Abstract
The present paper was focused on exploiting Plackett–Burman design to screen the effect of nine factors—poly (ethylene oxide) molecular weight (X1), poly (ethylene oxide) amount (X2), ethylcellulose amount (X4), drug solubility (X5), drug amount (X6), sodium chloride amount (X7), citric acid amount (X8), polyethylene glycol amount (X9), and glycerin amount (X11) on the release of drugs from the extended release extrudates, i.e., release rate and release mechanism. The experiments were carried out according to a nine-factor 12-run statistical model and subjected to an 8-h dissolution study in phosphate buffer pH 6.8. The significance of the model was indicated by the ANOVA and the residual analysis. Poly (ethylene oxide) amount, ethylcellulose amount and drug solubility had significant effect on the T90 values whereas poly (ethylene oxide) amount and ethylcellulose amount had significant effect on the n value.
KEY WORDS: ethylcellulose, extended release, hot melt extrusion, Plackett–Burman screening, poly (ethylene oxide)
INTRODUCTION
Hot melt extrusion (HME) is a technique in which during extrusion, a polymer melt is pumped through a shaping die and formed into a profile. This profile can be a plate, a film, a tube, or have any shape of its cross section (1,2). HME technology has been exploited in polymer industries since the 1930s (3). Since then, it has been extensively used in polymer (4), food (5,6), chemical (7), rubber (8), and metal industries (9). In pharmaceutical industries, this technology is exploited for the manufacturing of pellets (10,11), solid dispersion (12–14), topical dosage forms (15), powder coating (16), gastro retentive dosage forms (17), tablets (18), sustained release oral dosage forms (19,20), and ophthalmic inserts (21).
Polymer choice is the most critical factor to obtain the desired drug release profile during formulation development of hot melt extrudates. Most commonly, the hot melt extrudates are comprised of drug with one or more functional excipients (22). Polymer, a deformable carrier, is the most essential component of HME that carries the poor thermoplastic drug(s). The selection of the polymer as well as the drug to polymer ratio exhibits significant effect on the release profile of the dosage form (23). Hydrophilic polymers are most widely used for the development of extended release products. However, their use for controlling the release of highly water-soluble drug is restricted due to rapid diffusion of the dissolved drug through the hydrophilic gel layer (24). Literature reports the use of combinations of hydrophilic and hydrophobic matrices as the preferred choice for the preparation of extended release dosage forms (24). Plasticizers such as polyethylene glycol and glycerin, the second most important component of HME, are used to improve the processability of polymers by increasing their flexibility and reducing extrusion temperature (25,26). Few drugs have also reported as potential plasticizers (27). Recently, drug release modifying agents such as sodium chloride and citric acid have been reported in the literature (25–28). The mechanism of drug release modifying agents is varied and most often it is linked to increase in drug release rate by diffusion, erosion, or creating channels. Citric acid also promotes the thermal processability and matrix integrity by plasticization of polymer (25).
The impact of various factors like polymer concentration, drug loading, drug solubility, diluent, and ionic concentration on the release of the drug from the extended release formulations has been reported in the literature (29–31). In the development of extended release formulations, it becomes difficult to ascertain factors affecting the release of the drug from the extrudates. Screening designs are commonly used when little is known about a system or process. These designs, in general, are fractional factorial of a 2n design that can identify main factors from a large number of suspected variables. Statistical tools such as Plackett–Burman design helps in selecting the most important variables (32). The Plackett–Burman method allows evaluation of ‘N − 1’ variables by ‘N’ number of experiments (N must be a multiple of four). In the Plackett–Burman design, experiments are performed at various combinations of high and low values of the process variables and analyzed for their effect on the process (33,34). The Plackett–Burman design analyzes the input data and presents a rank ordering of the variables with magnitude of effect and designates signs to the effects to indicate whether an increase in factor values is advantageous or not (35).
Water solubility is one of the most important molecular properties of drugs for the development of extended release dosage forms as it is a key factor governing drug access to biological membranes (36). Therefore, in the present investigation, drugs having large difference in their solubility (theophylline and caffeine with solubility of 9.91 mg/mL and 136 mg/mL, respectively) was selected as model drugs. The objective of the study was to use the Plackett–Burman design to quantify the effect of amount and molecular weight of poly (ethylene oxide), amount and solubility of drug, ethylcellulose, sodium chloride and citric acid, polyethylene glycol and glycerin, amount on the mechanism, and rate of drug release from the extended release hot melt extrudates. In order to achieve the above-mentioned objective, a mathematical model that will work for a wide range of solubility of drugs will be developed and validated.
MATERIAL AND METHODS
Materials
Theophylline and caffeine were gifted by Bajaj Healthcare Ltd., India, poly (ethylene oxide) and ethylcellulose were gifted by Dow Chemical Company. All other chemicals and solvents used were of analytical grade and were procured from Merck India Ltd. Purified water was used throughout the study.
Experimental Design
The Plackett–Burman factorial design was employed in this study to correlate dependent and independent variables using the following polynomial model:
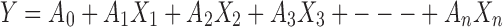 |
where Y is the response, A0 the constant and A1 to An are the coefficients of the response values.
The levels of independent and dependent variables evaluated in this study are listed in Table I. A nine-factor 12-run Plackett–Burman screening design was generated using Design-Expert® 6.0.10 (Version 2.05, Stat-Ease Inc., Minneapolis, USA; Table II). The software package was used to estimate the response of dependent variables and optimized conditions.
Table I.
Factors in the Plackett–Burman Screening Design
| Code | Low level | High level | |
|---|---|---|---|
| Independent factors | |||
| Poly (ethylene oxide) molecular weight | X 1 | 6 × 105 | 7 × 106 |
| Poly (ethylene oxide) amount (mg) | X 2 | 100.00 | 300.00 |
| Dummy | X 3 | −1.00 | 1.00 |
| Ethylcellulose amount (mg) | X 4 | 0.00 | 50.00 |
| Drug solubility (mg/mL) | X 5 | 9.91 | 136.00 |
| Drug amount (mg) | X 6 | 100.00 | 200.00 |
| Sodium chloride amount (mg) | X 7 | 0.00 | 20.00 |
| Citric acid amount (mg) | X 8 | 0.00 | 5.00 |
| Polyethylene glycol amount (mg) | X 9 | 0.00 | 5.00 |
| Dummy | X 10 | −1.00 | 1.00 |
| Glycerin amount (mg) | X 11 | 0.00 | 5.00 |
| Dependent factors | |||
| Time to release 90% of the drug | Y 1 | ||
| n value | Y 2 | ||
| % amount released in 4 h | Y 3 | ||
| % amount released in 8 h | Y 4 | ||
Table II.
