Abstract
Stilbenes are phytochemicals present in grapes, berries, peanuts and red wine. A widely studied stilbene, resveratrol (trans-3,5,4′-trihydroxystilbene), has been shown to exert antioxidant, anti-inflammatory, chemopreventive and antiaging effects in a number of biological systems. We reported earlier that pterostilbene (trans-3,5-dimethoxy-4′-hydroxystilbene), a structurally related stilbene found in blueberries, was effective in reducing the incidence and multiplicity of aberrant crypt foci formation in the colon of rats injected with azoxymethane (AOM). Our present study was to identify the chemopreventive potential of pterostilbene with colonic tumor formation as an end point and further to evaluate the mechanistic action of pterostilbene during colon carcinogenesis. F344 rats were given two AOM injections subcutaneously when they were 7 and 8 weeks old and continuously fed the control or 40 p.p.m. pterostilbene diet for 45 weeks. Overall analyses indicated that pterostilbene reduced colon tumor multiplicity of non-invasive adenocarcinomas, lowered proliferating cell nuclear antigen and downregulated the expression of β-catenin and cyclin D1. Pterostilbene decreased mucosal levels of the proinflammatory cytokines, tumor necrosis factor-α, interleukin (IL)-1β and IL-4. Colon tumors from pterostilbene-fed animals showed reduced expression of inflammatory markers as well as nuclear staining for phospho-p65, a key molecule in the nuclear factor-kappaB pathway. In HT-29 cells, pterostilbene reduced the protein levels of β-catenin, cyclin D1 and c-MYC, altered the cellular localization of β-catenin and inhibited the phosphorylation of p65. Our data with pterostilbene in suppressing colon tumorigenesis, cell proliferation as well as key inflammatory markers in vivo and in vitro suggest the potential use of pterostilbene for colon cancer prevention.
Introduction
Colorectal cancer is a significant global health concern and is the third leading cause of death from cancer among both men and women worldwide (World Health Organization statistics, 2009). It is predicted to be responsible for the death of almost 50 000 people each year in the USA alone (1). There has been increasing awareness and focus on the prevention of cancer over the past few decades since it is now known that 90–95% of the cause is attributable to lifestyle and diet, whereas the other smaller portion is due to genetic factors (2,3). Data collected from numerous epidemiological, preclinical and clinical studies reveal that several bioactive components in fruits and vegetables can reduce both colonic inflammation and colon cancer risk (4–6).
Stilbenes are phytochemicals present in grapes, berries, peanuts and red wine (7). Resveratrol (trans-3,5,4′-trihydroxystilbene), a widely studied stilbene, exhibits antioxidant, anti-inflammatory and antiaging action and displays chemopreventive effects in a number of biological systems (8–11). Resveratrol has been shown to reduce formation of preneoplastic lesions [aberrant crypt foci (ACF)] in rats (12), to decrease the incidence and size of tumors in the 1,2-dimethylhydrazine-induced model of colon cancer in rats (13) and to prevent the formation of colon and small intestinal tumors in APCMin/+ mice when administered in the drinking water (14).
Recently, pterostilbene (trans-3,5-dimethoxy-4′-hydroxystilbene; Figure 1A), a structurally similar compound to resveratrol, has been shown to exhibit significant effects against cell proliferation (15,16), inflammation (17,18), invasion and metastasis (19) and to reverse the effects of aging in rats (20). The presence of chronic inflammatory conditions in the colonic environment has been implicated in the development of colorectal cancer, and treatment regimens against inflammatory markers, such as inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2), have reduced the risk of colon cancer (21–23). In a previous study, we demonstrated that pterostilbene lowered the expression of iNOS and COX-2, and downregulated the messenger RNA levels of proinflammatory cytokines, such as tumor necrosis factor-α (TNF-α), interleukin (IL)-1β and IL-6 in HT-29 colon cancer cells (18).
Fig. 1.
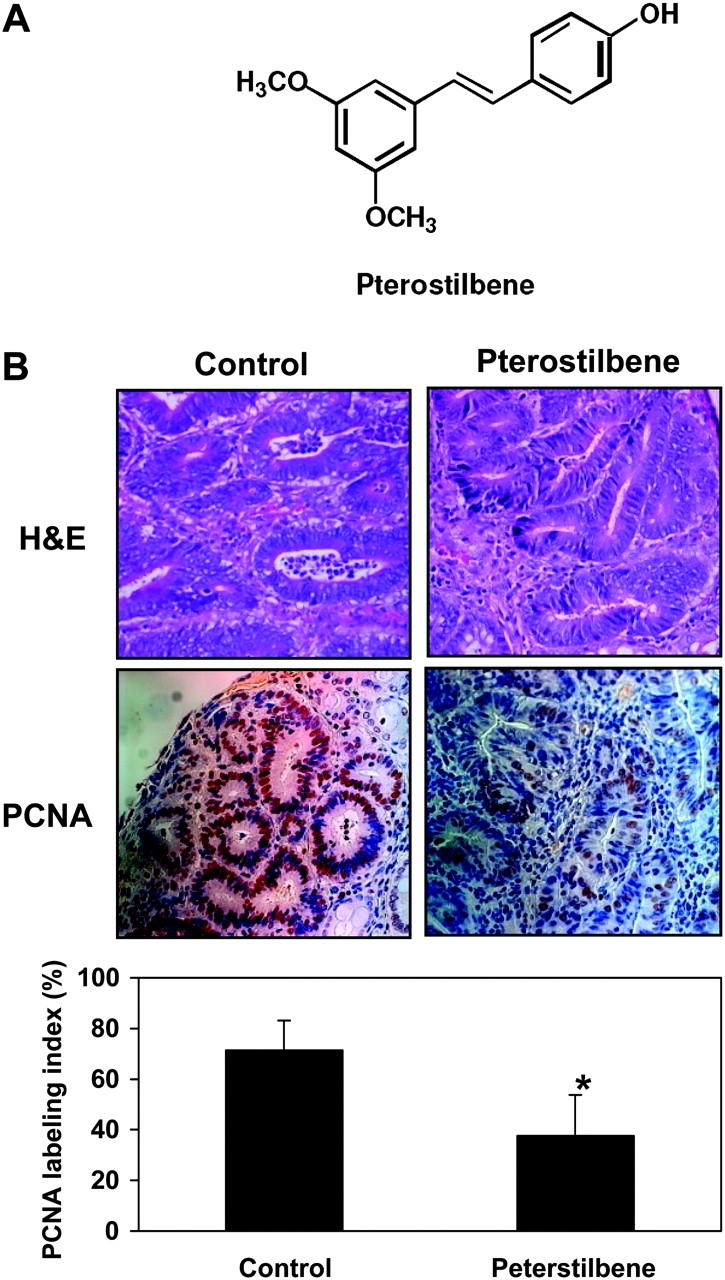
(A) Structure of pterostilbene. (B) Hematoxylin and eosin staining (top panels), PCNA staining (middle panels), and PCNA counting (bottom panels) of the colon tumors. For PCNA counting, four independent sections of the colon per animal with three animals per group were stained, and ∼1000 cells were counted from each section in total. The PCNA labeling index (PI) was calculated as the [(number of positive cells)/(total number of epithelial cells)] × 100 for each field. These PI values for different colon sections from the animals belonging to same group were then averaged. Statistical significance of treatment between the groups was analyzed by Student’s t-test. * P < 0.05.
