Abstract
The use of chronic opioid therapy (COT) for chronic non-cancer pain (CNCP) has increased dramatically in the past two decades. There has also been a marked increase in abuse of prescribed opioids and in accidental opioid overdose. Misuse of prescribed opioids may link these trends, but has thus far only been studied in small clinical samples. We therefore sought to validate an administrative indicator of opioid misuse among large samples of recipients of COT and determine the demographic, clinical, and pharmacological risks associated with possible and probable opioid misuse. 21,685 enrollees in commercial insurance plans and 10,159 in Arkansas Medicaid who had at least 90 days of continuous opioid use 2000–5 were studied for one year. Criteria were developed for possible and probable opioid misuse using administrative claims data concerning excess days supplied of shortacting and long-acting opioids, opioid prescribers and opioid pharmacies. We estimated possible misuse at 24% of COT recipients in the commercially insured sample and 20% in the Medicaid sample and probable misuse at 6% in commercially insured and at 3% in Medicaid. Among non-modifiable factors, younger age, back pain, multiple pain complaints and substance abuse disorders identify patients at high risk for misuse. Among modifiable factors, treatment with high daily dose opioids (especially>120mg MED per day) and short-acting Schedule II opioids appears to increase risk of misuse. The consistency of the findings across diverse patient populations and varying levels of misuse suggests that these results will generalize broadly, but awaits confirmation in other studies.
1. Introduction
The use of chronic opioid therapy (COT) for chronic non-cancer pain (CNCP) has increased dramatically in the past two decades [13, 28]. There has also been a marked increase in abuse of prescribed opioids [9, 13]. Indeed, in the U.S. prescription opioid abuse is the fastest growing form of drug abuse [9] and prescription opioids the most common cause of accidental drug overdose [24]. Misuse of prescribed opioids, defined by the National Institute of Drug Abuse as “taking a medication in a manner other than that prescribed or for a different condition than that for which the medication is prescribed,” [20] may contribute to these negative outcomes. Misuse may be a sign of developing or established opioid abuse. Misuse is also thought to contribute to the risk of accidental overdose [10].
Results from the population-based U.S. National Survey on Drug Use and Health (NSDUH) from 2002–2005 show that 4.8% of the population over age 12 has used prescription pain reliever non-medically in the previous 12 months [3]. These surveys provide estimates of non-prescribed use of opioids, but do not show its relationship to the use of COT for CNCP. Clinical surveys of patients on COT have provided widely varying (3% to 62%) estimates of the prevalence of opioid misuse according to a recent review. This review concluded that “the psychometric properties of the published questionnaires and interview protocols are weak; moreover, the samples included in the studies are often small and unrepresentative” [29]. It is also not clear that these surveys are valid or generalizable to the entire population of patients on COT.
Opioid misuse can be understood as part of a set of aberrant drug related behaviors [26]. A recent review of evidence on aberrant drug-related behaviors for the American Pain Society and American Academy of Pain Medicine noted shortcomings of research done on aberrant behaviors to date, such as: lack of linkage to dose of opioids prescribed, lack of adjustment for demographic variables, and focus on pain clinic populations that may not be applicable to primary care [8]. The College on Problems of Drug Dependence has called for additional research to identify those patients and populations at greatest risk for misusing prescription opioids [32].
Administrative claims data offers a means to monitor opioid misuse within large clinical populations. These populations are more representative of all patients receiving COT for CNCP and the data does not depend on completion of surveys by providers and patients. We therefore sought to validate administrative indicators of possible and probable opioid misuse among recipients of COT with CNCP and to determine the demographic, clinical, and pharmacological risks associated with possible and probable misuse. We studied two disparate populations, a commercially-insured multi-state population and a state-based Medicaid population, to identify risk factors common across these populations that differ in geography and socioeconomic status. These results could be used to monitor clinical populations for opioid misuse, design risk stratification algorithms, and provide the basis for quality improvement initiatives within integrated systems of care.
2. Methods
2.1. Data Source
Data were obtained from claims records from January 2000 through December 2005 from two sources: HealthCore (N=3,768,223), the country’s largest private health network, and Arkansas Medicaid (N=127,866). The HealthCore data included plans from five states in the West, Mid West, and South East regions of the United States. The two populations were chosen to describe the range of opioid use and its consequences in disparate patient groups. Due to the large number of persons included, the retrospective nature of the study, and the use of de-identified data, a waiver of the requirements for informed consent was granted from the Human Subject’s Review Committees at the participating institutions.
2.2. Study Sample
The study sample for this analysis consisted of adult enrollees (18 years and older) in the two health insurers that were chronic opioid users with greater than 90 days of opioid use in any 6-month period between 1/1/2001 and 12/31/2004, and less than a 32-day gap. The first day of the opioid use episode was defined as the index date. Eligible individuals needed 12 months of continuous enrollment prior to and following the index date. Individuals with a cancer diagnosis at any time in the year before or after the index date (other than non-melanoma skin cancer), residents of nursing homes, and hospice patients were excluded. Therefore the sample consisted of patients without cancer or at the end of life who were receiving chronic opioid therapy. Not all of these individuals received one of our tracer chronic pain diagnoses. There were 21,685 enrollees in HealthCore and 10,159 in Arkansas Medicaid who met these criteria. Additional details concerning the study have been reported elsewhere [5, 28].
2.3. Sociodemographic and Clinical Variables
Data on sociodemographic and clinical characteristics of the sample members were collected from claims records and enrollment summary files in the 12-month period prior to the start of the opioid use episode, i.e. the index date. The Charlson comorbidity index [7] was used as a measure of overall medical comorbidity. We recorded ICD-9CM pain diagnoses made during the 12 months before the index date. Arthritis/joint pain, back pain, neck pain, and headache were selected as tracer pain diagnoses and tracked individually because these were the most commonly reported pain sites in the WHO Collaborative Study of Psychological Problems in General Health Care [14]. We also collected information on the presence of the following other (“non-tracer”) pain diagnoses: extremity pain, abdominal pain, chest pain, kidney stones/gallstones, pelvic pain, rheumatoid arthritis, fractures, neuropathic pain, fibromyalgia, and tempormandibular joint pain. Mental health and substance use disorders were classified into the following groups based on ICD-9CM diagnoses using validated grouping software developed by the Agency for Healthcare Research and Quality [1]: adjustment disorders, anxiety disorders, mood disorders, personality disorders, miscellaneous disorder, and substance use disorders. Substance use disorders were further classified as alcohol use disorder, non-opioid drug use disorder, or opioid use disorder. Adjustment, anxiety, mood, personality and miscellaneous disorders were summed to create a variable identifying the number of mental health disorder types.
