Abstract
The production of oxygen and the supply of energy for life on earth rely on the process of photosynthesis using sunlight. Paradoxically, sunlight damages the photosynthetic machinery, primarily photosystem II (PSII), leading to photoinhibition and loss of plant performance. However, there is uncertainty about which wavelengths are most damaging to PSII under sunlight. In this work we examined this in a simple experiment where Arabidopsis (Arabidopsis thaliana) leaves were exposed to different wavelengths of sunlight by dispersing the solar radiation across the surface of the leaf via a prism. To isolate only the process of photodamage, the repair of photodamaged PSII was inhibited by infiltration of chloramphenicol into the exposed leaves. The extent of photodamage was then measured as the decrease in the maximum quantum yield of PSII using an imaging pulse amplitude modulation fluorometer. Under the experimental light conditions, photodamage to PSII occurred most strongly in regions exposed to ultraviolet (UV) or yellow light. The extent of UV photodamage under incident sunlight would be greater than we observed when one corrects for the optical efficiency of our system. Our results suggest that photodamage to PSII under sunlight is primarily associated with UV rather than photosynthetically active light wavelengths.
Plants absorb sunlight to power the productive photochemical reactions of photosynthesis. Absorption of sunlight may also lead to deleterious photochemistry that damages the photosynthetic machinery. The PSII protein complex is important in this regard as it seems to be most susceptible to photodamage that results in photoinhibition and ultimately suppresses photosynthetic CO2 assimilation, growth, and productivity (Long et al., 1994; Takahashi and Murata, 2008). Although plants have photoprotection mechanisms (Niyogi, 1999) and can effectively repair photodamaged PSII through the PSII repair cycle (Aro et al., 1993), photoinhibition still occurs under stressful environmental conditions (Nishiyama et al., 2006; Murata et al., 2007; Takahashi and Murata, 2008).
The onset of photoinhibition is strongly correlated with the absorption of excessive excitation energy for photosynthesis. Therefore, photodamage to PSII was most readily assumed to be attributed to the excess light absorbed by photosynthetic pigments (Melis, 1999). However, the extent of photodamage that is measured under conditions where the repair of photodamaged PSII is prevented by inhibiting chloroplast protein synthesis (i.e. lincomycin or chloramphenicol) is directly proportional to the intensity of light (Mattoo et al., 1984; Tyystjärvi and Aro, 1996; Nishiyama et al., 2001, 2004; Allakhverdiev and Murata, 2004; Chow et al., 2005). Furthermore, recent studies have demonstrated that interruption of the Calvin cycle (Hakala et al., 2005; Takahashi and Murata, 2005; Takahashi et al., 2007) and inhibition of electron transfer between QA and QB (Jegerschöld et al., 1990; Kirilovsky et al., 1994; Allakhverdiev et al., 2005) have no effect on the rate of photodamage to PSII, but in fact cause inhibition of the repair of photodamaged PSII due to suppression of the de novo synthesis of PSII proteins (Allakhverdiev et al., 2005; Takahashi and Murata, 2005, 2006). Thus, photodamage to PSII is paradoxically not associated with the excess light absorbed by photosynthetic pigments (Nishiyama et al., 2006; Murata et al., 2007; Takahashi and Murata, 2008).
Studies of the effect of monochromatic light on the photodamage process have suggested that photodamage to PSII primarily occurs at the manganese cluster of the oxygen-evolving complex (OEC) through a direct photoexcitation of manganese (Hakala et al., 2005; Ohnishi et al., 2005). Release of manganese ions (Mn2+) from thylakoid membranes is accompanied by photodamage to PSII (Hakala et al., 2005; Zsiros et al., 2006), suggesting that disruption of the manganese cluster upon absorption of light might be a primary event in photodamage. It is likely that the reaction center of PSII is secondarily damaged by light absorbed by photosynthetic pigments after inactivation of the OEC (Hakala et al., 2005; Ohnishi et al., 2005), if an alternative electron transfer donor from lumenal ascorbate is not available (Mano et al., 2004; Tóth et al., 2009). These findings have lead to a recent photodamage model called the manganese (or two-step; Ohnishi et al., 2005) mechanism of photoinhibition (Tyystjärvi, 2008).
