Abstract
Asthma and allergy are characterized by dysregulation of inflammatory responses toward Th2 responses and high serum levels of IgE. IgE plays a role in the effector phase by triggering the degranulation of mast cells after antigen-cross-linking but its role in the induction of helper T cell differentiation is unknown. We have previously shown lymphotoxin is required for maintaining physiological levels of serum IgE which minimize spontaneous Th1-mediated airway inflammation, suggesting a physiological role for IgE in the regulation of T helper cell differentiation. We describe the mechanism in which IgE modulates inflammation by regulating dendritic cell cytokine production. Physiological levels of IgE suppress IL-12 production in the spleen and lung, suggesting IgE limits Th1 responses in vivo. IgE directly stimulates dendritic cells through FcγRIII to suppress IL-12 in vitro and influences APC to skew CD4+ T cells toward Th2 differentiation. We demonstrate a novel role for IgE in regulating differentiation of adaptive inflammatory responses through direct interaction with FcγRIII on dendritic cells.
Keywords: IgE, helper T cells, dendritic cells, airway inflammation
1. Introduction
Allergic asthma is characterized by T helper type 2 (Th2) inflammation, airway hyperreactivity, eosinophilia, and elevated levels of serum IgE which correlate with the severity of the disease [1; 2; 3; 4]. The pathological role of IgE has been studied extensively and shown to mediate anaphylaxis, uticaria, and allergic rhinitis at the effector phase of inflammation [5; 6]. In these models it is proposed that IgE triggers mast cell degranulation through antigen crosslinking [7]. The high affinity receptor for IgE, FcεRI, is expressed on mast cells (MC) and basophils in the mouse, and also dendritic cells (DC) in humans [7]. Through this receptor, IgE mediates MC activation, survival and production of many inflammatory mediators in both antigen dependent and independent manners [8; 9; 10]. The role of mast cells in regulating components of allergic airway inflammation is controversial with a primary focus on their role to mediate airway hyperreactivity [11; 12; 13; 14].
Dendritic cells are the initiators and modulators of the immune response and translate innate signals from TLR ligands into antigen specific adaptive responses by stimulating T and B cells [15]. The production of IL-12 from dendritic cells is critical for the development of Th1 responses and can be regulated by toll like receptor (TLR) signaling [16; 17; 18; 19]. The mechanisms for how DC promote Th2 differentiation are less well understood.
Our previous studies have shown lymphotoxin is required for IgE production necessary for lung homeostasis and the prevention of spontaneous Th1-mediated airway inflammation [20]. Furthermore, mice that have been depleted of IgE show similar spontaneous Th1 inflammatory responses in the lung. Together, these data suggest IgE plays a physiological role in the homeostasis of T helper cell differentiation. In this study we show IgE signals through FcγRIII on DC to suppress IL-12 production and promote Th2 differentiation demonstrating a novel target cell for IgE activity. IgE may therefore, maintain T helper cell homeostasis, influencing CD4+ T helper cells during initiation of the immune response and at peripheral sites to maintain or amplify the differentiation of an antigen specific response.
2. Materials and Methods
2.1 Mice
Six- to 10-wk-old C57BL/6 mice were purchased from The Division of Cancer Treatment at the National Cancer Institute (Frederick, MD). LTα−/− mice were purchased from Jackson Laboratories (Bar Harbor, ME) and subsequently bred in our facilities, the Animal Resources Center (Chicago, IL). LTβR−/− were a kind gift from Klaus Pfeiffer and were subsequently bred in our facilities. FcεRγ−/− mice were purchased from Taconic (Hudson, NY). OTII TCR transgenic mice were kindly provided by Charles Surh, Scripps Research Institute La Jolla, CA, originated by William Heath, Melbourne, Australia. FcγRI−/− and FcγRIII−/− were originally created by Dr. JS Verbeek and were a kind gift from Dr. Anita Chong. Animals were housed in a specific pathogen-free facility maintained by The University of Chicago. All animal protocols were approved by the Institutional Animal Care and Use Committee (IACUC) at The University of Chicago.
2.2 IgE depletion
Mice were depleted of IgE in vivo with non-anaphylactic monoclonal antibodies as previously described [20]. Briefly, mice were injected i.p. with 50 ug Control Ig or anti-IgE mAb (RIE4, kindly provided by D.H. Conrad) weekly for 4–5 weeks [21]. Serum was collected using retro-orbital eye bleeding after anesthetizing with ketamine and subjected to ELISA (BD PharMingen, San Diego, CA) to quantify IgE levels.
2.3 Antibodies and flow cytometry
All antibodies for flow cytometric analysis were purchased from BD PharMingen, San Diego, CA. Cells were incubated in Fc-block, 2.4G2, for 10 min, stained with different fluorochrome-labeled antibodies in PBS containing 1% FBS (GIBCO) plus 0.01% NaN3 for 30 min on ice, and analyzed by flow cytometry on a FACScan™ or FACSCantos™ (BD Biosciences). Purified mAb used in these studies are: CD11b-PE-Cy7 (clone M1/70), CD11c-PE (clone N148), IgE-bio (clone R3-72), Streptavidin-PerCP, IgE-FITC (clone R3-72), IL-4-APC (clone 11B11), IFN-γ-FITC (clone XMG1.2), CD4-PE (clone L3T4) and CD4-PerCP (clone L3T4).
2.4 IL-12 analysis of spleen and lung
Single cell suspensions of splenocytes were obtained by physical disruption using frosted slides. Lung tissues were finely chopped and then digested 2–3 times by shaking (175 revolutions/min) for 30 min at 37°C in RPMI 1640 medium (Life Technologies) containing 1.5 mg/ml collagenase VIII (Sigma) and 2% heat-inactivated FCS. Lung cells were passed through a nytex filter, and RBCs were depleted with ammonium chloride-potassium lysing buffer. Cells were plated at 1×106 cell/ml in 500 ul with 1 ug/ml LPS (Sigma) in a 24 well plate. Supernatant was collected after 24 hours and IL-12 p70 was quantified by ELISA (BD PharMingen, San Diego, CA)
2.5 Generation of bone marrow derived dendritic cells
Bone marrow derived dendritic cells (BMDC) were generated according to Lutz et al [22]. Briefly, 2 × 105 cell/ml were plated in 10 mls on bacterial culture dishes in DMEM with 10% FCS, 50 uM 2-ME and 20 ng/ml GM-CSF (R and D Systems). On day 3, 10 mls fresh media containing GM-CSF was added to cultures. On day 6 and 8, half the supernatant was removed, spun down and cells were replaced with fresh GM-CSF containing media before returning to the culture dish. Dendritic cells were used at day 8 or 10 for experiments and were at least 85% CD11c+.
