Abstract
Homo- and heteromerization of 7 transmembrane spanning (7TM)/G-protein coupled receptors (GPCRs) has been an important field of study. Whereas initial studies were performed in artificial cell systems, recent publications are shifting the focus to the in vivo relevance of heteromerization. This is especially apparent for the field of opioid receptors. Drugs have been identified that selectively target opioid heteromers of the delta opioid receptor with the kappa and the mu opioid receptors, that influence nociception and ethanol consumption, respectively. In addition, in several cases, the specific physiological response produced by the heteromer may be directly attributed to a difference in receptor trafficking properties of the heteromers compared to their homomeric counterparts. This review attempts to highlight some of the latest developments with regard to opioid receptor heteromer trafficking and pharmacology.
Introduction
Opioid receptors
Opioids, and the receptors to which they bind, have been extensively studied and are well-known for their roles in pain modulation/analgesia and reward. The peptidergic opioid receptors belong to class A of the family of 7 transmembrane spanning (7TM)/G-protein coupled receptors (GPCRs). For many years opioid receptors were subdivided into three classes: the mu opioid receptor (MOP-R), the delta opioid receptor (DOP-R) and the kappa opioid receptor (KOP-R), each of which displays at least two pharmacological “subtypes” in vivo [1-3]. In the early 1990's sequence homology cloning efforts resulted in the identification of a fourth “opioid” receptor, the nociceptin (orphanin FQ/ORL1) receptor NOP-R, which despite its high homology to the other opioid receptors does not bind “classic” opioid ligands with high affinity [4]. Continuing research at the molecular level has resulted in the identification of receptor splice variants of each of these receptors [5]. An additional layer of complexity is added by the ability of opioid receptors to engage in receptor-receptor interactions, forming receptor homomers and heteromers with altered pharmacological properties [6,7], first described more than a decade ago [8]. Hence, although only four opioid receptor genes have been identified, the pharmacological diversity in opioid response is much greater.
An important aspect of opioid pharmacology is the establishment of tolerance. Opioid tolerance can have multiple causes, but often include mechanisms involving receptor trafficking. These mechanisms, such as receptor phosphorylation/desensitization, internalization, recycling or degradation, for a large part, rely on receptor interactions with non-7TM/GPCRs, such as protein kinases and β-arrestins [9,10].
This review focuses on recent advances in understanding opioid receptor heteromerization and trafficking.
Importance of opioid receptor trafficking
Not only do the opioid receptor subtypes vary in their signaling and ligand binding properties, but importantly, they also display different trafficking properties. The most studied receptors in this respect are the MOP-Rs, which recycle after ligand activation, and the DOP-Rs, which degrade after activation [11]. However, this is not as straightforward as it seems, since the MOP-R agonist morphine does not induce receptor internalization and/or recycling and there are endogenous ligands that both do and do not differentiate between MOP-R and DOP-R [4]. Both the ability and the inability of opioid receptors to desensitize and endocytose have been proposed as mechanisms responsible for opioid tolerance [12]. Nevertheless, it is clear that desensitization alone cannot explain morphine tolerance, since active receptors still remain in the “tolerant state” and are revealed as signs of physical withdrawal upon removal of drug.
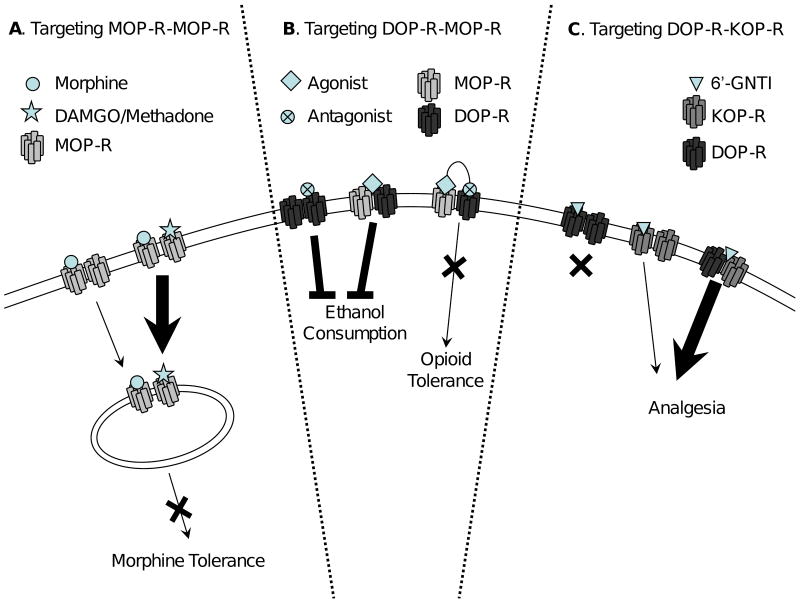
Importantly, several studies have suggested that enhancing MOP-R desensitization and endocytosis can delay tolerance [13,14]. Indeed, endogenous endorphins [14], and exogenous DAMGO [15] or methadone [16] all appear to enhance the ability of morphine to promote endocytosis of the MOP-R. One possible explanation for these findings is that morphine-occupied MOP-Rs are endocytosed because they form homomers with MOP-Rs occupied by an endocytosis-promoting ligand. Thus, these findings hint at the possible existence of MOP-R homomers [15] (Figure 1A).
Figure 1. Targeting receptor homo- and heteromers.
