Abstract
Female reproductive aging in rats is characterized by reduced gonadotropin releasing hormone (GnRH) neuronal activation under estradiol positive feedback conditions and a delayed and attenuated luteinizing hormone (LH) surge. The newly identified excitatory neuropeptide kisspeptin is proposed to be a critical mediator of the pubertal transition and the ovarian steroid-induced LH surge. We previously showed that estradiol induces less kisspeptin mRNA expression in the anterior hypothalamus [anatomical location of anteroventral periventricular nucleus (AVPV)] in middle-aged than in young rats and intrahypothalamic infusion of kisspeptin restores LH surge amplitude in middle-aged females. Thus, reduced kisspeptin neurotransmission may contribute to age-related LH surge abnormalities. This study tested the hypothesis that middle-aged females will also exhibit reduced numbers of kisspeptin immunopositive neurons in the AVPV under estradiol positive feedback conditions. Using immunohistochemistry, we demonstrate that middle-aged females primed with ovarian steroids have fewer AVPV kisspeptin immunopositive neurons than young females. Age did not affect kisspeptin mRNA expression in the pituitary, numbers of kisspeptin immunopositive neurons in the arcuate nucleus, or estradiol-dependent reductions in kisspeptin mRNA expression in the posterior hypothalamus (containing the arcuate nucleus). These data strongly suggest that age-related LH surge dysfunction results, in part, from a reduced sensitivity of AVPV kisspeptin neurons to estradiol and hence decreased availability of AVPV kisspeptin neurons to activate GnRH neurons under positive feedback conditions.
Keywords: Aging, Kisspeptin, GPR54, Hypothalamus, AVPV, Arcuate nucleus
Introduction
Recent evidence in humans and other species suggests that female reproductive senescence is a complex process that involves progressive ovarian dysfunction and an altered capacity of neuropeptides that control the hypothalamic-pituitary axis to respond to estradiol (Downs and Wise, 2009). The preovulatory luteinizing hormone (LH) surge of middle-aged rats typically has a delayed onset and reduced peak amplitude (Cooper et al., 1980; Scarbrough and Wise, 1990) and middle-aged women exhibit frequent failure of the LH surge despite serum estradiol levels appropriate for induction of a surge in young individuals (Weiss et al., 2004). This LH surge dysfunction does not result from a reduced density of gonadotropin releasing hormone (GnRH) neurons (Krajnak et al., 2001; Rance et al., 1990), reduced ability of GnRH neurons to release GnRH when depolarized (Rubin, 1992), or reduced pituitary responsiveness to GnRH in either rodents (Wise and Ratner, 1980) or perimenopausal women (Neal-Perry G., 2005). Age related LH surge abnormalities are associated with reduced GnRH neuron activation on the day of the LH surge, suggesting a failure of estrogen positive feedback conditions to increase excitation of GnRH neurons (Krajnak et al., 2001).
The recently discovered excitatory neuropeptide kisspeptin (KiSS1) plays an integral role in reproductive function (for review Kauffman, 2009). Initially produced as a 145 amino acid peptide, KiSS1 is subsequently cleaved into kisspeptin-54 and three shorter fragments, all of which demonstrate similar affinity and efficacy for the KiSS1 receptor (also known as GPR54) (Kotani et al., 2001). Kisspeptin is the most potent excitatory neuropeptide for GnRH neurons (Han et al., 2005). Kisspeptin neurons are found in the anteroventral periventricular nucleus (AVPV) and the arcuate nucleus (ARC), and KiSS1 expression in these hypothalamic regions is differentially regulated by estrogens (for review Roa et al., 2009). Estradiol increases the number of KiSS1 immunopositive neurons and KiSS1 mRNA in the AVPV under conditions that promote the LH surge, referred to as positive feedback (Adachi et al., 2007; Smith et al., 2005). In contrast, estradiol decreases numbers of KiSS1 immunopositive neurons and KiSS1 mRNA in the ARC, and this is thought to mediate estradiol suppression of LH release, referred to as negative feedback (Adachi et al., 2007; Smith et al., 2005). Mutations of the KiSS1 or KiSS1 receptor gene in humans or mice produce hypogonadotropic hypogonadism and failure to initiate the pubertal transition (for review Kauffman, 2009). Lastly, an autosomal dominant KiSS1 receptor mutation can result in idiopathic central precocious puberty (Teles et al., 2008).
Most KiSS1 research focuses on puberty and the regulation of reproduction in young adults, with few studies addressing the importance of this neuropeptide in reproductive aging. Recent evidence suggests that expression of KiSS1 mRNA is altered in the medial basal hypothalamus of aging female human and non-human primates (Kim et al., 2009; Rometo et al., 2007). We recently reported that estradiol induces less KiSS1 mRNA expression in the anterior hypothalamus (containing the AVPV) of middle-aged compared to young rats and that intrahypothalamic administration of kisspeptin-10 to middle-aged female rats restores LH surge amplitude (Neal-Perry et al., 2009). Therefore, we hypothesize that reduced KiSS1 activation of GnRH neurons underlies age-related changes in the LH surge. The objective of this study was to determine if age affects the ability of ovarian steroids to modulate the expression of hypothalamic and/or pituitary KiSS1 mRNA, KiSS1 receptor mRNA or the number of KiSS1 immunopositive neurons in the AVPV and/or ARC on the day of the LH surge.
Materials and Methods
Drugs
Estradiol benzoate (EB) and progesterone (P) were purchased from Steraloids, Inc. (Newport, RI, USA), dissolved in peanut oil and injected subcutaneously (sc). Ketamine (80 mg/kg) and xylazine (4 mg/kg) were purchased from Fort Dodge Animal Health (Fort Dodge, IA, USA) and Lloyd Laboratories (Shenandoah, IA, USA), respectively. Colchicine was purchased from Sigma-Aldrich (St. Louis, MO, USA). Paraformaldehyde was purchased from Sigma-Aldrich, and acrolein was purchased from Polysciences, Inc. (Warrington, PA, USA).
Animals
Young adult (3–4 months) and middle-aged (9–11 months, retired breeders) female Sprague-Dawley rats purchased from Taconic Farms (Germantown, NY, USA) were allowed free access to both food and water and maintained in a 14 h light:10 h dark cycle with lights off at 2000 h. Only young and middle-aged rats that demonstrated at least one or two consecutive estrous cycles, respectively, were included in the study. Estrous cyclicity was verified with daily vaginal lavage and cytology. All young and middle-aged rats were anesthetized with intramuscular injections of ketamine/xylazine, ovariohysterectomized (OVX) and allowed to recover for 7 days before ovarian steroid priming. All experiments were conducted in accordance with the guidelines set forth by the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at the Albert Einstein College of Medicine.
