Abstract
Human papillomavirus (HPV) E6/E7 mRNA has been proposed as a more specific marker for cervical dysplasia and cancer than HPV DNA. This study evaluated the RNA specificity of nucleic acid sequence-based amplification (NASBA)-based HPV detection using HPV DNA plasmids (HPV type 16 [HPV16], HPV18, HPV31, HPV33, and HPV45) and nucleic acid extracts of several cell lines, which were systematically subjected to enzymatic treatments with DNase and RNase. HPV plasmid dilutions (106 to 100 copies/μl) and nucleic acid extracts (total DNA, RNA-free DNA, total RNA, and DNA-free RNA) of unfixed and fixed (PreServCyt and SurePath) HaCaT, HeLa, and CaSki cells were tested with the NucliSENS EasyQ HPV test. The RNA-free DNA extracts of HeLa and CaSki cells could be amplified by HPV18 and -16 NASBA, respectively. Fixation of the cells did not influence NASBA. All HPV plasmids could be detected with NASBA. Based on the plasmid dilution series, a lower detection limit of 5 × 103 HPV DNA copies could be determined. Our study identified viral double-stranded DNA as a possible target for NASBA-based HPV detection. The differences in diagnostic accuracy between the NASBA-based tests and conventional HPV DNA detection assays seem to be attributable not to the more specific amplification of viral mRNA but to the limited type range and the lower analytical sensitivity for HPV DNA.
The causal relationship between a persistent infection with high-risk human papillomavirus (HR-HPV) and cervical cancer has resulted in the development of HPV detection systems (4, 5). The use of HPV DNA detection has been suggested for primary screening (26, 30), the triage of equivocal Pap smears (1, 3), and the follow-up of patients after treatment for high-grade cervical intraepithelial neoplasia (CIN2+) (2, 27, 41). Primary screening with Hybrid Capture 2 (HC2) (Qiagen, Hilden, Germany) generally detects more than 90% of all CIN2+ and is 25% (95% confidence interval [CI], 15 to 36%) relatively more sensitive than cytology at a cutoff of atypical squamous cells of undetermined significance (ASCUS) (or of low-grade squamous intraepithelial lesions [LSIL] if ASCUS is unavailable). However, because of the high prevalence of transient, asymptomatic infections, viral DNA detection has a lower specificity for CIN2+ than cytology, especially in young women (11). When HPV DNA testing is used as a primary screening test, additional, more specific tests should be used to minimize patient anxiety, overreferral for colposcopy and treatment, and increased costs.
In productive HPV infections, which appear cytologically as LSIL and histologically as CIN1, the expression of the viral E6 and E7 oncogenes is tightly regulated, with high-level expression only in suprabasal postmitotic cells (21, 33). On the other hand, in high-grade CIN and cancer, E6 and E7 are expressed throughout the thickness of the cervical epithelium. Therefore, E6/E7 mRNA has been proposed as a more specific marker for cervical dysplasia and cancer than HPV DNA (15). Multiplex nucleic acid sequence-based amplification (NASBA) assays, which utilize molecular beacon probes for the real-time detection and typing of E6/E7 mRNA from HPV type 16 (HPV16), HPV18, HPV31, HPV33 and HPV45, are commercially available (PreTect HPV-Proofer [NorChip AS, Klokkarstua, Norway] and NucliSENS EasyQ [bioMérieux, Marcy l'Etoile, France]). In theory, the isothermal (41°C) NASBA technology only amplifies single stranded nucleic acids (NA) or RNA equivalents, even in a background of double-stranded DNA (dsDNA) (12). However, unexpected dsDNA amplification by NASBA has been reported, which demonstrates the necessity of verifying the origin of a NASBA signal when specific RNA detection is the objective (29).
Our group, among others, reported that RNA extracted from cervical cells fixed in BD SurePath (BD Diagnostics, Burlington, NC) liquid-based cytology (LBC) medium using standard extraction techniques is of insufficient quality for real-time reverse transcription-PCR (RT-PCR) applications (16, 28). However, in our laboratory SurePath-fixed samples tested HPV positive with the NucliSENS EasyQ test. The poor recovery and quality of RNA from these samples, as experimentally established by spectrophotometry and use of the Agilent 2100 Bioanalyser (Agilent Technologies, Santa Clara, CA), suggested that RNA did not function as a template in the NASBA reaction.
Several studies established an association between HPV RNA detection and the severity of cervical lesions (19, 25, 31, 32) and assessed the analytical and clinical performance of commercial HPV RNA detection assays (6, 7, 10, 17, 18, 20, 22-24, 35, 36). In comparison to HPV DNA tests, NASBA-based HPV detection showed better results in terms of specificity for high-grade cervical lesions, while its sensitivity was lower. Currently, it is difficult to know whether the differences in diagnostic accuracy are a result of the more specific detection of RNA or whether they are due to the larger type detection range and high analytical sensitivity of the HPV DNA tests. To elucidate this issue, experimental confirmation that RNA is the sole target of HPV NASBA is required.
In this study, HPV DNA plasmids and NA extracts of several cell lines, which were systematically subjected to enzymatic treatments with DNase and RNase, were used to assess whether the NucliSENS EasyQ HPV v1.0 test amplifies DNA.
