Abstract
The Middle East is the home of ethnic groups from three main backgrounds: Semitic (Arabs and Jews), Indo-European (Persians and Kurdish) and Turkic (Turkish and Turkmens). Its geographic location, which has been under continuous influences from Asia, Europe and Africa, has made it an ideal site for epidemiological studies on Helicobacter pylori (H. pylori) infection and genotyping. The gastric cancer rate differs in this region from very high in Iran (26.1/105) to low in Israel (12.5/105) and very low in Egypt (3.4/105). Epidemiological studies showed that the prevalence of H. pylori is almost similar in those countries with a high level of infection in childhood. Importantly, the frequency of vacA s1 and m1 regions and cagA+ genotypes were higher in non Semitic populations who inhabit the North than Semitic populations, the inhabitants of Southern parts of the Middle East. H. pylori infection prevalence, distribution pattern of virulence factors, diet and smoking could not have explained the difference in cancer rate. This reflects the multifactorial aetiology of gastric cancer and suggests that H. pylori infection does not always directly correlate with the risk for gastrointestinal disease, such as gastric cancer. Further detailed investigations and international comparative studies of each risk factor need to be performed to investigate whether this represents a true enigma.
Keywords: Helicobacter pylori, Middle East, Gastric cancer, dupA, cagA, vacA, iceA
INTRODUCTION
Helicobacter pylori (H. pylori) causes gastritis and peptic ulceration and it is an important risk factor for gastric adenocarcinoma, the second highest cause of cancer deaths worldwide. The disease process is thought to have a multifactorial aetiology, and bacterial strain type, pattern of gastritis, and environmental conditions, are all thought to contribute[1]. H. pylori strains differ, and possession of specific virulence factors greatly increases the risk of disease. The best recognised of these are the cag pathogenicity island and active forms of the vacuolating cytotoxin (VacA). Duodenal ulcer promoting gene A (dupA) is a recently described gene shown to be associated with duodenal ulceration and protective against gastric cancer[2]. It was observed that the early acquisition of H. pylori infection in childhood resulted in pangastritis in adulthood. This pattern of gastritis is usually associated with mucosal atrophy which is a precancerous condition[3]. Therefore, acquiring the infection at an early age is a recognised risk factor for the development of gastric cancer[4]. Additionally, antral predominant gastritis is usually associated with duodenal ulcer. Furthermore, different environmental factors such as high salt intake and inadequate consumption of fruit and vegetables containing vitamin C has been regarded as risk a factor for development of gastric cancer[5].
The Middle East is home to ethnic groups from three main backgrounds: Semitic (Arabs and Jews), Indo-European (Persians and Kurdish) and Turkic (Turkish and Turkmens)[6-8]. Its geographic location, which has been under continuous influences from Asia, Europe and Africa, has made it an ideal site for epidemiological studies on H. pylori infection and genotyping. The prevalence of H. pylori infection has been reported from many Middle Eastern countries, including Iraq, Iran, Turkey, Libya, Egypt, Israel, Bahrain, Oman, Saudi Arabia, and the United Arab Emirates. It has been shown that the prevalence rates of infection in these countries are almost similar to each other and to the reported prevalence from Europe and United States. However, gastric cancer and other H. pylori related diseases rates vary from very high in Iran to rare in Iraq and Egypt (Table 1)[9]. It is not known whether the difference is due to host, environment, or bacterial factors or a combination of these factors. In this review I tried to find a reason for the difference in H. pylori related diseases amongst Middle Eastern countries by discussing the prevalence of H. pylori and its virulence determinants, the pattern of gastritis, and environmental factors that might influence the disease process in the Middle East.
Table 1.
Prevalence of atrophy, gastric cancer and the distribution of vacA allelic types and cagA status among H. pylori strains isolated in the Middle East
| Country | s1 (%) | s2 (%) | m1 (%) | m2 (%) | i1 (%) | i2 (%) | cagA (%) | Atrophy (%) | Male gastric cancer |
| Saudi Arabia | 58.3[61] | 41.7[61] | 12.6[61] | 87.4[61] | No data | No data | 52.0[43] | 3-19[77] | 5.7/105 |
| Kuwait | 46.4[23] | 53.6[23] | No data | No data | No data | No data | 41.0[23] | 28[75] | 4.8/105 |
| Jordan | 45.3[22] | 54.7[22] | 48.9[22] | 51.1[22] | No data | No data | 26.4[22] | 65[76] | 6.6/105 |
| Iran | 69.2[39] | 30.8[39] | 30.8[39] | 69.2[39] | 36.5[39] | 63.5[39] | 76.0[39] | 22-39[74] | 26.1/105 |
| Iraq | 88.6[39] | 11.4[39] | 25.7[39] | 74.3[39] | 28.5[39] | 71.5[39] | 71.0[39] | 3[69] | 4.5/105 |
| Turkey | 94.8[40] | 5.2[40] | 22.2[40] | 77.8[40] | No data | No data | 78.0[40] | 43-75[72,73] | 12.2/105 |
| Egypt | 42.9[41] | 57.1[41] | 14.3[41] | 85.7[41] | No data | No data | 35.7[41] | 54[24] | 3.4/105 |
PREVALENCE OF H. PYLORI
The prevalence of H. pylori infection varies between countries; generally, the prevalence is about 30% in developed and up to 80% in developing countries[5]. Diagnosis of H. pylori can be achieved by taking biopsies by endoscopy. However, this procedure is invasive and might not give accurate results if colonisation is patchy[10]. Furthermore, it does not suit population based studies. For population screening, serodiagnosis remains one of the methods of choice for detecting the prevalence of infection[11]. Systemic humoral immunoglobulin G (IgG) immune responses to the organism are developed by humans infected with H. pylori[12-14]. Serological tests are useful tools for the diagnosis of H. pylon infection because all H. pylori-infected patients produce an antibody response which can be detected in the serum[14]. The technique of choice is currently enzyme-linked immunosorbent assay because it is a simple, quick, and low-cost technique that permits immunoglobulin class-specific determinations[14].
In a study conducted in Iraq, the prevalence varied in various ages (age: percentage, 6 mo: 0%; 6-24 mo: 27%; 2-18 years: 58%). In the same study, it was shown that 78% of adults were infected with H. pylori which was significantly higher than children. The prevalence of H. pylori increased markedly with age with the maximum colonization (81.5%) occurring in adults (40-60 years)[15]. The same scenario was found in Saudi Arabia, Iran, Libya and Israel. In Saudi, the prevalence of H. pylori infection markedly increased with age. The prevalence of H. pylori infection rose from 32.4% in those aged 0-10 years to more than 66.4% in those aged 20-30 years and 75% in those over 50 years[16]. In Iran, the prevalence of H. pylori infection increased with age [age (years): percentage, 6-10: 46%; 10-20: 50%; 20-30: 67%; 30-40: 80%; 40-50: 85%; 50-60: 84%; 60-70: 81%; over 70: 83%][17]. In Libya, overall prevalence was 67% with a steady increase with age [age (years): percentage, 0-10: 50%; 10-20: 84%; 20-30: 66%; 30-40: 80%; 40-50: 88%; 50-60: 83%; 60-70: 83%; over 70: 94%][18]. In a study conducted in Israeli rural communal settlements with an age range from 6 to 90 years[19], the prevalence H. pylori infection was shown to be 72%. It was also found that the prevalence of H. pylori increased with age [age (years): percentage, 6-10: 10%; 10-20: 39%; 30-40: 60%; 40-60: 70%; over 60: 85%]. In the same study, a significant association was shown between H. pylori infection and the country of origin of Israeli migrants. The highest prevalence (85%) was found in migrants from the Mediterranean and Asia. While 80% of East European migrants were H. pylori positive, the prevalence in West Europeans was 57%. The prevalence in people born in Israel was 66%. The association between H. pylori infection and country of origin was not changed after age adjustment[19].
