (Fig. 7B), although nob and WT data superimposed at the higher two intensities. In comparison to these earlier response components, there was little difference between the LP and off-response of nob and WT mice.
FIG. 7.
Intensity–response functions for the amplitude of the major ERG components generated by the RPE in response to light. The different panels plot the amplitude of the c-wave (A), fast oscillation (B), light peak (C), or off-response (D) as a function of flash intensity for C57BL/6J mice (●) or nob mutant mice (♦). Data points indicate the average (±SE) of 5 to 12 responses obtained from different mice.
Relative contribution of rods and cones
Although it is clear that generation of RPE-derived components requires light absorption by photoreceptors, the relative roles of rods and cones in generating the various components have not been well defined. The main preparations that have been used are the cat retina, which is rod-dominated (Steinberg et al. 1973), and the chicken retina, which is cone-dominated (Meyer and May 1973). Since a robust response is recorded from both preparations, it appears that both rod and cone photoreceptors can initiate RPE activity. While approximately 97% of the photoreceptors in the mouse retina are rods (Carter-Dawson and LaVail 1979; Jeon et al. 1998), the cone ERG can be several hundred microvolts in amplitude (Xu et al. 2000), supporting the possibility that cones may provide an effective stimulus to the mouse RPE. To address the relative contribution of rod and cone activity, we have examined transducin mutant mice, in which rod photoreceptors do not respond to light, although overall retinal anatomy is normal (Calvert et al. 2000).
Figure 8A presents ERGs recorded from representative Tr+/− and Tr−/− mice under stimulus conditions that are used to examine rod- and cone-mediated retinal function (cf. Xu et al. 2000). As shown in the upper two records, dark-adapted ERGs are markedly abnormal in Tr−/− animals. The response to a dim flash (upper records), which evokes a clear ERG b-wave in WT and Tr+/− mice, was indistinguishable from the baseline noise in Tr−/− animals. When a high-intensity stimulus was used (middle records), the negative polarity a-wave was absent in Tr−/− mice, and the b-wave was reduced in comparison to WT or Tr+/− responses. In comparison to these differences in the dark-adapted ERG, the waveform and amplitude of the cone ERG were very similar between Tr+/− and Tr−/− mice (lower records). As noted by Calvert et al. (2000), these results indicate that rod-mediated function is lost in Tr−/− mice while cone function is retained. As a consequence, the Tr−/− mouse provides an opportunity to determine whether rod or cone activity is required to evoke the ERG components generated by the RPE.
FIG. 8.
ERGs of Tr mutant mice. (A) ERGs recorded from a representative Tr+/− mouse (left) and a Tr−/− littermate (right). The upper two pairs of records were obtained to a −3.6 (top records) or a 1.4 (middle records) log cd s/m2 strobe flash presented to the dark-adapted eye. The lower records were obtained to a 1.4 log cd s/m2 strobe flash superimposed on a steady adapting field. (B) ERGs recorded to 7-min duration stimulus flashes from Tr+/− (left) and Tr−/− (right) mice. Each record indicates the grand average of responses obtained from ≥5 individual mice. Note the small positive component present at stimulus onset in the Tr−/− responses (open arrows)
Figure 8B presents a series of responses obtained from Tr mutant mice. In Tr+/− mice, it is clear that each of the ERG components generated by the RPE is present. In comparison, Tr−/− mice do not generate a clear c-wave, FO, LP, or off-response, although there is a distinct transient component (open arrows) present at stimulus onset, which is likely to reflect the cone pathway activity that is spared in this mouse model (Calvert et al. 2000). Throughout the stimulus presentation, there was a small positive step in Tr−/− mice. This may be related to the positive step observed in rodent recordings to stimuli of relative long duration (Xu et al. 2003). However, as the maximum stimulus duration used by Xu et al. (2003) was only 400 ms, further analysis will be required to determine the nature of the positive plateau generated to the stimulus durations used here. While these data do not exclude some minor contribution from cone activity to the slow ERG components studied here, it is clear that they are dominated by rod-mediated activity across a broad range of stimulus intensity.
