Abstract
Redirecting the tropism of viral vectors enables specific transduction of selected cells by direct administration of vectors. We previously developed targeting lentiviral vectors by pseudotyping with modified Sindbis virus envelope proteins. These modified Sindbis virus envelope proteins have mutations in their original receptor-binding regions to eliminate their natural tropisms, and they are conjugated with targeting proteins, including antibodies and peptides, to confer their tropisms on target cells. We investigated whether our targeting vectors interact with DC-SIGN, which traps many types of viruses and gene therapy vectors by binding to the N-glycans of their envelope proteins. We found that these vectors do not interact with DC-SIGN. When these vectors were produced in the presence of deoxymannojirimycin, which alters the structures of N-glycans from complex to high mannose, these vectors used DC-SIGN as their receptor. Genetic analysis demonstrated that the N-glycans at E2 amino acid (aa) 196 and E1 aa 139 mediate binding to DC-SIGN, which supports the results of a previous report of cryoelectron microscopy analysis. In addition, we investigated whether modification of the N-glycan structures could activate serum complement activity, possibly by the lectin pathway of complement activation. DC-SIGN-targeted transduction occurs in the presence of human serum complement, demonstrating that high-mannose structure N-glycans of the envelope proteins do not activate human serum complement. These results indicate that the strategy of redirecting viral vectors according to alterations of their N-glycan structures would enable the vectors to target specific cells types expressing particular types of lectins.
The ultimate goal of gene therapy is cell- and tissue-specific targeted delivery of therapeutic genes. A targeted system increases the therapeutic effects of transgenes at the site of action while reducing adverse effects in surrounding cells and tissues that commonly occur through nonspecific modes of gene delivery (5-8). Gene therapy vectors that can home to specific cells and tissues after intravenous administration, also known as targeting vectors, are ideal for targeted delivery (62). In the past, many attempts have been made to develop targeting viral vectors by using adenovirus, adeno-associated virus, oncoretrovirus, lentivirus, measles virus, and alphavirus (70, 89).
To create targeting viral vectors, the natural tropisms of the viruses must first be eliminated and new binding specificities conferred (89). The binding of envelope viruses, such as oncoretrovirus, lentivirus, measles virus, and alphavirus, is mediated by envelope proteins. To redirect the tropisms of these viruses, the original receptor-binding regions of their envelope proteins must be eliminated. We have developed targeting oncoretroviral and lentiviral vectors by pseudotyping them with modified Sindbis virus envelope proteins and by mutating the receptor-binding regions of the envelope proteins, thereby reducing the nonspecific transduction of untargeted cells (61, 63-66). The mutated regions of the envelope protein originally interact directly with other receptors, including heparan sulfate, laminin receptor, and/or unknown molecules (10, 46, 67, 90). These mutations reduced the nonspecific transduction of the liver and spleen when the vectors were administered intravenously (66). By conjugating the virus with targeting ligands, including antibodies and peptides, the virus can transduce specific cells and tissues both in vitro and in vivo (53, 61, 63-66, 71, 72). These results demonstrated that we can eliminate the natural tropism of the Sindbis virus envelope protein while maintaining its fusion activity.
However, the N-glycans of the envelope proteins are still intact and possibly interact with cell surface lectins. DC-SIGN is the best-known cell surface lectin expressed on dendritic cells, certain macrophages, and activated B cells (27, 29, 30).
Structural and biochemical studies show flexible modes of DC-SIGN binding to cognate saccharides. The trimannose core unit of high-mannose N-glycans is the primary binding site for DC-SIGN (23), while nonreducing alpha1-2-linked terminal mannose moieties contribute to the high avidity seen when DC-SIGN binds the Man8 or Man9 structures common to many viral envelope glycoproteins (22). DC-SIGN traps a wide variety of viruses and viral vectors (HIV [29, 30], simian immunodeficiency virus [50], human T-cell leukemia virus type 1 [12], measles virus [17, 18], dengue virus [86], feline corona virus [77], herpes simplex virus type 1 [16], human cytomegalovirus [36], human herpesvirus type 8 [76], Ebola virus [1], West Nile virus [15], influenza virus [91], Marburg virus [57], and severe acute respiratory syndrome virus [93]) by binding to the N-glycans of the viruses and viral vectors. Binding of DC-SIGN with virus and viral vectors results in enhanced infection and/or transduction of DC-SIGN-positive cells (cis infection/transduction) and/or neighboring cells (trans infection/transduction).
If any targeting vector can be trapped by DC-SIGN, it is necessary to eliminate its binding to DC-SIGN to increase the targeting specificity of the virus in vivo (28, 49, 73). In addition to enhanced infection/transduction, binding to DC-SIGN causes signaling that can activate DC-SIGN-expressing antigen-presenting cells (32, 38). Activation of antigen-presenting cells can lead to adverse effects, including systemic inflammation and immune reactions to viral vectors and their transgene products (7, 8, 32, 59, 88). Therefore, investigation of the interactions between viral vectors and DC-SIGN, identification of N-glycans that mediate binding to DC-SIGN, and elimination of interactions with DC-SIGN are important aspects of reducing adverse effects of vector administration and prolonging transgene expression.
The envelope protein of our targeting lentiviral vectors, the Sindbis virus envelope protein, contains four N-linked glycans (9, 48). Sindbis virus can replicate in insect and mammalian cells, which have different types of enzymes to process N-glycans (3). Therefore, the structures of N-glycans differ between the virus produced in insect cells and that produced in mammalian cells (40, 58). The N-glycans of the virus produced in insect cells have either the high-mannose or the paucimannosidic structure. Paucimannosidic structure N-glycans, as well as high-mannose structure N-glycans, have terminal mannose residues, and all N-glycans produced in insect cells are predicted to be able to bind DC-SIGN (Fig. 1 a) (39, 47). On the other hand, two N-glycans of the virus produced in mammalian cells have the high-mannose structure, while two others have the complex structure (40, 58). The two complex structure N-glycans have been shown to be exposed on the surface of the envelope protein, while the two high-mannose structure N-glycans are buried within the center of the trimer of the envelope proteins (74, 94). Therefore, the virus produced in insect cells can access DC-SIGN as its receptor while the virus produced in mammalian cells cannot (47). Because our targeting vectors are produced in mammalian cells, they should not bind DC-SIGN efficiently. However, one group demonstrated that lentiviral vectors pseudotyped with a modified Sindbis virus envelope protein bind to DC-SIGN and target DC-SIGN-positive cells (92), in contrast to the results seen with replication-competent Sindbis virus. Both Sindbis virus and the pseudotyped lentiviral vectors were produced in mammalian cells; Sindbis virus was produced in baby hamster kidney (BHK) cells, chicken embryonic fibroblasts, and hamster fibroblast cells; and the pseudotyped vector was produced in human embryonic kidney fibroblast (293T) cells (69). Because it is known that the N-glycans of the HIV envelope protein produced in lymphocytes have structures different from those produced in macrophages, the different producer cells may account for the differences between the N-glycan structures of the virus and Sindbis virus envelope-pseudotyped lentivectors (54, 55). It is also known that the N-glycan structure of dengue virus can be altered by the presence of viral capsid (35). Thus, the capsid of Sindbis virus and HIV could also affect the structures of the N-glycans of envelope proteins differently.
FIG. 1.
(a) N-glycan structures and processing pathway. All N-glycans are first produced as the high-mannose structure in both mammalian cells and insect cells. In mammalian cells, certain N-glycans are further processed to the complex structure. In insect cells, certain N-glycans are further processed to the paucimannosidic structure. DMNJ inhibits mannosidase I, which is necessary for the formation of the complex structure; thus, all N-glycans have the high-mannose structure when generated in the presence of DMNJ. One representative structure of each N-glycan is shown. Man, mannose; GlcNAc, N-acetylglucosamine; SA, sialic acid; Gal, galactose. (b) Schematic representation of chimeric Sindbis virus envelope proteins. The Sindbis virus envelope protein is first synthesized as a polypeptide and subsequently cleaved by cellular proteases to generate the E3, E2, 6K, and E1 proteins. E1 and E2 are incorporated into the viral envelope, and E3 and 6K are leader sequences for E2 and E1, respectively. The N-linked glycosylation sites of the envelope proteins are shown. 2.2 is a modified Sindbis virus envelope protein in which the IgG-binding domain of protein A (ZZ) was inserted into the E2 region at aa 70. 2.2 1L1L has two flexible linkers (Gly-Gly-Gly-Gly-Ser) at aa 70 of the E2 protein. 2.2 ΔE2-196N does not have the N-glycan at E2 aa 196, 2.2 ΔE1-139N does not have the N-glycan at E1 aa 139, and 2.2 ΔE2-196N E1-139N does not have the N-glycans at either E2 aa 196 or E1 aa 139.
In this study, we investigated whether our targeting vector binds DC-SIGN. We found that DC-SIGN does not mediate the transduction of our targeting vectors efficiently. The vectors can be redirected to DC-SIGN by modifying the structures of the N-glycans of the envelope proteins by using the mannosidase I inhibitor deoxymannojirimycin (DMNJ) (25, 47, 51).
MATERIALS AND METHODS
Plasmids, antibodies, and chemicals.
