Abstract
Background
Major depressive disorder (MDD) is associated with coronary heart disease (CHD), but the mechanisms are unclear. The presence of MDD may increase CHD risk by affecting microvascular circulation. It is also plausible that genetic factors influencing MDD may overlap with those for CHD. We sought to examine the relationship between MDD and coronary flow reserve (CFR), the ratio of maximum flow during stress to flow at rest measured in milliliters per minute per gram of tissue.
Methods
We examined 289 male middle-aged twins, including 106 twins (53 twin pairs) discordant for a lifetime history of MDD and 183 control twins (unrelated to any twins in the experimental group) without MDD. To calculate CFR, we used positron emission tomography with nitrogen 13 (13N) ammonia to evaluate myocardial blood flow at rest and after adenosine stress. A standard perfusion defect score was also used to assess myocardial ischemia.
Results
There was no difference in myocardial ischemia between twins with and without MDD. Among the dizygotic twin pairs discordant for MDD, the CFR was 14% lower in the twins with MDD than in their brothers without MDD (2.36 vs 2.74) (P=.03). This association was not present in the monozygotic discordant pairs who were genetically matched (2.86 vs 2.64) (P = .19). The zygosity-MDD interaction after adjustment was significant (P=.006). The CFR in the dizygotic twins with MDD was also lower than in the control twins.
Conclusions
Our results provide evidence for a shared genetic pathway between MDD and microvascular dysfunction. Common pathophysiologic processes may link MDD and early atherosclerosis.
Substantial evidence has linked depression to cardiac morbidity and mortality. In recent meta-analyses, major depressive disorder (MDD) was found to be associated with greater than twice the risk of new coronary heart disease (CHD) events among initially healthy subjects.1,2 However, results are heterogeneous, and the exact mechanisms whereby MDD increases CHD risk are not clear. Many studies have found little correlation between MDD, or depressive symptoms, and measures of coronary artery disease severity such as angiographically determined coronary stenosis,3-5 systolic dysfunction,4-11 and exercise-induced ischemia.8 An unanswered question is whether MDD increases CHD risk by affecting the microvascular circulation rather than the conduit coronary arteries.
Coronary microvascular disease refers to abnormal vasomotor regulation of the small coronary arterioles (<200 μm in diameter), which are not visualized by coronary angiography and are the main determinants of coronary vascular resistance. It is now appreciated that coronary microvascular disease may precede the development of frank CHD and has independent prognostic value.12 Since no technique enables the direct visualization of coronary microcirculation in humans in vivo, the study of coronary microcirculation is indirect and relies on functional parameters. Coronary flow reserve (CFR) is the ratio of maximum flow during stress (in response to adenosine in the present study) to flow at rest measured in milliliters per minute per gram of tissue, an integrated measure of whole coronary circulation. In the absence of coronary stenosis, CFR provides an index of coronary vasodilator capacity and microvascular function.13-16
The study of twins offers a unique opportunity to investigate the relationship between MDD and CHD. Both MDD and CHD are highly heritable, with genetic factors explaining about 40% of the variation in MDD,17 and more than 50% of the variation in early-onset CHD.18 Thus, it is plausible that genetic factors influencing MDD may overlap with genetic factors for CHD; this common genetic substrate could explain the association between MDD and CHD.19,20
The purpose of this study was to examine the relationship between MDD and CFR, a global measure of coronary ischemia and microvascular function, taking into account potential shared genetic effects by studying a genetically informative sample of twins.
METHODS
SUBJECTS
The Twins Heart Study (THS)21,22 is an investigation of psychological, behavioral, and biological risk factors for subclinical cardiovascular disease in twins. Briefly, it includes 93 monozygotic and 87 dizygotic twin pairs from the Vietnam Era Twin (VET) Registry,23 a large registry of middle-aged male-male twins who served in the United States military during the Vietnam War. Twin pairs were selected for the THS if they were born between 1946 and 1956 and were free of symptomatic cardiovascular disease based on information collected by the VET Registry. Two groups of twin pairs were randomly sampled: one group included MDD-discordant twins, where one member of the pair had a lifetime history of MDD previously assessed with the Diagnostic Interview Schedule (DIS),24 and the other did not. The second group of twins included control pairs where neither had a history of MDD. Twin pairs were examined together at the Emory University General Clinical Research Center (GCRC). During their GCRC stay, their medical history, including psychiatric history, was confirmed and updated. The final THS sample included 68 MDD-discordant pairs, 7 MDD-concordant pairs (both twins had MDD), and 105 MDD-free control pairs. The protocol was approved by the institutional review board at Emory University, and informed consent was obtained from all subjects.
ASSESSMENT OF MDD AND OTHER PSYCHIATRIC DIAGNOSES
Lifetime history of MDD was assessed using the Structured Clinical Interview for DSM-IV–Patient Edition (SCID-P),25 which yields a clinical diagnosis of MDD based on a lifetime history. Because the SCID-P is considered the gold standard for a diagnosis of MDD, twins were reclassified according to the SCID-P when the earlier DIS diagnosis did not agree. The SCID-P also provided a diagnosis of other psychiatric disorders, including a lifetime history of posttraumatic stress disorder and of alcohol and drug abuse or dependence. Current substance abuse and other psychiatric diagnoses were infrequent. We also administered the Beck Depression Inventory-II (BDI-II),26 a standardized scale providing a continuous measure of depressive symptoms.
PET PROTOCOL
Twins underwent imaging of myocardial blood flow (MBF) with PET nitrogen 13 (13N) ammonia at rest and following pharmacologic (adenosine) stress during a single imaging session. All twins were admitted to the GCRC in the late morning or early afternoon on the day prior to the PET scan and stayed overnight in the GCRC facility. They all received a similar low-fat dinner and then remained fasting until the PET scan was completed. They were instructed to abstain from smoking and drinking alcoholic or caffeinated beverages and from eating any food other than what was served to them. All medications were withheld the morning of the PET scan. The entire imaging protocol was performed by personnel blinded to the MDD status of the twins.
The PET data were collected in 2-dimensional mode using a CTI ECAT Exact 47 (921) camera (5-mm resolution) (Siemens, Knoxville, Tennessee). Initially, a 2- to 3-mCi dose of 13N ammonia was injected, and a 4-minute static scan was collected and reconstructed without any corrections to verify subject position. Then, rest and pharmacologic stress (adenosine) ammonia imaging was performed. The rest and stress imaging protocols were identical except that a 4-minute infusion of adenosine (0.14 mg/kg/min) was started 2 minutes prior to the ammonia injection for the stress imaging session. 20 mCi of 13N ammonia were injected, and a 5-minute, 31-frame dynamic acquisition was started (12 frames × 5 seconds; 3 frames × 20 seconds; and 1 frame × 300 seconds). Data were collected in 47 planes, 3.375 mm thick, covering a range of 16 cm. Immediately after the conclusion of the dynamic sequence, a 15-minute gated acquisition was started. Finally, transmission data were collected for 5 minutes in the windowed mode using germanium 68 (68Ge) rods for segmented attenuation correction. The process was repeated, including a second transmission scan, for the stress study. Images were reconstructed with filtered back projection using a Hann filter cutoff at 1 cycle/cm. The electrocardiographic output was monitored continuously, and blood pressure and heart rate measurements were taken before, during (every minute), and after the adenosine infusion. The rate-pressure product was calculated as the mean systolic blood pressure during adenosine infusion multiplied by the mean heart rate during adenosine infusion divided by 100.
