Abstract
Cyclo(Phe-Pro) is a cyclic dipeptide produced by multiple Vibrio species. In this work, we present evidence that cyclo(Phe-Pro) inhibits the production of the virulence factors cholera toxin (CT) and toxin-coregulated pilus (TCP) in O1 El Tor Vibrio cholerae strain N16961 during growth under virulence gene-inducing conditions. The cyclo(Phe-Pro) inhibition of CT and TCP production correlated with reduced transcription of the virulence regulator tcpPH and was alleviated by overexpression of tcpPH.
Vibrio cholerae is an aquatic organism and a facultative human pathogen that causes the disease cholera. Cholera is an acute diarrheal disease that is endemic in many parts of the world. Cholera is acquired by ingestion of V. cholerae-contaminated food or water. Following V. cholerae ingestion, a complicated regulatory cascade is initiated in the proximal small intestine which leads to the production of a number of important virulence factors, including toxin-coregulated pilus (TCP) and cholera toxin (CT) (4). TCP is an adhesin that is required for colonization of the intestinal tract. CT is an enterotoxin that is responsible for the secretory diarrhea, which can rapidly lead to hypotensive shock and death.
CT and TCP production in V. cholerae is under the control of a hierarchical regulatory system known as the ToxR regulon (reviewed in reference 4). There are three primary regulatory proteins in this regulon, ToxR (19), TcpP (8), and ToxT (10). In response to unknown environmental cues, ToxR and TcpP, acting with their respective membrane-associated partners ToxS and TcpH, bind together at the toxT promoter to activate toxT transcription (8, 14, 17). ToxT then directly binds to the promoter of the tcpA-tcpF operon to activate the expression of the genes required for TCP production and to the ctxAB promoter to activate the production of CT (6, 10). ToxR also regulates the expression of additional genes that are important for pathogenesis, including the OmpU and OmpT porins, independently of TcpP and ToxT (18).
Recently, it was reported that V. cholerae produced a cyclic dipeptide (CDP), cyclo(Phe-Pro) (cFP), that exhibited characteristics that were consistent with it being a novel signaling molecule (20). This included the finding that cFP accumulated in culture media in a growth-dependent fashion and that the addition of cFP to the culture medium of an O1 El Tor V. cholerae strain grown in AB broth at 28°C resulted in enhanced OmpU and CT production (20). The induction of CT production by cFP in AB broth was intriguing, as virulence gene expression in El Tor vibrios is usually only induced under very specific growth conditions that are referred to as AKI conditions (defined below) (11). In light of these data, we sought to assess the effect of cFP on CT and TCP production in V. cholerae O1 El Tor strain N16961 (9) during growth under in vitro virulence gene-inducing conditions (i.e., AKI conditions).
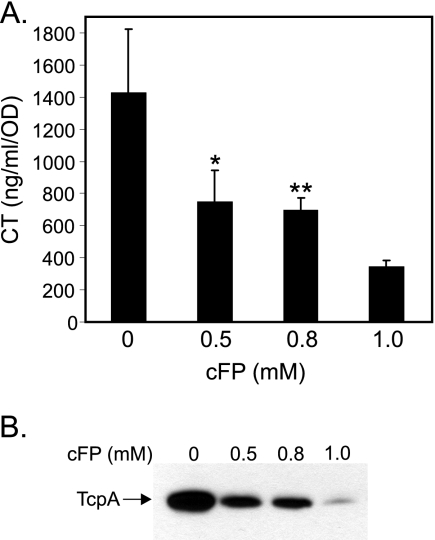
We first tested the hypothesis that cFP was an inducer of CT and TCP production by adding synthetic cFP to AKI broth prior to growth of strain N16961 under AKI growth conditions. cFP had been reported to accumulate in V. cholerae culture supernatants to a concentration of ∼0.8 mM (20). We therefore added cFP to the AKI broth at 0, 0.5, 0.8, and 1 mM concentrations. Control cultures received an equivalent volume of the solvent dimethyl sulfoxide (DMSO). AKI conditions were as follows. A saturated overnight V. cholerae culture was diluted 1:10,000 into 10 ml of AKI broth (1.5% Bacto peptone, 0.4% Difco yeast extract, 0.5% NaCl, pH 7.4) in a 15- by 150-mm test tube. The inoculated culture was then incubated statically at 37°C for 4 h before being transferred to a 125 ml-Erlenmeyer flask and incubated at 37°C with shaking. Following growth under AKI conditions, the cultures were assayed for virulence factor production by CT enzyme-linked immunosorbent assay (ELISA) and TcpA Western blot analyses, as previously described (3). These experiments showed that