Plackett–Burman Screening Design with Nine Variables Generated Using Stat-Ease Software
| Formula | X 1 | X 2 | X 3 | X 4 | X 5 | X 6 | X 7 | X 8 | X 9 | X 10 | X 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 7 × 106 | 100.00 | 1.00 | 0.00 | 9.91 | 100.00 | 20.00 | 5.00 | 5.00 | -1.00 | 5.00 |
| 2 | 7 × 106 | 300.00 | -1.00 | 50.00 | 9.91 | 100.00 | 0.00 | 5.00 | 5.00 | 1.00 | 0.00 |
| 3 | 6 × 105 | 300.00 | 1.00 | 0.00 | 136.0 | 100.00 | 0.00 | 0.00 | 5.00 | 1.00 | 5.00 |
| 4 | 7 × 106 | 100.00 | 1.00 | 50.00 | 9.91 | 200.00 | 0.00 | 0.00 | 0.00 | 1.00 | 5.00 |
| 5 | 7 × 106 | 300.00 | -1.00 | 50.00 | 136.00 | 100.00 | 20.00 | 0.00 | 0.00 | -1.00 | 5.00 |
| 6 | 7 × 106 | 300.00 | 1.00 | 0.00 | 136.00 | 200.00 | 0.000 | 5.00 | 0.00 | -1.00 | 0.00 |
| 7 | 6 × 105 | 300.00 | 1.00 | 50.00 | 9.91 | 200.00 | 20.00 | 0.00 | 5.00 | -1.00 | 0.00 |
| 8 | 6 × 105 | 100.00 | 1.00 | 50.00 | 136.00 | 100.00 | 20.00 | 5.00 | 0.00 | 1.00 | 0.00 |
| 9 | 6 × 105 | 100.00 | -1.00 | 50.00 | 136.00 | 200.00 | 0.00 | 5.00 | 5.00 | -1.00 | 5.00 |
| 10 | 7 × 106 | 100.00 | -1.00 | 0.00 | 136.00 | 200.00 | 20.00 | 0.00 | 5.00 | 1.00 | 0.00 |
| 11 | 6 × 105 | 300.00 | -1.00 | 0.00 | 9.91 | 200.00 | 20.00 | 5.00 | 0.00 | 1.00 | 5.00 |
| 12 | 6 × 105 | 100.00 | -1.00 | 0.00 | 9.91 | 100.00 | 0.00 | 5.00 | 0.00 | -1.00 | 0.00 |
Each variable was represented at two levels, namely, “high” and “low”. These levels define the upper and lower limits of the range covered by each variable. In addition to the variables of real interest, the Plackett–Burman design considers insignificant dummy variables, whose number should be one-third of all variables. The dummy variables, which are not assigned any values, introduce some redundancy required by the statistical procedure. Incorporation of the dummy variables into an experiment allows an estimation of the variance (experimental error) of an effect.
Hot Melt Extrusion
The composition of the hot melt extrudates was selected based on the statistical design presented in Table I. All the ingredients were passed through a #30 sieve and mixed in a blender for 10 min. The blend was fed into a single-screw hot melt extruder (fabricated by S.B. Panchal and Co., India) equipped with a 0.8-mm die. The screw speed was kept constant at 20 rpm. The temperature of the system was gradually increased till the extrusion process started and then it was kept constant at 80°C to 100°C. The extrudates were allowed to cool to room temperature and then were uniformly cut to the size of 5-mm length and filled into the size ‘0’ hard gelatin capsule shells such that each capsule contains 400 mg of extrudates.
In Vitro Release Studies
In vitro release studies were performed using USP dissolution apparatus 1 at 100 rpm in 900 mL of phosphate buffer pH 6.8 (Electrolab India Ltd., India) at 37 ± 0.5°C. Aliquots were withdrawn at predetermined time intervals, filtered and analyzed spectrophotometrically. All the studies were carried out in triplicates. T90 values were calculated by least square linear regression analysis.
Release Exponent Estimation
Release exponent (n) was estimated by fitting dissolution data to the Korsmeyer’s equation (37) as shown below.
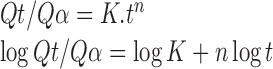 |
Validation of the Model
The developed model was validated by conducting two additional experiments. The practical responses obtained were compared with those generated by the software.
RESULT AND DISCUSSION
Statistical Design and Analysis
The Plackett–Burman screening design was used to evaluate the effect of the nine independent variables on the release of the drug from the extrudates. Low and high values for each factor tested in screening design were identified in preliminary experiments. The magnitude of responses for each 12 experiments (observed and predicted) is given in Table III along with residual values. The observed and predicted values were found to be in close agreement with each other. All the residual values were found to be less than 1.5 for all the four responses which ensures the suitability of the model.
Table III.
Observed and Predicted Values of the Responses
| No. | Y 1 | Y 2 | Y 3 | Y 4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OV | PV | R | OV | PV | R | OV | PV | R | OV | PV | R | |
| 1 | 3.49 | 3.28 | 0.21 | 0.100 | 0.10 | 0.00 | 94.63 | 94.73 | −0.1 | 97.12 | 97.25 | −0.13 |
| 2 | 11.09 | 11.30 | −0.21 | 0.79 | 0.81 | −0.02 | 62.03 | 60.88 | 1.15 | 80.67 | 80.54 | 0.13 |
| 3 | 3.93 | 3.94 | −0.01 | 0.33 | 0.35 | −0.02 | 91.85 | 91.95 | −0.1 | 99.29 | 98.61 | 0.68 |
| 4 | 5.72 | 5.73 | − 0.01 | 0.34 | 0.34 | 0.00 | 86.90 | 88.05 | −1.15 | 93.37 | 92.69 | 0.68 |
| 5 | 7.87 | 7.86 | 0.01 | 0.75 | 0.73 | 0.02 | 67.94 | 66.79 | 1.15 | 88.92 | 89.60 | −0.68 |
| 6 | 3.88 | 3.67 | 0.21 | 0.26 | 0.24 | 0.02 | 87.18 | 88.33 | −1.15 | 99.54 | 99.67 | −0.13 |
| 7 | 14.18 | 13.97 | 0.21 | 0.94 | 0.94 | 0.00 | 43.54 | 44.69 | −1.15 | 73.64 | 73.77 | −0.13 |
| 8 | 4.06 | 4.07 | −0.01 | 0.41 | 0.41 | 0.00 | 88.78 | 88.88 | −0.1 | 99.33 | 98.65 | 0.68 |
| 9 | 3.13 | 3.12 | 0.01 | 0.10 | 0.11 | −0.01 | 94.20 | 94.10 | 0.1 | 99.07 | 99.75 | −0.68 |
| 10 | 4.24 | 4.45 | −0.21 | 0.09 | 0.10 | −0.01 | 92.34 | 92.24 | 0.1 | 95.03 | 94.90 | 0.13 |
| 11 | 4.94 | 5.15 | −0.21 | 0.40 | 0.40 | 0.00 | 89.34 | 89.24 | 0.1 | 95.39 | 95.26 | 0.13 |
| 12 | 2.75 | 2.74 | 0.01 | 0.30 | 0.28 | 0.02 | 94.55 | 93.40 | 1.15 | 99.94 | 100.62 | −0.68 |
OV observed value, PV predicted value, R residual
Polynomial equations were generated for all the responses which are listed in Table IV. The magnitude and direction of the factor coefficient in the all the four equations explains the nature of the effect of factors on the responses. Factors with coefficients of greater magnitude show a high effect on the response suggesting that poly (ethylene oxide) amount, ethylcellulose amount, and solubility of drug demonstrated very significant effect on all four responses. Polyethylene glycol amount and glycerin amount also illustrated considerable effect on all four responses. The response value is directly proportional to the positive coefficients in the equations and inversely to the negative coefficients.