The plasmids expressing the wild-type Sindbis virus envelope protein, 2.2, and 2.2 1L1L have been previously described (63, 65, 72). The plasmids expressing 2.2 ΔE1-139N, 2.2 ΔE2-196N, and 2.2 ΔE2-196N E1-139N were constructed from 2.2 by introducing a point mutation at E1 amino acid (aa) 139 of N to E, at E2 aa 196 of N to Q, or both by using a site-directed mutagenesis kit. PAX2, a packaging plasmid of lentiviral vectors, was purchased from Addgene (Cambridge, MA). Cppt2e, a self-inactivating lentiviral vector containing the MLV (murine leukemia virus) promoter as its internal promoter (79), was used for transduction in all experiments, except for the transduction of human primary dendritic cells. Because the MLV promoter was inefficiently expressed in human primary dendritic cells, we used SIN18.cPPT.hEF1α.EGFP.WPRE (33), which contains the human EF-1α promoter as its internal promoter, for the transduction of human primary dendritic cells. The DC-SIGN-expressing lentiviral vector cppt2 DC-SIGN was constructed from lentiviral vector cppt2e by replacing its enhanced green fluorescent protein (EGFP) transgene with the cDNA of DC-SIGN (79). The CD20-expressing lentiviral vector CCR-CD20 was constructed from lentiviral vector pRRL-cPPT-CMV-X-PRE (2) by inserting cDNA of human CD20. The unconjugated anti-DC-SIGN monoclonal antibody clone DC28, clone 120612, and phycoerythrin (PE)-conjugated anti-DC-SIGN monoclonal antibody clone 120612 were purchased from R&D Systems (Minneapolis, MN). Anti-HLA class I monoclonal antibody was purified from culture supernatant of hybridomas using a protein A column. Anti-human CD20, CD11c, CD86, and HLA-DR were purchased from BD Bioscience (San Diego, CA). Mannan was purchased from Sigma-Aldrich (St. Louis, MO). The reverse transcriptase inhibitor zidovudine was obtained from the NIH AIDS Research and Reference Reagent Program. Complement human serum was purchased from Sigma, and DMNJ was purchased from Tocris Bioscience (Ellisville, MO).
Cells and viruses.
DC-SIGN 293T and DC-SIGN Jurkat cells were generated by transducing 293T or Jurkat cells with cppt2 DC-SIGN, followed by magnetic cell sorting, using anti-DC-SIGN monoclonal antibody. CD20 293T cells were produced by transducing 293T cells with CCR-CD20, followed by magnetic cell sorting using the anti-CD20 monoclonal antibody. 293T, DC-SIGN 293T, and CD20 293T cells were cultured in Iscove's modified Dulbecco's medium (Sigma-Aldrich) containing 10% fetal calf serum (FCS) and antibiotics. Jurkat and DC-SIGN Jurkat cells were cultured in RPMI medium (Invitrogen, Carlsbad, CA) containing 10% FCS (Sigma-Aldrich). All lentiviral vectors were produced in 293T cells by the previously described calcium phosphate transfection method (61). Briefly, 293T cells (1.8 × 107) were transfected with one envelope protein expression vector, packaging plasmid (PAX2), and a lentiviral vector (cppt2e or SIN18.cPPT.hEF1α.EGFP.WPRE). After transfection, the cells were cultured in AIM-V (Invitrogen) with or without 2 mM DMNJ. DMNJ was added immediately after the transfection period and kept in the culture medium until the harvesting of viral vectors. The supernatant was subjected to ultracentrifugation, and the pellet containing the virus was resuspended in phosphate-buffered saline (PBS). The concentrations of virus were quantitated by measuring amounts of viral capsid protein p24. The virus was frozen at −70°C until use.
Transduction of cells.
293T, DC-SIGN 293T, CD20 293T, Jurkat, and DC-SIGN Jurkat cells (1 × 105) were incubated with lentiviral vectors pseudotyped with various types of envelope proteins for 2 h. The amount of virus used for transduction was adjusted by the amount of HIV capsid protein p24. In one experiment shown in Table 1, the 2.2 pseudotype was conjugated with anti-DC-SIGN monoclonal antibody (2 μg/ml; R&D Systems). EGFP expression was assayed by flow cytometry 3 days posttransduction. All experiments were performed in triplicate, and the average values with standard deviations are shown.
TABLE 1.
| Envelope | Virus amta | % of target cells EGFP positive |
||||
|---|---|---|---|---|---|---|
| Jurkat | DC-SIGN Jurkat | 293T | DC-SIGN 293T | CD20 293T | ||
| Sindbis virus | 100/20b | 3.1 ± 0.8 | 5.3 ± 0.9 | 2.5 ± 0.3 | 3.5 ± 0.9 | 1.7 ± 0.3 |
| Sindbis virus DMNJ | 100/20b | 1.2 ± 0.7 | 25.4 ± 1.3 | 2.3 ± 0.3 | 11.1 ± 1.3 | 1.8 ± 0.8 |
| 2.2 1L1L | 2 | 0.2 ± 0.05 | 1.8 ± 0.2 | 6.6 ± 0.2 | 8.6 ± 0.4 | 4.6 ± 0.5 |
| 2.2 1L1L DMNJ | 2 | 0.2 ± 0.04 | 32.1 ± 2 | 6.3 ± 0.2 | 71.2 ± 4.3 | 3.7 ± 0.4 |
| 2.2 | 2 | 0.2 ± 0.08 | 0.3 ± 0.03 | 8.1 ± 1.4 | 7.5 ± 1 | 6.1 ± 1.8 |
| 2.2 + anti-DC-SIGN antibody | 2 | 0.08 ± 0.03 | 2.9 ± 0.03 | 7.6 ± 1 | 38.3 ± 2.6 | 4 ± 0.3 |
| 2.2 DMNJ | 2 | 0.13 ± 0.03 | 27 ± 5.6 | 4.3 ± 0.1 | 52.4 ± 0.4 | 2.7 ± 0.4 |
| VSV-G | 4 | 45.2 ± 1.8 | 37.9 ± 3.7 | 20.2 ± 3.7 | 26.2 ± 1.4 | 13.1 ± 0.5 |
The virus amount shown is the number of nanograms of HIV p24 per milliliter used for transduction. Jurkat and DC-SIGN Jurkat cells were transduced with 100 μl of each virus. 293T, DC-SIGN 293T, and CD20 293T cells were transduced with 200 μl of virus.
Jurkat and DC-SIGN Jurkat cells were transduced with Sindbis or Sindbis DMNJ virus at a concentration of 100 ng of HIV p24/ml. 293T, DC-SIGN 293T, and CD20 293T cells were transduced with Sindbis or Sindbis DMNJ virus at a concentration of 20 ng of HIV p24/ml.
Blocking of transduction by mannan and an anti-DC-SIGN antibody.
DC-SIGN 293T and DC-SIGN Jurkat cells (1 × 105) were incubated with mannan (200 μg/ml), control anti-HLA class I antibody clone W6/32 (20 μg/ml), or anti-DC-SIGN antibody clone 120615 (R&D Systems) (20 μg/ml) for 30 min before transduction. The cells were then infected with the 2.2 1L1L DMNJ pseudotype for 2 h in the presence of blocking reagents. EGFP expression was assayed by flow cytometry 3 days posttransduction. All experiments were performed in triplicate, and the average values with standard deviations are shown.
Analysis of N-glycan mutants.
The lentiviral vectors pseudotyped with 2.2 DMNJ, 2.2 ΔE1-139N DMNJ, 2.2 ΔE2-196N DMNJ, or 2.2 ΔE2-196N E1-139N DMNJ were conjugated with anti-HLA class I antibody (2 μg/ml) and used for the transduction of Jurkat cells to adjust the transduction units of each virus in an antibody-dependent and N-glycan-independent manner. The same titer of each virus was used for the transduction of DC-SIGN Jurkat cells without antibody conjugation to quantitate their titers in an N-glycan-dependent and antibody-independent manner. EGFP expression was analyzed by flow cytometry 3 days posttransduction. The N-glycan-dependent titers were assessed by three independent transduction experiments, and the average values with standard deviations are shown.
Transduction of human primary dendritic cells.
Human peripheral blood mononuclear cells were obtained from healthy normal donors by density gradient centrifugation, and dendritic cells were prepared by culturing the adherent fraction with 800 IU/ml granulocyte-macrophage colony-stimulating factor (GM-CSF; Bayer Healthcare, Seattle, WA) and 63 ng/ml interleukin-4 (IL-4; R&D Systems) for 7 days at 37°C as previously described (44). Dendritic cells were recovered on day 7 and purified by negative depletion using a MAb cocktail (anti-CD3, anti-CD19, and anti-CD56; BD Biosciences) and anti-mouse Ig-conjugated immunomagnetic beads (Invitrogen). The surface markers of isolated cells were confirmed by flow cytometry after staining the cells with antibodies against various dendritic cell markers, including the anti-HLA DR antibody conjugated with fluorescein isothiocyanate (FITC), anti-CD86 antibody conjugated with PE, anti-CD11c antibody conjugated with allophycocyanin (APC), and anti-DC-SIGN antibody conjugated with APC. The dendritic cells (2 × 105) were incubated for 1 h with mannan (200 μg/ml), control antibody (20 μg/ml), anti-DC-SIGN antibody (20 μg/ml), or nevirapine (5 μM). The cells were then infected with the vesicular stomatitis virus glycoprotein (VSV-G), 2.2 1L1L, or 2.2 1L1L DMNJ pseudotype (2.5 μg p24) for 6 h in the presence of blocking reagents. EGFP expression was assayed by flow cytometry 4 days posttransduction.
Inactivation of the vectors by complement.
Two lots of human AB serum (Sigma-Aldrich) were used. Heat-inactivated serum was prepared by incubating the serum at 56°C for 40 min. The vectors pseudotyped with the wild-type Sindbis virus envelope, 2.2 1L1L DMNJ, or 2.2 conjugated with anti-DC-SIGN antibody (50 μg/ml) (virus amount, 100 μl; 5 μg HIV p24/ml) were incubated with 100 μl of serum or heat-inactivated serum at 37°C for 1 h. Each virus was diluted 50-fold with PBS supplemented with calcium and magnesium [PBS (+)] and then incubated with DC-SIGN 293T cells (1 × 105) at 37°C for 2 h. EGFP expression was assayed by flow cytometry 3 days posttransduction. All experiments were performed in triplicate. The transduction efficiency of the virus treated with heat-inactivated serum was considered 100%. The relative transduction efficiency was calculated as follows: (% EGFP transduction with blocking agent/% EGFP transduction without blocking agent) × 100.
