Abstract
Rationale:
Superoxide (O2∸) has been implicated in the pathogenesis of many human diseases including hypertension, however commonly employed antioxidants have proven ineffective in clinical trials. It is possible that these agents are not adequately delivered to the subcellular sites of superoxide production.
Objective:
Because the mitochondria are important sources of reactive oxygen species, we postulated that mitochondrial targeting of superoxide scavenging would have therapeutic benefit.
Methods and Results:
In this study, we found that the hormone angiotensin II increased endothelial mitochondrial superoxide production. Treatment with the mitochondrial targeted antioxidant mitoTEMPO decreased mitochondrial O2∸, inhibited the total cellular O2∸, reduced cellular NADPH oxidase activity and restored the level of bioavailable NO. These effects were mimicked by overexpressing the mitochondrial MnSOD (SOD2), while SOD2 depletion with siRNA increased both basal and angiotensin II-stimulated cellular O2∸. Treatment of mice in vivo with mitoTEMPO attenuated hypertension when given at the onset of angiotensin II infusion and decreased blood pressure by 30 mm Hg following establishment of both angiotensin II-induced and DOCA-salt hypertension, while a similar dose of non-targeted TEMPOL was not effective. In vivo, mitoTEMPO decreased vascular O2∸, increased vascular NO• production and improved endothelial-dependent relaxation. Interestingly, transgenic mice overexpressing mitochondrial SOD2 demonstrated attenuated angiotensin II-induced hypertension and vascular oxidative stress similar to mice treated with mitoTEMPO.
Conclusions:
These studies show that mitochondrial O2∸ is important for the development of hypertension and that antioxidant strategies specifically targeting this organelle could have therapeutic benefit in this and possibly other diseases.
Keywords: hypertension, mitochondria, superoxide, mitochondrial targeted antioxidant
Introduction
During normal mitochondrial function, a small percent of electrons from the electron transport chain reduce oxygen to form superoxide (O2∸). In several common conditions, such as atherosclerosis 1, 2, ischemia reperfusion injury and aging 3-7, the mitochondria become dysfunctional and this leak of electrons is increased 1. The mitochondria contain a unique form of superoxide dismutase, the manganese containing SOD2, which is critical in protecting against excessive production of O2∸. Mice lacking this enzyme die of a cardiomyopathy within 10 days of birth and mice lacking one allele of SOD2, (SOD2+/− mice) develop hypertension with aging and in response to a high salt diet 8.
The development of hypertension in SOD2+/− mice is in keeping with a role of reactive oxygen species (ROS) in the pathogenesis of this and many other vascular diseases 9. Hypertension has been associated with increased ROS production in the vasculature, the kidney and in portions of the central nervous system that control blood pressure. The hormone angiotensin II, commonly implicated in hypertension, increases ROS production in the sites. Moreover, ROS overproduction leads to decreased bioavailability of NO•, impairs endothelium-dependent vasodilatation and promotes vasoconstriction. These alterations occur early in the development of vascular disease 10.
There is substantial interest in the enzymatic source of ROS in hypertension. Angiotensin II stimulates the NADPH oxidase in many mammalian cells via pathways involving protein kinase C and the tyrosine kinase c-Src 11. Angiotensin II also activates the NADPH oxidase in vivo and mice lacking components of this enzyme are resistant to both angiotensin II and salt-dependent hypertension. Specific inhibitors of the NADPH oxidase have anti-hypertensive effects 12, 13. Another potential source of ROS in hypertension is the mitochondria. We have previously found that angiotensin II increases production of mitochondrial ROS, decreases mitochondrial membrane potential and reduces the respiratory control ratio 14. These deleterious effects of angiotensin II on mitochondrial function were associated with increased cellular O2∸ production and decreased endothelial NO• bioavailability. These studies further indicated that angiotensin II activation of the NADPH oxidase led to oxidant disruption of mitochondrial function, supporting an important interplay between these two sources of ROS 14, and suggest that mitochondria-derived ROS could contribute to endothelial dysfunction and hypertension. In keeping with this concept, Widder et al. recently showed that mice transgenic for the mitochondrial antioxidant enzyme thioredoxin 2,, are resistant to angiotensin II-induced hypertension and endothelial dysfunction 15. Taken together, these studies suggest that mitochondrial-produced ROS could play an important role in hypertension.
We therefore performed the present study to test the hypothesis that mitochondrial-targeted antioxidant therapy would be effective in both preventing and treating hypertension. To gain further insight into the role of mitochondrial O2∸ in endothelial dysfunction and hypertension we examined the effects of depleting or overexpressing mitochondrial superoxide dismutase (SOD2) in cultured endothelial cells and transgenic mice with angiotensin II-induced hypertension. Our data strongly indicate that mitochondrial O2∸ is an important, previously largely ignored, therapeutic target to treat endothelial dysfunction and high blood pressure.
Materials and Methods
Reagents
MitoTEMPO, mitoTEMPO-H, 1-hydroxy-3-carboxy-pyrrolidine (CPH) and nitroxide 3-carboxy-proxyl (CP) were purchased from Alexis Corporation (San Diego, CA). Xanthine oxidase was purchased from Roche Molecular Biochemicals (Indianapolis, IN). All other reagents were obtained from Sigma (St Louis, MO).
Cell culture
Bovine aortic endothelial cells (BAEC, passage 4 to 8) were cultured on 100 mm plates in Media 199 containing 10% fetal calf serum supplemented with 2 mM L-glutamine, and 1% vitamins. Confluent cells were used for the experiments 16. Human aortic endothelial cells (HAEC) purchased from Lonza (Chicago, IL) and cultured in EGM-2 medium supplemented with 2% FBS but without antibiotics. On the day before the study, the FBS concentration was reduced to 1%. In preliminary experiments we examined the effect of varying doses of angiotensin II on cellular O2∸ production. We found that 4 hours of angiotensin II increased cellular O2∸ in a dose-dependent manner with maximum stimulation at 200 nM (Supp Figure I). This concentration was therefore used in the remainder of the experiments. It should be noted that due to degradation in culture, the steady state concentration of angiotensin II is substantially lower than that initially added 17.
Mitochondrial isolation and study
Mitochondria were isolated as previously described 18. Complex I (glutamate + malate as substrate) or complex II (succinate as the substrate) dependent mitochondrial respiration was studied using intact mitochondria 19 and fluorescence oxygen monitoring system (Instech Laboratories, Inc, Plymouth Meeting, PA). Mitochondrial H2O2 was measured by mixing 20 μg of mitochondrial protein with horseradish peroxidase (2 U/ml), peroxidase substrate acetamidophenol (1 mM), SOD (50 U/ml), and spin probe CAT1H (1 mM) 20. Production of mitochondrial O2∸ was visualized in intact cultured HAEC using the fluorescent probe MitoSOX (Ex/Em: 510/580 nm, Invitrogen) 21. HAEC were incubated with 2 μM MitoSOX in KHB for 20 minutes at 37° C in CO2 incubator. The mitochondrial subcellular location of MitoSOX was confirmed by co-labeling with 50 nM MitoTracker Green FM (Ex/Em: 490/516 nm).
Measurement of cellular O2∸, NADPH oxidase activity and NO levels
Superoxide was measured using dihydroethidium (DHE) and an HPLC-based assay with minor modification as described previously 22. NADPH oxidase activity was measured in membrane preparations prepared as described previously using ESR and the spin probe CPH 23, and was quantified as NADPH dependent O2∸ production. NO• levels in endothelial cells and vessels were quantified by ESR and colloid Fe(DETC)2 as described previously 24.
