Abstract
The human cytomegalovirus (HCMV) virion protein pUL83 (also termed pp65) inhibits the expression of interferon-inducible cellular genes. In this work we demonstrate that pUL83 is also important for efficient induction of transcription from the viral major immediate-early promoter. Infection with a mutant virus containing a premature translation termination codon in the UL83 open reading frame (ORF) (UL83Stop) resulted in decreased transcription from the major immediate-early promoter in a time- and multiplicity-dependent manner. Expression of pUL83 alone is capable of transactivating the promoter in a reporter assay, and pUL83 associates with the promoter in infected cells. To investigate the mechanism by which the protein regulates the major immediate-early promoter, we utilized a mutant virus expressing an epitope-tagged pUL83 from its own promoter to identify protein binding partners for pUL83 during infection. We identified and confirmed the interaction of pUL83 with cellular IFI16 family members throughout the course of HCMV infection. pUL83 recruits IFI16 to the major immediate-early promoter, and IFI16 binding at the promoter is dependent upon the presence of pUL83. Consistent with the results obtained with the UL83Stop virus, infection of IFI16 knockdown cells with wild-type virus resulted in decreased levels of immediate-early transcripts compared to those of control cells. These data identify a previously unknown role for pUL83 in the initiation of the human cytomegalovirus gene expression cascade.
Viral infection is marked by a race between the competing interests of the virus and the host cell. Efficient initiation of viral gene expression is critical to circumvent host defenses aimed at blocking viral gene expression. Human cytomegalovirus (HCMV), a betaherpesvirus encoding nearly 200 predicted proteins (57, 59), has evolved multiple means to evade the initial host cell response to infection. The first viral proteins expressed, the immediate-early proteins, play an important role in this process. Immediate-early proteins are detected in fibroblasts within 4 h of infection and thus are available to function at very early stages in the viral life cycle to block antiviral signaling events. For example, the IE1 protein binds to and inhibits STAT1 and STAT2 (64), two host cell proteins critical for the activation of interferon-inducible gene expression; and IE2 has also been implicated in regulation of transcription of antiviral genes (80). pTRS1 blocks the activation of protein kinase R (PKR), an important regulator of protein translation in response to innate immune signals (16, 33, 54, 85), and pUS3 inhibits antigen presentation by infected cells by sequestering and degrading the major histocompatibility complex (MHC) class I heavy-chain complex (39, 48, 55).
In addition to their role in subverting the host response to viral infection, immediate-early proteins are critical for the induction of viral gene expression. IE1 binds to and inhibits histone deacetylases (HDACs) to ensure a chromatin structure on viral genomes (60) that is conducive to transcription. IE2 binds to HDACs (60) and is a broad-acting transcriptional activator, increasing transcription from the promoters of HCMV early genes as well as cellular promoters (5, 38, 51, 60, 65, 66, 83). pTRS1 regulates viral gene transcription by increasing the activity of the major immediate-early promoter (MIEP) (71). Thus, immediate-early proteins block the cellular innate immune response, and they also play crucial roles in regulating the progression of the HCMV lytic cycle.
While the immediate-early proteins regulate functions that block the antiviral response of the infected cell, the cellular response to infection begins prior to the onset of their synthesis. Binding of HCMV glycoproteins to receptors on the surface of the cell is sufficient to activate the innate immune response (23, 75, 88). In fact, glycoprotein B (gB) alone can induce activation of the Toll-like receptor (TLR) signaling pathway within minutes of binding the plasma membrane (8, 10, 23). In order to effectively counter this response and facilitate viral gene expression, HCMV packages virus-coded proteins into its virions that block the activation of the cellular innate immune response.
One such HCMV protein, pUL83 (also termed pp65), is the most abundant component of the tegument domain of HCMV particles (7, 31). It is also the primary component of dense bodies, which are noninfectious particles produced by HCMV-infected cells (7, 37). Infection with a virus lacking the UL83 open reading frame (ORF) results in increased expression of interferon-responsive genes (1, 12). Expression of pUL83 alone is sufficient to reduce the expression of interferon-responsive genes in uninfected cells. The mechanism by which pUL83 blocks transcription of these genes appears to be multifaceted, involving regulation of both interferon response factor 3 (IRF3) (1) and the NF-κB subunit p65 (12). An additional role for pUL83 has been described for natural killer cells, in which it blocks the function of the NKp30 protein to inhibit natural-killer cell cytotoxicity (49).
While the contribution of pUL83 to subversion of the host immune response is well established, its potential impact on viral gene expression has not been examined. In this work we describe a role for pUL83 in the efficient and timely expression of the HCMV immediate-early gene products IE1 and IE2. Consistent with its effect on immediate-early gene expression within infected cells, pUL83 can directly activate the HCMV MIEP in transfection assays. To investigate the mechanism by which the virion protein influences gene expression, we identified protein binding partners for pUL83 in the context of HCMV infection and show that one of them, IFI16, is involved in the control of immediate-early gene transcription by pUL83. These results identify a role for pUL83 and IFI16 in the activation of immediate-early gene expression and viral growth and lead us to speculate that pUL83 may coopt the cellular protein IFI16 to simultaneously block the host cell interferon response and induce viral protein expression.
MATERIALS AND METHODS
Cells, viruses, and reagents.
Primary human foreskin fibroblasts (HFFs) were cultured in medium containing 10% newborn calf serum and used between passages 5 and 15 for all infections. IFI16 knockdown cells were generated by transduction of MRC5 fibroblasts with a lentivirus expressing a short hairpin RNA (shRNA) specific to IFI16. Briefly, the sequence (5′-GCUGGUCCUAACCAAACGU-3′) (30) was cloned into the pH1P shRNA cassette, a lentiviral vector was constructed by inserting the H1P cassette into the PacI site of pFUGW, and a lentivirus stock was produced by cotransfecting pFUGW with the Δ8.9 HIV-1 packaging vector and the vesicular stomatitis virus envelope glycoprotein (VSVG) into 293T cells (50). Transduced cells were selected by incubation with puromycin for 7 days prior to confirmation of knockdown by Western blotting. Cells were used within four passages of transduction.
The laboratory-adapted HCMV strain AD169 containing green fluorescent protein (GFP) inserted in the UL21.5 locus (ADsubUL21GFP) (82) or AD169 derived from a bacterial artificial chromosome (BAC) clone (BADwt) (86) was used as the wild-type virus. The UL83STOP virus (81) was generously provided by Wade Bresnahan (University of Minnesota). Cells were infected in a minimal volume of medium with 10% newborn calf serum. After 1 h the inoculum was removed and replaced with fresh medium containing serum. Cells were harvested by scraping them into medium and washing them once in phosphate-buffered saline (PBS). Cell pellets were frozen at −80°C until used.
Linear recombination in Escherichia coli was used as described previously (58) to generate BADinUL83TAP, a derivative of the HCMV AD169 strain that expresses a pUL83TAP fusion protein. Briefly, a PCR was used to generate a targeting cassette for recombination. The template for amplification contained a tandem affinity purification (TAP) tag followed by a kanamycin cassette flanked by Frt sites or a similar plasmid in which the TAP tag was replaced with a GFP cassette. The primers used for PCR were as follows: 5′ TCGTGTACACGGCCGGGGAGGGCGACGTGGTACAGATGGTGGTCGTGGTCGCCGGAAGAAGATGGAAAAAG 3′ and 5′ ACTACAAAAAAAAAAGCTGAACATGGTCATCTAGCAGCAAAGTTCTCCTTCGTCGTGGAATGCCTTCG 3′. The underlined nucleotides correspond to the viral sequence at the 3′ end of the UL83 ORF. The same primers were used for the generation of both the BADinUL83TAP and BADinUL83GFP constructs. The PCR product was transformed into recombination-competent DY380 E. coli, and chloramphenicol-plus kanamycin-resistant bacteria were selected. Chloramphenicol resistance was provided by the AD169 BAC backbone. BAC DNA was purified from drug-resistant colonies and checked for gross rearrangement by restriction digest. Recombinant BAC DNA was transformed into DH10B to prevent spurious recombination that could occur in the DY380 strain. DH10B cells containing the pUL83 fusions were transformed with a temperature-sensitive plasmid expressing Flp recombinase to remove the KanR gene from the targeting cassette. Following removal of the Flp expression plasmid by culture at the nonpermissive temperature, bacteria were screened for loss of kanamycin resistance, retention of chloramphenicol resistance, and sensitivity to ampicillin (to ensure that the Flp expression plasmid was removed). BAC DNA was purified and checked again for genomic integrity by restriction digest. BADinUL83TAP BAC DNA was transfected into HFFs to generate stocks of BADinUL83TAP, which grew indistinguishably from wild-type AD169 virus. BADinUL83GFP virus stocks were generated in the same manner as that described for BADinUL83TAP. Universal type I interferon was purchased from Sigma Aldrich.
ChIP analysis of MIEP binding proteins.