In studies with HT-29 colon cancer cells, a marked reduction in cyclin D1 levels by pterostilbene was observed (18). Cyclin D1 is overexpressed in patients with adenomatous polyps, primary colorectal adenocarcinoma (AC) and familial adenomatous polyposis (24,25). Cyclin D1 is a target gene of the Wnt signaling pathway (26), and mutations in this pathway are responsible for ∼90% of colorectal cancer (27). Mutations in genes belonging to the Wnt pathway, such as inactivating mutations in the adenomatous polyposis coli (APC) gene or activating mutations in β-catenin, result in the nuclear accumulation of β-catenin and subsequent complex formation with T-cell transcription factor/lymphoid enhancing factor transcription factors to activate gene transcription (28). T-cell transcription factor/lymphoid enhancing factor-binding sites on promoters of cell proliferation genes, such as cyclin D1 and c-MYC (26,29), thus serve to transmit the aberrant mutations into tumorigenic signals within the colonic crypts.
In our earlier study, we showed that pterostilbene was effective in reducing the incidence and multiplicity of ACF in the colon of rats injected with the carcinogen, azoxymethane (AOM) (30). We also observed a significant reduction in the markers for colonic cell proliferation and inflammation by pterostilbene in the colon crypts (30). In our present study, we evaluated the chemopreventive potential of the stilbene with colonic tumor formation as the endpoint and further determined the action of pterostilbene in regulating the expression of key protein markers during colon carcinogenesis. Detailed mechanism-based studies in the colon cancer cell line HT-29 provided further insights into the signaling pathways affected by pterostilbene for key markers found relevant in vivo.
Materials and methods
Synthesis of pterostilbene
Pterostilbene (trans-3,5-dimethoxy-4′-hydroxystilbene; Figure 1A) was synthesized at the National Products Utilization Research Unit, United States Department of Agriculture, University, MS (purity >99.9%) (16).
Reagents and cell culture
Recombinant human interferon-γ and TNF-α were purchased from R&D Systems (Minneapolis, MN), lipopolysaccharide (from Escherichia coli 0111:B4 γ-irradiated) was from Sigma (St. Louis, MO) and Wnt agonists, SB203580 and H-89, were from Calbiochem (San Diego, CA). Human colon carcinoma HT-29 cells were obtained from the American Type Culture Collection (Manassas, VA) and maintained in Dulbecco’s modification of Eagle’s medium supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin at 37°C and 5% CO2.
Western blot analysis
Whole cell, cytosolic and nuclear protein fractions from cell culture experiments were collected and analyzed by western blotting, as described previously (18,30,31). The primary antibodies against IκBα, p65, p-p65, cyclin D1, c-MYC and β-catenin (Santa Cruz Biotechnology, Santa Cruz, CA), p-p38, p-MSK1 (Cell signaling, Beverly, MA) and β-actin (Sigma, St Louis, MO) and secondary antibodies (Santa Cruz Biotechnology) were used. Quantification of western blots was done by ImagePro 6.2 program.
Fluorescence microscopy
HT-29 cells were incubated in a chamber slide (Nunc, Rochester, NY) with or without pterostilbene and/or a Wnt agonist (Calbiochem). Cells were fixed with 4% paraformaldehyde, blocked with 10% bovine serum albumin and then incubated sequentially with primary antibody (1:100), fluorophore-conjugated secondary antibody (Alexa Fluor® 488; Invitrogen, Carlsbad, CA) and 4′,6-diamidino-2-phenylindole. The cells were irradiated with green laser at 488 nm for detection of β-catenin and with UV light at 364 nm for nuclear staining by 4′,6-diamidino-2-phenylindole.
Electrophoretic mobility shift assay
Electrophoretic mobility shift assays were performed using the Lightshift Chemiluminescent electrophoretic mobility shift assay kit (Pierce Biotechnology, Rockford, IL) for the nuclear factor-kappaB (NF-κB) consensus oligonucleotide probe, 5′-AGTTGAGGGGACTTTCCCAGGC-3′, 5′-end-labeled with biotin (Integrated DNA Technologies, Coralville, IA). Binding reactions were made in a total volume of 20 μl by adding 20 μg of nuclear extracts to 20 fmol of probe in binding buffer (20 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (pH 8.0); 50 mM NaCl; 1 mM ethylenediaminetetraacetic acid; 5% glycerol; 0.05 μg/μl poly [dI–dC] and 0.5 mM dithiothreitol). After incubation at room temperature for 20 min, the samples were loaded into 6% native gel. Competition assay with unlabeled oligonucleotide (cold DNA) was done in parallel. This was followed by transfer of the DNA–protein complex to polyvinylidene difluoride membrane, blocking and UV cross-linking. The membrane was incubated in the substrate for the peroxidase enzyme and the shifted bands were observed by chemiluminescence.
Animals, diet and in vivo experimental procedures
Weanling male F344 rats obtained from Charles River Breeding Laboratories (Kingston, NY) were randomly distributed by weight into control and experimental groups. Animals had access to food and water at all times. Food cups were replenished with fresh diet twice weekly. Experimental diets were prepared by Research Diets (New Brunswick, NJ) and stored at 4°C. Beginning at 5 weeks of age, all rats were fed the modified American Institute of Nutrition-76A (AIN-76A) diet. At 7 weeks of age, the animals were given subcutaneous injections of AOM (CAS no. 25843-45-2; Ash Stevens, Detroit, MI) at a dose rate of 15 mg/kg body weight or saline as solvent control once weekly for 2 weeks. One day after the second AOM injection, animals were maintained on AIN-76A diet or AIN-76A diet containing 40 p.p.m. (0.004%) of pterostilbene. The dose of pterostilbene was based on our previous 8 week study using ACF as an end point (30). At autopsy, animals were killed by CO2 asphyxiation and the colon was removed, rinsed in phosphate-buffered saline, opened longitudinally and flattened on a filter paper. The location and size of each tumor were noted. Mucosal scrapings were collected and stored at −80°C for further analysis. Tumors were removed, fixed in 10% buffered formalin for 24 h and transferred to 80% ethanol for histopathological analysis.
