Abstract
Steroids play fundamental roles regulating mammalian reproduction and development. Although sex steroids and their receptors are well characterized in vertebrates and several arthropod invertebrates, little is known about the hormones and receptors regulating reproduction in other invertebrate species. Evolutionary insights into ancient endocrine pathways can be gained by elucidating the hormones and receptors functioning in invertebrate reproduction. Using a combination of genomic analyses, receptor imaging, ligand identification, target elucidation, and exploration of function through receptor knockdown, we now show that comparable progesterone chemoreception exists in the invertebrate monogonont rotifer Brachionus manjavacas, suggesting an ancient origin of the signal transduction systems commonly associated with the development and integration of sexual behavior in mammals.
Keywords: receptor, imaging steroids
Sex steroids and their receptors have been well studied in vertebrate animals, but much less is known about the mechanisms by which they regulate physiology and behavior in invertebrates (1). Recent gene sequencing, bioinformatics, and protein synthesis approaches suggested that modern nuclear steroid receptors evolved from an ancient receptor that arose 0.6–1.2 billion y ago, before a common bilateral ancestor diverged into the deuterostomes and protostomes (2). This ancient receptor is postulated to have been activated by estrogen, the terminal product in steroid biosynthesis, with intermediates progesterone and testosterone acquiring uses as hormones later in evolutionary history (3). The discovery of estrogen receptor homologs in mollusks (4) and annelids (5) indicated that Lophotrochozoan lineages have not lost this type of hormone chemoreception, despite the use of nonandrogen steroid hormones in Ecdysozoans (e.g., ecdysteroids in insects; dafachronic acids in Caenorhabditis elegans). Recently, an estrogen-like receptor was identified in C. elegans (6), supporting the hypothesis that steroid receptors are ancient and widespread. In addition, progesterone activates nonnuclear, membrane-associated receptors in vertebrates and invertebrates with impacts on behavior and reproduction (7). However, substantial gaps still remain in the phylogenetic distribution of sex steroid receptors in many invertebrates and their functions in sexual differentiation, development, reproduction, and behavior are unclear.
The invertebrate monogonont rotifer Brachionus manjavacas belongs to the Lophotrochozoa, one of the three major animal clades whose origin predates the Cambrian period 543 million years ago (8). An essential attribute of monogonont rotifers is their ability to reproduce both asexually and sexually. Although this flexible reproductive strategy is central to their evolutionary success, little is known about how reproduction is regulated in rotifers or the molecules involved in reproductive signaling in most nonarthropod invertebrates (9).
The switch from asexual to sexual reproduction in B. manjavacas is triggered in a quorum sensing process by a secreted pheromone called the mixis-inducing protein (10). N-terminal sequencing of 17 amino acids from the mixis-inducing protein established that this fragment was identical to a putative steroidogenesis-inducing protein reported from humans (11). This observation led to the hypothesis that steroid hormones may be involved in regulating sexual reproduction in B. manjavacas. We were also encouraged by the fact that the sex steroid receptor family is ancient, arising early in metazoan evolution (2). Genome mining of a rotifer cDNA library led to the identification of a 799 nucleotide sequence (GenBank accession no. FJ829246) with 60–80% homology to multiple membrane-associated progesterone receptors, including that of the sea urchin Strongylocentrotus purpuratus and several Drosophila species. The presence of a progesterone receptor in B. manjavacas led us to explore its role in endocrine signaling and reproductive physiology.
Results
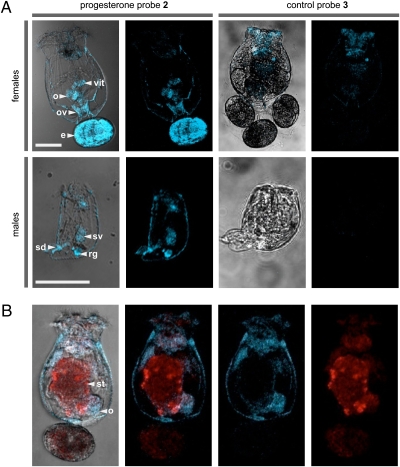
We designed and synthesized a molecular probe suitable for both histological analyses in live rotifers and subsequent protein isolation studies by immunoaffinity methods. By installing a 7-dimethylamino-4-coumarin tag (12), also called an immunoaffinity fluorescent (IAF) tag (13), onto progesterone (Fig. 1, 1), the resulting probe (Fig. 1, 2) could be used for both receptor imaging and in vitro isolation of a progesterone-binding receptor in B. manjavacas because of the availability of a selective monoclonal antibody (mAb) against the IAF tag. Incubation of live and dead rotifers with 2 consistently resulted in its localization in reproductive organs, including the ovaries, vitellarium (yolk gland), oviduct, and egg in females, and the seminal vesicle, rudimentary gut, and sperm duct in males (Fig. 2A). Eggs attached to females fluoresced only when punctured, suggesting that the eggshell prevented probe uptake. Using the natural auto-fluorescence of the algal food Tetraselmis suecica (excitation at 488 nm) to compare with localization of 2 (excitation at 375 nm), we confirmed that 2 was not localizing in the digestive system as a result of ingestion (Fig. 2B). Furthermore, male rotifers do not feed and lack stomachs, providing evidence that the staining of male reproductive organs is due to the membrane permeability of 2 rather than digestive absorption.