Chromatin immunoprecipitation (ChIP) was performed as described previously (26). HFFs were infected at a multiplicity of infection of 3 PFU/cell. DNA-protein complexes were cross-linked with 1% formaldehyde for 10 min, and the reaction was then quenched by the addition of glycine. Cells were resuspended in lysis buffer (10 mM Tris-HCl [pH 8.0], 100 mM NaCl, 1 mM EDTA, 0.5 mM EGTA, 0.1% sodium deoxycholate, 0.5% N-lauroylsarcosine, protease inhibitors) and then sonicated to fragment DNA. The number of viral genomes per sample was determined by quantitative PCR (qPCR), and equal genomes per sample were diluted in dilution buffer (0.01% sodium dodecyl sulfate [SDS], 1.1% Triton X-100, 1.2 mM EDTA, 16.7 mM Tris-HCl [pH 8.0], 167 mM NaCl, protease inhibitors). Samples were incubated overnight with the indicated antibody (5 μg), and the immune complexes were recovered by subsequent incubation with protein A/G Sepharose for 1 h at 4°C. The beads were collected and washed for 5 min at 4°C sequentially with the following buffers: low-salt buffer (0.1% SDS, 1% Triton X-100, 2 mM EDTA, 20 mM Tris-HCl [pH 8.0], 150 mM NaCl), high-salt buffer (0.1% SDS, 1% Triton X-100, 2 mM EDTA, 20 mM Tris-HCl [pH 8.0], 500 mM NaCl), LiCl buffer (250 mM LiCl, 1% Nonidet P-40, 1% sodium deoxycholate, 1 mM EDTA, 10 mM Tris-HCl [pH 8.0]), TE buffer (1 mM EDTA, 10 mM Tris-HCl [pH 8.0]), and TE-NaCl buffer (50 mM NaCl, 1 mM EDTA, 10 mM Tris-HCl [pH 8.0]). Following the final wash, the immune complexes were eluted with 1% SDS and 100 mM NaHCO3, and the eluted DNA-protein complexes were precipitated with ethanol. Cross-links were reversed by the addition of NaCl (200 mM) and incubation at 65°C for 5 h. Proteins were digested with proteinase K, and the DNA was purified by using QIAquick spin columns. DNA from the MIEP was detected by using primers (5′-AACAGCGTGGATGGCGTCTCC-3′ and 5′-GGCACCAAAATCAACGGGACTTT-3′), and the UL69 region was detected using primers (5′-CTCGTCGTGTGACAGCAGGATG-3′ and 5′-GAACTACAGCAACTCAGCCGTTTGA-3′). DNA was quantified using Power SYBR green PCR Master Mix (Applied Biosciences) on a 7800HT sequence detector system (Applied Biosystems).
Analysis of pUL83 binding proteins by mass spectrometry.
Infected cells were prepared for analysis as described previously (56). Briefly, cells were washed with PBS, harvested in PBS by scraping with a rubber policeman, collected by centrifugation at 3,000 rpm for 10 min, 4°C, and frozen in liquid nitrogen, as described previously (25). Cryogenic cell lysis was performed using a Retsch MM 301 mixer mill (Retsch, Newtown, PA) and homogenized in lysis buffer as described previously (25). Several buffers were tested to obtain an efficient protein extraction and maintain viral-host protein interactions, and the lysis buffer selected as optimal was 20 mM K-HEPES (pH 7.4), 110 mM KOAc, 2 mM MgCl2, 0.1% Tween-20, 1% Triton, 0.25 M NaCl, 1:100 (vol/vol) protease inhibitor mixture (20 mg/ml phenylmethylsulfonyl fluoride (PMSF) plus 0.4 mg/ml pepstatin A), and 1:200 (vol/vol) protease inhibitor cocktail (Sigma). M-270 epoxy Dynabeads (Dynal, NY) were coupled to IgG (MP Biomedicals, Solon, OH) or to custom-developed rabbit polyclonal anti-GFP antibodies, as previously described (25). Immunoaffinity purifications were performed via the PrA or GFP tags by using 1-h incubations of the cellular lysate with the conjugated magnetic beads. Isolated proteins were resolved by SDS-PAGE on 4 to 12% NuPAGE Novex Bis-Tris precast one-dimensional gel electrophoresis (1-DE) gel (Invitrogen) according to the manufacturer's specifications and stained with Coomassie blue stain (Pierce, Rockford, IL). Each entire gel lane was cut into 33- by 1-mm slices, and proteins were digested with trypsin (Promega, WI). The resulting peptides were extracted on reverse-phase resin (Poros 20 R2; PerSeptive Biosystems) as described previously (25) and subjected to matrix-assisted laser desorption ionization (MALDI) mass spectrometric (MS) analysis. The proteins isolated with pUL83TAP at 48 h postinfection (hpi) were analyzed by MS using a MALDI QqToF instrument (QqTOF Centaur; Sciex) and by tandem MS (MS/MS) using a MALDI ion trap (LCQ Deca XP Plus; Finnigan) (45, 46). The proteins isolated with pUL83GFP at 72 hpi were analyzed using a MALDI LTQ Orbitrap (Thermo, Bremen, Germany) as described previously (52). An XProteo computer algorithm was used to correlate the MS and MS/MS data with the National Center for Biotechnology Information nonredundant protein database, version 06/10/16, for Homo sapiens (152,010 sequences) and viruses (346,953 sequences). The parameters used for the database searching were previously described (52). Due to the reduced number of coisolated proteins, we manually inspected the MS/MS spectra and validated the peptide sequences of the candidate proteins. Only proteins that were confirmed by MS/MS analyses of at least two peptides were considered present.
Transfections and luciferase assay.
Cells were transfected using the Fugene 6 reagent (Roche) and the indicated plasmids. The MEIP-luciferase reporter construct was transfected using 0.5 μg/well of a six-well dish, while the amount of pUL83 expression vector (pCVS-pp65) was varied as described. The total amount of DNA in each transfection was kept constant by adding empty vector DNA. At 48 h posttransfection cells were lysed with 200 μl of passive lysis buffer (Promega), and supernatants clarified by centrifugation. Following the addition of luciferase assay reagent (Promega), relative light units were measured on a luminometer.
RNA and protein analysis.
For analysis of RNA by quantitative reverse transcription-PCR (qRT-PCR), total RNA was isolated using Trizol reagent (Invitrogen) according to the manufacturer's instructions, and contaminating DNA was removed using DNA-free reagents (Ambion). Relative quantitation was accomplished through a two-step qRT-PCR as previously described (82). Briefly, cDNA was synthesized with Superscript reverse transcription reagents and random hexamers according to the manufacturer's instructions. Real-time PCR was completed with SYBR green PCR Master Mix (Applied Biosystems) using primers to GAPDH (glyceraldehyde-3-phosphate dehydrogenase) (5′-CTGTTGCTGTAGCCAAATTCGT-3′ and 5′-ACCCACTCCTCCACCTTTGAC-3′) or UL122 (5′-ATGGTTTTGCAGGCTTTGATG-3′ and 5′-ACCTGCCCTTCACGATTCC-3′).
For immunofluorescent analysis, fibroblasts were grown on sterile glass coverslips in six-well plates to confluence. Cells were then infected with HCMV at a multiplicity of 0.1 PFU/cell. Following infection, cells were washed in PBS, fixed for 15 min in 2% paraformaldehyde, and permeabilized for 15 min in 0.1% Triton X-100. After washing with PBS-T (PBS plus 0.2% Tween 20), the cells were incubated for 30 min in PBS-T containing 2% bovine serum albumin (BSA) and incubated with either a mouse or rabbit primary antibody in PBS blocking buffer for 1 h at room temperature. After further washing with PBS-T, slides were incubated for 30 min at room temperature with either goat anti-mouse or goat-anti rabbit immunoglobulin Alexa 488, 546, or 633 (Molecular Probes). The second antibody was supplemented with 4′,6′-diamidino-2-phenylindole dihydrochloride (DAPI; Molecular Probes). Cells were washed with PBS-T, mounted with Slow Fade (Molecular Probes), and viewed using Zeiss LSM510 for laser scanning microscopy.
For immunoprecipitation experiments, frozen cell pellets were resuspended in 1 ml RIPA buffer (Tris-HCl, 50 mM, pH 7.4; 1% NP-40; 0.25% Na-deoxycholate; 150 mM NaCl; 1 mM EDTA; complete mini-EDTA-free protease inhibitors [Roche]). Lysates were sonicated and incubated on ice for 30 min, with vortexing for 5 s every 5 min. Insoluble material was pelleted by centrifugation at 14,000 rpm for 5 min at 4°C. Lysates were precleared for 30 min with protein A/G Sepharose (Santa Cruz) with rotation at 4°C. Antibodies were added, and the sample was incubated for 1 h at 4°C with rotation. Protein A/G Sepharose in RIPA buffer was added, and the sample was incubated for an additional 1 h at 4°C, with rotations. The Sepharose was pelleted and washed five times with RIPA buffer. Following the final wash, residual buffer was removed, the Sepharose was resuspended in sample buffer and boiled at 100°C for 5 min, and insoluble material was pelleted by spinning for 3 min at room temperature at 14,000 rpm. Samples were resolved on 10% SDS-PAGE gels, and protein binding was assessed by Western blotting.
For Western blotting the following antibodies were used: anti-UL83 (8F5) (62), anti-IE1 (clone 1B12) (89), anti-IE2 (clone 3A9) (60), anti-tubulin (Sigma; T6199), mouse anti-IFI16 (Santa Cruz; C-20), and goat anti-IFI16 (Santa Cruz). Antibodies were used at the dilution indicated by the manufacturer. Western blotting with rabbit polyclonal antibodies was performed in 1× TBST (1× TBS, 0.1% Tween 20) with 5% BSA overnight at 4°C, with rocking. Western blotting with mouse monoclonal antibodies was performed in 1× TBST with 1% BSA for 1 h at room temperature. Appropriate secondary antibody was used at a 1:5,000 dilution in 1× TBST plus 1% BSA for 1 h at room temperature. Blots were visualized using ECL reagent (Amersham). Unless otherwise noted, 30 μg of protein was loaded per lane for Western blots.
RESULTS
pUL83 is required for normal viral growth after infection at a low input multiplicity.