RESULTS
DC-SIGN does not mediate transduction of lentiviral vectors pseudotyped with the Sindbis virus envelope protein.
We used lentiviral vectors pseudotyped with the Sindbis virus envelope protein or modified Sindbis virus envelope proteins (Fig. 1b). Sindbis virus has two envelope proteins, E1 and E2. E2 mediates binding to its receptors, heparan sulfate, the laminin receptor, and other unknown receptors (10, 46, 67, 90). E1 mediates fusion between the target cell membrane and the viral envelope (80). E1 has an N-glycan at both aa 139 and 245. E2 also has an N-glycan at both aa 196 and 318 (9, 48). 2.2 1L1L is the envelope protein derived from wild-type Sindbis virus and contains mutations at E3 aa 61 to 64; E2 aa 70 to 74, 159, and 160; and E1 aa 247 and 248. Mutations at E3 aa 61 to 64 and E2 aa 70 to 74 eliminate the original receptor-binding regions of the wild-type Sindbis virus, and mutations at E2 aa 159 and 160 and E1 aa 226 and 227 increase the titers of the pseudotyped virus by unknown mechanisms when the pseudotyped virus is artificially bound to target cells (61, 63-66, 80). 2.2 is the envelope protein containing the same mutations as 2.2 1L1L, but it contains an insertion of the Fc-binding region (ZZ domain) of protein A (72). 2.2 can be conjugated with antibodies via interactions between the ZZ domain and the Fc region of antibodies. Conjugated antibodies can mediate the binding of pseudotyped vectors to target cells.
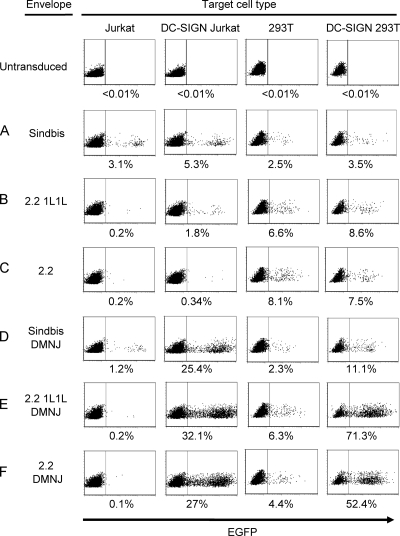
Previous studies have shown that N-glycans at E2 aa 196 and E1 aa 139 are exposed at the surface of the envelope protein, while those at E2 aa 318 and E2 aa 245 are inaccessible, in that they are buried within the trimer of the heterodimers of E1 and E2 (74, 94). The majority of the exposed N-glycans are known to have complex structures when the proteins are generated in mammalian cells, including BHK cells, hamster fibroblasts, and chicken embryonic fibroblasts (40, 58). If the N-glycan structures of lentiviral vectors pseudotyped with Sindbis virus, 2.2 1L1L, or 2.2 are the same as those of replication-competent Sindbis virus produced in the above-mentioned cells, those vectors will not efficiently bind to DC-SIGN, which is the lectin specific to the high-mannose structure of N-glycans (47). To confirm this, we transduced cells expressing DC-SIGN with the lentiviral vectors pseudotyped with these envelope proteins. We generated DC-SIGN-expressing 293T and Jurkat cells by transducing them with a DC-SIGN expression vector and designated them DC-SIGN 293T and DC-SIGN Jurkat cells, respectively (Fig. 2). As a control for the artificial expression of transduced molecules, we generated CD20 293T cells by the transduction of a CD20 expression vector that expresses human CD20. As expected, DC-SIGN expression does not significantly increase the transduction efficiency of 293T cells with the lentiviral vectors pseudotyped with Sindbis virus (Fig. 3 A and Table 1), 2.2 1L1L (Fig. 3B and Table 1), or 2.2 (Fig. 3C and Table 1). We observed slight increases in the transduction of DC-SIGN Jurkat cells compared to that of Jurkat cells, although this increase was not obvious in the experiments using 293T and DC-SIGN 293T cells. These observations are consistent with previous studies showing that only a small portion of the exposed N-glycans at E2 aa 196 and E1 aa 139 have high-mannose structure N-glycan cells (40, 58). The transduction mediated by the interaction between DC-SIGN and envelope proteins is 10-fold lower than the transduction efficiency mediated by anti-DC-SIGN antibodies conjugated with 2.2 (Table 1). We also examined lentiviral vectors pseudotyped with the various types of modified Sindbis virus envelope proteins that we previously described (61, 63-66). None of them efficiently transduced target cells through binding to DC-SIGN (data not shown).
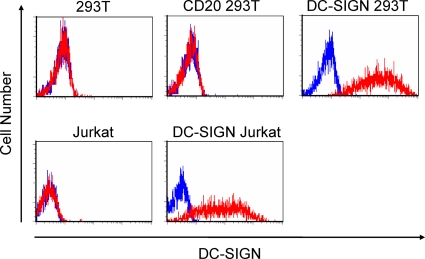
FIG. 2.
Expression of DC-SIGN on various cells, including Jurkat (human T-cell line), DC-SIGN Jurkat (Jurkat cells transduced with a DC-SIGN expression vector), 293T (human embryonic kidney cell line), CD20 293T (293T cells transduced with a CD20 expression vector), and DC-SIGN 293T (293T cells transduced with a DC-SIGN expression vector) cells. Each cell type was stained with either PE-conjugated isotype control antibody (blue line) or PE-conjugated anti-DC-SIGN antibody (red line).
FIG. 3.
Transduction of cells with lentiviral vectors pseudotyped with various types of envelope proteins. Jurkat, DC-SIGN Jurkat, 293T, DC-SIGN 293T, and CD20 293T cells were transduced with HIV vectors pseudotyped with (A) Sindbis virus, (B) 2.2 1L1L, (C) 2.2, (D) Sindbis virus DMNJ (Sindbis virus pseudotype produced in the presence of DMNJ), (E) 2.2 1L1L DMNJ (2.2 1L1L pseudotype produced in the presence of DMNJ), or (F) 2.2 DMNJ (2.2 pseudotype produced in the presence of DMNJ). EGFP expression was analyzed by flow cytometry 3 days posttransduction. The percentage of EGFP-positive cells is shown as an average of triplicate experiments. The y axis is forward scatter, and the x axis is EGFP expression. The virus amounts used for transduction are shown in Table 1.
Production of pseudotyped vectors in the presence of DMNJ redirects the vectors to DC-SIGN.
One study showed that replication-competent Sindbis virus cannot use DC-SIGN as its receptor when the virus is generated in mammalian cells but can when the virus is instead generated in insect cells. This difference is explained by the fact that insect cells do not produce N-glycans of the complex structure; therefore, all of the N-glycans of the virus produced in insect cells have either the high-mannose or paucimannosidic structure (Fig. 1a) (3). Both structures contain terminal mannose residues that enable their envelopes to bind DC-SIGN (47). Since lentiviral vectors require cellular factors present in human cells for proper virion formation, the vector cannot be generated in insect cells (4).
N-glycans are first synthesized as high-mannose structures, and then certain N-glycans undergo several further steps to become complex structures. DMNJ is an inhibitor of mannosidase I that is required for the formation of N-glycan complex structures (25). Thus, N-glycans produced in the presence of DMNJ retain their high-mannose structure. In fact, replication-competent Sindbis virus produced from mammalian cells in the presence of DMNJ can use DC-SIGN as its receptor (46).
We hypothesized that lentiviral vectors pseudotyped with Sindbis virus envelope protein or its derivative produced in the presence of DMNJ will convert the structures of exposed N-glycans of the envelope proteins from complex to high mannose, so the virus would use DC-SIGN as its receptor. We therefore produced lentiviral vectors pseudotyped with the wild-type Sindbis virus envelope, 2.2 1L1L, or 2.2 in the presence of DMNJ and designated them Sindbis virus (DMNJ), 2.2 1L1L (DMNJ), and 2.2 (DMNJ), respectively. We infected 293T, CD20 293T, CD20 293T, Jurkat, and DC-SIGN Jurkat cells with these viruses.
Unlike the virus produced in the absence of DMNJ, expression of DC-SIGN drastically increased the transduction efficiency of Sindbis virus (DMNJ) (Fig. 3D), 2.2 1L1L (DMNJ) (Fig. 3E), and 2.2 (DMNJ) (Fig. 3F) (Table 1). 2.2 (DMNJ) transduced DC-SIGN-expressing cells more efficiently than 2.2 conjugated with the anti-DC-SIGN antibody. We also examined this phenomenon by using other derivatives of Sindbis virus envelope proteins (previously published), including BAP SINDBIS, 4C-RGD SINDBIS, m168, and ZZ SINDBIS. Although these envelope proteins have different amino acid mutations and insertions, none have any mutations in the N-glycosylation signals. DMNJ redirected all pseudotypes to DC-SIGN, regardless of their mutations and insertions (data not shown).
