Abstract
We have previously shown that there is a deficiency in the structural protein, nonerythroid α spectrin (αIISp), in cells from patients with Fanconi anemia (FA). These studies indicate that this deficiency is due to reduced stability of αIISp and correlates with decreased repair of DNA interstrand cross-links and chromosomal instability in FA cells. An important factor in the stability of αIISp is its susceptibility to cleavage by the protease, μ-calpain. We hypothesized that increased μ-calpain cleavage of αIISp in FA cells leads to increased breakdown of αIISp and that knocking-down expression of μ-calpain in FA cells should restore levels of αIISp and correct a number of the phenotypic defects observed. The results showed that there is increased μ-calpain activity in FA-A, FA-C, FA-D2, FA-F and FA-G cells which could account for the deficiency in αIISp in these FA cells. Protein interaction studies indicated that FANCA and FANCG bind directly to μ-calpain. We hypothesize that this binding may lead to inhibition of μ-calpain activity in normal cells. Knocking-down μ-calpain by siRNA in FA-A cells restored levels of αIISp to normal and reversed a number of the cellular deficiencies in these cells. It corrected the DNA repair defect and the chromosomal instability observed after exposure to a DNA interstrand cross-linking agent. These studies indicated that FA proteins may play an important role in maintaining stability of αIISp in the cell by regulating its cleavage by μ-calpain. Thus by reducing breakdown of αIISp in FA cells, it may be possible to reverse a number of the cellular deficiencies observed in this disorder.
Fanconi anemia (FA)1 is a genetic disorder characterized by bone marrow failure, diverse congenital abnormalities, genomic instability and a marked predisposition to development of cancer (1–4). Two cellular hallmarks of the disorder are chromosomal instability and hypersensitivity to DNA interstrand cross-linking agents (1, 2, 4, 5). This hypersensitivity correlates with a defect in ability to repair DNA interstrand cross-links produced by these agents (2, 4–9). We have shown that the structural protein, nonerythroid α spectrin (αIISp), is present in normal human cell nuclei and that it plays an important role in the repair of DNA interstrand cross-links. It preferentially binds to DNA containing an interstrand cross-link (10); it co-localizes with the crosslink repair protein, XPF, and the Fanconi anemia protein, FANCA, in cross-link induced nuclear foci (11); it co-immunoprecipitates with XPF (11, 12); and antibodies against it inhibit incisions produced by XPF at the site of a DNA interstrand cross-link (10). αIISp also plays an important role in chromosomal stability. This has been demonstrated by our recent studies which show that siRNA mediated silencing of αIISp gene expression in normal human cells results in chromosomal instability as evidenced by increased interchromatid exchanges, fusions/radials and breaks (13). Depletion of αIISp in normal human cells also leads to decreased survival of these cells and decreased formation of damage-induced nuclear foci after DNA interstrand cross-link damage (13). These studies further demonstrate the importance of αIISp in repair of DNA interstrand cross-links.
We have shown that there is a deficiency in αIISp in cells from FA patients and that this deficiency correlates with decreased levels of repair of DNA interstrand cross-links, measured as diminished unscheduled DNA synthesis (UDS) and decreased production of incisions at sites of DNA interstrand cross-links; it also correlates with decreased formation of damage-induced nuclear foci (7, 9, 11). We have shown that in FA cells decreased levels of αIISp are not due to reduced expression of this protein and have proposed that they are due to its reduced stability (14). Since transfection of cells from at least three FA complementation groups, FA-A, FA-C and FA-G, with the corresponding FA cDNAs restored levels of αIISp to normal, these studies further suggest that FA proteins play a role in maintaining αIISp stability in the cell (15). Based on these studies, we have proposed a model for the role of αIISp in repair of DNA interstrand cross-links and its role in the repair defect in FA (10, 11, 16, 17). In this model, in normal human cells, FA proteins are involved in regulation of αIISp stability. After cells are damaged, αIISp binds to DNA at the sites of damage and acts as a scaffold to aid in the recruitment of repair proteins, such as XPF/ERCC1, to these sites, thus enhancing the efficiency of the repair process. In FA cells, deficiencies in FA proteins lead to decreased stability of αIISp and thus to reduced levels of αIISp. This in turn leads to reduced binding of αIISp to damaged DNA and decreased recruitment of repair proteins to the sites of DNA damage, which in turn leads to decreased DNA repair in these cells. αIISp could be particularly important in some of the initial steps of the cross-link repair process which involve incision and unhooking of the cross-link via XPF/ERCC1 (17). This unhooking step is critical in the FA pathway (18). This could be followed by subsequent steps involving localization of monoubiquinitated FANCD2 and additional FA proteins to chromatin, translesion DNA synthesis, nucleotide excision repair (NER) to excise the monoadduct, and homologous recombination and repair (8, 17, 19–22)
αIISp is an essential protein in cells and complete depletion leads to cell death (13, 23–25). However, we have shown using siRNA that αIISp can be knocked down in normal cells to levels found in FA cells (35–38% of normal) and the cells survive (13). These cells display many of the deficiencies characteristic of FA cells such as chromosomal instability, decreased cell survival after crosslink damage, and decreased repair of DNA interstrand cross-links (13). These studies thus suggest that normal maintenance of αIISp is very important. Stability of αIISp in cells is dependent upon a number of factors. Among these is the susceptibility of αIISp to cleavage by the protease, μ-calpain. μ-Calpain cleaves αIISp into distinct cleavage products and this process plays an important role in the regulation of essential cellular functions mediated by αIISp (26–31). It is possible that in FA cells there is increased calpain cleavage of αIISp which leads to increased breakdown of αIISp in these cells. If so, then determining whether decreasing μ-calpain activity in FA cells can lead to restoration of levels of αIISp in these cells would be of importance.
The present studies were therefore carried out to determine whether there is increased activity and/or levels of μ-calpain in FA cells that could account for the deficiency in αIISp in these cells and, if so, whether knocking down μ-calpain in FA cells could restore levels of αIISp and correct some of the deficiencies in these cells. The results showed that in the FA cell lines tested, FA-A, FA-C, FA-D2, FA-F and FA-G, activity, but not levels, of calpain was increased approximately three fold compared to normal. This correlated with an increase in the μ-calpain breakdown product of αIISp observed in FA-A and FA-G cells. Studies on the interaction of several FA proteins with μ-calpain showed that FANCA and FANCG bind directly to μ-calpain. We postulate that this binding may lead to inhibition of μ-calpain activity. This view is strengthened by the observation that in corrected FA-A and FA-G cells, which express the FANCA and FANCG proteins, respectively, μ-calpain activity is decreased to normal levels. Knocking down μ-calpain by siRNA in one of the FA cell lines examined, FA-A, restored levels of αIISp to those found in normal cells and reversed a number of the cellular deficiencies examined in these cells. After the transfected cells were damaged with a DNA interstrand cross-linking agent, cellular survival was enhanced, damage induced αIISp and XPF nuclear foci were observed, and chromosomal stability was increased. These studies thus indicate that enhanced breakdown of αIISp in FA cells may be important in production of a number of the cellular deficiencies observed in this disorder and that by reducing this breakdown it is possible to reverse a number of these deficiencies, which could be of potential therapeutic importance.
EXPERIMENTAL PROCEEDURES
Cell culture and protein extraction
Normal human lymphoblastoid cells (GM3299) were obtained from the Coriell Institute for Medical Research (Camden, NJ) and HeLa S3 cells were obtained from American Type Culture Collection (ATCC) (Manassas, VA). Several FA lymphoblastoid cell lines were examined: FA complementation group A (FA-A) cells (HSC 72) (a gift from Dr. Manuel Buchwald, Hospital for Sick Children, Toronto, Canada); corrected FA-A cells (HSC 72 cells stably transduced with a retroviral vector expressing the FANCA cDNA) (32); FA complementation group C (FA-C) cells (HSC 536) (a gift from Dr. Manuel Buchwald); corrected FA-C cells (HSC 536 cells stably transduced with a retroviral vector expressing the FANCC cDNA) (9); FA complementation group D2 (FA-D2) cells (PD 20) (a gift from Dr. Alan d’Andrea, Harvard Medical School, Boston, MA); FA complementation group F cells (GM 16757) (Coriell Institute for Medical Research, Camden, NJ); FA complementation group G (FA-G) cells (EUFA143) (a gift from Dr. Hans Joenje, Free University Amsterdam, The Netherlands); corrected FA-G cells (EUFA143 cells stably transduced with a retroviral vector expressing the FANCG cDNA) (9). Lymphoblastoid cells were grown in RPMI 1640 medium (Hyclone, Thermo Scientific, Waltham, MA) and HeLa cells were grown in F12K medium (Sigma-Aldrich, St. Louis, MO) as previously described (13).
Chromatin-associated protein extracts and nuclear extracts were prepared as previously described (13, 15). Whole cell lysates were obtained by resuspending cells in an Extraction Buffer (Biovision Inc., Mountain View, CA) following the manufacturer’s protocol.