PET FLOW MEASURES
To calculate CFR, measurements of MBF at rest and during stress were obtained. The last frame of the dynamic sequence was used as a template for sectorial region-of-interest analysis. The input function was generated by drawing a region of interest in the left ventricle chamber on a midventricular slice, and flow was calculated (expressed in milliliters per minute per gram of tissue) using established techniques.27,28 The left ventricle was sampled radially from 40 different angles, and 40 samples of flow were obtained for each short axis slice. The resulting hundreds of samples were grouped into 20 segments.
Our main outcome was the overall measure of CFR for the entire myocardium (across all 20 regions), defined as the ratio of maximum flow during stress to flow at rest. Secondarily, regional CFR was also calculated (ie, left anterior descending, left circumflex, and right coronary artery).
MYOCARDIAL PERFUSION DEFECT SCORE
In addition to flow measures, we constructed a summation score describing the number and severity of visible perfusion defects across the 20 segments of acquisition. In each segment, the defect severity was quantified on a 4-point scale, and these points were then summed up across the 20 segments yielding a total score. Separate scores were obtained for the rest (summed rest score) and stress (summed stress score) scans. A reversible defect score (summed difference score) was obtained by subtracting the rest score from the stress score. These scores, and a dichotomous indicator of perfusion abnormalities (defined as a summed stress score ≥429), represented secondary perfusion outcomes.
OTHER MEASUREMENTS
All twins provided a medical history and underwent a physical examination, including a fasting blood test. Standard CHD risk factors were evaluated as previously described.29 Physical activity was assessed with a modified version of the Baecke Questionnaire of Habitual Physical Activity used in the Atherosclerosis Risk in Communities (ARIC) Study,30 yielding a global score of physical activity at work and during sports and nonsports activities.
STATISTICAL ANALYSIS
All P values were corrected for the correlation between co-twins using generalized estimating equations or mixed-effects models with a random intercept for pair, depending on variable distribution. We first compared myocardial perfusion data within brothers in the MDD-discordant twin pairs. This was a matched co-twin analysis of MDD status that controlled by design for all factors shared by the twins. Twins share maternal factors, genes (50% if dizygotic and 100% if monozygotic), and childhood and adolescent environment if they are raised together, as they were in our sample. The within-pair comparison minimizes confounding by these factors. Control pairs were also compared with MDD twins and with non-MDD twins of the discordant twin pairs; because this analysis compared unrelated twins, it was not controlled for familial factors.
The within-pair analyses were further stratified by zygosity to examine the potential influence of genetic factors on the association between MDD and myocardial perfusion. Since monozygotic twins share 100% of their segregating genes, the analysis of within-pair differences in monozygotic twins is fully controlled for genetic factors shared between MDD and myocardial perfusion. Dizygotic twins only share on average 50% of genes, and differences among these twins are only partially controlled for shared genetic factors. In general, shared genetic factors would be indicated if the within-pair difference of myocardial perfusion in MDD-discordant pairs is smaller in monozygotic than in dizygotic pairs.
Because we were interested in microvascular dysfunction, in a subsequent step we adjusted for the presence of perfusion abnormalities. Analyses were further adjusted for CHD risk factors included in the Framingham risk score, which incorporates age, low-density lipoprotein cholesterol level, high-density lipoprotein cholesterol level, blood pressure, diabetes mellitus status, smoking status,31 use of statins, use of antidepressants, and study year. Sensitivity analyses were conducted by also adjusting for education level, marital status, habitual physical activity (Baecke score), body mass index, other medications (aspirin, β-blockers, and angiotensin-converting enzyme inhibitors), rate-pressure product during the adenosine infusion, and individual CHD risk factors in place of the Framingham risk score.
Since few twins met the criteria for current depressive episode, we also analyzed discordance using a score of 10 in the BDI-II as a proxy for current MDD. In addition, we examined ordinal levels of increasing BDI-II score severity with cut points at 10, 14, and 20.26,32 Twins were considered discordant if they fell into different BDI-II categories. All analyses were conducted using SAS software, version 9.1 (SAS Institute Inc, Chicago, Illinois). Significance level was set at .05, 2 sided.
RESULTS
STUDY SAMPLE
Of the 360 THS twins, 54 were excluded because PET imaging was not performed owing to scheduling conflicts or technical problems with the PET equipment or the imaging acquisition. Because we wanted complete MDD-discordant pairs for analysis, we further excluded 5 twins whose pairs became incomplete after the same exclusions, leaving 53 complete twin pairs who were discordant for lifetime history of MDD (25 dizygotic and 28 monozygotic). Of the remaining non–MDD discordant twins, 6 pairs were excluded because they were concordant for MDD, leaving 183 twin pairs both free of MDD who served as controls in the analysis (76 dizygotic and 107 monozygotic twin pairs). In most cases, the MDD in twins with the disease was in remission; only 4 subjects met the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) criteria for a current major depressive episode. Three discordant twin pairs (2 dizygotic and 1 monozygotic), and 3 twins from the control sample, were further excluded from the analyses of quantitative MBF because of missing flow data.
Compared with their co-twins without MDD, twins with MDD were less educated, less often married, less often taking statins, and more likely to smoke (Table 1). The medication regimens within MDD-discordant twin pairs did not otherwise differ substantially except for antidepressants. Results were similar when twins with MDD were compared with control twins. There were no substantial differences between MDD-free twins in the discordant pairs and the control twins. In the MDD-discordant pairs, there was no significant difference by zygosity in the association between any risk factor and MDD, except that current smokers were somewhat more represented in the dizygotic twins with MDD. However, the total number of current smokers was small, and there was no significant difference between monozygotic and dizygotic twin pairs in the association between MDD and pack-years of smoking.
Table 1.
