Abstract
The translocon at the outer membrane of the chloroplast assists the import of a large class of preproteins with amino-terminal transit sequences. The preprotein receptors Toc159 and Toc33 in Arabidopsis (Arabidopsis thaliana) are specific for the accumulation of abundant photosynthetic proteins. The receptors are homologous GTPases known to be regulated by phosphorylation within their GTP-binding domains. In addition to the central GTP-binding domain, Toc159 has an acidic N-terminal domain (A-domain) and a C-terminal membrane-anchoring domain (M-domain). The A-domain of Toc159 is dispensable for its in vivo activity in Arabidopsis and prone to degradation in pea (Pisum sativum). Therefore, it has been suggested to have a regulatory function. Here, we show that in Arabidopsis, the A-domain is not simply degraded but that it accumulates as a soluble, phosphorylated protein separated from Toc159. However, the physiological relevance of this process is unclear. The data show that the A-domain of Toc159 as well as those of its homologs Toc132 and Toc120 are targets of a casein kinase 2-like activity.
The Toc and Tic complexes cooperate to import nuclear-encoded chloroplast preproteins from the cytosol (Jarvis, 2008; Kessler and Schnell, 2009). Initially, incoming preproteins encounter the receptors Toc159 and Toc34 at the chloroplast surface. Both are GTP-binding proteins and share sequence homology in their G-domains. While Toc34 is anchored in the outer membrane by a short hydrophobic C-terminal tail, the triple-domain Toc159 is inserted via a largely hydrophilic 52-kD M-domain. In addition to the G- and M-domains, Toc159 has a large acidic A-domain covering the N-terminal half of the protein. Arabidopsis (Arabidopsis thaliana) encodes two isoforms of Toc34 (Toc33 and Toc34) and four of Toc159 (Toc159, Toc132, Toc120, and Toc90; Jackson-Constan and Keegstra, 2001). The Toc159 isoforms have a similar domain structure, but they differ from each other in length and sequence of their A-domain (Hiltbrunner et al., 2001a). However, Toc90 does not have an acidic domain at all and only consists of the G- and M-domains (Hiltbrunner et al., 2004). It has been demonstrated that the A-domain of AtToc159 and AtToc132 have properties of intrinsically disordered proteins (Hernández Torres et al., 2007; Richardson et al., 2009), suggesting an involvement of the A-domain in transient and multiple protein-protein interactions possibly with the transit peptides of preproteins. Toc34 and Toc159 together with the Toc75 channel constitute the Toc-core complex (Schleiff et al., 2003) and are required for the accumulation of highly abundant photosynthesis-associated proteins in the chloroplast. The Arabidopsis deletion mutants of Toc33 (ppi1; Jarvis et al., 1998) and Toc159 (ppi2; Bauer et al., 2000) have indicative phenotypes of their role in chloroplast biogenesis, respectively pale green and albino. Complementation experiments of the ppi2 mutant have established that the G- and M-domains have essential functions whereas the A-domain is dispensable (Lee et al., 2003; Agne et al., 2009). In preceding studies, possibly influenced by the model organism and experimental tools, Toc159 occurred in different forms. Initially, Toc159 was identified in pea (Pisum sativum) as an 86-kD protein lacking the entire A-domain (Hirsch et al., 1994; Bolter et al., 1998). In addition to its membrane-associated form, Arabidopsis Toc159 has been found as a soluble protein (Hiltbrunner et al., 2001b). However, the function and the fate of the A-domain as well as that of soluble Toc159 remain unknown and a matter of debate.
Not only GTP binding and hydrolysis by the Toc GTPases but also phosphorylation is known as a regulatory mechanism of chloroplast protein import at the Toc complex level (Oreb et al., 2008b). First, some precursor proteins, such as the small subunit of Rubisco, may be phosphorylated in their transit sequence by a cytosolic kinase (Martin et al., 2006). Phosphorylation promotes binding to a 14-3-3 protein and cytosolic Hsp70 in the guidance complex that delivers the phosphorylated preprotein to the Toc complex (May and Soll, 2000). Second, both Toc159 and Toc34 are known to be phosphorylated and independently so by distinct kinases, OEK70 and OEK98, respectively (Fulgosi and Soll, 2002). These two kinase activities have been located to the outer envelope membrane, but their molecular identification is still pending. Phosphorylation of the Toc GTPases may occur in the GTP-binding domains (Oreb et al., 2008a). For Toc34, data on the site (Ser-113 in pea and Ser-181 in Arabidopsis) and effects of phosphorylation are available (Jelic et al., 2002, 2003). It imposes a negative regulation on the Toc complex by inhibiting GTP and preprotein binding to Toc34, reducing its ability to bind Toc159 and to assemble into the Toc complex (Oreb et al., 2008a). The in vivo mutational analysis in Arabidopsis indicated that phosphorylation at Toc34 represents a nonessential mechanism (Aronsson et al., 2006; Oreb et al., 2007). Despite the 86-kD proteolytic fragment of Toc159 being a major phosphoprotein in the pea outer chloroplast membrane (Fulgosi and Soll, 2002), little is known of the molecular and regulatory mechanisms of Toc159 phosphorylation. In this study, we report that the A-domain of Toc159 can be purified as a stable fragment. Moreover, it is hyperphosphorylated, hinting at an important and highly regulated functional role. Our data suggest that Toc159 is the target of casein kinase 2 (CK2)-like and membrane-associated kinase activities.
RESULTS
TAP-Tagged Toc159
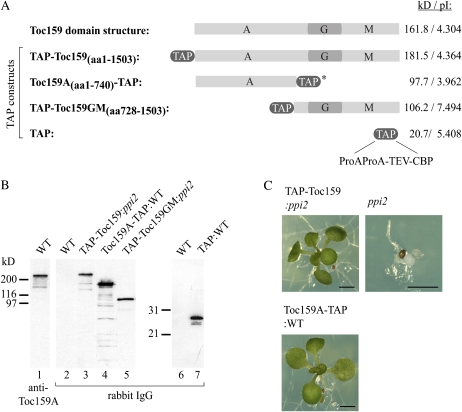
For the study of molecular interactions of Toc159 and its subdomains, we tagged Toc159 and domains of it with a Tandem Affinity Purification (TAP) tag (Fig. 1A). The TAP tag consists of two Protein A IgG-binding domains and calmodulin-binding protein separated by a tobacco etch virus (TEV) protease cleavage site. TEV cleavage can be used to release TAP-tagged protein from an IgG column. Transgenic Arabidopsis plants expressing full-length TAP-Toc159 (TAP-Toc159:ppi2) and TAP-Toc159GM lacking the A-domain (TAP-Toc159GM:ppi2) have been described before (Agne et al., 2009). Both fusion proteins were functional and able to complement the albino phenotype of the ppi2 mutant. In contrast, a fusion of the Toc159 A-domain to a C-terminal TAP tag as well as the TAP tag alone (data not shown) did not complement. Therefore, only wild-type plants expressing those fusion proteins were isolated for this study (Fig. 1C). Western blotting of total plant extracts of all of the transgenic lines revealed expression of all fusion proteins (Fig. 1B). Anomalous SDS-PAGE migration of the fusion proteins of the A-domain not correlating with the calculated molecular mass was observed (Fig. 1B). This anomalous migration behavior is probably due to the high content (26.9%) of acidic amino acid residues in the A-domain. The Toc159A-TAP fusion protein appeared more susceptible to protein degradation than the full-length protein (Fig. 1B, compare lanes 3 and 4). Several lower molecular mass bands were detected with unspecific rabbit IgG in western blotting of the Toc159A-TAP:WT extract. For full-length TAP-Toc159, only two additional lower molecular mass bands migrating between 116 and 200 kD were detected. The recognition of these bands by IgG and thus the presence of the N-terminal TAP tag indicated the N-terminal nature of the Toc159 fragments. Affinity-purified antibodies against the Toc159 A-domain revealed a similar pattern in extracts of wild-type plants, resulting in a band at 150 kD in addition to full-length Toc159 at 220 kD (Fig. 1B, lane 1). However, only one band was observed at 150 kD in the wild type. The detection of the 150-kD band in the wild type total extract by the anti-A-domain antibody is likewise consistent with an N-terminal fragment of Toc159.
Figure 1.
Transgenic plants for TAP of AtToc159. A, Schematic representation of the Toc159-TAP fusion proteins. *, In the C-terminally fused TAP tag, the order of its segments is inversed and reads CBP-TEV-ProAProA. B, Immunoblot of plant extracts of transgenic lines with unspecific rabbit IgG to detect the TAP-Toc159 fusion proteins (lanes 3–5) or the TAP tag alone (lane 7). Anti-Toc159A antibodies were used to detect endogenous AtToc159 in wild-type (WT) plants (lane 1). C, TAP-Toc159:ppi2 and Toc159A-TAP:WT plants reveal a green phenotype. Seedlings were grown for 21 d under short-day conditions on medium containing phosphinotricin before the photographs were taken. Bars = 0.2 cm.
