Abstract
Context
Amyloid-β peptide (Aβ42) has been implicated in the pathogenesis of Alzheimer disease (AD). Tarenflurbil, a selective Aβ42-lowering agent, demonstrated encouraging results on cognitive and functional outcomes among mildly affected patients in an earlier phase 2 trial.
Objective
To determine the efficacy, safety, and tolerability of tarenflurbil.
Design, Setting, and Patients
A multicenter, randomized, double-blind, placebo-controlled trial enrolling patients with mild AD was conducted at 133 trial sites in the United States between February 21, 2005, and April 30, 2008. Concomitant treatment with cholinesterase inhibitors or memantine was permitted.
Intervention
Tarenflurbil, 800 mg, or placebo, administered twice a day.
Main Outcome Measures
Co-primary efficacy end points were the change from baseline to month 18 in total score on the subscale of the Alzheimer Disease Assessment Scale–Cognitive Subscale (ADAS-Cog, 80-point version) and Alzheimer Disease Cooperative Studies–activities of daily living (ADCS-ADL) scale. Additional prespecified slope analyses explored the possibility of disease modification.
Results
Of the 1684 participants randomized, 1649 were included in the analysis, and 1046 completed the trial. Tarenflurbil had no beneficial effect on the co-primary outcomes (difference in change from baseline to month 18 vs placebo, based on least squares means: 0.1 for ADAS-Cog; 95% CI, −0.9 to 1.1; P=.86 and −0.5 for ADCS-ADL; 95% CI, −1.9 to 0.9; P=.48) using an intent-to-treat analysis. No significant differences occurred in the secondary outcomes. The ADAS-Cog score decreased by 7.1 points over 18 months. The tarenflurbil group had a small increase in frequency of dizziness, anemia, and infections.
Conclusion
Tarenflurbil did not slow cognitive decline or the loss of activities of daily living in patients with mild AD.
Leading theories on the patho-physiology of Alzheimer disease (AD) implicate overproduction of amyloid-β (Aβ), particularly 42 amino acid peptide Aβ42.1–3 Compounds modulating γ-secretase enzyme cleaving β-amyloid precursor protein (APP) to release various forms of Aβ are candidates for treatment of AD. One such compound is tarenflurbil (formerly R-flurbiprofen), a selective Aβ42-lowering agent that has been shown in vitro and in vivo to modulate γ-secretase activity and reduce Aβ42 production in favor of shorter less toxic forms of Aβ (eg, Aβ38 and Aβ37).4,5 In mouse models of AD, tarenflurbil prevents learning and memory deficits and reduces Aβ42 brain concentrations.4,6
A phase 2 trial of tarenflurbil suggested that patients with mild AD and moderate AD responded differently to treatment, that patients with mild AD had a dose-related slower rate of decline than those treated with placebo, and that the drug was well tolerated with few adverse effects.7 On the basis of these results, a large phase 3, randomized, placebo-controlled trial of tarenflurbil was conducted in patients with mild AD.
METHODS
Study Design
A randomized, double-blind, 2-group parallel study was conducted to compare tarenflurbil with placebo for 18 months involving 133 participating trial sites. Written informed consent was obtained from participants, their legally authorized representatives, or both. The institutional review board of participating institutions approved the study.
Patients were predominantly recruited from dementia clinics and were enrolled from February 21, 2005, through April 30, 2008. Initial enrollment criteria included community-dwelling patients with mild to moderate AD severity (Mini-Mental State Examination [MMSE] scores of 15–26, inclusive). Patients were assigned to treatment with tarenflurbil at doses of either 400 mg or 800 mg twice a day. After analysis of phase 2 data indicated that patients with mild AD appeared to have a more robust response to 800 mg of the tarenflurbil twice daily7 and after discussion with the US Food and Drug Administration, the enrollment criteria were changed on May 27, 2005, to enroll only patients with mild AD (MMSE score of 20–26, inclusive) with treatment modified by ending the 400-mg group: 42 patients with mild AD were switched to the 800-mg group and 32 patients with moderate AD were discontinued from the trial; thus, the revised analysis plan excluded those with moderate AD.