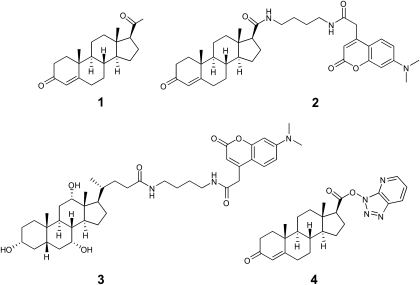
Fig. 1.
Progesterone (1), and a panel of probes including the IAF-labeled progesterone probe 2, an IAF-labeled control steroid probe 3, and a reactive steroid probe 4.
Fig. 2.
Imaging studies with the rotifer Brachionus manjavacas. (A) Confocal fluorescence microscopic images depicting rotifers treated with progesterone probe 2 or control probe 3. (B) Two-color confocal images showing a female rotifer fed T. suecica then treated with 2; T. suecica autofluoresces in the red channel, and 2 fluoresces in the blue channel. (Scale bars: 100 μm.) Blue and red fluorescence were collected with excitation at 375 ± 10 nm and emission at 458 ± 10 nm and excitation at 488 ± 10 nm and emission at 518 ± 10 nm, respectively. Organelles are noted by: vit, vitellarium (yolk gland); o, ovaries; ov, oviduct; e, egg; sv, seminal vesicle; rg, rudimentary gut; st, stomach; sd, sperm duct.
The specificity of the rotifer progesterone receptor for progestin-like molecules was investigated. An IAF-labeled control steroid probe (Fig. 1, 3) was synthesized in a similar method as 2 in Appendix S1, Scheme S1, but using cholic acid as a starting material (Appendix S1, Scheme S2). Imaging experiments with live rotifers resulted in a lack of localization of 3 (Appendix S1, Scheme S2) or a dye control (Appendix S1, Fig. S1) in the reproductive organs of either B. manjavacas females or males (Fig. 2A). These results suggest that the rotifer progesterone receptor exhibits strong specificity for its ligand.
These observations led us to screen for the presence of steroid hormones in rotifers. B. manjavacas biomass was extracted and lipophilic (i.e., steroid-containing) fractions were subjected to normal-phase high performance liquid chromatography (HPLC). Three sterols were isolated in sufficient quantities for characterization, identified as campesterol (Appendix S1, Fig. S2, 5) at 18 mg/g dry biomass, campesta-5,7-dien-3-β-ol (Appendix S1, Fig. S2, 6) at 12 mg/g, and 5-α-campest-7-en-3-β-ol (Appendix S1, Fig. S2, 7) at 4.1 mg/g (Appendix S1, Fig. S2). Campesterol (5) is a known plant sterol found in the green alga T. suecica, the food of B. manjavacas (14); thus, it was concluded that sterols 5–7 were of dietary origin. When rotifer extracts were screened by using a progesterone enzyme immunoassay (EIA), we found that 1 was present at a concentration of 3.1 ng/g dry biomass. To further support these data, we analyzed partially purified rotifer extracts by using LC-tandem mass spectrometry (LC-MS/MS) in multiple reaction monitoring mode and unequivocally showed that 1 was present at a concentration of 4.4 ng/g dry rotifer biomass (Appendix S1, Fig. S3), comparable to the progesterone EIA result.
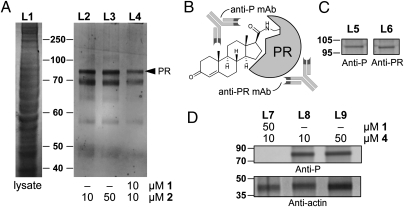
We then applied an immunoprecipitation (IP) technique (13) to screen for progesterone-binding proteins in crude lysates of B. manjavacas. Lysates were incubated with 2 and immunoprecipitated with an Affigel-Hz resin containing a mouse mAb engineered with affinity against the IAF tag. Two bands at ≈70 and 82 kDa were observed by SDS/PAGE over several repeated experiments (Fig. 3A, L2 and L3). These bands were confirmed to arise from selective binding to 2 because the addition of 1 to the lysate before IP reduced the isolation of the two proteins (Fig. 3A, L4).
Fig. 3.
