Abstract
The aerospace industry requires the development of a range of chemical sensor technologies for such applications as leak detection, emission monitoring, fuel leak detection, environmental monitoring, and fire detection. A family of chemical sensors are being developed based on micromachining and microfabrication technology to fabricate microsensors with minimal size, weight, and power consumption, and the use of nanomaterials and structures to develop sensors with improved stability combined with higher sensitivity. However, individual sensors are limited in the amount of information that they can provide in environments that contain multiple chemical species. Thus, sensor arrays are being developed to address detection needs in such multi-species environments. These technologies and technical approaches have direct relevance to breath monitoring for clinical applications. This paper gives an overview of developing cutting-edge sensor technology and possible barriers to new technology implementation. This includes lessons learned from previous microsensor development, recent work in development of a breath monitoring system, and future directions in the implementation of cutting edge sensor technology. Clinical applications and the potential impact to the biomedical field of miniaturized smart gas sensor technology are discussed.
Breath analysis techniques offer a potential revolution in health care diagnostics. In principle, each breath contains information regarding the internal state of a patient [1–6]. The technical challenge facing both clinicians and measurement system providers is to extract from that breath meaningful data which can be correlated to patient health. Benchtop instrumentation such as mass spectrometers can use laboratory settings to provide a baseline correlation between exhaled species and physical conditions. However, to fully realize the potential of breath analysis as a patient diagnostic tool, its application must take place not only in the laboratory, but also in the clinic and at home.
A long-term vision for breath diagnoses in standard clinical settings and at home is a portable, smart breath health diagnostic system (SBHDS) with comprehensive capabilities. It is envisioned that, at any time or place as needed, a patient could breathe into this device and information regarding the patient’s health be obtained. While no single technique can provide complete diagnosis of a patient, a wide range of conditions may be diagnosed using the SBHDS (see for example table 1). The SBHDS should be as compact and as easy to use as a cell phone, and like a cell phone should be able to transmit not only status reports but also detailed information regarding patient health. The overall idea is to provide a simple-to-use tool which can enable comprehensive patient diagnosis where it is needed, not just where it is convenient. However, in order to achieve this vision, advancements in measurement technology and miniaturized sensor systems are necessary.
Table 1.
A sampling of the correlation between chemical species, physical ailment and possible cause [5, 6, 17–20].
| Chemical species detected | Physical ailment | Possible cause |
|---|---|---|
| NO | Asthma, airway inflammation | Triggered condition due to irritants or exposures, lung inflammation |
| CO | Lung inflammation, hemolysis, smoke exposure | Asthma, hemolytic anemia, various exposures |
| Hydrocarbons | Lung cancer, other cancers, other lung or systemic diseases |
Radiation exposure, previous smoking |
| H2S | Airway inflammation | Lung inflammation |
| pH | Gastric acid reflux | GERD/peptic ulcer disease |
The application of silicon processing or micro-electromechanical systems (MEMS) and even nanotechnology in the field of sensor technology can enable new capabilities and possibilities [7–10]. However, significant technological barriers remain in order for the vision of a SBHDS to become a reality. This paper briefly discusses trends in sensor technology and offers suggestions toward the implementation of sensor systems. A smart ‘Lick and Stick’ microsystem will be discussed, illustrating both the sensor technology trends and lessons learned. While this example involves an aerospace application, the technology approaches discussed have broad implications and the implementation of these approaches in biomedical applications will be discussed. The paper concludes that systems such as the SBHDS are viable, but to realize this vision would require a multidisciplinary effort of clinicians, sensor technology developers and system manufacturers. Once systems are developed, they need to be tested in standard clinical and specific application settings.
Approaches to enable sensor implementation
The implementation of sensor technology often faces a range of both technology and system engineering barriers. While each application is different, a common thread of development approaches and technology attributes to enable the sensor technology to be implemented have been suggested [11–14]. These include the following:
Interaction with the developer to tailor the sensor for the application
Sensor technology is best applied with strong interaction with the user. If at all possible, sensor implementation should be considered in the design phase of the system application. A sensor developer should strongly interact with the user to make sure that the sensor system meets the needs of the application and to tailor the sensor development in order to meet those needs. The sensor system should not just provide raw parameters to the user, but as a whole tell the user the information they need to know.
