Abstract
Vibrio parahaemolyticus inhabits marine, brackish, and estuarine waters worldwide, where fluctuations in salinity pose a constant challenge to the osmotic stress response of the organism. Vibrio parahaemolyticus is a moderate halophile, having an absolute requirement for salt for survival, and is capable of growth at 1 to 9% NaCl. It is the leading cause of seafood-related bacterial gastroenteritis in the United States and much of Asia. We determined whether growth in differing NaCl concentrations alters the susceptibility of V. parahaemolyticus O3:K6 to other environmental stresses. Vibrio parahaemolyticus was grown at a 1% or 3% NaCl concentration, and the growth and survival of the organism were examined under acid or temperature stress conditions. Growth of V. parahaemolyticus in 3% NaCl versus that in 1% NaCl increased survival under both inorganic (HCl) and organic (acetic acid) acid conditions. In addition, at 42°C and −20°C, 1% NaCl had a detrimental effect on growth. The expression of lysine decarboxylase (encoded by cadA), the organism's main acid stress response system, was induced by both NaCl and acid conditions. To begin to address the mechanism of regulation of the stress response, we constructed a knockout mutation in rpoS, which encodes the alternative stress sigma factor, and in toxRS, a two-component regulator common to many Vibrio species. Both mutant strains had significantly reduced survival under acid stress conditions. The effect of V. parahaemolyticus growth in 1% or 3% NaCl was examined using a cytotoxicity assay, and we found that V. parahaemolyticus grown in 1% NaCl was significantly more toxic than that grown in 3% NaCl.
Vibrio parahaemolyticus is a Gram-negative bacterium that inhabits coastal waters worldwide. Vibrio parahaemolyticus grows optimally in warmer waters and is most commonly isolated during the summer months, often in association with plankton, crustaceans, mollusks, and fish (16, 17). During the winter months, the organism is typically scarce and usually is isolated from sediment samples (16). While V. parahaemolyticus has been shown to be the etiological agent of disease in several kinds of crustaceans and shellfish, it is most notably a pathogen of humans (17). Vibrio parahaemolyticus was first discovered in Japan during an outbreak of gastroenteritis in 1950 (12). It is the leading cause of seafood-related bacterial gastroenteritis in the United States and much of Asia (6, 39). Infection is most frequently associated with the consumption of oysters harvested from warm waters, particularly along the U.S. Gulf Coast, where vibrios grow to high levels during the summer months (6, 7, 42). Newly released data from the CDC comparing the incidence rates of laboratory-confirmed infections by gastrointestinal pathogens in 1996 to 2008 revealed an increase of 47% for Vibrio infections, of which V. parahaemolyticus accounted for 55%, while rates for all other enteric pathogens decreased or remained the same (5). An outbreak of V. parahaemolyticus infections which caused rapid hospitalization of those infected occurred in India in 1995 (28). These infections were caused by a single serogroup, a new, highly virulent O3:K6 strain, which has now disseminated globally (1, 6, 20, 26, 34, 38). Recent studies report the recovery of O3:K6 isolates from the water in southern Chile, a region that previously was considered too cold to support the growth of this organism (4, 11, 13).
All V. parahaemolyticus strains inhabit marine, brackish, and estuarine waters, where fluctuations in salinity pose a constant challenge to the adaptive response of the organism. Vibrio parahaemolyticus is moderately halophilic in nature and requires a minimum of 0.086 M (0.5%) NaCl for growth (29). It has also been demonstrated that this organism has the ability to grow in medium containing NaCl concentrations upwards of 1.5 M, making V. parahaemolyticus more osmotolerant than many other Vibrio species, such as V. cholerae, V. vulnificus, and V. fischeri, which occupy similar niches (27). In a recent study, we examined the genome of V. parahaemolyticus O3:K6 (designated RIMD2210633) and identified homologues of ectoine and betaine synthesis genes, as well as homologues of four single-component compatible solute transporters and two multicomponent compatible solute transporters (27). The large compendium of compatible solute systems in V. parahaemolyticus suggests that they might play an additional role(s) in survival.
Within offshore waters, V. parahaemolyticus is generally faced with NaCl concentrations of 3.5% salinity (35 ppt), but in estuarine systems and within oysters (which are osmoconformers), it must adapt to changes in salinity. In addition, as a human pathogen, once inside the human host, like most enteric pathogens, V. parahaemolyticus must overcome the inorganic-pH challenge presented by gastric acid from the stomach and organic acids found within the intestine, as well as decreasing salinity (salinity in the intestine is approximately 300 mM NaCl). Organic acids have the ability to cross the cell membrane and enter the cytoplasm of the cell, whereas inorganic acids remain in the extracellular environment. Once in the cells, the organic acids can disassociate, decreasing the cytoplasmic pH and increasing the turgor pressure within the cell due to increases in anions from the acids (9). Thus, inorganic and organic acids can affect cells very differently.
We suggest that the ability to grow at different NaCl concentrations, such as those vibrios would encounter in estuarine environments, allows V. parahaemolyticus to adapt more effectively to other environmental stresses (temperature fluctuations) and to the challenges that occur upon invasion of the human host (low pH). In this study, we show that V. parahaemolyticus RIMD2210633 cells grown at 3% NaCl are more resistant to acid and temperature stresses than cells grown at 1% NaCl. We demonstrate that V. parahaemolyticus grown in 3% NaCl is better able to survive sublethal and lethal acid shock conditions, as well as persistent high- and low-temperature conditions. We determined possible regulatory mechanisms involved in stress responses by examining the global regulator genes toxRS and rpoS. Last, we examined how changing environmental conditions, such as high and low NaCl and low pH, might affect the virulence of V. parahaemolyticus by determining its cytotoxicity toward human intestinal (Caco-2) cells.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
All bacterial strains and plasmids used in this study are listed in Table 1. Genetic manipulations used Escherichia coli strains DH5α λpir and the diaminopimelic acid (DAP) auxotroph β2155 λpir. Unless otherwise noted, all strains were grown in 5 ml of the appropriate medium aerobically (250 rpm) at 37°C in Luria-Bertani (LB) broth (Fisher Scientific, Fair Lawn, NJ) with the final NaCl (Fisher Scientific) concentration adjusted to percentages as appropriate. The E. coli β2155 DAP auxotroph was grown on medium supplemented with 0.3 mM DAP (Sigma Aldrich, St. Louis, MO). The V. parahaemolyticus O3:K6 strain was selected for streptomycin resistance as described below.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain, genotype, or plasmid | Relevant characteristic(s) | Source or reference |
|---|---|---|
| Vibrio parahaemolyticus | ||
| RIMD2210633 | O3:K6 clinical isolate | 19 |
| ΔtoxRS | RIMD2210633 ΔtoxRS(VP0812-813) | This study |
| ΔrpoS | RIMD2210633 ΔrpoS (VP2553) | This study |
| Escherichia coli | ||
| DH5α λpir | Δlac pir | |
| β2155 DAP | ΔdapA::erm pir for bacterial conjugation | |
| DH5α λpir ΔtoxRS | DH5α λpir containing pDS132ΔtoxRS | This study |
| DH5α λpir ΔrpoS | DH5α λpir containing pDS132ΔrpoS | This study |
| β2155 DAP-ΔtoxRS | β2155 harboring pDS132ΔtoxRS | This study |
| β2155 DAP-ΔrpoS | β2155 harboring pDS132ΔrpoS | This study |
| Plasmids | ||
| pDS132 | Suicide plasmid; Cmr; SacB | 31 |
| pDS132ΔtoxRS | pDS132 harboring truncated toxRS region | This study |
| pDS132ΔrpoS | pDS132 harboring truncated rpoS gene | This study |
Growth under acidic conditions.
