Abstract
Programmed cardiac myocyte death via the intrinsic, or mitochondrial, pathway is a mechanism of pathological ventricular remodeling after myocardial infarction and during chronic pressure overload hypertrophy. Transcriptional upregulation of the closely related proapoptotic Bcl2 family members BNip3 in ischemic myocardium and Nix in hypertrophied myocardium suggested a molecular mechanism by which programmed cell death can be initiated by cardiac stress and lead to dilated cardiomyopathy. Studies using transgenic and gene knockout mice subsequently demonstrated that expression of BNip3 and Nix is both sufficient for cardiomyopathy development and necessary for cardiac remodeling after reversible coronary occlusion and transverse aortic banding, respectively. Here, these data are reviewed in the context of recent findings showing that Nix not only stimulates cardiomyocyte apoptosis but also induces mitochondrial autophagy (mitophagy) and indirectly activates the mitochondrial permeability transition pore, causing cell necrosis. New findings are presented suggesting that Nix and BNip3 have an essential function, “mitochondrial pruning,” that restrains mitochondrial proliferation in cardiomyocytes and without which an age-dependent mitochondrial cardiomyopathy develops.
Keywords: Apoptosis, Heart Failure, Mitochondria, Autophagy
BNip3 and Nix are Inducible Cardiomyocyte Death Factors
The cellular hallmark of acquired dilated cardiomyopathy is loss of functioning cardiomyocytes, frequently with their replacement by fibrotic tissue [1]. In the case of myocardial infarction, this “cardiomyocyte drop-out” is early and localized and constitutes the primary injury. In chronically ischemic hearts and nonischemic cardiomyopathies, cardiomyocyte drop-out (typically characterized in the literature as apoptosis) is widespread and induced as a programmed secondary response to the primary injury [2–5]. Because adult cardiac myocytes are incapable of meaningful cellular regeneration [6], chronic persistent apoptosis at a rate of 0.1–1% of total cardiomyocytes is sufficient to produce cardiac dilation and heart failure [7]. The workload placed on the heart by programmed cardiac myocyte loss initiates a feed-forward cycle of unfavorable geometrical remodeling that further induces cardiomyocyte death genes, resulting in a downward functional spiral progressing to dilated cardiomyopathy and end-stage heart failure [8, 9].
Cardiomyocyte apoptosis can be mediated either through the extrinsic cytokine death receptor pathway, or the intrinsic mitochondrial pathway. The focus here is on mitochondrial apoptosis, regulated by the Bcl2 family of mitochondrial-targeted pro- and anti-“apoptotic” proteins. These factors unambiguously regulate caspase-dependent apoptosis and so the moniker of “pro-” or “anti-apoptotic” is at least partially correct. As explained in more detail below, however, evidence is accumulating that Bcl2 family proteins also regulate nonapoptotic forms of programmed cell death that occur concomitantly and can be confused with apoptosis. Here, I characterize Bcl2 family members as “pro”- or “anti-apoptotic” according to convention, with the caveat that this is a functional generalization that does not suggest one specific mechanism for cell death.
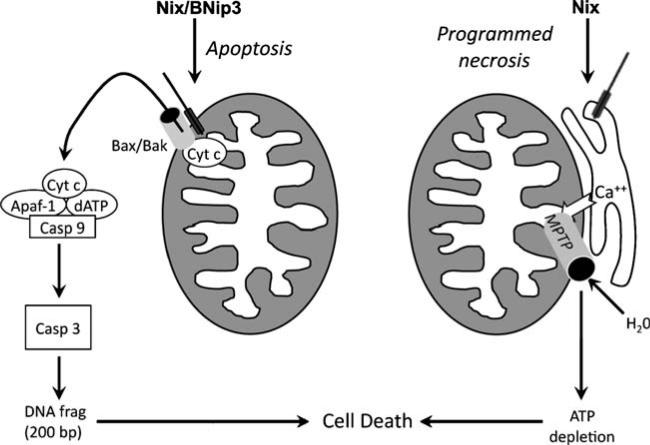
Bcl2 family proteins are classified according to their structural features and function in cell death [10]. The “multidomain” proteins, Bax and Bak, are pore-forming proteins that permeabilize mitochondrial outer membranes, leading to cytochrome c release and intrinsic apoptosis signaling. Pore formation by Bax and Bak is facilitated by proapoptotic BH3 domain-only factors, including cardiac-expressed BNip3 and Nix (also called BNip3L). BNip3, Nix, and related BH3-only factors heterodimerize with antiapoptotic factors like Bcl2 and BclXl in the heart, which prevents activation of Bax and Bak. While the pore-forming proteins tend to be constitutively expressed, the regulatory factors are under transcriptional and posttranslational controls [10–15]. Thus, the canonical function of proapoptotic BNip3 and Nix in programmed cardiomyocyte death is to sense cardiac stress and initiate outer mitochondrial membrane pore formation through activation of Bax and Bak. The latter factors permit mitochondrial cytochrome c release into the cytosol, leading to caspase activation and apoptosis (Fig. 1, left).
Fig. 1.
Schematic diagram depicting canonical apoptotic function of Nix and BNip3 and atypical MTPT opening by Nix. Left, Nix and BNip3 localize to mitochondrial outer membranes via carboxyl-terminal hydrophobic domains and stimulate membrane permeabilization by Bax and/or Bak. Cytochrome c released into the cytoplasm binds in a functional “apoptosome” with other factors to activate initiator caspase 9, resulting in cascade activation of effector caspase 3, DNA fragmentation, and apoptosis. Right, Nix localizes to endoplasmic and sarcoplasmic reticulum where it increases reticular calcium via a poorly defined mechanism. Reticular calcium transferred to mitochondria through calcium “hot spots” is takenupbythe mitochondrial calcium uniporter and opens mitochondrial permeability transition pores, resulting in osmotic swelling and mitochondrial rupture. Although cytochrome c is released by this mechanism, cell death occurs because of metabolic shutdown, not apoptosis
Although BNip3 and Nix are structurally and functionally similar, they are regulated in the heart by entirely distinct stimuli: BNip3 is transcriptionally upregulated in the heart by hypoxia [14, 16]. Although Nix is also upregulated by hypoxia in some tissues [17–19], this is not the case in the heart or in cultured cardiomyocytes. Instead, myocardial Nix upregulation appears to be a specific transcriptional response to pathological hypertrophy and is mediated by activation of Gq/protein kinase C signaling pathways [13, 15].