Ease of application
Sensor system development, including the use of micro/nano fabrication, to enable multipoint inclusion of complete sensor systems is needed without significantly increasing overall size, weight and power consumption. If adding sensor systems to an application becomes as easy as ‘licking and sticking’ like postage stamps, smart sensor systems that are self-contained, self-powered and do not require significant integration, then one major barrier to inclusion of sensors for intelligence is significantly lessened.
Reliability
Sensor systems have to be reliable and rugged. Users must be able to believe the data reported by these systems and have trust in the ability of the sensor system to respond to changing situations. Broad use of sensor systems will also have a much better chance of occurring if sensor systems are highly reliable.
Redundancy and cross-correlation
If the sensor systems are reliable and easy to install, while minimally increasing the weight or complexity of a vehicle subsystem, the application of a large number of sensor systems is not problematic. This allows redundant sensors systems to spread throughout the application and cross-correlation between the sensor systems to improve reliability of both the sensor data and the vehicle system information.
Orthogonality
The information provided by the various sensory systems should be orthogonal, that is, each provides a different piece of information on the state of the final system. A single measurement is often not enough to give situational awareness. Thus, the mixture of different techniques to ‘see, feel, smell, and hear’ can combine to give complete information on a system and improve the capability to understand the environment.
While not exhaustive, this list of attributes combined together significantly addresses a range of sensor system shortcomings. A new generation of sensor technology with new capabilities is necessary to incorporate these attributes as a whole. For example, the use of integrated electronics, networking systems and micro/nano processing technology can produce smaller, multifunctional and smarter systems with improved capabilities. In addition, they show a current direction of sensor technology that leads to the ability to make measurements in ways not previously possible.
Sensor system development example
‘Lick and Stick’ leak sensor technology
An example of sensor development which demonstrates the above technology trends is an integrated smart leak detection system for a range of launch vehicle propulsion systems [15, 16]. This leak detection system is an example of a smart microsensor system that is also a multifunctional system. A microsensor array fabricated by microfabrication (MEMS)-based technology, including hydrogen, oxygen and hydrocarbon sensors, is used to detect hazardous conditions on the launch pad. Thus, a range of potential launch vehicle fuels (hydrogen or hydrocarbons) and oxygen can be measured simultaneously. Each sensor operates in a manner which maximizes the orthogonality of the measurements performed. Thus, the oxygen sensor has minimal response to hydrogen or hydrocarbons, and the hydrogen sensor typically does not respond to the vast majority of hydrocarbons. Although the hydrocarbon sensor can respond to hydrogen as well as hydrocarbons, the cross-interferences can be accounted for. The sensors can detect a fuel leak and combine that measurement with a determination of the oxygen concentration to ascertain if an explosive condition exists.
The array has been incorporated with signal conditioning electronics, power, data storage and telemetry. The final system will be self-contained with the surface area comparable to a postage stamp. Thus, this postage stamp-sized ‘Lick and Stick’ type gas sensor technology can enable a matrix of leak detection sensors placed throughout a region with minimal size and weight as well as with no power consumption from the vehicle. The electronics can be programmed to provide the user with certain information required on a regular basis, but much further diagnostic information when needed. It is envisioned that a number of these sensors would be placed in a region and the resulting measurements fed into a central processing hub to determine the magnitude and location of a leak. Sensor outputs can be fed to a data processing station, enabling real-time visual images of leaks and enhancing vehicle safety.