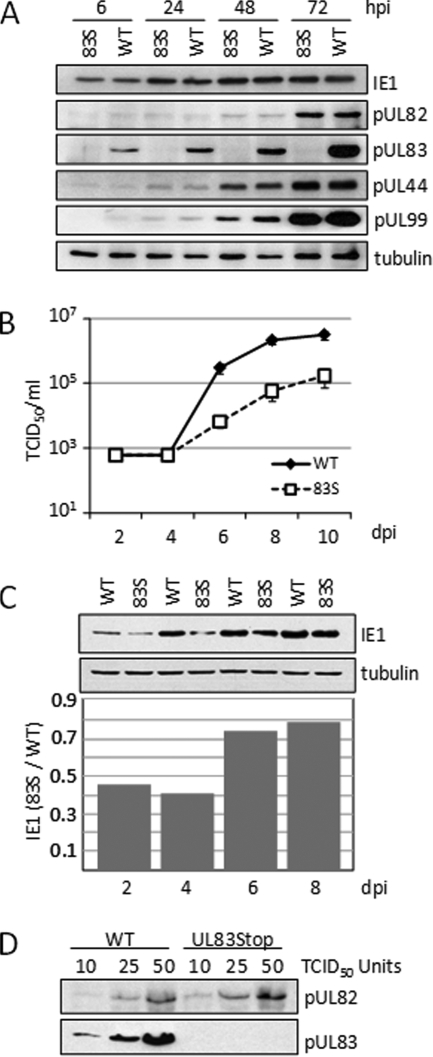
A previous report (81) has shown that an HCMV deletion mutant lacking the UL83 open reading frame (ORF) accumulates reduced levels of the UL123-coded IE1, UL122-coded IE2, and pUL82 proteins, whereas a mutant virus termed UL83Stop, with 10 single-base-pair substitutions generating stop codons near the start of the UL83 ORF, accumulated normal amounts of all three proteins. The authors proposed that the different phenotypes for the two pUL83-deficient viruses could result from an inhibitory effect of the UL83 deletion on expression of the UL82 ORF, because the two ORFs reside on the same bicistronic mRNA (72). Reduced expression of pUL82 would then impact expression of the immediate-early RNAs, because pUL82 helps to activate immediate-early RNA accumulation (11). These experiments were performed at a multiplicity of 5 PFU/cell, and consistent with the earlier report (81), we observed normal accumulation of representative immediate-early, early, and late viral proteins after infection of fibroblasts with UL83Stop at this relatively high input multiplicity (Fig. 1A). However, the mutant virus displayed delayed growth kinetics after infection at a multiplicity of infection of 0.05 PFU/cell (Fig. 1B), indicating that UL83Stop exhibits a multiplicity-dependent growth defect. The UL83Stop virus reproducibly exhibited an ∼10-fold reduced yield between days 6 and 10 after infection at the lower input multiplicity. In experiments in which virus growth was monitored for an extended period, the mutant virus initially exhibited a growth defect and then either partially or completely matched the wild-type yield between days 12 to 15 postinfection (data not shown). A reduction in the accumulation of the IE1 protein was also observed after infection at this low input multiplicity (Fig. 1C). At 4 days postinfection, fibroblasts infected with the UL83Stop virus exhibited a 60% reduction in the level of the IE1 protein compared to wild-type virus infection. UL83Stop virions contained levels of pUL82 similar to those of wild-type virions (Fig. 1D), ruling out the possibility that the mutation in the UL83 ORF influenced expression of the adjacent UL82 ORF or that a lack of pUL83 affected incorporation of pUL82 into the virion.
FIG. 1.
A pUL83-deficient virus exhibits a growth defect following infection at a low multiplicity of infection. (A) At a relatively high input multiplicity, normal levels of viral proteins accumulated after infection with 83STOP virus. Human fibroblasts were infected at a multiplicity of 5 PFU/cell with either wild-type (WT) or 83STOP (83S) virus, and viral protein expression was assayed by Western blotting using antibodies to the indicated proteins. Tubulin was assayed as a loading control. (B) At a relatively low input multiplicity, 83STOP virus displayed a growth defect. Fibroblasts were infected at a multiplicity of 0.05 PFU/cell. Cell-free supernatants were harvested on the indicated days postinfection (dpi), and the amount of HCMV present was determined by a 50% tissue culture infective dose (TCID50) assay of fibroblasts. (C) At a relatively low input multiplicity, UL83STOP virus failed to accumulate normal levels of an immediate-early protein. Fibroblasts were infected at a multiplicity of 0.05 PFU/cell, and IE1 protein expression was analyzed by Western blotting and quantified using a phosphoimager. (D) UL83Stop virions contain amounts of pUL82 similar to those of wild-type virions. Equivalent infectious units of the wild type and UL83Stop were assayed for pUL82 content by Western blotting. The filter was stripped and reprobed with antibody to pUL83 to confirm its absence in UL83Stop virions.
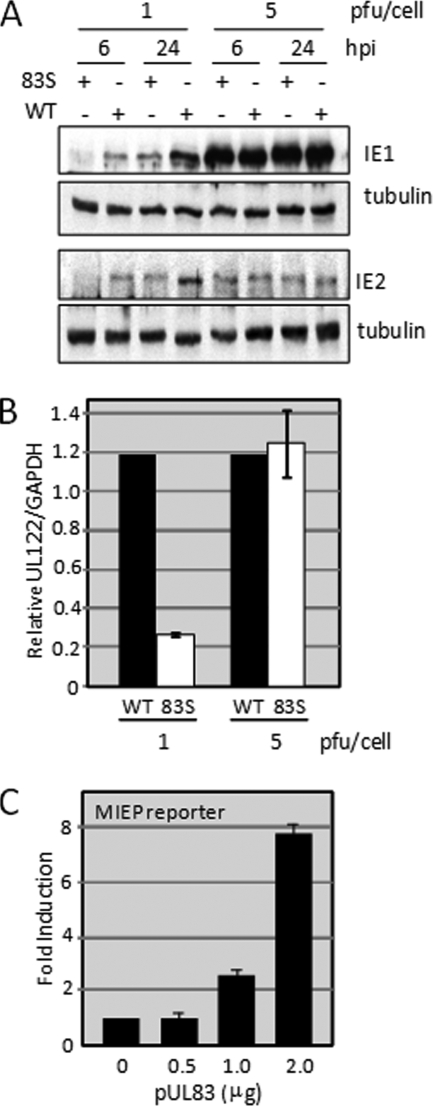
To investigate this multiplicity-dependent effect during a single cycle of replication, we compared expression of the IE1 and IE2 proteins following infection with UL83Stop and its wild-type parent at a multiplicity of 1 versus 5 PFU/cell (Fig. 2A). At the higher multiplicity, the two viruses accumulated similar levels of the IE1 and IE2 proteins at both 6 and 24 hpi. However, the mutant generated less IE1 and IE2 than the wild-type virus at 6 h after infection at the lower multiplicity. The aberrant protein accumulation was reflected at the RNA level (Fig. 2B). qRT-PCR demonstrated that UL83Stop accumulated 5-fold less UL122-specific RNA than wild-type virus at 6 h after infection at a multiplicity of 1 PFU/cell. Also consistent with the protein expression levels, similar accumulations of the UL122 transcript were observed following infection with either virus at the higher multiplicity. Together these results suggest that the delayed replication of the UL83Stop virus is the result of delayed accumulation of immediate-early transcripts and that the requirement for pUL83 can be overcome by increasing the multiplicity of infection.
FIG. 2.
pUL83 is important for complete activation of the MIEP during the immediate-early phase of the replication cycle. (A) pUL83 stimulates IE1 accumulation. Fibroblasts were infected at a multiplicity of 1 or 5 PFU/cell with either WT or 83STOP (83S) virus. Cell lysates were analyzed by Western blotting for IE1 and IE2 expression at 6 and 24 hpi. The results are representative of two independent experiments. (B) pUL83 stimulates the accumulation of an immediate-early RNA. Fibroblasts were infected as described in the legend for panel A, and RNA was isolated and assayed by real-time PCR to determine the levels of IE1 and IE2 transcripts. Levels of virus RNA are presented relative to the levels of the cellular GAPDH RNA. (C) pUL83 alone stimulates MIEP activity outside the context of an infection. U2OS cells were transfected with an MIEP-luciferase reporter construct together with increasing amounts of a pUL83 expression vector. The amount of transfected DNA was kept constant by the addition of empty expression vector.
pUL83 activates the MIEP.
A UL83-deficient deletion mutant has been shown to deliver reduced amounts of pUL82 to infected cells (81), and reduced pUL82 levels might then impact immediate-early gene expression in this variant. In contrast, UL83Stop-infected fibroblasts contain normal levels of pUL82 (Fig. 1A) (81), and UL83Stop virions contain a normal amount of pUL82 (Fig. 1D), demonstrating that a pUL82 deficiency is not responsible for reduced expression of immediately-early genes. However, pUL83-deficient virus particles have recently been shown to lack additional tegument proteins (20). Therefore, rather than monitor the levels of multiple virion proteins in UL83Stop particles, we tested whether pUL83 exerts a direct effect on the activity of the MIEP (Fig. 2C). U2OS cells were transfected with a reporter construct containing the MIEP, driving expression of the luciferase gene together with increasing amounts of a pUL83 expression construct. Expression of the MIEP reporter gene was increased in the presence of pUL83 in a dose-responsive manner, reaching an 8-fold induction at the highest dose tested. This experiment demonstrates that pUL83 alone is sufficient to induce the activity of an MIEP reporter.
pUL83 interacts with IFI16.
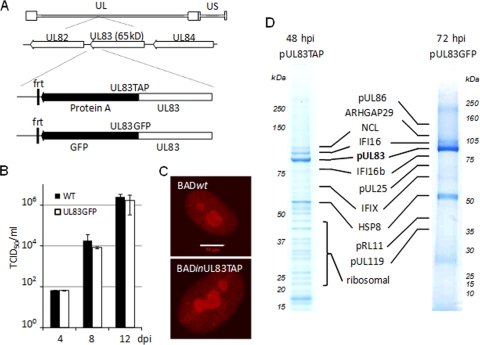
The ability of pUL83 to modulate the MIEP reporter construct outside the context of an infection demonstrated that the induction was dependent on the interaction of the viral protein with cellular, rather than viral, processes. To identify cellular factors important for this functional role, we searched for pUL83 binding partners within infected fibroblasts by using a mass spectrometry-based approach. Two mutant viruses, BADinUL83TAP and BADinUL83GFP, in which a sequence encoding a tandem affinity purification tag (TAP tag) or the GFP coding sequence was fused to the 3′ end of the UL83 ORF (Fig. 3A), were generated. These viruses expressed a pUL83TAP or pUL83GFP fusion protein from the UL83 ORF at its normal location within the viral genome. We have used both of these tags in the past to identify interacting proteins (24, 56), and we arbitrarily chose to use viruses carrying the two different tags in this experiment. The variants as well as wild-type virus replicated (Fig. 3B and data not shown), and incorporation of the tags did not affect the localization of the modified pUL83 (Fig. 3C and data not shown).
FIG. 3.