Forced Expression of BNip3 and Nix is Sufficient to Induce Dilated Cardiomyopathy in Mice
Our laboratory employed conventional and conditional cardiac transgenesis to determine whether the increased BNip3 or Nix observed in ischemic and hypertrophied hearts was sufficient to induce the cardiomyocyte death and ventricular remodeling that lead to dilated cardiomyopathies in these syndromes. Our initial foray into this area of investigation expressed Nix in mouse hearts using the standard α-myosin heavy chain (αMHC) transgene promoter [13]. This widely used promoter confers cardiomyocyte-specific expression that begins shortly after the birth of the mouse pup. αMHC-Nix mice were normal at birth (i.e., just before Nix began to be expressed) but failed to thrive thereafter, and all died at the age of 7–8 days. Echocardiography performed on day6 revealed dilated and hypocontractile left ventricles, and TUNEL staining of these hearts showed massive cardiomyocyte apoptosis (apoptotic indices of 15–20%). Thus, forced cardiomyocyte Nix expression is sufficient to cause rapidly progressive apoptotic cardiomyopathy and premature death. In a follow-up study that used a conditional (tetracycline suppressible) cardiac Nix expression system, we observed that cardiomyocyte apoptosis was more prevalent and dilated cardiomyopathy more severe after neonatal than adult Nix expression. However, simultaneous superimposition of microsurgical aortic coarctation and Nix induction in the adult hearts greatly accelerated the progression to heart failure [20]. These studies demonstrated a connection between cardiomyocyte growth and programmed death, revealing that the susceptibility of cardiomyocytes to programmed death is increased to a similar degree by normal postnatal cardiac growth and pathological cardiac hypertrophy. Based on the studies of Nix regulation and the consequences of transgenic Nix expression on the heart, we suggested that Nix coordinates transcriptional and physiological cues leading to cardiomyocyte drop-out and left ventricular remodeling [21].
We have also examined the in vivo consequences of cardiomyocyte BNip3 overexpression using a conditional, tetracycline-suppressible cardiac expression system [22]. Whereas the heart was exquisitely sensitive to Nix-mediated cardiomyocyte death (see above), neonatal BNip3 expression at approximately 50 times the normal myocardial levels increased the rate of cardiomyocyte TUNEL positivity to only ~1%. However, simultaneous superimposition of microsurgical left anterior descending coronary artery occlusion and BNip3 induction in adult hearts created markedly larger myocardial infarctions (~35% of the left ventricle) than in identically treated nontransgenic littermates (~20% of the left ventricle) [22]. These results show that forced BNip3 expression is sufficient to modestly increase cardiomyocyte apoptosis in neonatal hearts but not to cause the aggressive cardiomyopathy seen with Nix expression at similar levels and under identical conditions. We concluded that transcriptional upregulation is only one factor that leads to BNip3-induced cardiomyocyte death and that posttranslational regulation, either through ischemia-stimulated translocation to mitochondria [23, 24] or nuclei [25, 26], is required to fully activate BNip3 death promoting activity in the heart.
Ablation of BNip3 and Nix Prevents Ventricular Remodeling after MI and Pressure Overload, Respectively
Since BNip3 and Nix are transcriptionally upregulated in the heart in a stimulus-specific manner, we hypothesized that preventing their expression might have benefits in the particular conditions wherein they are induced. Our approach was to create BNip3 and Nix gene knockout mice, subject them to the specific stimulus that normally induces the ablated gene (ischemia–reperfusion for BNip3 and pressure overload or Gq-mediated hypertrophy for Nix), and compare cardiomyocyte apoptosis, ventricular remodeling, and cardiac function between the knockout mice and identically treated wild-type controls.
Germ-line ablation of the BNip3 gene resulted in a mouse that was without any detectable cardiac, hematological, neurological, or other phenotype at baseline. Accordingly, we felt comfortable analyzing its response to the stress of cardiac ischemia–reperfusion injury [22]. We employed a model of reversible left anterior descending coronary artery ligation that mimics early reperfusion after myocardial infarction. BNip3 ablation did not affect infarct size. This is not surprising because for BNip3 ablation to have an effect, it must first be expressed, and it is only expressed and activated in the heart in response to ischemia (see above). Because cardiomyocytes within the infarct area die a necrotic death shortly after interruption of blood flow and before they can regulate BNip3 gene and protein expression, BNip3 ablation does not diminish infarct size. In the peri-infarct region, however, cardiomyocytes are injured rather than killed outright and presumably survive long enough to induce BNip3 (that kills them later on). Indeed, in the days following the primary ischemia–reperfusion injury, cardiomyocyte apoptosis was induced in both the peri-infarct region and the remote myocardium but was ~50% less prevalent in BNip3 null mice, compared to wild-type controls. Decreased cardiomyocyte apoptosis caused by BNip3 ablation was associated with striking declines in left ventricular remodeling and improved cardiac performance, as measured by magnetic resonance imaging. Together, these results show that BNip3 is an important factor mediating cardiomyocyte loss and adverse ventricular remodeling in postischemic hearts.
Because Nix is upregulated by cardiac hypertrophy [13, 15], we postulated that Nix gene ablation would prevent ventricular remodeling and the progression to heart failure in chronically pressure-overloaded hearts. However, germ-line Nix knockout mice produced by three independent groups, including our own, exhibited a profound hematological phenotype that was a potential confounder for our proposed cardiac studies: increased blood reticulocyte counts associated with decreased in vivo and in vitro apoptosis of splenic erythroid precursors and extreme hypersensitivity to erythropoietin [27–29]. For this reason, we employed a Nkx-2.5 Cre-lox strategy to create mice in which the Nix gene was selectively deleted in cardiac myocytes. The cardiac-specific Nix knockout mice then underwent surgical transverse aortic banding to create chronic pressure overloading [30]. Pressure overloaded mouse hearts develop characteristically increased cardiomyocyte apoptosis that is associated with progressive ventricular dilatation and cardiac failure. However, cardiac-specific Nix knockout mice exhibited only half as much cardiomyocyte apoptosis (TUNEL positivity) and developed less late replacement fibrosis, than pressure overloaded hearts from control mice. Furthermore, while the pressure overload hypertrophy response was not affected by cardiomyocyte Nix ablation, the progressive ventricular dilation and wall thinning that typically follow after aortic banding were largely prevented, which preserved systolic function. These findings showed that apoptosis is a cause of functional cardiac decompensation after pressure overload and established that Nix is a critical inducible factor mediating this response.