Modulation of SOD2 expression
To manipulate mitochondrial O2∸ we inhibited the expression of mitochondrial SOD2 using siRNA from Qiagen or overexpressed SOD2 using transfection-ready expression plasmid for human SOD2 (Addgene Inc). As a control we used non-silencing siRNA (Qiagen AllStars negative control) and a GFP empty plasmid (Lonza). The plasmids were grown in bacteria using standard techniques and purified with a kit (Sigma).
Animal experiments
Hypertension was induced by angiotensin II (490 ng/kg/min) as described previously 25 using either C57Bl/6 or mice transgenic for human SOD2 (tgSOD2 mice). In addition, mice received a separate minipump for co-infusion of either TEMPOL, mitoTEMPO or vehicle as described in the figure legends. In other animals, mitoTEMPO treatment was started seven days after saline or angiotensin II minipump placement. Blood pressure was monitored using either the tail cuff method or telemetry as previously described 26, 27. Following 14 days of angiotensin II infusion the animals were sacrificed by CO2 inhalation and aortas were extracted for the analysis of nitric oxide and O2∸ production, and endothelial functions. DOCA-salt induced hypertension was induced as described previously 28 using C57Bl/6 mice. Ten days after surgery the mice were implanted with osmotic pumps containing saline or mitoTEMPO (0.7 mg/kg/day). Seventeen days after surgery the animals were sacrificed by CO2 inhalation and segments of mouse aorta were used for analysis of vascular nitric oxide and O2∸ production. Endothelium-dependent vasodilatation was analyzed in isolated 3-mm aortic segments in organ chambers as we have previously described 27.
Statistics
Experiments were analyzed using the Student Neuman Keuls post-hoc test and analysis of variance (ANOVA). P levels < 0.05 were considered significant.
Results
Mitochondrial accumulation of mitoTEMPO
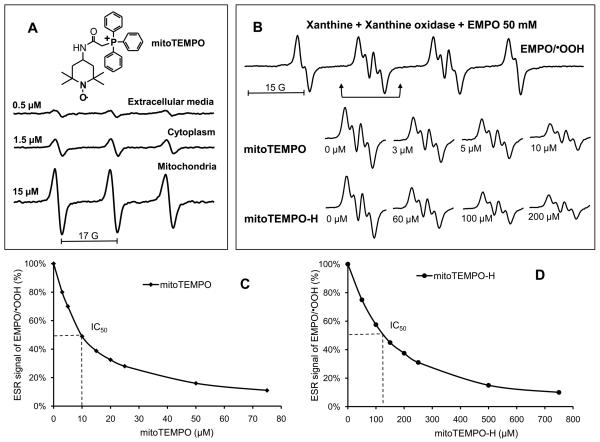
It has been previously suggested that conjugation to a lipophilic triphenylphosphonium cation allows targeting of an antioxidant to the mitochondria and can prevent mitochondrial oxidative damage and mitochondrial dysfunction 29, 30. We have developed a new mitochondria-targeted superoxide dismutase mimetic mitoTEMPO (Figure 1A). To confirm mitochondrial accumulation of mitoTEMPO we used ESR to examine the relative intensities of the nitroxide signal in the cell media, mitochondria and cytoplasm following one hour incubation with mitoTEMPO. As evident in figure 1A, the mitoTEMPO accumulation in the cytoplasm fraction was three-fold greater than that present in the extracellular media, while analysis of the mitochondria revealed substantial accumulation of mitoTEMPO up to 15 μM.
Figure 1.
Accumulation of mitoTEMPO in mitochondria and scavenging of O2∸ by mitoTEMPO. (A) ESR spectra of various compartments from BAEC following incubation with mitoTEMPO (1 μM) for 1 hour. Cells were lysed cytoplasmic and mitochondrial fractions isolated by centrifugation. Spectra of the extracellular media, cellular cytoplasm or mitochondria (10 mg weight for each sample) are shown. (B) Spin trapping of O2∸ using the spin trap EMPO and xanthine/xanthine oxidase O2∸ generating system in the absence or presence of mitoTEMPO and mitoTEMPO-H. The efficacy of O2∸ scavenging was derived from dose-dependent decrease of ESR amplitude of EMPO-OOH in the presence of mitoTEMPO (C) or mitoTEMPO-H (D).
Scavenging of O2∸ by mitoTEMPO
In additional experiments we examined the capacity of mitoTEMPO or its reduced form, mitoTEMPO-H, to scavenge O2∸. We employed the spin trap EMPO and generated O2∸ using xanthine and xanthine oxidase. As evident in figure 1B, exposure of EMPO to xanthine/xanthine oxidase produced an ESR spectrum typical of the EMPO-OOH radical adduct. Addition of either mitoTEMPO or mitoTEMPO-H inhibited formation of EMPO-OOH radical adduct in dose dependent manner (Figure 1B and 1C). Control experiments showed that neither mitoTEMPO nor mitoTEMPO-H had a direct effect on xanthine oxidase activity (Supp Figure II). The estimated IC50's for mitoTEMP0 and mitoTEMPO-H were 10 μM and 123 μM, respectively. Based on competition with 50 mM EMPO, which has a rate constant of 74 M−1s−1 for reaction with O2∸, the rate constants of reactions of mitoTEMPO and mitoTEMPO-H with O2∸ were estimated to be 3.7×105 M−1s−1 and 3.0×104 M−1s−1, respectively. These data are similar to the previously reported rate constants for TEMPOL (6.5×105 M−1s−1) and TEMPONE-H (1.2×104 M−1s−1) 31, 32.
The above data indicate that mitoTEMPO would be an effective O2∸ scavenger in intact cells. We therefore investigated O2∸ dismutation in the cytoplasm and mitochondria of BAEC treated with mitoTEMPO using a commercially available kit (Cayman). Incubation of cells with 25 nM mitoTEMPO increased mitochondrial O2∸ dismutation by 3-fold while not affecting cytoplasmic dismutation (Table 1). These data are consistent with mitochondrial accumulation of mitoTEMPO shown in figure 1A and demonstrate specificity of this agent for mitochondrial protection against O2∸.
Table 1.
Effect of transfection with SOD2 plasmid or depletion of SOD2 with siSOD2 or treatment with mitoTEMPO (mT) on superoxide dismutation.
| Superoxide dismutation rate (units/mg protein) | ||||||||
|---|---|---|---|---|---|---|---|---|
| BAEC | HAEC | |||||||
| Group | Control | mT | AngII | AngII+mT | GFP | SOD2 | NS siRNA | siSOD2 |
| Mitochondria | 0.56±0.06 | 1.75±0.15* | 0.59±0.07 | 1.74±0.12* | 0.55±0.06 | 1.3±0.11§ | 0.52±0.07 | 0.19±0.05§ |
| Cytoplasm | 5.7±0.3 | 5.4±0.4 | 5.6±0.4 | 5.4±0.5 | 1.3±0.1 | 1.2±0.2 | 1.3±0.2 | 1.1±0.1 |
Results represent mean for 3-5 experiments per group,
P< 0.05 vs control,
P < 0.01 vs control transfection with GFP of NS siRNA.