Identification of UL83 binding partners in the context of infection. (A) Diagram showing the UL83 locus in BADinUL83TAP and BADinUL83GFP. (B) BADinUL83GFP grew normally. HFFs were infected with BADinUL83GFP at a multiplicity of 0.1, and the amount of HCMV in cell-free supernatants was determined by a TCID50 assay at the indicated times postinfection. (C) pUL83TAP was localized normally during infection. pUL83 localization was determined during infection with BADwt (top) or BADinUL83TAP (bottom). For unmodified pUL83, anti-UL83 monoclonal antibody was used for immunofluorescence, while anti-protein A was used to visualize pUL83TAP. The white bar indicates 10 μm. (D) Fibroblasts were infected at a multiplicity of 3 PFU/cell with the indicated viruses and harvested at 48 or 72 hpi. pUL83TAP, pUL83GFP, and associated proteins were isolated on magnetic beads, resolved on 4 to 12% SDS-PAGE gradient gels, stained with Coomassie blue, and analyzed by MALDI MS and MS/MS. Identified proteins are indicated.
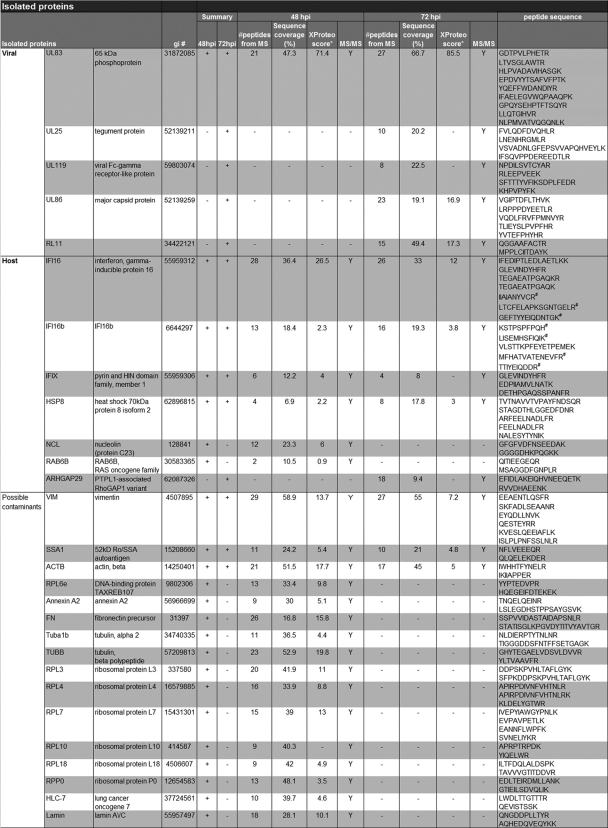
To identify interacting proteins, fibroblasts were harvested at 48 hpi with BADinUL83TAP or at 72 hpi with BADinUL83GFP; and pUL83TAP- and pUL83GFP-containing protein complexes were isolated by using magnetic beads covalently coupled to rabbit IgG or GFP-specific antibody, respectively. The coisolated proteins were resolved by 1-DE, enzymatically digested, and identified by mass spectrometry. As shown in Fig. 3D, several potential pUL83-interacting proteins were identified in the experiment. Table 1 displays a complete list of the isolated proteins at the two times of infection. Although their capture with the fusion proteins suggests that they interact with pUL83, confirmation is needed to definitively identify the proteins as interacting partners. Therefore, the proteins in Table 1 must be considered potential pUL83-interacting proteins.
TABLE 1.
List of proteins identified in the immunoisolates of TAP- and GFP-tagged pUL83a
pUL83 was isolated via the protein A or GFP tag at 48 hpi and 72 hpi, respectively. Coisolated proteins were analyzed using MALDI MS and MS/MS analyses. The gi number, number of peptides detected, sequence coverage (%), XProteo score (d′) following database searching using the MS data, and peptide sequences confirmed by MS/MS are indicated for each identified protein. Only the proteins that were confirmed via the fragmentation of at least two peptides were included in the table. # indicates peptides unique to either IFI16 or IFI16b. The proteins classified as possible contaminants indicate proteins that we routinely observe as likely nonspecific associations in isolates from mammalian cells.
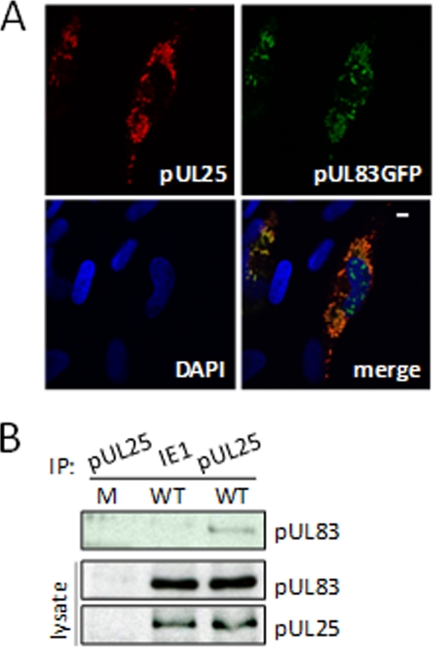
Several HCMV proteins were identified in the capture experiment (Table 1 and Fig. 3D): pUL86, pUL25, pUL119, and pRL11. These proteins were identified in the samples prepared at 72 but not 48 hpi, and we confirmed the interaction of pUL25 with pUL83GFP by colocalization (Fig. 4A) and coimmunoprecipitation (Fig. 4B). pUL83 (7, 31) and pUL25 (90) are both virion tegument proteins, so it is not surprising that they interact. We have not yet tested the other predicted interactions of pUL83 with viral proteins. It is not clear why we did not detect these interactions at 48 as well as 72 hpi. Although UL119 is expressed with early kinetics, the others are late proteins (18), so we might have preferentially detected their interaction with pUL83 at the later time because the proteins had accumulated to higher levels at this point in the infection. Alternatively, time-dependent modification of the proteins might influence their interactions. It is also possible that the GFP tag provided greater sensitivity than the TAP tag in the experiment.
FIG. 4.
Confirmation of the pUL83-pUL25 interaction. (A) pUL25 and pUL83 substantially colocalize within infected cells. Fibroblasts infected with BADinUL83GFP at a multiplicity of 0.5 PFU/cell were fixed and stained with antibody specific for pUL25 (red) to test for colocalization with pUL83GFP (green). The nucleus was stained with DAPI to provide context, and the white bar indicates 10 μm. (B) pUL83 interacts with pUL25 during infection. HFFs were mock infected or infected with wild-type virus at a multiplicity of 3 PFU/cell, and pUL25-specific immune complexes were isolated by immunoprecipitation (IP) 72 h later. Lysates were also immunoprecipitated with antibody to IE1 as a specificity control. The presence of pUL83 in pUL25-specific immune complexes was determined by Western blotting (top), and total pUL83 and pUL25 in cell lysates were monitored as controls (bottom).
Among the prominent cell-coded proteins identified in the analysis at both times after infection were members of the HIN-200 family of proteins (hematopoietic interferon-inducible nuclear antigens with 200 amino acid repeats): IFI16, IFI16b, and IFIX. IFI16 has previously been shown to interact with double-stranded and single-stranded DNA, but with no apparent sequence specificity. The interferon-inducible protein has been shown to be involved in the regulation of p53 transcriptional activity (2, 30, 32, 44, 47, 63, 78), and murine orthologs of IFI16 have recently been shown to regulate the innate immune response (13, 68, 69, 74). Given the similar role of pUL83 in the regulation of the cellular interferon response during HCMV infection (1, 12), we chose to pursue this potential interaction in more detail.
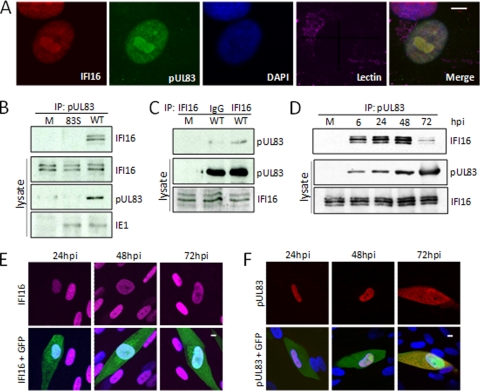
Consistent with previous reports of their separate localizations (3, 4, 27), immunofluorescent analysis revealed that IFI16 and pUL83 were colocalized within the nucleus and nucleolus in infected cells at 48 hpi following infection at a low multiplicity of infection (Fig. 5A). Both proteins were present in both the nucleoplasm and nucleoli. We were able to confirm the interaction by coimmunoprecipitation of IFI16 with an antibody to pUL83 from extracts of cells infected with wild-type virus but not pUL83-deficient virus (Fig. 5B). This experiment provides an important control for the specificity of the pUL83-IFI16 interaction, because it utilizes native pUL83 and therefore demonstrates that the interactions detected in BADinUL83TAP- and BADinUL83GFP-infected cells are not artifacts of the tags. Multiple IFI16 species arising from three differentially spliced mRNAs have been described (41), and three IFI16 isoforms were detected in mock-infected and virus-infected lysates. The levels of IFI16 isoforms were not affected by infection, and all three were evident in the pUL83-specific immunoprecipitates. In a reciprocal experiment, IFI16-specific antibody, but not nonspecific IgG, coimmunoprecipitated pUL83 from infected cells (Fig. 5C). IFI16 coprecipitated with UL83 throughout infection (Fig. 5D). The interaction was evident at the first time tested, 6 hpi, and the amount of IFI16 associated with UL83 peaked at 48 hpi and decreased by 72 hpi. Immunofluorescent analysis provided a possible explanation for the decrease in association of the viral and cellular proteins late after infection. At 72 hpi, IFI16 remained in the nucleus (Fig. 5E), while pUL83 was partially localized to the cytoplasm (Fig. 5F).
FIG. 5.