2.6 LPS induced APC and BMDC cytokine production
Bulk splenocytes and LN were collected from RAG1−/− mice were plated at 3 × 105 cell/ml in 500 ul DMEM with 10% FCS and 50 uM 2-ME on DNP-HSA (Sigma) coated 24 well plates (2 ug/ml) in the presence of 1 ug/ml SPE-7, a murine monoclonal antibody of IgE isotype specific for DNP (Sigma). For some experiments, increasing concentrations of purified 2.4G2 (BD PharMingen San Diego, CA) was added to block FcγRII/III. After 18–20 hours, 1 ug/ml LPS was added to induce IL-12 production. Twenty-four hours later, cell supernatants were collected and cytokines were analyzed by ELISA. For experiments using bone marrow derived dendritic cells, BMDC were plated at 5 × 105 cell/ml in 500 ul on DNP-HSA coated plates in the presence of 10 ng/ml LPS (Sigma). Twenty-four hours later, supernatant was collected and IL-12 p70 and IL-10 production was analyzed by ELISA (BD PharMingen, San Diego, CA).
2.7 T cell differentiation using RAG1−/− APC
CD4+ T cells from spleen and LN of OTII Tg mice were isolated as described above using Miltenyi CD4+ T cell isolation kit. 2.5 × 103 cell/ml APC were co-cultured with 1 × 104 CD4+ OTII T cells in the presence of 1.0 ug/ml OVA peptide 323–339, 1 ug/ml LPS, 1 ug/ml anti-DNP IgE and 5 ug/ml DNP-HSA in U-bottom 96 well plate. After 7 days, live cells were isolated by Ficol separation and restimulated on 1 ug/ml anti-CD3 coated plates. 48 hours later, culture supernatants were measured by mouse Th1/Th2 cytokine beads array (CBA) kit according to the manufacturer’s protocol (BD PharMingen, San Diego, CA.).
2.8 Statistical analysis
Student’s t test (two tailed) was used to calculate P values for all calculations of statistical significance.
3. Results
3.1 IgE influences IL-12 production
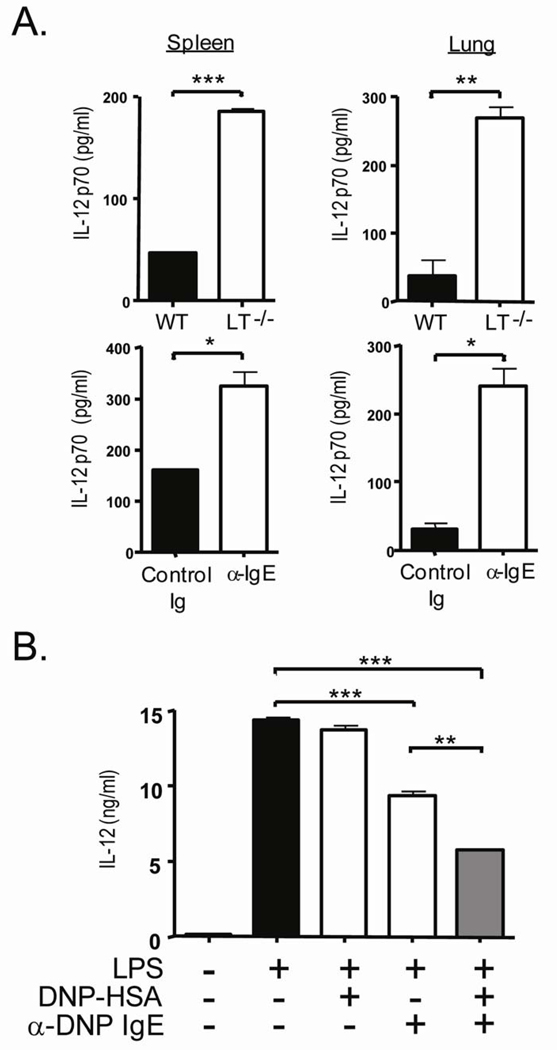
We have previously shown lymphotoxin (LT) is required for physiological serum levels of IgE and that LT−/− mice, IgE−/− mice and WT mice depleted of IgE develop spontaneous Th1 inflammation, suggesting IgE negatively regulates Th1 responses in addition to positively directing Th2 responses [20]. Th1 inflammatory responses require IL-12 production from antigen presenting cells to promote IFN-γ production from CD4+ T cells [23]. We therefore examined IL-12 production in the lung and spleen of LT−/− mice (Figure 1A). Bulk splenocytes after LPS stimulation from LT−/− mice produced a four-fold increase in IL-12 compared to cells from WT mice and similarly, collagenase digested lung cells also had a threefold increase in IL-12 production (285 ± 38.7 pg/ml from LT−/− and 85.9 ± 3.8 pg/ml from WT, P=0.0014). IgE neutralization with non-anaphylactic anti-IgE has been used to examine the role of IgE in immediate and late responses in allergic airway inflammation, late phases of contact hypersensitivity, airway responses in respiratory syncitial virus, behavioral correlates and ulcerative colitis [24; 25; 26; 27; 28]. Monoclonal antibodies neutralize free serum IgE without crosslinking IgE bound to mast cells therefore, reducing the overall serum level of IgE without triggering mast cell mediated events such as histamine, leukotriene and cytokine release. To determine whether IL-12 production was a direct consequence of the lack of IgE in the LT−/− mice, IgE was depleted from WT mice for 4 weeks in vivo and IL-12 production from splenocytes and lung cells was determined after LPS stimulation (Figure 1A). Likewise, IL-12 production from the spleen of IgE depleted mice was 2 fold higher than the control treated mice ( 323.7 ± 29.6 pg/ml compared to 159.6 ± 1.5 pg/ml) and a similar increase was seen in the lung (240.6 ± 25.7 pg/ml from α-IgE compared to 30.92 ± 9.45 pg/ml from Control Ig mice). These data show the importance of IgE in the regulation of the Th1 initiating cytokine, IL-12.
Fig 1.
IgE suppresses IL-12 production in vivo and in vitro. (A) Splenocytes and cells from collagenase digested lung from 12 week old WT and LT−/− mice were stimulated with LPS for 24 hours. After 24 hours supernatant was collected and subjected ELISA to measure IL-12p70. *** P<0.0001, ** P=0.0007 and * P<0.040. (B) APC from RAG-1−/− spleens and LN were sensitized with or without 5 ug/ml anti-DNP IgE on DNP-HSA coated plates. After 24 hours supernatant was collected and subjected ELISA to measure IL-12p70. Data are reported as a mean and representative of at least 4 experiments where *** P<0.0004 and ** P=0.0010.