the presence of cFP at 0.5 and 0.8 mM resulted in an approximately 2-fold reduction in CT production (Fig. 1A). The presence of cFP at 1 mM resulted in an ∼4.5-fold reduction in CT production. The results of the CT assays were confirmed by a TcpA Western blot analysis (Fig. 1B), which showed a cFP concentration-dependent reduction in TcpA production. Samples from the DMSO control cultures did not show any changes in CT or TcpA production, as previously documented (2) (data not shown). In contrast to the previous report, our data showed that cFP inhibited CT and TCP production in a concentration-dependent manner during growth under virulence gene-inducing conditions. One possible explanation for the disparate CT results of these two studies could be the culture conditions. To test this, we quantified CT and TcpA production in strain N16961 and O1 El Tor strain C6706 following growth in the presence and absence of cFP in AB broth at 28°C as described in the previous study (20). Using these growth conditions, we did not detect CT or TcpA production in either of these two strains (data not shown). In agreement with the published data (20), we did observe increased ompU expression in N16961 during growth under AKI conditions in AKI broth supplemented with 1 mM cFP (data not shown). This observation suggests that strain-specific variation could account for the contrasting CT production results obtained in these two studies. Alternatively, the differences in CT production could be due to the methods that were used for CT detection and quantification in each respective study.
FIG. 1.
cFP inhibits CT and TCP production. V. cholerae was cultured under AKI conditions in the presence of the indicated concentrations of cFP before CT and TcpA production was assayed. (A) Cholera toxin production. The results are the averages of the results of three or more independent experiments ± standard deviations. Statistical analysis was performed using a Tukey-Kramer multiple comparison test. *, P < 0.05; **, P < 0.01. (B) Results of TcpA Western blot analysis of whole-cell lysates.
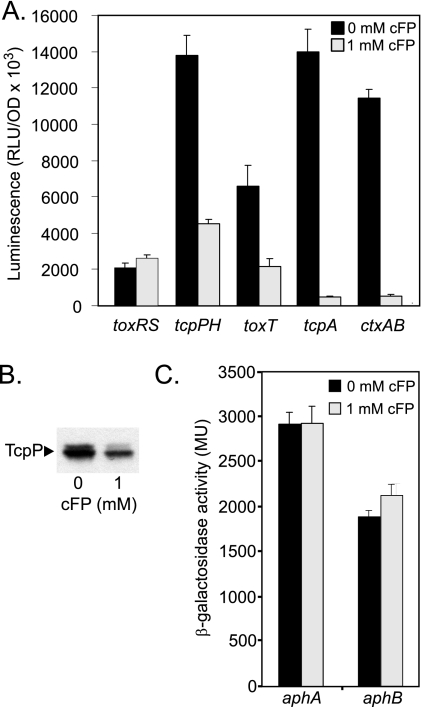
Since CT and TCP production are controlled by the ToxR regulon, we assayed to determine if the presence of cFP in AKI broth affected the induction of the ToxR regulon. This was accomplished by using bioluminescence reporters (kindly provided by Jun Zhu, University of Pennsylvania) (22) to quantify toxR, tcpP, toxT, ctxAB, and tcpA transcription. Strain N16961 containing the indicated reporters was cultured under AKI growth conditions in the presence or absence of 1 mM cFP for 5 h, at which time aliquots of the cultures were transferred to white 96-well microtiter plates for quantification of luminescence production using a BioTek Synergy HT spectrophotometer. Luminescence production was reported as the relative light units divided by the optical density at 630 nm (OD630). We first examined the effect of cFP on ctxAB and tcpA transcription. We found that the addition of 1 mM cFP resulted in ∼21- and 29-fold reductions in ctxAB and tcpA promoter activities, respectively, suggesting that cFP inhibition of CT and TcpA occurred at the transcription level (Fig. 2A). Because the ctxAB and tcpA promoters are directly regulated by ToxT, which is itself regulated by ToxR and TcpP, we next assayed to determine if cFP affected the expression of any of these regulatory genes during growth under AKI growth conditions. The results showed that cFP had little, if any, effect on toxR transcription (Fig. 2A). As enhanced ompU expression in the presence of cFP appears to be toxR dependent (20), this result suggests the possibility that cFP could affect the activation state of ToxR and thereby enhance ompU expression, but additional work will be required to test this hypothesis. In contrast, tcpPH and toxT expression were both reduced by ∼3.5-fold. As toxT, ctxAB, and tcpA are all downstream of tcpPH in the toxR regulon, these results suggested that cFP inhibits tcpPH expression during growth under AKI conditions.