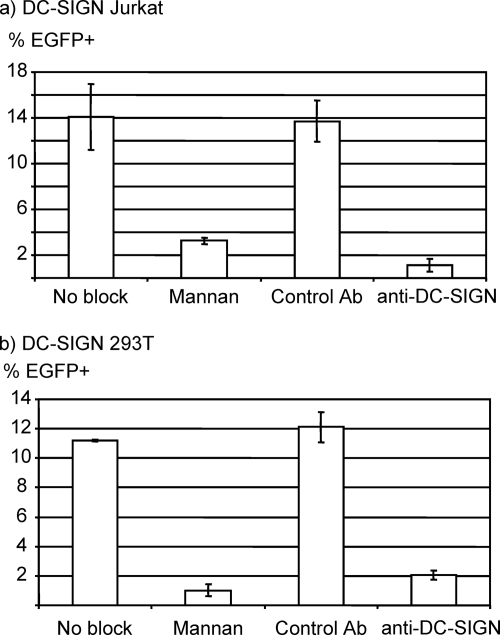
We next confirmed that this enhanced transduction was mediated by the interaction between DC-SIGN and N-glycans. We examined whether anti-DC-SIGN antibody or mannan (a polymer of mannose) can inhibit this enhanced transduction (Fig. 4). Anti-DC-SIGN antibody and mannan both specifically blocked the transduction of DC-SIGN-expressing cells by DMNJ viruses, demonstrating that the binding of these viruses is mediated by the interaction between N-glycans and DC-SIGN. Transduction of DC-SIGN 293T cells with the 2.2 1L1L DMNJ pseudotypes was also blocked by bafilomycin A (data not shown), which prevents acidification of the endosomes, demonstrating that the DMNJ-treated virus-DC-SIGN-expressing cell fusion step occurs in a pH-dependent manner, similar to the fusion of Sindbis virus and its target cells (52, 31).
FIG. 4.
Blocking of transduction by mannan and anti-DC-SIGN antibody. DC-SIGN Jurkat (a) and DC-SIGN 293T (b) cells were each incubated with mannan (polymer of mannose sugar), isotype control antibody, or anti-DC-SIGN antibody before and during transduction with the HIV vector pseudotyped with 2.2 1L1L DMNJ (1 ng of p24 for DC-SIGN Jurkat and 200 pg of p24 for DC-SIGN 293T cells). EGFP expression was analyzed 3 days posttransduction. The percentage of EGFP-positive cells is shown as an average of triplicate experiments with standard deviations.
N-glycans at E1 aa 139 and E2 aa 196 mediate binding to DC-SIGN.
Envelope viruses and viral vectors incorporate a wide variety of cell surface molecules on their envelopes, and these cellular molecules have been shown to mediate the binding of virus to target cells. Because DMNJ can change the N-glycan structures of incorporated cellular molecules to the high-mannose structure, binding to DC-SIGN could be mediated by incorporated cellular molecules rather than viral envelope proteins. To investigate whether the N-glycans of the modified Sindbis virus envelope proteins mediate binding to DC-SIGN, we eliminated the N-glycans of the envelope protein.
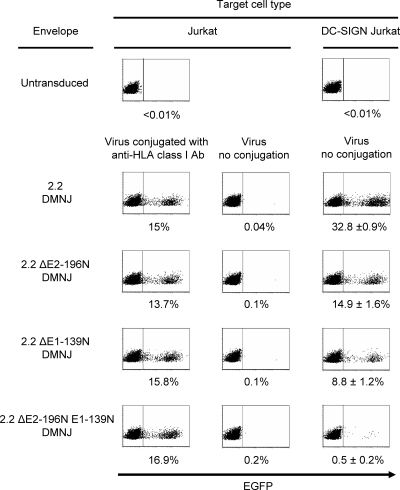
Cryoelectron microscopy (cryo-EM) analysis showed that E1 aa 139 and E2 aa 196 are exposed on the surface of the Sindbis virus envelope protein (74, 94). We investigated whether N-glycans at these positions are exposed and mediate binding to DC-SIGN. We introduced mutations in 2.2 at E1 aa 139, E2 aa 196, or both and designated them 2.2 ΔE1-139N, 2.2 ΔE2-196N, and 2.2 ΔE2-196N E1-139N, respectively (Fig. 1b). We generated lentiviral vectors pseudotyped with 2.2, 2.2 ΔE1-139N, 2.2 ΔE2-196N, or 2.2 ΔE2-196N E1-139N in the presence of DMNJ and designated them 2.2 (DMNJ), 2.2 ΔE1-139N (DMNJ), 2.2 ΔE2-196N (DMNJ), and 2.2 ΔE2-196N E1-139N (DMNJ), respectively. Each virus was titrated on Jurkat cells, with conjugation of anti-HLA class I antibody to confirm that each virus had the same infectivity in a DC-SIGN-independent manner (Fig. 5). We transduced DC-SIGN Jurkat cells with each virus, without antibody conjugation, using the same titers as calculated for the transduction of Jurkat cells. Elimination of N-glycans at E1 aa 139 and E2 aa 196 reduced the transduction of DC-SIGN Jurkat cells 73% (from 33% to 9%) and 55% (from 33% to 15%), respectively. When both N-glycans were eliminated, the transduction of DC-SIGN Jurkat cells was reduced 98% (from 33% to 0.5%). These results demonstrated that N-glycans at E1 aa 139 and E2 aa 196, but not cellular molecules incorporated into virions, mediate binding to DC-SIGN. The results also confirmed the cryo-EM analysis showing that N-glycans at those two sites are exposed on the surface of the Sindbis virus envelope protein. These results and data in Fig. 3 and Table 1 also suggest that the structures of N-glycans at E1 aa 139 and E2 aa 196 are complex if DMNJ is absent during virus production.
FIG. 5.
Identification of N-glycans that bind DC-SIGN. HIV vectors pseudotyped with 2.2, 2.2 ΔE2-196N, 2.2 ΔE1-139N, or 2.2 ΔE2-196N E1-139N were produced in the presence of DMNJ and designated 2.2 DMNJ, 2.2 ΔE2-196N DMNJ, 2.2 ΔE1-139N DMNJ, and 2.2 ΔE2-196N E1-139N DMNJ, respectively. The viruses were titrated on Jurkat cells by conjugating them with the antibody against HLA class I, which is abundantly expressed on Jurkat cells. The same titer of each virus was used to transduce DC-SIGN Jurkat cells without conjugation of an antibody. The p24 values of the viruses used for transduction are 2, 2, 8, and 8 ng for 2.2 DMNJ, 2.2 ΔE2-196N DMNJ, 2.2 ΔE1-139N DMNJ, and 2.2 ΔE2-196N E1-139N DMNJ pseudotype, respectively. EGFP expression was analyzed by flow cytometry 3 days posttransduction. The percentage of EGFP-positive cells is shown as an average of triplicate experiments with standard deviations. The y axis is forward scatter, and the x axis is EGFP expression.
Lentiviral vectors redirected by N-glycan modification can efficiently transduce human primary dendritic cells.
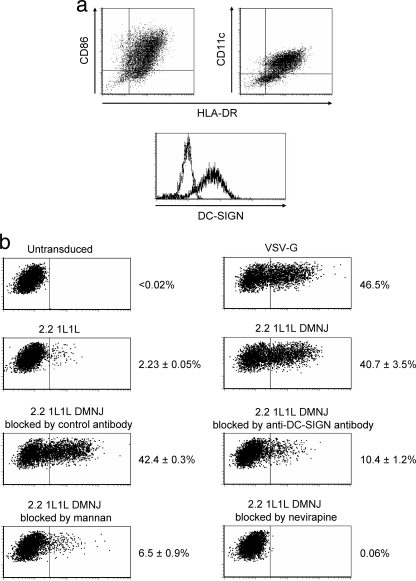
We examined whether modification of N-glycans can redirect lentiviral vectors to human primary dendritic cells, which physiologically express DC-SIGN both in vivo and in vitro. Human primary dendritic cells were prepared by in vitro differentiation of monocytes using GM-CSF and IL-4. On the day of transduction, most of the cells express the dendritic cell surface markers (HLA-DR, CD11c, and CD86) and DC-SIGN (Fig. 6 a). The cells were transduced with the same amount of lentiviral vectors pseudotyped with VSV-G, 2.2 1L1L, or 2.2 1L1L DMNJ (Fig. 6b). The transduction efficiency of the 2.2 1L1L pseudotype was 20-fold less than that of the VSV-G pseudotype. The 2.2 1L1L DMNJ pseudotype transduced cells 18-fold more than the 2.2 1L1L pseudotype, demonstrating that modification of N-glycan increased the transduction efficiency of primary DC-SIGN-expressing cells. The EGFP expression of cells transduced with the 2.2 1L1L pseudotype was completely blocked by the reverse transcriptase inhibitor nevirapine, which excludes the possibility of pseudotransduction mediated by the binding of virion-associated EGFP to target cells. Transduction of dendritic cells with the 2.2 1L1L DMNJ pseudotype was blocked by mannan and the anti-DC-SIGN antibody, demonstrating that the high-mannose structure N-glycans and DC-SIGN are involved in this transduction. These experiments also showed that the targeting lentiviral vectors redirected by N-glycan modification have titers similar to those of VSV-G-pseudotyped lentiviral vectors, which have been most commonly used due to their high titers on wide varieties of cell types.
FIG. 6.
(a) Expression of surface markers on human dendritic cells. Dendritic cells were prepared by culturing the adherent fraction of peripheral blood mononuclear cells in GM-SCF and IL-4 and then depleting T, B, and NK cells using anti-CD3, -CD19, and -CD56 antibodies and anti-mouse IgG-conjugated immunomagnetic beads. The cells were costained with the anti-HLA DR antibody conjugated with FITC, the anti-CD86 antibody conjugated with PE, and the anti-CD11c antibody conjugated with APC. The cells were also stained with the anti-DC-SIGN antibody conjugated with APC. All staining was performed in the presence of human AB serum to block nonspecific staining of Fc receptors, and isotype control antibodies conjugated with FITC, PE, or APC were used as negative controls for staining. (b) Dendritic cells (2 × 105) were incubated for 1 h with mannan (200 μg/ml), control antibody (20 μg/ml), anti-DC-SIGN antibody (20 μg/ml), or nevirapine (5 μM). The cells were then infected with the VSV-G, 2.2 1L1L, or 2.2 1L1L DMNJ pseudotype (2.5 μg p24) for 6 h in the presence of blocking reagents. EGFP expression was assayed by flow cytometry 4 days posttransduction. Experiments necessary to show statistically significant differences were performed in triplicate, and averages of the triplicate experiments are shown with standard deviations.