Confirmation of the pUL83-IFI16 interaction. (A) IFI16 and pUL83 were partially colocalized within infected cells. Fibroblasts infected with wild-type HCMV at a multiplicity of 0.5 PFU/cell were fixed and stained with antibodies specific for UL83 and IFI16 to test for colocalization. The nucleus and Golgi were monitored to provide context, and the white bar indicates 10 μm. (B) IFI16 coprecipitates with antibody to pUL83. Fibroblasts were mock infected (M) or infected with wild-type HCMV (WT) at a multiplicity of 3 PFU/cell and harvested 72 h later. pUL83-specific immune complexes were isolated by immunoprecipitation (IP), and the presence of IFI16 in those complexes was determined by Western blotting using antibodies specific to IFI16. Lysates were assayed by Western blotting for the presence of the indicated proteins as controls. (C) pUL83 coprecipitates with antibody to IFI16. IFI16-specific immune complexes were isolated by immunoprecipitation from mock-infected fibroblasts or at 72 hpi of fibroblasts with wild-type HCMV at a multiplicity of 3 PFU/cell. Immunoprecipitation was also performed with nonspecific antibody (IgG). The presence of pUL83 in precipitates was confirmed by Western blotting with a pUL83-specific antibody. Lysates were assayed by Western blotting for the presence of the indicated proteins as controls. (D) IFI16 interacts with pUL83 throughout the course of infection. Lysates of cells infected at a multiplicity of 3 PFU/cell were prepared at the indicated times and subjected to immunoprecipitation with antibody to pUL83. Lysates were assayed by Western blotting for the presence of the indicated proteins as controls. (E) IFI16 remained in the nucleus during HCMV infection. Fibroblasts were infected at a multiplicity of 0.5 PFU/cell with a derivative of wild-type HCMV expressing a GFP marker protein (green). Cells were fixed and processed for immunofluorescence using an antibody to IFI16 (red) at the indicated times after infection. Nuclei were stained with DAPI to provide context, and the white bar indicates 10 μm. (F) pUL83 was initially localized to the nucleus but was also in the cytoplasm by 72 hpi. Fibroblasts were infected at a multiplicity of 0.5 PFU/cell with a derivative of wild-type HCMV expressing a GFP marker protein (green). Cells were fixed and processed for immunofluorescence using an antibody to pUL83 (red) at the indicated times after infection. Nuclei were stained with DAPI to provide context, and the white bar indicates 10 μm.
These experiments identify several candidate proteins likely to interact with pUL83 in the context of viral infection and confirm the newly discovered interaction between pUL83 and three IFI16 isoforms.
pUL83 cooperates with IFI16 to activate the MIEP.
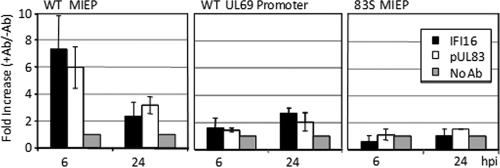
We next asked if IFI16 is involved in the regulation of the MIEP by pUL83. We initially employed chromatin immunoprecipitation (ChIP) analysis to test whether the two proteins are present at the promoter. DNA sequences from the MIEP were found in pUL83- and IFI16-specific immune complexes at 6 hpi and to a lesser extent at 24 hpi (Fig. 6, left). The proteins were not detected at the UL69 viral promoter at 6 hpi, although they were present to a limited extent at the later time (Fig. 6, middle). Importantly, neither protein was detected at the MIEP after infection with the pUL83-deficient mutant (Fig. 6, right), demonstrating a dependence of the IFI16 association on pUL83.
FIG. 6.
pUL83 and IFI16 were present at the MIEP, and IFI16 required pUL83 for this association. Fibroblasts were infected at a multiplicity of 3 PFU/cell with BADwt (WT) or UL83STOP virus (83S). Prior to harvesting at the indicated times after infection, cells were cross-linked with formaldehyde, and IFI16 and pUL83 immune complexes were isolated by immunoprecipitation. Immunoprecipitations carried out without a primary antibody (Ab) were included as a control. The presence of specific DNA sequences (MIEP or UL69 promoter) in the immunoprecipitates was assessed by qRT-PCR.
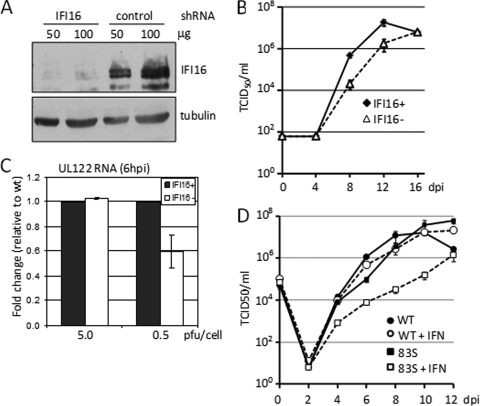
To test the functional consequence of IFI16 binding to the MIEP during the immediate-early stage of HCMV infection, we examined the growth properties of HCMV in cells lacking IFI16. Primary human fibroblasts were infected with a lentivirus vector expressing a short hairpin RNA (shRNA) specific for the IFI16 transcript or a vector containing a scrambled control shRNA. shRNA-mediated knockdown of IFI16 resulted in an approximately 80% decrease in the level of IFI16 protein (Fig. 7A). Multistep growth analysis of wild-type virus replication in these cells revealed that knockdown of IFI16 expression caused a delay in the accumulation of infectious progeny (Fig. 7B), which was almost identical to that observed following infection with the UL83Stop virus (Fig. 1B). As recruitment of IFI16 to the MIEP depends on the presence of pUL83 (Fig. 6), this result suggests that the growth defect observed for wild-type HCMV in IFI16 knockdown cells is the result of decreased expression from the MIEP. When IE2 RNA expressed from the MIEP after infection with wild-type virus was examined by qRT-PCR in IFI16 knockdown cells, we observed a multiplicity-dependent defect in its accumulation at 6 hpi (Fig. 7C). The defect in IE2 RNA accumulation mirrors that observed during infection with the UL83Stop virus (Fig. 2B) and argues that recruitment of IFI16 to the MIEP by pUL83 is necessary for efficient transcription from the MIEP at low multiplicities of infection.
FIG. 7.
IFI16 is required for efficient HCMV replication. (A) Knockdown of IFI16 levels by using shRNA. The level of IFI6 in cells expressing control or IFI16-sepcific shRNA was assayed by Western blotting. Tubulin was monitored as a loading control. (B) Wild-type HCMV exhibits a growth defect in IFI16-deficient cells. Normal fibroblasts (diamonds) and IFI16-deficient fibroblasts (triangles) were infected at a multiplicity of 0.1 PFU/cell. Titers of cell-free virus were determined by TCID50 assay. The experiment was performed in triplicate, and standard errors were determined. (C) Accumulation of immediate-early UL122 RNA in IFI16 knockdown cells. Following infection with wild-type HCMV at a multiplicity of 5 or 0.5 PFU/cell, the level of HCMV UL122 RNA was quantified by qRT-PCR in normal (black bars) and IFI16-deficient cells (white bars). The results are the average of two independent experiments. (D) Growth of 83Stop (83S) virus is sensitive to interferon. Fibroblasts were infected at a multiplicity of 0.1 PFU/cell wild-type HCMV or 83S in the absence or presence of 500 U/ml alpha interferon (IFN). Titers of cell-free virus were determined by TCID50 assay. The experiment was performed in triplicate, and standard errors were determined.
As pUL83 and IFI16 have both been implicated in the interferon response, we tested the possibility that the UL83Stop virus might be differentially susceptible to the effects of interferon treatment (Fig. 7D). When cells infected with wild-type virus were treated with 500 U/ml type I interferon, no significant effect on viral replication was observed. Under these same conditions, the growth of the UL83Stop virus was reduced more than 50-fold. These results confirm that HCMV pUL83 is involved in preventing the antiviral action of interferon during HCMV replication.
DISCUSSION
We have identified an additional role for pUL83 at the start of HCMV infection. UL83Stop, which contains a translational termination codon in the UL83 ORF, replicates with delayed kinetics (Fig. 1B). Accumulation of IE1 and IE2 proteins is delayed in UL83Stop-infected cells compared to the wild-type parent (Fig. 1C and 2A). pUL83 alone is capable of inducing expression from the promoter that controls IE1 and IE2 transcription, the MIEP (Fig. 2C), consistent with a multiplicity-dependent reduction of IE2 RNA within cells infected by the pUL83-deficient virus (Fig. 2B). pUL83 binds specifically to the cellular IFI16 protein (Fig. 3D and 5) and recruits IFI16 to the MIEP (Fig. 6). The pUL83-IFI16 interaction plays an important role in the control of transcription from the MIEP, as cells with reduced IFI16 accumulate reduced levels of IE2 RNA and produce progeny with delayed kinetics following infection with wild-type HCMV (Fig. 7). The reduced accumulation of IE2 mirrors that seen during infection with UL83Stop (Fig. 2B) and supports the view that the delayed kinetics of HCMV replication in IFI16-knockdown cells is at least partly the result of a requirement for pUL83 to recruit IFI16 to the MIEP.
It is noteworthy that a UL83-deficient virus has recently been shown to infect macrophages poorly (20). The defect was localized to a very early event in the infectious cycle, and the authors observed that the mutant failed to incorporate detectable levels of pUL69 and pUL97 into virions. This study raises the possibility that the lack of additional virion proteins at the start of infection could contribute to the multiplicity-dependent growth defect that we have observed for UL83Stop. In this regard, we have previously demonstrated that a pUL69-deficient virus displays a very early defect after infection of fibroblasts (34).
Many herpesviruses have been shown to encode virion proteins that facilitate the expression of viral immediate-early genes in the absence of de novo viral protein synthesis (17, 42, 43), so it is not surprising that HCMV encodes several such proteins, including pUL82 (also termed pp71). pUL82 is delivered by the virion to the newly infected cell together with other tegument proteins during the initial stages of infection, and it enhances expression from the MIEP (6, 11, 73). Previously it has been shown that a deletion mutant lacking the UL83 ORF fails to incorporate pUL82 into the virion (79), and a delay in expression of the IE1 and IE2 proteins was attributed to the absence of pUL82 at the start of infection. Here we show that a virus which lacks pUL83 in the virion but contains pUL82 (Fig. 1D) is also defective for immediate-early gene expression. Our work demonstrates that pUL83 directly contributes to active transcription from the MIEP. We present several lines of evidence in support of this conclusion: (i) IE2 transcript levels are reduced following infection with UL83Stop virus compared to wild-type virus (Fig. 2B); (ii) pUL83 alone induces transcription from a reporter construct controlled by the MIEP in a dose-responsive manner (Fig. 2C); and (iii) pUL83 is physically associated with the MIEP during the immediate-early phase of infection (Fig. 6). Together, these results show that pUL83 regulates transcription from the MIEP in a manner distinguishable from its effects on the availability of pUL82. pUL83 might also directly influence the expression of additional viral promoters, but we have not yet tested this possibility.