Vibrio parahaemolyticus RIMD2210633 was grown overnight in 5 ml of LB (approximately 5 × 109 CFU/ml) containing either 0.5% NaCl, 1% NaCl, or 3% NaCl (all at pH 7) and diluted 1:100 into 5 ml of fresh LB containing either 0.5%, 1%, or 3% NaCl, respectively, which was acidified to pH 5 using 1 M HCl. For growth under neutral conditions (pH 7), LB was adjusted using 1 M NaOH. To determine the effects of other cations on growth, LB containing various concentrations of KCl was examined. For growth analysis, 200 μl of the diluted cultures were transferred to a 96-well microtiter plate and incubated at 37°C with shaking at high speed. Optical densities at 595 nm were measured hourly for 24 h using a Tecan Sunrise microplate reader and Magellan plate reader software (Tecan Systems Inc., San Jose, CA). Graphs based on the data obtained from the plate reader were plotted using the SigmaPlot 8 software program.
Bacterial stress survivability assays.
To determine V. parahaemolyticus RIMD2210633 survivability under lethal acid conditions, cells were grown overnight under low-salt conditions (LB, 1% NaCl), and 100 μl of these overnight cultures were diluted into 5 ml of fresh LB (1% NaCl) and allowed to grow to mid-log phase (optical density at 595 nm [OD595] of 0.4). The OD was measured at 595 nm throughout this study. At this point, the cells were harvested by centrifugation (5,000 rpm for 5 min) and resuspended in either LB (1% NaCl) or LB (3% NaCl) (to mimic the salt concentrations of V. parahaemolyticus' ocean environment) and then incubated for 30 min at 37°C. Both cultures were harvested by centrifugation and resuspended in fresh LB (1.5% NaCl) with the pH adjusted to 4 with HCl. At 0, 30, and 60 min, the cells were serially diluted in phosphate-buffered saline (PBS) (Sigma Aldrich) and plated on LB plates (1.5% agar) supplemented with 3% NaCl. Colony counts were performed to determine the concentrations of the bacteria at the various time points, and the percent survival was determined by dividing the number of bacterial cells at 30 or 60 min by the initial starting concentration (0 min). In a separate set of experiments, survivability was also assessed as described above but with the bacteria grown overnight in LB (3% NaCl) and resuspended in LB (3% NaCl) throughout the remainder of the experiment. All cultures were grown in triplicate, and each experiment was performed at least twice. Next, we investigated the effect of organic acid stress on V. parahaemolyticus, since vibrios encounter both inorganic acid stress (in the stomach) and organic acid stress (in the intestine) during infection. Second, we wanted to examine the effects of both inorganic and organic acids on the adaptive acid tolerance response of V. parahaemolyticus. We followed the procedure outlined in studies conducted with V. cholerae and with Salmonella and other enteric pathogens for performing acid adaptation prior to examining survival in lethal acid (2, 9, 23). We used both inorganic and organic acids in the same assay, since it is known that organic acid stress alone does not elicit an adaptive acid stress response (2, 9). Briefly, V. parahaemolyticus was grown to mid-log phase with the addition of a 30-min adaptive phase in either pH 5.5 (inorganic acid stress) or 1 mM acetic acid (mixed organic and inorganic acid stress) that was adjusted to pH 5.5 with HCl before the cells were resuspended in fresh LB (1.5% NaCl) with the pH adjusted to 4 with HCl or 4 mM acetic acid.
To examine growth at high temperatures, the above protocol was repeated with the organism grown overnight in LB with either 1% or 3% NaCl at 37°C and then resuspended in fresh LB with 1% NaCl or 3% NaCl, respectively, under neutral conditions at 42°C. Vibrio parahaemolyticus is very sensitive to heat stress (47°C is lethal), so 42°C was chosen as a sublethal high-temperature stress. To determine survival under cold stress, cultures were grown overnight in LB with either 1% or 3% NaCl at 37°C and then resuspended in fresh LB with 1% or 3% NaCl, respectively, and grown to mid-exponential phase at 37°C. These cultures were then subjected to −20°C for a period of 24 h. Prior to plating at the 24-h time point, the cultures were thawed at room temperature.
Isolation of a streptomycin-resistant mutant strain of V. parahaemolyticus.
In order to isolate streptomycin-resistant mutants of V. parahaemolyticus, a 5-ml overnight culture was centrifuged for 10 min at 4,000 × g, and the bacterial pellet was resuspended in 100 μl of fresh LB (3% NaCl). The resuspended culture was plated on LB plates containing 3% NaCl and 1,000 μg/ml of streptomycin (Fisher Scientific) and incubated for 24 h at 37°C. Streptomycin-resistant colonies were plated and maintained on LB (3% NaCl) plates containing 200 μg/ml of streptomycin.
Construction of V. parahaemolyticus ΔtoxRS and ΔrpoS mutants from RIMD2210633.
In-frame deletion mutants were created using splicing by overlap extension (SOE) PCR and allelic exchange (15). Using the V. parahaemolyticus RIMD2210633 genome sequence (19) as a template, primers were designed and purchased from Integrated DNA Technologies (Coralville, IA) to perform SOE PCR and obtain single knockout mutants for VP0819-VP0820 (toxRS) and VP2553 (rpoS) (Table 2). We constructed 669-bp and 394-bp truncated versions of the toxRS and rpoS genes, respectively. Briefly, the ΔtoxRS PCR fragment was cloned into the suicide vector pDS132 (31), which was designated pDSΔtoxRS. pDSΔtoxRS was subsequently electroporated into the Escherichia coli strain DH5α λpir. pDSΔtoxRS was then plasmid purified and transformed into the E. coli strain β2155, a DAP auxotroph, and pDSΔtoxRS was then conjugated into V. parahaemolyticus RIMD2210633 via cross streaking on LB plates containing 0.3 mM DAP. Growth from these plates was then transferred to LB (3% NaCl) plates, which allows for optimal growth of V. parahaemolyticus, containing streptomycin (200 μg/ml) and chloramphenicol (25 μg/ml) to select only for V. parahaemolyticus containing pDSΔtoxRS. Exconjugate colonies were cultured overnight in the absence of antibiotics, and serial dilutions were plated on LB (3% NaCl)-10% sucrose to select for cells which had lost pDSΔtoxRS. Double-crossover deletion mutants were then screened by PCR using the SOEFLtoxRSF and SOEFLtoxRSR primers. This procedure was similarly carried out for the deletion of the rpoS gene.
TABLE 2.
Primers used in this study
| Use and primer | Sequence (5′-3′) | Tm (°C) | Product size (bp) |
|---|---|---|---|
| Splice overlap extension PCR | |||
| SOEAtoxRS | ACC GTA GAA CCG TGA TTT AGG | 54 | 289 |
| SOEBtoxRS | ATG GCG ATT ACA TTC GCG T | 55 | |
| SOECtoxRS | ACG CGA ATG TAA TCG CCA T | 67 | 353 |
| TTG AGT CTG AAG AAA GCG GG | |||
| SOEDtoxRS | CGG CAC CAA ATT TCT ACT TG | 52 | |
| SOEArpoS | TCA GCT CAG CTT AAT CCC TGA | 56 | 507 |
| SOEBrpoS | CCT GAC GCA AAC TCT GA | 55 | |
| SOECrpoS | TCA GAG TTG TTT GAG TCA GG | 68 | 315 |
| C GTC AAACTCTTCACGGACA | |||
| SOEDrpoS | ATT GCG ACC ATG GGT AGT TC | 55 | |
| SOEFLtoxRSF | AGC CAC TTT ATG AGT GCC TA | 53 | 1,840 |
| SOEFLtoxRSR | GGA CGA CTT TGT GAT TTA GC | 51 | |
| SOEFLrpoSF | ACA GCA TTG CTT TCA TGA AC | 55 | 1,950 |
| SOEFLrpoSR | AAA GAG CCG CCA AAG AAA GT | 55 | |
| Real-time PCR | |||
| cadA Forward | TTG TAT GCC TCA GTC GCT TG | 55 | 138 |
| cadA Reverse | TCA CGC ATT TGC TAA CGA AC | 54 | |
| toxR Forward | GAG ATT CCG CTG GGT TTG TA | 55 | 101 |
| toxR Reverse | TGT GGC TTC TGC TGT GAA TC | 55.5 | |
| rpoS Forward | ATT TGA CGT ACA CGC TCA CG | 55.5 | 165 |
| rpoS Reverse | CGT CTT TGA TCC ATT GGT TG | 52 | |
| 16S Forward | ACG GCC TGG GGA GTA CGG TC | 60 | 234 |
| 16S Reverse | TTG CGC TCG TTG CGG GAC TT | 60 |
RNA extraction, cDNA synthesis, and qPCR.