Lentiviral vectors redirected by N-glycan modification are resistant to human serum complement.
Serum complements can attack pathogens by several mechanisms, including classical, lectin, and alternative pathways (78). The complement can also inactivate lentiviral vectors by these mechanisms (34, 42, 81). Inactivation of viral vectors by complement should be avoided to maintain the titers of vectors in vivo. High-mannose structure N-glycans can be related to pathogens derived from nonmammalian cells and could activate complement via the lectin pathway (41). Mannose-binding lectin (MBL), which plays important roles in the lectin pathway of complement activation, is a serum C-type lectin that can bind to mannose residues of N-glycans (24). It was also shown that MBL binds to N-glycans of the Ebola and Marburg virus envelope proteins at the sites where DC-SIGN binds (42). Therefore, there was concern as to whether redirecting lentiviral vectors by modification of N-glycans could change the susceptibility to inactivation by serum complement.
To investigate this, the titers of virus treated with complement serum or with complement-inactivated serum were compared. Some (35 to 50%) lentiviral vectors pseudotyped with wild-type Sindbis virus envelope protein were inactivated after 1 h of incubation with the same volume of human complement serum (Fig. 7). However, the 2.2 pseudotype redirected by the conjugation of an anti-DC-SIGN antibody was more severely inactivated by serum complement (more than 90%), probably via the classical pathway activated by the Fc region of the conjugated antibody. Therefore, redirecting vectors by antibody conjugation would be problematic when used in immunocompetent animals, including humans. In contrast, redirecting the virus by modification of N-glycans did not increase the susceptibility to complements, indicating that this targeting strategy can be applied to targeting in immunocompetent animals.
FIG. 7.
Two lots of human AB serum were used. Heat-inactivated serum was made by incubation at 56°C for 40 min. Wild-type Sindbis virus envelope protein, 2.2 1L1L DMNJ pseudotypes, and 2.2 pseudotype conjugated with anti-DC-SIGN antibody (Ab; 50 μg/ml) (virus amount, 100 μl; 5 μg HIV p24/ml) were incubated with 100 μl of serum or heat-inactivated serum at 37°C for 1 h. Each virus was diluted 50-fold with PBS (+) and then incubated with DC-SIGN 293T. EGFP expression was assayed by flow cytometry 3 days posttransduction. All experiments were performed in triplicate. The transduction efficiency of the virus treated with heat-inactivated serum was considered 100%. The relative transduction efficiency was calculated by the formula presented in Materials and Methods.
DISCUSSION
In this study, we showed that lentiviral vectors pseudotyped with wild-type or modified Sindbis virus envelope proteins could be redirected to bind to DC-SIGN by changing their N-glycan structures from complex to high mannose when produced in the presence of DMNJ. Using this redirecting strategy, we determined that the N-glycans at E2 aa 196 and E1 aa 139 of the envelope proteins are accessible to DC-SIGN, which supports previous cryo-EM imaging studies of the Sindbis virus envelope protein (74, 94). We further found that N-glycans modified by DMNJ do not activate serum complements. Our results suggest that this strategy to target DC-SIGN can be applied to target antigen-presenting dendritic cells for immunotherapy. Conversely, because this study showed that our Sindbis virus targeting envelope proteins do not interact with DC-SIGN unless generated in the presence of DMNJ, DC-SIGN-bearing cells will not be a target and will not contribute to the nonspecific transduction of our current targeting vectors.
We demonstrated that our targeting lentiviral vectors specifically transduced DC-SIGN-expressing cells when their N-glycan structure was modified to high mannose using DMNJ. This result is consistent with the results obtained with replication-competent Sindbis virus, which demonstrated that the virus cannot use DC-SIGN as its receptor when replicated in mammalian cells but can do so when replicated in insect cells, wherein all N-glycans contain terminal mannose residues (47). DMNJ has been used to change the N-glycan structures of other viral envelope proteins, which enables the envelope proteins to bind to DC-SIGN (47, 51, 55). The location of N-glycans of the Sindbis virus envelope protein was previously analyzed by cryo-EM. Our mutation analysis of N-glycan-linked amino acid residues is consistent with the cryo-EM structure (74, 94). Cryo-EM data demonstrated that the N-glycans at E2 aa 196 and E1 aa 139 are exposed while those at E2 aa 318 and E1 aa 245 are not. With replication-competent Sindbis virus, it is believed that the E1 protein mediates fusion between the envelope and the cellular membrane (80), whereas the E2 protein mediates binding to viral receptors. However, our functional analysis demonstrated that the E1 protein's N-glycan also mediates binding to the cellular receptor, DC-SIGN, when the N-glycan at aa 139 has the high-mannose structure.
Although modification of N-glycans efficiently redirected our targeting vectors, there was concern that changing N-glycans to high-mannose structures could potentially activate serum complement. MBL in serum can bind to high-mannose structure N-glycans on pathogens such as HIV and activate serum complement (24, 41, 42). Our results showed that modification of N-glycans did not activate complement. Resistance to complement will enable the use of viral vectors in immunocompetent animals; thus, N-glycan modification could be useful for targeted transduction in vivo.
One group demonstrated that lentiviral vectors pseudotyped with modified Sindbis virus envelope proteins without DMNJ treatment can use DC-SIGN as their receptor (92). The mutations in their envelope proteins were identical to those in ours, and no further modifications were made at or near the N-glycosylation sites. Thus, according to our studies, their envelope proteins should not be able to efficiently bind DC-SIGN. It is unclear why their results differ from ours. One possibility is that transduction in their experimental setting may be mediated by a mechanism other than interactions between high-mannose structure N-glycans and DC-SIGN. For example, lectins such as CD301, which is able to bind to complex-structure N-glycans, may bind lentiviral vectors pseudotyped with Sindbis virus envelope protein and its derivatives (85).
Dendritic cells are potent antigen-presenting cells and provide signals to stimulate immune cells, including cytotoxic T cells and antibody-producing B-cells, and play crucial roles in regulating immune reactions (84). Ex vivo transduction and in vivo transduction of antigen-presenting cells in a nonspecific fashion using VSV-G-pseudotyped lentiviral vectors have been shown to induce strong immune reactions against transgene products, including HIV proteins and tumor-specific antigens (37). Efficient transduction of antigen-presenting cells by intravenous injection of targeting vectors could be a convenient and efficient method for immunization against infectious diseases and cancers. Several groups also showed that transduction of antigen-presenting cells by lentiviral vectors induced tolerance to transgene products, although the mechanism by which this occurs is still not clear (14, 20, 45, 56). If antigen-presenting cell-targeting vectors can induce tolerance to self-antigens, they may also be a means to treat autoimmune diseases.
The results reported here demonstrated specific transduction of DC-SIGN-expressing cells by the redirected vectors in vitro; however, many types of cells express a variety of lectins that bind to high-mannose structure N-glycans in vivo (19, 87). For example, one DC-SIGN homologue, DC-SIGN R, is expressed on endothelial cells and liver sinusoidal cells (75). Therefore, intravenous administration of the DMNJ-treated virus would transduce not only macrophages and dendritic cells, which express DC-SIGN, but also other cell types expressing other lectins such as DC-SIGN R. Thus, for in vivo experiments using targeting vectors redirected by N-glycan modification, it would be difficult to predict precisely which cell types are transduced. To achieve even more specific transduction of antigen-presenting cells, targeting by N-glycans should be combined with other targeting strategies which we previously described (61, 63, 65), such as those using peptides and antibodies specific to antigen-presenting cells.
In contrast to targeting of DC-SIGN for purposes of immunization, one would want to avoid DC-SIGN to enhance the specificity of targeting of other cell types. Many attempts have been made to develop targeting retroviral vectors by using various types of envelope proteins, including avian sarcoma and leukosis virus (83), MLV (43) spleen necrosis virus (13), and measles virus (26) envelope proteins. Although the interactions between these envelope proteins and their native receptors have been intensively studied, interactions between lectins and the vectors have not been fully investigated. Several research groups have successfully redirected measles and lentiviral vectors by using modified measles virus envelope proteins that had mutations in the regions of binding for their original receptors, CD46 and CD150 (68). The measles virus envelope proteins produced in mammalian cells not treated with DMNJ have been shown to bind to DC-SIGN, in addition to CD46 and CD150 (17, 18). Since N-glycans of the targeting measles virus envelope proteins were not modified, the viruses and pseudotyped vectors are still able to bind to DC-SIGN-positive cells, potentially limiting the effectiveness of targeting in vivo. Indeed, several pathogens were shown to be captured in spleen marginal zones by DC-SIGN in vivo (28, 49, 73). Thus, preventing interaction with DC-SIGN is important for targeting vectors to specifically transduce target cells and tissues in vivo.
DC-SIGN captures pathogens and processes them for presentation on both major histocompatibility complex class I and II molecules to induce immune responses to the pathogens (21, 60, 82). Binding of DC-SIGN with pathogens also triggers signaling and cytokine production (11, 32, 38). Thus, binding of gene therapy vectors with DC-SIGN would enhance immune reactions to vectors and transgene products, followed by elimination of transduced cells. Thus, identifying the specific N-glycans mediating that interaction and eliminating them will be necessary to increase the effectiveness of targeting vectors and prolong transgene expression.
Because elimination of the original tropism of the Sindbis virus envelope protein has been the most important factor for decreasing nonspecific transduction by targeting vectors, the finding that our original targeting vectors do not interact efficiently with DC-SIGN is important for designing highly specific targeting vectors. Understanding the interactions between lectins and targeting viral vectors is important for both targeting and avoidance of specific cell types in vivo. Further studies of the interactions between targeting vectors and lectins would facilitate increasing the specificity of transduction by the vectors in in vivo settings.