Immunoprecipitation (IP) of a rotifer progesterone receptor (PR). (A) SDS/PAGE analysis depicting the IP of B. manjavacas lysate (L1) in the presence of progesterone probe 2 (L2 and L3). IP of lysates treated with progesterone (1) showed a reduction in the isolated proteins (L4). (B) Reactive probe 4 was designed to form a covalent adduct with a PR by formation of an amide with the side-chain amine of a lysine residue. The resulting covalent adducts can be selectively identified by using an anti-progesterone mAb (anti-P) and can be validated by using an anti-progesterone receptor mAb (anti-PR). (C) Validation of 4 using mammalian MCF-7 cell lysates. Western blot analyses depicting the presence of a 100-kDa protein in MCF-7 cell lysates incubated with 4. Comparable bands corresponding to the MCF-7 PR were identified after incubation with an anti-P mAb in L5 or an established anti-PR mAb in L6. (D) Western blot analysis of B. manjavacas lysate treated with 4 showed a single major band at 82 kDa after treatment with 4 (L8 and L9). This band could be ablated by the cotreatment with 1 (L7). Loading control was conducted with actin (A).
Tryptic-digest LC-MS/MS analyses were conducted on crude IP mixtures incubated with 2 and compared with crude lysates obtained without 2. Over three repetitions, we identified two key tryptic fragments with m/z of 1,239.57 and 1,589.74 appearing only in IP lysates treated with 2. Subsequent in-gel digestion gave comparable fragments m/z of 1,239.12 and 1,589.48 from the band at 82 kDa (Fig. 3A, PR). Analyses of the digested peptides indicated that these masses arose from the respective peptides EWEMQFLEK and DFYGPGGPYSVFAGR (Appendix S1, Fig. S4), as predicted by the nucleotide sequence of FJ829246, and were up to 92% identical to peptide sequences observed in related progesterone receptors (BLAST searches provided in Appendix S1, Fig. S5). These data provided strong evidence for the presence of a progesterone receptor within B. manjavacas.
To further support this conclusion, we designed and synthesized a probe containing a reactive 7-aza-benzotriazole ester (Fig. 1, 4 and Appendix S1, Scheme S3), with the goal of covalently labeling the active site of a progesterone receptor through formation of an amide bond by displacement of the 7-aza-benzotriazole ester in 4 via nucleophilic side-chain residues proximal to the steroidal binding pocket of the receptor. This approach was validated by Western blot analysis of a human cell lysate (MCF-7), which delivered a band at 100 kDa when treated with 4 and developed with an anti-progesterone mAb (anti-P mAb). A comparable band corresponding to the MCF-7 progesterone receptor was identified after staining with an established anti-progesterone receptor mAb (Fig. 3C, L5 and L6), thereby confirming the assignment. Reactive probe 4 was presented to protein lysates prepared from whole B. manjavacas, which were then immunoprecipitated by using the anti-P mAb to isolate proteins that bound to or were modified by 4 (Fig. 3D). Over multiple repetitions, Western blot analyses identified a band at 82 kDa (Fig. 3D, L8 and L9) corresponding to the rotifer progesterone receptor, which could be blocked by pretreating the lysates with 1 (Fig. 3D, L7).
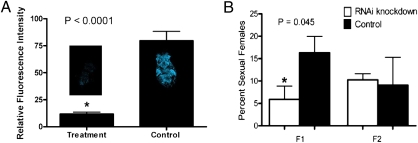
The function of the rotifer progesterone receptor was explored by using RNA interference (RNAi) by transfection of female rotifers with dsRNA representing a 507-bp fragment of the progesterone receptor gene (FJ829246). Quantitative real-time PCR demonstrated a 62% decrease (n = 12) in expression of this gene relative to PBS-control-treated animals. Using confocal fluorescence microscopy, we observed an 85% reduction in binding of 2 (n = 6, two sample, one-tailed paired Student's t test, P < 0.0001; Fig. 4A) when compared with rotifers treated with dsRNA from the rotifer elongation factor gene (control). The decrease in binding of 2 and, therefore, progesterone in RNAi experiments confirms that the rotifer gene FJ829246 functions as a progesterone receptor.
Fig. 4.
RNAi experiments with female rotifers transfected with dsRNA from the rotifer progesterone receptor gene (treatment), dsRNA from the rotifer enlongation factor gene (control for A), or with PBS (control for B). (A) Relative fluorescence intensity of female rotifers incubated with progesterone probe 2 (n = 6, two sample, one-tailed paired Student's t test, P < 0.0001; error bars denote SE). (B) Percent sexual females in first-generation rotifer daughters (F1) and second generation rotifer daughters (F2) (n = 4, two sample, one-tailed paired Student's t test, P = 0.045 for F1; n = 4, two sample, one-tailed paired Student's t test, P = 0.434 for F2; error bars denote SE).