Distribution of Demographic, Behavioral, and Coronary Risk Factors in Dizygotic and Monozygotic Twinsa
| Risk Factor | Discordant Twin Pairs (n=53 Pairs) |
Control Twin Pairs (n=183 Pairs) |
|
|---|---|---|---|
| Twin Without MDD | Twin With MDD | ||
| Demographic Factors | |||
| Age, y | 53.7 (2.9) | 53.7 (2.9) | 54.8 (2.8) |
| Greater than high school education | 35.8 | 20.7 | 33.3 |
| Married | 83.0 | 66.0 | 82.5 |
|
| |||
| Medical History and Risk Factors | |||
| Systolic blood pressure, mm Hg | 127 (17) | 126 (14) | 132 (15) |
| Diastolic blood pressure, mm Hg | 79.9 (10.3) | 79.3 (9.0) | 81.9 (11.1) |
| LDL-C, mg/dL | 123 (33) | 126 (33) | 123 (32) |
| HDL-C, mg/dL | 38.5 (8.2) | 36.8 (9.5) | 38.6 (9.2) |
| Diabetes | 11.5 | 13.2 | 6.0 |
| BMI | 29.3 (4.4) | 28.9 (4.5) | 29.2 (4.6) |
| Current smoker | 17.0 | 34.0 | 17.5 |
| Framingham risk score | 5.3 (2.5) | 5.8 (2.1) | 5.9 (2.1) |
| Baecke physical activity score30 | 7.5 (1.8) | 7.3 (2.2) | 7.4 (1.6) |
| Alcoholic beverages consumed in a typical week | 4.0 (6.0) | 5.1 (8.3) | 4.8 (8.0) |
| Prior coronary heart disease | 9.4 | 9.4 | 8.2 |
| Lifetime history of PTSD | 5.7 | 11.3 | 4.9 |
| Lifetime history of alcohol abuse or dependence | 41.5 | 49.1 | 41.0 |
| Lifetime history of drug abuse or dependence | 15.1 | 20.7 | 16.4 |
| BDI-II score26 | 3.8 (4.4) | 8.2 (9.2) | 3.9 (5.2) |
|
| |||
| Medications Taken | |||
| Antidepressants | 7.5 | 34.0 | 9.8 |
| ACE inhibitors | 18.9 | 9.4 | 10.9 |
| Statins | 37.7 | 28.3 | 20.2 |
| β-Blockers | 9.4 | 13.2 | 6.0 |
| Aspirin | 20.7 | 18.9 | 25.1 |
Abbreviations: ACE, angiotensin-converting enzyme; BDI, Beck Depression Inventory; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; MDD, major depressive disorder; PTSD, posttraumatic stress disorder.
SI conversion factor: To convert HDL-C and LDL-C to millimoles per liter, multiply by 0.0259.
All data are reported as either mean (SD) values or percentages of subjects.
IMAGING AND HEMODYNAMICS
Few individuals had a summed stress score of 4 or higher, which would indicate a significant perfusion abnormality (Table 2). There were no statistical differences between MDD-discordant twins in perfusion defects, either during adenosine stress testing or at baseline. Hemodynamic responses during adenosine infusion were also similar, including maximum systolic blood pressure, maximum heart rate, and average and maximum rate-pressure product (Table 2). These results did not vary substantially after stratification by zygosity. Discordant twin pairs did not differ from control pairs in any of these imaging and hemodynamic variables (data not shown).
Table 2.
Within-Pair Comparison of Myocardial Perfusion Imaging Data in MDD-Discordant Twin Pairsa
| Dizygotic |
Monozygotic |
|||||
|---|---|---|---|---|---|---|
| Characteristic | Twin Without MDD |
Twin With MDD |
P Valueb |
Twin Without MDD |
Twin With MDD |
P Valueb |
| Summed stress score | 2.04 (1.35) | 2.16 (1.01) | .92 | 2.54 (0.84) | 2.36 (0.66) | .89 |
| Summed stress score ≥4, No. (%) | 4 (16) | 6 (24) | .42 | 9 (32) | 9 (32) | >.99 |
| Summed rest score | 0.48 (0.35) | 0.84 (0.42) | .40 | 0.61 (0.31) | 0.93 (0.38) | .43 |
| Summed difference score | 1.56 (1.33) | 1.32 (0.75) | .84 | 1.93 (0.68) | 1.43 (0.44) | .66 |
| Maximum heart rate during AST | 85.3 (3.5) | 82.5 (3.6) | .48 | 87.1 (3.6) | 81.4 (2.3) | .17 |
| Maximum systolic blood pressure during AST | 134 (5) | 127 (4) | .12 | 131 (4) | 128 (3) | .63 |
| Average rate-pressure product during AST | 90.9 (5.7) | 89.0 (4.6) | .65 | 91.8 (5.0) | 84.7 (3.0) | .24 |
| Maximum rate-pressure product during AST | 116 (9) | 108 (6) | .23 | 114 (6) | 104 (4) | .22 |
Abbreviations: AST, adenosine stress testing; MDD, major depressive disorder.
Unless otherwise indicated, all data are reported as mean (SE) values.
P values were calculated using mixed models for continuous variables or generalized estimating equations for categorical variables.
MYOCARDIAL BLOOD FLOW
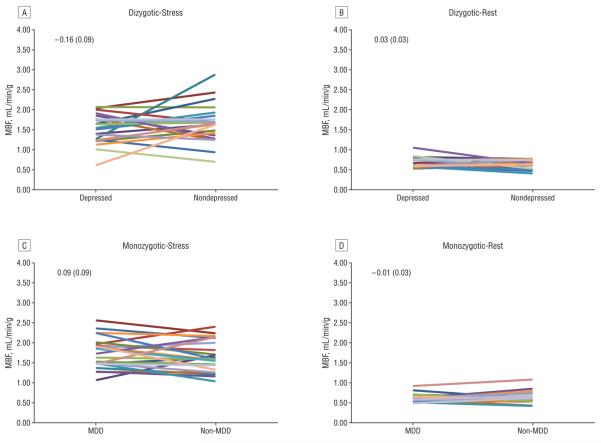
Resting MBF did not differ significantly between depressed twins of either zygosity and their nondepressed brothers in the MDD-discordant twin pairs (Figure 1). However, dizygotic twins with MDD had a blunted hyperemic MBF response to adenosine (0.16 mL/mg/g of tissue less than their brothers without MDD) (P=.03 for the change in MBF with adenosine from baseline). As a result, CFR was on average 14% lower in dizygotic twins with MDD than in their brothers without MDD (Table 3). Such a difference was not present in the monozygotic brothers, who are genetically identical (P=.01 for the MDD × zygosity interaction).
Figure 1.
Within-pair major depressive disorder (MDD)-discordant twin comparisons of myocardial blood flow (MBF), averaged over the entire left ventricle. A, Dizygotic hyperemia (after adenosine infusion); B, dizygotic at rest; C, monozygotic hyperemia; and D, monozygotic at rest. Each colored line represents a twin pair. The mean (SEM) within-pair differences are reported for each condition.