In Turkey[20], the overall prevalence of H. pylori infection was 81%. There is no marked difference in H. pylori prevalence in different ages [age (years): percentage, 0-10: 70%; 10-20: 83%; 20-30: 77%; 30-40: 87%; 40-50: 88%; 50-60: 90%].
In some countries in the Middle East, the prevalence of H. pylori infection has been studied using polymerase chain reaction and histopathology. The prevalence of H. pylori infection in Jordan and Bahrain was 77.5% and 79%, respectively[21,22]. In Kuwait and Egypt, H. pylori, as detected by H and E and H. pylori special stains, was present in 84% and 86% of the biopsy samples, respectively[23,24]. There was no significant difference in the prevalence of infection between male and female subjects in this region. In a study conducted in Western Saudi, the prevalence of VacA and CagA were significantly elevated in males vs females[25]. In another study in Jordan, there was a clear trend that females were infected with less virulent H. pylori strains, though the correlation was not significant[22].
INCIDENCE OF GASTRIC CANCER IN THE MIDDLE EAST
Despite declining incidence rates in Western countries, gastric cancer remains the second most common cancer type and second cause of cancer-related death worldwide. H. pylori infection is strongly associated with gastric cancer risk. Gastric cancer rate varies from country to country and from region to region. For example, it is very high in Japan (62.7/105) and estimated to be 12 times higher than India[9]. Gastric cancer occurs nearly 7 times more frequently in Iran than in Iraq (Table 1). These data might not be very accurate because of incompetent diagnostic methods, limitation of medical services, and the lack of unique reporting systems. However, I searched through all published literature and could not find any major discordance. Despite the geographical proximity, the gastric cancer rate varies from very low in Iraq and Egypt to intermediate in Israel and Turkey to high in Iran (Table 1)[9,26-28]. Interestingly, H. pylori infection prevalence in these countries is relatively high and almost the same. This discordance between gastric cancer incidence and H. pylori seroprevalence might imply that H. pylori infection is not the only factor related to gastric cancer risk[29,30]. A 6-fold variation was found in the association between gastric cancer risk and H. pylori infection[31]. H. pylori virulence factors, immune response, diet, environment, and other factors should be considered.
BACTERIAL VIRULENCE FACTORS
Cytotoxin associated gene A
The cytotoxin associated gene A (CagA) protein, which is encoded by the cagA gene, is a highly immunogenic protein. H. pylori strains possessing cagA are associated with a significantly increased risk for the development of atrophic gastritis, peptic ulcer diseases and gastric cancer[32-36]. The cagA gene is situated at one end of a 40 kb DNA insertion called the cag PAI and may have been acquired from a non-Helicobacter origin[33,37]. The cag PAI contains approximately 30 genes which are multicistronic. The difference in the ability of H. pylori strains to trigger chemokines from gastric mucosa depends upon the expression of genes within the cag PAI[33,37,38].
Strains from Iraq, Turkey and Iran possessing cagA were found in 71%, 78% and 76% of the samples analysed, respectively. cagA presence was significantly associated with peptic ulcer disease incidence in Iraq and Turkey but not in Iran[39,40]. In Iraq, the majority of the population are Arabs. However in a study by Hussein et al[39], samples were collected from Kurdish majority (Kurdistan) region.
In Jordan, the cagA genotype was detected in 26.4%[22]. While Kuwaitis and other Arabian Gulf Arabs had essentially the same prevalence rate of about 41%, Egyptians had a modest positivity of 35.7%[23,41]. In a study conducted in Israel, cagA and cagE genes were present in only 25.5% and 24.5%, respectively[42]. The prevalence of cagA positivity in Saudi was 52%[43]. cagA was associated with gastric cancer and/or peptic ulceration in Iran, Iraq, Saudi, Turkey and Israel[39,42-45]. However, Hussein et al[39] could not find significant relationships between cagA status and disease risk in the Iranian population.
Argent et al[46], suggested that the presence or absence of cagA is not enough to understand the relationship between cagA and clinical outcomes. It was found that there was a size variation of the CagA protein and this variation was shown to be related to the presence of the repeat tyrosine phosphorylation motifs (TPM) sequences containing the EPIYA within the 3’ variable ends[47]. It was found that H. pylori strains in Western and East Asian countries carry the EPIYA-A, EPIYA-B. While Western H. pylori strains carry Western cagA-specific EPIYA-C segments which vary in number ranging from 1-3[48], East Asian strains carry the CagA-specific EPIYA-D motif. We previously studied the TPM of the cagA in Iraq and Iran. The presence of cagA alleles with more than 3 phosphorylation motifs was significantly higher amongst Iranian strains than those from Iraq (there was no Iraqi cagA-positive strain with more than 3 TPM, 12% in Iran). We thought that the presence of cagA with more phosphorylation motifs in Iran may help explain the higher cancer incidence rate in Iran[39]. However, a recent study from Turkey, where the gastric cancer rate is much lower than Iran, found that 34% of cagA-positive Turkish strains carried more than three motifs. For the first time, in this study, they reported a cagA positive strain with 5 C motifs[49]. The absence of cagA with more than 3 motifs in Iraq can be due to a type 2 error.
There is clear discrimination in cagA distribution between Semitic (Arab and Jew) and non Semitic (Kurd, Turk and Persian) populations[39,40,50]. Semitic populations tend to carry less virulent H. pylori[22,23,41]. The cagA positivity in Saudi Arabia is higher than other Semitic countries. This might be due the fact that Saudi society has been influenced by Hajj.
VacA
The VacA is another H. pylori virulence factor[35]. Unlike cagA, almost all H. pylori strains posses the vacuolating cytotoxin gene (vacA). Vacuolating cytotoxin activity is related to the mosaic structure of vacA. In general, type s1/m1 and s1/m2 strains produce high and moderate levels of toxin activity, respectively, whereas s2/m2 strains produce no vacuolating activity[51]. A 12-amino-acid hydrophilic amino-terminal segment, present in type s2 but absent from type s1 VacA proteins, slows the capacity of VacA to form membrane channels and abolishes vacuolation. Some type s1/m2 VacA toxins show cytotoxic activity toward selected cell types, including RK-13, but relatively little activity for HeLa or AGS cells[51-53]. Heterogeneity among vacA alleles may be an important factor in understanding variations in clinical manifestations among H. pylori-infected subjects. Several studies have demonstrated that gastric infection with H. pylori strains containing type s1 vacA alleles is associated with a higher risk for development of peptic ulcer disease than infection with strains containing type s2 vacA alleles[51]. This relation is not seen in East Asia as the vast majority of East Asian strains are vacA type s1[51,54-56]. Thus in these countries, s1 cannot be used as a marker for the presence of peptic ulcer disease because the prevalence of the s1 genotype is uniformly high.