Distribution of TAP-Toc159
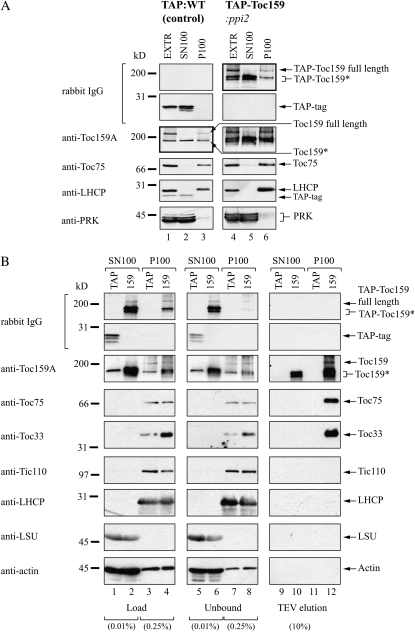
When total extracts of both TAP:WT control plants and TAP-Toc159:ppi2 plants were separated into soluble (SN100) and total (P100) membrane fractions by centrifugation at 100,000g (Fig. 2A), a similar immunodetection pattern was obtained when probing the western blot with anti-Toc159A and unspecific IgG, respectively (Fig. 2A, thick-framed immunoblots). Using anti-Toc159A wild type, full-length Toc159 was detected in the total extract as well as the P100 membrane fraction of TAP:WT plants. In contrast, a lower Mr band (Toc159*) was almost equally present in the P100 membrane and the SN100 soluble fraction. Similarly, using unspecific IgG full length, TAP-Toc159 was detected in the total extract and the P100 membrane fraction, and one to two prominent lower Mr bands (TAP-Toc159*) were present in both the P100 membrane and SN100 soluble fractions. In control western blots, the outer membrane marker Toc75 and the thylakoid marker chlorophyll a/b-binding protein were only detectable in the P100 membrane fraction. The soluble marker phosphoribulokinase as well as the TAP tag expressed in TAP:WT plants were detected almost exclusively in the soluble fraction. The membrane and soluble markers indicate good separation of the respective fractions. In conclusion, these results suggested the existence of an N-terminal fragment of Toc159 with a dual localization and to some extent properties of a soluble protein. Considering the high Mr of this fragment as well as its solubility, we hypothesize that it might constitute a large proportion of the A-domain.
Figure 2.
IgG affinity purification of TAP-Toc159 species from soluble and membrane fractions. A, Immunoblot analysis of total protein extracts (EXTR), soluble fraction (SN100), and membrane fraction (P100) of wild-type plants expressing only the TAP tag (lanes 1–3) and ppi2 plants expressing TAP-Toc159 (lanes 4–6). Fifty micrograms of protein from each fraction was separated by SDS-PAGE and subjected to western-blot analysis with antibodies as indicated. B, Immunoblot analysis of different fractions obtained during purification of the TAP tag/TAP-Toc159 from the soluble and membrane fractions. Fifty milligrams of soluble protein (SN100) and 20 mg of Triton X-100-solubilized membrane-associated protein (P100) were loaded on an IgG Affi-Gel column. Proteins were eluted using TEV protease. The percentage indicates the amount of protein loaded relative to the total amount of protein in the load, unbound and eluate fractions (100%). LHCP, Chlorophyll a/b-binding protein; LSU, large Rubisco subunit; PRK, phosphoribulokinase; TAP, samples originating from TAP:WT plants; 159, samples originating from TAP-Toc159:ppi2 plants; TAP-Toc159*/Toc159*, proteolytic fragments of TAP-Toc159/Toc159.
Isolation of N-Terminally TAP-Tagged Toc159 Species
We purified TAP-Toc159 as well as its N-terminal fragment from the soluble SN100 fraction and from the Triton X-100-solubilized P100 membrane fraction of TAP-Toc159:ppi2 plants using IgG affinity chromatography followed by TEV protease elution (Fig. 2B, lane 159). As a negative control, we used the corresponding fractions from TAP:WT plants (Fig. 2B, lane TAP). The load and unbound fractions as well as the TEV eluates of the IgG chromatography experiment were analyzed by SDS-PAGE and western blotting. From the P100 fraction of TAP-Toc159:ppi2 plants, we purified full-length Toc159 as well as the two lower Mr fragments (Toc159*) together with known components of the Toc complex, Toc75 and Toc33 (Fig. 2B, lane 12). From the SN100 fraction of the same plants, only the lower Mr fragments of TAP-Toc159 were obtained (Fig. 2B, lane 10). On control western blots, neither Tic110, chlorophyll a/b-binding protein, the large Rubisco subunit, nor actin was detected in the TEV eluates. No Toc proteins were detected in the negative control TEV eluates (Fig. 2B, lanes 9 and 11).
Identification of Different Toc159 Molecular Species
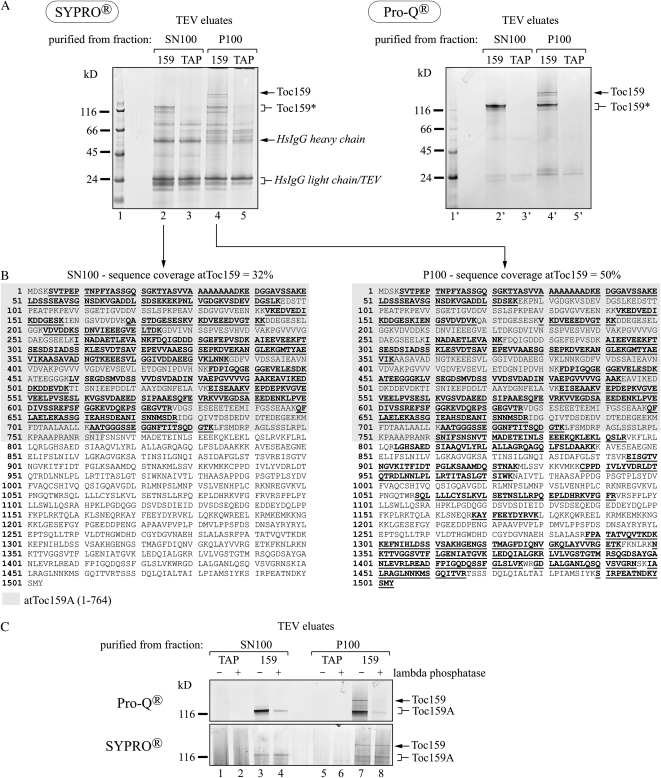
We used mass spectrometry to determine the molecular nature of the different forms of Toc159 in the TEV eluates. The TEV eluates (Fig. 2B) were separated on a Bis-Tris gel and stained with SyproRuby (Fig. 3A). The band corresponding in mass to full-length Toc159 was only detected in the TEV eluate of the P100 fraction (Fig. 3A, lane 4). The lower molecular mass fragment was present in the TEV eluate of both the P100 and the SN100 fractions (Fig. 3A, lanes 2 and 4). Some apparently nonspecific bands occurred in the control eluates (Fig. 3A, lanes 3 and 5) as well as the eluates derived from TAP-Toc159:ppi2 plants (Fig. 3A, lanes 2 and 4). Bands of interest were excised, digested in-gel with trypsin, eluted, and subjected to mass spectrometry. The P100 fraction contained full-length Toc159; peptides along the entire sequence of Toc159 were identified, giving an overall coverage of 50% (Fig. 3B, right panel). The SN100 fraction yielded peptides mapping only to amino acids 1 to 733. This corresponds to a coverage of 32% of the total Toc159 sequence and of 57% of the A-domain sequence (defined here as amino acids 1–764 of Toc159 based on data from Bolter et al. [1998]; Fig. 3B, left panel). This indicates that the Toc159 fragment in the SN100 fraction represents the entire A-domain as a separate soluble protein.
Figure 3.
The Toc159 species purified from the soluble fraction constitute the A-domain. A, TEV eluates after purification of TAP-Toc159 from TAP-Toc159:ppi2 (159) and of the control TAP tag from TAP:WT tag plants (TAP) were separated on a NuPAGE Novex 4% to 12% BisTris gel. The gel was stained with the phosphospecific fluorescent dye ProQ-Diamond and with the total protein stain SyproRuby. The PeppermintStick molecular mass marker (Invitrogen) contains two phosphorylated proteins of 45 kD (ovalbumin) and 24 kD (β-casein). Toc159*, Proteolytic fragment of Toc159. B, Peptides (boldface and underlined) and sequence coverage obtained by tandem mass spectrometry analysis of TAP-Toc159 species. The Toc159 A-domain sequence, inferred from the experimentally determined start of the Toc86 fragment of pea Toc159 (Hirsch et al., 1994; Bolter et al., 1998), is highlighted in gray. C, Phosphatase treatment demonstrates phosphospecific staining of Toc159 species. TEV eluates derived from TAP:WT tag plants (TAP) or TAP-Toc159:ppi2 plants (159) were treated (+) or not (−) with λ-phosphatase prior to separation on a NuPAGE Novex 4% to 12% BisTris gel and ProQ-Diamond and SyproRuby staining. SN100, Protein purified from the soluble fraction; P100, protein purified from the 100,000g pellet solubilized with Triton X-100.