The remainder of the inclusion and exclusion criteria remained unchanged throughout the trial: 55 years or older and living in the community, meeting criteria for dementia by the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) (DSM-IV), and having probable AD by National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer's Disease and Related Disorders Association criteria.8 Additional inclusion criteria at screening included no clinically significant focal intracranial pathology as assessed by CT or MRI within the previous 12 months, a modified Hachinski ischemic score9 of less than 4, at least 6 years of education or sufficient work history to exclude mental retardation, adequate vision and hearing to participate in study assessments, and a reliable caregiver who saw the patient at least 4 days a week and could accompany the patient to each clinic visit.
Participants taking an acetylcholinesterase inhibitor were enrolled provided they had been taking that specific medication for at least 6 months before taking the study drug. Participants taking memantine were enrolled if they had been taking stable doses for at least 3 months before taking the study drug. Randomization was stratified by use or nonuse of cholinesterase inhibitors and memantine. Participants taking antidepressant, anti-psychotic, or anxiolytic drugs, vitamin E, or Ginkgo biloba were eligible, provided that the dose was stable for at least 3 months prior to randomization. Chronic aspirin use for cardioprotective therapy was allowed.
Participants were excluded if they had evidence of epilepsy; focal brain lesion; head injury with loss of consciousness or confusion after the injury; DSM-IV-TR (Text Revision) criteria for any major psychiatric disorder including psychosis, major depression, bipolar disorder, or alcohol or substance abuse; history of upper gastrointestinal tract bleeding requiring surgery, transfusion, or both within 3 years or documented evidence of active gastric or duodenal ulcer disease within 3 months; history or evidence of active malignancy, except for prostate cancer, basal cell carcinoma, or squamous cell carcinoma of the skin within 24 months of entry; a chronic or acute renal, hepatic, or metabolic disorder; any use of AD immunotherapy or recent use of any investigational therapy or major surgery; an uncontrolled cardiac condition (New York Heart Association class III or IV); anticoagulant therapy such as warfarin within 12 weeks of enrollment; use of any CYP2C9 enzyme inhibitor or the CYP2C9 enzyme substrates losartan, phenytoin, tamoxifen, torsemide, and fluvastatin within 2 weeks of enrollment; recent history of chronic use of nonsteroidal anti-inflammatory drugs (NSAIDs) at any dose or aspirin greater than 325 mg/d; or history of hypersensitivity to any NSAIDs including cyclooxygenase 2 (COX-2)–specific inhibitors. Race was determined by self-report and was assessed to evaluate possible drug effect modification.
Eligible participants were randomized by a central randomization schema generated by the sponsor. The randomization tables were maintained in a locked file room of the head of the Quality Assurance department. The clinical system was used to assign blinded drug treatment kits. Both dosages of tarenflurbil were administered as 2 tablets twice a day: a single tarenflurbil tablet for the 400-mg and 2 tarenflurbil tablets for the 800-mg groups, then after the protocol amendment only at doses of 800 mg twice daily. Participants in the placebo group took 2 tablets identical to the tarenflurbil tablets twice a day to ensure blinding. Participants were not asked to guess their randomization group.
Adverse event monitoring, physical examinations including vital signs measurement, standard resting 12-lead electrocardiograms, and blood and urine sample collection for clinical laboratory analysis and determination of plasma tarenflurbil concentration were performed at the screening visit and at months 1, 3, 6, 9, 12, 15, and 18. Additional adverse event monitoring was performed via telephone with caregivers at week 2 and every month between scheduled visits. All participants were assessed 30 days after the last dose of study medication. A central laboratory was used throughout the study.
Outcome Measures and Power Estimates
Co-primary efficacy outcomes were cognition as assessed by the Alzheimer Disease Assessment Scale–Cognitive Subscale (ADAS-Cog, 80-point version)10 and functional ability as assessed by the Alzheimer Disease Cooperative Study activities of daily living (ADCS-ADL, 78-point scale).11 A key secondary outcome measure assessed global function with the Clinical Dementia Rating (CDR) sum of boxes (CDR-sb, 18-point scale).12 Additional secondary outcomes included the MMSE (30-point scale),13 Neuropsychiatric Inventory (144-point scale),14 quality of life scale (QOL-AD, 13–52 points),15 Caregiver Burden Inventory (96-point scale),16 and 70-point version of ADAS-Cog. Blood samples were collected and stored for population pharmacokinetic analysis and for apolipoprotein E (APOE) genotype testing.