Effect of mitoTEMPO on production of mitochondrial ROS and respiration
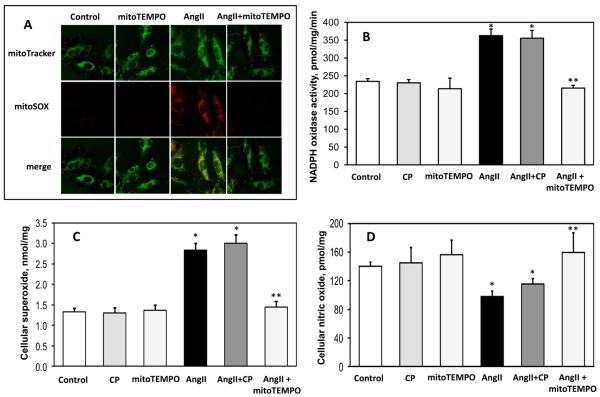
We previously reported that angiotensin II increases mitochondrial ROS production and impairs mitochondrial respiration in endothelial cells 14. We therefore sought to determine if mitoTEMPO would ameliorate these effects. To monitor mitochondrial O2∸ levels in intact cells, we employed the mitochondria specific fluorescent probe MitoSOX. As expected, stimulation of HAEC with angiotensin II (200 nM, 4 hours) significantly increased mitochondrial O2∸ as reflected by MitoSOX fluorescence (Figure 2A). MitoSOX fluorescence co-localized with the mitochondria as detected using the probe mitoTracker (Figure 2A). HPLC analysis of the O2∸ specific product of MitoSOX (2-OH-Mito-E+) 33 confirmed the specificity of O2∸ measurements with mitoSOX (Supp Figure III). Experiments with PMA and antimycin A confirmed site-specific detection of cellular and mitochondrial O2∸ by DHE and mitoSOX (Supp Figure IV). Interestingly, supplementation of HAEC with 25 nM mitoTEMPO for 15-minutes after angiotensin II-stimulation abolished the MitoSOX signal indicating that mitoTEMPO decreases mitochondrial O2∸ in intact cells (Figures 2A, Supp. Figure III).
Figure 2.
Effect of mitoTEMPO on mitochondrial O2∸, endothelial O2∸, nitric oxide and NADPH oxidase activity. (A) Mitochondrial O2∸ was measured in control or angiotensin II (Ang II)-stimulated HAEC using fluorescent probe MitoSOX. Mitochondrial localization of MitoSOX signal was confirmed by colocalization with MitoTracker. (B) Activity of NADPH oxidase measured in membrane fractions isolated from unstimulated or angiotensin II (Ang II) stimulated BAEC (4 hours, 200nM) and supplemented for 15 minutes with saline, the mitochondria-impermeable SOD mimetic 3-carboxyproxyl (CP), or the mitochondria-targeted SOD mimetic mitoTEMPO (25 nM). (C) Cellular O2∸ was measured in intact BAEC using DHE and HPLC. (D) Nitric oxide was measured in intact cells after treatment with saline, CP or mitoTEMPO using ESR and the NO spin trap Fe(DETC)2 24. Results are mean±SEM, n=5-8 each. *P<0.05 vs control, ** P <0.05 vs Ang II.
Scavenging mitochondrial O2∸ by mitoTEMPO could improve mitochondrial function. To determine if this is correct, we measured respiration of isolated mitochondria in the presence of complex I substrates malate/glutamate or the complex II substrate succinate. Coupling of mitochondrial respiration was estimated by measurements of State 3 and State 4 oxygen consumption in the presence or absence of ADP. In unstimulated cells, treatment with mitoTEMPO did not affect mitochondrial respiration (Table 2). Angiotensin II increased state 4 respiration (without ADP) and reduced state 3 respiration (in the presence of ADP). These changes were reflected in marked reduction of respiratory control ratio (RCR), indicating uncoupling of mitochondrial respiration. Supplementation with mitoTEMPO markedly improved these parameters for both complex I and complex II substrates, indicating that the mitochondrial impairment caused by angiotensin II could be reversed by mitoTEMPO.
Table 2.
Mitochondrial H2O2 and respiration in AngII-stimulated BAEC treated with mitoTEMPO.
| H2O2, pmol/mg/min | Respiration, nmol/mg/min | |||||||
|---|---|---|---|---|---|---|---|---|
| Malate + Glutamate |
Succinate | Malate + Glutamate | Succinate | |||||
| State 3 | Sate 4 | RCR | State 3 | State 4 | RCR | |||
| Control | 67±4 | 234±20 | 11.6±0.7 | 3.3±0.4 | 3.5±0.1 | 9.5±0.3 | 4.7±0.6 | 2.0±0.4 |
| mitoTEMPO | 72±5 | 235 ±19 | 11.5±0.6 | 3.2±0.5 | 3.6±0.4 | 9.3±0.4 | 4.5±0.7 | 2.1±0.4 |
| Ang II * | 133 ±12 | 377±30 | 8.2±0.5 | 5.3±1.0 | 1.5±0.6 | 7.9±0.2 | 6.9±0.5 | 1.1±0.3 |
| Ang II+mitoTEMPO§ | 79±4 | 227±24 | 9.8±0.7 | 3.7±0.5 | 2.7±0.5 | 9.1±0.3 | 4.8±0.6 | 1.9±0.3 |
Results are mean ± SEM. Ang II = angiotensin II.
P < 0.05 vs control,
P < 0.05 vs AngII.
Improvement of respiratory coupling could reduce electron leakage in mitochondria resulting in diminished total mitochondrial ROS production. To address this, we measured total H2O2 production by mitochondria isolated from either control or angiotensin II-stimulated cells. Angiotensin II increased H2O2 production in response to complex I and complex II substrates. Treatment of cells with mitoTEMPO completely reversed this effect of angiotensin II (Table 2). MitoTEMPO had no effect on H2O2 production in mitochondria in cells not treated with angiotensin II. These data indicate that in addition to O2∸ scavenging, mitoTEMPO has the capacity to reduce mitochondrial ROS production by normalizing respiration. Thus, treatment of angiotensin II-stimulated cells with mitoTEMPO inhibits mitochondrial oxidative stress, improves respiration and inhibits production of mitochondrial H2O2.
Effect of mitoTEMPO on endothelial O2∸, NO and NADPH oxidase activity
The studies described above indicate that angiotensin II increases mitochondrial H2O2 and mitoTEMPO prevents this. H2O2 is freely diffusible and can stimulate the extra-mitochondrial NADPH oxidase via c-Src-mediated mechanisms 34. We therefore tested the hypothesis that inhibition of mitochondrial H2O2 by mitoTEMPO would decrease activity of the NADPH oxidase, reducing cellular O2∸ and improving NO production.
To perform these studies, we stimulated BAEC with angiotensin II (200 nM for 4 hours) and then exposed the cells to mitoTEMPO (25 nM) for 15 minutes. Membrane fractions were then produced by centrifugation and NADPH oxidase activity measured using ESR. It was found that angiotensin II significantly increased non-mitochondrial NADPH oxidase activity (Figure 2B). Treatment of cells with mitoTEMPO completely blocked the increase in NADPH oxidase activity caused by angiotensin II but did not affect basal NADPH oxidase activity in unstimulated cells (Figure 2B). Importantly, supplementation with a mitochondria impermeable SOD-mimetic CP did not affect NADPH oxidase activity. Direct addition of mitoTEMPO (0.5 μM) to membrane fractions did not affect NADPH oxidase activity (Supp Figure V).