Phosphorylation of the Toc159 A-Domain
In vitro phosphorylation of the 86-kD fragment of Toc159 (comprising the G- and M-domains) and the isolated G-domain has been reported (Fulgosi and Soll, 2002; Oreb et al., 2008a). More recently, phosphopeptides derived from the A-domain have also been reported (de la Fuente van Bentem et al., 2008; Sugiyama et al., 2008; Whiteman et al., 2008; Reiland et al., 2009). To determine whether Toc159 and the separated A-domain occur as phosphoproteins in vivo, the TEV eluates were stained with the phosphospecific dye ProQ-Diamond after SDS-PAGE (Fig. 3A, right panel). ProQ-Diamond stained the bands identified by mass spectrometry as full-length Toc159 and its A-domain (Fig. 3A, lanes 2′ and 4′). The nonspecific bands present in the eluates and nonphosphorylated bands of the Mr standard were not or only weakly stained. Treatment of TEV eluates with λ-phosphatase prior to ProQ-Diamond staining strongly reduced the intensity of the bands, demonstrating phosphospecificity (Fig. 3C). Therefore ProQ-Diamond staining of full-length Toc159 and of the A-domain is indeed due to phosphorylation and not to unspecific binding of the dye to the acidic A-domain.
Summary of Phosphorylation Sites in the Toc159 A-Domain
To identify phosphorylation sites in Toc159, two different approaches were used: (1) isolation of TAP-Toc159 and identification of phosphorylation sites by mass spectrometry analysis; and (2) identification of Toc159 phosphopeptides in the available Arabidopsis phosphoproteome data. In the first approach, full-length Toc159 and its A-domain fragment were isolated from the P100 or the soluble SN100 fraction as described above and subjected to mass spectrometry analysis. Thereby, three Toc159 phosphosites (S71, S281, and S589) and an additional phosphopeptide of Toc159 were identified (Table I; Supplemental Table S1). In a large-scale phosphoproteomics experiment, the same phosphosites plus nine additional ones (S210, S288, S448, S609, S630, S632, T684, S686, and T692) were assigned (Reiland et al., 2009). The data of the latter study are available in the PhosPhAt database (Heazlewood et al., 2008). PhosPhAt contains data from four other phosphoproteomics studies (de la Fuente van Bentem et al., 2008; Sugiyama et al., 2008; Whiteman et al., 2008; Jones et al., 2009), identifying four phosphosites (S71, S281, S609, and S632) conforming with the data of Reiland et al. (2009). Interestingly, all 12 phosphosites map to the A-domain of Toc159 (Fig. 4), and most of them are predicted to be a target of CK2 (Table I). Phosphoproteomics yielded more phosphopeptides mapping to the A-domain to which the phosphosites could not be assigned. No phosphopeptides mapping to either the G- or M-domain were identified in any of these studies. In summary, the results identify the A-domain of Toc159 as a highly phosphorylated protein in vivo.
Table I. Summary of Toc159 phosphopeptides.
The phosphopeptide spectra of this study are available in Supplemental Spectra S1. Phosphorylation motif prediction was done with NetPhosK 1.0 (Blom et al., 2004). n.d., Not determined.
| Study | Peptide Sequence | Position of Phosphorylated Amino Acids | Predicted Kinase |
| This study | VGADDLpSDSEK | S71 | CK2 |
| VGADDLSDSEKEKPNLVGDGK | n.d. | ||
| FDQIGDDDpSGEFEPVSDK | S281 | CK2 | |
| VVEGDpSAEEDENKLPVEDIVSSR | S589 | CK2 | |
| VVEGDpSAEEDENKLPVEDIVSSR | S589 | CK2 | |
| KVVEGDpSAEEDENKLPVEDIVSSR | S589 | CK2 | |
| ASSGIEAHSDEANISNNMSDR | n.d. | ||
| ASSGIEAHSDEANISNNMSDR | n.d. | ||
| Reiland et al. (2009) | VGADDLpSDSEK | S71 | CK2 |
| VGADDLSDSEK | n.d. | ||
| ELDSSSEAVSGNSDKVGADDLSDSEK | n.d. | ||
| VDVDDKpSDNVIEEEGVELTDK | S210 | CK2 | |
| FDQIGDDDpSGEFEPVSDK | S281 | CK2 | |
| FDQIGDDDSGEFEPVpSDK | S288 | CK2 | |
| INADAETLEVANKFDQIGDDDSGEFEPVSDK | n.d. | ||
| FTSESDSIADSSK | n.d. | ||
| FDPIGQGEGGEVELEpSDK | S448 | CK2, PKC | |
| KVVEGDpSAEEDENK | S589 | CK2 | |
| EFpSFGGKEVDQEPSGEGVTR | S609 | CK1 | |
| VDGSESEEETEE°MIFGSSEAAK | n.d. | ||
| VDGpSEpSEEETEE°MIFGSSEAAK | S630/S632 | CK2, PKA/CK2, CK1 | |
| EVDQEPSGEGVTRVDGSESEEETEE°MIFGSSEAAK | n.d. | ||
| ASSGIEAHSDEANISNNMSDR | n.d. | ||
| IDGQIVTDSDEDVDTEDEGEEK | n.d. | ||
| IDGQIVpTDpSDEDVDpTEDEGEEK | T684/S686/T692 | CK2/CK2/CK2, CK1 | |
| de la Fuente van Bentem et al. (2008); Sugiyama et al. (2008) | VGADDLpSDSEK | S71 | CK2 |
| Sugiyama et al. (2008); Whiteman et al. (2008) | FDQIGDDDpSGEFEPVSDK | S281 | CK2 |
| Sugiyama et al. (2008) | VGVEVEELPVSESLK | n.d. | |
| Sugiyama et al. (2008) | EFpSFGGK | S609 | CK1 |
| Whiteman et al. (2008) | VDGSEpSEEETEE°MIFGSSEAAK | S632 | CK2, CK1 |
| Jones et al. (2009) | ASSGIEAHSDEANISNNMSDR | n.d. |
Figure 4.
Toc159 phosphopeptides map to the A-domain. A, Sequence of AtToc159 with phosphopeptides (boldface and underlined) and phosphorylation sites (red) according to Table I. B, WebLogo analysis (Crooks et al., 2004) of Toc159 phosphorylation sites. Color scheme is as follows: S/T, red; D/E, blue; K/R, orange; L/I/V/M, green.
In Vivo Phosphorylation of Toc159A-TAP
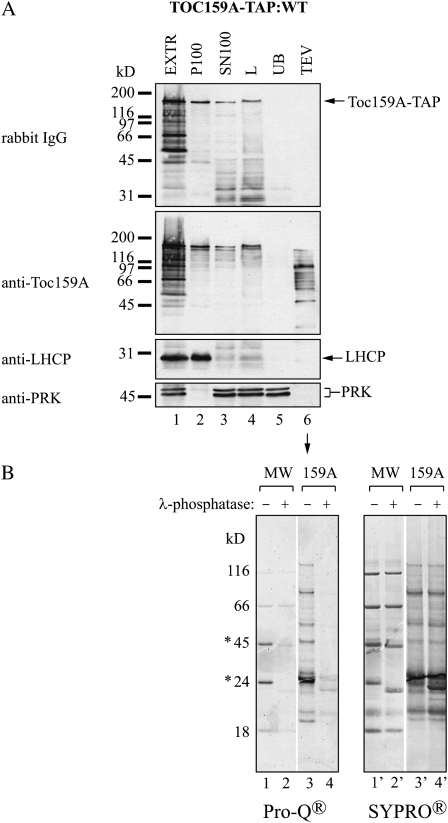
To determine whether phosphorylation of the A-domain of Toc159 depended on being part of full-length Toc159, we made use of Toc159A-TAP:WT plants expressing the first 740 amino acids of Toc159 fused to a C-terminal TAP tag (Fig. 1). When analyzed by western blotting, Toc159A-TAP was present in both the SN100 and P100 fractions, indicating partial association of Toc159A-TAP with membranes (Fig. 5A, lanes 2 and 3). We purified Toc159A-TAP from the SN100 fraction (Fig. 5). Anti-Toc159A antibodies detected multiple bands on the western blot of the TEV eluate (Fig. 5A, lane 6). Apparently, Toc159A-TAP was more degraded than the A-domain originating from TAP-Toc159. Equal volumes of the Toc159A-TAP eluate were subjected to SyproRuby and ProQ-Diamond staining with or without prior phosphatase treatment (Fig. 5B). The SyproRuby stain confirmed equal loading of the two samples. Phosphatase treatment prior to the analysis strongly reduced ProQ-Diamond staining when compared with untreated Toc159A-TAP eluate. This demonstrates that the soluble Toc159A-TAP alone is an in vivo kinase target independent of being part of the full-length protein.
Figure 5.
Toc159A overexpressed in planta is phosphorylated. A, Immunoblot analysis of different fractions obtained during purification of Toc159A(aa1-740)-TAP from the soluble fraction (SN100). Soluble protein (lanes 3 and 4) was loaded on a HsIgG-Affi-Gel column. After removal of unbound protein (UB; lane 5) and washing, elution was carried out using AcTEV protease (TEV; lane 6). EXTR, Plant extract; P100, 100,000g pellet; SN100, 100,000g supernatant; L, 100,000g supernatant at the time of loading on HsIgG-Affi-Gel. B, The PeppermintStick phosphoprotein molecular mass standard (MW) and the TEV eluate derived from the soluble fraction of WT:Toc159A-TAP plants (159A) were treated (+) or not (−) with λ-phosphatase prior to separation on a NuPAGE Novex 4% to 12% BisTris gel and ProQ-Diamond and SyproRuby staining. Asterisks indicate phosphorylated proteins of the molecular mass standard.
