Abstract
Exhaled NO (FENO) measurements have been utilized as a marker to diagnose asthma as well as a non-invasive tool for monitoring airway inflammation and the response to anti-inflammatory medications. One area where this non-invasive monitoring may be helpful is for asthmatic athletes as they train for competitive events. We hypothesized that in the course of training an asthmatic individual may experience worsening of lung inflammation reflected in FENO levels that may be too subtle to detect by conventional methods like spirometry. Data were collected from an asthmatic patient (n = 1) over the course of endurance training using both the desktop (NIOX) and the portable NO (MINO) analyzers daily for eight weeks. We found that average NO levels measured in the desktop system correlated well with the two portable analyzers (r2 =0.73, r2 = 0.74 p < 0.0001); additionally, there was a strong correlation between the two MINO devices (r2 = 0.88; p < 0.0001). A strong negative relationship existed between the number of miles run and NO, regardless of the device used. FEV1 and PEF, however, did not change significantly as the miles run increased. Exercise training in asthmatics was associated with a decrease (improvement) in NO levels but no significant change in FEV1 and PEF. This suggests that exhaled NO levels may be more sensitive to changes in the airway as a result of exercise than traditional pulmonary function testing.
Exhaled nitric oxide (FENO) measurements have been utilized as a non-invasive tool for monitoring airway inflammation [1]. In asthma, one of the conditions most examined by this technique, FENO values have been used to understand the pathophysiology [2], guide diagnosis [3], predict exacerbations [4–6] and guide treatment [7, 8]. One area where this non-invasive monitoring may be helpful is for asthmatic athletes as they train for competitive events. The effect of exercise training on airway inflammation in asthma is not clear. Exercise can increase ventilation by up to 200 L min−1. Hyperpnea has been shown to lead to the loss of water and heat from the airways; this drying and cooling can lead to bronchial hyperesponsiveness and the symptoms associated with the clinical syndrome of asthma [9, 10].
Exercise-induced bronchoconstriction impacts the ability of athletes to train and compete at high levels of physical exertion [11, 12]. Perhaps even more concerning than the continual rise in asthmatics at the Olympic level is the recent data indicating a high prevalence of asthma at the level of college varsity athletics and the high risk of death during these athletic events due to asthma [13, 14]. While athletes do not always have classical asthma, it is important to have an objective test to monitor exercise-induced bronchoconstriction. Athletes need to carefully monitor their asthma, especially when training to compete at levels approaching their physical limits. Asthma symptoms have been shown to be poor predictors of asthma [15, 16]. Thus, athletes need a more reliable method for monitoring their asthma in order to prevent the onset of exacerbations during training and competition as well as to optimize their performance. Spirometry and peak flow measurement can be difficult to interpret in the realm of elite athletes, who often operate at lung function well above normal: lung function classified as ‘normal’ for the general population may actually be a significant decrease in function for a competitive swimmer. With these issues in mind, we considered that FENO would be an excellent non-invasive method for monitoring inflammation in the airways of athletes during training.
In this case study we hypothesized that in the course of training an asthmatic individual (n = 1) may experience worsening of lung inflammation reflected in FENO levels that may be too subtle to detect by conventional methods like spirometry and peak flow measurements. This patient had a borderline obstructive ventilatory defect with normal gas exchange but with air trapping and hyperventilation. Decreased expiratory gas flows were present at all lung volumes in this patient. Detailed baseline characteristics of this patient are given in table 1. The patient’s asthma was well managed during this study with inhaled corticosteroids and salmeterol.
Table 1.
Demographic and lung function information of the patient.
| Age | 23 |
| Gender | Female |
| Race | Caucasian |
| FVC (L) | 3.86 |
| % predicted | 97% |
| FEV1 (L) | 2.99 |
| % predicted | 88% |
| FEV1/FVC (%) | 77 |
| DLCO (mL min−1 mmHg−1) | 24.42 |
| % predicted | 86% |
| FENO measured by desktop NIOX (ppb) | 26.9 |
| FENO measured by portable analyzer (ppb) | 32.5 |
Abbreviations: forced vital capacity (FVC); forced expiratory volume (FEV1); forced expiratory flow (FEF); diffusion capacity of lung for carbon monoxide (DLCO); fractional exhaled nitric oxide (FENO); parts per billion (ppb).