Total RNA was extracted from V. parahaemolyticus RIMD2210633, using the RNAprotect bacterial reagent (Qiagen, Valencia, CA) and an RNeasy minikit (Qiagen) according to the manufacturer's protocols. Prior to RNA isolation, bacteria were cultured overnight in LB containing 3% NaCl at pH 7, and diluted in fresh LB (3% NaCl) at pH 7, and grown for 4 h or for 4 h in LB (3% NaCl) plus 30 min in LB (3% NaCl) at pH 5. Similarly, bacteria were cultured overnight in LB containing 1% NaCl at pH 7 and diluted in fresh LB (1% NaCl) at pH 7 and grown for 4 h in LB (1% NaCl) and 4 h in LB 1% NaCl plus 30 min in LB (1% NaCl) at pH 5. RNA quantity was assessed by measurement on a Nanodrop spectrophotometer (Thermo Scientific, Waltham, MA), and samples were subsequently treated with DNase to remove any contaminating genomic DNA (Turbo DNase; Invitrogen, Carlsbad, CA) as per the manufacturer's protocol. One μg of each sample of RNA was run on a 1% agarose gel in 1× Tris-borate-EDTA (TBE) buffer (Mediatech Inc., Herndon, VA) to ensure quality of the samples. cDNA was synthesized by using SuperScript II reverse transcriptase (Invitrogen) according to the manufacturer's protocol, with 500 μg of RNA as a template and primed by 200 ng of random hexamers. cDNA samples were diluted 1:250 and used in quantitative real-time PCR (qPCR) using Fast SYBR green PCR master mix (Invitrogen) on an Applied Biosystems 7500 fast real-time PCR system (Foster City, CA). All assays were performed in triplicate at least twice. The gene-specific primers were designed using the Primer3 software program according to the real-time PCR guidelines and are listed in Table 2. The data were analyzed using the Applied Biosystems 7500 software program, and differences in the ratio of expression were extrapolated using the delta-delta threshold cycle (CT) method (30). The levels of expression of each gene, as determined from their CT values, were normalized to the level of 16S rRNA to correct for sampling errors.
Cell culture conditions and cytotoxicity assays.
Caco-2 cells were purchased from the American Type Culture Collection (Manassas, VA) and maintained in a monolayer in complete Dulbecco's modified Eagle's medium with 10% fetal bovine serum, incubated at 37°C with 5% CO2. The ability of V. parahaemolyticus to lyse Caco-2 cells was determined using the CytoTox 96 nonradioactive cytotoxicity assay (Promega, Madison, WI), which measures the release of lactate dehydrogenase (LDH) from lysed cells. Briefly, a 96-well plate was seeded with 1 × 103 Caco-2 cells diluted in Hanks buffered salt solution (HBSS) (Mediatech). Vibrio parahaemolyticus wild-type cells and the ΔtoxRS mutant cells were grown under the following conditions: (i) in LB containing 3% NaCl at pH 7 or (ii) in LB containing 1% NaCl at pH 7. Vibrio parahaemolyticus cells were washed in HBSS once and subsequently resuspended in HBSS. Bacteria were then added to the wells containing Caco-2 cells at a multiplicity of infection of 100:1 and incubated for 4 h at 37°C with 5% CO2. To account for the spontaneous loss of LDH from cells, wells containing Caco-2 cells only and wells containing bacterial cells alone were measured as controls. Supernatant from each well (50 μl) was transferred to a fresh 96-well plate, and LDH was assessed according to the manufacturer's protocol. Percent cytotoxicity was measured using the following equation: (Aexperimental − Aeffector spontaneous − Atarget spontaneous)/(A100% lysis − Atarget spontaneous) × 100, where Aexperimental stands for the absorbance of Caco-2 cells treated with V. parahaemolyticus, Aeffector spontaneous is the spontaneous release of LDH from wells with only bacterial cells, Atarget spontaneous is the spontaneous release of LDH from untreated Caco-2 cells, and A100% lysis indicates cells that have been treated for maximal release of LDH.
RESULTS
Growth at sublethal pH is dependent upon NaCl concentration.
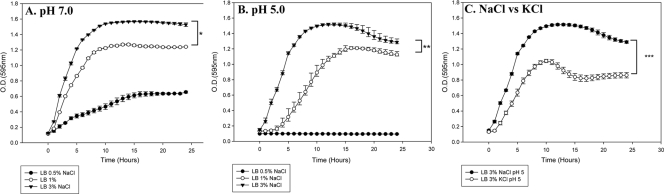
Vibrio parahaemolyticus is a moderately halophilic organism with an absolute requirement for salt. Under our test conditions, V. parahaemolyticus grew best in neutral medium containing 3% NaCl (Fig. 1). When the concentration of NaCl in the medium is decreased to 1 or 0.5% NaCl, the organism reached final OD595s of 1.2 and 0.6, respectively, at pH 7.0 (Fig. 1A). To determine the effects of the NaCl concentration on the growth of V. parahaemolyticus under different stress conditions, we first examined growth under a sublethal (pH 5) acid condition. Wild-type V. parahaemolyticus RIMD2210633 was grown in LB containing 3%, 1%, and 0.5% NaCl at pH 5 (Fig. 1B). At pH 5, V. parahaemolyticus grew better in LB containing 3% NaCl, reaching an OD595 of 1.5 (compared to 1.6 at pH 7). In contrast, when V. parahaemolyticus was grown in LB containing 1% NaCl at pH 5, the organism exhibited a 4-h lag time before the onset of log-phase growth and reached a final OD595 of 1 (Fig. 1B). When the NaCl concentration of the medium was reduced to 0.5% NaCl, V. parahaemolyticus failed to grow at pH 5 (Fig. 1B). These data suggest that decreasing amounts of NaCl in the culture medium stresses the cells and hinders the ability of V. parahaemolyticus to resist acid stress conditions. We also determined whether or not growth in 1% NaCl or 3% NaCl and at pH 7 or pH 5 varied among different strains of V. parahaemolyticus. In addition to RIMD2210633, we repeated the growth experiments using the strains UCMV493 (an environmental isolate lacking tdh and trh), AQ4235 (a trh+ clinical isolate lacking tdh), and AN-5034 (tdh+; lacking trh) and found that there was no significant difference among strains for growth under the different NaCl concentrations and for growth at pH 5 (data not shown).
FIG. 1.
Effect of NaCl concentration on the ability of wild-type V. parahaemolyticus RIMD2210633 to grow under mild, sublethal acid stress (pH 5). Vibrio parahaemolyticus was grown aerobically at 37°C in either LB with 3% NaCl (closed triangles), LB with 1% NaCl (open circles), or LB with 0.5% NaCl (closed circles) at pH 7 (A) or pH 5 (B). The organism was also grown in LB with 3% KCl at pH 5 (C), and growth was determined at OD595. All cultures were grown in triplicate, and each experiment was performed at least twice. Error bars indicate standard deviations. An unpaired Student t test was used to infer statistical difference between cells grown in 3% NaCl and cells grown in either 1% or 0.5% NaCl for 24 h. *, P < 0.02; **, P < 0.002; ***, P < 0.0001.
Next, we investigated whether it was specifically the sodium ion that conveyed an advantage to the organism under acidic conditions. When V. parahaemolyticus was grown in LB (3% KCl) adjusted to pH 5, the organism was able to reach a final OD595 of only 0.8, compared with 1.4 when grown in LB (3% NaCl) (Fig. 1C). This suggests that V. parahaemolyticus has the ability to grow in the presence of different cations, which was previously shown (29). However, under mildly acidic conditions, cells grown in sodium are less susceptible to low-pH stress than those grown in potassium. After 24 h of bacterial growth, the pHs of LB (1% NaCl) at pH 5 and LB (3% NaCl) at pH 5 had both increased to approximately pH 7 in all of the experiments performed.