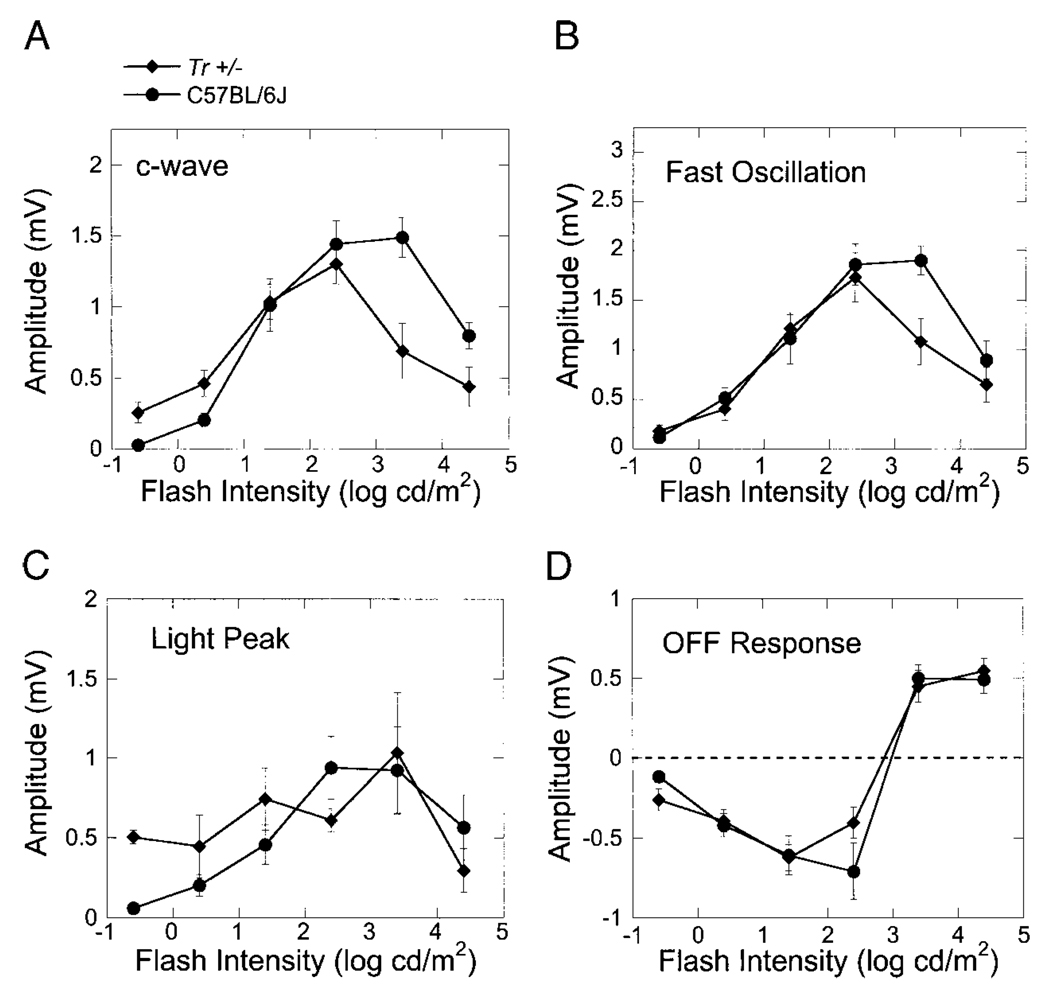
Peak-to-trough measures from individual Tr-mutant mouse recordings are summarized in Fig. 9 for each of the major ERG components. Although responses from Tr−/− mice were markedly reduced in amplitude, there was no difference between Tr+/− and C57BL/6J WT mice with respect to any of the major components generated by the RPE.
FIG. 9.
Intensity–response functions for the amplitude of the major ERG components generated by the RPE in response to light. The different panels plot the amplitude of the c-wave (A), fast oscillation (B), light peak (C), or off- response (D) as a function of flash intensity for C57BL/6J mice (●) or Tr+/− mice (♦). Data points indicate the average (±SE) of 5 to 12 responses obtained from different mice.
DISCUSSION
In defining a noninvasive recording procedure to monitor light-evoked electrical activity of the mouse RPE, we characterized the stimulus–response characteristics of the underlying components in three mouse strains that are commonly used in retinal research. In addition, we incorporated mutant mouse strains, to begin to take advantage of the potential power of mutant mice in addressing basic questions regarding ERG components that are generated by the RPE. Taken together, these results indicate that it is possible to examine RPE function in mice and to examine how this may be compromised by a disease process or affected by gene manipulation. In addition, application of this approach to mutant mouse lines provides an opportunity to evaluate the role of specific molecular events in generating the different response components.
