Summary
Pept2 knockout mice are an important tool to evaluate the evolving role and relevance of this proton-coupled oligopeptide transporter beyond drug disposition, where the transporter also modulates the pharmacodynamic and toxicodynamic effects of drug substrates. Our in vivo studies with glycylsarcosine in Pept2 knockout mice have established “proof of concept” that PEPT2 can have a significant effect on dipeptide disposition. Subsequent studies with the aminocephalosporin antibiotic cefadroxil have shown relevance to pharmacology and infectious disease. Finally, studies with the endogenous peptidomimetic 5-aminolevulinic acid have demonstrated relevance to toxicology in the framework of porphyria- and lead-induced neurotoxicity. These studies have consistently demonstrated the dual action of PEPT2 with respect to its apical localization in choroid plexus epithelium and kidney in: 1) effluxing substrates from CSF into choroid plexus, thereby affecting regional pharmacokinetics in brain; and 2) reabsorbing substrates from renal tubular fluid into proximal tubules, thereby affecting systemic pharmacokinetics and exposure. Moreover, these studies have shown that the regional effect of PEPT2 in limiting substrate concentrations in the CSF is more dramatic than its effect in increasing systemic exposure. In the case of 5-aminolevulinic acid, such regional modulation of drug disposition translates directly into significant changes in neurotoxicity.
Keywords: PEPT2, knockout mice, glycylsarcosine, cefadroxil, 5-aminolevulinic acid, disposition, dynamics, toxicity
Introduction
Heterogeneity of Proton-Coupled Oligopeptide Transporters (POTs)
a) Molecular Biology of POTs
The superfamily of POTs is characterized by the ability to transport small peptides and peptide-mimetic molecules across biological membranes.1,2) Uptake of peptides into epithelial cells by these transporters is driven by an inwardly-directed proton gradient and negative membrane potential. In mammals, the POT family consists of four members: PEPT1 (SLC15A1), PEPT2 (SLC15A2), PHT1 (SLC15A4) and PHT2 (SLC15A3) which vary in size from 572–729 amino acids and contain 12 transmembrane domains, with the N- and C-termini facing the cytosol. PEPT1 was the first mammalian POT cloned, using expression-cloning strategies from a rabbit intestinal cDNA library.3) PEPT2 was next identified and cloned from a human kidney cDNA library4). The more recent members PHT15) and PHT26) were cloned from a rat brain cDNA library. These transporters differ from PEPT1 and PEPT2 in that they recognize the amino acid L-histidine as a substrate. While both PEPT1 and PEPT2 have high inter-species homology (about 80% in rat, rabbit, human, and mouse), the sequence homology between these transporters for a given species is low (about 50%).7) Rat PHT1 and PHT2 have an amino acid identity of about 50%, but they show little sequence homology to either PEPT1 or PEPT2 (less then 20%).
PEPT1 and PEPT2 proteins are believed to share a high degree of overlapping substrate specificity, possessing the capability for sequence-independent transport of 400 different dipeptides and 8000 different tripeptides. It is unclear whether or not the PHT1 and PHT2 proteins can transport the same spectrum of di-/tripeptides. However, the ability of PHT1/PHT2 to transport L-histidine marks a distinct difference in functionality from PEPT1/PEPT2. Since a three-dimensional structure of the transporter proteins has not been developed, no precise pharmacophore model is currently available. However, preferred configurations and conformational features of PEPT1 and PEPT2 substrates include: 1) a peptide backbone of 2–3 amino acid residues, 2) both a free amino acid and carboxy terminus with the free amino group in the α or β positions, 3) the presence of hydrophobic side chains, and 4) stereoselectivity with L-amino acids and trans-conformers being preferred. It must be noted that these are not absolute criteria and some notable exceptions have been reported in the literature.1,2,8)
b) Expression of POTs
Several studies have shown unique tissue distribution and expression patterns for the different POTs. PEPT1 protein is localized in the brush border (apical) membrane of absorptive epithelia cells of the small intestine9,10) and kidney.11) Intestinal PEPT1 is confined to duodenum, jejunum and ileum of the small intestine in rat9) and human,10) while renal PEPT1 is localized predominantly in S1 segments of the early convoluted proximal tubule in rat (i.e., pars convoluta).11) PEPT1 mRNA is expressed at lower levels in the rabbit and human liver3,12) and the human pancreas.12)
PEPT2 exhibits a different tissue expression pattern compared to PEPT1.4,13) In kidney, immunolocalization studies show it is localized predominantly in S3 segments of the latter proximal tubule in rat (i.e., pars recta)11). PEPT2 mRNA also exhibits strong levels of expression in the rabbit brain, lung and mammary gland, with weaker signals detected in the pancreas, skeletal muscle, heart, liver, spleen and colon.14) In rat brain, PEPT2 mRNA has been specifically localized to astrocytes, subependymal cells, ependymal cells and epithelial cells of the choroid plexus.15) With immunohistochemistry, PEPT2 protein has been found in astrocytes, ependyma and choroid plexus epithelium, as well as in some neurons of rat.16) The same study also found that PEPT2 expression in cerebral cortex (probably astrocytic) decreased with age. The choroid plexus epithelium is the site of the blood-cerebrospinal fluid barrier (BCSFB) and the presence of PEPT2 at that barrier has been demonstrated in rat by immunoblot17,18) and functional analyses.18–20) PEPT2 appears to be absent at the blood-brain barrier of rat cerebral capillaries.16) Immunoblots show that PEPT2 protein is expressed in whole brain homogenates16) as well as in peripheral nervous system glial cells of rat.21) In the eye, in situ hybridization studies have localized PEPT2 mRNA to the retina of rat.15) In the lung, PEPT2 protein was expressed in alveolar type II pneumocytes, bronchial epithelium, and endothelium of small vessels of rat.22) Relatively less is known about the expression and distribution of PHT1 and PHT2. PHT1 mRNA has been found in the rat brain and eye, particularly in the choroid plexus and retina.5) PHT2 transcripts were expressed primarily in the rat lymphatic system, lung, and spleen and detected faintly in the brain.6)
c) Function of POTs
As mentioned previously, an inwardly-directed electrochemical gradient for protons provides POTs with the driving force needed for active transport of peptides and peptide-mimetic molecules across biological membranes.1,2) The pH of the extracellular microenvironment, therefore, plays an important role in determining the rate of transport. This is important in the intestine and kidney where low pH microenvironments are established by ion transporters at the microvilli of apical membranes in intestinal and renal epithelial cells.