Table IV.
Regression Equations of the Fitted Models
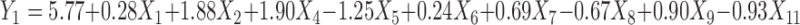
|
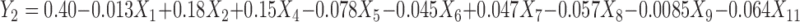
|
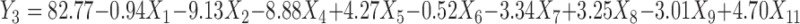
|
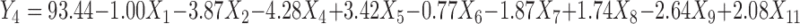
|
Using analysis of variance (ANOVA), the significance (F value <0.05) of the ratio of mean square variation due to regression coefficient and residual error was tested. The regression coefficient and probability values obtained for all the four responses were as shown in Table V. The value of more than 0.99 for regression coefficient and less than 0.05 for probability indicated the significance of the model except for the response Y3 (% of drug release in 4 h). The analysis of variance for all the four responses was as shown in the Tables VI, VII, VIII, and IX.
Table V.
Probability and Correlation Coefficient Values for the Responses
| Factors | Responses | |||
|---|---|---|---|---|
| Y 1 | Y 2 | Y 3 | Y 4 | |
| Prob > F | 0.0089 | 0.0045 | 0.1127 | 0.0164 |
| Regression | 0.9980 | 0.9990 | 0.9738 | 0.9963 |
Table VI.
Analysis of Variance for Response Y 1
| Source | Sum of squares | df | Mean square | F value | Prob > F |
|---|---|---|---|---|---|
| X 1 | 0.91 | 1 | 0.91 | 6.64 | 0.1233 |
| X 2 | 42.19 | 1 | 42.19 | 308.69 | 0.0032 |
| X 4 | 43.40 | 1 | 43.40 | 317.53 | 0.0031 |
| X 5 | 18.90 | 1 | 18.90 | 138.29 | 0.0072 |
| X 6 | 0.70 | 1 | 0.70 | 5.13 | 0.1518 |
| X 7 | 5.71 | 1 | 5.71 | 41.80 | 0.0231 |
| X 8 | 5.47 | 1 | 5.47 | 40.01 | 0.0241 |
| X 9 | 9.79 | 1 | 9.79 | 71.65 | 0.0137 |
| X 11 | 10.30 | 1 | 10.30 | 75.40 | 0.0130 |
| Residual | 0.27 | 2 | 0.14 | ||
| Total (corrected) | 137.64 | 11 |
Standard deviation of the residuals = 0.37. Explained variation about the mean = 99.80
Table VII.
Analysis of Variance for Response Y 2
| Source | Sum of squares | df | Mean square | F value | Prob > F |
|---|---|---|---|---|---|
| X 1 | 0.00218 | 1 | 0.00219 | 5.02 | 0.1543 |
| X 2 | 0.39 | 1 | 0.38 | 861.96 | 0.0012 |
| X 4 | 0.29 | 1 | 0.29 | 656.57 | 0.0015 |
| X 5 | 0.073 | 1 | 0.073 | 166.99 | 0.0059 |
| X 6 | 0.025 | 1 | 0.025 | 56.65 | 0.0172 |
| X 7 | 0.027 | 1 | 0.027 | 60.89 | 0.0160 |
| X 8 | 0.039 | 1 | 0.039 | 89.04 | 0.0110 |
| X 9 | 0.000867 | 1 | 0.00086 | 1.99 | 0.2936 |
| X 11 | 0.049 | 1 | 0.049 | 112.32 | 0.0088 |
| Residual | 0.00087 | 2 | 0.00043 | ||
| Total (corrected) | 0.88 | 11 |
Standard deviation of the residuals = 0.021. Explained variation about the mean = 99.90
Table VIII.
Analysis of Variance for Response Y 3
| Source | Sum of squares | df | Mean square | F value | Prob > F |
|---|---|---|---|---|---|
| X 1 | 10.53 | 1 | 10.53 | 0.28 | 0.6507 |
| X 2 | 999.55 | 1 | 999.55 | 26.39 | 0.0359 |
| X 4 | 945.19 | 1 | 945.19 | 24.95 | 0.0378 |
| X 5 | 219.31 | 1 | 219.31 | 5.79 | 0.1379 |
| X 6 | 3.29 | 1 | 3.29 | 0.087 | 0.7961 |
| X 7 | 134.27 | 1 | 134.27 | 3.54 | 0.2005 |
| X 8 | 127.01 | 1 | 127.01 | 3.35 | 0.2086 |
| X 9 | 108.60 | 1 | 108.60 | 2.87 | 0.2325 |
| X 11 | 265.46 | 1 | 265.46 | 7.01 | 0.1180 |
| Residual | 75.77 | 2 | 37.88 | ||
| Total (corrected) | 2,888.96 | 11 |
Table IX.