IgE does not bind to T cells directly but whether it can bind to murine antigen presenting cells is unclear. DC express members of the family of multi-chain immune recognition receptors (MIRR) which tranduce signals into the cell resulting in functional outcomes [29]. IL-12 production from DC is required for the development of Th1 responses and concordant suppression of a Th2 response by the regulation of key transcription factors Tbet and GATA-3 in activated T cells [30; 31]. To investigate whether IgE has a direct effect in IL-12 suppression from antigen presenting cells (APC), bulk splenocytes and LN cells from RAG−/− deficient mice were stimulated with LPS (Figure 1B). RAG−/− mice were used due to their lack of T and B cells and therefore had no previous exposure to IgE. IgE mediated stimulation was accomplished by incubation cells with anti-DNP IgE monoclonal antibody followed by antigen crosslinking with multivalent DNP-HSA. After overnight stimulation with or without antigen-IgE crosslinking, bulk RAG−/− cells were stimulated with 1 ug/ml LPS to stimulate IL-12 p70 production. Compared to LPS stimulation alone, the addition of IgE mediated stimulation reduces IL-12 production from APC by nearly 70%. Furthermore, IgE without Ag crosslinking has a substantial effect on the inhibition of IL-12 production as well; suggesting this clone of IgE may have cytokinergic properties [8]. Therefore, IgE-mediated stimulation of antigen presenting cells, either through crosslinking or on it’s own, has a direct effect to suppress TLR4-mediated IL-12 production.
3.2 IgE crosslinking on APC promotes Th2 differentiation
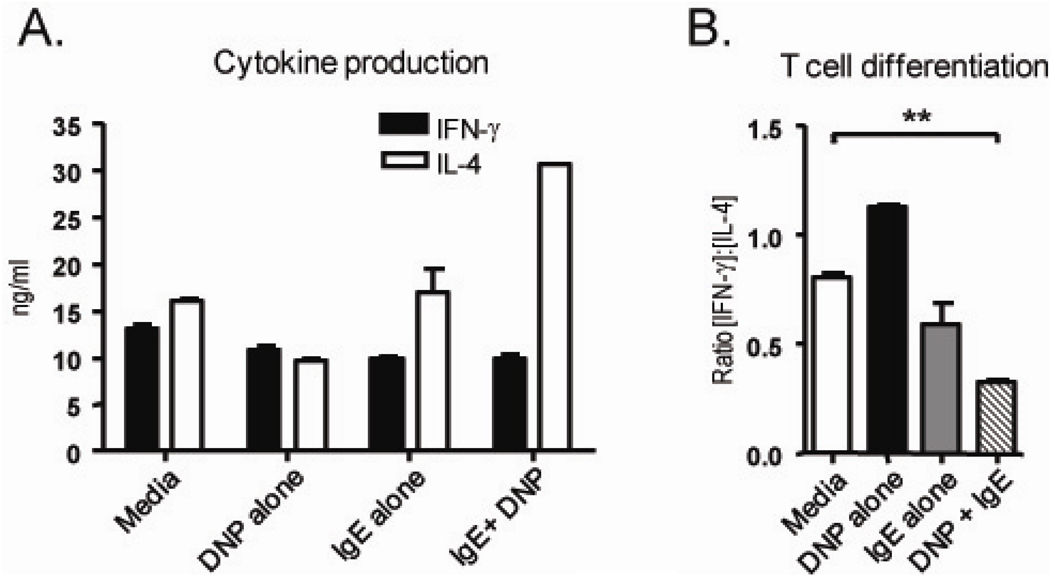
To determine whether IgE mediated IL-12 suppression had a functional consequence in T helper cell differentiation, IgE crosslinked APC from RAG−/− spleen and LN were used as antigen presenting cells in a CD4+ T cell differentiation assay (Figure 2). To allow for T cell differentiation, CD4+ OTII T cells specific for a peptide from the OVA antigen, were incubated with OVA peptide and APC that had been treated by IgE crosslinking. After 4–5 days, the primed T cells were collected, normalized for cell number, restimulated with anti-CD3 for 48 hours and the cytokine profile was analyzed. OTII cells which had been incubated with only peptide and APC (Media) produce ample amounts of IFN-γ (12.9 ng/ml) and IL-4 (16.03 ng/ml). However, in the presence of IgE-crosslinked APC (IgE+DNP), Ag specific OTII cells produce two-fold the amount of IL-4, thus shifting the ratio of IFN-γ to IL-4, characteristic of Th2 polarized inflammation (Figure 2A). IgE alone reduced the ratio of [IFN-γ]:[IL-4] from 0.89 to 0.59 but this difference was not statistically significant; suggesting IgE alone may have influence on antigen presenting cells and T cell polarization (Figure 2B). The ratio of [IFN-γ]:[IL-4] from T cells primed with APC which had been treated with Ag-IgE crosslinking (DNP + IgE) had a significant effect however, proposing IgE crosslinking on APC modifies their ability to promote antigen specific T cell differentiation.
Fig 2.
IgE influences APC to promote Th2 differentiation. APC from RAG-1−/− spleens and LN were sensitized with or without 5 ug/ml anti-DNP IgE on DNP-HSA coated plates. After 24 hours of sensitization, purified CD4+ OTII T cells to APC with OVAp. After priming, live cells were normalized for cell number and restimulated with anti-CD3. After 48 hours restimulation, the supernatant was collected and subjected to CBA for cytokine quantification (A). T cell differentiation was determined by a ratio of [IFN-γ]: [IL-4] (B). Data are representative of at leaset 3 experiments where ** P=0.0026 for untreated (media) vs. IgE crosslinked (DNP+ IgE).