Changes in lung function during training were monitored in this patient. An increase in inflammation (FENO) and a decrease in lung function (FEV1, PEF) during training for long-distance runs were expected. The number of consecutive miles run in the past 12 h, asthma-related symptoms (cough, etc), food and drink consumed in the past 4 h and medications taken in the past 4 h were noted every weekday in the morning (8–10 am) and afternoon (2–4 pm) at least 2 h after completing a run. The Aerocrine NIOX online system was used as the gold standard for FENO measurements; two portable online devices (MINO) were also used to assess the validity of portable devices for measuring FENO. A baseline was set before training began: average NO using the desktop system was 26.9 ppb while that for the portable device was 32.5 ppb. Peak flows were determined by Piko-1 (Pulmonary Data Services, Inc.); spirometry was performed once a week and FVC, FEV1, PEF and FEF25–75% were noted. All measurements were done at least 2 h after exercise. This was all done for a total of 7 weeks.
Average FENO levels measured on the standard desktop system correlated well with the two portable MINO analyzers (r2 = 0.73, r2 = 0.74; p < 0.0001); additionally, there was a strong correlation between the two MINO devices (r2 = 0.88; p < 0.0001). Although a strong relationship existed between the types of devices, we found that one of the portable devices consistently produced higher NO values: the mean MINO B reading was 7.2 ppb greater than that of the desktop machine (p = 0.26). The same was not true for the other MINO device (MINO A): no statistically significant difference was found between the NO values in MINO A and the desktop NIOX machine. Michils et al [17] recently found similar results: MINO results highly correlated to the desktop analyzer but MINO results were consistently higher than that of the desktop analyzer [17]. This group found that differences in expiratory pressure explain the difference observed in FENO levels measured with the two devices [17].
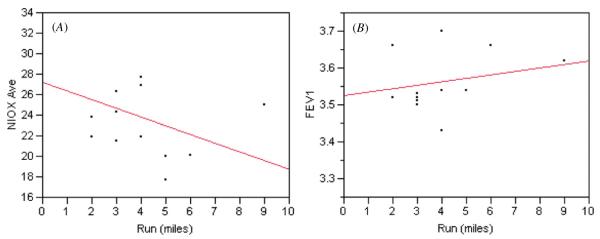
As the miles run by our patient increased, FENO measured by the standard desktop system decreased while FEV1 increased (figure 1). As the number of miles run increased, average NO levels determined by the desktop system decreased; a strong negative correlation was found between the number of miles run and FENO, regardless of the device used (NIOX r2 = −0.57; MINO A r2 = −0.53; MINO B r2 = −0.53; all p < 0.05 except NIOX p = 0.084). FEV1 and PEF, however, did not change significantly as the miles run increased (FEV1 r2 = 0.20 p = 0.40; PEF r2 = 0.11 p = 0.17). Relationships were very significant (p < 0.0001) between MINO A and MINO B, NIOX and MINO A, NIOX and MINO B, distance run and NIOX, distance run and MINO A, distance run and MINO B (table 2).
Figure 1.
Effect of miles run on average FENO measured by the desktop NIOX system (A) and peak flow FEV1 (B). (This figure is in colour only in the electronic version)
Table 2.
Multivariate analysis of the correlations between miles run, FENO measured by a desktop analyzer (NIOX average) and two portable analyzers (MINO A average, MINO B average), FEV1, and PEF. Significance probabilities are indicated in parenthesis.
| Run (miles) | NIOX average | MINO A average | MINO B average | FEV1 | PEF | |
|---|---|---|---|---|---|---|
| Run (miles) | 1 | −0.57 (p = 0.084) |
−0.53 (p = 0.046) |
−0.53 (p = 0.021) |
0.24 (p = 0.40) |
0.11 (p = 0.17) |
| NIOX average | −0.57 (p = 0.084) |
1 | 0.73 (p < 0.0001) |
0.74 (p < 0.0001) |
−0.37 (p = 0.19) |
0.23 (p = 0.073) |
| MINO A average | −0.53 (p = 0.046) |
0.73 (p < 0.0001) |
1 | 0.88 (p < 0.0001) |
−0.14 (p = 27) |
0.22 (p = 0.45) |
| MINO B average | −0.53 (p = 0.021) |
0.74 (p < 0.0001) |
0.88 (p < 0.0001) |
1 | −0.25 (p = 0.27) |
0.34 (p = 0.17) |
| FEV1 | 0.24 (p = 0.40) |
−0.37 (p = 0.19) |
−0.14 (p = 0.27) |
−0.25 (p = 0.27) |
1 | −0.22 (p = 0.66) |
| PEF | 0.11 (p = 0.17) |
0.23 (p = 0.073) |
0.22 (p = 0.45) |
0.34 (p = 0.17) |
−0.22 (p = 0.66) |
1 |
Abbreviations: average FENO measured by desktop analyzer (NIOX average); average FENO measured by portable analyzer A (MINO A average); average FENO measured by portable analyzer B (MINO B average); forced expiratory volume in 1 s (FEV1); peak expiratory flow (PEF).