Histopathology and immunohistochemistry
The tumor tissues were dehydrated, embedded in paraffin and cut into 4 μm thick sections. For histopathology, the sections were hydrated and stained with hematoxylin and eosin according to the standard protocol. The stained sections were analyzed for tumor grades by a pathologist, who was unaware of the treatments. For immunohistochemical analysis, only non-invasive ACs (NIAs) were selected for the evaluation of protein markers. The detailed procedures for immunohistochemical analysis were reported previously (30). The primary antibodies against proliferating cell nuclear antigen (PCNA) (1:1500 diluted; BD Pharmingen, San Diego, CA), cyclin D1 (1:500 diluted), β-catenin (1:500 diluted), p-p65 (1:250 diluted), iNOS (1:500 diluted) (Santa Cruz Biotechnology) and COX-2 (1:200 diluted; Cayman Chemical, Ann Arbor, MI) were treated on the sections. The images were taken randomly at ×400 using Zeiss AxioCam HRc camera fitted to a Zeiss Axioskope 2 Plus microscope (Carl Zeiss USA, Thornwood, NY). Nuclear staining was observed with PCNA, whereas cyclin D1, iNOS and COX-2 showed cytoplasmic staining, and β-catenin was observed in the cytoplasm and cell membrane.
Measurement of cytokine production by enzyme-linked immunosorbent assay
Colonic mucosa samples (n = 12 in control group, n = 6 in pterostilbene group) were homogenized in a phosphate buffered saline-based buffer solution (phosphate-buffered saline, 0.4 M NaCl, 10 mM ethylenediaminetetraacetic acid, 0.1 mM phenylmethylsulfonyl fluoride, 0.1 M benzethonium ion, 0.5% bovine serum albumin, 3.0% Aprotinin, 0.05% Tween 20) on ice using a Tekmar Tissuemiser (Fisher Scientific International, Pittsburgh, PA). The homogenized solution was centrifuged at 10 000 r.p.m., 4°C for 10 min. The supernatant was collected for determination of protein concentration and stored at −20°C. For determination of the levels of IL-1β, IL-4 and TNF-α, tissue homogenates were normalized down to a concentration of 1.0 mg/ml of total protein and then diluted 10-fold in diluent buffer for analysis, following the manufacturer’s protocols. Invitrogen Immunoassay kits (BioSource International, Carmillo, CA) were used to determine the levels of IL-1β (catalog no. KRC0012), IL-4 (catalog no. KRC0042) and TNF-α (catalog no. KRC3012).
Tissue/serum extraction procedures and gas chromatography-mass spectrometry analysis of pterostilbene
Colon tissues and serum samples were frozen and kept at −80°C until extraction. For colon tissue extraction, tissues were homogenized with sodium phosphate buffer pH 7.4 (0.2 M NaH2PO4: 0.2 M Na2HPO4) (80:20) and centrifuged. The supernatant was collected and the tissue pellet was re-extracted with sodium phosphate buffer. The supernatants were extracted with ethyl acetate, combined, dried under a stream of nitrogen, then derivatized with N,O-bis[trimethylsilyl]-trifluoroacetamide:dimethylformamide (1:1; Pierce Biotechnology) and heated. The derivatized samples were used for gas chromatography-mass spectrometry (GC-MS). For serum extraction, serum was mixed with potassium phosphate buffer. Ice-cold high-performance liquid chromatography grade acetonitrile was then added, vortexed and centrifuged. The supernatant was dried under a stream of nitrogen and derivatized following procedures used for the colon tissues. GC-MS analysis was performed on a JEOL GCMate II Instrument (JEOL USA, Peabody, MA) using a J&W DB-5 capillary column (0.25 mm internal diameter, 0.25 μm film thickness and 30 m length; Agilent Technologies, Foster City, CA). The GC temperature program was: initial 190°C, increased to 240°C at 20°C/min rate, then increased to 280°C at 2.5°C /min rate, then finally increased to 300°C at the rate of 30°C/min and held at this temperature for 30 s. The carrier gas was ultrahigh purity helium, at 1 ml/min flow rate. The injection port, GC-MS interface and ionization chamber were at 250, 250 and 250°C, respectively. The volume of injection was 1 μl, splitless injection. The mass spectrum was acquired in the positive, electron impact (70 eV), low-resolution mode. Pterostilbene was determined and quantified from a reconstructed ion chromatogram using m/z 328 (and qualifying ions 313 and 280). GC-MS analyses were carried out in duplicates, and quantitation was performed from a calibration curve of a standard sample of pterostilbene (retention time, 9.3 min).
Statistical analysis
Statistical analysis was performed using the Student’s t-test unless otherwise noted.
Results
Pterostilbene reduces the tumor multiplicity of colonic tumors in AOM-injected rats
The effects of dietary administration of pterostilbene on AOM-induced colon tumorigenesis were evaluated, and the results are summarized in Table I. Body weights of the animals fed with 40 p.p.m. pterostilbene throughout 45 weeks were comparable with those of the animals on the control diet. None of the rats in the saline group (without AOM injection, n = 6) developed tumors when autopsied at week 45 (data not shown). At the termination of the study, the AOM control rats and AOM–pterostilbene-treated rats had tumor incidence of 87.5 and 67.8%, respectively. Histopathological analysis by hematoxylin and eosin staining revealed that ∼95% of the tumors from the control group were AC, and the remaining 5% were carcinoma in situ. All tumors from the pterostilbene group were identified as ACs. In both the control and pterostilbene-treated groups, ∼90% of the total ACs belonged to the NIA grade, whereas the remaining 10% was invasive AC (Table I). Pterostilbene treatment reduced the number of NIA by 40.2% (P = 0.04). Also, the mean number of invasive AC was decreased in pterostilbene group but there were not enough tumors per animals to achieve statistical significance. Since there was no report on serum or tissue levels of pterostilbene from long-term studies, we analyzed levels of pterostilbene in the serum and colon mucosa in this study. As shown in Table I, there was no detectable level of pterostilbene in the control group, whereas serum and colon mucosa levels in the pterostilbene-treated group were 48.0 ± 6.9 (ng/ml) and 10.9 ± 3.8 (ng/g), respectively.
Table I.