Due to their unique tissue distribution and expression patterns, the POTs are thought to have distinct functions in vivo. Being the predominant (and perhaps only) POT located on the brush border membrane of the small intestine, PEPT1, a high-capacity and low-affinity transporter, is the transporter responsible for the absorption of small peptide fragments from the digestion of dietary proteins.3) It may also be the primary transporter responsible for absorption of peptide-mimetic drugs such as some ACE inhibitors and the antiviral prodrug valacyclovir. Despite the sequential expression of PEPT1 and PEPT2 in the proximal tubule of the nephron,11) recent in vivo studies have shown PEPT1 plays a relatively minor role in the reabsorption of a dipeptide and an aminocephalosporin from tubular fluid.23,24) In contrast, these studies have shown that PEPT2, a high-affinity and low-capacity transporter, is the major player involved in the renal handling and reabsorption of peptide substrates and peptide-mimetic drugs. The localization of PEPT2 on the apical membrane of choroid plexus epithelial cells at the BCSFB is thought to facilitate its mediation of neuropeptide homeostasis and removal of neurotoxins from the brain. This localization of PEPT2 also makes it an attractive target for manipulating delivery of peptide-mimetic drugs to the brain.25) Because little is known about the cellular localization, tissue distribution, and transport properties of PHT1 and PHT2, little is also known about their functional activity in vivo. Functional studies17–20) in the choroid plexus have failed to show inhibition of dipeptide uptake by excess L-histidine, suggesting that PHT1/PHT2 are unlikely to be involved in peptide transport at the BCSFB. Some studies suggest they may play a role in intracellular trafficking of small peptides.6,26)
d) Relevance to physiology, pharmacology and toxicology
While in vitro studies are convenient to probe mechanism, they are limited by experimental designs that employ nonphysiologic conditions, such as the lack of an intact blood supply. Moreover, the presence of multiple transport systems with overlapping substrate specificities in a tissue or organ of interest confounds an accurate assessment of the significance of a specific transporter relative to other transporters. Studies using wild-type and knockout mice offer a unique opportunity to study the role and relevance of a particular POT under physiological in vivo conditions. By challenging in vivo knockout models, unique phenotypes can be discovered, demonstrating the role and relevance of a particular POT with respect to drug disposition, dynamics, and toxicity. The rest of this review will address in vivo findings of PEPT2 with respect to the three model substrates glycylsarcosine,23) cefadroxil,24) and 5-aminolevulinic acid27), and attempt to illustrate the evolving relevance of this transporter to physiology, pharmacology, and toxicology using Pept2 knockout mice developed by our laboratory.28) As reported previously,23) the Pept2 knockout mice do not appear to have an up-regulation in the expression levels of related POT genes, at least in kidney and brain.
Disposition of Glycylsarcosine (GlySar)
Our first study investigated the in vivo pharmacokinetics, tissue distribution, and renal handling of a synthetic dipeptide following an intravenous bolus dose (0.05 µmol/g body weight) of [14C]GlySar in wild-type and gender-matched Pept2 knockout mice.23) These findings showed that, in Pept2 knockout mice, the clearance of GlySar was markedly increased (2-fold), resulting in significantly lower systemic exposure of GlySar. In addition, renal reabsorption was almost abolished and GlySar was eliminated almost exclusively by glomerular filtration. Of the 46% of GlySar reabsorbed in wild-type mice, PEPT2 accounted for 86% of this process. Null mice also had lower choroid plexus concentrations of GlySar and a 5-fold lower choroid plexus-to-cerebrospinal fluid (CSF) ratio compared with wild-type mice at 60 min. Despite a 2-fold lower systemic exposure, null mice exhibited a greater CSF/blood ratio at 60 min (0.9 versus 0.2) and area under the curve (AUCCSF/AUCblood) ratio over 60 min (0.45 versus 0.12), indicating that PEPT2 significantly impacts GlySar exposure in the CSF compartment.
These findings were consistent with our hypothesis that PEPT2 is the predominant peptide transporter in kidney and that it acts as an efflux transporter in the choroid plexus (clearing peptides from CSF). However the next logical step was to determine whether or not these in vivo results hold when PEPT2 is challenged with a substrate of pharmacologic relevance.