Calpain activity assay
Calpain activity was determined using a calpain activity assay kit (Biovision) according to the manufacturer’s protocol. Briefly, chromatin-associated protein extracts from FA-A, FA-C, FA-D2, FA-F, FA-G, corrected FA-A, corrected FA-C, corrected FA-G and normal cells (10 μg) were mixed with a fluorogenic calpain substrate and incubated for 1 hour in a 96 well plate at 37°C in the dark. The fluorescence intensity at 400 nm excitation and 505 nm emission wavelengths was measured using a Perkin Elmer Victor3 V plate reader (Perkin Elmer, Waltham, MA).
Immunoblot analysis
For analysis of levels of μ-calpain in normal, FA, and corrected FA cells, chromatin-associated protein extracts were subjected to SDS-PAGE and western blot analysis was carried out as previously described (10, 15). Levels of μ-calpain αIISp breakdown product were determined by western blot analysis of chromatin-associated extracts from normal, FA-A and FA-G cells. For analysis of levels of μ-calpain and αIISp in μ-calpain siRNA and nontarget siRNA transfected cells, whole cell lysates were prepared and processed as above. Immunoblots were probed with anti-μ-calpain (Santa Cruz Biotechnology, Santa Cruz, CA), anti α-spectrin (mAb 1622, Chemicon, Temecula, CA), anti μ-calpain αIISp breakdown product against a sequence at the carboxyl end of the amino-terminal fragment generated by μ-calpain cleavage of αII spectrin (28) (gift from Dr. Jon Morrow, Yale University School of Medicine), anti-tubulin (Santa Cruz Biotechnology), or anti-topoisomerase (Calbiochem, Gibbstown, NJ). Immunoblots were developed using Pierce Ultra chemiluminescent substrate (Pierce, Thermo Scientific, Rockford, IL) and then exposed to X-ray film (10, 15). Images were scanned using a Hewlett-Packard ScanJet 4c/T scanner and analyzed with ImageQuant (Molecular Dynamics, Sunnyvale, CA).
siRNA analysis
siRNA against the μ-calpain nucleotides (GUGAAGGAGUUGCGGACAA)was purchased from Dharmacon (Lafayette, CO) and a control non-target siRNA was from Qiagen (Valencia, CA). FA-A and normal cells were transfected with μ-calpain siRNA or non-target siRNA using Lipofectamine 2000 Transfection Reagent (Invitrogen Inc., Carlsbad, CA) as previously described (13). Cell survival was assessed using trypan blue exclusion (13). Cells were harvested at 24, 48 and 72 hoursafter transfection and whole cell protein lysates prepared.
Treatment of cells with mitomycin C and cell survival analysis
FA-A cells transfected with either μ-calpain siRNA (200 pM, 240 pM or 300 pM) or nontarget siRNA and normal cells transfected with 300 pM μ-calpain siRNA or non-target siRNA were treated with mitomycin C (MMC) (Sigma-Aldrich, Corp) (30 – 400 nM) 24 hours after transfection. Cell survival was assessed at 16, 24 and 48 hours after MMC treatment using trypan blue exclusion.
Chromosome analysis
FA-A cells, transfected with either μ-calpain or non-target siRNA, and normal cells, transfected with non-target siRNA, were incubated for 48 hours at 37 °C, 5 % CO2. 24 hours after siRNA transfection, a group of these cells was treated with MMC (30–100 nM) and incubated for another 24 hours. Colcemid (Sigma–Aldrich Corp.) (0.1 μg per ml) was then added to both groups of cells and incubation continued for 2 hours. The cells were harvested and subjected to chromosome analysis as previously described (13). At least 100 metaphases from either μ-calpain or non-target siRNA transfected cells were scored for chromosomal abnormalities. Metaphases were viewed using a Leitz DMRB microscope (Leica, Bannockburn, IL) equipped with a DEI-750 analog camera (Optronics, Goleta, CA). Images were obtained using Image Pro-Plus 6.0 software (Media Cybernetics, Bethesda, MD).
Coimmunoprecipitation of proteins
For examination of coimmunoprecipitation of FA proteins with μ-calpain, nuclear extracts from normal cells were prepared and FA proteins (FANCA, FANCC, FANCF or FANCG) coimmunoprecipitated with anti-μ-calpain or mouse IgG. For this anti-μ-calpain or mouse IgG (Sigma-Aldrich Corp.) was bound to protein G-coated agarose beads (Sigma-Aldrich Corp.) and the binding reactions and IPs were carried out as previously described (10, 15). The IPs were subjected to SDS-PAGE, transferred to nitrocellulose membranes, and immunoblotted as previously described (10, 15). The primary antibodies used were anti-FANCA, anti-FANCC, anti-FANCF, and anti-FANCG (Bethyl Laboratories, Montgomery, TX).
Indirect immunofluorescence
FA-A and normal cells, transfected with either μ-calpain (300 pM) or non-target siRNA, were treated with 400 nM MMC 24 hours after transfection. The cells were harvested 16 hours post damage and examined for nuclear localization of aIISp and XPF foci using indirect immunofluorescence as previously described (11, 13). Primary antibodies used were anti-α-spectrin (mAb 1622, Chemicon), or anti-XPF (Santa Cruz Biotech). Secondary antibodies were Alexafluor 488 goat anti-mouse IgG conjugate or Alexafluor 594 rabbit anti-goat IgG conjugate (Molecular Probes, Invitrogen, Carlsbad, CA). Stained cells were viewed with a Leitz DMRB microscope at 40x and images were captured using a DEI-750 analog camera. Images were imported into a computerized imaging system using Image Pro-Plus 6.0 software and Adobe Photoshop CS and the number of nuclear foci quantitated. 300 cells were counted for each group of cells.
Yeast Two-Hybrid Analysis
Escherichia coli strain DH5α (Invitrogen) was used in the construction and propagation of all plasmid constructs. E. coli were grown in Luria broth at 37 °C. Plasmids were maintained in DH5α under the selection of ampicillin. Saccharomyces cerevisiae strain EGY48 (Origene Technologies, Rockville, MD) was grown in liquid YPD (Q-Biogene, MP Biomedicals, Solon, OH) or YPD agar plates at 30 °C.
Expression constructs were made in vectors supplied by the DupLexA yeast two-hybrid system (OriGene Technologies). LexA DNA-binding domain (BD) constructs were made in the pEG202 vector, and the B42 transcriptional activation domain (AD) constructs were made in the pJG4-5 vector as previously described (16). Vectors containing FANCA, FANCC, FANCG cDNAs were constructed as previously described (16). μ-Calpain cDNA (GeneBank accession number NM_005186) was amplified by PCR frompCMV-XL5 (OriGene Technologies) using Accuprime Pfx DNA polymerase supermix (Invitrogen), and restriction sites were added to the 5′ and 3′ ends of the cDNAs. Primers designed for PCR amplification of the FA and αIISp cDNAs have been previously described (16). Primers for PCR amplification of μ-calpain were flanked by EcoR1 and Xho1 restriction sites (forward primer 5′-GCGCGAATTCATGTCGGAGGAGATC-3′, reverse primer 5′-GCGCCTCGAGTCATGCAAACATGGT-3′).
The DupLEX-A yeast two-hybrid system (OriGene Technologies) was used for yeast two-hybrid analysis as previously described (16). All of the cDNAs were subcloned into the pEG202 (BD) and pJG4-5 (AD) vectors. Transformation of the yeast strains with these vectors and reporter vectors was as previously described (16). After transformation, six yeast colonies were randomly selected and inoculated in rows onto YNB (glucose-His-Trp-ura) X-gal plates and the colonies grown for 2–5 days. Positive interactions between the AD and BD fusion proteins were documented by the presence of blue colonies, which indicated that the reporter gene, β-galactosidase, had been activated. Each experiment was repeated eight to nine times.
RESULTS
Calpain activity in FA cells is greater than in corrected FA cells or normal cells
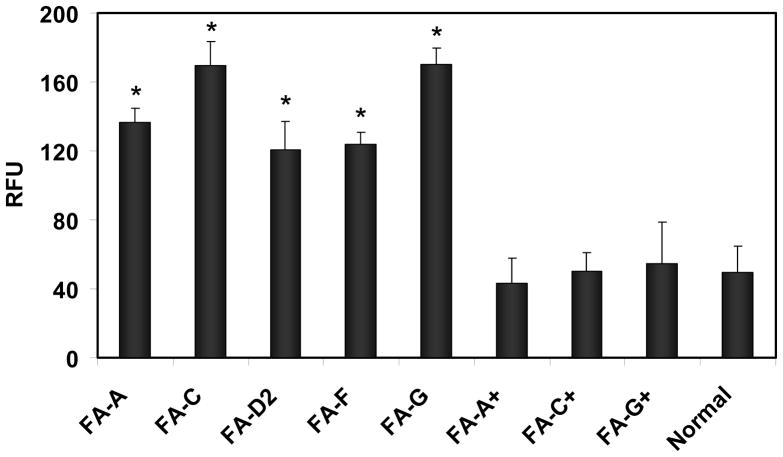
Studies were carried out to determine whether there were differences in the levels of calpain activity in FA cells compared to those found in normal cells. Chromatin-associated protein extracts from FA-A, -C, -D2, -F, -G and normal cells were analyzed for calpain activity, which was determined by measuring cleavage of a fluorogenic substrate and was represented as relative fluorescence units (RFU). The results showed that calpain activity was increased 2.5 fold in FA-A, FA-D2, and FA-F cells as compared to normal cells and 3.5 fold in FA-C and FA-G cells compared to normal cells (Figure 1). These increases in calpain activity in FA cells were statistically significant(p <0.0001).