Rhead et al[45] have described a novel determinant of VacA toxicity, called the intermediate or i-region. They showed that two allelic variants of this region existed, i1, and i2. Furthermore, only s1/m2 strains varied in i-type; s1/m1 and s2/m2 strains were exclusively i1 and i2, respectively. This novel region determines vacuolating activity among these s1/m2 strains. More importantly, a significant correlation has been established between the i1 region and gastric cancer[45]. In contrast to Rhead et al[45], no disease association between vacA i genotypes and outcome was found in East Asian and Southeast Asian countries. More studies, from other countries, are needed to determine whether this region is a true virulence determinant[57].
The studies from Turkey, Iran and Iraq (non-Semitic countries) had a high prevalence of vacA s1 genotype of more than 70%, whereas strains from Semitic countries such as Egypt, Jordan, Saudi Arabia, Kuwait and Israel, had a low prevalence of less than 60%. The prevalence of vacA s1 genotype in the non Semitic countries was significantly higher than that in the Semitic countries[41,58].
Reports from Turkey, Iraq and Iran showed that vacA m2 was found in around 70% of typed strains. In Egypt, Saudi and Israel, the percentage of vacA m2 was between 85% and 92%. 51.1% of Jordanian strains typed vacA m2. vacA i region was studied in Iran and Iraq only. vacA i1 was associated with gastric cancer and gastric ulcer in Iran and Iraq, respectively.
Studies from Iraq, Kuwait, Jordan, Israel and Iran did not show any association between vacA s and m genotypes and gastroduodenal diseases[22,23,39,42,59]. In Iraq, an association with gastroduodenal diseases and vacA i-region genotype was shown. Studies from Iran and Turkey[44,60] reported a significant relationship between vacA s1 genotype and peptic ulcers. The vacA m1 genotype was linked to an increased risk for peptic ulcers in Turkey and Saudi Arabia[61,62].
Studies from Iran, Iraq, Jordan, Turkey and Israel have shown a significant association between cagA status and vacA s1, m1 and/or i1 genotypes[22,39,42,44,45]. In cagA-negative strains, most of the vacA genotypes were i2 genotypes[58].
Other virulence factors
dupA: Recently, a novel virulence factor dupA (duodenal ulcer promoting gene A) (jhp0917-jhp0918) was shown to be associated with duodenal ulceration and increased epithelial cell interleukin-8 secretion[2]. The dupA gene is located in the region of the bacterial genome that encodes surface proteins. A significant relationship between dupA and duodenal ulcer was found, and the presence of dupA was associated with neutrophil infiltration. These findings, however, were not confirmed in a study of Brazilian children and adults[63], thus indicating possible geographic differences. More recently, it has been found that dupA was not significantly associated with duodenal ulceration in populations from Belgium, South Africa, China, and the USA, but was significantly associated with gastric cancer development[64]. In the Middle East, dupA was studied in Iraq and Iran. dupA was found to be associated with duodenal ulcers in Iraq but not Iran[39]. In addition, dupA-negative H. pylori strains were found to associate with pre-malignant lesions in Iran[65]. No other studies have been conducted in the Middle East. More studies are needed to address the prevalence of dupA-negative strains and the association between this gene and clinical outcome.
iceA: iceA (induced by contact with epithelium) exists in allelic variants including iceA1 and iceA2. iceA1 only can be induced in the gastric epithelium. The iceA1-positive H. pylori strains were shown to be associated with peptic ulceration and increased mucosal IL-8 secretion, while a higher prevalence of iceA2 strains was found among patients with non-ulcer dyspepsia[66,67]. In Turkey, iceA1 was found to be positive in 32.2% of the strains[68]. In Jordan, analyses of virulence genes revealed that iceA2 (73.6%) was the predominant genotype[22]. In a study conducted in Saudi, it was shown that all ulcer cases were infected with iceA1, while 94.6% of gastritis and 90.9% of normal subjects were infected with iceA2[43]. In Israel, iceA1 was found in 37% and iceA2 in 52% of cases. Both iceA alleles were found in 11%[42]. More research is needed to study iceA and its association with diseases in this region.
HISTOPATHOLOGICAL CHANGES
All strains of H. pylori induce a marked inflammation in the gastric mucosa which is characterised by neutrophil, lymphocyte and other inflammatory cell infiltration. While antral-predominant gastritis leads to increased acid production from the uninflamed corpus and predisposes to duodenal ulceration, corpus-predominant gastritis leads to hypochlorhydria and predisposes to gastric ulceration and adenocarcinoma[3]. In studies conducted in Iraq, Turkey and UAE, it was found there is antral-predominant mononuclear cell infiltration[59,69,70]. In a study conducted in Iran, where the gastric cancer rate is very high, it was found that mononuclear cell infiltration was similar throughout the stomach; on average, patients had pangastritis[71].
Gradual loss of gastric glandular tissue as a consequence of long term mucosal destruction is called atrophic gastritis[3]. The tissue damage may involve progressive loss of all specific mucosal cells including the acid producing parietal cells, pepsinogen producing chief cells and mucus producing gland cells. When these cell types have shrunk, the protective mucus layer will gradually disappear and acid secretion will cease[5]. Such pathological changes increase the risk of gastric ulceration and development of gastric adenocarcinoma[72]. However, this protects against duodenal ulcers because of low acid secretion[5]. In a study conducted in Turkey, histological evidence of mucosal atrophy was found in 43% of H. pylori-infected subjects[72] while in another study in Turkey atrophy was found in 75% of the subjects[73]. In UAE, gastric atrophy in Helicobacter associated gastritis was seen in 54% of cases[70]. In a study conducted in Iran, where the gastric cancer rate is much higher than Turkey, histological evidence of mucosal atrophy was found in 39% and 22% of antral and corpus biopsies, respectively[74]. In Iraq, glandular atrophy was found in only one (3%) specimen taken from the antrum. In Kuwait, H. pylori were found in 81.7% patients, of which 28.3% had atrophic gastritis and 15.1% intestinal metaplasia[75]. Atrophy was found in 65% and 54% of examined subjects in Jordan and Egypt, respectively[24,76] (Table 1).
In a study conducted in Saudi where a comparison of Sydney scores from younger and older patients was made, no significant differences were seen in the scores of H. pylori density, neutrophilic activity, or chronic inflammatory cell infiltration between the two groups. While intestinal metaplasia was not found in any young patient, 22% of older patients had focal metaplastic changes. The atrophic changes were seen in 19% of older patients and one (3%) younger patient[77].
In Turkey, while a significant relationship was found between cagA positivity and neutrophil activity and glandular atrophy in antral specimens, corpus neutrophil infiltration was found to be more severe in the vacA m1 group than in the vacA m2 group[59]. No association between virulence factors and histopathology was found in Iraq[69]. In Iran, dupA-negative strains were associated with premalignant histological changes[65].
In most of the studies conducted in the Middle East, histological changes seen in the antral sections (such as neutrophil infiltration of the lamina propria and the glands and the increase in the number of lymphocytes and plasma cells) were on average of mild scores.