Table 3.
Within-Pair Comparison of CFR in MDD-Discordant Twin Pairsa
| Adjustment Characteristic |
Dizygotic |
Monozygotic |
MDD × Zygosity Interaction P Value |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Twin Without MDD, Mean (95% CI) |
Twin With MDD, Mean (95% CI) |
Within-Pair Difference |
Twin Without MDD, Mean (95% CI) |
Twin With MDD, Mean (95% CI) |
Within-Pair Difference |
||||
| Mean (SE) [%] | P Value | Mean (SE) [%] | P Value | ||||||
| Unadjusted | 2.74 (2.44 to 3.05) |
2.36 (2.06 to 2.67) |
−0.38 (0.17) [−13.9] |
.03 | 2.64 (2.35 to 2.93) |
2.86 (2.58 to 3.14) |
0.22 (0.16) [8.3] |
.19 | .01 |
| Adjusted for presence of perfusion defects | 2.74 (2.43 to 3.05) |
2.36 (2.06 to 2.66) |
−0.38 (0.17) [−13.9] |
.03 | 2.64 (2.35 to 2.93) |
2.86 (2.58 to 3.15) |
0.22 (0.16) [8.3] |
.18 | .01 |
| Adjusted for other factorsb | 2.72 (2.43 to 3.01) |
2.30 (2.01 to 2.60) |
−0.41 (0.18) [−15.4] |
.02 | 2.66 (2.34 to 2.94) |
2.92 (2.65 to 3.20) |
0.26 (0.17) [9.8] |
.13 | .006 |
Abbreviations: CFR, coronary flow reserve; CI, confidence interval; MDD, major depressive disorder.
Coronary flow reserve is defined as the ratio of myocardial blood flow during adenosine stress testing to flow at baseline. All values in the table are derived from mixed models that included a random intercept for pair.
Adjusted for presence of perfusion defects, the Framingham risk score (including age, current smoking, systolic blood pressure, low-density lipoprotein cholesterol level, high-density lipoprotein cholesterol level, and diabetes), use of statins, use of antidepressants, and study year. Adjusting for additional covariables, including education, marital status, habitual physical activity, body mass index, other medications, history of coronary heart disease, and rate-pressure product during the adenosine infusion did not materially change the results.
These results were not affected by adjusting for the presence of myocardial perfusion defects, defined either as a summed stress score of 4 or higher or as a continuous score. Further adjusting for the Framingham risk score and additional covariables also did not substantially change the study estimates (Table 3). Nor did adjusting for individual CHD risk factors in place of the Framingham risk score generally affect the study estimates. When current smoking was added to the model, the within-pair difference in CFR in the dizygotic twins was somewhat reduced to −0.26 (9.8%), but the interaction between MDD and zygosity remained significant (P=.04).
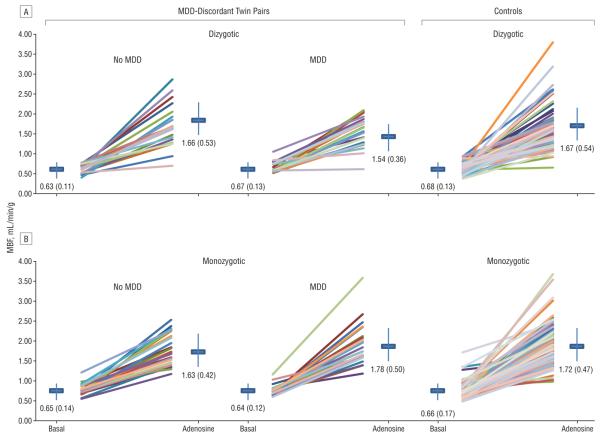
Resting MBF was similar in control twins and MDD-discordant twins (Figure 2). Myocardial blood flow during adenosine-induced hyperemia was also similar in control twins and non-MDD twins of either zygosity. The CFR in the control twins was 2.68 (95% confidence interval [CI], 2.55-2.80), which was similar to the CFR in the non-MDD twins of the discordant pairs, either monozygotic (2.64; 95% CI, 2.35-2.93) or dizygotic (2.74; 95% CI, 2.44-3.05). However, the CFR of the control twins was significantly higher than the CFR of the MDD dizygotic twins (P=.04) but not the MDD monozygotic twins (P=.26), after adjusting for CHD risk factors and statin use.
Figure 2.
Myocardial blood flow (MBF), averaged over the entire left ventricle, at rest (basal) and after adenosine infusion in major depressive disorder (MDD)-discordant and control twin pairs. Each colored line represents a twin pair. The mean (SD) within-pair differences are reported for each condition.
Results were similar when regional myocardial territories (left anterior descending, left circumflex, and right coronary artery) were examined. Major depressive disorder was associated with lower regional CFR in dizygotic but not in monozygotic twin pairs, and the MDD × zygosity interaction was significant (P<.05) in all 3 regions. These results indicate that global myocardial perfusion, rather than specific vascular territories, was reduced in dizygotic brothers with MDD.
ELEVATED DEPRESSIVE SYMPTOMS
There were 35 twin pairs (15 monozygotic and 20 dizygotic) who were discordant for a BDI-II score of 10 or higher. Analysis of these pairs yielded results similar to those of the MDD-discordant pairs. The mean CFR was 17.6% lower in the dizygotic twins with a BDI-II score of 10 or higher than in their brothers with a BDI-II score lower than 10 (2.44 vs 2.96) (P=.02), while no significant difference was found in the monozygotic twin pairs (2.54 vs 2.53) (P=.99). When ordinal BDI-II categories of increasing depressive symptom severity were considered, results were similar. For each increasing category of BDI-II difference between brothers, the mean CFR was 0.19 units lower in the twin with the higher BDI-II score if the twins were dizygotic (P=.049). No difference was found in monozygotic pairs (P=.64). On average, dizygotic twins with a higher BDI-II score than their brothers had a CFR that was 14% lower than their brothers (P=.03).
COMMENT
Using PET myocardial perfusion imaging to evaluate a genetically informative sample of twin pairs discordant for MDD, we found that MDD is associated with lower CFR in dizygotic twin pairs but not in monozygotic twin pairs. Since monozygotic twins are 100% matched on their segregating genes, and dizygotic twins are only 50% matched, this finding suggests a shared genetic pathway between MDD and CFR. In addition, we found that the association is independent of perfusion defects, suggesting that the microvascular circulation is involved. Our results are consistent with the hypothesis of a common genetic substrate linking MDD and microvascular dysfunction. These results have substantial implications for our understanding of the link between MDD and CHD. First, microvascular disease may be an important link between MDD and CHD risk. Second, genetic susceptibility plays a role. Thus, not all individuals with MDD may be at increased risk of CHD.