In response to a flash of light, the mouse RPE generates all of the ERG components that have been identified in other vertebrate species (Steinberg et al. 1985). Overall, the present data agree with the earlier report of Kikiwada (1968), who evaluated RPE-derived ERG components in a wide range of species using a single stimulus condition. When stimulus intensity was varied, we noted that each component increased in amplitude to a maximum and then declined at higher intensities. As mice were tested only once on a given day, and more than one day typically elapsed between successive recordings, these amplitude decreases observed at high stimulus intensities cannot reflect a cumulative effect of light adaptation. While the explanation for this decline will require further investigation, it is possible that the decrease seen at the higher stimulus intensities may reflect an increase in the kinetics of underlying generators such that positive and negative polarity components that normally occur one after the other coincide in time at the higher stimulus intensities, resulting in an apparent reduction in response amplitude. This possibility was supported by analysis of response timing parameters. All measurements of response timing were substantially shorter at the highest stimulus intensities, consistent with the hypothesis that the decline observed at high intensities reflects to some extent destructive interference between the underlying response components, which are normally separated in time. Moreover, the magnitude of this response acceleration was greatest in albino mouse strains, which showed the greatest overall decline at the highest stimulus intensities. Finally, the rising phase of the intensity–response functions in albino strains were also shifted to the left from that for C57BL/6J mice. Based on analysis of the conventional ERG a- and b-waves, it appears that some, but not all, of this difference reflects a greater sensitivity to light in albino mouse strains. Rhodopsin bleaching may also contribute to the amplitude decrease observed at high stimulus intensities. To address this possibility, we plan to examine some of the mouse mutants that have been developed that display abnormally slow rhodopsin regeneration (Peachey and Ball 2003).
An additional interesting feature of the mouse response is that the polarity of the off-response inverted from negative to positive as stimulus intensity was increased. The explanation for this inversion is not known, and it is worth noting that a similar result was not obtained in two WT strains of rat studied using this general technique (Peachey et al. 2002) nor in prior studies of cat (Linsenmeier and Steinberg 1982). The intensity-dependent inversion of the off-response indicates that the mouse off-response reflects a complex interaction of several components of different polarity.
The present data were obtained using a system modeled after that used recently to study two strains of WT rat (Peachey et al. 2002). We, nevertheless, noted several differences between the responses of the two species. In addition to the intensity-dependent inversion of the off-response noted above, the overall amplitudes of the c-wave and the LP were much larger in the mouse than in the rat. This difference cannot reflect the use of a different reference electrode position (unstimulated fellow eye in the mouse versus orbit of the stimulated eye in the rat), as rat recordings were not substantially different when the fellow eye was used as a reference (AD Marmorstein, NS Peachey, and J Yocum, unpublished data). Instead, these results indicate the mouse has an advantage over the rat, in terms of signal to noise for these response components.
Paralytic agents were not required to make stable recordings from the mouse or the rat (Peachey et al. 2002). This difference from other species, such as cat, where residual eye movements must be eliminated pharmacologically, confers distinct advantages to rodent-based recordings. First, by reducing the degree of support required to maintain the mouse or rat in a stable physiological state, the overall experimental setup is simplified. In addition, it is possible to expand the experimental design to allow for recordings to be made on more than one occasion.
To evaluate specific issues regarding the initial mechanisms that evoke these slow responses from the RPE, recordings were made from two lines of mutant mice with well-defined functional defects in the absence of cellular degeneration. The findings obtained with these begin to tap into the potential of mutant mouse lines to dissect electrophysiological activity of the RPE. A basic question concerns the relative roles of rod and cone activity in evoking RPE contributions to the ERG. To examine this issue, we examined mice lacking rod transducin, resulting in an interruption of the phototransduction cascade in rods but not cones (Calvert et al. 2000). Because the gene deletion spares cone function, any light-evoked electrophysiological response obtained from these animals must originate with cone activity. Although the RPE responses of Tr+/− mice appeared normal in all respects, those of Tr−/− animals were markedly abnormal. That Tr−/− responses lacked the c-wave, FO, LP, and off-response indicates that each of these components is evoked by rod activity in the mouse. We cannot, however, exclude a minor contribution from the cone photoreceptors to the overall response. Under the present recording conditions, responses of Tr−/− mice appeared to include a small response that was evidenced as a positive deflection throughout stimulus presentation. The complete definition of this cone contribution will require additional studies of other mutant lines affecting rod and/or cone function selectively (e.g., Seeliger et al. 2001).