Efficacy of pterostilbene in the AOM-induced colon cancer model in F344 male rats: tumor incidence and tumor multiplicity
| Experimental groupa | No. of animals at autopsy | Body weight at autopsy (mean ± SE) (g) | Tumor incidenceb (rats with colon tumors/total no. of rats) | Tumor multiplicityc (no. of NIA per rat) (mean ± SE) | Tumor multiplicityc (no. of invasive AC per rat) (mean ± SE) | Serum level of pterostilbened (mean ± SE) (ng/ml) | Colon mucosa level of pterostilbened (mean ± SE) (ng/g) |
| Control diet | 24 | 439 ± 6.6 | 21/24 (87.5%) | 1.79 ± 0.28 | 0.21 ± 0.08 | N.D.e | N.D.e |
| Pterostilbene | 28 | 445 ± 5.5 | 19/28 (67.8%) | 1.07 ± 0.21(P = 0.04) | 0.07 ± 0.05 | 48.0 ± 6.9 | 10.9 ± 3.8 |
Pterostilbene (40 p.p.m.) was administered in the diet starting at one day after the second AOM treatment and continuously thereafter for 45 weeks.
Tumor incidence was analyzed by two-tailed Fisher’s exact probability test. No statistical significance was observed.
Tumor multiplicity was analyzed by the Student’s t-test.
Samples were randomly selected from the control (serum, n = 10; colon mucosa, n = 10) or pterostilbene-fed groups (serum, n = 8; colon mucosa, n = 6) for analysis.
N.D.: Not detectable.
Pterostilbene decreases a cell proliferation marker, PCNA, in the colon ACs
As shown in Figure 1B, the histological evaluation revealed that the majority of tumors were NIAs. The expression of PCNA, a marker for cell proliferation, was further determined in the NIAs from the control and pterostilbene groups. The colon tumors from the pterostilbene-fed group showed significant reduction of PCNA nuclear staining compared with the control group. PCNA-positive nuclei in the colon tumors were 71% of the cells in the control group and 38% of the cells in the pterostilbene-treated group (P = 0.02) (Figure 1B).
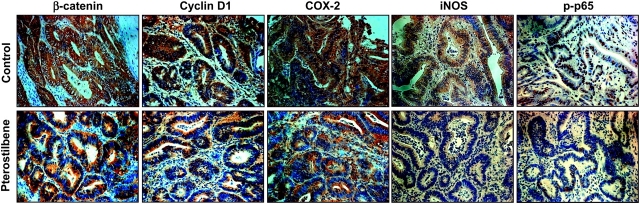
Pterostilbene downregulates the expression of β-catenin and cyclin D1 in the colon ACs
Aberrant expression of β-catenin can be regarded as a key event during colorectal tumorigenesis and is linked to the increased transcription of a number of genes, such as cyclin D1 (24,32). β-Catenin was identified along the membrane of the epithelial cells in the control group (Figure 2). The colonic crypt cells in the control group showed homogeneous and intense staining for β-catenin in the cytosol as well as in the membrane, with lower and scattered staining in the nucleus. In contrast, the tumors from the pterostilbene group had no observable nuclear staining (Figure 2). The cytoplasmic expression of β-catenin was also markedly inhibited by the treatment with pterostilbene (Figure 2). Since cyclin D1 is a downstream signaling target of β-catenin, and overexpression of cyclin D1 is reported in patients with colorectal tumors where its lowering has therapeutic significance (24,32), we determined whether pterostilbene reduced cyclin D1 levels in colon tumors. Positive brownish staining of cyclin D1 in the control group or pterostilbene-fed group was predominantly localized in the cytoplasm. The colon tumors from the control group showed stronger staining for cyclin D1 than those from the pterostilbene group (Figure 2).
Fig. 2.
Pterostilbene inhibits the expression of β-catenin, cyclin D1, COX-2, iNOS and phospho-p65 protein in the colon tumors. The colon tumor sections were processed and incubated with the respective primary antibodies as described in the Materials and Methods. β-Catenin and cyclin D1 staining was high in the cytosol and also present in the nucleus to a lower extent. iNOS and COX-2 showed cytoplasmic staining, whereas nuclear staining was predominant with phospho-p65. n = 3 per group for each analysis. A representative section is shown. Image magnification, ×400.
Pterostilbene reduces the expression of inflammatory enzymes, iNOS and COX-2, and decreases nuclear staining of phospho-p65 in colon ACs
Overexpression of inflammatory markers is a hallmark of colorectal tumors. This knowledge, as well as our previous observations on the efficacy of pterostilbene against inflammation (18,30), led us to examine the effects of long-term feeding of pterostilbene on the inflammatory markers in the AOM-injected rats. As shown in Figure 2, there was significant inhibition of the expression of iNOS and COX-2 proteins within the crypts in the ACs from the pterostilbene group, compared with those from the control group. We next determined the effects of pterostilbene on a key NF-κB signaling molecule, p65, because NF-κB is an upstream factor of both iNOS and COX-2 transcription, and it is critical during tumorigenesis where ablation of the proteins in this pathway causes regression of tumors in animal models (33). The activated form of NF-κB subunit p65, i.e. phospho-p65, was markedly reduced in the nucleus of the colon tumors from the pterostilbene group, when compared with those from the control group (Figure 2).
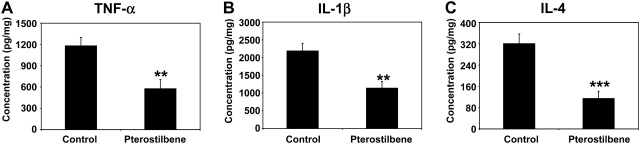
Pterostilbene inhibits mucosal levels of the inflammatory cytokines TNF-α, IL-1β and IL-4
Inflammatory cytokines are found to be present in human cancers, including those of the colorectum, breast, prostate and bladder (34,35). The action of cytokines to facilitate carcinogenesis is multifold: DNA damage by reactive oxygen species and reactive nitrogen species, inhibition of DNA repair by reactive oxygen species, functional inactivation of tumor suppressor genes, tissue remodeling via activation of matrix metalloproteinases (MMPs), stimulation of angiogenesis and control of cell adhesion molecules (35). Results of enzyme-linked immunosorbent assay conducted for inflammatory cytokines on mucosal scrapings derived from the AOM-injected rats are shown in Figure 3. Dietary administration of pterostilbene significantly lowered the levels of cytokines, TNF-α by 51.0% (P = 0.009), IL-1β by 47.7% (P = 0.008) and IL-4 by 64.2% (P = 0.002).
Fig. 3.