A. Targeting the MOP-R homomer using a “cocktail” consisting of morphine (circle) and DAMGO or Methadone (star) to promote internalization and prevent tolerance. B. Targeting the DOP/MOP-R heteromer using an agonist (e.g. TAN-67, diamond) to decrease ethanol consumption, or using a bivalent ligand, consisting of a MOP-R agonist and a DOP-R antagonist (e.g. MDAN-19), to decrease morphine tolerance. C. Targeting the DOP/KOP-R heteromer using 6′-GNTI (triangle), which produces potent analgesia through the heteromer when administered i.t.
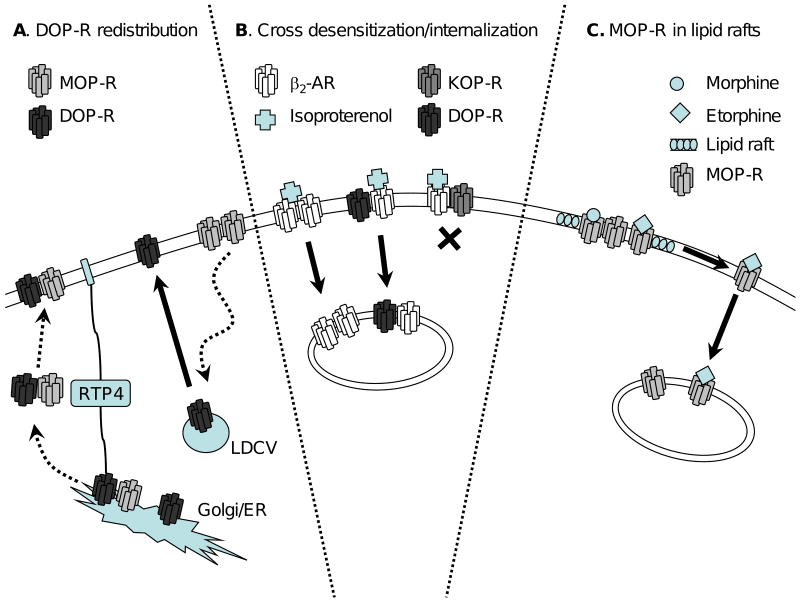
Additionally, recent studies propose that DOP-Rs are located predominantly intracellularly, but can be translocated to the cell surface under several conditions (for review see [17] & Figure 2A), e.g. chronic morphine exposure, stress, inflammation or more recently, ethanol consumption [18]. It appears that both substance P [19] and the presence of MOP-Rs may play a role in the translocation of DOP-Rs to the surface [20,21]. However, the redistribution of intracellular DOP-Rs remains controversial, with recent findings arguing against this hypothesis [22].
Figure 2. Opioid receptor heteromerization affects trafficking.
A. A population of DOP-R is located intracellularly. MOP-R and/or receptor transporter proteins (RTP4) may assist in the translocation of DOP-R from large dense core vesicles (LDCV) or the Golgi/endoplasmatic reticulum (ER) to the cell surface. B. Heteromers of the β2-AR/DOP-R and the β2-AR/KOP-R have altered endocytic properties compared to the respective receptor homomers: β2-AR activation by isoproterenol (cross) induces DOP-R internalization, whereas KOP-R prevents β2-AR internalization. C. Differential trafficking of activated MOP-Rs out of lipid rafts. MOP-Rs are located in lipid rafts on the cell surface. Activation of MOP-R with etorphine (diamond) but not morphine (circle) causes MOP-Rs to move away from the lipid rafts, which could potentially affect homomer formation.
Opioid receptor heteromerization
7TM/GPCRs were initially thought to function solely as monomeric entities. However, a vast amount of data has been amassed over the last two decades suggesting that 7TM/GPCRs can interact with themselves or each other to form receptor homo- and heteromers [6]. The opioid receptors were some of the first heteromers that were comprehensively studied using functional (changes in pharmacology) as well as a biochemical (immunoprecipitation, cross linking) and biophysical (FRET, BRET) methods [23]. The DOP-R can form heteromers with both the MOP-R [24-27] as well as the KOP-R [27,28]. The MOP-R can also heteromerize with the NOP-R [29]. The existence of MOP/KOP-R heteromers is uncertain; Wang et al showed that all opioid receptors have a similar affinity to form receptor homo- and heteromers [27]. However, other studies found no such interactions [28].
It is possible that opioid heteromers could represent some of the opioid receptor subtypes that have been pharmacologically defined, but not attributed to splice variants, such as DOP-R1 (with a preference for DPDPE and BNTX) and DOP-R2 (with a preference for deltorphin II and naltriben) [3]. For example, there is some evidence that the DOP-R1 could be a DOP/KOP-R heteromer [30,31], while the DOP-R2 could be a DOP/MOP-R heteromer [32]. However, Gomes et al. found that the DOP/MOP-R heteromer showed enhanced affinity for some DOP-R1 (BNTX) but also some DOP-R2 (deltorphin II) ligands but not others (DPDPE and naltriben) [33]. Intriguingly, some behavioural effects of DOP-R1, but not DOP-R2 ligands are affected by disruption of MOP-R, again suggesting that the DOP-R1 is a DOP/MOP-R heteromer [34,35].