Steroid priming
On postoperative day 7 at 0900 h, all rats were injected sc with either 2 µg of EB or peanut oil (control); a second injection was given 24 h later. Forty-eight h after the first injection (day 9), 500 µg of P or control was administered. These doses of EB and P reliably induce LH surges in rats of this strain (Neal-Perry et al., 2005).
Hypothalamic and pituitary dissection and reverse transcription, real time PCR
Young and middle-aged rats underwent OVX and hormone priming as described above. On postoperative day 9, animals were anesthetized with ketamine/xylazine and killed by decapitation. Young rats (n=13–19) and middle-aged rats (n=11–21) were killed 4 h (1300 h) after their last injection of P or oil, the onset time of the LH surge in young rats (Neal-Perry et al., 2008). Because LH surge onset is delayed in middle-aged rats (Neal-Perry et al., 2008), some middle-aged rats (n=9–15) were killed 7 h (1600 h; approximates time of LH surge onset in middle-aged rats) following their last injection of P or oil. After decapitation, the pituitary and the entire hypothalamus were rapidly isolated. The original block of hypothalamic tissue was bordered laterally by the hypothalamic sulci, rostrally 2 mm anterior to the optic chiasm, and caudally at the mammillary bodies. The hypothalamus was divided just posterior to the optic chiasm to obtain a sample, which contained the ARC, one of the anatomical locations for hypothalamic KiSS1 neurons. Specimens were immediately frozen on dry ice, and expression of KiSS1 mRNA in the ARC and pituitary, and expression of KiSS1 receptor mRNA in the pituitary was later determined by semi-quantitative, reverse transcription (RT), real time PCR utilizing the protocol previously described (Neal-Perry et al., 2009).
Briefly, DNA-free total RNA was purified according to the manufacturer’s instructions using the RNeasy Lipid Mini kit, including a DNase step (Qiagen, Valencia, CA, USA). Gene expression assays were performed in a two-step reaction. The first step involved RT, where single-stranded cDNA was reverse transcribed from RNA according to the manufacturer’s recommendations using the High Capacity cDNA Reverse Transcription Kit with RNase inhibitor (Applied Biosystems, Foster City, CA, USA) with 500 ng of RNA per 20 µl of RT reaction. In the second step, PCR products were synthesized from cDNA samples using the TaqMan Gene Expression Assay and Master Mix (Applied Biosystems). TaqMan gene expression assays contained the proprietary TaqMan probe and primers (Applied Biosystems) for the housekeeping gene and endogenous control, GAPDH (primer limited, VIC®/MGB probe, part number 4374966, context sequence NM_017008.3) and the specific targets KiSS1 (KiSS1, primer limited, Fam probe, assay ID Rn00710914_m1, context sequence NM_181692.1) or KiSS1 receptor (GPR54, primer limited, Fam probe, assay ID Rn00710914_m1, context sequence: MN_181692.1). When the RT step was performed without reverse transcriptase, no PCR signal was detected, demonstrating that the mRNA samples were not contaminated with genomic DNA. Real-time PCR was performed using ABI PRISM 7900HT (PE Applied Biosystems) in multiplex conditions using 50 ng of cDNA per 20 µl of total reaction mix.
Amplified transcripts were quantified using the comparative threshold cycle method and using GAPDH as a normalizer. Fold changes in either KiSS1 mRNA or KiSS1 receptor mRNA expression were calculated as 2−ΔΔCT with CT = threshold cycle, ΔCT = CT (KiSS1) - CT (GADPH) or ΔCT = CT (KiSS1 receptor) - CT (GADPH), and ΔΔCT = ΔCT (experimental) - ΔCT (reference). The young control group served as the specific reference control for each of the ΔΔCT calculations.
Immunohistochemistry
Rats used for immunohistochemistry (IHC) received an intracerebroventricular (icv) cannula stereotaxically implanted in the third ventricle at time of OVX. Anesthetized rats were placed in a Kopf stereotaxic apparatus with the nose bar set at +5.0 mm. Using stereotaxic coordinates provided by the atlas of Pellegrino et al. (Pellegrino L.J., 1979) and Bregma as a landmark (anterior/posterior, +0.2 mm; medial/lateral, +0.0 mm; dorsal/ventral, −7.8 mm), a 22 gauge guide cannula (Plastics One, Roanoke, VA, USA) was implanted into the third ventricle. Using a protocol adapted form Adachi et al. (2007) KiSS1 immunopositive neurons were visualized (Adachi et al., 2007). On the first day of injections (EB or oil), rats were lightly anesthetized with ketamine/xylazine at 0900 h and then infused through an icv cannula with 75 µg of colchicine dissolved in 15 µl of normal saline over 15 minutes. Four hours after the P or oil injection (postoperative day 9 at 1300 h), rats were killed with ketamine/xylazine and perfused with 2.5% acrolein and 4% paraformaldehyde. Brains were removed, post-fixed in 4% paraformaldehyde overnight at 4°C, and then transferred to 30% sucrose for cryoprotection. After the brains sank they were frozen in methylbutane and stored frozen at −80°C until sectioning. Using a cryostat, 25 µm coronal sections through the AVPV and the ARC were collected and stored in cryoprotectant at −20°C until processed for IHC. Placement of the infusion cannula within the third ventricle was confirmed during sectioning, and only rats with confirmed cannula placement were included for IHC. A 1-in-3 series of sections from the AVPV (4 sections/ rat) and a 1-in-6 series from the ARC (16 sections/rat) were selected for IHC.
IHC was performed using a protocol modified from Lapatto et al. (2007) (Lapatto et al., 2007). Briefly, free floating sections were rinsed several times in 0.05 M potassium phosphate buffered saline (KPBS), followed by reduction of the aldehydes with 1% sodium borohydride for 20 min and further rinsing. Sections were then incubated in 0.014% phenylhydrazine for 15 min, rinsed and incubated with the anti-KiSS1 rabbit polyclonal primary antibody (1:300,000; antiserum #566, a generous gift from Dr. A. Caraty, Toulouse Cedex, France) in KPBS with 0.4% Triton X-100 for 1 h at room temperature and then 48–72 h at 4°C. After rinsing, sections were incubated in secondary biotinylated goat anti-rabbit IgG (1:600; Vector Laboratories, Burlingame, CA, USA) for 1 h at room temperature, rinsed again and then incubated for 1 h in ABC Elite Kit (Vector Laboratories) at room temperature. Following incubation, sections were rinsed in KBPS, then in 0.175 M sodium acetate. Staining was performed with a DAB/nickel chromogen solution for 10 min, and sections were sequentially rinsed in sodium acetate and KPBS. Sections were mounted on Super Frost/Plus mounting slides (Fisher Scientific, Pittsburgh, PA, USA), air-dried overnight, dehydrated in ascending alcohol concentrations, cleared by xylenes, and cover slipped with Permount (Fisher Scientific). Tissue sections treated in identical fashion except without primary antibody served to control for antibody specificity.
