Abstract
This study used 1-methyl-4-phenyl-1,2,3,6,-tetrahydropyridine (MPTP) in mice to determine if exercise improves behavior and dopamine (DA) and serotonin (5HT) content. Male C57BL/6 mice received MPTP (4×20 mg/kg) or saline. They remained sedentary or exercised by treadmill or voluntary running wheel for 6 weeks (n=8/group). Saline-treated mice ran significantly faster on running wheels (22.8±1.0 m/min) than on treadmill (8.5±0.5 m/min), and MPTP lesion did not reduce voluntary exercise (19.3±1.5 m/min, p>0.05). There was a significant effect of both lesion and exercise on overall Rotarod performance (ORP): MPTP lesion reduced ORP, while treadmill exercise increased ORP vs sedentary mice (p<0.05). MPTP increased anxiety in the marble-burying test: sedentary lesioned mice buried more marbles (74.0±5.2%) than sedentary controls (34.8±11.8%, p<0.05). Conversely, exercise reduced anxiety on the elevated plus maze. Among saline-treated mice, those exposed to voluntary wheel-running showed an increased percent of open arm entries (49.8±3.5%, p<0.05) relative to relative to sedentary controls (36.2±4.0%, p<0.05). Neither MPTP nor exercise altered symptoms of depression measured by sucrose preference or tail suspension. MPTP significantly reduced DA in striatum (in sedentary lesioned mice to 42.1±3.0% of saline controls), and lowered 5HT in amygdala and striatum (in sedentary lesioned mice to 86.1±4.1% and 66.5±8.2% of saline controls, respectively); exercise had no effect. Thus, exercise improves behavior in a model of DA depletion, without changes in DA or 5HT.
Keywords: MPTP, anxiety, depression, affective behavior, mood, motor, C57BL/6, male, mouse
INTRODUCTION
Parkinson s Disease (PD) is a progressive neurodegenerative disease with impaired motor function including slow execution of movement, rigidity, postural instability, and resting tremor [22]. However non-motor behavioral deficits are also common, occurring in approximately 60% of PD patients [1, 11, 50]. Depression affects ca. 25–40% of PD patients [46, 51], and anxiety affects approximately 40% [85]. The degree of psychiatric dysfunction does not appear to be related to the presence or extent of motor impairment [47, 61, 79]. In fact, affective disorders can be observed years before movement difficulties arise [72]. Understanding the neuropsychiatric contribution to PD symptoms will inform treatment options aimed at improving patient quality of life [11, 71].
The 1-methyl-4-phenyl-1,2,3,6,-tetrahydropyridine (MPTP) mouse model replicates many neuropathologic and neurochemical features of PD [38]. MPTP causes selective loss of dopaminergic (DA) neurons in the substantia nigra pars compacta (SNpc) leading to DA depletion in the caudate putamen (CPu [37]) and selective motor impairment [19, 66]. A recent study from our laboratories determined that MPTP also impairs cognitive function and elements of affective behavior [82]. C57BL/6 mice are highly susceptible to MPTP lesioning [77], and are commonly used in behavioral pharmacology research [15, 16, 52]. The present study applied MPTP lesioning in C57BL/6 mice to determine if nigrostriatal lesions induce anxiety and depression.
The second part of this study addressed the potential positive effects of exercise on symptoms of anxiety and depression in MPTP-lesioned mice. Recent studies have demonstrated that exercise improves motor function in PD patients and in animal models of PD [13, 25, 54, 59, 78]. Exercise also improves neuropsychiatric symptoms in non-PD patients and in mice [3, 5, 7, 20, 21, 56, 58]. Thus, it may similarly confer mental health benefits in PD patients.
Mice were subject to daily exercise for 30 days, either by running on a motorized treadmill or via exposure to a running wheel. Including voluntary exercise addresses the potential confounds of psychological stress imposed by forced exercise. We hypothesized that MPTP lesion would produce deficits in motor function and affective behavior, and that exercise would improve motor function and ameliorate anxiety and depression. Modulation of behavior would be accompanied by corresponding changes in brain levels of DA and serotonin (5HT), transmitters important for mood and motor function.
METHODS
Animals
Forty-eight young adult male C57BL/6 mice (8–10 weeks of age, 22–26g BW at the start of the study) were obtained from Charles River Laboratories (Wilmington, MA). Mice were group-housed 4/cage on a 12:12 reversed light-dark schedule (lights off at 11AM) in a temperature-controlled vivarium. Water and food were provided ad libitum. For 5 days before lesioning, all mice were handled daily for ca. 3 minutes. Half of the mice (n=24) received 80 mg/kg MPTP (Sigma-Aldrich, St. Louis, MO) as 4 i.p. injections of 20mg/kg free base in 0.1 ml saline every 2 hours. This dosing schedule produces approximately 90–95% striatal dopamine depletion 7 days post-lesion, along with 60–70% loss of SNpc DA neurons [37, 38]. The remaining mice (n=24) received equivalent injections of 0.9% saline. MPTP-lesioned and saline-treated mice were randomly assigned to one of 3 groups (n=8/group): forced treadmill exercise, voluntary wheel running or sedentary. All procedures were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals, and were approved by the Institutional Animal Care and Use Committee at the University of Southern California.
Experimental Design
Mice were subject to 30 days of daily exercise or handling 5 days per week, beginning 5 days after saline or MPTP when both MPTP-induced cell death is complete [37, 38]. We delayed the onset of exercise training to focus on the potential for exercise-induced neurorehabilitation, rather than neuroprotective effects of exercise on MPTP lesioning. Furthermore, the delayed onset of exercise training avoids the hyperactivity expressed transiently within the first few hours after MPTP [53, 60]. All exercise and behavioral testing was conducted in the animal room. Daily exercise began 2 hours after lights-off under red light during the mouse active phase. The duration of daily exercise or handling was the same for all mice, and followed the methods of Petzinger et al [59]: 30 min on the first day (5 days after lesion), increasing incrementally to 60 min by 19 days of exercise (25 days post-MPTP). The last day of exercise was 42 days post-lesion. In addition, mice were tested weekly for sucrose preference as a measure of depression (see below), beginning the day before MPTP lesioning and continuing weekly thereafter throughout exercise training.
After the last day of exercise, mice were tested for motor function and affective behavior according to established methods. Each test has been shown in published literature to be sensitive to anxious/depressive/locomotor changes in the C57BL/6 mouse, and each addresses a different facet of behavioral recovery following MPTP lesion [17, 35, 57, 69]. Mice were subject to 2 tests per day in a set order with at least 2 hours between tests: marble burying and Rotarod on the first day, followed by elevated plus maze and tail suspension on the second day. With this schedule, motor function (Rotarod) and symptoms of depression (sucrose preference, tail-suspension) were evaluated at the beginning of the dark phase, while symptoms of anxiety (elevated plus maze, marble burying) were measured at the end of the light phase. With the exception of weekly sucrose preference tests, each mouse was subject only once to each test, and all mice were tested in the same order. Although this approach does not eliminate the potential for carryover effects of previous tests, carryover effects were the same for saline- and MPTP-treated mice. Mice were sacrificed 45 days after MPTP.
Exercise
Forced Treadmill Exercise
Mice were exercised on a 6-lane treadmill with a 45-cm runway inclined 5° (EXER-6M, Columbus Instruments, Columbus, OH). Each daily exercise session started with an initial warm-up at slower speeds, after which treadmill speeds increased incrementally. In addition, hour-long treadmill sessions included 5 min of rest after 30 min. On the first day of exercise, treadmill speed averaged 6.7 m/min for 30 min. After 19 days, mice ran at 8.5 m/min for 60 min. To encourage running, a metal-beaded curtain was fixed as a tactile stimulus 20 cm before the shockplate at the end of each runway. Mice that failed to maintain their position on the treadmill received a mild foot-shock. Nonetheless, although this treadmill regimen provides sustained high-intensity exercise training, both saline- and MPTP-treated mice are able to maintain a forward position on the treadmill [59].
Voluntary Wheel Running
Voluntary wheel running was tested in separate cages each containing a Fast-Trac running wheel (18 cm diameter, Bio-serv Inc, Frenchtown, NJ) equipped with bicycle odometer (CatEye, Boulder, CO) to measure distance and speed. To equate handling and exercise duration with mice undergoing forced treadmill exercise, each mouse had access to the running wheel for the same duration (up to 60 min) as the forced treadmill exercise group.
Sedentary
Sedentary mice were exposed to either a non-moving treadmill or a fixed running wheel on the same daily schedule as exercising mice [29, 43].
Behavior
Sucrose Preference
Mice prefer to drink dilute sucrose solutions [4, 28]. A reduction in sucrose preference is thought to reflect anhedonia, the lack of pleasure in normally-pleasurable activities [76]. Anhedonia is a key component of depression [40, 42]. Baseline sucrose preference was measured pre-lesion, and the test was repeated weekly thereafter, including the last day of exercise. To minimize neophobia, mice were exposed to the sucrose solution overnight two days before testing. The water bottle in each home-cage was replaced with two 50-ml bottles fitted with ball-point drinking spouts, each containing 2% sucrose. On the day before testing, mice were water-deprived overnight to increase subsequent fluid consumption. On the following day at the onset of the dark phase, mice were individually housed for 1 hour with access to 2 bottles, one containing 2% sucrose and the other with water. Fluid consumption was determined by weight. The location of the sucrose bottle (to the right or left side of the cage) was alternated to minimize side preferences. Sucrose preference was calculated as a percentage of total fluid intake.