Pterostilbene lowers the production of the inflammatory cytokines, TNF-α (A), IL-1β (B) and IL-4 (C), in the colonic mucosa. The mucosa samples were homogenized and assayed by enzyme-linked immunosorbent assay for the different cytokines, as described under Materials and Methods section. Colon mucosa samples were randomly selected from each group and cytokine levels were analyzed (n = 12 in control group, n = 6 in pterostilbene group). The mean ± SD values are shown. **P < 0.01, ***P < 0.005.
Pterostilbene reduces the protein levels of β-catenin, cyclin D1 and c-MYC and alters the cellular localization of β-catenin in HT-29 colon cancer cells
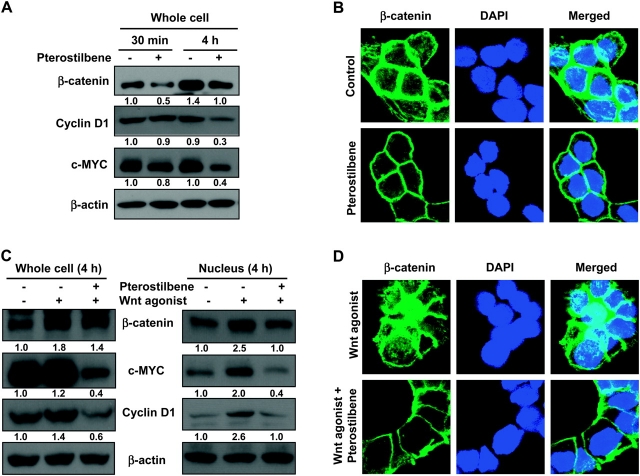
To investigate the effects of pterostilbene on β-catenin expression in colon cancer, we employed the HT-29 colon cancer cells, which are known to possess the wild-type β-catenin and truncated Apc gene (36). As shown in Figure 4A, we observed lowering of the protein levels of β-catenin after 30 min of treatment with pterostilbene. Cyclin D1 and c-MYC proteins, two well-known downstream targets of β-catenin, were decreased by pterostilbene at a later time point, 4 h (Figure 4A). In confocal microscopy, the untreated cells showed intense staining for β-catenin predominantly in the cytoplasm and the membrane, and the treatment with pterostilbene markedly lowered the levels of β-catenin (Figure 4B). The β-catenin transcriptional activity is known to be regulated not only through the levels of protein degradation but also through its nuclear localization (37). In order to better understand the inhibitory effects of pterostilbene on the β-catenin pathway, we used a Wnt agonist that mimics the effect of Wnt in inducing β-catenin/T-cell transcription factor-dependent transcriptional activity. The Wnt agonist increased the expression of cyclin D1, c-MYC and β-catenin proteins, whereas cotreatment of the Wnt agonist with pterostilbene lowered the expression level of these proteins in the nucleus (Figure 4C). In confocal microscopy, treatment with the Wnt agonist increased β-catenin predominantly, and the cotreatment of Wnt agonist with pterostilbene lowered Wnt agonist-induced β-catenin in the membrane and nucleus (Figure 4D).
Fig. 4.
Pterostilbene downregulates the expression of β-catenin and its downstream targets, cyclin D1 and c-MYC, in HT-29 colon cancer cells. (A) Effect of pterostilbene on levels of β-catenin, cyclin D1 and c-MYC. HT-29 cells (1.5 × 106 per 100 mm dish) were treated with pterostilbene (50 μM) for 30 min or 4 h. The cells were harvested for whole cell protein and samples were immunoblotted. (B) Action of pterostilbene on cellular localization of β-catenin. HT-29 cells (30 000 per chamber in a four-well chamber slide) were incubated with pterostilbene (50 μM) for 4 h. Green, staining for β-catenin; blue, nuclear staining by 4′,6-diamidino-2-phenylindole (DAPI). (C) Effects of pterostilbene on Wnt/β-catenin signaling proteins in the presence of Wnt agonist. HT-29 cells were treated with a Wnt agonist (10 μM) with or without pterostilbene (50 μM) for 4 h. The whole-cell and nuclear proteins were harvested and immunoblotted for β-catenin, cyclin D1, c-MYC and β-actin. (D) Effects of pterostilbene on the nuclear localization of β-catenin induced by Wnt agonist. HT-29 cells were incubated with a Wnt agonist (10 μM) with or without pterostilbene (50 μM) for 4 h. Green, staining for β-catenin; blue, nuclear staining by DAPI. Quantification of western blots was done by ImagePro 6.2 program, and the numbers are given at the bottom of each western blot.
Pterostilbene regulates the NF-κB pathway by inhibiting the phosphorylation of p65 in HT-29 colon cancer cells
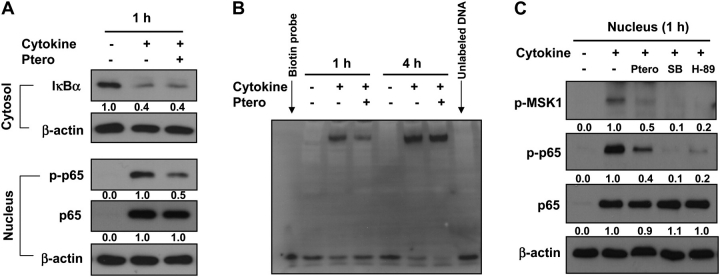
The NF-κB pathway is often recognized as a critical link between inflammatory processes and cancer development (38). HT-29 cells, when treated with a cytokine mixture comprising 10 ng/ml each of TNF-α, interferon-γ and lipopolysaccharide, activated the NF-κB signaling, as shown by the increased degradation of IκBα in the cytoplasm and increased p65 in the nucleus (Figure 5A). As depicted in Figure 5A, pterostilbene neither blocked the degradation of IκBα nor affected the accumulation of p65 in the nucleus caused by the treatment with cytokines. However, phosphorylated p65 (Ser 276) levels were markedly downregulated by pterostilbene in the nucleus (Figure 5A). Analysis of nuclear protein fractions by electrophoretic mobility shift assay revealed that pterostilbene lowered the NF-κB-binding activity (Figure 5B). The effects of pterostilbene on phospho-p65 levels as well as NF-κB-binding activity were pronounced at 1 h time point (Figure 5A and B). In our previous report, we identified p38 mitogen-activated protein kinase as a major target regulated by pterostilbene in HT-29 colon cancer cells (18). It is known that p38 is responsible for the phosphorylation of MSK-1 (39) and that this activated MSK-1 phosphorylates p65 at serine 276 (40). Therefore, we determined the effect of pterostilbene on p38/MSK-1/p65 signaling. When HT-29 cells were treated with cytokine mixture (TNF-α, interferon-γ and lipopolysaccharide) together with pterostilbene, an MSK-1 inhibitor (H-89) or a p38 mitogen-activated protein kinase inhibitor (SB203580), the reduction of phospho-p65 was shown by pterostilbene as well as by both MSK-1 and p38 mitogen-activated protein kinase inhibitors. Further, pterostilbene decreased the level of phospho-MSK-1 protein, suggesting the role of MSK-1 in mediating the action of pterostilbene to lower the phosphorylation of p65 (Figure 5C).