Similar to the DOP-R, two MOP-R subtypes exist: MOP-R1, which is inhibited by naloxanazine, and MOP-R2 which is not [1]. Three KOP-R subtypes have thus far been identified pharmacologically: KOP-R1 binds arylacetamides and KOP-R2 does not [2], while KOP-R3 [36] is insensitive to the KOP-R ligand U50,488. Some of these subtypes may actually represent the same receptors e.g. MOP-R2 may be DOP-R2 [37], whereas DOP-R1 and KOP-R2 may be the same DOP/KOP-R heteromer [30]. Additionally, opioid receptors may engage in the formation of heteromers with receptors outside the opioid receptor family. MOP-R has been described to form heteromers with many receptors including NK1 [38], CCR5 [39] and the α2A-adrenergic receptors [40], whereas both DOP-R and KOP-R can form receptor heteromers with the β2- adrenergic receptor [41]. Importantly, these heteromers typically exhibit unique pharmacological properties, showing differences in ligand affinities, signalling and receptor trafficking.
It is difficult to distinguish whether the need for two opioid receptors to produce a biological effect is due to heteromerization or convergence in a circuit. Importantly, in at least one case the MOP-R1 and DOP-R1 have been shown to co-immunoprecipitate in CNS tissue [25] and antibodies that selectively recognize the DOP/MOP-R heteromer in vitro recognize a receptor complex in vivo as well [42]. Furthermore, in at least one case, a ligand has been identified that selectively activates a heteromer in vitro and produces a biological effect in vivo [43]. Thus, while there is still some debate as to the in vivo role of DOP/MOP-R or DOP/KOP-R heteromers, there is mounting evidence that these targets do exist, at least in some tissues, in vivo.
Opioid receptor heteromer trafficking
There is still much ongoing debate on the ontogeny and fate of receptor heteromers. It is yet to be verified whether receptor homo- and heteromers are formed i) in the endoplasmatic reticulum and Golgi apparatus during receptor synthesis and maturation or ii) while expressed on the cell surface. As evidence of this debate, early studies suggested that the heteromerization of DOP/MOP-R may only occur at the cell surface [26], while other reports have shown that DOP/MOP-R heteromers are formed in the ER [44]. In addition, a group of receptor chaperone proteins, called receptor transporting proteins (RTPs) and receptor expression enhancing proteins (REEPs) have recently been identified, which may assist in the transport of heteromers to the cell surface [42,45] (2A).
Following their endocytosis, the MOP-R and DOP-R appear to be sorted to different compartments. Specifically, while the MOP-R is recycled to the plasma membrane [46,47], the DOP-R is targeted for degradation [11]. Thus, from a post-endocytic trafficking viewpoint, the DOP/MOP-R heteromer could display a unique trafficking profile, although there is some question as to whether the DOP/MOP-R internalizes as receptor heteromer in vitro [26]. In addition, heteromerization of the KOP-R and DOP-R also appears to affect trafficking. For example, while DOP-Rs are rapidly endocytosed in response to activation by etorphine, KOP-Rs are not, and when the two receptors are co-expressed KOP-Rs prevent the endocytosis of DOP-Rs [48].
Heteromers between the β2-AR and either the DOP-R or the KOP-R also display altered trafficking properties compared to receptor homomers/monomers. For example, when the β2-AR and DOP-R are co-expressed, activation of either receptor leads to co-internalization of the other receptor. On the other hand, the β2-AR/KOP-R heteromer does not endocytose when activated by agonists for either protomer [41] (Figure 2B). Similarly, when the MOP-R and NK1 receptor [38] are co-expressed, the receptors co-internalize with activation by agonists selective for either protomer. Heteromerization between MOP-R and NK1-R could explain why there is a loss of morphine place preference in NK1 −/− mice [49] and why chronic morphine treatment alters substance P-induced internalization of the NK1-R [50]. On the other hand, MOP-R heteromerization with CCR5 only produces cross desensitization but not cross internalization [39].
A recent finding suggests that MOP-Rs are located in lipid rafts on the cell surface, but when activated by specific ligands (etorphine, but not morphine) move out of these rafts [51]. Mechanisms like this could play a role in the formation or breakup of heteromers and shed more light on receptor heteromer ontogeny (Figure 2C).
Targeting opioid heteromers
One method of targeting homomers and heteromers is the co-administration of two drugs that can interact with the two binding pockets of the homomer/heteromer (Figure 1 A). For instance, it was suggested that the DOP-R antagonist TIPPψ enhances morphine analgesia in vivo, by acting on the DOP/MOP-R heteromer [25]. In addition, several efforts have been made to design single ligands that bridge opioid heteromers and, thus, potentially bring together individual receptors into heteromeric complexes and/or stabilize an existing heteromer. The early work in this area targeted homomers and was motivated by a desire to develop both tools to distinguish between different types of opioid receptors and more potent ligands. These ligands often did exhibit enhanced potency (presumably due to their higher affinity) [52]. Recently, these efforts have been expanded to probe the existence of heteromers, receptor allosteric coupling, and potential novel antinociceptive sites [53-55]. For example, the mu-delta agonist-antagonist (MDAN) series of ligands consists of a MOP-R agonist (oxymorphone) and a DOP-R antagonist (naltrindole) moiety linked by a spacer of variable length. The underlying hypothesis behind these ligands is the finding that DOP-R anatagonists (naltrindole) attenuate acute morphine tolerance and dependence [56]. Some of the ligands from the MDAN series show activities in vivo consistent with a unique activity at a heteromer, i.e. reduced tolerance and naloxone precipitated withdrawal signs. This is exemplified by the finding that the novel properties of one molecule in this series, MDAN-19, can not be recapitulated by adding the two pharmacaphores separately, and that its novel properties are dependent on the spacer length (Figure 1B). The proposed mechanism for these bivalent ligands is the ability of the DOP-R antagonist to negatively modulate the MOP-R receptor [54]. In addition, the bivalent ligand KDN-21 is a DOP/KOP-R bivalent antagonist that blocks antinociception produced by both the DOP-R1 agonist DPDPE and the KOP-R2 agonist bremazocine in the spinal cord [30]. Several bivalent ligands have been synthesized that have dual affinity for MOP-R and KOP-R [57,58] as well as “tri-functional” ligands harbouring DOP-R antagonistic, MOP-R agonistic and KOP-R partial agonistic properties [59]. Some of these ligands have recently been tested in vivo [60]. However, while these ligands were potent analgesics, activity did not differ from the monovalent parent compounds, and therefore are not uniquely targeting KOP/MOP-R heteromers. In short, since all bivalent ligands described to date retain activity at homomers/monomers, it has been difficult to draw conclusions as to the existence or functional importance of heteromers using these tools.