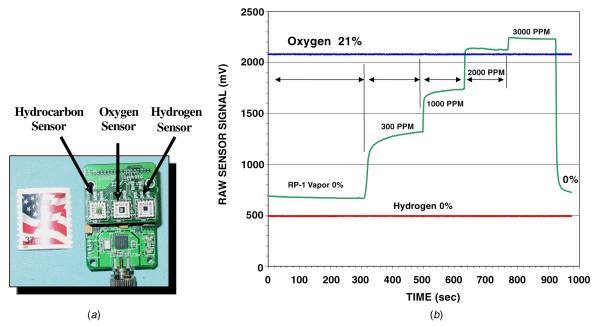
A prototype model of the ‘Lick and Stick’ leak detection sensor system is shown in figure 1(a). The complete system has signal conditioning electronics, power, data storage and telemetry with hydrogen, hydrocarbon and oxygen sensors. Figure 1(b) shows the operation of the electronics with the three-sensor system simultaneously. The data highlight the response of the SiC-based gas sensor at various hydrocarbon fuel (RP-1) concentrations. The oxygen concentration is held constant at 21% and the hydrogen sensor signal shows no response, suggesting a lack of cross-sensitivity between the hydrogen and hydrocarbon sensors to the detection of this hydrocarbon. The hydrocarbon sensor is able to detect fuel concentrations from 300 ppm to 3000 ppm although lower concentrations are possible. Thus, these three sensors exhibit a high degree of orthogonality with each one significantly tailored to a specific species.
Figure 1.
(a) A prototype version of a ‘Lick and Stick’ leak sensor system with hydrogen, hydrocarbon and oxygen detection capabilities combined with supporting electronics. (b) Response of the three sensors of this system to a constant oxygen environment and varying hydrocarbon (RP-1) concentrations. The sensor signal shown is the output from the signal conditioning electronics which processes the measured sensor current [15, 16].
This example demonstrates the combination of multiple sensor types into a complete system giving more full-field information than would be available individually. The modular ‘Lick and Stick’ approach allows sensors to be placed where they are needed without the addition of lead wires for power and communication. In application, these ‘Lick and Stick’ sensors can be placed through a region creating a wireless network system. The output of each sensor can be used to compare with others in a region and provide a more complete picture of the environment. This is an example of a complete ‘Lick and Stick’ smart, multi-parameter sensor microsystem that is usable wherever and whenever needed, thus opening a range of monitoring applications illustrating a number of the sensor implementation approaches discussed above.
Relevance to biological applications
Advancements in sensor system technology as shown in figure 1 have relevance to possible breath analysis applications, but the sensor system needs to be tailored for the application. This section briefly describes the use of microsensor technology for general astronaut health monitoring applications.
Assessing astronaut health, and as necessary applying appropriate countermeasures, is central for realizing long-duration flight missions. Long-term exposure to microgravity is known to have a range of negative effects including loss of aerobic capability, muscle degradation, redistribution of fluids and loss of bone mass. This health degradation intensifies with exposure time to microgravity, and maintaining astronaut health in microgravity during long-duration missions, e.g. a voyage to Mars, is necessary for mission success. Early warning of changes in astronaut health is vital to identifying health issues and providing proper treatment to avoid a major health crisis. However, the use of large and cumbersome instruments in space applications is prohibitive due to burdens on the vehicle in mass and power consumption. Further, equipment which requires significant human intervention is problematic given the limited crew time for this type of activity [13]. Thus, the development and implementation of multiuse microsystems which provide the same capability, and more, than larger systems but are portable with minimal size, weight and power consumption is necessary.
One area where microsystems can impact astronaut health monitoring is in exercise equipment. Astronauts are presently placed on a regular exercise program designed to countermeasure the physical degradation due to microgravity exposure. This is a period of time where the astronaut, whose work overload is already considerable, is already involved in health-related activities and monitoring would have limited effect on schedule. Optimization of the astronaut’s time, the effectiveness of the workout and the information derived on the astronaut’s health is highly beneficial. The detection of oxygen (O2) and that of carbon dioxide (CO2) are fundamental measurements for exercise monitoring. A non-intrusive system that can maximize the effect of the workout is an enabling technology for long-term space missions. This system would need to be easily integrated into astronaut exercise equipment, operate for long durations without recalibration while providing reliable data, have little effect on vehicle mass or power and provide the data to either the astronaut or ground systems in an easy-to-use manner.