Nix Induces Apoptotic and Nonapoptotic Forms of Death
There is no question that Nix induces conventional caspase-dependent apoptosis through mitochondrial outer membrane permeabilization. In our first studies, we demonstrated mitochondrial localization of Nix and showed that it induces mitochondrial cytochrome c release, caspase activation, and oligonucleosomal DNA degradation, leading to cell death [13]. More recently, however, we observed that only ~80% of transfected Nix localizes to mitochondria in neonatal rat cardiomyocytes or HEK293 cells, with the remainder localizing to the endoplasmic reticulum (ER)/sarcoplasmic reticulum (SR) [31]. In the same studies, we noted that transcriptional upregulation of Nix after experimental pressure overload was associated with preferential increases in SR- (rather than mitochondrial-) associated Nix. The SR is the dominant site for cardiomyocyte calcium storage, and we also found that ER/SR-localized Nix can affect intracellular calcium stores, as had previously been described for Bcl2 and Bax [32–34]. In the case of Nix, there was a direct relationship between Nix and ER/SR calcium: Nix overexpression increased reticular calcium stores, while Nix ablation decreased reticular calcium stores [31].
Calcium transport from reticulum to mitochondria through junctional “calcium hot-spots” [35] is a potent stimulus for the mitochondrial permeability transition, leading to a form of cell death referred to as “programmed necrosis” [36, 37]. Accordingly, we hypothesized that reticular–mitochondrial cross-talk stimulated by ER/SR-localized Nix might induce nonapoptotic, or “necrotic”, cell death that had previously been attributed to Nix, but never mechanistically defined [38]. To examine this notion, we created and expressed mitochondria-specific, ER/SR-specific, and cytosolic Nix mutants and assessed in vitro cell viability, caspase activation, and the mitochondrial inner membrane potential, Δψm (which is lost upon opening of the permeability transition pore) [31]. The nontargeted cytosolic Nix mutant did not induce cell death. Mitochondrial-directed Nix produced cell death associated with caspase activation but with no mitochondrial permeability transition, i.e., Δψm was maintained. In contrast, reticular-directed Nix produced cell death with opening of the mitochondrial permeability transition pore (MPTP), as well as with caspase activation (a secondary consequence of cytochrome c released after MPTP opening and outer mitochondrial membrane rupture) [38]. We interpreted these results as evidence that Nix stimulates conventional intrinsic pathway apoptosis but that also induces programmed necrosis by localizing to ER/SR, increasing reticular calcium stores and calcium delivery to mitochondria and causing mitochondrial permeability pore transition (Fig. 1, right). The in vivo relevance of these events to Nix-mediated cardiomyocyte death and ventricular remodeling has not yet been established.
Mitochondrial Pruning: The Essential Function of Cardiac-Expressed Nix and BNip3
An important unresolved question about cardiac-expressed programmed cell death factors is “Why are they in the heart in the first place?” At best, the heart has limited ability to regenerate lost cardiac myocytes [6]. There seems to be no evolutionary advantage to programmed cardiomyocyte death, and the studies reviewed above show only benefits from deleting BNip3 or Nix in the heart. The conventional response to the “Why?” question is that apoptosis is necessary for normal embryonic development of the heart, and while temporally and spatially restricted apoptosis is observed during cardiac development, our studies showed that germ-line deletion of Nix [27, 30], BNip3 [22], and even both in combination (see below) has no effect on cardiac development or young adult heart structure. Thus, these two death genes seem to have no critical developmental function in the heart.
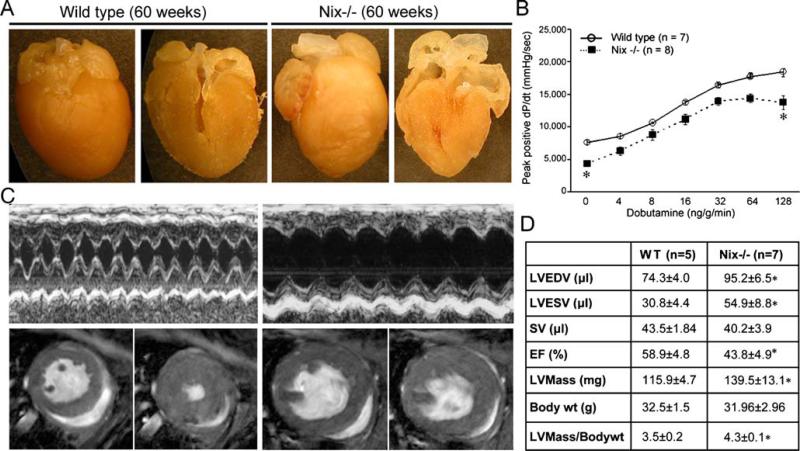
The nuances of cardiac physiology are sometimes best revealed by instruments less crude than acute aortic banding or experimental myocardial infarction. We considered that long-term observation of the Nix and BNip3 knockout models might reveal a different aspect of the phenotype, and therefore, we maintained a small cohort of these mice for over a year, with periodic noninvasive assessment of cardiac size and contractility. These heretofore unpublished studies revealed that hearts of BNip3 knockout mice were structurally and functionally normal throughout the study period, i.e., in mice of all ages. By contrast, germ-line Nix knockout mice developed cardiac enlargement with depressed left ventricular contractile performance at 60 weeks, compared to age-matched wild-type controls (Fig. 2a, b). Interestingly, cardiac enlargement in senescent Nix knockout mice was not associated with typical wall thinning by echocardiography or magnetic resonance imaging (MRI; Fig. 2c). Indeed, left ventricular mass assessed by MRI was significantly increased (Fig. 2d).
Fig. 2.