Phosphorylation of Recombinant Toc159A by Arabidopsis Protein in Vitro
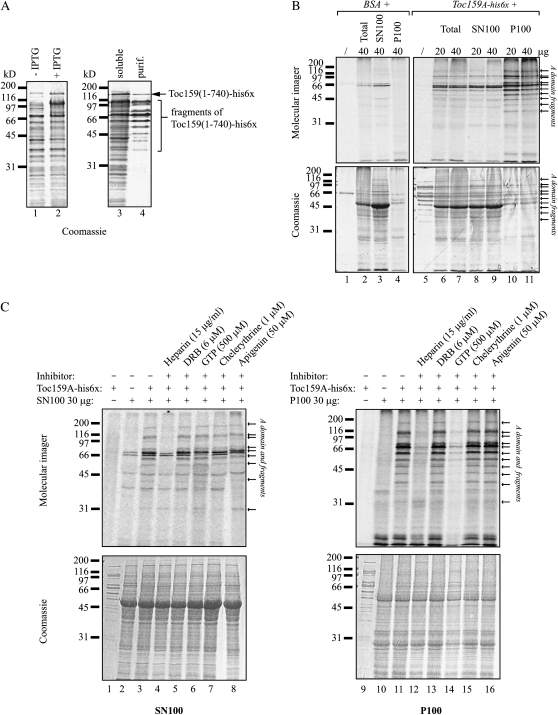
To investigate Arabidopsis kinase activity phosphorylating Toc159A, we overexpressed and purified Toc159A-His-6x from bacteria (Fig. 6A) and used it as a substrate in in vitro phosphorylation assays. Arabidopsis extracts were used as a kinase source (Fig. 6B). Similar to the A-domain purified from Toc159A-TAP:WT plants, recombinant Toc159A-His-6x expressed in bacteria could only be purified as multiple fragments. Toc159A-His-6x was incubated with [γ-33P]ATP in the presence of either 20 or 40 μg of total protein extract (Total; Fig. 6B, lanes 6 and 7), total soluble protein (SN100; Fig. 6B, lanes 8 and 9), and Triton X-100-solubilized total membrane proteins (P100; Fig. 6B, lanes 10 and 11). As a negative control, the same fractions were incubated with [γ-33P]ATP in the presence of bovine serum albumin instead of Toc159A-His-6x to reveal phosphorylation of endogenous proteins in the extract (Fig. 6B, lanes 2–4). The experiments were analyzed by SDS-PAGE followed by phosphorimager analysis. Multiple bands derived from recombinant Toc159A-His-6x were phosphorylated. In the case of the P100 Triton X-100 solubilisate, phosphorylation appeared entirely specific to Toc159A-His-6x. In the total extract and SN100 samples, Toc159A-His-6x was phosphorylated to a lesser extent and some of the phosphoprotein bands were due to phosphorylation of endogenous plant proteins. Phosphorylation of the A-domain was not due to autophosphorylation, as incubation of Toc159A-His-6x with [γ-33P]ATP alone did not yield any phosphobands (Fig. 6B, lane 5).
Figure 6.
In vitro phosphorylation of Toc159A by an Arabidopsis kinase activity sensitive to heparin and using GTP. A, Toc159A-His-6x (Toc159aa1-740-His-6x) was overexpressed in E. coli BL21(DE3) and purified under native conditions from the bacterial soluble protein fraction using nickel-nitrilotriacetic acid agarose affinity chromatography. Samples of noninduced cultures (−IPTG [isopropylthio-β-galactoside]), induced cultures (+IPTG), the soluble protein fraction (soluble), and the purified protein (purif.) were separated by SDS-PAGE followed by Coomassie Brilliant Blue staining. B, Bovine serum albumin (BSA; control) or purified Toc159A-His-6x was incubated in the presence of [γ-33P]ATP with different amounts of Arabidopsis plant cell extract (Total; lanes 2, 6, and 7), the supernatant (SN100; lanes 3, 8, and 9), or the pellet (P100; lanes 4, 10, and 11) fraction after 100,000g centrifugation of the extract. Incubation in the absence of plant protein (lanes 1 and 5) was used to monitor autophosphorylation. The samples were analyzed by SDS-PAGE followed by Coomassie Brilliant Blue staining (bottom) and phosphorimager analysis (top). To ease detection of phosphorylated A-domain fragments in the plant samples, the Mr values of the characteristic fragments of Toc159A-His-6x, observed after bacterial overexpression and purification, are marked by arrows. C, In vitro phosphorylation of Toc159A-His-6x by the SN100 (left) and P100 (right) fractions in the presence of the kinase inhibitors heparin, DRB, chelerythrine chloride, and apigenin or unlabeled GTP as an alternative phosphate donor.
To roughly characterize the identified kinase activities, a similar experiment was repeated in the presence of kinase inhibitors: heparin (inhibiting CK2, protein kinase C [PKC], and Ca2+/calmodulin-dependent protein kinase), 5,6-dichlorobenzimidazole riboside (DRB; inhibiting CK2 and cyclin-dependent kinase), apigenin (inhibiting CK2 and mitogen-activated protein kinase), chelerythrine (inhibiting PKC), or nonradioactive GTP as a potential competitor (CK2; Fig. 6C). Phosphorylation of Toc159A-His-6x by the SN100 and the P100 fractions was effectively inhibited by heparin at a concentration of 15 μg mL−1 (Fig. 6C, lanes 4 and 12). Cold GTP at 500 μm clearly competed [γ-33P]ATP phosphorylation of Toc159A-His-6x by P100 (Fig. 6C, lane 14) but interestingly not by the SN100 fraction (Fig. 6C, lane 6). All other inhibitors tested had no apparent effect on Toc159A-His-6x phosphorylation. Heparin inhibition and the ability to use GTP as well as ATP as the phosphodonor are characteristic of CK2.
The NetPhosK 1.0 server (Blom et al., 2004) predicts 57 CK2 sites (score 0.5) in Toc159, 48 of which locate to the A-domain. Of the 12 identified in vivo phosphosites, seven were predicted to be phosphorylated by CK2 (S71, S210, S281, S288, S589, T684, and S686) only, whereas the remaining five phosphosites (S448, S609, S630, S632, and T692) could be targets of CK2 and/or of members of a different kinase family. The results of the kinase inhibitor experiment (Fig. 6C) as well as the observation that most of the Toc159A in vivo phosphorylation sites (Table I) are predicted CK2 targets strongly suggest that Toc159 is phosphorylated by a CK2-like activity in vivo. Furthermore, the in vitro A-domain phosphorylation experiments (Fig. 6, B and C) indicate distinct kinase activities localized either in the membrane or the soluble compartment.
Phosphorylation by CK2
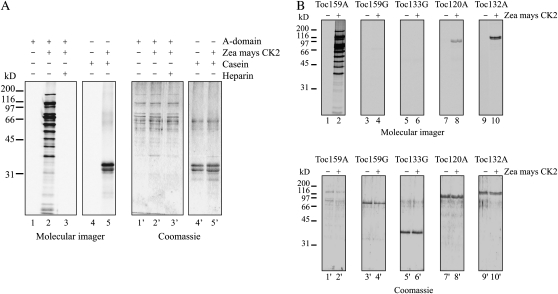
To demonstrate that Toc159A is an in vitro substrate of CK2, we incubated Toc159A-His-6x (Fig. 7A, lane 2) as well as the control substrate casein (Fig. 7A, lane 5) with recombinant maize (Zea mays ) CK2 α-subunit in the presence of [γ-33P]ATP. The experiment was analyzed by SDS-PAGE followed by phosphorimaging. The Toc159A-His-6x as well as casein were strongly phosphorylated by maize CK2 (Fig. 7A, lanes 2 and 5). Heparin, the known CK2 inhibitor, completely abolished phosphorylation (Fig. 7A, lane3).
Figure 7.
Phosphorylation of recombinant A-domains by CK2. A, One microgram of purified Toc159A-His-6x or casein as a control was incubated without (lanes 1 and 4) or with (lanes 2 and 5) recombinant maize CK2 α-subunit and ATP/[γ-33P]ATP as the phosphate donor. In addition, phosphorylation of Toc159A-His-6x with CK2 was performed in the presence of 15 μg mL−1 heparin as an inhibitor (lane 3). The samples were separated by SDS-PAGE followed by Coomassie Brilliant Blue staining (lanes 1′–5′) and phosphorimager analysis (lanes 1–5). B, One microgram of purified Toc33G-His-6x, Toc120A-His-6x, Toc132A-His-6x, Toc159A-His-6x, or GST-Toc159G was incubated without (even lanes) or with (odd lanes) recombinant CK2 and ATP/[γ-33P]ATP as the phosphate donor. The samples were separated by SDS-PAGE followed by Coomassie Brilliant Blue staining (lanes 1′–10′) and phosphorimager analysis (lanes 1–10).