MDD AND MYOCARDIAL PERFUSION
Major depressive disorder is associated with CHD incidence and mortality, but results are heterogeneous across studies.1,2 Furthermore, in previous research MDD has shown little relationship with indicators of ischemia or coronary disease severity. For example, a recent study in patients with stable CHD found little association between MDD and systolic or diastolic function, inducible ischemia, or wall motion abnormalities.8 Other studies have similarly reported no relationship between MDD and measures of disease severity, such as left ventricula rejection fraction and Killip class,4-6,9-11 angiographically determined coronary stenosis,3-5 and coronary calcification,7,33 with few exceptions.34-37
To our knowledge, this is the first study to examine the relationship between MDD and inducible ischemia in a predominantly healthy sample. Using PET imaging, we found that MDD was not associated with myocardial perfusion defects, either at rest or after pharmacologic stress with adenosine. However, the myocardial vasodilator response to adenosine was blunted and the CFR lower in the dizygotic twins with MDD than in their brothers without MDD. A reduction in global hyperemic response despite no difference in perfusion defects suggests an involvement of the coronary microcirculation.14
An impairment of coronary circulatory function reflects a proatherosclerotic state with substantial diagnostic and prognostic implications.38,39 Positron emission tomography is considered the gold standard for the noninvasive assessment of coronary circulatory function that can be assessed by pharmacologically increasing MBF.40 It is now well established that abnormalities in coronary perfusion may be altered by pathophysiologic conditions unrelated to stenosis,41,42 and patients with CHD risk factors such as hypercholesterolemia, hypertension, and diabetes mellitus have perfusion abnormalities even in the absence of coronary occlusion, suggesting coronary microcirculatory dysfunction.16,43-46 Our results suggest that, similar to traditional risk factors, MDD is associated with perfusion abnormalities compatible with microvascular dysfunction.
The underlying causes of microvascular disease are multifactorial, and adenosine-induced hyperemic response (and CFR) is a mixture of both endothelial-dependent and independent pathways.40 Oxidant stress is a modulator of vasomotor tone and likely plays a role.47 Other active processes may be involved, such as inflammation, smooth muscle cell proliferation, apoptosis, and the expression of vascular cellular adhesion molecules, creating a state of endothelial activation that may contribute to atherosclerosis40 and plaque vulnerability.48,49 In addition to playing a role in cardiovascular diseases, oxidant stress, inflammation, and immune activation are associated with MDD.50-52 Thus, it is reasonable to hypothesize that these processes may represent the common link between MDD and coronary vascular dysfunction.
GENETIC INFLUENCES
Our results suggest that the link between MDD and CFR is, at least in part, due to shared genes: no difference was found in the monozygotic twin pairs who are fully matched for genetic factors. Our data substantiate the hypothesis that shared genes underlie the association between MDD and CHD.20 An earlier study from the VET Registry53 based on survey data collected in 1990 and 1992 found a significant genetic correlation of 0.42 between MDD, assessed with the DIS, and self-reported heart disease. Our findings are in line with those of this earlier study, which was limited by self-reported data. Our results, however, provide important new information pointing to microvascular disease as a key component of the shared genetic pathway between MDD and CHD. The fact that adjusting for smoking somewhat weakened the association in the dizygotic pairs suggests that inherited factors may act in concert with behavioral factors to increase microvascular disease in subjects with MDD.
In addition to having a lower CFR than their twin brothers, dizygotic, but not monozygotic, twins with MDD had a lower CFR than unrelated control twins. This finding confirms that shared genes are implicated in the association rather than familial environment, which is postulated to be shared to a similar extent in monozygotic and dizygotic twins. In fact, shared genetic liability between MDD and CFR can only involve cases of MDD that are heritable. The case mix of MDD in the discordant dizygotic twin pairs is on average more likely to be linked to genetic factors than the mix in the monozygotic pairs, since by definition any differences between monozygotic twins are due to the environment.
Although to our knowledge, no studies examining linkage or candidate gene associations have studied both MDD and CHD, 2 linkage intervals appear to overlap, 2q33 to 35 and 15q25.3 to 26.2. These regions contain candidate genes related to serotonin and inflammation, 2 pathways that are biologically relevant to both MDD and CHD.20 In addition, genes that encode key elements of the autonomic nervous system or the hypothalamic-pituitary-adrenal axis may be related to both MDD and cardiac function.54-59 Additional pathways may include the methylenetetrahydrofolate reductase (MTHFR) gene and in the apolipoprotein Egene, which have been associated with both MDD and CHD in separate studies.60-62
Shared genetic pathways may define common etiologic mechanisms across multiple chronic conditions. For example, genetic liability toward inflammation, oxidative stress, metabolic abnormalities, or autonomic dysfunction may predispose to cardiovascular diseases, diabetes, cancer, and psychiatricand/or neurodegenerative diseases.50,63,64 Consistent with this hypothesis, our research group recently found evidence for shared genetic pathways between MDD and inflammation22 and between MDD and autonomic function.21 A shared genetic liability with MDD has even been described for behavioral risk factors such as physical activity65 and smoking.66
Our results also provide a clue to the inconsistencies in previous literature concerning the association between MDD and CHD. If genetically predisposed individuals with MDD are those at highest risk, the genetic admixture of the population may be the reason for the inconsistent findings.
LIMITATIONS
Our study is cross-sectional and thus limited in its ability to evaluate the temporal relationship between MDD and CFR. However, the covariation of these 2 phenotypes may mostly depend on a common genetic precursor rather than be a cause-effect relationship.
Another limitation is that few twins met the criteria for a current major depressive episode, preventing us from examining the data in relation to current vs past MDD. However, we did have information on current depressive symptoms, and the analysis of depressive symptoms confirmed the MDD results. On the other hand, the fact that a relationship between MDD and CFR is seen in persons mostly in remission is clinically important because it indicates that such a relationship persists over time and may be of significance for chronic processes such as CHD.
Residual confounding is always a possible limitation of any observational study. However, we had information on a variety of potential confounding factors. Furthermore, the analysis of twin pairs matched for family background represents an effective strategy to control for many unmeasured confounding factors.
Although our analysis provides evidence for a common genetic substrate between MDD and CFR, we were not able to quantify the proportion of the phenotypic correlation that is due to heritable factors. Our research design systematically selected MDD-discordant pairs. This precluded genetic modeling of heritability for MDD because we did not have a random sample of the full registry. By design, our study targets the portion of heritability of MDD that might be shared with CFR, which is likely only a portion of the total heritability.
Finally, because our twins were middle-aged male military veterans, caution should be used in generalizing our results to different populations. Our study provides the foundation for future investigations in diverse sociodemographic groups.