Disposition of Cefadroxil
Our second study investigated the in vivo pharmacokinetics, renal tubular reabsorption, and brain penetration of cefadroxil, a broad spectrum, first-generation aminocephalosporin antibiotic.24) In these experiments, [3H]cefadroxil was administered by a single intravenous bolus injection in wild-type and gender-matched Pept2 knockout mice over a wide range of doses (i.e., 100, 50, 12.5, and 1 nmol/g body weight). Results showed that cefadroxil disposition was clearly nonlinear over the dose range studied, due to both saturable renal tubular secretion and reabsorption of the antibiotic. After the 1 nmol/g dose of cefadroxil, Pept2 null mice exhibited a 3-fold greater total clearance and 3-fold lower systemic concentrations of drug compared to wild-type animals. Further, the cefadroxil plasma concentrations produced at this dose (i.e., approximately 0.01–10 µM) are clinically relevant since they are in the minimal inhibitory concentration range of most bacteria.29) Renal reabsorption of cefadroxil was almost completely abolished in Pept2 null mice versus wild-type animals (i.e., 3% versus 70%, respectively; p<0.001). Of the 70% of cefadroxil reabsorbed in wild-type mice, PEPT2 accounted for 95% of reabsorbed substrate. Tissue distribution studies indicated that PEPT2 had a dramatic effect on cefadroxil tissue exposure, especially in brain where the CSF-to-blood concentration ratio of cefadroxil was 6-fold greater in Pept2 null mice compared with wild-type animals.
The results were consistent with our hypothesis that PEPT2 significantly limits the exposure of cefadroxil in CSF, despite its role in increasing systemic exposure of the cephalosporin by renal reabsorption. Thus, cefadroxil (and possibly other aminocephalosporins) may be ineffective in the treatment of meningitis, at least in part, because of PEPT2-mediated efflux of antibiotic from CSF, thereby resulting in sub-therapeutic levels of drug at its active site. However, while the results were encouraging, a question that remained unanswered was whether or not PEPT2-mediated changes in drug disposition would result in significant changes to the pharmacological or toxicological effect of a drug.
Disposition and Neurotoxicity of 5-Aminolevulinic Acid (5-ALA)
Our third study investigated the role of PEPT2 in modulating 5-ALA concentrations in the CSF and brain, and whether or not these changes would translate into greater neuroprotection in vivo against challenge doses of 5-ALA, an endogenous heme precursor. 5-ALA was chosen for study because of its known neurotoxicity in patients with hepatic porphyria30–32) or lead toxicity33, and because it is a PEPT2 substrate.14,17,34) Studies below report the PEPT2-mediated changes in 5-ALA pharmacokinetics and pharmacodynamics under different experimental conditions.
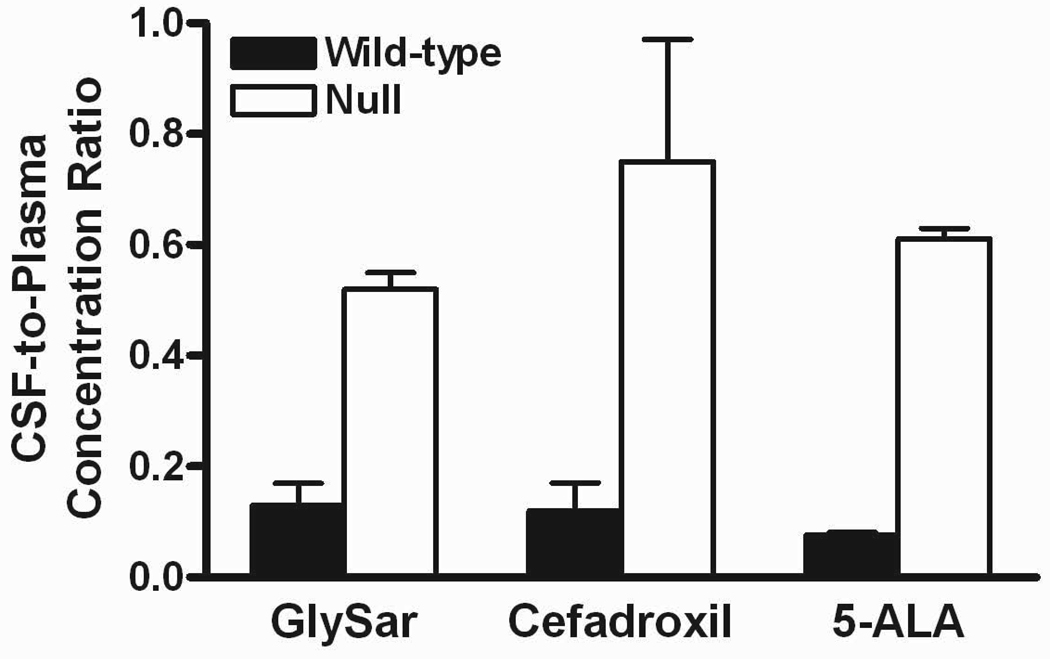
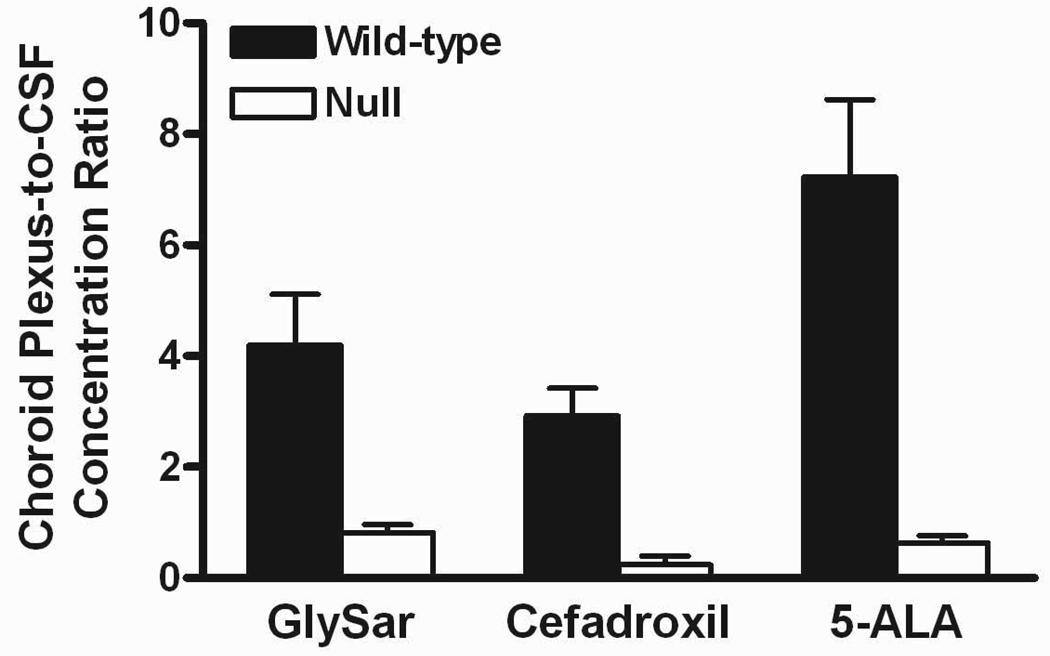
Preliminary studies were first performed to probe the pharmacokinetics of [14C]5-ALA after an intravenous bolus dose of drug (10 nmol/g body weight) in gender-matched wild-type and Pept2 knockout mice27). Results indicated that Pept2 knockout mice had a 2-fold higher clearance resulting in a 2-fold lower systemic exposure. Despite the reduction in systemic concentrations of 5-ALA, knockout mice showed a 5-fold greater concentration of drug in CSF, an 8-fold greater CSF/blood concentration ratio, and significantly lower concentrations of drug in choroid plexus. These results are very consistent with that of GlySar and cefadroxil, as described previously and in Figure 1. As shown in this figure, the CSF-to-plasma ratios increased to a similar extent for GlySar, cefadroxil, and 5-ALA in Pept2 knockout mice as compared to wild-type animals. Similar reductions in the choroid plexus-to-CSF ratio were also observed in Pept2 null vs. wild-type mice for all three PEPT2 substrates. These findings underscore the impact of PEPT2 in limiting exposure of these substrates to the CSF compartment and emphasize the directionality of PEPT2 transport from CSF into the choroid plexus epithelium in vivo.