Cell Counting
To quantify KiSS1 immunopositive neurons in the AVPV and ARC, an Olympus BX41 microscope attached to an Olympus digital camera (Olympus, Melville, NY, USA) was utilized. Sections were analyzed using Pictureframe™ 2.2 software (Optronics, Goleta, CA, USA), and cell counting was performed by two researchers blinded to the treatment utilizing ImageJ software (available at http://rsbweb.nih.gov/ij/). Bilateral cell counts for each section were averaged between researchers, and variation in cell counts between researchers was 10.5%. Total cell counts for both the AVPV (4 sections/rat) and the ARC (16 sections/rat) were summed and analyzed. KiSS1 immunoreactive cells were counted if they displayed a round or oval morphology with a diameter of approximately 18 ± 0.1 µm, and blue/black cytoplasmic staining.
Statistical Analysis
Mean KiSS1 and KiSS1 receptor mRNA fold changes, and mean total cell counts for the AVPV and ARC were calculated using Intercooled Stata 9.2 software (StataCorp., College Station, TX, USA). All data were normally distributed and expressed as mean ± SEM. Two-way ANOVA (age × treatment) was utilized to detect differences in either KiSS1 or KiSS1 receptor mRNA fold changes and to detect differences in the total cell counts for the AVPV and ARC. P < 0.05 was considered statistically significant. Newman Keuls post-hoc tests were performed as appropriate to determine specific interactions.
Results
Reproductive age does not influence posterior hypothalamic KiSS1 mRNA
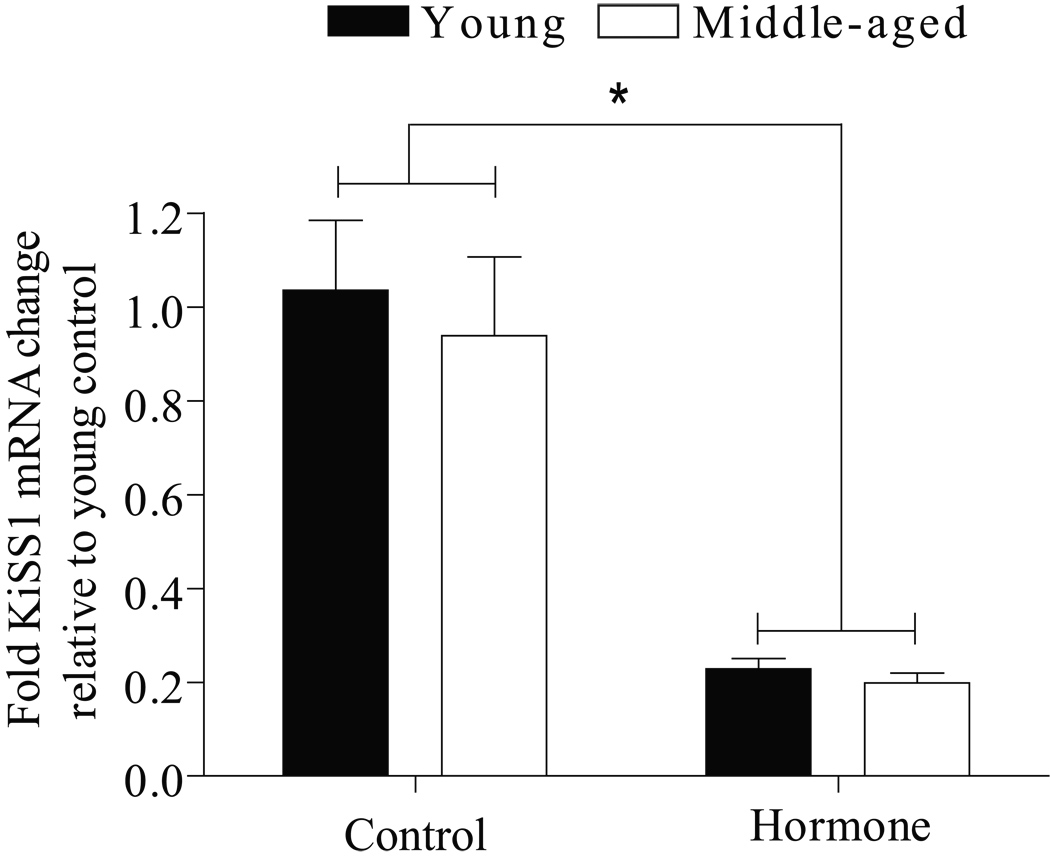
Effects of age and hormone treatment on the expression of KiSS1 mRNA in the posterior hypothalamus (containing the ARC) were examined using real time RT-PCR. We recently reported that middle-aged rats have reduced levels of KiSS1 mRNA expression in the anterior hypothalamus compared to young rats under estrogen positive feedback conditions (Neal-Perry et al., 2009). This study reports data on the posterior hypothalamus (containing the ARC). There was no difference in KiSS1 mRNA expression in either young or middle-aged females treated with EB or EB and P; therefore, data from all hormone treatments were pooled for each age group. Likewise, there was no difference in KiSS1 mRNA in middle-aged rats killed at 4 or 7 h after their last injection; therefore, these data were pooled. Ovarian steroid treatment (EB ± P) significantly decreased posterior hypothalamic KiSS1 mRNA (P<0.0001) in both young and middle-aged rats (Figure 1). Reproductive age did not influence expression of KiSS1 mRNA in the posterior hypothalamus in either control or hormone-primed rats.
Figure 1. Age does not influence posterior hypothalamic KiSS1 mRNA.
KiSS1 mRNA data are expressed relative to young OVX controls and are reported as mean ± SEM. All young rats were killed 4 h after their last injection (control, N=4; EB, N=4; EB + P, N=5); middle-aged rats were killed at either 4 (control, N=2; EB, N=5; EB + P, N=4) or 7 h (control, N=5; EB, N=5; EB + P, N=5) after their last injection. KiSS1 mRNA levels did not differ in middle-aged rats killed at 4 or 7 h relative to their last injection therefore, theses data were pooled (control, N=7; EB, N=10; EB + P, N=9). KiSS1 mRNA expression did not differ in rats primed with EB or EB + P in either young or middle-aged rats; therefore, the data from each age was pooled (young hormone-treated, N=9; middle-aged hormone-treated, N=19). There was a significant main effect of hormone treatment [F (1,35)=94, P<0.0001] and no interaction between treatment and age. * = <0.0001 hormone vs. control.