Cardiomegaly and contractile depression in 60-week-old Nix knockout mice. a Images of intact (left panels) and halved (right panels) hearts from 60-week-old wild-type and germ-line Nix knockout (Nix−/−). b Invasive hemodynamic assessment of isovolumic contractility as a function of dobutamine dose in 60-week-old mice. c Representative M-mode echocardiograms (top) and MRI (bottom) demonstrating left ventricular enlargement and diminished ejection performance but absence of wall thinning. For MRI, left panels are diastole, right panels are systole. d Quantitative morpho-metric and functional data from MRI studies. LVEDV left ventricular end diastolic volume, LVESD left ventricular end systolic volume, SV stroke volume, EF ejection fraction
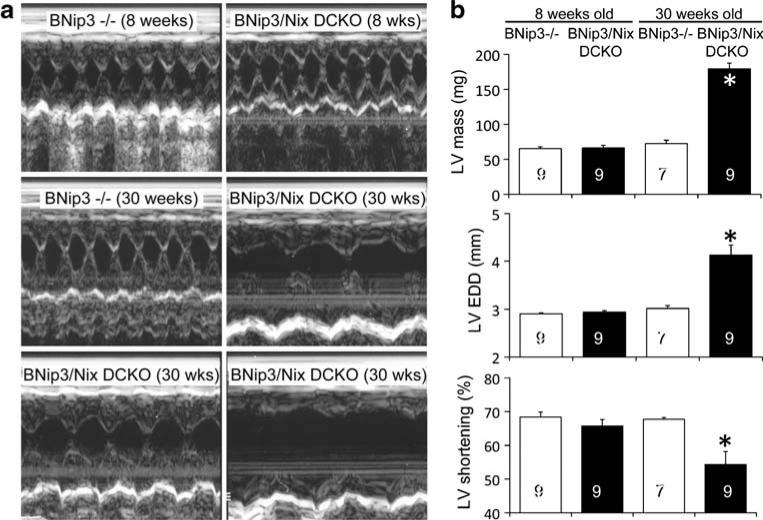
The above observation that absence of Nix produces cardiac enlargement with aging was made in germ-line Nix knockout mice. It was therefore possible that cardiomegaly and cardiomyopathy developed as a secondary response to some systemic influence, such as the hematological abnormalities that are characteristic of this gene knockout [27, 28], rather than because of a direct protective effect of Nix in cardiac myocytes. We wanted to know whether loss of Nix specifically from cardiac myocytes would reproduce this pathology and, in so doing, demonstrate a cell-autonomous protective function for cardiomyocyte-expressed Nix. We also considered that the near functional identity of Nix and BNip3 in mediating cell death [38] might reflect a similar functional redundancy for any putative cardioprotective effect. For these reasons, we initiated a second series of long-term studies in cardiac-specific Nix knockout mice (Nix flox/flox + Nkx2.5 Cre) crossed onto the BNip3 knockout background (BNip3−/−), termed BNip3/Nix double cardiac knockout (DCKO). At 8 weeks of age, echocardiographic cardiac structure and function were normal in both BNip3−/− and BNip3/Nix DCKO mice (Fig. 3a, top and Fig. 3b). However, by 30 weeks, the DCKO mice had developed massive cardiac enlargement with depressed left ventricular ejection performance but without wall thinning (Fig. 3a, middle and bottom, and Fig. 3b). In other words, the DCKO mice developed the same age-dependent cardiac phenotype as did the germ-line Nix knockout mice but in half the time. This finding indicates that BNip3 and Nix have similar or synergistic protective effects on the heart and that these effects are cardiomyocyte autonomous.
Fig. 3.
Age-dependent cardiomyopathy in 30-week-old Nix/BNip3 double cardiac knockout (DCKO) mice. a Representative M-mode echocardiograms from 8-week BNip3−/− and DCKO mice (top), from a 30-week BNip3−/− mouse (middle, left), and from three 30-week DCKO mice (middle, right, and bottom). b Quantitative group echocardiographic data. LVEDD left ventricular end diastolic dimension
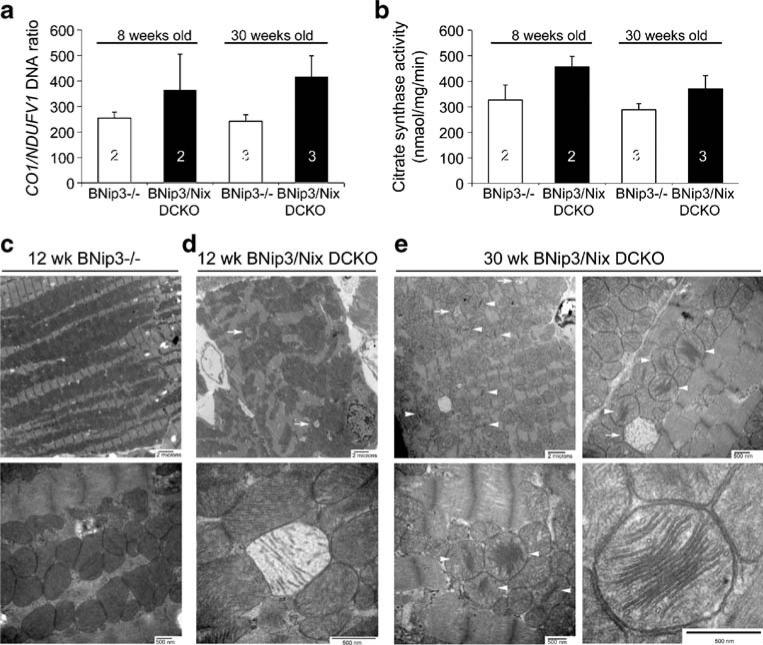
At the cellular and subcellular level, the two known functions of Nix are to stimulate programmed cell death and to stimulate mitochondrial clearance [38, 39]. As reviewed above, the programmed death effect has been demonstrated in a variety of cell types, in vitro and in vivo. To date, mitoautophagy has only been shown in erythroid cells, where elimination of mitochondria before release of newly developed reticulocytes (young red blood cells) into the blood stream appears to be essential to prevent hemolytic anemia. However, uncontrolled mitochondrial proliferation has previously been demonstrated as a cause of cardiomyopathy that is remarkably similar to that which developed in the aging germ-line Nix knockout and DCKO mice [40, 41]. Thus, we examined cardiac mitochondria in young and old DCKO mice: Assays of mitochondrial DNA content (RT-qPCR of mitochondrial cytochrome c oxidase 1 (CO1) DNA compared to nuclear-encoded NADH dehydrogenase (NDUFV1) DNA) and mitochondrial protein (citrate synthase activity) suggested increased mitochondrial content in DCKO hearts at both 8 and 30 weeks of age, compared to normal values in BNip3−/−hearts (Fig. 4a, b). This impression was confirmed by ultrastructural studies: Young (12-week-old) BNip3−/− hearts showed normal mitochondrial size, density, and pattern of distribution within the sarcoplasm, with normal mitochondrial cristae and lamellar structures (Fig. 4c). In contrast, mitochondrial distribution within DCKO hearts of the same age lacked the highly organized pattern that is usual for cardiac myocytes, and there were numerous examples of degenerated mitochondria with almost complete loss of cristae (Fig. 4d, arrows). Strikingly, hearts of older (30-week) DCKO mice showed marked increases in mitochondrial content, with almost complete loss of normal subcellular organization. Mitochondrial size showed dramatic heterogeneity, with both giant and dwarf mitochondria present (Fig. 4e, top left panel). As in young DCKO hearts, degenerated mitochondria were observed (Fig. 4e, arrows). In addition, however, there was widespread atypical mitochondrial morphology consisting of peripheral loss and dilation of cristae, with sparing of the central lamellae (Fig. 4e, arrow heads; Fig. 4e, bottom right panel).
