Abstract
Plasmodium falciparum resistance to chloroquine and sulphadoxine–pyrimethamine has led to the recent adoption of artemisinin-based combination therapies (ACTs) as the first line of treatment against malaria. ACTs comprise semisynthetic artemisinin derivatives paired with distinct chemical classes of longer acting drugs. These artemisinins are exceptionally potent against the pathogenic asexual blood stages of Plasmodium parasites and also act on the transmissible sexual stages. These combinations increase the rates of clinical and parasitological cures and decrease the selection pressure for the emergence of antimalarial resistance. This Review article discusses our current knowledge about the mode of action of ACTs, their pharmacological properties and the proposed mechanisms of drug resistance.
Six decades ago, hopes of malaria eradication were raised by the discovery and implementation of chloroquine (CQ). The use of this highly effective, fast-acting and inexpensive 4-aminoquinoline, along with the potent insecticide dichlorodiphenyltrichloroethane (DDT), quickly proved successful in substantially reducing the incidence and severity of malaria worldwide. Initial successes were achieved primarily in regions with temperate climates and seasonal malaria transmission. However, in many parts of the world eradication efforts were effectively thwarted by multiple issues, including insecticide resistance in Anopheles mosquito vectors, high rates of Plasmodium parasite transmission, logistical hurdles to implementing control strategies, wars and population displacements, and a lack of sustained funding. Subsequently, resistance to CQ and the cost-effective replacement drug sulphadoxine–pyrimethamine (SP) emerged in the most lethal human malarial pathogen, Plasmodium falciparum1,2. In some areas, the switch to either mefloquine (MFQ) or quinine resulted in the appearance of multidrug-resistant parasites, particularly in Southeast Asia3. The global consequence was a resurgence of malaria morbidity and mortality. With nearly 40% of the global population at risk, 300–660 million episodes of clinical P. falciparum malaria occur annually and there are an estimated one million deaths4. Most of these occur in sub-Saharan Africa (FIG. 1a), where rates of transmission can reach 1,500 mosquito-delivered parasite inoculations per individual per year5.
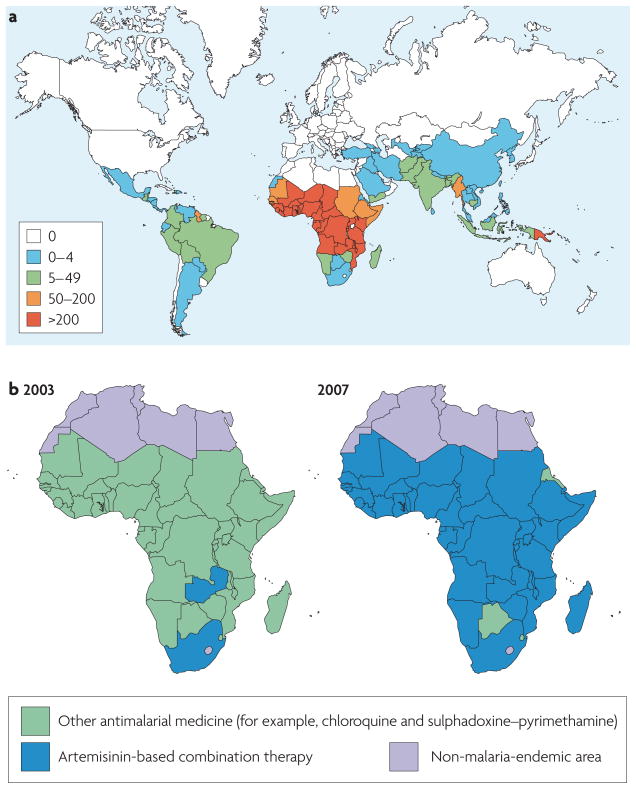
Figure 1. The worldwide incidence of malaria and the rapid adoption of artemisinin-based combination therapies across sub-Saharan Africa.
a | The estimated incidence of malaria worldwide in 2006, stratified per 1, 000 population. Cases in Africa constituted 86% of the global total, Southeast Asia accounted for 9% and the eastern Mediterranean region had 3%. Plasmodium falciparum was found to be responsible for over 75% of the cases in most sub-Saharan African countries but was second to Plasmodium vivax in most countries outside Africa. b | The official first-line antimalarial policy in Africa in 2003 and 2007, demonstrating the dramatic shift from a diversity of first-line antimalarials (typically chloroquine or sulphadoxine–pyrimethamine) towards the adoption of artemisinin-based combination therapies. Part a image is modified, with permission, from REF. 106 © (2008) WHO. Part b image is modified, with permission, from REF. 140 © (2007) UNICEF.
Now there is hope that the tide may turn again with the implementation of artemisinin-based combination therapies (ACTs). Their success in treating CQ- and SP-resistant malaria has prompted the WHO to recommend ACTs as the preferred first-line antimalarials against P. falciparum malaria and has elicited substantial funding and logistical support from, among others, The Global Fund to Fight AIDS, Tuberculosis, and Malaria, The World Bank, Roll Back Malaria, the President’s Malaria Initiative, the Medicines for Malaria Venture, and the Bill and Melinda Gates Foundation. ACTs have now been widely adopted across sub-Saharan Africa (FIG. 1b). Their outstanding efficacy, together with complementary interventions that reduce transmission, such as long-lasting insecticide-treated bed nets and indoor residual insecticide spraying, has led to a renewed call for eradicating malaria6.
Is eradication, or even progressive elimination, feasible? To begin to address this, we review artemisinin (ART) derivatives and their partner drugs in terms of their modes of action, pharmacokinetic properties and proposed mechanisms of antimalarial resistance. We also refer to therapeutic strategies that might decrease the emergence of drug resistance and, finally, we present a perspective on the current ACT-based efforts to reduce the burden of malaria.
ARTs
Discovery and synthesis
A major advance in the search for effective treatments for drug-resistant malaria came with the isolation of ART (also known as qinghaosu) from the Chinese wormwood Artemisia annua7 (Supplementary information S1 (figure)). This sesquiterpene lactone endoperoxide is extremely potent against CQ- and SP-resistant P. falciparum in vitro and in vivo and can produce faster parasite clearance and fever resolution times than any other licensed antimalarial, including quinine8. However, ART must first be extracted and then chemically modified to produce semisynthetic derivatives with improved pharmacological properties (see below), which adds significantly to the cost of ACT therapy. As an alternative, Saccharomyces cerevisiae has been engineered to synthesize artemisinic acid, a precursor of ART9. This scalable production system has the potential to reduce the cost of goods and ensure a steady supply of the drug, thereby possibly alleviating two major concerns as the world transitions to first-line therapy with ACTs.
Artemisinin derivatives
Current ACTs use the ART derivatives artemether (ATM), artesunate (AS) or dihydroartemisinin (DHA) (Supplementary information S1 (figure)), which have improved oral bioavailability compared with that of ART. In humans, these derivatives rapidly achieve peak plasma levels and possess elimination half-lives of approximately 1–3 hrs8,10 (TABLE 1). Their metabolism is partly mediated by the cytochrome P450 enzymes CYP2B6 and CYP3A4 (REF. 10). Bioconversion of ATM and AS produces DHA along with other metabolites.
Table 1.
Plasma half-lives of drugs used in artemisinin-based combination therapies
| Antimalarial | t1/2 of artemisinin derivative | t1/2of partner drug | Regions currently in use* |
|---|---|---|---|
| Artemether–lumefantrine | ~3 hr | 4–5 days | Africa, EM, SE Asia, WP and SA |
| Artesunate–mefloquine | <1 hr | 14–21 days | Africa, SE Asia, WP and SA |
| Artesunate–amodiaquine | <1 hr | 9–18 days‡ | Africa and EM |
| Dihydroartemisinin–piperaquine | 45 min | ~5 weeks | SE Asia |
| Artesunate–pyronaridine§ | <1 hr | 16 days | NA |
| Chloroquine|| | NA | 1–2 months | Africa, EM, SE Asia, WP and SA |
| Sulphadoxine–pyrimethamine|| | NA | ~4 days (S) or ~8 days (P) | Africa, EM (IPT in Africa, EM and WP) |
This refers to the t1/2 of the active metabolite monodesethylamodiaquine; the t1/2 of amodiaquine is ~3hr.
Recently completed Phase III trials.
These former first-line antimalarials are included as a reference. EM, eastern Mediterranean; IPT, intermittent preventive treatment; NA, not applicable; P, pyrimethamine; S, sulphadoxine; SA, South America; SE Asia, Southeast Asia; t1/2, half-life; WP, Western Pacific.
Mechanisms of action and parasite resistance
The mechanisms by which ARTs exert their antimalarial activity remain contentious11. Nevertheless, most studies concur that the activity of ART and many if not all of its potent derivatives results from reductive scission of the peroxide bridge by reduced haem iron, which is produced inside the highly acidic digestive vacuole (DV) as it digests haemoglobin. In support of this, a recent study with fluorescent ART trioxane derivatives provided evidence for their rapid accumulation in the DV and their activation by neutral lipid-associated haem12. Other studies with fully synthetic endoperoxides (including trioxolanes and trioxaquines) as well as ART found that these compounds can alkylate haem, both in vitro with P. falciparum and in vivo in rodent malaria models, and identified a correlation between the degree of alkylation and the potency of trioxolanes against cultured P. falciparum asexual blood-stage parasites13–16. In addition to the formation of potentially toxic haem-adducts, activated ART (for which the site of action remains unclear; FIG. 2) might in turn generate free radicals that alkylate and oxidize proteins and possibly lipids in parasitized erythrocytes17–19. In agreement with this, ART activity can be potentiated by oxygen and oxidizing agents and attenuated by reducing agents17. Alternatively, ART derivatives might be activated by peroxide bond cleavage by intracellular iron–sulphur redox centres, which are found in multiple Plasmodium spp. enzymes. Subsequent alkylation of these iron–sulphur-containing enzymes could result in parasite death20. A role for non-haem iron sources in the activation of ART is supported by the antagonism between multiple endoper-oxides (including ART) and chelators that are specific to nonhaem iron21. Recent transmission electron microscopy studies also indicate that ART and other endoperoxides can compromise the integrity of the DV in a dose- and time-dependent manner, before any effect is observed on the endoplasmic reticulum and the mitochondria22.