2.4. Opioid Misuse Score
A misuse score was generated based on four variables: days supplied of short-acting opioids, days supplied of long-acting opioids, number of opioid pharmacies, and number of opioid prescribers. Short and long-acting opioids were counted separately so that patients prescribed both types to allow for treatment of breakthrough pain did not necessarily receive higher misuse scores. The misuse score was designed to detect patients receiving excess days supply of opioids, those utilizing multiple prescribers or multiple pharmacies to obtain these opioids. The score was designed so that a higher score would reflect a higher probability of potential misuse. This is consistent with the recommendations of an expert panel who recommended multiple prescribers and multiple pharmacies as the top two administrative criteria for potential misuse of prescribed controlled substances [23]. The components of our score also parallel items in validated survey measures such as the Prescription Opioid Misuse Index [17].
Each of the component variables was categorized into 3 groups. For long-acting and short-acting days supplied, a ‘0’ score was assigned if days supplied over a six month post-index period was less than or equal to 185 days, a ‘1’ score was assigned if between 186 and 240 days (up to 50% excess days), and a ‘2’ score was assigned if greater than 240 days was supplied in a 180 day period (>50% excess days). The number of opioid pharmacies and number of opioid prescribers during a 180 day period were separately categorized into 3 groups empirically, based on distribution of these measures: 0–2 prescribers/pharmacies, 3–4 prescribers/pharmacies, and >=5 prescribers/pharmacies. The misuse score was then derived by summing over these 4 variables, with a possible range from 0 to 8. The misuse score for a 1-year period was derived by adding the two 6-month misuse scores together (range 0–16). The 1-year misuse score was then categorized by 0–1 ‘no misuse’, 2–4 ‘possible misuse’, and >=5 ‘probable misuse’ to create the 3-categorical misuse outcome. To validate this misuse score, we examined the likelihood of receiving a diagnosis of opioid abuse or dependence during up to nine six month periods of follow-up for all observed misuse scores (0–16).
2.4.1. Medication Variables
Data on opioid and sedative-hypnotic medication use were collected for the six-month period after the index date in which all recipients had opioid use. Morphine equivalent dose (MED) for a single prescription was calculated by multiplying the quantity of each prescription by the strength of the prescription (milligrams of opioid per unit dispensed), and multiplying this total by a conversion factor [28]. Opioid dose per day supplied was calculated by adding the total morphine equivalents for the three major opioid groups and dividing by the sum of the total days supply (assuming maximum authorized use as calculated by the dispensing pharmacist). If the total days supply exceeded the number of days in the period (180 days), suggesting concurrent use of different opioid types, the daily dose was calculated by dividing the total dose dispensed by 180 days. Types of opioid received were determined based on opioid class (as defined by DEA schedule and duration of action). Subjects were coded as receiving a particular opioid class if they received at least 30 days supply of that class within a six-month period. Seven mutually exclusive categories were thus derived: Non-Schedule II short-acting, Schedule II short-acting, Schedule II long-acting, Non-schedule II plus Schedule II short acting, Schedule II short-acting plus Schedule II long-acting, Non-Schedule II short-acting plus Schedule II long-acting, and all three opioid types. We also collected data on days supply for other non-opioid medications, often used in the treatment of non-cancer pain conditions: sedative/hypnotics (includes benzodiazepines as well as other hypnotic drugs), muscle relaxants, stimulants, Cox-II inhibitors, anticonvulsants, tricyclic antidepressants, serotonin or serotonin-norepinephrine re-uptake inhibitors (SSRI/SNRI), and miscellaneous analgesic medications. We only present data on sedative-hypnotic medications only since the others did not have significant effects on risks for opioid misuse.
3. Statistical Analysis
Descriptive statistics such as mean, standard deviation, and frequency were provided for the demographic and clinical variables. We used polytomous logistic regression models to evaluate the association between the 3-level categorical misuse outcome, with “no misuse” as the reference group, and the independent variables that were hypothesized to be associated with opioid misuse. Independent variables included: (1) demographic variables (age and gender), (2) medical and pain clinical variables (pre-index joint pain, back pain, headache, neck pain, number of non-tracer pain conditions (range 0–10), and Charlson score), (3) mental health/substance abuse diagnoses (pre-index number of mental health disorders by AHRQ (range 0–5), alcohol use disorder, opioid use disorder, and non-opioid drug use disorder), and (4) pharmacological variables (pre-index sedative / hypnotic days supply, post-index opioid daily dose, and post-index types of opioid received). All the analyses were performed using SAS 9.1 [2].
4. Results
4.1. Sociodemographic and Clinical Characteristics
Table 1 describes the socio-demographic characteristics of adult enrollees in HealthCore and Arkansas Medicaid with at least 90 days of uninterrupted opioid use within a six-month period. While 0.6% of the HealthCore population was included, 7.7% of the Arkansas Medicaid population was included. This is likely due to much higher rates of medical and psychiatric co-morbidity in Medicaid populations, but may also reflect geographic differences in opioid prescribing patterns. On average, chronic opioid users were middle-aged and predominantly female in both samples. The Arkansas sample was predominantly female as is typical of Medicaid populations. Subjects had a mean of 0.85 (HealthCore) and 1.25 (Arkansas Medicaid) tracer pain conditions. Back pain was the most common CNCP diagnosis in both samples. However, 45% (n=16551) of the HealthCore sample and 26% (n=2479) of Arkansas Medicaid enrollees with chronic opioid use had not been diagnosed with one of our four tracer CNCP diagnoses. Among patients with no tracer CNCP condition, in HealthCore 5961 (36%) and in Arkansas Medicaid 1437 (58%) had some other pain conditions. The most common other pain conditions in both samples were extremity, abdominal and chest pain. In HealthCore, 10590 (29%) COT patients have no CNCP conditions or any other tracked pain conditions. 7354 (20%) had 1 CNCP or other pain condition, and 18661 (51%) have >=2. In our Arkansas sample, 1042 (11%) COT patients had no tracer CNCP conditions or any other tracked pain conditions, while 1615 (17%) had 1 CNCP or other pain condition, and 6994 (72%) have >=2. Arkansas Medicaid chronic opioid users had a higher mean Charlson comorbidity index. Among chronic opioid users, 14% in HealthCore and 20% in Arkansas Medicaid received a diagnosis of a comorbid mental health or substance use disorder. The most common mental health diagnoses in both samples were mood disorders (HealthCore 11.3%, Medicaid 21.2%) and anxiety disorders (HealthCore 7.2%, Medicaid 17.0%). Substance related disorders were diagnosed before COT index date in 2.5% of HealthCore COT recipients and 6% of Arkansas Medicaid COT recipients.
Table 1.