However, there is at least one case in which a ligand has been described that shows novel activity at a opioid heteromer distinct from that on either homomer, and is an analgesic in vivo. 6′-GNTI is a small molecule with high affinity for DOP-R and KOP-R. However, while this ligand is an antagonist at DOP-R and a weak partial agonist at KOP-R, it is a potent agonist on DOP/KOP-R heteromers and produces antinociception when administered intrathecally [43], consistent with the localization of DOP/KOP-R heteromers in the spinal cord but not in the brain [43] (Figure 1C).
Therapeutic potential of opioid heteromers
The availability of ligands with selective or novel activity at receptor heteromers would not only enable the study of heteromer localization in vivo, but their dynamics as well. In addition, if opioid receptor heteromers are expressed in a tissue-selective manner, they could be exploited to prevent the side effects of opiates, such as respiratory depression, constipation and dependence that arise as a consequence of systemic use of these drugs. In addition, if the expression of select heteromers is altered during the development of morphine tolerance, they could represent unexplored and selective targets for reversing, preventing or modulating tolerance and dependence. Furthermore, receptor heteromers could be expressed in a gender-specific manner, and thereby explain some of the gender specific effects of opioids, in particular KOP-R opioids [61].
By way of example, 6′-GNTI was an effective analgesic only when administered in the spinal cord but not when injected intracerebroventricularly [43]. Thus, a drug like 6′-GNTI could show reduced side effects such as respiratory depression if the target of 6′-GNTI (the DOP/KOP-R heteromer) is selectively expressed in the spinal cord. In another example, TAN-67, a DOP-R1 selective agonist, reduces ethanol consumption in mice. The activity of TAN-67 is dependent on the presence of DOP-R as well as MOP-R (Figure 1B). These results suggest that DOP-R1 is a DOP/MOP-R heteromer that can be selectively targeted with an agonist to reduce ethanol consumption [34], without the side effects of disphoria produced by opioid receptor antagonists.
In addition, drug “cocktails” that target either homomer or heteromers could be therapeutically valuable. For example, an opioid cocktail consisting of morphine and either methadone or DAMGO promotes morphine-induced endocytosis and thus takes advantage of the homomeric nature of the MOP-R, leading to reduced tolerance and dependence [15,16], Figure 1A. Another example is a cocktail of morphine and serotonin, which drives the endocytosis of the MOP-R/5-HT2A heteromer, suggesting that serotonin might be expected to reduce the development of tolerance and dependence to morphine also by altering receptor trafficking [62].
Conclusions
Although evidence in support of the existence of opioid receptor homo- and heteromers has and continues to accumulate, their functional relevance remains ambiguous [63]. In most studies to date, receptor heteromers have either been recombinantly co-expressed in a cell line or they have been studied in cell lines that endogenously express receptors at physiological levels. However, utilizing these systems for the detection of novel activities at heteromers is challenging at best, since in all cases both homomers and heteromers are expressed. Although some recent effort has been undertaken in cloning obligatory receptor heteromers to circumvent this problem [64], only drugs with novel activity at heteromers will truly enable our ability to delineate the existence and therapeutic relevance of opioid receptor heteromers. Attempts to model receptor heteromers have shown promise [65,66]. Hopefully, these endeavours will decrease the serendipity factor in designing receptor heteromer selective drugs. Indeed, the potential of receptor heteromers as novel pharmacological targets, possibly with select tissue distribution and thus reduced side effects, warrants the large effort that continues in this area.
Research on opioid receptors has been conducted for more than a century. The discovery of opioid receptor homo- and heteromers has opened up a new way of investigating how opioids and opioid receptors function, as well as how receptor trafficking affects heteromerization and vice versa. A better understanding of these processes may lead to the development of analgesic drugs that produce less tolerance, and potentially reduced side effects.