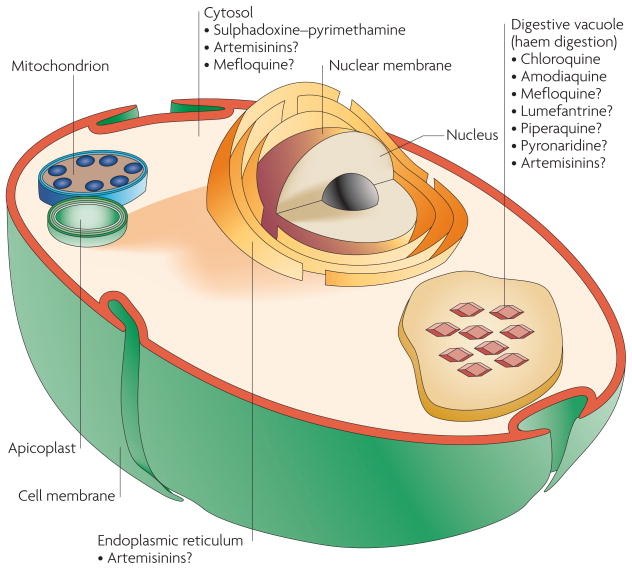
Figure 2. Site of action of antimalarial drugs.
Depiction of an intra-erythrocytic Plasmodium falciparum parasite and the proposed target sites of several key antimalarials.
Investigations into potential protein targets of ARTs have included studies with radiolabelled ART that identified several covalently modified proteins, suggesting that their alkylation and inactivation might account for parasite death23,24. Separate studies have focused on PfATP6, a P. falciparum SERCA-type calcium-dependent ATPase25 in the endoplasmic reticulum. In transfected Xenopus laevis oocytes, PfATP6 activity is abolished by ART but is unaffected by other antimalarials, including the inactive compound desoxyartemisinin, which lacks the endoperoxide bridge25. Single nucleotide polymorphisms (SNPs) in pfatp6 were observed in some P. falciparum isolates from French Guiana and Senegal that displayed reduced in vitro susceptibility to ARTs, suggesting a causal association26. However, a recent analysis of pfatp6 sequence conservation in 388 clinical isolates from 17 different countries identified 33 SNPs, 29 of which were non-synonymous mutations, implying a high degree of genetic diversity27. A direct association between pfatp6 SNPs and decreased ART susceptibility has yet to be experimentally documented in P. falciparum.
Studies with transgenic P. falciparum asexual blood-stage parasites cultured in vitro have found that ART susceptibility can be influenced by genetic changes in the loci encoding P. falciparum multidrug resistance protein 1 (PfMDR1; also known as Pgh-1) and P. falciparum chloroquine resistance transporter (PfCRT). Point mutations in both genes, as well as pfmdr1 gene duplications, are known to affect parasite responses to diverse antimalarials. The potencies of these drugs can be altered by their degree of accumulation inside the DV, which is the site of haem detoxification28. Both of these putative transporters are located on the membrane of the DV in intra-erythrocytic parasites, suggesting that they are important regulators of drug accumulation in the DV. An association between increased pfmdr1 copy number and a higher risk of treatment failure using AS paired with MFQ has been observed in a clinical study conducted in Thailand29. Transfection studies have since confirmed this association between pfmdr1 amplification and reduced susceptibility to ART and MFQ30. Recent gene disruption evidence now suggests that P. falciparum multidrug resistance-associated protein 1 (PfMRP1), which resides on the parasite plasma membrane, can additionally influence ART susceptibility in vitro31.
Now, evidence has emerged of decreased ART efficacy in P. falciparum isolates from Thailand and Cambodia32,33. This has raised concerns of a potentially imminent spread of ART resistance, especially given that both CQ and SP resistance emerged first in Southeast Asia and subsequently migrated to Africa34,35. This pattern of migration might reflect the substantial drug pressure that is applied to malarial infections in Southeast Asia, where hosts have minimal immunity and there is little competition between parasite clones in vivo because of the low rates of transmission (BOX 1). This would favour the dissemination of drug-resistant parasites even if they harboured mutations that conferred a fitness cost, because they would rarely encounter drug-sensitive parasites in the same host and would be frequently subjected to drug pressure36. Southeast Asian parasites might also have a particular capacity to hypermutate in response to drug pressure37. Clinical groups are currently documenting cases of delayed parasite clearance times in patients who were given ART monotherapy or ACTs. Genomic analysis of such isolates, using the recently developed methods of rapid genome sequencing, haplotype maps and genome tiling arrays, should yield candidate genes that can be evaluated using transfection-based approaches38–40. In a separate approach using the rodent parasite model Plasmodium chabaudi, researchers have now selected genetically stable parasite lines that are resistant to ART and AS41. These parasites lacked mutations in the P. chabaudi homologues of pfatp6 and pfmdr1. Further genetic analysis identified a SNP in each of the resistant clones in the gene that encodes a putative deubiquitinating protease termed UBP-1 (REF. 42). Such studies with human parasites and rodent models should enable the identification of molecular markers that can be used to track the emergence and spread of P. falciparum parasites with reduced ART susceptibility.
Box 1. A complex disease.
One of the main challenges that affects malaria control efforts is the diversity of epidemiological contexts in different malaria-endemic regions. One key variable involves disease transmission. The entomological inoculation rate (that is, the number of infectious bites per person per year) varies from 1 to 2 in many parts of Southeast Asia up to as high as 1,500 in some settings in Africa115. Factors that influence this rate include human proximity to mosquito larval habitats116, the local mosquito vector (Anopheles gambiae is the main African vector and is widely regarded as the most dangerous because of its vector competence, singular preference for humans over other animals and prolific breeding117) and environmental conditions (including temperature, altitude, humidity and patterns of seasonal rainfall118).
Differences in the transmission and endemicity in malaria-endemic regions have a direct influence on clinical outcomes of infection. Owing to the low transmission rate in Southeast Asia, most infections are symptomatic and therefore treated with antimalarials. This is in contrast to Africa, where more frequent transmission leads to the acquisition of immunity. However, this immunity is not sterilizing and typically manages to only lower the parasite burden and reduce the clinical impact. Immunity acquired in Africans who live in areas of moderate to high levels of transmission is referred to as premunition. This refers to an immune state that is contingent on chronic infection and that rapidly wanes if the resident moves to a non-malarious region for a period of months to years119. As a result of the partial immunity that typically develops in repeatedly infected Africans, many cases of malaria on that continent are asymptomatic and are therefore not treated with antimalarials120. This in turn substantially influences the dynamics of intrahost parasite competition and the spread of drug resistance121.
In addition to mosquito, environmental and parasite factors, host factors affect both the burden and severity of disease. For a discussion of the key host factors, readers are directed to recent comprehensive reviews122–124.
ACTs
The poor pharmacokinetic properties of ART and its derivatives, including the short half-lives of this chemical class, translate into substantial treatment failure rates when used as monotherapy43. Combining a member of this class with a longer-lasting partner drug assures sustained anti-malarial pressure after the plasma concentrations of the ART derivatives have fallen below therapeutic levels. This increases the antimalarial treatment efficacy and reduces the selective pressure for resistance. Ideally, antimalarial combination drug partners would have similar pharmacokinetic properties so that no drug is left ‘unprotected’ by the other. That said, ACTs benefit substantially from the ability of the ART derivative to rapidly reduce the parasite biomass, resulting in few parasites to be cleared by the partner drug and reducing the pool of parasites from which resistance can emerge. Below, we present the key partner drugs that are currently in use. In addition, SP has also been used in combination with the derivative AS44. However, this combination is not reviewed, owing to the increasing prevalence of SP-resistant parasites that reduce treatment efficacy and the availability of a recent and comprehensive review2.
Mefloquine
MFQ, a fluorinated 4-quinoline, is moderately well absorbed orally and possesses an elimination half-life of 2–3 weeks, partly owing to its high lipophilicity and extensive tissue distribution. Earlier work showed that MFQ associates with intra-erythrocytic haemozoin45. However, evidence that this association might be secondary to a primarily cytosolic mode of action comes from studies with transgenic P. falciparum lines expressing different pfmdr1 copy numbers, which observed that reduced parasite susceptibility to MFQ (and ART) was associated with increased PfMDR1-mediated solute import into the DV46. As alluded to earlier, human clinical data, as well as in vitro studies, reveal that pfmdr1 gene amplification is a major determinant of MFQ resistance and is associated with an increased risk of treatment failure and recrudescence with MFQ monotherapy or AS–MFQ combination therapy29,47. Point mutations in pfmdr1 can also alter P. falciparum susceptibility to MFQ in vitro, although this does not seem to substantially affect the treatment outcome29.
MFQ was first introduced as a first-line antimalarial in Thailand in the mid 1980s, but resistance emerged in a few years3. Therefore, in 1994 MFQ was paired with AS (AS–MFQ) as a 3-day combination regimen. The main clinical benefits of AS–MFQ were made evident by its 100% cure rate measured in 1998, versus a cure rate of 71% with MFQ monotherapy measured in 1990 (REF. 48). Further investigations into the pharmacokinetic–pharmacodynamic properties of AS–MFQ are required to better understand why this combination has remained so effective, even against highly MFQ-resistant parasites49,50.
Lumefantrine
Lumefantrine (LMF; also called benflumetol) is structurally related to the hydrophobic arylamino-alcohol antimalarials, including MFQ, quinine and halofantrine (Supplementary information S1 (figure)), suggesting that they have similar modes of action51. The selection of ATM and LMF as a combination (known as Coartem; Novartis/Chinese Academy of Medical Military Sciences) stems in part from their reported synergistic effects against P. falciparum in vitro52. The 3-day, 6-dose ATM–LMF regime has proven highly effective in treating P. falciparum infections50 and is prioritized by the WHO as a replacement for CQ and SP monotherapies. The pharmacokinetic properties of LMF include a large apparent volume of distribution and a terminal elimination half-life of 4–5 days53. Human pharmacokinetic and pharmacodynamic studies correlate the risk of clinical failure (the likelihood to recrudesce) with plasma LMF concentrations falling below 280 ng per ml54. These findings, combined with the report of up to 15-fold variability in LMF plasma concentrations in clinical trial volunteers55, highlight the importance of appropriate dosing with LMF-containing combinations.