Demographic table
| Variables | category | Arkansas Medicaid N= 10,159 |
Health Core N=21,865 |
|---|---|---|---|
| Demographics | |||
| Age | 18–30 | 660 (6.84%) | 1857 (5.07%) |
| 31–40 | 1669 (17.29%) | 6193 (16.92%) | |
| 41–50 | 2469 (25.58%) | 11731 (32.05%) | |
| 51–64 | 2588 (26.82%) | 12344 (33.72%) | |
| >=65 | 2265 (23.47%) | 4480 (12.24%) | |
| Gender | Male | 2744 (28.43%) | 15002 (40.98%) |
| Female | 6907 (71.57%) | 21603 (59.02%) | |
| Medical clinical variables | |||
| CNCP: back | Yes | 4842 (50.17%) | 13414 (36.65%) |
| CNCP: neck | Yes | 1620 (16.79%) | 5510 (15.05%) |
| CNCP: arth | Yes | 3530 (36.58%) | 6398 (17.48%) |
| CNCP: head | Yes | 2057 (21.31%) | 5875 (16.05%) |
| # other pain | Mean(SD) | 1.6 (1.4) | 1.1 (1.2) |
| Charlson score | Mean(SD) | 1.1 (1.4) | 0.4 (0.9) |
| MH clinical variables | |||
| # MH disorders | 0 | 6619 (68.58%) | 29965 (81.86%) |
| 1 | 1990 (20.62%) | 4995 (13.65%) | |
| >=2 | 1042 (10.80%) | 1645 (4.49%) | |
| SA - Alcohol | Yes | 278 (2.88%) | 445 (1.22%) |
| No | 9373 (97.12%) | 36160 (98.78%) | |
| SA - Opioid | Yes | 59 (0.61%) | 258 (0.70%) |
| No | 9592 (99.39%) | 36347 (99.30%) | |
| SA - Nonopioid | Yes | 356 (3.69%) | 440 (1.20%) |
| No | 9295 (96.31%) | 36165 (98.80%) | |
| Pharmacological variables | |||
| Sedative/hypnotics days supply |
Mean(SD) | 78.5 (128.8) | 71.8 (136.5) |
| Median opioid daily dose (MED) | 35.27mg. | 31.73mg. | |
| Opioid daily dose | < median | 4945 (51.24%) | 18304 (50.00%) |
| median ~ 120mg/day |
4115 (42.64%) | 15731 (42.98%) | |
| > 120mg/day | 591 (6.12%) | 2570 (7.02%) | |
| Opioid days supply | 91 ~ 160 days | 5222 (54.11%) | 21923 (59.89%) |
| 161 ~ 185 days |
1729 (17.92%) | 5498 (15.02%) | |
| >185 days | 2700 (27.98%) | 9184 (25.09%) | |
| Opioid types | non-schedule II only |
7615 (78.90%) | 28621 (78.19%) |
| schedule II short only |
239 (2.48%) | 754 (2.06%) | |
| schedule II long only |
502 (5.20%) | 1939 (5.30%) | |
| non-schedule II + schedule II short |
284 (2.94%) | 1058 (2.89%) | |
| non-schedule II + schedule II long |
622 (6.44%) | 2748 (7.51%) | |
| schedule II short + long |
263 (2.73%) | 908 (2.48%) | |
| All 3 types | 126 (1.31%) | 577 (1.58%) |
4.2. Pharmacological variables
Mean opioid daily dose was approximately 53 mg. MED in both samples. Median dose was 31mg MED in HealthCore and 35mg MED in Arkansas. Mean opioid days supply was 160 days in HealthCore and 166 days in Arkansas Medicaid among this sample receiving at least 90 days supply. In both samples, 78% of subjects received only Non-Schedule II opioids. Approximately 5% of both samples received only Schedule II long-acting opioids. From 6–7% of both samples received Non-Schedule II short-acting and Schedule II long-acting opioids. Sedative-hypnotics were the most common non-opioid symptom relief medications prescribed in both samples (HC mean 72, AR mean 78 days supplied).
4.3. Misuse Scores
In the HealthCore sample, 70% were categorized as showing no evidence of misuse, with 24% categorized as possible misuse and 6% as probable misuse. The components of the misuse score most likely to be coded in the possible misuse range (i.e., 10–20%), were days supply of short-acting and long-acting opioids and number of prescribers. Spearman correlations between total opioid days supplied and unique opioid pharmacies (r=0.15, 95%CI 0.14 to 0.16, p<.0001) and between total opioid days supplied and unique opioid prescribers (r=0.06, 95%CI −0.017 to 0.04, p=.22) were low, suggesting independent dimensions of misuse. However, Spearman correlations between unique opioid pharmacies and unique opioid prescribers were higher (r=0.27, 95%CI 0.26 to 0.28, p<.0001) suggesting related dimensions of misuse.
In the Arkansas Medicaid sample, 76% were categorized as showing no evidence of misuse, with 20% categorized as possible misuse and 3% as probable misuse. The components of the misuse score most likely to be coded in the possible misuse range (i.e., 10–20%), were days supply of short-acting opioids and number of prescribers. Spearman correlations between total opioid days supplied and unique opioid pharmacies (r=0.01, 95%CI − 0.009 to 0.031, p= 0.27) and between total opioid days supplied and unique opioid prescribers (r=−0.01, 95%CI −0.031 to 0.008, p=0.27) were low, suggesting independent dimensions of misuse. However, Spearman correlations between unique opioid pharmacies and unique opioid prescribers were higher (r=0.45, 95%CI 0.43 to 0.46, p<.0001) suggesting related dimensions of misuse.