The power estimates were based on the joint power for detecting a difference between treatment groups in the changes from baseline to month 18 on the ADAS-Cog and the ADCS-ADL scales. Statistical power for each end point was calculated separately assuming an effect size of 20%, ie, treatment difference divided by standard deviation, using a 2-sided test and a 5% significance level. Assuming SD of changes from baseline to month 18 is approximately 10.0 for ADAS-Cog and 13.0 for ADCS-ADL, with 800 patients per group, the study would have had at least 98% power to detect a treatment difference in the changes from baseline to month 18 of 2.0 points for ADAS-Cog and 2.6 points for ADCS-ADL. No adjustment for dropouts was made in this calculation because the co-primary analyses use the z score last-observation-carried-forward imputation algorithm. Because the co-primary end points were expected to be correlated, the joint power would have been in excess of 0.96 (0.982) for detecting treatment differences.
Statistical Analysis
The primary analysis was performed on changes from baseline to month 18 in total score for ADAS-Cog and ADCS-ADL. Slopes of total scores for both scales were evaluated as a secondary outcome. The key secondary efficacy end point was change from baseline to month 18 CDR-sb score, and slopes were also evaluated. Other secondary efficacy end points were changes from baseline to month 18 for MMSE, Neuropsychiatric Inventory, QOL-AD, and Caregiver Burden Inventory. Safety end points included incidence of adverse events, clinical laboratory tests, vital signs, electrocardiogram, and physical examination.
All efficacy analyses were performed using the intent-to-treat population, which in this instance consisted of all participants who were randomized, had mild AD at screening, and received at least 1 dose of study medication. Participants initially randomized to the 400-mg group were pooled with the 800-mg group. A z score last-observation-carried-forward method was used to impute missing data for the main change-from-baseline analysis of each efficacy end point. A missing value was replaced with a value that was the same number of SDs from the treatment group mean at that time point as that participant's last observed value [z score=(observed value – treatment group mean)/ treatment group SD]. This imputation method accounts for AD being a progressive disease and for data that may not be missing at random (ie, patients who progress more quickly may be more likely to withdraw). No imputation was used for comparison of slopes. A per-protocol analysis that included only those participants who completed double-blind therapy and who had no major protocol violations was also performed for the co-primary and key secondary end points. Participants with moderate AD were not included in any of these analyses.
Change-from-baseline analyses were conducted using an analysis of covariance model with treatment group, clinical site, and current use of acetylcholinesterase inhibitor, memantine, or both as fixed effects with the baseline score as the covariate. The slopes analyses were conducted using a repeated measures linear mixed model, with random intercepts and slopes, baseline score and time as covariates, factors for treatment group, clinical site, and current use of acetylcholinesterase inhibitor, memantine, or both, and a term for treatment group × time interaction. Time was treated as a continuous variable. Because both co-primary change-from-baseline analyses had to be statistically significant at the .05 level to meet study objectives, no adjustment for multiple comparisons was made for co-primary analyses.
A gatekeeper approach was used to control for multiple comparisons for the slopes analyses of the ADAS-Cog and ADCS-ADL and for the change-from-baseline and slopes analyses for the CDR-sb. After performing the primary analysis, treatment comparisons were planned in this order: (1) slopes for the ADAS-Cog; (2) slopes for the ADCS-ADL; (3) change from baseline to month 18 in the CDR-sb; (4) slopes for the CDR-sb. Each comparison could only be considered statistically significant if it, and all preceding analyses, were statistically significant at the .05 level. No multiple comparison adjustments were used for other efficacy end points.