Acknowledgments
We thank Benjamin E. Reubinoff and Michal Gropp (Hadassah-Hebrew University Medical Center, Jerusalem, Israel) for providing SIN18.cPPT.hEF1α.EGFP.WPRE. We thank the UCLA CFAR Virology Core Laboratory for the p24 ELISA.
This work was supported by NIH grants AI069350, CA120327, and NS055212; a UCLA Human Gene Medicine grant; and a CFAR Mentorship grant.
Footnotes
Published ahead of print on 19 May 2010.
REFERENCES
- 1.Alvarez, C. P., F. Lasala, J. Carrillo, O. Muniz, A. L. Corbi, and R. Delgado. 2002. C-type lectins DC-SIGN and L-SIGN mediate cellular entry by Ebola virus in cis and in trans. J. Virol. 76:6841-6844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barry, S. C., B. Harder, M. Brzezinski, L. Y. Flint, J. Seppen, and W. R. Osborne. 2001. Lentivirus vectors encoding both central polypurine tract and posttranscriptional regulatory element provide enhanced transduction and transgene expression. Hum. Gene Ther. 12:1103-1108. [DOI] [PubMed] [Google Scholar]
- 3.Betenbaugh, M. J., N. Tomiya, S. Narang, J. T. Hsu, and Y. C. Lee. 2004. Biosynthesis of human-type N-glycans in heterologous systems. Curr. Opin. Struct. Biol. 14:601-606. [DOI] [PubMed] [Google Scholar]
- 4.Bieniasz, P. D., and B. R. Cullen. 2000. Multiple blocks to human immunodeficiency virus type 1 replication in rodent cells. J. Virol. 74:9868-9877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown, B. D., A. Cantore, A. Annoni, L. S. Sergi, A. Lombardo, P. Della Valle, A. D'Angelo, and L. Naldini. 2007. A microRNA-regulated lentiviral vector mediates stable correction of hemophilia B mice. Blood 110:4144-4152. [DOI] [PubMed] [Google Scholar]
- 6.Brown, B. D., B. Gentner, A. Cantore, S. Colleoni, M. Amendola, A. Zingale, A. Baccarini, G. Lazzari, C. Galli, and L. Naldini. 2007. Endogenous microRNA can be broadly exploited to regulate transgene expression according to tissue, lineage and differentiation state. Nat. Biotechnol. 25:1457-1467. [DOI] [PubMed] [Google Scholar]
- 7.Brown, B. D., G. Sitia, A. Annoni, E. Hauben, L. S. Sergi, A. Zingale, M. G. Roncarolo, L. G. Guidotti, and L. Naldini. 2007. In vivo administration of lentiviral vectors triggers a type I interferon response that restricts hepatocyte gene transfer and promotes vector clearance. Blood 109:2797-2805. [DOI] [PubMed] [Google Scholar]
- 8.Brown, B. D., M. A. Venneri, A. Zingale, L. Sergi Sergi, and L. Naldini. 2006. Endogenous microRNA regulation suppresses transgene expression in hematopoietic lineages and enables stable gene transfer. Nat. Med. 12:585-591. [DOI] [PubMed] [Google Scholar]
- 9.Burke, D., and K. Keegstra. 1979. Carbohydrate structure of Sindbis virus glycoprotein E2 from virus grown in hamster and chicken cells. J. Virol. 29:546-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Byrnes, A. P., and D. E. Griffin. 1998. Binding of Sindbis virus to cell surface heparan sulfate. J. Virol. 72:7349-7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caparrós, E., P. Munoz, E. Sierra-Filardi, D. Serrano-Gomez, A. Puig-Kroger, J. L. Rodriguez-Fernandez, M. Mellado, J. Sancho, M. Zubiaur, and A. L. Corbi. 2006. DC-SIGN ligation on dendritic cells results in ERK and PI3K activation and modulates cytokine production. Blood 107:3950-3958. [DOI] [PubMed] [Google Scholar]
- 12.Ceccaldi, P. E., F. Delebecque, M. C. Prevost, A. Moris, J. P. Abastado, A. Gessain, O. Schwartz, and S. Ozden. 2006. DC-SIGN facilitates fusion of dendritic cells with human T-cell leukemia virus type 1-infected cells. J. Virol. 80:4771-4780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chu, T. H., I. Martinez, W. C. Sheay, and R. Dornburg. 1994. Cell targeting with retroviral vector particles containing antibody-envelope fusion proteins. Gene Ther. 1:292-299. [PubMed] [Google Scholar]
- 14.Cui, Y., J. Golob, E. Kelleher, Z. Ye, D. Pardoll, and L. Cheng. 2002. Targeting transgene expression to antigen-presenting cells derived from lentivirus-transduced engrafting human hematopoietic stem/progenitor cells. Blood 99:399-408. [DOI] [PubMed] [Google Scholar]
- 15.Davis, C. W., H. Y. Nguyen, S. L. Hanna, M. D. Sanchez, R. W. Doms, and T. C. Pierson. 2006. West Nile virus discriminates between DC-SIGN and DC-SIGNR for cellular attachment and infection. J. Virol. 80:1290-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Jong, M. A., L. de Witte, A. Bolmstedt, Y. van Kooyk, and T. B. Geijtenbeek. 2008. Dendritic cells mediate herpes simplex virus infection and transmission through the C-type lectin DC-SIGN. J. Gen. Virol. 89:2398-2409. [DOI] [PubMed] [Google Scholar]
- 17.de Witte, L., M. Abt, S. Schneider-Schaulies, Y. van Kooyk, and T. B. Geijtenbeek. 2006. Measles virus targets DC-SIGN to enhance dendritic cell infection. J. Virol. 80:3477-3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Witte, L., R. D. de Vries, M. van der Vlist, S. Yuksel, M. Litjens, R. L. de Swart, and T. B. Geijtenbeek. 2008. DC-SIGN and CD150 have distinct roles in transmission of measles virus from dendritic cells to T-lymphocytes. PLoS Pathog. 4:e1000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Witte, L., A. Nabatov, M. Pion, D. Fluitsma, M. A. de Jong, T. de Gruijl, V. Piguet, Y. van Kooyk, and T. B. Geijtenbeek. 2007. Langerin is a natural barrier to HIV-1 transmission by Langerhans cells. Nat. Med. 13:367-371. [DOI] [PubMed] [Google Scholar]
- 20.Dresch, C., S. L. Edelmann, P. Marconi, and T. Brocker. 2008. Lentiviral-mediated transcriptional targeting of dendritic cells for induction of T cell tolerance in vivo. J. Immunol. 181:4495-4506. [DOI] [PubMed] [Google Scholar]
- 21.Engering, A., T. B. Geijtenbeek, S. J. van Vliet, M. Wijers, E. van Liempt, N. Demaurex, A. Lanzavecchia, J. Fransen, C. G. Figdor, V. Piguet, and Y. van Kooyk. 2002. The dendritic cell-specific adhesion receptor DC-SIGN internalizes antigen for presentation to T cells. J. Immunol. 168:2118-2126. [DOI] [PubMed] [Google Scholar]
- 22.Feinberg, H., R. Castelli, K. Drickamer, P. H. Seeberger, and W. I. Weis. 2007. Multiple modes of binding enhance the affinity of DC-SIGN for high mannose N-linked glycans found on viral glycoproteins. J. Biol. Chem. 282:4202-4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feinberg, H., D. A. Mitchell, K. Drickamer, and W. I. Weis. 2001. Structural basis for selective recognition of oligosaccharides by DC-SIGN and DC-SIGNR. Science 294:2163-2166. [DOI] [PubMed] [Google Scholar]
- 24.Fraser, I. P., H. Koziel, and R. A. Ezekowitz. 1998. The serum mannose-binding protein and the macrophage mannose receptor are pattern recognition molecules that link innate and adaptive immunity. Semin. Immunol. 10:363-372. [DOI] [PubMed] [Google Scholar]
- 25.Fuhrmann, U., E. Bause, G. Legler, and H. Ploegh. 1984. Novel mannosidase inhibitor blocking conversion of high mannose to complex oligosaccharides. Nature 307:755-758. [DOI] [PubMed] [Google Scholar]
- 26.Funke, S., A. Maisner, M. D. Muhlebach, U. Koehl, M. Grez, R. Cattaneo, K. Cichutek, and C. J. Buchholz. 2008. Targeted cell entry of lentiviral vectors. Mol. Ther. 16:1427-1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geijtenbeek, T. B., J. den Dunnen, and S. I. Gringhuis. 2009. Pathogen recognition by DC-SIGN shapes adaptive immunity. Future Microbiol. 4:879-890. [DOI] [PubMed] [Google Scholar]
- 28.Geijtenbeek, T. B., P. C. Groot, M. A. Nolte, S. J. van Vliet, S. T. Gangaram-Panday, G. C. van Duijnhoven, G. Kraal, A. J. van Oosterhout, and Y. van Kooyk. 2002. Marginal zone macrophages express a murine homologue of DC-SIGN that captures blood-borne antigens in vivo. Blood 100:2908-2916. [DOI] [PubMed] [Google Scholar]
- 29.Geijtenbeek, T. B., D. S. Kwon, R. Torensma, S. J. van Vliet, G. C. van Duijnhoven, J. Middel, I. L. Cornelissen, H. S. Nottet, V. N. KewalRamani, D. R. Littman, C. G. Figdor, and Y. van Kooyk. 2000. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell 100:587-597. [DOI] [PubMed] [Google Scholar]
- 30.Geijtenbeek, T. B., R. Torensma, S. J. van Vliet, G. C. van Duijnhoven, G. J. Adema, Y. van Kooyk, and C. G. Figdor. 2000. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell 100:575-585. [DOI] [PubMed] [Google Scholar]
- 31.Glomb-Reinmund, S., and M. Kielian. 1998. The role of low pH and disulfide shuffling in the entry and fusion of Semliki Forest virus and Sindbis virus. Virology 248:372-381. [DOI] [PubMed] [Google Scholar]