FIG. 2.
cFP inhibits tcpPH, toxT, tcpA, and ctxAB transcription. V. cholerae containing the indicated promoter-lux reporters was grown under AKI conditions in the presence or absence of 1 mM cFP for 5 h, at which time luminescence production was quantified. (A) Effects of 1 mM cFP on gene expression. RLU, relative light units. (B) Results of TcpP Western blot analysis of strain N16961 grown in the presence or absence of 1 mM cFP. (C) Effects of 1 mM cFP on aphA and aphB expression at 5 h. MU, Miller units. The reported results are the averages of the results of three or more experiments ± standard deviations.
If cFP inhibits tcpPH transcription, we hypothesized that cFP treatment should also result in a corresponding reduction in the amount of TcpP protein. To test this, we performed a TcpP Western blot analysis. Strain N16961 was grown under AKI conditions in the presence or absence of 1 mM cFP for 5 h, at which time aliquots of the cultures were collected and normalized to equivalent OD600. The cells from equal volumes of each culture were then collected by centrifugation, resuspended in equal volumes of SDS-PAGE solubilization buffer, and heated at 100°C for 10 min. Equal volumes of each sample were then resolved by SDS-12.5% PAGE before being transferred to a polyvinylidene fluoride membrane. The membrane was incubated with rabbit polyclonal antisera against TcpP (kind gift of Vic DiRita, University of Michigan) (16), and immunoreactive proteins were visualized as previously described (3). The results of the Western blot analysis showed that the addition of 1 mM cFP inhibited TcpP production (Fig. 2B). This result is consistent with that of the tcpPH reporter assay and provides further evidence indicating that cFP is an inhibitor of tcpPH transcription.
The expression of tcpPH is positively regulated by two cytoplasmic regulators, AphA (21) and AphB (13), which bind cooperatively to the tcpPH promoter to activate its expression. Thus, one mechanism by which cFP could affect tcpPH expression is by altering aphA or aphB expression. To test this hypothesis, we constructed transcriptional reporters for aphA and aphB in pTL61T (15), as previously described (3). N16961 cells containing pXB203 (aphA-lacZ) or pXB204 (aphB-lacZ) were then grown under AKI conditions in the presence or absence of 1 mM cFP for 5 h, at which time the β-galactosidase activity was quantified. The results showed that the expression levels of both aphA and aphB in cFP-treated cultures were similar to their expression levels in the cFP-negative control cultures (Fig. 2C), suggesting that cFP probably does not affect tcpPH transcription via the expression of aphA or aphB.
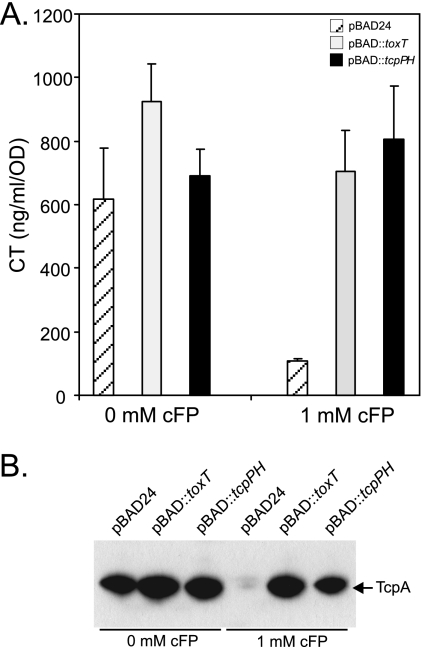
We hypothesized that if the negative effects of cFP on CT and TCP production resulted from the inhibition of tcpPH expression, then the overexpression of tcpPH or the downstream regulatory gene toxT should complement for CT and TCP production during growth in the presence of inhibitory concentrations of cFP. To test this, we overexpressed tcpPH and toxT from the arabinose promoter in pBAD24 (7). N16961 containing pBAD24, pBAD24::toxT, or pBAD24::tcpPH (8) was cultured in the presence and absence of 1 mM cFP under AKI conditions in AKI broth supplemented with 0.004% arabinose to induce gene expression from the arabinose promoter in pBAD24. The cultures were then assayed for CT and TCP production as previously described (3). The results showed that 1 mM cFP strongly inhibited CT production in the pBAD24 control culture (Fig. 3 A). In contrast, the overexpression of toxT (gray bars) or tcpPH in the cFP-exposed cultures complemented CT production to the levels observed in the cFP-negative control cultures. Corresponding results were observed in the TcpA Western blot analysis, where 1 mM cFP strongly inhibited TCP production in the vector control culture (Fig. 3B) and the overexpression of toxT or tcpPH alleviated the inhibitory effects of cFP on TcpA production in the cFP-treated cultures. Overall, these results showed that ectopic overexpression of tcpPH or toxT alleviated the inhibitory effects of cFP on CT and TCP production. Based on these results, we concluded that the negative effects of cFP on CT and TCP production resulted from inhibition of tcpPH transcription in the strains assayed.