Enhancement of MIEP activity by pUL83 also can account for the kinetic replication defect of the UL83Stop mutant (Fig. 1B). After an initial infection at a low input multiplicity, progeny spread to neighboring cells. However, cells infected in the second and subsequent waves of spread receive higher doses of virus than those that occurred in the initial round of infection, allowing UL83Stop to more efficiently express immediate-early proteins. Therefore, as the infection spreads, the replication defect is ameliorated. While pUL82 clearly plays an important role activating the viral transcriptional program, pUL83 independently contributes to transcription of immediate-early genes by acting directly at the MIEP.
The pUL83 interaction partner, IFI16, is a member of the p200 family of proteins (84) that was originally identified in a screen for proteins whose expression was increased by interferon (84). Since that time, it has been recognized that IFI16 acts as a modulator of transcription during cell stress (9, 21, 22, 63). Indeed, its role in the regulation of both the DNA damage response and the innate immune response indicates that IFI16 is an important regulator of stress responses in general. Murine homologs of IFI16 have recently been shown to bind DNA in response to interferon treatment, consistent with the ability of IFI16 to modulate transcription during cell stress. Its role as a transcription modulator is consistent with the presence of IFI16 together with pUL83 at the HCMV MIEP (Fig. 6). pUL83 must be present in order for IFI16 to reside at the MIEP (Fig. 6), but we do not know if either protein directly contacts viral DNA or whether their association with the MIEP is bridged by other viral or cellular proteins.
IFI16 from extracts of uninfected HeLa cells has previously been found to bind to the HCMV UL54 promoter (53), and IFI16 inhibits activity of this viral promoter in a reporter assay performed with uninfected cells (40). We have not yet tested how IFI16 might influence activity of this early promoter within infected cells in which pUL83 is present.
A p200 protein has previously been shown to play an important role during murine cytomegalovirus (MCMV) infection (35, 70). Replication of MCMV is decreased in cells containing a dominant negative form of the murine p200 family member p204. The replication defect was found to include delayed immediate-early gene expression (35), suggesting that regulation of viral immediate-early genes by p200 family members is a conserved feature of cytomegalovirus infection. Given the role of p200 family members in regulating the cellular stress response, it seems likely that viruses must subvert the function of these innate immune sensors to successfully circumvent host cell defenses, and it is possible that additional p200 family members are targeted by HCMV proteins.
IFI16 may affect transcription by modulating the NF-κB pathway (14, 76). Loss of IFI16 expression resulted in decreased levels of NF-κB DNA binding activity in response to tumor necrosis alpha (TNF-α) treatment (76). In addition, overexpression of IFI16 resulted in decreased expression of the NF-κB regulatory protein IκBα, independent of IκB kinase (IKK) activation (14). NF-κB activity is induced by HCMV infection (15, 19, 28, 29, 61, 67, 87), and infection with a pUL83-deficient virus results in enhanced NF-κB activity, as evidenced by increased nuclear localization of NF-κB subunits and increased NF-κB DNA binding activity (12). Given the ability of both pUL83 and IFI16 to modulate NF-κB activity, it is tempting to speculate that the regulation of NF-κB activity during HCMV infection results from the interaction of pUL83 with IFI16. The HCMV MIEP contains four NF-κB sites (77), which have been shown to stimulate the MIEP under some conditions (reference 77 and references therein). Thus, the pUL83-IFI16 complex at the MIEP might act to stimulate transcription through NF-κB.
In addition to the ability of pUL83 to activate the HCMV MIEP described here, pUL83 inhibits the ability of infected cells to respond to interferon and limits the expression of cellular inflammatory genes during the immediate-early phase of HCMV infection (1, 12) (Fig. 7D). How does pUL83 simultaneously antagonize expression of inflammatory genes and activate the MIEP? The promoters of inflammatory genes generally include NF-κB binding elements (36), as does the MIEP (77). Possibly, then, pUL83 redirects IFI16 from NF-κB at inflammatory genes to the MIEP, or it could modify the activity of IFI16 so that it stimulates NF-κB activity in the context of the MIEP but blocks activity at inflammatory genes. Uncharacterized pUL83 interactions might also contribute to the two seemingly contradictory effects. Whatever the mechanism, pUL83 might hijack a cellular innate protective response, NF-κB activation, simultaneously inducing the expression of HCMV immediate-early proteins and blocking the expression of cellular inflammatory genes.
In summary, we provide multiple lines of evidence supporting the conclusion that the HCMV pUL83 protein enhances transcription from the MIEP during HCMV infection and that this regulation requires the cellular IFI16 protein.
Acknowledgments
This work was supported by grants from the National Cancer Institute (CA82396 and CA85786) to T.S. and the National Institute on Drug Abuse (DP1DA026192) to I.M.C., Princeton University Start-Up funding to I.M.C., and grants from the U.S. National Institutes of Health to B.T.C. (RR00862), B.T.C. and M.P.R. (CA89810 and RR22220), and M.P.R. (GM62427). N.J.M. was the recipient of an American Cancer Society postdoctoral fellowship, and E.S.O. received a predoctoral fellowship from the New Jersey Commission on Cancer Research.
Footnotes
Published ahead of print on 26 May 2010.
REFERENCES
- 1.Abate, D. A., S. Watanabe, and E. S. Mocarski. 2004. Major human cytomegalovirus structural protein pp65 (ppUL83) prevents interferon response factor 3 activation in the interferon response. J. Virol. 78:10995-11006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aglipay, J. A., S. W. Lee, S. Okada, N. Fujiuchi, T. Ohtsuka, J. C. Kwak, Y. Wang, R. W. Johnstone, C. Deng, J. Qin, and T. Ouchi. 2003. A member of the Pyrin family, IFI16, is a novel BRCA1-associated protein involved in the p53-mediated apoptosis pathway. Oncogene 22:8931-8938. [DOI] [PubMed] [Google Scholar]
- 3.Arcangeletti, M. C., F. De Conto, F. Ferraglia, F. Pinardi, R. Gatti, G. Orlandini, A. Calderaro, F. Motta, M. C. Medici, M. Martinelli, P. Valcavi, S. V. Razin, C. Chezzi, and G. Dettori. 2003. Human cytomegalovirus proteins PP65 and IEP72 are targeted to distinct compartments in nuclei and nuclear matrices of infected human embryo fibroblasts. J. Cell Biochem. 90:1056-1067. [DOI] [PubMed] [Google Scholar]
- 4.Arcangeletti, M. C., I. Rodighiero, F. De Conto, R. Gatti, G. Orlandini, F. Ferraglia, F. Motta, S. Covan, S. V. Razin, G. Dettori, and C. Chezzi. 2009. Modulatory effect of rRNA synthesis and ppUL83 nucleolar compartmentalization on human cytomegalovirus gene expression in vitro. J. Cell Biochem. 108:415-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arlt, H., D. Lang, S. Gebert, and T. Stamminger. 1994. Identification of binding sites for the 86-kilodalton IE2 protein of human cytomegalovirus within an IE2-responsive viral early promoter. J. Virol. 68:4117-4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baldick, C. J., Jr., A. Marchini, C. E. Patterson, and T. Shenk. 1997. Human cytomegalovirus tegument protein pp71 (ppUL82) enhances the infectivity of viral DNA and accelerates the infectious cycle. J. Virol. 71:4400-4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baldick, C. J., Jr., and T. Shenk. 1996. Proteins associated with purified human cytomegalovirus particles. J. Virol. 70:6097-6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boehme, K. W., M. Guerrero, and T. Compton. 2006. Human cytomegalovirus envelope glycoproteins B and H are necessary for TLR2 activation in permissive cells. J. Immunol. 177:7094-7102. [DOI] [PubMed] [Google Scholar]
- 9.Bourette, R. P., and G. Mouchiroud. 2008. The biological role of interferon-inducible P204 protein in the development of the mononuclear phagocyte system. Front. Biosci. 13:879-886. [DOI] [PubMed] [Google Scholar]
- 10.Boyle, K. A., R. L. Pietropaolo, and T. Compton. 1999. Engagement of the cellular receptor for glycoprotein B of human cytomegalovirus activates the interferon-responsive pathway. Mol. Cell. Biol. 19:3607-3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bresnahan, W. A., and T. E. Shenk. 2000. UL82 virion protein activates expression of immediate early viral genes in human cytomegalovirus-infected cells. Proc. Natl. Acad. Sci. U. S. A. 97:14506-14511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Browne, E. P., and T. Shenk. 2003. Human cytomegalovirus UL83-coded pp65 virion protein inhibits antiviral gene expression in infected cells. Proc. Natl. Acad. Sci. U. S. A. 100:11439-11444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bürckstümmer, T., C. Baumann, S. Bluml, E. Dixit, G. Durnberger, H. Jahn, M. Planyavsky, M. Bilban, J. Colinge, K. L. Bennett, and G. Superti-Furga. 2009. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat. Immunol. 10:266-272. [DOI] [PubMed] [Google Scholar]
- 14.Caposio, P., F. Gugliesi, C. Zannetti, S. Sponza, M. Mondini, E. Medico, J. Hiscott, H. A. Young, G. Gribaudo, M. Gariglio, and S. Landolfo. 2007. A novel role of the interferon-inducible protein IFI16 as inducer of proinflammatory molecules in endothelial cells. J. Biol. Chem. 282:33515-33529. [DOI] [PubMed] [Google Scholar]