FIGURE 1.
Calpain activity in FA cells is greater than in corrected FA cells or normal cells. Calpain activity was measured in chromatin-associated protein extracts from FA-A, FA-C, FA-D2, FA-F and FA-G cells and from corrected FA-A (FA-A+), FA-C (FA-C+), and FA-G (FAG+) and compared with this activity from normal cells. Activity was determined by measuring cleavage of a fluorogenic substrate and expressed as relative fluorescence units (RFU). Vertical lines represent ± s.e.m. for 5–8 experiments (p <0.0001).
Calpain activity in corrected FA cells was also examined. The results showed that levels of calpain activity in corrected FA-A, FA-C and FA-G cells were similar to those found in normal cells. No statistical difference was observed between the levels found in corrected FA cells compared to those observed in normal cells (p ≥ 0.6) (Figure 1). We have previously shown that in these corrected FA cells levels of the FANCA, FANCC and FANCG proteins return to normal, respectively (15, 32). Thus when levels of these FA proteins were restored to normal, calpain activity returned to levels similar to those found in normal cells. This suggests that the increase in μ-calpain activity found in FA cells could be due to their deficiency in FA proteins which may play a role in modulating μ-calpain activity.
Protein levels of μ-calpain in FA cells are the same as in corrected FA cells and normal cells
Protein levels of μ-calpain in FA cells were also examined and compared to those found in normal cells and corrected FA cells. For these studies, chromatin-associated protein extracts from FA, corrected FA and normal human lymphoblastoid cells were subjected to western blot analysis. The results showed that there were similar levels of μ-calpain in FA-A, FA-C, FA-D2, FA-F and FA-G cells compared to normal cells (Figure S1A and S1C). No significant differences in μ-calpain levels were observed between the FA cells and normal cells (p ≥ 0.7) (Figure S1C). Similarly, no significant differences were observed in levels of μ-calpain in corrected FA-A, FA-C or FA-G cells compared to normal cells (p ≥ 0.5) (Figure S1B and S1C).
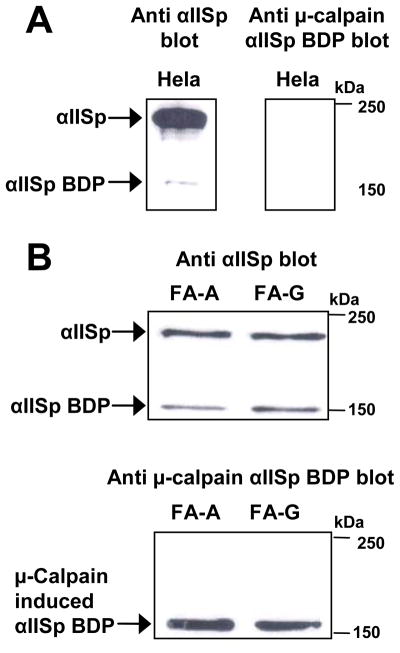
Increased μ-calpain αIISp breakdown product in FA cells
Since μ-calpain activity was shown to be increased in FA cells (Figure 1), studies were undertaken to determine whether there was an increase in μ-calpain cleavage of αIISp in FA cells. Chromatin-associated protein extracts from normal, FA-A and FA-G cells were examined for the presence of the μ-calpain induced αIISp breakdown product. Western blot analysis, using an antibody which specifically recognized the μ-calpain αIISp breakdown product, showed that in HeLa cells there were only very low levels of the 150 kDa αIISp breakdown product produced by μ-calpain (Figure 2A). Similar results were observed in normal lymphoblastoid cells. However, in both FA-A and FA-G cells there were greatly enhanced levels of this 150 kDa breakdown product, which correlated with reduced levels of full length αIISp in these cells compared to normal cells (Figure 2B). These results indicate that in cells from at least two of the FA complementation groups, FA-A and FA-G, there is increased breakdown of αIISp by μ-calpain. This correlates with the increase in μ-calpain activity in these cells (Figure 1).
FIGURE 2.
Increased μ-calpain breakdown product of αIISp in FA cells. Chromatin-associated extracts from (A) HeLa cells and (B) FA-A and FA-G cells were examined for presence of the μ-calpain breakdown product of αIISp (BDP) using western blot analysis. Blots were probed with anti-α spectrin or an antibody specific for the 150 kDa N-terminal μ-calpain cleavage product of αIISp.
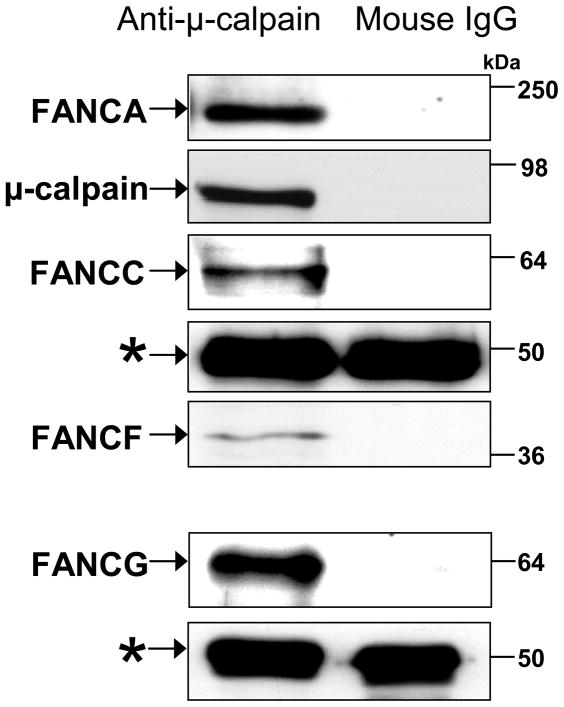
FA proteins coimmunoprecipitate with μ-calpain
If FA proteins are involved in inhibition of μ-calpain activity in normal cells, it is possible that they do so by interacting with this protein. Coimmunoprecipitation studies were therefore carried out in order to ascertain whether FA proteins interact with μ-calpain,. The results showed that FANCA, FANCC, FANCF and FANCG co-immunoprecipitated with μ-calpain in nuclear extracts from normal human lymphoblastoid cells (GM3299) (Figure 3). This indicates that these FA proteins interact either directly or indirectly with μ-calpain. These immunoprecipitations were repeated 8–10 times with similar results.
FIGURE 3.
FA proteins coimmunoprecipitated with μ-calpain. FA proteins that coimmunoprecipitated with μ-calpain from normal human lymphoblastoid (GM3299) cell nuclear extracts were examined by western blot analysis. FA proteins were immunoprecipitated with anti-μ-calpain or mouse IgG1. Immunoblots were probed with anti-FANCA, anti-FANCC, anti-FANCF, anti-FANCG and anti-μ-calpain. The IgG1 heavy chain (*) was used as a loading control. Molecular weight markers are indicated to the right.
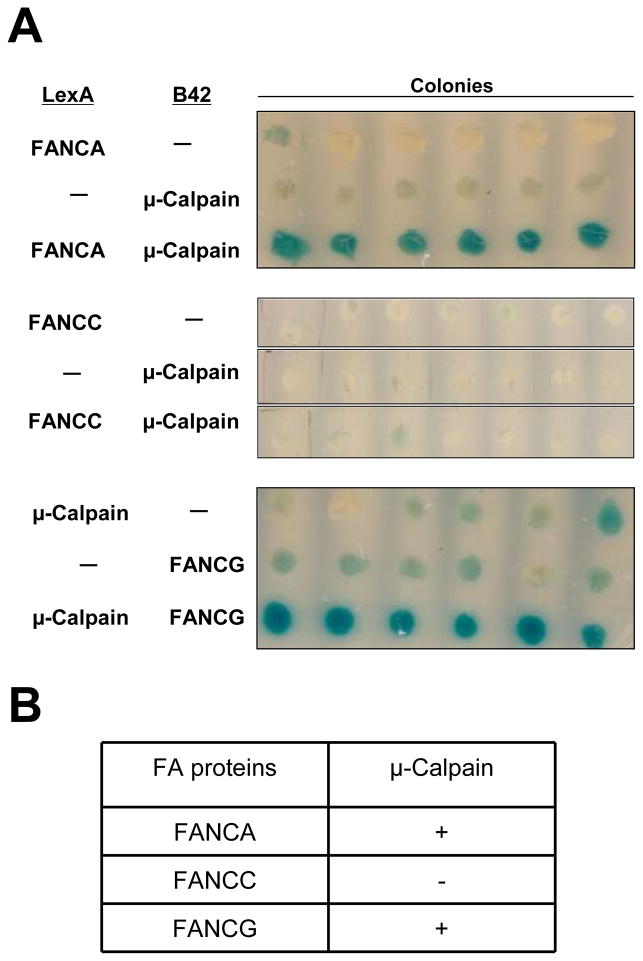
Direct interaction between FA proteins and μ-calpain
To determine whether the FA proteins directly interacted with μ-calpain, yeast two-hybrid analysis was carried out. Three FA proteins were examined, FANCA, FANCC and FANCG. Direct interaction was detected between FANCA and μ-calpain and between FANCG and μ-calpain as evidenced by the development of blue color in the colonies, which indicted activation of the β-galactosidase reporter (Figure 4A). Direct interaction was not detected between FANCC and μ-calpain as seen by the presence of white colonies, which indicated the reporter gene had not been activated (Figure 4A). The results shown are from a single experiment in which six randomly chosen colonies were examined. Each of these binding experiments was repeated eight to nine times with similar results (Figure 4B).