This decrease of NADPH oxidase activity in mitoTEMPO treated cells was accompanied by reduced production of cellular O2∸ measured in intact cells using dihydroethidium and HPLC (Figure 2C) and an increase in endothelial NO production as detected by ESR and the spin trap Fe[DETC]2 (Figure 2D). Treatment of angiotensin II-stimulated cells with the mitochondria impermeable analog 3-carboxyproxyl 30 had no effect on these parameters (Figure 2C,D). These data indicate that scavenging of mitochondrial O2∸ with mitoTEMPO results in decrease of cellular O2∸ and recovery of endothelial NO.
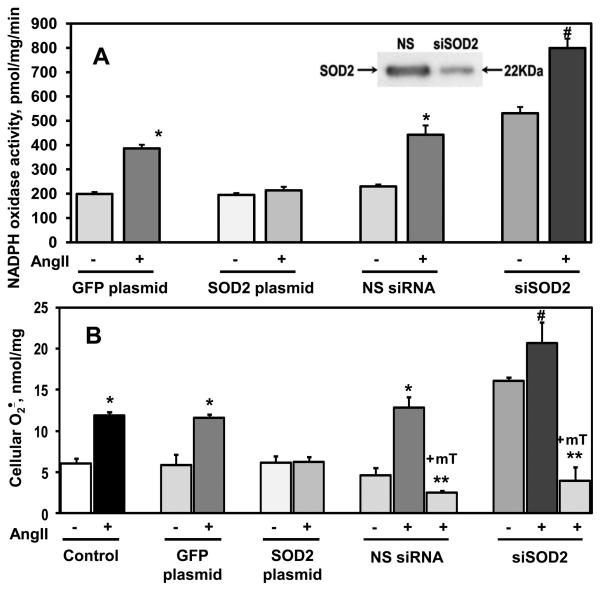
SOD2 modulates Angiotensin II-stimulated cellular O2∸ and NADPH oxidase activity
It is conceivable that the effects of mitoTEMPO are not mediated by O2∸ scavenging but are due to non-specific effects. We therefore performed additional experiments to manipulate the levels of mitochondrial superoxide dismutase (SOD2) and measured NADPH oxidase activity and cellular O2∸ production. Transfection of HAEC with an SOD2 plasmid increased mitochondrial SOD2 activity by 2.4-fold, while cytoplasmic SOD1 activity was not changed (Table 1). Depletion of SOD2 with siRNA decreased SOD2 activity by 2.7-fold (Table 1). In cells transfected with a GFP control plasmid, angiotensin II stimulation doubled NADPH oxidase activity. In contrast, angiotensin II had no effect on NADPH oxidase activity in HAEC transfected with the SOD2 plasmid (Figure 3A). Interestingly, depletion of SOD2 using siRNA transfection increased NADPH oxidase activity in unstimulated cells compared to cells transfected with nonsilencing control RNA. Furthermore, angiotensin II stimulation of SOD2 depleted cells resulted in higher activity of NADPH oxidase compared to cell treated with either non-silencing RNA or a control GFP plasmid (Figure 3A).
Figure 3.
SOD2 modulates angiotensin II stimulated O2∸ production. SOD2 was overexpressed or depleted by 72-hours transfection with a SOD2-expressing plasmid or siRNA. (A) Activity of NADPH oxidase was measured in the membrane fractions of unstimulated or angiotensin II (200 nM, 4 hours) stimulated HAEC using ESR and spin probe CPH 23. MitoTEMPO (+mT, 25 nM) was added to HAEC after angiotensin II (Ang II) stimulation, 15-minutes prior to isolation of membrane fraction. The insert shows a typical Western blot of mitochondrial fractions isolated from siSOD2 or non-silencing siRNA treated HAEC indicating significant depletion of SOD2 in siSOD2 treated cells. (B) Superoxide was measured by DHE/HPLC 22. Results are mean±SEM, n=4-8 each, *P<0.01 vs no angiotensin II, **P < 0.01 vs Ang II , §P<0.05 vs NS control, #P < 0.05 vs NS+Ang II .
Analysis of intact cells showed that angiotensin II increased the O2∸ to a similar extent in non-transfected cells or cells treated with a GFP control plasmid (Figure 3B). SOD2 overexpression completely prevented angiotensin II-stimulated O2∸ production while not affecting O2∸ production in unstimulated cells. In keeping with its effect on NADPH oxidase activity, SOD2 depletion enhanced both basal and angiotensin II-stimulated O2∸ in intact cells (Figure 3B). These data confirmed that modulation of mitochondrial O2∸ by mitoTEMPO or changing SOD2 levels affects production of cellular O2∸ by NADPH oxidase. Fluorescent microscopy with mitoSOX showed that SOD2 depletion increased both basal and angiotensin II – stimulated mitochondrial superoxide production, and that this could be inhibited by mitoTEMPO (Supp Figure VI). It is important to note that mitoTEMPO treatment inhibited cellular O2∸ and mimicked SOD2 overexpression (Figure 3B) in SOD2 depleted cells, further validating mitoTEMPO as an SOD2 mimetic.
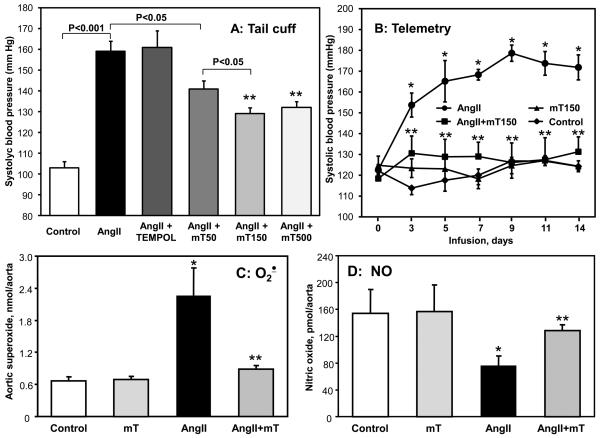
Antihypertensive effect of mitoTEMPO
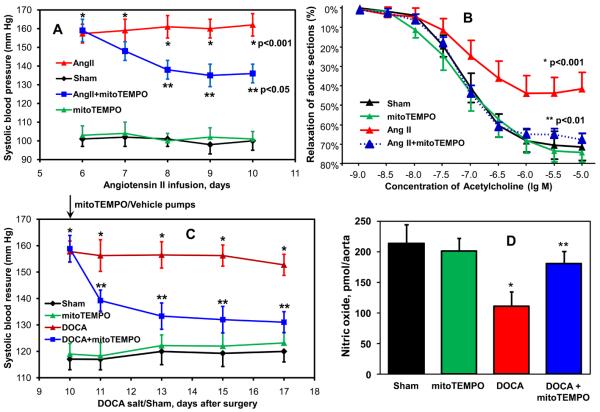
Increased vascular O2∸ production has been implicated in the pathogenesis of endothelial dysfunction and hypertension 9, 35. Because mitoTEMPO diminished mitochondrial ROS, inhibited angiotensin II-stimulated endothelial O2∸ and prevented inactivation of NO caused by angiotensin II in cultured endothelial cells (Figure 2, 3), we hypothesized that it could improve endothelial function and decrease hypertension in vivo. Co-infusion of mitoTEMPO (Figure 4A) significantly attenuated the angiotensin II-induced hypertension in a dose-dependent manner (50, 150 and 500 μg/kg/day), while not affecting blood pressure in normal mice. Telemetric measurements of blood pressure confirmed that co-infusion of mitoTEMPO (150 μg/kg/day) with angiotensin II markedly attenuated hypertension (Figure 4B). In addition, mitoTEMPO inhibited the increase in vascular O2∸ (Figure 4C) and prevented the decrease of vascular NO (Figure 4D) caused by angiotensin II infusion. Importantly, infusion of the same dose of the non-targeted SOD mimetic TEMPOL (286 nmol/kg/day) did not affect angiotensin II-induced hypertension (Figure 4A). These data demonstrate that the mitochondrial-targeted SOD2 mimetic mitoTEMPO attenuates angiotensin II-induced hypertension at an extremely low dose.