We expected that lung inflammation, as reflected by NO levels, would increase with the amount of miles run for training. We also expected that this change in the inflammatory status of the airways would be too subtle to detect with traditional methods like spirometry and peak flow. We found no significant change in FEV1 and PEF during training, supporting our hypothesis that these methods were not sensitive in detecting small changes in lung function over the course of training. However, exercise training was associated with a decrease (improvement) in NO levels in this individual with mild asthma, the opposite of what was expected to occur in inflammatory processes associated with asthma and exercise. The fact that NO levels were strongly related to the amount of training while PEF and FEV1 were not, suggests that exhaled NO levels may be more sensitive to changes in the airway as a result of exercise than traditional pulmonary function testing. The negative relationship between number of miles run and NO levels suggests that inflammation in the lungs of an asthmatic may actually decrease during a well-managed training regimen or that inflammation is not a major mechanism in exercise-induced asthma. Larger studies need to be done in order to determine the inflammatory status in the lungs during endurance training in athletes with more severe asthma.
The decrease in exhaled nitric oxide during a training regimen may also be a result of the increased cardiac output that occurs during exercise: as the cardiac output increases, the ability of the pulmonary vasculature to take up NO may increase, thereby leading to an overall decrease in the amount of NO exhaled in breath. The traditional medical concern is that asthma may limit the athlete’s ability to compete. While the exact role of NO in asthma remains elusive, the major known physiologic role of NO is to relax smooth muscle cells through the activation of guanylate cyclase to produce cyclic GMP (cGMP) [18]. This makes it the most potent endogenous vasodilator, but it can also act as a bronchodilator. This dual function of vasodilation and bronchodilation improves ventilation perfusion matching and possibly exercise performance [19]. This beneficial function of NO has to be balanced, however, against the potential harm of very high NO levels associated with airway inflammation and poorly controlled asthma [2]. While the optimal NO level that is associated with improved performance is not clear, recent studies clearly suggest that using FENO as a guide to treat asthma can result in optimal control with less medication [20]. This idea of optimal control with least medications is an important goal in all individuals with asthma, but it has special importance in athletes who have to deliver peak physical performance. While this FENO based management approach makes intuitive physiologic sense, more data are needed.
Interestingly, the number of elite Olympic athletes with asthma has increased steadily since the Olympic Committee began monitoring use of β2-agonists in 1996 [15]. Exhaled NO measurement represents an opportunity for the scientific community to gain information about what is occurring at the level of the airways in elite athletes. As a non-invasive method, exhaled NO measurement should not interfere with athletic performance, while providing invaluable information about airway inflammation that cannot be obtained by other traditional methods used to assess lung function. The recent availability of portable NO measurement devices and their validity make exhaled NO measurements accessible in the field, at the pool and on the track. Collection of these data would help current athletes safely monitor their asthma control during competition and provide valuable data for reference ranges that will assist clinicians in understanding the inflammatory status of airways in athletes during training and competition.
Footnotes
Conflict of interest The authors declare no conflict of interest in this article.