Figure 1.
Top panel: cerebrospinal fluid (CSF)-to-plasma concentration ratios in Pept2 null mice were 4.0, 6.2 and 8.0 times that of values in wild-type mice for glycylsarcosine (GlySar), cefadroxil and 5-aminolevulinic acid (5-ALA), respectively. Bottom panel: choroid plexus-to-CSF concentration ratios in Pept2 null mice were 0.2, 0.08 and 0.09 times that of values in wild-type mice for GlySar, cefadroxil and 5-ALA, respectively. Samples were obtained 60 min after dosing GlySar (50 nmol/g body weight)23), 120 min after dosing cefadroxil (1 nmol/g body weight)24), and 60 min after dosing 5-ALA (10 nmol/g body weight).27) Data are expressed as mean ± SE (n=6–9).
With respect to pharmacodynamics, PEPT2 had a major impact on the ability of Pept2 null mice to survive the toxic insult of a single high dose of 5-ALA (4000 mg/kg s.c.).27) The time at which 50% of the animals died was 21 hr in wild-type mice as compared to only 4 hr in null mice (p<0.001), providing strong evidence that PEPT2 confers a neuroprotective advantage against the toxicity of 5-ALA. Further evidence of a neuroprotective role of PEPT2 was demonstrated under chronic dosing conditions of 5-ALA (500 mg/kg s.c. each day) for 7 days, where wild-type mice showed no sign of a reduced ability to maintain balance on a rotating rod while for Pept2 knockout mice, rotary rod times were progressively lower in response to chronic 5-ALA administration.27) Neuromuscular dysfunction (i.e., shorter balance times) in the null mice was particularly evident after 4 days of 5-ALA dosing, and balance times were reduced to 58% of control values at 7 days. This finding was even more obvious when chronic dosing conditions of 5-ALA (100 mg/kg s.c each day) were examined for 30 days. Specifically, by 30 days, wild-type mice had balance times that were 91% of control values while Pept2 knockout mice had balance times that were only 60% of control values. Two-way ANOVA showed these correlations were highly significant as a function of both time and genotype (p<0.001 for both factors). These differences could not be explained by plasma concentrations as there was little difference in the systemic exposure of 5-ALA (after a single dose of 100 mg/kg s.c.) in wild-type and Pept2 null mice. In contrast, the CSF concentrations were 8- and 30-fold greater in Pept2 null mice at 30 min and 240 min (p<0.001 for both times), respectively, indicating that the observed pharmacodynamic differences between genotypes were the result of differences in brain, and not systemic, levels of 5-ALA. Moreover, the results are clinically relevant since chronic dosing of 5-ALA at 100 mg/kg s.c. produced plasma concentrations (i.e. ~ 23 µM, on average) which are similar to those observed in patients during acute attacks of porphyria (i.e. 2–13 µM).31)
These findings are novel in that they demonstrate not only a neuroprotective phenotype for the POT family member PEPT2, but that PEPT2-mediated effects on disposition in the brain translate into significant changes in toxicity.27) This phenomenon demonstrates the ability of a transporter to modulate drug effects beyond the conventional role of mediating drug disposition. The ability of PEPT2 to limit 5-ALA exposure in CSF suggests that it may act as a secondary genetic modifier in the sensitivity of the brain to diseases such as hepatic porphyria or to environmental challenges such as lead poisoning. Figure 2 shows a proposed model of 5-ALA neurotoxicity and the protective role of PEPT2 in reducing 5-ALA concentrations in CSF and the interstitial fluid (ISF) surrounding parenchymal cells. As a result of higher 5-ALA concentrations in the ISF of Pept2 knockout mouse (or in conditions where choroid plexus expression of PEPT2 is reduced), there would be more interactions of this neurotoxin with extracellular receptors (e.g., glutamatergic or GABAergic receptors30,35)), leading to an increased risk of toxicity. This scenario was demonstrated phenotypically as reduced survival and balancing times in our transgenic Pept2 null mice.
