Abstract
Novel dietary agents for prevention and therapy of prostate cancer (PCa) are desired. The aim of this study was to determine the effect of fisetin, a tetrahydroxyflavone, on inhibition of cell growth and induction of apoptosis in human PCa cells. Treatment of fisetin (10–60 μM, 48 h) was found to result in a decrease in the viability of LNCaP, CWR22Rυ1 and PC-3 cells but had only minimal effects on normal prostate epithelial cells as assessed by 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazoliumbromide assay. Treatment of LNCaP cells with fisetin also resulted in G1-phase arrest that was associated with a marked decrease in the protein expression of cyclins D1, D2 and E and their activating partner cyclin-dependent kinases 2, 4 and 6 with concomitant induction of WAF1/p21 and KIP1/p27. Fisetin treatment also resulted in induction of apoptosis, poly (ADP-ribose) polymerase (PARP) cleavage, modulation in the expressions of Bcl-2 family proteins, inhibition of phosphatidyl inositol 3-kinase and phosphorylation of Akt at Ser473 and Thr308. There was also induction of mitochondrial release of cytochrome c into cytosol, downregulation of X-linked inhibitor of apoptosis protein and upregulation of second mitochondria-derived activator of caspase/direct inhibitor of apoptosis-binding protein with low pI on treatment of cells with fisetin. Treatment of cells with fisetin also resulted in significant activation of caspases-3, -8 and -9. Pretreatment of cells with caspase inhibitor (Z-VAD-FMK) blocked fisetin-induced activation of caspases. These data provide the first evidence that fisetin could be developed as an agent against PCa.
Introduction
Prostate cancer (PCa) is the most common cancer amongst men in the USA and the second most common malignant cause of male death worldwide after lung cancer (1). The substantial mortality and morbidity associated with PCa and its poor treatment options have led a surge to develop novel means for its prevention. Diet-based agent for prevention and therapy is an attractive option in PCa because of its incidence, prevalence, disease-related mortality, long latency between premalignant lesions and clinically evident cancer and molecular pathogenesis, the known hormonal influence on the manifestations of the disease and epidemiologic data indicating that modifiable environmental factors may decrease risk (2,3).
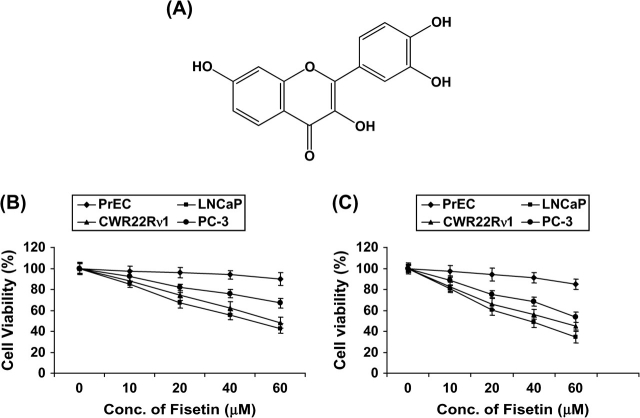
Fisetin (3,3′,4′,7-tetrahydroxyflavone, Figure 1A) is found in fruits and vegetables, such as strawberry, apple, persimmon, grape, onion and cucumber (4). Fisetin has been reported to inhibit cell cycle progression in HT-29 human colon cancer cells (5). It has been shown to have antiproliferative effect on the human PCa and breast cancer cell lines (6). Fisetin showed dose-dependent cytotoxic effects on SK-HEP-1 cells, accompanied by DNA fragmentation, induction of CPP32 activity and increase of p53 protein (7). Fisetin caused a decrease in intracellular peroxide level, activation of endonuclease and suppression of Mcl-1 proteins in the human leukemia cell line, HL-60 (8). Fisetin activated extracellular signal-regulated kinase and induced cyclic adenosine 3′,5′-monophosphate response element-binding protein phosphorylation in rat hippocampal slices and enhanced object recognition in mice (9). Fisetin was found to suppress tumor necrosis factor, various inflammatory agents and carcinogen-induced nuclear factor-κB (NF-κB) activation, blocked the phosphorylation and degradation of IκBα by inhibiting IKKα activation and suppression of the phosphorylation and nuclear translocation of p65. Fisetin also suppressed NF-κB-dependent reporter gene expression and NF-κB reporter activity induced by TNFR1, TRADD, TRAF2, NIK and IKK (10). Recently, fisetin along with quercetin, myricetin, quercetagetin, (−)-epigallocatechin gallate and theaflavins markedly inhibited epidermal growth factor-induced cell transformation of mouse epidermal JB6 Cl 41 cells (11). Fisetin was found to induce phase II enzymes such as reduced nicotinamide adenine dinucleotide phosphate:quinone oxidoreductase activity in murine hepatoma 1c1c7 cells. Furthermore, transfection studies using a human quinone oxidoreductase antioxidant/electrophile response element reporter construct demonstrated that fisetin activated the antioxidant response element/electrophile response element (12). Fisetin treatment of HL60 cells caused high expression of NF-κB, activation of mitogen-activated protein kinase p38, an increase of phosphoprotein levels and inhibition of enzymes involved in redox status maintenance (13).
Fig. 1.
(A) Structure of fisetin. (B and C) Effect of fisetin on cell growth. As detailed in Materials and methods, LNCaP, CWR22Rυ1, PC-3 and PrEC cells were treated with fisetin (10–60 μM) for 24 and 48 h and the viability of cells was determined by the 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazoliumbromide assay. The data are expressed as the percentage of cell viability and represent the means ± standard errors of three experiments in which each treatment was performed in multiple wells.
We hypothesized that fisetin may afford chemopreventive as well as cancer chemotherapeutic effects against PCa. Here, we show that fisetin, through modulations in cki–cyclin–cyclin-dependent kinase (cdk) network, inhibition of phosphatidyl inositol 3-kinase (PI3K) and Akt, results in inhibition of cell growth followed by apoptosis of human PCa LNCaP cells.
Materials and methods
Materials
Anti-cyclins D1, D2, E, Bad and Bcl-xL, active caspases-3, -8 and -9, Akt, phospho-Akt (Ser473 and Thr308), PI3K (p85), X-linked inhibitor of apoptosis (XIAP) and second mitochondria-derived activator of caspase (Smac)/direct inhibitor of apoptosis-binding protein with low pI (DIABLO) antibodies were obtained from Cell Signaling Technology (Beverly, MA). The mono and polyclonal antibodies cdks 2, 4 and 6, WAF1/p21, KIP1/p27 and Bcl-2 were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). PARP (116) and Bak were procured from Upstate (Lake Placid, NY). PARP (85) was obtained from Promega (Madison, WI). Anti-mouse and anti-rabbit secondary antibody horseradish peroxidase conjugate was obtained from Amersham Life Science (Arlington Height, IL). Akt-small interfering RNA (siRNA) and scrambled siRNA were purchased from Dharmacon (Lafayette, CO). Fisetin was purchased from Sigma Chemical Co. (St Louis, MO). BCA Protein Assay Kit was obtained from Pierce (Rockford, IL). Novex precast Tris–glycine gels were obtained from Invitrogen (Carlsbad, CA). The Apo-Direct Kit for flow cytometry was purchased from Phoenix Flow Systems (San Diego, CA). Magic Red™ Caspase Detection Kit was purchased from Immunochemistry Technologies, LLC (Bloomington, MN). Annexin-V-Fluos Staining Kit and Cell Death Detection ELISAPLUS Kit were from Roche Diagnostics Corporation (Indianapolis, IN). ApoAlert Cell Fractionation Kit was purchased from BD Biosciences Clontech (Palo Alto, CA).