Figure 4.
Attenuation of angiotensin II-induced hypertension in C57Blk/6 mice by mitoTEMPO. (A) Systolic blood pressure in mice infused for 14 days with saline, angiotensin II (Ang II, 0.7 mg/kg/day) or co-infused with mitoTEMPO (50, 150 and 500 μg/kg/day) or TEMPOL. Systolic blood pressure was measured using tail-cuff plethysmography. (B) Telemetric measurements of systolic blood pressure in mice infused with saline, angiotensin II or co-infused with mitoTEMPO (150 μg/kg/day). Blood pressure values represent mean ± SEM for 5-7 animals per group. *P<0.01 vs control, ** P <0.01 vs Ang II. Following 14 days infusion with saline, angiotensin II or co-infusion with mitoTEMPO (150 μg/kg/day) aortic tissue was isolated for measurements of O2∸ with DHE and HPLC (C) or nitric oxide ESR and Fe(DETC)2 (D). Results represent mean ± SEM for 5-7 animals per group. *P<0.05 vs control, ** P <0.05 vs Ang II.
The above studies showing that mitoTEMPO can prevent hypertension do not provide insight into whether it could lower blood pressure after hypertension is established. We therefore performed additional studies in which mitoTEMPO was administered after the onset of angiotensin II-induced hypertension. Following seven days of angiotensin II infusion (0.7 mg/kg/day) systolic blood pressure reached 160 mm Hg (Figure 5A). The subsequent addition of mitoTEMPO (1.5 μmol/kg/day) resulted in a significant time-dependent decrease of blood pressure. MitoTEMPO treatment of hypertensive mice not only reduced blood pressure but also improved endothelial function as evidenced by measurements of endothelium-dependent relaxation evoked by acetylcholine (Figure 5B). Of note, mitoTEMPO did not affect endothelium-dependent vasodilatation in normotensive mice (Figure 5B) and did not alter the endothelium-independent responses to sodium nitroprusside (Supp Figure VII).
Figure 5.
Effects of mitoTEMPO treatment after the onset of hypertension on blood pressure, vascular function and NO production. (A) Blood pressure of mice treated with mitoTEMPO (0.7 mg/kg/day) after onset of angiotensin II (Ang II)-induced hypertension. (B) Endothelial-dependent relaxation in aortic vessels isolated from mice infused with saline (Sham), mitoTEMPO, angiotensin II or angiotensin II-infused mice treated with mitoTEMPO. Vessels were preconstricted with PGF2α and relaxations to cumulative concentrations of acetylcholine were examined. (C) Blood pressure of mice treated with mitoTEMPO (0.7 mg/kg/day) after onset of DOCA-salt induced hypertension. (D) Production of aortic nitric oxide measured in isolated aorta by ESR and Fe(DETC)2. Results represent mean ± SEM for 6-8 animals per group. *P<0.001 vs Sham, ** P <0.05 vs angiotensin II / DOCA.
Because the above findings were made in mice treated with angiotensin II, there was a question as to whether the effect of mitoTEMPO is limited to the angiotensin II model. We therefore performed additional experiments in mice with DOCA-salt hypertension 36. This model of hypertension differs from angiotensin II-induced hypertension because it is largely volume dependent and is associated with suppressed plasma renin activity. In these experiments, mitoTEMPO was administered 10 days after the DOCA-salt surgery. As in the case of angiotensin II-induced hypertension, mitoTEMPO reduced blood pressure in DOCA-salt mice but did not affect blood pressure in sham-operated mice (Figure 5C). ESR studies demonstrated that DOCA-salt hypertension decreased endothelial NO production and that mitoTEMPO treatment restored this, while not affecting vascular NO in sham mice (Figure 5D).
Overexpression of SOD2 attenuates angiotensin II-induced endothelial dysfunction and hypertension
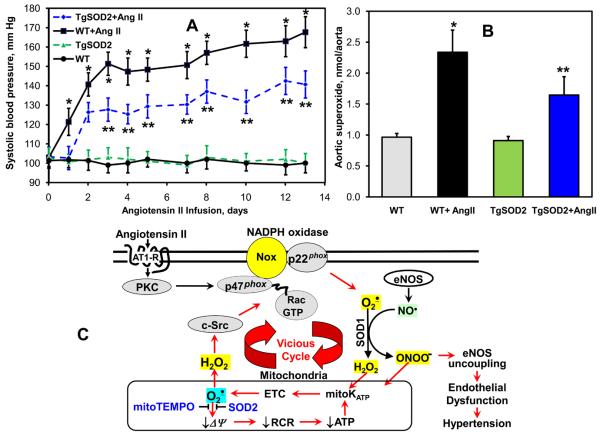
The above experiments with mitoTEMPO suggest that reducing mitochondrial O2∸ improves endothelial function and hypertension. To confirm this using non-pharmacological means, we investigated production of vascular O2∸ and the development of hypertension in tgSOD2 mice. These animals have a 2-fold increase in SOD2 activity and protein levels 37. While basal blood pressures in tgSOD2 and C57Bl/6 mice were similar, the hypertensive response to angiotensin II was attenuated and delayed in tgSOD2 mice (Figure 6A). The production of vascular O2∸ in angiotensin II-infused tgSOD2 mice was significantly lower compared with C57Blk/6 mice while basal O2∸ level was not different (Figure 6B). These data are in keeping with our findings with mitoTEMPO treatment and confirmed the role of mitochondrial O2∸ in vivo.
Figure 6.
Analysis of blood pressure and vascular O2∸ in SOD2 overexpressing transgenic mice infused with angiotensin II. (A) Systolic blood pressure in TgSOD2 and C57Blk/6 mice infused with saline or angiotensin II (0.7 mg/kg/day). (B) Production of aortic O2∸ measured with DHE and HPLC. Results represent mean ± SEM for 4-8 animals per group. * P<0.05 vs Ang II. (C) Proposed role of mitochondrial O2∸ in endothelial dysfunction and hypertension.
Discussion
The present study provides the first evidence that scavenging of mitochondrial O2∸ improves endothelial function and reduces hypertension. In this work we found that treatment with either the mitochondria-targeted SOD mimetic mitoTEMPO or overexpression of SOD2 inhibited oxidative stress and prevented the loss of endothelial nitric oxide caused by angiotensin II both in cultured endothelial cells and intact mice. Furthermore, treatment of hypertensive mice with mitoTEMPO after the onset of either angiotensin II- or DOCA salt-induced hypertension significantly reduced blood pressure and substantially improved endothelium-dependent vasodilatation. Analysis of SOD2 overexpressing transgenic mice confirmed an important role of mitochondrial O2∸ in endothelial function and hypertension.