MATERIALS AND METHODS
Plasmids.
DNA plasmids containing the HPV16 or -18 genome were purchased (Clonit, Milan, Italy), and DNA plasmids containing the HPV31 (pT713), -33 (pBR322), and -45 (pGEM4) genomes were kindly supplied by A. Lorincz (Qiagen, Gaithersburg, MD), G. Orth (Institut Pasteur, Paris, France), and E.-M. de Villiers (DKFZ, Heidelberg, Germany).
Tenfold dilution series of HPV plasmids for HPV16, -18, -31, -33, and -45 were made, ranging from 1 × 106 to 1 × 100 copies/μl, to assess the lower detection limit of the NucliSENS EasyQ HPV test. Each plasmid dilution was tested by real-time quantitative type-specific PCR as described previously (14) to confirm the presence of the expected amount of the appropriate plasmid.
To assess the influence of the T7 promoter, which was present in the HPV31 and -45 plasmids, an HPV45 plasmid without the T7 promoter was constructed. Simultaneously, 1 μg of the original HPV45 plasmid (HPV45 genome cloned in pGEM4) and 1 μg of pBR322 cloning plasmid (Fermentas GmbH, St. Leon-Rot, Germany) were digested with HindIII restriction enzyme (Fermentas) in a 20-μl volume for 30 min at 37°C. The digested products were analyzed by gel electrophoresis (0.8% [wt/vol] agarose in Tris-acetate-EDTA buffer) and visualized with GelRed (Biotium, Hayward, CA). The desired products (7.8 kb for HPV45 and 4.3 kb for pBR322) were excised from the agarose gel and purified with the QIAquick gel extraction kit (Qiagen) according to the manufacturer's instructions. The HPV45 genome (56 ng) and the pBR322 vector (64 ng) were ligated using Ready-To-Go T4 DNA ligase (GE Healthcare Life sciences, Waukesha, WI) in a 20-μl volume for 45 min at 16°C. Next, 5 μl of the ligated vector/insert DNA solution was transformed into competent Escherichia coli GT116. After overnight incubation at 37°C, nine randomly picked colonies were subjected to colony PCR to verify the presence of HPV45. Amplification was performed in a 20-μl reaction mixture containing 1× REDTaq ReadyMix PCR mix (Sigma-Aldrich, St. Louis, MO), 10 μM forward primer (5′-CGT CGG GCT GGT AGT TGT G-3′), and 10 μM reverse primer (5′-ATT GCA TTT GGA ACC TCA GAA TG-3′) (13). A single PCR-positive colony was grown overnight at 37°C in 5 ml Luria-Bertani medium supplemented with ampicillin (0.5%, vol/vol). HPV45 DNA plasmid without the T7 promoter sequence was purified using the QIAprep Spin Miniprep kit (Qiagen) according to the manufacturer's instructions. To elute the plasmid DNA (>10 kb), 50 μl preheated elution buffer (70°C) was used. The concentration and purity of the plasmid DNA were determined by UV spectrophotometry. A 10-fold dilution series of the HPV45 plasmid without T7 promoter was made, ranging from 1 × 106 to 1 × 100 copies/μl, to assess the lower detection limit of the NucliSENS EasyQ HPV test. Each plasmid dilution was tested by real-time quantitative type-specific PCR as described previously (14) to confirm the presence of the expected amount of plasmid.
Cell lines.
HeLa cells (containing 10 to 50 copies HPV18 per cell) and CaSki cells (containing 500 to 600 copies HPV16 per cell) were purchased from the American Type Culture Collection. HaCaT cells (HPV-negative keratinocytes) were kindly provided by P. Boukamp (DKFZ, Heidelberg, Germany). Cultures were harvested at 90% confluence, and approximately 8 × 106 cells were resuspended in 8 ml phosphate-buffered saline (PBS) for immediate NA extraction or fixed in 8 ml SurePath or PreservCyt (Hologic Inc., Marlborough, MA) medium for storage at 4°C for 1 week. Worldwide, the SurePath method, which uses an ethanol-based preservative, and the Thinprep technique (Hologic Inc., Marlborough, MA), which applies the methanol-based fixative PreservCyt, are the most commonly used methods in LBC. Residual material from PreservCyt-fixed samples is suitable for DNA and RNA isolation and subsequent analysis by reverse transcriptase PCR (RT-PCR) (16).
Extraction and purification of NA.
Total DNA was extracted from 2 ml cell suspension with the GenElute Mammalian Genomic DNA Miniprep kit (Sigma-Aldrich Corp., St-Louis, MO) according to the manufacturer's instructions. The protocol included an optional RNase A treatment step to obtain RNA-free genomic DNA.
Total RNA was extracted from 2 ml cell suspension by the reagent-based TRIzol method (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. To remove residual DNA, the total RNA was treated with amplification-grade DNase I (Sigma-Aldrich Corp., St. Louis, MO) according to the manufacturer's instructions.
Total DNA and total RNA were extracted from the unfixed and fixed (PreServCyt and SurePath) HeLa cells. HeLa cell RNA-free DNA and DNA-free RNA were obtained in triplicate. RNA-free DNA and DNA-free RNA were purified from unfixed and fixed CaSki and HaCaT cells. The concentrations of NA extracts were assessed by UV spectrophotometry.