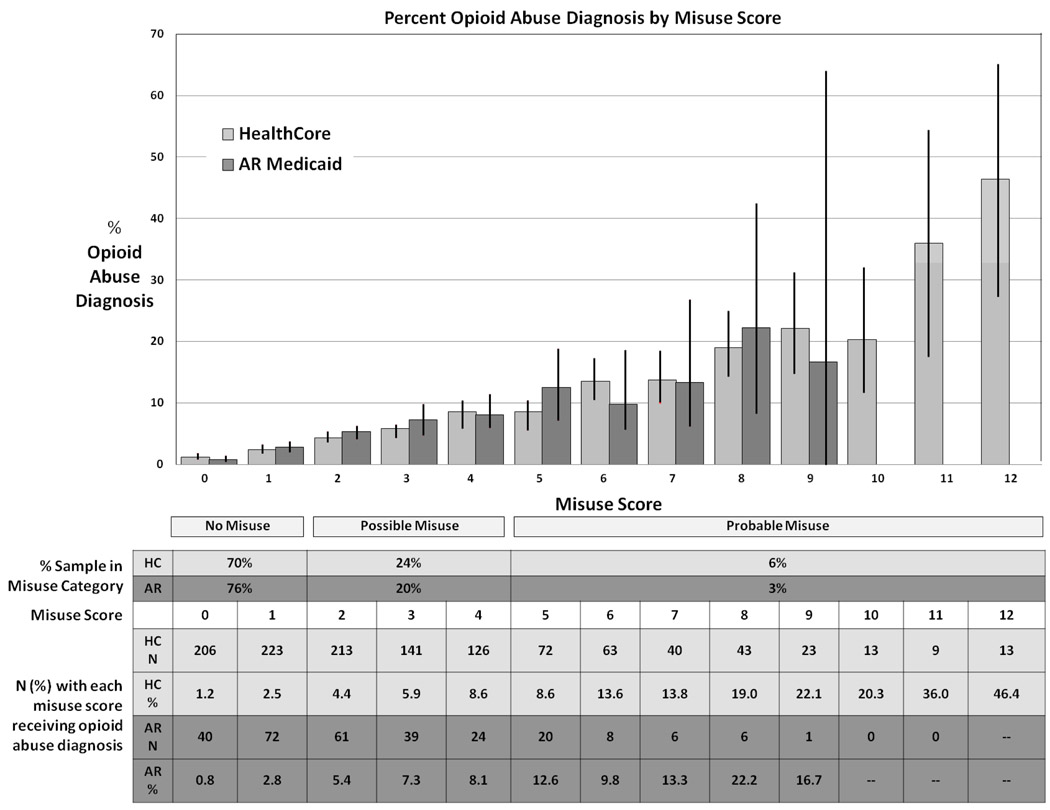
In both samples, the likelihood of receiving a post-index opioid abuse or dependence diagnosis increased linearly with increasing misuse scores. (Tests for linear trend: HealthCore: Wald’s Chi Square=1008.0, p<.0001; OR= 1.41, (95%CI, 1.380, 1.440); AR: Wald’s Chi Square=223.0, p<.0001; OR= 1.51 (95%CI, 1.43, 1.60). Thus, the risk of receiving an opioid abuse diagnosis increased 41% in HealthCore and 51% in Arkansas Medicaid for each single point increase in misuse score. In HealthCore, abuse diagnoses increased from 1.23% among those with a misuse score of zero to 46% for those with a misuse score of 12. (Less than 10 subjects received any score above 12 and percents become unstable.) In Arkansas Medicaid, abuse diagnoses increased from 0.8% among those with a misuse score of zero to 22% for those with a misuse score of 8. (Less than 10 subjects received any score above 8 and percents become unstable.) Figure 1 displays the distribution of the misuse scores for HealthCore and Medicaid samples.
Fig. 1.
Distribution of misuse scores and abuse diagnoses
Table 2 displays the polytomous logistic regression results for possible (score 3–4) and probable (score ≥ 5) misuse groups in the HealthCore sample. Risks for these two levels of misuse are quite similar. Age is strongly inversely related to risk of misuse. Persons with a claim diagnosis with headache and back pain have modestly increased risk of misuse, while a claim diagnosis of joint pain is modestly protective. Increasing numbers of other pain conditions modestly increases risk. The diagnosis of two or more mental health disorder types increases risk for probable misuse. Any pre-index substance abuse diagnosis increases risk of misuse, with non-opioid drug abuse most significant for possible misuse and opioid abuse most significant for probable misuse. Each month of sedative-hypnotic medication use increases risk for misuse.
Table 2.
Factors associated with possible and probable opioid misuse; HealthCore.
| Possible Misuse | Probable Misuse | |||||||
|---|---|---|---|---|---|---|---|---|
| Variables | Odds Ratio |
95% CI | Wald's Chisq |
P-value | Odds Ratio |
95% CI | Wald's Chisq |
P-value |
| Age: 18–30 | 3.56 | 3.11, 4.09 | 326.819 | <0.001 | 15.95 | 11.73, 21.67 | 313.043 | <0.001 |
| Age: 31–40 | 2.89 | 2.60, 3.21 | 382.312 | <0.001 | 9.86 | 7.43, 13.07 | 252.119 | <0.001 |
| Age: 41–50 | 2.15 | 1.95, 2.37 | 234.928 | <0.001 | 5.31 | 4.02, 7.00 | 139.624 | <0.001 |
| Age: 51–64 | 1.45 | 1.32, 1.60 | 56.561 | <0.001 | 2.21 | 1.66, 2.93 | 29.824 | <0.001 |
| reference: age ≥65 | ||||||||
| Female | 1.06 | 1.01, 1.12 | 5.027 | 0.025 | 1.09 | 0.98, 1.21 | 2.740 | <0.001 |
| reference: male | ||||||||
| CNCP: Joint | 0.91 | 0.85, 0.98 | 6.479 | 0.011 | 0.94 | 0.82, 1.07 | 0.899 | <0.001 |
| CNCP: Back | 1.13 | 1.07, 1.19 | 17.902 | <0.001 | 1.11 | 1.00, 1.23 | 3.782 | <0.001 |
| CNCP: Head | 1.22 | 1.14, 1.31 | 32.069 | <0.001 | 1.58 | 1.41, 1.78 | 58.891 | <0.001 |
| CNCP: Neck | 1.00 | 0.93, 1.07 | 0.004 | 0.952 | 0.93 | 0.81, 1.06 | 1.295 | <0.001 |
| reference: no CNCP diagnosis | ||||||||
| # non-tracer pain | 1.08 | 1.06, 1.11 | 41.601 | <0.001 | 1.19 | 1.14, 1.24 | 63.131 | <0.001 |
| reference: no non-tracer pain | ||||||||
| Charlson score | 0.98 | 0.95, 1.00 | 2.888 | 0.089 | 0.96 | 0.91, 1.02 | 1.814 | <0.001 |
| MH: 1 | 1.07 | 0.99 , 1.15 | 3.144 | 0.076 | 1.04 | 0.91, 1.18 | 0.279 | <0.001 |
| MH: ≥2 | 1.12 | 0.99 , 1.26 | 3.390 | 0.066 | 1.15 | 0.95, 1.40 | 2.046 | <0.001 |
| reference: no mental health diagnosis | ||||||||
| SA / alcohol | 1.38 | 1.10, 1.72 | 8.080 | 0.004 | 1.77 | 1.27, 2.47 | 11.303 | <0.001 |
| SA / opioid | 1.39 | 1.00, 1.93 | 3.929 | 0.047 | 3.53 | 2.39, 5.21 | 40.113 | <0.001 |
| SA / non-opioid | 1.75 | 1.38, 2.22 | 21.199 | <0.001 | 1.78 | 1.27, 2.48 | 11.291 | <0.001 |
| reference: no SA diagnosis | ||||||||
| Sedative/hypnotics days supply/30d |
1.02 | 1.02, 1.03 | 64.585 | <0.001 | 1.04 | 1.03, 1.05 | 65.140 | <0.001 |
| reference: <30d supply | ||||||||
| Opioid daily dose: median-120 | 1.65 | 1.56, 1.74 | 315.925 | <0.001 | 2.68 | 2.39, 3.00 | 284.748 | <0.001 |
| Opioid daily dose: >120 | 2.37 | 2.13, 2.65 | 241.722 | <0.001 | 6.70 | 5.60, 8.03 | 426.878 | <0.001 |
| reference: opioid daily dose < median | ||||||||
| Opioid type: schedule II short only |
1.35 | 1.14, 1.59 | 12.437 | <0.001 | 1.08 | 0.77, 1.50 | 0.197 | <0.001 |
| Opioid type: schedule II long only |
0.99 | 0.88, 1.11 | 0.040 | 0.842 | 0.42 | 0.33, 0.54 | 47.152 | <0.001 |
| Opioid type: non-schedule II + schedule II short |
3.06 | 2.66, 3.52 | 248.668 | <0.001 | 5.19 | 4.26, 6.32 | 267.503 | <0.001 |
| Opioid type: non-schedule II + schedule II long |
1.66 | 1.51, 1.82 | 113.453 | <0.001 | 1.88 | 1.61, 2.19 | 65.532 | <0.001 |
| Opioid type: schedule II short + long |
1.65 | 1.41, 1.92 | 40.230 | <0.001 | 1.39 | 1.08, 1.78 | 6.725 | <0.001 |
| Opioid type: All 3 types | 3.47 | 2.83, 4.26 | 142.631 | <0.001 | 7.44 | 5.81, 9.52 | 254.018 | <0.001 |
| reference: non-schedule II only | ||||||||
In the HealthCore sample, opioid daily dose above the median is significantly related to both possible and probable misuse. Risk is further increased in those over 120mg MED. Compared to those who took only Non-schedule II opioids, those who took any regimen including short-acting schedule II opioids show increased risk of misuse. Risk of probable misuse is significantly reduced in those receiving only long-acting Schedule II opioids.