There has been considerable debate about the probability of parasites developing resistance to LMF in Africa. Some suggest that the emergence of resistance will be slower in Africa than in Southeast Asia because of the intrinsic differences of the parasites in intrahost parasite competition, drug exposure and host immunity (BOX 1). Alternatively, the high transmission rates in Africa and subsequent exposure of many parasites to subtherapeutic concentrations of this drug might repeatedly select for the emergence and subsequent dissemination of resistant parasites56. In vitro investigations have reported some cross-resistance between LMF and MFQ and have identified copy number changes and SNPs in pfmdr1 that can decrease parasite susceptibility to both agents30,57. ATM–LMF treatment failure has been associated in Asia with pfmdr1 amplification and in Africa with selection for certain PfMDR1 polymorphic residues (Asn86, Phe184 and Asp1246)57–59. In a study from Zanzibar, selection for pfmdr1 variants seemed to be operating mainly in re-infections that occurred during the elimination phase of LMF60. Recent clinical and in vitro studies have also observed that mutant pfcrt alleles that confer CQ resistance can enhance susceptibility of the parasite to LMF and ART61. These findings are consistent with the excellent clinical efficacy of this combination in regions where CQ resistance remains highly prevalent. In 2008, Coartem was used to treat 70 million cases of malaria, most of which were in Africa. A dispersible formulation of Coartem has also been developed for babies and children through a partnership between Novartis and the Medicines for Malaria Venture. This dispersible formulation has been found to be highly effective, achieving a 98% clinical cure rate in a large study involving several African countries62. It has a sweet fruit flavour and costs under US$0.40 per child for public-sector buyers, making this an attractive ACT for the treatment of malaria in children.
Amodiaquine
Amodiaquine (AQ), a potent 4-amino-quinoline antimalarial, is used infrequently because of reported toxicity. This toxicity includes instances of drug-induced agranulocytosis and hepatitis, which are thought to result from AQ bio-activation to a protein-reactive quinoneimine metabolite63. These adverse events, however, were generally associated with the use of AQ as a prophylactic. A meta-analysis of clinical studies found that therapeutic AQ regimes with a total dose of up to 35 mg per kg body weight over 3 days were as well tolerated as CQ or SP for the treatment of uncomplicated P. falciparum malaria64. However, one recent study reported that an increased risk of neutropenia was associated with AQ therapy in patients with HIV who were receiving antiretroviral therapy65.
In vivo AQ is rapidly converted by hepatic P450 enzymes into monodesethyl-AQ. This metabolite, which retains substantial antimalarial activity, has a half-life in blood plasma of 9–18 days and reaches a peak concentration of ~500 nM 2 hours after oral administration. By contrast, AQ has a half-life of ~3 hours, attaining a peak concentration of ~25 nM within 30 minutes of oral administration66. In vivo clearance rates of AQ, however, display a variation between individuals that ranges from 78 to 943 ml per minute per kg67. One study recently reported an increased risk of treatment failure with parasites that had in vitro monodesethyl-AQ half-maximal inhibitory concentration (IC50) values of above 60 nM68.
AQ is structurally closely related to CQ and might also prevent haem detoxification (see below). Indeed, AQ has been reported to interact with μ-oxo dimers of haem ((FeIII-protoporphyrin IX)2O) in vitro, although this has not been confirmed with cultured parasites69. PfCRT Lys76Thr and PfMDR1 Asn86Tyr substitutions are both associated with decreased susceptibility to AQ and CQ, and CQ-resistant parasites can have reduced accumulation of AQ70–72. However, both in vitro and in vivo evidence suggest that cross-resistance between the two drugs is incomplete and that AQ can remain effective against some CQ-resistant parasites64,73. Analysis of the selective pressure on pfmdr1 before and after treatment with AQ found a positive selection for the Asn86Tyr mutation, contrasting with selection against this mutant allele following ATM–LMF treatment58,59,74.
Currently, AQ is partnered with AS and is available as fixed-dose tablets. Clinical investigations have shown AS–AQ to be an effective ACT with an acceptable safety profile, with the exception of infrequent instances of drug-induced anaemia75. In a comparative ACT trial in Angola, AS–AQ resulted in a lower level of gametocytes than ATM–LMF76. However, a clinical trial in Papua, Indonesia, found that AS–AQ-treated patients with mixed P. falciparum and Plasmodium vivax infections had a higher parasitological failure rate, gametocyte carriage and risk of anaemia than those treated with DHA and piperaquine (PQP)77.
Piperaquine
PQP is a bisquinoline and is also structurally related to CQ. This potent and well-tolerated drug was adopted as the primary antimalarial in China during the 1970s and 1980s in response to increasing CQ treatment failure rates78. Although studies on the mode of action of PQP in P. falciparum remain limited, investigations in the rodent parasite Plasmodium berghei have found that this drug acts mainly on mature asexual blood-stage trophozoites79. PQP is postulated to accumulate in the DV and bind to haem-containing species, inhibiting haem detoxification. PQP-treated P. berghei parasites have swollen DVs and abnormal haemozoin clumping80. PQP is currently being evaluated in combination with DHA (DHA–PQP). Pharmacologically, PQP is characterized by a large volume of distribution and reduced rates of excretion after multiple doses. This lipophilic drug is rapidly absorbed, with a Tmax (time to reach the highest concentration) of 2 hours after a single dose81. In clinical trials, the cure rates, fever and parasite clearance times82,83 of DHA–PQP were similiar to those of AS–MFQ. DHA–PQP was also better tolerated, with no clinically significant cardiovascular or metabolic effects84. One concern was that the percentages of circulating erythrocytes that harboured gametocytes were reportedly higher in patients who received DHA–PQP than in those treated with AS–MFQ, suggesting that DHA–PQP may not be as effective in reducing parasite transmission to mosquito vectors as other combinations83,85.
PQP-resistant P. falciparum has been reported in China, presumably as a result of its one-time widespread use as monotherapy and perhaps worsened by its long elimination half-life (~5 weeks)80. Debate continues on the genetic basis of PQP resistance and the extent to which this affects CQ susceptibility86,87. Clarifying this will provide important biomarkers of resistance to monitor clinical DHA–PQP efficacy.
Pyronaridine
Pyronaridine (PYR) is an acridine-type (benzonaphthyridine) Mannich base. It was first synthesized in China and was introduced as a new anti-malarial drug in 1970. PYR is highly potent against P. falciparum, with mean fever subsidence times of 1–2 days and parasite clearance times of 2–3 days. PYR has an excellent therapeutic index in treating mice infected with P. berghei88. PYR activity is limited to the asexual blood stages, with no apparent efficacy against the preceding asymptomatic liver stages or the intra-erythrocytic gametocyte forms89,90.
PYR inhibits the formation of β-haematin (a synthetic form of haemozoin91) in vitro, forms complexes with haematin, inhibits glutathione-dependent degradation of haem-containing species and enhances haematin-induced lysis of infected erythrocytes92. Furthermore, treatment of P. falciparum and P. berghei with PYR leads to rapid changes in the morphology of their DV93. Additional morphological changes include the formation of multilamellate whorls, swelling of the pellicular complexes and distended and granulated mitochondria93. These effects were also observed in a PYR-treated CQ-resistant line of P. berghei, whereas the DV showed no significant change, suggesting that PYR might also possess a secondary DV-independent mode of action93.
In China, clinical trials against both CQ-sensitive and CQ-resistant P. falciparum infections found PYR monotherapy to be highly effective when administered orally over 2–3 days94. In a trial in Cameroon, PYR was 100% effective at eliminating malarial symptoms after 3 days, versus 44% efficacy for CQ95. However, patient follow-up extended to only 14 days, and a subsequent trial that examined infections that occur more than 28 days after treatment observed a small percentage of recrudescent infections96. AS–PYR has recently completed several large-scale Phase III clinical trials, and initial results demonstrate excellent efficacy97 (I. Borghini-Fuhrer, personal communication).
Artemisinin-based combination therapy efficacy against P. vivax
ACTs also constitute an excellent treatment for P. vivax, the cause of benign tertian malaria and an important malarial pathogen with highest prevalence outside Africa98. In one trial in Papua, Indonesia, DHA–PQP and ATM–LMF were both highly effective in resolving uncomplicated P. vivax malaria, reducing anaemia and decreasing the number of gametocytes99. Late relapses were attributed to the emergence of previously dormant hypnozoites from the liver, a biological characteristic of P. vivax that is not shared by P. falciparum and that is only responsive to primaquine treatment. AS–PYR was also reported to be highly effective in treating P. vivax malaria, with similar cure rates and faster parasite and fever clearance times than those of those of CQ (which continues to be widely used to treat P. vivax malaria)100.
Artemisinin toxicity concerns
Earlier animal studies with ARTs had raised concerns about toxicity, including reports of increased embryo lethality or malformations early post-conception in pregnant rats and rabbits101. Studies in dogs, rats and monkeys also found that ARTs could cause occult brainstem neurotoxicity102. As a precaution, the WHO does not recommend ACT treatment for children weighing ≤5 kg or women in their first trimester of pregnancy. Clinical evidence nevertheless supports the safety of ACTs in children weighing ≥5 kg and pregnant women in subsequent trimesters. One prospective study in a cohort of nearly 500 pregnant women with acute P. falciparum malaria indicated that ARTs were well tolerated during pregnancy, including during the first trimester, with no evidence of adverse effects103. Birth outcomes among treated individuals did not differ greatly from community rates for abortion, stillbirth, congenital abnormality and mean gestation at delivery. A more recent study with 50 pregnant women treated with DHA–PQP found no serious adverse events and no evidence of toxicity for either the fetus or the mother104. Additional studies are required to further evaluate the safety of ARTs and the principal ACTs in these populations105.
Further issues for ACT implementation
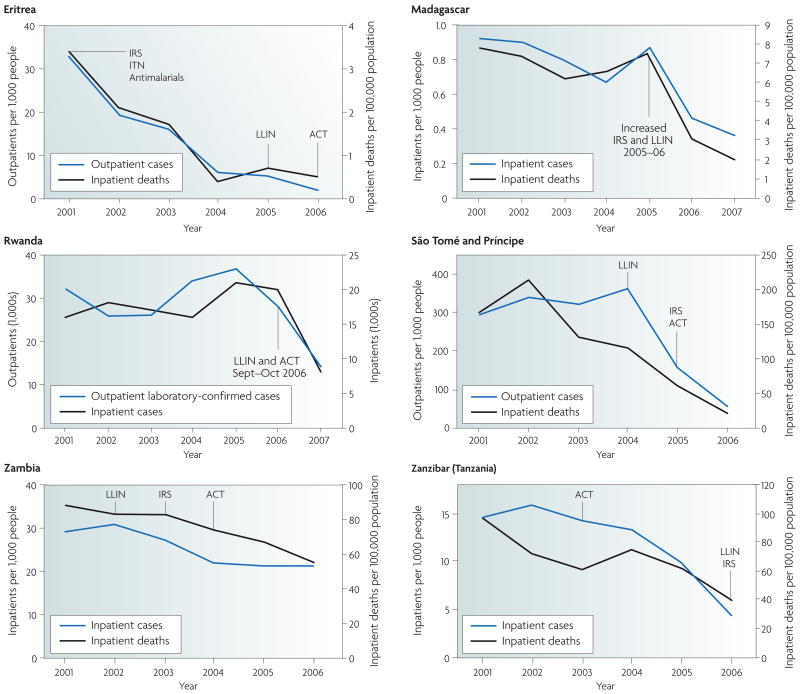
The application of ACTs to combat CQ- and SP-resistant P. falciparum malaria has produced demonstrable improvements in morbidity and mortality rates in Africa106. Several examples of this are illustrated in FIG. 3. In addition to parasite resistance, multiple other factors affect the effectiveness of these ACTs, especially their pharmacokinetic properties. A notable example is LMF, the clinical cure rates of which were found to significantly improve when the drug was administered with a high fat meal54, and ATM–LMF efficacy was found to be dramatically enhanced using a six-dose regimen as opposed to a four-dose regimen57.