Additional efficacy analyses included categorization of participants who improved from baseline at each visit for ADAS-Cog, ADCS-ADL, and CDR-sb. For ADAS-Cog, these criteria had to be met on 2 consecutive visits to qualify as an improver. Blood samples were collected for the measurement of tarenflurbil and S-flurbiprofen. A population pharmacokinetic (PK) model was developed using the plasma concentration data collected and the tarenflurbil PK parameters area under the curve (AUC(0–12 h)) and Cmax (maximum plasma drug concentration) for each participant were estimated and used to explore potential relationships between tarenflurbil plasma concentrations and the primary efficacy outcomes using a mixed model.
Safety analyses were conducted using all participants, including those with mild or moderate AD, who received at least 1 dose of the study drug (safety population). The Pearson χ2 test was used for all treatment comparisons based on categorical variables (eg, demographic and baseline characteristics; proportions of improvers). Analysis of variance or analysis of covariance was used to compare treatment groups for continuous variables. Statistical analyses used SAS software version 9.1.3 (SAS Institute Inc, Cary, North Carolina). P<.05 was considered significant.
RESULTS
Study Participants and Follow-up
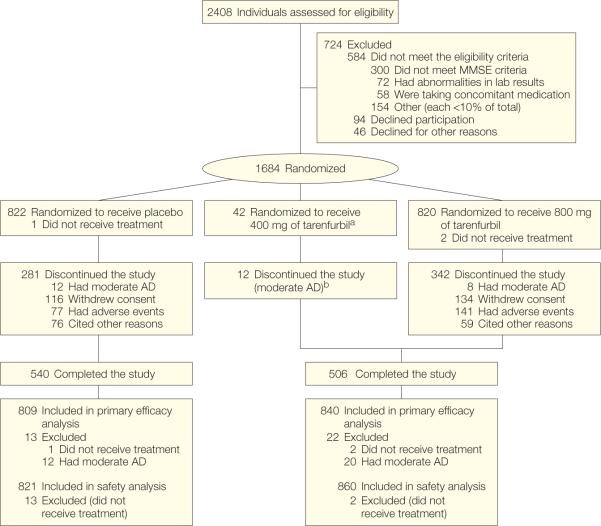
Of 2408 patients screened, 724 were excluded (Figure 1). Demographic characteristics of patients enrolled (51.3% women; mean age, 74.7 years) were similar to those excluded.
Figure 1.
Participant Flow Chart
aPatients originally assigned to receive twice daily 400 mg of tarenflurbil were incorporated into the twice daily 800-mg group.
bExcluded from the main efficacy analyses using the intent-to-treat population but were included in the safety analyses. Rates of adverse events leading to study discontinuation are based on the intent-to-treat population. The numbers differ from those in the text, which are based on the safety population.
The modified intention-to-treat population comprised 809 patients in the placebo group and 840 in the treatment group. Baseline characteristics were well balanced between the 2 groups (Table 1).
Table 1.
Baseline Demographics and Assessment Scores
| Demographics and Clinical Features | All Participants (N = 1649) | Placebo (n = 809) | Tarenflurbil (n = 840) | P Valuea |
|---|---|---|---|---|
| Age, mean (SD) [range], y | 74.6 (8.4) [53−100] | 74.7 (8.4) [53−100] | 74.6 (8.5)[53−100] | .93 |
|
| ||||
| Age range, y, No. (%) | ||||
| <55 | 9 (0.5) | 3 (0.4) | 6 (0.7) | .67 |
|
| ||||
| ≥55−<65 | 218 (13.2) | 110 (13.6) | 108 (12.9) | |
|
| ||||
| ≥65−<75 | 496 (30.1) | 233 (28.8) | 263 (31.3) | |
|
| ||||
| ≥75−<85 | 752 (45.6) | 378 (46.7) | 374 (44.5) | |
|
| ||||
| ≥85 | 174 (10.6) | 85 (10.5) | 89 (10.6) | |
|
| ||||
| Female sex, No. (%) | 840 (50.9) | 425 (52.5) | 415 (49.4) | .20 |
|
| ||||
| Any college, No. (%) | 1028 (62.3) | 499 (61.7) | 529 (63.0) | .59 |
|
| ||||
| White race, No. (%) | 1558 (94.5) | 761 (94.1) | 797 (94.9) | .47 |
|
| ||||