Analysis of Variance for Response Y 4
| Source | Sum of squares | df | Mean square | F value | Prob > F |
|---|---|---|---|---|---|
| X 1 | 12.02 | 1 | 12.02 | 8.43 | 0.1010 |
| X 2 | 179.49 | 1 | 179.49 | 125.90 | 0.0078 |
| X 4 | 219.39 | 1 | 219.39 | 153.89 | 0.0064 |
| X 5 | 140.43 | 1 | 140.43 | 98.50 | 0.0100 |
| X 6 | 7.10 | 1 | 7.10 | 4.98 | 0.1553 |
| X 7 | 42.00 | 1 | 42.00 | 29.46 | 0.0323 |
| X 8 | 36.51 | 1 | 36.51 | 25.61 | 0.0369 |
| X 9 | 83.58 | 1 | 83.58 | 58.63 | 0.0166 |
| X 11 | 52.13 | 1 | 52.13 | 36.56 | 0.0263 |
| Residual | 185.927 | 2 | 1.43 | ||
| Total (corrected) | 5,279.80 | 11 |
Standard deviation of the residuals = 1.19. Explained variation about the mean = 99.63
Effect of Individual Factor in Presence of Other Factors on Drug Release and Release Exponent
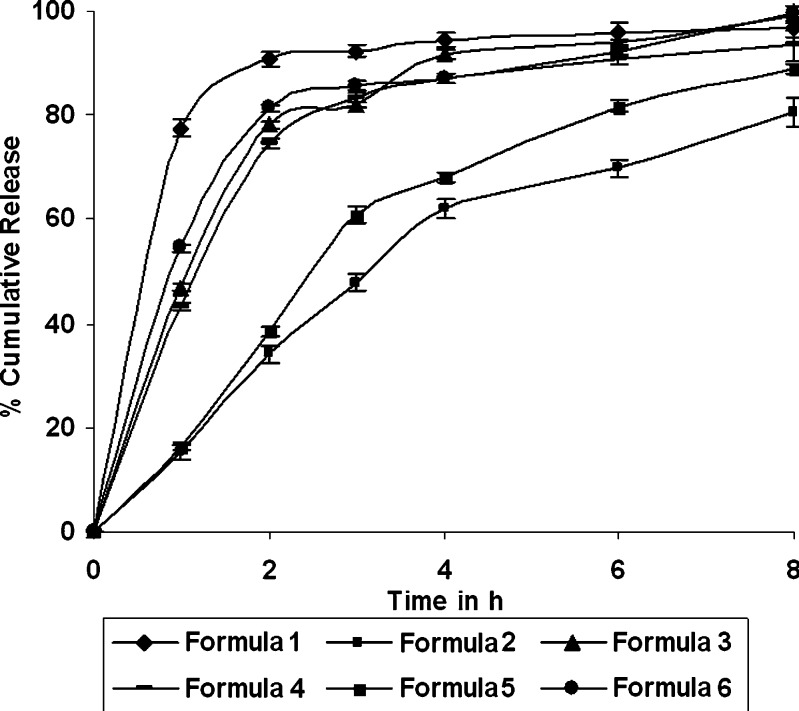
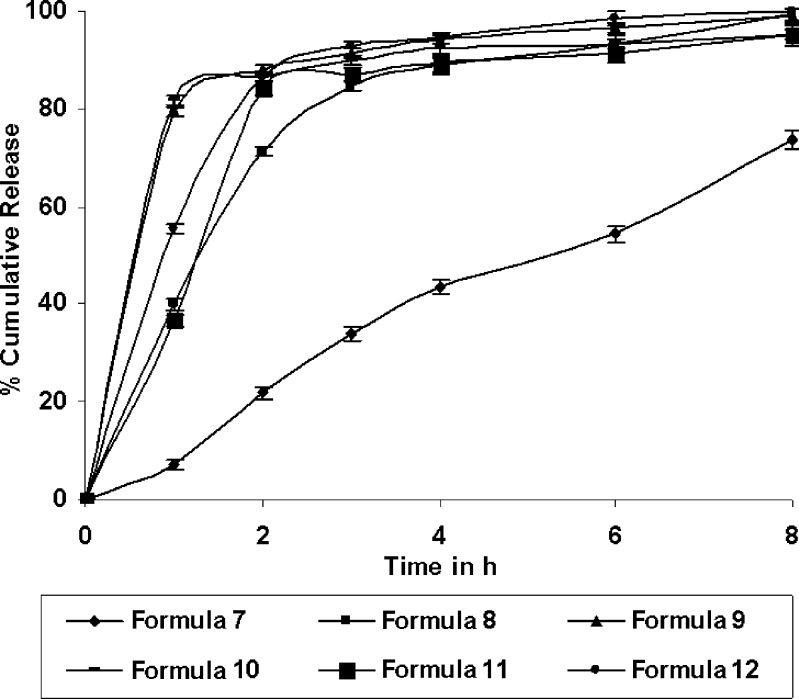
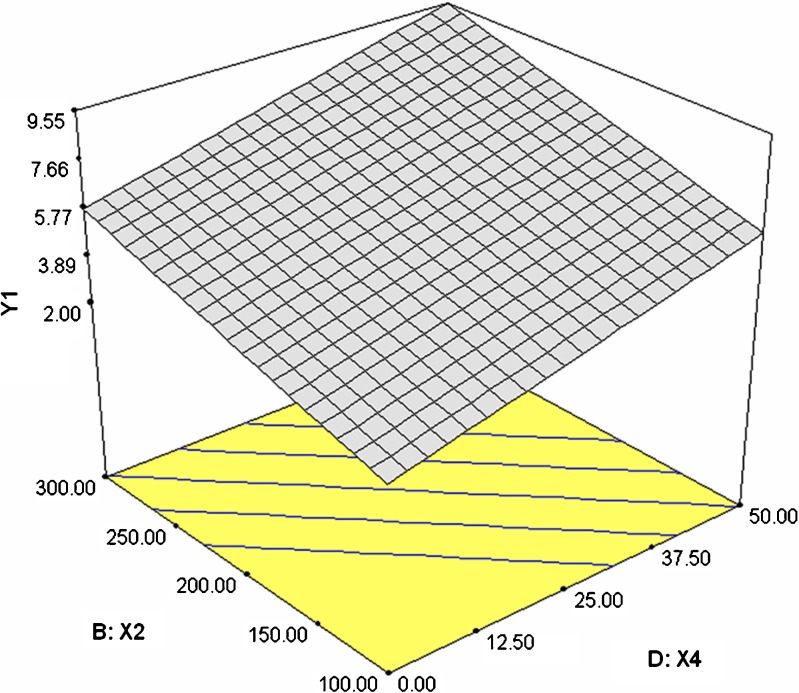
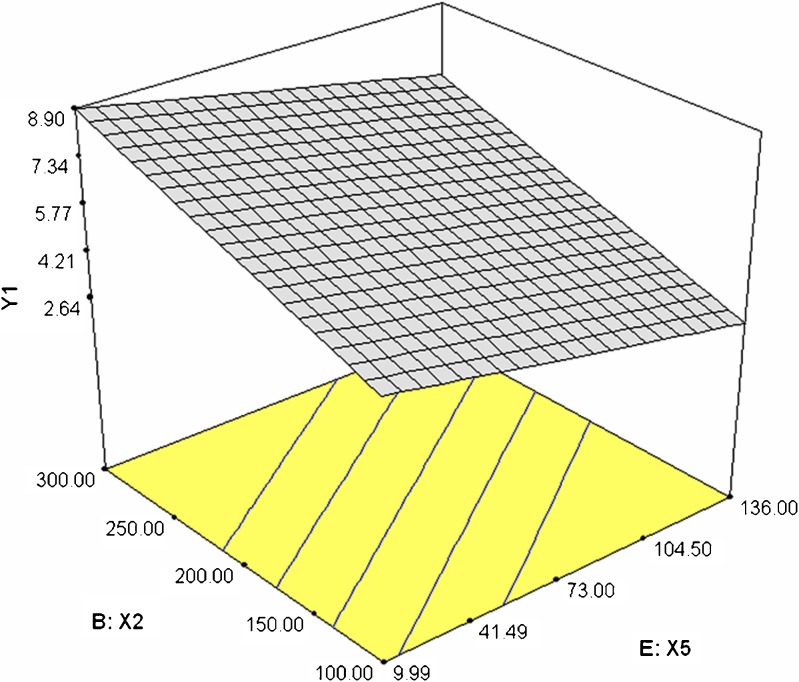
Dissolution profiles of the formulations 1–6 and 7–12 were as shown in the Figs. 1 and 2 respectively. Time required for 90% of drug released (T90) ranged between 2.75 h (formulation 12) and 14.18 h (formulation 7). Release exponent (n) values varied from 0.1 (formulation 1) and 0.94 (formulation 7). The interrelationships of the three major parameters poly (ethylene oxide) amount, ethylcellulose amount and solubility of drug) in presence of middle level of remaining parameters are illustrated in Figs. 3, 4, and 5.