Table 3 displays the polytomous logistic regression results for possible (score 3–4) and probable (score ≥ 5) misuse groups in Arkansas Medicaid. Risks for these two levels of misuse are similar. Age is strongly inversely related to risk of misuse. Back and neck pain increase risk of misuse, as does increasing numbers of other pain conditions. Increasing number of mental health disorder types is not associated with possible misuse, but is negatively associated with probable misuse. Alcohol abuse is marginally associated with possible misuse, but is negatively associated with probable misuse. Pre-index diagnosis of opioid drug abuse is not associated with possible misuse, but is significantly associated with probable misuse. Pre-index non-opioid drug abuse is more significantly associated with possible and probable opioid misuse.
Table 3.
Factors associated with possible and probable opioid misuse; Arkansas Medicaid.
| Possible Misuse | Probable Misuse | |||||||
|---|---|---|---|---|---|---|---|---|
| Variables | Odds Ratio |
95% CI | Wald's Chisq |
P-value | Odds Ratio |
95% CI | Wald's Chisq |
P-value |
| Age: 18–30 | 4.13 | 3.23, 5.28 | 128.510 | <0.001 | 16.77 | 8.60, 32.73 | 68.341 | <0.001 |
| Age: 31–40 | 3.58 | 2.93, 4.37 | 155.235 | <0.001 | 10.16 | 5.40, 19.12 | 51.663 | <0.001 |
| Age: 41–50 | 2.53 | 2.09, 3.05 | 92.067 | <0.001 | 5.13 | 2.73, 9.64 | 25.838 | <0.001 |
| Age: 51–64 | 1.86 | 1.54, 2.24 | 42.716 | <0.001 | 2.92 | 1.53, 5.56 | 10.616 | <0.001 |
| reference: age ≥65 | ||||||||
| Female | 0.92 | 0.82, 1.03 | 1.956 | 0.162 | 0.69 | 0.53, 0.88 | 8.492 | <0.001 |
| reference: male | ||||||||
| CNCP: Joint | 0.90 | 0.80, 1.02 | 2.818 | 0.093 | 0.87 | 0.66, 1.14 | 1.008 | <0.001 |
| CNCP: Back | 1.35 | 1.20, 1.51 | 26.731 | <0.001 | 1.70 | 1.31, 2.20 | 15.762 | <0.001 |
| CNCP: Head | 1.07 | 0.94, 1.21 | 0.904 | 0.342 | 1.09 | 0.83, 1.43 | 0.361 | <0.001 |
| CNCP: Neck | 1.26 | 1.10, 1.44 | 10.686 | 0.001 | 1.29 | 0.97, 1.70 | 3.094 | <0.001 |
| reference: no CNCP diagnosis | ||||||||
| # non-tracer pain | 1.09 | 1.04, 1.13 | 14.879 | <0.001 | 1.27 | 1.16, 1.39 | 27.632 | <0.001 |
| reference: no non-tracer pain | ||||||||
| Charlson score | 0.97 | 0.93, 1.01 | 2.118 | 0.146 | 0.89 | 0.81, 0.98 | 5.351 | <0.001 |
| MH: 1 | 1.02 | 0.89, 1.16 | 0.050 | 0.823 | 0.89 | 0.66, 1.19 | 0.648 | <0.001 |
| MH: ≥2 | 0.94 | 0.79, 1.12 | 0.456 | 0.500 | 0.73 | 0.50, 1.05 | 2.897 | <0.001 |
| reference: no mental health diagnosis | ||||||||
| SA / alcohol | 1.41 | 1.06, 1.86 | 5.639 | 0.018 | 0.98 | 0.53, 1.82 | 0.004 | <0.001 |
| SA / opioid | 1.48 | 0.81, 2.69 | 1.626 | 0.202 | 2.66 | 1.14, 6.23 | 5.087 | <0.001 |
| SA / non-opioid | 1.60 | 1.24, 2.06 | 13.227 | <0.001 | 2.29 | 1.48, 3.54 | 13.796 | <0.001 |
| reference: no SA diagnosis | ||||||||
| Sedative/hypnotics days supply/30d |
1.00 | 0.99, 1.01 | 0.007 | 0.931 | 0.98 | 0.95, 1.01 | 1.791 | <0.001 |
| reference: <30d supply | ||||||||
| Opioid daily dose: median-120 | 1.21 | 1.08, 1.35 | 10.261 | 0.001 | 1.80 | 1.38, 2.35 | 18.665 | <0.001 |
| Opioid daily dose: >120 | 2.02 | 1.61, 2.54 | 36.582 | <0.001 | 4.69 | 3.03, 7.24 | 48.448 | <0.001 |
| reference: opioid daily dose < median | ||||||||
| Opioid: schedule II short only | 1.55 | 1.14, 2.11 | 7.719 | 0.005 | 1.94 | 1.06, 3.56 | 4.581 | <0.001 |
| Opioid: schedule II long only | 1.38 | 1.10, 1.73 | 7.827 | 0.005 | 0.71 | 0.41, 1.23 | 1.484 | <0.001 |
| Opioid: non-schedule II + schedule II short |
3.70 | 2.85, 4.80 | 96.235 | <0.001 | 4.69 | 2.93, 7.50 | 41.415 | <0.001 |
| Opioid: non-schedule II + schedule II long |
2.24 | 1.84, 2.71 | 66.598 | <0.001 | 2.20 | 1.50, 3.23 | 16.323 | <0.001 |
| Opioid: schedule II short + long | 2.12 | 1.58, 2.85 | 24.970 | <0.001 | 1.44 | 0.81, 2.55 | 1.546 | <0.001 |
| Opioid: All 3 types | 4.22 | 2.79, 6.38 | 46.559 | <0.001 | 8.00 | 4.53, 14.13 | 51.331 | <0.001 |
| reference: non-schedule II only | ||||||||
In the Arkansas sample, opioid daily dose is associated with misuse, especially when the dose exceeds 120mg MED per day. Compared to those who took only Non-schedule II opioids, those who took any Schedule II opioids show increased risk of misuse with the exception of those receiving only Schedule II long-acting opioids who show decreased risk for probable misuse.