Such a system could be combined with other health evaluation techniques for a more full-field approach for evaluating an astronaut’s health. In addition to O2 and CO2 for gas exchange measurements, a range of other molecules are of interest to determine astronaut health. The detection of organic and chemical markers and the correlation of these markers to patient health conditions are a powerful broad-base to health diagnostics. Table 1 shows a brief list of species that have been identified as disease markers [5, 6, 17–20]. An informatics tool which can non-invasively alert crew in space of changes in the health state and help target further investigation is also an enabling technology for long-duration missions. It could be suggested that beyond exercise evaluation, such a tool could be used while the astronaut is having physical examinations or even long term to diagnose health status while in the field, e.g. on extravehicular activities (EVAs).
Thus, in order then to meet the needs to non-invasively monitor astronaut fitness as well as provide indicators of physiological change, measurement of CO2, O2 and a range of other species is necessary. The system must be small, portable, reliable and able to measure a range of parameters. With this information, astronaut fitness and health monitoring programs can be individually tailored to maximize the physical well-being of each astronaut, and therefore maximize the task performing capabilities of each astronaut and the likelihood of mission success.
A preliminary effort to produce such a microfabricated breath monitoring system has been concluded [21]. The project fabricated, tested and delivered an integrated breath monitoring sensor system including an array of gas microsensors, data acquisition and display unit, sample pump and mouthpiece. The core species monitored were CO2, O2 with other species such as carbon monoxide (CO), nitrogen oxides (NO, NO2), hydrogen sulfide (H2S), pH and temperature (T). These sensors were produced for other applications but were evaluated for breath monitoring.
Significant miniaturization of the testing system occurred and the complete system was characterized including patient testing. Overall, this work has evaluated the maturity and capability of microsensors to replace rack-sized lab equipment with a miniaturized metabolic gas monitor system. Testing demonstrated that the higher temperature O2 and CO2 sensors provide the functionality required for a breath monitoring system and can in principle be used to replace rack-sized lab equipment with portable systems. Tests with less mature sensor technology were either inconclusive or pointed to specific design changes to specifically meet the needs of biomonitoring. This work has helped outline a path to achieve a miniaturized metabolic gas monitor system for use in exercise feedback and personal health monitoring for both in space and on the ground which includes modifications of the sensors for breath monitoring applications.
Clinical implications
Benefits of developing miniaturized breath analysis devices for use in astronauts go beyond use in space. One of the most promising potential uses of such devices is the advancement of personalized health by allowing home monitoring of certain diseases by the patients themselves, or by allowing remote monitoring. One such example could be the use of a portable miniaturized analyzer for monitoring nitric oxide levels in asthmatics at home [1, 3, 5, 17, 18].
Asthma affects 15 million Americans, including almost 5 million children. Each year, asthma is responsible for 2 million emergency room visits, 500 000 hospitalizations and 4500 deaths [19, 20, 22]. It is a disease characterized by inflammation in the airway and airway hyper-responsiveness and constriction [23]. Effective treatment is directed at both components. Inflammation is more important and is treated by anti-inflammatory medications. Although the precise mechanism of airway hyper-responsiveness remains unknown, it is thought to be dependent on and the result of the other main feature of asthma, airway inflammation. Once inflammation is controlled, the hyper-responsiveness is much easier to control by bronchodilators [23]. Thus, anti-inflammatory therapy is the cornerstone for asthma management, but not all patients respond, and there is no good way to determine when inflammation is well controlled. Symptoms are displayed late in some individuals and do not always correlate with objective findings. This may explain cases of sudden deaths in asthma, which are likely due to poor symptom perception by the patients and over-reliance on bronchodilators. Pulmonary function tests like spirometry, and peak flows only monitor the airway hyper-responsiveness part but not the inflammation, which is the real problem in asthma. While there is general agreement that pulmonary function tests are not the best way to determine asthma control, until recently there were no other good and practical alternatives. Lung biopsies are too invasive and not justified and thus are rarely if ever done outside of a research setting. But this research showed us that even in mild or seemingly controlled asthma (by the conventional tests) the airways still show evidence of inflammation [24–28]. Currently, a major limitation to identifying and treating asthma is that there are no widely applicable commercialized methods for accurate monitoring of inflammation in this disease [24–28]. Thus, there is a vital need for accurate measures of airway inflammation that are easy to perform, reliable and can be performed repeatedly preferably by the patients at home. This is where breath analysis and monitoring comes in.