Fig. 4.
Mitochondrial abnormalities in Nix/BNip3 double cardiac knockout mice. a Quantitative PCR of cardiac mitochondrial CO1 and nuclear NDUFV1 gene content. b Myocardial citrate synthase activity. c–e Transmission EM of myocardial samples from different genotypes and ages: c Twelve-week-old BNip3−/−. Top, 6,000×; bottom, 30,000×. d Twelve-week-old DCKO. Top, 6,000×; bottom, 70,000×. e Thirty-week-old DCKO. Top left, 6,000×; top right, 25,000×; bottom left, 30,000×; bottom right, 50,000×
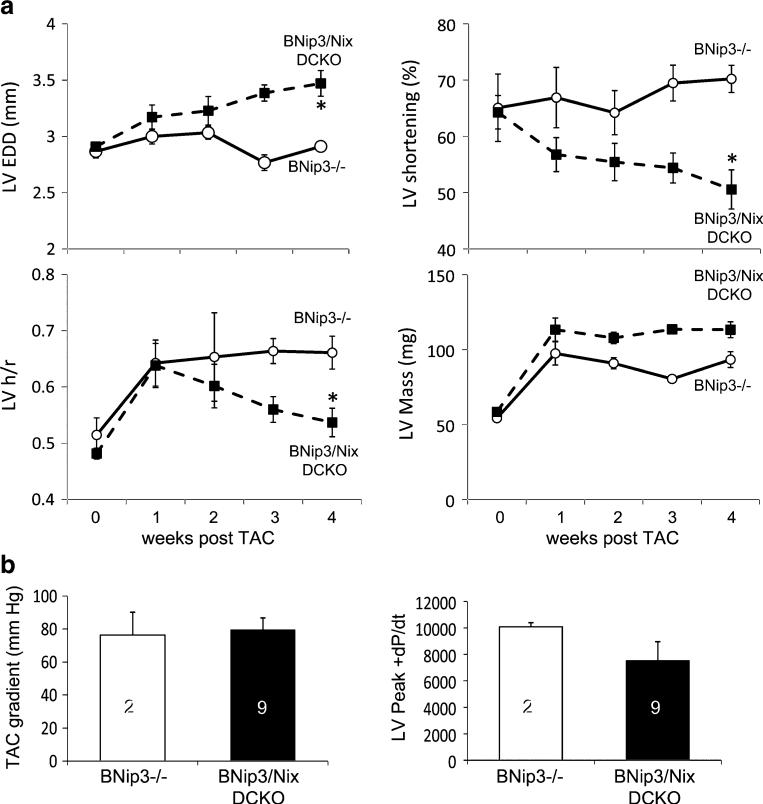
Mitochondrial abnormalities in young DCKO mice that have not yet developed a cardiac phenotype suggested either that the cardiomyopathy was unrelated to the mitochondrial findings or that the phenotype simply was not evident at baseline in younger animals. To differentiate between these possibilities, we performed aortic banding on three 8-week-old BNip3−/− mice (who responded to aortic banding exactly as wild-type mice do [30]) and seven DCKO mice and followed their response to pressure overloading for 4 weeks. This is similar to the experiment in which we previously showed that cardiac-specific ablation of Nix alone decreased left ventricular remodeling 10 weeks after aortic banding [30]. However, with concomitant BNip3 ablation in the DCKO mice, the hearts began to dilate and fail within 1 week of pressure overloading (Fig. 5). Thus, the mitochondrial abnormalities observed with concomitant genetic ablation of both Nix and BNip3 in cardiac myocytes are associated with a dysfunctional response to pressure overloading and ultimately result in spontaneous cardiomyopathy with many of the morphological, functional, and ultrastructural characteristics of cardiomyopathies previously attributed to increased mitochondrial biogenesis [40, 41].
Fig. 5.