Studies of the action spectrum of photodamage to PSII have shown that UV damages PSII more effectively than visible light (Jones and Kok, 1966; Jung and Kim, 1990; Hakala et al., 2005; Ohnishi et al., 2005). Thus, under identical light intensity, UV is the most damaging wavelength to PSII. However, inferring damage under natural sunlight is not straight forward as there is a need to account for the spectral distribution and intensity of sunlight. It is unclear which wavelengths of sunlight are most damaging to PSII and we cannot discount the premise that significant primary photodamage to PSII is caused by light absorbed by photosynthetic pigments (Vass and Cser, 2009). To identify which wavelengths of sunlight are most damaging to PSII, sunlight was spectrally dispersed via a prism onto an Arabidopsis (Arabidopsis thaliana) leaf infiltrated with chloramphenicol and decrease in the maximum quantum yield of PSII (Fv/Fm) was measured using an imaging pulse amplitude modulation (PAM) fluorometer. This simple but powerful approach revealed the in vivo spectral dependence of photodamage that had two peaks at UV and yellow wavelengths. Since the spectral efficiency of our optical system decreased below 400 nm, we calculated photodamage to PSII under incident sunlight. Our results show that photodamage to PSII was primarily associated with UV wavelengths and secondarily with yellow light wavelengths. This finding indicates that photodamage to PSII is less associated with light absorbed by photosynthetic pigments under sunlight and suggest that most of photodamage to PSII is potentially avoidable during photosynthesis.
RESULTS
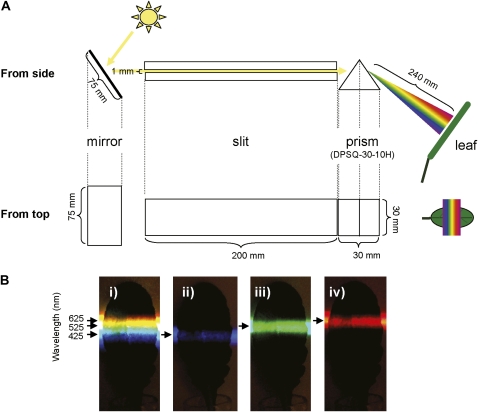
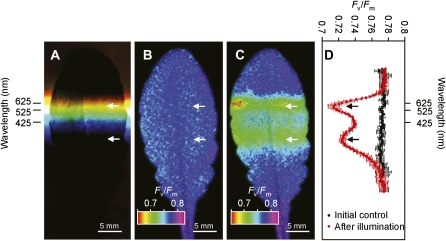
We set out to examine the photodamage in Arabidopsis leaves caused by exposure to different wavelengths of sunlight. To investigate only the process of photodamage, the repair of PSII was inhibited by infiltration of chloramphenicol via the leaf petiole to block the synthesis of PSII proteins, primarily the D1 protein. The extent of photodamage after a period of exposure was then measured as the decrease in Fv/Fm using an imaging PAM fluorometer (Imaging-PAM; Walz). To expose the leaf to different wavelengths of light, an equilateral prism (DPSQ-30-10H; Sigma Koki) evenly dispersed the sunlight from 200 to 2,000 nm (T = 90%) across the surface of the leaf (Fig. 1A). Wavelengths (425, 525, and 625 nm) of dispersed light were defined using pairs of short-pass (LS) and long-pass (LL) cutoff filters (Fig. 1B). The initial Fv/Fm value was 0.776, which was uniform across the whole leaf (Fig. 2, B and D). After exposure of the leaf to spectrally dispersed sunlight for 30 min, the Fv/Fm value decreased to minimum values of 0.724 and 0.714 at leaf regions corresponding to UV and yellow light wavelengths, respectively (Fig. 2, C and D). Similar results were obtained in a number of replicate experiments and even after light exposure for 15 min (Supplemental Fig. S1).
Figure 1.
A, A diagram showing the apparatus used for exposure of the leaf to dispersed sunlight. The sunlight was reflected by a mirror, passed through a slit, and dispersed by a prism. The leaf was placed 240 mm away from the prism, perpendicular to the light beam in the dark box. B, Wavelengths of dispersed light, defined using pairs of short-pass LS and long-pass LL cutoff filters. i, In the absence of cutoff filters. ii, In the presence of LL-400 and LS-450 filters. iii, In the presence of LL-500 and LS-550 filters. iv, In the presence of LL-600 and LS-650 filters.