Figure 3. Recent trends in malaria cases and deaths.
The graphs show the recent trends in malaria cases and deaths in six African countries following the implementation of malaria control programmes based on the use of: indoor residual spraying (IRS); insecticide-treated bed nets (ITN), including the more recent long-lasting insecticide-treated bed nets (LLIN); and an artemisinin-based combination therapy (ACT), typically artesunate–amodiaquine or artemether–lumefantrine. Figure is modified, with permission, from REF. 106 © (2008) WHO.
As ACTs become the first-line antimalarial therapy, increased selection pressure will be placed on this valuable resource. Comprehensive and standardized monitoring programmes will be essential to assess therapeutic efficacies, determine drug susceptibilities in vitro, screen for resistance markers and distinguish recrudescences from reinfections in treated patients107,108. These programmes should benefit from the WorldWide Antimalarial Resistance Network (WWARN), which was established to coordinate antimalarial resistance monitoring109. Maximizing the efficacy and longevity of ACTs will require the continued development of supply and distribution infrastructures, adequate health care worker training in ACT usage, sustained financial support of implementation programmes and a detailed understanding of antimalarial efficacy.
However, the prevalence of counterfeit or clinically substandard ACTs that contain small quantities of the ART derivative threatens to subvert ACT efficacy. This provides an ideal mechanism for the selection of resistance. Recent estimates are that 33%–53% of all ACT tablets in mainland Southeast Asia are counterfeit110. One study carried out in six African countries also documented substandard medicine in 35% of ACTs purchased from private pharmacies and found ART monotherapy to be common despite the appeal by the WHO in January 2006 to halt its production111. In addition, although ACTs have clearly proven effective for the treatment of malaria in Southeast Asia, where transmission is typically low, concerns remain about their long-term implementation as first-line therapy in high-transmission areas in Africa. This is exacerbated by the crucial dependence on ART derivatives in virtually all of the existing antimalarial combination therapies, creating a substantial selection advantage for parasites with decreased susceptibility to ARTs. Evidence for the emergence of these parasites in parts of Southeast Asia32,33 necessitates urgent containment measures and underscores the need for the continual development of alternative antimalarials112 and new treatments using existing drugs (BOX 2).
Box 2. Drug rotations and chemosensitizing agents.
If faced with the spread of artemisinin-resistant Plasmodium falciparum, one potential strategy could be to introduce chloroquine (CQ)-containing combinations that maintain efficacy against CQ-resistant strains. CQ is inexpensive and has a good safety profile. It has a well-characterized mode of action (inhibiting haem detoxification by binding to haemozoin, an immutable host factor51,91), and resistance to CQ has been clearly attributed to multiple point mutations in the P. falciparum CQ resistance transporter (PfCRT)125,126. The PfCRT Lys76Thr mutation in particular has proved to be a highly sensitive molecular marker of CQ resistance, providing an effective epidemiologic tool to assess CQ efficacy28. Recent studies in Malawi showed that the absolute removal of CQ for a decade led to the virtual disappearance of parasite strains harbouring mutant pfcrt and restoration of CQ clinical efficacy to >98% (REF. 127). This has been attributed to a fitness cost associated with the Lys76Thr mutation, as recovery of the CQ-sensitive phenotype was associated with the expansion of wild-type pfcrt from the endogenous population rather than a genetic reversion128. Decreases in the prevalence of parasites possessing PfCRT Lys76Thr have also been observed in Gabon, Vietnam, China and Thailand129. Resistance reversal agents would potentially reduce the large selection pressure that CQ would exert on parasite reservoirs with mutant pfcrt alleles. One such agent is the antihistamine chlorpheniramine, which has been demonstrated clinically to ameliorate CQ efficacy130. Indeed, a recent study found comparable mean fever clearance times between groups treated with CQ–chlorpheniramine or amodiaquine partnered with sulphadoxine–pyrimethamine (AQ–SP). However, CQ–chlorpheniramine produced delayed parasite clearance times and increased failure rates relative to those of AQ–SP131. More potent antimalarials with reversal properties will need to be identified for this strategy to be widely implemented.
The eradication of malaria?
The success of the ACTs has led to a new call for the eradication of malaria. Is this possible? The introduction of CQ and DDT once raised hopes of malaria eradication, and substantial gains were made throughout Asia and South America. However, it was quickly realized that elimination of the disease in Africa was not achievable at the time.
Now, malaria control and progressive elimination is moving up the political agenda, led in no small part by the Bill and Melinda Gates Foundation. This has caused economists to present cogent arguments regarding the cost-effectiveness of tackling malaria. The means to do this are available in the form of vector control (including the use of long-lasting insecticide-treated bed nets and indoor residual spraying; BOX 3), intermittent preventive treatment in pregnancy and, increasingly, infancy, (BOX 4) and early diagnosis and treatment with effective ACTs. This has once again raised the possibility of eradication. In October 2007, Bill and Melinda Gates called on global leaders to embrace “an audacious goal — to reach a day when no human being has malaria, and no mosquito on earth is carrying it.” This goal has been enthusiastically supported by the director general of the WHO, Dr Margaret Chan. UN Secretary-General Ban Ki-moon called for “ensuring universal coverage by the end of 2010”, supported by the Roll Back Malaria Partnership. The substantial commitment and financial infusion is already providing beneficial results for those living in malaria-endemic regions (FIG. 3). However, the difficult task remains of acquiring, distributing and implementing the tools that are required to reduce malaria-related morbidity and mortality and interrupt disease transmission113 — namely ACTs, vector control measures and effective education of community health workers.
Box 3. Vector control measures.
Alongside chemotherapeutic interventions, Anopheles vector control programmes are an essential component of efforts to decrease the malaria burden. These include the use of long-lasting insecticide-treated bed nets and insecticides to interrupt transmission. Currently, only pyrethroid insecticides are approved for treating bed nets. Careful monitoring of insecticide-resistant mosquitoes is necessary to ensure continued effective- ness. In addition, indoor residual spraying (using either dichlorodiphenyltrichloroethane (DDT) or pyrethroid insecticides) is a key vector control method that has been proven to decrease malaria transmission and reduce the risk of malaria-related illness and death132.
Box 4. Intermittent preventive treatment.
The WHO advocates intermittent preventive treatment (IPT) in areas with a high prevalence of malaria as a means to prevent or reduce the adverse outcomes that are associated with malarial infection during pregnancy133,134. This involves the treatment of asymptomatic pregnant women, regardless of their parasite infection status, with regularly spaced therapeutic doses. Currently the only antimalarial approved for IPT during pregnancy (IPTp) is sulphadoxine–pyrimethamine (SP), which is also the primary antimalarial used for IPT of infants (IPTi). This is mainly due to the limited safety, toxicology and efficacy data of other antimalarials in these highly vulnerable patient populations. The benefit of IPT stems from the clearance or suppression of asymptomatic infections combined with a prophylactic effect during the long drug elimination phase. IPTp clinical studies recently observed that among HIV-negative women, a two-dose regimen of SP led to a reduction in the risk of placental malaria, low birth weight and maternal anaemia compared with risks in women given a placebo135. To achieve similar benefits in HIV-positive women required more frequent dosing135. Studies in Tanzania, Ghana and Mozambique have demonstrated that IPTi also led to a significant decrease in the incidence of cases of malaria in children136–138.
The major concern with SP-based IPTp and IPTi is the increasing prevalence of SP-resistant parasites, which decreases the treatment and prophylactic efficacy139. Therefore, there is an urgent need to identify replacements for SP. Ideal candidates should have a long half-life (as current evidence suggests that prophylaxis might be the most important determinant of IPT efficacy), be well tolerated to ensure a high degree of compliance, be easy and safe to administer during pregnancy and be inexpensive.
A key part of the strategy to decrease the malaria burden is to ensure universal access to ACTs. Although subsidized antimalarials are currently available through public facilities in most malaria-endemic areas, only a percentage of these populations has access to these sources, and those ACTs available through the private sector are priced according to market demands. To address this issue, the AMFm (Affordable Medicines Facility — malaria) has been formed to increase patient access to ACTs, following a recommendation from the US Institute of Medicine114. Phase 1 of the AMFm is being hosted and managed by the Roll Back Malaria Partnership, by invitation from the Global Fund. To achieve its stated goal, AMFm is expected to provide a payment to manufacturers of ACTs that meet recognized quality standards to reduce the price to first-line buyers (that is, governments, importers and wholesalers). This is intended to make these drugs more affordable to patients, even after mark-ups by distributors, taking costs to $0.50 or less for a full treatment course. This should not only increase the distribution of affordable ACTs throughout endemic regions but also increase their use compared with the less expensive but less effective CQ, SP and ART monotherapies (as well as the counterfeit ACTs). AMFm should also improve predictability in terms of supply demands, thus providing stability to the ACT production market. An important part of AMFm is in-country supporting interventions, designed to ensure that those suffering from malaria benefit from the reduced pricing. These not only include public education and awareness programmes but also provide training, pharmacovigilance and resistance monitoring programmes.
We conclude that at the very least, existing tools can reduce malaria morbidity and mortality worldwide to levels that have never been achieved before. However, this will require sustained financial support from implementation programmes and a detailed understanding of the pharmacological and genetic factors that affect antimalarial chemotherapy, specifically pharmacokinetic and pharmacodynamic properties, drug–drug interactions and mechanisms of resistance. If executed properly, these measures could yield an important achievement in global infectious diseases control and public health.
Supplementary Material
Acknowledgments
We thank I. Borghini-Fuhrer and C. Li for their critical reading of the manuscript. R.T.E. is supported in part by the Training Program in Microbiology for Infectious Diseases (T32 AI007161, Department of Microbiology & Immunology, Columbia University Health Sciences, New York, USA). Funding for this work was also provided in part by the National Institute of Allergy and Infectious Diseases (R01 AI079709). We also thank T. Harris (Graphic Arts Center, Albert Einstein College of Medicine, Bronx, New York) for her initial input into developing figure 2. Our thanks extend also to A. Guilloux (WHO, Geneva) and P. Salama and E. White Johansson (UNICEF, New York) for providing the information for figures 1 and 3.