3.3 Role of FcγR in IgE-mediated IL-12 suppression from RAG−/− APC
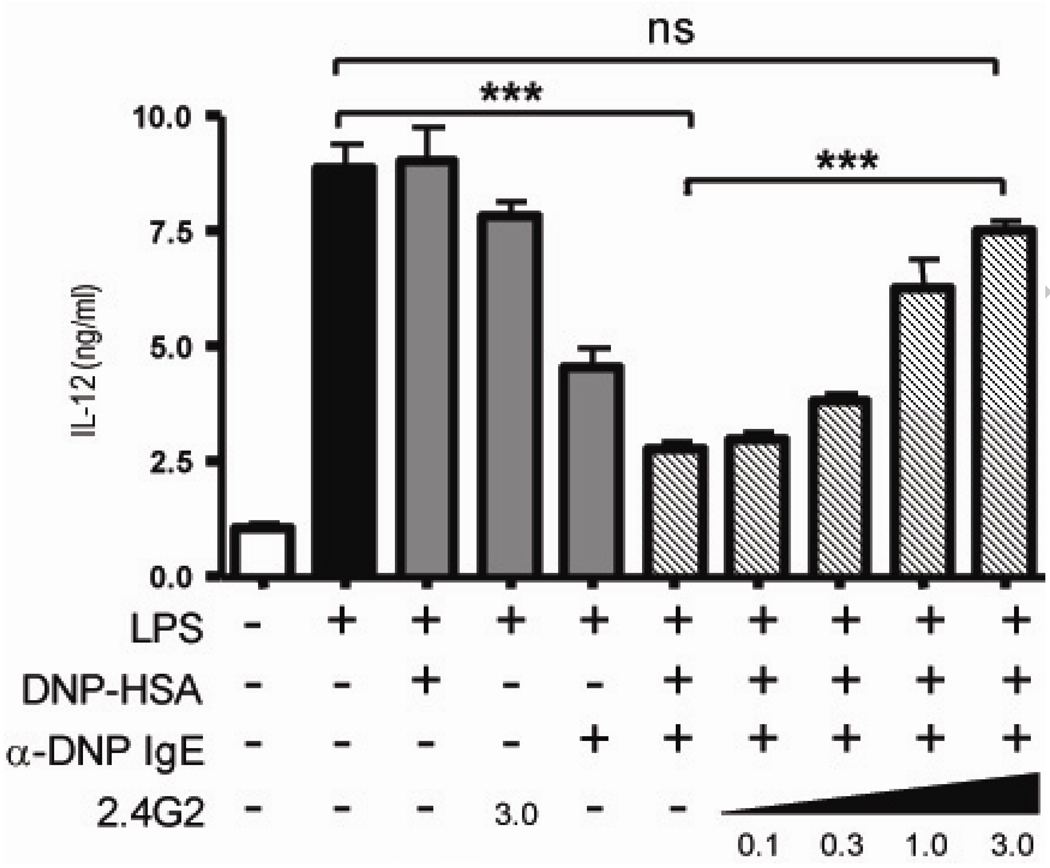
Unlike humans, the murine high affinity receptor for IgE, FcεRI, is not expressed on antigen presenting cells [32]. To determine whether IgE could directly suppress IL-12 production from APC through Fc receptors specific for the IgG isotype, FcγRII and FcγRIII were blocked using soluble monoclonal antibody, 2.4G2 (Figure 3). As seen before, the addition of LPS to RAG−/− APC induced IL-12 production and antigen alone or 2.4G2 alone (in absence of antigen or IgE) had no significant difference on this production. Crosslinking IgE in an antigen dependent manner with the addition of plate bound DNP-HSA, however resulted in a 3 fold decrease in IL-12 production compared to APC treated with LPS alone. The addition of 2.4G2 blocked the IgE mediated IL-12 suppression in a dose dependent manner, almost completely preventing IgE mediated IL-12 suppression with the addition of 3.0 ug/ml 2.4G2 (Figure 3 striped bars). These data demonstrate blocking FcγRII/III with the use of 2.4G2 prevents the IgE mediated IL-12 suppression.
Fig 3.
IgE mediates cytokine modulation via an FcγR from dendritic cells. APC from RAG1−/− spleens and LN were sensitized with or without 5 ug/ml anti-DNP IgE on DNP-HSA coated plates and stimulated with LPS and for some samples increasing amounts of soluble 2.4G2. After 24 hours incubation, supernatant was collected and IL-12 p70 was measured by ELISA. Data are reported as mean ± SEM and representative of 3 experiments where *** P<0.0003 and ns P=0.0594.
3.4 IgE signals directly on DC to regulate cytokine production and requires FcγRIII
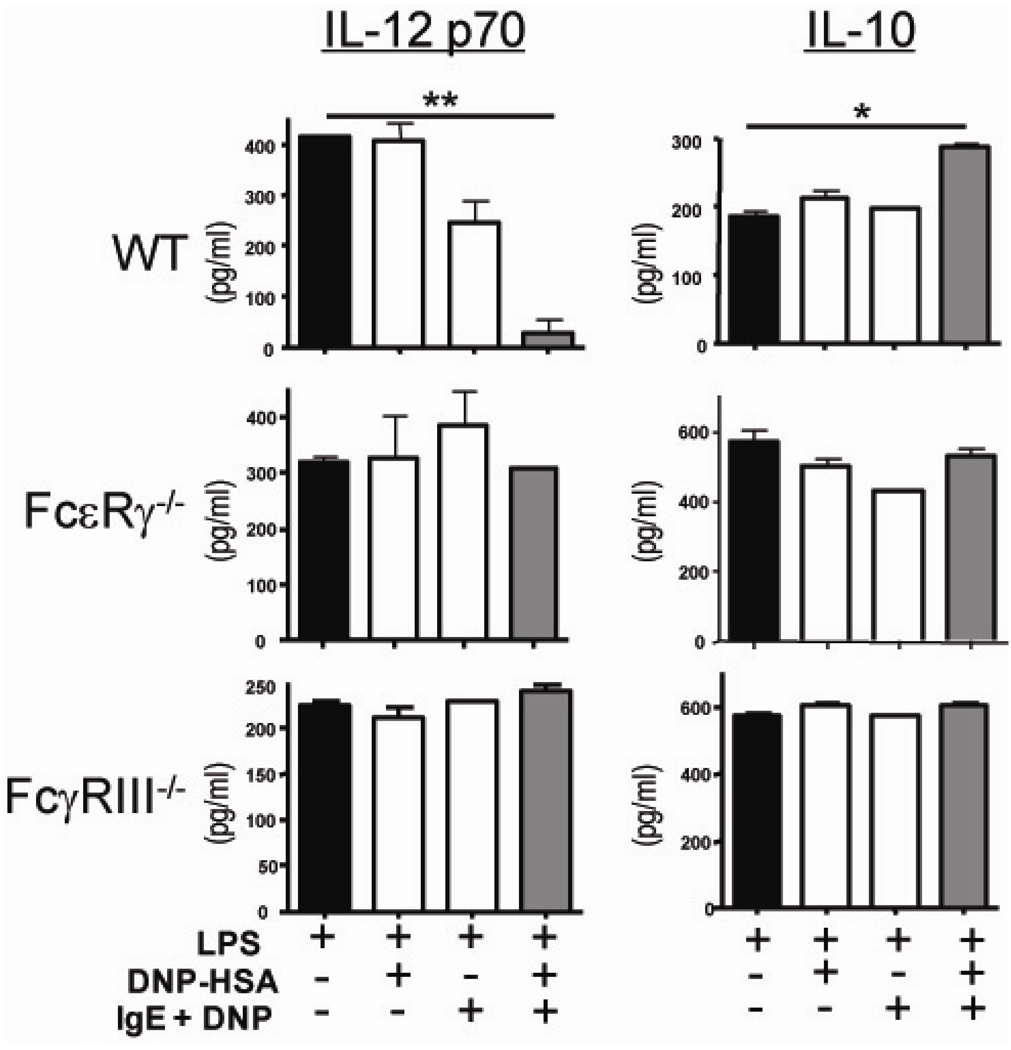
As integral antigen presenting cells, we hypothesized DC are potential targets of IgE mediated IL-12 suppression. To test this, bone marrow derived dendritic cells (BMDC) were generated with GM-CSF and bone marrow from the femurs of WT mice (Figure 4). To ensure the population of cells were indeed DC and did not have contaminants such as basophils or mast cells, FACS analysis was performed and confirmed a homogeneous population of CD11c+ CD11b+ cells with no CD117 or FcεRI expression (data not shown). The BMDC were treated with monomeric and antigen crosslinked IgE and stimulated with LPS to produce IL-12. Similar to the RAG−/− APC population, crosslinking of IgE on WT BMDC results in a significant suppression of IL-12 (413.1 ± 5.73 pg/ml from LPS stimulated alone compared to 38.38 ± 16.98 pg/ml with IgE crosslinking p = 0.0023) and interestingly induces IL-10 production (Figure 4, right panel). A similar reproducible trend in IL-12 suppression is seen with monomeric IgE but data are not statistically significantly different than LPS stimulation alone. To confirm that Fc receptors were in fact required for IgE mediated regulation of DC produced cytokines, BMDC were derived from FcεRγ−/− mice (Figure 4 middle graphs). This chain is the common chain to activating Fc receptors including FcγRI, FcγRIII, FcεRI and FcαRI. In the absence of activating FcR on dendritic cells, IgE crosslinking does not influence IL-12 production or IL-10 production.