Rapid decompensation of Nix/BNip3 double cardiac knockout (DCKO) mice after acute pressure overloading. a Time-dependent changes in echocardiographically determined left ventricular end diastolic dimension (LVEDD, upper left), fractional shortening (upper right), ratio of wall thickness to radius (h/r, bottom left), and mass (lower right) after transverse aortic constriction (TAC). b Transverse aortic gradient (left) and peak positive dP/dt (right) at terminal invasive hemodynamic studies 4 weeks after TAC
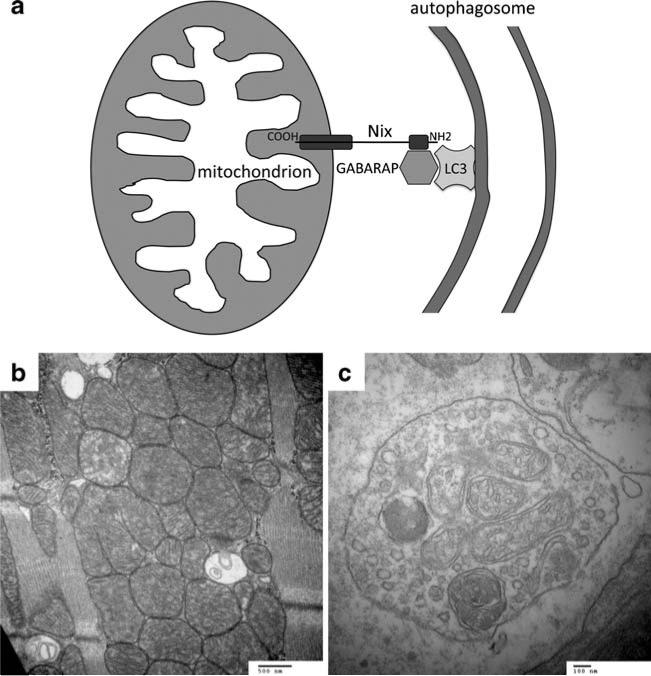
We know of no evidence that Nix or BNip3 regulate mitochondrial biogenesis. However, Nix-mediated mitochondrial clearance during reticulocyte maturation [28, 42] and the current findings suggest that Nix might regulate mitochondrial content by targeting senescent or damaged mitochondria for autophagic elimination. Two nearly simultaneous, but independent, studies have identified the molecular mechanism by which Nix can identify damaged mitochondria and target them for autophagic clearance: recruitment of LC3/GABARAP proteins that promote autophagic membrane formation. In the first report [43], phage display screening for binding partners of the autophagic protein GABARAP (γ-aminobutyric acid receptor-associated protein) identified Nix as a relatively low-affinity (Kd~100 μM) binding factor. These investigators modified the putative GABARAP binding site in Nix, amino acids 31–43 (at the opposite end of the Nix protein from the C-terminal mitochondrial targeting and transmembrane domains), and demonstrated that a critical tryptophan (W36) is essential for Nix–GABARAP binding. Interestingly, BNip3 shares a similar sequence near its amino terminus. A nearly concurrent report identified the Nix-GABARAP interaction in a yeast two-hybrid screen using the autophagy factor Atg8 as bait [44]. Confirmatory studies of human Atg8 family members showed Nix binding to all LC3/GABARAP proteins, except LC3B. As in the first study, the Nix-GABARAP binding domain was localized to the amino terminus (resides 35–38), and a critical tryptophan was identified (W35 of murine Nix). The functional significance of Nix-GABARAP binding for mitophagy was then established in an elegant experiment that used the hallmark reticulocyte mitochondrial clearance defect [28, 29, 41] as an assay for in vivo mitophagy. Bone marrow from Nix null mice was retrovirally transfected to express either wild-type or mutant (W35 and others) Nix and transplanted into irradiated mice. Both wild-type and GABARAP binding-deficient Nix was capable of rescuing the reticulocyte mitochondrial clearance defect under normal conditions. This result suggests that there is a redundant mitochondrial clearance mechanism, which is likely BNip3-mediated mitophagy. However, in mice where reticulocytosis was provoked by inducing a hemolytic anemia with hydralazine (a model we had previously used to exacerbate the reticular–mitochondrial abnormality in Nix−/− mice [41]), the W35 GABARAP binding-deficient Nix mutant gave only an incomplete rescue. This result indicates that Nix-mediated autophagic clearance of mitochondria can be especially important under conditions of cell stress. This proposed mechanism, summarized schematically in Fig. 6 (top), could explain our findings in Nix- and Nix/BNip3-deficient hearts. Indeed, our ultrastructural studies also suggest an abnormality in mitophagy and autophagic vesicle formation in senescent hearts lacking Nix and/or BNip3 (Fig. 6, bottom). However, definitive proof connecting cardiomyopathy with absence of Nix/BNip3, defective autophagic mitochondrial clearance and resulting mitochondrial overabundance, will require experimental models that are not currently available.
Fig. 6.
Schematic diagram depicting proposed molecular mechanism for mitochondrial pruning by Nix and BNip3. a Nix localizes to mitochondrial outer membranes via hydrophobic C-terminal domain (COOH) and to GABARAP via amino terminal domain (NH2). GABARAP acts as a docking protein with autophagic membrane-associated LC3 to target Nix-labeled mitochondrion for autophagic clearance. b Mitochondrial replaced by double membrane delimited autophagic vesicles in a senescent BNip3 knockout mouse heart (30,000×). c High magnification (80,000×) of autophagic vesicle containing mitochondrial remnants
In conclusion, accumulated data indicate that Nix and BNip3 exert multiple effects, all of which involve direct or indirect interactions with mitochondria. Apoptosis is stimulated by Nix/BNip3-mediated outer membrane permeabilization. Mitochondrial permeability transition is likely induced via calcium cross-talk between endoplasmic reticulum/sarcoplasmic reticulum and mitochondria, caused by the ability of Nix to increase reticular calcium content (a function likely shared by BNip3, although this has not formally been demonstrated), and “mitochondrial pruning” may be a constitutive, homeostatic function of these two death proteins, where damaged or senescent mitochondria are recognized and preferentially eliminated by Nix and/or BNip3. The latter function would be expected to have special impact in the heart, which has the highest metabolic rate of any human organ, and is especially rich in mitochondria [45]. In the absence of normal Nix- and BNip3-mediated mitochondrial pruning, we observe accumulation of abnormal mitochondria. If mitochondrial pruning is indeed the necessary physiological function of these two mitochondrial “death genes,” then Nix- (and BNip3-) mediated cardiomyocyte death represents malfunction of normal homeostasis caused by stress-mediated increases in expression. This conceptual paradigm explains the presence of these death proteins in cardiac myocytes and also warrants caution for therapies designed to inhibit programmed cell death by directly attacking these factors.
Acknowledgments
Supported by NIH HL059888. The author would like to express his deep appreciation to the many members of his laboratory who contributed in various ways to the Nix story over the past decade, especially to those who were tolerant of the idea that we should maintain some mice for long periods of time just to see what might develop.