- Artemisinin-based combination therapy
A combination of artemisinin or one of its derivatives with one or more antimalarials of a different chemical class
- Pharmacokinetic properties
Characteristics of a drug, including its mechanisms of absorption and distribution, the rate at which a drug action begins and the duration of the effect, the chemical changes of the agent in the body, and the effects and routes of excretion of drug metabolites
- Antimalarial resistance
The ability of a parasite strain to survive and multiply despite the administration and adsorption of a drug given in doses equal to or higher than those usually recommended but within tolerance of the subject. The form of the drug that is active against the parasite must be able to gain access to the parasite or to the infected red blood cell for the duration that is necessary for its normal action
- Recrudescence
The reappearance of asexual parasitaemia, after initial parasite clearance, that results from the same infection that caused the original illness
- Pharmacodynamic properties
These include the physiological effects of a drug on the body, on microorganisms or on parasites in or on the body; the mechanisms of drug action; and the relationship between drug concentration and effect. Pharmacodynamics is often summarized as the study of what a drug does to the body; whereas pharmacokinetics is the study of what the body does to a drug
- Gametocyte
A sexual form of the intra-erythrocytic Plasmodium parasite that matures over a 2-week period, after which it can transmit to Anopheles mosquito vectors. Following ingestion during the insect blood meal, a gametocyte transforms rapidly into a female or male gamete that can undergo sexual recombination in the mosquito midgut
- Asexual blood-stage trophozoite
An asexual form of the intra-erythrocytic Plasmodium parasite that is undergoing cell growth and nuclear division, in preparation for parasite differentiation into a mature schizont that produces individual progeny (known as merozoites). These merozoites burst from the infected cell, ready to initiate new rounds of intracellular development
- Selection pressure
Evolutionary pressure that allows certain genotypes to outcompete others. In the case of malaria, resistance to antimalarials disseminates owing to the selective survival advantage that resistant parasites have in the presence of the drug. In a given population, the greater the proportion of parasites that are exposed to antimalarials at concentrations that allow proliferation only of resistant parasites, the greater the selection pressure
- Pharmacovigilance
The pharmacological science relating to the detection, assessment, understanding and prevention of adverse effects resulting from the short- or long-term use of medicines
Footnotes
DATABASES
Entrez Genome Project: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=genomeprj
Anopheles gambiae | Plasmodium berghei|
Plasmodium chabaudi | Plasmodium falciparum |
Plasmodium vivax | Saccharomyces cerevisiae | Xenopus laevis
UniProtKB: http://www.uniprot.org
PfATP6 | PfCRT | PfMDR1 | PfMRP | UBP-1
FURTHER INFORMATION
David A. Fidock’s homepage: http://microbiology.columbia.edu/fidock/Fidocklab/Fidock_lab_home.html
Affordable Medicines Facility – malaria: http://www.theglobalfund.org/en/amfm
Bill and Melinda Gates Foundation: http://www.gatesfoundation.org/Pages/home.aspx
The Global Fund to Fight AIDS, Tuberculosis and Malaria: http://www.theglobalfund.org/en
Medicines for Malaria Venture: http://www.mmv.org
President’s Malaria Initiative: http://www.fightingmalaria.gov
Roll Back Malaria Partnership: http://www.rollbackmalaria.org
World Bank: http://www.worldbank.org
WWARN: http://www.wwarn.org
Contributor Information
Richard T. Eastman, Email: re2180@columbia.edu.
David A. Fidock, Email: df2260@columbia.edu.
References
- 1.Wellems TE, Plowe CV. Chloroquine-resistant malaria. J Infect Dis. 2001;184:770–776. doi: 10.1086/322858. [DOI] [PubMed] [Google Scholar]
- 2.Gregson A, Plowe CV. Mechanisms of resistance of malaria parasites to antifolates. Pharmacol Rev. 2005;57:117–145. doi: 10.1124/pr.57.1.4. [DOI] [PubMed] [Google Scholar]
- 3.Wongsrichanalai C, Pickard AL, Wernsdorfer WH, Meshnick SR. Epidemiology of drug-resistant malaria. Lancet Infect Dis. 2002;2:209–218. doi: 10.1016/s1473-3099(02)00239-6. [DOI] [PubMed] [Google Scholar]
- 4.Snow RW, Guerra CA, Noor AM, Myint HY, Hay SI. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature. 2005;434:214–217. doi: 10.1038/nature03342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Talisuna AO, Okello PE, Erhart A, Coosemans M, D’Alessandro U. Intensity of malaria transmission and the spread of Plasmodium falciparum resistant malaria: a review of epidemiologic field evidence. Am J Trop Med Hyg. 2007;77:170–180. [PubMed] [Google Scholar]
- 6.Feachem R, Sabot O. A new global malaria eradication strategy. Lancet. 2008;371:1633–1635. doi: 10.1016/S0140-6736(08)60424-9. Discusses the need to coordinate a global strategy to progressively eliminate malaria. [DOI] [PubMed] [Google Scholar]
- 7.Jiang JB, Li GQ, Guo XB, Kong YC, Arnold K. Antimalarial activity of mefloquine and qinghaosu. Lancet. 1982;2:285–288. doi: 10.1016/s0140-6736(82)90268-9. [DOI] [PubMed] [Google Scholar]
- 8.White NJ. Qinghaosu (artemisinin): the price of success. Science. 2008;320:330–334. doi: 10.1126/science.1155165. Reviews artemisinins, including a historical perspective, their pharmacological properties, their clinical efficacy and initial evidence of emerging resistance. [DOI] [PubMed] [Google Scholar]
- 9.Ro DK, et al. Production of the antimalarial drug precursor artemisinic acid in engineered yeast. Nature. 2006;440:940–943. doi: 10.1038/nature04640. [DOI] [PubMed] [Google Scholar]
- 10.German PI, Aweeka FT. Clinical pharmacology of artemisinin-based combination therapies. Clin Pharmacokinet. 2008;47:91–102. doi: 10.2165/00003088-200847020-00002. [DOI] [PubMed] [Google Scholar]
- 11.Golenser J, Waknine JH, Krugliak M, Hunt NH, Grau GE. Current perspectives on the mechanism of action of artemisinins. Int J Parasitol. 2006;36:1427–1441. doi: 10.1016/j.ijpara.2006.07.011. Summarizes a large and sometimes conflicting body of investigations into the mode of action of artemisinins, their metabolism, suggested mechanisms of resistance and adverse events. [DOI] [PubMed] [Google Scholar]
- 12.Hartwig CL, et al. Accumulation of artemisinin trioxane derivatives within neutral lipids of Plasmodium falciparum malaria parasites is endoperoxide-dependent. Biochem Pharmacol. 2009;77:322–336. doi: 10.1016/j.bcp.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robert A, Benoit-Vical F, Claparols C, Meunier B. The antimalarial drug artemisinin alkylates heme in infected mice. Proc Natl Acad Sci USA. 2005;102:13676–13680. doi: 10.1073/pnas.0500972102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bousejra-El Garah F, Claparols C, Benoit-Vical F, Meunier B, Robert A. The antimalarial trioxaquine DU1301 alkylates heme in malaria-infected mice. Antimicrob Agents Chemother. 2008;52:2966–2969. doi: 10.1128/AAC.00165-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Creek DJ, et al. Relationship between antimalarial activity and heme alkylation for spiro- and dispiro-1,2,4-trioxolane antimalarials. Antimicrob Agents Chemother. 2008;52:1291–1296. doi: 10.1128/AAC.01033-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Creek DJ, et al. Stability of peroxide antimalarials in the presence of human hemoglobin. Antimicrob Agents Chemother. 2009;53:3496–3500. doi: 10.1128/AAC.00363-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krungkrai SR, Yuthavong Y. The antimalarial action on Plasmodium falciparum of qinghaosu and artesunate in combination with agents which modulate oxidant stress. Trans R Soc Trop Med Hyg. 1987;81:710–714. doi: 10.1016/0035-9203(87)90003-4. [DOI] [PubMed] [Google Scholar]
- 18.Asawamahasakda W, Ittarat I, Pu YM, Ziffer H, Meshnick SR. Reaction of antimalarial endoperoxides with specific parasite proteins. Antimicrob Agents Chemother. 1994;38:1854–1858. doi: 10.1128/aac.38.8.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kannan R, Kumar K, Sahal D, Kukreti S, Chauhan VS. Reaction of artemisinin with haemoglobin: implications for antimalarial activity. Biochem J. 2005;385:409–418. doi: 10.1042/BJ20041170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu Y. How might qinghaosu (artemisinin) and related compounds kill the intraerythrocytic malaria parasite? A chemist’s view. Acc Chem Res. 2002;35:255–259. doi: 10.1021/ar000080b. [DOI] [PubMed] [Google Scholar]
- 21.Stocks PA, et al. Evidence for a common non-heme chelatable-iron-dependent activation mechanism for semisynthetic and synthetic endoperoxide antimalarial drugs. Angew Chem Int Edn Engl. 2007;46:6278–6283. doi: 10.1002/anie.200604697. [DOI] [PubMed] [Google Scholar]
- 22.del Pilar Crespo M, et al. Artemisinin and a series of novel endoperoxide antimalarials exert early effects on digestive vacuole morphology. Antimicrob Agents Chemother. 2008;52:98–109. doi: 10.1128/AAC.00609-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhisutthibhan J, Meshnick SR. Immunoprecipitation of [3H]-dihydroartemisinin translationally controlled tumor protein (TCTP) adducts from Plasmodium falciparum-infected erythrocytes by using anti-TCTP antibodies. Antimicrob Agents Chemother. 2001;45:2397–2399. doi: 10.1128/AAC.45.8.2397-2399.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olliaro PL, Haynes RK, Meunier B, Yuthavong Y. Possible modes of action of the artemisinin-type compounds. Trends Parasitol. 2001;17:122–126. doi: 10.1016/s1471-4922(00)01838-9. [DOI] [PubMed] [Google Scholar]
- 25.Eckstein-Ludwig U, et al. Artemisinins target the SERCA of Plasmodium falciparum. Nature. 2003;424:957–961. doi: 10.1038/nature01813. [DOI] [PubMed] [Google Scholar]