Fig 4.
IgE mediated DC cytokine regulation requires FcγRIII. Bone marrow derived dendritic cells were derived from WT, FcεRγ−/− and FcγRIII−/− bone marrow with GM-CSF. BMDC were sensitized with or without 5 ug/ml anti-DNP IgE on DNP-HSA coated plates and stimulated with LPS. After 24 hours incubation, supernatant was collected and IL-12 p70 was measured by ELISA. Data are reported as mean ± SEM and representative of 2 experiments where ** P=0.0054 and * P=0.014.
Although the absence of IgE mediated IL-12 regulation in the FcεRγ−/− and 2.4G2 treated cells strongly suggest the responsible FcR to be FcγRIII, we sought to test this directly using BMDC generated from FcγRIII−/− mice (Figure 4, bottom graphs). After IgE crosslinking and LPS stimulation, FcγRIII−/− DC produce similar amounts of IL-12 and IL-10 as the non-IgE treated cells. IL-12 production from DC that lack FcγRI was also suppressed by IgE crosslinking, further confriming IgE does require this receptor to regulate DC cytokine production (data not shown). Taken together, these data point to FcγRIII as the required FcR for IgE mediated DC cytokine modulation and Th2 promotion.
4. Discussion
Despite its well-characterized pathological role and strong correlation with allergy and asthma, the physiological role of IgE for T cell differentiation remains less clear. Our previous work demonstrates a regulatory role for IgE in T helper cell differentiation as seen by spontaneous Th1 airway inflammation in its absence. Here we describe a mechanism for which IgE influences Th1/2 differentiation by altering dendritic cell cytokine production through FcγRIII.
The ability of IgE to influence and control mast cell activation and survival and to regulate secretion of inflammatory mediators in vitro is well established and previously described [5; 9]. Although murine DC do not express the high affinity IgE receptor, FcεRI, they do express multiple FcγR which influence inflammation. [32]. This raises an intriguing question as to whether these receptors on DC are required for IgE-mediated Th1 inhibition. IgE may have additional cellular targets, such as DC, for the regulation of Th1 responses; we propose that IgE regulates helper T cell differentiation and the resulting inflammatory response through modulation of DC mediated cytokines. LT−/− mice lack detectable IgE and interestingly develop a spontaneous Th1 mediated airway inflammation. This is specifically due to IgE and not other LT mediated defects such as the lack of LN or central tolerance issues since reconstitution of IgE restores lung homeostasis and a Th2 environment similar to WT mice; suggesting IgE directly influences the regulation of Th1 inflammation [20]. IL-12 production from DC is required for the development of a Th1 response and concordant suppression of a Th2 response by the regulation of key transcription factors Tbet and GATA-3 in activated T cells [30; 31].
Our data suggest that IgE influences IL-12 production at both lymphoid and peripheral sites such as the spleen and lung as shown by increased levels of IL-12 from LT−/− and IgE depleted WT mice. We determined IgE could directly influence T cell differentiation through the alteration of antigen presenting cell cytokine production, namely IL-12 suppression. Indeed, cytokine production from both macrophages and dendritic cells are subject to regulation by FcγR [33; 34]. Although murine DC do not express the high affinity IgE receptor, FcεRI, they do express multiple FcγR that influence inflammation [35]. We have now demonstrated that IgE has regulatory effects on dendritic cells through FcγRIII that could be especially important in the development and maintenance allergic disease or asthma. Indeed, our current data suggesting IgE binds to FcγRIII is consistent with previous studies that show FcγRIII on mast cells and macrophages can bind IgE-immunc complexes and this interaction is inhibited with 2.4G2 [36]. Interestingly, human DC and Langerhans' cells express the high affinity receptor for IgE, FcεRI, which has been demonstrated to participate as a receptor for IgE mediated allergen presentation [37; 38; 39]. It remains unclear as to whether FcεRI crosslinking via IgE-Ag complexes results in cytokine alteration from these cells, suggesting IgE binding to different Ig receptors may have distinct outcomes that influence the initiation and polarization of an immune response. Similarly, the subtype of APC that receives this signal could also determine the fate of the inflammatory response in and IgE dependent mechanism. Indeed, FcεRI activated inflammatory epidermal dendritic cells polarize a CD4+ T cell response toward Th1 whereas FcεRI activated Langherhans' cell like dendritic cells produce chemotactic factors such as MCP-1 [40]. Clearly, IgE has the ability to influence adaptive responses via multiple mechanisms in dendritic cells in addition to the well-described roles on basophils and mast cells.
Interestingly, the reduction in IL-12 production from LPS activated DC in the presence of IgE crosslinking does not correlate with an equal decrease in IFN-γ production from CD4+ T cells activated with DC under these conditions, however a significant effect was seen on IL-4 production. It is possible that under these conditions, the Th1 polarizing cytokine IL-12 may have a stronger effect regulating Th2 induction while still providing adequate IL-12 for Th1 differentiation. Indeed, IL-12 negatively regulates the development of Th2 through GATA-3 and promotes Th1 differentiation through the upregulation of t-bet [41]. Additionally, IFN-γ production has been seen in the absence of IL-12 [42; 43]. Under IgE-mediated stimulation conditions, the reduced IL-12 production may be above the threshold needed to induce Tbet and IFN-γ production but below the that needed for the negative regulatory function to suppress GATA-3 and IL-4 production, thus the cells produce more IL-4 but similar levels of IFN-γ.
DC from the lung have been shown to specifically promote a Th2 response and local DC have been shown to mediate the initiation of an immune response in situ and are required for features of allergic airway inflammation during the challenge phase [44; 45]. In the absence of IgE, our previous data showed that naïve CD4+ T cell homeostasis is disrupted as seen by a spontaneous Th1 inflammatory response [20]. Baseline cytokine levels are restored with the addition of IgE, suggesting a regulatory role for IgE in homeostasis of a tissue that is a site for mass antigen entry. Indeed, DC in the lung are sentinels for airborne antigen and exhibit tolerant states compared to their splenic counterparts [46]. Local dendritic cells play a central role in the development and maintenance of Th2 responses in the lung [44; 47; 48; 49]. Located in areas of the lung to easily pick up antigen, a careful balance of DC subsets regulates either tolerance or immunity to airborne antigens [46; 49]. Interestingly, local class switching and production of IgE has been found in the nasal mucosa of patients with allergic rhinitis and a bias towards IgE+ B cells and plasma cells in the nasal mucosa compared to B cells in circulation exists [50; 51]. Therefore, DC in the lung exposed to innate airborne signals have an increased opportunity to interact with IgE and are subject to IgE mediated regulation, especially when allergens are increased in the local area. These current data shed light on a new model regarding the critical role of FcγRIII on dendritic cells in a positive loop for allergy and asthma in the lung.