Middle-aged OVX rats have increased KiSS1 receptor mRNA in the pituitary
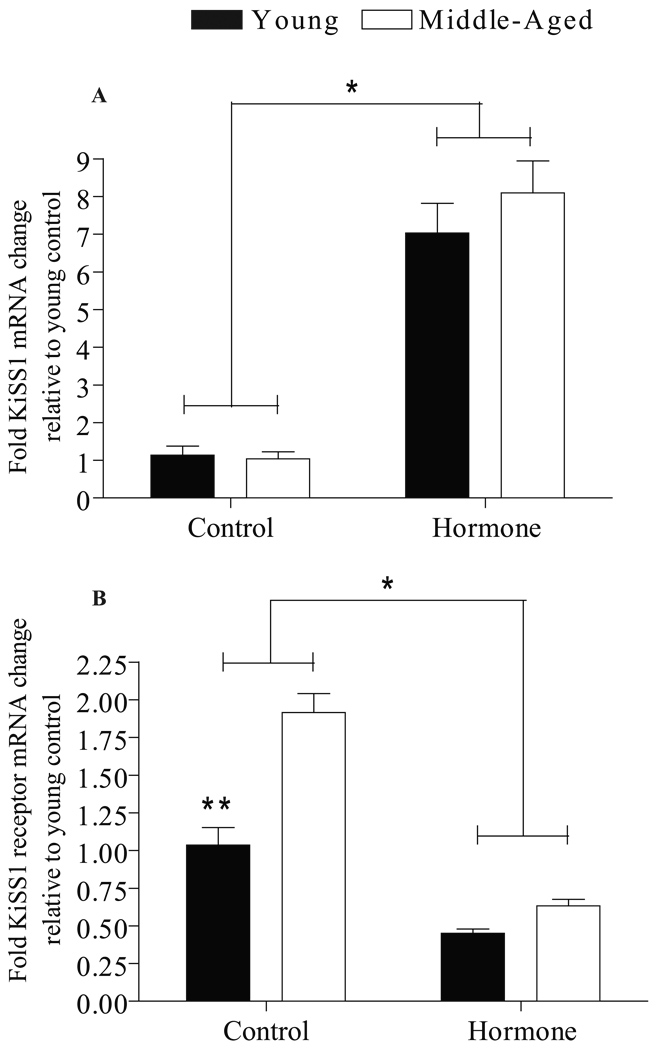
Effects of age and hormone treatment on expression of KiSS1 and KiSS1 receptor mRNA in the pituitary are shown in Figure 2. There was no difference in pituitary KiSS1 and KiSS1 receptor mRNA expression in either young or middle-aged rats treated with EB or EB and P; therefore, the data from all hormone treatments were pooled for each age group. Likewise, there was no difference in KiSS1 or KiSS1 receptor mRNA in middle-aged rats killed at 4 or 7 h after their last injection; therefore, these data were pooled. Ovarian steroid treatment significantly increased pituitary KiSS1 mRNA (P<0.001) (Figure 2A) and decreased pituitary KiSS1 receptor mRNA (P<0.001) (Figure 2B) in young and middle-aged rats. Middle-aged OVX rats injected with oil (controls) had significantly more pituitary KiSS1 receptor mRNA than young OVX controls (P<0.001). In contrast, reproductive age did not influence KiSS1 mRNA in the pituitary from control or hormone-treated rats.
Figure 2. Effects of age and hormone treatment on KiSS1 and KiSS1 receptor mRNA in the pituitary.
KiSS1 (A) and KiSS1 receptor (B) mRNA data are expressed relative to young OVX controls and are reported as mean ± SEM. All young rats were killed 4 h after their last injection (control, N=6; EB, N=7; EB + P, N=6); middle-aged rats were killed at either 4 (control, N=8; EB, N=6; EB + P, N=7) or 7 h (control, N=3; EB, N=3 EB + P, N=3) after their last injection. KiSS1 and KiSS1 receptor mRNA levels did not differ in middle-aged rats killed at 4 or 7 h relative to their last injection; therefore, the data for each group were pooled (control, N=11; EB, N=9; EB + P, N=10). KiSS1 and KiSS1 receptor mRNA expression did not differ in rats primed with EB or EB + P in either young or middle-aged rats; therefore, the hormone data from each age group were pooled (young hormone-treated, N=13; middle-aged hormone-treated, N=19). There was a significant main effect of treatment [F (1,44)=59, P<0.001] and no interaction between treatment and age on KiSS1 mRNA expression in the pituitary. There was a significant main effect of treatment [F (1,45)=135, P<0.001], age [F (1,45)=45, P<0.001) and an interaction between treatment and age [F (1,45)=19, P<0.001] on KiSS1 receptor mRNA expression in the pituitary. * = <0.001 hormone vs. control, ** = <0.001 Middle-aged control vs. young control.
AVPV KiSS1 immunopositive neurons are reduced in middle-aged rats under estradiol positive feedback conditions
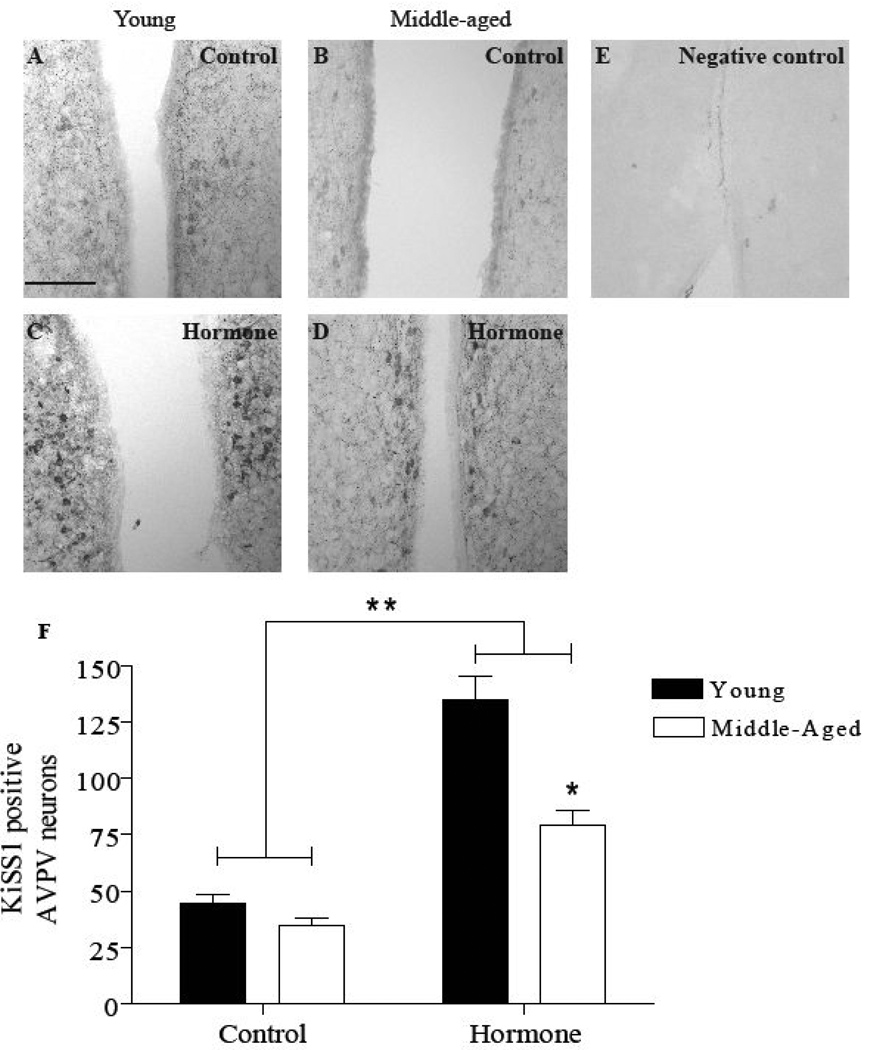
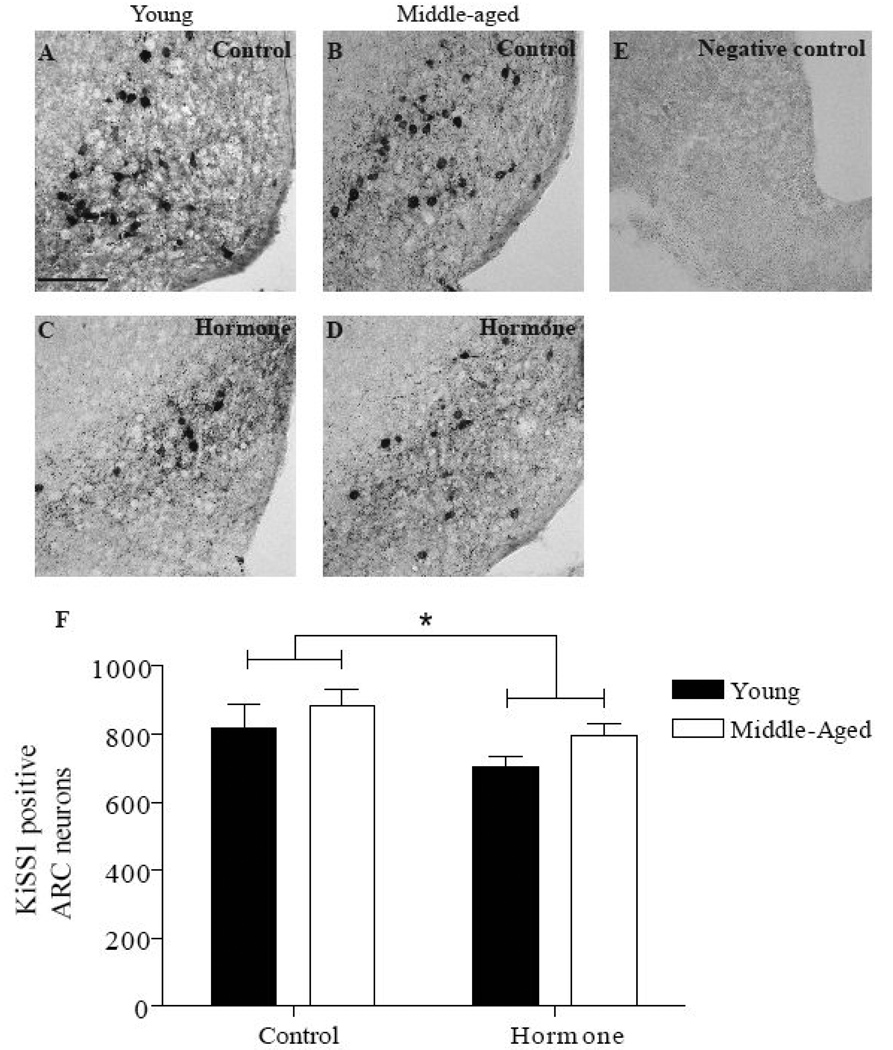