Cell culture and treatment
The LNCaP, CWR22Rυ1 and PC-3 cells were obtained from ATCC (Manassas, VA). LNCaP cells were cultured in Dulbecco's modified Eagle's medium supplemented with 5% heat-inactivated fetal bovine serum and 1% penicillin/streptomycin. CWR22Rυ1 and PC-3 cells were grown in RPMI 1640 (Invitrogen) supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin. Human prostate epithelial cells (PrECs) were obtained from Cambrex Bioscience (Walkersville, MD) and grown in prostate epithelial basal cell medium (Cambrex Bioscience) according to the manufacturer's instructions. The cells were maintained under standard cell culture conditions at 37°C and 5% CO2 in a humid environment. Fisetin dissolved in dimethyl sulfoxide (final concentration 0.1% vol/vol) was used for the treatment of cells. The cells (60–70% confluent) were treated with fisetin (10–60 μM) for 24 and 48 h in complete growth medium.
Cell viability
The effect of fisetin on the viability of cells was determined by 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazoliumbromide assay. LNCaP, CWR22Rυ1, PC-3 and PrEC cells were plated at 1 × 104 cells per well in 200 μl of complete culture medium containing 10–60 μM concentrations of fisetin in 96-well microtiter plates for 24 and 48 h. After incubation for specified times at 37°C in a humidified incubator, 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazoliumbromide [5 mg/ml in phosphate-buffered saline (PBS)] was added to each well and incubated for 2 h, after which the plate was centrifuged at 1800g for 5 min at 4°C. The absorbance was recorded on a microplate reader at the wavelength of 540 nm. The effect of fisetin on growth inhibition was assessed as percentage of cell viability where dimethyl sulfoxide-treated cells were taken as 100% viable. Dimethyl sulfoxide at the concentrations used was without any effect on cell viability.
DNA cell cycle analysis
The LNCaP cells (50–60% confluent) were treated with fisetin (10–60 μM, 48 h) in complete medium. The cells were trypsinized thereafter, washed twice with cold PBS and centrifuged. The cell pellet was resuspended in 50 μl cold PBS to which cold ethanol (450 μl) was added and the cells were incubated for 1 h at 4°C. The cells were centrifuged at 1100 r.p.m. for 5 min, pellet washed twice with cold PBS, suspended in 500 μl PBS and incubated with 5 μl RNase (20 μg/ml final concentration) for 30 min. The cells were chilled over ice for 10 min and incubated with propidium iodide (50 μg/ml final concentration) for 1 h for analysis by flow cytometry. Flow cytometry was performed with a FACScan (Becton Dickinson, Franklin Lakes, NJ). A minimum of 10 000 cells per sample were collected and the DNA histograms were further analyzed by using ModiFitLT software (Verily Software House, Topsham, ME) for cell cycle analysis.
Protein extraction and western blotting
Following the treatment of LNCaP cells with fisetin (10–60 μM, 48 h), the media was aspirated, the cells were washed with cold PBS (pH 7.4) and ice-cold lysis buffer (50 mM Tris–HCl, 150 mM NaCl, 1 mM ethyleneglycol-bis(aminoethylether)-tetraacetic acid, 1 mM ethylenediaminetetraacetic acid, 20 mM NaF, 100 mM Na3VO4, 0.5% NP-40, 1% Triton X-100, 1 mM phenylmethylsulfonyl fluoride, pH 7.4) with freshly added protease inhibitor cocktail (Protease Inhibitor Cocktail Set III, Calbiochem, La Jolla, CA) over ice for 30 min. The cells were scraped and the lysate was collected in a microfuge tube and passed through needle to break up the cell aggregates. The lysate was cleared by centrifugation at 14 000g for 15 min at 4°C and the supernatant (whole-cell lysate) was used or immediately stored at −80°C.
For western blotting, 30–50 μg protein was resolved over 8–12% polyacrylamide gels and transferred to a nitrocellulose membrane. The blot was blocked in blocking buffer (5% non-fat dry milk/1% Tween 20; in 20 mM Tris-buffered saline, pH 7.6) for 1 h at room temperature and incubated with appropriate monoclonal or polyclonal primary antibody in blocking buffer for 1.5 h to overnight at 4°C followed by incubation with anti-mouse or anti-rabbit secondary antibody horseradish peroxidase conjugate obtained from Amersham Life Science and detected by chemiluminescence and autoradiography using XAR-5 film obtained from Eastman Kodak Co. (Rochester, NY). Densitometric measurements of the band in western blot analysis were performed using digitalized scientific software program UN-SCAN-IT (Silk Scientific Corporation, Orem, UT).
Apoptosis assessment by annexin-V staining
The annexin-V-Fluos Staining Kit was used for the detection of apoptotic cells according to vendor's protocol. This kit uses a staining protocol in which the apoptotic cells are stained with annexin-V (green fluorescence). The LNCaP cells were grown to 70% confluency and treated with fisetin (10–60 μM) for 48 h. The fluorescence was measured by a Zeiss 410 confocal microscope (Thornwood, NY). Confocal images of green annexin–fluorescein isothiocyanate fluorescence were collected using 488 nm excitation light from an argon/krypton laser, a 560 nm dichroic mirror and a 514–540 nm band-pass barrier filter.
Apoptosis by enzyme-linked immunosorbent assay
Following treatment of LNCaP cells with fisetin (10–60 μM, 48 h), the extent of apoptosis was determined by Cell Death Detection ELISAPLUS assay, according to the manufacturer's protocol. The whole-cell lysate (40 μg of total protein) was added to the lysis buffer and pipetted on a streptavidin-coated 96-well microtiter plate to which the immunoreagent mix was added and incubated for 2 h at room temperature, with continuous shaking at 500 r.p.m. The wells were then washed with washing buffer, the substrate solution added and the color developed (10–20 min) was read at 405 nm against the blank, reference wavelength of 490 nm. The enrichment factor (total amount of apoptosis) was calculated by dividing the absorbance of the sample (A405 nm) by the absorbance of the controls without treatment (A490 nm).