Table 4 displays distribution of opioid misuse scores by opioid daily dose and predominant opioid type. Number and percent (of chronic opioid users) receiving that opioid dose range in MED or opioid types are displayed in the first column for both HealthCore and Arkansas. Number and percent of users receiving that opioid dose or type receiving scores in the 0–1 ‘no misuse’, 2–4 ‘possible misuse’, and 5+ ‘probable misuse’ ranges are provided in the next three columns. Mean misuse scores by dose level and predominant type are displayed in the last column. Opioid dose and opioid type groups all have significantly different misuse scores (p<0.0001 by chi-square and ANOVA). As a sensitivity analysis, we calculated misuse scores using only the numbers of opioid prescribers and opioid pharmacies. These scores ranged from low to high in the same order of opioid types as those calculated from days supplied, prescribers and pharmacies (data not shown).
Table 4.
Misuse scores by opioid daily dose and predominant opioid types.
| HealthCore | Medicaid | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | Score 0–1 |
Score 2–4 |
Score 5+ |
Mean Misuse Scores |
Total | Score 0–1 |
Score 2–4 |
Score 5+ |
Mean Misuse Scores |
|
| DAILY DOSE | ||||||||||
| Up to 35mg (median dose) | 18,291 50.0% |
14,438 78.9% |
3,347 18.3% |
506 2.8% |
0.88 | 4,945 51.2% |
3,969 80.3% |
866 17.5% |
110 2.2% |
0.85 |
| 35–120mg MED | 15,719 43.0% |
10,053 64.0% |
4,486 28.5% |
1,180 7.5% |
1.49 | 4115 42.6% |
3,085 75.0% |
874 21.2% |
156 3.8% |
1.05 |
| 120mg+ | 2,569 7.0% |
1,289 50.2% |
916 35.7% |
364 14.2% |
2.17 | 591 6.1% |
310 52.5% |
225 38.1% |
56 9.5% |
1.80 |
|
PREDOMINANT OPIOID TYPES |
||||||||||
| Non-schedule II- only | 28,598 78.2% |
21,460 75.0% |
5,957 20.8% |
1,181 4.1% |
1.04 | 7,615 78.9% |
6,192 81.3% |
1,245 16.4% |
178 2.3% |
0.82 |
| Schedule II - short only | 754 2.1% |
489 64.9% |
222 29.4% |
43 5.7% |
1.42 | 239 2.5% |
164 68.6% |
62 25.9% |
13 5.4% |
1.26 |
| Schedule II - long only | 1,938 5.3% |
1,273 65.7% |
578 29.8% |
87 4.5% |
1.36 | 502 5.2% |
340 67.7% |
145 28.9% |
17 3.4% |
1.25 |
| Non-schedule II + Schedule II short |
1,057 2.9% | 438 41.4% | 446 42.2% | 173 16.4% | 2.47 | 284 2.9% | 137 48.2% | 122 43% | 25 8.8% | 1.89 |
| 2,747 7.5% |
1,502 54.7% |
947 34.5% |
298 10.9% |
1.90 | 622 6.4% |
354 56.9% |
223 35.9% |
45 7.2% |
1.64 | |
| Non-schedule II + Schedule II long |
908 2.5% |
448 49.3% |
353 38.9% |
107 11.8% |
2.06 | 263 2.7% |
134 51% |
109 41.4% |
20 7.6% |
1.79 |
| Schedule II short + long | 577 1.6% |
170 29.5% |
246 42.6% |
161 27.9% |
3.39 | 126 1.3% |
43 34.1% |
59 46.8% |
24 19.1% |
2.65 |
| All 3 Opioid Types | ||||||||||
Column percents presented in total column, Row percents presented in other columns. Chi-square tests with p <0.0001 for all cross-tabulations. Mean misuse scores all differ by ANOVA (p <0.001).
5. Discussion
This study is the first large-scale study estimating risks of opioid misuse in the general medical population. Based on rates of excess opioid days supplied, opioid prescribers and opioid pharmacies, we estimated possible misuse at 24% in HealthCore and 20% in Arkansas Medicaid and estimated probable misuse at 6% in HealthCore and 3% in Arkansas Medicaid. While the Arkansas Medicaid sample was more prone to misuse due to high rates of chronic physical and mental illness, the HealthCore sample likely had more opportunity for misuse due to a less regulated pharmacy benefit. Our misuse score shows a significant linear relation to the risk of receiving an opioid abuse diagnosis. The risks for possible and probable misuse are similar, suggesting that opioid misuse is a single dimension. The most important risks appear to be: age, substance abuse diagnosis, daily opioid dose, and opioid types used. Among Schedule II opioids, use of long-acting opioids alone appears to be associated with lower levels of misuse. Since these findings are based on large samples of patients on chronic opioid therapy (COT) (>90d) from two dissimilar health plans, findings that are consistent between plans are likely broadly generalizable to publicly and privately-insured general medical populations.
Our study points to the importance of both non-modifiable risk factors (that may be useful for risk stratification) and modifiable risk factors (that may serve as the focus of risk reduction efforts). Among demographic risk factors, age appears to be much more important than sex. The odds of possible and probable opioid misuse declined significantly with increasing age with patients age 18–30 at least 4–5 times the risk of those over 65 years. This is consistent with previous studies [29], and patterns of substance abuse in general. The type and number of pain complaints was modestly associated with misuse. Back pain was associated with increased risk of misuse in both samples. Headache was associated with increased risk in HealthCore, while neck pain was associated with increased risk in Medicaid. Increasing number of non-tracer pain conditions was associated with increased risk in both samples. Mental health diagnoses and alcohol abuse were not consistently related to misuse risk, but pre-index non-opioid drug abuse and opioid abuse diagnoses increased risk of misuse in both samples. Others have shown that stimulant abusers, for example, have high rates of prescription opioid abuse [16]. Alcohol abuse increased risk in the HealthCore sample, but not in the Medicaid sample. Increasing numbers of types of mental health diagnoses was associated with increased risk in the HealthCore sample but decreased risk in the Medicaid sample. These contrasting results concerning the risks for misuse associated with alcohol abuse and mental health disorders likely result from the two-fold higher base rates of these disorders in the Medicaid population. An earlier study by Edlund et al showed increased risk of new substance abuse diagnoses among CNCP patients treated with COT who had a history of mental health disorders [11]. But not all studies have showed increased risk of misuse in patients with mental health disorders [12, 18].