DIET IN THE MIDDLE EAST
Diet pattern correlates with gastric diseases. Most populations in the Mediterranean region (including Middle Eastern populations) adhere to the Mediterranean dietary pattern. Mediterranean food has several common features including low consumption of meat and animal products, a high consumption of fish, vegetables, fruit, and cereal, and olive oil as the main source of fat. A Mediterranean diet, particularly olive oil, vegetable and fruit consumption, has been shown to be related to a low risk of cardiovascular disease and several cancers including upper gastrointestinal tract cancer[78,79]. Whole grain is also related to low risk of gastric cancer[78]. In contrast, intake of refined grains by some Mediterranean populations was associated with increased risk of stomach cancer[78]. Overall, the Mediterranean dietary pattern is associated with better health and protects against various chronic diseases. After revision of most recently published papers about diet and food in the Middle East, I did not find any significant differences in the diet pattern amongst the countries of this region[80,81].
SMOKING IN THE MIDDLE EAST
The association between smoking and increased risk of gastric cancer has been observed. In an epidemiological study conducted in Portugal it was shown that smoking is associated with gastric intestinal metaplasia[82]. In other studies, it was shown that tobacco can increase the risk of gastric cancer[83], and epidemiological evidence linking smoking and gastric cancer has been found[84]. The prevalence of smoking in Iraq where the gastric cancer rate is very low is twice as the prevalence in Iran where the gastric cancer is very high. On the other hand, the highest prevalence was found in Turkey[85]. The prevalence of smoking in other Middle Eastern countries can be seen in Table 2.
Table 2.
Smoking prevalence in different countries (%)
| Males | Females | |
| Saudi: Male Adult (30-70 yr and older), 1996-2001 | 19.1 | 8.3 |
| Kuwait: Adult, 1996 | 29.6 | 1.5 |
| Italy: Adult (15 yr and older), 2002 | 31.1 | 22.3 |
| Iraq: Adult (16 yr and older), 1990 | 40 | 5 |
| Jordan: Adult, 1999 | 48 | 10 |
| Japan: Adult (15 yr and older), 2002 | 51 | 10 |
| Iran: Adult (15 yr and older), 1999-2000 | 22.2 | 2.1 |
| Egypt: Adult (15 yr and older), 2000 | 40 | 18 |
| Spain: Adult (16 yr and older), 2001 | 39.1 | 24.6 |
| Turkey: Adult (20 yr and older), 1997-1998 | 50.9 | 10.9 |
| Israel: Adult (25-64 yr and older), 1999-2001 | 38.6 | 22.1 |
| Costa Rica: Adult (20-49 yr and older), 2001 | 29 | 9.7 |
GENERAL CONSIDERATIONS
H. pylori is a causative agent of peptic ulcer disease and an important risk factor of gastric cancer. Despite the high H. pylori infection rate in Middle Eastern countries, gastric cancer incidence is low in all countries but Iran. Previous reports have shown that the age of patients at the onset of infection may help predict the disease development process as the early acquisition of infection carries more risk of disease development. As shown above, H. pylori infection is acquired early in life in this region. In spite of this, the cancer rate is low.
In the Middle East, the populations can ethnically be divided into two main groups: Semitic populations (Arab and Jew who are southern region inhabitants) and non Semitic populations (Kurd, Turk, and Persian who are northern region inhabitants).
The prevalence of virulence factors in countries inhabited by non Semitic populations (Turkey, Iran and Iraq) is similar to what was found in South Asian and Europe[41] and significantly more than what was found in countries with a Semitic background. This might be because populations which lived in the northern part of the Middle East near Europe and south Asia might correlate with European and south Asian populations. Additionally, this provides more evidence of the ethnical tropism of H. pylori infection. It is noticed that virulence factor rates in Saudi H. pylori are higher than other Arabic countries. This might be due the fact that Saudi society has been an open society due to continued population movement into this region to perform pilgrimage (Hajj).
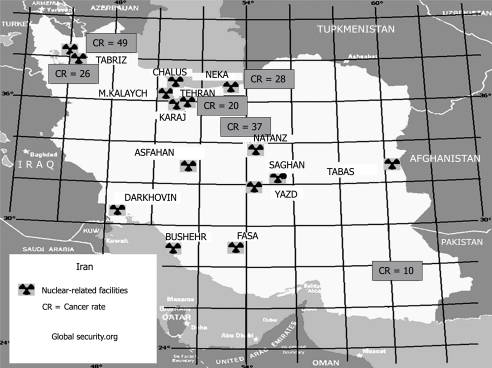
Graham et al[86] suggested a better strategy for the explanation of the relationship between H. pylori and diseases by focusing on underlying patterns of gastritis. This, to a certain extent, is true in this region because all histopathology reports have shown that antral predominant gastritis is the main pattern of gastritis in this region apart from Iran where corpus or pan gastritis is the main pattern. However, if atrophic gastritis, which is precancerous and shown to be associated with cancer, is considered, the high cancer rate in Iran cannot be explained because atrophic gastritis is very high in Turkey, Jordan, UAE, and Egypt with much lower cancer rates. Yet another question to be answered: why this difference in gastritis pattern? Previous reports have blamed environmental factors and diet. For the sake of argument, one would accept the blame but which environmental factor can play such a role in a region which has almost the same environment and traditions. Moreover, as seen above, most Mediterranean countries (including Middle Eastern and South European countries) adhere to a Mediterranean diet pattern and neither diet pattern nor smoking rates seem to explain the difference in cancer rate. The only difference, however, that can be found between Iran and other countries (apart from Israel) is the presence of nuclear facilities in Iran. Examining the map of Iran’s nuclear sites, Iranian uranium mines, nuclear reactors, and uranium processing facilities that include three known uranium enrichment plants can be found in Iran’s most populous urban areas especially in the northwest and central region where there is a very high gastric cancer rate (Figure 1)[87]. On the other hand, there is no nuclear facility in the southeast where the gastric cancer rate is similar to that found in neighboring countries[87]. In Israel, these facilities are in the desert and relatively isolated areas. But if the theory of the relationship between those facilities and cancer is true, one would expect high rates of other cancers in Iran and this is not true[28].
Figure 1.
Map showing Iran’s nuclear sites and annual incidence of gastric cancer in Iran reported from different cancer registries presented as male age standardized rate per 100 000.
Host genetics play an essential role in the inflammatory process and in the interactions between the host and H. pylori (see review by Kusters et al[88]). It has been shown that proinflammatory genetic polymorphisms tend to increase the risk of development of gastric cancer. Hence, would the genetic make-up explain the dilemma of cancer rate in this region? Proinflammatory host genetic facilitates gastric cancer through the development of hypochlorhydric and atrophic gastritis which has been studied and could not have explained the difference in cancer rate. Graham et al[86], suggested that host genetics affect individuals and generally cannot explain widespread changes. In addition, the genetic markers we have at present are not sensitive or specific enough to form the basis of a screening strategy[89]. According to Canedo et al[89], any genetic association studies should fulfil specific criteria among which there should be no evidence of population admixture. This criterion is almost impossible to fulfil in this region. When looking back through history, you will find that Indo-European races (Kurd and Iranian) are not indigenous. In addition, after the rise of Islam and the Arab conquest of the surrounding countries, there was much intermixture between Indo-European and Semitic populations. Further evidence for this intermixture is that in a study of human genetics, a close relatedness of Semitic and Indo-Europeans with each other and with neighbouring geographic groups was shown. In the same study it was shown that Semitic North African groups are more distant genetically from Semitic-speaking groups from the Near East and Iran[90]. Hence, avoiding a mixed population is insuperable in this region. However, this does not negate the importance of the host genetic analyses of cytokine polymorphisms affecting mucosal inflammation and gastric acid secretion. Carefully planned projects would provide additional information to identify predictive markers for an individual’s risk for gastric atrophy and malignancy.