We have previously shown that angiotensin II stimulates production of mitochondrial O2∸ 14. This was dependent on NADPH oxidase activity because siRNA-induced depletion of the NADPH oxidase subunit p22phox, or inhibition of NADPH oxidase activity by apocynin prevented mitochondrial impairment and attenuated mitochondrial O2∸ production 14, demonstrating an upstream role of the NADPH oxidase in modulation of mitochondrial O2∸. In the current study we have additionally found that mitochondrial O2∸ stimulates extramitochondrial NADPH oxidase activity in a feed-forward fashion. Taken together, these studies indicate that the interplay between mitochondrial and NADPH oxidase-derived O2∸ constitutes a vicious cycle (Figure 6C) in which the NADPH oxidase increases mitochondrial ROS, which further activates the cytoplasmic NADPH oxidase and increases cellular O2∸ production, diminishing NO• bioavailability and uncoupling eNOS 38. The effect of mitochondrial ROS on NADPH oxidase activity is quite likely mediated by c-Src 39 which can be stimulated by H2O2 34. Indeed, activation of NADPH oxidase has been reported to be a biphasic process in which the first phase requires direct activation by angiotensin II followed by a second phase of sustained activation that is H2O2 dependent 40. This could explain why inhibition of mitochondrial H2O2 by mitoTEMPO (Table 2) results in decrease of NADPH oxidase activity (Figure 2C). Our current findings also indicate that scavenging of mitochondrial O2∸ using mitochondria-targeted antioxidants can interrupt this vicious cycle.
Our work is in keeping with prior findings that SOD2+/− mice are prone to age-associated and salt-induced hypertension 8 and that treatment with the mitochondria-targeted antioxidant mQ10 attenuates hypertension in spontaneously hypertensive rats 41. Our findings also provide additional insight into the role of SOD2 in development of these pathological conditions. We suggest that SOD2 depletion increases mitochondrial O2∸ levels which upregulates NADPH oxidase activity. Indeed, the key role of NADPH oxidase in hypertension and atherosclerosis has been well documented 35. The synergism between mitochondrial and cellular O2∸ production reported in our work may explain these pathological effects in SOD+/− mice.
Previous studies have shown that the nontargeted SOD mimetic TEMPOL prevents hypertension, renal and vascular dysfunction in several models of hypertension 25, 42. Importantly, we found that targeting of SOD mimetic to mitochondria provided beneficial effects at a dose 1000-fold lower than previously reported for TEMPOL. This finding is important in two respects. First, it confirms a critical role of the mitochondria in hypertension and endothelial dysfunction. Second, our data demonstrate the feasibility of using very low doses of mitochondrial-targeted antioxidants for therapeutic purposes.
Importantly, mitoTEMPO not only attenuated development of hypertension but was also effective in treating hypertension after it was established. This is clinically important because treatment is commonly started in humans after hypertension has developed. Our findings indicate that targeting mitochondrial ROS with agents like mitoTEMPO might therefore be effective in treating human hypertension. This is potentially important because many patients' blood pressure remains poorly controlled despite treatment with multiple drugs. MitoTEMPO and other mitochondria-targeted agents might therefore represent a new class of anti-hypertensive agent that could add to the currently available therapeutic armamentarium.
MitoTEMPO had no effect on blood pressure in normotensive animals. This is in keeping with the concept that O2∸ does not affect hemodynamics under normal physiological conditions but begins to play a role in pathophysiological states 43. Indeed, SOD2 overexpression and mitoTEMPO supplementation did not affect basal activity of NADPH oxidase, production of vascular O2∸ or endothelium-dependent relaxation. This is potentially important, because unlike some currently employed anti-hypertensive agents, mitoTEMPO would unlikely cause hypotension in normotensive subjects.
In addition to hypertension, there are many other common conditions including aging, atherosclerosis, diabetes and degenerative neurological disorders in which mitochondrial oxidative stress seems to play a role 44, 45. Of note, large clinical trials have failed to show a benefit of often-employed antioxidants such as vitamin E and vitamin C in many of these conditions 46, 47, and have paradoxically shown deleterious effects in some trials 47. There are many potential explanations why these antioxidants have proven ineffective in these studies, but one relates to the fact that agents such as vitamin E and vitamin C are not targeted to sites of ROS generation that are most important in pathological conditions. It is conceivable that the use of SOD mimetics such as mitoTEMPO, targeted to compartments where ROS is generated such as the mitochondria, would be more effective in these conditions. The ability to achieve these effects in relatively low doses might also limit potential untoward effects of antioxidant therapy observed with other agents.
Novelty and Significance.
“What is known?”
Oxidative stress is strongly implicated in the pathogenesis of hypertension.
Angiotensin II increases superoxide production by NADPH oxidases.
Angiotensin II causes mitochondrial dysfunction.
“What new information does this article contribute?”
Scavenging of mitochondrial superoxide significantly improves endothelial function.
Inhibition of mitochondrial superoxide reduces the activity of NADPH oxidases.
Mitochondria-targeted antioxidants can be used as antihypertensive agents.
Despite the fact that the mitochondria are an important source of superoxide in vascular cells, the role of mitochondrial superoxide in endothelial dysfunction remains unclear. We have found that stimulation of endothelial cells with angiotensin II increases the production of mitochondrial superoxide. Overexpression of SOD2 or treatment with mitochondria-targeted SOD mimetic mitoTEMPO attenuates activation of vascular NADPH oxidases, inhibits production of cellular superoxide, restores nitric oxide production, improves endothelium-dependent vasodilatation and reduces blood pressure in angiotensin II infused mice. This work demonstrates that angiotensin II-induced superoxide production by NADPH oxidase stimulates mitochondrial superoxide that in turn provides redox-dependent feed-forward stimulation of NADPH oxidase. This vicious cycle can be interrupted at the mitochondrial site by mitochondria targeted antioxidants. For the first time we have found that angiotensin II-induced hypertension was attenuated in SOD2 overexpressing transgenic mice. Furthermore, mitoTEMPO treatment after the onset of DOCA-salt or angiotensin II-induced hypertension significantly decreased blood pressure. These studies show that mitochondrial superoxide is important for the development of hypertension and that antioxidant strategies specifically targeting this organelle could have therapeutic benefit in this and possibly other diseases.
Supplementary Material
Acknowledgements
We thank Dr. Kathy K. Griendling for fruitful discussion, Bernard Lassègue, Lula L. Hilenski, Alexander V. Panov and Vladimir I. Mayorov for technical assistance.
Sources of Funding
This work was supported by funding from National Institute of Health grants HL094469, HL38206, HL058863, HL06072808, PO-1 HL058000, PO-1 HL075209, American Heart Association SDG 0430201N and Grant-in-Aid 09GRNT2220128.