The results obtained with Tr-mutant mice allow us to address the potential role of all-trans retinol as the light peak substance, which has eluded identification to date. A number of potential candidates for the light peak substance have been proposed, including dopamine (Dawis and Niemeyer 1986; Gallemore and Steinberg 1990), epinephrine (Joseph and Miller 1992), adrenergic agents (Quinn et al. 2001), melatonin (Dawis and Niemeyer 1988), and adrenergic agents (Quinn et al. 2001). Of these, however, only adrenergic agents are still considered likely candidates. In response to light, all-trans retinol is released by the outer segment into the subretinal space, where it is taken up at the apical membrane of the RPE. Although these characteristics implicate all-trans retinol as a candidate for the light peak substance, the absence of a LP in Tr−/− mice is inconsistent with this hypothesis.
Recordings were also made from nob mice, which lack the b-wave and presumably most light-evoked activity of the inner retina. There was no major difference in waveform between nob and WT animals. This result indicates that inner retinal activity is not required to evoke any of the ERG components studied here. This general conclusion agrees with the report of Gallemore et al. (1988), who noted that the c-wave, FO, and LP were retained in a chicken retina/RPE/choroid preparation following the application of pharmacological agents that interfere with postreceptoral transmission. In the nob mouse, however, there was a reproducible increase in the amplitude of the c-wave and FO. This increase indicates that the amplitudes of these components are diminished by a response originating in the depolarizing bipolar cell pathway. The increase observed in nob c-waves may indicate that the positive polarity c-wave is normally countered by a component of negative polarity. Since the photoreceptor response is not impaired in nob mice (Pardue et al. 1998), it appears that the positive polarity contribution to the c-wave from the apical membrane of the RPE is normally offset by two negative potentials, slow PIII generated by Müller cells and a second negative polarity contribution that may originate in the rod depolarizing bipolar cell. To further examine this possibility, it will be useful to examine additional mouse mutants in which the ERG b-wave is selectively reduced in amplitude.
In sum, the present results indicate that the ERG can be used to reliably record RPE-generated potentials from mice. Further application to mutant mice will provide a useful approach toward defining the cellular origin of each component and to completely define the effect of gene manipulation on the function of the retina and RPE.
ACKNOWLEDGMENT
We are grateful to Dr. Janis Lem, Tufts University School of Medicine, for providing transducin mutant mice for this study.
GRANTS
This work was supported by the Medical Research Service, Department of Veterans Affairs (to N. S. Peachey), by National Institutes of Health Grants EY-14456 (to N. S. Peachey) and EY-13160 (to A. D. Marmorstein), and by an unrestricted grant from Research to Prevent Blindness to the University of Arizona.
REFERENCES
- Armington JC. The Electroretinogram. New York: Academic Press; 1974. [Google Scholar]
- Ball SL, Pardue MT, McCall MA, Gregg RG, Peachey NS. Immunohistochemical analysis of the outer plexiform layer in the nob mouse shows no abnormalities. Vis Neurosci. 2003;20:267–272. doi: 10.1017/s0952523803203059. [DOI] [PubMed] [Google Scholar]
- Bok D. The retinal pigment epithelium: a versatile partner in vision. J Cell Sci. 1993;17 Suppl:189–195. doi: 10.1242/jcs.1993.supplement_17.27. [DOI] [PubMed] [Google Scholar]