Fig. 1.
Release profile of the formulation 1 to formulation 6
Fig. 2.
Release profile of the formulation 7 to formulation 12
Fig. 3.
3-D plot illustrating the effect of poly (ethylene oxide) amount (X 2) and ethylcellulose amount (X 4) on response Y 1
Fig. 4.
3-D plot illustrating the effect of poly (ethylene oxide) amount (X 2) and drug solubility (X 5) on response Y 1
Fig. 5.
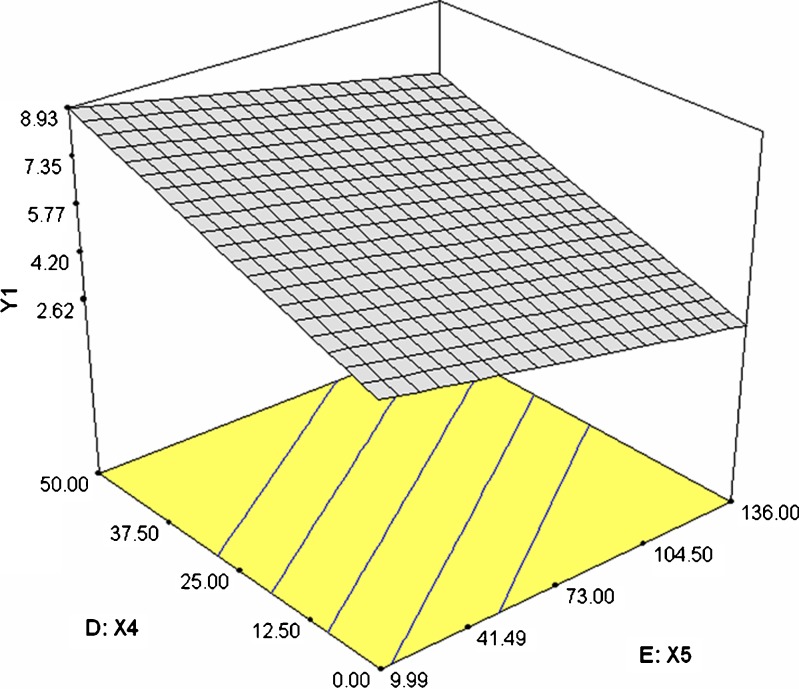
3-D plot illustrating the effect of ethylcellulose amount (X 4) and drug solubility (X 5) on response Y 1
In order to study the behavior of the individual parameters in the presence of other parameters, each parameter was treated alternatively at the highest and lowest level while keeping all other remaining parameters constant at their middle levels in equations given in Table IV. The effect of the individual independent variables in the presence of the middle level of the remaining factors is described in Table X and is further discussed in the following sections. Factors having positive coefficient (in equations of Table IV) for Y1 and negative coefficient for Y3 and Y4 will be the ones which will help in retarding the release of the drug and vice versa. Response Y2 is an indicator of release mechanism and was calculated using Korsmeyer’s equation. To confirm the diffusion mechanism, the data were fit to the Korsmeyer’s equation (37);
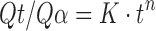 |
where Qt is the amount released at time t, Qα is overall released amount, K is a constant incorporating the properties of macromolecular polymeric system and the drug, and n is a kinetic constant that depends on the transport mechanism. The exponent n gives information about the release mechanism; whereby, a value of n < 0.5 illustrates Fickian diffusion, n = 0.5 characterizes diffusion-controlled release, 0.5 < n < 1.0 indicates anomalous (non-Fickian transport), and n = 1.0 indicates swelling controlled release (zero order kinetics). Drug diffusion and polymer erosion control the release process in equal parts, if n = 0.66 (38).
Table X.
Response Values at High and Low Level of Individual Factor at Middle Level of Remaining Factors
| Sr. No. | Factor | Code | Level | Response Value at middle level of other factors | |||
|---|---|---|---|---|---|---|---|
| Y 1 | Y 2 | Y 3 | Y 4 | ||||
| 1 | Poly (ethylene oxide) | X 1 | Low | 5.5 | 0.41 | 83.71 | 94.44 |
| molecular weight | X 1 | High | 6.05 | 0.39 | 81.84 | 92.44 | |
| 2 | Poly (ethylene oxide) | X 2 | Low | 3.9 | 0.22 | 91.9 | 97.31 |
| amount (mg) | X 2 | High | 7.65 | 0.58 | 73.65 | 89.57 | |
| 3 | Ethylcellulose | X 1 | Low | 3.87 | 0.25 | 91.65 | 97.71 |
| amount (mg) | X 1 | High | 7.68 | 0.56 | 73.9 | 89.16 | |
| 4 | Drug solubility | X 2 | Low | 7.03 | 0.50 | 78.5 | 90.02 |
| (mg/mL) | X 2 | High | 4.52 | 0.32 | 87.04 | 96.86 | |
| 5 | Drug amount (mg) | X 1 | Low | 5.53 | 0.45 | 83.3 | 94.21 |
| X 1 | High | 6.01 | 0.36 | 82.25 | 92.67 | ||
| 6 | Sodium chloride | X 2 | Low | 5.08 | 0.35 | 86.11 | 95.31 |
| amount (mg) | X 2 | High | 6.46 | 0.45 | 79.43 | 91.57 | |
| 7 | Citric acid amount | X 1 | Low | 6.45 | 0.46 | 79.52 | 91.69 |
| (mg) | X 1 | High | 5.1 | 0.34 | 86.02 | 95.18 | |
| 8 | Polyethylene glycol | X 2 | Low | 4.87 | 0.41 | 85.78 | 96.08 |
| amount (mg) | X 2 | High | 6.68 | 0.39 | 79.77 | 90.8 | |
| 9 | Glycerin amount | X 2 | Low | 6.7 | 0.46 | 78.07 | 91.35 |
| (mg) | X 2 | High | 4.85 | 0.34 | 87.48 | 95.52 | |
X 1–Poly (ethylene oxide) molecular weight
X 2–Poly (ethylene oxide) amount
Effect of Poly (ethylene oxide) Molecular Weight (X1)
As suggested by the polynomial equations in Table IV, molecular weight of poly (ethylene oxide) had a very insignificant effect on all the four responses. The effect of poly (ethylene oxide) molecular weight on Y1, at mid level of the remaining factors can be seen from Table X. Increasing poly (ethylene oxide) molecular weight from 6 × 105 to 7 × 106 resulted in increasing T90 value from 5.5 to 6.05 h indicating that poly (ethylene oxide) molecular weight had slight effect on retarding the release of drug. There was no change in the release mechanism with n value decreasing from 0.41 (Fickian diffusion) to 0.39 (Fickian diffusion) with increase in molecular weight.