To confirm the presence of amplifiable DNA and to assess the effectiveness of the DNase treatments, the NA extracts were assayed by real-time PCR for the β-globin gene (forward primer, 5′-CAG GTA CGG CTG TCA TCA CTT AGA-3′; reverse primer, 5′-CAT GGT GTC TGT TTG AGG TTG CTA-3′) using the LightCycler based on SYBR green I methodology (34). Different steps, such as DNA extraction, sample preparation, amplification, and post-PCR treatment, were performed in strictly separated rooms. Positive (total HeLa cell DNA) and negative (all PCR components but no NA) PCR controls were used.
Real-time multiplex NASBA.
HPV plasmid dilutions and NA extracts (total DNA, RNA-free DNA, total RNA, and DNA-free RNA) of unfixed and fixed (PreServCyt and SurePath) HaCaT, HeLa, and CaSki cells were tested with the NucliSENS EasyQ HPV test according to the manufacturer's instructions. Briefly, three premixes were made by the reconstitution of reagent spheres in the reagent sphere diluent followed by the addition of either U1A/HPV16, HPV18/31, or HPV33/45 primer/molecular beacon mixes and KCl stock solution. Ten microliters of premix was distributed to each well, followed by 5 μl sample, and incubated for 4 min at 65°C and 2 min at 41°C. The reaction was started by the addition of enzymes and measured in real time using the NucliSENS EasyQ analyzer at 41°C. Data analysis was performed using the NucliSENS EasyQ Director software.
The NucliSENS EasyQ HPV test includes primer pairs targeting U1A mRNA as intrinsic control to determine the sample validity. If U1A is positive or one or more HPV targets are positive, the sample is valid. If U1A is negative and no HPV targets are positive, the sample is invalid. To evaluate the run validity, positive controls for U1A/HPV16, HPV18/HPV31, and HPV33/45 are included.
RESULTS
Absorbance measurements allowed the estimation of the quantity of the extracted and purified NA extracts. Table 1 shows the input of NA (in ng/5 μl) in the NucliSENS EasyQ HPV test. All total DNA, total RNA, and RNA-free DNA extracts showed positive results for the β-globin PCR, which indicated the presence of amplifiable DNA. The DNase treatment did not consistently abolish the PCR signal. When the DNase-treated RNA showed a positive PCR result, the DNase treatment was repeated. Only DNA-free RNA was used for NucliSENS EasyQ testing.
TABLE 1.
Results of the NucliSENS EasyQ HPV test for NA extracts
| NA extract | Fixationa | Cell type | NA input (ng/5μl) | NucliSENS EasyQ resultb |
||
|---|---|---|---|---|---|---|
| U1A | HPV18 | HPV16 | ||||
| Total RNA | Unfixed | HeLa | 410 | Pos | Pos | Neg |
| PSC | HeLa | 3033 | Pos | Pos | Neg | |
| SP | HeLa | 163 | Neg | Pos | Neg | |
| Total DNA | Unfixed | HeLa | 81 | Pos | Pos | Neg |
| PSC | HeLa | 123 | Pos | Pos | Neg | |
| SP | HeLa | 35 | Neg | Pos | Neg | |
| DNA-free RNA | Unfixed | HeLa | 451 | Neg | Pos | Neg |
| Unfixed | HeLa | 359 | Pos | Pos | Neg | |
| Unfixed | HeLa | 949 | Pos | Pos | Neg | |
| Unfixed | CaSki | 478 | Neg | Neg | Neg | |
| Unfixed | HaCaT | 465 | Pos | Neg | Neg | |
| PSC | HeLa | 2572 | Pos | Pos | Neg | |
| PSC | HeLa | 2493 | Pos | Pos | Neg | |
| PSC | HeLa | 990 | Neg | Pos | Neg | |
| PSC | CaSki | 1241 | Neg | Neg | Pos | |
| PSC | HaCaT | 493 | Neg | Neg | Neg | |
| SP | HeLa | 236 | Neg | Pos | Neg | |
| SP | HeLa | 58 | Neg | Pos | Neg | |
| SP | HeLa | 117 | Neg | Pos | Neg | |
| SP | CaSki | 87 | Neg | Neg | Neg | |
| SP | HaCaT | 79 | Neg | Neg | Neg | |
| RNA-free DNA | Unfixed | HeLa | 30 | Neg | Pos | Neg |
| Unfixed | HeLa | 18 | Neg | Pos | Neg | |
| Unfixed | HeLa | 47 | Neg | Pos | Neg | |
| Unfixed | CaSki | 10 | Neg | Neg | Pos | |
| Unfixed | HaCaT | 27 | Neg | Neg | Neg | |
| PSC | HeLa | 11 | Neg | Pos | Neg | |
| PSC | HeLa | 36 | Neg | Pos | Neg | |
| PSC | HeLa | 10 | Neg | Pos | Neg | |
| PSC | CaSki | 10 | Neg | Neg | Pos | |
| PSC | HaCaT | 14 | Neg | Neg | Neg | |
| SP | HeLa | 11 | Neg | Pos | Neg | |
| SP | HeLa | 15 | Neg | Pos | Neg | |
| SP | HeLa | 20 | Neg | Pos | Neg | |
| SP | CaSki | 13 | Neg | Neg | Pos | |
| SP | HaCaT | 17 | Neg | Neg | Neg | |
PSC, PreServCyt; SP, SurePath.