The effect of age and hormone treatment on numbers of KiSS1 immunopositive neurons in the AVPV (Figure 3) and the ARC (Figure 4) were examined using IHC. There was no difference in the number of KiSS1 immunopositive neurons in the AVPV or ARC in EB or EB and P treated animals; therefore, these data were pooled in each age group. Ovarian steroid treatment significantly increased the number of KiSS1 immunopositive neurons in the AVPV of both age groups relative to OVX controls (P<0.0001; Figure 3F). Consistent with the previous mRNA findings in the anterior hypothalamus (Neal-Perry et al., 2009), middle-aged females treated with ovarian steroids had significantly fewer KiSS1 immunopositive neurons (80 ± 7) in the AVPV compared to young females (135 ± 10; P<0.001). In the ARC (Figure 4), hormone treatment modestly but significantly reduced the number of KiSS1 immunopositive neurons in both age groups (P<0.05; Figure 4F) relative to OVX controls. Reproductive age did not affect the number of KiSS1 immunopositive neurons in the ARC.
Figure 3. Middle-aged rats have fewer KiSS1 immunopositive neurons in the AVPV under estradiol positive feedback conditions.
Total KiSS1 immunopositive cell numbers in the AVPV are reported as mean ± SEM (F). The number of KiSS1 immunopositive neurons did not differ in rats primed with EB or EB + P in either young (EB, N=8; EB + P, N=6) or middle-aged rats (EB, N=6; EB + P, N=7); therefore, the data from each age group were pooled (young hormone-treated, N=14; middle-aged hormone-treated, N=13). Representative photomicrographs of KiSS1 immunopositive neurons (A–E) in the AVPV; A: young control (OVX, oil-primed, N=6), B: middle-aged control (OVX, oil-primed, N=6), C: young hormone, D: middle-aged hormone, E: negative control incubated without primary antibody. There was a significant main effect of hormone treatment [F (1,35)=51, P<0.0001], age [F (1,35)=12, P<0.005) and an interaction between treatment and age [F (1,35)=6, P<0.05] on KiSS1 immunopositive neurons in the AVPV. * = P<0.001 Middle-aged hormone vs. young hormone, ** = P<0.0001 hormone vs. control. Scale bar = 100 µm.
Figure 4. Reproductive age does not affect the numbers of KiSS1 immunopositive neurons or their response to hormone treatment in the ARC.
Total KiSS1 immunopositive neurons in the ARC are reported as mean ± SEM (F). Cell counts in rats primed with EB or EB + P were combined for both young (EB, N=7; EB + P, N=6) and middle-aged rats (EB, N=5; EB + P, N=7); as described above (young hormone-treated, N=13; middle-aged hormone-treated, N=12). Representative photomicrographs of KiSS1 immunopositive neurons (A–E) in the ARC; A: young control (OVX, oil-primed, N=6), B: middle-aged control (OVX, oil-primed, N=6), C: young hormone, D: middle-aged hormone, E: negative control incubated without primary antibody. There was a significant main effect of hormone treatment [F (1,33)=5, P<0.05]. * = <0.05 hormone vs. control. Scale bar = 100 µm.