References
- 1.Diwan A, Dorn GW., II Decompensation of cardiac hypertrophy: Cellular mechanisms and novel therapeutic targets. Physiology (Bethesda) 2007;22:56–64. doi: 10.1152/physiol.00033.2006. [DOI] [PubMed] [Google Scholar]
- 2.Berry JJ, Hoffman JM, Steenbergen C, Baker JA, Floyd C, Van Trigt P, et al. Human pathologic correlation with PET in ischemic and nonischemic cardiomyopathy. Journal of Nuclear Medicine. 1993;34:39–47. [PubMed] [Google Scholar]
- 3.Olivetti G, Abbi R, Quaini F, Kajstura J, Cheng W, Nitahara JA, et al. Apoptosis in the failing human heart. Journal of Nuclear Medicine. 1997;336:1131–1141. doi: 10.1056/NEJM199704173361603. [DOI] [PubMed] [Google Scholar]
- 4.Francis GS. Changing the remodeling process in heart failure: Basic mechanisms and laboratory results. Current Opinion in Cardiology. 1998;13:156–161. [PubMed] [Google Scholar]
- 5.Olivetti G, Capasso JM, Sonnenblick EH, Anversa P. Side-to-side slippage of myocytes participates in ventricular wall remodeling acutely after myocardial infarction in rats. Circulation Research. 1990;67:23–34. doi: 10.1161/01.res.67.1.23. [DOI] [PubMed] [Google Scholar]
- 6.Rubart M, Field LJ. Cardiac regeneration: Repopulating the heart. Annual Review of Physiology. 2006;68:29–49. doi: 10.1146/annurev.physiol.68.040104.124530. [DOI] [PubMed] [Google Scholar]
- 7.Hayakawa Y, Chandra M, Miao W, Shirani J, Brown JH, Dorn GW, II, et al. Inhibition of cardiac myocyte apoptosis improves cardiac function and abolishes mortality in the peripartum cardiomyopathy of Galpha(q) transgenic mice. Circulation. 2003;108:3036–3041. doi: 10.1161/01.CIR.0000101920.72665.58. [DOI] [PubMed] [Google Scholar]
- 8.Foo RS, Mani K, Kitsis RN. Death begets failure in the heart. Journal of Clinical Investigation. 2005;115:565–571. doi: 10.1172/JCI24569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dorn GW., II Apoptotic and non-apoptotic programmed cardiomyocyte death in ventricular remodelling. Cardiovascular Research. 2009;81:465–473. doi: 10.1093/cvr/cvn243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Youle RJ, Strasser A. The BCL-2 protein family: Opposing activities that mediate cell death. Nature Reviews. Molecular Cell Biology. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 11.Chen Z, Chua CC, Ho YS, Hamdy RC, Chua BH. Overexpression of Bcl-2 attenuates apoptosis and protects against myocardial I/R injury in transgenic mice. American Journal of Physiology. Heart and Circulatory Physiology. 2001;280:H2313–H2320. doi: 10.1152/ajpheart.2001.280.5.H2313. [DOI] [PubMed] [Google Scholar]
- 12.Condorelli G, Morisco C, Stassi G, Notte A, Farina F, Sgaramella G, et al. Increased cardiomyocyte apoptosis and changes in proapoptotic and antiapoptotic genes bax and bcl-2 during left ventricular adaptations to chronic pressure overload in the rat. Circulation. 1999;99:3071–3078. doi: 10.1161/01.cir.99.23.3071. [DOI] [PubMed] [Google Scholar]
- 13.Yussman MG, Toyokawa T, Odley A, Lynch RA, Wu G, Colbert MC, et al. Mitochondrial death protein Nix is induced in cardiac hypertrophy and triggers apoptotic cardiomyopathy. Nature Medicine. 2002;8:725–730. doi: 10.1038/nm719. [DOI] [PubMed] [Google Scholar]
- 14.Regula KM, Ens K, Kirshenbaum LA. Inducible expression of BNIP3 provokes mitochondrial defects and hypoxia-mediated cell death of ventricular myocytes. Circulation Research. 2002;91:226–231. doi: 10.1161/01.res.0000029232.42227.16. [DOI] [PubMed] [Google Scholar]
- 15.Galvez AS, Brunskill EW, Marreez Y, Benner BJ, Regula KM, Kirschenbaum LA, et al. Distinct pathways regulate proapoptotic Nix and BNip3 in cardiac stress. Journal of Biological Chemistry. 2006;281:1442–1448. doi: 10.1074/jbc.M509056200. [DOI] [PubMed] [Google Scholar]
- 16.Yurkova N, Shaw J, Blackie K, Weidman D, Jayas R, Flynn B, et al. The cell cycle factor E2F-1 activates Bnip3 and the intrinsic death pathway in ventricular myocytes. Circulation Research. 2008;102:472–479. doi: 10.1161/CIRCRESAHA.107.164731. [DOI] [PubMed] [Google Scholar]
- 17.Bruick RK. Expression of the gene encoding the proapoptotic Nip3 protein is induced by hypoxia. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:9082–9087. doi: 10.1073/pnas.97.16.9082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sowter HM, Ratcliffe PJ, Watson P, Greenberg AH, Harris AL. HIF-1-dependent regulation of hypoxic induction of the cell death factors BNIP3 and NIX in human tumors. Cancer Research. 2001;61:6669–6673. [PubMed] [Google Scholar]
- 19.Birse-Archbold JL, Kerr LE, Jones PA, McCulloch J, Sharkey J. Differential profile of Nix upregulation and traslocation during hypxia/ischaemia in vivo versus in vitro. Journal of Cerebral Blood Flow and Metabolism. 2005;25:1356–1365. doi: 10.1038/sj.jcbfm.9600133. [DOI] [PubMed] [Google Scholar]
- 20.Syed F, Odley A, Hahn HS, Brunskill EW, Lynch RA, Marreez Y, et al. Physiological growth synergizes with pathological genes in experimental cardiomyopathy. Circulation Research. 2004;95:1200–1206. doi: 10.1161/01.RES.0000150366.08972.7f. [DOI] [PubMed] [Google Scholar]
- 21.Dorn GW., II Physiologic growth and pathologic genes in cardiac development and cardiomyopathy. Trends in Cardiovascular Medicine. 2005;15:185–189. doi: 10.1016/j.tcm.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 22.Diwan A, Krenz M, Syed FM, Wansapura J, Ren X, Koesters AG, et al. Inhibition of ischemic cardiomyocyte apoptosis through targeted ablation of Bnip3 restrains postinfarction remodeling in mice. Journal of Clinical Investigation. 2007;117:2825–2833. doi: 10.1172/JCI32490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kubasiak LA, Hernandez OM, Bishopric NH, Webster KA. Hypoxia and acidosis activate cardiac myocyte death through the Bcl-2 family protein BNIP3. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:12825–12830. doi: 10.1073/pnas.202474099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kubli DA, Quinsay MN, Huang C, Lee Y, Gustafsson AB. Bnip3 functions as a mitochondrial sensor of oxidative stress during myocardial ischemia and reperfusion. American Journal of Physiology. Heart and Circulatory Physiology. 2008;295:H2025–H2031. doi: 10.1152/ajpheart.00552.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmidt-Kastner R, Aguirre-Chen C, Kietzmann T, Saul I, Busto R, Ginsberg MD. Nuclear localization of the hypoxia-regulated pro-apoptotic protein BNIP3 after global brain ischemia in the rat hippocampus. Brain Research. 2004;1001:133–142. doi: 10.1016/j.brainres.2003.11.065. [DOI] [PubMed] [Google Scholar]