FIGURE 4.
FANCA and FANCG, but not FANCC bind directly to μ-calpain. Yeast two-hybrid analysis of the interaction of FANCA, FANCC and FANCG to μ-calpain was examined. (A) LexA-FANCA or LexA-FANCC fusion proteins were co-expressed in yeast with either an empty vector (−) or the B42 construct containing μ-calpain; B42-FANCG fusion protein was coexpressed in yeast with an empty vector or a LexA-μ-calpain construct. Six randomly selected colonies from each of these yeast transformations were replica plated in a row to plates containing X-gal to test for activity for the reporter gene, β-galactosidase. Positive interaction between fusion proteins is seen by the presence of blue colonies, which indicates that the reporter gene has been activated. White colonies indicate no positive protein interactions. (B) Summary of the results of yeast two-hybrid analysis of the interaction of FANCA, FANCC and FANCG with μ-calpain: (+) good binding; (−) no binding.
Knocking down μ-calpain in FA-A cells by siRNA restores levels of αIISp to those found in normal cells
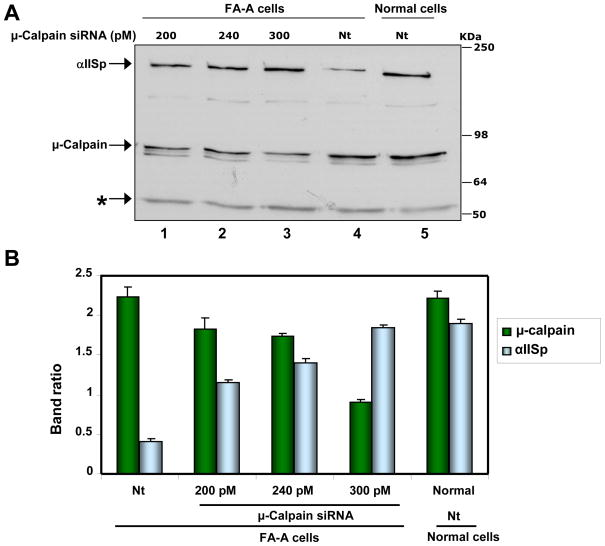
Studies were undertaken to determine whether knocking down expression of μ-calpain in cells from one of the FA complementation groups, FA-A, could increase the levels of αIISp in these cells. The results showed that transient transfection of FA-A cells withμ-calpain siRNA led to an increase in levels of αIISp. This increase was dependent upon the concentration of μ-calpain siRNA transfected: as the amount increased, the levels of μ-calpain observed decreased and the levels of αIISp increased (Figure 5A, lanes 1–4; Figure 5B). Immunoblot analysis showed that 24 hours after transfection with 300 pmol of μ-calpain siRNA levels of μ-calpain were slightly decreased (Figure S2). However, by 48 hours after transfection with 300 pmol of μ-calpain siRNA, levels of μ-calpain were reduced to 44% (Figure 5A, lane 3; Figure 5B; Figure S2, lane 5) of those found in FA-A cells transfected with non-target (Nt) siRNA (Figure 5A, lane 4; Figure 5B; Figure S2, lane 2) or in Nt siRNA transfected normal cells (Figure 5A, lane 5; Figure 5B; Figure S2, lane 11). This decrease in μ-calpain levels in μ-calpain siRNA transfected FA-A cells was significant compared to levels found in Nt siRNA transfected FA-A and normal cells (p < 0.0001). This decrease in μ-calpain levels was associated with an increase in αIISp levels. After transfection of FA-A cells with 300 pmole of μ-calpain siRNA, levels of αIISp had significantly increased by 48 hours (Figure 5A, lane 3; Figure 5B; Figure S2, lane 5) compared to the levels of αIISp found in Nt siRNA transfected FA-A cells (p > 0.001) (Figure 5A, lane 4; Figure 5B; Figure S2, lane 2). These levels of αIISp were similar to those found in Nt siRNA transfected normal cells; no significant difference was observed (p ≥ 0.5) (Figure 5A, lane 5; Figure 5B; Figure S2, lane 11). Thus by knocking down μ-calpain in FA-A cells, it was possible to restore levels of αIISp to those found in normal cells. By 72 hours after μ-calpain siRNA transfection, levels of μ-calpain in FA-A cells had started to increase again and this was accompanied by a decrease in the levels of αIISp (Figure S2).
FIGURE 5.
Knocking down μ-calpain in FA-A cells by siRNA restores levels of αIISp to those found in normal cells. (A) FA-A cells were transiently transfected with 200, 240 or 300 pM μ-calpain siRNA or nontarget (Nt) siRNA. 48 hours after transfection, levels of μ-calpain and αIISp in the FA-A and normal cells were examined by western blot analysis. Immunoblots were probed with anti-μ-calpain and anti-αIISp. Tubulin (*) was used as a loading control. (B) Immunoblots were scanned and levels of μ-calpain and αIISp quantitated. Vertical lines represent ± s.e.m. for 5–8 experiments.
Knocking down levels of μ-calpain in FA-A cells by siRNA did not lead to an increase in the levels of FANCA (Figure S2). It also did not lead to any change in FA-A cells in levels of FANCC, which were lower than in normal cells (Figure S2), as has been shown by other investigators (4, 33).
Cell survival of these FA-A cells at 24 hours, 48 hours and 72 hours after transfection with 300 pmol μ-calpain siRNA or non-target siRNA was 96% (data not shown). Thus reducing μ-calpain in FA-A cells to levels which allowed restoration of αIISp to levels found in normal cells had little effect on the viability of these cells.
siRNA knockdown of μ-calpain in FA-A cells leads to increased cell viabilityl and nuclear foci formation after damage with a DNA interstrand cross-linking agent
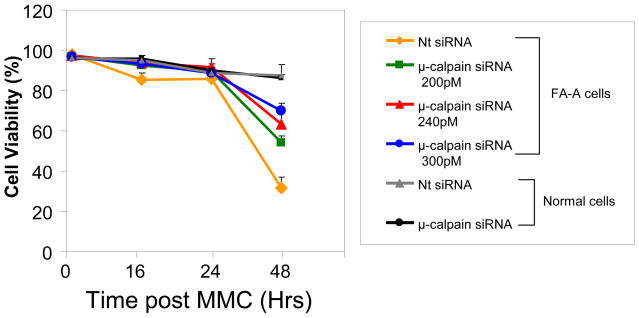
Whether knockdown of μ-calpain by siRNA in FA-A cells could enhance viability of these cells after damage with a DNA interstrand crosslinking agent, MMC, was examined. Non-target siRNA transfected FA-A cells showed a dramatic decrease in cell viability 48 hours after treatment with MMC (400 nM). Cell viability was 36%of that of MMC treated normal cells transfected with nontartet siRNA (Figure 6). Transfection of FA-A cells with μ-calpain siRNA increased the resistance of these cells to MMC. 48 hours after treatment with MMC (400 nM) viability of FA-A cells transfected with 300 pM of μ-calpain siRNA was increased to 85% of that of MMC treated normal cells transfected with Nt siRNA or μ-calpain siRNA (Figure 6).
FIGURE 6.
siRNA knockdown of μ-calpain in FA-A cells leads to increased cell viability after damage with MMC. FA-A cells were transfected with 200, 240 or 300 pM μ-calpain siRNA or nontarget (Nt) siRNA. Normal cells were transfected with Nt siRNA or 300 pM μ-calpain siRNA. Cells were treated with MMC (400 nM) 24 hr after transfection and cell viability was examined 0 hr, 16 hr, 24 hr, and 48 hr after MMC treatment. Vertical lines represent ± s.e.m. for 5–8 experiments.