Immunofluorescence staining for active caspases-3 and -7
LNCaP cells were incubated with 40 μM concentration of the general caspase inhibitor Z-VAD-FMK for 2 h followed by the addition of fisetin (40 μM, 48 h) and then harvested. ICT's Magic Red™ substrate-based MR-Caspase assay kit was used for the immunofluorescence staining for caspases-3 and 7. This kit utilizes the fluorophore, cresyl violet. As the four-amino acid sequence, aspartylglutamylalanylaspartic acid (DEVD), is the optimal sequence for caspase-3 as well as -7, it was coupled to cresyl violet to create the caspase-3/7 substrate, MR-(DEVD)2. When bi-substituted via amide linkage to two target caspase sequence groups [(DEVD)2], cresyl violet does not fluoresce. Following enzymatic hydrolysis at one or both of the aspartic acid amide linkage sites, the mono and non-substituted cresyl violet fluorophores fluoresce red and fluorescence photographs were obtained at 510–560 nm excitation and 610 nm emission.
Detection of cytochrome c release
After the treatment of LNCaP cells with fisetin (10–60 μM, 48 h), the mitochondrial and cytosolic fractions were prepared according to manufacturer's instructions. After the separation of cytosolic and mitochondrial fractions, 40 μg protein was resolved over 12% polyacrylamide gels and immunoblotted with the cytochrome c and cytochrome c oxidase-4 antibodies.
Silencing of Akt by siRNA
LNCaP cells were transfected with Akt-siRNA (75 nmol) and scrambled siRNA (75 nmol) procured from Dharmacon using the Nucleofection Kit R specific for LNCaP transfection from Amaxa Biosystems (Gaithersburg, MD). Cells were resuspended in a solution from Nucleofector Kit R following the manufacturer's guidelines. One hundred microliters of nucleofector solution R was mixed with 2 × 106 cells and siRNA. They were then transferred to the cuvette provided with the kit and were nucleofected with an Amaxa Nucleofector apparatus. Cells were transfected using the T-001 pulsing parameter and were transferred into 100 mm plates containing 37°C prewarmed culture medium. After transfection, cells were cultured and the medium was replaced with fresh medium. Cells were treated with 40 μM fisetin for 48 h, and protein lysates were prepared. Using 2 μg of green fluorescent protein, we observed 70–80% transfection efficiency with this protocol.
Statistical analysis
Results were analyzed using a two-tailed Student's t-test to assess statistical significance and P values <0.05 were considered significant.
Results
Inhibition of cell growth by fisetin in LNCaP cells but not in PrECs
We first investigated the dose- and time-dependent effect of treatment with fisetin (10–60 μM) on the growth of human PCa (LNCaP, CWR22Rυ1 and PC-3) cells and normal PrECs. We evaluated the effect of fisetin on the growth of these cells by 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazoliumbromide assay. We compared the antiproliferative effects of fisetin on LNCaP, CWR22Rυ1, PC-3 and PrEC cells. Treatment with fisetin (10–60 μM) for 24 h decreased cell viability in LNCaP (15, 33, 45 and 57%), CWR22Rυ1 (12, 26, 38 and 52%) and PC-3 (7, 18, 24 and 33%) cells but had minimal effect on PrEC cells at these doses (Figure 1B). At 48 h, there was much pronounced decrease in cell viability on treatment with fisetin (10–60 μM) in LNCaP (19, 40, 49 and 62%), CWR22Rυ1 (18, 34, 44 and 55%) and PC-3 (11, 25, 32 and 46%) cells but had minimal effect on PrECs (Figure 1C). Based on the results of these data, we selected LNCaP cells for our study since fisetin treatment caused maximum decrease in cell viability in LNCaP cells as compared with CWR22Rυ1 and PC-3 cells.
G1-phase cell cycle arrest by fisetin in LNCaP cells
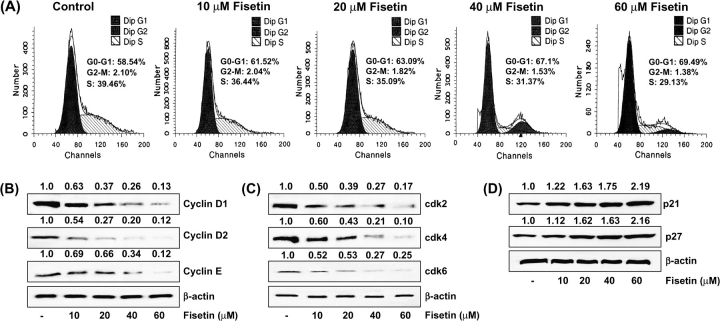
To assess whether fisetin-induced growth inhibition of the cells is mediated via alterations in cell cycle, we evaluated the effect of fisetin on cell cycle distribution. We performed DNA cell cycle analysis using LNCaP cells, and fisetin treatment was found to result in a significant dose-dependent increase of cell population in the G1 phase of the cell cycle. The G1-phase cell cycle distribution was 61, 63, 67 and 69% at 10, 20, 40 and 60 μM concentrations of fisetin, respectively (Figure 2A). This increase in the G0/G1-phase cell population was accompanied by a concomitant decrease in the S-phase and G2/M-phase cell populations.
Fig. 2.
(A) Effect of fisetin on cell cycle distribution in LNCaP cells. The cells treated with fisetin (10–60 μM, 48 h) were collected and stained with propidium iodide by using an apoptosis Apo-Direct Kit obtained from Phoenix Flow Systems as per vendor's protocol followed by flow cytometry. Following fluorescence-activated cell sorter analysis, cellular DNA histograms were further analyzed by ModiFitLT V3.0. The data are representative examples for duplicate tests. The details are described in Materials and methods. (B) Effect of fisetin on protein expression of cyclin D1, cyclin D2 and cyclin E in LNCaP cells. (C) Effect of fisetin on protein expression of cdk 2, cdk 4 and cdk 6 in LNCaP cells. (D) Effect of fisetin on protein expression of WAF1/p21 and KIP1/p27 in LNCaP cells. As detailed in Materials and methods, the cells were treated with fisetin (10–60 μM, 48 h) and then harvested. Total cell lysates were prepared and 40 μg protein was subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis followed by immunoblot analysis and chemiluminescence detection. Equal loading of protein was confirmed by stripping the immunoblot and reprobing it for β-actin. The immunoblots shown here are representative of three independent experiments with similar results. The values above the figures represent relative density of the bands normalized to β-actin.