CONCLUSION
To conclude, there is unexplained variation in the distribution of virulence factors and gastritis patterns in the Middle East. These variations fail to explain the discordance between H. pylori infection rates and the variations in gastric cancer prevalence. Although detailed studies are needed to investigate dietary pattern, generally diet is unlikely to contribute because all the countries are following the same Mediterranean pattern. Smoking rates could not explain the variation in cancer rates as we have seen countries with a very high smoking rate but low cancer rate. This might indicate the presence of an enigma similar to or part of that reported (controversially) in Africa and Asia[91,92]. Further detailed investigations and international comparative studies of each risk factor need to be performed to investigate whether this phenomenon represents a true enigma.
Footnotes
Supported by The University of Nottingham
Peer reviewer: Ulrich KS Peitz, MD, Department of Gastroenterology, Hepatology and Infectious Disease, Otto-von-Guericke University Magdeburg, Leipziger Str. 44, D 39120 Magdeburg, Germany
S- Editor Wang YR L- Editor O’Neill M E- Editor Ma WH
References
- 1.Peek RM Jr, Blaser MJ. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat Rev Cancer. 2002;2:28–37. doi: 10.1038/nrc703. [DOI] [PubMed] [Google Scholar]
- 2.Lu H, Hsu PI, Graham DY, Yamaoka Y. Duodenal ulcer promoting gene of Helicobacter pylori. Gastroenterology. 2005;128:833–848. doi: 10.1053/j.gastro.2005.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asaka M, Kato M, Kudo M, Katagiri M, Nishikawa K, Koshiyama H, Takeda H, Yoshida J, Graham DY. Atrophic changes of gastric mucosa are caused by Helicobacter pylori infection rather than aging: studies in asymptomatic Japanese adults. Helicobacter. 1996;1:52–56. doi: 10.1111/j.1523-5378.1996.tb00008.x. [DOI] [PubMed] [Google Scholar]
- 4.Blaser MJ, Nomura A, Lee J, Stemmerman GN, Perez-Perez GI. Early-life family structure and microbially induced cancer risk. PLoS Med. 2007;4:e7. doi: 10.1371/journal.pmed.0040007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atherton JC. The pathogenesis of Helicobacter pylori-induced gastro-duodenal diseases. Annu Rev Pathol. 2006;1:63–96. doi: 10.1146/annurev.pathol.1.110304.100125. [DOI] [PubMed] [Google Scholar]
- 6. Available from: http://www.britannica.com/EBchecked/topic/286417/Indo-Iranian-languages Indo-Iranian languages. Accessed on November 12, 2009.
- 7. Available from: http://www.britannica.com/EBchecked/topic/31677/Arabic-language Arabic language. Accessed on November 12, 2009.
- 8. Available from: http://www.britannica.com/EBchecked/topic/609790/Turkey Turkey. Accessed on November 12, 2009.
- 9.GLOBOCAN IARC. Cancer Map: Male Stomach Cancer, Age-Standardized Incidence Rate per 100,000 in 2002. Available from: http://www-dep.iarc.fr/. Accessed on May 30, 2007.
- 10.Ormand JE, Talley NJ. Helicobacter pylori: controversies and an approach to management. Mayo Clin Proc. 1990;65:414–426. doi: 10.1016/s0025-6196(12)62541-5. [DOI] [PubMed] [Google Scholar]
- 11.Dooley CP, Cohen H, Fitzgibbons PL, Bauer M, Appleman MD, Perez-Perez GI, Blaser MJ. Prevalence of Helicobacter pylori infection and histologic gastritis in asymptomatic persons. N Engl J Med. 1989;321:1562–1566. doi: 10.1056/NEJM198912073212302. [DOI] [PubMed] [Google Scholar]
- 12.Talley NJ, Newell DG, Ormand JE, Carpenter HA, Wilson WR, Zinsmeister AR, Perez-Perez GI, Blaser MJ. Serodiagnosis of Helicobacter pylori: comparison of enzyme-linked immunosorbent assays. J Clin Microbiol. 1991;29:1635–1639. doi: 10.1128/jcm.29.8.1635-1639.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Veenendaal RA, Peña AS, Meijer JL, Endtz HP, van der Est MM, van Duijn W, Eulderink F, Kreuning J, Lamers CB. Long term serological surveillance after treatment of Helicobacter pylori infection. Gut. 1991;32:1291–1294. doi: 10.1136/gut.32.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perez-Perez GI, Dworkin BM, Chodos JE, Blaser MJ. Campylobacter pylori antibodies in humans. Ann Intern Med. 1988;109:11–17. doi: 10.7326/0003-4819-109-1-11. [DOI] [PubMed] [Google Scholar]
- 15.Hussein NR, Robinson K, Atherton JC. A study of age-specific Helicobacter pylori seropositivity rates in Iraq. Helicobacter. 2008;13:306–307. doi: 10.1111/j.1523-5378.2008.00618.x. [DOI] [PubMed] [Google Scholar]
- 16.Marie MA. Seroprevalence of Helicobacter pylori Infection in Large Series of Patients in an Urban Area of Saudi Arabia. Korean J Gastroenterol. 2008;52:226–229. [PubMed] [Google Scholar]
- 17.Alizadeh AH, Ansari S, Ranjbar M, Shalmani HM, Habibi I, Firouzi M, Zali MR. Seroprevalence of Helicobacter pylori in Nahavand: a population-based study. East Mediterr Health J. 2009;15:129–135. [PubMed] [Google Scholar]
- 18.Bakka AS, Salih BA. Prevalence of Helicobacter pylori infection in asymptomatic subjects in Libya. Diagn Microbiol Infect Dis. 2002;43:265–268. doi: 10.1016/s0732-8893(02)00411-x. [DOI] [PubMed] [Google Scholar]
- 19.Gilboa S, Gabay G, Zamir D, Zeev A, Novis B. Helicobacter pylori infection in rural settlements (Kibbutzim) in Israel. Int J Epidemiol. 1995;24:232–237. doi: 10.1093/ije/24.1.232. [DOI] [PubMed] [Google Scholar]
- 20.Novis BH, Gabay G, Naftali T. Helicobacter pylori: the Middle East scenario. Yale J Biol Med. 1998;71:135–141. [PMC free article] [PubMed] [Google Scholar]
- 21.Fakhro AR, Fateha Bel D, Amin Farid IM, Jamsheer HM. The association between Helicobacter pylori infection and lymphoid reaction in patients suffering from dyspepsia in Bahrain. Saudi J Gastroenterol. 1999;5:129–133. [PubMed] [Google Scholar]
- 22.Nimri LF, Matalka I, Bani Hani K, Ibrahim M. Helicobacter pylori genotypes identified in gastric biopsy specimens from Jordanian patients. BMC Gastroenterol. 2006;6:27. doi: 10.1186/1471-230X-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al Qabandi A, Mustafa AS, Siddique I, Khajah AK, Madda JP, Junaid TA. Distribution of vacA and cagA genotypes of Helicobacter pylori in Kuwait. Acta Trop. 2005;93:283–288. doi: 10.1016/j.actatropica.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 24.Mahmoud RAK, Morcos HH, Hegazi AA, Abo Seif MA, El-Hadidy KS. The serological gastric biopsy: a non-endoscopical/histopathologic diagnostic approach in management of the dyspeptic patients. Am J Immunol. 2006;2:88–96. [Google Scholar]