Marble Burying
Marble burying is a test of defensive burying in mice, similar to shock-prod burying in rats [24]. A greater number of buried marbles reflects higher levels of anxiety. The test was conducted as described previously [57, 80]. Briefly, each mouse was placed in a novel cage filled with a 10-cm thick layer of pine bedding containing a cluster of 25 small blue glass marbles (10–12 mm diam.). After 30 minutes, the mouse was returned to its home cage, and the number of buried marbles was scored by 2 observers blinded to the treatment group. A marble was considered partially buried if more than 70% of its surface was covered by bedding. Marbles that were no longer visible were considered completely buried.
Motor Endurance
Mice were tested for motor endurance on an accelerating Rotarod (Columbus Instruments, Columbus, OH, USA) according to the methods of Rozas et al [65]. This protocol uses a spindle designed for rats (7.3 cm diam) to minimize dependence on motor coordination. Before lesioning, mice were acclimatized at low speed (5 rpm, 1.9 cm/s) for 10 minutes during the dark phase. At the end of the study (day 43 post-lesion), mice were subject to 7 trials at increasing speeds from 12 to 24 rpm at 2 rpm intervals [65]. Each trial lasted 150 seconds with 150 seconds rest between trials. The latency to fall in each trial was recorded, and the overall Rotarod performance (ORP) was calculated for each animal as the area under the curve of latency to fall versus cylinder speed [66, 68].
Elevated Plus Maze
The elevated plus maze is a test of anxiety which takes advantage of the mouse’s preference for enclosed spaces versus the drive to explore a novel environment [14, 15]. The maze consisted of 2 open arms (5 × 30 cm) perpendicular to 2 closed arms enclosed by 15 cm clear Plexiglas walls. The arms converged at a 5 × 5 cm central platform. To facilitate entry, open arms were fitted with 1 cm Plexiglas railings. Each plus maze was located 50 cm above the floor, and visually isolated within a separate enclosure in the animal room. For testing, a mouse was placed on the central platform facing an open arm, and behavior was viewed remotely via webcam for 6 minutes. The number of open and closed arm entries and duration of time on the open arms was scored live by an observer blinded to the treatment groups. An entry was recorded when all four paws entered the arm.
Tail Suspension
Similar to the forced swim test, tail suspension measures behavioral despair as a symptom of depression [75]. Mice placed in an aversive and inescapable situation alternate between movement and immobility; stillness is thought to reflect “despair” [62]. Mice were individually suspended from their tails at a height of 20 cm using a piece of adhesive tape wrapped around the tail 2 cm from the tip. Behavior was videotaped for 6 min. The duration of immobility was measured by an observer blinded to the treatment groups. Mice were considered immobile only when completely motionless, and mice that climbed their tails were excluded from the data. Results are expressed as percent of time spent immobile.
Plasma Corticosterone
To measure “stressor-induced” plasma corticosterone concentrations immediately following the tail suspension test [9], a single 200 ul retro-orbital blood sample was collected from each mouse into heparinized microcentrifuge tubes. Samples were chilled on ice, and then centrifuged at 13,000 rpm for 20 min. Plasma was collected and stored at 20°C until assayed. Plasma corticosterone concentrations were measured in duplicate using a commercially-available radioimmunoassay kit (MP Biomedical, Irvine CA) with minor modifications [31]. Assay sensitivity was 6.25 ng/ml corticosterone.
Tissue Collection and Analysis
Mice were sacrificed by cervical dislocation the day after the final behavioral test (45 days post-lesion). The CPu, nucleus accumbens (Acb, includes core and shell), basolateral region of the amygdala (BLA, includes the basolateral and lateral nuclei, and portions of the basomedial nucleus), and prefrontal cortex (PFC) were dissected, frozen on dry ice, and stored at −80°C. These tissues include key efferent targets of midbrain DA (SNpc, ventral tegmental area) and 5HT neurons (dorsal raphe). Tissue was homogenized in 0.4 M HClO4, and centrifuged to separate precipitated protein. The pellet was resuspended in 0.5 M NaOH and used to determine total protein concentration with the CoomassiePlus protein assay (Pierce, Rockford, IL) and microplate reader EL×800 (BioTek Instruments Inc., Winooski, VT) equipped with KCjunior software. Concentrations of DA and 5HT were determined in the supernatant by HPLC with electrochemical detection as previously described [36, 41, 59]. The system consisted of an auto-sampler (ESA Inc., Chelmsford, MA) equipped with a 150 × 3.2 mm reverse phase C-18 column (3μm diameter) regulated at 28°C, and an ESA CoulArray 5600A detector equipped with a 4-channel analytical cell with potentials set at −100 mV, −30 mV, 220 mV and 350 mV. The HPLC was integrated with a computer running the CoulArray analytical program for Windows (ESA Inc). Mobile phase consisted of acetonitrile in phosphate buffer and an ion-pairing agent, and was delivered at 0.6 ml/min.
Statistical Analysis
Behavioral and neurochemical data were analyzed by two-factor ANOVA. Where effects of lesion or exercise were identified, we conducted additional comparisons of exercise treatment in MPTP and saline-treated mice (by ANOVA with post-hoc analysis by Fisher PLSD) and the effects of MPTP lesion within each exercise group (by t-test). Daily exercise and sucrose preference were evaluated by repeated measures ANOVA. Statistical analysis was performed by Graphpad Prism (v. 5.0c; GraphPad Software, San Diego, CA). Significance was set at p<0.05.
RESULTS
Exercise
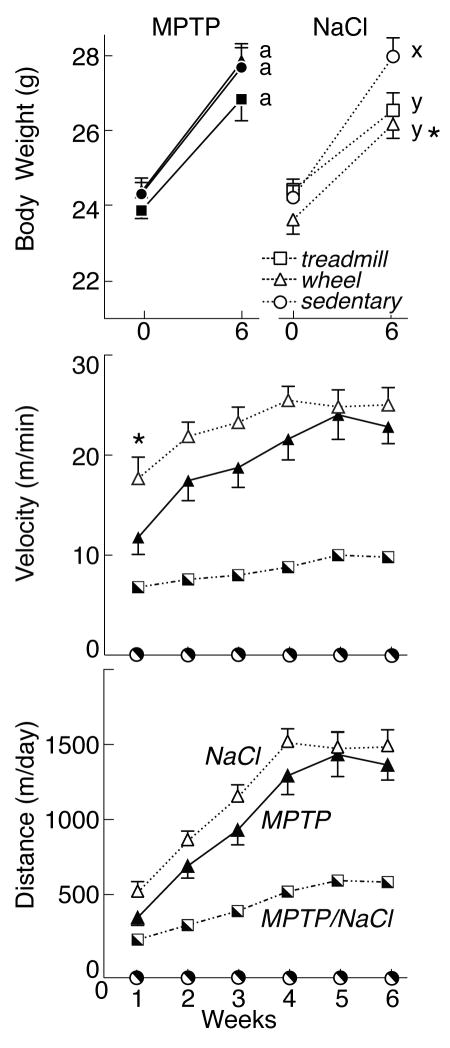
Figure 1 depicts body weight and exercise measures for saline- and MPTP-lesioned mice. At the start of the study, there were no differences in body weight among treatment groups. MPTP lesion did not impair weight gain. Forty-five days after lesioning, MPTP-lesioned mice (all groups) gained an average of 3.3±0.2 g to reach 27.5±0.3 g BW, while BW in saline-treated mice increased by 2.8±0.3 g to 27.0±0.3 g (p>0.05). Among saline-treated mice, exercise reduced final bodyweight (treadmill: 26.7±0.3 g; wheel-running: 27.1±0.4 g), compared with sedentary controls (27.9±0.4 g, p<0.05).
Figure 1.
Top: Body weights (mean±SEM) in MPTP- (left, filled symbols) and saline-treated (NaCl) mice (right, open symbols) before lesion (Week 0) and at the end of the study (Week 6). Bottom: Exercise velocity (m/min) and distance (m/day) in mice subject to forced treadmill exercise (squares) and in MPTP- and saline-treated mice exercising voluntarily on running wheels (triangles). Exercise effects are denoted by letters (MPTP: a, b; saline: x, y); bars with common letters in the same lesion group are not different. * identifies differences between MPTP- and saline-treated mice in the same exercise group.
All groups of exercising mice increased their average velocity and distance traveled during the study period. Since the duration of daily exercise lengthened progressively throughout the study, distance reflects the increase in both speed and duration; velocity provides a consistent measure of running behavior throughout the study. As reported previously (Petzinger et al, 2007), MPTP and saline-treated mice undergoing forced treadmill exercise ran at an average speed of 8.5±0.5 m/min throughout the study, with peak speeds of 20 m/min. Initially, these mice ran 201 m during 30 min of exercise. During the last week of exercise training, they ran 590 m over 60 min (9.8 m/min).
Somewhat surprisingly, exercise distance and velocity were significantly greater with voluntary wheel-running than for forced treadmill exercise. In fact, average velocity on the Fast-trac running wheels throughout the 6 weeks of exercise was over twice that for treadmill exercise in both MPTP-lesioned mice (19.3±1.5 m/min) and saline-treated mice (22.8±1.0 m/min). During the first week of voluntary exercise, MPTP-lesioned mice ran significantly less (11.8±1.7 m/min) than saline-treated mice (17.7±2.1 m/min, p<0.05). With this exception, MPTP lesion did not reduce wheel-running (p>0.5). Throughout the course of the study, there was a significant increase in exercise velocity on running wheels, as determined by repeated measures ANOVA [F(5, 70) =11.27; p<0.05]. During the final week of exercise, MPTP-lesioned mice achieved an average velocity of 22.9±1.7 m/min, running 1371.2±100.5 m in 60 min. Saline-treated mice ran 1493.6±102.9 m, for an average speed of 24.9±1.7 m/min.