| Time since original diagnosis, mean (SD), mo | 20.4 (19.8) (n = 1238) | 20.5 (21.5) (n = 608) | 20.4 (18.0) (n = 630) | .94 |
|
| ||||
| Apolipoprotein ε4 positive, No. (%) | 750 (58.1) (n = 1292) | 367 (57.9) (n = 634) | 383 (58.2) (n = 658) | .91 |
|
| ||||
| Concomitant medications for AD, No. (%) | ||||
| None | 307 (18.6) | 146 (18.0) | 161 (19.2) | .81b |
|
| ||||
| Acetylcholinesterase inhibitor only | 548 (33.2) | 268 (33.1) | 280 (33.3) | |
|
| ||||
| Memantine only | 103 (6.2) | 48 (5.9) | 55 (6.5) | |
|
| ||||
| Acetylcholinesterase inhibitor and memantine | 690 (41.8) | 347 (42.9) | 343 (40.8) | |
|
| ||||
| Cognitive and functional measure scores, mean (SD)c | ||||
| MMSE | 23.3 (2.0) (n = 1649) | 23.3 (2.0) (n = 809) | 23.3 (2.0) (n = 840) | .63 |
|
| ||||
| ADAS-Cog score, points | ||||
| 80 | 25.9 (8.7) (n = 1643) | 25.7 (8.9) (n = 806) | 26.1 (8.5) (n = 837) | .36 |
|
| ||||
| 70 | 18.0 (7.5) (n = 1644) | 17.8 (7.7) (n = 806) | 18.2 (7.4) (n = 838) | .35 |
|
| ||||
| ADCS-ADL | 63.6 (11.3) (n = 1580) | 63.6 (11.1) (n = 777) | 63.6 (11.5) (n = 803) | .93 |
|
| ||||
| CDR-sb | 4.9 (2.3) (n = 1643) | 5.0 (2.4) (n = 806) | 4.9 (2.3) (n = 837) | .37 |
|
| ||||
| Neuropsychiatric Inventory | 8.4 (9.9) (n = 1606) | 8.6 (10.2) (n = 785) | 8.1 (9.6) (n = 821) | .31 |
|
| ||||
| QOL-AD | 36.9 (6.0) (n = 1370) | 36.7 (6.0) (n = 666) | 37.1 (6.0) (n = 704) | .17 |
|
| ||||
| Caregiver Burden Inventory | 16.1 (13.8) (n= 1421) | 16.3 (14.1) (n = 701) | 15.8 (13.4) (n = 720) | .48 |
Abbreviations: AD, Alzheimer disease; ADAS-Cog, AD Assessment Scale; ADCS-ADL, AD Cooperative Studies-activities of daily living scale; Cognitive Subscale; CDR-sb, Clinica Dementia Rating-sum of boxes; MMSE, Mini-Mental State Examination; QOL, quality of life
P value based on Pearson χ2 test.
Comparison of the distribution across the 4 categories of concomitant medication use, based on a Pearson χ2 test.
See “Methods” section for test total points.
During the 18-month trial, 269 patients (33%) discontinued from the placebo group and 334 patients (40%) discontinued from the tarenflurbil group (P=.006). The 75th percentile of the follow-up distribution was 12.1 months for the placebo group and 10.0 months for the tarenflurbil group. In the tarenflurbil group, patients' mean percentage of pills taken relative to those that were supposed to be taken was 92.7% and 92.9% for placebo. Only 11.2% taking tarenflurbil and 11.4% taking placebo took less than 80% of their dosages.
Virtually all patients (99.1%) took at least 1 concomitant medication during the study (mean number of medications, 12.8). The most frequent concomitant medications were psychoanaleptic agents (86.6%), vitamins (62.6%), serum lipidreducing agents (55.3%), antithrombotic agents (51.0%), antibacterials (34.1%), analgesics (32.7%), mineral supplements (31.6%), agents acting on the reninangiotensin system (31.1%), psycholeptics (26.7%), drugs for acid-related disorders (25.9%), β-blockers (22.5%), and urologic agents (20.1%). Types and frequencies of concomitant medications were not significantly different between groups.