Effect of Poly (ethylene oxide) Amount (X2)
Having the second highest coefficient of +1.88, poly (ethylene oxide) amount exhibited a very significant effect on all the four responses. About two times decrease in T90 value was observed when the poly (ethylene oxide) amount was increased from its lower value of 100 mg to a higher value of 300 mg. As the amount of poly (ethylene oxide) increased, the number of entangling polymer chains and consequent entrapment of the drug inside the polymer network increased, which cause a delay in drug release. Similar effects were also observed for response Y3 and Y4, where in the amount of drug released was decreased with increase in poly (ethylene oxide) amount. The amount of poly (ethylene oxide) also had a significant effect on the release exponent (n). Increasing the amount of poly (ethylene oxide) from 100 mg to 300 mg increased the n values from 0.22 to 0.58. Increasing the amount of poly (ethylene oxide) resulted in decreasing the fluid-filled channels through which the drug may diffuse and increasing the transpolymer diffusional pathway of matrix. Fluid-filled channels are characteristic of the Fickian square root of time release pattern whereas transpolymer diffusion is pertinent to anomalous drug release where n > 0.5 (39).
To study the effect of poly (ethylene oxide) amount in presence of other two significant factors ethylcellulose amount and drug solubility, a three-dimensional surface graph was constructed (Figs. 3 and 4). Increasing poly (ethylene oxide) and ethylcellulose amount increased response Y1. Thus, both these factors act together in harmony to retard the release of the drug (Fig. 3). However, drug solubility act in a reverse manner as that of poly (ethylene oxide) and ethylcellulose amount, thereby by enhancing the release of drug with increase in its solubility.
Effect of Ethylcellulose Amount (X4)
Ethylcellulose is an inert and hydrophobic polymer prepared by treating purified cellulose with an alkaline solution followed by ethylation of the alkali cellulose with chloroethane (40). It has been used as matrix-forming material for sustained release dosage forms (41, 42). The mechanism of drug release from the ethylcellulose matrix is simple diffusion for water-soluble drugs and diffusion followed by polymer relaxation for slightly soluble and practically insoluble drugs (43). Ethylcellulose, being a hydrophobic matrix, further retards release of the drugs from the matrix and was found to be the major controlling factor for the system. When concentration of ethylcellulose was increased from 0 to 50 mg; T90 values were increased from 3.87 to 7.68 h at medium levels of all the other variables.
It also affected mechanism of drug release. At medium level of all the other factors, as concentration of ethylcellulose was increased from 0 mg to 50 mg, n value increased from 0.25 to 0.56. Thus, with increase in ethylcellulose amount, the mechanism of drug release changed from Fickian diffusion to anomalous. These results are in agreement with the reported literature (43).
To study the effect of ethylcellulose amount in the presence of two other significant factors, poly (ethylene oxide) amount and drug solubility, a three-dimensional surface graph was constructed (Figs. 3 and 5). As discussed above, ethylcellulose amount and poly (ethylene oxide) amount together helped in reducing the release of the drug and thereby increasing the T90 value
Effect of Drug Solubility (X5)
Drug solubility had significant effect on the release of the drug from the matrix. However, unlike the above three parameters discussed, drug solubility with negative coefficient in the polynomial equation is inversely related to T90 value and helps in enhancing the release of the drug. At medium level of all the other factors, T90 value varied from 7.03 to 4.52 h with increase in drug solubility from 9.91 mg/mL to 136 mg/mL. This was in accordance with the literature (44,45). Drug solubility also contributed towards the release mechanism by decreasing the n value from 0.50 to 0.32 changing the mechanism from diffusion-controlled release to Fickian diffusion.
Effect of Drug Amount (X6)
The T90 value changes from 5.53 to 6.01 h when drug amount was changed from 100 mg to 200 mg. The data itself indicate the minimal effect of drug amount on the Y1. Similarly, it showed minimum effect on remaining three responses.
Effect of Sodium Chloride (X7)
Sodium chloride showed positive effect on the T90 values, thereby reducing the release rate. Its effect on the Y1 and Y2 was found to be insignificant. An increase in amount of sodium chloride creates more osmotic pressure difference and pulls more water into the dosage form. In the presence of more water, poly (ethylene oxide) forms a highly viscous gel and subsequently controls the drug release. The results are in agreement with those reported by Sastry et al. (46).
Effect of Citric Acid (X8)
Citric acid was added to the formulation so as to modulate the pH of the microenvironment, which would have an effect on the drug release. Data revealed that citric acid has insignificant effect on all responses. Apart from modulating micro environmental pH, increased drug release is due to its solubility in water and thereby its ability to form channels within the polymer matrix.
Effect of Polyethylene Glycol (X9)
Polyethylene glycol was incorporated in the formulation as plasticizer. Plasticizers are used to increase the flexibility of the polymer. At medium levels of the all the other variables, as the concentration of polyethylene glycol was increased from 0 to 5 mg, the T90 value changes from 4.87 to 6.68 h. Also, the n value was changed from 0.41 to 0.39 suggesting that there was no change in the release mechanism.
Effect of Glycerin (X11)
At medium levels of all the other variables, as the concentration of glycerin was increased from 0 to 5 mg, the T90 value changes from 6.70 to 4.85 h. This was in accordance to the literature (47). Also, the n value was changed from 0.46 to 0.34. From the data, it is clear that the effect of glycerin and polyethylene glycol was of similar magnitude but in opposite directions.
Validation of the Model
The formulations used for the development of the validation batches were as shown in the Table XI. The results of the developed formulations were as shown in Table XII. The difference between the predicted and experimental values was within the limits of ±1.0, as required by the model.
Table XI.
Composition of the Validation Batches
| Formula | Quantities in mg | |
|---|---|---|
| Ingredients | Formulation 1 | Formulation 2 |
| Poly (ethylene oxide) | ||
| WSR 303 (molecular | 292.21 | 298.86 |
| weight 7 × 106) | ||
| Ethylcellulose N–7 | 47.66 | 47.50 |
| Theophylline | 134.70 | 125.97 |
| Sodium chloride | 19.87 | 15.86 |
| Citric acid | 0.36 | 0.12 |
| Polyethylene Glycol | 4.08 | 4.94 |
| Glycerin | 0.28 | 0.81 |
| Total | 499.16 | 494.06 |
Table XII.