Like Toc159, the Arabidopsis homologs Toc120 and Toc132 have acidic domains. The three A-domains differ in length and amino acid composition. CK2 phosphorylation is predicted for all three by the NetPhosK 1.0 server (Supplemental Fig. S1). The PhosPhAt database lists three A-domain phosphorylation sites for Toc132 (S195, S296, and S298) as well as two for Toc120 (S318 and T320; Heazlewood et al., 2008; Sugiyama et al., 2008). Toc132 S296 and S298 are predicted CK2 phosphorylation sites (Supplemental Fig. S1). To test whether the A-domains of Toc120 and Toc132 are CK2 substrates, an in vitro phosphorylation experiment using the recombinant CK2 was carried out (Fig. 7B, lanes 8′ and 10′). The purified G-domains of Toc159 (Fig. 7B, lane 4′) and Toc33 (Fig. 7B, lane 6′) were also tried as substrates because they also contain predicted CK2 sites. Toc159A (Fig. 7B, lane 2), Toc120A (Fig. 7B, lane 8), and Toc132A (Fig. 7B, lane 10) were phosphorylated by the recombinant CK2, and neither of them revealed autophosphorylation activity in the absence of kinase (Fig. 7B, lanes 1, 7, and 9). Phosphorylation of Toc159 and Toc33 G-domains did not occur. In summary, the results demonstrate that the A-domains of Toc159, Toc120, and Toc132 behave as in vitro CK2 substrates, suggesting that A-domain phosphorylation by CK2 is a conserved feature of the Toc159 homologs having an A-domain.
Inhibition of in Vitro Chloroplast Protein Import by Heparin
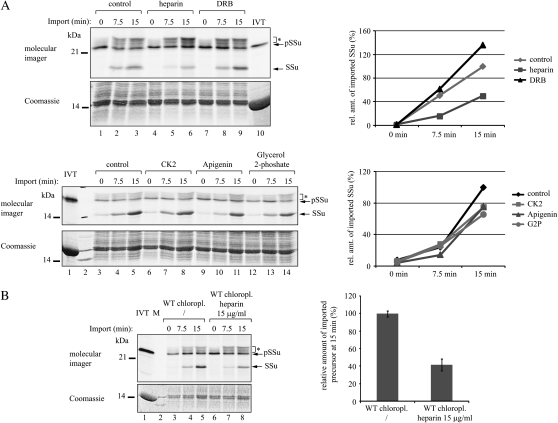
To test for a functional role of A-domain phosphorylation in chloroplast protein import, we preincubated wild-type chloroplasts with CK2, various kinase inhibitors (heparin, DRB, apigenin), or a phosphatase inhibitor (glycerol-2-phosphate) prior to in vitro import experiments using the small subunit of Rubisco (pSSu) preprotein (Fig. 8A). None of the treatments, with the exception of heparin, had a significant effect on import efficiency. Heparin reduced the amount of imported preprotein (SSu) at 15 min to 40% in comparison with untreated chloroplasts at the same time point (Fig. 8B). This result suggests that phosphorylation at the A-domain does not directly affect the preprotein translocation reaction.
Figure 8.
Inhibition of in vitro chloroplast protein import by heparin. A, Isolated Arabidopsis chloroplasts were preincubated without or with 15 μg mL−1 heparin, 6 μm DRB, 3 units μL−1 recombinant maize CK2 α-subunit, 50 μm apigenin, or 10 mm glycerol-2-phosphate for 20 min at 25°C in the dark. Then, in vitro-translated, [35S]Met-labeled preprotein of the small subunit of Rubisco (pSSu) was added and import was allowed to proceed for 0, 7.5, and 15 min. B, Quantification of the effect of heparin on chloroplast protein import. The graph shows the quantification of the amount of imported SSu at 15 min in three independent experiments. In both panels, the amount of SSu imported into wild-type (WT) chloroplasts without the addition of inhibitor at 15 min was set to 100%. IVT, In vitro translate; M, molecular mass standard. Asterisks indicate pSSu modified in the course of the import reactions.
DISCUSSION
In a previous study, we demonstrated the occurrence of a lower molecular mass fragment of Toc159 in the soluble fraction (Agne et al., 2009). By combining TAP tagging and mass spectrometry analysis, we demonstrate here that this soluble fragment corresponds to the A-domain of Toc159. Regarding the chemical properties of the A-domain, its solubility is not surprising. However, based on the literature, the occurrence of an abundant and stable A-domain is unexpected. The initial isolation of pea Toc159 as an 86-kD protein lacking the A-domain (Hirsch et al., 1994; Bolter et al., 1998) led to the general assumption that the A-domain is sensitive to proteolysis and completely degraded. In line with this assumption, we earlier attributed lower molecular mass bands in the total extract and in the soluble fraction to N-terminal proteolytic products of Toc159 lacking the A-domain (Hiltbrunner et al., 2001b). This study now suggests that the lower molecular mass, soluble form of Toc159 at the time was most likely identical to the A-domain of Toc159.
Surprisingly, a portion of the A-domain was present in the membrane fraction (Figs. 2 and 3, P100), indicating that this population of the A-domain remains associated with chloroplast membranes despite the separation of the anchoring M-domain. Considering the hydrophilic profile of the A-domain, its association with membranes is probably extrinsic, either due to protein-protein interactions or interactions with polar lipid head groups. It is tempting to speculate that the A-domain remains associated with the Toc complex, where it continues to exert an unknown regulatory function. Even when expressed alone in Arabidopsis plants, the recombinant A-domain associated with the membrane fraction (Fig. 5A, P100), suggesting an affinity for the chloroplast independent of being part of the full-length Toc159 protein.
So far, no specific interaction partners of the A-domain are known. A recent paper described the association of the Toc complex with actin and a direct interaction of recombinant full-length Toc159 with actin (Jouhet and Gray, 2009). The authors suggested that this interaction is likely to involve the A-domain of Toc159. However, actin was absent from the eluates obtained after TAP tag purification of Toc159 from the SN100 as well as the P100 fractions as monitored by western blotting (Fig. 2B). Mass spectrometry revealed only two or three unique peptides of actin (At3g46520 and At1g49240) in the P100 TAP-Toc159 eluate (data not shown). However, tryptic peptides of actin were also identified in the control eluates; thus, our data do not provide further insight on the actin-Toc159 interaction. Our data do not exclude a dynamic interaction of actin with a small portion of cellular Toc159. Any copurified actin may then have been under the detection limit.
Interestingly, the recombinant A-domain when expressed separately in Arabidopsis appeared much more degraded than the form released from Toc159 (Fig. 5). Similarly, only a small portion of the recombinant A-domain when expressed in Escherichia coli was present as an intact protein. This suggests that the A-domain is stabilized by being or having been part of Toc159. The A-domain of Toc159 has recently been shown to behave as an intrinsically disordered protein (Richardson et al., 2009). Therefore, it could potentially undergo induced folding by interaction with other protein components at the outer membrane or the Toc complex while still being part of Toc159. This may render the endogenous A-domain more stable than the separate, recombinant protein.
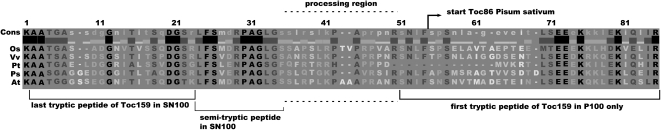
Toc159 was first isolated from pea chloroplasts as an 86-kD fragment lacking the A-domain, and its N-terminal amino acid (Ala-737) was determined (Hirsch et al., 1994). The corresponding amino acid in Arabidopsis Toc159 (Ser-765) is located 31 amino acids downstream of the last peptide identified in the isolated A-domain (Figs. 3B and 9). Together with identification of the intact, separated A-domain, these data now suggest that the formation of Toc86 is the result of the processing of Toc159 at a specific cleavage site. Cleavage of Toc159 at the border between the A- and G-domains was apparent in a number of previous studies. When isolated chloroplasts were incubated with in vitro-translated Toc159, a portion of it was converted to Toc86 (Toc159GM; Smith et al., 2002; Supplemental Fig. S2A). The first experiments of this type were performed to study the targeting and insertion of Toc159 into chloroplasts. In several studies of pea Toc86, the cleavage was attributed to the removal of an “unusual” targeting sequence or presequence (Hirsch et al., 1994; Muckel and Soll, 1996). However, once it became clear that Toc86 is only part of a protein still much larger at its N terminus, this hypothesis was discarded.
Figure 9.
Alignment of the junction region between A- and G-domains of the Toc159 proteins of Oryza sativa (Os), Vitis vinifera (Vv), Populus trichocarpa (Pt), Pisum sativum (Ps), and Arabidopsis thaliana (At). Tryptic peptides identified in the soluble (SN100) or membrane (P100) fraction and the N terminus of pea Toc86 are shown. In addition, the positions of a semitryptic peptide identified in the soluble fraction and the region where Toc159 processing is likely to occur are shown.
The presumed cleavage site in AtToc159 appears to be close to amino acid 728, as a truncated version of AtToc159 (AtToc159GM) starting at 728 was not cleaved to an obvious extent (Agne et al., 2009; Supplemental Fig. S2A). In line with this observation, the most C-terminal peptide of the soluble A-domain in the SN100 fraction ended with Lys-733. In addition, we identified a semitryptic peptide ending at Ser-744, suggesting that this amino acid is at or close to the processing site (Fig. 9; Supplemental Fig. S2C). Furthermore, the closest peptide identified in the P100 fraction but not in the SN100 fraction started with Ser-761 (Fig. 9). Therefore, we hypothesize that cleavage of AtToc159 occurs in the region between Ser-744 and Arg-760 or slightly beyond (Fig. 9). This region contains residues conserved between Toc159 orthologs, so that a site-specific cleavage at a conserved sequence motif may be envisaged. This hypothesis is in good agreement with the N-terminal Ala-737 identified in pea Toc86 (Hirsch et al., 1994). Currently, we are attempting to identify the Toc159 processing site and activity, which, considering the cleavage by isolated chloroplasts, is expected to be located in the outer envelope.