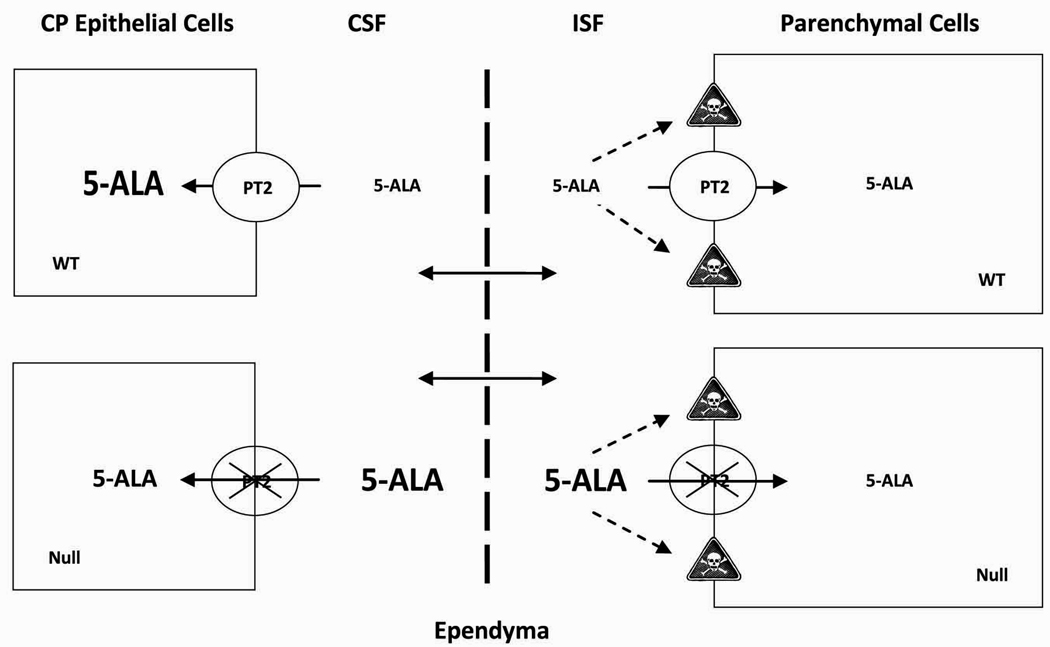
Figure 2.
Schematic of how the proton-coupled oligopeptide transporter SLC15A2 (PEPT2 displayed as PT2) affects the distribution of 5-aminolevulinic acid (5-ALA) in different compartments of the brain.27) In particular, the loss of PEPT2 results in substantially lower concentrations of 5-ALA in choroid plexus (CP) epithelial cells, and substantially higher concentrations of 5-ALA in cerebrospinal fluid (CSF) and interstitial fluid (ISF) surrounding the parenchymal cells. As a result of the higher concentrations of 5-ALA in ISF, there may be more interactions with extracellular receptors, thereby, leading to an increased risk of neurotoxicity (as displayed by the “skull and crossbones” symbol). The top-half of the figure represents a scenario in wild-type (WT) mice while the bottom-half of the figure represents a scenario in Pept2-deficient (Null) mice.
Translation to humans
Translation of the role and relevance of PEPT2 in drug disposition, dynamics, and toxicity from the mouse to human will depend on four important factors: 1) the degree of inter-species sequence homology between mouse and human PEPT2 protein, 2) the conservation of PEPT2 transport functionality between these species; 3) the concordance in cellular localization, expression levels and tissue distribution patterns of PEPT2 in both species; and 4) the concordance in cellular localization, expression levels and distribution patterns of other transporters with overlapping substrate specificities to PEPT2. These criteria, with the exception of criterion 4, have been tested when comparing the human and monkey peptide transporters, PEPT1 and PEPT2.36) As mentioned previously, the sequence homology between the mouse and human PEPT2 is high at about 80%. The molecular and structural features of the POT superfamily is highly conserved37) and studies have shown the ability of human cell lines expressing PEPT2 to transport the same range of substrates as mouse PEPT2.38–40) Moreover, the apical localization of PEPT2 in the kidney38,41) and lung42) cells of human has been shown indicating the same directionality of transport. Further studies, however, will be needed to demonstrate the inter-species concordance in expression levels and tissue distribution patterns of PEPT2. Since more than one transporter may affect the tissue distribution and organ elimination of a drug, additional studies will need to be performed to probe the influence of transporters with overlapping substrate specificity.
Another point to consider is that while systemic drug exposure may not be that different between individuals, there may still be dramatic changes in the local exposure of CSF or brain to drug. As a result, plasma monitoring alone may be insufficient to predict whether or not patients will have the desired drug response because of variations in systemic versus regional transporter activity.
Pharmacogenomic Implications
As the PEPT2 transporter is continued to be challenged with various substrates or conditions, more phenotypes will be elucidated, further demonstrating the relevance of PEPT2 in mediating drug disposition, dynamics, and toxicity. To the extent that more PEPT2-mediated therapeutic agents are discovered, the transporter will become an important target for manipulating the delivery of drugs to intended sites of action (e.g., the brain), or manipulating the overall kinetic, dynamic, or toxic profiles of drugs. What is less clear, however, is the extent to which genetic variants of PEPT2 exist in the human population, and whether or not these variants may lead to functional polymorphisms in drug disposition, dynamics, and toxicity.