Growth in low salt enhances susceptibility to lethal pH stress.
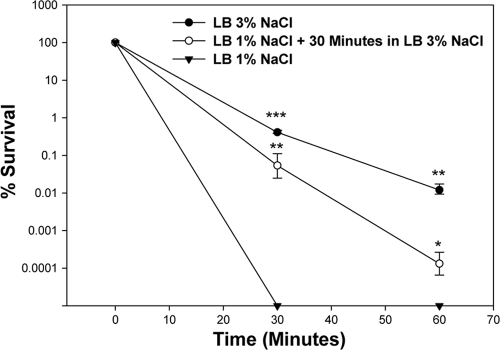
Next, we wanted to determine the effect of NaCl concentrations on V. parahaemolyticus when it was subjected to lethal pH stress (Fig. 2). The organism was grown to mid-log phase in LB containing either 1% NaCl or 3% NaCl to a concentration of approximately 108 cells. These cells were then resuspended in fresh LB containing either 1% NaCl or 3% NaCl, respectively, which was adjusted to pH 4, about 10× more acidic than the minimum pH this organism can grow at (pH 5). After 30 min, plating of cells originally grown in LB containing 1% NaCl showed a >6-log decrease in counts, where no surviving cells remained (limit of detection of 10 CFU/ml) (Fig. 2). However, plating of cells grown in LB containing 3% NaCl showed significantly higher survival, with an approximate 2-log decrease in viable cells after exposure to acid stress for 30 min (P < 0.001) and an approximate 4-log decrease in viable cells after 60 min (P < 0.01) (Fig. 2). To determine if the addition of a resuscitation phase in 3% NaCl would be sufficient to protect the organism, we grew V. parahaemolyticus cells to mid-log phase and then resuspended them in LB containing 3% NaCl for 30 min before subjecting them to pH 4. These cells showed a significantly higher survival than cells grown only in LB containing 1% NaCl, with a 4-log increase in survivability at the 30-min time point (P < 0.01) and a 2-log increase at the 60-min time point (P < 0.02) (Fig. 2). These data show that a short resuscitation phase in 3% NaCl increases resistance of V. parahaemolyticus to low-pH conditions. Throughout the 60 min in lethal acid, the pH of the medium remained at 4.0.
FIG. 2.
Effect of NaCl concentration on wild-type V. parahaemolyticus RIMD2210633 exposed to lethal acid stress (pH 4). Closed circles indicate cells grown exponentially in LB with 3% NaCl followed by growth in LB with 3% NaCl at pH 4 for up to 60 min. Closed triangles indicate cells grown exponentially in LB with 1% NaCl followed by growth in LB with 3% NaCl at pH 4 for up to 60 min. Open circles indicate cells grown in LB with 1% NaCl plus a 30-min resuscitation phase in LB with 3% NaCl prior to acidification to pH 4. Survivability was determined by dividing the surviving population at various time points by the initial population. All cultures were grown in triplicate, and each experiment was performed at least twice. Error bars indicate standard deviations. An unpaired Student t test was used to determine statistical differences between cells grown in 1% NaCl and cells grown in either 1% NaCl with the 30-min resuscitation in 3% NaCl or cells grown completely in 3% NaCl. *, P < 0.01; **, P < 0.02; ***, P < 0.001.
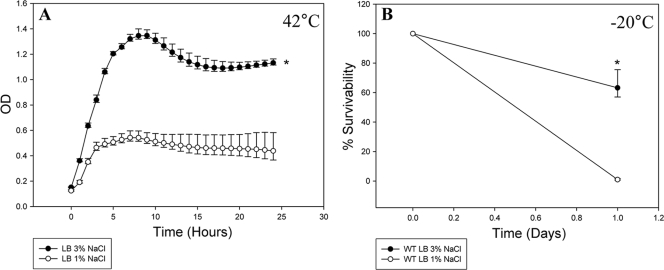
To determine whether the NaCl concentration plays a role in the organism's ability to withstand other stresses, we tested both growth at high temperature and survival at low temperature. First, we grew V. parahaemolyticus at 42°C in LB containing either 1% NaCl or 3% NaCl (Fig. 3 A). When grown in LB with 3% NaCl, the organism reached a maximum OD595 of 1.4. However, when V. parahaemolyticus was grown in LB containing 1% NaCl, it reached a maximum OD of 0.4, which is a highly significant difference (P < 0.0001). We also examined survival of the organism at −20°C (Fig. 3B). After 24 h of exposure to −20°C, we were able to recover 65% of the cells cultured in LB containing 3% NaCl; however, there was a significant decrease in survival for cells cultured in LB containing 1% NaCl (P < 0.0001), with a survival rate of <1% (limit of detection of 10 CFU/ml) (Fig. 3B). Taken together, these data demonstrate that V. parahaemolyticus grown in 3% NaCl is better suited to withstand temperature stress than cells grown in 1% NaCl. Overall the data indicate that growth in 1% NaCl is a stress condition for the organism and reduces its ability to tolerate other stresses.
FIG. 3.
Effect of high- and low-temperature stress on the growth and survival of V. parahaemolyticus RIMD2210633 at different NaCl concentrations. The organism was grown in either LB with 1% NaCl at pH 7 or LB with 3% NaCl at pH 7 at 42°C for 24 h (A). To study the role NaCl plays in low-temperature survival, exponential cultures of V. parahaemolyticus grown in either LB with 1% NaCl at pH 7 or LB with 3% NaCl at pH 7 were subjected to −20°C for 24 h (B). Survivability was determined by dividing the surviving population after 24 h by the initial population. All cultures were grown in triplicate, and each experiment was performed at least twice. Error bars indicate standard deviations. An unpaired Student t test was used to determine statistical differences between cells grown in 3% NaCl and cells grown in 1% NaCl. *, P < 0.0001.
Transcriptional analysis of cadA, a known acid tolerance gene.
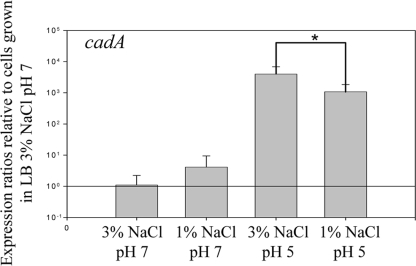
In order to decipher the role of NaCl in acid survival, we determined the effect of NaCl on expression of the main acid response system in V. parahaemolyticus (Fig. 4). The cadA gene (VP2890) encodes lysine decarboxylase, an enzyme responsible for the removal of CO2 from the amino acid lysine, which has been previously shown to play a major role in the acid tolerance of V. parahaemolyticus (40). We examined transcript levels of cadA in cells grown for 4 h in LB containing either 1% NaCl or 3% NaCl under strictly neutral conditions or after a subsequent 30-min period in LB containing either 1% or 3% NaCl at pH 5. In neutral-pH cultures containing 1% NaCl, the expression level for cadA was approximately 0.54 log (3.5-fold) higher than that observed in cells grown in 3% NaCl; however, this change was not statistically significant (P > 0.05) (Fig. 4). Following exposure to acidic conditions (pH 5), cadA transcript levels exhibited approximately a 3.6-log (3,800-fold) and 3.1-log (1,350-fold) increase for cells grown in LB containing either 3% NaCl or 1% NaCl, respectively, which are significantly different from their pH 7 counterparts (P < 0.01) (Fig. 4). Cells grown in 3% NaCl and then subjected to acid stress expressed cadA at an approximately 0.47-log (3-fold) higher level than cells that were grown in LB containing 1% NaCl and then acid stressed, and these differences were significantly different (P < 0.01). These data suggest that 3% NaCl may be important in the induction of the acid stress response, since cells grown in LB (3% NaCl) elicited a stronger cadA response after acidification than cells grown in LB (1% NaCl). Vibrio parahaemolyticus contains two pathways for the synthesis of the compatible solutes ectoine and betaine. In addition to its two synthesis systems, V. parahaemolyticus also contains six compatible solute transporters (27). Thus, V. parahaemolyticus encodes triple the number of compatible solute systems found in V. cholerae that may have an additional role beyond the osmotic tolerance response in V. parahaemolyticus. We therefore examined the expression levels of the main compatible solute synthesis system genes ectA (VP1722) and betA (VP1111) and the betaine/carnitine/choline transporter 1 (BCCT1) gene (VP1723), proU1 (VP1726), BCCT3 gene (VPA0356), and proU2 (VPA1109) transport systems after inorganic acid stress shock and found no significant changes in expression levels (P ≥ 0.05, data not shown). These data suggest that the compatible solute systems do not play a direct role in inorganic acid stress response.
