Abstract
Monoclonal antibodies to Histoplasma capsulatum can modify pathogenesis. We now show that monoclonal antibody H1C to a 70-kDa antigen increases intracellular fungal growth and reduces macrophage nitric oxide release but has no effect on fungal burden or survival in murine infection. This further demonstrates the complexities of host-pathogen interactions.
Histoplasma capsulatum is a dimorphic fungal pathogen that is the causative agent of histoplasmosis. After Candida species, H. capsulatum is the most common causative agent of systemic mycoses in North America and either the first- or second-most-common cause in Central and South America (4, 7). We previously identified an IgG1 isotype monoclonal antibody (MAb), H1C, that reacts with a 70-kDa H. capsulatum cell wall antigen (6). The MAb is highly specific for H. capsulatum, as it did not react with yeast cell antigens from related microbes. An inhibition enzyme-linked immunosorbent assay (ELISA) system using H1C is also highly specific for the detection of antigenemia and antigenuria in patients with histoplasmosis (6). Furthermore, the inhibition ELISA may be useful for monitoring treatment responses in patients with histoplasmosis (5).
We have shown that MAbs to the H. capsulatum cell surface proteins histone 2B (H2B) and heat shock protein 60 (Hsp60) can alter the intracellular fate of the fungus, modify the inflammatory response to infection, and prolong the survival of lethally infected mice (8, 9). In particular, we demonstrated the marked protective efficacy of IgG1 and IgG2a MAbs to Hsp60. In the present study, we assessed the capacity of H1C to modify host-pathogen interactions.
H1C was purified from cell culture supernatants using protein G-agarose beads (Pierce Biotechnology) according to the manufacturer's instructions. Aliquots of H1C were screened to ensure the absence of endotoxin with the Limulus amebocyte lysate assay (BioWhittaker Inc.). An irrelevant, isotype-matched mouse IgG1 MAb (SouthernBiotech) was used as a control for all experiments. H. capsulatum G217B was used as the reference strain for all the studies (gift from G. Deepe, University of Cincinnati). H. capsulatum yeast cells were grown in Ham's F-12 medium at 37°C with rotary shaking and collected as described previously (9). Enumeration of the yeast cells was accomplished with a hemacytometer.
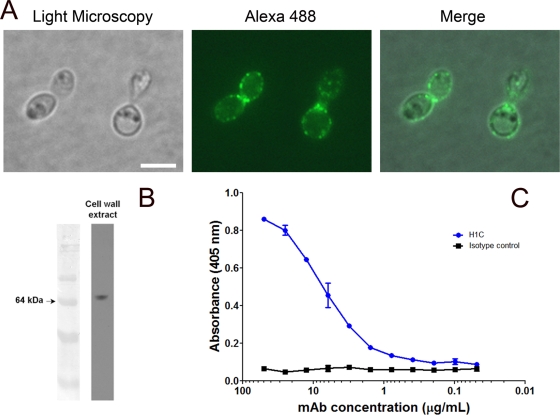
Immunofluorescence and immunoblotting were performed as described previously (8), except that H1C labeled with Alexa 488 was used in lieu of MAbs to Hsp60. Fluorescence analysis revealed that H1C diffusely labeled the fungal cell surface in a punctuate manner (Fig. 1 A), and H1C reacted with a 70-kDa protein, as shown by immunoblotting (Fig. 1B). A whole-cell H. capsulatum yeast ELISA was also used to examine the binding of H1C to H. capsulatum, as described previously (9). Significant binding occurred at concentrations >1 μg/ml H1C (Fig. 1C). Hence, H1C readily interacted with the 70-kDa protein at the H. capsulatum cell surface.
FIG. 1.
MAb H1C binds to a 70-kDa protein on the cell surface of H. capsulatum. (A) Immunofluorescence analysis demonstrates that H1C diffusely reacts with the fungal cell surface. (B) H1C reacts with a 70-kDa protein in cell wall extracts from H. capsulatum. (C) Yeast cell ELISA demonstrates the reactivity of H1C with intact fungal cells. Experiments were repeated at least three times, with consistent results.