RNAi experiments were also used to gain mechanistic insights of the rotifer progesterone receptor and effects on rotifer reproduction. Females transfected with the dsRNA progesterone receptor gene fragment or with PBS buffer were incubated for 72 h, followed by counting of sexual and asexual females. Rotifers exposed to the dsRNA rotifer progesterone receptor gene fragment experienced a 64% decrease in induction of sexuality in first-generation daughters (F1) when compared with controls (n = 4, two sample, one-tailed paired Student's t test, P = 0.045), but no significant effect on induction of sexuality was observed in second-generation females (F2) after an additional 96 h of incubation (n = 4, two sample, one-tailed paired Student's t test, P = 0.434; Fig. 4B). Mating behavior of males toward sexual females was also explored by counting the number of males circling females, and no significant difference was observed between RNAi treatments and controls (36% circling for RNAi treatments, 39% circling for buffer-only controls; n = 3, two-sample, one-tailed paired Student's t test, P = 0.374). Almost twice as many asexual maternal females failed to produce clonal daughters when transfected with the dsRNA rotifer progesterone receptor gene fragment compared with controls (n = 9, two sample, one-tailed Student's t test, P = 0.022).
Discussion
These studies identify a rotifer progesterone receptor and provide evidence that supports progesterone chemoreception as a key regulatory step involved in rotifer reproduction. Using a dual fluorescent and affinity probe (2), we demonstrated localization of a progesterone-specific chemoreceptor to sex organs within female and male rotifers, while also establishing ligand-selective binding using a control steroid probe (Fig. 2). These data are consistent with specific recognition of 3-keto steroids by their receptor, as illustrated by progesterone binding interactions to the human progesterone receptor (15). Because of the structural similarity of progesterone (1) and testosterone, 2 may have been expected to detect testosterone receptors in our imaging experiments. However, testosterone receptors were not detected in the transcriptomes of either female or male B. manjavacas, although these transcriptomes are not yet fully sequenced.
Two complementary methods, progesterone EIA and LC-MS/MS, established the presence of 1 in rotifer tissues at 3–4 ng/g dry biomass (Appendix S1, Fig. S3), consistent with its role as a natural ligand for the progesterone receptor. Progesterone (1) is present in mammals at very low levels, generally <2 ng/mL in human blood with the exception of pregnant females (16), comparable with the low concentration of progesterone observed in rotifers. Although we conclude that rotifers use progesterone, the involvement of additional very closely related invertebrate steroids, such as 6β-hydroxyprogesterone found in hemolymph of the crayfish Astacus leptodactylus (17), cannot be ruled out.
In vitro target elucidation studies with fluorescent probe 2 and reactive probe 4 suggested the presence of an 82-kDa rotifer progesterone receptor through the use of two affinity methods (18) and were supported by the peptides EWEMQFLEK and DFYGPGGPYSVFAGR found via MS/MS analyses (Fig. 3). Although the 82-kDa band is consistent with the size of many membrane progesterone receptors belonging to the progestin and adiponectin receptor family (PAQR; ref. 19), it did not match the predicted ≈20-kDa size of our rotifer nucleotide sequence (FJ829246). The rotifer progesterone receptor gene is most closely related to progesterone receptor membrane components (PGRMC); previously identified PGRMCs range in size from 18 to 22 kDa but form complexes with larger proteins such as cytochrome b5, thus resulting in complexes of much higher molecular masses (20, 21). The observed 82-kDa band may represent a protein complex containing the rotifer PGRMC or a larger rotifer membrane progesterone receptor related to the PAQR family. Complete identification of the gene sequences for additional steroid receptors in B. manjavacas will be available upon the full sequencing of its genome.
RNAi knockdown of the gene FJ829246 further confirmed the identification of the rotifer progesterone receptor, with a 62% decrease in gene expression measured by qPCR and an 85% decrease in progesterone binding to the receptor in intact rotifers (Fig. 4A), establishing the function of our gene. The 64% decrease in induction of sexuality for daughters of female rotifers exposed to RNAi (Fig. 4B), whereas other biological functions such as mating behavior were unaffected, indicated that the progesterone signaling is necessary for hormonal regulation in these animals. Furthermore, RNAi-treated maternal females showed a doubling of the rate of reproductive failure among asexual females. Together, these data suggest that the rotifer progesterone receptor plays a central role in rotifer sexual reproduction, including the transition from asexual to sexual reproduction. These findings build on the growing evidence of evolutionary conservation of progesterone receptor function and mechanism over a wide span of eukaryotes (22, 23).
Collectively, our data demonstrate that progesterone (1), a well-studied hormone with known importance in mammalian reproduction, plays a central role in reproduction of the ancestral microinvertebrate B. manjavacas (Rotifera) and, thus, progesterone and its receptor exhibits a conservation of function over a broad expanse of animal phylogeny. This study provides further evidence to support the ancient origin of steroid hormonal regulation and suggests that endocrine regulation of mammalian reproduction may be derived from primitive regulatory pathways.