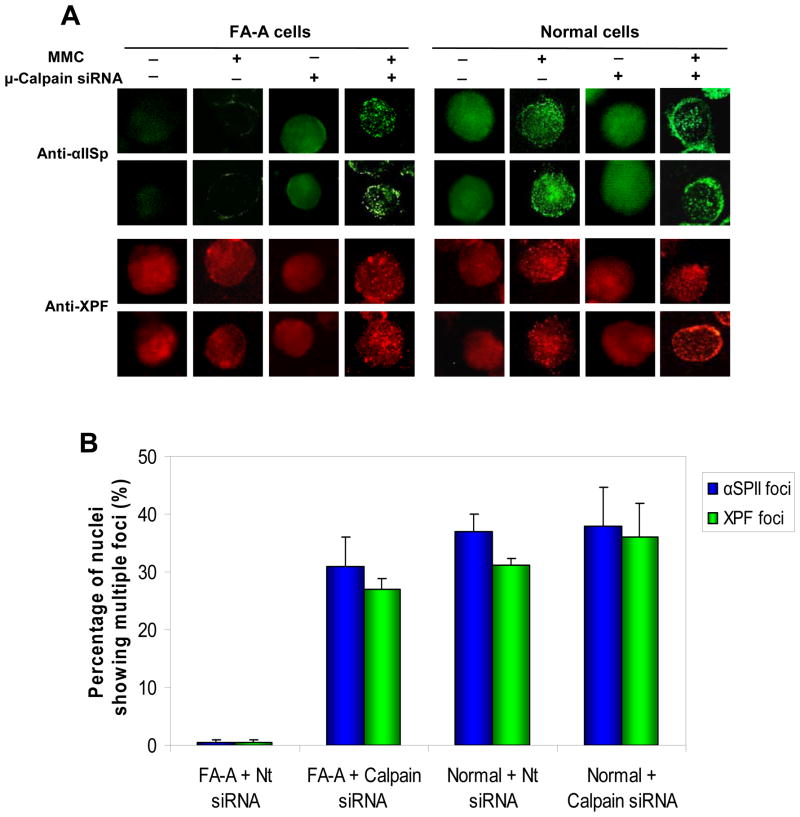
Whether knockdown of μ-calpain had an influence on the ability of FA-A cells to form damage-induced nuclear foci was also examined. Immunofluorescence studies showed that in undamaged normal cells, after transfection with nontarget siRNA or μ-calpain siRNA (40 hours post transfection), αIISp and the cross-link repair protein XPF were present in the nucleus in a diffuse pattern (Figure 7A). However, in the nontarget siRNA transfected and μ-calpain siRNA transfected normal cells, 16 hours after treatment with 400 nM MMC (40 hours post transfection) both αIISp and XPF were localized in nuclear foci (Figure 7A). These results are similar to those we have previously obtained in normal cells in which we have shown that 15 hours after damage with another DNA interstrand cross-linking agent, 8-methoxypsoralen (8-MOP) plus UVA light, both αIISp and XPF nuclear foci are observed (11). In contrast, in undamaged, non-target transfected FA-A cells, 40 hours after transfection, the nuclear staining pattern of αIISp was diffuse and markedly less intense than in undamaged normal cells (Figure 7A). This is consistent with reduced levels of αIISp in these cells (15). Examination of XPF in the undamaged, non-target transfected FA-A cell nuclei showed that there was a diffuse pattern of XPF staining, which was similar to that seen in normal cells (Figure 7A). This correlates with our previous studies which have shown that there are similar levels of XPF in FA-A and normal cells (11). Only a few XPF foci were observed in the nuclei of non-target siRNA transfected FA-A cells 16 hours after treatment with MMC (400 nM) (40 hours post transfection) (Figure 7A), which is similar to our previous findings (11).
FIGURE 7.
siRNA knockdown of μ-calpain in FA-A cells leads to formation of αIISp and XPF nuclear foci after damage with MMC. (A) FA-A cells were transfected with non-target siRNA (−) or 300 pM μ-calpain siRNA (+). Normal cells were transfected with Nt siRNA (−) or 300 pM μ-calpain siRNA (+). 24 hr after transfection, cells were either undamaged or treated with MMC (400 nM). Formation of αIISp and XPF nuclear foci was examined 16 hr after MMC treatment using indirect immunoflourescence and staining with anti-αIISp or anti-XPF. (B) The percentage of the nuclei showing multiple αIISp and XPF nuclear foci (> 10 foci) in normal and FA-A cells after transfection with non-target (Nt) siRNA or μ-calpain siRNA and MMC treatment was quantitated. 300 cells were counted in each group for each experiment. Vertical lines represent ± s.e.m. for 5 experiments.
When FA-A cells were transfected with 300 pM μ-calpain siRNA, adiffuse αIISp staining pattern was observed in the undamaged cells 40 hours post transfection, which was similar to that observed in undamaged normal cells (Figure 7A). This correlates with our results which showed that there were increased levels of αIISp in μ-calpain siRNA transfected FA-A cells. 16 hours after treatment of μ-calpain siRNA transfected FA-A with MMC (400 nM) (40 hours post transfection), both αIISp and XPF localized to damage-induced nuclear foci (Figure 7A). Quantitation of these results showed that the percentage of nuclei in the μ-calpain siRNA transfected FA-A cells showing multiple foci (>10 foci) after MMC treatment was similar to that observed in the nontarget siRNA transfected or μ-calpain siRNA transfected normal cells after MMC treatment (Figure 7B). No significant difference was observed in the percentage of nuclei showing multiple foci in μ-calpain siRNA transfected FA-A cells compared to normal cells transfected with either Nt siRNA or μ-calpain siRNA (p ≥ 0.5). Thus restoring levels of αIISp in FA-A cells leads to ability of these cells to form damage-induced αIISp and XPF foci just as occurs in normal cells.
Knockdown of μ-calpain in FA-A cells by siRNA restores chromosomal stability
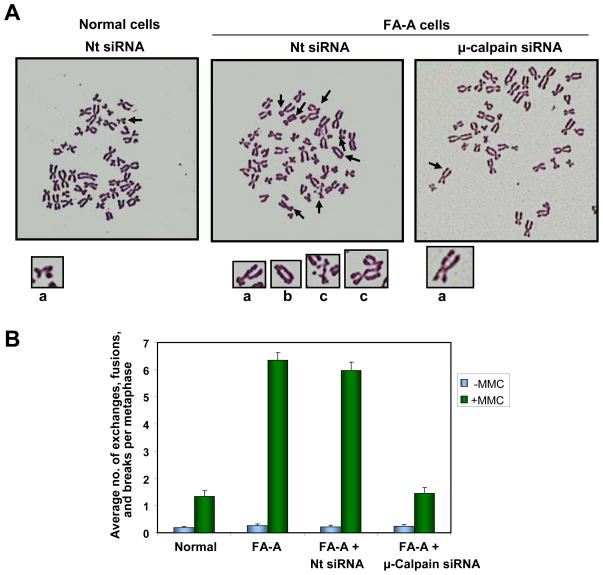
Studies were carried out to determine whether restoring levels of αIISp in FA-A cells could increase the chromosomal stability in these cells. After transfection of FA-A cells with non-target siRNA and subsequent treatment with MMC (30 nM) for 24 hours (a total of 48 hours post transfection), metaphase spreads were examined for chromosomal aberrations. In the 100 metaphases counted, the number of FA-A cells treated with MMC (either not transfected or transfected with non-target siRNA), which showed chromosomal aberrations, was 3 fold greater than in the MMC treated normal cells (Table S1). In the FA-A cells transfected with non-target siRNA, there was a significant increase in the production of interchromatid exchanges, fusions/radials and breaks per metaphase, compared to production of these aberrations in normal cells treated with MMC and transfected with non-target siRNA (p < 0.001) (Figure 8A). This is similar to the number of these aberrations observed in Nt siRNA transfected FA-A cells after MMC treatment (Figure 8A and B) Quantitation of these chromosomal aberrations showed that in the 100 metaphases counted, the average number of interchromatid exchanges, fusions/radials and breaks per metaphase in MMC treated FA-A cells or non-target siRNA transfected FA-A cells was approximately 4 fold greater than those observed in damaged normal cells (Figure 8B). This increase in chromosomal aberrations after DNA interstrand cross-link damage in FA-A cells is similar to results reported by other laboratories (34–36).
FIGURE 8.
Knockdown of μ-calpain in FA-A cells by siRNA restores chromosomal stability. (A) Normal and FA-A cells were transfected with non-target (Nt) siRNA and FA-A cells transfected with μ-calpain siRNA (300 pM) and subsequently treated (24 hours post transfection) with MMC (30 nM) for 24 hours. Metaphase spreads were prepared and examined for chromosomal aberrations (interchromatid exchanges, fusions/radials and breaks). Arrows indicate these aberrations. Several of the chromosome aberrations indicated with arrows are magnified below each metaphase spread: a. chromatid breaks, b. interchromatid exchange, c. fusion/radial, b. interchromatid exchange. (B) One hundred metaphase spreads were scored for interchromatid exchanges, fusions/radials and breaks and the average number of these aberrations per metaphase was quantitated. Vertical lines represent ± s.e.m.
After transfection of FA-A cells with μ-calpainsiRNA (300 pM) and subsequent treatment with MMC (30 nM) (as above), there was a significant decrease in the average number of these chromosomal aberrations per metaphase compared to the number of these aberrations in MMC treated Nt siRNA transfected FA-A cells (p < 0.001) (Figure 8A) and in the total number of cells showing these aberrations (Table S1). Quantitation of these results showed the average number of chromosomal aberrations per metaphase (i.e. interchromatid exchanges, fusions/radials, and breaks) was reduced to levels similar to those foundin normal cells after MMC treatment and the differences were not significant (p ≥ 0.5) (Figure 8B). Analysis of these types of chromosomal aberrations showed that in the μ-calpain siRNA transfected FA-A cells, there were decreases in each of these types of aberrations: interchromatid exchanges, fusions/radials and breaks (Table S1). These studies indicate that increasing levels of αIISp in FA-A cells, by knocking down μ-calpain, restoreschromosomal stability after DNA interstrand crosslink damage.
DISCUSSION
Two hallmarks of FA are chromosome instability and a defect in repair of DNA interstrand cross-links (1, 2, 4, 5). We have shown that the structural protein, αIISp, plays an important role in both of these processes (10–13). We have additionally demonstrated that there are decreased levels of αIISp in FA cells, which our studies indicate are due to increased breakdown of this protein rather than to decreased expression (14). The diminished levels of αIISp in FA cells correlate with increased sensitivity to DNA interstrand cross-linking agents and decreased DNA repair (6, 9). We therefore now endeavored to determine whether, by decreasing breakdown of αIISp in FA cells, the DNA repair defect can be corrected and chromosome stability enhanced.