Non-standard Abbreviations and Acronyms
- Ang II
angiotensin II
- DHE
dihydroethidium
- EMPO
2-ethoxycarbonyl-2-methyl-3,4-dihydro-2H-pyrrole-1-oxide
- ESR
electron spin resonance
- mitoTEMPO
(2-(2,2,6,6-tetramethylpiperidin-1-oxyl-4-ylamino)-2-oxoethyl) triphenylphosphonium
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- TEMPOL
4-hydroxy-2,2,6,6-tetramethylpiperidinyloxy
Footnotes
Disclosure
None.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Madamanchi NR, Runge MS. Mitochondrial dysfunction in atherosclerosis. Circ Res. 2007;100:460–473. doi: 10.1161/01.RES.0000258450.44413.96. [DOI] [PubMed] [Google Scholar]
- 2.Puddu P, Puddu GM, Galletti L, Cravero E, Muscari A. Mitochondrial dysfunction as an initiating event in atherogenesis: a plausible hypothesis. Cardiology. 2005;103:137–141. doi: 10.1159/000083440. [DOI] [PubMed] [Google Scholar]
- 3.Perier C, Tieu K, Guegan C, Caspersen C, Jackson-Lewis V, Carelli V, Martinuzzi A, Hirano M, Przedborski S, Vila M. Complex I deficiency primes Bax-dependent neuronal apoptosis through mitochondrial oxidative damage. Proc Natl Acad Sci U S A. 2005;102:19126–19131. doi: 10.1073/pnas.0508215102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rego AC, Oliveira CR. Mitochondrial dysfunction and reactive oxygen species in excitotoxicity and apoptosis: implications for the pathogenesis of neurodegenerative diseases. Neurochem Res. 2003;28:1563–1574. doi: 10.1023/a:1025682611389. [DOI] [PubMed] [Google Scholar]
- 5.Wall JA, Wei J, Ly M, Belmont P, Martindale JJ, Tran D, Sun J, Chen WJ, Yu W, Oeller P, Briggs S, Gustafsson AB, Sayen MR, Gottlieb RA, Glembotski CC. Alterations in oxidative phosphorylation complex proteins in the hearts of transgenic mice that overexpress the p38 MAP kinase activator, MAP kinase kinase 6. Am J Physiol Heart Circ Physiol. 2006;291:H2462–72. doi: 10.1152/ajpheart.01311.2005. [DOI] [PubMed] [Google Scholar]
- 6.Ballinger SW. Mitochondrial dysfunction in cardiovascular disease. Free Radic Biol Med. 2005;38:1278–1295. doi: 10.1016/j.freeradbiomed.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 7.Bulteau AL, Szweda LI, Friguet B. Mitochondrial protein oxidation and degradation in response to oxidative stress and aging. Exp Gerontol. 2006;41:653–657. doi: 10.1016/j.exger.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez-Iturbe B, Sepassi L, Quiroz Y, Ni Z, Wallace DC, Vaziri ND. Association of mitochondrial SOD deficiency with salt-sensitive hypertension and accelerated renal senescence. J Appl Physiol. 2007;102:255–260. doi: 10.1152/japplphysiol.00513.2006. [DOI] [PubMed] [Google Scholar]
- 9.Harrison DG. Endothelial function and oxidant stress. Clin Cardiol. 1997;20(11 Suppl 2):II-11–17. [PubMed] [Google Scholar]
- 10.Harrison DG, Cai H. Endothelial control of vasomotion and nitric oxide production. Cardiol Clin. 2003;21:289–302. doi: 10.1016/s0733-8651(03)00073-0. [DOI] [PubMed] [Google Scholar]
- 11.Lavoie JL, Sigmund CD. Minireview: overview of the renin-angiotensin system--an endocrine and paracrine system. Endocrinology. 2003;144:2179–2183. doi: 10.1210/en.2003-0150. [DOI] [PubMed] [Google Scholar]
- 12.Zhou MS, Hernandez Schulman I, Pagano PJ, Jaimes EA, Raij L. Reduced NAD(P)H oxidase in low renin hypertension: link among angiotensin II, atherogenesis, and blood pressure. Hypertension. 2006;47:81–86. doi: 10.1161/01.HYP.0000197182.65554.c7. [DOI] [PubMed] [Google Scholar]
- 13.Tian N, Moore RS, Phillips WE, Lin L, Braddy S, Pryor JS, Stockstill RL, Hughson MD, Manning RD., Jr NADPH oxidase contributes to renal damage and dysfunction in Dahl salt-sensitive hypertension. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1858–1865. doi: 10.1152/ajpregu.90650.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doughan AK, Harrison DG, Dikalov SI. Molecular mechanisms of angiotensin II mediated mitochondrial dysfunction. Linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ Res. 2008;102:488–496. doi: 10.1161/CIRCRESAHA.107.162800. [DOI] [PubMed] [Google Scholar]
- 15.Widder JD, Fraccarollo D, Galuppo P, Hansen JM, Jones DP, Ertl G, Bauersachs J. Attenuation of Angiotensin II-Induced Vascular Dysfunction and Hypertension by Overexpression of Thioredoxin 2. Hypertension. 2009;54:338–44. doi: 10.1161/HYPERTENSIONAHA.108.127928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuzkaya N, Weissmann N, Harrison DG, Dikalov S. Interactions of peroxynitrite with uric acid in the presence of ascorbate and thiols: implications for uncoupling endothelial nitric oxide synthase. Biochem Pharmacol. 2005;70:343–354. doi: 10.1016/j.bcp.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 17.Hoch NE, Guzik TJ, Chen W, Deans T, Maalouf SA, Gratze P, Weyand C, Harrison DG. Regulation of T-cell function by endogenously produced angiotensin II. Am J Physiol Regul Integr Comp Physiol. 2009;296:R208–216. doi: 10.1152/ajpregu.90521.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trounce IA, Kim YL, Jun AS, Wallace DC. Assessment of mitochondrial oxidative phosphorylation in patient muscle biopsies, lymphoblasts, and transmitochondrial cell lines. Methods Enzymol. 1996;264:484–509. doi: 10.1016/s0076-6879(96)64044-0. [DOI] [PubMed] [Google Scholar]
- 19.Doughan AK, Harrison DG, Dikalov SI. Molecular mechanisms of angiotensin II-mediated mitochondrial dysfunction: linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ Res. 2008;102:488–496. doi: 10.1161/CIRCRESAHA.107.162800. [DOI] [PubMed] [Google Scholar]
- 20.Doughan AK, Dikalov SI. Mitochondrial redox cycling of mitoquinone leads to superoxide production and cellular apoptosis. Antioxid Redox Signal. 2007;9:1825–1836. doi: 10.1089/ars.2007.1693. [DOI] [PubMed] [Google Scholar]
- 21.Csiszar A, Labinskyy N, Zhao X, Hu F, Serpillon S, Huang Z, Ballabh P, Levy RJ, Hintze TH, Wolin MS, Austad SN, Podlutsky A, Ungvari Z. Vascular superoxide and hydrogen peroxide production and oxidative stress resistance in two closely related rodent species with disparate longevity. Aging Cell. 2007;6:783–797. doi: 10.1111/j.1474-9726.2007.00339.x. [DOI] [PubMed] [Google Scholar]
- 22.Dikalov S, Griendling KK, Harrison DG. Measurement of reactive oxygen species in cardiovascular studies. Hypertension. 2007;49:717–727. doi: 10.1161/01.HYP.0000258594.87211.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dikalov SI, Dikalova AE, Bikineyeva AT, Schmidt HH, Harrison DG, Griendling KK. Distinct roles of Nox1 and Nox4 in basal and angiotensin II-stimulated superoxide and hydrogen peroxide production. Free Radic Biol Med. 2008;45:1340–1351. doi: 10.1016/j.freeradbiomed.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dikalov S, Fink B. ESR techniques for the detection of nitric oxide in vivo and in tissues. Methods Enzymol. 2005;396:597–610. doi: 10.1016/S0076-6879(05)96052-7. [DOI] [PubMed] [Google Scholar]