References
- [1].Grob NM, Dweik RA. Exhaled nitric oxide in asthma. From diagnosis, to monitoring, to screening: are we there yet? Chest. 2008;133:837–9. doi: 10.1378/chest.07-2743. [DOI] [PubMed] [Google Scholar]
- [2].Dweik RA, et al. NO chemical events in the human airway during the immediate and late antigen-induced asthmatic response. Proc. Natl Acad. Sci. USA. 2001;98:2622–7. doi: 10.1073/pnas.051629498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Taylor DR. Nitric oxide as a clinical guide for asthma management. J. Allergy Clin. Immunol. 2006;117:259–62. doi: 10.1016/j.jaci.2005.11.010. [DOI] [PubMed] [Google Scholar]
- [4].Jones SL, et al. The predictive value of exhaled nitric oxide measurements in assessing changes in asthma control. Am. J. Respir. Crit. Care Med. 2001;164:738–43. doi: 10.1164/ajrccm.164.5.2012125. [DOI] [PubMed] [Google Scholar]
- [5].Harkins MS, Fiato KL, Iwamoto GK. Exhaled nitric oxide predicts asthma exacerbation. J. Asthma. 2004;41:471–6. doi: 10.1081/jas-120033990. [DOI] [PubMed] [Google Scholar]
- [6].Pijnenburg MW, et al. Exhaled nitric oxide predicts asthma relapse in children with clinical asthma remission. Thorax. 2005;60:215–8. doi: 10.1136/thx.2004.023374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Beck-Ripp J, et al. Changes of exhaled nitric oxide during steroid treatment of childhood asthma. Eur. Respir. J. 2002;19:1015–9. doi: 10.1183/09031936.02.01582001. [DOI] [PubMed] [Google Scholar]
- [8].Yates DH, et al. Effect of a nitric oxide synthase inhibitor and a glucocorticosteroid on exhaled nitric oxide. Am. J. Respir. Crit. Care Med. 1995;152:892–6. doi: 10.1164/ajrccm.152.3.7663801. [DOI] [PubMed] [Google Scholar]
- [9].Anderson SD, et al. Sensitivity to heat and water loss at rest and during exercise in asthmatic patients. Eur. J. Respir. Dis. 1982;63:459–71. [PubMed] [Google Scholar]
- [10].Godfrey S. Controversies in the pathogenesis of exercise-induced asthma. Eur. J. Respir. Dis. 1986;68:81–8. [PubMed] [Google Scholar]
- [11].Haahtela T, Malmberg P, Moreira A. Mechanisms of asthma in Olympic athletes—practical implications. Allergy. 2008;63:685–94. doi: 10.1111/j.1398-9995.2008.01686.x. [DOI] [PubMed] [Google Scholar]
- [12].Carlsen KH, Kowalski ML. Asthma, allergy, the athlete and the Olympics. Allergy. 2008;63:383–6. doi: 10.1111/j.1398-9995.2008.01630.x. [DOI] [PubMed] [Google Scholar]
- [13].Parsons JP, et al. Prevalence of exercise-induced bronchospasm in a cohort of varsity college athletes. Med. Sci. Sports Exercise. 2007;39:1487–92. doi: 10.1249/mss.0b013e3180986e45. [DOI] [PubMed] [Google Scholar]
- [14].Becker JM, et al. Asthma deaths during sports: report of a 7-year experience. J. Allergy Clin. Immunol. 2004;113:264–7. doi: 10.1016/j.jaci.2003.10.052. [DOI] [PubMed] [Google Scholar]
- [15].Bousquet J, et al. Allergic rhinitis and its impact on asthma (ARIA) 2008 Update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen) Allergy. 2008;63(Suppl. 86):8–160. doi: 10.1111/j.1398-9995.2007.01620.x. [DOI] [PubMed] [Google Scholar]
- [16].Rundell KW, et al. Self-reported symptoms and exercise-induced asthma in the elite athlete. Med. Sci. Sports Exercise. 2001;33:208–13. doi: 10.1097/00005768-200102000-00006. [DOI] [PubMed] [Google Scholar]
- [17].Michils A, et al. Comparisons between portable and chemoluminescence exhaled nitric oxide measurements. Eur. Respir. J. 2008;32:243–4. doi: 10.1183/09031936.00025308. [DOI] [PubMed] [Google Scholar]
- [18].Dweik RA. Pulmonary hypertension and the search for the selective pulmonary vasodilator. Lancet. 2002;360:886–7. doi: 10.1016/S0140-6736(02)11067-1. [DOI] [PubMed] [Google Scholar]
- [19].Dweik RA, et al. Nitric oxide synthesis in the lung. Regulation by oxygen through a kinetic mechanism. J. Clin. Invest. 1998;101:660–6. doi: 10.1172/JCI1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Smith AD, et al. Use of exhaled nitric oxide measurements to guide treatment in chronic asthma. N. Engl. J. Med. 2005;352:2163–73. doi: 10.1056/NEJMoa043596. [DOI] [PubMed] [Google Scholar]