Acknowledgments
This work was supported by grants from the Austrian Science Fund (P18723), the Jubiläumsfonds of the Austrian National Bank and the Lanyar Stiftung Graz (all to MW). JLW and RvR were supported by funds provided by the State of California for medical research through the University of California San Francisco, by the NIH National Institute on Drug Abuse grants DA015232 and DA019958 and DOD grant W81XWH-08-1-0005.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and Recommended Reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Ling GS, Simantov R, Clark JA, Pasternak GW. Naloxonazine actions in vivo. Eur J Pharmacol. 1986;129:33–38. doi: 10.1016/0014-2999(86)90333-x. [DOI] [PubMed] [Google Scholar]
- 2.Zukin RS, Eghbali M, Olive D, Unterwald EM, Tempel A. Characterization and visualization of rat and guinea pig brain kappa opioid receptors: evidence for kappa 1 and kappa 2 opioid receptors. Proc Natl Acad Sci U S A. 1988;85:4061–4065. doi: 10.1073/pnas.85.11.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zaki PA, Bilsky EJ, Vanderah TW, Lai J, Evans CJ, Porreca F. Opioid receptor types and subtypes: the delta receptor as a model. Annu Rev Pharmacol Toxicol. 1996;36:379–401. doi: 10.1146/annurev.pa.36.040196.002115. [DOI] [PubMed] [Google Scholar]
- 4.Waldhoer M, Bartlett SE, Whistler JL. Opioid receptors. Annu Rev Biochem. 2004;73:953–990. doi: 10.1146/annurev.biochem.73.011303.073940. [DOI] [PubMed] [Google Scholar]
- 5.Pasternak GW. Multiple opiate receptors: deja vu all over again. Neuropharmacology. 2004;47 1:312–323. doi: 10.1016/j.neuropharm.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 6.Ferre S, Baler R, Bouvier M, Caron MG, Devi LA, Durroux T, Fuxe K, George SR, Javitch JA, Lohse MJ, et al. Building a new conceptual framework for receptor heteromers. Nat Chem Biol. 2009;5:131–134. doi: 10.1038/nchembio0309-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levac BA, O'Dowd BF, George SR. Oligomerization of opioid receptors: generation of novel signaling units. Curr Opin Pharmacol. 2002;2:76–81. doi: 10.1016/s1471-4892(02)00124-8. [DOI] [PubMed] [Google Scholar]
- 8.Cvejic S, Devi LA. Dimerization of the delta opioid receptor: implication for a role in receptor internalization. J Biol Chem. 1997;272:26959–26964. doi: 10.1074/jbc.272.43.26959. [DOI] [PubMed] [Google Scholar]
- 9.Gainetdinov RR, Premont RT, Bohn LM, Lefkowitz RJ, Caron MG. Desensitization of G protein-coupled receptors and neuronal functions. Annu Rev Neurosci. 2004;27:107–144. doi: 10.1146/annurev.neuro.27.070203.144206. [DOI] [PubMed] [Google Scholar]
- 10.Martini L, Whistler JL. The role of mu opioid receptor desensitization and endocytosis in morphine tolerance and dependence. Curr Opin Neurobiol. 2007;17:556–564. doi: 10.1016/j.conb.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 11.Whistler JL, Enquist J, Marley A, Fong J, Gladher F, Tsuruda P, Murray SR, Von Zastrow M. Modulation of postendocytic sorting of G protein-coupled receptors. Science. 2002;297:615–620. doi: 10.1126/science.1073308. [DOI] [PubMed] [Google Scholar]
- 12.Williams JT, Christie MJ, Manzoni O. Cellular and synaptic adaptations mediating opioid dependence. Physiol Rev. 2001;81:299–343. doi: 10.1152/physrev.2001.81.1.299. [DOI] [PubMed] [Google Scholar]
- 13.Kim JA, Bartlett S, He L, Nielsen CK, Chang AM, Kharazia V, Waldhoer M, Ou CJ, Taylor S, Ferwerda M, et al. Morphine-induced receptor endocytosis in a novel knockin mouse reduces tolerance and dependence. Curr Biol. 2008;18:129–135. doi: 10.1016/j.cub.2007.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Using a knock-in mouse model the authors provide in vivo evidence to support the hypothesis that the inability of MOP-Rs to endocytose after morphine activation lies at the base of the establishment of morphine tolerance
- 14.Zollner C, Mousa SA, Fischer O, Rittner HL, Shaqura M, Brack A, Shakibaei M, Binder W, Urban F, Stein C, et al. Chronic morphine use does not induce peripheral tolerance in a rat model of inflammatory pain. J Clin Invest. 2008;118:1065–1073. doi: 10.1172/JCI25911. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This study shows that, in a clinically relevant model of inflammatory pain, the presence of endogenous opioids prevents the establishment of peripheral morphine tolerance
- 15.He L, Fong J, von Zastrow M, Whistler JL. Regulation of opioid receptor trafficking and morphine tolerance by receptor oligomerization. Cell. 2002;108:271–282. doi: 10.1016/s0092-8674(02)00613-x. [DOI] [PubMed] [Google Scholar]
- 16.He L, Whistler JL. An opiate cocktail that reduces morphine tolerance and dependence. Curr Biol. 2005;15:1028–1033. doi: 10.1016/j.cub.2005.04.052. [DOI] [PubMed] [Google Scholar]; • Describes the development of a morphine-methadone cocktail that drives MOP-R homomer endocytosis. Shows that promoting endoctyosis reduces tolerance and dependence
- 17.Bie B, Pan ZZ. Trafficking of central opioid receptors and descending pain inhibition. Mol Pain. 2007;3:37. doi: 10.1186/1744-8069-3-37. [DOI] [PMC free article] [PubMed] [Google Scholar]; • A review of the mechanism and different conditions under which DOP-Rs redistribute from large dense core vesicles to the cell surface
- 18.Margolis EB, Fields HL, Hjelmstad GO, Mitchell JM. Delta-opioid receptor expression in the ventral tegmental area protects against elevated alcohol consumption. J Neurosci. 2008;28:12672–12681. doi: 10.1523/JNEUROSCI.4569-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guan JS, Xu ZZ, Gao H, He SQ, Ma GQ, Sun T, Wang LH, Zhang ZN, Lena I, Kitchen I. Interaction with Vesicle Luminal Protachykinin Regulates Surface Expression of δ-Opioid Receptors and Opioid Analgesia. Cell. 2005;122:619–631. doi: 10.1016/j.cell.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 20.Cahill CM, Morinville A, Lee MC, Vincent JP, Collier B, Beaudet A. Prolonged morphine treatment targets delta opioid receptors to neuronal plasma membranes and enhances delta-mediated antinociception. J Neurosci. 2001;21:7598–7607. doi: 10.1523/JNEUROSCI.21-19-07598.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morinville A, Cahill CM, Esdaile MJ, Aibak H, Collier B, Kieffer BL, Beaudet A. Regulation of delta-opioid receptor trafficking via mu-opioid receptor stimulation: evidence from mu-opioid receptor knock-out mice. J Neurosci. 2003;23:4888–4898. doi: 10.1523/JNEUROSCI.23-12-04888.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scherrer G, Imamachi N, Cao YQ, Contet C, Mennicken F, O'Donnell D, Kieffer BL, Basbaum AI. Dissociation of the opioid receptor mechanisms that control mechanical and heat pain. Cell. 2009;137:1148–1159. doi: 10.1016/j.cell.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Elaborate study showing that spinal MOP-R and DOP-R each have a distinct location and a distinct function in the spinal cord.