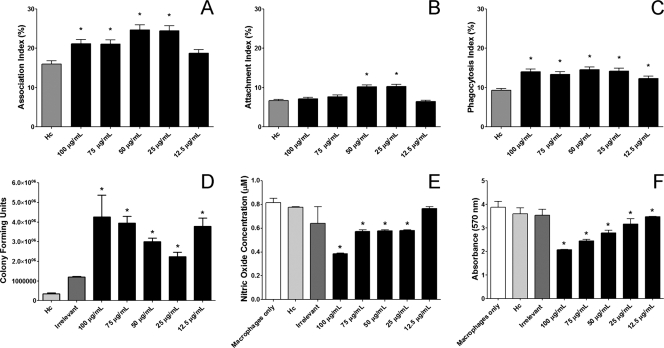
Phagocytosis and killing assays were accomplished as described by confocal microscopy and fluorescence-activated cell sorting (9) using J774.16 macrophagelike cells. H1C increased the association of H. capsulatum with the J774.16 cells (Fig. 2 A). Although increases in yeast cell attachment occurred at 25 and 50 μg/ml of H1C (Fig. 2B), phagocytosis of H. capsulatum increased at all the concentrations tested (Fig. 2C). Further, H. capsulatum opsonized with H1C had significantly increased intracellular survival after 24 h compared to controls (Fig. 2D). GraphPad Prism version 5.00 for Windows (GraphPad Software) was used for statistical analyses. Kruskal-Wallis nonparametrical tests were used for one-way analysis of variance to compare differences between groups, and individual comparisons of groups were performed with the Bonferroni posttest.
FIG. 2.
MAb H1C alters H. capsulatum-macrophage interactions. (A) H1C alters H. capsulatum association with J774.16 macrophage-like phagocytosis. H1C opsonization affects H. capsulatum attachment (B) and phagocytosis (C). (D) H1C-opsonized H. capsulatum has significantly improved intracellular survival. (E) Opsonization of H. capsulatum with H1C reduces nitric oxide production. (F) Opsonization of H. capsulatum with H1C increases macrophage death. Macrophage toxicity data are at 2 h of coculture, and similar results were present at 4 h. For each graph, three independent experiments were performed, with each condition tested in triplicate, except for the nitric oxide experiments, which were performed twice with triplicates. Graphs were generated with pooled data from the experiments. The bars are the mean values, with error bars representing the standard deviations. *, P < 0.05, which indicates a comparison between the condition and H. capsulatum in the absence of antibody. “Hc” represents yeast without antibody in the presence of macrophages, “irrelevant” indicates an IgG1 isotype control antibody with yeast cells and macrophages, and the concentrations listed represent the specific amounts of H1C MAb present with H. capsulatum yeast and macrophages.
Since intracellular growth increased in the presence of H1C, we examined the production of nitric oxide and superoxide by J774.16 cells cocultured with H. capsulatum exposed to H1C, irrelevant antibody, or phosphate-buffered saline (PBS), as described previously (10), and also assessed J774.16 viability. Nitric oxide formation was assessed with a commercial Griess reagent kit (Promega), and superoxide dismutase activity was quantified with a kit (Cayman Chemical). H1C-opsonized H. capsulatum induced significantly less nitric oxide release than controls at concentrations ≥25 μg/ml after 2 h of coculture (Fig. 2E). In contrast, there were no differences in superoxide radicals (data not shown). J774.16 viability after exposure to H. capsulatum was measured using an MTT [3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide] assay (Sigma-Aldrich). H1C concentrations ≥25 μg/ml in the presence of H. capsulatum yeast cells resulted in a dose-dependent toxicity on the J774.16 cells at 2 h of coculture (Fig. 2D). H1C alone in the presence of the J774.16 cells had no effect on the macrophages (data not shown). Given the damage to the macrophages caused by the opsonized H. capsulatum yeast cells, it is possible that the reduction in nitric oxide was a direct effect of the reduction in viable macrophages.
C57BL/6 mice (6 to 8 weeks old, female; NCI) were used for survival studies, which were approved by the Animal Institute Committee of the Albert Einstein College of Medicine. C57BL/6 mice were injected intraperitoneally with 250 μg of H1C, an isotype-matched control MAb, or PBS. Two hours later, the mice were intranasally challenged with either 5 × 106 CFU (sublethal dose) or 1.25 × 107 CFU (lethal dose for survival studies) of H. capsulatum yeast. The mice were closely monitored. CFU studies revealed that there was a slight trend toward an increase in numbers of CFU at day 3 after infection in animals given H1C, but the differences at days 3 and 7 in the fungal burdens between H1C-treated animals and controls were not significant (Fig. 3 A). Lethally challenged mice receiving H1C died at day 10 ± 1, whereas control mice died between days 10 and 12, which results are not statistically different by Kaplan-Meier analysis (P > 0.05). We also tested the efficacy of 100 and 500 μg of H1C in the lethal-challenge model, but these doses similarly failed to alter survival (data not shown).
FIG. 3.
Pretreatment with intraperitoneal injections of MAb H1C. A dose of 100 μg does not alter the pulmonary fungal burden in sublethally infected mice (A) or prolong survival in mice receiving lethal challenge (B). CFU studies were preformed with three mice per condition for each day assessed. Survival studies were performed using groups of 10 mice, and the experiment was repeated, with similar results.