FIG. 3.
Overexpression of tcpPH and toxT alleviates cFP-dependent inhibition of CT and TCP production. V. cholerae containing the indicated expression vector was grown under AKI conditions in the presence or absence of 1 mM cFP. Arabinose (0.004%) was added to the AKI broth to induce expression of toxT and tcpPH. (A) Cholera toxin production. The CT results are the averages of the results of three experiments ± standard deviations. (B) Results of TcpA Western blot analysis of whole-cell lysates.
The collective findings presented here indicate that cFP inhibits the production of CT and TCP during the growth of O1 El Tor strains under virulence gene-inducing conditions by negatively affecting tcpPH transcription. There are a number of known mechanisms by which cFP could affect tcpPH expression. For example, it is possible that cFP alters AphA or AphB activity. The crystal structure of AphA showed that it had an N-terminal winged-helix DNA-binding domain similar to that of MarR family proteins (5). As MarR activity has been shown to be modulated by effector molecules (1), it is possible that cFP could affect AphA activity at the tcpPH promoter. AphB is a LysR family transcriptional regulator that has been hypothesized to activate tcpPH expression in response to yet-unknown environmental signals (13). Thus, cFP could function by altering AphB activity at the tcpPH promoter in a similar manner. Alternatively, cFP could affect cyclic AMP (cAMP) production and thereby affect tcpPH expression, since cAMP receptor protein-cAMP has been shown to be a negative regulator of tcpPH expression (12). Lastly, it is also possible that cFP affects tcpPH transcription through a novel mechanism. Work is ongoing in our laboratory to determine the mechanism of cFP action in virulence gene expression and its significance in V. cholerae pathogenesis.
Footnotes
Published ahead of print on 7 May 2010.
REFERENCES
- 1.Alekshun, M. N., and S. B. Levy. 1999. Alteration of the repressor activity of MarR, the negative regulator of the Escherichia coli marRAB locus, by multiple chemicals in vitro. J. Bacteriol. 181:4669-4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bina, X. R., J. A. Philippart, and J. E. Bina. 2009. Effect of the efflux inhibitors 1-(1-naphthylmethyl)-piperazine and phenyl-arginine-beta-naphthylamide on antimicrobial susceptibility and virulence factor production in Vibrio cholerae. J. Antimicrob. Chemother. 63:103-108. [DOI] [PubMed] [Google Scholar]
- 3.Bina, X. R., D. Provenzano, N. Nguyen, and J. E. Bina. 2008. Vibrio cholerae RND family efflux systems are required for antimicrobial resistance, optimal virulence factor production, and colonization of the infant mouse small intestine. Infect. Immun. 76:3595-3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Childers, B. M., and K. E. Klose. 2007. Regulation of virulence in Vibrio cholerae: the ToxR regulon. Future Microbiol. 2:335-344. [DOI] [PubMed] [Google Scholar]
- 5.De Silva, R. S., G. Kovacikova, W. Lin, R. K. Taylor, K. Skorupski, and F. J. Kull. 2005. Crystal structure of the virulence gene activator AphA from Vibrio cholerae reveals it is a novel member of the winged helix transcription factor superfamily. J. Biol. Chem. 280:13779-13783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DiRita, V. J., C. Parsot, G. Jander, and J. J. Mekalanos. 1991. Regulatory cascade controls virulence in Vibrio cholerae. Proc. Natl. Acad. Sci. U. S. A. 88:5403-5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guzman, L. M., D. Belin, M. J. Carson, and J. Beckwith. 1995. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 177:4121-4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hase, C. C., and J. J. Mekalanos. 