- Calvert PD, Krasnoperova NV, Lyubarsky AL, Isayama T, Nicolo M, Kosaras B, Wong G, Gannon KS, Margolskee RF, Sidman RL, Pugh EN, Jr, Makino CL, Lem J. Phototransduction in transgenic mice after targeted deletion of the rod transducin α-subunit. Proc Natl Acad Sci USA. 2000;97:13913–13918. doi: 10.1073/pnas.250478897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candille S, Pardue MT, McCall MA, Peachey NS, Gregg RG. Localization of the mouse nob (no b-wave) gene to the centromeric region of the X chromosome. Invest Ophthalmol Vis Sci. 1999;40:2748–2751. [PubMed] [Google Scholar]
- Carter-Dawson LD, LaVail MM. Rods and cones in the mouse retina. I. Structural analysis using light and electron microscopy. J Comp Neurol. 1979;188:245–262. doi: 10.1002/cne.901880204. [DOI] [PubMed] [Google Scholar]
- Dawis SM, Niemeyer G. Dopamine influences the light peak in the perfused mammalian eye. Invest Ophthalmol Vis Sci. 1986;27:330–335. [PubMed] [Google Scholar]
- Dawis SM, Niemeyer G. Similarity and diversity of monoamines in their effects on the standing potential, light peak and electroretinogram of the perfused cat eye. Clin Vision Sci. 1988;3:108–119. [Google Scholar]
- Fishman GA, Birch DG, Holder GE, Brigell MG. Electrophysiologic Testing in Disorders of the Retina, Optic Nerve, and Visual Pathway. 2nd ed. San Francisco, CA: American Academy of Ophthalmology; 2001. [Google Scholar]
- Gallemore RP, Griff ER, Steinberg RH. Evidence in support of a photoreceptoral origin for the ‘light-peak substance’. Invest Ophthalmol Vis Sci. 1988;29:566–571. [PubMed] [Google Scholar]
- Gallemore RP, Steinberg RH. Effects of dopamine on the chick retinal pigment epithelium: membrane potentials and light-evoked responses. Invest Ophthalmol Vis Sci. 1990;31:67–80. [PubMed] [Google Scholar]
- Gregg RG, Lukasiewicz PD, Peachey NS, Sagdullaev BT, McCall MA. Nyctalopin is required for signaling through depolarizing bipolar cells in the murine retina. Invest Ophthalmol Vis Sci. 2003a;44:4180. ARVO E-Abstract. [Google Scholar]
- Gregg RG, Mukhopadhyay S, Candille SI, Ball SL, Pardue MT, McCall MA, Peachey NS. Identification of the gene and the mutation responsible for the nob (no b-wave) phenotype. Invest Ophthalmol Vis Sci. 2003b;44:378–384. doi: 10.1167/iovs.02-0501. [DOI] [PubMed] [Google Scholar]
- Griff ER, Steinberg RH. Changes in apical [K+] produce delayed basal membrane responses of the retinal pigment epithelium in the gecko. J Gen Physiol. 1984;83:193–211. doi: 10.1085/jgp.83.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu SM, Thompson DA, Srikumari CR, Lorenz B, Finckh U, Nicoletti A, Murthy KR, Rathmann M, Kumaramanickavel G, Denton MJ, Gal A. Mutations in RPE65 cause autosomal recessive childhood-onset severe retinal dystrophy. Nature Genet. 1997;17:194–197. doi: 10.1038/ng1097-194. [DOI] [PubMed] [Google Scholar]
- Hetling JR, Pepperberg DR. Sensitivity and kinetics of mouse rod flash responses determined in vivo from paired-flash electroretinograms. J Physiol. 1999;516:593–609. doi: 10.1111/j.1469-7793.1999.0593v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon CJ, Strettoi E, Masland RH. The major cell populations of the mouse retina. J Neurosci. 1998;18:8936–8946. doi: 10.1523/JNEUROSCI.18-21-08936.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph DP, Miller SS. Alpha-1-adrenergic modulation of K and Cl transport in bovine retinal pigment epithelium. J Gen Physiol. 1992;99:263–290. doi: 10.1085/jgp.99.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashani Z, Chang B, Hawes N, Hurd R, Heckenlively JR, Nusinowitz S. Comparison of electroretinographic responses across eleven normal inbred mouse strains. Invest Ophthalmol Vis Sci. 2003;44:1896. ARVO E-Abstract. [Google Scholar]