Isolation of the A-domain as a separate molecule and cleavage of Toc159 by isolated chloroplasts suggest a specific proteolytic activity. However, it is difficult to prove beyond doubt that formation of the free A-domain is a genuine cellular phenomenon and not somehow enhanced by the experimental manipulation, especially the homogenization conditions. For the TAP tag purification experiments, leaf tissue was ground using a mortar and pestle, a rather harsh method. When using a much more gentle method (Supplemental Fig. S2B), forcing Arabidopsis protoplasts through a double layer of 23- and 18-μm nylon mesh, the A-domain fragment was also observed (Supplemental Fig. S2B). This is in support of the cleavage occurring in the cell and not during the course of experimentation. In some experiments (Fig. 2), the free A-domain appeared to increase over the course of the experiment. It may well be that the Toc159 processing activity copurifies with TAP-Toc159 and remains active during the preparation despite the addition of a protease inhibitor cocktail. Indeed, in the TAP-Toc159 eluate, some chloroplast-localized peptidases were identified by mass spectrometry (data not shown). Characterization of these candidates is part of our strategy to identify the Toc159 processing activity and thereby to determine the physiological relevance of Toc159 cleavage (see above).
Recent studies (de la Fuente van Bentem et al., 2008; Sugiyama et al., 2008; Whiteman et al., 2008; Jones et al., 2009; Reiland et al., 2009) as well as our own results (Fig. 3; Table I) indicate that full-length Toc159 as well as the A-domain alone are phosphorylated. In fact, all of the phosphopeptides identified belonged to the A-domain, and most of them are predicted to be phosphorylated by CK2. Even when expressed alone, the A-domain was phosphorylated (Fig. 5B), suggesting that it does not need to be part of Toc159 for the modification to occur. In contrast, the recombinant A-domain was not phosphorylated when expressed in E. coli (data not shown). Earlier studies demonstrated that the Toc159GM (Toc86) fragment is a phosphoprotein and that phosphorylation may occur at the G-domain (Fulgosi and Soll, 2002; Oreb et al., 2008a). This study as well as the recent phosphoproteomic studies have not demonstrated in vivo phosphorylation at the G-domain. The new data are not necessarily inconsistent with the earlier results on Toc86/Toc159G phosphorylation. Predicted phosphorylation sites in the G-domain are less numerous, and their phosphorylation may occur at a lower rate compared with the A-domain, thus preventing their detection by mass spectrometry.
The recombinant A-domain in vitro behaved as a substrate of kinases localized in the membrane and soluble fractions of Arabidopsis (Fig. 6C). The kinase activity in the membrane fraction resembled a CK2-like activity, because it was inhibited by heparin and its ATP-dependent phosphorylation activity was reduced in the presence of 500 μm GTP. The soluble kinase activity was not changed in the presence of nonradioactive GTP and therefore is not likely to be identical to the membrane-bound activity. A kinase inhibited by heparin and capable of using GTP as the phosphate donor has been identified in the outer envelope of pea chloroplasts (Soll et al., 1988). The major phosphorylated products of this GTP-dependent kinase were proteins of 23 and 32.5 kD. However, a protein migrating at a molecular mass greater than 220 kD was among the set of phosphorylated envelope proteins (of a total around 10) and may be identical to pea Toc159. It is conceivable, therefore, that the GTP-dependent kinase is related to the A-domain-phosphorylating activity in Arabidopsis membranes.
Using recombinant CK2, we demonstrate that all A-domains present in members of the Toc159 family are in vitro CK2 substrates. It is tempting to speculate that phosphorylation at the A-domain by CK2 or a CK2-like kinase is a general feature of Toc GTPases having an A-domain. However, CK2 is a constitutively active and pleiotropic kinase activity, and the function of the A-domain is so far unknown. Therefore, assigning a role for CK2 phosphorylation remains a challenge for the future. Phosphorylation sites, which are not predicted to be targets of CK2, have also been identified in vivo in the A-domains of Toc120, Toc132, and Toc159. Consequently, another kinase activity unlike CK2 is involved in phosphorylation of the A-domains.
To determine whether phosphorylation at the A-domain directly affects preprotein import, we carried out in vitro import experiments in the presence of soluble CK2, various kinase inhibitors, and a phosphatase inhibitor. Our current knowledge suggests that the A-domain is exposed at the surface of the chloroplast and therefore should be accessible during experimentation. None of the treatments, with the exception of heparin, had an effect on import efficiency. While we cannot entirely exclude that the various inhibitor treatments were ineffective in modifying phosphorylation, we favor the hypothesis that phosphorylation of the A-domain does not affect protein import in vitro. Regarding heparin, it is a known inhibitor of CK2 and inhibits phosphorylation of the A-domain as well as in vitro protein import in this study. However, heparin is also known to inhibit a large number of enzymatic and cellular activities. Therefore, it may well be that heparin inhibits CK2 as well as other kinases independent of its inhibitory effect on chloroplast protein import in vitro.
In summary, these results give, to our knowledge, first answers regarding the cellular fate of the Toc159 A-domain. While the loss of the A-domain was observed earlier, it was attributed to rapid degradation. This study is in agreement with a regulatory function of the A-domain in protein import. We hypothesize that the regulatory function of the A-domain is modulated by at least two mechanisms: phosphorylation and proteolytic processing. We are currently experimentally addressing this hypothesis together with the regulatory role of the A-domain in the chloroplast import process.
MATERIALS AND METHODS
Generation of Transgenic Lines
Transgenic lines expressing TAP-Toc159GM and TAP-Toc159 fusion proteins have been described elsewhere (Agne et al., 2009). To obtain vector pCHF8-CTAPi, the sequence encoding the TAP tag was amplified using primers 5′-CAGAAGATCTTCTAGATCCATGGAGAGCAGCA-3′ and 5′-CCCTAGGGCCTCACTTTGGGGCTTG-3′ from plasmid pPTN289-CTAPi (Rohila et al., 2004), digested with BglII/AvrII, and cloned using the BglII/XbaI sites into pCHF8 (Dr. C. Fankhauser, Center for Integrative Genomics, University of Lausanne). To obtain vector pCHF8-NTAPi, the sequence encoding the TAP tag was amplified using primers 5′-CATGCCATGGTGGTCGACAA-3′ and 5′-TGCTCTAGAAGTCATGAGCCCTCCACTAGACAGT-3′ from pPTN289-NTAPi (Rohila et al., 2004) and ligated after NcoI/BspHI digestion into the BspHI-digested pCHF8. The binary vector pCHF8 contains two cauliflower mosaic virus 35S eukaryotic promoters, one of them having a duplicated enhancer region, an rbcs terminator, and the phosphinotricin acetyltransferase gene for transgene selection. A DNA fragment encoding the A-domain of Toc159 (Toc159A, amino acids 1–740) was amplified from pET21d-Toc159 (Bauer et al., 2000) using primers 5′-GATATGGATCCATGGCATCAAAGTCGG-3′ and 5′-CAATACTAGTAGGTCGATCCATAGAG-3′ and digested and cloned using BamHI/SpeI into pCHF8-CTAP, resulting in pCHF8-Toc159A-CTAP. The constructs pCHF8-NTAPi and pCHF8-TOC159A-CTAP were used to transform heterozygous ppi2 plants by floral dipping (Clough and Bent, 1998). Transgenic plants were selected by Basta treatment of the T1 generation, either by spraying a solution containing 150 mg L−1 herbicide or plating on medium containing 30 μg mL−1. The transgenic lines expressing the TAP tag alone or Toc159A-TAP in a wild-type genetic background are named TAP:WT or Toc159A-TAP:WT, respectively, whereas the line expressing TAP-Toc159 in the ppi2 genetic background is named TAP-Toc159:ppi2.
Plant Growth Conditions
Arabidopsis (Arabidopsis thaliana) plants were grown on soil (Rasenerde Top Dressing; Ricoter) or in vitro on 0.8% (w/v) Phytoagar (Duchefa) containing 0.5× Murashige and Skoog medium (Duchefa) under short-day conditions (8/16-h photoperiod at 120 μmol m−2 s−1, 21°C). For import experiments, the in vitro growth medium was complemented with 0.8% (w/v) Suc.