Primary Outcomes Analysis
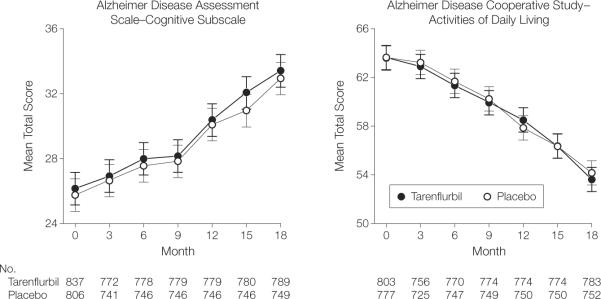
Results of the primary outcome are shown in Table 2 and Figure 2. (The number of participants shown differs because Table 2 excludes those with missing baseline values.) Based on least squares means, the tarenflurbil and placebo groups did not differ in change from baseline to month 18. The mean treatment difference for the ADAS-Cog score was 0.1 (95% confidence interval [CI], −0.9 to 1.1; P=.86) and −0.5 for ADCSADL score (95% CI, −1.9 to 0.9; P=.48). Similarly, the change from baseline did not differ in months 3 through 15. The slopes did not differ for either of the primary outcomes. Results did not differ in the observed case or per-protocol analyses (eTable 1, available at http//:www.jama.com).
Table 2.
Change from Baseline in Participants With at Least 1 Baseline Neuropsychiatric Test Result
| Intention to Treat Population, Mean (SD) |
||||||||
|---|---|---|---|---|---|---|---|---|
| Measure | 3 Month | 6 Month | 9 Month | 12 Month | 15 Month | 18 Month | Slope (SD) | P Valuea |
| ADAS-Cog 80, T/P sample sizes: | 769/738 | 775/743 | 776/743 | 776/743 | 777/743 | 786/746 | 786/746 | |
|
| ||||||||
| Tarenflurbil (n = 786) | 0.85 (5.66) | 1.84 (6.08) | 2.02 (7.45) | 4.24 (7.99) | 5.91 (9.19) | 7.27 (10.52) | 5.22 (0.272) | |
|
| ||||||||
| Placebo (n = 746) | 0.81 (4.94) | 1.73 (5.74) | 2.01 (6.57) | 4.28 (7.50) | 5.11 (8.18) | 7.08 (9.24) | 5.06 (0.274) | |
|
| ||||||||
| CFB P Value | .83 | .77 | .93 | .74 | .097 | .86 | .69 | |
|
| ||||||||
| ADCS-ADL, T/P sample sizes: | 727/706 | 740/721 | 742/722 | 742/723 | 742/723 | 751/725 | 751/725 | |
|
| ||||||||
| Tarenflurbil (n = 751) | −1.02 (6.49) | −2.55 (7.84) | −3.92 (8.93) | −5.36 (10.18) | −7.48 (11.65) | −10.20 (13.31) | −7.12 (0.394) | |
|
| ||||||||
| Placebo (n = 725) | −0.65 (6.42) | −2.14 (7.72) | −3.59 (8.78) | −6.05 (10.72) | −7.54 (11.86) | −9.74 (14.01) | −7.08 (0.395) | |
|
| ||||||||
| CFB P Value | .38 | .36 | .58 | .19 | .80 | .48 | .95 | |
|
| ||||||||
| ADAS-Cog 70, T/P sample sizes: | 770/738 | 776/743 | 777/743 | 777/743 | 778/743 | 787/746 | 787/746 | |
|
| ||||||||
| Tarenflurbil (n = 787) | 0.87 (4.86) | 1.73 (5.35) | 1.76 (6.59) | 3.84 (7.29) | 5.45 (8.48) | 6.68 (9.85) | 4.71 (0.259) | |
|
| ||||||||
| Placebo (n = 746) | 0.88 (4.39) | 1.58 (5.09) | 1.72 (5.94) | 3.81 (6.99) | 4.78 (7.57) | 6.44 (8.69) | 4.55 (0.262) | |
|
| ||||||||
| CFB P Value | >.99 | .77 | .89 | .77 | .18 | .87 | .67 | |