Predicted and Observed Value of the Validation Batches
| Response | Formulation 1 | Formulation 2 | ||
|---|---|---|---|---|
| Predicted | Experimental | Predicted | Experimental | |
| Y 1 | 13.33 | 13.74 | 13.34 | 14.26 |
| Y 2 | 0.93 | 0.97 | 0.94 | 0.92 |
| Y 3 | 47.06 | 47.59 | 47.08 | 46.52 |
| Y 4 | 74.95 | 74.83 | 74.98 | 74.59 |
CONCLUSION
Within the limits commonly used to prepare controlled release matrices by melt extrusion technology, some formulation variables are expected to have significant effect on the amount and pattern of drug release. Poly (ethylene oxide) amount, ethylcellulose amount and drug solubility had significant effect on the T90 values whereas poly (ethylene oxide) amount and ethylcellulose amount had a significant effect on the mechanism of release. From this study it can be concluded that sustained release matrices can be prepared easily by combining ethylcellulose and poly (ethylene oxide). Ethylcellulose plays a major role in controlling the drug release for longer duration. Poly (ethylene oxide) content is expected to have an impact on the pattern by which the drug is released from the matrix. Poly (ethylene oxide) amount, drug loading, electrolytes, buffers and plasticizers on the other hand are expected to have marginal effect on both the responses, assuming they were within the limits used in this study.
References
- 1.Mcginity JW, Koleng JJ, Repta MA, Zhang, F. Melt extrusion. In: Swarbrick J, Boylan C, editors. Encyclopedia of Pharmaceutical Technology. Marcel Dekker Inc., New York; 2000;19:203–226.
- 2.Breitenbach J. Melt extrusion: from process to drug delivery technology. Eur J Pharm Biopharm. 2002;54:107–117. doi: 10.1016/S0939-6411(02)00061-9. [DOI] [PubMed] [Google Scholar]
- 3.Chokshi R, Hossein Z. Hot-melt extrusion technique: a review. Iranian J Pharm Res. 2004;3:3–16. [Google Scholar]
- 4.Dangtungee R, Desai SS, Tantayanon S, Supaphol P. Melt rheology and extrudate swell of low-density polyethylene/ethylene–octene copolymer blends. Polym Test. 2006;25:888–895. doi: 10.1016/j.polymertesting.2006.05.007. [DOI] [Google Scholar]
- 5.Bengoechea C, Arrachid A, Guerrero A, Hill SE, Mitchell JR. Relationship between the glass transition temperature and the melt flow behavior for gluten, casein and soya. J Cereal Sci. 2007;45:275–284. doi: 10.1016/j.jcs.2006.08.011. [DOI] [Google Scholar]
- 6.Babin P, Valle GD, Dendievel R, Lourdin D, Salvo L. X-ray tomography study of the cellular structure of extruded starches and its relations with expansion phenomenon and foam mechanical properties. Carbohydr Polym. 2007;68:329–340. doi: 10.1016/j.carbpol.2006.12.005. [DOI] [Google Scholar]
- 7.Chen L, Pang XJ, Yu ZL. Study on polycarbonate/multi-walled carbon nanotubes composite produced by melt processing. Material Sci Eng: A. 2007;457:287–291. doi: 10.1016/j.msea.2007.01.107. [DOI] [Google Scholar]
- 8.Griffon JM. A new elastomer for construction and building. Constr Build Mater. 1988;2:73–84. doi: 10.1016/0950-0618(88)90019-0. [DOI] [Google Scholar]
- 9.Tang Y, Tan D, Li W, Pan Z, Liu L, Hu W. Preparation of Al–Fe–V–Si alloy by spray co-deposition with added its over-sprayed powders. J Alloys Compd. 2007;439:103–108. doi: 10.1016/j.jallcom.2006.08.233. [DOI] [Google Scholar]
- 10.Mehuys E, Vervaet C, Remon JP. Hot-melt extruded ethylcellulose cylinders containing a HPMC–Gelucire® core for sustained drug delivery. J Control Release. 2004;94:273–280. doi: 10.1016/j.jconrel.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 11.Siepmann F, Muschert S, Flament MP, Leterme P, Gayot A, Siepmann J. Controlled drug release from Gelucire-based matrix pellets: experiment and theory. Int J Pharm. 2006;317:136–143. doi: 10.1016/j.ijpharm.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 12.Ghebremeskel AN, Vemavarapu C, Lodaya M. Use of surfactants as plasticizers in preparing solid dispersions of poorly soluble API: selection of polymer–surfactant combinations using solubility parameters and testing the processability. Int J Pharm. 2007;328:119–129. doi: 10.1016/j.ijpharm.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 13.Patterson JE, James MB, Forster AH, Lancaster RW, Butler JM, Rades T. Preparation of glass solutions of three poorly water soluble drugs by spray drying, melt extrusion and ball milling. Int J Pharm. 2007;336:22–34. doi: 10.1016/j.ijpharm.2006.11.030. [DOI] [PubMed] [Google Scholar]
- 14.Verreck G, Decorte A, Heymans K, Adriaensen J, Liu D, Tomasko D. Hot stage extrusion of p-amino salicylic acid with EC using CO2 as a temporary plasticizer. Int J Pharm. 2006;327:45–50. doi: 10.1016/j.ijpharm.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 15.Mididoddi PK, Repka MA. Characterization of hot-melt extruded drug delivery systems for onychomycosis. Eur J Pharm Biopharm. 2007;66:95–105. doi: 10.1016/j.ejpb.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 16.Sauer D, Zheng W, Coots LB, McGinity JW. Influence of processing parameters and formulation factors on the drug release from tablets powder-coated with Eudragit® L 100-55. Eur J Pharm Biopharm. 2007;67:464–475. doi: 10.1016/j.ejpb.2007.02.021. [DOI] [PubMed] [Google Scholar]
- 17.Fukuda M, Peppas NA, McGinity JW. Floating hot-melt extruded tablets for gastroretentive controlled drug release system. J Control Release. 2006;115:121–129. doi: 10.1016/j.jconrel.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 18.Fukuda M, Peppas NA, McGinity JW. Properties of sustained release hot-melt extruded tablets containing chitosan and xanthan gum. Int J Pharm. 2006;310:90–100. doi: 10.1016/j.ijpharm.2005.11.042. [DOI] [PubMed] [Google Scholar]
- 19.Verhoeven E, Vervaet C, Remon JP. Xanthan gum to tailor drug release of sustained-release ethylcellulose mini-matrices prepared via hot-melt extrusion: in vitro and in vivo evaluation. Eur J Pharm Biopharm. 2006;63:320–330. doi: 10.1016/j.ejpb.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 20.Lyons JG, Hallinan M, Kennedy JE, Devine DM, Geever LM, Blackie P, et al. Preparation of monolithic matrices for oral drug delivery using a supercritical fluid assisted hot melt extrusion process. Int J Pharm. 2007;329:62–71. doi: 10.1016/j.ijpharm.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 21.Jain SP, Shah SP, Rajadhyaksha NS, Singh PP. Twice a day ocular inserts of acyclovir by melt extrusion technique. Indian J Pharm Sci. 2007;69:562–567. doi: 10.4103/0250-474X.36945. [DOI] [Google Scholar]