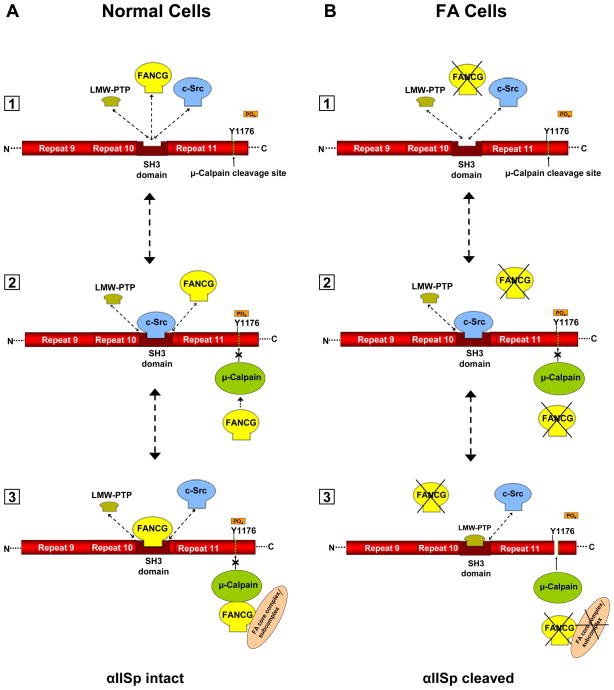
Breakdown of αIISp in human cells can occur by proteolytic cleavage by μ-calpain (26, 28, 31). αIISp, which is composed of 21 triple-helical repeats, contains a μ-calpain cleavage site in repeat 11 (Figure 9) (26, 28, 37–39). Activation of μ-calpain leads to cleavage of αIISp at this site and to the production of specific cleavage products (26, 28, 31). The aim of the present study was to determine whether there is an increase in μ-calpain activity and/or levels in FA cells which may account for the increased breakdown of αIISp in these cells, and, if so, whether knocking down expression of this protein in FA cells can restore αIISp levels to normal and reverse many of the cellular phenotypic deficiencies observed.
FIGURE 9.
Proposed model for involvement of FA proteins in cleavage of αIISp by μ-calpain. FANCG is used as an example in this figure; other FA proteins, such as FANCA, may be substituted into this figure. A portion of αIISp is shown containing repeats 9–11. A. [1] In normal cells an equilibrium exists between LMW-PTP, FANCG and c-Src for binding to the SH3 domain of αIISp. [2] When c-Src binds to the SH3 domain of αIISp it phosphorylates Tyr1176 (Y1176) and prevents cleavage of αIISp by μ-calpain. It also prevents binding of LMW-PTP to the SH3 domain of αIISp. [3] When FANCG binds to the SH3 domain, this prevents the binding of LMW-PTP to this site and the dephosphorylation of Tyr1176. This inhibits the ability of μ-calpain to cleave αIISp at this site, thus preventing the cleavage of αIISp. FANCG (or another FA protein) may also separately bind to calpain and inhibit its ability to cleave αIISp as shown. Both FA proteins may be FANCG, as shown, or one or the other, or both, may be FANCA or another FA protein. These proteins may also be bound to the FA core complex or to a sub-complex of the FA core proteins. B. [1] In FA cells (FA-G cells are used here as an example), there is absence of the FANCG protein and thus there is no binding of FANCG to the SH3 domain of αIISp. [2] Binding of c-Src to the SH3 domain of αIISp leads to phosphorylation Tyr1176 (Y1176) and prevents cleavage of αIISp by μ-calpain. It also prevents binding of LMW-PTP to the SH3 domain of αIISp. [3] In the absence of FANCG, LMW-PTP can bind to the SH3 domain of αIISp and dephosphorylate Tyr1176, allowing μ-calpain to cleave αIISp at its cleavage site. There is also no FANCG, or associated FA core complex or subcomplex, to bind separately to μ-calpain and inhibit its ability to cleave αIISp. This results in μ-calpain breakdown of αIISp in FA-G cells. Similar events may occur in different FA complementation groups (e.g. FA-A).
The present results show that in the five FA complementation groups tested, FA-A, FA-C, FA-D2, FA-F and FA-G, there is a 2.5 to 3.5 fold increase in μ-calpain activity, but not in expression of this protein, compared to its activity in normal cells. In FA-A and FA-G cells, this increase in activity correlates with an increase in the μ-calpain 150 kDa αIISp breakdown product. It is thus possible that in FA cells reduced levels of αIISp are due to its increased cleavage by μ-calpain. Our previous studies have indicated that FA proteins play a role in maintaining αIISp stability in the cell. We have shown that in corrected FA-A, FA-C, and FA-G cells, which express the appropriate FA protein, levels of αIISp are restored to those found in normal cells (15). The present studies provide evidence that FA proteins aid in maintaining αIISp stability via regulation of the activity of μ-calpain. In corrected FA-A, FA-C, and FA-G cells, the activity of μ-calpain is reduced to that found in normal cells. This suggests that the FANCA, FANCC and FANCG proteins play a role in inhibiting μ-calpain activity. We postulate that they may do this by binding to μ-calpain. In support of this view, the present studies showed that FANCA and FANCG bound directly to μ-calpain. It is thus possible to speculate that binding of FANCA and FANCG to μ-calpain has an inhibitory effect on μ-calpain activity and that this is one way in which these FA proteins play a role in regulating stability of αIISp in cells and reducing its breakdown. Since FANCC did not bind directly to μ-calpain, even though it co-immunoprecipitated with it, this would indicate that its interaction with μ-calpain and its effect on μ-calpain activity is indirect.
If, as these studies indicate, increased activity of μ-calpain in FA cells leads to increased breakdown of αIISp, then knocking down μ-calpain should lead to enhanced levels of αIISp. This was found to be the case. Knocking down μ-calpain by siRNA in FA-A cells led to restoration of levels of αIISp to normal. Of particular importance was the finding that reduction in expression of μ-calpain by siRNA lead to correction of a number of the cellular deficiencies present in FA-A cells. Cell survival in μ-calpain siRNA transfected FA-A cells treated with MMC was increased from 36% to 85% of levels found in normal cells. The defect in ability of FA-A cells to form damage-induced nuclear foci in response to MMC was also corrected. μ-Calpain siRNA transfected FA-A cells showed increased αIISp foci, which correlated with increased levels of αIISp in these cells. XPF nuclear foci were also observed in transfected FA-A cells treated with MMC, just as they were in normal cells. We have previously shown that αIISp and XPF foci colocalize in nuclei of normal cells after they are treated with a DNA interstrand cross-linking agent (11). These foci are presumed to represent localization of proteins to sites of DNA damage. Their presence correlates with increased levels of UDS or DNA repair synthesis in normal cells after damage with a DNA interstrand cross-linking agent (9). These foci were absent in MMC damaged non-target siRNA transfected FA-A cells, similar to our previous finding of lack of formation of XPF foci in FA-A cells treated with 8-MOP plus UVA light (11). In addition, we have previously shown that knockdown of αIISp by siRNA in normal human cells leads to loss of formation of XPF foci after damage with MMC (13). These studies collectively demonstrate that αIISp is critical for the localization of XPF into nuclear foci after cells are damaged with a DNA interstrand cross-linking agent and further support our model that αIISp acts as a scaffold in the nucleus where it is involved in recruitment of repair proteins, such as XPF, to sites of damage (10, 11, 17).
We have previously shown that αIISp is also critical for chromosomal stability (13). Knockdown of αIISp in normal cells leads to formation of chromosomal aberrations similar to those found in FA cells (13). In FA cells chromosome instability is particularly evident after damage with a DNA interstrand cross-linking agent. We therefore hypothesized that by restoring levels of αIISp in FA cells, it should be possible to enhance chromosome stability in these cells. The present results verified this hypothesis. When μ-calpain was knocked down by siRNA to levels that lead to restoration of αIISp to normal levels, the total number of chromosomal aberrations (i.e., interchromatid exchanges, fusions/radials and breaks) observed after cells were treated with MMC were reduced to levels that were similar to those found in normal cells. The present studies, which show that restoring levels of αIISp in FA-A cells to normal levels leads to correction of the chromosomal aberrations in these cells, combined with our previous work, which showed that knocking down αIISp in normal cells leads to increased chromosomal aberrations (13), demonstrate that αIISp plays an important role in maintenance of chromosome stability. These studies thus show that maintaining αIISp stability is critical for a number of cellular processes which include not only maintenance of chromosome stability but also cell survival and DNA repair after exposure to a DNA interstrand cross-linking agent.