1998. TcpP protein is a positive regulator of virulence gene expression in Vibrio cholerae. Proc. Natl. Acad. Sci. U. S. A. 95:730-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heidelberg, J. F., J. A. Eisen, W. C. Nelson, R. A. Clayton, M. L. Gwinn, R. J. Dodson, D. H. Haft, E. K. Hickey, J. D. Peterson, L. Umayam, S. R. Gill, K. E. Nelson, T. D. Read, H. Tettelin, D. Richardson, M. D. Ermolaeva, J. Vamathevan, S. Bass, H. Qin, I. Dragoi, P. Sellers, L. McDonald, T. Utterback, R. D. Fleishmann, W. C. Nierman, O. White, S. L. Salzberg, H. O. Smith, R. R. Colwell, J. J. Mekalanos, J. C. Venter, and C. M. Fraser. 2000. DNA sequence of both chromosomes of the cholera pathogen Vibrio cholerae. Nature 406:477-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Higgins, D. E., E. Nazareno, and V. J. DiRita. 1992. The virulence gene activator ToxT from Vibrio cholerae is a member of the AraC family of transcriptional activators. J. Bacteriol. 174:6974-6980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iwanaga, M., K. Yamamoto, N. Higa, Y. Ichinose, N. Nakasone, and M. Tanabe. 1986. Culture conditions for stimulating cholera toxin production by Vibrio cholerae O1 El Tor. Microbiol. Immunol. 30:1075-1083. [DOI] [PubMed] [Google Scholar]
- 12.Kovacikova, G., and K. Skorupski. 2001. Overlapping binding sites for the virulence gene regulators AphA, AphB and cAMP-CRP at the Vibrio cholerae tcpPH promoter. Mol. Microbiol. 41:393-407. [DOI] [PubMed] [Google Scholar]
- 13.Kovacikova, G., and K. Skorupski. 1999. A Vibrio cholerae LysR homolog, AphB, cooperates with AphA at the tcpPH promoter to activate expression of the ToxR virulence cascade. J. Bacteriol. 181:4250-4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krukonis, E. S., R. R. Yu, and V. J. Dirita. 2000. The Vibrio cholerae ToxR/TcpP/ToxT virulence cascade: distinct roles for two membrane-localized transcriptional activators on a single promoter. Mol. Microbiol. 38:67-84. [DOI] [PubMed] [Google Scholar]
- 15.Linn, T., and R. St. Pierre. 1990. Improved vector system for constructing transcriptional fusions that ensures independent translation of lacZ. J. Bacteriol. 172:1077-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matson, J. S., and V. J. DiRita. 2005. Degradation of the membrane-localized virulence activator TcpP by the YaeL protease in Vibrio cholerae. Proc. Natl. Acad. Sci. U. S. A. 102:16403-16408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller, V. L., and J. J. Mekalanos. 1985. Genetic analysis of the cholera toxin-positive regulatory gene toxR. J. Bacteriol. 163:580-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller, V. L., and J. J. Mekalanos. 1988. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J. Bacteriol. 170:2575-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller, V. L., and J. J. Mekalanos. 1984. Synthesis of cholera toxin is positively regulated at the transcriptional level by toxR. Proc. Natl. Acad. Sci. U. S. A. 81:3471-3475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park, D. K., K. E. Lee, C. H. Baek, I. H. Kim, J. H. Kwon, W. K. Lee, K. H. Lee, B. S. Kim, S. H. Choi, and K. S. Kim. 2006. Cyclo(Phe-Pro) modulates the expression of ompU in Vibrio spp. J. Bacteriol. 188:2214-2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skorupski, K., and R. K. Taylor. 1999. A new level in the Vibrio cholerae ToxR virulence cascade: AphA is required for transcriptional activation of the tcpPH operon. Mol. Microbiol. 31:763-771. [DOI] [PubMed] [Google Scholar]
- 22.Xu, X., A. M. Stern, Z. Liu, B. Kan, and J. Zhu. 2010. Virulence regulator AphB enhances toxR transcription in Vibrio cholerae. BMC Microbiol. 10:3. [DOI] [PMC free article] [PubMed] [Google Scholar]