Fig. 5.
Effects of pterostilbene on the NF-κB pathway. (A) Pterostilbene lowers nuclear phospho-p65 levels in HT-29 cells. HT-29 cells (1.5 × 106 cells per 100 mm dish) were incubated with a mixture of TNF-α, interferon (IFN)-γ and lipopolysaccharide (LPS) (each at 10 ng/ml) in the presence or absence of pterostilbene (30 μM) for 1 h, and cytosolic and nuclear protein fractions were collected and immunoblotted. (B) DNA binding of NF-κB is reduced by pterostilbene at 1 h. HT-29 cells were incubated with a mixture of TNF-α, IFN-γ and LPS together with pterostilbene (30 μM) for 1 or 4 h. NF-κB DNA-binding activity was determined by electrophoretic mobility shift assay. (C) Phosphorylation of p65 mediated through the p38/MSK-1 pathway is inhibited by pterostilbene in HT-29 cells. HT-29 cells were incubated with a mixture of TNF-α, IFN-γ and LPS in the presence or absence of pterostilbene (30 μM), MSK-1 inhibitor (H-89, 10 μM) or p38 mitogen-activated protein kinase inhibitor (SB203580, 10 μM) for 1 h. The nuclear proteins were harvested and immunoblotted for phospho-MSK-1, phospho-p65 and p65. Quantification of western blots was done by ImagePro 6.2 program, and the numbers are given at the bottom of each western blot.
Discussion
The present study is an extension of our previous work, which identified pterostilbene as an effective agent in suppressing the formation of ACF in the colons of rats injected with the colon-specific carcinogen, AOM (30). The results from the current research conducted in the same animal model of colon cancer, but with tumors as an end point, revealed that dietary administration of pterostilbene reduced the colon tumor multiplicity and regulated intermediate signaling pathways of proliferation and inflammation in the colon.
A comparison of tumor numbers across the different grades of tumor showed an overall reduction by the treatment with pterostilbene, although statistical difference was shown only with tumor multiplicity with NIA (40.2% reduction). The moderate reduction of tumor multiplicity and tumor incidence may be due to a low dose of pterostilbene used. In our study, pterostilbene was given at 40 p.p.m. (0.004% in the diet), and ∼40–50 ng/ml range of pterostilbene was detected in the serum from the animals. This number is low when considering that many of the reported studies on tumorigenesis with stilbenes have used much higher doses. Using colon adenoma as an end point, a study conducted by Sale et al. (41) indicated that resveratrol and its analogue DMU212 (3,4,5,4′-tetramethoxystilbene) given as 0.2% in the diet significantly decreased the number of adenomas, whereas 0.05% of either stilbenes given in the diet did not . Since pterostilbene at 0.004% dose showed reduction in tumor multiplicity, pterostilbene may be more effective than resveratrol in the inhibition of colon cancer, although dose–response studies are needed to fully understand and design effective chemoprevention strategies with stilbenes.
AOM-induced tumors result from mutations in the Wnt/β-catenin pathway (42) as does the APCMin/+ model. However, unlike the APCMin/+ model, AOM-induced tumors are caused by mutations in the β-catenin gene (43,44). These mutations result in β-catenin stabilization and aberrant expression of β-catenin, which is considered as a key event during colon tumorigenesis (45). Immunohistochemical analysis revealed abundance of β-catenin mostly in the cytoplasm and relatively low nuclear staining in the ACs of rats injected with AOM, whereas administration of pterostilbene markedly reduced the staining for β-catenin in both the cytoplasm and the nucleus (Figure 2). In addition, pterostilbene was effective in reducing the abundance of β-catenin in the cytosol and to some extent in the nucleus of HT-29 cells (Figure 4).
Cyclin D1 is a very well-known cell cycle protein targeted by β-catenin (26) and is known to be overexpressed in colonic tumors (24,25). c-MYC is yet another important protein for cell proliferation regulated by β-catenin and Wnt pathway (29). These observations on cyclin D1 and c-MYC were corroborated by the potency of pterostilbene to affect the β-catenin levels in the colon tumors and in HT-29 cells. In the tumor studies, pterostilbene lowered the level of cyclin D1 protein in the colon tumor tissues induced with AOM (Figure 2). In cultured colon cancer cells, pterostilbene markedly lowered the protein levels of cyclin D1 and c-MYC in cells both unstimulated (Figure 4A) and stimulated with a Wnt agonist (Figure 4C). More importantly, nuclear levels of β-catenin and cyclin D1 are reported to play more important role in tumorigenesis than the total protein levels (37,46). In our studies, pterostilbene reduced the Wnt agonist-induced levels of cyclin D1 and β-catenin in the nucleus (Figure 4C).
In addition to the effects on β-catenin and cell proliferation, our results indicate the anti-inflammatory property of pterostilbene. We observed marked reduction in the staining intensities for iNOS, COX-2 and phospho-p65 in the colon tumors from the rats fed pterostilbene diet (Figure 2). Mucosal levels of inflammatory cytokines, such as TNF-α, IL-1β and IL-4, were also significantly downregulated by pterostilbene (Figure 3). In HT-29 cells, the phosphorylation of p65 subunit of NF-κB was downregulated by pterostilbene (Figures 2 and 5), possibly leading to lower binding potential of NF-κB (47,48). This may eventually affect the transcription of a number of target genes predominantly involved in inflammatory cell responses. Several anti-inflammatory agents that target the nitric oxide or the prostaglandin pathway are reported to present chemopreventive action in the colon (3,23,49). A clinical trial on celecoxib, the selective COX-2 inhibitor, at a dose of 400 mg once daily, reduced advanced adenoma formation in the colon by almost 50% compared with the placebo through a 3 year treatment period (50). Promising results with other agents such as the use of low concentrations of difluromethylornithine and sulindac as chemopreventive agents in colorectal cancer highlight the potential role of inflammation in its pathogenesis and the importance of combination strategies (49). Since pterostilbene at a low dose in the diet (0.004%) was shown to decrease the inflammatory markers, the development of this natural compound against colorectal cancer may be promising.