- 26.Jambou R, et al. Resistance of Plasmodium falciparum field isolates to in vitro artemether and point mutations of the SERCA-type PfATPase6. Lancet. 2005;366:1960–1963. doi: 10.1016/S0140-6736(05)67787-2. [DOI] [PubMed] [Google Scholar]
- 27.Dahlstrom S, et al. Diversity of the sarco/endoplasmic reticulum Ca2+-ATPase orthologue of Plasmodium falciparum (PfATP6) Infect Genet Evol. 2008;8:340–345. doi: 10.1016/j.meegid.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 28.Valderramos SG, Fidock DA. Transporters involved in resistance to antimalarial drugs. Trends Pharmacol Sci. 2006;27:594–601. doi: 10.1016/j.tips.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Price RN, et al. Mefloquine resistance in Plasmodium falciparum and increased pfmdr1 gene copy number. Lancet. 2004;364:438–447. doi: 10.1016/S0140-6736(04)16767-6. Demonstrates that amplification of pfmdr1 is a major mediator of resistance to mefloquine in P. falciparum malaria. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sidhu AB, et al. Decreasing pfmdr1 copy number in Plasmodium falciparum malaria heightens susceptibility to mefloquine, lumefantrine, halofantrine, quinine, and artemisinin. J Infect Dis. 2006;194:528–535. doi: 10.1086/507115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raj DK, et al. Disruption of a Plasmodium falciparum multidrug resistance-associated protein (PfMRP) alters its fitness and transport of antimalarial drugs and glutathione. J Biol Chem. 2009;284:7687–7696. doi: 10.1074/jbc.M806944200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Noedl H, Socheat D, Satimai W. Artemisinin-resistant malaria in Asia. N Engl J Med. 2009;361:540–541. doi: 10.1056/NEJMc0900231. [DOI] [PubMed] [Google Scholar]
- 33.Dondorp AM, et al. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2009;361:455–467. doi: 10.1056/NEJMoa0808859. Provides clinical evidence for delayed parasite clearance times in patients with a P. falciparum infection who are treated with AS in western Cambodia, and calls for urgent containment methods to halt the spread of resistance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wootton JC, et al. Genetic diversity and chloroquine selective sweeps in Plasmodium falciparum. Nature. 2002;418:320–323. doi: 10.1038/nature00813. [DOI] [PubMed] [Google Scholar]
- 35.Roper C, et al. Intercontinental spread of pyrimethamine-resistant malaria. Science. 2004;305:1124. doi: 10.1126/science.1098876. [DOI] [PubMed] [Google Scholar]
- 36.Klein EY, Smith DL, Boni MF, Laxminarayan R. Clinically immune hosts as a refuge for drug-sensitive malaria parasites. Malar J. 2008;7:67. doi: 10.1186/1475-2875-7-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rathod PK, McErlean T, Lee P-C. Variations in frequencies of drug resistance in Plasmodium falciparum. Proc Natl Acad Sci USA. 1997;94:9389–9393. doi: 10.1073/pnas.94.17.9389. Provides compelling evidence that some P. falciparum strains harbour the ability to rapidly acquire antimalarial drug resistance, termed the ‘accelerated resistance to multiple drugs’ phenotype. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ekland EH, Fidock DA. Advances in understanding the genetic basis of antimalarial drug resistance. Curr Opin Microbiol. 2007;10:363–370. doi: 10.1016/j.mib.2007.07.007. Summarizes recent developments in genetic and genomic tools to explore the resistance of Plasmodium spp. to antimalarial drugs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dharia NV, et al. Use of high-density tiling microarrays to identify mutations globally and elucidate mechanisms of drug resistance in Plasmodium falciparum. Genome Biol. 2009;10:R21. doi: 10.1186/gb-2009-10-2-r21. Describes a rapid method, based on hybridizations of a P. falciparum 4.8-million-feature tiled array that covers 90% of coding regions, to identify SNPs and copy number variations in drug-pressured mutant parasites. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kozarewa I, et al. Amplification-free Illumina sequencing-library preparation facilitates improved mapping and assembly of (G+C)-biased genomes. Nature Methods. 2009;6:291–295. doi: 10.1038/nmeth.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Afonso A, et al. Malaria parasites can develop stable resistance to artemisinin but lack mutations in candidate genes atp6 (encoding the sarcoplasmic and endoplasmic reticulum Ca2+ ATPase), tctp, mdr1, and cg10. Antimicrob Agents Chemother. 2006;50:480–489. doi: 10.1128/AAC.50.2.480-489.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hunt P, et al. Gene encoding a deubiquitinating enzyme is mutated in artesunate- and chloroquine-resistant rodent malaria parasites. Mol Microbiol. 2007;65:27–40. doi: 10.1111/j.1365-2958.2007.05753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Woodrow CJ, Krishna S. Antimalarial drugs: recent advances in molecular determinants of resistance and their clinical significance. Cell Mol Life Sci. 2006;63:1586–1596. doi: 10.1007/s00018-006-6071-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bukirwa H, Critchley J. Sulfadoxine-pyrimethamine plus artesunate versus sulfadoxine-pyrimethamine plus amodiaquine for treating uncomplicated malaria. Cochrane Database Syst Rev. 2006;2006:CD004966. doi: 10.1002/14651858.CD004966.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sullivan DJ, Jr, Matile H, Ridley RG, Goldberg DE. A common mechanism for blockade of heme polymerization by antimalarial quinolines. J Biol Chem. 1998;273:31103–31107. doi: 10.1074/jbc.273.47.31103. Demonstrates that antimalarial–haem complex formation is potentially a common mechanism of drug action. [DOI] [PubMed] [Google Scholar]
- 46.Rohrbach P, et al. Genetic linkage of pfmdr1 with food vacuolar solute import in Plasmodium falciparum. EMBO J. 2006;25:3000–3011. doi: 10.1038/sj.emboj.7601203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alker AP, et al. Pfmdr1 and in vivo resistance to artesunate-mefloquine in falciparum malaria on the Cambodian-Thai border. Am J Trop Med Hyg. 2007;76:641–647. [PubMed] [Google Scholar]
- 48.Nosten F, et al. Effects of artesunate-mefloquine combination on incidence of Plasmodium falciparum malaria and mefloquine resistance in western Thailand: a prospective study. Lancet. 2000;356:297–302. doi: 10.1016/s0140-6736(00)02505-8. [DOI] [PubMed] [Google Scholar]
- 49.Nosten F, et al. Treatment of multidrug-resistant Plasmodium falciparum malaria with 3-day artesunate-mefloquine combination. J Infect Dis. 1994;170:971–977. doi: 10.1093/infdis/170.4.971. [DOI] [PubMed] [Google Scholar]
- 50.Jansen FH, et al. Assessment of the relative advantage of various artesunate-based combination therapies by a multi-treatment Bayesian random-effects meta-analysis. Am J Trop Med Hyg. 2007;77:1005–1009. [PubMed] [Google Scholar]
- 51.Fitch CD. Ferriprotoporphyrin IX, phospholipids, and the antimalarial actions of quinoline drugs. Life Sci. 2004;74:1957–1972. doi: 10.1016/j.lfs.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 52.Hassan Alin M, Bjorkman A, Wernsdorfer WH. Synergism of benflumetol and artemether in Plasmodium falciparum. Am J Trop Med Hyg. 1999;61:439–445. doi: 10.4269/ajtmh.1999.61.439. [DOI] [PubMed] [Google Scholar]
- 53.Ezzet F, Mull R, Karbwang J. Population pharmacokinetics and therapeutic response of CGP 56697 (artemether + benflumetol) in malaria patients. Br J Clin Pharmacol. 1998;46:553–561. doi: 10.1046/j.1365-2125.1998.00830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ezzet F, van Vugt M, Nosten F, Looareesuwan S, White NJ. Pharmacokinetics and pharmacodynamics of lumefantrine (benflumetol) in acute falciparum malaria. Antimicrob Agents Chemother. 2000;44:697–704. doi: 10.1128/aac.44.3.697-704.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Checchi F, et al. Supervised versus unsupervised antimalarial treatment with six-dose artemether-lumefantrine: pharmacokinetic and dosage-related findings from a clinical trial in Uganda. Malar J. 2006;5:59. doi: 10.1186/1475-2875-5-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bloland PB, Ettling M, Meek S. Combination therapy for malaria in Africa: hype or hope? Bull World Health Organ. 2000;78:1378–1388. [PMC free article] [PubMed] [Google Scholar]
- 57.Price RN, et al. Molecular and pharmacological determinants of the therapeutic response to artemether-lumefantrine in multidrug-resistant Plasmodium falciparum malaria. Clin Infect Dis. 2006;42:1570–1577. doi: 10.1086/503423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sisowath C, et al. In vivo selection of Plasmodium falciparum pfmdr1 86N coding alleles by artemether-lumefantrine (Coartem) J Infect Dis. 2005;191:1014–1017. doi: 10.1086/427997. [DOI] [PubMed] [Google Scholar]
- 59.Dokomajilar C, Nsobya SL, Greenhouse B, Rosenthal PJ, Dorsey G. Selection of Plasmodium falciparum pfmdr1 alleles following therapy with artemether-lumefantrine in an area of Uganda where malaria is highly endemic. Antimicrob Agents Chemother. 2006;50:1893–1895. doi: 10.1128/AAC.50.5.1893-1895.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sisowath C, et al. The role of pfmdr1 in Plasmodium falciparum tolerance to artemether-lumefantrine in Africa. Trop Med Int Health. 2007;12:736–742. doi: 10.1111/j.1365-3156.2007.01843.x. [DOI] [PubMed] [Google Scholar]
- 61.Sisowath C, et al. In vivo selection of Plasmodium falciparum parasites carrying the chloroquine-susceptible pfcrt K76 allele after treatment with artemether-lumefantrine in Africa. J Infect Dis. 2009;199:750–757. doi: 10.1086/596738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Abdulla S, et al. Efficacy and safety of artemether-lumefantrine dispersible tablets compared with crushed commercial tablets in African infants and children with uncomplicated malaria: a randomised, single-blind, multicentre trial. Lancet. 2008;372:1819–1827. doi: 10.1016/S0140-6736(08)61492-0. First clinical report of the dispersible formulation of ATM–LMF, showing excellent clinical efficacy in young African children with uncomplicated malaria. This paediatric formulation should substantially improve administration and dosing of this widely used ACT. [DOI] [PubMed] [Google Scholar]