While NO has long been known as an atmospheric pollutant present in vehicle exhaust emissions and cigarette smoke, its clinical importance as a biological mediator in animals and humans has been recognized over the past decade [1, 29–33]. NO is present in virtually all mammalian organ systems and in the exhaled breath of humans. Patients with asthma have high levels of NO in their exhaled breath which is closely related to airway inflammation, a concept that is supported by several observations [1, 29, 32, 33]. Administration of anti-inflammatory drugs (corticosteroids, leukotriene antagonists) results in a decrease in exhaled NO, while viral upper respiratory infections and allergen challenge are associated with increased exhaled NO levels. Furthermore, through its vasodilating properties, endogenous NO may increase the exudation of plasma by increasing blood flow to the bronchial circulation, thus increasing airway edema.
Thus, NO is involved in the pathogenesis of airway inflammation in asthma and measuring exhaled NO has clinical utility in monitoring the inflammatory component of asthma. The measurement is non-invasive and easy to use, and the testing can be done very quickly. An individual simply exhales for about 10 s into the device and the NO level is displayed immediately providing rapid assessment of airway inflammation. The major advantage of this test is that it is as simple and non-invasive as the traditional pulmonary function tests but provides information about inflammations that was previously only available from invasive testing like biopsies [5, 18].
Several improvements are still needed, however, to make exhaled NO a useful clinical tool in routine asthma monitoring and management. Specifically, technological improvements are needed to make equipment for measuring NO more portable, less expensive and easier to maintain and calibrate [5, 18]. Since NO levels in exhaled breath are in the parts per billion (ppb) range, a sensitive method utilizing chemiluminescence was initially needed to detect these low levels. Old standard electrochemical methods based on liquid electrolytes-detected levels in the parts per million (ppm) range, while the chemiluminescence method is far more sensitive. The chemiluminescence method depends on the reaction of NO with ozone to form in an excited state which then emits the extra energy as light that can be detected by a photomultiplier tube [30]. Several commercial chemiluminescence analyzers are available to measure NO levels. One such device for exhaled breath was approved by the FDA in 2003 [18]. However, the chemiluminescence approach, while appropriate for some clinical settings, has multiple problems. The technology and the devices are very expensive, their use is restricted to the physician’s office and they are not portable and therefore inappropriate for home testing. Thus, while the detection of NO is a mature approach having received FDA approval, several improvements are still needed to make exhaled NO a useful clinical tool in routine asthma monitoring and management. Technological improvements are needed to make equipment for measuring NO more portable, less expensive and easier to maintain and calibrate. In turn, a major reason that such a product does not presently exist for NO is a lack of adequate sensor technology. Thus, development of such miniaturized portable sensors is needed to address this problem. Such an ideal product would be a portable breath monitoring device that can be used at home [5].
Suggestions for sensor system development and application
These results emphasize the point that while users might leverage sensor technology being developed in other applications for their application, unique problems require specialized solutions. The field of biomedical applications presents significant, unique challenges to sensor system implementation. Nonetheless, the discussion above and related experience might suggest some general directions related to the implementation of sensor technology in biomedical applications [14]. These include the following.
Understand the requirements early and use a complete team approach, including both developers and users, during all steps of system development.
Apply microfabrication techniques across the complete system to minimize size, weight and power consumption.
Design the sensor system to optimize measurement of multiple parameters simultaneously to improve full-field system information and measurement reliability.