- 15.Caposio, P., A. Luganini, G. Hahn, S. Landolfo, and G. Gribaudo. 2007. Activation of the virus-induced IKK/NF-kappaB signalling axis is critical for the replication of human cytomegalovirus in quiescent cells. Cell. Microbiol. 9:2040-2054. [DOI] [PubMed] [Google Scholar]
- 16.Cassady, K. A. 2005. Human cytomegalovirus TRS1 and IRS1 gene products block the double-stranded-RNA-activated host protein shutoff response induced by herpes simplex virus type 1 infection. J. Virol. 79:8707-8715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castillo, J. P., and T. F. Kowalik. 2002. Human cytomegalovirus immediate early proteins and cell growth control. Gene 290:19-34. [DOI] [PubMed] [Google Scholar]
- 18.Chambers, J., A. Angulo, D. Amaratunga, H. Guo, Y. Jiang, J. S. Wan, A. Bittner, K. Frueh, M. R. Jackson, P. A. Peterson, M. G. Erlander, and P. Ghazal. 1999. DNA microarrays of the complex human cytomegalovirus genome: profiling kinetic class with drug sensitivity of viral gene expression. J. Virol. 73:5757-5766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan, G., E. R. Bivins-Smith, M. S. Smith, and A. D. Yurochko. 2008. Transcriptome analysis of NF-kappaB- and phosphatidylinositol 3-kinase-regulated genes in human cytomegalovirus-infected monocytes. J. Virol. 82:1040-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chevillotte, M., S. Landwehr, L. Linta, G. Frascaroli, A. Luske, C. Buser, T. Mertens, and J. von Einem. 2009. Major tegument protein pp65 of human cytomegalovirus is required for the incorporation of pUL69 and pUL97 into the virus particle and for viral growth in macrophages. J. Virol. 83:2480-2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choubey, D., R. Deka, and S. M. Ho. 2008. Interferon-inducible IFI16 protein in human cancers and autoimmune diseases. Front. Biosci. 13:598-608. [DOI] [PubMed] [Google Scholar]
- 22.Choubey, D., and R. Panchanathan. 2008. Interferon-inducible Ifi200-family genes in systemic lupus erythematosus. Immunol. Lett. 119:32-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Compton, T. 2004. Receptors and immune sensors: the complex entry path of human cytomegalovirus. Trends Cell Biol. 14:5-8. [DOI] [PubMed] [Google Scholar]
- 24.Cristea, I. M., J. W. Carroll, M. P. Rout, C. M. Rice, B. T. Chait, and M. R. MacDonald. 2006. Tracking and elucidating alphavirus-host protein interactions. J. Biol. Chem. 281:30269-30278. [DOI] [PubMed] [Google Scholar]
- 25.Cristea, I. M., R. Williams, B. T. Chait, and M. P. Rout. 2005. Fluorescent proteins as proteomic probes. Mol. Cell. Proteomics 4:1933-1941. [DOI] [PubMed] [Google Scholar]
- 26.Cuevas-Bennett, C., and T. Shenk. 2008. Dynamic histone H3 acetylation and methylation at human cytomegalovirus promoters during replication in fibroblasts. J. Virol. 82:9525-9536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dawson, M. J., and J. A. Trapani. 1995. The interferon-inducible autoantigen, IFI 16: localization to the nucleolus and identification of a DNA-binding domain. Biochem. Biophys. Res. Commun. 214:152-162. [DOI] [PubMed] [Google Scholar]
- 28.DeMeritt, I. B., L. E. Milford, and A. D. Yurochko. 2004. Activation of the NF-kappaB pathway in human cytomegalovirus-infected cells is necessary for efficient transactivation of the major immediate-early promoter. J. Virol. 78:4498-4507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DeMeritt, I. B., J. P. Podduturi, A. M. Tilley, M. T. Nogalski, and A. D. Yurochko. 2006. Prolonged activation of NF-kappaB by human cytomegalovirus promotes efficient viral replication and late gene expression. Virology 346:15-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fujiuchi, N., J. A. Aglipay, T. Ohtsuka, N. Maehara, F. Sahin, G. H. Su, S. W. Lee, and T. Ouchi. 2004. Requirement of IFI16 for the maximal activation of p53 induced by ionizing radiation. J. Biol. Chem. 279:20339-20344. [DOI] [PubMed] [Google Scholar]
- 31.Gibson, W. 1981. Structural and nonstructural proteins of strain Colburn cytomegalovirus. Virology 111:516-537. [DOI] [PubMed] [Google Scholar]
- 32.Gugliesi, F., M. Mondini, R. Ravera, A. Robotti, M. de Andrea, G. Gribaudo, M. Gariglio, and S. Landolfo. 2005. Up-regulation of the interferon-inducible IFI16 gene by oxidative stress triggers p53 transcriptional activity in endothelial cells. J. Leukoc. Biol. 77:820-829. [DOI] [PubMed] [Google Scholar]
- 33.Hakki, M., E. E. Marshall, K. L. De Niro, and A. P. Geballe. 2006. Binding and nuclear relocalization of protein kinase R by human cytomegalovirus TRS1. J. Virol. 80:11817-11826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hayashi, M. L., C. Blankenship, and T. Shenk. 2000. Human cytomegalovirus UL69 protein is required for efficient accumulation of infected cells in the G1 phase of the cell cycle. Proc. Natl. Acad. Sci. U. S. A. 97:2692-2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hertel, L., M. De Andrea, B. Azzimonti, A. Rolle, M. Gariglio, and S. Landolfo. 1999. The interferon-inducible 204 gene, a member of the Ifi 200 family, is not involved in the antiviral state induction by IFN-alpha, but is required by the mouse cytomegalovirus for its replication. Virology 262:1-8. [DOI] [PubMed] [Google Scholar]
- 36.Hiscott, J. 2007. Convergence of the NF-kappaB and IRF pathways in the regulation of the innate antiviral response. Cytokine Growth Factor Rev. 18:483-490. [DOI] [PubMed] [Google Scholar]
- 37.Irmiere, A., and W. Gibson. 1983. Isolation and characterization of a noninfectious virion-like particle released from cells infected with human strains of cytomegalovirus. Virology 130:118-133. [DOI] [PubMed] [Google Scholar]
- 38.Jenkins, D. E., C. L. Martens, and E. S. Mocarski. 1994. Human cytomegalovirus late protein encoded by ie2: a trans-activator as well as a repressor of gene expression. J. Gen. Virol. 75(9):2337-2348. [DOI] [PubMed] [Google Scholar]
- 39.Johnson, D. C., and N. R. Hegde. 2002. Inhibition of the MHC class II antigen presentation pathway by human cytomegalovirus. Curr. Top. Microbiol. Immunol. 269:101-115. [DOI] [PubMed] [Google Scholar]
- 40.Johnstone, R. W., J. A. Kerry, and J. A. Trapani. 1998. The human interferon-inducible protein, IFI 16, is a repressor of transcription. J. Biol. Chem. 273:17172-17177. [DOI] [PubMed] [Google Scholar]
- 41.Johnstone, R. W., M. H. Kershaw, and J. A. Trapani. 1998. Isotypic variants of the interferon-inducible transcriptional repressor IFI 16 arise through differential mRNA splicing. Biochemistry 37:11924-11931. [DOI] [PubMed] [Google Scholar]
- 42.Kalejta, R. F. 2008. Functions of human cytomegalovirus tegument proteins prior to immediate early gene expression. Curr. Top. Microbiol. Immunol. 325:101-115. [DOI] [PubMed] [Google Scholar]
- 43.Kalejta, R. F. 2008. Tegument proteins of human cytomegalovirus. Microbiol. Mol. Biol. Rev. 72:249-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim, K. S., K. W. Kang, Y. B. Seu, S. H. Baek, and J. R. Kim. 2009. Interferon-gamma induces cellular senescence through p53-dependent DNA damage signaling in human endothelial cells. Mech. Ageing Dev. 130:179-188. [DOI] [PubMed] [Google Scholar]
- 45.Krutchinsky, A. N., M. Kalkum, and B. T. Chait. 2001. Automatic identification of proteins with a MALDI-quadrupole ion trap mass spectrometer. Anal. Chem. 73:5066-5077. [DOI] [PubMed] [Google Scholar]
- 46.Krutchinsky, A. N., W. Zhang, and B. T. Chait. 2000. Rapidly switchable matrix-assisted laser desorption/ionization and electrospray quadrupole-time-of-flight mass spectrometry for protein identification. J. Am. Soc. Mass Spectrom. 11:493-504. [DOI] [PubMed] [Google Scholar]
- 47.Kwak, J. C., P. P. Ongusaha, T. Ouchi, and S. W. Lee. 2003. IFI16 as a negative regulator in the regulation of p53 and p21(Waf1). J. Biol. Chem. 278:40899-40904. [DOI] [PubMed] [Google Scholar]
- 48.Liu, Z., M. Winkler, and B. Biegalke. 2009. Human cytomegalovirus: host immune modulation by the viral US3 gene. Int. J. Biochem. Cell Biol. 41:503-506. [DOI] [PubMed] [Google Scholar]
- 49.Lodoen, M., K. Ogasawara, J. A. Hamerman, H. Arase, J. P. Houchins, E. S. Mocarski, and L. L. Lanier. 2003. NKG2D-mediated natural killer cell protection against cytomegalovirus is impaired by viral gp40 modulation of retinoic acid early inducible 1 gene molecules. J. Exp. Med. 197:1245-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lois, C., E. J. Hong, S. Pease, E. J. Brown, and D. Baltimore. 2002. Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science 295:868-872. [DOI] [PubMed] [Google Scholar]