- Kikiwada N. Variations in the corneo-retinal standing potential of the vertebrate eye during light and dark adaptation. Jpn J Physiol. 1968;18:687–702. doi: 10.2170/jjphysiol.18.687. [DOI] [PubMed] [Google Scholar]
- Kofuji P, Ceelen P, Zahs KR, Surbeck LW, Lester HA, Newman EA. Genetic inactivation of an inwardly rectifying potassium channel (Kir4.1 subunit) in mice: phenotypic impact in retina. J Neurosci. 2000;21:5733–5740. doi: 10.1523/JNEUROSCI.20-15-05733.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsenmeier RA, Steinberg RH. Origin and sensitivity of the light peak of the intact cat eye. J Physiol. 1982;331:653–673. doi: 10.1113/jphysiol.1982.sp014396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsenmeier RA, Steinberg RH. Delayed basal hyperpolarization of the cat retinal pigment epithelium, and its relation to the fast-oscillation of the DC ERG. J Gen Physiol. 1984;83:213–222. doi: 10.1085/jgp.83.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlhens F, Bareil C, Griffin JM, Zrenner E, Amalric P, Eliaou C, Liu SY, Harris E, Redmond TM, Arnaud B, Claustres M, Hamel CP. Mutations in RPE65 cause Leber’s congenital amaurosis. Nature Genet. 1997;17:139–141. doi: 10.1038/ng1097-139. [DOI] [PubMed] [Google Scholar]
- Marmorstein AD. The polarity of the retinal pigment epithelium. Traffic. 2001;2:867–872. doi: 10.1034/j.1600-0854.2001.21202.x. [DOI] [PubMed] [Google Scholar]
- Marmorstein AD, Marmorstein LY, Rayborn M, Wang X, Hollyfield JG, Petrukhin K. Bestrophin, the product of the Best vitelliform macular dystrophy gene (VMD2), localizes to the basolateral plasma membrane of the retinal pigment epithelium. Proc Natl Acad Sci USA. 2000;97:12758–12763. doi: 10.1073/pnas.220402097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmorstein LY, Munier FL, Arsenijevic Y, Schorderet DF, McLaughlin PJ, Chung D, Traboulsi E, Marmorstein AD. Aberrant accumulation of EFEMP1 underlies drusen formation in Malattia Leventinese and age-related macular degeneration. Proc Natl Acad Sci USA. 2002;99:13067–13072. doi: 10.1073/pnas.202491599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maw MA, Kennedy B, Knight A, Bridges R, Roth KE, Mani EJ, Mukkadan JK, Nancarrow D, Crabb JW, Denton MJ. Mutation of the gene encoding cellular retinaldehyde-binding protein in autosomal recessive retinitis pigmentosa. Nature Genet. 1997;17:198–200. doi: 10.1038/ng1097-198. [DOI] [PubMed] [Google Scholar]
- Meyer DB, May HC., Jr The topographical distribution of rods and cones in the adult chicken retina. Exp Eye Res. 1973;17:347–355. doi: 10.1016/0014-4835(73)90244-3. [DOI] [PubMed] [Google Scholar]
- Oakley B, 2nd, Green DG. Correlation of light-induced changes in retinal extracellular potassium concentration with the c-wave of the electroretinogram. J Neurophysiol. 1976;39:1117–1133. doi: 10.1152/jn.1976.39.5.1117. [DOI] [PubMed] [Google Scholar]
- Pardue MT, McCall MA, LaVail MM, Gregg RG, Peachey NS. A naturally-occurring mouse model of X-linked congenital stationary night blindness. Invest Ophthalmol Vis Sci. 1998;39:2443–2449. [PubMed] [Google Scholar]
- Peachey NS, Ball SL. Electrophysiological analysis of visual function in mutant mice. Doc Ophthalmol. 2003;107:13–36. doi: 10.1023/a:1024448314608. [DOI] [PubMed] [Google Scholar]
- Peachey NS, Goto Y, Al-Ubaidi MR, Naash MI. Properties of the mouse cone-mediated electroretinogram during light adaptation. Neurosci Lett. 1993;162:9–11. doi: 10.1016/0304-3940(93)90547-x. [DOI] [PubMed] [Google Scholar]
- Peachey NS, Stanton JB, Marmorstein AD. Noninvasive recording and response characteristics of the rat DC electroretinogram. Vis Neurosci. 2002;19:693–701. doi: 10.1017/s0952523802196015. [DOI] [PubMed] [Google Scholar]