Materials and Methods
General Methods.
Commercially available reagents were purchased from Sigma-Aldrich, AK Scientific, or VWR Scientific and used as received unless otherwise noted. All reactions were performed under a nitrogen atmosphere unless otherwise noted. Reactions were monitored by using TLC using Silica Gel 60 F254 plates from EMD Chemicals and visualized with a UV lamp at 254 nm or by staining with Hannesian's stain [90 mL of water, 2.5 g of (NH4)6Mo7O24 · 4H2O, 1 g of Ce(NH4)4(SO4)4 · 2H2O, 10 mL of concentrated H2SO4].
Compound Purification and Analyses.
Flash chromatography was performed by using Geduran Silica gel 40- to 63-μm particle size from EMD Chemicals. HPLC was performed by using an Alltima C18 column (10 × 250 mm, 5 μm) from Alltech Associates with a linear gradient of acetonitrile and water for IAF-labeled control steroid probe synthesis. NMR spectra were recorded at 500 MHz and 125 MHz for 1H and 13C-NMR, respectively, and referenced to residual CHCl3 (7.24 and 77.0 ppm, for 1H and 13C, respectively). High-resolution mass spectra (HRMS) were obtained from the mass spectral facilities at Georgia Institute of Technology or the University of California at San Diego.
Rotifer Strains.
We used Brachionus manjavacas (24) as test animals, a species originally collected from the Azov Sea (25), and formerly known as B. plicatilis Russian (11). This species has been cultured continuously in the laboratory since 1983, with periodic collection and storage of resting eggs.
Rotifer Culturing.
Rotifers were obtained by hatching resting eggs in 15 ppt artificial seawater (ASW) made from Instant Ocean sea salts at 25 °C under constant fluorescent illumination of 2,000 lx. Rotifers were fed the green alga T. suecica cultured in f/2 medium (26, 27) at 25 °C and ASW in 5-L bags under constant fluorescent illumination. Flask cultures were inoculated by transferring about 100 females into a dense T. suecica culture of 250 mL and gently aerating at 25 °C. Males were obtained from the 5 day-old cultures in log phase growth.
Confocal Fluorescence Microscopy.
B. manjavacas ovigerous females and males were transferred to 24-well plates in 1 mL of 15 ppt ASW with or without T. suecica, and 1 μg of 2 or 3 was added to each well in DMSO (0.5 mg/mL solution). The rotifers were allowed to incubate with 2 or 3 at 25 °C in the dark for 30 min. The rotifers were anesthetized with 0.5 mL of seltzer water and then fixed with 50 μL of 10% formalin. The rotifers were imaged immediately by using a Zeiss LSM/NLO 510 confocal/multiphoton microscope at Georgia Institute of Technology, with magnifications of ×10 (females) or ×20 (males) with excitiation at 375 ± 10 nm and emission at 458 ± 10 nm, and excitiation at 488 ± 10 nm and emission at 518 ± 10 nm for DAPI and FITC, respectively.
Isolation of Dietary Steroids from B. manjavacas Extracts.
B. manjavacas bodies were exhaustively extracted with MeOH and MeOH/DCM mixtures (1:1 and 1:2). The combined extracts were filtered, reduced in vacuo, and partitioned between MeOH/H2O (9:1) and petroleum ether. The aqueous fraction was adjusted to MeOH/H2O (3:2) and then partitioned against CHCl3. The petroleum ether fraction was separated by normal-phase HPLC (Agilent Zorbax RX-SIL; 9.4 × 250 mm, 5 μm) using a linear gradient of hexanes/ethyl acetate to yield campesterol (5), campesta-5,7-dien-3-β-ol (6), and 5-α-campest-7-en-3-β-ol (7). Crude fractions used in the progesterone EIA were generated by saponification of the petroleum ether fraction using 0.3 M KOH in 90% aqueous MeOH. After heating for 2 h at 90 °C, steroids were extracted by using hexanes, combined, and concentrated in vacuo, and used without further purification.
LC-MS/MS Rotifer Steroid Analysis.
LC-MS/MS analyses were carried out by using normal-phase HPLC fractions generated from the petroleum ether fraction. A Shimadzu LC-10 AD VP binary pump system coupled to a Perkin-Elmer Series 200 autoinjector was interfaced to a 4000 quadrupole linear-ion trap operating in multiple reaction monitoring (MRM) mode. An Ascentis C18 column (1.0 × 150 mm, 3 μm) was held at 30 °C. The effulent was directed to the ion source needle held at 5500 V and a heated desolvation gas of 200 °C. Two MRM channels were optimized with regard to declustering potential (DP), collision energy (CE), and collision exit potential (CXP). They were as follows: m/z, 315.3/109.0; DP, 80 V; CE, 38 V; CXP, 8 V; and m/z, 315.3/97.1; DP, 80 V; CE, 35 V; CXP, 6 V. Serial dilutions of known concentrations of progesterone were used to generate a calibration curve for determination of concentration of progesterone in the extracted sample.