FIG. 4.
Relative expression of cadA in 1% and 3% NaCl and at pH 7 and pH 5. Wild-type V. parahaemolyticus RIMD2210633 was grown for 4 h prior to RNA extraction under the following conditions: (i) LB with 3% NaCl at pH 7, (ii) LB with 3% NaCl at pH 7 plus an additional 30 min at pH 5, (iii) LB with 1% NaCl at pH 7, and (iv) LB with 1% NaCl at pH 7 plus an additional 30 min at pH 5. Bars represent expression levels of cadA normalized to 16S rRNA and relative to the expression levels found in V. parahaemolyticus grown in LB with 3% NaCl at pH 7 as determined by use of qPCR. RNA was extracted on at least two separate occasions for all of the conditions examined, and qPCR was carried out in duplicate for each gene. Error bars indicate standard deviations. An unpaired Student t test was used to determine statistical differences between results for LB with 3% NaCl at pH 5 and those for LB with 1% NaCl at pH 5. *, P < 0.01.
Roles of toxR and rpoS in stress response.
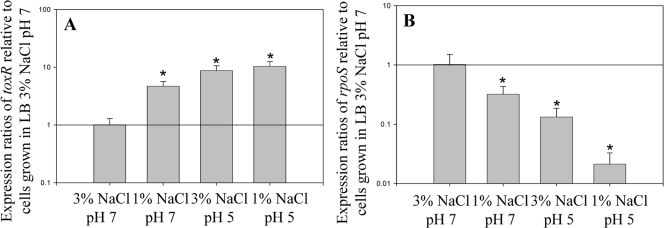
To explore possible regulators in the stress response of V. parahaemolyticus, we examined the two-component regulatory operon toxRS (VP0819-VP0820) and the alternative σ38 sigma factor rpoS (VP2553). First, we examined the expression patterns of both toxR and rpoS under different NaCl concentrations and pH conditions to determine whether there are differences in expression patterns (Fig. 5). We evaluated mRNA transcript levels of toxR in LB (1% NaCl) at pH 7 or pH 5 or in LB (3% NaCl) at pH 5 relative to transcript levels in cells grown in LB with 3% NaCl at pH 7 (Fig. 5A). When V. parahaemolyticus was grown in LB containing 1% NaCl at neutral pH, toxR expression increased approximately 5-fold compared to that when the organism was grown in LB containing 3% NaCl (Fig. 5A). When LB with 3% NaCl was acidified to pH 5, the expression of toxR increased approximately 9-fold (Fig. 5A). In LB containing 1% NaCl at pH 5, expression of toxR was approximately 10-fold higher. In all cases, the changes in cellular toxR expression were found to be statistically significant compared to results with LB (3% NaCl) at pH 7 (P < 0.0001) (Fig. 5A).
FIG. 5.
Expression analysis of toxR (VP0820) and rpoS (VP2553) at 1% and 3% NaCl and at pH 7 and pH 5. Vibrio parahaemolyticus RIMD2210633 was grown under the following conditions: (i) LB with 3% NaCl at pH 7, (ii) LB with 3% NaCl at pH 7 plus 30 min at pH 5, (iii) LB with 1% NaCl at pH 7, and (iv) LB with 1% NaCl at pH 7 plus 30 min at pH 5. Cultures were allowed to grow for 4 h prior to extraction of RNA. Bars represent expression ratios of the toxR (A) or rpoS (B) gene relative to its expression levels found in V. parahaemolyticus grown in LB with 3% NaCl at pH 7 as determined by use of qPCR. RNA was extracted on at least two separate occasions for the conditions examined, and qPCR was run in duplicate for each gene. Error bars indicate standard deviations. An unpaired Student t test was used to determine statistical differences between the samples examined. The asterisk denotes expression ratios significantly greater than the ratio for wild-type cells grown in 3% NaCl at pH 7 (P < 0.00001) for toxR (A) or significantly less (P < 0.00001) for rpoS (B).
The mRNA transcript levels of rpoS were also examined in cells grown in LB (1% NaCl) at pH 7 or pH 5 or in LB (3% NaCl) at pH 5 relative to rpoS transcript levels for cells grown in LB (3% NaCl) at pH 7 (Fig. 5B). The expression level of rpoS decreased approximately 8-fold in cells grown in 3% NaCl at pH 5 relative to that in cells grown in 3% NaCl at pH 7. In cells grown in 1% NaCl at pH 7, the expression of rpoS in cells decreased approximately 3-fold, while in 1% NaCl at pH 5, expression of rpoS decreased approximately 37-fold relative to that in cells grown in LB with 3% NaCl at pH 7 (Fig. 5B). In all cases, the decrease in rpoS expression levels was significant (P < 0.0001) compared to results with LB (3% NaCl) at pH 7 (Fig. 5B). These data demonstrate that both the NaCl concentration and acid conditions have an effect on expression of these regulators, with both stress conditions inducing toxR and repressing rpoS expression.
Effect of ΔtoxRS and ΔrpoS on acid stress survival.
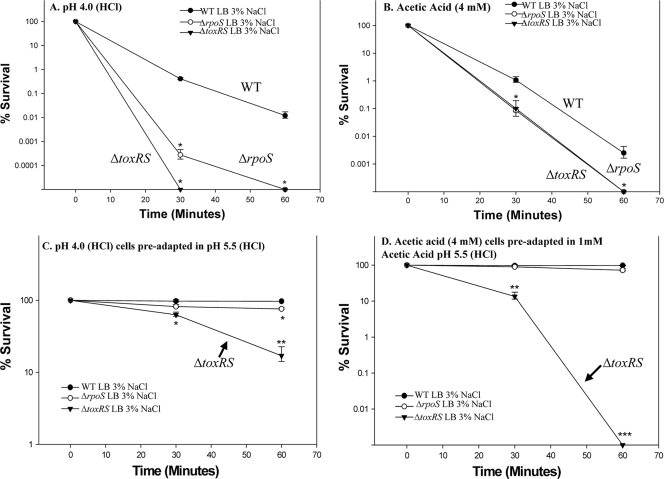
To further assess the role these regulators might play in the stress response, we created deletions in either the toxRS operon or the rpoS gene in V. parahaemolyticus RIMD2210633. The rpoS gene encodes a homologue of the stress/stationary-phase sigma factor, and toxRS encodes a regulatory system, ToxRS, that in V. cholerae is required for the acid stress response (21-24). The ΔtoxRS and ΔrpoS mutants and the wild type were grown in LB with 1% NaCl, 3% NaCl, or 6% NaCl at 37°C to evaluate growth under low-NaCl conditions, optimal NaCl conditions, and a higher-than-optimal NaCl condition, which is rarely seen in the natural environment. For all three of the NaCl concentrations tested, the growth of the two mutants was comparable to that of the wild-type strain, suggesting that neither toxRS nor rpoS is essential for regulation of the osmotic stress response (data not shown). We performed a lethal acid survivability assay with both mutant strains grown in LB containing 3% NaCl subjected to inorganic (HCl, pH 4) acid stress and compared the survivability of the two mutants against that of wild-type V. parahaemolyticus. Again, the pH remained at 4 throughout the 60 min in lethal acid. Also, we performed the survivability assay using organic acid (4 mM acetic acid), since there is evidence that organic acids affect cells in a different manner from inorganic acid and in some cases require different stress response systems. Both mutants demonstrated significantly higher mortality in the presence of inorganic acid (Fig. 6 A). By 30 min, we were not able to recover any viable ΔtoxRS mutant cells (P < 0.01). At the same time point, the ΔrpoS mutant exhibited an approximately 3-log decrease in survival compared with the wild type (P < 0.01), and by 60 min, there were no recoverable ΔrpoS cells (P < 0.01) (Fig. 6A). Both mutants also demonstrated an approximately 1-log decrease in survival compared with the wild type at 30 min of exposure to 4 mM acetic acid (P < 0.01) (Fig. 6B). By 60 min, we were not able to recover any viable cells for either of the mutants (P < 0.01). This suggests that both the toxRS operon and the rpoS gene play important roles in V. parahaemolyticus survival in lethal levels of both inorganic and organic acids. Next, we examined whether an acid-adaptive phase could rescue the ΔtoxRS and ΔrpoS mutant strains to wild-type levels in acid survivability assays. Here we included an additional 30 min of acid adaptation either in pH 5.5 (HCl) or with the addition of 1 mM acetic acid after adjustment to pH 5.5 (HCl). This 30-min acid adaptation phase rescued the ΔrpoS mutant to wild-type levels (Fig. 6C and D). In contrast, the ΔtoxRS mutant was not completely rescued to wild-type levels and continued to show significantly reduced mortality (P < 0.001) even though it demonstrated better survival after preadaptation in sublethal acid than when it was not preadapted (Fig. 6C and D). These data suggest that ToxRS plays a more significant role in the response to both inorganic and mixed inorganic/organic stress than does RpoS.