Discussion
We demonstrate the novel findings that middle-aged female rats have fewer KiSS1 immunopositive neurons in the AVPV on the day of the LH surge than young rats. These findings are consistent with our previous finding of decreased KiSS1 mRNA in the anterior hypothalamus of middle-aged rats compared to young rats on the day of the LH surge (Neal-Perry et al., 2009). In contrast, reproductive age did not affect expression of KiSS1 mRNA in the posterior hypothalamus (anatomical location of the ARC), pituitary KiSS1 mRNA, or the number of KiSS1 immunopositive neurons in the ARC on the day of the LH surge. Additionally, OVX control middle-aged rats express more KiSS1 receptor mRNA in the pituitary than young OVX controls. These data strongly suggest that age-related LH surge dysfunction most likely results from reduced responsiveness of AVPV KiSS1 neurons to estradiol positive feedback conditions, leading to reduced KiSS1 release, and consequently attenuated activation of GnRH neurons and GnRH/LH release.
This work clearly demonstrates that estradiol regulation of KiSS1 expression is selectively altered by reproductive aging in the AVPV and not the ARC. There is compelling evidence that KiSS1 activates GnRH neurons and is required for induction of the preovulatory LH surge (Clarkson et al., 2008). For example, central administration of an anti-KiSS1 antibody attenuates the proestrous LH surge in rats (Kinoshita et al., 2005), and KiSS1 administration induces LH release and stimulates cFos expression in GnRH neurons (Gottsch et al., 2004; Irwig et al., 2004; Shahab et al., 2005). Although not assessed in the present study, alterations in KiSS1 receptor expression in GnRH neurons, leading to reduced responsiveness to KiSS1, could account for the delayed and attenuated LH surge associated with reproductive aging. However, this is unlikely because unilateral kisspeptin-10 infusion into the medial preoptic area restores both total and peak LH release in ovarian steroid-primed, middle-aged rats (Neal-Perry et al., 2009). These data demonstrate that the hypothalamus of middle-aged females maintains responsiveness to KiSS1 and suggests that KiSS1 receptor function or number is not reduced or compromised in middle-aged females. This hypothesis is consistent with Navarro et al. (2004) who reported hypothalamic KiSS1 receptor mRNA expression did not change between young adult and 18-month-old rats (Navarro et al., 2004). Similarly, Kim et al. (2009) reported equivalent KiSS1 receptor mRNA levels in the preoptic area of intact premenopausal and postmenopausal rhesus monkeys (Kim et al., 2009).
This is the first study to demonstrate that advanced reproductive age negatively affects the ability of estradiol positive feedback conditions to increase numbers of KiSS1 neurons in the AVPV. It is possible that the methods used to detect KiSS1 immunopositive neurons were not sensitive enough to identify KiSS1 neurons with very low levels of KiSS1 expression. Consequently, the absolute number of KiSS1 neurons in the AVPV may not change in middle-aged rats. Alternatively, it is also possible that LH surge dysfunction in middle-aged rats reflect an age-related decrease in the density of kisspeptin fibers in contact with GnRH neurons and other neurons critical to the LH surge. Nonetheless, our data clearly show a reduced ability of estradiol positive feedback conditions to upregulate KiSS1 mRNA (Neal-Perry et al., 2009) and the number of visible KiSS1 immunopositive neurons in the AVPV of middle-aged compared to young rats. When viewed as a whole, these data suggest that reduced KiSS1 availability in the AVPV, and not differences in KiSS1 receptor expression or function, most likely contribute to age-related LH surge abnormalities.
This is also the first study to evaluate and compare pituitary expression of KiSS1 and KiSS1 receptor in young and middle-aged female rats. Although rats express both KiSS1 receptor and KiSS1 mRNA in the pituitary (Kinoshita et al., 2005; Kotani et al., 2001; Muir et al., 2001), the significance of KiSS1 signaling in the regulation of pituitary gonadotropin release is controversial. For example, KiSS1 does not alter gonadotropin release from rat pituitary fragments (Matsui et al., 2004; Thompson et al., 2004) or OVX, hypothalamo-pituitary-disconnected ewes (Smith et al., 2008). In contrast, KiSS1 increased LH release from ewe-derived pituitary cell cultures in the follicular phase but not the luteal phase (Smith et al., 2008). Still others report increased LH release following direct application of KiSS1 to rat pituitary cell cultures (Gutierrez-Pascual et al., 2007; Navarro et al., 2005). These discrepancies could be explained by differences in reproductive phase, experimental design (in vivo vs. in vitro) and animal models used in these studies. Consistent with our findings in the female rat pituitary, Richard et al. (2008) demonstrated a decrease in KiSS1 mRNA and an increase in KiSS1 receptor mRNA in OVX, oil-treated rats compared to OVX, estradiol-primed rats (Richard et al., 2008). Thus, pituitary KiSS1 and KiSS1 receptor mRNA appears to be differentially regulated by ovarian steroids. Moreover, our new data indicate that the expression of pituitary KiSS1 receptor mRNA increases with reproductive age in OVX rats. The physiological significance of KiSS1 receptor mRNA upregulation in OVX, middle-aged rats is currently unknown. It is possible that the upregulation of KiSS1 receptor mRNA in the pituitary of OVX, middle-aged rats reflects age-related differences in KiSS1 release when ovarian steroids are withdrawn.
In agreement with several studies (Adachi et al., 2007; Kauffman et al., 2007; Smith et al., 2005), we found that estradiol priming decreases KiSS1 mRNA expression and the numbers of KiSS1 immunopositive neurons in the ARC relative to OVX, oil-treated rats. However, the decrease in KiSS1 immunopositive neurons was less pronounced than the decrease in KiSS1 mRNA levels. We found it difficult to visualize KiSS1 immunopositive cell bodies in the AVPV and ARC under standard IHC conditions (although cell processes were readily identified); therefore, we adapted methods to enhance KiSS1 detection described by Adachi et al. (2007), and infused colchicine into the third ventricle (Adachi et al., 2007). Colchicine disrupts microtubules thereby impairing KiSS1 transport to nerve terminals and causing KiSS1 to concentrate in the soma. Hence, it is possible that colchicine increased the number of KiSS1 immunopositive neurons detected in the ARC. Nonetheless, the trend was the same in that KiSS1 mRNA and numbers of KiSS1 immunopositive neurons were decreased by estradiol. Moreover, reproductive age did not appear to affect the number of KiSS1 immunopositive neurons in the ARC of OVX females or their response to ovarian steroids. As these neurons are hypothesized to participate in estrogen negative feedback, such findings are consistent with our observations that LH levels are similar in young and middle-aged female rats under negative feedback conditions (Neal-Perry et al., 2005; Neal-Perry et al., 2008).