Behavior
Motor Endurance
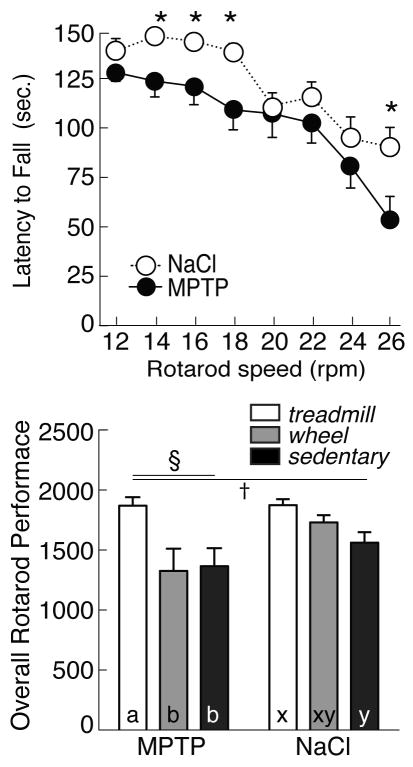
Figure 2 presents ORP for all groups of mice, as well as the latency to fall from the Rotarod at increasing speeds. Although MPTP did not reduce treadmill or wheel-running exercise, there was a significant effect of lesion [F(1, 46) =5.40; p<0.05] and exercise [F(2, 45) =7.03; p<0.05] on ORP when mice were tested 43 days post-lesion. MPTP lesion (all groups) reduced the latency to fall from the Rotarod, both at slow speeds (MPTP: 127.0±7.9 sec vs saline: 146.7±1.9 sec at 14 rpm, p<0.05) and at higher speeds (MPTP: 56.0±11.6 sec vs saline: 90.6±10.0 sec at 26 rpm, p<0.05). However, compared with sedentary animals (ORP for MPTP: 1365.3±150.8, saline: 1596.9±71.5, p<0.05), treadmill exercise significantly increased ORP in both MPTP (1869.4±71.7) and saline-treated mice (1874.8±49.8). By contrast, wheel-running failed to improve performance on the Rotarod in either MPTP (1325.9±186.5 ORP) or saline-treated mice (1730.0±59.7 ORP; p>0.05 vs sedentary mice). Furthermore, although there was considerable individual variability among both wheel-running (range: 10.6 to 26.2 m/min) and ORP (range: 616 to 1964), there was no substantial correlation between voluntary exercise and Rotarod performance (p>0.05).
Figure 2.
Top: Latency to fall (in seconds) from Rotarod spindle at increasing speeds (12–26 rpm) in MPTP- (filled symbols) and saline-treated mice (NaCl, open symbols). Asterisks indicate significant difference between NaCL and MPTP-lesioned mice. Bottom: Overall Rotarod performance (ORP) in MPTP (left) or NaCl unlesioned mice (right), and in mice undergoing forced treadmill exercise (open bars), voluntary wheel running exercise (grey bars) or that were sedentary (filled bars). By 2-factor ANOVA, § indicates effect of lesion, † indicates effect of exercise. In post-hoc analyses, exercise effects are denoted by letters (MPTP: a, b; saline: x, y); bars with common letters in the same lesion group are not different.
Marble Burying
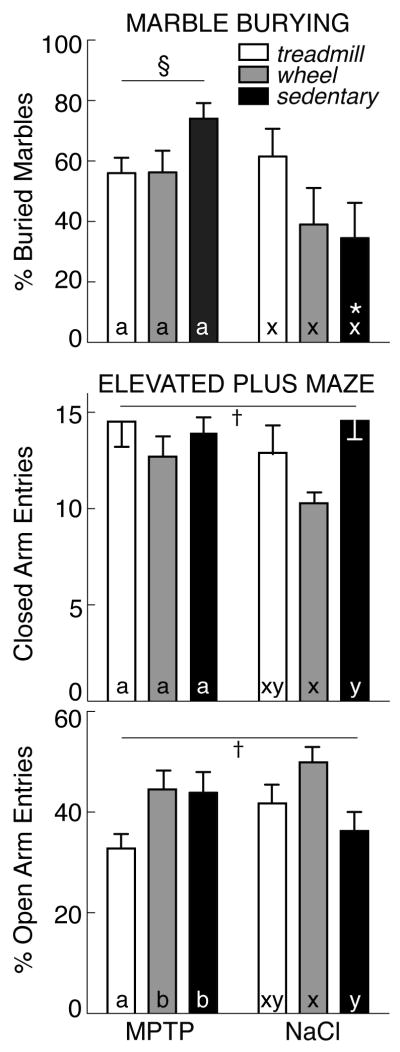
Performance in two tests of anxiety (marble burying, elevated plus maze) is shown in Figure 3. By 2-factor ANOVA, there was a significant effect of lesion on marble burying [F(1, 46) =9.23; p<0.05], but no effect of exercise. MPTP-lesioned mice (all groups) buried more marbles (15.6±0.9 of 25 marbles completely buried, 62.3±3.8%) compared with saline-treated animals (45.1±6.6%). This was particularly evident among sedentary mice: MPTP-lesioned animals buried 74.0±5.2% of the marbles, while saline-treated mice buried only 34.8±11.8% (p<0.05). However, when subject to treadmill or wheel-running exercise, MPTP and saline-treated mice buried equivalent numbers of marbles (56.1±4.4% for MPTP, 50.3±7.9% for saline).
Figure 3.
Top: Percent of marbles buried (mean±SEM) in MPTP- (left) and saline-treated mice (right) that were exercised on a treadmill (open bars) or running wheel (grey bars) or that remained sedentary (filled bars). Bottom: Percent of open arm entries on the elevated plus maze. By 2-factor ANOVA, § indicates effect of lesion, † indicates effect of exercise. In post-hoc analyses, exercise effects are denoted by letters (MPTP: a, b; saline: x, y); bars with common letters in the same lesion group are not different. * identifies differences between MPTP- and saline-treated mice in the same exercise group.
Elevated Plus Maze
In the elevated plus maze, the number of closed arm entries reflects voluntary motor activity, while the percent of open arm entries is a measure of anxiety [84]. There was no significant effect of MPTP lesion on either the number of closed arm entries (13.8±0.7 entries/6 min, all groups) or the percent of open arm entries (40.6±2.4%) compared with saline treatment (12.6±0.7 entries/6 min, 42.9±2.4% p>0.05). However, there was a significant effect of exercise on both the number of closed arm entries [F(2, 45) =3.48; p<0.05], and the percent of open arm entries [F(2, 45)=3.48; p<0.05] (Figure 3). Among saline-treated mice, those exposed to voluntary wheel-running showed an increased percent of open arm entries (49.8±3.5%, p<0.05) relative to sedentary controls (36.2±4.0%, p<0.05). Among MPTP-treated subjects, treadmill exercise reduced the percent of open arm entries (32.6±2.8%) vs sedentary animals (43.9±4.2%, p<0.05). With wheel-running after MPTP lesion, the percent of open arm entries (44.2±3.9%) was not different from sedentary mice (p>0.05).
Sucrose Preference
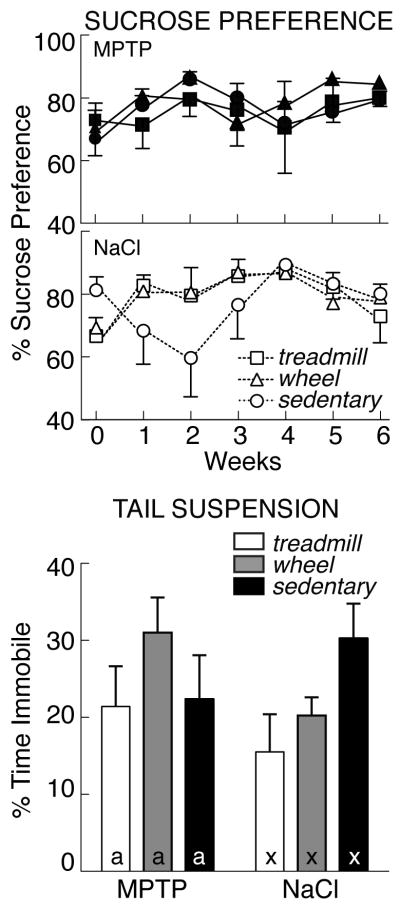
Performance in two tests of depression (sucrose preference, tail suspension) is shown in Figure 4. In a baseline test before lesioning, mice consumed 1.4±0.1 ml, with a 71.2±2.6% preference for a dilute sucrose solution, similar to previous reports [76]. During subsequent weekly tests, there was no effect of MPTP or exercise on sucrose preference. Likewise, there was no difference in preference for sucrose solutions over time. However, as measured by repeated measures ANOVA, there was a significant increase in total fluid consumption during the study (p<0.05). In the final preference test conducted after 6 weeks of exercise or handling, mice drank 2.0±0.1 ml during the 1-hour test, with a 79.1±1.9% sucrose preference.
Figure 4.