We also observe in the absence of antigen, IgE alone has effects on DC cytokine production and resultant T helper cell differentiation. Various monomeric IgE clones, including the clone used in this study, SPE-7, are able to mediate signals to murine mast cells without crosslinking however, we can not completely rule out potential cross-linking of IgE due to aggregates [8; 9]. Interestingly, this phenomenon has been seen in recent studies that propose “natural” IgE production that is independent of exogenous Ag or MHC II [52]. Antigen independent IgE activity has also been shown in vivo [53]. Antigen-mediated crosslinking does result in a significant reduction in IL-12 compared to IgE alone suggesting both “natural” IgE-and antigen specific IgE-mediated regulation of cytokine production by DC (Figure 1), however antigen specific IgE mediated stimulation has a greater effect on the resulting Th2 response (Figure 2a). Therefore, In the presence of IgE and antigen, an allergic or pathogenic challenge which could then further stimulate Th2 responses and the resulting Ag specific IgE production could feed back in a positive fashion resulting in the exacerbated phenotype seen in models of airway inflammation or chronic asthma. In the absence of adequate IgE mediated homeostatic signals, environmental antigen stimulation may result in the lack of Th2 signals and ultimately Th1 inflammation [20]. Therefore it will be important to investigate whether patients with less than baseline levels of serum IgE have defects in inflammatory responses.
An interesting set of findings was published regarding the coordination of TLRs and FcγR in T cell differentiation [54]. In this paper, FcγR crosslinking on DC results in a similar cytokine modulation as we see with IgE. There are differences however between the isotypes IgG and IgE in the engagement of antigen/allergen, tissue distribution and regulation. We believe these findings to be complementary to our study and provide further support for that IgE influences DC via FcγR.
IgE has a very strong correlation with Th2 mediated diseases such as asthma and allergy and previous work focuses primarily on IgE mediated regulation of basophils and mast cells to amplify an ongoing Th2 response [7]. Our work presents a previously unappreciated role for IgE to regulate professional antigen presenting cells such as DC and thus influencing the initiation and maintenance of CD4+ T cell responses. Local IgE class switching had been found at the site of antigen entry suggesting it plays a primary effector role in allergic or airway responses. After inhalation of the allergen, one can imagine crosslinking on the DC to occur at a local setting therefore influencing subsequent T cell responses. Baseline levels of IgE are considerably lower than that of total IgG such that with the induction of a response the ratio of antigen specific IgE to total IgE is much greater than that of antigen specific IgG to total IgG, thus more assigning more potency to the allergen specific IgE [55].
Attempts to understand the role of IgE in asthma have primarily focused on single downstream targets such as the FcεRI, MC, basophils or individual FcγR. The results of these studies have uncovered only portions of the role for IgE or particular cell types, mainly mast cell activation, eosinophil recruitment and airway hyperreactivity. The current study, however, implicates dendritic cells; the directors of the adaptive response are also subject to IgE mediated regulation, resulting in skewing T helper cell inflammation. These present data are consistent with the cytokinergic properties of some IgE clones and the newly described “natural” IgE which is present in the absence of an inflammatory response and could influence the homeostasis of CD4+ T cells through dendritic or mast cell regulation before antigen challenge.
We have uncovered an unknown mechanism for how IgE regulates the homeostasis of T helper cell differentiation. Through FcγRIII, IgE influences dendritic cell cytokine production to provide adequate environment for CD4+ T cell polarization towards Th2. We demonstrate that IgE has regulatory effects on antigen presenting cells that could be especially important in the development and maintenance allergic disease or asthma. This knowledge may help to modulate current therapies or to create new ones for Th2 mediated disease such as asthma and allergy as well as draw attention to using IgE levels as a diagnostic tool for Th1 mediated inflammation in addition to the well-characterized relationship to Th2 diseases. Furthermore, we draw an important connection between two key components of allergic airway disease, IgE and dendritic cells.
Acknowledgements
We would like to acknowledge critical comments by Drs. Anne Sperling, Ping Yu and Yonglian Sun.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Robinson DS, Hamid Q, Ying S, Tsicopoulos A, Barkans J, Bentley AM, Corrigan C, Durham SR, Kay AB. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 1992;326:298–304. doi: 10.1056/NEJM199201303260504. [DOI] [PubMed] [Google Scholar]
- 2.Yazdanbakhsh M, Kremsner PG, van Ree R. Allergy, parasites, and the hygiene hypothesis. Science. 2002;296:490–494. doi: 10.1126/science.296.5567.490. [DOI] [PubMed] [Google Scholar]
- 3.Brown WG, Halonen MJ, Kaltenborn WT, Barbee RA. The relationship of respiratory allergy, skin test reactivity, and serum IgE in a community population sample. J Allergy Clin Immunol. 1979;63:328–335. doi: 10.1016/0091-6749(79)90127-1. [DOI] [PubMed] [Google Scholar]
- 4.Burrows B, Martinez FD, Halonen M, Barbee RA, Cline MG. Association of asthma with serum IgE levels and skin-test reactivity to allergens. N Engl J Med. 1989;320:271–277. doi: 10.1056/NEJM198902023200502. [DOI] [PubMed] [Google Scholar]
- 5.Galli SJ, Nakae S, Tsai M. Mast cells in the development of adaptive immune responses. Nat Immunol. 2005;6:135–142. doi: 10.1038/ni1158. [DOI] [PubMed] [Google Scholar]
- 6.Maezawa Y, Nakajima H, Kumano K, Kubo S, Karasuyama H, Iwamoto I. Role of IgE in Th2 cell-mediated allergic airway inflammation. Int Arch Allergy Immunol. 2003;131 Suppl 1:2–6. doi: 10.1159/000070473. [DOI] [PubMed] [Google Scholar]