- 32.Gringhuis, S. I., J. den Dunnen, M. Litjens, B. van Het Hof, Y. van Kooyk, and T. B. Geijtenbeek. 2007. C-type lectin DC-SIGN modulates Toll-like receptor signaling via Raf-1 kinase-dependent acetylation of transcription factor NF-kappaB. Immunity 26:605-616. [DOI] [PubMed] [Google Scholar]
- 33.Gropp, M., P. Itsykson, O. Singer, T. Ben-Hur, E. Reinhartz, E. Galun, and B. E. Reubinoff. 2003. Stable genetic modification of human embryonic stem cells by lentiviral vectors. Mol. Ther. 7:281-287. [DOI] [PubMed] [Google Scholar]
- 34.Guibinga, G. H., and T. Friedmann. 2005. Baculovirus GP64-pseudotyped HIV-based lentivirus vectors are stabilized against complement inactivation by codisplay of decay accelerating factor (DAF) or of a GP64-DAF fusion protein. Mol. Ther. 11:645-651. [DOI] [PubMed] [Google Scholar]
- 35.Hacker, K., L. White, and A. M. de Silva. 2009. N-linked glycans on dengue viruses grown in mammalian and insect cells. J. Gen. Virol. 90:2097-2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Halary, F., A. Amara, H. Lortat-Jacob, M. Messerle, T. Delaunay, C. Houles, F. Fieschi, F. Arenzana-Seisdedos, J. F. Moreau, and J. Dechanet-Merville. 2002. Human cytomegalovirus binding to DC-SIGN is required for dendritic cell infection and target cell trans-infection. Immunity 17:653-664. [DOI] [PubMed] [Google Scholar]
- 37.He, Y., and L. D. Falo, Jr. 2007. Lentivirus as a potent and mechanistically distinct vector for genetic immunization. Curr. Opin. Mol. Ther. 9:439-446. [PMC free article] [PubMed] [Google Scholar]
- 38.Hodges, A., K. Sharrocks, M. Edelmann, D. Baban, A. Moris, O. Schwartz, H. Drakesmith, K. Davies, B. Kessler, A. McMichael, and A. Simmons. 2007. Activation of the lectin DC-SIGN induces an immature dendritic cell phenotype triggering Rho-GTPase activity required for HIV-1 replication. Nat. Immunol. 8:569-577. [DOI] [PubMed] [Google Scholar]
- 39.Hsieh, P., and P. W. Robbins. 1984. Regulation of asparagine-linked oligosaccharide processing. Oligosaccharide processing in Aedes albopictus mosquito cells. J. Biol. Chem. 259:2375-2382. [PubMed] [Google Scholar]
- 40.Hubbard, S. C. 1988. Regulation of glycosylation. The influence of protein structure on N-linked oligosaccharide processing. J. Biol. Chem. 263:19303-19317. [PubMed] [Google Scholar]
- 41.Ji, X., H. Gewurz, and G. T. Spear. 2005. Mannose binding lectin (MBL) and HIV. Mol. Immunol. 42:145-152. [DOI] [PubMed] [Google Scholar]
- 42.Ji, X., G. G. Olinger, S. Aris, Y. Chen, H. Gewurz, and G. T. Spear. 2005. Mannose-binding lectin binds to Ebola and Marburg envelope glycoproteins, resulting in blocking of virus interaction with DC-SIGN and complement-mediated virus neutralization. J. Gen. Virol. 86:2535-2542. [DOI] [PubMed] [Google Scholar]
- 43.Kasahara, N., A. M. Dozy, and Y. W. Kan. 1994. Tissue-specific targeting of retroviral vectors through ligand-receptor interactions. Science 266:1373-1376. [DOI] [PubMed] [Google Scholar]
- 44.Kiertscher, S. M., and M. D. Roth. 1996. Human CD14+ leukocytes acquire the phenotype and function of antigen-presenting dendritic cells when cultured in GM-CSF and IL-4. J. Leukoc. Biol. 59:208-218. [DOI] [PubMed] [Google Scholar]
- 45.Kimura, T., R. C. Koya, L. Anselmi, C. Sternini, H. J. Wang, B. Comin-Anduix, R. M. Prins, E. Faure-Kumar, N. Rozengurt, Y. Cui, N. Kasahara, and R. Stripecke. 2007. Lentiviral vectors with CMV or MHCII promoters administered in vivo: immune reactivity versus persistence of expression. Mol. Ther. 15:1390-1399. [DOI] [PubMed] [Google Scholar]
- 46.Klimstra, W. B., H. W. Heidner, and R. E. Johnston. 1999. The furin protease cleavage recognition sequence of Sindbis virus PE2 can mediate virion attachment to cell surface heparan sulfate. J. Virol. 73:6299-6306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Klimstra, W. B., E. M. Nangle, M. S. Smith, A. D. Yurochko, and K. D. Ryman. 2003. DC-SIGN and L-SIGN can act as attachment receptors for alphaviruses and distinguish between mosquito cell- and mammalian cell-derived viruses. J. Virol. 77:12022-12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Knight, R. L., K. L. Schultz, R. J. Kent, M. Venkatesan, and D. E. Griffin. 2009. Role of N-linked glycosylation for Sindbis virus infection and replication in vertebrate and invertebrate systems. J. Virol. 83:5640-5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Koppel, E. A., C. W. Wieland, V. C. van den Berg, M. Litjens, S. Florquin, Y. van Kooyk, T. van der Poll, and T. B. Geijtenbeek. 2005. Specific ICAM-3 grabbing nonintegrin-related 1 (SIGNR1) expressed by marginal zone macrophages is essential for defense against pulmonary Streptococcus pneumoniae infection. Eur. J. Immunol. 35:2962-2969. [DOI] [PubMed] [Google Scholar]
- 50.Lee, B., G. Leslie, E. Soilleux, U. O'Doherty, S. Baik, E. Levroney, K. Flummerfelt, W. Swiggard, N. Coleman, M. Malim, and R. W. Doms. 2001. cis expression of DC-SIGN allows for more efficient entry of human and simian immunodeficiency viruses via CD4 and a coreceptor. J. Virol. 75:12028-12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Levroney, E. L., H. C. Aguilar, J. A. Fulcher, L. Kohatsu, K. E. Pace, M. Pang, K. B. Gurney, L. G. Baum, and B. Lee. 2005. Novel innate immune functions for galectin-1: galectin-1 inhibits cell fusion by Nipah virus envelope glycoproteins and augments dendritic cell secretion of proinflammatory cytokines. J. Immunol. 175:413-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liang, M., K. Morizono, N. Pariente, M. Kamata, B. Lee, and I. S. Chen. 2009. Targeted transduction via CD4 by a lentiviral vector uses a clathrin-mediated entry pathway. J. Virol. 83:13026-13031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liang, M., N. Pariente, K. Morizono, and I. S. Chen. 2009. Targeted transduction of CD34+ hematopoietic progenitor cells in nonpurified human mobilized peripheral blood mononuclear cells. J. Gene Med. 11:185-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liedtke, S., M. Adamski, R. Geyer, A. Pfutzner, H. Rubsamen-Waigmann, and H. Geyer. 1994. Oligosaccharide profiles of HIV-2 external envelope glycoprotein: dependence on host cells and virus isolates. Glycobiology 4:477-484. [DOI] [PubMed] [Google Scholar]
- 55.Lin, G., G. Simmons, S. Pöhlmann, F. Baribaud, H. Ni, G. J. Leslie, B. S. Haggarty, P. Bates, D. Weissman, J. A. Hoxie, and R. W. Doms. 2003. Differential N-linked glycosylation of human immunodeficiency virus and Ebola virus envelope glycoproteins modulates interactions with DC-SIGN and DC-SIGNR. J. Virol. 77:1337-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marodon, G., S. Fisson, B. Levacher, M. Fabre, B. L. Salomon, and D. Klatzmann. 2006. Induction of antigen-specific tolerance by intrathymic injection of lentiviral vectors. Blood 108:2972-2978. [DOI] [PubMed] [Google Scholar]
- 57.Marzi, A., T. Gramberg, G. Simmons, P. Moller, A. J. Rennekamp, M. Krumbiegel, M. Geier, J. Eisemann, N. Turza, B. Saunier, A. Steinkasserer, S. Becker, P. Bates, H. Hofmann, and S. Pöhlmann. 2004. DC-SIGN and DC-SIGNR interact with the glycoprotein of Marburg virus and the S protein of severe acute respiratory syndrome coronavirus. J. Virol. 78:12090-12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mayne, J. T., J. R. Bell, E. G. Strauss, and J. H. Strauss. 1985. Pattern of glycosylation of Sindbis virus envelope proteins synthesized in hamster and chicken cells. Virology 142:121-133. [DOI] [PubMed] [Google Scholar]
- 59.Mingozzi, F., M. V. Maus, D. J. Hui, D. E. Sabatino, S. L. Murphy, J. E. Rasko, M. V. Ragni, C. S. Manno, J. Sommer, H. Jiang, G. F. Pierce, H. C. Ertl, and K. A. High. 2007. CD8(+) T-cell responses to adeno-associated virus capsid in humans. Nat. Med. 13:419-422. [DOI] [PubMed] [Google Scholar]
- 60.Moris, A., C. Nobile, F. Buseyne, F. Porrot, J. P. Abastado, and O. Schwartz. 2004. DC-SIGN promotes exogenous MHC-I-restricted HIV-1 antigen presentation. Blood 103:2648-2654. [DOI] [PubMed] [Google Scholar]
- 61.Morizono, K., G. Bristol, Y. M. Xie, S. K. Kung, and I. S. Chen. 2001. Antibody-directed targeting of retroviral vectors via cell surface antigens. J. Virol. 75:8016-8020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Morizono, K., and I. S. Chen. 2005. Targeted gene delivery by intravenous injection of retroviral vectors. Cell Cycle 4:854-856. [DOI] [PubMed] [Google Scholar]