Progesterone EIA.
The progesterone EIA kit (catalog no. 900–011) was purchased from Assay Designs. The assay was run according to the provided protocol. Both fractions generated from saponification of the petroleum ether fraction (i.e., steroid-containing fraction and saponified fatty acid fraction) were tested in the assay, along with controls. A progesterone standard curve was generated as outlined in the protocol. The steroid-containing fraction (4.8 mg, 0.06% crude extract) was taken up in 400 mL of the provided assay buffer, and the fraction was serially diluted by half twice, to provide a total of three samples, each sample assayed in duplicate. The fatty acid-containing fraction (29 mg, 1.5% crude extract) was also treated in a similar manner, to provide a total of three samples, with each tested in duplicate. Progesterone was present only in the treatment wells containing the steroid-containing fraction.
B. manjavacas Lysate.
Adult rotifer biomass (1 g of wet weight) was placed in modified RIPA buffer (150 mM NaCl, 50 mM Tris·HCl at pH 7.4, 1% Triton X-100, 1 mM EDTA, 1 mM PMSF, 5 μg/mL aprotinin, 1 μg/mL pepstatin-A, 2 μg/mL lLeupeptin, 1 mM Na3VO4, and 1 mM NaF). The cells were ruptured by sonication for 5 min at 4 °C and centrifuged for 5 min at 16,060 × g at 4 °C. The soluble fraction was spin dialyzed 3 volumes of RIPA buffer on an Ultracel YM-3 3-kDa filter and concentrated to 1.3 mg/mL by Bradford analysis.
Immunoprecipitation Studies with Probe 2.
Samples of the resulting lysate (200 μL) were treated with 10 μL of a 0.1 mg/mL DMSO stock of probe 2. The sample was incubated with rotary shaking for 4 h at 4 °C. The sample was then immunoprecipitated by the addition of Affigel 10 resin containing 3.5 mg·mL−1 of the XRI-TF35 mAb (40 μL). The resulting mixture was agitated for 12 h at 4 °C with the resin. The resin was then washed with ice cold PBS (3 × 2 mL). The bound protein was eluted from the resin incubating with 35 μL of 1 mg·mL−1 of dimethylamino-4-coumarin acetic acid in RIPA buffer for 1 h at room temperature. SDS/PAGE analysis was run on a 4–12% gradient gel (Novex) with Mops running buffer and silver stained (Invitrogen). Samples of the identified bands were excised and submitted to Nano-LC/MS/MS analysis conducted by contract services from the Center for Functional Genomics at the University of Albany, State University of New York or the University of California at San Diego.
Western Blotting Studies Using Probe 4.
See Appendix S1 file for human cell lysate validation methods. An aliquot of rotifer lysate (400 μL) was treated with 20 μL of a 1 mg/mL stock of 4 in DMSO for 4 h at 4 °C. The sample was then immunoprecipitated by the addition of 15 μL of a ≈0.01 mg/mL stock of a mouse anti-progesterone IgG mAb containing 1 mg/mL of BSA for 2 h at 4 °C. This procedure was then followed by the addition of 50 μL of protein A fast flow resin from GE Healthcare. The resulting mixture was agitated for 12 h at 4 °C with the resin. The resin was then washed with ice cold PBS (2 × 1 mL). The bound protein was boiled in SDS/PAGE loading buffer S3401-1VL (Sigma-Aldrich). SDS/PAGE analysis was conducted on a Novex X-cell station by using NuPage 4–12% Bis-Tris gels and Mops SDS running buffer. Western blotting was conducted by transfer to Hybond-P PVDF membrane by GE Healthcare followed by blocking for 2 h with a solution of 0.1% Tween-20 with 5% wt/vol nonfat dry milk 20 in Tris-buffered saline (TBS, pH 7.6), staining with the primary antibody, a mouse anti-progesterone IgG mAb from Assay Designs, in 0.1% Tween-20 with 5% BSA in TBS, mouse anti-progesterone mAb, and a Goat anti-mouse IgG HRP mAb conjugate from Promega in 0.1% Tween-20 with 5% BSA in TBS. The primary and secondary antibodies were applied at 1:100 and 1:2,000 dilution, respectively, from their manufactures preparation. The total protein content in each gel was determined by staining with ponceau S from Promega.
RNAi Knockdown Methods.