A few investigators have reported that certain genetic variants of human PEPT2 (hPEPT2) may lead to functional polymorphisms in transport. For example, Terada et al.43) showed that a single amino acid substitution (Arg57His) of hPEPT2 caused the complete loss of functional activity when expressed in Xenopus oocytes or HEK293 cells, in spite of PEPT2 having a conserved protein expression at the plasma membrane. This phenotype, although believed to be rare in humans, is analogous to our Pept2 knockout mouse. Pinsonneault et al.44) conducted a haplotype analysis of 27 single nucleotide polymorphisms of hPEPT2 and found two main variants containing several phased amino acid substitutions (i.e., hPEPT2*1 and hPEPT2*2; about 45% each), being present in all ethnic groups tested. They found that CHO cells, transfected with both variants, displayed similar Vmax values for GlySar but significantly different values for Km (83 µM vs. 233 µM for hPEPT2*1 and hPEPT2*2, respectively). The two haplotypes also differed in their pH sensitivity of GlySar uptake. While these two in vitro studies43,44) point to an attenuation (or complete abolition) of PEPT2-mediated transport in some individuals, the in vivo relevance of these genetic variants in the human population remains unclear. Further studies will be needed to determine the frequency and phenotypic significance of these (and other) genetic polymorphisms in PEPT2.
Conclusions
The Pept2 knockout mouse has become an important tool to evaluate the evolving role and relevance of this transporter in drug disposition, dynamics and toxicity. Although disruption of the PEPT2 gene itself does not result in obvious phenotypic changes in the knockout mouse, our studies emphasize the fact that challenging the knockout in a certain manner may bring about phenotypic abnormalities. Our studies have challenged the Pept2 knockout model with various substrates of physiological, pharmacological and toxicological relevance, and have consistently demonstrated the dual action of this transporter with respect to its apical localization in kidney and choroid plexus epithelial cells. The results have clearly shown that in vivo: 1) PEPT2 effluxes GlySar, cefadroxil and 5-ALA from the CSF into choroid plexus, thereby affecting regional disposition in the brain; and 2) PEPT2 reabsorbs these substrates from renal tubular fluid, thereby affecting systemic pharmacokinetics and exposure. It also appears that the regional effect of PEPT2 in limiting exposure of substrates to the CSF and ISF of brain may be of more importance for some compounds than its effect in increasing systemic exposure. Specifically, in the case of 5-ALA, the modulation of regional brain disposition by PEPT2 translates directly into significant changes in neurotoxicity.
Acknowledgments
This study was partially funded by NIH Grants R01 GM035498 (DES) and R01 NS034709 and P01 HL018575 (RFK). MAK was supported by the Pharmacological Sciences Training Program of the NIH Grant T32 GM007767.
References
- 1.Daniel H, Kottra G. The proton oligopeptide cotransporter family SLC15 in physiology and pharmacology. Pflugers Arch. 2004;447:610–618. doi: 10.1007/s00424-003-1101-4. [DOI] [PubMed] [Google Scholar]
- 2.Herrera-Ruiz D, Knipp GT. Current perspectives on established and putative mammalian oligopeptide transporters. J. Pharm. Sci. 2002;92:691–714. doi: 10.1002/jps.10303. [DOI] [PubMed] [Google Scholar]
- 3.Fei Y, Kanai Y, Boron WF, Heidiger MA. Expression cloning of a mammalian proton-coupled oligopeptide transporter. Nature (Lond) 1994;368:563–566. doi: 10.1038/368563a0. [DOI] [PubMed] [Google Scholar]
- 4.Lui W, Liang R, Ganapathy V, Leibach FH. Molecular cloning of PEPT2, a new member of the H+/peptide cotransporter family from human kidney. Biochem. Biophys. Acta. 1995;1235:461–466. doi: 10.1016/0005-2736(95)80036-f. [DOI] [PubMed] [Google Scholar]
- 5.Yamashita T, Shimada S, Tagaki T, Tohyama M. Cloning and functional expression of a brain peptide/histidine transporter. J Biol. Chem. 1997;272:10205–10211. doi: 10.1074/jbc.272.15.10205. [DOI] [PubMed] [Google Scholar]
- 6.Sakata K, Yamashita T, Maeda M, Shimada S. Cloning of a lymphatic peptide/histidine transporter. J. Biochem. 2001;356:53–60. doi: 10.1042/0264-6021:3560053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Botka C, Wittig T, Graul R, Nielson C, Amidon G, Sadée W. Human proton/oligopeptide transporter (POT) genes: identification of putative human genes using bioinformatics. AAPS Pharm. Sci. 2000;2:1–22. doi: 10.1208/ps020216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rubio-Aliaga I, Daniel H. Mammalian peptide transporters as targets for drug delivery. Trends pharmacol. Sci. 2002;23:434–440. doi: 10.1016/s0165-6147(02)02072-2. [DOI] [PubMed] [Google Scholar]