Pos, positive; Neg, negative.
Real-time multiplex NASBA.
The positive controls for HPV16, -18, -31, -33, and -45 consistently showed positive results, which indicated the run validity. Table 1 shows the results for the U1A control of the NucliSENS EasyQ HPV test for the NA extracts (total RNA, total DNA, DNA-free RNA, and RNA-free DNA) of unfixed and fixed (PreServCyt and SurePath) cells (HeLa, CaSki, and HaCaT). Each RNA-free DNA extract appeared to be negative for the U1A control, regardless of the cell type or fixation. The NA extracts of cells fixed in SurePath were consistently negative for U1A. The total DNA and total RNA extracts of unfixed or PreServCyt-fixed HeLa showed positive U1A signals. Only half of the DNA-free RNA extracts of unfixed cells or PreServCyt-fixed cells were U1A positive.
Table 1 shows the results for the HPV targets of the NucliSENS EasyQ HPV test for the NA extracts (total RNA, total DNA, DNA-free RNA, and RNA-free DNA) of unfixed and fixed (PreServCyt and SurePath) cells (HeLa, CaSki, and HaCaT). All NA extracts of unfixed and fixed HeLa cells were consistently positive for HPV18. For CaSki cells, all RNA-free DNA extracts were HPV16 positive, irrespective of fixation. The DNA-free RNA extracts of the PreServCyt-fixed CaSki cells were positive for HPV16, while those of the unfixed and the SurePath-fixed CaSki cells did not show a positive HPV16 signal. All NA extracts of the HaCaT cells were negative for all HPV types.
All HPV plasmids could be detected with NASBA (Table 2). For HPV16, -18, and -33 NASBA, the lower detection limit was 5 × 103 copies of the corresponding plasmids. HPV31 and HPV45 NASBA detected 5 × 102 copies of the corresponding plasmids. HPV31 and -45 plasmids showed multiple positive test results (Table 2). HPV31 plasmids showed positive signals for HPV16, -31, and -45 NASBA. HPV45 plasmids showed positive signals for HPV16 and -45 NASBA.
TABLE 2.
Results of the NucliSENS EasyQ HPV test for HPV plasmids
| Plasmid | Sensitivity | NucliSENS EasyQ resulta |
||||
|---|---|---|---|---|---|---|
| HPV16 | HPV18 | HPV31 | HPV33 | HPV45 | ||
| HPV16 | 5 × 103 | Pos | Neg | Neg | Neg | Neg |
| HPV18 | 5 × 103 | Neg | Pos | Neg | Neg | Neg |
| HPV31 | 5 × 102 | Pos | Neg | Pos | Neg | Pos |
| HPV33 | 5 × 103 | Neg | Neg | Neg | Pos | Neg |
| HPV45 | 5 × 102 | Pos | Neg | Neg | Neg | Pos |
Pos, positive; Neg, negative.
The HPV45 plasmid without the T7 promoter was tested with the NucliSENS EasyQ HPV test. HPV45 NASBA showed a lower detection limit of 5 × 103 copies. HPV16 NASBA was negative for the HPV45 plasmid without the T7 promoter.
DISCUSSION
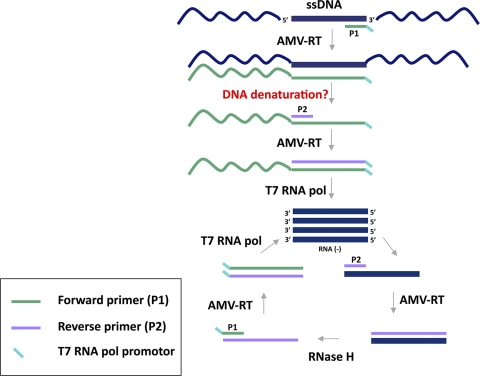
The necessity of E6/E7 expression for incurring and maintaining a malignant phenotype suggested a promising concept for E6/E7 mRNA detection to identify progressive HPV infections. Several in-house HPV RNA detection methods based on RT-PCR have been described (25, 31, 32, 39). Their performance is determined by the targeted transcript, i.e., full-length or spliced E6/E7 transcripts, and is often hampered by the lack of an internal control (9). Moreover, RT-PCR can give false-positive results if the RNA sample contains traces of genomic DNA. Strategies to cope with this problem are DNase digestion prior to RT-PCR, the performance of “no-RT control” PCRs, or the selection of intron-spanning primer pairs, which is impossible for intronless genes or when full-length transcripts are amplified. NASBA has been described as an alternative approach to specifically detect RNA targets and is based on the activity of avian myeloblastosis virus (AMV) reverse transcriptase, RNase H, and T7 DNA-dependent RNA polymerase (T7 RNA pol) together with two primers. The process occurs at 41°C and results in the exponential accumulation of single-stranded RNA (ssRNA products), which can be detected through molecular beacon technology (12). Unexpected NASBA of bacterial genomic DNA (29) and the development of a NASBA-based DNA detection and quantification system have been described (40), although the molecular mechanism of isothermal amplification of dsDNA has never been elucidated (12). dsDNA is not expected to denature at 41°C or during the 65°C incubation step, which is required to allow annealing of the primers to the target and is performed before NASBA enzymes are added to the reaction mixture. It is possible that single-stranded replicate intermediates of dsDNA can function as a template for NASBA. Moreover, for HPV DNA plasmids and viral episomal DNA, the accessibility of the target is higher than that for genomic DNA, which has a more complex conformational organization. However, it remains unclear how the newly synthesized DNA strand denatures from the template strand to allow extension of the second primer P2 to render a double-stranded T7 RNA pol promoter sequence (Fig. 1). From that point on, the reaction continues with synthesis of the RNA used for entry in the amplification phase of the NASBA process.