Top: Percent sucrose preference (mean±SEM) measured weekly in MPTP- (left) and saline-treated mice (right) that were exercised on a treadmill (open bars) or running wheel (grey bars) or that remained sedentary (filled bars). Bottom: Percent immobility during tail suspension. There was no effect of lesion or exercise in either test of depression. Exercise effects are denoted by letters (MPTP: a, b; saline: x, y); bars with common letters in the same lesion group are not different.
Tail Suspension
The tail suspension test is a measure of behavioral despair. There were no significant effects of MPTP or exercise on immobility in the tail suspension test and no interaction (p>0.05). Immobility in sedentary, saline-treated controls (90.3±17.3 sec, 25.1±4.8% of the 6-minute test) was similar to that reported previously [17]. MPTP-lesioned mice spent an equivalent amount of time immobile (25.0±3.0%, all groups, p>0.05). Likewise, exercise did not alter immobility (treadmill and wheel-running: 22.0±2.7%) compared with sedentary mice (23.6±3.7%, p>0.05).
Endocrine Measurements
Corticosterone
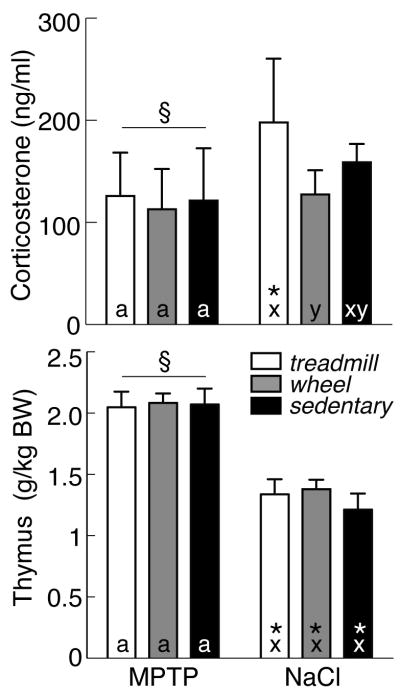
Figure 5 presents endocrine measures of MPTP lesion and exercise. As measured in a single plasma sample collected immediately after the tail suspension test, sedentary saline-treated control mice showed elevated concentrations of circulating corticosterone (158.8±6.4 ng/ml) relative to basal levels reported previously for C57BL/6 mice [8]. By 2-factor ANOVA, there was a significant effect of lesion [F(1, 46)=11.00; p<0.05], but no effect of exercise. Somewhat surprisingly, stress-induced corticosterone was lower in MPTP-treated mice (119.8±9.0 ng/ml, all groups) relative to saline-treated animals (159.7±8.8 ng/ml). In particular, saline-treated mice subject to treadmill exercise had the highest levels of corticosterone (190.0±19.1 ng/ml), significantly exceeding that in both MPTP-treated mice with treadmill exercise (125.9±16.1 ng/ml, p<0.05), and in saline-treated wheel-running animals (130.4±10.3 ng/ml, p<0.05).
Figure 5.
Top: Serum corticosterone concentrations (mean±SEM) in MPTP- (left) and saline-treated mice (right) that were exercised on a treadmill (open bars) or running wheel (grey bars) or that remained sedentary (filled bars). Bottom: Thymus weights. By 2-factor ANOVA, § indicates effect of lesion. In post-hoc analyses, exercise effects are denoted by letters (MPTP: a, b; saline: x, y); bars with common letters in the same lesion group are not different. * identifies differences between MPTP- and saline-treated mice in the same exercise group.
Thymus
Thymus mass is inversely proportional to circulating levels of corticosterone [18, 86]. As with corticosterone, there was a significant effect of lesion on thymus weight [F(1, 46)=67.45; p<0.05], but no effect of exercise. Thymus weight in saline-treated mice averaged 1.3±0.1 g/kg BW (all groups) vs 2.1±0.1 g/kg BW for MPTP-lesioned animals. Furthermore, for each group of saline-treated mice (sedentary, treadmill or wheel-running), thymus weight was significantly below that in the equivalent group of MPTP-treated mice.
Neurochemistry
DA
Figure 6 presents tissue levels of DA expressed as a percent of sedentary saline-treated controls. In controls, CPu and Acb had the highest DA levels: 464.3±61.1 and 179.6±16.6 ng/mg protein, respectively. DA levels in BLA (90.6±11.2 ng/mg protein) and PFC (84.3±12.6 ng/mg protein) were considerably less. As in previous studies from our laboratories [59, 82], MPTP lesion reduced striatal DA, as measured 45 days after lesioning. However, there was no effect of exercise on tissue DA levels in any brain region.
Figure 6.
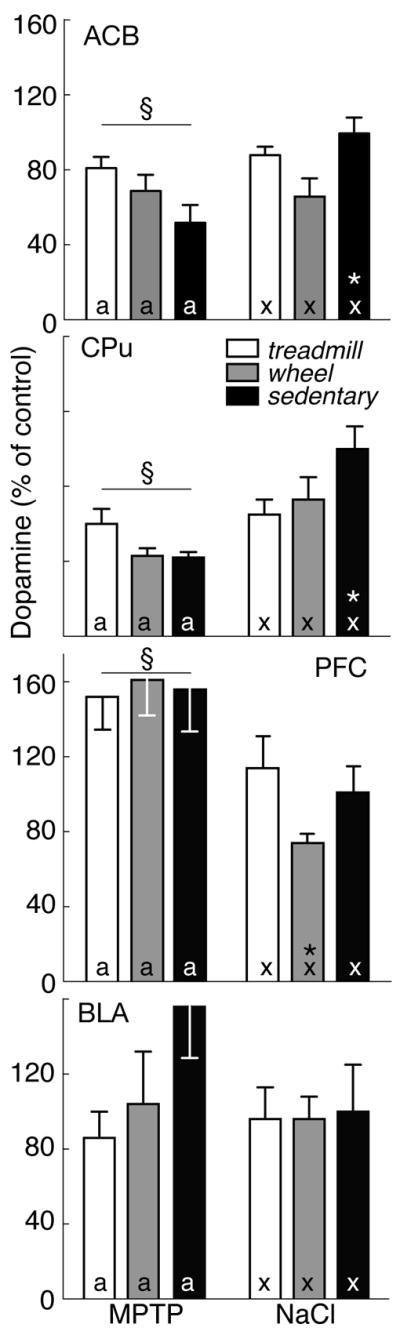
DA levels in four brain regions expressed as percent of saline-treated sedentary controls (mean±SEM) in MPTP- (left) and saline-treated mice (right) that were exercised on a treadmill (open bars) or running wheel (grey bars) or that remained sedentary (filled bars). By 2-factor ANOVA, § indicates effect of lesion. In post-hoc analyses, exercise effects are denoted by letters (MPTP: a, b; saline: x, y); bars with common letters in the same lesion group are not different. * identifies differences between MPTP- and saline-treated mice in the same exercise group.
In CPu, MPTP lesion significantly reduced DA [F(1, 46)=19.39; p<0.05], along with a lesion x exercise interaction. After MPTP, DA levels in CPu of sedentary mice were 42.1±3.1% of saline-treated controls (p<0.05). However, exercise minimized the inhibitory effect of MPTP on DA content. With treadmill exercise, striatal DA was similar in MPTP and saline-treated mice (MPTP: 50.1±11.4%; saline: 64.8±8.5% of sedentary controls). Wheel running produced an equivalent response (MPTP: 43.1±4.3% vs saline: 72.5±12.3%). Thus, DA levels of exercising mice did not differ between MPTP- and saline-treated groups (p>0.05).
MPTP lesion had a similar effect on DA in Acb [F(1, 46) =6.06; p<0.05], as well as an interaction with exercise [F(5, 42) =3.637; p<0.05]. In sedentary mice with MPTP lesion, Acb DA was 51.5±9.0% of that in saline-treated sedentary controls. Although exercise reduced DA levels in Acb of saline-treated mice (treadmill: 87.7±5.3%; wheel-running: 66.4±10.1% of sedentary controls), exercise also prevented further loss of DA with MPTP lesion (treadmill: 80.6±6.9%; wheel-running: 65.0±8.8% of saline controls). Conversely, MPTP increased DA in PFC to 161.1±11.4% of control [F(1, 46) =12.55; p<0.05], but there was no interaction of lesion and exercise. The effect of MPTP was particularly evident in mice exposed to voluntary wheel-running (MPTP: 160.5±20.0% vs saline: 74.1±4.7% of control, p<0.05). There was no effect of MPTP or exercise on DA in BLA (p>0.05).
5HT
Figure 7 presents tissue content of 5HT in the same brain regions. In sedentary, saline-treated controls, levels of 5HT were highest in PFC (2.6±0.2 ng/mg protein) and BLA (2.5±0.2 ng/mg protein). Similar to the DA levels presented above, MPTP altered 5HT content, but exercise had no significant effect. In CPu and BLA, MPTP reduced 5HT to 70.4±6.2% [F(1, 46) =3.85; p<0.05] and 73.0±3.9% [F(1, 46)=7.34; p<0.05] of saline-treated mice, respectively (p<0.05). 5HT increased after MPTP lesion in Acb (to 158.5±13.0% of saline controls) [F(1, 46)=14.37; p<0.05]. Treadmill exercise enhanced Acb 5HT in MPTP-lesioned mice (211.0±18.9%), compared with saline-treated animals (113.4±15.7% of sedentary controls, p<0.05)
Figure 7.