In conclusion, pterostilbene inhibits colon tumorigenesis by regulating the Wnt/β-catenin-signaling pathway and the inflammatory responses. Pterostilbene alone or in combination with other known chemopreventive agents can be of great importance for colon cancer prevention. Overall, the data indicate that pterostilbene, an active constituent in blueberries, holds great promise in the field of chemoprevention by dietary agents.
Funding
National Institute of Environmental and Health Sciences (P30 ES005022); Trustees Research Fellowship Program at Rutgers, The State University of New Jersey.
Acknowledgments
The authors thank Maria Hyra and Lamberto R.Navoa of the Animal Facility in the Department of Chemical Biology for their technical assistance in taking care of the animals, and Dr Allan Conney for helpful comments on our manuscript. We also thank Dr Yong Lin at the University of Medicine and Dentistry of New Jersey for the statistical analysis and Dr Gloria Hervey of the USDA, ARS, Natural Products Utilization Research Unit for her excellent technical help. This work is dedicated to the late Dr Bandaru S.Reddy.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- AC
adenocarcinoma
- ACF
aberrant crypt foci
- AIN-76A
American Institute of Nutrition-76A
- AOM
azoxymethane
- COX-2
cyclooxygenase-2
- GC-MS
gas chromatography-mass spectrometry
- IL
interleukin
- iNOS
inducible nitric oxide synthase
- NF-κB
nuclear factor-kappaB
- NIA
non-invasive AC
- PCNA
proliferating cell nuclear antigen
- TNF-α
tumor necrosis factor-α
References
- 1.Jemal A, et al. Cancer statistics, 2009. CA Cancer J. Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Center MM, et al. International trends in colorectal cancer incidence rates. Cancer Epidemiol. Biomarkers Prev. 2009;18:1688–1694. doi: 10.1158/1055-9965.EPI-09-0090. [DOI] [PubMed] [Google Scholar]
- 3.Half E, et al. Colon cancer: preventive agents and the present status of chemoprevention. Expert Opin. Pharmacother. 2009;10:211–219. doi: 10.1517/14656560802560153. [DOI] [PubMed] [Google Scholar]
- 4.Millen AE, et al. Fruit and vegetable intake and prevalence of colorectal adenoma in a cancer screening trial. Am. J. Clin. Nutr. 2007;86:1754–1764. doi: 10.1093/ajcn/86.5.1754. [DOI] [PubMed] [Google Scholar]
- 5.Rimando AM, et al. Biological/chemopreventive activity of stilbenes and their effect on colon cancer. Planta Med. 2008;74:1635–1643. doi: 10.1055/s-0028-1088301. [DOI] [PubMed] [Google Scholar]
- 6.van Duijnhoven FJB, et al. Fruit, vegetables, and colorectal cancer risk: the European Prospective Investigation into Cancer and Nutrition. Am. J. Clin. Nutr. 2009;89:1441–1452. doi: 10.3945/ajcn.2008.27120. [DOI] [PubMed] [Google Scholar]
- 7.Rimando AM, et al. Resveratrol, pterostilbene, and piceatannol in vaccinium berries. J. Agric. Food Chem. 2004;52:4713–4719. doi: 10.1021/jf040095e. [DOI] [PubMed] [Google Scholar]
- 8.Aggarwal BB, et al. Role of resveratrol in prevention and therapy of cancer: preclinical and clinical studies. Anticancer Res. 2004;24:2783–2840. [PubMed] [Google Scholar]
- 9.Baur JA, et al. Therapeutic potential of resveratrol: the in vivo evidence. Nat. Rev. Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 10.Gescher AJ. Resveratrol from red grapes—pedestrian polyphenol or useful anticancer agent? Planta Med. 2008;74:1651–1655. doi: 10.1055/s-2008-1074516. [DOI] [PubMed] [Google Scholar]
- 11.Bishayee A. Cancer prevention and treatment with resveratrol: from rodent studies to clinical trials. Cancer Prev. Res. 2009;2:409–418. doi: 10.1158/1940-6207.CAPR-08-0160. [DOI] [PubMed] [Google Scholar]
- 12.Tessitore L, et al. Resveratrol depresses the growth of colorectal aberrant crypt foci by affecting bax and p21CIP expression. Carcinogenesis. 2000;21:1619–1622. [PubMed] [Google Scholar]
- 13.Sengottuvelan M, et al. Dietary supplementation of resveratrol suppresses colonic tumour incidence in 1,2-dimethylhydrazine-treated rats by modulating biotransforming enzymes and aberrant crypt foci development. Br. J. Nutr. 2006;96:145–153. doi: 10.1079/bjn20061789. [DOI] [PubMed] [Google Scholar]
- 14.Schneider Y, et al. Resveratrol inhibits intestinal tumorigenesis and modulates host-defense-related gene expression in an animal model of human familial adenomatous polyposis. Nutr. Cancer. 2001;39:102–107. doi: 10.1207/S15327914nc391_14. [DOI] [PubMed] [Google Scholar]
- 15.Pan M-H, et al. Pterostilbene induces apoptosis and cell cycle arrest in human gastric carcinoma cells. J. Agric. Food Chem. 2007;55:7777–7785. doi: 10.1021/jf071520h. [DOI] [PubMed] [Google Scholar]
- 16.Rimando AM, et al. Cancer chemopreventive and antioxidant activities of pterostilbene, a naturally occurring analogue of resveratrol. J. Agric. Food Chem. 2002;50:3453–3457. doi: 10.1021/jf0116855. [DOI] [PubMed] [Google Scholar]
- 17.Pan M-H, et al. Pterostilbene suppressed lipopolysaccharide-induced up-expression of iNOS and COX-2 in murine macrophages. J. Agric. Food Chem. 2008;56:7502–7509. doi: 10.1021/jf800820y. [DOI] [PubMed] [Google Scholar]
- 18.Paul S, et al. Anti-inflammatory action of pterostilbene is mediated through the p38 mitogen-activated protein kinase pathway in colon cancer cells. Cancer Prev. Res. 2009;2:650–657. doi: 10.1158/1940-6207.CAPR-08-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pan M-H, et al. Pterostilbene inhibited tumor invasion via suppressing multiple signal transduction pathways in human hepatocellular carcinoma cells. Carcinogenesis. 2009;30:1234–1242. doi: 10.1093/carcin/bgp121. [DOI] [PubMed] [Google Scholar]
- 20.Joseph JA, et al. Cellular and behavioral effects of stilbene resveratrol analogues: implications for reducing the deleterious effects of aging. J. Agric. Food Chem. 2008;56:10544–10551. doi: 10.1021/jf802279h. [DOI] [PubMed] [Google Scholar]