Develop sensor systems with electronics and software to provide, e.g., data processing, data interpretation, built-in calibration, self-test, etc, to produce reliable intelligent systems.
Bring system intelligence down to the lowest component level feasible to avoid complex, centralized processing systems and allow a versatile, modular system.
Tailor the system interface to provide the various users what they want to know in an easy-to-use fashion. While having more in-depth material available on demand is necessary, often all a user is trying to understand is as straightforward as a yes or no answer.
Supporting technologies often determine success of a sensor system so the importance of packaging, power and communication must be emphasized.
Demonstrate sensor system technology reliability and durability extensively before implementation.
Overall, the approach is to bring a broad team together to make a smart, small SBHDS which can be applied where and when needed and reliably measure a range of parameters.
Summary and conclusion
This paper has presented an overview of technology trends in sensor technology and suggestions related to implementation in space and health applications. These include improved ease of application through stand-alone, small, low-powered and smart sensor systems; improved reliability of sensor data; cross-correlation and redundancy of sensor elements and the use of orthogonal measurements. An example was given of a ‘Lick and Stick’ leak detection system which embodies a number of these properties. An example of the relevance of the use of smart microsystems to biological applications was given related to astronaut health monitoring for long-duration missions, and an example of technology development for this application was briefly discussed. Further development of these devices for biological applications is a system level problem involving a team approach, integration of multiple component systems and extensive testing.
In conclusion, the field of breath analysis for health applications is potentially a paradigm change in health care. In order for this potential to become a reality, the ability to field smart, miniaturized sensor systems to measure a range of relevant parameters is necessary. The development is a multidisciplinary process that involves a range of expertise and evolving system maturation and testing. This paper has suggested a number of approaches to advance the process of realizing a Smart Breath Health Diagnostic System. Advances in the exhaled nitric oxide field may result in the availability of miniaturized portable analyzers that can be used for personalized home or remote monitoring in the very near future.
Acknowledgments
The authors would like to acknowledge the invaluable contributions of Professor C C Liu of Case Western Reserve University who has been central to this development. The authors would also like to acknowledge the contributions of Dr J Xu, Dr L Matus, Dr G Beheim, Dr P Neudeck of NASA GRC; Dr C Chang and D Lukco of ASRC Aerospace/NASA GRC; A Trunek, D Spry, and Dr L Chen of OAI; Dr D Makel, Dr B Ward and S Carrazana of Makel Engineering, Inc.; and Prof. P Dutta of Ohio State University.
References
- [1].Dweik RA. The promise and reality of nitric oxide in the diagnosis and treatment of lung disease. Cleve. Clin. J. Med. 2001;68:486, 488, 490, 493. doi: 10.3949/ccjm.68.6.486. [DOI] [PubMed] [Google Scholar]