- 7.Gould HJ, Sutton BJ, Beavil AJ, Beavil RL, McCloskey N, Coker HA, Fear D, Smurthwaite L. The biology of IGE and the basis of allergic disease. Annu Rev Immunol. 2003;21:579–628. doi: 10.1146/annurev.immunol.21.120601.141103. [DOI] [PubMed] [Google Scholar]
- 8.Kalesnikoff J, Huber M, Lam V, Damen JE, Zhang J, Siraganian RP, Krystal G. Monomeric IgE stimulates signaling pathways in mast cells that lead to cytokine production and cell survival. Immunity. 2001;14:801–811. doi: 10.1016/s1074-7613(01)00159-5. [DOI] [PubMed] [Google Scholar]
- 9.Kitaura J, Song J, Tsai M, Asai K, Maeda-Yamamoto M, Mocsai A, Kawakami Y, Liu FT, Lowell CA, Barisas BG, Galli SJ, Kawakami T. Evidence that IgE molecules mediate a spectrum of effects on mast cell survival and activation via aggregation of the FcepsilonRI. Proc Natl Acad Sci U S A. 2003;100:12911–12916. doi: 10.1073/pnas.1735525100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vangelista L, Soprana E, Cesco-Gaspere M, Mandiola P, Di Lullo G, Fucci RN, Codazzi F, Palini A, Paganelli G, Burrone OR, Siccardi AG. Membrane IgE binds and activates Fc epsilon RI in an antigen-independent manner. J Immunol. 2005;174:5602–5611. doi: 10.4049/jimmunol.174.9.5602. [DOI] [PubMed] [Google Scholar]
- 11.Takeda K, Hamelmann E, Joetham A, Shultz LD, Larsen GL, Irvin CG, Gelfand EW. Development of eosinophilic airway inflammation and airway hyperresponsiveness in mast cell-deficient mice. J Exp Med. 1997;186:449–454. doi: 10.1084/jem.186.3.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams CM, Galli SJ. Mast cells can amplify airway reactivity and features of chronic inflammation in an asthma model in mice. J Exp Med. 2000;192:455–462. doi: 10.1084/jem.192.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ogawa K, Kaminuma O, Kikkawa H, Nakata A, Asahina M, Egan RW, Akiyama K, Mori A. Transient contribution of mast cells to pulmonary eosinophilia but not to hyper-responsiveness. Clin Exp Allergy. 2002;32:140–148. doi: 10.1046/j.0022-0477.2001.01248.x. [DOI] [PubMed] [Google Scholar]
- 14.Henz BM, Maurer M, Lippert U, Worm M, Babina M. Mast cells as initiators of immunity and host defense. Exp Dermatol. 2001;10:1–10. doi: 10.1034/j.1600-0625.2001.100101.x. [DOI] [PubMed] [Google Scholar]
- 15.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 16.Hsieh CS, Macatonia SE, Tripp CS, Wolf SF, O'Garra A, Murphy KM. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 17.Macatonia SE, Hosken NA, Litton M, Vieira P, Hsieh CS, Culpepper JA, Wysocka M, Trinchieri G, Murphy KM, O'Garra A. Dendritic cells produce IL-12 and direct the development of Th1 cells from naive CD4+ T cells. J Immunol. 1995;154:5071–5079. [PubMed] [Google Scholar]
- 18.Hemmi H, Kaisho T, Takeda K, Akira S. The roles of Toll-like receptor 9, MyD88, and DNA-dependent protein kinase catalytic subunit in the effects of two distinct CpG DNAs on dendritic cell subsets. J Immunol. 2003;170:3059–3064. doi: 10.4049/jimmunol.170.6.3059. [DOI] [PubMed] [Google Scholar]
- 19.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 20.Kang HS, Blink SE, Chin RK, Lee Y, Kim O, Weinstock J, Waldschmidt T, Conrad D, Chen B, Solway J, Sperling AI, Fu YX. Lymphotoxin is required for maintaining physiological levels of serum IgE that minimizes Th1-mediated airway inflammation. J Exp Med. 2003;198:1643–1653. doi: 10.1084/jem.20021784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keegan AD, Fratazzi C, Shopes B, Baird B, Conrad DH. Characterization of new rat anti-mouse IgE monoclonals and their use along with chimeric IgE to further define the site that interacts with Fc epsilon RII and Fc epsilon RI. Mol Immunol. 1991;28:1149–1154. doi: 10.1016/0161-5890(91)90030-n. [DOI] [PubMed] [Google Scholar]
- 22.Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 23.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 24.Haile S, Lefort J, Eum SY, Dumarey C, Huerre M, Heusser C, Vargaftig BB. Suppression of immediate and late responses to antigen by a non-anaphylactogenic anti-IgE antibody in a murine model of asthma. Eur Respir J. 1999;13:961–969. doi: 10.1034/j.1399-3003.1999.13e06.x. [DOI] [PubMed] [Google Scholar]
- 25.Chai OH, Lee HK, Lee YC, Lee MS, Han EH, Kim HT, Song CH. Roles of TNF-alpha and IgE in the late phase of contact hypersensitivity induced by trimellitic anhydride. Exp Mol Med. 2005;37:408–417. doi: 10.1038/emm.2005.51. [DOI] [PubMed] [Google Scholar]
- 26.Dakhama A, Lee YM, Ohnishi H, Jing X, Balhorn A, Takeda K, Gelfand EW. Virus-specific IgE enhances airway responsiveness on reinfection with respiratory syncytial virus in newborn mice. J Allergy Clin Immunol. 2009;123:138 e5–145 e5. doi: 10.1016/j.jaci.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 27.Costa-Pinto FA, Basso AS, Russo M. Role of mast cell degranulation in the neural correlates of the immediate allergic reaction in a murine model of asthma. Brain Behav Immun. 2007;21:783–790. doi: 10.1016/j.bbi.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 28.Kang OH, Kim DK, Choi YA, Park HJ, Tae J, Kang CS, Choi SC, Nah YH, Lee HK, Lee YM. Suppressive effect of non-anaphylactogenic anti-IgE antibody on the development of dextran sulfate sodium-induced colitis. Int J Mol Med. 2006;18:893–899. [PubMed] [Google Scholar]
- 29.Sigalov AB. Multichain immune recognition receptor signaling: different players, same game? Trends Immunol. 2004;25:583–589. doi: 10.1016/j.it.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 30.Heufler C, Koch F, Stanzl U, Topar G, Wysocka M, Trinchieri G, Enk A, Steinman RM, Romani N, Schuler G. Interleukin-12 is produced by dendritic cells and mediates T helper 1 development as well as interferon-gamma production by T helper 1 cells. Eur J Immunol. 1996;26:659–668. doi: 10.1002/eji.1830260323. [DOI] [PubMed] [Google Scholar]
- 31.Murphy KM, Reiner SL. The lineage decisions of helper T cells. Nat Rev Immunol. 2002;2:933–944. doi: 10.1038/nri954. [DOI] [PubMed] [Google Scholar]
- 32.Ravetch JV, Kinet JP. Fc receptors. Annu Rev Immunol. 1991;9:457–492. doi: 10.1146/annurev.iy.09.040191.002325. [DOI] [PubMed] [Google Scholar]