B. manjavacas resting eggs were decapsulated to render them more permeable to dsRNA (28). Resting eggs were then hatched in 15 ppt ASW with transfection solution for the progesterone receptor treatment or elongation factor 1α control (for binding assay), and the hatchlings were isolated in fresh 15 ppt ASW and exposed to freshly prepared transfection solution overnight. The transfection solution consisted of 2 μL of FuGENE HD lipofection transfection reagent (Roche Diagnostics), 43 μL of sterile PBS, plus 5 μL of sterile water containing 10–20 ng progesterone receptor or elongation factor dsRNA. dsRNA derived from B. manjavacas progesterone receptor and elongation factor gene was synthesized in vitro (29). Intensity of fluorescence in confocal microscopy images was quantified by using ImageJ, and a two sample, one-tailed paired Student's t test (n = 8) was calculated.
Gene Expression Analysis.
After 24 h of exposure of rotifer hatchlings to transfection solution, individual rotifers were preserved in RNAlater (Qiagen) for up to 5 d at −80 °C before RNA extraction. RNA extractions were performed immediately before qPCR. Total RNA was isolated from individual rotifers by using RNeasy Micro Kit (Qiagen) with the modification that rotifer cells were disrupted by vortexing with glass beads (425–600 μm). A 1:9 dilution of rotifer RNA was used as a template for triplicate one-step reverse transcription and qPCR reactions by using EXPRESS One-Step SYBR GreenER Kit (Invitrogen). qPCR primers for the target progesterone receptor gene (qPR1for 5′-CGGTCCGTATTCCGTATTTG-3′, qPR1rev 5′-TTCGGCTGACTCTTCTTCGT-3′) were designed from B. manjavacas progesterone receptor membrane component 1-like protein nucleotide sequence (FJ829246). qPCR primers for the actin reference housekeeping gene (actinQBfor 5′-GCATCCACGAGACCACCTAT-3′, actinQBrev 5′-TAGGATCGAACCACCAATCC-3′) were designed from B. plicatilis actin mRNA nucleotide sequence (GenBank accession no. AB111352). Amplification and detection of progesterone receptor and actin genes were conducted by using a Mastercycler Realplex 2 (Eppendorf). Expression levels of progesterone receptor were normalized to actin levels, and relative expression of progesterone receptor gene in progesterone receptor-transfected rotifers to PBS control rotifers was determined by using the ΔΔCT method (30).
Mixis and Mating Bioassays.
Mixis (11) and mating (29) bioassays were performed as previously described. RNAi knockdown for treatments and controls for each bioassay were performed as described in the previous section.
Supplementary Material
Acknowledgments
We thank David Mark Welch for providing access to the rotifer transcriptome database (http://gmod.mbl.edu/) and Hilary Smith for insightful discussions and comments regarding the manuscript. This work was supported by National Science Foundation Grant BE/GenEn MCB-0412674 (to T.W.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1006074107/-/DCSupplemental.
References
- 1.Mizuta T, Kubokawa K. Presence of sex steroids and cytochrome P450 genes in amphioxus. Endocrinology. 2007;148:3554–3565. doi: 10.1210/en.2007-0109. [DOI] [PubMed] [Google Scholar]
- 2.Thornton JW, Need E, Crews D. Resurrecting the ancestral steroid receptor: ancient origin of estrogen signaling. Science. 2003;301:1714–1717. doi: 10.1126/science.1086185. [DOI] [PubMed] [Google Scholar]
- 3.Thornton JW. Evolution of vertebrate steroid receptors from an ancestral estrogen receptor by ligand exploitation and serial genome expansions. Proc Natl Acad Sci USA. 2001;98:5671–5676. doi: 10.1073/pnas.091553298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Cosmo A, Di Cristo C, Paolucci M. A estradiol-17β receptor in the reproductive system of the female of Octopus vulgaris: characterization and immunolocalization. Mol Reprod Dev. 2002;61:367–375. doi: 10.1002/mrd.10014. [DOI] [PubMed] [Google Scholar]
- 5.Keay J, Thornton JW. Hormone-activated estrogen receptors in annelid invertebrates: implications for evolution and endocrine disruption. Endocrinology. 2009;150:1731–1738. doi: 10.1210/en.2008-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mimoto A, et al. Identification of an estrogenic hormone receptor in Caenorhabditis elegans. Biochem Biophys Res Commun. 2007;364:883–888. doi: 10.1016/j.bbrc.2007.10.089. [DOI] [PubMed] [Google Scholar]