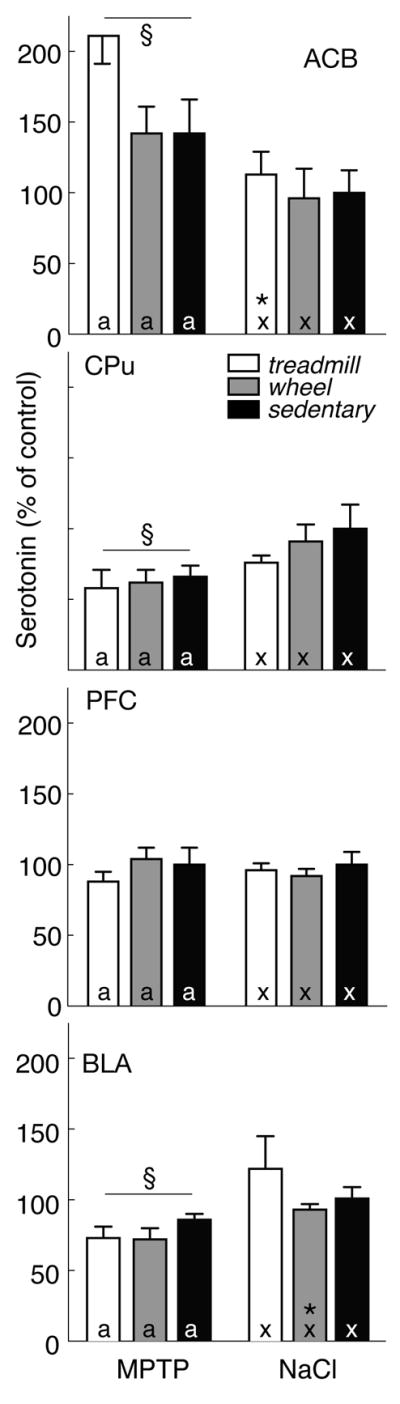
5HT levels in four brain regions expressed as percent of saline-treated sedentary controls (mean±SEM) in MPTP- (left) and saline-treated mice (right) that were exercised on a treadmill (open bars) or running wheel (grey bars) or that remained sedentary (filled bars). By 2-factor ANOVA, § indicates effect of lesion. In post-hoc analyses, exercise effects are denoted by letters (MPTP: a, b; saline: x, y); bars with common letters in the same lesion group are not different. * identifies differences between MPTP- and saline-treated mice in the same exercise group.
DISCUSSION
The present study evaluated the ability of forced or voluntary exercise to overcome the inhibitory effects of MPTP lesions on motor and affective behavior, as well as dopamine and serotonin content in male C57BL/6 mice. Because exercise has been shown to enhance motor function in patients with PD [13, 26, 54] and also improves mood in non-PD patients [3, 5, 7, 20, 56, 58], the present study determined if exercise could manifest similar benefits in a mouse model of PD. For 6 wks after injection of MPTP or saline, mice were subject to vigorous exercise 5 d/wk, up to 1h/day on a treadmill or running wheel, or remained sedentary. MPTP did not reduce voluntary exercise, except during the first week after lesioning. Nonetheless, MPTP lesion impaired performance on the Rotarod. MPTP-induced deficits in motor behavior were paralleled by a reduction in striatal content of DA and 5HT, as reported previously [82]. MPTP was anxiogenic, as measured by increased marble burying. This was accompanied by 5HT depletion in BLA. In contrast, exercise was anxiolytic, as reflected by open arm entries on the elevated plus maze. However, exercise had no effect on brain levels of DA or 5HT. As determined by sucrose preference or tail suspension, there was no effect of MPTP or exercise on depression. Although mice exercising on a running wheel ran further than those exposed to the treadmill, performance on the Rotarod improved only in mice subject to forced exercise. These results demonstrate anxiogenic effects of MPTP lesioning, and anxiolytic effects of exercise. Similarly, MPTP reduces and forced exercise selectively improves motor function. Thus, exercise has the potential to improve both affective and motor behavior in PD patients.
At face value, MPTP is an appropriate animal model for PD because MPTP lesions replicate the loss of dopamine neurons in SNpc that occurs spontaneously in PD [37]. Indeed, MPTP lesions in monkeys and humans also demonstrate key symptoms of motor dysfunction seen in PD patients, including rigidity, bradykinesia, and resting tremor [27, 44]. Although MPTP faithfully and selectively lesions nigrostriatal DA neurons in mice and reduces striatal DA content, the resulting behavioral phenotype demonstrates relatively subtle motor deficits, many of which resolve rapidly [48]. In the present study, there were no differences in running velocity or distance traveled during voluntary wheel-running exercise in control and MPTP-lesioned mice, except during the first week. Likewise, MPTP did not impair voluntary motor activity in the elevated plus maze, as measured by the number of closed arm entries.
Nonetheless, MPTP lesions do impair motor function. Previous studies have demonstrated lasting deficits in performance on the Rotarod [68], as well as on tests of balance, gait [49, 63], and swimming [34]. Different lesioning protocols with MPTP make it difficult to directly compare among published studies. In particular, studies of Rozas et al [68], Haobam et al [34], Liebetanz et al [49], and Pothakos et al [63] use repeated injection with MPTP at intervals of days or weeks to mimic chronic Parkinsonism. By contrast, acute MPTP lesions in the present study (4 injections of 20 mg/kg free base MPTP, each 2h apart) allow behavioral and neurochemical recovery over time [38]. In this manner, our injection paradigm represents a dynamic model, but in the opposite direction from PD, where symptoms become progressively worse. Despite the potential for recovery during 44 days after MPTP, motor and neurochemical deficits persist in the present study, as demonstrated by reductions in both ORP and dopamine content in CPu and Acb.
Fewer studies have examined the effects of MPTP lesions on affective behavior. Previous studies have demonstrated increased immobility in the tail suspension test 30 days post-MPTP [55, 82], as well as mild cognitive impairment in the social transmission of food preference test [82]. In a similar manner, anxiety is increased in parkin-deficient mice, a knockout model of early-onset familial PD [88]. The present study expands upon these findings, revealing a selective increase in anxiety as demonstrated with marble burying, along with decreased levels of 5HT in BLA. 5HT in BLA is strongly implicated in fear and anxiety [10]. The MPTP-induced reduction in 5HT content is consistent with this role. Importantly, anxiogenic responses in the marble burying test are not confounded by any MPTP-induced motor deficits, since anxious mice bury more marbles. However, 5HT content was selectively decreased by MPTP in BLA and CPu. By contrast, 5HT content in Acb of MPTP-lesioned mice was increased above that in saline-treated animals, as reported previously [39, 67, 89].
Interestingly, MPTP failed to elicit similar anxiogenic responses in the elevated plus maze. In this regard, defensive burying represents an active coping strategy in response to a discrete threat. By contrast, anxious behavior in the elevated plus maze is expressed as a passive avoidance response to a potential threat. Different brain circuits may regulate responses to these two challenges [33]. Our findings suggest that DA depletion in SNpc selectively increases anxiety in response to proximal discrete threat stimuli. As reflected in performance on the elevated plus maze, exploratory behavior involving reward circuits through the ventral striatum and lateral hypothalamus is unaffected by MPTP, at least when tested 44 days after MPTP. The absence of anhedonia in the sucrose preference test also supports this conclusion. Alternatively, it is possible that faster recovery of the ventral striatal circuits post-lesion accounts for the lack of anxiety symptoms in the elevated plus maze. In this regard, sucrose preference was tested weekly throughout the study, and we did not observe evidence of transient depressive symptoms that resolved before mice were tested at 44 days post-lesion.
Investigating potential mood effects of MPTP in mice has clinical relevance for understanding neurologic function in PD patients. Disturbance of mood is a common condition in PD, with an average prevalence of 25–40% in outpatient settings for major depressive disorders [46, 51], and up to 40% for anxiety disorders [85]. During early stages of illness, when DA cell loss and/or dysfunction are insufficient to elicit prominent motor deficits, mood symptoms may be the first sign of brain degeneration [45, 64, 74]. Later, with the initiation of DA replacement therapy, mood symptoms are seen in as many as 75% of patients with motor fluctuations. In particular, depression or anxiety is reported in the ‘off’ period (when there is an absence of the normal motor response to antiparkinsonian medications) and a neutral or elevated mood in the ‘on’ state (the subjective sense that they are having a motor response to their medication). In addition, depressive and anxiety disorders occur frequently independent of motor fluctuations.
Since PD is a movement disorder, it stands to reason that exercise training improves motor function in PD patients and MPTP-lesioned mice [25, 26, 78]. Clinical trials and epidemiologic studies have supported the potential benefits of exercise in PD patients [12, 23]. Epidemiologic studies suggest a role of exercise in lowering the risk for PD, while clinical trials in patients with PD have shown functional benefits, including the unified PD rating scale, functional limitations (walking, stair climbing), gait training, daily activities, and breathing [13, 26, 70]. Using MPTP lesions in mice, previous studies have shown improvement in gait and balance, with exercise training [63, 80]. However, in the present study, we were surprised to find that ORP improved only with treadmill exercise training, and not after voluntary wheel-running. This finding was particularly unexpected since both MPTP-lesioned mice and saline controls ran further and faster during voluntary exercise on a running wheel than when subject to forced treadmill exercise. Vigorous running wheel activity after MPTP lesion is consistent with the large number of closed arm entries and lack of sucrose anhedonia in these mice, and suggests that exploratory drive and basic locomotor activity is not compromised by MPTP. However, performance on the Rotarod reflects more than exercise capacity, and may have a learning component. In fact, the Rotarod ressembles a round treadmill. To avoid falling, mice must maintain a constant and consistent pace, similar to forced exercise. Ultimately, tests of exercise on motor function must take into account the specific exercise training regimen.