- 25.Jaber SM. The pattern of CagA and VacA proteins in Helicobacter pylori seropositive asymptomatic children in western Saudi Arabia. Saudi Med J. 2005;26:1372–1377. [PubMed] [Google Scholar]
- 26.Nouarie M, Pourshams A, Kamangar F, Sotoudeh M, Derakhshan MH, Akbari MR, Fakheri H, Zahedi MJ, Caldwell K, Abnet CC, et al. Ecologic study of serum selenium and upper gastrointestinal cancers in Iran. World J Gastroenterol. 2004;10:2544–2546. doi: 10.3748/wjg.v10.i17.2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sadjadi A, Malekzadeh R, Derakhshan MH, Sepehr A, Nouraie M, Sotoudeh M, Yazdanbod A, Shokoohi B, Mashayekhi A, Arshi S, et al. Cancer occurrence in Ardabil: results of a population-based cancer registry from Iran. Int J Cancer. 2003;107:113–118. doi: 10.1002/ijc.11359. [DOI] [PubMed] [Google Scholar]
- 28.Sadjadi A, Nouraie M, Mohagheghi MA, Mousavi-Jarrahi A, Malekezadeh R, Parkin DM. Cancer occurrence in Iran in 2002, an international perspective. Asian Pac J Cancer Prev. 2005;6:359–363. [PubMed] [Google Scholar]
- 29.Kikuchi S, Wada O, Nakajima T, Nishi T, Kobayashi O, Konishi T, Inaba Y. Serum anti-Helicobacter pylori antibody and gastric carcinoma among young adults. Research Group on Prevention of Gastric Carcinoma among Young Adults. Cancer. 1995;75:2789–2793. doi: 10.1002/1097-0142(19950615)75:12<2789::aid-cncr2820751202>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 30.Nomura A, Stemmermann GN, Chyou PH, Kato I, Perez-Perez GI, Blaser MJ. Helicobacter pylori infection and gastric carcinoma among Japanese Americans in Hawaii. N Engl J Med. 1991;325:1132–1136. doi: 10.1056/NEJM199110173251604. [DOI] [PubMed] [Google Scholar]
- 31.Forman D. Helicobacter pylori and gastric cancer. Scand J Gastroenterol Suppl. 1996;220:23–26. [PubMed] [Google Scholar]
- 32.Rokkas T, Ladas S, Liatsos C, Petridou E, Papatheodorou G, Theocharis S, Karameris A, Raptis S. Relationship of Helicobacter pylori CagA status to gastric cell proliferation and apoptosis. Dig Dis Sci. 1999;44:487–493. doi: 10.1023/a:1026636803101. [DOI] [PubMed] [Google Scholar]
- 33.Atherton JC. CagA: a role at last. Gut. 2000;47:330–331. doi: 10.1136/gut.47.3.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Atherton JC. CagA, the cag pathogenicity island and Helicobacter pylori virulence. Gut. 1999;44:307–308. doi: 10.1136/gut.44.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Atherton JC. H. pylori virulence factors. Br Med Bull. 1998;54:105–120. doi: 10.1093/oxfordjournals.bmb.a011662. [DOI] [PubMed] [Google Scholar]
- 36.Peek RM Jr, Moss SF, Tham KT, Pérez-Pérez GI, Wang S, Miller GG, Atherton JC, Holt PR, Blaser MJ. Helicobacter pylori cagA+ strains and dissociation of gastric epithelial cell proliferation from apoptosis. J Natl Cancer Inst. 1997;89:863–868. doi: 10.1093/jnci/89.12.863. [DOI] [PubMed] [Google Scholar]
- 37.Censini S, Lange C, Xiang Z, Crabtree JE, Ghiara P, Borodovsky M, Rappuoli R, Covacci A. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc Natl Acad Sci USA. 1996;93:14648–14653. doi: 10.1073/pnas.93.25.14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tomb JF, White O, Kerlavage AR, Clayton RA, Sutton GG, Fleischmann RD, Ketchum KA, Klenk HP, Gill S, Dougherty BA, et al. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature. 1997;388:539–547. doi: 10.1038/41483. [DOI] [PubMed] [Google Scholar]
- 39.Hussein NR, Mohammadi M, Talebkhan Y, Doraghi M, Letley DP, Muhammad MK, Argent RH, Atherton JC. Differences in virulence markers between Helicobacter pylori strains from Iraq and those from Iran: potential importance of regional differences in H. pylori-associated disease. J Clin Microbiol. 2008;46:1774–1779. doi: 10.1128/JCM.01737-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saribasak H, Salih BA, Yamaoka Y, Sander E. Analysis of Helicobacter pylori genotypes and correlation with clinical outcome in Turkey. J Clin Microbiol. 2004;42:1648–1651. doi: 10.1128/JCM.42.4.1648-1651.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van Doorn LJ, Figueiredo C, Mégraud F, Pena S, Midolo P, Queiroz DM, Carneiro F, Vanderborght B, Pegado MD, Sanna R, et al. Geographic distribution of vacA allelic types of Helicobacter pylori. Gastroenterology. 1999;116:823–830. doi: 10.1016/s0016-5085(99)70065-x. [DOI] [PubMed] [Google Scholar]
- 42.Benenson S, Halle D, Rudensky B, Faber J, Schlesinger Y, Branski D, Rabinowitz N, Wilschanski M. Helicobacter pylori genotypes in Israeli children: the significance of geography. J Pediatr Gastroenterol Nutr. 2002;35:680–684. doi: 10.1097/00005176-200211000-00018. [DOI] [PubMed] [Google Scholar]
- 43.Momenah AM, Tayeb MT. Helicobacter pylori cagA and iceA genotypes status and risk of peptic ulcer in Saudi patients. Saudi Med J. 2007;28:382–385. [PubMed] [Google Scholar]
- 44.Erzin Y, Koksal V, Altun S, Dobrucali A, Aslan M, Erdamar S, Dirican A, Kocazeybek B. Prevalence of Helicobacter pylori vacA, cagA, cagE, iceA, babA2 genotypes and correlation with clinical outcome in Turkish patients with dyspepsia. Helicobacter. 2006;11:574–580. doi: 10.1111/j.1523-5378.2006.00461.x. [DOI] [PubMed] [Google Scholar]
- 45.Rhead JL, Letley DP, Mohammadi M, Hussein N, Mohagheghi MA, Eshagh Hosseini M, Atherton JC. A new Helicobacter pylori vacuolating cytotoxin determinant, the intermediate region, is associated with gastric cancer. Gastroenterology. 2007;133:926–936. doi: 10.1053/j.gastro.2007.06.056. [DOI] [PubMed] [Google Scholar]
- 46.Argent RH, Hale JL, El-Omar EM, Atherton JC. Differences in Helicobacter pylori CagA tyrosine phosphorylation motif patterns between western and East Asian strains, and influences on interleukin-8 secretion. J Med Microbiol. 2008;57:1062–1067. doi: 10.1099/jmm.0.2008/001818-0. [DOI] [PubMed] [Google Scholar]