TAP Tag Purification
TAP:WT and TAP-Toc159:ppi2 seedlings (fresh weight, 10 g) grown on soil for 28 d under short-day conditions were crushed in a mortar in a total volume of 30 mL of grinding buffer (100 mm Tris-HCl, pH 7.5, 200 mm NaCl, 1 mm phenylmethylsulfonyl fluoride, 5 mm NaF, 0.2% [v/v] protease inhibitor cocktail [Sigma P9599], and PhosSTOP phosphatase inhibitor cocktail [Roche]), filtered through two layers of Miracloth, and centrifuged at 1,500g for 10 min at 4°C. The supernatant (plant extract) was centrifuged at 100,000g for 1 h at 4°C (Beckmann SW28). Pellet-associated proteins were solubilized in grinding buffer containing 10% glycerol and 0.75% (v/v) Triton X-100 followed by centrifugation at 100,000g for 1 h at 4°C (Beckmann SW55Ti). Roughly 50 mg of soluble protein (supernatant of the first ultracentrifugation; SN100) and 20 mg of solubilized membrane protein (supernatant of the second ultracentrifugation; P100) were gently rotated with a 200-μL bed volume of human IgG coupled to Affi-Gel (Bio-Rad) overnight at 4°C. IgG beads were washed with grinding buffer without (SN100) or with (P100) glycerol and Triton X-100 and preceding elution once with buffer devoid of protease inhibitors. Proteins were eluted by incubating recovered beads with 30 units of AcTEV Protease (Invitrogen) for 2 h at 16°C. Proteins of each fraction were concentrated by CHCl3-methanol precipitation and separated on Tris-Gly SDS-PAGE gels (immunoblotting) or on NuPAGE Novex 4% to 12% BisTris gels (protein staining and mass spectrometry analysis). Dephosphorylation of TEV eluates prior to loading was done for 20 min at 30°C with 4 units μL−1 Lambda Protein Phosphatase (New England Biolabs).
Mass Spectrometry Analysis
Proteins separated on NuPAGE Novex 4% to 12% BisTris gels were stained using ProQ-Diamond and SyproRuby (Invitrogen). Each chosen gel slice was diced into small pieces. In-gel digestion was performed according to a modified protocol from Shevchenko et al. (1996, 2006). Before mass spectrometric analysis, samples were desalted using SepPak cartridges (Waters). Dried peptides were resuspended in 3% (v/v) acetonitrile (ACN), 0.2% (v/v) formic acid and analyzed on a LTQ FT-ICR mass spectrometer (Thermo Fisher Scientific) coupled with an Eksigent nano-liquid chromatography system (Eksigent Technologies). Peptide mixtures were loaded onto laboratory-made capillary columns (75 μm i.d.; BGB Analytik), 8 cm length, packed with Magic C18 AQ beads (3 μm, 100 Å; Michrom BioResources). Peptides were eluted from the column by an increased ACN concentration in the mobile phase from 5% (v/v) ACN, 0.2% (v/v) formic acid to 40% (v/v) ACN, 0.2% (v/v) formic acid over 74 min, followed by a 10-min wash step at 5% (v/v) ACN, 0.2% (v/v) formic acid. Peptide ions were detected in a survey scan from 300 to 1,600 atomic mass units at 100,000 full width half-maximum nominal resolution followed by three data-dependent tandem mass spectrometry scans (isolation width 2 atomic mass units, relative collision energy 35%, dynamic exclusion enabled, repeat count 1, followed by peak exclusion for 2 min). Tandem mass spectrometry spectra were searched using Mascot 2.1.04 (Matrix Science) against The Arabidopsis Information Resource 8 protein database (download on December 14, 2007) supplemented with contaminants. The search parameters were as follows: requirement for tryptic ends, one missed cleavage allowed, mass tolerance = 5 ppm. Besides carbamylation of Cys residues as fixed modification, oxidation of Met and phosphorylation of Ser, Thr, and Tyr were included as variable modifications. Phosphopeptide identifications were accepted with a minimal Mascot ion score of 30 and a Mascot expect value of 0.01 or less. A normalized delta ions score (ΔI) was calculated for all phosphopeptides containing more than one Ser, Thr, or Tyr residue by taking the difference of the two top-ranking phosphopeptide ion scores and dividing that difference by the ion score of the top-ranking phosphopeptide. Phosphorylation site assignments with ΔI ≥ 0.4 were accepted. Further manual spectra inspection was conducted as follows: verifying neutral loss by assigning the parent ion −98-D peak.
Phosphorylation Assays
One microgram of recombinant protein or casein was incubated with or without 37.5 units of recombinant maize (Zea mays) CK2 α-subunit (Biaffin) in the presence of 25 mm Tris-HCl, pH 8.0, 5 mm MgCl2, 1 mm dithiothreitol (DTT), 50 μm ATP, and 1 μCi of [γ-33P]ATP for 30 min at 25°C. Reactions were stopped by diluting in ice-cold buffer and CHCl3-methanol precipitation. The samples were separated by SDS-PAGE and examined by autoradiography. For in vitro phosphorylation using plant extract, 4-week-old seedlings of Arabidopsis wild-type Wassilewskija (4 g) grown under short-day conditions on soil were ground in extraction buffer (50 mm HEPES KOH, pH 7.5, 100 mm NaCl, 0.5% polyvinylpyrrolidone, 5 mm NaF, 1 mm DTT, 0.1% [v/v] protease inhibitor cocktail, and 1 mm phenylmethylsulfonyl fluoride). Samples were filtered through a layer of Miracloth and centrifuged for 10 min at 1,500g to remove debris. The supernatant (total) was subjected to centrifugation for 1 h at 100,000g (SW55Ti). Proteins associated with the pellet (P100) were solubilized with extraction buffer containing 0.5% (v/v) Triton X-100. Any nonsolubilized matter was removed by centrifugation for 1 h at 100,000g (SW55Ti). Twenty to 40 μg of protein of the extract before ultracentrifugation (total), the supernatant of the first ultracentrifugation (SN100), and protein solubilized from the pellet (P100) were incubated with 5 μg of recombinant Toc159A-His-6x in phosphorylation buffer (50 mm HEPES, pH 7.5, 5 mm MgCl2, 0.5 mm MnCl2, 50 mm NaCl, and 0.1 mm CaCl2), 100 μm ATP, and 2 μCi of [γ-33P]ATP for 40 min at 25°C. As a control, a similar experiment was performed with 40 μg of each fraction containing 1 μg of bovine serum albumin. In another series of experiments, kinase inhibitors heparin (15 μg mL−1), DRB (6 μm), chelerythrine chloride (1 μm), and 4′,5,7-trihydroxyflavone (apigenin; 50 μm) or unlabeled GTP (500 μm) was added. Reactions were stopped by diluting with ice-cold buffer and CHCl3-methanol precipitation. The samples were separated by SDS-PAGE and examined by autoradiography.
Chloroplast Protein Import Assay
Chloroplast import assays were performed as described (Agne et al., 2009). To study the role of phosphorylation in import, chloroplasts were preincubated with or without 15 μg mL−1 heparin sodium salt (172 units mg−1), 6 μm DRB, 3 units μL−1 recombinant maize CK2 α-subunit, 50 μm apigenin, or 10 mm glycerol-2-phosphate in the presence of 20 μm ATP for 20 min at 25°C. Then, ATP was added to a final concentration of 5 mm and import was allowed to proceed for 0, 7.5, and 15 min.
Purification of Recombinant Proteins
Toc33(aa1-265)-His-6x (Toc33G-His-6x; Weibel et al., 2003), Toc120(aa1-343)-His-6x (Toc120A-His-6x), Toc132(aa1-431)-His-6x (Toc132A-His-6x; Ivanova et al., 2004), Toc159(aa1-740)-His-6x (Toc159A-His-6x; Bauer et al., 2000), and GST-Toc159(aa727-1093) (GST-Toc159G; Rahim et al., 2009) were overexpressed in Escherichia coli strain BL21(DE3) transformed with expression vectors. Bacterial pellets were lysed using lysozyme and sonication in 50 mm Tris-HCl, pH 8, 300 mm NaCl, and 5 mm imidazol followed by centrifugation for 45 min at 14,000 rpm (SS34). His-6x-fused proteins were purified from the supernatant fraction by nickel-nitrilotriacetic acid agarose affinity chromatography and dialyzed against 50 mm HEPES, pH 7.5, 100 mm NaCl, and 1 mm DTT. GST-Toc159G was purified as described before (Rahim et al., 2009).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Distribution of the predicted CK2 phosphorylation sites along the A-domains of Toc120, Toc132, and Toc159 as well as the G-domains of Toc33 and Toc159.
Supplemental Figure S2. Toc159 cleavage.
Supplemental Table S1. List of identified phosphopeptides.
Supplemental Spectra S1. Tandem mass spectrometry spectra for identified phosphopeptides.
Supplementary Material
Acknowledgments
We thank Jana Smutny (Université de Neuchâtel) for technical assistance, Bernd Roschitzki (Functional Genomics Center Zurich) for support in mass spectrometry, and Danny Schnell (University of Massachusetts) for providing plasmids encoding the A-domains of Toc120 and Toc132.