FIG. 1.
Proposed mechanism for NASBA amplification of dsDNA.
This study evaluated whether the NucliSENS EasyQ HPV test enables amplification of viral DNA, based on HPV DNA plasmids and enzymatically treated NA extracts of several cell lines. NucliSENS EasyQ HPV version 1 was launched in 2007 and was based on the original PreTect Proofer assay developed by NorChip, except for the NucliSENS hardware platform and the software for NASBA measurements and data analysis (NucliSENS Director software) (17). The HPV positivity of the RNA-free DNA extracts and the HPV DNA plasmids indicated that viral dsDNA can function as a template for NASBA with the NucliSENS EasyQ HPV test. Fixation did not interfere with the ability to amplify the RNA-free DNA extracts. For the RNA-free DNA extracts, the U1A signal was consistently absent, which suggests that the U1A primers and probe are specific for RNA and indicate the presence of amplifiable RNA. SurePath fixation consistently abolished the U1A signal of DNA-free RNA extracts, which is in accordance with the previously described poor recovery and quality of RNA from SurePath-fixed samples using standard extraction techniques (16, 28). The U1A negativity of some of the unfixed and PreServCyt-fixed DNA-free RNA extracts could be the result of other factors that influenced RNA quantity and/or integrity, such as inefficient RNA extraction or repeated DNase treatment. The absence of amplifiable RNA could also explain the HPV16 negativity of the CaSki cell DNA-free RNA extracts. Several DNA-free RNA extracts appeared to be U1A negative and HPV positive, which could be the result of the longer length of the U1A mRNA or its low expression level compared to the HPV18 target.
Based on the plasmid dilution series, a lower detection limit of 5 × 103 plasmid copies could be determined for HPV16, -18, and -33 NASBA. Similar results have been shown by another study that showed amplification of homologous plasmid DNA using more than 104 molecules per NASBA assay under nondenaturing conditions (38). For HPV31 and -45, NASBA could detect 5 × 102 copies. However, both these plasmids contained a double-stranded T7 RNA pol promoter sequence and therefore could not be considered representative controls (Fig. 1).
As explained above, the double-stranded T7 promoter can be immediately used by the T7 RNA pol to produce many new RNA molecules that are complementary to the target mRNA. After this initiation phase, NASBA enters the cyclic or amplification phase. An HPV45 plasmid without the T7 promoter was constructed as an appropriate control for NASBA-based HPV45 DNA amplification. This plasmid showed HPV45 positivity (lower detection limit, 5 × 103), but unlike with the original HPV45 plasmid, the HPV16 NASBA was negative. These findings suggest that the molecular beacon probe for HPV16 cross-hybridizes with amplified HPV45 ssRNA, while the HPV16 primers do not anneal to the HPV45 DNA. The same could apply to HPV31 ssRNA and the molecular beacon probes for HPV16 and -45.
Our results show that the NASBA detection limit for HPV DNA is high (between 5 × 102 and 5 × 103 HPV plasmids). The sensitivity of the NASBA assay decreases significantly when DNA is used as a target rather than the corresponding RNA (23). This indicates that even in the presence of identical amounts of DNA and RNA, the RNA target will outcompete the DNA target for the enzymes of NASBA.
Quite a few studies have assessed the clinical performance of commercial NASBA-based HPV RNA detection assays in comparison to HPV DNA tests and showed a higher specificity for the mRNA test (6, 7, 10, 18, 20, 22, 24, 35, 36). However, some of these studies (6, 10) do not constitute a direct comparison of HPV DNA and RNA for the detection of clinical end points but have been correlative; i.e., the samples were already selected due to their HPV DNA positivity so that the corresponding transcript could be sought. Furthermore, several other factors hamper the establishment of consensus findings across these studies: different types of clinical specimens tested, lack of demographic data, and different type detection ranges of the mRNA and DNA assays (9). The NucliSENS EasyQ HPV test and the technologically related PreTect Proofer test detect only five HR-HPV types, while the HC2 and Amplicor tests, to which they are often compared, detect 13 HR-HPV types. Consequently, it is currently not clear whether the increased specificity of NASBA-based testing is driven by true detection of transcripts or by detection of a more limited range of HPV types (36). The HR-HPV types detected by the NASBA-based tests are the most commonly identified carcinogenic types and can be found in more than 90% of cervical tumors worldwide (8). The low positivity rates of the NASBA-based tests compared to the DNA tests in women with normal cytology or low-grade cervical lesions could be explained by the presence of other HR-HPV types, which are less likely to persist and progress to CIN2+ lesions. In this context, it should also be mentioned that the HC2 test is known to show cross-hybridization with low-risk types not included in the probe cocktails (37), which further undermines its specificity for CIN2+.