- 9.Ogihara H, Saito H, Shin BC, Terado T, Takenoshita S, Nagamachi Y, Inui K, Takata K. Immunolocalization of H+/peptide cotransporter in rat digestive tract. Biochem. Biophys. Res. Commun. 1996;220:848–852. doi: 10.1006/bbrc.1996.0493. [DOI] [PubMed] [Google Scholar]
- 10.Groneberg DA, Döring F, Eynott PR, Fischer A, Daniel H. Intestinal peptide transport: ex vivo uptake studies and localization of peptide carrier PEPT1. Am. J. Physiol. Gastrointest. Liver Physiol. 2001;281:G697–G704. doi: 10.1152/ajpgi.2001.281.3.G697. [DOI] [PubMed] [Google Scholar]
- 11.Shen H, Smith DE, Yang T, Brosius F. Localization of PEPT1 and PEPT2 proton-coupled oligopeptide transporter mRNA and protein in rat kidney. Am. J. Physiol. 1999;276:F658–F665. doi: 10.1152/ajprenal.1999.276.5.F658. [DOI] [PubMed] [Google Scholar]
- 12.Liang R, Fei Y-J, Prasad PD, Ramamoorthy S, Han H, Yang-Feng TL, Hediger MA, Ganapathy V, Leibach FH. Human intestinal H+/peptide cotransporter. J. Biol. Chem. 1995;270:6456–6463. doi: 10.1074/jbc.270.12.6456. [DOI] [PubMed] [Google Scholar]
- 13.Boll M, Herget M, Wagener M, Weber WM, Markovich D, Biber J, Clauss W, Murer H, Daniel H. Expression cloning and functional characterization of the kidney cortex high-affinity proton-coupled peptide transporter. Proc. Natl. Acad. Sci. USA. 1996;93:284–289. doi: 10.1073/pnas.93.1.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Döring F, Walter J, Will J, Focking M, Boll M, Amasheh S, Clauss W, Daniel H. Delta-aminolevulinic acid transport by intestinal and renal peptide transporters and its physiological and clinical implications. J Clin. Invest. 1998;101:2761–2767. doi: 10.1172/JCI1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berger U, Hediger M. Distribution of peptide transporter PEPT2 mRNA in the rat nervous system. Anat. Embryol. 1999;199:439–449. doi: 10.1007/s004290050242. [DOI] [PubMed] [Google Scholar]
- 16.Shen H, Smith DE, Keep RF, Brosius FC. Immunolocalization of the proton-coupled oligopeptide transporter PEPT2 in developing rat brain. Mol. Pharm. 2004;1:248–256. doi: 10.1021/mp049944b. [DOI] [PubMed] [Google Scholar]
- 17.Novotny A, Xiang J, Stummer W, Teuscher NS, Smith DE, Keep R. Mechanisms of 5-aminolevulinic acid uptake at the choroid plexus. J. Neurochem. 2000;75:321–328. doi: 10.1046/j.1471-4159.2000.0750321.x. [DOI] [PubMed] [Google Scholar]
- 18.Shu C, Shen H, Keep RF, Smith DE. Role of PEPT2 in peptide/mimetic trafficking at the blood-cerebral fluid barrier: studies in rat choroid plexus epithelial cells in primary culture. J. Phar. Exp. Ther. 2002;301:820–829. doi: 10.1124/jpet.301.3.820. [DOI] [PubMed] [Google Scholar]
- 19.Tuescher NS, Novotny A, Keep RF, Smith DE. Functional evidence for the presence of PEPT2 in rat choroid plexus: Studies with glycylsarcosine. J. Pharmacol. Exp. Ther. 2000;294:494–499. [PubMed] [Google Scholar]
- 20.Teuscher NS, Keep RF, Smith DE. PEPT2-mediated uptake of neuropeptides in rat choroid plexus. Pharm. Res. 2001;18:807–813. doi: 10.1023/a:1011088413043. [DOI] [PubMed] [Google Scholar]
- 21.Groneberg DA, Döring F, Nickolaus M, Daniel H, Fischer A. Expression of PEPT2 peptide transporter mRNA and protein in glial cells of rat dorsal root ganglia. Neurosci. Lett. 2001;304:181–184. doi: 10.1016/s0304-3940(01)01794-3. [DOI] [PubMed] [Google Scholar]
- 22.Groneberg DA, Nickolaus M, Springer J, Döring F, Daniel H, Fischer A. Localization of the peptide transporter PEPT2 in the lung: implications for pulmonary oligopeptide uptake. Am. J. Pathol. 2001;158:707–714. doi: 10.1016/S0002-9440(10)64013-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ocheltree M, Shen H, Hu Y, Keep RF, Smith DE. Role and relevance of peptide transporter 2 (PEPT2) in the kidney and choroid plexus: in vivo studies with glycylsarcosine in wild-type and PEPT2 knockout mice. J. Pharmacol. Exp. Ther. 2005;35:240–247. doi: 10.1124/jpet.105.089359. [DOI] [PubMed] [Google Scholar]
- 24.Shen H, Ocheltree S, Hu Y, Keep RF, Smith DE. Impact of genetic knockout of PEPT2 on cefadroxil pharmacokinetics, renal tubular reabsorption, and brain penetration in mice. Drug Metab. & Disp. 2007;35:1209–1216. doi: 10.1124/dmd.107.015263. [DOI] [PubMed] [Google Scholar]
- 25.Smith DE, Johanson CE, Keep RF. Peptide and peptide analog transport systems at the blood-CSF barrier. Adv. Drug Deliv. Rev. 2004;56:1765–1791. doi: 10.1016/j.addr.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 26.Ocheltree M, Keep RF, Shen H, Yang D, Hughes B, Smith DE. Preliminary investigation into the expression of proton-coupled oligopeptide transporters in neural retina and retinal pigment epithelium (RPE): lack of functional activity in RPE plasma membranes. Pharm. Res. 2003;20:1364–1372. doi: 10.1023/a:1025741723724. [DOI] [PubMed] [Google Scholar]