- 47.Argent RH, Zhang Y, Atherton JC. Simple method for determination of the number of Helicobacter pylori CagA variable-region EPIYA tyrosine phosphorylation motifs by PCR. J Clin Microbiol. 2005;43:791–795. doi: 10.1128/JCM.43.2.791-795.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ren S, Higashi H, Lu H, Azuma T, Hatakeyama M. Structural basis and functional consequence of Helicobacter pylori CagA multimerization in cells. J Biol Chem. 2006;281:32344–32352. doi: 10.1074/jbc.M606172200. [DOI] [PubMed] [Google Scholar]
- 49.Salih BA, Bolek BK, Arikan S. DNA sequence analysis of cagA 3’ motifs of Helicobacter pylori strains from patients with peptic ulcer diseases. J Med Microbiol. 2010;59:144–148. doi: 10.1099/jmm.0.014894-0. [DOI] [PubMed] [Google Scholar]
- 50.Salih BA, Abasiyanik MF, Ahmed N. A preliminary study on the genetic profile of cag pathogenicity-island and other virulent gene loci of Helicobacter pylori strains from Turkey. Infect Genet Evol. 2007;7:509–512. doi: 10.1016/j.meegid.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 51.Atherton JC, Cao P, Peek RM Jr, Tummuru MK, Blaser MJ, Cover TL. Mosaicism in vacuolating cytotoxin alleles of Helicobacter pylori. Association of specific vacA types with cytotoxin production and peptic ulceration. J Biol Chem. 1995;270:17771–17777. doi: 10.1074/jbc.270.30.17771. [DOI] [PubMed] [Google Scholar]
- 52.Letley DP, Lastovica A, Louw JA, Hawkey CJ, Atherton JC. Allelic diversity of the Helicobacter pylori vacuolating cytotoxin gene in South Africa: rarity of the vacA s1a genotype and natural occurrence of an s2/m1 allele. J Clin Microbiol. 1999;37:1203–1205. doi: 10.1128/jcm.37.4.1203-1205.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van Doorn LJ, Figueiredo C, Sanna R, Pena S, Midolo P, Ng EK, Atherton JC, Blaser MJ, Quint WG. Expanding allelic diversity of Helicobacter pylori vacA. J Clin Microbiol. 1998;36:2597–2603. doi: 10.1128/jcm.36.9.2597-2603.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pan ZJ, Berg DE, van der Hulst RW, Su WW, Raudonikiene A, Xiao SD, Dankert J, Tytgat GN, van der Ende A. Prevalence of vacuolating cytotoxin production and distribution of distinct vacA alleles in Helicobacter pylori from China. J Infect Dis. 1998;178:220–226. doi: 10.1086/515601. [DOI] [PubMed] [Google Scholar]
- 55.Ito Y, Azuma T, Ito S, Miyaji H, Hirai M, Yamazaki Y, Sato F, Kato T, Kohli Y, Kuriyama M. Analysis and typing of the vacA gene from cagA-positive strains of Helicobacter pylori isolated in Japan. J Clin Microbiol. 1997;35:1710–1714. doi: 10.1128/jcm.35.7.1710-1714.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Han SR, Schreiber HJ, Bhakdi S, Loos M, Maeurer MJ. vacA genotypes and genetic diversity in clinical isolates of Helicobacter pylori. Clin Diagn Lab Immunol. 1998;5:139–145. doi: 10.1128/cdli.5.2.139-145.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ogiwara H, Graham DY, Yamaoka Y. vacA i-region subtyping. Gastroenterology. 2008;134:1267; author reply 1268. doi: 10.1053/j.gastro.2007.11.062. [DOI] [PubMed] [Google Scholar]
- 58.Sugimoto M, Zali MR, Yamaoka Y. The association of vacA genotypes and Helicobacter pylori-related gastroduodenal diseases in the Middle East. Eur J Clin Microbiol Infect Dis. 2009;28:1227–1236. doi: 10.1007/s10096-009-0772-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Umit H, Tezel A, Bukavaz S, Unsal G, Otkun M, Soylu AR, Tucer D, Otkun M, Bilgi S. The relationship between virulence factors of Helicobacter pylori and severity of gastritis in infected patients. Dig Dis Sci. 2009;54:103–110. doi: 10.1007/s10620-008-0316-9. [DOI] [PubMed] [Google Scholar]
- 60.Mohammadi M, Oghalaie A, Mohajerani N, Massarrat S, Nasiri M, Bennedsen M, Colding H, Andersen LP. Prevalence of Helicobacter pylori vacuolating cytotoxin and its allelic mosaicism as a predictive marker for Iranian dyspeptic patients. Bull Soc Pathol Exot. 2003;96:3–5. [PubMed] [Google Scholar]
- 61.Momenah AM, Tayeb MT. Relationship between Helicobacter pylori vacA genotypes status and risk of peptic ulcer in Saudi patients. Saudi Med J. 2006;27:804–807. [PubMed] [Google Scholar]
- 62.Salih BA, Abasiyanik MF, Saribasak H, Huten O, Sander E. A follow-up study on the effect of Helicobacter pylori eradication on the severity of gastric histology. Dig Dis Sci. 2005;50:1517–1522. doi: 10.1007/s10620-005-2871-7. [DOI] [PubMed] [Google Scholar]
- 63.Gomes LI, Rocha GA, Rocha AM, Soares TF, Oliveira CA, Bittencourt PF, Queiroz DM. Lack of association between Helicobacter pylori infection with dupA-positive strains and gastroduodenal diseases in Brazilian patients. Int J Med Microbiol. 2008;298:223–230. doi: 10.1016/j.ijmm.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 64.Argent RH, Burette A, Miendje Deyi VY, Atherton JC. The presence of dupA in Helicobacter pylori is not significantly associated with duodenal ulceration in Belgium, South Africa, China, or North America. Clin Infect Dis. 2007;45:1204–1206. doi: 10.1086/522177. [DOI] [PubMed] [Google Scholar]
- 65.Douraghi M, Mohammadi M, Oghalaie A, Abdirad A, Mohagheghi MA, Hosseini ME, Zeraati H, Ghasemi A, Esmaieli M, Mohajerani N. dupA as a risk determinant in Helicobacter pylori infection. J Med Microbiol. 2008;57:554–562. doi: 10.1099/jmm.0.47776-0. [DOI] [PubMed] [Google Scholar]
- 66.Figueiredo C, Quint WG, Sanna R, Sablon E, Donahue JP, Xu Q, Miller GG, Peek RM Jr, Blaser MJ, van Doorn LJ. Genetic organization and heterogeneity of the iceA locus of Helicobacter pylori. Gene. 2000;246:59–68. doi: 10.1016/s0378-1119(00)00054-8. [DOI] [PubMed] [Google Scholar]
- 67.van Doorn LJ, Figueiredo C, Sanna R, Plaisier A, Schneeberger P, de Boer W, Quint W. Clinical relevance of the cagA, vacA, and iceA status of Helicobacter pylori. Gastroenterology. 1998;115:58–66. doi: 10.1016/s0016-5085(98)70365-8. [DOI] [PubMed] [Google Scholar]