In summary, we demonstrate that estradiol-treated, middle-aged female rats have reduced numbers of KiSS1 immunopositive neurons in the AVPV on the day of the LH surge. In contrast, reproductive age does not affect the expression of KiSS1 mRNA in the posterior hypothalamus (containing the ARC), and ovarian steroids produce comparable decreases in KiSS1 mRNA and in the number of KiSS1 immunopositive neurons in the ARC of young and middle-aged females. We also demonstrate that reproductive age does not affect pituitary KiSS1 or KiSS1 receptor mRNA levels under estrogen positive feedback conditions. Similarly, reproductive age does not affect pituitary KiSS1 mRNA levels in OVX control rats. In contrast, middle-aged OVX control female rats expressed more KiSS1 receptor mRNA in the pituitary than young controls. Because KiSS1 infusion into the medial preoptic area rescues LH surge amplitude in middle-aged female rats (Neal-Perry et al., 2009), we propose that age-related LH surge dysfunction results in part from a reduced ability of estradiol to increase KiSS1 expression in AVPV neurons, resulting in reduced release of KiSS1 and reduced KiSS1 activation of GnRH neurons on the day of the LH surge. Equally important, our findings also suggest that GnRH neurons in middle-aged female rats retain their capacity to respond to excitatory stimuli such as KiSS1.
Supplementary Material
Acknowledgements
We thank Drs. Brigitte Todd, Andrea Reyna-Neyra, Ewa Borys, and Xianchun Huang for technical support and critical evaluation of this manuscript; Dr. Gloria Hoffman for technical support with IHC; and Dr. Alain Caraty for the generous gift of the KiSS1 rabbit polyclonal antibody. This work was supported by DHHS grants R01 HD29856 and T32 AG23475, The Robert Wood Johnson Foundation, the American Federation for Aging Research, and the D.P. Department of Neuroscience and the Department of Obstetrics and Gynecology and Women’s Health, Albert Einstein College of Medicine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adachi S, Yamada S, Takatsu Y, Matsui H, Kinoshita M, Takase K, Sugiura H, Ohtaki T, Matsumoto H, Uenoyama Y, Tsukamura H, Inoue K, Maeda K. Involvement of anteroventral periventricular metastin/kisspeptin neurons in estrogen positive feedback action on luteinizing hormone release in female rats. J Reprod Dev. 2007;53:367–378. doi: 10.1262/jrd.18146. [DOI] [PubMed] [Google Scholar]
- Clarkson J, d'Anglemont de Tassigny X, Moreno AS, Colledge WH, Herbison AE. Kisspeptin-GPR54 signaling is essential for preovulatory gonadotropin-releasing hormone neuron activation and the luteinizing hormone surge. J Neurosci. 2008;28:8691–8697. doi: 10.1523/JNEUROSCI.1775-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper RL, Conn PM, Walker RF. Characterization of the LH surge in middle-aged female rats. Biol Reprod. 1980;23:611–615. doi: 10.1095/biolreprod23.3.611. [DOI] [PubMed] [Google Scholar]
- Downs JL, Wise PM. The role of the brain in female reproductive aging. Mol Cell Endocrinol. 2009;299:32–38. doi: 10.1016/j.mce.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology. 2004;145:4073–4077. doi: 10.1210/en.2004-0431. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Pascual E, Martinez-Fuentes AJ, Pinilla L, Tena-Sempere M, Malagon MM, Castano JP. Direct pituitary effects of kisspeptin: activation of gonadotrophs and somatotrophs and stimulation of luteinising hormone and growth hormone secretion. J Neuroendocrinol. 2007;19:521–530. doi: 10.1111/j.1365-2826.2007.01558.x. [DOI] [PubMed] [Google Scholar]
- Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci. 2005;25:11349–11356. doi: 10.1523/JNEUROSCI.3328-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwig MS, Fraley GS, Smith JT, Acohido BV, Popa SM, Cunningham MJ, Gottsch ML, Clifton DK, Steiner RA. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology. 2004;80:264–272. doi: 10.1159/000083140. [DOI] [PubMed] [Google Scholar]
- Kauffman AS. Sexual differentiation and the Kiss1 system: hormonal and developmental considerations. Peptides. 2009;30:83–93. doi: 10.1016/j.peptides.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffman AS, Gottsch ML, Roa J, Byquist AC, Crown A, Clifton DK, Hoffman GE, Steiner RA, Tena-Sempere M. Sexual differentiation of Kiss1 gene expression in the brain of the rat. Endocrinology. 2007;148:1774–1783. doi: 10.1210/en.2006-1540. [DOI] [PubMed] [Google Scholar]
- Kim W, Jessen HM, Auger AP, Terasawa E. Postmenopausal increase in KiSS-1, GPR54, and luteinizing hormone releasing hormone (LHRH-1) mRNA in the basal hypothalamus of female rhesus monkeys. Peptides. 2009;30:103–110. doi: 10.1016/j.peptides.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita M, Tsukamura H, Adachi S, Matsui H, Uenoyama Y, Iwata K, Yamada S, Inoue K, Ohtaki T, Matsumoto H, Maeda K. Involvement of central metastin in the regulation of preovulatory luteinizing hormone surge and estrous cyclicity in female rats. Endocrinology. 2005;146:4431–4436. doi: 10.1210/en.2005-0195. [DOI] [PubMed] [Google Scholar]
- Kotani M, Detheux M, Vandenbogaerde A, Communi D, Vanderwinden JM, Le Poul E, Brezillon S, Tyldesley R, Suarez-Huerta N, Vandeput F, Blanpain C, Schiffmann SN, Vassart G, Parmentier M. The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J Biol Chem. 2001;276:34631–34636. doi: 10.1074/jbc.M104847200. [DOI] [PubMed] [Google Scholar]
- Krajnak K, Rosewell KL, Wise PM. Fos-induction in gonadotropin-releasing hormone neurons receiving vasoactive intestinal polypeptide innervation is reduced in middle-aged female rats. Biol Reprod. 2001;64:1160–1164. doi: 10.1095/biolreprod64.4.1160. [DOI] [PubMed] [Google Scholar]
- Lapatto R, Pallais JC, Zhang D, Chan YM, Mahan A, Cerrato F, Le WW, Hoffman GE, Seminara SB. Kiss1−/− mice exhibit more variable hypogonadism than Gpr54−/− mice. Endocrinology. 2007;148:4927–4936. doi: 10.1210/en.2007-0078. [DOI] [PubMed] [Google Scholar]