The present study supports and extends previous studies suggesting the potential for exercise to improve affective behavior in animal models of PD. In particular, our study is the first to compare forced vs voluntary exercise after MPTP lesion. It is interesting to note that voluntary wheel-running reduced anxiety, as measured by an increase in percent of open arm entries in the elevated plus maze. Treadmill exercise was without effect. Circulating levels of stress-induced corticosterone were elevated in mice subject to treadmill exercise, compared with those exposed to voluntary wheel-running. Thus, the stress of forced exercise may diminish the potential benefits of exercise on affective behavior.
The potential benefits of exercise on mental health are widely accepted, even in the popular press. A variety of studies have reported beneficial effects of exercise on depression and anxiety in humans, including improvements in mood disturbances associated with chronic disease [3, 5, 7, 20, 56, 58]. Furthermore, many studies show that the effects of exercise on mood may also be sustained. Considering the number of studies investigating exercise effects on anxiety and depression in humans, it is remarkable that we know so little about the underlying mechanisms. It has been hypothesized that the beneficial effects of exercise may reflect increased levels of brain-derived neurotrophic factor in the hippocampus [6, 87] or improvement in circadian rhythmicity [73]. It seems likely that exercise also has a variety of effects on neurotransmitters and neurotrophic factors, including glutamate [81], 5-HT, and DA. An earlier study in aged mice subject to MPTP lesion found an increase in striatal DA with exercise compared to sedentary lesioned animals [78]. However, exercise was initiated 12h post-MPTP, thereby including both neuroprotective and neurorehabilitative effects. In the present study, exercise beginning 5 days after MPTP had minimal impact on DA or 5HT content in brain regions important for mood or motor function. Similar results have been reported recently with forced exercise after MPTP in mice [2, 59], and with treadmill exercise in humans [83]. Nonetheless, at some level exercise improves mood and motor behavior through dynamics of neurotransmitter content and/or release.
Acknowledgments
We thank Drs. Satoru M. Sato and Eleni Antzoulatos for assistance with behavioral testing and tissue preparation. This work supported by grants from the NIH (DC009125 to RIW, NS44327 to MWJ) and the US Army (NETRP W81XWH-04-1-0444 to MWJ).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aarsland D, Larsen JP, Lim NG, Janvin C, Karlsen K, Tandberg E, Cummings JL. Range of neuropsychiatric disturbances in patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1999;67(4):492–496. doi: 10.1136/jnnp.67.4.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Jarrah M, Pothakos K, Novikova, Smirnova IV, Kurz MJ, Stehno-Bittel L, Lau YS. Endurance exercise promotes cardiorespiratory rehabilitation without neurorestoration in the chronic mouse model of parkinsonism with severe neurodegeneration. Neuroscience. 2007;149(1):28–37. doi: 10.1016/j.neuroscience.2007.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Babyak M, Blumenthal JA, Herman S, Khatri P, Doraiswamy M, Moore K, Craighead WE, Baldewicz TT, Krishnan KR. Exercise treatment for major depression: maintenance of therapeutic benefit at 10 months. Psychosom Med. 2000;62:633–638. doi: 10.1097/00006842-200009000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Bachmanov AA, Li X, Reed DR, Ohmen JD, Li S, Chen Z, Tordoff MG, de Jong PJ, Wu C, West DB, Chatterjee A, Ross DA, Beauchamp GK. Positional cloning of the mouse saccharin preference (Sac) locus. Chem Senses. 2001;26(7):925–933. doi: 10.1093/chemse/26.7.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbour KA, Blumenthal JA. Exercise training and depression in older adults. Neurobiol Aging. 2005;26 (Suppl 1):119–123. doi: 10.1016/j.neurobiolaging.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 6.Berchtold NC, Chinn G, Chou M, Kesslak JP, Cotman CW. Exercise primes a molecular memory for brain-derived neurotrophic factor protein induction in the rat hippocampus. Neuroscience. 2005;133(3):853–861. doi: 10.1016/j.neuroscience.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 7.Blumenthal JA, Babyak MA, Moore KA, Craighead WE, Herman S, Khatri P, Waugh R, Napolitano MA, Forman LM, Appelbaum M, Doraiswamy PM, Krishnan KR. Effects of exercise training on older patients with major depression. Arch Intern Med. 1999;159:2349–2356. doi: 10.1001/archinte.159.19.2349. [DOI] [PubMed] [Google Scholar]
- 8.Brinks V, de Kloet ER, Oitzl MS. Strain specific fear behaviour and glucocorticoid response to aversive events: modelling PTSD in mice. Prog Brain Res. 2008;167:257–261. doi: 10.1016/S0079-6123(07)67019-8. [DOI] [PubMed] [Google Scholar]
- 9.Brown MR, Rivier C, Vale W. Central nervous system regulation of adrenocorticotropin secretion: role of somatostatins. Endocrinology. 1984;114(5):1546–1549. doi: 10.1210/endo-114-5-1546. [DOI] [PubMed] [Google Scholar]
- 10.Charney DS. Neuroanatomical circuits modulating fear and anxiety behaviors. Acta Psychiatr Scand. 2003;108(Suppl 417):38–50. doi: 10.1034/j.1600-0447.108.s417.3.x. [DOI] [PubMed] [Google Scholar]
- 11.Chaudhuri KR, Healy DG, Schapira AH National Institute for Clinical Excellence. Non-motor symptoms of Parkinson’s disease: diagnosis and management. Lancet Neurol. 2006;5(3):235–245. doi: 10.1016/S1474-4422(06)70373-8. [DOI] [PubMed] [Google Scholar]
- 12.Chen H, Zhang SM, Schwarzschild MA, Hernán MA, Ascherio A. Physical activity and the risk of Parkinson disease. Neurology. 2005;64(4):664–669. doi: 10.1212/01.WNL.0000151960.28687.93. [DOI] [PubMed] [Google Scholar]
- 13.Comella CL, Stebbins GT, Brown-Toms N, Goetz CG. Physical therapy and Parkinson’s disease: a controlled clinical trial. Neurology. 1994;44(3 Pt 1):376–378. doi: 10.1212/wnl.44.3_part_1.376. [DOI] [PubMed] [Google Scholar]
- 14.Crawley JN. Neuropharmacologic specificity of a simple animal model for the behavioral actions of benzodiazepines. Pharmacol Biochem Behav. 1981;15(5):695–659. doi: 10.1016/0091-3057(81)90007-1. [DOI] [PubMed] [Google Scholar]
- 15.Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, Hitzemann RJ, Maxson SC, Miner LL, Silva AJ, Wehner JM, Wynshaw-Boris A, Paylor R. Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacology (Berl) 1997;132(2):107–124. doi: 10.1007/s002130050327. [DOI] [PubMed] [Google Scholar]
- 16.Crawley JN, Davis LG. Baseline exploratory activity predicts anxiolytic responsiveness to diazepam in five mouse strains. Brain Res Bull. 1982;8(6):609–612. doi: 10.1016/0361-9230(82)90087-9. [DOI] [PubMed] [Google Scholar]
- 17.Cryan JF, Mombereau C, Vassout A. The tail suspension test as a model for assessing antidepressant activity: Review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev. 2005;29(4–5):571–625. doi: 10.1016/j.neubiorev.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 18.Dallman MF, Akana SF, Cascio CS, Darlington DN, Jacobson L, Levin N. Regulation of ACTH secretion: variations on a theme of B. Recent Prog Horm Res. 1987;43:113–173. doi: 10.1016/b978-0-12-571143-2.50010-1. [DOI] [PubMed] [Google Scholar]
- 19.Dauer W, Przedborski S. Parkinson’s disease: mechanisms and models. Neuron. 2003;39(6):889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 20.De Moor MH, Beem AL, Stubbe JH, Boomsma DI, De Geus EJ. Regular exercise, anxiety, depression and personality: a population-based study. Prev Med. 2006;42:273–279. doi: 10.1016/j.ypmed.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 21.Duman CH, Schlesinger L, Russell DS, Duman RS. Voluntary exercise produces antidepressant and anxiolytic behavioral effects in mice. Brain Res. 2008;1199:148–158. doi: 10.1016/j.brainres.2007.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fahn S. Description of Parkinson’s disease as a clinical syndrome. Ann NY Acad Sci. 2003;991:1–14. doi: 10.1111/j.1749-6632.2003.tb07458.x. [DOI] [PubMed] [Google Scholar]
- 23.Falvo MJ, Schilling BK, Earhart GM. Parkinson’s disease and resistive exercise: rationale, review, and recommendations. Mov Disord. 2008;23(1):1–11. doi: 10.1002/mds.21690. [DOI] [PubMed] [Google Scholar]
- 24.File SE, Lippa AS, Beer B, Lippa MT. Animal tests of anxiety. Curr Protoc Neurosci. 2004:8318–8322. doi: 10.1002/0471142301.ns0803s26. [DOI] [PubMed] [Google Scholar]