- 23.Gomes I, Filipovska J, Jordan B, Devi L. Oligomerization of opioid receptors. Methods. 2002;27:358. doi: 10.1016/s1046-2023(02)00094-4. [DOI] [PubMed] [Google Scholar]
- 24.George SR, Fan T, Xie Z, Tse R, Tam V, Varghese G, O'Dowd BF. Oligomerization of mu- and delta-opioid receptors. Generation of novel functional properties. J Biol Chem. 2000;275:26128–26135. doi: 10.1074/jbc.M000345200. [DOI] [PubMed] [Google Scholar]
- 25.Gomes I, Gupta A, Filipovska J, Szeto HH, Pintar JE, Devi LA. A role for heterodimerization of mu and delta opiate receptors in enhancing morphine analgesia. Proc Natl Acad Sci U S A. 2004;101:5135–5139. doi: 10.1073/pnas.0307601101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Law PY, Erickson-Herbrandson LJ, Zha QQ, Solberg J, Chu J, Sarre A, Loh HH. Heterodimerization of mu- and delta-opioid receptors occurs at the cell surface only and requires receptor-G protein interactions. J Biol Chem. 2005;280:11152–11164. doi: 10.1074/jbc.M500171200. [DOI] [PubMed] [Google Scholar]
- 27.Wang D, Sun X, Bohn LM, Sadee W. Opioid receptor homo- and heterodimerization in living cells by quantitative bioluminescence resonance energy transfer. Mol Pharmacol. 2005;67:2173–2184. doi: 10.1124/mol.104.010272. [DOI] [PubMed] [Google Scholar]
- 28.Jordan BA, Devi LA. G-protein-coupled receptor heterodimerization modulates receptor function. Nature. 1999;399:697–700. doi: 10.1038/21441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pan Y, Bolan E, Pasternak G. Dimerization of morphine and orphanin FQ/nociceptin receptors: generation of a novel opioid receptor subtype. Biochem Biophys Res Commun. 2002;297:659. doi: 10.1016/s0006-291x(02)02258-1. [DOI] [PubMed] [Google Scholar]
- 30.Bhushan RG, Sharma SK, Xie Z, Daniels DJ, Portoghese PS. A bivalent ligand (KDN-21) reveals spinal delta and kappa opioid receptors are organized as heterodimers that give rise to delta(1) and kappa(2) phenotypes. Selective targeting of delta-kappa heterodimers. J Med Chem. 2004;47:2969–2972. doi: 10.1021/jm0342358. [DOI] [PubMed] [Google Scholar]
- 31.Portoghese PS, Lunzer MM. Identity of the putative delta1-opioid receptor as a delta-kappa heteromer in the mouse spinal cord. Eur J Pharmacol. 2003;467:233–234. doi: 10.1016/s0014-2999(03)01599-1. [DOI] [PubMed] [Google Scholar]
- 32.Porreca F, Takemori AE, Sultana M, Portoghese PS, Bowen WD, Mosberg HI. Modulation of mu-mediated antinociception in the mouse involves opioid delta-2 receptors. J Pharmacol Exp Ther. 1992;263:147–152. [PubMed] [Google Scholar]
- 33.Gomes I, Jordan BA, Gupta A, Trapaidze N, Nagy V, Devi LA. Heterodimerization of mu and delta opioid receptors: A role in opiate synergy. J Neurosci. 2000;20:RC110. doi: 10.1523/JNEUROSCI.20-22-j0007.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Rijn RM, Whistler JL. The delta-1 opioid receptor is a heterodimer that opposes the actions of the delta-2 receptor on alcohol intake. Biol Psychiatry. 2009 doi: 10.1016/j.biopsych.2009.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Shows that DOP-R1 and DOP-R2s have opposing effects on alcohol consumption and represent distinct pharmacological targets that can work synergistically. Also shows that the effects of DOP-R1 requires both DOP-R and MOP-R, suggesting that DOP-R1 could be a DOP/MOP-R heteromer.