- 51.Lukac, D. M., J. R. Manuppello, and J. C. Alwine. 1994. Transcriptional activation by the human cytomegalovirus immediate-early proteins: requirements for simple promoter structures and interactions with multiple components of the transcription complex. J. Virol. 68:5184-5193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Luo, Y., T. Li, F. Yu, T. Kramer, and I. M. Cristea. 2010. Resolving the composition of protein complexes using a MALDI LTQ Orbitrap. J. Am. Soc. Mass Spectrom. 21:34-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Luu, P., and O. Flores. 1997. Binding of SP1 to the immediate-early protein-responsive element of the human cytomegalovirus DNA polymerase promoter. J. Virol. 71:6683-6691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marshall, E. E., C. J. Bierle, W. Brune, and A. P. Geballe. 2009. Essential role for either TRS1 or IRS1 in human cytomegalovirus replication. J. Virol. 83:4112-4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Misaghi, S., Z. Y. Sun, P. Stern, R. Gaudet, G. Wagner, and H. Ploegh. 2004. Structural and functional analysis of human cytomegalovirus US3 protein. J. Virol. 78:413-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moorman, N. J., I. M. Cristea, S. S. Terhune, M. P. Rout, B. T. Chait, and T. Shenk. 2008. Human cytomegalovirus protein UL38 inhibits host cell stress responses by antagonizing the tuberous sclerosis protein complex. Cell Host Microbe 3:253-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Murphy, E., I. Rigoutsos, T. Shibuya, and T. E. Shenk. 2003. Reevaluation of human cytomegalovirus coding potential. Proc. Natl. Acad. Sci. U. S. A. 100:13585-13590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Murphy, E., J. Vanicek, H. Robins, T. Shenk, and A. J. Levine. 2008. Suppression of immediate-early viral gene expression by herpesvirus-coded microRNAs: implications for latency. Proc. Natl. Acad. Sci. U. S. A. 105:5453-5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Murphy, E., D. Yu, J. Grimwood, J. Schmutz, M. Dickson, M. A. Jarvis, G. Hahn, J. A. Nelson, R. M. Myers, and T. E. Shenk. 2003. Coding potential of laboratory and clinical strains of human cytomegalovirus. Proc. Natl. Acad. Sci. U. S. A. 100:14976-14981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nevels, M., C. Paulus, and T. Shenk. 2004. Human cytomegalovirus immediate-early 1 protein facilitates viral replication by antagonizing histone deacetylation. Proc. Natl. Acad. Sci. U. S. A. 101:17234-17239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nogalski, M. T., J. P. Podduturi, I. B. DeMeritt, L. E. Milford, and A. D. Yurochko. 2007. The human cytomegalovirus virion possesses an activated casein kinase II that allows for the rapid phosphorylation of the inhibitor of NF-kappaB, IkappaBalpha. J. Virol. 81:5305-5314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nowak, B., C. Sullivan, P. Sarnow, R. Thomas, F. Bricout, J. C. Nicolas, B. Fleckenstein, and A. J. Levine. 1984. Characterization of monoclonal antibodies and polyclonal immune sera directed against human cytomegalovirus virion proteins. Virology 132:325-338. [DOI] [PubMed] [Google Scholar]
- 63.Ouchi, M., and T. Ouchi. 2008. Role of IFI16 in DNA damage and checkpoint. Front. Biosci. 13:236-239. [DOI] [PubMed] [Google Scholar]
- 64.Paulus, C., S. Krauss, and M. Nevels. 2006. A human cytomegalovirus antagonist of type I IFN-dependent signal transducer and activator of transcription signaling. Proc. Natl. Acad. Sci. U. S. A. 103:3840-3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pizzorno, M. C., M. A. Mullen, Y. N. Chang, and G. S. Hayward. 1991. The functionally active IE2 immediate-early regulatory protein of human cytomegalovirus is an 80-kilodalton polypeptide that contains two distinct activator domains and a duplicated nuclear localization signal. J. Virol. 65:3839-3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pizzorno, M. C., P. O'Hare, L. Sha, R. L. LaFemina, and G. S. Hayward. 1988. trans-activation and autoregulation of gene expression by the immediate-early region 2 gene products of human cytomegalovirus. J. Virol. 62:1167-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Poole, E., I. Groves, A. MacDonald, Y. Pang, A. Alcami, and J. Sinclair. 2009. Identification of TRIM23 as a cofactor involved in the regulation of NF-kappaB by human cytomegalovirus. J. Virol. 83:3581-3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ranjan, P., J. B. Bowzard, J. W. Schwerzmann, V. Jeisy-Scott, T. Fujita, and S. Sambhara. 2009. Cytoplasmic nucleic acid sensors in antiviral immunity. Trends Mol. Med. 15:359-368. [DOI] [PubMed] [Google Scholar]
- 69.Roberts, T. L., A. Idris, J. A. Dunn, G. M. Kelly, C. M. Burnton, S. Hodgson, L. L. Hardy, V. Garceau, M. J. Sweet, I. L. Ross, D. A. Hume, and K. J. Stacey. 2009. HIN-200 proteins regulate caspase activation in response to foreign cytoplasmic DNA. Science 323:1057-1060. [DOI] [PubMed] [Google Scholar]
- 70.Rolle, S., M. De Andrea, D. Gioia, D. Lembo, L. Hertel, S. Landolfo, and M. Gariglio. 2001. The interferon-inducible 204 gene is transcriptionally activated by mouse cytomegalovirus and is required for its replication. Virology 286:249-255. [DOI] [PubMed] [Google Scholar]
- 71.Romanowski, M. J., E. Garrido-Guerrero, and T. Shenk. 1997. pIRS1 and pTRS1 are present in human cytomegalovirus virions. J. Virol. 71:5703-5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rüger, B., S. Klages, B. Walla, J. Albrecht, B. Fleckenstein, P. Tomlinson, and B. Barrell. 1987. Primary structure and transcription of the genes coding for the two virion phosphoproteins pp65 and pp71 of human cytomegalovirus. J. Virol. 61:446-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Saffert, R. T., and R. F. Kalejta. 2006. Inactivating a cellular intrinsic immune defense mediated by Daxx is the mechanism through which the human cytomegalovirus pp71 protein stimulates viral immediate-early gene expression. J. Virol. 80:3863-3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schroder, K., D. A. Muruve, and J. Tschopp. 2009. Innate immunity: cytoplasmic DNA sensing by the AIM2 inflammasome. Curr. Biol. 19:R262-R265. [DOI] [PubMed] [Google Scholar]
- 75.Simmen, K. A., J. Singh, B. G. Luukkonen, M. Lopper, A. Bittner, N. E. Miller, M. R. Jackson, T. Compton, and K. Fruh. 2001. Global modulation of cellular transcription by human cytomegalovirus is initiated by viral glycoprotein B. Proc. Natl. Acad. Sci. U. S. A. 98:7140-7145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sponza, S., M. De Andrea, M. Mondini, F. Gugliesi, M. Gariglio, and S. Landolfo. 2009. Role of the interferon-inducible IFI16 gene in the induction of ICAM-1 by TNF-alpha. Cell. Immunol. 257:55-60. [DOI] [PubMed] [Google Scholar]
- 77.Stinski, M. F., and H. Isomura. 2008. Role of the cytomegalovirus major immediate early enhancer in acute infection and reactivation from latency. Med. Microbiol. Immunol. 197:223-231. [DOI] [PubMed] [Google Scholar]
- 78.Tawara, H., N. Fujiuchi, J. Sironi, S. Martin, J. Aglipay, M. Ouchi, M. Taga, P. L. Chen, and T. Ouchi. 2008. Loss of p53-regulatory protein IFI16 induces NBS1 leading to activation of p53-mediated checkpoint by phosphorylation of p53 SER37. Front. Biosci. 13:240-248. [DOI] [PubMed] [Google Scholar]
- 79.Taylor, R. T., and W. A. Bresnahan. 2006. Human cytomegalovirus IE86 attenuates virus- and tumor necrosis factor alpha-induced NF-kappaB-dependent gene expression. J. Virol. 80:10763-10771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Taylor, R. T., and W. A. Bresnahan. 2005. Human cytomegalovirus immediate-early 2 gene expression blocks virus-induced beta interferon production. J. Virol. 79:3873-3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Taylor, R. T., and W. A. Bresnahan. 2006. Human cytomegalovirus immediate-early 2 protein IE86 blocks virus-induced chemokine expression. J. Virol. 80:920-928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Terhune, S. S., J. Schroer, and T. Shenk. 2004. RNAs are packaged into human cytomegalovirus virions in proportion to their intracellular concentration. J. Virol. 78:10390-10398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tevethia, M. J., D. J. Spector, K. M. Leisure, and M. F. Stinski. 1987. Participation of two human cytomegalovirus immediate early gene regions in transcriptional activation of adenovirus promoters. Virology 161:276-285. [DOI] [PubMed] [Google Scholar]
- 84.Trapani, J. A., M. Dawson, V. A. Apostolidis, and K. A. Browne. 1994. Genomic organization of IFI16, an interferon-inducible gene whose expression is associated with human myeloid cell differentiation: correlation of predicted protein domains with exon organization. Immunogenetics 40:415-424. [DOI] [PubMed] [Google Scholar]
- 85.Valchanova, R. S., M. Picard-Maureau, M. Budt, and W. Brune. 2006. Murine cytomegalovirus m142 and m143 are both required to block protein kinase R-mediated shutdown of protein synthesis. J. Virol. 80:10181-10190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yu, D., G. A. Smith, L. W. Enquist, and T. Shenk. 2002. Construction of a self-excisable bacterial artificial chromosome containing the human cytomegalovirus genome and mutagenesis of the diploid TRL/IRL13 gene. J. Virol. 76:2316-2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yurochko, A. D., M. W. Mayo, E. E. Poma, A. S. Baldwin, Jr., and E. S. Huang. 1997. Induction of the transcription factor Sp1 during human cytomegalovirus infection mediates upregulation of the p65 and p105/p50 NF-kappaB promoters. J. Virol. 71:4638-4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhu, H., J. P. Cong, and T. Shenk. 1997. Use of differential display analysis to assess the effect of human cytomegalovirus infection on the accumulation of cellular RNAs: induction of interferon-responsive RNAs. Proc. Natl. Acad. Sci. U. S. A. 94:13985-13990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhu, H., Y. Shen, and T. Shenk. 1995. Human cytomegalovirus IE1 and IE2 proteins block apoptosis. J. Virol. 69:7960-7970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zini, N., M. C. Battista, S. Santi, M. Riccio, G. Bergamini, M. P. Landini, and N. M. Maraldi. 1999. The novel structural protein of human cytomegalovirus, pUL25, is localized in the viral tegument. J. Virol. 73:6073-6075. [DOI] [PMC free article] [PubMed] [Google Scholar]