- 25.Fisher BE, Petzinger GM, Nixon K, Hogg E, Bremmer S, Meshul CK, Jakowec MW. Exercise-induced behavioral recovery and neuroplasticity in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse basal ganglia. J Neurosci Res. 2004;77(3):378–390. doi: 10.1002/jnr.20162. [DOI] [PubMed] [Google Scholar]
- 26.Fisher BE, Wu AD, Salem GJ, Song J, Lin CH, Yip J, Cen S, Gordon J, Jakowec M, Petzinger G. The effect of exercise training in improving motor performance and corticomotor excitability in people with early Parkinson’s disease. Arch Phys Med Rehabil. 2008;89(7):1221–1229. doi: 10.1016/j.apmr.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Forno LS, DeLanney LE, Irwin I, Langston JW. Neuropathology of MPTP-treated monkeys. Comparison with the neuropathology of human idiopathic Parkinson’s Disease. In: Markey SP, Castagnoli NJ, Trevor AJ, Kopin IJ, editors. MPTP: A Neurotoxin Producing a Parkinsonian Syndrome. San Diego: Academic Press; 1986. pp. 119–139. [Google Scholar]
- 28.Frazier CR, Mason P, Zhuang X, Beeler JA. Sucrose exposure in early life alters adult motivation and weight gain. PLoS ONE. 2008;3(9):e3221. doi: 10.1371/journal.pone.0003221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fukai T, Siegfried MR, Ushio-Fukai M, Cheng Y, Kojda G, Harrison DG. Regulation of the vascular extracellular superoxide dismutase by nitric oxide and exercise training. J Clin Invest. 2000;105(11):1631–1639. doi: 10.1172/JCI9551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gerlach M, Riederer P. Animal models of Parkinson’s disease: an empirical comparison with the phenomenology of the disease in man. J Neural Transm. 1996;103(8–9):987–1041. doi: 10.1007/BF01291788. [DOI] [PubMed] [Google Scholar]
- 31.Gorton LM, Khan AM, Bohland M, Sanchez-Watts G, Donovan CM, Watts AG. A role for the forebrain in mediating time-of-day differences in glucocorticoid counterregulatory responses to hypoglycemia in rats. Endocrinology. 2007;148(12):6026–6039. doi: 10.1210/en.2007-0194. [DOI] [PubMed] [Google Scholar]
- 32.Gudelsky GA, Yamamoto BK. Actions of 3,4-methylenedioxymethamphetamine (MDMA) on cerebral dopaminergic, serotonergic and cholinergic neurons. Pharmacol Biochem Behav. 2008;90(2):198–207. doi: 10.1016/j.pbb.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hakvoort Schwerdtfeger RM, Menard JL. The lateral hypothalamus and anterior hypothalamic nucleus differentially contribute to rats’ defensive responses in the elevated plus-maze and shock-probe burying tests. Physiol Behav. 2008;93(4–5):697–705. doi: 10.1016/j.physbeh.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 34.Haobam R, Sindhu KM, Chandra G, Mohanakumar KP. Swim-test as a function of motor impairment in MPTP model of Parkinson’s disease: a comparative study in two mouse strains. Behav Brain Res. 2005;163(2):159–167. doi: 10.1016/j.bbr.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 35.Hogg S. A review of the validity and variability of the elevated plus-maze as an animal model of anxiety. Pharmacol Biochem Behav. 1996;54(1):21–30. doi: 10.1016/0091-3057(95)02126-4. [DOI] [PubMed] [Google Scholar]
- 36.Irwin I, Finnegan KT, Delanney LE, Di Monte D, Langston JW. The relationships between aging, monoamine oxidase, striatal dopamine and the effects of MPTP in C57BL/6 mice: a critical reassessment. Brain Res. 1992;572(1–2):224–231. doi: 10.1016/0006-8993(92)90473-m. [DOI] [PubMed] [Google Scholar]
- 37.Jackson-Lewis V, Jakowec MW, Burke RE, Przedborski S. Time course and morphology of dopaminergic neuronal death caused by the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Neurodegeneration. 1995;4(3):257–269. doi: 10.1016/1055-8330(95)90015-2. [DOI] [PubMed] [Google Scholar]
- 38.Jakowec MW, Petzinger GM. 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned model of parkinson’s disease, with emphasis on mice and nonhuman primates. Comp Med. 2004;54(5):497–513. [PubMed] [Google Scholar]
- 39.Kapitza IG, Kalinina TS, Nerobkova LN, Voronina TA, Klodt PM, Narkevich VB, Kudrin VS. Relationship between the severity of hypokinesia induced by neurotoxin 1-methyl-4-phenyl-1,2,3,6-tretrahydropyridine and neurochemical changes in brain structures of C57BL/6 mice. Bull Exp Biol Med. 2008;146(1):52–55. doi: 10.1007/s10517-008-0204-5. [DOI] [PubMed] [Google Scholar]
- 40.Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, Wittchen HU, Kendler KS. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- 41.Kilpatrick IC, Jones MW, Phillipson OT. A semiautomated analysis method for catecholamines, indoleamines, and some prominent metabolites in microdissected regions of the nervous system: an isocratic HPLC technique employing coulometric detection and minimal sample preparation. J Neurochem. 1986;46(6):1865–1876. doi: 10.1111/j.1471-4159.1986.tb08506.x. [DOI] [PubMed] [Google Scholar]
- 42.Klein DF. Endogenomorphic depression A conceptual and terminological revision. Arch Gen Psychiatry. 1974;31:447–454. doi: 10.1001/archpsyc.1974.01760160005001. [DOI] [PubMed] [Google Scholar]
- 43.Kojda G, Cheng YC, Burchfield J, Harrison DG. Dysfunctional regulation of endothelial nitric oxide synthase (eNOS) expression in response to exercise in mice lacking one eNOS gene. Circulation. 2001;103(23):2839–2844. doi: 10.1161/01.cir.103.23.2839. [DOI] [PubMed] [Google Scholar]
- 44.Langston JW, Ballard P, Tetrud JW, Irwin I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 1983;219(4587):979–980. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]
- 45.Lauterbach EC, Duvoisin RC. Anxiety disorders in familial parkinsonism. Am J Psychiatry. 1991;148:274. [PubMed] [Google Scholar]
- 46.Leentjens AF. Depression in Parkinson’s disease: conceptual issues and clinical challenges. J Geriatr Psychiatry Neurol. 2004;17:120–126. doi: 10.1177/0891988704267456. [DOI] [PubMed] [Google Scholar]
- 47.Lemke MR. Depressive symptoms in Parkinson’s disease. Eur J Neurol. 2008;15 (Suppl 1):21–25. doi: 10.1111/j.1468-1331.2008.02058.x. [DOI] [PubMed] [Google Scholar]
- 48.Leng A, Mura A, Hengerer B, Feldon J, Ferger B. Effects of blocking the dopamine biosynthesis and of neurotoxic dopamine depletion with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) on voluntary wheel running in mice. Behav Brain Res. 2004;154(2):375–383. doi: 10.1016/j.bbr.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 49.Liebetanz D, Baier PC, Paulus W, Meuer K, Bahr M, Weishaupt JH. A highly sensitive automated complex running wheel test to detect latent motor deficits in the mouse MPTP model of Parkinson’s disease. Exp Neurol. 2007;205(1):207–213. doi: 10.1016/j.expneurol.2007.01.030. [DOI] [PubMed] [Google Scholar]
- 50.Macht M, Schwarz R, Ellgring H. Patterns of psychological problems in Parkinson’s disease. Acta Neurol Scand. 2005;111(2):95–101. doi: 10.1111/j.1600-0404.2005.00375.x. [DOI] [PubMed] [Google Scholar]
- 51.Marsh L, McDonald WM, Cummings J, Ravina B. Provisional diagnostic criteria for depression in Parkinson’s disease: Report of an NINDS/NIMH Work Group. Mov Disord. 2005;21(2):148–158. doi: 10.1002/mds.20723. [DOI] [PubMed] [Google Scholar]
- 52.Mathis C, Neumann PE, Gershenfeld H, Paul SM, Crawley JN. Genetic analysis of anxiety-related behaviors and responses to benzodiazepine-related drugs in AXB and BXA recombinant inbred mouse strains. Behav Genet. 1995;25(6):557–568. doi: 10.1007/BF02327579. [DOI] [PubMed] [Google Scholar]
- 53.Mitra N, Mohanakumar KP, Ganguly DK. Dissociation of serotoninergic and dopaminergic components in acute effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in mice. Brain Res Bull. 1992;28(3):355–364. doi: 10.1016/0361-9230(92)90035-v. [DOI] [PubMed] [Google Scholar]
- 54.Miyai I, Fujimoto Y, Ueda Y, Yamamoto H, Nozaki S, Saito T, Kang J. Treadmill training with body weight support: its effect on Parkinson’s disease. Arch Phys Med Rehabil. 2000;81(7):849–852. doi: 10.1053/apmr.2000.4439. [DOI] [PubMed] [Google Scholar]
- 55.Mori A, Ohashi S, Nakai M, Moriizumi T, Mitsumoto Y. Neural mechanisms underlying motor dysfunction as detected by the tail suspension test in MPTP-treated C57BL/6 mice. Neuroscience Res. 2005;51(3):265–274. doi: 10.1016/j.neures.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 56.Motl RW, Konopack JF, McAuley E, Elavsky S, Jerome GJ, Marquez DX. Depressive symptoms among older adults: long-term reduction after a physical activity intervention. J Behav Med. 2005;28:385–394. doi: 10.1007/s10865-005-9005-5. [DOI] [PubMed] [Google Scholar]