- 22.Crowley MM, Zhang F, Repka MA, Thumma S, Upadhye SB, Battu SK, et al. Pharmaceutical applications of hot-melt extrusion: part I. Drug Dev Ind Pharm. 2007;33:909–926. doi: 10.1080/03639040701498759. [DOI] [PubMed] [Google Scholar]
- 23.Sprockel OL, Sen M, Shivanand P, Prapaitrakul W. A melt-extrusion process for manufacturing matrix drug delivery systems. Int J Pharm. 1997;26:191–199. doi: 10.1016/S0378-5173(97)00165-8. [DOI] [Google Scholar]
- 24.Ganesh S, Radhakrishnan M, Ravi M, Prasannakumar B, Kalyani J. In vitro evaluation of the effect of combination of hydrophilic and hydrophobic polymers on controlled release zidovudine matrix tablets. Indian J Pharm Sci. 2008;70:461–465. doi: 10.4103/0250-474X.44594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schilling SU, Bruce CD, Shah NH, Malick AW, McGinity JW. Citric acid monohydrate as a release-modifying agent in melt extruded matrix tablets. Int J Pharm. 2008;361:158–168. doi: 10.1016/j.ijpharm.2008.05.035. [DOI] [PubMed] [Google Scholar]
- 26.Chamarthy SP, Pinal R. Plasticizer concentration and the performance of a diffusion-controlled polymeric drug delivery system. Colloids Surf, A Physicochem Eng Asp. 2008;331:25–30. doi: 10.1016/j.colsurfa.2008.05.047. [DOI] [Google Scholar]
- 27.Siepmanna F, Bruna VL, Siepmanna J. Drugs acting as plasticizers in polymeric systems: a quantitative treatment. J Control Release. 2006;27:298–306. doi: 10.1016/j.jconrel.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 28.Hamed E, Gerson MC, Millard RW, Sakr A. A study of the pharmacodynamic differences between immediate and extended release bumetanide formulations. Int J Pharm. 2003;267:129–140. doi: 10.1016/j.ijpharm.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 29.Zaghloul AA, Faltinek J, Vaithiyalingam SR, Reddy IK, Khan MA. Naproxen-Eudragit microspheres: screening of process and formulation variables of extended release tablets. Pharmazie. 2001;56:321–324. [PubMed] [Google Scholar]
- 30.Verma M, Singla AK, Dhawan S. Release of diltiazem hydrochloride from hydrophilic matrices of poly (ethylene oxide) and carbopol. Drug Dev Ind Pharm. 2004;30:545–553. doi: 10.1081/DDC-120037485. [DOI] [PubMed] [Google Scholar]
- 31.El-Malah Y, Nazzal S. Hydrophilic matrices: application of Plackett–Burman screening design to model the effect of POLYOX–carbopol blends on drug release. Int J Pharm. 2006;309:163–170. doi: 10.1016/j.ijpharm.2005.11.032. [DOI] [PubMed] [Google Scholar]
- 32.Plackett RL, Burmann JP. The design of optimum multifactorial experiments. Biometrika. 1946;33:305–325. doi: 10.1093/biomet/33.4.305. [DOI] [Google Scholar]
- 33.Haaland PD. Experimental design in biotechnology. New York: Marcel Dekker; 1989. [Google Scholar]
- 34.Stanbury PA, Whitaker SJ. Hall media for industrial fermentation. In: Stanbury PA, Whitaker SJ, editors. Principles of fermentation technology. Oxford: Butterworth Heinemann; 1995. pp. 110–122. [Google Scholar]
- 35.Murray J. X-Stat, Version 2.02: statistical experimental design, data analysis and nonlinear optimization. John Wiley and Sons, NY. 1994.
- 36.Faller B, Ertla P. Computational approaches to determine drug solubility. Adv Drug Del Rev. 2007;59:533–545. doi: 10.1016/j.addr.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 37.Korsmeyer M, Gurny R, Doelker E, Buri P, Peppas N. Mechanisms of solute release from porous hydrophilic polymers. Int J Pharm. 1983;15:25–35. doi: 10.1016/0378-5173(83)90064-9. [DOI] [PubMed] [Google Scholar]
- 38.Mockel J, Lippold B. Zero order drug release from hydrocolloid matrices. Pharm Res. 1993;10:1066–1070. doi: 10.1023/A:1018931210396. [DOI] [PubMed] [Google Scholar]
- 39.Siepmann J, Streubel A, Peppas NA. Understanding and prediction of drug delivery from hydrophilic matrix tablets using the sequential layer model. Pharm Res. 2002;19:306–313. doi: 10.1023/A:1014447102710. [DOI] [PubMed] [Google Scholar]
- 40.Ethylcellulose Premium Polymers, Technical bulletin, Colorcon Asia Pvt Ltd, 2007.
- 41.Agrawal AM, Neau SH, Bonate PL. Wet granulation fine particle ethylcellulose tablets: effect of production variables and mathematical modelling of drug release. AAPS PharmaSci. 2003;5: article 13. [DOI] [PMC free article] [PubMed]
- 42.Tiwari SB, Murthy K, Pai MR, Mehta PR, Chowdhary PB. Controlled release formulation of tramadol hydrochloride using hydrophilic and hydrophobic matrix systems. AAPS PharmaSciTech. 2003;4:article 31. [DOI] [PMC free article] [PubMed]
- 43.Neau SH, Howard MA, Claudius JS, Howard DS. The effect of aqueous solubility of xanthine derivatives on the release mechanism from ethylcellulose matrix tablets. Int J Pharm. 1999;179:97–105. doi: 10.1016/S0378-5173(98)00391-3. [DOI] [PubMed] [Google Scholar]
- 44.Kumar K, Shah MH, Ketkar A, Mahadik KR, Paradkar A. Effect of drug solubility and different excipients on floating behaviour and release from glyceryl monooleate matrices. Int J Pharm. 2004;272:151–160. doi: 10.1016/j.ijpharm.2003.12.025. [DOI] [PubMed] [Google Scholar]
- 45.Sriamornsak P, Kennedy RA. Effect of drug solubility on release behaviour of calcium polysaccharide gel coated pellets. Eur J Pharm Sci. 2007;32:231–239. doi: 10.1016/j.ejps.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 46.Sastry SV, DeGennaro MD, Reddy IK, Khan MA. Atenolol gastrointestinal therapeutic system: I. Screening of formulation variables. Drug Dev Ind Pharm. 1997;23:157–165. doi: 10.3109/03639049709149789. [DOI] [Google Scholar]
- 47.He W, Du Y, Fan L. Study on volume ratio and plasticiser screening of free coating membranes composed of ethylcellulose and chitosan. J Appl Polym Sci. 2006;100:1932–1939. doi: 10.1002/app.22949. [DOI] [Google Scholar]