Our study identified viral dsDNA as a possible target for commercial NASBA-based HPV detection, but this does not affect the potential clinical value of these assays. The results suggest that redefinition of the biological significance of the assays is appropriate. The differences in diagnostic accuracy between the NASBA-based tests and conventional HPV DNA detection assays seem attributable not to the more specific amplification of viral mRNA but to the limited type range and the lower analytical sensitivity for HPV DNA. Detection of a confined array of HR-HPV types could be used in primary screening or in triage of patients with abnormal cytology or a positive wide-spectrum HPV DNA test but requires further investigation in prospective, longitudinal studies.
Acknowledgments
G.A.V.B. has a Ph.D. fellowship from the Research Foundation-Flanders (FWO). I.M.M. is supported by the Industrial Research Fund of the University of Antwerp. C.A.J.H. is supported by the Foundation Emmanuel van der Schueren. J.J.B. is supported by the Research Foundation-Flanders (FWO) and the Belgian Cancer Foundation.
Special thanks go to laboratory technicians Brenda Gabriels, Ludo Boels, and Karen Ileghems.
Footnotes
Published ahead of print on 12 May 2010.
REFERENCES
- 1.Arbyn, M., F. Buntinx, M. Van Ranst, E. Paraskevaidis, P. Martin-Hirsch, and J. Dillner. 2004. Virologic versus cytologic triage of women with equivocal Pap smears: a meta-analysis of the accuracy to detect high-grade intraepithelial neoplasia. J. Natl. Cancer Inst. 96:280-293. [DOI] [PubMed] [Google Scholar]
- 2.Arbyn, M., E. Paraskevaidis, P. Martin-Hirsch, W. Prendiville, and J. Dillner. 2005. Clinical utility of HPV-DNA detection: triage of minor cervical lesions, follow-up of women treated for high-grade CIN: an update of pooled evidence. Gynecol. Oncol. 99:S7-S11. [DOI] [PubMed] [Google Scholar]
- 3.Atypical Squamous Cells of Undetermined Significance/Low-Grade Squamous Intraepithelial Lesions Triage Study Group. 2000. Human papillomavirus testing for triage of women with cytologic evidence of low-grade squamous intraepithelial lesions: baseline data from a randomized trial. J. Natl. Cancer Inst. 92:397-402. [DOI] [PubMed] [Google Scholar]
- 4.Bosch, F. X., A. Lorincz, N. Munoz, C. J. Meijer, and K. V. Shah. 2002. The causal relation between human papillomavirus and cervical cancer. J. Clin. Pathol. 55:244-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boulet, G. A., C. A. Horvath, S. Berghmans, and J. Bogers. 2008. Human papillomavirus in cervical cancer screening: important role as biomarker. Cancer Epidemiol. Biomarkers Prev. 17:810-817. [DOI] [PubMed] [Google Scholar]
- 6.Cattani, P., A. Siddu, S. D'Onghia, S. Marchetti, R. Santangelo, V. G. Vellone, G. F. Zannoni, and G. Fadda. 2009. RNA (E6 and E7) assays versus DNA (E6 and E7) assays for risk evaluation for women infected with human papillomavirus. J. Clin. Microbiol. 47:2136-2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cattani, P., G. F. Zannoni, C. Ricci, S. D'Onghia, I. N. Trivellizzi, A. Di Franco, V. G. Vellone, M. Durante, G. Fadda, G. Scambia, G. Capelli, and R. De Vincenzo. 2009. Clinical performance of human papillomavirus E6 and E7 mRNA testing for high-grade lesions of the cervix. J. Clin. Microbiol. 47:3895-3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clifford, G., S. Franceschi, M. Diaz, N. Munoz, and L. L. Villa. 2006. HPV type-distribution in women with and without cervical neoplastic diseases. Vaccine 24(Suppl. 3):S3/26-S3/34. [DOI] [PubMed] [Google Scholar]
- 9.Cuschieri, K., and N. Wentzensen. 2008. Human papillomavirus mRNA and p16 detection as biomarkers for the improved diagnosis of cervical neoplasia. Cancer Epidemiol. Biomarkers Prev. 17:2536-2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cuschieri, K. S., M. J. Whitley, and H. A. Cubie. 2004. Human papillomavirus type specific DNA and RNA persistence—implications for cervical disease progression and monitoring. J. Med. Virol. 73:65-70. [DOI] [PubMed] [Google Scholar]
- 11.Cuzick, J., M. Arbyn, R. Sankaranarayanan, V. Tsu, G. Ronco, M. H. Mayrand, J. Dillner, and C. J. Meijer. 2008. Overview of human papillomavirus-based and other novel options for cervical cancer screening in developed and developing countries. Vaccine 26(Suppl. 10):K29-K41. [DOI] [PubMed] [Google Scholar]