Stability of αIISp in cells is dependent upon its proteolytic cleavage by μ-calpain. μ-Calpain has been shown to cleave αIISp at Tyr1176 in repeat 11 and this cleavage is controlled by phophorylation of this reside by c-Src, a kinase that binds to a flanking scr-homology 3 (SH3) domain in the 10th repeat of αIISp (Figure 9) (26, 27, 40). SH3 domains are modular domains that are important in mediating protein-protein interactions and formation of protein networks (41–45). When Tyr1176 is phosphorylated, αIISp becomes resistant to μ-calpain cleavage (30, 40). It has also been shown that Tyr1176 can be dephosphorylated by low-molecular-weight phosphotyrosine phosphatase (LMW-PTP) when the latter binds to the SH3 domain of αIISp (30). This in turn leads to cleavage of αIISp by μ-calpain (30). Based on our present and previous studies as well as these cited findings from other investigators, we have developed a model for maintance of αIISp stability in normal human cells and the increased breakdown of αIISp in FA cells (Figure 9). In this model, a FA protein (e.g., FANCG or FANCA) binds to αIISp via the SH3 domain of αIISp. We have evidence that both FANCG (16) and FANCA (in preparation) bind directly to the SH3 domain of αIISp via motifs in these proteins which have specificity for SH3 domains. In addition, six other FA proteins (FANCD1, FANCD2, FANCI, FANCL, FANCM, and FANCN) contain similar SH3 domain binding motifs and could also potentially bind to αIISp via them (16). We propose that in normal cells an equilibrium exists between specific FA proteins, LMW-PTP and c-Src for binding to the SH3 domain of αIISp (Figure 9). When a FA protein (e.g., FANCG or FANCA) binds to the SH3 domain of αIISp, this prevents binding of LMW-PTP to this domain, thus inhibiting dephosphorylation of Tyr1176 and preventing cleavage of αIISp by μ-calpain at this site. In addition, FA proteins, including specifically FANCG and FANCA, also directly bind to μ-calpain and in this way may inhibit its ability to cleave αIISp. Thus one or more FA proteins could play an important role in maintaining normal levels of full length αIISp in the cell in one or more ways. An FA protein could bind to the SH3 domain of αIISp inhibiting the ability of μ-calpain to cleave αIISp and/or it could bind directly to μ-calpain, inhibiting its activity and ability to cleave αIISp. These FA proteins (e.g., FANCG or FANCA) could also be associated with the FA core complex or a subcomplex of FA core proteins and this interaction could be important for the binding of FA proteins to αIISp and μ-calpain. In FA cells, for example FA-G and FA-A cells, where there is a deficiency in FANCG and FANCA, respectively, there is a deficiency in binding of these proteins to the SH3 domain of αIISp and LMW-PTP can bind without interference. LMW-PTP dephosphorylates Tyr1176, which then allows μ-calpain to cleave αIISp at its μ-calpain cleavage site, leading to increased breakdown of αIISp and decreased levels of the full length protein in these cells. FANCG and FANCA would also not be present to bind to μ-calpain and inhibit its activity, which could lead to increased breakdown of αIISp. It is possible that other FA proteins may be similarly involved in this process, either directly or indirectly. Calpain cleavage of αIISp can also be enhanced by binding of calmodulin to αIISp at its site adjacent to the calpain cleavage site (27, 46). It is possible that one or more of the FA proteins may regulate αIISp stability by modulating calmodulin binding to αIISp. This will be examined in future studies.
The question then arises as to whether if a number of the FA proteins could be involved in αIISp stability, why in a particular FA cell line, lack of one FA protein cannot be compensated by the presence of another one. There are a number of possible explanations. One is that FANCG or FANCA, for example, are associated with the FA core complex or a subcomplex of FA core proteins and this entire group of proteins is required to maintain αIISp stabiltiy in normal cells. This is suggested by data which show that FANCC coimmunoprecipitates with αIISp (12) and μ-calpain, as we show here, but does not directly interact with either of these proteins (16 and present results); thus, the effect of FANCC on αIISp stability would likely be mediated by other FA core complex proteins. Loss of or defects in one of these proteins would consequently affect the stability of αIISp. It is thus possible that more than one FA protein is needed to maintain αIISp stability in the cell. Extending this model, one may further speculate that different FA proteins directly binding to αIISp or μ-calpain could mediate different effects. For example, binding of a FA protein complex via FANCG may be different than its binding via FANCA. These possibilities will be explored in further studies.
Thus, by knocking down μ-calpain in FA-A cells and thereby restoring αIISp levels to normal, it is possible to correct a number of the phenotypic defects observed in FA cells. These include decreasing sensitivity to DNA interstrand cross-linking agents, increasing DNA repair as observed by the presence of damage-induced nuclear foci, and enhancing chromosome stability. FA proteins may play an important role in maintaining stability of αIISp in the cell by regulating its cleavage by μ-calpain. Thus, increased breakdown of αIISp in FA cells, due to loss of functional FA proteins needed to maintain its stability, may be a critical factor in both the increased chromosome instability and defective DNA repair observed. Reversing this breakdown by knocking down μ-calpain, as demonstrated in the present study, may have therapeutic relevance.
Supplementary Material
Footnotes
This work was supported by NHLBI, National Institutes of Health, Grant RO1 HL054860 (to M.W.L)
Abbreviations: FA, Fanconi anemia; αIISp, nonerythroid α spectrin; NER, nucleotide excision repair; UDS, unscheduled DNA synthesis; RFU, relative fluorescence units; BD, DNA-binding domain; AD, transcriptional activation domain; MMC, mitomycin C; SH3, src-homology 3; LMW-PTP, low-molecular-weight phosphotyrosine phosphatase
SUPPORTING INFORMATION AVAILABLE
A figure showing the levels of μ-calpain protein in FA cells, corrected FA cells and normal cells, a figure showing that knocking down μ-calpain in FA-A cells has no effect on levels of FANCA or FANCC, and a table giving quantitation of MMC-induced chromosomal aberrations in normal cells and FA-A cells transfected with μ-calpain siRNA. This material is available free of charge via the Internet at http://pubs/acs/org.
References
- 1.Joenje H, Patel KJ. The emerging genetic and molecular basis of Fanconi anaemia. Nat Rev Genet. 2001;2:446–457. doi: 10.1038/35076590. [DOI] [PubMed] [Google Scholar]
- 2.Taniguchi T, D’Andrea AD. Molecular pathogenesis of Fanconi anemia: recent progress. Blood. 2006;107:4223–4233. doi: 10.1182/blood-2005-10-4240. [DOI] [PubMed] [Google Scholar]
- 3.Mathew CG. Fanconi anaemia genes and susceptibility to cancer. Oncogene. 2006;25:5875–5884. doi: 10.1038/sj.onc.1209878. [DOI] [PubMed] [Google Scholar]
- 4.de Winter JP, Joenje H. The genetic and molecular basis of Fanconi anemia. Mutat Res: Fundament Mol Mech Mutagen. 2009;668:11–19. doi: 10.1016/j.mrfmmm.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 5.Moldovan GL, D’Andrea AD. How the Fanconi anemia pathway guards the genome. Ann Rev Genet. 2009;43:223–249. doi: 10.1146/annurev-genet-102108-134222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lambert MW, Tsongalis GJ, Lambert WC, Parrish DD. Correction of the DNA repair defect in Fanconi anemia complementation groups A and D cells. Biochem Biophys Res Commun. 1997;230:587–591. doi: 10.1006/bbrc.1996.6008. [DOI] [PubMed] [Google Scholar]
- 7.Kumaresan KR, Lambert MW. Fanconi anemia, complementation group A, cells are defective in ability to produce incisions at sites of psoralen interstrand cross-links. Carcinogenesis. 2000;21:741–751. doi: 10.1093/carcin/21.4.741. [DOI] [PubMed] [Google Scholar]
- 8.Niedernhofer LJ, Lalai AS, Hoeijmakers JH. Fanconi anemia (cross)linked to DNA repair. Cell. 2005;123:1191–1198. doi: 10.1016/j.cell.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 9.Kumaresan K, Sridharan D, McMahon L, Lambert MW. Deficiency in incisions produced by XPF at the site of a DNA interstrand cross-link in Fanconi anemia cells. Biochemistry. 2007;46:14359–14368. doi: 10.1021/bi7015958. [DOI] [PubMed] [Google Scholar]
- 10.McMahon LW, Sangerman J, Goodman SR, Kumaresan K, Lambert MW. Human alpha spectrin II and the FANCA, FANCC, and FANCG proteins bind to DNA containing psoralen interstrand cross-links. Biochemistry. 2001;40:7025–7034. doi: 10.1021/bi002917g. [DOI] [PubMed] [Google Scholar]