Figure 2.
Photodamage to PSII under different wavelengths of sunlight. An Arabidopsis leaf was preincubated with 1 mm chloramphenicol at 20 μmol photons m−2 s−1 for 4 h. The leaf was exposed to spectrally dispersed sunlight for 30 min. Fv/Fm was measured with an imaging PAM before and after the light exposure. A, Photograph of Arabidopsis leaf exposed to sunlight dispersed using a prism. B, Image of the Fv/Fm value before exposure to dispersed sunlight. C, Image of the Fv/Fm value after exposure to dispersed sunlight for 30 min. D, Quantified inhibitory effects of wavelengths of light on Fv/Fm obtained from B and C. The values are mean ± sd (bars) from three measurement sites (right and left sides and center of the leaf along to midrib) in the same experiment. The pairs of arrows in each section are aligned with the two photoinhibition peaks. Approximate wavelengths of light regions incident on the leaf were determined with pairs of LS and LL cutoff filters (see Fig. 1B) and are shown in A and D.
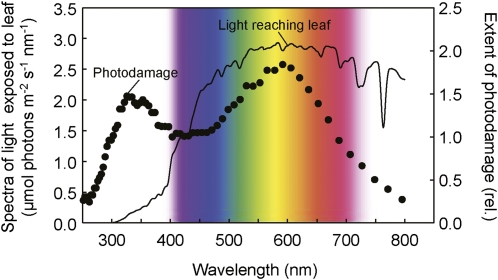
To examine the relationship between photodamage to PSII and light absorbed by the leaf, the absorptance spectrum of Arabidopsis leaves (from 350–700 nm) was measured with a spectroradiometer interfaced with an integrating sphere attachment (Supplemental Fig. S2A). The absorptance spectrum of the Arabidopsis leaf showed maximum absorptance in the UV-blue spectral region (350–500 nm) and around the red spectral region (660–680 nm; Supplemental Fig. S2A). Almost 90% of incident irradiance was absorbed in these two spectral regions. The region of green-yellow wavelengths (500–600 nm) showed the lowest absorptance (60% of incident irradiance was absorbed at 550 nm). The spectrum of light absorbed by the leaf during the photodamage experiment to the leaf in Figure 2 showed peaks in the blue and red wavebands (Supplemental Fig. S2B). Importantly, peak absorptance wavebands did not coincide with the peaks of photodamage to PSII, observed in UV (330 nm) and yellow (600 nm) wavebands (Fig. 3). These results indicate that photodamage to PSII is not associated with light absorbed by photosynthetic pigments.
Figure 3.
Relative (rel.) extent of photodamage to PSII at different wavelengths of dispersed sunlight. The extent of photodamage to PSII was calculated from data in Figure 2 and normalized to a value of one at 400 nm. The spectrum of incident sunlight measured during the photodamage treatment to the leaf in Figure 2 was used to calculate the spectrum of light reaching the leaf (see “Materials and Methods”).
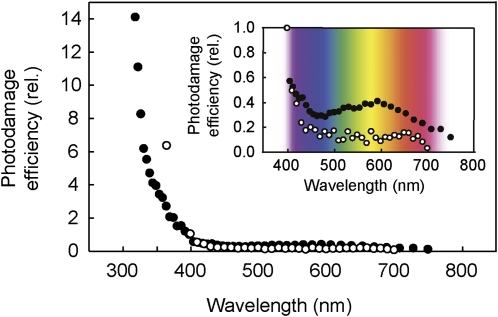
Since the extent of photodamage is directly proportional to the intensity of light (Tyystjärvi and Aro, 1996; Sarvikas et al., 2006), the photodamage efficiency at different wavelengths can be calculated by dividing the extent of photodamage to PSII (the extent of decreased Fv/Fm) by intensity of incident light at each wavelength (Fig. 4). Since we do not know how much light is absorbed by the target of photodamage, we calculated photodamage efficiency using incident light intensity. The absorptance of the leaf (see Supplemental Fig. S2) is mainly due to absorption by photosynthetic pigments and may not represent the sites of photodamage. Photodamage efficiency was highest in UV and nearly constant through the visible waveband, consistent with data observed in Arabidopsis intact leaves (Sarvikas et al., 2006). Interestingly, our result showed that the quantum efficiency of photodamage has a small but apparent peak at yellow (600 nm) wavelength in the visible light region (Fig. 4).