Inhibition of cyclins, cdks and induction of WAF1/p21 and KIP1/p27 by fisetin in LNCaP cells
We determined the protein expression of the cyclins and cdks, which are known to be operative in G1 phase of the cell cycle. Fisetin treatment of LNCaP cells caused a dose-dependent decrease in the protein expression of cyclins D1, D2 and E (Figure 2B). The decrease in cyclin D2 protein expression was more pronounced than that of cyclin D1 and cyclin E. Using immunoblot analysis, we also found that treatment of LNCaP cells with fisetin resulted in a dose-dependent decrease in cdks 2, 4 and 6 (Figure 2C). We next assessed the effect of fisetin treatment on the induction of WAF1/p21, which is known to regulate the entry of cells at G1 to S transition checkpoint. Immunoblot analysis and relative density of the bands revealed that treatment with fisetin resulted in induction of WAF1/p21 and KIP1/p27 even at the lowest concentration of 10 μM with a significant increase at the highest concentration of 60 μM (Figure 2D).
Induction of apoptosis by fisetin in LNCaP cells
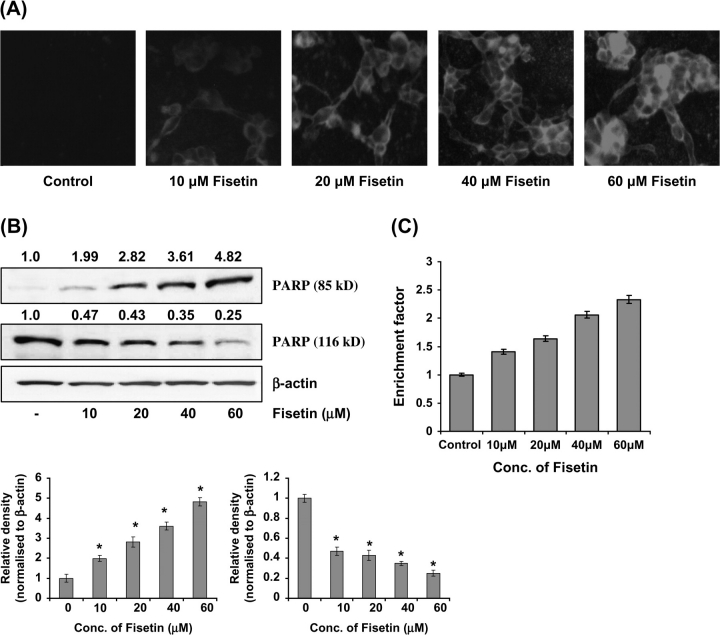
Annexin-V staining was performed to determine apoptotic cells following fisetin treatment to LNCaP cells. Annexin-V specially binds to phosphatidylserine and has been employed for determination of apoptotic cells. When LNCaP cells were stained with annexin-V and examined under a fluorescence microscope, apoptotic cells were found to be increased in fisetin (10–60 μM, 48 h)-treated cells (Figure 3A). Additionally, PARP cleavage analysis showed that the full-size PARP (116 kD) protein was cleaved to yield an 85 kD fragment after treatment of cells with fisetin (Figure 3B) as shown by immunoblot analysis and relative density of the bands. We also performed enzyme-linked immunosorbent assay to evaluate the induction of apoptosis by fisetin. Treatment with fisetin resulted in significant apoptosis in LNCaP cells (40, 64, 103 and 106% at 10, 20, 40 and 60 μM concentrations of fisetin, respectively, 48 h), as compared with control in a dose-dependent fashion (Figure 3C).
Fig. 3.
(A) Effect of fisetin on induction of apoptosis in LNCaP cells as assessed by fluorescence microscopy. LNCaP cells were treated with fisetin (10–60 μM, 48 h). The fluorescence was measured by a Zeiss 410 confocal microscope. The details are described under Materials and methods and the data shown here are from one representative experiment repeated two times with similar results, magnification ×400. (B) Effect of fisetin on cleavage of PARP. As detailed in Materials and methods, the cells were treated with fisetin (10–60 μM, 48 h) and then harvested. Total cell lysates were prepared and 40 μg protein was subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis followed by immunoblot analysis and chemiluminescence detection. Equal loading of protein was confirmed by stripping the immunoblot and reprobing it for β-actin. The immunoblots shown here are representative of three independent experiments with similar results. The values above the figures represent relative density of the bands normalized to β-actin. (C) Effect of fisetin on induction of apoptosis in LNCaP cells. Apoptosis was determined by cell death ELISAPLUS, as per the vendor's protocol. Data are expressed as enrichment factors. Statistical analysis was performed by Student's t-test, and P < 0.05 was considered significant.
Modulation of Bcl-2 family proteins by fisetin in LNCaP cells
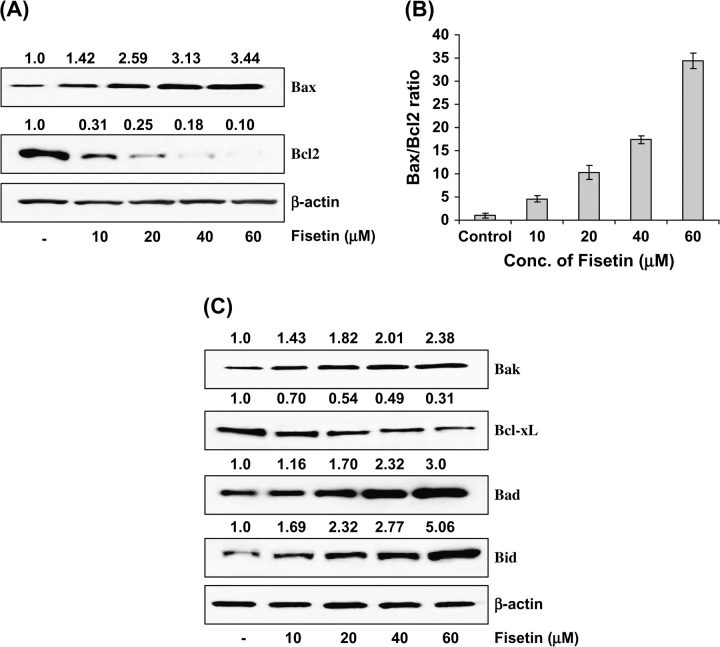
Bax and Bcl-2 play significant role in apoptosis; therefore, we next studied the effect of treatment with fisetin on the constitutive protein levels of Bax and Bcl-2 in LNCaP cells. The immunoblot analysis and relative density of the bands exhibited a significant increase in the protein expression of Bax at 10–60 μM concentration of fisetin (Figure 4A). In sharp contrast, the protein expression of Bcl-2 was significantly decreased by fisetin treatment in a dose-dependent fashion (Figure 4A). A significant dose-dependent shift in the ratio of Bax to Bcl-2 was observed after treatment with fisetin indicating the induction of apoptotic process (Figure 4B). Furthermore, we assessed the protein levels of Bak, Bad, Bid (pro-apoptotic) and Bcl-xL (antiapoptotic). The immunoblots revealed a significant increase in the protein expression of Bak, Bad and Bid and a significant decrease in Bcl-xL expression by treatment with fisetin in a dose-dependent fashion in LNCaP cells (Figure 4C), thus further confirming the induction of apoptotic process.