- 27.Hu Y, Shen H, Keep RF, Smith DE. Peptide transporter 2 (PEPT2) expression in brain protects against 5-aminolevulinic acid neurotoxicity. J. Neurochem. 2007;103:2058–2065. doi: 10.1111/j.1471-4159.2007.04905.x. [DOI] [PubMed] [Google Scholar]
- 28.Shen H, Smith D, Keep R, Xiang J, Brosius FC., III Targeted disruption of the PEPT2 gene markedly reduces dipeptide uptake in the choroid plexus. J. Biol. Chem. 2003;278:4786–4791. doi: 10.1074/jbc.M207397200. [DOI] [PubMed] [Google Scholar]
- 29.Courtieu L, Drugeon H. Compared sensitivities of 532 bacterial strains to six cephalosporins. Int. J. Clin. Pharmacol. Res. 1983;3:195–201. [PubMed] [Google Scholar]
- 30.Anderson E, Sassa A, Bishop F, Desnick J. Disorders of heme biosynthesis: X-linked siderolblastic anemia and the porphyrias. In: Scriver R, Beaudet L, Sly S, Valle D, editors. The Metabolic & Molecular Bases of Inherited Disease. New York: McGraw-Hill; 2001. pp. 2991–3062. [Google Scholar]
- 31.Lindberg P, Martini R, Baumgartner M, Erne B, Borg J, Zielasek J, Ricker K, Steck A, Toyka KV, Meyer UA. Motor neuropathy in porphobilinogen deaminase-deficient mice imitates the peripheral neuropathy of human acute porphyria. J. Clin. Invest. 1999;103:1127–1134. doi: 10.1172/JCI5986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Albers W, Fink K. Porphyric neuropathy. Muscle Nerve. 2004;30:410–422. doi: 10.1002/mus.20137. [DOI] [PubMed] [Google Scholar]
- 33.Klassen D. Heavy metals and heavy-metal antagonists. In: Brunton L, Lazo S, Parker L, editors. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. New York: McGraw-Hill; 2006. pp. 1753–1775. [Google Scholar]
- 34.Ocheltree M, Shen H, Hu Y, Xiang J, Keep RF, Smith DE. Role of PEPT2 in the choroid plexus uptake of glycylsarcosine and 5-aminolevulinic acid: studies in wild-type and null mice. Pharm. Res. 2004;21:1680–1685. doi: 10.1023/b:pham.0000041465.89254.05. [DOI] [PubMed] [Google Scholar]
- 35.Adhikari A, Penatti CA, Resende R, Ulrich H, Britto G, Bechara H. 5-Aminolevulinate and 4, 5-diox-ovalerate ions decrease GABAA receptor density in neuronal cells, synaptosomes and rat brain. Brain Res. 2006;1093:95–104. doi: 10.1016/j.brainres.2006.03.103. [DOI] [PubMed] [Google Scholar]
- 36.Zhang E, Emerick R, Pak Y, Wrighton S, Hillgren K. Comparison of human and monkey peptide transporters: PEPT1 and PEPT2. Mol. Pharmaceutics. 2004;1:201–210. doi: 10.1021/mp0499712. [DOI] [PubMed] [Google Scholar]
- 37.Fei J, Ganapathy V, Leibach F. Molecular and structural features of the proton-coupled oligopeptide transporter superfamily. Prog. Nucleic Acid Res. & Mol. Biol. 1998;58:239–261. doi: 10.1016/s0079-6603(08)60038-0. [DOI] [PubMed] [Google Scholar]
- 38.Ganapathy M, Brandsch M, Prasad P, Ganapathy V, Leibach F. Differential recognition of β-lactam antibiotics by intestinal and renal peptide transporters, PEPT1 and PEPT2. J. Biol. Chem. 1995;270:25672–25677. doi: 10.1074/jbc.270.43.25672. [DOI] [PubMed] [Google Scholar]
- 39.Biegel A, Knutter I, Hartrodt B, Gabauer S, Theis S, Luckner P, Kottra G, Rastetter M, Zebisch K, Thondorf I, Daniel H, Neubert K, Brandsch M. The renal type H+/peptide symporter PEPT2: structure-affinity relationships. Amino Acids. 2006;31:137–156. doi: 10.1007/s00726-006-0331-0. [DOI] [PubMed] [Google Scholar]
- 40.Sugawara M, Huang W, Fei Y, Leibach F, Ganapathy V, Ganapathy M. Transport of valganciclovir, a ganciclovir prodrug, via peptide transporters PEPT1 and PEPT2. J. Pharm. Sci. 2000;89:781–789. doi: 10.1002/(SICI)1520-6017(200006)89:6<781::AID-JPS10>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 41.Ramamoorthy S, Liu W, Ma Y, Yang-Feng T, Ganapathy V, Leibach F. Proton/peptide cotransporter (PEPT2) from human kidney: functional characterization and chromosomal localization. Biochemica. et. Biophysica. Acta. 1995;1240:1–4. doi: 10.1016/0005-2736(95)00178-7. [DOI] [PubMed] [Google Scholar]
- 42.Bahadurri P, D’Souza V, Pinsonneault J, Sadée W, Bao S, Knoell D, Swaan P. Functional characterization of the peptide transporter PEPT2 in primary cultures of human upper airway epithelium. Am. J. Resp. Cell & Mol. Biol. 2005;32:319–325. doi: 10.1165/rcmb.2004-0322OC. [DOI] [PubMed] [Google Scholar]
- 43.Terada T, Irie M, Okuda M, Inui K. Genetic variant Arg57His in human H+/peptide cotransporter 2 causes a complete loss of transport function. Biochem. Biophys. Res. Commun. 2004;316:416–420. doi: 10.1016/j.bbrc.2004.02.063. [DOI] [PubMed] [Google Scholar]
- 44.Pinsonneault J, Nielsen U, Sadée W. Genetic variants of the human H+/dipeptide transporter PEPT2: analysis of haplotype functions. J. Pharmacol. Exp. Ther. 2004;311:1088–1096. doi: 10.1124/jpet.104.073098. [DOI] [PubMed] [Google Scholar]