- 26.Burton TR, Henson ES, Baijal P, Eisenstat DD, Gibson SB. The pro-cell death Bcl-2 family member, BNIP3, is localized to the nucleus of human glial cells: Implications for glioblastoma multiforme tumor cell survivial under hypoxia. International Journal of Cancer. 2005;118:1660–1669. doi: 10.1002/ijc.21547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diwan A, Koesters AG, Odley AM, Pushkaran S, Baines CP, Spike BT, et al. Unrestrained erythroblast development in Nix−/− mice reveals a mechanism for apoptotic modulation of erythropoiesis. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:6794–6799. doi: 10.1073/pnas.0610666104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schweers RL, Zhang J, Randall MS, Loyd MR, Li W, Dorsey FC, et al. NIX is required for programmed mitochondrial clearance during reticulocyte maturation. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:19500–19505. doi: 10.1073/pnas.0708818104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sandoval H, Thiagarajan P, Dasgupta SK, Schumacher A, Prchal JT, Chen M, et al. Essential role for Nix in autophagic maturation of erythroid cells. Nature. 2008;454:232–235. doi: 10.1038/nature07006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diwan A, Wansapura J, Syed FM, Matkovich SJ, Lorenz JN, Dorn GW., II Nix-mediated apoptosis links myocardial fibrosis, cardiac remodeling, and hypertrophy decompensation. Circulation. 2008;117:396–404. doi: 10.1161/CIRCULATIONAHA.107.727073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Diwan A, Matkovich SJ, Yuan Q, Zhao W, Yatani A, Brown JH, et al. Endoplasmic reticulum-mitochondria crosstalk in NIX-mediated murine cell death. Journal of Clinical Investigation. 2009;119:203–212. doi: 10.1172/JCI36445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Foyouzi-Youssefi R, Arnaudeau S, Borner C, Kelley WL, Tschopp J, Lew DP, et al. Bcl-2 decreases the free Ca2+ concentration within the endoplasmic reticulum. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:5723–5728. doi: 10.1073/pnas.97.11.5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nutt LK, Pataer A, Pahler J, Fang B, Roth J, McConkey DJ, et al. Bax and Bak promote apoptosis by modulating endoplasmic reticular and mitochondrial Ca2+ stores. Journal of Biological Chemistry. 2002;277:9219–9225. doi: 10.1074/jbc.M106817200. [DOI] [PubMed] [Google Scholar]
- 34.Scorrano L, Oakes SA, Opferman JT, Cheng EH, Sorcinelli MD, Pozzan T, et al. BAX and BAK regulation of endoplasmic reticulum Ca2+: A control point for apoptosis. Science. 2003;300:135–139. doi: 10.1126/science.1081208. [DOI] [PubMed] [Google Scholar]
- 35.Rizzuto R, Pozzan T. Microdomains of intracellular Ca2+: Molecular determinants and functional consequences. Physiological Reviews. 2006;86:369–408. doi: 10.1152/physrev.00004.2005. [DOI] [PubMed] [Google Scholar]
- 36.Henriquez M, Armisen R, Stutzin A, Quest AFG. Cell death by necrosis, a regulated way to go. Current Molecular Medicine. 2008;8:187–206. doi: 10.2174/156652408784221289. [DOI] [PubMed] [Google Scholar]
- 37.Nakayama H, Chen X, Baines CP, Klevitsky R, Zhang X, Zhang H, et al. Ca2+- and mitochondrial-dependent cardiomyocyte necrosis as a primary mediator of heart failure. Journal of Clinical Investigation. 2007;117:2431–2444. doi: 10.1172/JCI31060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dorn GW, II, Kirshenbaum LA. Cardiac reanimation: Targeting cardiomyocyte death by BNIP3 and NIX/ BNIP3L. Oncogene. 2008;27(Suppl 1):S158–S167. doi: 10.1038/onc.2009.53. [DOI] [PubMed] [Google Scholar]
- 39.Zhang J, Ney PA. Role of BNIP3 and NIX in cell death, autophagy, and mitophagy. Cell Death and Differentiation. 2009;16:939–946. doi: 10.1038/cdd.2009.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lehman JJ, Barger PM, Kovacs A, Saffitz JE, Medeiros D, Kelly DP. PPARg coactivator-1 (PGC-1) promotes cardiac mitochondrial biogenesis. Journal of Clinical Investigation. 2000;106:847–856. doi: 10.1172/JCI10268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Russell LK, Mansfield CM, Lehman JJ, Kovacs A, Courtois M, Saffitz JE, et al. Cardiac-specific induction of the transcriptional coactivator peroxisome proliferator-activated receptor g coactivator-1a promotes mitochondrial biogenesis and reversible cardiomyopathy in a developmental stage-dependent manner. Circulation Research. 2004;94:525–533. doi: 10.1161/01.RES.0000117088.36577.EB. [DOI] [PubMed] [Google Scholar]
- 42.Zhang J, Ney PA. NIX induces mitochondrial autophagy in reticulocytes. Autophagy. 2008;4:354–356. doi: 10.4161/auto.5552. [DOI] [PubMed] [Google Scholar]
- 43.Schwarten M, Mohrluder J, Ma P, Stoldt M, Thielmann Y, Stangler T, et al. Nix directly binds to GABARAP: A possible crosstalk between apoptosis and autophagy. Autophagy. 2009;5:690–698. doi: 10.4161/auto.5.5.8494. [DOI] [PubMed] [Google Scholar]
- 44.Novak I, Kirkin V, McEwan DG, Zhang J, Wild P, Rozenknop A, et al. Nix is a selective autophagy receptor for mitochondrial clearance. EMBO Rep. 2010;11:45–51. doi: 10.1038/embor.2009.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goffart S, Kleist-Retzow JC, Wiesner RJ. Regulation of mitochondrial proliferation in the heart: Power-plant failure contributes to cardiac failure in hypertrophy. Cardiovascular Research. 2004;64:198–207. doi: 10.1016/j.cardiores.2004.06.030. [DOI] [PubMed] [Google Scholar]