Fig. 4.
(A) Effect of fisetin treatment of LNCaP cells on protein expression of Bax and Bcl-2. (B) Bax/Bcl-2 ratio. (C) Protein expressions of Bak, Bcl-xL, Bad and Bid. As detailed in Materials and methods, the cells were treated with fisetin (10–60 μM, 48 h) and then harvested. Total cell lysates were prepared and 40 μg protein was subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis followed by immunoblot analysis and chemiluminescence detection. Equal loading of protein was confirmed by stripping the immunoblot and reprobing it for β-actin. The immunoblots shown here are representative of three independent experiments with similar results. The values above the figures represent relative density of the bands normalized to β-actin.
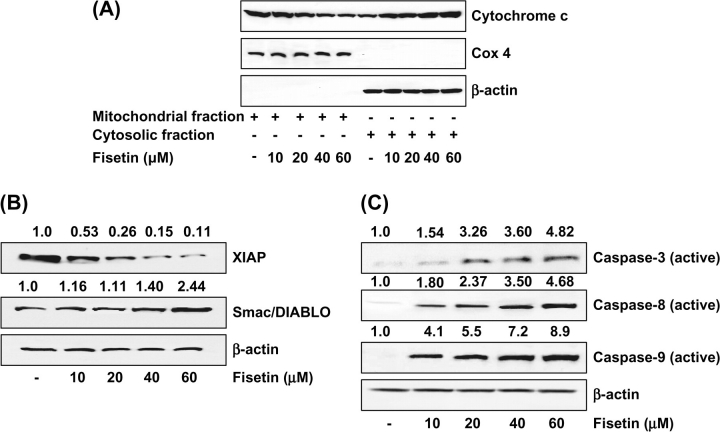
Induction of mitochondrial release of cytochrome c into cytosol by fisetin in LNCaP cells
The release of cytochrome c, one of the most important respiratory chain proteins located in the mitochondria, into the cytosol is the hallmark of apoptosis of cells treated with certain apoptosis inducers (14). We, therefore, measured the cytosolic cytochrome c using immunoblotting in LNCaP cells that were treated for 48 h with 10–60 μM of fisetin. As shown in Figure 5A, the levels of cytochrome c in the cytosol were elevated dose dependently after treatment with fisetin. To verify that the observed cytoplasmic cytochrome c was not due to mechanical disruption of the mitochondria, simultaneous analysis was done for cytochrome c oxidase-4, which was not detected in the cytosolic extracts of any samples (Figure 5A).
Fig. 5.
(A) Effect of fisetin on mitochondrial release of cytochrome c into cytosol. As detailed in Materials and methods, the cells were treated with fisetin (10–60 μM, 48 h) and then harvested. Mitochondrial and cytosolic fractions were prepared according to vendor's protocol and analyzed for cytochrome c and cytochrome c oxidase-4 by immunoblot analysis and chemiluminescence detection. The immunoblots shown here are representative of three independent experiments with similar results. (B) Effect of fisetin on protein expression of XIAP and Smac/DIABLO in LNCaP cells. (C) Effect of fisetin on protein expression of caspases-3, -8 and -9 in LNCaP cells. As detailed in Materials and methods, the cells were treated with fisetin (10–60 μM, 48 h) and then harvested. Total cell lysates were prepared and 40 μg protein was subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis followed by immunoblot analysis and chemiluminescence detection. Equal loading of protein was confirmed by stripping the immunoblot and reprobing it for β-actin. The immunoblots shown here are representative of three independent experiments with similar results. The values above the figures represent relative density of the bands normalized to β-actin.
Inhibition of XIAP and induction of Smac/DIABLO by fisetin in LNCaP cells
Dysregulation of apoptosis plays an important role in cancer and resistance to chemotherapy. The XIAP is considered to be the most potent caspase inhibitor of all known inhibitor of apoptosis family members. Only recently, an antagonist of XIAP has been identified, termed Smac/DIABLO (15). To explore the relevance of antiapoptotic XIAP and pro-apoptotic Smac/DIABLO in PCa, we analyzed XIAP and Smac/DIABLO protein expression in LNCaP cells after treatment with fisetin. By immunoblot analysis and relative density of the bands, we found that treatment with fisetin (10–60 μM, 48 h) caused inhibition of XIAP and induction of Smac/DIABLO in LNCaP cells (Figure 5B).
Induction of active caspases-3, -8 and -9 by fisetin in LNCaP cells
The apoptotic program is executed by a family of highly conserved cysteinyl aspartate-specific proteases known as caspases, which dismantle the cell in an orderly fashion by cleaving a large number of cellular substrates. Modulating the mechanisms of caspase activation and suppression is a critical molecular target in chemoprevention since these processes lead to apoptosis (14). As shown by immunoblot analysis and relative density of the bands in Figure 5C, treatment of cells with fisetin resulted in a dose-dependent increase in the activation of caspases-3, -8 and -9.
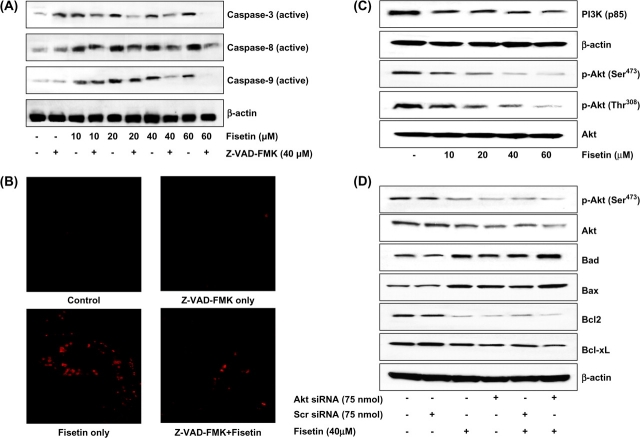
Inhibition of fisetin-induced induction of caspases by Z-VAD-FMK in LNCaP cells
The above results indicated that fisetin treatment of LNCaP cells resulted in apoptosis via activation of caspases. Since Z-VAD-FMK inhibits caspase activation, we next studied whether Z-VAD-FMK could block fisetin-mediated apoptosis. Compared with fisetin treatment (10–60 μM), preincubation of LNCaP cells with Z-VAD-FMK (40 μM) for 2 h before fisetin treatment resulted in significant decrease in the protein expression of active caspases-3, -8 and -9 as observed by immunoblot analysis (Figure 6A).
Fig. 6.