References
- Agne B, Infanger S, Wang F, Hofstetter V, Rahim G, Martin M, Lee DW, Hwang I, Schnell D, Kessler F. (2009) A toc159 import receptor mutant, defective in hydrolysis of GTP, supports preprotein import into chloroplasts. J Biol Chem 284: 8670–8679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronsson H, Combe J, Patel R, Jarvis P. (2006) In vivo assessment of the significance of phosphorylation of the Arabidopsis chloroplast protein import receptor, atToc33. FEBS Lett 580: 649–655 [DOI] [PubMed] [Google Scholar]
- Bauer J, Chen K, Hiltbunner A, Wehrli E, Eugster M, Schnell D, Kessler F. (2000) The major protein import receptor of plastids is essential for chloroplast biogenesis. Nature 403: 203–207 [DOI] [PubMed] [Google Scholar]
- Blom N, Sicheritz-Ponten T, Gupta R, Gammeltoft S, Brunak S. (2004) Prediction of post-translational glycosylation and phosphorylation of proteins from the amino acid sequence. Proteomics 4: 1633–1649 [DOI] [PubMed] [Google Scholar]
- Bolter B, May T, Soll J. (1998) A protein import receptor in pea chloroplasts, Toc86, is only a proteolytic fragment of a larger polypeptide. FEBS Lett 441: 59–62 [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Crooks GE, Hon G, Chandonia JM, Brenner SE. (2004) WebLogo: a sequence logo generator. Genome Res 14: 1188–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Fuente van Bentem S, Anrather D, Dohnal I, Roitinger E, Csaszar E, Joore J, Buijnink J, Carreri A, Forzani C, Lorkovic ZJ, et al. (2008) Site-specific phosphorylation profiling of Arabidopsis proteins by mass spectrometry and peptide chip analysis. J Proteome Res 7: 2458–2470 [DOI] [PubMed] [Google Scholar]
- Fulgosi H, Soll J. (2002) The chloroplast protein import receptors Toc34 and Toc159 are phosphorylated by distinct protein kinases. J Biol Chem 277: 8934–8940 [DOI] [PubMed] [Google Scholar]
- Heazlewood JL, Durek P, Hummel J, Selbig J, Weckwerth W, Walther D, Schulze WX. (2008) PhosPhAt: a database of phosphorylation sites in Arabidopsis thaliana and a plant-specific phosphorylation site predictor. Nucleic Acids Res 36: D1015–D1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández Torres J, Maldonado MAA, Chomilier J. (2007) Tandem duplications of a degenerated GTP-binding domain at the origin of GTPase receptors Toc159 and thylakoidal SRP. Biochem Biophys Res Commun 364: 325–331 [DOI] [PubMed] [Google Scholar]
- Hiltbrunner A, Bauer J, Alvarez-Huerta M, Kessler F. (2001a) Protein translocon at the Arabidopsis outer chloroplast membrane. Biochem Cell Biol 79: 629–635 [DOI] [PubMed] [Google Scholar]
- Hiltbrunner A, Bauer J, Vidi PA, Infanger S, Weibel P, Hohwy M, Kessler F. (2001b) Targeting of an abundant cytosolic form of the protein import receptor at Toc159 to the outer chloroplast membrane. J Cell Biol 154: 309–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiltbrunner A, Grunig K, Alvarez-Huerta M, Infanger S, Bauer J, Kessler F. (2004) AtToc90, a new GTP-binding component of the Arabidopsis chloroplast protein import machinery. Plant Mol Biol 54: 427–440 [DOI] [PubMed] [Google Scholar]
- Hirsch S, Muckel E, Heemeyer F, von Heijne G, Soll J. (1994) A receptor component of the chloroplast protein translocation machinery. Science 266: 1989–1992 [DOI] [PubMed] [Google Scholar]
- Ivanova Y, Smith MD, Chen K, Schnell DJ. (2004) Members of the Toc159 import receptor family represent distinct pathways for protein targeting to plastids. Mol Biol Cell 15: 3379–3392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson-Constan D, Keegstra K. (2001) Arabidopsis genes encoding components of the chloroplastic protein import apparatus. Plant Physiol 125: 1567–1576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis P. (2008) Targeting of nucleus-encoded proteins to chloroplasts in plants. New Phytol 179: 257–285 [DOI] [PubMed] [Google Scholar]
- Jarvis P, Chen LJ, Li H, Peto CA, Fankhauser C, Chory J. (1998) An Arabidopsis mutant defective in the plastid general protein import apparatus. Science 282: 100–103 [DOI] [PubMed] [Google Scholar]
- Jelic M, Soll J, Schleiff E. (2003) Two Toc34 homologues with different properties. Biochemistry 42: 5906–5916 [DOI] [PubMed] [Google Scholar]
- Jelic M, Sveshnikova N, Motzkus M, Horth P, Soll J, Schleiff E. (2002) The chloroplast import receptor Toc34 functions as preprotein-regulated GTPase. Biol Chem 383: 1875–1883 [DOI] [PubMed] [Google Scholar]
- Jones AME, MacLean D, Studholme DJ, Serna-Sanz A, Andreasson E, Rathjen JP, Peck SC. (2009) Phosphoproteomic analysis of nuclei-enriched fractions from Arabidopsis thaliana. J Proteomics 72: 439–451 [DOI] [PubMed] [Google Scholar]
- Jouhet J, Gray JC. (2009) Interaction of actin and the chloroplast protein import apparatus. J Biol Chem 284: 19132–19141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler F, Schnell D. (2009) Chloroplast biogenesis: diversity and regulation of the protein import apparatus. Curr Opin Cell Biol 21: 494–500 [DOI] [PubMed] [Google Scholar]
- Lee KH, Kim SJ, Lee YJ, Jin JB, Hwang I. (2003) The M domain of atToc159 plays an essential role in the import of proteins into chloroplasts and chloroplast biogenesis. J Biol Chem 278: 36794–36805 [DOI] [PubMed] [Google Scholar]
- Martin T, Sharma R, Sippel C, Waegemann K, Soll J, Vothknecht UC. (2006) A protein kinase family in Arabidopsis phosphorylates chloroplast precursor proteins. J Biol Chem 281: 40216–40223 [DOI] [PubMed] [Google Scholar]
- May T, Soll J. (2000) 14-3-3 proteins form a guidance complex with chloroplast precursor proteins in plants. Plant Cell 12: 53–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muckel E, Soll J. (1996) A protein import receptor of chloroplasts is inserted into the outer envelope membrane by a novel pathway. J Biol Chem 271: 23846–23852 [DOI] [PubMed] [Google Scholar]
- Oreb M, Hofle A, Mirus O, Schleiff E. (2008a) Phosphorylation regulates the assembly of chloroplast import machinery. J Exp Bot 59: 2309–2316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oreb M, Tews I, Schleiff E. (2008b) Policing Tic ‘n’ Toc, the doorway to chloroplasts. Trends Cell Biol 18: 19–27 [DOI] [PubMed] [Google Scholar]
- Oreb M, Zoryan M, Vojta A, Maier UG, Eichacker LA, Schleiff E. (2007) Phospho-mimicry mutant of atToc33 affects early development of Arabidopsis thaliana. FEBS Lett 581: 5945–5951 [DOI] [PubMed] [Google Scholar]
- Rahim G, Bischof S, Kessler F, Agne B. (2009) In vivo interaction between atToc33 and atToc159 GTP-binding domains demonstrated in a plant split-ubiquitin system. J Exp Bot 60: 257–267 [DOI] [PubMed] [Google Scholar]
- Reiland S, Messerli G, Baerenfaller K, Gerrits B, Endler A, Grossmann J, Gruissem W, Baginsky S. (2009) Large-scale Arabidopsis phosphoproteome profiling reveals novel chloroplast kinase substrates and phosphorylation networks. Plant Physiol 150: 889–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson L, Jelokhani-Niaraki M, Smith M. (2009) The acidic domains of the Toc159 chloroplast preprotein receptor family are intrinsically disordered protein domains. BMC Biochem 10: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohila JS, Chen M, Cerny R, Fromm ME. (2004) Improved tandem affinity purification tag and methods for isolation of protein heterocomplexes from plants. Plant J 38: 172–181 [DOI] [PubMed] [Google Scholar]
- Schleiff E, Soll J, Kuchler M, Kuhlbrandt W, Harrer R. (2003) Characterization of the translocon of the outer envelope of chloroplasts. J Cell Biol 160: 541–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevchenko A, Tomas H, Havlis J, Olsen JV, Mann M. (2006) In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat Protoc 1: 2856–2860 [DOI] [PubMed] [Google Scholar]
- Shevchenko A, Wilm M, Vorm O, Mann M. (1996) Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal Chem 68: 850–858 [DOI] [PubMed] [Google Scholar]
- Smith MD, Hiltbrunner A, Kessler F, Schnell DJ. (2002) The targeting of the atToc159 preprotein receptor to the chloroplast outer membrane is mediated by its GTPase domain and is regulated by GTP. J Cell Biol 159: 833–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll J, Fischer I, Keegstra K. (1988) A guanosine 5′-triphosphate-dependent protein kinase is localized in the outer envelope membrane of pea chloroplasts. Planta 176: 488–496 [DOI] [PubMed] [Google Scholar]
- Sugiyama N, Nakagami H, Mochida K, Daudi A, Tomita M, Shirasu K, Ishihama Y. (2008) Large-scale phosphorylation mapping reveals the extent of tyrosine phosphorylation in Arabidopsis. Mol Syst Biol 4: 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weibel P, Hiltbrunner A, Brand L, Kessler F. (2003) Dimerization of Toc-GTPases at the chloroplast protein import machinery. J Biol Chem 278: 37321–37329 [DOI] [PubMed] [Google Scholar]
- Whiteman SA, Serazetdinova L, Jones AM, Sanders D, Rathjen J, Peck SC, Maathuis FJ. (2008) Identification of novel proteins and phosphorylation sites in a tonoplast enriched membrane fraction of Arabidopsis thaliana. Proteomics 8: 3536–3547 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.