- 35.Hosohata Y, Vanderah TW, Burkey TH, Ossipov MH, Kovelowski CJ, Sora I, Uhl GR, Zhang X, Rice KC, Roeske WR, et al. delta-Opioid receptor agonists produce antinociception and [35S]GTPgammaS binding in mu receptor knockout mice. Eur J Pharmacol. 2000;388:241–248. doi: 10.1016/s0014-2999(99)00897-3. [DOI] [PubMed] [Google Scholar]
- 36.Clark JA, Liu L, Price M, Hersh B, Edelson M, Pasternak GW. Kappa opiate receptor multiplicity: evidence for two U50,488-sensitive kappa 1 subtypes and a novel kappa 3 subtype. J Pharmacol Exp Ther. 1989;251:461–468. [PubMed] [Google Scholar]
- 37.Wolozin BL, Pasternak GW. Classification of multiple morphine and enkephalin binding sites in the central nervous system. Proc Natl Acad Sci U S A. 1981;78:6181–6185. doi: 10.1073/pnas.78.10.6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pfeiffer M, Kirscht S, Stumm R, Koch T, Wu D, Laugsch M, Schroder H, Hollt V, Schulz S. Heterodimerization of substance P and mu-opioid receptors regulates receptor trafficking and resensitization. J Biol Chem. 2003;278:51630–51637. doi: 10.1074/jbc.M307095200. [DOI] [PubMed] [Google Scholar]
- 39.Chen C, Li J, Bot G, Szabo I, Rogers TJ, Liu-Chen LY. Heterodimerization and cross-desensitization between the micro-opioid receptor and the chemokine CCR5 receptor. Eur J Pharmacol. 2004;483:175–186. doi: 10.1016/j.ejphar.2003.10.033. [DOI] [PubMed] [Google Scholar]
- 40.Jordan BA, Gomes I, Rios C, Filipovska J, Devi LA. Functional interactions between mu opioid and alpha 2A-adrenergic receptors. Mol Pharmacol. 2003;64:1317–1324. doi: 10.1124/mol.64.6.1317. [DOI] [PubMed] [Google Scholar]
- 41.Jordan BA, Trapaidze N, Gomes I, Nivarthi R, Devi LA. Oligomerization of opioid receptors with beta 2-adrenergic receptors: a role in trafficking and mitogen-activated protein kinase activation. Proc Natl Acad Sci U S A. 2001;98:343–348. doi: 10.1073/pnas.011384898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Decaillot FM, Rozenfeld R, Gupta A, Devi LA. Cell surface targeting of mu-delta opioid receptor heterodimers by RTP4. Proc Natl Acad Sci U S A. 2008;105:16045–16050. doi: 10.1073/pnas.0804106105. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Shows that members of the receptor transporter protein family may act as chaperones to assist cell surface targeting of DOP/MOP-R heteromers.
- 43.Waldhoer M, Fong J, Jones RM, Lunzer MM, Sharma SK, Kostenis E, Portoghese PS, Whistler JL. A heterodimer-selective agonist shows in vivo relevance of G protein-coupled receptor dimers. Proc Natl Acad Sci U S A. 2005;102:9050–9055. doi: 10.1073/pnas.0501112102. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Describes a ligand with novel properties on a DOP/KOP-R heteromer, distinct from those at the homomeric receptors. Demonstrates that this ligand is a tissue-selective analgesic
- 44.Hasbi A, Nguyen T, Fan T, Cheng R, Rashid A, Alijaniaram M, Rasenick MM, O'Dowd BF, George SR. Trafficking of preassembled opioid mu-delta heterooligomer-Gz signaling complexes to the plasma membrane: coregulation by agonists. Biochemistry. 2007;46:12997–13009. doi: 10.1021/bi701436w. [DOI] [PubMed] [Google Scholar]; •• An elegant study showing how DOP/MOP-R heteromers are formed only in the ER, and not after translocation to the cell surface.
- 45.Van Rijn RM, Whistler JL. The only way is up: preventing opioid tolerance by promoting cell surface expression of MOR-DOR heterodimers? Mol Interv. 2008;8:277–280. doi: 10.1124/mi.8.6.4. [DOI] [PubMed] [Google Scholar]
- 46.Koch T, Widera A, Bartzsch K, Schulz S, Brandenburg LO, Wundrack N, Beyer A, Grecksch G, Hollt V. Receptor endocytosis counteracts the development of opioid tolerance. Mol Pharmacol. 2005;67:280–287. doi: 10.1124/mol.104.004994. [DOI] [PubMed] [Google Scholar]
- 47.Qiu Y, Loh HH, Law PY. Phosphorylation of the delta-opioid receptor regulates its beta-arrestins selectivity and subsequent receptor internalization and adenylyl cyclase desensitization. J Biol Chem. 2007;282:22315–22323. doi: 10.1074/jbc.M611258200. [DOI] [PubMed] [Google Scholar]
- 48.Chu P, Murray S, Lissin D, von Zastrow M. Delta and kappa opioid receptors are differentially regulated by dynamin-dependent endocytosis when activated by the same alkaloid agonist. J Biol Chem. 1997;272:27124–27130. doi: 10.1074/jbc.272.43.27124. [DOI] [PubMed] [Google Scholar]
- 49.Murtra P, Sheasby AM, Hunt SP, De Felipe C. Rewarding effects of opiates are absent in mice lacking the receptor for substance P. Nature. 2000;405:180–183. doi: 10.1038/35012069. [DOI] [PubMed] [Google Scholar]
- 50.Gu G, Kondo I, Hua XY, Yaksh TL. Resting and evoked spinal substance P release during chronic intrathecal morphine infusion: parallels with tolerance and dependence. J Pharmacol Exp Ther. 2005;314:1362–1369. doi: 10.1124/jpet.105.087718. [DOI] [PubMed] [Google Scholar]