In conclusion, our results suggest a link between MDD and microvascular dysfunction that is largely due to a shared genetic substrate. Thus, common pathophysiologic processes may link MDD and early atherosclerosis. Our data indicate that the influence of MDD on CHD risk is at least in part heritable and that genetically predisposed individuals could be at risk for both MDD and CHD.
Acknowledgments
Funding/Support: This study was supported by grants K24HL077506, R01 HL68630, R01 AG026255 (Dr Vaccarino), and K24 MH076955 (Dr Bremner) from the National Institutes of Health (NIH); Emory University General Clinical Research Center grant MO1-RR00039; and grant 0245115N from the American Heart Association (Dr Vaccarino). The US Department of Veterans Affairs has provided financial support for the development and maintenance of the Vietnam Era Twin (VET) Registry.
Role of the Sponsors: The funding agencies had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Financial Disclosure: Dr Faber is a consultant for, shareholder in, and receives royalties from Syntermed Inc, which licenses the Emory Cardiac Toolbox, used for some analyses in this study.
REFERENCES
- 1.Nicholson A, Kuper H, Hemingway H. Depression as an aetiologic and prognostic factor in coronary heart disease: a meta-analysis of 6362 events among 146 538 participants in 54 observational studies. Eur Heart J. 2006;27(23):2763–2774. doi: 10.1093/eurheartj/ehl338. [DOI] [PubMed] [Google Scholar]
- 2.Rugulies R. Depression as a predictor for coronary heart disease: a review and meta-analysis. Am J Prev Med. 2002;23(1):51–61. doi: 10.1016/s0749-3797(02)00439-7. [DOI] [PubMed] [Google Scholar]
- 3.Vaccarino V, Johnson BD, Sheps DS, et al. National Heart, Lung, and Blood Institute Depression, inflammation, and incident cardiovascular disease in women with suspected coronary ischemia: the National Heart, Lung, and Blood Institute-sponsored WISE study. J Am Coll Cardiol. 2007;50(21):2044–2050. doi: 10.1016/j.jacc.2007.07.069. [DOI] [PubMed] [Google Scholar]
- 4.Lespérance F, Frasure-Smith N, Theroux P, Irwin M. The association between major depression and levels of soluble intercellular adhesion molecule 1, interleukin-6, and C-reactive protein in patients with recent acute coronary syndromes. Am J Psychiatry. 2004;161(2):271–277. doi: 10.1176/appi.ajp.161.2.271. [DOI] [PubMed] [Google Scholar]
- 5.Barefoot JC, Helms MJ, Mark DB, et al. Depression and long-term mortality risk in patients with coronary artery disease. Am J Cardiol. 1996;78(6):613–617. doi: 10.1016/s0002-9149(96)00380-3. [DOI] [PubMed] [Google Scholar]
- 6.Lane D, Ring C, Lip GYH, Carroll D. Depression, indirect clinical markers of cardiac disease severity, and mortality following myocardial infarction. Heart. 2005;91(4):531–532. doi: 10.1136/hrt.2004.036392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diez Roux AV, Ranjit N, Powell L, et al. Psychosocial factors and coronary calcium in adults without clinical cardiovascular disease. Ann Intern Med. 2006;144(11):822–831. doi: 10.7326/0003-4819-144-11-200606060-00008. [DOI] [PubMed] [Google Scholar]
- 8.Lett H, Ali S, Whooley M. Depression and cardiac function in patients with stable coronary heart disease: findings from the Heart and Soul Study. Psychosom Med. 2008;70(4):444–449. doi: 10.1097/PSY.0b013e31816c3c5c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blumenthal JA, Lett HS, Babyak MA, et al. NORG Investigators Depression as a risk factor for mortality after coronary artery bypass surgery. Lancet. 2003;362(9384):604–609. doi: 10.1016/S0140-6736(03)14190-6. [DOI] [PubMed] [Google Scholar]
- 10.Jiang W, Alexander J, Christopher E, et al. Relationship of depression to increased risk of mortality and rehospitalization in patients with congestive heart failure. Arch Intern Med. 2001;161(15):1849–1856. doi: 10.1001/archinte.161.15.1849. [DOI] [PubMed] [Google Scholar]
- 11.Dickens C, McGowan L, Percival C, et al. New onset depression following myocardial infarction predicts cardiac mortality. Psychosom Med. 2008;70(4):450–455. doi: 10.1097/PSY.0b013e31816a74de. [DOI] [PubMed] [Google Scholar]
- 12.Kaul S, Ito H. Microvasculature in acute myocardial ischemia, I: evolving concepts in pathophysiology, diagnosis, and treatment. Circulation. 2004;109(2):146–149. doi: 10.1161/01.CIR.0000111582.02736.CD. [DOI] [PubMed] [Google Scholar]
- 13.de Silva R, Camici PG. Role of positron emission tomography in the investigation of human coronary circulatory function. Cardiovasc Res. 1994;28(11):1595–1612. doi: 10.1093/cvr/28.11.1595. [DOI] [PubMed] [Google Scholar]
- 14.Kaufmann PA, Camici PG. Myocardial blood flow measurement by PET: technical aspects and clinical applications. J Nucl Med. 2005;46(1):75–88. [PubMed] [Google Scholar]
- 15.Hasdai D. Endothelium-independent coronary microvascular function: all vessels are not created equally. Am Heart J. 2003;145(4):567–568. doi: 10.1067/mhj.2003.96. [DOI] [PubMed] [Google Scholar]
- 16.Di Carli MF, Janisse J, Grunberger G, Ager J. Role of chronic hyperglycemia in the pathogenesis of coronary microvascular dysfunction in diabetes. J Am Coll Cardiol. 2003;41(8):1387–1393. doi: 10.1016/s0735-1097(03)00166-9. [DOI] [PubMed] [Google Scholar]
- 17.Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: review and meta-analysis. Am J Psychiatry. 2000;157(10):1552–1562. doi: 10.1176/appi.ajp.157.10.1552. [DOI] [PubMed] [Google Scholar]
- 18.Scheuner MT. Genetic predisposition to coronary artery disease. Curr Opin Cardiol. 2001;16(4):251–260. doi: 10.1097/00001573-200107000-00006. [DOI] [PubMed] [Google Scholar]
- 19.de Geus EJ. Genetic pleiotropy in depression and coronary artery disease. Psychosom Med. 2006;68(2):185–186. doi: 10.1097/01.psy.0000208628.90274.bc. [DOI] [PubMed] [Google Scholar]