Figure 4.
Spectrum of photodamage efficiency. The relative (rel.) efficiency of photodamage to PSII was calculated by dividing the extent of photodamage by the number of photons at each wavelength (black circles). The action spectrum of photodamage to PSII in Arabidopsis published by Sarvikas et al. (2006) is shown (white circles). Both spectra were normalized to a value of one at 400 nm.
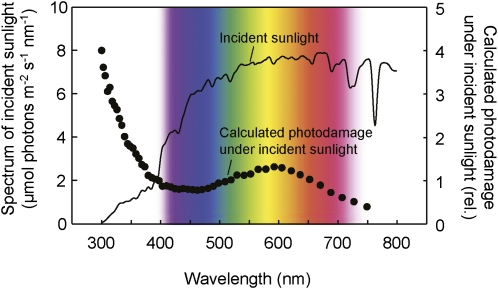
Due to the optics of our experimental setup, the proportion of UV to visible light was lower in the spectrum of light reaching the leaf than that of incident sunlight (Supplemental Fig. S3). Consequently, the extent of photodamage by UV shown in Figure 3 is an underestimate. To correct for this, we multiplied the quantum efficiency of photodamage by the intensity of incident sunlight at each wavelength (Fig. 5). This emphasized that photodamage to PSII was primarily associated with UV wavelengths under sunlight.
Figure 5.
Calculated relative (rel.) photodamage to PSII in the leaf under incident sunlight. The spectrum of photodamage to PSII was calculated by multiplying photodamage efficiency by the incident sunlight at each wavelength, which was then normalized to one at 400 nm. The spectrum of incident sunlight was measured during the light exposure in Figure 2.
DISCUSSION
The sunlight illumination results indicate that for an intact Arabidopsis leaf the PSII photodamage is primarily associated with UV wavelengths of sunlight (Fig. 5). The spectrum of photodamage to PSII differed from that of light absorption by the leaf (Supplemental Fig. S2B). Therefore, the major photodamage to PSII under sunlight appears not to be associated with light absorbed by photosynthetic pigments (either via acceptor or donor side photoinhibition mechanisms). Recent studies have hypothesized that initial photodamage to PSII occurs at the manganese cluster of the OEC presumably via direct excitation of manganese (manganese mechanism of photoinhibition; Tyystjärvi, 2008), with UV being more effective at inducing photodamage compared to visible light (Hakala et al., 2005; Ohnishi et al., 2005; Fig. 4). The peak of photodamage in the UV region on the leaf (Fig. 5) is therefore consistent with this manganese-based mechanism. On the other hand, it is unclear why yellow light causes photodamage (Fig. 5).
In considering the adverse effects of the yellow wavelengths of sunlight, for a number of reasons we suggest this might also be attributed to the manganese mechanism. First, although visible light excites manganese much less effectively than UV and blue wavelengths (Hakala et al., 2005; Sarvikas et al., 2006), yellow light is much more abundant in the solar spectrum than UV and blue wavelengths (Fig. 5). Second, there is less absorption of yellow light by chlorophylls (primary absorption blue and red) and carotenoids (primary absorption blue and green) associated with the photosystems (Supplemental Fig. S2A). Third, the nonabsorbed light reflects and scatters within leaves, resulting in an increase in light intensity near the leaf surface and at depth (Vogelmann et al., 1996). Thus, the weakly absorbed yellow light may be more likely to excite manganese than other wavelengths of visible light, resulting in higher efficiency (Fig. 4) and extent of photodamage to PSII (Fig. 5). Finally, also consistent with this hypothesis, a decrease in leaf chlorophyll content has been demonstrated to enhance the sensitivity of PSII to photodamage in the presence of lincomycin that suppresses the repair of photodamaged PSII (Pätsikkä et al., 2002). The spectrum of photodamage efficiency shown in Arabidopsis intact leaves had no visible light peak (Sarvikas et al., 2006). The difference with our results may be due to a different content of leaf pigments (the leaf used in our study might have higher amount of photosynthetic pigments or lower amounts of green-yellow absorbing pigments such as carotenoids and phenolic compounds). Further studies are necessary to verify this hypothesis.