- 63.Naisbitt DJ, et al. Metabolism-dependent neutrophil cytotoxicity of amodiaquine: a comparison with pyronaridine and related antimalarial drugs. Chem Res Toxicol. 1998;11:1586–1595. doi: 10.1021/tx980148k. [DOI] [PubMed] [Google Scholar]
- 64.Olliaro P, Mussano P. Amodiaquine for treating malaria. Cochrane Database Syst Rev. 2003;2003:CD000016. doi: 10.1002/14651858.CD000016. [DOI] [PubMed] [Google Scholar]
- 65.Gasasira AF, et al. High risk of neutropenia in HIV-infected children following treatment with artesunate plus amodiaquine for uncomplicated malaria in Uganda. Clin Infect Dis. 2008;46:985–991. doi: 10.1086/529192. [DOI] [PubMed] [Google Scholar]
- 66.Pussard E, et al. Disposition of monodesethylamodiaquine after a single oral dose of amodiaquine and three regimens for prophylaxis against Plasmodium falciparum malaria. Eur J Clin Pharmacol. 1987;33:409–414. doi: 10.1007/BF00637639. [DOI] [PubMed] [Google Scholar]
- 67.White NJ, et al. Pharmacokinetics of intravenous amodiaquine. Br J Clin Pharmacol. 1987;23:127–135. doi: 10.1111/j.1365-2125.1987.tb03020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Legrand E, Volney B, Meynard JB, Mercereau-Puijalon O, Esterre P. In vitro monitoring of Plasmodium falciparum drug resistance in French Guiana: a synopsis of continuous assessment from 1994 to 2005. Antimicrob Agents Chemother. 2008;52:288–298. doi: 10.1128/AAC.00263-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.de Dios AC, Casabianca LB, Kosar A, Roepe PD. Structure of the amodiaquine-FPIX mu oxo dimer solution complex at atomic resolution. Inorg Chem. 2004;43:8078–8084. doi: 10.1021/ic0489948. [DOI] [PubMed] [Google Scholar]
- 70.Bray PG, Hawley SR, Mungthin M, Ward SA. Physicochemical properties correlated with drug resistance and the reversal of drug resistance in Plasmodium falciparum. Mol Pharmacol. 1996;50:1559–1566. [PubMed] [Google Scholar]
- 71.Ochong EO, van den Broek IV, Keus K, Nzila A. Short report: association between chloroquine and amodiaquine resistance and allelic variation in the Plasmodium falciparum multiple drug resistance 1 gene and the chloroquine resistance transporter gene in isolates from the upper Nile in southern Sudan. Am J Trop Med Hyg. 2003;69:184–187. [PubMed] [Google Scholar]
- 72.Holmgren G, et al. Amodiaquine resistant Plasmodium falciparum malaria in vivo is associated with selection of pfcrt 76T and pfmdr1 86Y. Infect Genet Evol. 2006;6:309–314. doi: 10.1016/j.meegid.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 73.Pradines B, et al. Prevalence of in vitro resistance to eleven standard or new antimalarial drugs among Plasmodium falciparum isolates from Pointe-Noire, Republic of the Congo. J Clin Microbiol. 2006;44:2404–2408. doi: 10.1128/JCM.00623-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Picot S, et al. A systematic review and meta-analysis of evidence for correlation between molecular markers of parasite resistance and treatment outcome in falciparum malaria. Malar J. 2009;8:89. doi: 10.1186/1475-2875-8-89. Reviews many studies assessing the impact of known P. falciparum drug resistance determinants on the risk of treatment failure. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Faye B, et al. Efficacy and tolerability of four antimalarial combinations in the treatment of uncomplicated Plasmodium falciparum malaria in Senegal. Malar J. 2007;6:80. doi: 10.1186/1475-2875-6-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Guthmann JP, et al. High efficacy of two artemisinin-based combinations (artesunate + amodiaquine and artemether + lumefantrine) in Caala, Central Angola. Am J Trop Med Hyg. 2006;75:143–145. [PubMed] [Google Scholar]
- 77.Hasugian AR, et al. Dihydroartemisinin-piperaquine versus artesunate-amodiaquine: superior efficacy and posttreatment prophylaxis against multidrug-resistant Plasmodium falciparum and Plasmodium vivax malaria. Clin Infect Dis. 2007;44:1067–1074. doi: 10.1086/512677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hien TT, et al. Dihydroartemisinin-piperaquine against multidrug-resistant Plasmodium falciparum malaria in Vietnam: randomised clinical trial. Lancet. 2004;363:18–22. doi: 10.1016/s0140-6736(03)15163-x. [DOI] [PubMed] [Google Scholar]
- 79.Vennerstrom JL, et al. Bisquinolines. 1 N,N-bis(7-chloroquinolin-4-yl)alkanediamines with potential against chloroquine-resistant malaria. J Med Chem. 1992;35:2129–2134. doi: 10.1021/jm00089a025. [DOI] [PubMed] [Google Scholar]
- 80.Davis TM, Hung TY, Sim IK, Karunajeewa HA, Ilett KF. Piperaquine: a resurgent antimalarial drug. Drugs. 2005;65:75–87. doi: 10.2165/00003495-200565010-00004. [DOI] [PubMed] [Google Scholar]
- 81.Liu C, et al. Pharmacokinetics of piperaquine after single and multiple oral administrations in healthy volunteers. Yakugaku Zasshi. 2007;127:1709–1714. doi: 10.1248/yakushi.127.1709. [DOI] [PubMed] [Google Scholar]
- 82.Ashley EA, et al. A randomized, controlled study of a simple, once-daily regimen of dihydroartemisinin-piperaquine for the treatment of uncomplicated, multidrug-resistant falciparum malaria. Clin Infect Dis. 2005;41:425–432. doi: 10.1086/432011. [DOI] [PubMed] [Google Scholar]
- 83.Smithuis F, et al. Efficacy and effectiveness of dihydroartemisinin-piperaquine versus artesunate-mefloquine in falciparum malaria: an open-label randomised comparison. Lancet. 2006;367:2075–2085. doi: 10.1016/S0140-6736(06)68931-9. [DOI] [PubMed] [Google Scholar]
- 84.Karunajeewa H, et al. Safety evaluation of fixed combination piperaquine plus dihydroartemisinin (Artekin) in Cambodian children and adults with malaria. Br J Clin Pharmacol. 2004;57:93–99. doi: 10.1046/j.1365-2125.2003.01962.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Warhurst DC, Duraisingh MT. Rational use of drugs against Plasmodium falciparum. Trans R Soc Trop Med Hyg. 2001;95:345–346. doi: 10.1016/s0035-9203(01)90177-4. [DOI] [PubMed] [Google Scholar]
- 86.Basco LK, Ringwald P. In vitro activities of piperaquine and other 4-aminoquinolines against clinical isolates of Plasmodium falciparum in Cameroon. Antimicrob Agents Chemother. 2003;47:1391–1394. doi: 10.1128/AAC.47.4.1391-1394.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Muangnoicharoen S, Johnson DJ, Looareesuwan S, Krudsood S, Ward SA. Role of known molecular markers of resistance in the antimalarial potency of piperaquine and dihydroartemisinin in vitro. Antimicrob Agents Chemother. 2009;53:1362–1366. doi: 10.1128/AAC.01656-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vivas L, et al. Anti-malarial efficacy of pyronaridine and artesunate in combination in vitro and in vivo. Acta Trop. 2008;105:222–228. doi: 10.1016/j.actatropica.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 89.Basco LK, Ringwald P, Franetich JF, Mazier D. Assessment of pyronaridine activity in vivo and in vitro against the hepatic stages of malaria in laboratory mice. Trans R Soc Trop Med Hyg. 1999;93:651–652. doi: 10.1016/s0035-9203(99)90085-8. [DOI] [PubMed] [Google Scholar]
- 90.Ringwald P, Meche FS, Basco LK. Short report: effects of pyronaridine on gametocytes in patients with acute uncomplicated falciparum malaria. Am J Trop Med Hyg. 1999;61:446–448. doi: 10.4269/ajtmh.1999.61.446. [DOI] [PubMed] [Google Scholar]
- 91.Pagola S, Stephens PW, Bohle DS, Kosar AD, Madsen SK. The structure of malaria pigment β-haematin. Nature. 2000;404:307–310. doi: 10.1038/35005132. A landmark study that elucidated the structure of β-haematin, the proposed target of several antimalarials, including chloroquine. This structure revealed dimer linkages that are formed through reciprocal iron-carboxylate bonds, which are in turn linked into chains via hydrogen bonds in the haematin crystal. [DOI] [PubMed] [Google Scholar]
- 92.Auparakkitanon S, Chapoomram S, Kuaha K, Chirachariyavej T, Wilairat P. Targeting of hematin by the antimalarial pyronaridine. Antimicrob Agents Chemother. 2006;50:2197–2200. doi: 10.1128/AAC.00119-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wu LJ, Rabbege JR, Nagasawa H, Jacobs G, Aikawa M. Morphological effects of pyronaridine on malarial parasites. Am J Trop Med Hyg. 1988;38:30–36. doi: 10.4269/ajtmh.1988.38.30. [DOI] [PubMed] [Google Scholar]
- 94.Chang C, Lin-Hua T, Jantanavivat C. Studies on a new antimalarial compound: pyronaridine. Trans R Soc Trop Med Hyg. 1992;86:7–10. doi: 10.1016/0035-9203(92)90414-8. [DOI] [PubMed] [Google Scholar]
- 95.Ringwald P, Bickii J, Basco L. Randomised trial of pyronaridine versus chloroquine for acute uncomplicated falciparum malaria in Africa. Lancet. 1996;347:24–28. doi: 10.1016/s0140-6736(96)91558-5. [DOI] [PubMed] [Google Scholar]
- 96.Basco LK, Ringwald P. Molecular epidemiology of malaria in Yaounde, Cameroon. VII Analysis of recrudescence and reinfection in patients with uncomplicated falciparum malaria. Am J Trop Med Hyg. 2000;63:215–221. [PubMed] [Google Scholar]
- 97.Ramharter M, et al. Fixed-dose pyronaridine-artesunate combination for treatment of uncomplicated falciparum malaria in pediatric patients in Gabon. J Infect Dis. 2008;198:911–919. doi: 10.1086/591096. [DOI] [PubMed] [Google Scholar]
- 98.Price RN, et al. Vivax malaria: neglected and not benign. Am J Trop Med Hyg. 2007;77:79–87. Highlights the burden and impact of the often-overlooked disease, P. vivax malaria. [PMC free article] [PubMed] [Google Scholar]