- 51.Zheng H, Chu J, Qiu Y, Loh HH, Law PY. Agonist-selective signaling is determined by the receptor location within the membrane domains. Proc Natl Acad Sci U S A. 2008;105:9421–9426. doi: 10.1073/pnas.0802253105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Portoghese PS. From models to molecules: opioid receptor dimers, bivalent ligands, and selective opioid receptor probes. J Med Chem. 2001;44:2259–2269. doi: 10.1021/jm010158+. [DOI] [PubMed] [Google Scholar]
- 53.Daniels DJ, Kulkarni A, Xie Z, Bhushan RG, Portoghese PS. A bivalent ligand (KDAN-18) containing delta-antagonist and kappa-agonist pharmacophores bridges delta2 and kappa1 opioid receptor phenotypes. J Med Chem. 2005;48:1713–1716. doi: 10.1021/jm034234f. [DOI] [PubMed] [Google Scholar]
- 54.Daniels DJ, Lenard NR, Etienne CL, Law PY, Roerig SC, Portoghese PS. Opioid-induced tolerance and dependence in mice is modulated by the distance between pharmacophores in a bivalent ligand series. Proc Natl Acad Sci U S A. 2005;102:19208–19213. doi: 10.1073/pnas.0506627102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Peng X, Knapp BI, Bidlack JM, Neumeyer JL. Pharmacological properties of bivalent ligands containing butorphan linked to nalbuphine, naltrexone, and naloxone at mu, delta, and kappa opioid receptors. J Med Chem. 2007;50:2254–2258. doi: 10.1021/jm061327z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Abdelhamid EE, Sultana M, Portoghese PS, Takemori AE. Selective blockage of delta opioid receptors prevents the development of morphine tolerance and dependence in mice. J Pharmacol Exp Ther. 1991;258:299–303. [PubMed] [Google Scholar]
- 57.Neumeyer JL, Zhang A, Xiong W, Gu XH, Hilbert JE, Knapp BI, Negus SS, Mello NK, Bidlack JM. Design and synthesis of novel dimeric morphinan ligands for kappa and micro opioid receptors. J Med Chem. 2003;46:5162–5170. doi: 10.1021/jm030139v. [DOI] [PubMed] [Google Scholar]
- 58.Peng X, Knapp BI, Bidlack JM, Neumeyer JL. Synthesis and preliminary in vitro investigation of bivalent ligands containing homo- and heterodimeric pharmacophores at mu, delta, and kappa opioid receptors. J Med Chem. 2006;49:256–262. doi: 10.1021/jm050577x. [DOI] [PubMed] [Google Scholar]
- 59.Neumeyer JL, Peng X, Knapp BI, Bidlack JM, Lazarus LH, Salvadori S, Trapella C, Balboni G. New opioid designed multiple ligand from Dmt-Tic and morphinan pharmacophores. J Med Chem. 2006;49:5640–5643. doi: 10.1021/jm0605785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mathews JL, Fulton BS, Negus SS, Neumeyer JL, Bidlack JM. In vivo characterization of (-)(-)MCL-144 and (+)(-)MCL-193: isomeric, bivalent ligands with mu/kappa agonist properties. Neurochem Res. 2008;33:2142–2150. doi: 10.1007/s11064-008-9752-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gear RW, Miaskowski C, Gordon NC, Paul SM, Heller PH, Levine JD. Kappa-opioids produce significantly greater analgesia in women than in men. Nat Med. 1996;2:1248–1250. doi: 10.1038/nm1196-1248. [DOI] [PubMed] [Google Scholar]
- 62.Lopez-Gimenez JF, Vilaro MT, Milligan G. Morphine desensitization, internalization, and down-regulation of the mu opioid receptor is facilitated by serotonin 5-hydroxytryptamine2A receptor coactivation. Mol Pharmacol. 2008;74:1278–1291. doi: 10.1124/mol.108.048272. [DOI] [PubMed] [Google Scholar]; • Shows that heteromers of MOP-R and 5HT2A are endocytosed when coactivated by serotonin and morphine, but not by either drug alone
- 63.Kuszak AJ, Pitchiaya S, Anand JP, Mosberg HI, Walter NG, Sunahara RK. Purification and functional reconstitution of monomeric mu-opioid receptors: Allosteric modulation of agonist binding by Gi2. J Biol Chem. 2009 doi: 10.1074/jbc.M109.026922. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Using reconstituted MOP-R and single molecule imaging, the authors show that MOP-R monomers are the minimal functional unit for receptor activation
- 64.Terpager M, Scholl DJ, Kubale V, Martini L, Elling EC, Schwartz TW. Construction of Covalently Coupled, Concatameric dimers of 7TM Receptors. Journal of Receptors and Signal Transduction. 2009 doi: 10.1080/10799890903154217. in press. [DOI] [PubMed] [Google Scholar]
- 65.Filizola M, Weinstein H. Structural models for dimerization of G-protein coupled receptors: the opioid receptor homodimers. Biopolymers. 2002;66:317–325. doi: 10.1002/bip.10311. [DOI] [PubMed] [Google Scholar]
- 66.Filizola M, Weinstein H. The study of G-protein coupled receptor oligomerization with computational modeling and bioinformatics. Febs J. 2005;272:2926–2938. doi: 10.1111/j.1742-4658.2005.04730.x. [DOI] [PubMed] [Google Scholar]