- 57.Njung’e K, Handley SL. Evaluation of marble-burying behavior as a model of anxiety. Pharmacol Biochem Behav. 1991;38(1):63–67. doi: 10.1016/0091-3057(91)90590-x. [DOI] [PubMed] [Google Scholar]
- 58.Penninx BW, Rejeski WJ, Pandya J, Miller ME, Di Bari M, Applegate WB, Pahor M. Exercise and depressive symptoms: a comparison of aerobic and resistance exercise effects on emotional and physical function in older persons with high and low depressive symptomatology. J Gerontol B Psychol Sci Soc Sci. 2002;57:124–132. doi: 10.1093/geronb/57.2.p124. [DOI] [PubMed] [Google Scholar]
- 59.Petzinger GM, Walsh JP, Akopian G, Hogg E, Abernathy A, Arevalo P, Turnquist P, Vucković M, Fisher BE, Togasaki DM, Jakowec MW. Effects of treadmill exercise on dopaminergic transmission in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse model of basal ganglia injury. J Neurosci. 2007;27(20):5291–5300. doi: 10.1523/JNEUROSCI.1069-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pileblad E, Nissbrandt H, Carlsson A. Biochemical and functional evidence for a marked dopamine releasing action of N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (NMPTP) in mouse brain. J Neural Transm Gen Sect. 1984;60(3–4):199–203. doi: 10.1007/BF01249093. [DOI] [PubMed] [Google Scholar]
- 61.Poewe W. Non-motor symptoms in Parkinson’s disease. Eur J Neurol. 2008;15 (Suppl 1):14–20. doi: 10.1111/j.1468-1331.2008.02056.x. [DOI] [PubMed] [Google Scholar]
- 62.Porsolt RD, Brossard G, Hautbois C, Roux S. Rodent models of depression: forced swimming and tail suspension behavioral despair tests in rats and mice. Curr Prot Neurosci. 2004:8.10.A1–8.10.A10. doi: 10.1002/0471142301.ns0810as14. [DOI] [PubMed] [Google Scholar]
- 63.Pothakos K, Kurz MJ, Lau YS. Restorative effect of endurance exercise on behavioral deficits in the chronic mouse model of Parkinson’s disease with severe neurodegeneration. BMC Neuroscience. 2009;10:6. doi: 10.1186/1471-2202-10-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Richard IH, Schiffer RB, Kurlan R. Anxiety and Parkinson’s disease. J Neuropsychiatry Clin Neurosci. 1996;8:383–392. doi: 10.1176/jnp.8.4.383. [DOI] [PubMed] [Google Scholar]
- 65.Rozas G, Guerra MJ, Labandeira Garcia JL. An automated Rotarod method for quantitative drug-free evaluation of overall motor deficits in rat models of parkinsonism. Brain Res Protoc. 1997;2(1):75–84. doi: 10.1016/s1385-299x(97)00034-2. [DOI] [PubMed] [Google Scholar]
- 66.Rozas G, Labandeira Garcia JL. Drug-free evaluation of rat models of parkinsonism and nigral grafts using a new automated Rotarod test. Brain Res. 1997;749(2):188–199. doi: 10.1016/S0006-8993(96)01162-6. [DOI] [PubMed] [Google Scholar]
- 67.Rozas G, Liste I, Guerra MJ, Labandeira-García JL. Sprouting of the serotonergic afferents into the striatum after selective lesions of the dopaminergic system by MPTP in adult mice. Neurosci Letters. 1998;245(3):151–154. doi: 10.1016/s0304-3940(98)00198-0. [DOI] [PubMed] [Google Scholar]
- 68.Rozas G, Lopez-Martin E, Guerra MJ, Labandeira-García JL. The overall rod performance test in the MPTP-treated-mouse model of Parkinsonism. J Neurosci Methods. 1998;83(2):165–175. doi: 10.1016/s0165-0270(98)00078-8. [DOI] [PubMed] [Google Scholar]
- 69.Rygula R, Abumaria N, Flügge G, Fuchs E, Rüther E, Havemann-Reinecke U. Anhedonia and motivational deficits in rats: impact of chronic social stress. Behav Brain Res. 2005;162(1):127–134. doi: 10.1016/j.bbr.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 70.Schenkman M, Hall D, Kumar R, Kohrt WM. Endurance exercise training to improve economy of movement of people with Parkinson disease: three case reports. Phys Ther. 2008;88(1):63–76. doi: 10.2522/ptj.20060351. [DOI] [PubMed] [Google Scholar]
- 71.Schrag A, Jahanshahi M, Quinn N. How does Parkinson’s disease affect quality of life? A comparison with quality of life in the general population. Mov Disord. 2000;15(6):1112–1118. doi: 10.1002/1531-8257(200011)15:6<1112::aid-mds1008>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 72.Schuurman AG, van den Akker M, Ensinck KT, Metsemakers JF, Knottnerus JA, Leentjens AF, Buntinx F. Increased risk of Parkinson’s disease after depression: a retrospective cohort study. Neurology. 2002;58(10):1501–1504. doi: 10.1212/wnl.58.10.1501. [DOI] [PubMed] [Google Scholar]
- 73.Solberg LC, Horton TH, Turek FW. Circadian rhythms and depression: effects of exercise in an animal model. Am J Physiol. 1999;276:R152–161. doi: 10.1152/ajpregu.1999.276.1.R152. [DOI] [PubMed] [Google Scholar]
- 74.Stein MB, Heuser IJ, Juncos JL, Uhde TW. Anxiety disorders in patients with Parkinson’s disease. Am J Psychiatry. 1990;147:217–220. doi: 10.1176/ajp.147.2.217. [DOI] [PubMed] [Google Scholar]
- 75.Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology (Berl) 1985;85(3):367–170. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- 76.Strekalova T, Spanagel R, Bartsch D, Henn FA, Gass P. Stress-induced anhedonia in mice is associated with deficits in forced swimming and exploration. Neuropsychopharmacology. 2004;29(11):2007–2017. doi: 10.1038/sj.npp.1300532. [DOI] [PubMed] [Google Scholar]
- 77.Sundstrom E, Stromberg I, Tsutsumi T, Olson L, Jonsson G. Studies on the effect of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) on central catecholamine neurons in C57BL/6 mice Comparison with three other strains of mice. Brain Res. 1987;405(1):26–38. doi: 10.1016/0006-8993(87)90986-3. [DOI] [PubMed] [Google Scholar]
- 78.Tillerson JL, Caudle WM, Reverón ME, Miller GW. Exercise induces behavioral recovery and attenuates neurochemical deficits in rodent models of Parkinson’s disease. Neuroscience. 2003;119(3):899–911. doi: 10.1016/s0306-4522(03)00096-4. [DOI] [PubMed] [Google Scholar]
- 79.Tolosa E, Compta Y, Gaig C. The premotor phase of Parkinson’s disease. Parkinsonism Relat Disord. 2007;13 (Suppl):S2–7. doi: 10.1016/j.parkreldis.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 80.Uday G, Pravinkumar B, Manish W, Sudhir U. LHRH antagonist attenuates the effect of fluoxetine on marble-burying behavior in mice. Eur J Pharmacol. 2007;563(1–3):155–159. doi: 10.1016/j.ejphar.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 81.VanLeeuwen JE, Petzinger GM, Walsh JP, Akopian GK, Vuckovic M, Jakowec MW. J Neurosci Res. 2010;88(3):650–668. doi: 10.1002/jnr.22216. [DOI] [PubMed] [Google Scholar]
- 82.Vuckovic MG, Wood RI, Holschneider DP, Abernathy A, Togasaki DM, Smith A, Petzinger GM, Jakowec MW. Memory, mood, dopamine, and serotonin in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse model of basal ganglia injury. Neurobiol Dis. 2008;32(2):319–327. doi: 10.1016/j.nbd.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang GJ, Volkow ND, Fowler JS, Franceschi D, Logan J, Pappas NR, Wong CT, Netusil N. PET studies of the effects of aerobic exercise on human striatal dopamine release. J Nuclear Med. 2000;41(8):1352–1356. [PubMed] [Google Scholar]
- 84.Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc. 2007;2(2):322–328. doi: 10.1038/nprot.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Walsh K, Bennett G. Parkinson’s disease and anxiety. Postgrad Med J. 2001;77:89–93. doi: 10.1136/pmj.77.904.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Watts AG, Sanchez-Watts G. Interactions between heterotypic stressors and corticosterone reveal integrative mechanisms for controlling corticotropin-releasing hormone gene expression in the rat paraventricular nucleus. J Neurosci. 2002;22(14):6282–6289. doi: 10.1523/JNEUROSCI.22-14-06282.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zheng H, Liu Y, Li W, Yang B, Chen D, Wang X, Jiang Z, Wang H, Wang Z, Cornelisson G, Halberg F. Beneficial effects of exercise and its molecular mechanisms on depression in rats. Behav Brain Res. 2006;168:47–55. doi: 10.1016/j.bbr.2005.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhu XR, Maskri L, Herold C, Bader V, Stichel CC, Gunturkun O, Lubbert H. Non-motor behavioural impairments in parkin-deficient mice. Eur J Neuroscience. 2007;26(7):1902–1911. doi: 10.1111/j.1460-9568.2007.05812.x. [DOI] [PubMed] [Google Scholar]
- 89.Zigmond MJ, Berger TW, Grace AA, Stricker EM. Compensatory responses to nigrostriatal bundle injury Studies with 6-hydroxydopamine in an animal model of parkinsonism. Mol Chem Neuropathol. 1989;10(3):185–200. doi: 10.1007/BF03159728. [DOI] [PubMed] [Google Scholar]