To prevent photoinhibition, photoprotective mechanisms are used by the plant to both suppress the photodamage to PSII and to minimize inhibition of the repair of photodamaged PSII. The reduced extent of photodamage at the light absorption peaks of photosynthetic pigments (Fig. 5) could therefore be associated with activation of photoprotective mechanisms. In the previous photodamage models, photodamage to PSII was proposed to be attributed to excess light absorbed by photosynthetic pigments (Melis, 1999). Therefore, utilization and dissipation of absorbed light energy through the photosynthetic carbon fixation, thermal energy dissipation, water-water cycle (hydrogen peroxide scavenging), and the photorespiratory pathway were assumed to suppress photodamage to PSII (Melis, 1999). However, recent studies have demonstrated utilization and dissipation of excessive absorbed light energy through such photoprotective mechanisms (reactive oxygen scavenging system, thermal energy dissipation, and photorespiratory pathway) are primarily associated with minimizing inhibition of the repair of photodamaged PSII but not preventing photodamage to PSII (Nishiyama et al., 2001; Takahashi et al., 2007, 2009). Thus, such photoprotection mechanisms have no influence on the extent of photodamage at any wavelengths of sunlight. However, chloroplast movement to avoid light might be partially associated with suppressing photodamage to PSII by blue light as chloroplast movement responds to strong blue light and prevents photodamage to PSII (Kasahara et al., 2002). Furthermore, proton gradients across the thylakoid membrane from linear and cyclic electron flows might be also associated with suppressing photodamage to PSII from light absorbed by photosynthetic pigments, primarily blue and red wavelengths (Fig. 5), as it prevents photodamage to PSII (Takahashi et al., 2009). We need to note that the prevention of photodamage to PSII by generation of proton gradients across thylakoid membranes is not associated with thermal energy dissipation and its mechanism has not yet been clarified (Takahashi et al., 2009).
Our results show that photodamage to PSII by sunlight is primarily associated with UV and yellow wavelengths (Fig. 5), suggesting that photodamage to PSII should be suppressed by mechanisms that attenuate these wavelengths, i.e. leaf (Satter and Galston, 1981) and chloroplast (Kasahara et al., 2002) movements and accumulation of compounds that absorb UV and/or yellow wavelengths (i.e. phenolic compounds in the epidermal cells; Li et al., 1993; Landry et al., 1995; Booij-James et al., 2000; Winkel-Shirley, 2001, 2002). The spectrum of sunlight photodamage might vary among plant species and growth conditions depending on the nature and the amount of light-absorbing compounds. Indeed, plants grown under sunlight induce protective screening from UV wavelengths compared to those grown under artificial light lacking in UV, which lowers their photosynthetic quantum efficiency at and below 400 nm (McCree, 1972). Furthermore, photodamage might also vary through the day and with changing weather and season through their influence on the spectrum of sunlight reaching leaves. Given that UV effectively damages PSII (Fig. 4), small increases in UV radiation through thinning of the stratospheric ozone layer caused by an artificial release of chlorofluorocarbons and other ozone antagonists might strongly enhance the extent of photodamage.
Photodamage to PSII has long been believed to be directly attributable to light absorbed by photosynthetic pigments. Therefore, photodamage to PSII was long assumed to be an unavoidable consequence for photosynthetic organisms. However, these results suggest that artificial or in situ filtering of UV wavelengths could help in reducing photodamage to PSII that causes photoinhibition, with little detriment to photosynthetic CO2 fixation. Consequently, the growth and productivity of plants under sunlight may be increased using these strategies.
MATERIALS AND METHODS
Plant and Growth Condition
Arabidopsis (Arabidopsis thaliana) ecotype Columbia was grown in a growth cabinet at 22°C to 25°C under light at 100 μmol photons m−2 s−1 with a light/dark cycle of 10/14 h. Four weeks after germination, fully expanded leaves were used for experiments. To inhibit the repair of photodamaged PSII, the leaf petiole was incubated in 1 mm chloramphenicol under a light of 20 μmol photons m−2 s−1 for 4 h before experiments.