(A) Effect of Z-VAD-FMK on fisetin-induced activation of caspases in LNCaP cells. As detailed in Materials and methods, cells were incubated with 40 μM concentration of the general caspase inhibitor Z-VAD-FMK for 2 h followed by addition of fisetin (10–60 μM, 48 h) and then harvested. (B) Immunofluorescence staining for caspases-3 and -7. LNCaP cells were incubated with 40 μM concentration of the general caspase inhibitor Z-VAD-FMK for 2 h followed by addition of fisetin (40 μM, 48 h) and then harvested. Caspase activity was detected within whole living cells using ICT's Magic Red™ substrate-based MR-Caspase assay kit and fluorescence photographs were obtained at 510–560 nm excitation and 610 nm emission. The photomicrographs shown here are from one representative experiment repeated two times with similar results. (C). Inhibitory effects of fisetin on PI3K (p85) and phosphorylation of Akt (Ser473 and Thr308) in LNCaP cells. As detailed in Materials and methods, the cells were treated with fisetin (10–60 μM, 48 h) and then harvested. (D) Akt-dependent modulation in the Bcl-2 family proteins by fisetin in LNCaP cells. The LNCaP cells were transfected with Akt-siRNA (75 nM) or scrambled siRNA (75 nM) and were then treated with 40 μM fisetin for 48 h. Whole-cell lysate was prepared and 40 μg protein was subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis followed by immunoblot analysis and chemiluminescence detection. Equal loading of protein was confirmed by stripping the immunoblot and reprobing it for β-actin. The immunoblots shown here are representative of three independent experiments with similar results.
Active caspases-3 and -7 activity was also detected by immunofluorescence staining within whole living LNCaP cells using ICT's Magic Red™ substrate-based MR-Caspase assay kit. The MR-Caspase photostable fluorogenic substrate easily penetrates the cell membrane and the membranes of the internal cellular organelles, entering the cell in the non-fluorescent state. In the presence of caspase-3 and -7 enzymes (DEVDases), the four-amino acid (DEVD) caspase target sequences are cleaved off yielding a red fluorescent product. DEVDase-mediated production of the red fluorophore signals apoptotic activity within that particular cell. On treatment with Z-VAD-FMK (40 μM) for 2 h before fisetin treatment (40 μM), there was significant decrease in the immunofluorescence staining of active caspases-3 and -7 (Figure 6B). Similarly, when cells were treated with fisetin only (40 μM), there was intense staining of active caspases-3 and -7 as detected by red fluorescent product (Figure 6B).
Inhibition of PI3K and phosphorylation of Akt protein expression by fisetin in LNCaP cells
The PI3K/Akt signaling pathway and its downstream transcription factors have been studied in detail for their role in cell proliferation, survival, cycle control, as well as other cellular functions. Accumulating evidence shows that dysregulation of this pathway also plays an essential role in cancer development. We first determined the effect of fisetin on PI3K protein expression in LNCaP human prostate carcinoma cells. Western blot analysis revealed that fisetin (10–60 μM) caused inhibition in the expression of regulatory (p85) subunit of PI3K in LNCaP cells (Figure 6C). Treatment of LNCaP cells with fisetin (10–60 μM) also inhibited phosphorylation of Akt at both Ser473 and Thr308 (Figure 6C).
Akt-dependent modulation in the Bcl-2 family proteins by fisetin in LNCaP cells
To assess whether fisetin-induced apoptosis was Akt dependent, we silenced Akt by siRNA. Silencing of Akt by siRNA resulted in increase in the protein expressions of proapoptotic Bad and Bax and decrease in the protein expressions of Bcl-2 and Bcl-xL (Figure 6D). Fisetin treatment (40 μM) to Akt-siRNA-treated cells further augmented the increase in Bad and Bax and decrease in Bcl-2 and Bcl-xL, suggesting that these effects are mediated in part through Akt (Figure 6D).
Discussion
PCa remains one of the most common malignancies in Western countries and is also increasing in Asian countries. The number of new PCa cases expected to be diagnosed in 2007 were 218 890 with 27 000 deaths in USA (1). Asian men have much lower incidence and mortality due to PCa than men in North America and Europe. This difference is attributed to lifestyle changes and environmental-related factors. Incidence of hormone-related cancers is also on the rise in Asians who migrated to USA and adopted western lifestyles and eating habits, which suggests that diet in their native countries may account for their low incidence of hormone-related cancers. It is estimated that nearly one-third of all cancer deaths in USA could be prevented through appropriate dietary modification (2,16,17). The goal of primary chemoprevention is to decrease the incidence of a given cancer, simultaneously reducing both treatment-related adverse events and mortality. The development of chemoprevention strategies against PCa would have a huge impact, both medically and economically. Because of its ubiquity and disease burden, PCa is an attractive target for chemoprevention.
This study was designed to show the chemopreventive/chemotherapeutic potential of fisetin against PCa. Treatment of LNCaP, CWR22Rυ1 and PC-3 cells with fisetin resulted in decrease in cell viability but had minimal effect on PrECs (Figure 1B and C). Fisetin treatment of LNCaP cells also resulted in dose-dependent arrest of cells in G1 phase of the cell cycle (Figure 2A). Various studies have shown the involvement of cell cycle regulation-mediated apoptosis as a mechanism of cell growth inhibition (18–22); we investigated the involvement of the cki–cyclin–cdk machinery during the induction of cell cycle arrest and apoptosis by fisetin in LNCaP cells. In eukaryotes, passage through the cell cycle is governed by the function of a family of protein kinase complexes (23). Each complex is composed minimally of a catalytic subunit, the cdk, and its essential activating partner, the cyclin. Under normal conditions, these complexes are activated at specific intervals and through a series of events and result in the progression of cells through different phases of cell cycle, thereby ensuring normal cell growth (24). Any defect in this machinery causes an altered cell cycle regulation that may result in unwanted cellular proliferation ultimately culminating in the development of cancer. Since our study has demonstrated that fisetin treatment of LNCaP cells resulted in a G1-phase arrest of the cell cycle, we examined the effect of fisetin on cell cycle regulatory molecules operative in the G1 phase of the cell cycle. Fisetin treatment of the cells was found to result in significant downmodulation of cyclins and cdks (Figure 2B and C). Our data also demonstrated a significant upregulation of the cki WAF1/p21 and KIP1/p27 by fisetin (Figure 2D).
The induction of apoptosis is a physiological process that functions as an essential mechanism of tissue homeostasis and is regarded as the preferred way to eliminate unwanted cells. This observation was verified by fluorescence microscopy and PARP cleavage (Figure 3A and B). Collectively, these results suggest that fisetin inhibits the growth of prostate carcinoma cells through cell cycle arrest and induction of apoptosis.