MAbs have been shown to protect against several fungal diseases, including candidiasis, cryptococcosis, aspergillosis, and pneumocystosis (2, 3). We have previously demonstrated that IgM, IgG2a, and IgG1 MAbs to H. capsulatum H2B or Hsp60 modify histoplasmosis (8, 9), but an IgG2b to Hsp60 is disease enhancing (8, 9). Hence, this report presents the second nonprotective MAb to H. capsulatum. Isotype has been shown to influence the outcome of cryptococcosis with human and murine MAbs to the major polysaccharide of Cryptococcus neoformans; in addition, a recent study showed that human IgG1-treated mice had accelerated death (1). Our findings do not rule out the possibility that a protective antibody to the 70-kDa protein could be generated, either one with a different isotype or one corresponding to a different epitope on the protein. Notably, although we found no differences in fungal burden or survival in our murine infection model, our results nevertheless suggest a possible role for the 70-kDa molecule in the virulence of the fungus, since H1C-opsonized H. capsulatum had increased intracellular survival, modified nitric oxide production, and increased macrophage damage. Our data further demonstrate the complexities involved in antibody-pathogen interactions.
(The data in this paper are from a thesis to be submitted by A. J. Guimarães in partial fulfillment of the requirements for a Ph.D. degree from the Sue Golding Graduate Division of Medical Science, Albert Einstein College of Medicine, Yeshiva University, Bronx, NY.)
Acknowledgments
A.J.G., M.D.C., and L.C.L.L. were supported in part by an Interhemispheric Research Training Grant in Infectious Diseases, Fogarty International Center (NIH grant D43-TW007129). L.C.L.L. was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico—Brasil (CNPq). J.D.N. is supported in part by NIH grants AI52733 and AI056070-01A2 and by the Center for AIDS Research at the Albert Einstein College of Medicine and Montefiore Medical Center (NIH grant AI-51519).
Footnotes
Published ahead of print on 19 May 2010.
REFERENCES
- 1.Beenhouwer, D. O., E. M. Yoo, C. W. Lai, M. A. Rocha, and S. L. Morrison. 2007. Human immunoglobulin G2 (IgG2) and IgG4, but not IgG1 or IgG3, protect mice against Cryptococcus neoformans infection. Infect. Immun. 75:1424-1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Casadevall, A. 1995. Antibody immunity and invasive fungal infections. Infect. Immun. 63:4211-4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casadevall, A., and L. A. Pirofski. 2007. Antibody-mediated protection through cross-reactivity introduces a fungal heresy into immunological dogma. Infect. Immun. 75:5074-5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Couppie, P., C. Aznar, B. Carme, and M. Nacher. 2006. American histoplasmosis in developing countries with a special focus on patients with HIV: diagnosis, treatment, and prognosis. Curr. Opin. Infect. Dis. 19:443-449. [DOI] [PubMed] [Google Scholar]
- 5.Gomez, B. L., J. I. Figueroa, A. J. Hamilton, S. Diez, M. Rojas, A. Tobon, A. Restrepo, and R. J. Hay. 1999. Detection of the 70-kilodalton histoplasma capsulatum antigen in serum of histoplasmosis patients: correlation between antigenemia and therapy during follow-up. J. Clin. Microbiol. 37:675-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gomez, B. L., J. I. Figueroa, A. J. Hamilton, B. L. Ortiz, M. A. Robledo, A. Restrepo, and R. J. Hay. 1997. Development of a novel antigen detection test for histoplasmosis. J. Clin. Microbiol. 35:2618-2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goodwin, R. A., Jr., J. L. Shapiro, G. H. Thurman, S. S. Thurman, and R. M. Des Prez. 1980. Disseminated histoplasmosis: clinical and pathologic correlations. Medicine (Baltimore) 59:1-33. [PubMed] [Google Scholar]
- 8.Guimaraes, A. J., S. Frases, F. J. Gomez, R. M. Zancope-Oliveira, and J. D. Nosanchuk. 2009. Monoclonal antibodies to heat shock protein 60 alter the pathogenesis of Histoplasma capsulatum. Infect. Immun. 77:1357-1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nosanchuk, J. D., J. N. Steenbergen, L. Shi, G. S. Deepe, Jr., and A. Casadevall. 2003. Antibodies to a cell surface histone-like protein protect against Histoplasma capsulatum. J. Clin. Invest. 112:1164-1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi, L., P. C. Albuquerque, E. Lazar-Molnar, X. Wang, L. Santambrogio, A. Gacser, and J. D. Nosanchuk. 2008. A monoclonal antibody to Histoplasma capsulatum alters the intracellular fate of the fungus in murine macrophages. Eukaryot. Cell 7:1109-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]