- Matsui H, Takatsu Y, Kumano S, Matsumoto H, Ohtaki T. Peripheral administration of metastin induces marked gonadotropin release and ovulation in the rat. Biochem Biophys Res Commun. 2004;320:383–388. doi: 10.1016/j.bbrc.2004.05.185. [DOI] [PubMed] [Google Scholar]
- Muir AI, Chamberlain L, Elshourbagy NA, Michalovich D, Moore DJ, Calamari A, Szekeres PG, Sarau HM, Chambers JK, Murdock P, Steplewski K, Shabon U, Miller JE, Middleton SE, Darker JG, Larminie CG, Wilson S, Bergsma DJ, Emson P, Faull R, Philpott KL, Harrison DC. AXOR12, a novel human G protein-coupled receptor, activated by the peptide KiSS-1. J Biol Chem. 2001;276:28969–28975. doi: 10.1074/jbc.M102743200. [DOI] [PubMed] [Google Scholar]
- Navarro VM, Castellano JM, Fernandez-Fernandez R, Barreiro ML, Roa J, Sanchez-Criado JE, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. Developmental and hormonally regulated messenger ribonucleic acid expression of KiSS-1 and its putative receptor, GPR54, in rat hypothalamus and potent luteinizing hormone-releasing activity of KiSS-1 peptide. Endocrinology. 2004;145:4565–4574. doi: 10.1210/en.2004-0413. [DOI] [PubMed] [Google Scholar]
- Navarro VM, Castellano JM, Fernandez-Fernandez R, Tovar S, Roa J, Mayen A, Nogueiras R, Vazquez MJ, Barreiro ML, Magni P, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. Characterization of the potent luteinizing hormone-releasing activity of KiSS-1 peptide, the natural ligand of GPR54. Endocrinology. 2005;146:156–163. doi: 10.1210/en.2004-0836. [DOI] [PubMed] [Google Scholar]
- Neal-Perry G, Lebesgue D, Lederman M, Shu J, Zeevalk GD, Etgen AM. The excitatory peptide kisspeptin restores the luteinizing hormone surge and modulates amino acid neurotransmission in the medial preoptic area of middle-aged rats. Endocrinology. 2009 doi: 10.1210/en.2008-1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal-Perry G, A T, Aubuchon M, Santoro N. Evidence for a hypothalamic basis for the female reproductive senescence in the human; The Endocrine society’s 87th Annual Meeting; San Diego, CA. 2005. [Google Scholar]
- Neal-Perry GS, Zeevalk GD, Santoro NF, Etgen AM. Attenuation of preoptic area glutamate release correlates with reduced luteinizing hormone secretion in middle-aged female rats. Endocrinology. 2005;146:4331–4339. doi: 10.1210/en.2005-0575. [DOI] [PubMed] [Google Scholar]
- Neal-Perry GS, Zeevalk GD, Shu J, Etgen AM. Restoration of the luteinizing hormone surge in middle-aged female rats by altering the balance of GABA and glutamate transmission in the medial preoptic area. Biol Reprod. 2008;79:878–888. doi: 10.1095/biolreprod.108.069831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrino LJ, P AS, Cushman AJ. A stereotaxic atlas of the rat brain. New York: Plenum Press; 1979. [Google Scholar]
- Rance NE, McMullen NT, Smialek JE, Price DL, Young WS., 3rd Postmenopausal hypertrophy of neurons expressing the estrogen receptor gene in the human hypothalamus. J Clin Endocrinol Metab. 1990;71:79–85. doi: 10.1210/jcem-71-1-79. [DOI] [PubMed] [Google Scholar]
- Richard N, Galmiche G, Corvaisier S, Caraty A, Kottler ML. KiSS-1 and GPR54 genes are co-expressed in rat gonadotrophs and differentially regulated in vivo by oestradiol and gonadotrophin-releasing hormone. J Neuroendocrinol. 2008;20:381–393. doi: 10.1111/j.1365-2826.2008.01653.x. [DOI] [PubMed] [Google Scholar]
- Roa J, Castellano JM, Navarro VM, Handelsman DJ, Pinilla L, Tena-Sempere M. Kisspeptins and the control of gonadotropin secretion in male and female rodents. Peptides. 2009;30:57–66. doi: 10.1016/j.peptides.2008.08.009. [DOI] [PubMed] [Google Scholar]
- Rometo AM, Krajewski SJ, Voytko ML, Rance NE. Hypertrophy and increased kisspeptin gene expression in the hypothalamic infundibular nucleus of postmenopausal women and ovariectomized monkeys. J Clin Endocrinol Metab. 2007;92:2744–2750. doi: 10.1210/jc.2007-0553. [DOI] [PubMed] [Google Scholar]
- Rubin BS. Isolated hypothalami from aging female rats do not exhibit reduced basal or potassium-stimulated secretion of luteinizing hormone-releasing hormone. Biol Reprod. 1992;47:254–261. doi: 10.1095/biolreprod47.2.254. [DOI] [PubMed] [Google Scholar]
- Scarbrough K, Wise PM. Age-related changes in pulsatile luteinizing hormone release precede the transition to estrous acyclicity and depend upon estrous cycle history. Endocrinology. 1990;126:884–890. doi: 10.1210/endo-126-2-884. [DOI] [PubMed] [Google Scholar]
- Shahab M, Mastronardi C, Seminara SB, Crowley WF, Ojeda SR, Plant TM. Increased hypothalamic GPR54 signaling: a potential mechanism for initiation of puberty in primates. Proc Natl Acad Sci U S A. 2005;102:2129–2134. doi: 10.1073/pnas.0409822102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology. 2005;146:3686–3692. doi: 10.1210/en.2005-0488. [DOI] [PubMed] [Google Scholar]
- Smith JT, Rao A, Pereira A, Caraty A, Millar RP, Clarke IJ. Kisspeptin is present in ovine hypophysial portal blood but does not increase during the preovulatory luteinizing hormone surge: evidence that gonadotropes are not direct targets of kisspeptin in vivo. Endocrinology. 2008;149:1951–1959. doi: 10.1210/en.2007-1425. [DOI] [PubMed] [Google Scholar]
- Teles MG, Bianco SD, Brito VN, Trarbach EB, Kuohung W, Xu S, Seminara SB, Mendonca BB, Kaiser UB, Latronico AC. A GPR54-activating mutation in a patient with central precocious puberty. N Engl J Med. 2008;358:709–715. doi: 10.1056/NEJMoa073443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson EL, Patterson M, Murphy KG, Smith KL, Dhillo WS, Todd JF, Ghatei MA, Bloom SR. Central and peripheral administration of kisspeptin-10 stimulates the hypothalamic-pituitary-gonadal axis. J Neuroendocrinol. 2004;16:850–858. doi: 10.1111/j.1365-2826.2004.01240.x. [DOI] [PubMed] [Google Scholar]
- Weiss G, Skurnick JH, Goldsmith LT, Santoro NF, Park SJ. Menopause and hypothalamic-pituitary sensitivity to estrogen. Jama. 2004;292:2991–2996. doi: 10.1001/jama.292.24.2991. [DOI] [PubMed] [Google Scholar]
- Wise PM, Ratner A. LHRH-induced LH and FSH responses in the aged female rat. J Gerontol. 1980;35:506–511. doi: 10.1093/geronj/35.4.506. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.