FIG. 6.
Effect of acid stress on the ΔtoxRS and ΔrpoS deletion mutants. Wild-type (closed circles), ΔtoxRS (closed triangles), or ΔrpoS (open circles) cells were grown in LB with 3% NaCl and subjected for 60 min to either inorganic (pH 4) stress (A) or organic (4 mM acetic acid, pH 4.5) stress (B) or given a preadaptation step prior to the assay of either 30 min at pH 5.5 (HCl) (C) or 1 mM acetic acid adjusted to pH 5.5 (HCl) (D). Survivability was determined by dividing the surviving population at various time points by the initial population. All cultures were grown in triplicate, and each experiment was performed at least twice. Error bars indicate standard deviations. An unpaired Student t test was used to determine statistical differences in survival between the wild-type cells and both the ΔtoxRS and ΔrpoS deletion mutants. *, P < 0.01; **, P < 0.001; ***, P < 0.0001.
Cytotoxicity toward Caco-2 cells.
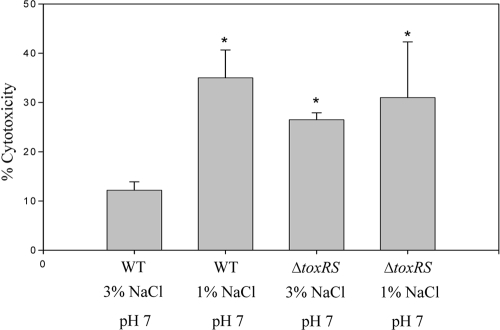
To begin to determine the physiological role that changing NaCl conditions might play on the virulence of V. parahaemolyticus, we investigated cytotoxicity toward Caco-2 cells. For these assays, V. parahaemolyticus was grown in either LB with 1% NaCl at pH 7 or LB with 3% NaCl at pH 7 (Fig. 7). Wild-type cells grown in LB (3% NaCl) at pH 7 produced the lowest levels of cellular lysis (10.4%). When the organism was grown in LB (1% NaCl) at pH 7, we observed 35% cytotoxicity, which was a statistically significant increase in percent cytotoxicity (P < 0.01) (Fig. 7). We next examined the ΔtoxRS deletion mutant, since we expected it to exhibit reduced cytotoxicity to Caco-2 cells since the toxRS operon has been linked previously to positive regulation of expression of the thermostable direct hemolysin gene in V. parahaemolyticus (18). In addition, there is also evidence that the toxRS operon responds to environmental stimuli, such as changing salinity (18). When the ΔtoxRS mutant was grown in LB with 3% NaCl at pH 7, we observed 27% cytotoxicity, compared with 10.4% cytotoxicity for the wild type under the same conditions, while the ΔtoxRS mutant grown in LB with 1% NaCl at pH 7 showed 32% cytotoxicity. The cytotoxicity of the ΔtoxRS mutant grown in both LB (1% NaCl) at pH 7 and LB (3% NaCl) at pH 7 was significantly higher than that for the wild type grown in LB (3% NaCl) at the same pH (P < 0.01). No significant difference in cytotoxicity was observed between wild-type cells grown in 1% NaCl and the ΔtoxRS mutant. The rpoS mutant was also examined, and it showed cytotoxicity levels that were similar to those for wild-type cells (data not shown).
FIG. 7.
Cytotoxicity of V. parahaemolyticus toward Caco-2 cells. Wild-type cells and ΔtoxRS cells were grown in LB with 1% NaCl at pH 7 or LB with 3% NaCl at pH 7 before being incubated with Caco-2 cells at an MOI of 100:1. Each condition was examined in triplicate, and the experiment was repeated at least twice. Error bars indicate standard deviations. The paired t test was performed to determine statistical difference between the samples tested. The asterisk denotes significant difference between results for wild-type cells grown in LB with 1% NaCl or ΔtoxRS cells grown in either LB with 1% NaCl or LB with 3% NaCl (P < 0.01) compared with results for wild-type cells grown in LB with 3% NaCl.
DISCUSSION
In order to determine the role that changes in osmolarity play in the stimulation of acid tolerance and thermotolerance in V. parahaemolyticus, we examined the effects of growth in 1% or 3% NaCl on the ability of the organism to resist these additional stresses. We found that growth of V. parahaemolyticus at 3% NaCl protects the organism from low organic and inorganic pHs and from both low and high temperatures. We found that 1% NaCl is a stress condition that prevents adaptation to additional stresses. These findings could lead to harvesting and processing interventions to ameliorate V. parahaemolyticus O3:K6 levels in shellfish. Most bivalves are classified as osmoconformers, that is, their internal osmolarity fluctuates in accordance with the osmolarity of their external environment, which may be either estuarine or oceanic or any level in between (14, 36). The effect of growth in 3% NaCl was quite pronounced under acid conditions and was correlated with increased expression of cadA. Recently Tanaka and colleagues identified cadA (VP2890), which encodes lysine decarboxylase, in V. parahaemolyticus and showed transcriptional expression of the lysine decarboxylase after acid induction and in the presence of external lysine (40). The cadA-mutated strain constructed in their study showed reduced tolerance to acidic conditions compared to the wild type (40). In E. coli, there are at least three known inducible systems for acid resistance (10, 35). The first and second systems are glutamate dependent and arginine dependent, requiring glutamate decarboxylase and arginine decarboxylase, respectively. Neither of these systems is present in V. parahaemolyticus, and it appears that Cad is the sole decarboxylase system in this organism. We found that cadA expression was induced by low pH regardless of the NaCl concentrations of the growth media. However, cadA expression was significantly higher (P < 0.01) in LB with 3% NaCl at pH 5 than was the case for cells in LB with 1% NaCl at pH 5, which may explain why cells grown under these conditions better tolerate low-pH stress.