Our study shows that there is increased risk of possible and probable misuse in both samples of patients at doses over 120mg MED per day, the dose at which caution and consultation are advised in the Washington State Opioid Dosing Guideline [30] and the British Pain Society Guideline [6]. Only 6–7% of COT recipients achieved this dose, so we did not examine risk associated with the higher 200mg MED dose mentioned in the APS/AAPM opioid guideline which was achieved by only 1–2% of recipients. This finding of increased risk is important, as the Washington State guideline has been controversial, with professional pain organizations arguing that there is no evidence of increased harm at doses above 120mg [4, 25]. These patients may be receiving high doses for a variety of reasons including difficult to control or opioid insensitive pain. Our study shows that there is markedly increased risk of possible and probable misuse in both samples of patients at doses over 120mg MED per day. This suggests that increased monitoring for misuse is appropriate for individuals on high dose COT.
Use of long-acting opioids alone was generally associated with reduced risk of misuse. The effect was not significant for possible misuse in the Arkansas sample, but was significant for both possible and probable misuse in the larger HealthCore sample. The reasons for this protective effect are unclear. It could be due to lower rates of excess days supply in those receiving only long acting opioids. However, those receiving only long-acting opioids also tended to have fewer prescribers and pharmacies. The lower risk of misuse could be due to more stable opioid blood levels resulting in improved pain control as has been claimed by manufacturers of long-acting opioid preparations [21]. Or use of long-acting opioids could be a marker of a more explicit and organized approach to COT [22]. It may also be that long-acting opioids are more often prescribed in specialty pain care, where there is greater expertise and closer follow-up than in primary care. Our results cast some doubt on the proposed focus on long-acting opioids in the FDA Risk Evaluation and Management System (REMS) under development. The primary targets of the FDA REMS are accidental overdose and drug abuse, which are not the same issue as the possible and probable misuse that is the focus of our study. Nevertheless, our data suggest that increased restrictions on prescribing of long-acting opioids might increase risk of misuse.
Our study has several limitations. First, our misuse outcome is limited to administrative claims data. We did not document opioid misuse through clinical observation or surveys. Our misuse measure focused on excess use of opioids, as indicated by excess opioid days supply, excess opioid prescribers, and excess opioid pharmacies, but not other aberrant behaviors (such as obtaining opioids from illicit sources, friends, or the Internet). Excess days supply could be due to factors other than misuse, such as an effort to control inadequately treated pain. However, there is actually no consensus gold standard measure of opioid misuse, and all methods of measurement have limitations. For example, surveys are subject to response bias, especially for stigmatized behavior such as medication misuse. A recent review of misuse concluded that “no one procedure or set of predictor variables is sufficient to identify chronic pain patients at-risk for opioid misuse or abuse ” [29]. We have used the labels ‘possible misuse’ and ‘probable misuse’ to indicate that our claims data findings should be considered suggestive rather than definitive indicators of misuse. We were able to show that our misuse score had a significant linear relation to the likelihood of receiving a diagnosis of opioid abuse/dependence. Pain researchers and clinicians have argued that the criteria used for opioid abuse and dependence in the DSM-IV and ICD-9 are not appropriate for patients prescribed COT [19, 27]. Nevertheless, these abuse diagnoses have been subject to extensive field testing and are the best available validating variable from administrative data [15]. Administrative substance abuse diagnoses have generally been shown to have low sensitivity but high specificity when compared with structured interview clinical diagnoses [31]. Both clinical and administrative indicators of misuse are likely valuable tools in efforts to reduce possible harms from COT.
Second, our clinical and pharmacological risk factors are also derived from claims data. We did not independently verify chart diagnoses of pain, mental health or substance diagnoses. We did not have access to laboratory data such as urine drug screens. Our medication data was derived from pharmacy claims records. If patients paid out of pocket for medications, we would have no record of this use. Third, we monitored outcomes for only the year after our index date (defined as the first days of 90 days of near-continuous opioid use). Thus our estimated misuse rates may be conservative. Finally, we did not assess pain levels, amount of pain relief, or satisfaction with COT, so we are unable to address the role of pseudo-addiction or the overall balance of benefits and harms for these COT recipients.
In summary, we have examined administrative claims data from two large samples of chronic opioid users from dissimilar health plans to identify risks of possible and probable opioid misuse. This represents the first large-scale population-based study of risks for opioid misuse. Among non-modifiable factors, younger age, back pain, multiple pain complaints and substance abuse disorders identify patients at high risk for misuse. Among modifiable factors, treatment with high daily dose opioids (especially >120mg MED per day) and short-acting Schedule II opioids appears to increase risk of misuse. Whether policies that target these factors can reduce the harms associated with chronic opioid therapy, needs to be tested in a separate study. The consistency of the findings across diverse patient populations and varying levels of misuse suggests that these results will generalize broadly, but awaits confirmation in other studies.
Acknowledgements
This research was supported by a grant from the National Institute on Drug Abuse (R01 DA 022560) to Mark Sullivan. We wish to thank Michael VonKorff for comments on an earlier draft of this manuscript and Alison Sattler for secretarial assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts of interest to report,
References
- 1.Clinical Classification Software (CCS) for ICD-9 CM. Rockville, MD: Agency for Healthcare Research and Quality; [Google Scholar]
- 2.SAS 9.1. Cary, NC: SAS Institute, Inc; [Google Scholar]
- 3.Results from the 2006 National Survey on Drug Use and Health: National Findings. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2007. [Google Scholar]
- 4.APF position statement on Washington State interagency guideline on opioid dosing for chronic non-cancer pain: an educational pilot to improve care and safety with opioid treatment. American Pain Foundation; 2007. May, [Google Scholar]
- 5.Braden JB, Fan MY, Edlund MJ, Martin BC, DeVries A, Sullivan MD. Trends in use of opioids by noncancer pain type 2000–2005 among Arkansas Medicaid and HealthCore enrollees: results from the TROUP study. J Pain. 2008;9:1026–1035. doi: 10.1016/j.jpain.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.British Pain Society Consensus Statement. Opioids for persistent pain: good practice. [Accessed on: February 25, 2010];British Pain Society. 2010 :1–34. Available at: http://www.britishpainsociety.org/book_opioid_main.pdf.