- 63.Morizono, K., N. Pariente, Y. Xie, and I. S. Chen. 2009. Redirecting lentiviral vectors by insertion of integrin-tageting peptides into envelope proteins. J. Gene Med. 11:549-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Morizono, K., G. E. Ringpis, N. Pariente, Y. Xie, and I. S. Chen. 2006. Transient low pH treatment enhances infection of lentiviral vector pseudotypes with a targeting Sindbis envelope. Virology 355:71-81. [DOI] [PubMed] [Google Scholar]
- 65.Morizono, K., Y. Xie, G. Helguera, T. R. Daniels, T. F. Lane, M. L. Penichet, and I. S. Chen. 2009. A versatile targeting system with lentiviral vectors bearing the biotin-adaptor peptide. J. Gene Med. 11:655-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Morizono, K., Y. Xie, G. E. Ringpis, M. Johnson, H. Nassanian, B. Lee, L. Wu, and I. S. Chen. 2005. Lentiviral vector retargeting to P-glycoprotein on metastatic melanoma through intravenous injection. Nat. Med. 11:346-352. [DOI] [PubMed] [Google Scholar]
- 67.Myles, K. M., D. J. Pierro, and K. E. Olson. 2003. Deletions in the putative cell receptor-binding domain of Sindbis virus strain MRE16 E2 glycoprotein reduce midgut infectivity in Aedes aegypti. J. Virol. 77:8872-8881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nakamura, T., K. W. Peng, M. Harvey, S. Greiner, I. A. Lorimer, C. D. James, and S. J. Russell. 2005. Rescue and propagation of fully retargeted oncolytic measles viruses. Nat. Biotechnol. 23:209-214. [DOI] [PubMed] [Google Scholar]
- 69.Naldini, L., U. Blomer, P. Gallay, D. Ory, R. Mulligan, F. H. Gage, I. M. Verma, and D. Trono. 1996. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science 272:263-267. [DOI] [PubMed] [Google Scholar]
- 70.Ohno, K., K. Sawai, Y. Iijima, B. Levin, and D. Meruelo. 1997. Cell-specific targeting of Sindbis virus vectors displaying IgG-binding domains of protein A. Nat. Biotechnol. 15:763-767. [DOI] [PubMed] [Google Scholar]
- 71.Pariente, N., S. H. Mao, K. Morizono, and I. S. Chen. 2008. Efficient targeted transduction of primary human endothelial cells with dual-targeted lentiviral vectors. J. Gene Med. 10:242-248. [DOI] [PubMed] [Google Scholar]
- 72.Pariente, N., K. Morizono, M. S. Virk, F. A. Petrigliano, R. E. Reiter, J. R. Lieberman, and I. S. Chen. 2007. A novel dual-targeted lentiviral vector leads to specific transduction of prostate cancer bone metastases in vivo after systemic administration. Mol. Ther. 15:1973-1981. [DOI] [PubMed] [Google Scholar]
- 73.Pereira, C. F., R. Torensma, K. Hebeda, A. Kretz-Rommel, S. J. Faas, C. G. Figdor, and G. J. Adema. 2007. In vivo targeting of DC-SIGN-positive antigen-presenting cells in a nonhuman primate model. J. Immunother. 30:705-714. [DOI] [PubMed] [Google Scholar]
- 74.Pletnev, S. V., W. Zhang, S. Mukhopadhyay, B. R. Fisher, R. Hernandez, D. T. Brown, T. S. Baker, M. G. Rossmann, and R. J. Kuhn. 2001. Locations of carbohydrate sites on alphavirus glycoproteins show that E1 forms an icosahedral scaffold. Cell 105:127-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pöhlmann, S., E. J. Soilleux, F. Baribaud, G. J. Leslie, L. S. Morris, J. Trowsdale, B. Lee, N. Coleman, and R. W. Doms. 2001. DC-SIGNR, a DC-SIGN homologue expressed in endothelial cells, binds to human and simian immunodeficiency viruses and activates infection in trans. Proc. Natl. Acad. Sci. U. S. A. 98:2670-2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rappocciolo, G., F. J. Jenkins, H. R. Hensler, P. Piazza, M. Jais, L. Borowski, S. C. Watkins, and C. R. Rinaldo, Jr. 2006. DC-SIGN is a receptor for human herpesvirus 8 on dendritic cells and macrophages. J. Immunol. 176:1741-1749. [DOI] [PubMed] [Google Scholar]
- 77.Regan, A. D., and G. R. Whittaker. 2008. Utilization of DC-SIGN for entry of feline coronaviruses into host cells. J. Virol. 82:11992-11996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Roozendaal, R., and M. C. Carroll. 2006. Emerging patterns in complement-mediated pathogen recognition. Cell 125:29-32. [DOI] [PubMed] [Google Scholar]
- 79.Sander, W. E., M. E. Metzger, K. Morizono, A. Bonifacino, S. R. Penzak, Y. M. Xie, I. S. Chen, J. Bacon, S. G. Sestrich, L. P. Szajek, and R. E. Donahue. 2006. Noninvasive molecular imaging to detect transgene expression of lentiviral vector in nonhuman primates. J. Nucl. Med. 47:1212-1219. [PubMed] [Google Scholar]
- 80.Sanz, M. A., M. T. Rejas, and L. Carrasco. 2003. Individual expression of Sindbis virus glycoproteins. E1 alone promotes cell fusion. Virology 305:463-472. [DOI] [PubMed] [Google Scholar]
- 81.Schauber-Plewa, C., A. Simmons, M. J. Tuerk, C. D. Pacheco, and G. Veres. 2005. Complement regulatory proteins are incorporated into lentiviral vectors and protect particles against complement inactivation. Gene Ther. 12:238-245. [DOI] [PubMed] [Google Scholar]
- 82.Singh, S. K., J. Stephani, M. Schaefer, H. Kalay, J. J. Garcia-Vallejo, J. den Haan, E. Saeland, T. Sparwasser, and Y. van Kooyk. 2009. Targeting glycan modified OVA to murine DC-SIGN transgenic dendritic cells enhances MHC class I and II presentation. Mol. Immunol. 47:164-174. [DOI] [PubMed] [Google Scholar]
- 83.Snitkovsky, S., and J. A. Young. 1998. Cell-specific viral targeting mediated by a soluble retroviral receptor-ligand fusion protein. Proc. Natl. Acad. Sci. U. S. A. 95:7063-7068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Steinman, R. M. 2007. Dendritic cells: understanding immunogenicity. Eur. J. Immunol. 37(Suppl. 1):S53-S60. [DOI] [PubMed] [Google Scholar]
- 85.Takada, A., K. Fujioka, M. Tsuiji, A. Morikawa, N. Higashi, H. Ebihara, D. Kobasa, H. Feldmann, T. Irimura, and Y. Kawaoka. 2004. Human macrophage C-type lectin specific for galactose and N-acetylgalactosamine promotes filovirus entry. J. Virol. 78:2943-2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tassaneetrithep, B., T. H. Burgess, A. Granelli-Piperno, C. Trumpfheller, J. Finke, W. Sun, M. A. Eller, K. Pattanapanyasat, S. Sarasombath, D. L. Birx, R. M. Steinman, S. Schlesinger, and M. A. Marovich. 2003. DC-SIGN (CD209) mediates dengue virus infection of human dendritic cells. J. Exp. Med. 197:823-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Turville, S. G., P. U. Cameron, A. Handley, G. Lin, S. Pöhlmann, R. W. Doms, and A. L. Cunningham. 2002. Diversity of receptors binding HIV on dendritic cell subsets. Nat. Immunol. 3:975-983. [DOI] [PubMed] [Google Scholar]
- 88.Vandenberghe, L. H., L. Wang, S. Somanathan, Y. Zhi, J. Figueredo, R. Calcedo, J. Sanmiguel, R. A. Desai, C. S. Chen, J. Johnston, R. L. Grant, G. Gao, and J. M. Wilson. 2006. Heparin binding directs activation of T cells against adeno-associated virus serotype 2 capsid. Nat. Med. 12:967-971. [DOI] [PubMed] [Google Scholar]
- 89.Waehler, R., S. J. Russell, and D. T. Curiel. 2007. Engineering targeted viral vectors for gene therapy. Nat. Rev. Genet. 8:573-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang, K. S., R. J. Kuhn, E. G. Strauss, S. Ou, and J. H. Strauss. 1992. High-affinity laminin receptor is a receptor for Sindbis virus in mammalian cells. J. Virol. 66:4992-5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang, S. F., J. C. Huang, Y. M. Lee, S. J. Liu, Y. J. Chan, Y. P. Chau, P. Chong, and Y. M. Chen. 2008. DC-SIGN mediates avian H5N1 influenza virus infection in cis and in trans. Biochem. Biophys. Res. Commun. 373:561-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yang, L., H. Yang, K. Rideout, T. Cho, K. I. Joo, L. Ziegler, A. Elliot, A. Walls, D. Yu, D. Baltimore, and P. Wang. 2008. Engineered lentivector targeting of dendritic cells for in vivo immunization. Nat. Biotechnol. 26:326-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yang, Z. Y., Y. Huang, L. Ganesh, K. Leung, W. P. Kong, O. Schwartz, K. Subbarao, and G. J. Nabel. 2004. pH-dependent entry of severe acute respiratory syndrome coronavirus is mediated by the spike glycoprotein and enhanced by dendritic cell transfer through DC-SIGN. J. Virol. 78:5642-5650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang, W., S. Mukhopadhyay, S. V. Pletnev, T. S. Baker, R. J. Kuhn, and M. G. Rossmann. 2002. Placement of the structural proteins in Sindbis virus. J. Virol. 76:11645-11658. [DOI] [PMC free article] [PubMed] [Google Scholar]