- 25.Dikalova A, Clempus R, Lassegue B, Cheng G, McCoy J, Dikalov S, San Martin A, Lyle A, Weber DS, Weiss D, Taylor WR, Schmidt HH, Owens GK, Lambeth JD, Griendling KK. Nox1 overexpression potentiates angiotensin II-induced hypertension and vascular smooth muscle hypertrophy in transgenic mice. Circulation. 2005;112:2668–2676. doi: 10.1161/CIRCULATIONAHA.105.538934. [DOI] [PubMed] [Google Scholar]
- 26.Krege JH, Hodgin JB, Hagaman JR, Smithies O. A noninvasive computerized tail-cuff system for measuring blood pressure in mice. Hypertension. 1995;25:1111–1115. doi: 10.1161/01.hyp.25.5.1111. [DOI] [PubMed] [Google Scholar]
- 27.Widder JD, Guzik TJ, Mueller CF, Clempus RE, Schmidt HH, Dikalov SI, Griendling KK, Jones DP, Harrison DG. Role of the multidrug resistance protein-1 in hypertension and vascular dysfunction caused by angiotensin II. Arterioscler Thromb Vasc Biol. 2007;27:762–768. doi: 10.1161/01.ATV.0000259298.11129.a2. [DOI] [PubMed] [Google Scholar]
- 28.Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest. 2003;111:1201–1209. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murphy MP, Smith RA. Drug delivery to mitochondria: the key to mitochondrial medicine. Adv Drug Deliv Rev. 2000;41:235–250. doi: 10.1016/s0169-409x(99)00069-1. [DOI] [PubMed] [Google Scholar]
- 30.Dhanasekaran A, Kotamraju S, Karunakaran C, Kalivendi SV, Thomas S, Joseph J, Kalyanaraman B. Mitochondria superoxide dismutase mimetic inhibits peroxide-induced oxidative damage and apoptosis: role of mitochondrial superoxide. Free Radic Biol Med. 2005;39:567–583. doi: 10.1016/j.freeradbiomed.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 31.Krishna MC, Russo A, Mitchell JB, Goldstein S, Dafni H, Samuni A. Do nitroxide antioxidants act as scavengers of O2-. or as SOD mimics? J Biol Chem. 1996;271:26026–26031. doi: 10.1074/jbc.271.42.26026. [DOI] [PubMed] [Google Scholar]
- 32.Dikalov S, Skatchkov M, Bassenge E. Quantification of peroxynitrite, superoxide, and peroxyl radicals by a new spin trap hydroxylamine 1-hydroxy-2,2,6,6-tetramethyl-4-oxo-piperidine. Biochem Biophys Res Commun. 1997;230:54–57. doi: 10.1006/bbrc.1996.5880. [DOI] [PubMed] [Google Scholar]
- 33.Zielonka J, Hardy M, Kalyanaraman B. HPLC study of oxidation products of hydroethidine in chemical and biological systems: ramifications in superoxide measurements. Free Radic Biol Med. 2009;46:329–338. doi: 10.1016/j.freeradbiomed.2008.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ushio-Fukai M, Griendling KK, Becker PL, Hilenski L, Halleran S, Alexander RW. Epidermal growth factor receptor transactivation by angiotensin II requires reactive oxygen species in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2001;21:489–495. doi: 10.1161/01.atv.21.4.489. [DOI] [PubMed] [Google Scholar]
- 35.Harrison DG, Cai H, Landmesser U, Griendling KK. Interactions of angiotensin II with NAD(P)H oxidase, oxidant stress and cardiovascular disease. J Renin Angiotensin Aldosterone Syst. 2003;4:51–61. doi: 10.3317/jraas.2003.014. [DOI] [PubMed] [Google Scholar]
- 36.Katori M, Majima M. A missing link between a high salt intake and blood pressure increase. J Pharmacol Sci. 2006;100:370–390. doi: 10.1254/jphs.crj06003x. [DOI] [PubMed] [Google Scholar]
- 37.Kowluru RA, Kowluru V, Xiong Y, Ho YS. Overexpression of mitochondrial superoxide dismutase in mice protects the retina from diabetes-induced oxidative stress. Free Radic Biol Med. 2006;41:1191–1196. doi: 10.1016/j.freeradbiomed.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 38.Boulden BM, Widder JD, Allen JC, Smith DA, Al-Baldawi RN, Harrison DG, Dikalov SI, Jo H, Dudley SC., Jr Early determinants of H2O2-induced endothelial dysfunction. Free Radic Biol Med. 2006;41:810–817. doi: 10.1016/j.freeradbiomed.2006.05.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Griendling KK, Sorescu D, Lassegue B, Ushio-Fukai M. Modulation of protein kinase activity and gene expression by reactive oxygen species and their role in vascular physiology and pathophysiology. Arterioscler Thromb Vasc Biol. 2000;20:2175–2183. doi: 10.1161/01.atv.20.10.2175. [DOI] [PubMed] [Google Scholar]
- 40.Seshiah PN, Weber DS, Rocic P, Valppu L, Taniyama Y, Griendling KK. Angiotensin II stimulation of NAD(P)H oxidase activity: upstream mediators. Circ Res. 2002;91:406–413. doi: 10.1161/01.res.0000033523.08033.16. [DOI] [PubMed] [Google Scholar]
- 41.Graham D, Huynh NN, Hamilton CA, Beattie E, Smith RA, Cocheme HM, Murphy MP, Dominiczak AF. Mitochondria-targeted antioxidant MitoQ10 improves endothelial function and attenuates cardiac hypertrophy. Hypertension. 2009;54:322–328. doi: 10.1161/HYPERTENSIONAHA.109.130351. [DOI] [PubMed] [Google Scholar]
- 42.Welch WJ, Mendonca M, Blau J, Karber A, Dennehy K, Patel K, Lao YS, Jose PA, Wilcox CS. Antihypertensive response to prolonged tempol in the spontaneously hypertensive rat. Kidney Int. 2005;68:179–187. doi: 10.1111/j.1523-1755.2005.00392.x. [DOI] [PubMed] [Google Scholar]
- 43.Laursen JB, Rajagopalan S, Galis Z, Tarpey M, Freeman BA, Harrison DG. Role of superoxide in angiotensin II-induced but not catecholamine-induced hypertension. Circulation. 1997;95:588–593. doi: 10.1161/01.cir.95.3.588. [DOI] [PubMed] [Google Scholar]
- 44.de Cavanagh EM, Inserra F, Ferder M, Ferder L. From mitochondria to disease: role of the renin-angiotensin system. Am J Nephrol. 2007;27:545–553. doi: 10.1159/000107757. [DOI] [PubMed] [Google Scholar]
- 45.Fridovich I. Mitochondria: are they the seat of senescence? Aging Cell. 2004;3:13–16. doi: 10.1046/j.1474-9728.2003.00075.x. [DOI] [PubMed] [Google Scholar]
- 46.Song Y, Cook NR, Albert CM, Van Denburgh M, Manson JE. Effects of vitamins C and E and beta-carotene on the risk of type 2 diabetes in women at high risk of cardiovascular disease: a randomized controlled trial. Am J Clin Nutr. 2009;90:429–437. doi: 10.3945/ajcn.2009.27491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sesso HD, Buring JE, Christen WG, Kurth T, Belanger C, MacFadyen J, Bubes V, Manson JE, Glynn RJ, Gaziano JM. Vitamins E and C in the prevention of cardiovascular disease in men: the Physicians' Health Study II randomized controlled trial. Jama. 2008;300:2123–2133. doi: 10.1001/jama.2008.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.