- 11.Sridharan D, Brown M, Lambert WC, McMahon LW, Lambert MW. Nonerythroid alpha II spectrin is required for recruitment of FANCA and XPF to nuclear foci induced by DNA interstrand cross-links. J Cell Science. 2003;116:823–835. doi: 10.1242/jcs.00294. [DOI] [PubMed] [Google Scholar]
- 12.Sridharan DM, McMahon LW, Lambert MW. αII-Spectrin interacts with five groups of functionally important proteins in the nucleus. Cell Biol Intl. 2006;30:866–878. doi: 10.1016/j.cellbi.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 13.McMahon LW, Zhang P, Sridharan DM, Lefferts JA, Lambert MW. Knockdown of αII spectrin in normal human cells by siRNA leads to chromosomal instability and decreased DNA interstrand cross-link repair. Biochem Biophys Res Commun. 2009;381:288–293. doi: 10.1016/j.bbrc.2009.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lefferts JA, Lambert MW. Fanconi anemia cell lines deficient in αII spectrin express normal levels of αII spectrin mRNA. Biochem Biophys Res Comm. 2003;307:510–515. doi: 10.1016/s0006-291x(03)01213-0. [DOI] [PubMed] [Google Scholar]
- 15.McMahon LW, Walsh CE, Lambert MW. Human α spectrin II and the Fanconi anemia proteins FANCA and FANCC interact to form a nuclear complex. J Biol Chem. 1999;274:32904–32980. doi: 10.1074/jbc.274.46.32904. [DOI] [PubMed] [Google Scholar]
- 16.Lefferts JA, Wang C, Sridharan D, Baralt M, Lambert MW. The SH3 domain of αII spectrin is a target for the Fanconi anemia protein, FANCG. Biochemistry. 2009;48:254–263. doi: 10.1021/bi801483u. [DOI] [PubMed] [Google Scholar]
- 17.Wang C, Lambert MW. The Fanconi anemia protein, FANCG, binds to the ERCC1-XPF endonuclease via its tetratricopeptide repeats and the central domain of ERCC1. Biochemistry. 2010 doi: 10.1021/bi100584c. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhagwat N, Olsen AL, Wang AT, Hanada K, Stuckert P, Kanaar R, D’Andrea A, Niedernhofer LJ, McHugh PJ. XPF-ERCC1 participates in the Fanconi anemia pathway of cross-link repair. Mol Cell Biol. 2009;29:6427–6437. doi: 10.1128/MCB.00086-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thompson LH, Hinz JM. Cellular and molecular consequences of defective Fanconi anemia proteins in replication-coupled DNA repair: mechanistic insights. Mutat Res. 2009;668:54–72. doi: 10.1016/j.mrfmmm.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dronker MLG, Kanaar R. Repair of DNA interstrand cross-links. Mutat Res. 2001;486:217–247. doi: 10.1016/s0921-8777(01)00092-1. [DOI] [PubMed] [Google Scholar]
- 21.McHugh PJ, Spanswick VJ, Hartley JA. Repair of DNA interstrand crosslinks: Molecular mechanisms and clinical relevance. Lancet. 2001;2:483–490. doi: 10.1016/S1470-2045(01)00454-5. [DOI] [PubMed] [Google Scholar]
- 22.Patel KJ, Joenje H. Fanconi anemia and DNA replication repair. DNA Repair. 2007;6:885–890. doi: 10.1016/j.dnarep.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 23.Lee JK, Coyne RS, Dubreuil RR, Goldstein LS, Branton D. Cell shape and interaction defects in alpha-spectrin mutants of Drosophila melanogaster. J Cell Biol. 1993;123:1797–1809. doi: 10.1083/jcb.123.6.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Norman KR, Moerman DG. α Spectrin is essential for morphogenesis and body wall muscle formation in Caenorhabditis elegans. J Cell Biol. 2002;157:665–677. doi: 10.1083/jcb.200111051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Metral S, Machnicka B, Bigot S, Colin Y, Dhermy D, Lecomte MC. αII-Spectrin is critical for cell adhesion and cell cycle. J Biol Chem. 2009;284:2409–2418. doi: 10.1074/jbc.M801324200. [DOI] [PubMed] [Google Scholar]
- 26.Harris AS, Croall DE, Morrow JS. The calmodulin-binding site in α-fodrin is near the calcium-dependent protease-I cleavage site. J Biol Chem. 1988;263:15754–15761. [PubMed] [Google Scholar]
- 27.Harris AS, Croall DE, Morrow JS. Calmodulin regulates fodrin susceptibility to cleavage by calcium-dependent protease I. J Biol Chem. 1989;264:17401–17408. [PubMed] [Google Scholar]
- 28.Huh GY, Glant SB, Je S, Morrow JS, Kim JH. Calpain proteolysis of αII-spectrin in the normal adult human brain. Neurosci Lett. 2001;316:41–44. doi: 10.1016/s0304-3940(01)02371-0. [DOI] [PubMed] [Google Scholar]
- 29.Pike BR, Flint J, Dutta S, Johnson E, Wang KKW, Hayes RL. Accumulation of non-erythroid αII-spectrin and calpain-cleaved αII-spectin breakdown products in cerebrospinal fluid after traumatic brain injury in rats. J Neurochem. 2001;78:1297–1306. doi: 10.1046/j.1471-4159.2001.00510.x. [DOI] [PubMed] [Google Scholar]
- 30.Nicolas G, Fournier CM, Galand C, Malbert-Colas L, Bournier O, Kroviarski Y, Bourgeois M, Camonis JH, Dhermy D, Grandchamp B, Lecomte MC. Tyrosine phosphorylation regulates alpha II spectrin cleavage by calpain. Mol Cell Biol. 2002;22:3527–3536. doi: 10.1128/MCB.22.10.3527-3536.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Czogalla A, Sikorski AF. Spectrin and calpain: a’target’ and a ‘sniper’ in the pathology of neuronal cells. Cell Mol Life Sci. 2005;62:1913–1924. doi: 10.1007/s00018-005-5097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brois DW, McMahon LW, Ramos NJ, Anglin LM, Walsh CE, Lambert MW. A deficiency in a 230 kDa DNA repair protein in Fanconi anemia complementation group A cells is corrected by the FANCA cDNA. Carcinogenesis. 1999;20:1845–1853. doi: 10.1093/carcin/20.9.1845. [DOI] [PubMed] [Google Scholar]
- 33.Yamashita T, Kupfer GM, Naf D, Suliman A, Joenje H, Asano S, D’Andrea AD. The Fanconi anemia pathway requires FAA phosphorylation and FAA/FAC nuclear accumulation. Proc Natl Acad Sci USA. 1998;95:13085–13090. doi: 10.1073/pnas.95.22.13085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Howlett NG, Taniguchi T, Durkin SG, D’Andrea AD, Glover TW. The Fanconi anemia pathway is required for the DNA replication stress response and for the regulation of common fragile site stability. Human Mol Genet. 2005;14:693–701. doi: 10.1093/hmg/ddi065. [DOI] [PubMed] [Google Scholar]
- 35.Godthelp BC, van Buul PPW, Jaspers NGJ, Elghalbzouri-Maghrini E, van Duijn-Goedhart A, Arwert F, Joenje H, Zdzienicka MZ. Cellular characterization of cells from Fanconi anemia complementation group, FA-D1/BRCA2. Mutat Res. 2006;601:191–201. doi: 10.1016/j.mrfmmm.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 36.Ciccia A, Ling C, Coulthard R, Yan Z, Xue Y, Meetel AR, Laghmani EH, Joenje H, McDonald N, de Winter JP, Wang W, West SC. Identification of FAAP24, a Fanconi anemia core complex protein that interacts with FANCM. Mol Cell. 2007;25:331–343. doi: 10.1016/j.molcel.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 37.Winkelmann JC, Forget BG. Erythroid and nonerythroid spectrins. Blood. 1993;81:3173–3185. [PubMed] [Google Scholar]
- 378.Goodman SR, Zimmer WE, Clark MB, Zagon IS, Barker JE, Bloom ML. Brain spectrin: of mice and men. Brain Res Bull. 1995;36:593–606. doi: 10.1016/0361-9230(94)00264-2. [DOI] [PubMed] [Google Scholar]
- 39.De Matteis MA, Morrow JS. Spectrin tethers and mesh in the biosynthetic pathway. J Cell Sci. 2000;113:2331–2343. doi: 10.1242/jcs.113.13.2331. [DOI] [PubMed] [Google Scholar]
- 40.Nearelow JH, Cianci CD, Morrow JS. c-Src binds alpha II spectrin’s Src homology 3 (SH3) domain and bocks calpain susceptibility by phosphorylating Tyr1176. J Biol Chem. 2003;278:7735–7741. doi: 10.1074/jbc.M210988200. [DOI] [PubMed] [Google Scholar]
- 41.Musacchio A, Gibson T, Lehto VP, Saraste M. SH3 - an abundant protein domain in search of a function. FEBS Lett. 1992;307:55–61. doi: 10.1016/0014-5793(92)80901-r. [DOI] [PubMed] [Google Scholar]
- 42.Cohen GB, Ren R, Baltimore D. Modular binding domains in signal transduction proteins. Cell. 1995;80:237–248. doi: 10.1016/0092-8674(95)90406-9. [DOI] [PubMed] [Google Scholar]
- 43.Kuriyan J, Cowburn D. Modular peptide recognition domains in eukaryotic signaling. Annu Rev Biophys Biomol Struct. 1997;26:259–288. doi: 10.1146/annurev.biophys.26.1.259. [DOI] [PubMed] [Google Scholar]
- 44.Stein R. SH2 and SH3 domains. Unraveling signaling networks with peptide antagonists. Methods Mol Biol. 1998;88:187–195. doi: 10.1385/0-89603-487-9:187. [DOI] [PubMed] [Google Scholar]
- 45.Mayer BJ. SH3 domains: complexity in moderation. J Cell Sci. 2001;114:1253–1263. doi: 10.1242/jcs.114.7.1253. [DOI] [PubMed] [Google Scholar]
- 46.Simonovic M, Zhang Z, Cianci CD, Steitz TA, Morrow JS. Structure of the calmodulin αII-spectrin complex provides insight into the regulation of cell plasticity. J Biol Chem. 2006;281:34333–34340. doi: 10.1074/jbc.M604613200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.