- 12.Deiman, B., P. van Aarle, and P. Sillekens. 2002. Characteristics and applications of nucleic acid sequence-based amplification (NASBA). Mol. Biotechnol. 20:163-179. [DOI] [PubMed] [Google Scholar]
- 13.Depuydt, C. E., I. H. Benoy, E. J. Bailleul, J. Vandepitte, A. J. Vereecken, and J. J. Bogers. 2006. Improved endocervical sampling and HPV viral load detection by Cervex-Brush Combi. Cytopathology 17:374-381. [DOI] [PubMed] [Google Scholar]
- 14.Depuydt, C. E., G. A. Boulet, C. A. Horvath, I. H. Benoy, A. J. Vereecken, and J. J. Bogers. 2007. Comparison of MY09/11 consensus PCR and type-specific PCRs in the detection of oncogenic HPV types. J. Cell Mol. Med. 11:881-891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gravitt, P. E., F. Coutlee, T. Iftner, J. W. Sellors, W. G. Quint, and C. M. Wheeler. 2008. New technologies in cervical cancer screening. Vaccine 26(Suppl. 10):K42-K52. [DOI] [PubMed] [Google Scholar]
- 16.Horvath, C. A., G. Boulet, S. Sahebali, C. Depuydt, T. Vermeulen, D. Vanden Broeck, A. Vereecken, and J. Bogers. 2008. Effects of fixation on RNA integrity in a liquid-based cervical cytology setting. J. Clin. Pathol. 61:132-137. [DOI] [PubMed] [Google Scholar]
- 17.Jeantet, D., F. Schwarzmann, J. Tromp, W. J. Melchers, A. A. van der Wurff, T. Oosterlaken, M. Jacobs, and A. Troesch. 2009. NucliSENS EasyQ HPV v1 test—testing for oncogenic activity of human papillomaviruses. J. Clin. Virol. 45(Suppl. 1):S29-S37. [DOI] [PubMed] [Google Scholar]
- 18.Keegan, H., J. McInerney, L. Pilkington, P. Gronn, I. Silva, F. Karlsen, N. Bolger, C. Logan, L. Furuberg, J. O'Leary, and C. Martin. 2009. Comparison of HPV detection technologies: hybrid capture 2, PreTect HPV-Proofer and analysis of HPV DNA viral load in HPV16, HPV18 and HPV33 E6/E7 mRNA positive specimens. J. Virol. Methods 155:61-66. [DOI] [PubMed] [Google Scholar]
- 19.Kraus, I., T. Molden, R. Holm, A. K. Lie, F. Karlsen, G. B. Kristensen, and H. Skomedal. 2006. Presence of E6 and E7 mRNA from human papillomavirus types 16, 18, 31, 33, and 45 in the majority of cervical carcinomas. J. Clin. Microbiol. 44:1310-1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lie, A. K., B. Risberg, B. Borge, B. Sandstad, J. Delabie, R. Rimala, M. Onsrud, and S. Thoresen. 2005. DNA- versus RNA-based methods for human papillomavirus detection in cervical neoplasia. Gynecol. Oncol. 97:908-915. [DOI] [PubMed] [Google Scholar]
- 21.Middleton, K., W. Peh, S. Southern, H. Griffin, K. Sotlar, T. Nakahara, A. El-Sherif, L. Morris, R. Seth, M. Hibma, D. Jenkins, P. Lambert, N. Coleman, and J. Doorbar. 2003. Organization of human papillomavirus productive cycle during neoplastic progression provides a basis for selection of diagnostic markers. J. Virol. 77:10186-10201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Molden, T., I. Kraus, F. Karlsen, H. Skomedal, J. F. Nygard, and B. Hagmar. 2005. Comparison of human papillomavirus messenger RNA and DNA detection: a cross-sectional study of 4,136 women >30 years of age with a 2-year follow-up of high-grade squamous intraepithelial lesion. Cancer Epidemiol. Biomarkers Prev. 14:367-372. [DOI] [PubMed] [Google Scholar]
- 23.Molden, T., I. Kraus, H. Skomedal, T. Nordstrom, and F. Karlsen. 2007. PreTect HPV-Proofer: real-time detection and typing of E6/E7 mRNA from carcinogenic human papillomaviruses. J. Virol. Methods 142:204-212. [DOI] [PubMed] [Google Scholar]
- 24.Molden, T., J. F. Nygard, I. Kraus, F. Karlsen, M. Nygard, G. B. Skare, H. Skomedal, S. O. Thoresen, and B. Hagmar. 2005. Predicting CIN2+ when detecting HPV mRNA and DNA by PreTect HPV-proofer and consensus PCR: a 2-year follow-up of women with ASCUS or LSIL Pap smear. Int. J. Cancer 114:973-976. [DOI] [PubMed] [Google Scholar]
- 25.Nakagawa, S., H. Yoshikawa, T. Yasugi, M. Kimura, K. Kawana, K. Matsumoto, M. Yamada, T. Onda, and Y. Taketani. 2000. Ubiquitous presence of E6 and E7 transcripts in human papillomavirus-positive cervical carcinomas regardless of its type. J. Med. Virol. 62:251-258. [DOI] [PubMed] [Google Scholar]
- 26.Naucler, P., W. Ryd, S. Tornberg, A. Strand, G. Wadell, K. Elfgren, T. Radberg, B. Strander, B. Johansson, O. Forslund, B. G. Hansson, E. Rylander, and J. Dillner. 2007. Human papillomavirus and Papanicolaou tests to screen for cervical cancer. N. Engl. J. Med. 357:1589-1597. [DOI] [PubMed] [Google Scholar]