- 20.McCaffery JM, Frasure-Smith N, Dube MP, et al. Common genetic vulnerability to depressive symptoms and coronary artery disease: a review and development of candidate genes related to inflammation and serotonin. Psychosom Med. 2006;68(2):187–200. doi: 10.1097/01.psy.0000208630.79271.a0. [DOI] [PubMed] [Google Scholar]
- 21.Vaccarino V, Lampert R, Bremner JD, et al. Depressive symptoms and heart rate variability: evidence for a shared genetic substrate in a study of twins. Psychosom Med. 2008;70(6):628–636. doi: 10.1097/PSY.0b013e31817bcc9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vaccarino V, Brennan ML, Miller AH, et al. Association of major depressive disorder with serum myeloperoxidase and other markers of inflammation: a twin study. Biol Psychiatry. 2008;64(6):476–483. doi: 10.1016/j.biopsych.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldberg J, Curran B, Vitek ME, Henderson WG, Boyko EJ. The Vietnam Era Twin Registry. Twin Res. 2002;5(5):476–481. doi: 10.1375/136905202320906318. [DOI] [PubMed] [Google Scholar]
- 24.Robins LN, Helzer JE, Croughan J, Ratcliff KS. National Institute of Mental Health Diagnostic Interview Schedule: its history, characteristics, and validity. Arch Gen Psychiatry. 1981;38(4):381–389. doi: 10.1001/archpsyc.1981.01780290015001. [DOI] [PubMed] [Google Scholar]
- 25.First MB, Spitzer RL, Williams JBW, Gibbon M. Structured Clinical Interview for DSM IV–Patient Edition (SCID-P-P) American Psychiatric Press; Washington, DC: 1995. [Google Scholar]
- 26.Beck AT, Steer RA, Brown GK. BDI-II: Beck Depression Inventory. 2nd ed. Psychological Corp; San Antonio, TX: 1996. [Google Scholar]
- 27.Hutchins GD, Schwaiger M, Rosenspire KC, Krivokapich J, Schelbert H, Kuhl DE. Noninvasive quantification of regional blood flow in the human heart using N-13 ammonia and dynamic positron emission tomographic imaging. J Am Coll Cardiol. 1990;15(5):1032–1042. doi: 10.1016/0735-1097(90)90237-j. [DOI] [PubMed] [Google Scholar]
- 28.El Fakhri G, Sitek A, Guerin B, Kijewski MF, Di Carli MF, Moore SC. Quantitative dynamic cardiac 82Rb PET using generalized factor and compartment analyses. J Nucl Med. 2005;46(8):1264–1271. [PubMed] [Google Scholar]
- 29.Hachamovitch R, Berman DS, Kiat H, et al. Exercise myocardial perfusion SPECT in patients without known coronary artery disease: incremental prognostic value and use in risk stratification. Circulation. 1996;93(5):905–914. doi: 10.1161/01.cir.93.5.905. [DOI] [PubMed] [Google Scholar]
- 30.Richardson MT, Ainsworth BE, Wu HC, Jacobs DR, Jr, Leon AS. Ability of the Atherosclerosis Risk in Communities (ARIC)/Baecke Questionnaire to assess leisure-time physical activity. Int J Epidemiol. 1995;24(4):685–693. doi: 10.1093/ije/24.4.685. [DOI] [PubMed] [Google Scholar]
- 31.Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97(18):1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 32.Di Benedetto M, Lindner H, Hare DL, Kent S. Depression following acute coronary syndromes: a comparison between the Cardiac Depression Scale and the Beck Depression Inventory II. J Psychosom Res. 2006;60(1):13–20. doi: 10.1016/j.jpsychores.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 33.O'Malley PG, Jones DL, Feuerstein IM, Taylor AJ. Lack of correlation between psychological factors and subclinical coronary artery disease. N Engl J Med. 2000;343(18):1298–1304. doi: 10.1056/NEJM200011023431803. [DOI] [PubMed] [Google Scholar]
- 34.Frasure-Smith N, Lesperance F, Juneau M, Talajic M, Bourassa MG. Gender, depression and one-year prognosis after myocardial infarction. Psychosom Med. 1999;61(1):26–37. doi: 10.1097/00006842-199901000-00006. [DOI] [PubMed] [Google Scholar]
- 35.Tiemeier H, van Dijck W, Hofman A, Witteman JCM, Stijnen T, Breteler MMB. Relationship between atherosclerosis and late-life depression: The Rotterdam Study. Arch Gen Psychiatry. 2004;61(4):369–376. doi: 10.1001/archpsyc.61.4.369. [DOI] [PubMed] [Google Scholar]
- 36.Agatisa PK, Matthews KA, Bromberger JT, Edmundowicz D, Chang YF, Sutton-Tyrrell K. Coronary and aortic calcification in women with a history of major depression. Arch Intern Med. 2005;165(11):1229–1236. doi: 10.1001/archinte.165.11.1229. [DOI] [PubMed] [Google Scholar]
- 37.Jones DJ, Bromberger JT, Sutton-Tyrrell K, Matthews KA. Lifetime history of depression and carotid atherosclerosis in middle-aged women. Arch Gen Psychiatry. 2003;60(2):153–160. doi: 10.1001/archpsyc.60.2.153. [DOI] [PubMed] [Google Scholar]
- 38.Schindler TH, Nitzsche EU, Schelbert HR, et al. Positron emission tomography-measured abnormal responses of myocardial blood flow to sympathetic stimulation are associated with the risk of developing cardiovascular events. J Am Coll Cardiol. 2005;45(9):1505–1512. doi: 10.1016/j.jacc.2005.01.040. [DOI] [PubMed] [Google Scholar]
- 39.Schächinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation. 2000;101(16):1899–1906. doi: 10.1161/01.cir.101.16.1899. [DOI] [PubMed] [Google Scholar]
- 40.Schindler TH, Zhang XL, Vincenti G, Mhiri L, Lerch R, Schelbert HR. Role of PET in the evaluation and understanding of coronary physiology. J Nucl Cardiol. 2007;14(4):589–603. doi: 10.1016/j.nuclcard.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gould KL, Kirkeeide RL, Buchi M. Coronary flow reserve as a physiologic measure of stenosis severity. J Am Coll Cardiol. 1990;15(2):459–474. doi: 10.1016/s0735-1097(10)80078-6. [DOI] [PubMed] [Google Scholar]
- 42.Beanlands RSB, Muzik O, Melon P, et al. Noninvasive quantification of regional myocardial flow reserve in patients with coronary atherosclerosis using nitrogen-13 ammonia positron emission tomography: determination of extent of altered vascular reactivity. J Am Coll Cardiol. 1995;26(6):1465–1475. doi: 10.1016/0735-1097(95)00359-2. [DOI] [PubMed] [Google Scholar]