Light Treatment
The experiment was undertaken outside at the Research School of Biology in the Australian National University (Canberra, Australia, 35°27′S, 149°10′E) in November, 2007. Light exposure commenced at noon and the intensity of direct sunlight during treatment was 2,070 to 2,144 μmol photons m−2 s−1 (LI-250; LI-COR Inc.). Sunlight was first reflected onto a 1-mm slit that was 200-mm deep. The emergent light entered into a quartz equilateral dispersing prism (DPSQ-30-10H; Sigma Koki). The leaf was placed 240 mm away from the prism and exposed perpendicularly to the dispersed light in the dark box (Fig. 1A). The temperature of the dark box was between 25°C and 27°C. To define approximate wavelengths of dispersed light incident on different treated regions of the leaf, pairs of LS and LL cutoff filters (Corion) were used. The center of each band was defined as 424, 525, and 625 nm with LL-400/LS-450, LL-500/LS-550, and LL-600/LS-650 filter pairs, respectively (Fig. 1B).
Measurement of Photoinhibition
Fv/Fm was measured after incubation in darkness for 15 min with an imaging PAM fluorometer (Imaging-PAM; Walz). The Fv/Fm value shown in Figure 2 was calculated using the software ImagingWin (Version 2.2.0.0, Walz).
Measurement of Leaf Absorptance
Leaf absorptance for Arabidopsis was calculated by measuring leaf reflectance and transmittance spectra with an LI-1800 spectroradiometer and the 1800-12S integrating sphere attachment (LI-COR Inc.). The sample scan was divided by its corresponding reference scan from 350 to 800 nm. Leaf absorptance was calculated as: 1 − reflectance − transmittance.
Measurement of Spectra of Incident Sunlight and Light Reaching the Leaf
The optical properties of the experimental setup were measured with a spectroradiometer (LI-1800; LI-COR Inc.) using the small cosine corrected head. A 0.33-mm slit formed by two razor blades was positioned 10 mm above the center of the head to enable the spectrum to be spatially resolved. A correction factor for the slit was made by multiplying the irradiance at each wavelength by the ratio of sunlight measured with and without the slit. Spectra were measured at 1-mm intervals along the position of the leaf in the light box. The frequency of the peak irradiance and half bandwidth frequency were linearly related to distance (half bandwidths increased from 26 nm at 359 nm, to 48 nm at 477 nm, and 144 nm at 693 nm). The efficiency of the optical system was calculated at each position as the ratio of the irradiance at the peak wavelength to that of incident sunlight (Supplemental Fig. S3B). The efficiency, which increased curvilinearly from 0.24 at 361 nm to reach a maximum of 0.38 at 477 nm, was used to calculate the actual quantum dose at each position along the leaf.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Photodamage to PSII under different wavelengths of sunlight.
Supplemental Figure S2. The spectrum of light absorbance of a leaf.
Supplemental Figure S3. Spectra of incident sunlight and light reaching the leaf.