- 21.Kawamori T, et al. Suppression of azoxymethane-induced colonic aberrant crypt foci by a nitric oxide synthase inhibitor. Cancer Lett. 2000;148:33–37. doi: 10.1016/s0304-3835(99)00310-9. [DOI] [PubMed] [Google Scholar]
- 22.Pereg D, et al. Non-steroidal anti-inflammatory drugs for the prevention and treatment of cancer. J. Intern. Med. 2005;258:115–123. doi: 10.1111/j.1365-2796.2005.01519.x. [DOI] [PubMed] [Google Scholar]
- 23.Rao CV, et al. Chemopreventive properties of a selective inducible nitric oxide synthase inhibitor in colon carcinogenesis, administered alone or in combination with celecoxib, a selective cyclooxygenase-2 inhibitor. Cancer Res. 2002;62:165–170. [PubMed] [Google Scholar]
- 24.Arber N, et al. Increased expression of cyclin D1 is an early event in multistage colorectal carcinogenesis. Gastroenterology. 1996;110:669–674. doi: 10.1053/gast.1996.v110.pm8608874. [DOI] [PubMed] [Google Scholar]
- 25.Zhang T, et al. Concurrent overexpression of cyclin D1 and cyclin-dependent kinase 4 (Cdk4) in intestinal adenomas from multiple intestinal neoplasia (Min) mice and human familial adenomatous polyposis patients. Cancer Res. 1997;57:169–175. [PubMed] [Google Scholar]
- 26.Tetsu O, et al. [beta]-Catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- 27.Giles RH, et al. Caught up in a Wnt storm: wnt signaling in cancer. Biochim. Biophys. Acta. 2003;1653:1–24. doi: 10.1016/s0304-419x(03)00005-2. [DOI] [PubMed] [Google Scholar]
- 28.Klaus A, et al. Wnt signalling and its impact on development and cancer. Nat. Rev. Cancer. 2008;8:387–398. doi: 10.1038/nrc2389. [DOI] [PubMed] [Google Scholar]
- 29.He T-C, et al. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 30.Suh N, et al. Pterostilbene, an active constituent of blueberries, suppresses aberrant crypt foci formation in the azoxymethane-induced colon carcinogenesis model in rats. Clin. Cancer Res. 2007;13:350–355. doi: 10.1158/1078-0432.CCR-06-1528. [DOI] [PubMed] [Google Scholar]
- 31.Suh N, et al. Novel triterpenoids suppress inducible nitric oxide synthase (iNOS) and inducible cyclooxygenase (COX-2) in mouse macrophages. Cancer Res. 1998;58:717–723. [PubMed] [Google Scholar]
- 32.Alao JP. The regulation of cyclin D1 degradation: roles in cancer development and the potential for therapeutic invention. Mol. Cancer. 2007;6:24. doi: 10.1186/1476-4598-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karin M. Nuclear factor-[kappa]B in cancer development and progression. Nature. 2006;441:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 34.Kundu JK, et al. Inflammation: gearing the journey to cancer. Mutat. Res. 2008;659:15–30. doi: 10.1016/j.mrrev.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 35.Balkwill F, et al. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 36.Yang J, et al. Adenomatous polyposis coli (APC) differentially regulates beta-catenin phosphorylation and ubiquitination in colon cancer cells. J. Biol. Chem. 2006;281:17751–17757. doi: 10.1074/jbc.M600831200. [DOI] [PubMed] [Google Scholar]
- 37.Nelson WJ, et al. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004;303:1483–1487. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vallabhapurapu S, et al. Regulation and function of NF-κB transcription factors in the immune system. Annu. Rev. Immunol. 2009;27:693–733. doi: 10.1146/annurev.immunol.021908.132641. [DOI] [PubMed] [Google Scholar]
- 39.Deak M, et al. Mitogen- and stress-activated protein kinase-1 (MSK1) is directly activated by MAPK and SAPK2/p38, and may mediate activation of CREB. EMBO J. 1998;17:4426–4441. doi: 10.1093/emboj/17.15.4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reber L, et al. Ser276 phosphorylation of NF-kB p65 by MSK1 controls SCF expression in inflammation. PLoS One. 2009;4:e4393. doi: 10.1371/journal.pone.0004393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sale S, et al. Comparison of the effects of the chemopreventive agent resveratrol and its synthetic analog trans 3,4,5,4′-tetramethoxystilbene (DMU-212) on adenoma development in the Apc(Min+) mouse and cyclooxygenase-2 in human-derived colon cancer cells. Int. J. Cancer. 2005;115:194–201. doi: 10.1002/ijc.20884. [DOI] [PubMed] [Google Scholar]
- 42.Takahashi M, et al. Frequent mutations of the {beta}-catenin gene in mouse colon tumors induced by azoxymethane. Carcinogenesis. 2000;21:1117–1120. [PubMed] [Google Scholar]
- 43.Kaiser S, et al. Transcriptional recapitulation and subversion of embryonic colon development by mouse colon tumor models and human colon cancer. Genome Biol. 2007;8:R131. doi: 10.1186/gb-2007-8-7-r131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang QS, et al. Altered expression of cyclin D1 and cyclin-dependent kinase 4 in azoxymethane-induced mouse colon tumorigenesis. Carcinogenesis. 1998;19:2001–2006. doi: 10.1093/carcin/19.11.2001. [DOI] [PubMed] [Google Scholar]
- 45.Morin PJ, et al. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- 46.Kim JK, et al. Nuclear cyclin D1: an oncogenic driver in human cancer. J. Cell. Physiol. 2009;220:292–296. doi: 10.1002/jcp.21791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ganchi PA, et al. A novel NF-kappa B complex containing p65 homodimers: implications for transcriptional control at the level of subunit dimerization. Mol. Cell. Biol. 1993;13:7826–7835. doi: 10.1128/mcb.13.12.7826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mosialos G, et al. v-Rel and c-Rel are differentially affected by mutations at a consensus protein kinase recognition sequence. Oncogene. 1993;8:721–730. [PubMed] [Google Scholar]
- 49.Reddy BS. Strategies for colon cancer prevention: combination of chemopreventive agents. Subcell. Biochem. 2007;42:213–225. doi: 10.1007/1-4020-5688-5_10. [DOI] [PubMed] [Google Scholar]
- 50.Arber N, et al. Celecoxib for the prevention of colorectal adenomatous polyps. N. Engl. J. Med. 2006;355:885–895. doi: 10.1056/NEJMoa061652. [DOI] [PubMed] [Google Scholar]