The third acid tolerance system in E. coli requires the rpoS stress response sigma factor, a homologue of which, VP2553, is present in V. parahaemolyticus RIMD2210633, sharing 76% and 80% amino acid identity with RpoS in E. coli and V. cholerae, respectively. RpoS was originally described as a stationary-phase stress response sigma factor in bacteria and has since been shown to be important for cell survival under stress conditions, such as oxidative stress and exposure to acid in many pathogens, and in both the exponential- and stationary-phase acid tolerance response of E. coli, Salmonella enterica serovar Typhimurium, and V. cholerae (41). In our study, we found that rpoS expression was higher in cells grown in 3% NaCl than in those grown in 1% NaCl regardless of pH conditions. Overall rpoS expression was reduced at pH 5 compared to that at pH 7. The rpoS mutant strain we constructed had significantly reduced resistance to both lethal inorganic and organic acid conditions compared to the wild type. However, a short adaptive phase restored the rpoS mutant to wild-type levels (Fig. 6D). These data suggest that in V. parahaemolyticus, RpoS may not be the main or only alternative stress response sigma factor. Our analysis of the V. parahaemolyticus RIMD2210633 genome identified at least 11 putative sigma factors, which include the open reading frames VP0055 (encoding extracytoplasmic sigma factor), VP0404 (encoding RpoD), VP2210 (encoding RpoD), VP2232 (encoding RpoF), VP2358 (encoding RpoD), VP2553 (encoding RpoS), VP2578 (encoding RpoE), VP2670 (encoding RpoN), VP2953 (encoding RpoH), VPA1555 (encoding RpoF), and VPA1690 (encoding RpoD). This is four more than are present in other enteric species, such as E. coli and S. enterica.
Vibrio cholerae, a pathogen closely related to V. parahaemolyticus, also encodes only the Cad system among the three decarboxylase systems described above. It uses an additional regulatory system, ToxRS (encoded by toxRS), a signal transduction system composed of the transmembrane proteins ToxR and ToxS (21-24), for the acid stress response. In V. cholerae, ToxR is a transcriptional activator which senses changes in the environment and cocoordinately regulates the expression of at least 60 genes, including those encoding multiple virulence factors and outer membrane proteins and those involved in both the acid and bile stress responses (3, 8, 21-25, 32, 33, 37). The toxR and toxS genes in V. parahaemolyticus share 51% and 65% amino acid identity, respectively, with those in V. cholerae. In this study, we found that expression of toxR was increased 10-fold at pH 5.0 compared to that at pH 7.0, regardless of the NaCl concentration. Compared to rpoS, the toxR gene was significantly induced under acid conditions (P < 0.0001). In similarity to the V. parahaemolyticus ΔrpoS mutant strain, the ΔtoxRS mutant had a reduced ability to survive under both lethal inorganic and organic acid conditions. An adaptive phase of 30 min at sublethal inorganic or organic acid concentrations did not restore the ΔtoxRS mutant to wild-type levels in the survivability assays; however, the ΔrpoS mutant was restored. These data indicate that ToxR plays an essential role in acid tolerance in V. parahaemolyticus for both inorganic and organic stress. It is known that in V. cholerae, ToxR directly and positively regulates the expression of ompU, which encodes the outer membrane protein OmpU (21, 22). A homolog of OmpU (VP2467) is present in V. parahaemolyticus, and it will be interesting to determine if ToxR plays a role in the regulation of OmpU and what its possible role may be in the acid stress response.
In summary, we show that growth of V. parahaemolyticus in different NaCl concentrations can greatly affect its response to pH and temperature stresses. Wild-type V. parahaemolyticus grew better in neutral-pH medium containing 3% NaCl than at pH 5.0 in medium containing 3% NaCl, suggesting that this pathogen may grow better in normal (neutral-pH) seawater. In medium containing 3% NaCl, there was a 65% survival of V. parahaemolyticus after freezing for 24 h at −20°C, but in medium containing only 1% NaCl, there was <1% survival. This suggests that oysters obtained from low-salinity waters may experience greater reductions of V. parahaemolyticus during frozen storage, particularly since oysters are osmoconformers, adjusting their tissue salinities to the levels found in the seawater. From a practical standpoint, short-term freezing may be a viable processing intervention to reduce V. parahaemolyticus O3:K6 levels in oysters regardless of the water conditions from which they were obtained, but our results suggest that greater benefit would likely be obtained for oysters obtained from low-salinity seawater. Tests for cytotoxicity of V. parahaemolyticus toward human intestinal cells showed greater cytotoxicity when V. parahaemolyticus was propagated in medium containing 1% NaCl (35% toxicity) than with 3% NaCl (10.4% toxicity). Our studies suggest that it may be possible to manipulate harvesting and storage conditions to reduce V. parahaemolyticus O3:K6 levels in shellfish; however, shellfish-based studies are needed to confirm these findings.
Acknowledgments
This research was supported by an NSF grant, IOS-0918429, and funding from the U.S. Department of Agriculture, NRI CSREES grant 2008-35201-04535.
Footnotes
Published ahead of print on 14 May 2010.
REFERENCES
- 1.Ansaruzzaman, M., M. Lucas, J. L. Deen, N. A. Bhuiyan, X. Y. Wang, A. Safa, M. Sultana, A. Chowdhury, G. B. Nair, D. A. Sack, L. von Seidlein, M. K. Puri, M. Ali, C. L. Chaignat, J. D. Clemens, and A. Barreto. 2005. Pandemic serovars (O3:K6 and O4:K68) of Vibrio parahaemolyticus associated with diarrhea in Mozambique: spread of the pandemic into the African continent. J. Clin. Microbiol. 43:2559-2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baik, H. S., S. Bearson, S. Dunbar, and J. W. Foster. 1996. The acid tolerance response of Salmonella typhimurium provides protection against organic acids. Microbiology 142(Pt. 11):3195-3200. [DOI] [PubMed] [Google Scholar]
- 3.Bina, J., J. Zhu, M. Dziejman, S. Faruque, S. Calderwood, and J. Mekalanos. 2003. ToxR regulon of Vibrio cholerae and its expression in vibrios shed by cholera patients. Proc. Natl. Acad. Sci. U. S. A. 100:2801-2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. 1999. Outbreak of Vibrio parahaemolyticus infection associated with eating raw oysters and clams harvested from Long Island Sound—Connecticut, New Jersey, and New York, 1998. MMWR Morb. Mortal. Wkly. Rep. 48:48-51. [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. 2009. Preliminary FoodNet data on the incidence of infection with pathogens transmitted commonly through food—10 states, 2008. MMWR Morb. Mortal. Wkly. Rep. 58:333-337. [PubMed] [Google Scholar]
- 6.Daniels, N. A., L. MacKinnon, R. Bishop, S. Altekruse, B. Ray, R. M. Hammond, S. Thompson, S. Wilson, N. H. Bean, P. M. Griffin, and L. Slutsker. 2000. Vibrio parahaemolyticus infections in the United States, 1973-1998. J. Infect. Dis. 181:1661-1666. [DOI] [PubMed] [Google Scholar]
- 7.Daniels, N. A., B. Ray, A. Easton, N. Marano, E. Kahn, A. L. McShan II, L. Del Rosario, T. Baldwin, M. A. Kingsley, N. D. Puhr, J. G. Wells, and F. J. Angulo. 2000. Emergence of a new Vibrio parahaemolyticus serotype in raw oysters: a prevention quandary. JAMA 284:1541-1545. [DOI] [PubMed] [Google Scholar]
- 8.DiRita, V. J., and J. J. Mekalanos. 1991. Periplasmic interaction between two membrane regulatory proteins, ToxR and ToxS, results in signal transduction and transcriptional activation. Cell 64:29-37. [DOI] [PubMed] [Google Scholar]