Supplementary Material
References
- Allakhverdiev SI, Murata N. (2004) Environmental stress inhibits the synthesis de novo of proteins involved in the photodamage-repair cycle of photosystem II in Synechocystis sp PCC 6803. Biochim Biophys Acta 1657: 23–32 [DOI] [PubMed] [Google Scholar]
- Allakhverdiev SI, Nishiyama Y, Takahashi S, Miyairi S, Suzuki I, Murata N. (2005) Systematic analysis of the relation of electron transport and ATP synthesis to the photodamage and repair of photosystem II in Synechocystis. Plant Physiol 137: 263–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aro EM, Virgin I, Andersson B. (1993) Photoinhibition of photosystem II: inactivation, protein damage and turnover. Biochim Biophys Acta 1143: 113–134 [DOI] [PubMed] [Google Scholar]
- Booij-James IS, Dube SK, Jansen MAK, Edelman M, Mattoo AK. (2000) Ultraviolet-B radiation impacts light-mediated turnover of the photosystem II reaction center heterodimer in Arabidopsis mutants altered in phenolic metabolism. Plant Physiol 124: 1275–1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow WS, Lee HY, He J, Hendrickson L, Hong YN, Matsubara S. (2005) Photoinactivation of photosystem II in leaves. Photosynth Res 84: 35–41 [DOI] [PubMed] [Google Scholar]
- Hakala M, Tuominen I, Keränen M, Tyystjärvi T, Tyystjärvi E. (2005) Evidence for the role of the oxygen-evolving manganese complex in photoinhibition of Photosystem II. Biochim Biophys Acta 1706: 68–80 [DOI] [PubMed] [Google Scholar]
- Jegerschöld C, Virgin I, Styring S. (1990) Light-dependent degradation of the D1 protein in photosystem II is accelerated after inhibition of the water splitting reaction. Biochemistry 29: 6179–6186 [DOI] [PubMed] [Google Scholar]
- Jones LW, Kok B. (1966) Photoinhibition of chloroplast reactions. I. Kinetics and action spectra. Plant Physiol 41: 1037–1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung J, Kim HS. (1990) The chromophores as endogenous sensitizers involved in the photogeneration of singlet oxygen in spinach thylakoids. Photochem Photobiol 52: 1003–1009 [Google Scholar]
- Kasahara M, Kagawa T, Oikawa K, Suetsugu N, Miyao M, Wada M. (2002) Chloroplast avoidance movement reduces photodamage in plants. Nature 420: 829–832 [DOI] [PubMed] [Google Scholar]
- Kirilovsky D, Rutherford AW, Etienne AL. (1994) Influence of DCMU and ferricyanide on photodamage in photosystem II. Biochemistry 33: 3087–3095 [DOI] [PubMed] [Google Scholar]
- Landry LG, Chapple CCS, Last RL. (1995) Arabidopsis mutants lacking phenolic sunscreens exhibit enhanced ultraviolet-B injury and oxidative damage. Plant Physiol 109: 1159–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JY, Oulee TM, Raba R, Amundson RG, Last RL. (1993) Arabidopsis flavonoid mutants are hypersensitive to UV-B irradiation. Plant Cell 5: 171–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long SP, Humphries S, Falkowski PG. (1994) Photoinhibition of photosynthesis in nature. Annu Rev Plant Physiol Plant Mol Biol 45: 633–662 [Google Scholar]
- Mano J, Hideg E, Asada K. (2004) Ascorbate in thylakoid lumen functions as an alternative electron donor to photosystem II and photosystem I. Arch Biochem Biophys 429: 71–80 [DOI] [PubMed] [Google Scholar]
- Mattoo AK, Hoffman-Falk H, Marder JB, Edelman M. (1984) Regulation of protein metabolism: coupling of photosynthetic electron transport to in vivo degradation of the rapidly metabolized 32-kilodalton protein of the chloroplast membranes. Proc Natl Acad Sci USA 81: 1380–1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCree KJ. (1972) Action spectrum, absorptance and quantum yield of photosynthesis in crop plants. Agric Meteorol 9: 191–216 [Google Scholar]
- Melis A. (1999) Photosystem II damage and repair cycle in chloroplasts: what modulates the rate of photodamage in vivo? Trends Plant Sci 4: 130–135 [DOI] [PubMed] [Google Scholar]
- Murata N, Takahashi S, Nishiyama Y, Allakhverdiev SI. (2007) Photoinhibition of photosystem II under environmental stress. Biochim Biophys Acta 1767: 414–421 [DOI] [PubMed] [Google Scholar]
- Nishiyama Y, Allakhverdiev SI, Murata N. (2006) A new paradigm for the action of reactive oxygen species in the photoinhibition of photosystem II. Biochim Biophys Acta 1757: 742–749 [DOI] [PubMed] [Google Scholar]