Members of the Bcl-2 family of proteins are critical regulators of the apoptotic pathway. Bcl-2 is an upstream effector molecule in the apoptotic pathway and is identified as a potent suppressor of apoptosis (25). Bcl-2 has been shown to form a heterodimer with the proapoptotic member Bax and might thereby neutralize its proapoptotic effects. Therefore, alterations in the levels of Bax and Bcl-2, i.e. the ratio of Bax/Bcl-2, are a decisive factor and play an important role in determining whether cells will undergo apoptosis under experimental conditions that promote cell death. In our study, a decrease in Bcl-2 and an increase in Bax protein expression were observed in LNCaP cells (Figure 4A); hence, the ratio of Bax to Bcl-2 was altered in favor of apoptosis (Figure 4B). Our results suggest that upregulation of Bax and downmodulation of Bcl-2 may be another molecular mechanism through which fisetin induces apoptosis. We also found upregulation in the protein expressions of proapoptotic Bak, Bad and Bid and downregulation of antiapoptotic Bcl-xL on treatment with fisetin in LNCaP cells (Figure 4C).
It was recently discovered that mitochondria contain and release proteins such as cytochrome c that are involved in the apoptotic cascade. Cell-free systems demonstrate that mitochondrial products are rate limiting for the activation of caspases and endonucleases in cell extracts (26). Functional studies indicate that drug-induced opening or closing of the mitochondrial megachannel (permeability transition pore) can induce or prevent apoptosis (27). These experiments indicate that cytochrome c is a key factor in apoptosis and that its release further activates caspases, resulting in the appearance of apoptosis. Our study confirms that cytosolic cytochrome c was increased in LNCaP cells after treatment with fisetin (Figure 5A). Suppression of apoptosis promotes tumor progression, immune evasion of neoplastic cells as well as resistance to chemotherapy and irradiation (28). Several genes critical in the regulation of apoptosis have been identified, including XIAP—a member of the IAP family. XIAP is thought to act as a key determinant of apoptosis resistance by effectively inhibiting the activation of caspases-3, -7 and -9 (15). Thus, high expression of XIAP has been reported in many malignant tumor types, such as carcinomas of the breast, ovaries, lung, pancreas, cervix and prostate (29).
Whereas antiapoptotic XIAP has been shown to be a potent caspase inhibitor (30), its antagonists Smac/DIABLO and Omi/HtrA2 promote apoptosis by binding to XIAP, thereby preventing them from inhibition of caspases. Only recently, an inverse relation between XIAP expression and Smac/DIABLO has been observed after apoptosis induction in colon cancer cells, lymphoma cells and keratinocytes (31). In our study, we also found that fisetin treatment (10–60 μM, 48 h) of LNCaP cells caused inhibition of XIAP and induction of Smac/DIABLO (Figure 5B).
Caspases, comprised of 12 proteins, are a family of cysteinyl aspartate-specific proteases involved in apoptosis and are subdivided into initiator (caspases-8, -9 and -10) and executioner (caspases-3, -6 and -7) caspases. The intrinsic and extrinsic pathways converge at caspase-3. Active caspases and 8 of the intrinsic and extrinsic pathways, respectively, have been shown to directly cleave and activate the effector protease caspase-3 (14). In the present study, we have shown that caspases-3, -8 and -9 are activated during fisetin-mediated apoptosis (Figure 5C). Our studies have further shown by immunoblot analysis (Figure 6A) and immunofluorescence staining (Figure 6B) that addition of Z-VAD-FMK, a general caspase inhibitor, significantly reduced fisetin-mediated apoptosis, providing evidence that fisetin-mediated apoptosis in LNCaP cells was caspase dependent.
The PI3K/Akt pathway is activated downstream of a variety of extracellular signals and activation of this signaling pathway impacts a number of cellular processes including cell growth, proliferation and survival. The alteration of components of this pathway, through either activation of oncogenes or inactivation of tumor suppressors, disrupts a signaling equilibrium and can thus lead to cellular transformation (32). We found that treatment of LNCaP cells with fisetin caused decrease in the protein expression of PI3K (p85) and phosphorylation of Akt at both Thr308 and Ser473 (Figure 6C). The Akt family has been shown to be the primary downstream mediator of the effects of PI3K and regulates a variety of cellular processes through the phosphorylation of a wide spectrum of downstream substrates. Indeed, dysregulation of the PI3K/Akt signaling pathway can lead to an alteration of all the aspects of cell physiology that comprise the hallmarks of cancer. Cell survival is influenced by Akt through a variety of effector proteins including inhibition of the proapoptotic Bcl-2 family member Bad and inhibition of the forkhead transcription factors that normally activate apoptosis-related genes. In our study, we also found that silencing of Akt by siRNA caused increase in the protein expressions of Bad and Bax and decrease in Bcl-2 and Bcl-xL, which was firther augmented on treatment with fisetin, suggesting that these effects are mediated in part through Akt (Figure 6D).
In summary, the present study demonstrates that treatment of LNCaP cells with fisetin, a novel dietary flavonoid caused inhibition of PCa by G1-phase cell cycle arrest, modulating cki–cyclin–cdk network and induction of apoptosis. We suggest that fisetin could be a useful chemotherapeutic agent against PCa.
Funding
United States Public Health Service (R01 CA 78809, R01 CA 101039, R01 CA 120451, P50 DK065303).
Acknowledgments
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- cdk
cyclin-dependent kinase
- DEVD
aspartylglutamylalanylaspartic acid
- DIABLO
direct inhibitor of apoptosis-binding protein with low pI
- NF-κB
nuclear factor-κB
- PARP
poly (ADP-ribose) polymerase
- PBS
phosphate-buffered saline
- PCa
prostate cancer
- PI3K
phosphatidyl inositol 3-kinase
- PrECs
prostate epithelial cells
- siRNA
small interfering RNA
- Smac
second mitochondria-derived activator of caspase
- XIAP
X-linked inhibitor of apoptosis protein
References
- 1.Jemal A, et al. Cancer statistics. CA Cancer J. Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Syed DN, et al. Chemoprevention of prostate cancer through dietary agents: progress and promise. Cancer Epidemiol. Biomarkers Prev. 2007;16:2193–2203. doi: 10.1158/1055-9965.EPI-06-0942. [DOI] [PubMed] [Google Scholar]