- 27.Paraskevaidis, E., M. Arbyn, A. Sotiriadis, E. Diakomanolis, P. Martin-Hirsch, G. Koliopoulos, G. Makrydimas, J. Tofoski, and D. H. Roukos. 2004. The role of HPV DNA testing in the follow-up period after treatment for CIN: a systematic review of the literature. Cancer Treat. Rev. 30:205-211. [DOI] [PubMed] [Google Scholar]
- 28.Powell, N., K. Smith, and A. Fiander. 2006. Recovery of human papillomavirus nucleic acids from liquid-based cytology media. J. Virol. Methods 137:58-62. [DOI] [PubMed] [Google Scholar]
- 29.Rodriguez-Lazaro, D., J. Lloyd, J. Ikonomopoulos, M. Pla, and N. Cook. 2004. Unexpected detection of DNA by nucleic acid sequence-based amplification technique. Mol. Cell Probes 18:251-253. [DOI] [PubMed] [Google Scholar]
- 30.Ronco, G., S. Brezzi, F. Carozzi, P. P. Dalla, P. Giorgi-Rossi, D. Minucci, C. Naldoni, N. Segnan, M. Zappa, M. Zorzi, and J. Cuzick. 2007. The New Technologies for Cervical Cancer Screening randomised controlled trial. An overview of results during the first phase of recruitment. Gynecol. Oncol. 107:S230-S232. [DOI] [PubMed] [Google Scholar]
- 31.Rose, B. R., C. H. Thompson, M. H. Tattersall, P. M. Elliott, C. Dalrymple, and Y. E. Cossart. 1995. Identification of E6/E7 transcription patterns in HPV 16-positive cervical cancers using the reverse transcription/polymerase chain reaction. Gynecol. Oncol. 56:239-244. [DOI] [PubMed] [Google Scholar]
- 32.Sotlar, K., A. Stubner, D. Diemer, S. Menton, M. Menton, K. Dietz, D. Wallwiener, R. Kandolf, and B. Bultmann. 2004. Detection of high-risk human papillomavirus E6 and E7 oncogene transcripts in cervical scrapes by nested RT-polymerase chain reaction. J. Med. Virol. 74:107-116. [DOI] [PubMed] [Google Scholar]
- 33.Stanley, M., L. Gissmann, and D. Nardelli-Haefliger. 2008. Immunobiology of human papillomavirus infection and vaccination-implications for second generation vaccines. Vaccine 26(Suppl. 10):K62-K67. [DOI] [PubMed] [Google Scholar]
- 34.Steinau, M., M. S. Rajeevan, and E. R. Unger. 2006. DNA and RNA references for qRT-PCR assays in exfoliated cervical cells. J. Mol. Diagn. 8:113-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Szarewski, A., L. Ambroisine, L. Cadman, J. Austin, L. Ho, G. Terry, S. Liddle, R. Dina, J. McCarthy, H. Buckley, C. Bergeron, P. Soutter, D. Lyons, and J. Cuzick. 2008. Comparison of predictors for high-grade cervical intraepithelial neoplasia in women with abnormal smears. Cancer Epidemiol. Biomarkers Prev. 17:3033-3042. [DOI] [PubMed] [Google Scholar]
- 36.Trope, A., K. Sjoborg, A. Eskild, K. Cuschieri, T. Eriksen, S. Thoresen, M. Steinbakk, V. Laurak, C. M. Jonassen, U. Westerhagen, M. B. Jacobsen, and A. K. Lie. 2009. Performance of human papillomavirus DNA and mRNA testing strategies for women with and without cervical neoplasia. J. Clin. Microbiol. 47:2458-2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vernon, S. D., E. R. Unger, and D. Williams. 2000. Comparison of human papillomavirus detection and typing by cycle sequencing, line blotting, and hybrid capture. J. Clin. Microbiol. 38:651-655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Voisset, C., B. Mandrand, and G. Paranhos-Baccala. 2000. RNA amplification technique, NASBA, also amplifies homologous plasmid DNA in non-denaturing conditions. Biotechniques 29:236-238, 240. [DOI] [PubMed] [Google Scholar]
- 39.Wang-Johanning, F., D. W. Lu, Y. Wang, M. R. Johnson, and G. L. Johanning. 2002. Quantitation of human papillomavirus 16 E6 and E7 DNA and RNA in residual material from ThinPrep Papanicolaou tests using real-time polymerase chain reaction analysis. Cancer 94:2199-2210. [DOI] [PubMed] [Google Scholar]
- 40.Yates, S., M. Penning, J. Goudsmit, I. Frantzen, B. van de Weijer, D. van Strijp, and B. van Gemen. 2001. Quantitative detection of hepatitis B virus DNA by real-time nucleic acid sequence-based amplification with molecular beacon detection. J. Clin. Microbiol. 39:3656-3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zielinski, G. D., A. G. Bais, T. J. Helmerhorst, R. H. Verheijen, F. A. de Schipper, P. J. Snijders, F. J. Voorhorst, F. J. van Kemenade, L. Rozendaal, and C. J. Meijer. 2004. HPV testing and monitoring of women after treatment of CIN 3: review of the literature and meta-analysis. Obstet. Gynecol. Surv. 59:543-553. [DOI] [PubMed] [Google Scholar]