- 7.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 8.Chou R, Fanciullo GJ, Fine PG, Miaskowski C, Passik SD, Portenoy RK. Opioids for chronic noncancer pain: prediction and identification of aberrant drug-related behaviors: a review of the evidence for an American Pain Society and American Academy of Pain Medicine clinical practice guideline. J Pain. 2009;10:131–146. doi: 10.1016/j.jpain.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 9.Compton WM, Volkow ND. Major increases in opioid analgesic abuse in the United States: concerns and strategies. Drug Alcohol Depend. 2006;81:103–107. doi: 10.1016/j.drugalcdep.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 10.Denisco RA, Chandler RK, Compton WM. Addressing the intersecting problems of opioid misuse and chronic pain treatment. Exp Clin Psychopharmacol. 2008;16:417–428. doi: 10.1037/a0013636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edlund MJ, Steffick D, Hudson T, Harris KM, Sullivan M. Risk factors for clinically recognized opioid abuse and dependence among veterans using opioids for chronic non-cancer pain. Pain. 2007;129:355–362. doi: 10.1016/j.pain.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 12.Friedman R, Li V, Mehrotra D. Treating pain patients at risk: evaluation of a screening tool in opioid-treated pain patients with and without addiction. Pain Med. 2003;4:182–185. doi: 10.1046/j.1526-4637.2003.03017.x. [DOI] [PubMed] [Google Scholar]
- 13.Gilson AM, Ryan KM, Joranson DE, Dahl JL. A reassessment of trends in the medical use and abuse of opioid analgesics and implications for diversion control: 1997–2002. J Pain Symptom Manage. 2004;28:176–188. doi: 10.1016/j.jpainsymman.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Gureje O, Von Korff M, Simon GE, Gater R. Persistent pain and well-being: a World Health Organization Study in Primary Care. JAMA. 1998;280:147–151. doi: 10.1001/jama.280.2.147. [DOI] [PubMed] [Google Scholar]
- 15.Hasin D, Hatzenbuehler ML, Keyes K, Ogburn E. Substance use disorders: Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV) and International Classification of Diseases, tenth edition (ICD-10) Addiction. 2006;101 Suppl 1:59–75. doi: 10.1111/j.1360-0443.2006.01584.x. [DOI] [PubMed] [Google Scholar]
- 16.Havens JR, Stoops WW, Leukefeld CG, Garrity TF, Carlson RG, Falck R, Wang J, Booth BM. Prescription opiate misuse among rural stimulant users in a multistate community-based study. Am J Drug Alcohol Abuse. 2009;35:18–23. doi: 10.1080/00952990802326298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knisely JS, Wunsch MJ, Cropsey KL, Campbell ED. Prescription Opioid Misuse Index: a brief questionnaire to assess misuse. J Subst Abuse Treat. 2008;35:380–386. doi: 10.1016/j.jsat.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 18.Michna E, Jamison RN, Pham LD, Ross EL, Janfaza D, Nedeljkovic SS, Narang S, Palombi D, Wasan AD. Urine toxicology screening among chronic pain patients on opioid therapy: frequency and predictability of abnormal findings. Clin J Pain. 2007;23:173–179. doi: 10.1097/AJP.0b013e31802b4f95. [DOI] [PubMed] [Google Scholar]
- 19.Miotto K, Compton P, Ling W, Conolly M. Diagnosing addictive disease in chronic pain patients. Psychosomatics. 1996;37:223–235. doi: 10.1016/S0033-3182(96)71561-X. [DOI] [PubMed] [Google Scholar]
- 20.National Institute on Drug Abuse. Pain and Opiophobia. [Accessed on: March 1, 2010];Research Report Series-Prescription Drugs: Abuse and Addiction. Available at: http://www.nida.nih.gov/ResearchReports/Prescription/Prescription6a.html.
- 21.Nicholson B. Benefits of extended-release opioid analgesic formulations in the treatment of chronic pain. Pain Pract. 2009;9:71–81. doi: 10.1111/j.1533-2500.2008.00232.x. [DOI] [PubMed] [Google Scholar]
- 22.Nwokeji ED, Rascati KL, Brown CM, Eisenberg A. Influences of attitudes on family physicians' willingness to prescribe long-acting opioid analgesics for patients with chronic nonmalignant pain. Clin Ther. 2007;(29 Suppl):2589–2602. doi: 10.1016/j.clinthera.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 23.Parente ST, Kim SS, Finch MD, Schloff LA, Rector TS, Seifeldin R, Haddox JD. Identifying controlled substance patterns of utilization requiring evaluation using administrative claims data. Am J Manag Care. 2004;10:783–790. [PubMed] [Google Scholar]
- 24.Paulozzi LJ, Ryan GW. Opioid analgesics and rates of fatal drug poisoning in the United States. Am J Prev Med. 2006;31:506–511. doi: 10.1016/j.amepre.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 25.Peppin J. Washington State Develops guideline for opioid dosing of chronic noncancer pain. In: American Academy of Pain Medicine, editor. Pain Medicine Network Newletter. American Academy of Pain Medicine; 2008. Winter. [Google Scholar]
- 26.Portenoy RK. Opioid therapy for chronic nonmalignant pain: a review of the critical issues. J Pain Symptom Manage. 1996;11:203–217. doi: 10.1016/0885-3924(95)00187-5. [DOI] [PubMed] [Google Scholar]
- 27.Sees KL, Clark HW. Opioid use in the treatment of chronic pain: assessment of addiction. J Pain Symptom Manage. 1993;8:257–264. doi: 10.1016/0885-3924(93)90154-n. [DOI] [PubMed] [Google Scholar]
- 28.Sullivan MD, Edlund MJ, Fan MY, DeVries A, Braden JB, Martin BC. Trends in use of opioids for non-cancer pain conditions 2000–2005 in Commercial and Medicaid insurance plans: the TROUP study. Pain. 2008;138:440–449. doi: 10.1016/j.pain.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turk DC, Swanson KS, Gatchel RJ. Predicting opioid misuse by chronic pain patients: a systematic review and literature synthesis. Clin J Pain. 2008;24:497–508. doi: 10.1097/AJP.0b013e31816b1070. [DOI] [PubMed] [Google Scholar]
- 30.Washington State Agency Medical Directors’ Group. Interagency guideline on opioid dosing for chronic non-cancer pain: an educational pilot to improve care and safety with opioid treatment. 2007 [Google Scholar]
- 31.Wilson CR, Sherritt L, Gates E, Knight JR. Are clinical impressions of adolescent substance use accurate? Pediatrics. 2004;114:e536–e540. doi: 10.1542/peds.2004-0098. [DOI] [PubMed] [Google Scholar]
- 32.Zacny J, Bigelow G, Compton P, Foley K, Iguchi M, Sannerud C. College on problems of drug dependence taskforce on prescription opioid non-medical use and abuse: position statement. Drug Alcohol Depend. 2003;69:215–232. doi: 10.1016/s0376-8716(03)00003-6. [DOI] [PubMed] [Google Scholar]