- 68.Baglan PH, Srinay E, Ahmed K, Ozkan M, Bozday G, Bozday AM, Ozden A. Turkish isolates of Helicobacter pylori belong to the Middle Eastern genotypes. Clin Microbiol Infect. 2006;12:97–98. doi: 10.1111/j.1469-0691.2005.01293.x. [DOI] [PubMed] [Google Scholar]
- 69.Hussein NR, Napaki SM, Atherton JC. A study of Helicobacter pylori-associated gastritis patterns in Iraq and their association with strain virulence. Saudi J Gastroenterol. 2009;15:125–127. doi: 10.4103/1319-3767.48971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zaitoun AM. Histological study of chronic gastritis from the United Arab Emirates using the Sydney system of classification. J Clin Pathol. 1994;47:810–815. doi: 10.1136/jcp.47.9.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sotoudeh M, Derakhshan MH, Abedi-Ardakani B, Nouraie M, Yazdanbod A, Tavangar SM, Mikaeli J, Merat S, Malekzadeh R. Critical role of Helicobacter pylori in the pattern of gastritis and carditis in residents of an area with high prevalence of gastric cardia cancer. Dig Dis Sci. 2008;53:27–33. doi: 10.1007/s10620-007-9817-1. [DOI] [PubMed] [Google Scholar]
- 72.Fikret D, Kaya Ö, Suna E, Vahap O, Mustafa A, Şebnem A. Relationship between atrophic gastritis, intestinal metaplasia, dysplasia and Helicobacter pylori infection. Turkish J Gastro. 2001;12:169–170. [Google Scholar]
- 73.Kaklikkaya N, Cubukcu K, Aydin F, Bakir T, Erkul S, Tosun I, Topbas M, Yazici Y, Buruk CK, Erturk M. Significance of cagA status and vacA subtypes of Helicobacter pylori in determining gastric histopathology: virulence markers of H. pylori and histopathology. J Gastroenterol Hepatol. 2006;21:1042–1047. doi: 10.1111/j.1440-1746.2006.04199.x. [DOI] [PubMed] [Google Scholar]
- 74.Malekzadeh R, Sotoudeh M, Derakhshan MH, Mikaeli J, Yazdanbod A, Merat S, Yoonessi A, Tavangar M, Abedi BA, Sotoudehmanesh R, et al. Prevalence of gastric precancerous lesions in Ardabil, a high incidence province for gastric adenocarcinoma in the northwest of Iran. J Clin Pathol. 2004;57:37–42. doi: 10.1136/jcp.57.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sarkar C, Anim JT, Ibrahim BH. Atrophic Gastritis and Intestinal Metaplasia in Helicobacter pylori-Associated Antral Gastritis. Medical Principles Practice. 1994;4:197–203. [Google Scholar]
- 76.Matalka II, Al-Omari FA, Al-Jarrah MA, Obeidat FN, Kanaan FM. Image-based discriminating morphological features for gastric atrophy assessment: a step to go further. Pathol Res Pract. 2008;204:235–240. doi: 10.1016/j.prp.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 77.Khan AR. Comparison of H. pylori-gastritis among young and old patients by using "the modified Sydney system of classification and grading". Saudi J Gastroenterol. 1999;5:81–84. [PubMed] [Google Scholar]
- 78.La Vecchia C. Mediterranean diet and cancer. Public Health Nutr. 2004;7:965–968. doi: 10.1079/phn2004562. [DOI] [PubMed] [Google Scholar]
- 79.Visioli F, Grande S, Bogani P, Galli C. The role of antioxidants in the mediterranean diets: focus on cancer. Eur J Cancer Prev. 2004;13:337–343. doi: 10.1097/01.cej.0000137513.71845.f6. [DOI] [PubMed] [Google Scholar]
- 80.Musaiger AO. Diet and prevention of coronary heart disease in the Arab Middle East countries. Med Princ Pract. 2002;11 Suppl 2:9–16. doi: 10.1159/000066415. [DOI] [PubMed] [Google Scholar]
- 81.da Silva R, Bach-Faig A, Raidó Quintana B, Buckland G, Vaz de Almeida MD, Serra-Majem L. Worldwide variation of adherence to the Mediterranean diet, in 1961-1965 and 2000-2003. Public Health Nutr. 2009;12:1676–1684. doi: 10.1017/S1368980009990541. [DOI] [PubMed] [Google Scholar]
- 82.Peleteiro B, Lunet N, Figueiredo C, Carneiro F, David L, Barros H. Smoking, Helicobacter pylori virulence, and type of intestinal metaplasia in Portuguese males. Cancer Epidemiol Biomarkers Prev. 2007;16:322–326. doi: 10.1158/1055-9965.EPI-06-0885. [DOI] [PubMed] [Google Scholar]
- 83.De Stefani E, Boffetta P, Carzoglio J, Mendilaharsu S, Deneo-Pellegrini H. Tobacco smoking and alcohol drinking as risk factors for stomach cancer: a case-control study in Uruguay. Cancer Causes Control. 1998;9:321–329. doi: 10.1023/a:1008829321668. [DOI] [PubMed] [Google Scholar]
- 84.González CA, Pera G, Agudo A, Palli D, Krogh V, Vineis P, Tumino R, Panico S, Berglund G, Simán H, et al. Smoking and the risk of gastric cancer in the European Prospective Investigation Into Cancer and Nutrition (EPIC) Int J Cancer. 2003;107:629–634. doi: 10.1002/ijc.11426. [DOI] [PubMed] [Google Scholar]
- 85.WHO. Smoking prevalence tobacco economy. Available from: http://www.who. int/tobacco/media/en. Accessed on December 7, 2009.
- 86.Graham DY, Lu H, Yamaoka Y. African, Asian or Indian enigma, the East Asian Helicobacter pylori: facts or medical myths. J Dig Dis. 2009;10:77–84. doi: 10.1111/j.1751-2980.2009.00368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Malekzadeh R, Derakhshan MH, Malekzadeh Z. Gastric cancer in Iran: epidemiology and risk factors. Arch Iran Med. 2009;12:576–583. [PubMed] [Google Scholar]
- 88.Kusters JG, van Vliet AH, Kuipers EJ. Pathogenesis of Helicobacter pylori infection. Clin Microbiol Rev. 2006;19:449–490. doi: 10.1128/CMR.00054-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Canedo P, Figueiredo C, Machado JC. After Helicobacter pylori, genetic susceptibility to gastric carcinoma revisited. Helicobacter. 2007;12 Suppl 2:45–49. doi: 10.1111/j.1523-5378.2007.00564.x. [DOI] [PubMed] [Google Scholar]
- 90.Nasidze I, Quinque D, Rahmani M, Alemohamad SA, Stoneking M. Close genetic relationship between Semitic-speaking and Indo-European-speaking groups in Iran. Ann Hum Genet. 2008;72:241–252. doi: 10.1111/j.1469-1809.2007.00413.x. [DOI] [PubMed] [Google Scholar]
- 91.Holcombe C. Helicobacter pylori: the African enigma. Gut. 1992;33:429–431. doi: 10.1136/gut.33.4.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Miwa H, Go MF, Sato N. H. pylori and gastric cancer: the Asian enigma. Am J Gastroenterol. 2002;97:1106–1112. doi: 10.1111/j.1572-0241.2002.05663.x. [DOI] [PubMed] [Google Scholar]