- 43.Seiler C, Hess O, Buechi M, Suter T, Krayenbuehl H. Influence of serum cholesterol and other coronary risk factors on vasomotion of angiographically normal coronary arteries. Circulation. 1993;88(5, pt 1):2139–2148. doi: 10.1161/01.cir.88.5.2139. [DOI] [PubMed] [Google Scholar]
- 44.Frielingsdorf J, Kaufmann PG, Seiler C, Vassalli G, Suter TM, Hess OM. Abnormal coronary vasomotion in hypertension: role of coronary artery disease. J Am Coll Cardiol. 1996;28(4):935–941. doi: 10.1016/s0735-1097(96)00260-4. [DOI] [PubMed] [Google Scholar]
- 45.Nitenberg A, Valensi P, Sachs R, Dali M, Aptecar E, Attali J. Impairment of coronary vascular reserve and ACh-induced coronary vasodilation in diabetic patients with angiographically normal coronary arteries and normal left ventricular systolic function. Diabetes. 1993;42(7):1017–1025. doi: 10.2337/diab.42.7.1017. [DOI] [PubMed] [Google Scholar]
- 46.Zeiher AM, Schachinger V, Minners J. Long-term cigarette smoking impairs endothelium-dependent coronary arterial vasodilator function. Circulation. 1995;92(5):1094–1100. doi: 10.1161/01.cir.92.5.1094. [DOI] [PubMed] [Google Scholar]
- 47.Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. 2000;87(10):840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- 48.Tomai F, Crea F, Gaspardone A, et al. Unstable angina and elevated C-reactive protein levels predict enhanced vasoreactivity of the culprit lesion. Circulation. 2001;104(13):1471–1476. doi: 10.1161/hc3801.096354. [DOI] [PubMed] [Google Scholar]
- 49.Topol EJ, Yadav JS. Recognition of the importance of embolization in atherosclerotic vascular disease. Circulation. 2000;101(5):570–580. doi: 10.1161/01.cir.101.5.570. [DOI] [PubMed] [Google Scholar]
- 50.Berk M, Ng F, Dean O, Dodd S, Bush AI. Glutathione: a novel treatment target in psychiatry. Trends Pharmacol Sci. 2008;29(7):346–351. doi: 10.1016/j.tips.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 51.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27(1):24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kiecolt-Glaser JK, Glaser R. Depression and immune function: central pathways to morbidity and mortality. J Psychosom Res. 2002;53(4):873–876. doi: 10.1016/s0022-3999(02)00309-4. [DOI] [PubMed] [Google Scholar]
- 53.Scherrer JF, Xian H, Bucholz KK, et al. A twin study of depression symptoms, hypertension, and heart disease in middle-aged men. Psychosom Med. 2003;65(4):548–557. doi: 10.1097/01.psy.0000077507.29863.cb. [DOI] [PubMed] [Google Scholar]
- 54.Federenko I, Wust S, Hellhammer DH, Dechoux R, Kumsta R, Kirschbaum C. Free cortisol awakening responses are influenced by awakening time. Psychoneuroendocrinology. 2004;29(2):174–184. doi: 10.1016/s0306-4530(03)00021-0. [DOI] [PubMed] [Google Scholar]
- 55.Finley JC, Jr, O'Leary M, Wester D, et al. A genetic polymorphism of the alpha2-adrenergic receptor increases autonomic responses to stress. J Appl Physiol. 2004;96(6):2231–2239. doi: 10.1152/japplphysiol.00527.2003. [DOI] [PubMed] [Google Scholar]
- 56.Li GH, Faulhaber HD, Rosenthal M, et al. Beta-2 adrenergic receptor gene variations and blood pressure under stress in normal twins. Psychophysiology. 2001;38(3):485–489. [PubMed] [Google Scholar]
- 57.McCaffery JM, Pogue-Geile MF, Ferrell RE, Petro N, Manuck SB. Variability within alpha- and beta-adrenoreceptor genes as a predictor of cardiovascular function at rest and in response to mental challenge. J Hypertens. 2002;20(6):1105–1114. doi: 10.1097/00004872-200206000-00021. [DOI] [PubMed] [Google Scholar]
- 58.Neumann SA, Lawrence EC, Jennings JR, Ferrell RE, Manuck SB. Heart rate variability is associated with polymorphic variation in the choline transporter gene. Psychosom Med. 2005;67(2):168–171. doi: 10.1097/01.psy.0000155671.90861.c2. [DOI] [PubMed] [Google Scholar]
- 59.Wüst S, Van Rossum EF, Federenko IS, Koper JW, Kumsta R, Hellhammer DH. Common polymorphisms in the glucocorticoid receptor gene are associated with adrenocortical responses to psychosocial stress. J Clin Endocrinol Metab. 2004;89(2):565–573. doi: 10.1210/jc.2003-031148. [DOI] [PubMed] [Google Scholar]
- 60.López-León S, Janssens AC, Gonzalez-Zuloeta Ladd AM, et al. Meta-analyses of genetic studies on major depressive disorder. Mol Psychiatry. 2008;13(8):772–785. doi: 10.1038/sj.mp.4002088. [DOI] [PubMed] [Google Scholar]
- 61.Klerk M, Verhoef P, Clarke R, Blom HJ, Kok FJ, Schouten EG, MTHFR Studies Collaboration Group MTHFR 677C→T polymorphism and risk of coronary heart disease: a meta-analysis. JAMA. 2002;288(16):2023–2031. doi: 10.1001/jama.288.16.2023. [DOI] [PubMed] [Google Scholar]
- 62.Song Y, Stampfer MJ, Liu S. Meta-analysis: apolipoprotein E genotypes and risk for coronary heart disease. Ann Intern Med. 2004;141(2):137–147. doi: 10.7326/0003-4819-141-2-200407200-00013. [DOI] [PubMed] [Google Scholar]
- 63.Elenkov IJ, Iezzoni DG, Daly A, Harris AG, Chrousos GP. Cytokine dysregulation, inflammation and well-being. Neuroimmunomodulation. 2005;12(5):255–269. doi: 10.1159/000087104. [DOI] [PubMed] [Google Scholar]
- 64.Tracy RP. Emerging relationships of inflammation, cardiovascular disease and chronic diseases of aging. Int J Obes Relat Metab Disord. 2003;27(suppl 3):S29–S34. doi: 10.1038/sj.ijo.0802497. [DOI] [PubMed] [Google Scholar]
- 65.De Moor MH, Boomsma DI, Stubbe JH, Willemsen G, de Geus EJ. Testing causality in the association between regular exercise and symptoms of anxiety and depression. Arch Gen Psychiatry. 2008;65(8):897–905. doi: 10.1001/archpsyc.65.8.897. [DOI] [PubMed] [Google Scholar]
- 66.Kendler KS, Neale MC, Maclean CJ, Heath AC, Eaves LJ, Kessler RC. Smoking and major depression: a causal analysis. Arch Gen Psychiatry. 1993;50(1):36–43. doi: 10.1001/archpsyc.1993.01820130038007. [DOI] [PubMed] [Google Scholar]