- Petrukhin K, Koisti MJ, Bakall B, Li W, Xie G, Marknell T, Sandgren O, Forsman K, Holmgren G, Andreasson S, Vujic M, Bergen AA, Mc-Garty-Dugan V, Figueroa D, Austin CP, Metzker ML, Caskey CT, Wadelius C. Identification of the gene responsible for Best macular dystrophy. Nature Genet. 1998;19:241–247. doi: 10.1038/915. [DOI] [PubMed] [Google Scholar]
- Quinn RH, Quong JN, Miller SS. Adrenergic receptor activated ion transport in human fetal retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2001;42:255–264. [PubMed] [Google Scholar]
- Ridder W, III, Nusinowitz S, Heckenlively JR. Causes of cataract development in anesthetized mice. Exp Eye Res. 2002;75:365–370. doi: 10.1006/exer.2002.2007. [DOI] [PubMed] [Google Scholar]
- Robson JG, Frishman LJ. Response linearity and kinetics of the cat retina: the bipolar cell component of the dark-adapted electroretinogram. Vis Neurosci. 1995;12:837–850. doi: 10.1017/s0952523800009408. [DOI] [PubMed] [Google Scholar]
- Robson JG, Saszik SM, Ahmed J, Frishman LJ. Rod and cone contributions to the a-wave of the electroretinogram of the macaque. J Physiol. 2003;547:509–530. doi: 10.1113/jphysiol.2002.030304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeliger MW, Grimm C, Stahlberg F, Friedburg C, Jaissle G, Zrenner E, Guo H, Reme CE, Humphries P, Hofmann F, Biel M, Fariss RN, Redmond TM, Wenzel A. New views on RPE65 deficiency: the rod system is the source of vision in a mouse model of Leber congenital amaurosis. Nature Genet. 2001;29:70–74. doi: 10.1038/ng712. [DOI] [PubMed] [Google Scholar]
- Sprecher E, Bergman R, Richard G, Lurie R, Shalev S, Petronius D, Shalata A, Anbinder Y, Leibu R, Perlman I, Cohen N, Szargel R. Hypotrichosis with juvenile macular dystrophy is caused by a mutation in CDH3, encoding P-cadherin. Nature Genet. 2001;29:134–136. doi: 10.1038/ng716. [DOI] [PubMed] [Google Scholar]
- Steinberg RH, Linsenmeier RA, Griff ER. Retinal pigment epithelial cell contributions to the electroretinogram and electrooculogram. Prog Ret Res. 1985;4:33–66. [Google Scholar]
- Steinberg RH, Oakley B, 2nd, Niemeyer G. Light-evoked changes in [K+]o in the retina of the intact cat eye. J Neurophysiol. 1980;44:897–921. doi: 10.1152/jn.1980.44.5.897. [DOI] [PubMed] [Google Scholar]
- Steinberg RH, Reid M, Lacy PL. The distribution of rods and cones in the retina of the cat (Felis domesticus) J Comp Neurol. 1973;148:229–248. doi: 10.1002/cne.901480209. [DOI] [PubMed] [Google Scholar]
- Steinberg RH, Schmidt R, Brown KT. Intracellular responses to light from cat pigment epithelium: origin of the electroretinogram c-wave. Nature. 1970;227:728–730. doi: 10.1038/227728a0. [DOI] [PubMed] [Google Scholar]
- Weber BH, Vogt G, Pruett RC, Stohr H, Felbor U. Mutations in the tissue inhibitor of metalloproteinases-3 (TIMP3) in patients with Sorsby’s fundus dystrophy. Nature Genet. 1994;8:352–356. doi: 10.1038/ng1294-352. [DOI] [PubMed] [Google Scholar]
- Witkovsky P, Dudek EF, Ripps H. Slow PIII component of the carp electroretinogram. J Gen Physiol. 1975;65:119–134. doi: 10.1085/jgp.65.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyszecki G, Stiles WS. Color Science: Concepts and Methods, Quantitative Data and Formulae. 2nd ed. New York: Wiley; 1982. [Google Scholar]
- Xu L, Ball SL, Alexander KR, Peachey NS. Pharmacological analysis of the rat cone electroretinogram. Vis Neurosci. 2003;20:297–306. doi: 10.1017/s0952523803203084. [DOI] [PubMed] [Google Scholar]
- Xu X, Quiambao AB, Roveri L, Pardue MT, Marx JL, Röhlich P, Peachey NS, Al-Ubaidi MR. Degeneration of cone photoreceptors induced by expression of the Mas1 oncogene. Exp Neurol. 2000;163:207–219. doi: 10.1006/exnr.2000.7370. [DOI] [PubMed] [Google Scholar]