- [2].Khatri SB, Ozkan M, McCarthy K, Laskowski D, Hammel J, Dweik RA, Erzurum SC. Alterations in exhaled gas profile during allergen-induced asthmatic response. Am.J.Respir.Crit.Care.Med. 2001;164(Pt 1):1844–8. doi: 10.1164/ajrccm.164.10.2106119. [DOI] [PubMed] [Google Scholar]
- [3].Khatri SB, Hammel J, Kavuru MS, Erzurum SC, Dweik RA. Temporal association of nitric oxide levels and airflow in asthma after whole lung allergen challenge. J. Appl. Physiol. 2003;95:436–40. doi: 10.1152/japplphysiol.01127.2002. discussion 435. [DOI] [PubMed] [Google Scholar]
- [4].Machado RF, Stoller JK, Laskowski D, Zheng S, Lupica JA, Dweik RA, Erzurum SC. Low levels of nitric oxide and carbon monoxide in alpha 1-antitrypsin deficiency. J. Appl. Physiol. 2002;93:2038–43. doi: 10.1152/japplphysiol.00659.2002. [DOI] [PubMed] [Google Scholar]
- [5].Grob NM, Dweik RA. Exhaled nitric oxide in asthma. From diagnosis, to monitoring, to screening: are we there yet? Chest. 2008;133:837–9. doi: 10.1378/chest.07-2743. [DOI] [PubMed] [Google Scholar]
- [6].Machado RF, et al. Detection of lung cancer by sensor array analyses of exhaled breath. Am.J.Respir.Crit.Care. Med. 2005;171:1286–91. doi: 10.1164/rccm.200409-1184OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Liu CC, Hesketh P, Hunter GW. Chemical microsensors. Interface Mag. 2004 Summer13:22–9. [Google Scholar]
- [8].Stetter J, Hesketh P, Hunter G. Sensors: engineering structure and materials from micro to nano. Interface Mag. 2006 Spring15:66–9. [Google Scholar]
- [9].Liu CC, O’Connor E, Strohl KP, Klann KP, Ghiurcan GA, Hunter G, Dudik L, Shao MJ. An assessment of microfabrication to sensor development and the integration of the sensor microsystem. Proc. Microfabricated System and MEMS VI. 2002 May;:1–8. 2002. [Google Scholar]
- [10].Hunter GW, Xu JC, Evans LJ, Vander Wal RL, Berger GM, Kulis MJ, Liu CC. Chemical sensors based on metal oxide nanostructures. ECS Trans. 2006;3:199–209. [Google Scholar]
- [11].Hunter GW, Liu CC, Makel DB. Microfabricated chemical sensors for aerospace applications. In: Gad-el-Hak M, editor. MEMS Handbook: Design and Fabrication. 2nd edn. CRC Press LLC; Boca Raton, FL: 2006. chapter 11. [Google Scholar]
- [12].Hunter GW, Xu JC, Makel DB. Case studies in chemical sensor development. In: Hesketh P, editor. BioNanoFluidic MEMS. Springer; New York: 2007. chapter 8. [Google Scholar]
- [13].Hunter GW. Morphing, self-repairing engines: a vision for the intelligent engine of the future. AIAA/ICAS Int. Air and Space Symposium, 100th Anniversary of Flight; Dayton, OH. 14–17 July 2003; 2003. AIAA paper 2003-3045. [Google Scholar]
- [14].Hunter GW, Oberle L, Baakalini G, Perotti J, Hong T. Intelligent sensor systems for integrated system health management in exploration applications. 1st Int. Forum on Integrated System Health Engineering and Management in Aerospace; Napa, CA. Nov, 2005. 2005. [Google Scholar]
- [15].Hunter GW, et al. Development of SiC-based gas sensors for aerospace applications. In: Dudley M, Gouma P, Kimoto T, Neudeck PG, Saddow SE, editors. Silicon Carbide 2004—Materials Processing and Devices (Mater. Res. Soc. Symp. Proc. 815) Materials Research Society; Warrendale, PA: 2004. pp. 287–98. [Google Scholar]
- [16].Hunter GW, Xu J, Neudeck PG, Makel DB, Ward B, Liu CC. Intelligent chemical sensor systems for in-space safety applications. 42nd AIAA/ASME/SAE/ASEE Joint Propulsion Conf. and Exhibit (Sacramento, CA, 10–12 July 2006) 2006 Tech. Rep AIAA-06-58419. [Google Scholar]