- 3.Adhami VM, et al. Polyphenols from green tea and pomegranate for prevention of prostate cancer. Free Radic. Res. 2006;40:1095–1104. doi: 10.1080/10715760600796498. [DOI] [PubMed] [Google Scholar]
- 4.Arai Y, et al. Dietary intakes of flavonols, flavones and isoflavones by Japanese women and the inverse correlation between quercetin intake and plasma LDL cholesterol concentration. J. Nutr. 2000;130:2243–2250. doi: 10.1093/jn/130.9.2243. [DOI] [PubMed] [Google Scholar]
- 5.Lu X, et al. Fisetin inhibits the activities of cyclin-dependent kinases leading to cell cycle arrest in HT-29 human colon cancer cells. J. Nutr. 2005;135:2884–2890. doi: 10.1093/jn/135.12.2884. [DOI] [PubMed] [Google Scholar]
- 6.Haddad AQ, et al. Novel antiproliferative flavonoids induce cell cycle arrest in human prostate cancer cell lines. Prostate Cancer Prostatic Dis. 2006;9:68–76. doi: 10.1038/sj.pcan.4500845. [DOI] [PubMed] [Google Scholar]
- 7.Chen YC, et al. Wogonin and fisetin induction of apoptosis through activation of caspase 3 cascade and alternative expression of p21 protein in hepatocellular carcinoma cells SK-HEP-1. Arch. Toxicol. 2002;76:351–359. doi: 10.1007/s00204-002-0346-6. [DOI] [PubMed] [Google Scholar]
- 8.Lee WR, et al. Wogonin and fisetin induce apoptosis in human promyeloleukemic cells, accompanied by a decrease of reactive oxygen species, and activation of caspase 3 and Ca(2+)-dependent endonuclease. Biochem. Pharmacol. 2002;63:225–236. doi: 10.1016/s0006-2952(01)00876-0. [DOI] [PubMed] [Google Scholar]
- 9.Maher P, et al. Flavonoid fisetin promotes ERK-dependent long-term potentiation and enhances memory. Proc. Natl Acad. Sci. USA. 2006;103:16568–16573. doi: 10.1073/pnas.0607822103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sung B, et al. Fisetin, an inhibitor of cyclin-dependent kinase 6, down-regulates nuclear factor-{kappa}B-regulated cell proliferation, antiapoptotic and metastatic gene products through the suppression of TAK-1 and receptor-interacting protein-regulated I{kappa}B{alpha} kinase activation. Mol. Pharmacol. 2007;71:1703–1714. doi: 10.1124/mol.107.034512. [DOI] [PubMed] [Google Scholar]
- 11.Ichimatsu D, et al. Structure-activity relationship of flavonoids for inhibition of epidermal growth factor-induced transformation of JB6 Cl 41 cells. Mol. Carcinog. 2007;46:436–445. doi: 10.1002/mc.20292. [DOI] [PubMed] [Google Scholar]
- 12.Hou DX, et al. Fisetin induces transcription of NADPH:quinone oxidoreductase gene through an antioxidant responsive element-involved activation. Int. J. Oncol. 2001;18:1175–1179. doi: 10.3892/ijo.18.6.1175. [DOI] [PubMed] [Google Scholar]
- 13.de Sousa RR, et al. Phosphoprotein levels, MAPK activities and NFkappaB expression are affected by fisetin. J. Enzyme Inhib. Med. Chem. 2007;22:439–444. doi: 10.1080/14756360601162063. [DOI] [PubMed] [Google Scholar]
- 14.Khan N, et al. Apoptosis by dietary factors: the suicide solution for delaying cancer growth. Carcinogenesis. 2007;28:233–239. doi: 10.1093/carcin/bgl243. [DOI] [PubMed] [Google Scholar]
- 15.Huang Y, et al. Structural basis of caspase inhibition by XIAP: differential roles of the linker versus the BIR domain. Cell. 2001;104:781–790. [PubMed] [Google Scholar]
- 16.Michels KB. The role of nutrition in cancer development and prevention. Int. J. Cancer. 2005;114:163–165. doi: 10.1002/ijc.20662. [DOI] [PubMed] [Google Scholar]
- 17.Khan N, et al. Cancer chemoprevention through dietary antioxidants: progress and promise. Antioxid. Redox. Signal. 2008;10:475–510. doi: 10.1089/ars.2007.1740. [DOI] [PubMed] [Google Scholar]
- 18.Malik A, et al. Pomegranate fruit juice for chemoprevention and chemotherapy of prostate cancer. Proc. Natl Acad. Sci. USA. 2005;102:14813–14818. doi: 10.1073/pnas.0505870102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kweon MH, et al. A novel antioxidant 3-O-caffeoyl-1-methylquinic acid enhances ultraviolet A-mediated apoptosis in immortalized HaCaT keratinocytes via Sp1-dependent transcriptional activation of p21(WAF1/Cip1) Oncogene. 2007;26:3559–3571. doi: 10.1038/sj.onc.1210135. [DOI] [PubMed] [Google Scholar]
- 20.Sarfaraz S, et al. Cannabinoid receptor agonist-induced apoptosis of human prostate cancer cells LNCaP proceeds through sustained activation of ERK1/2 leading to G1 cell cycle arrest. J. Biol. Chem. 2006;281:39480–39491. doi: 10.1074/jbc.M603495200. [DOI] [PubMed] [Google Scholar]
- 21.Sato M, et al. A natural peptide, dolastatin 15, induces G2/M cell cycle arrest and apoptosis of human multiple myeloma cells. Int. J. Oncol. 2007;30:1453–1459. [PubMed] [Google Scholar]
- 22.Tian Z, et al. Dulxanthone A induces cell cycle arrest and apoptosis via up-regulation of p53 through mitochondrial pathway in HepG2 cells. Int. J. Cancer. 2008;122:31–38. doi: 10.1002/ijc.23048. [DOI] [PubMed] [Google Scholar]
- 23.Ahmad N, et al. Photodynamic therapy results in induction of WAF1/CIP1/P21 leading to cell cycle arrest and apoptosis. Proc. Natl Acad. Sci. USA. 1998;95:6977–6982. doi: 10.1073/pnas.95.12.6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanchez I, et al. New insights into cyclins, CDKs and cell cycle control. Semin. Cell Dev. Biol. 2005;16:311–321. doi: 10.1016/j.semcdb.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 25.Oltersdorf T, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 26.Martinou JC, et al. Cytochrome c release from mitochondria: all or nothing. Nat. Cell Biol. 2000;2:E41–E43. doi: 10.1038/35004069. [DOI] [PubMed] [Google Scholar]
- 27.Tsujimoto Y, et al. Bcl-2 family: life-or-death switch. FEBS Lett. 2000;466:6–10. doi: 10.1016/s0014-5793(99)01761-5. [DOI] [PubMed] [Google Scholar]
- 28.Igney FH, et al. Death and anti-death: tumour resistance to apoptosis. Nat. Rev. Cancer. 2002;2:277–288. doi: 10.1038/nrc776. [DOI] [PubMed] [Google Scholar]
- 29.Hofmann HS, et al. Expression of inhibitors of apoptosis (IAP) proteins in non-small cell human lung cancer. J. Cancer Res. Clin. Oncol. 2002;128:554–560. doi: 10.1007/s00432-002-0364-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suzuki Y, et al. Ubiquitin-protein ligase activity of X-linked inhibitor of apoptosis protein promotes proteasomal degradation of caspase-3 and enhances its anti-apoptotic effect in Fas-induced cell death. Proc. Natl Acad. Sci. USA. 2001;98:8662–8667. doi: 10.1073/pnas.161506698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takasawa R, et al. Sustained release of Smac/DIABLO from mitochondria commits to undergo UVB-induced apoptosis. Apoptosis. 2003;8:291–299. doi: 10.1023/a:1023629023696. [DOI] [PubMed] [Google Scholar]
- 32.Hanahan D, et al. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]