- 9.Foster, J. W. 1999. When protons attack: microbial strategies of acid adaptation. Curr. Opin. Microbiol. 2:170-174. [DOI] [PubMed] [Google Scholar]
- 10.Foster, J. W., and M. Moreno. 1999. Inducible acid tolerance mechanisms in enteric bacteria. Novartis Found. Symp. 221:55-69,70-74. [DOI] [PubMed] [Google Scholar]
- 11.Fuenzalida, L., C. Hernandez, J. Toro, M. L. Rioseco, J. Romero, and R. T. Espejo. 2006. Vibrio parahaemolyticus in shellfish and clinical samples during two large epidemics of diarrhoea in southern Chile. Environ. Microbiol. 8:675-683. [DOI] [PubMed] [Google Scholar]
- 12.Fujino, T., Y. Okuno, D. Nakada, A. Aoyama, K. Fukai, T. Mukai, and T. Veho. 1953. On the bacteriological examination of shirasu food poisoning. Med. J. Osaka Univ. 4:299-304. [Google Scholar]
- 13.Gonzalez-Escalona, N., V. Cachicas, C. Acevedo, M. L. Rioseco, J. A. Vergara, F. Cabello, J. Romero, and R. T. Espejo. 2005. Vibrio parahaemolyticus diarrhea, Chile, 1998 and 2004. Emerg. Infect. Dis. 11:129-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gunter, G. 1955. Mortality of oysters and abundance of certain associates as related to salinity. Ecology 36:601-605. [Google Scholar]
- 15.Horton, R. M., H. D. Hunt, S. N. Ho, J. K. Pullen, and L. R. Pease. 1989. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene 77:61-68. [DOI] [PubMed] [Google Scholar]
- 16.Kaneko, T., and R. R. Colwell. 1973. Ecology of Vibrio parahaemolyticus in Chesapeake Bay. J. Bacteriol. 113:24-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krantz, G. E., R. R. Colwell, and E. Lovelace. 1969. Vibrio parahaemolyticus from the blue crab Callinectes sapidus in Chesapeake Bay. Science 164:1286-1287. [DOI] [PubMed] [Google Scholar]
- 18.Lin, Z., K. Kumagai, K. Baba, J. J. Mekalanos, and M. Nishibuchi. 1993. Vibrio parahaemolyticus has a homolog of the Vibrio cholerae toxRS operon that mediates environmentally induced regulation of the thermostable direct hemolysin gene. J. Bacteriol. 175:3844-3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Makino, K., K. Oshima, K. Kurokawa, K. Yokoyama, T. Uda, K. Tagomori, Y. Iijima, M. Najima, M. Nakano, A. Yamashita, Y. Kubota, S. Kimura, T. Yasunaga, T. Honda, H. Shinagawa, M. Hattori, and T. Iida. 2003. Genome sequence of Vibrio parahaemolyticus: a pathogenic mechanism distinct from that of V. cholerae. Lancet 361:743-749. [DOI] [PubMed] [Google Scholar]
- 20.Martinez-Urtaza, J., A. Lozano-Leon, A. DePaola, M. Ishibashi, K. Shimada, M. Nishibuchi, and E. Liebana. 2004. Characterization of pathogenic Vibrio parahaemolyticus isolates from clinical sources in Spain and comparison with Asian and North American pandemic isolates. J. Clin. Microbiol. 42:4672-4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Merrell, D. S., C. Bailey, J. B. Kaper, and A. Camilli. 2001. The ToxR-mediated organic acid tolerance response of Vibrio cholerae requires OmpU. J. Bacteriol. 183:2746-2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Merrell, D. S., and A. Camilli. 2002. Acid tolerance of gastrointestinal pathogens. Curr. Opin. Microbiol. 5:51-55. [DOI] [PubMed] [Google Scholar]
- 23.Merrell, D. S., and A. Camilli. 1999. The cadA gene of Vibrio cholerae is induced during infection and plays a role in acid tolerance. Mol. Microbiol. 34:836-849. [DOI] [PubMed] [Google Scholar]
- 24.Merrell, D. S., D. L. Hava, and A. Camilli. 2002. Identification of novel factors involved in colonization and acid tolerance of Vibrio cholerae. Mol. Microbiol. 43:1471-1491. [DOI] [PubMed] [Google Scholar]
- 25.Miller, V. L., R. K. Taylor, and J. J. Mekalanos. 1987. Cholera toxin transcriptional activator toxR is a transmembrane DNA binding protein. Cell 48:271-279. [DOI] [PubMed] [Google Scholar]
- 26.Nair, G. B., T. Ramamurthy, S. K. Bhattacharya, B. Dutta, Y. Takeda, and D. A. Sack. 2007. Global dissemination of Vibrio parahaemolyticus serotype O3:K6 and its serovariants. Clin. Microbiol. Rev. 20:39-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naughton, L. M., S. L. Blumerman, M. Carlberg, and E. F. Boyd. 2009. Osmoadaptation among Vibrio species and unique genomic features and physiological responses of Vibrio parahaemolyticus. Appl. Environ. Microbiol. 75:2802-2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okuda, J., M. Ishibashi, E. Hayakawa, T. Nishino, Y. Takeda, A. K. Mukhopadhyay, S. Garg, S. K. Bhattacharya, G. B. Nair, and M. Nishibuchi. 1997. Emergence of a unique O3:K6 clone of Vibrio parahaemolyticus in Calcutta, India, and isolation of strains from the same clonal group from Southeast Asian travelers arriving in Japan. J. Clin. Microbiol. 35:3150-3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Palasuntheram, C. 1981. The halophilic properties of Vibrio parahaemolyticus. J. Gen. Microbiol. 127:427-428. [DOI] [PubMed] [Google Scholar]
- 30.Pfaffl, M. W. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Philippe, N., J. P. Alcaraz, E. Coursange, J. Geiselmann, and D. Schneider. 2004. Improvement of pCVD442, a suicide plasmid for gene allele exchange in bacteria. Plasmid 51:246-255. [DOI] [PubMed] [Google Scholar]
- 32.Provenzano, D., and K. E. Klose. 2000. Altered expression of the ToxR-regulated porins OmpU and OmpT diminishes Vibrio cholerae bile resistance, virulence factor expression, and intestinal colonization. Proc. Natl. Acad. Sci. U. S. A. 97:10220-10224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Provenzano, D., D. A. Schuhmacher, J. L. Barker, and K. E. Klose. 2000. The virulence regulatory protein ToxR mediates enhanced bile resistance in Vibrio cholerae and other pathogenic Vibrio species. Infect. Immun. 68:1491-1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quilici, M. L., A. Robert-Pillot, J. Picart, and J. M. Fournier. 2005. Pandemic Vibrio parahaemolyticus O3:K6 spread, France. Emerg. Infect. Dis. 11:1148-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Richard, H. T., and J. W. Foster. 2003. Acid resistance in Escherichia coli. Adv. Appl. Microbiol. 52:167-186. [DOI] [PubMed] [Google Scholar]
- 36.Shumway, S. E. 1976. Effect of salinity fluctuation on the osmotic pressure and Na+, Ca2+ and Mg2+ ion concentrations in the hemolymph of bivalve molluscs. Mar. Biol. 41:153-177. [Google Scholar]
- 37.Skorupski, K., and R. K. Taylor. 1997. Control of the ToxR virulence regulon in Vibrio cholerae by environmental stimuli. Mol. Microbiol. 25:1003-1009. [DOI] [PubMed] [Google Scholar]
- 38.Smolikova, L. M., M. Lomov Iu, T. V. Khomenko, G. P. Murnachev, T. A. Kudriakova, O. P. Fetsailova, E. M. Sanamiants, L. D. Makedonova, G. V. Kachkina, and E. N. Golenishcheva. 2001. Studies on halophilic vibrios causing a food poisoning outbreak in the city of Vladivostok. Zh. Mikrobiol. Epidemiol. Immunobiol. 2001:3-7. (In Russian.) [PubMed] [Google Scholar]
- 39.Su, Y. C., and C. Liu. 2007. Vibrio parahaemolyticus: a concern of seafood safety. Food Microbiol. 24:549-558. [DOI] [PubMed] [Google Scholar]
- 40.Tanaka, Y., B. Kimura, H. Takahashi, T. Watanabe, H. Obata, A. Kai, S. Morozumi, and T. Fujii. 2008. Lysine decarboxylase of Vibrio parahaemolyticus: kinetics of transcription and role in acid resistance. J. Appl. Microbiol. 104:1283-1293. [DOI] [PubMed] [Google Scholar]
- 41.Yildiz, F. H., and G. K. Schoolnik. 1998. Role of rpoS in stress survival and virulence of Vibrio cholerae. J. Bacteriol. 180:773-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zimmerman, A. M., A. DePaola, J. C. Bowers, J. A. Krantz, J. L. Nordstrom, C. N. Johnson, and D. J. Grimes. 2007. Variability of total and pathogenic Vibrio parahaemolyticus densities in northern Gulf of Mexico water and oysters. Appl. Environ. Microbiol. 73:7589-7596. [DOI] [PMC free article] [PubMed] [Google Scholar]