- [17].Dweik RA. Nitric oxide reactions in the asthmatic airway. In: Marczin N, Yacoub MH, editors. Disease Markers in Exhaled Breath: Basic Mechanisms and Clinical Applications (NATO Science Series) IOS Press; Amsterdam: 2002. pp. 159–66. [Google Scholar]
- [18].Gill M, Graff GR, Adler AJ, Dweik RA. Validation study of fractional exhaled nitric oxide measurements using a handheld monitoring device. J. Asthma. 2006;43:731–4. doi: 10.1080/02770900601031045. [DOI] [PubMed] [Google Scholar]
- [19].Anonymous . Expert Panel Report II. National Institutes of Health; Bethesda, MD: 1997. Guidelines for the diagnosis and the management of asthma. [Google Scholar]
- [20].Wenzel SE. Asthma: defining of the persistent adult phenotypes. Lancet. 2006;368:804–13. doi: 10.1016/S0140-6736(06)69290-8. [DOI] [PubMed] [Google Scholar]
- [21].Ward BJ, Dweik RA, Laskowski D, Hunter GW, Liu CC, Stetter J. Integrated breath analysis system (to be published)
- [22].Kiley J, Smith R, Noel P. Asthma phenotypes. Curr. Opin. Pulm. Med. 2007;13:19–23. doi: 10.1097/MCP.0b013e328011b84b. [DOI] [PubMed] [Google Scholar]
- [23].Busse WW, Rosenwasser LJ. Mechanisms of asthma. J. Allergy. Clin. Immunol. 2003;111(Suppl):S799–804. doi: 10.1067/mai.2003.158. [DOI] [PubMed] [Google Scholar]
- [24].Wenzel SE. Phenotypes in asthma: useful guides for therapy, distinct biological processes, or both? Am. J. Respir. Crit. Care. Med. 2004;170:579–80. doi: 10.1164/rccm.2407005. [DOI] [PubMed] [Google Scholar]
- [25].Busse WW, Banks-Schlegel S, Wenzel SE. Pathophysiology of severe asthma. J. Allergy. Clin. Immunol. 2000;106:1033–42. doi: 10.1067/mai.2000.111307. [DOI] [PubMed] [Google Scholar]
- [26].Djukanovic R, Roche WR, Wilson JW, Beasley CR, Twentyman OP, Howarth RH, Holgate ST. Mucosal inflammation in asthma. Am. Rev. Respir. Dis. 1990;142:434–57. doi: 10.1164/ajrccm/142.2.434. [DOI] [PubMed] [Google Scholar]
- [27].Liu L, Jarjour NN, Busse WW, Kelly EA. Enhanced generation of helper T type 1 and 2 chemokines in allergen-induced asthma. Am. J. Respir. Crit. Care. Med. 2004;169:1118–24. doi: 10.1164/rccm.200312-1659OC. [DOI] [PubMed] [Google Scholar]
- [28].O’Byrne PM, Postma DS. The many faces of airway inflammation. Asthma and chronic obstructive pulmonary disease. Asthma research group. Am. J. Respir. Crit. Care. Med. 1999;159(Pt 2):S41–63. [PubMed] [Google Scholar]
- [29].Dweik RA, Comhair SA, Gaston B, Thunnissen FB, Farver C, Thomassen MJ, Kavuru M, Hammel J, Abu-Soud HM, Erzurum SC. NO chemical events in the human airway during the immediate and late antigen-induced asthmatic response. Proc. Natl Acad. Sci. USA. 2001;98:2622–7. doi: 10.1073/pnas.051629498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Dweik RA, Laskowski D, Abu-Soud HM, Kaneko F, Hutte R, Stuehr DJ, Erzurum SC. Nitric oxide synthesis in the lung. Regulation by oxygen through a kinetic mechanism. J. Clin. Invest. 1998;101:660–6. doi: 10.1172/JCI1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Dweik RA, Laskowski D, Ozkan M, Farver C, Erzurum SC. High levels of exhaled nitric oxide (NO) and NO synthase III expression in lesional smooth muscle in lymphangioleiomyomatosis. Am. J. Respir. Cell. Mol. Biol. 2001;24:414–8. doi: 10.1165/ajrcmb.24.4.4127. [DOI] [PubMed] [Google Scholar]
- [32].Ozkan M, Dweik RA. Nitric oxide and airway reactivity. Clin. Pulm. Med. 2001;8:199–206. [Google Scholar]
- [33].Dweik RA. Nitric oxide, hypoxia, and superoxide: the good, the bad, and the ugly! Thorax. 2005;60:265–7. doi: 10.1136/thx.2004.038471. [DOI] [PMC free article] [PubMed] [Google Scholar]