- 99.Ratcliff A, et al. Two fixed-dose artemisinin combinations for drug-resistant falciparum and vivax malaria in Papua, Indonesia: an open-label randomised comparison. Lancet. 2007;369:757–765. doi: 10.1016/S0140-6736(07)60160-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tjitra E, et al. Multidrug-resistant Plasmodium vivax associated with severe and fatal malaria: a prospective study in Papua, Indonesia. PLoS Med. 2008;5:e128. doi: 10.1371/journal.pmed.0050128. Describes severe and fatal malaria resulting from P. vivax infections in an area with high-grade chloroquine resistance in both P. falciparum and P. vivax. Dispels earlier assumptions that P. vivax infection was rarely lethal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Clark RL, et al. Developmental toxicity of artesunate and an artesunate combination in the rat and rabbit. Birth Defects Res B Dev Reprod Toxicol. 2004;71:380–394. doi: 10.1002/bdrb.20027. [DOI] [PubMed] [Google Scholar]
- 102.Brewer TG, Genovese RF, Newman DB, Li Q. Factors relating to neurotoxicity of artemisinin antimalarial drugs “listening to arteether”. Med Trop (Mars) 1998;58:22–27. [PubMed] [Google Scholar]
- 103.McGready R, et al. Artemisinin antimalarials in pregnancy: a prospective treatment study of 539 episodes of multidrug-resistant Plasmodium falciparum. Clin Infect Dis. 2001;33:2009–2016. doi: 10.1086/324349. [DOI] [PubMed] [Google Scholar]
- 104.Rijken MJ, et al. Dihydroartemisinin–piperaquine rescue treatment of multidrug-resistant Plasmodium falciparum malaria in pregnancy: a preliminary report. Am J Trop Med Hyg. 2008;78:543–545. [PubMed] [Google Scholar]
- 105.White NJ, McGready RM, Nosten FH. New medicines for tropical diseases in pregnancy: catch-22. PLoS Med. 2008;5:e133. doi: 10.1371/journal.pmed.0050133. Discusses the need for further studies on the use and evaluation of medicines for treatment of diseases during pregnancy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.WHO. World malaria report 2008. WHO; Geneva: 2008. [Google Scholar]
- 107.Laufer MK, Djimde AA, Plowe CV. Monitoring and deterring drug-resistant malaria in the era of combination therapy. Am J Trop Med Hyg. 2007;77:160–169. [PubMed] [Google Scholar]
- 108.Vestergaard LS, Ringwald P. Responding to the challenge of antimalarial drug resistance by routine monitoring to update national malaria treatment policies. Am J Trop Med Hyg. 2007;77:153–159. [PubMed] [Google Scholar]
- 109.Sibley CH, Barnes KI, Watkins WM, Plowe CV. A network to monitor antimalarial drug resistance: a plan for moving forward. Trends Parasitol. 2008;24:43–48. doi: 10.1016/j.pt.2007.09.008. Provides the rationale for the creation of WWARN, which is developing a group of open-access databases to aid antimalarial drug treatment and prevention decisions. [DOI] [PubMed] [Google Scholar]
- 110.Newton PN, et al. A collaborative epidemiological investigation into the criminal fake artesunate trade in South East Asia. PLoS Med. 2008;5:e32. doi: 10.1371/journal.pmed.0050032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bate R, Coticelli P, Tren R, Attaran A. Antimalarial drug quality in the most severely malarious parts of Africa — a six country study. PLoS ONE. 2008;3:e2132. doi: 10.1371/journal.pone.0002132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fidock DA, Eastman RT, Ward SA, Meshnick SR. Recent highlights in antimalarial drug resistance and chemotherapy research. Trends Parasitol. 2008;24:537–544. doi: 10.1016/j.pt.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hay SI, Smith DL, Snow RW. Measuring malaria endemicity from intense to interrupted transmission. Lancet Infect Dis. 2008;8:369–378. doi: 10.1016/S1473-3099(08)70069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Moon S, Perez Casas C, Kindermans JM, de Smet M, von Schoen-Angerer T. Focusing on quality patient care in the new global subsidy for malaria medicines. PLoS Med. 2009;6:e1000106. doi: 10.1371/journal.pmed.1000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hay SI, Rogers DJ, Toomer JF, Snow RW. Annual Plasmodium falciparum entomological inoculation rates (EIR) across Africa: literature survey, internet access and review. Trans R Soc Trop Med Hyg. 2000;94:113–127. doi: 10.1016/s0035-9203(00)90246-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Smith DL, Dushoff J, McKenzie FE. The risk of a mosquito-borne infection in a heterogeneous environment. PLoS Biol. 2004;2:e368. doi: 10.1371/journal.pbio.0020368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Coluzzi M. The clay feet of the malaria giant and its African roots: hypotheses and inferences about origin, spread and control of Plasmodium falciparum. Parassitologia. 1999;41:277–283. [PubMed] [Google Scholar]
- 118.Obsomer V, Defourny P, Coosemans M. The Anopheles dirus complex: spatial distribution and environmental drivers. Malar J. 2007;6:26. doi: 10.1186/1475-2875-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Struik SS, Riley EM. Does malaria suffer from lack of memory? Immunol Rev. 2004;201:268–290. doi: 10.1111/j.0105-2896.2004.00181.x. [DOI] [PubMed] [Google Scholar]
- 120.Breman JG, Alilio MS, Mills A. Conquering the intolerable burden of malaria: what’s new, what’s needed: a summary. Am J Trop Med Hyg. 2004;71:1–15. [PubMed] [Google Scholar]
- 121.Hastings IM, Watkins WM. Intensity of malaria transmission and the evolution of drug resistance. Acta Trop. 2005;94:218–229. doi: 10.1016/j.actatropica.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 122.Williams TN. Human red blood cell polymorphisms and malaria. Curr Opin Microbiol. 2006;9:388–394. doi: 10.1016/j.mib.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 123.Rogerson SJ, Hviid L, Duffy PE, Leke RF, Taylor DW. Malaria in pregnancy: pathogenesis and immunity. Lancet Infect Dis. 2007;7:105–117. doi: 10.1016/S1473-3099(07)70022-1. [DOI] [PubMed] [Google Scholar]
- 124.Langhorne J, Ndungu FM, Sponaas AM, Marsh K. Immunity to malaria: more questions than answers. Nature Immunol. 2008;9:725–732. doi: 10.1038/ni.f.205. [DOI] [PubMed] [Google Scholar]
- 125.Fidock DA, et al. Mutations in the P. falciparum digestive vacuole transmembrane protein PfCRT and evidence for their role in chloroquine resistance. Mol Cell. 2000;6:861–871. doi: 10.1016/s1097-2765(05)00077-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Djimde A, et al. A molecular marker for chloroquine-resistant falciparum malaria. N Engl J Med. 2001;344:257–263. doi: 10.1056/NEJM200101253440403. [DOI] [PubMed] [Google Scholar]
- 127.Laufer MK, et al. Return of chloroquine antimalarial efficacy in Malawi. N Engl J Med. 2006;355:1959–1966. doi: 10.1056/NEJMoa062032. Demonstrates that the strict removal of chloroquine from Malawi resulted in a decrease in the prevalence of drug-resistant parasites, suggesting that there is a fitness cost associated with chloroquine resistance. [DOI] [PubMed] [Google Scholar]
- 128.Mita T, et al. Expansion of wild type allele rather than back mutation in pfcrt explains the recent recovery of chloroquine sensitivity of Plasmodium falciparum in Malawi. Mol Biochem Parasitol. 2004;135:159–163. doi: 10.1016/j.molbiopara.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 129.Ginsburg H. Should chloroquine be laid to rest? Acta Trop. 2005;96:16–23. doi: 10.1016/j.actatropica.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 130.Sowunmi A, Adedeji AA, Gbotosho GO, Fateye BA, Happi TC. Effects of pyrimethamine-sulphadoxine, chloroquine plus chlorpheniramine, and amodiaquine plus pyrimethamine-sulphadoxine on gametocytes during and after treatment of acute, uncomplicated malaria in children. Mem Inst Oswaldo Cruz. 2006;101:887–893. doi: 10.1590/s0074-02762006000800011. [DOI] [PubMed] [Google Scholar]
- 131.Ogungbamigbe T, Ojurongbe O, Ogunro P, Okanlawon B, Kolawole S. Chloroquine resistant Plasmodium falciparum malaria in Osogbo Nigeria: efficacy of amodiaquine + sulfadoxine-pyrimethamine and chloroquine + chlorpheniramine for treatment. Mem Inst Oswaldo Cruz. 2008;103:79–84. doi: 10.1590/s0074-02762008000100012. [DOI] [PubMed] [Google Scholar]
- 132.Sadasivaiah S, Tozan Y, Breman JG. Dichlorodiphenyltrichloroethane (DDT) for indoor residual spraying in Africa: how can it be used for malaria control? Am J Trop Med Hyg. 2007;77:249–263. [PubMed] [Google Scholar]
- 133.Guyatt HL, Snow RW. Malaria in pregnancy as an indirect cause of infant mortality in sub-Saharan Africa. Trans R Soc Trop Med Hyg. 2001;95:569–576. doi: 10.1016/s0035-9203(01)90082-3. [DOI] [PubMed] [Google Scholar]
- 134.Steketee RW, Nahlen BL, Parise ME, Menendez C. The burden of malaria in pregnancy in malaria-endemic areas. Am J Trop Med Hyg. 2001;64:28–35. doi: 10.4269/ajtmh.2001.64.28. [DOI] [PubMed] [Google Scholar]
- 135.ter Kuile FO, van Eijk AM, Filler SJ. Effect of sulfadoxine-pyrimethamine resistance on the efficacy of intermittent preventive therapy for malaria control during pregnancy: a systematic review. JAMA. 2007;297:2603–2616. doi: 10.1001/jama.297.23.2603. [DOI] [PubMed] [Google Scholar]
- 136.Schellenberg D, et al. Intermittent treatment for malaria and anaemia control at time of routine vaccinations in Tanzanian infants: a randomised, placebo-controlled trial. Lancet. 2001;357:1471–1477. doi: 10.1016/S0140-6736(00)04643-2. [DOI] [PubMed] [Google Scholar]
- 137.Macete E, et al. Intermittent preventive treatment for malaria control administered at the time of routine vaccinations in Mozambican infants: a randomized, placebo-controlled trial. J Infect Dis. 2006;194:276–285. doi: 10.1086/505431. [DOI] [PubMed] [Google Scholar]
- 138.Kobbe R, et al. Malaria incidence and efficacy of intermittent preventive treatment in infants (IPTi) Malar J. 2007;6:163. doi: 10.1186/1475-2875-6-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.White NJ. How antimalarial drug resistance affects post-treatment prophylaxis. Malar J. 2008;7:9. doi: 10.1186/1475-2875-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.UNICEF. Malaria and children: progress in intervention coverage. UNICEF; New York: 2007. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.