- 33.Anderson CF, Lucas M, Gutierrez-Kobeh L, Field AE, Mosser DM. T cell biasing by activated dendritic cells. J Immunol. 2004;173:955–961. doi: 10.4049/jimmunol.173.2.955. [DOI] [PubMed] [Google Scholar]
- 34.Sutterwala FS, Noel GJ, Clynes R, Mosser DM. Selective suppression of interleukin-12 induction after macrophage receptor ligation. J Exp Med. 1997;185:1977–1985. doi: 10.1084/jem.185.11.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barnes N, Gavin AL, Tan PS, Mottram P, Koentgen F, Hogarth PM. FcgammaRI-deficient mice show multiple alterations to inflammatory and immune responses. Immunity. 2002;16:379–389. doi: 10.1016/s1074-7613(02)00287-x. [DOI] [PubMed] [Google Scholar]
- 36.Takizawa F, Adamczewski M, Kinet JP. Identification of the low affinity receptor for immunoglobulin E on mouse mast cells and macrophages as Fc gamma RII and Fc gamma RIII. J Exp Med. 1992;176:469–475. doi: 10.1084/jem.176.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maurer D, Ebner C, Reininger B, Fiebiger E, Kraft D, Kinet JP, Stingl G. The high affinity IgE receptor (Fc epsilon RI) mediates IgE-dependent allergen presentation. J Immunol. 1995;154:6285–6290. [PubMed] [Google Scholar]
- 38.Maurer D, Fiebiger E, Reininger B, Ebner C, Petzelbauer P, Shi GP, Chapman HA, Stingl G. Fc epsilon receptor I on dendritic cells delivers IgE-bound multivalent antigens into a cathepsin S-dependent pathway of MHC class II presentation. J Immunol. 1998;161:2731–2739. [PubMed] [Google Scholar]
- 39.Bieber T, de la Salle H, Wollenberg A, Hakimi J, Chizzonite R, Ring J, Hanau D, de la Salle C. Human epidermal Langerhans cells express the high affinity receptor for immunoglobulin E (Fc epsilon RI) J Exp Med. 1992;175:1285–1290. doi: 10.1084/jem.175.5.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Novak N, Valenta R, Bohle B, Laffer S, Haberstok J, Kraft S, Bieber T. FcepsilonRI engagement of Langerhans cell-like dendritic cells and inflammatory dendritic epidermal cell-like dendritic cells induces chemotactic signals and different T-cell phenotypes in vitro. J Allergy Clin Immunol. 2004;113:949–957. doi: 10.1016/j.jaci.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 41.Fields PE, Kim ST, Flavell RA. Cutting edge: changes in histone acetylation at the IL-4 and IFN-gamma loci accompany Th1/Th2 differentiation. J Immunol. 2002;169:647–650. doi: 10.4049/jimmunol.169.2.647. [DOI] [PubMed] [Google Scholar]
- 42.Piccotti JR, Chan SY, Goodman RE, Magram J, Eichwald EJ, Bishop DK. IL-12 antagonism induces T helper 2 responses, yet exacerbates cardiac allograft rejection. Evidence against a dominant protective role for T helper 2 cytokines in alloimmunity. J Immunol. 1996;157:1951–1957. [PubMed] [Google Scholar]
- 43.Piccotti JR, Li K, Chan SY, Ferrante J, Magram J, Eichwald EJ, Bishop DK. Alloantigen-reactive Th1 development in IL-12-deficient mice. J Immunol. 1998;160:1132–1138. [PubMed] [Google Scholar]
- 44.Constant SL, Brogdon JL, Piggott DA, Herrick CA, Visintin I, Ruddle NH, Bottomly K. Resident lung antigen-presenting cells have the capacity to promote Th2 T cell differentiation in situ. J Clin Invest. 2002;110:1441–1448. doi: 10.1172/JCI16109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Rijt LS, Jung S, Kleinjan A, Vos N, Willart M, Duez C, Hoogsteden HC, Lambrecht BN. In vivo depletion of lung CD11c+ dendritic cells during allergen challenge abrogates the characteristic features of asthma. J Exp Med. 2005;201:981–991. doi: 10.1084/jem.20042311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Heer HJ, Hammad H, Kool M, Lambrecht BN. Dendritic cell subsets and immune regulation in the lung. Semin Immunol. 2005;17:295–303. doi: 10.1016/j.smim.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 47.de Heer HJ, Hammad H, Soullie T, Hijdra D, Vos N, Willart MA, Hoogsteden HC, Lambrecht BN. Essential role of lung plasmacytoid dendritic cells in preventing asthmatic reactions to harmless inhaled antigen. J Exp Med. 2004;200:89–98. doi: 10.1084/jem.20040035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oriss TB, Ostroukhova M, Seguin-Devaux C, Dixon-McCarthy B, Stolz DB, Watkins SC, Pillemer B, Ray P, Ray A. Dynamics of dendritic cell phenotype and interactions with CD4+ T cells in airway inflammation and tolerance. J Immunol. 2005;174:854–863. doi: 10.4049/jimmunol.174.2.854. [DOI] [PubMed] [Google Scholar]
- 49.Hammad H, de Vries VC, Maldonado-Lopez R, Moser M, Maliszewski C, Hoogsteden HC, Lambrecht BN. Differential capacity of CD8+ alpha or CD8− alpha dendritic cell subsets to prime for eosinophilic airway inflammation in the T-helper type 2-prone milieu of the lung. Clin Exp Allergy. 2004;34:1834–1840. doi: 10.1111/j.1365-2222.2004.02133.x. [DOI] [PubMed] [Google Scholar]
- 50.KleinJan A, Vinke JG, Severijnen LW, Fokkens WJ. Local production and detection of (specific) IgE in nasal B-cells and plasma cells of allergic rhinitis patients. Eur Respir J. 2000;15:491–497. doi: 10.1034/j.1399-3003.2000.15.11.x. [DOI] [PubMed] [Google Scholar]
- 51.King CL, Thyphronitis G, Nutman TB. Enumeration of IgE secreting B cells. A filter spot-ELISA. J Immunol Methods. 1990;132:37–43. doi: 10.1016/0022-1759(90)90395-c. [DOI] [PubMed] [Google Scholar]
- 52.McCoy KD, Harris NL, Diener P, Hatak S, Odermatt B, Hangartner L, Senn BM, Marsland BJ, Geuking MB, Hengartner H, Macpherson AJ, Zinkernagel RM. Natural IgE production in the absence of MHC Class II cognate help. Immunity. 2006;24:329–339. doi: 10.1016/j.immuni.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 53.Bryce PJ, Miller ML, Miyajima I, Tsai M, Galli SJ, Oettgen HC. Immune sensitization in the skin is enhanced by antigen-independent effects of IgE. Immunity. 2004;20:381–392. doi: 10.1016/s1074-7613(04)00080-9. [DOI] [PubMed] [Google Scholar]
- 54.Bandukwala HS, Clay BS, Tong J, Mody PD, Cannon JL, Shilling RA, Verbeek JS, Weinstock JV, Solway J, Sperling AI. Signaling through Fc gamma RIII is required for optimal T helper type (Th)2 responses and Th2-mediated airway inflammation. J Exp Med. 2007;204:1875–1889. doi: 10.1084/jem.20061134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Davies DR, Metzger H. Structural basis of antibody function. Annu Rev Immunol. 1983;1:87–117. doi: 10.1146/annurev.iy.01.040183.000511. [DOI] [PubMed] [Google Scholar]