- 7.Thomas P. Characteristics of membrane progestin receptor alpha (mPRalpha) and progesterone membrane receptor component 1 (PGMRC1) and their roles in mediating rapid progestin actions. Front Neuroendocrinol. 2008;29:292–312. doi: 10.1016/j.yfrne.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Halanych KM. The new view of animal phylogeny. Annu Rev Ecol Evol Syst. 2004;35:229–256. [Google Scholar]
- 9.Köhler HR, et al. Sex steroid receptor evolution and signalling in aquatic invertebrates. Ecotoxicology. 2007;16:131–143. doi: 10.1007/s10646-006-0111-3. [DOI] [PubMed] [Google Scholar]
- 10.Kubanek J, Snell TW. In: Chemical Communication Among Microbes. Winans SC, Bassler BL, editors. Washington, DC: Am Soc Microbiol; 2008. pp. 453–463. [Google Scholar]
- 11.Snell TW, et al. A protein signal triggers sexual reproduction in Brachionus plicatilis (Rotifera) Mar Biol. 2006;149:763–773. [Google Scholar]
- 12.Alexander MD, et al. A central strategy for converting natural products into fluorescent probes. ChemBioChem. 2006;7:409–416. doi: 10.1002/cbic.200500466. [DOI] [PubMed] [Google Scholar]
- 13.Hughes CC, MacMillan JB, Gaudêncio SP, Fenical W, La Clair JJ. Ammosamides A and B target myosin. Angew Chem Int Ed Engl. 2009;48:728–732. doi: 10.1002/anie.200804107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patterson GW, et al. Sterols of Tetraselmis (Prasinophyceae) Comp Biochem Physiol B. 1993;105:253–256. [Google Scholar]
- 15.Williams SP, Sigler PB. Atomic structure of progesterone complexed with its receptor. Nature. 1998;393:392–396. doi: 10.1038/30775. [DOI] [PubMed] [Google Scholar]
- 16.NIH Clinical Center . Progesterone Historical Reference Ranges, May 16, 2004. Bethesda, MD: National Institutes of Health; 2004. [Google Scholar]
- 17.Ollevier F, De Clerck D, Diederik H, De Loof A. Identification of nonecdysteroid steroids in hemolymph of both male and female Astacus leptodactylus (Crustacea) by gas chromatography-mass spectrometry. Gen Comp Endocrinol. 1986;61:214–228. doi: 10.1016/0016-6480(86)90199-1. [DOI] [PubMed] [Google Scholar]
- 18.Gronemeyer H, Govindan MV. Affinity labelling of steroid hormone receptors. Mol Cell Endocrinol. 1986;46:1–19. doi: 10.1016/0303-7207(86)90064-x. [DOI] [PubMed] [Google Scholar]
- 19.Zhu Y, Hanna RN, Schaaf MJ, Spaink HP, Thomas P. Candidates for membrane progestin receptors—past approaches and future challenges. Comp Biochem Physiol C Toxicol Pharmacol. 2008;148:381–389. doi: 10.1016/j.cbpc.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 20.Hughes AL, et al. Dap1/PGRMC1 binds and regulates cytochrome P450 enzymes. Cell Metab. 2007;5:143–149. doi: 10.1016/j.cmet.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 21.Lösel RM, Besong D, Peluso JJ, Wehling M. Progesterone receptor membrane component 1—many tasks for a versatile protein. Steroids. 2008;73:929–934. doi: 10.1016/j.steroids.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 22.Lavery DN, McEwan IJ. Structure and function of steroid receptor AF1 transactivation domains: Induction of active conformations. Biochem J. 2005;391:449–464. doi: 10.1042/BJ20050872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gronemeyer H. Transcription activation by estrogen and progesterone receptors. Annu Rev Genet. 1991;25:89–123. doi: 10.1146/annurev.ge.25.120191.000513. [DOI] [PubMed] [Google Scholar]
- 24.Fontaneto D, Giordani I, Melone G, Serra M. Disentangling the morphological stasis in two rotifer species of the Brachionus plicatilis species complex. Hydrobiologia. 2007;583:297–307. [Google Scholar]
- 25.Rico-Martinez R, Snell TW. Comparative binding of antibody to a mate recognition pheromone on female Brachionus plicatilis and Brachionus rotundiformis (Rotifera) Hydrobiologia. 1997;358:71–76. [Google Scholar]
- 26.Guillard RRL, Ryther JH. Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt, and Detonula confervacea (cleve) Gran. Can J Microbiol. 1962;8:229–239. doi: 10.1139/m62-029. [DOI] [PubMed] [Google Scholar]
- 27.Berg CJ., Jr . Culture of Marine Invertebrates. Stroudsburg, PA: Hutchinson Ross; 1983. [Google Scholar]
- 28.Snell TW, Shearer TL, Smith HA. Exposure to dsRNA elicits RNA interference in Brachionus manjavacas (Rotifera) Mar Biotechnol (NY) 2010 doi: 10.1007/s10126-010-9295-x. in press. [DOI] [PubMed] [Google Scholar]
- 29.Snell TW, et al. Genetic determinants of mate recognition in Brachionus manjavacas (Rotifera) BMC Biol. 2009;7:60–72. doi: 10.1186/1741-7007-7-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.