- Nishiyama Y, Allakhverdiev SI, Yamamoto H, Hayashi H, Murata N. (2004) Singlet oxygen inhibits the repair of photosystem II by suppressing the translation elongation of the D1 protein in Synechocystis sp. PCC 6803. Biochemistry 43: 11321–11330 [DOI] [PubMed] [Google Scholar]
- Nishiyama Y, Yamamoto H, Allakhverdiev SI, Inaba M, Yokota A, Murata N. (2001) Oxidative stress inhibits the repair of photodamage to the photosynthetic machinery. EMBO J 20: 5587–5594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niyogi KK. (1999) Photoprotection revisited: genetic and molecular approaches. Annu Rev Plant Physiol Plant Mol Biol 50: 333–359 [DOI] [PubMed] [Google Scholar]
- Ohnishi N, Allakhverdiev SI, Takahashi S, Higashi S, Watanabe M, Nishiyama Y, Murata N. (2005) Two-step mechanism of photodamage to photosystem II: step 1 occurs at the oxygen-evolving complex and step 2 occurs at the photochemical reaction center. Biochemistry 44: 8494–8499 [DOI] [PubMed] [Google Scholar]
- Pätsikkä E, Kairavuo M, Šeršen F, Aro EM, Tyystjärvi E. (2002) Excess copper predisposes photosystem II to photoinhibition in vivo by outcompeting iron and causing decrease in leaf chlorophyll. Plant Physiol 129: 1359–1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarvikas P, Hakala M, Pätsikkä E, Tyystjärvi T, Tyystjärvi E. (2006) Action spectrum of photoinhibition in leaves of wild type and npq1-2 and npq4-1 mutants of Arabidopsis thaliana. Plant Cell Physiol 47: 391–400 [DOI] [PubMed] [Google Scholar]
- Satter RL, Galston AW. (1981) Mechanisms of control of leaf movements. Annu Rev Plant Physiol Plant Mol Biol 32: 83–110 [Google Scholar]
- Takahashi S, Bauwe H, Badger M. (2007) Impairment of the photorespiratory pathway accelerates photoinhibition of photosystem II by suppression of repair process and not acceleration of damage process in Arabidopsis thaliana. Plant Physiol 144: 487–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S, Milward SE, Fan DY, Chow WS, Badger MR. (2009) How does cyclic electron flow alleviate photoinhibition in Arabidopsis? Plant Physiol 149: 1560–1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S, Murata N. (2005) Interruption of the Calvin cycle inhibits the repair of photosystem II from photodamage. Biochim Biophys Acta 1708: 352–361 [DOI] [PubMed] [Google Scholar]
- Takahashi S, Murata N. (2006) Glycerate-3-phosphate, produced by CO2 fixation in the Calvin cycle, is critical for the synthesis of the D1 protein of photosystem II. Biochim Biophys Acta 1757: 198–205 [DOI] [PubMed] [Google Scholar]
- Takahashi S, Murata N. (2008) How do environmental stresses accelerate photoinhibition? Trends Plant Sci 13: 178–182 [DOI] [PubMed] [Google Scholar]
- Tóth SZ, Puthur JT, Nagy V, Garab G. (2009) Experimental evidence for ascorbate-dependent electron transport in leaves with inactive oxygen-evolving complexes. Plant Physiol 149: 1568–1578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyystjärvi E. (2008) Photoinhibition of photosystem II and photodamage of the oxygen evolving manganese cluster. Coord Chem Rev 252: 361–376 [Google Scholar]
- Tyystjärvi E, Aro EM. (1996) The rate constant of photoinhibition, measured in lincomycin-treated leaves, is directly proportional to light intensity. Proc Natl Acad Sci USA 93: 2213–2218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vass I, Cser K. (2009) Janus-faced charge recombinations in photosystem II photoinhibition. Trends Plant Sci 14: 200–205 [DOI] [PubMed] [Google Scholar]
- Vogelmann TC, Nishio JN, Smith WK. (1996) Leaves and light capture: light propagation and gradients of carbon fixation within leaves. Trends Plant Sci 1: 65–70 [Google Scholar]
- Winkel-Shirley B. (2001) Flavonoid biosynthesis: a colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol 126: 485–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkel-Shirley B. (2002) Biosynthesis of flavonoids and effects of stress. Curr Opin Plant Biol 5: 218–223 [DOI] [PubMed] [Google Scholar]
- Zsiros O, Allakhverdiev SI, Higashi S, Watanabe M, Nishiyama Y, Murata N. (2006) Very strong UV-A light temporally separates the photoinhibition of photosystem II into light-induced inactivation and repair. Biochim Biophys Acta 1757: 123–129 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.