|
| ||||||||
| CDR-sb, T/P sample sizes: | 767/738 | 771/743 | 772/743 | 773/743 | 773/744 | 782/746 | 782/746 | |
|
| ||||||||
| Tarenflurbil (n = 782) | 0.41 (1.36) | 0.84 (1.76) | 1.30 (2.09) | 1.67 (2.35) | 2.19 (2.71) | 2.91 (3.21) | 1.95 (0.092) | |
|
| ||||||||
| Placebo (n = 746) | 0.32 (1.43) | 0.64 (1.74) | 1.02 (2.08) | 1.49 (2.47) | 1.99 (2.80) | 2.43 (3.12) | 1.69 (0.093) | |
|
| ||||||||
| CFB P Value | .52 | .091 | 041 | .26 | .24 | .0036 | .046 | |
|
| ||||||||
| MMSE, T/P sample sizes: | 569/588 | 630/615 | ||||||
|
| ||||||||
| Tarenflurbil (n = 630) | NA | NA | NA | −2.36 (4.10) | NA | −3.65 (5.13) | ||
|
| ||||||||
| Placebo (n = 615) | NA | NA | NA | −2.04 (4.11) | NA | −3.27 (5.09) | ||
|
| ||||||||
| CFB P Value | NA | NA | NA | 0.37 | NA | .27 | b | |
|
| ||||||||
| Neuropsychiatric Inventory, T/P sample sizes: | 747/712 | 755/723 | 757/724 | 757/724 | 757/725 | 767/727 | 767/727 | |
|
| ||||||||
| Tarenflurbil (n = 767) | 0.38 (7.53) | 0.39 (8.35) | 1.57 (9.38) | 2.63 (10.36) | 3.47 (11.76) | 3.99 (12.32) | 3.12 (0.364) | |
|
| ||||||||
| Placebo (n = 727) | −0.28 (8.23) | 0.44 (9.55) | 1.34 (10.41) | 1.74 (10.61) | 2.59 (11.58) | 3.37 (11.88) | 2.63 (0.365) | |
|
| ||||||||
| CFB P Value | .33 | .37 | .71 | .26 | .30 | .47 | .34 | |
|
| ||||||||
| QOL participant, T/P sample sizes: | 538/541 | 556/549 | 570/553 | |||||
|
| ||||||||
| Tarenflurbil (n = 570) | NA | −0.37 (4.96) | NA | −0.18 (5.05) | NA | −0.86 (5.49) | ||
|
| ||||||||
| Placebo (n = 553) | NA | −0.22 (4.81) | NA | −0.54 (5.05) | NA | −0.76 (5.41) | ||
|
| ||||||||
| CFB P Value | NA | .91 | NA | .093 | NA | .84 | b | |
|
| ||||||||
| QOL caregiver, T/P sample sizes: | 546/542 | 563/552 | 578/557 | |||||
|
| ||||||||
| Tarenflurbil (n = 578) | NA | −1.06 (5.08) | NA | −1.71 (5.33) | NA | −2.82 (5.52) | ||
|
| ||||||||
| Placebo (n = 557) | NA | −0.95 (5.18) | NA | −2.07 (4.97) | NA | −2.54 (5.61) | ||
|
| ||||||||
| CFB P Value | NA | .73 | NA | .11 | NA | .51 | b | |
|
| ||||||||
| Caregiver Burden Inventory, T/P sample sizes: | 543/536 | 572/566 | 591/574 | |||||
|
| ||||||||
| Tarenflurbil (n = 591) | NA | 1.88 (11.01) | NA | 4.67 (12.71) | NA | 6.57 (13.40) | ||
|
| ||||||||
| Placebo (n = 574) | NA | 1.54 (10.88) | NA | 4.28 (12.36) | NA | 5.93 (13.04) | ||
|
| ||||||||
| CFB P Value | NA | .92 | NA | .57 | NA | .31 | b | |
Abbreviations: ADAS-Cog, Alzheimer Disease Assessment Scale-Cognitive Subscale; Alzheimer Disease Cooperative Studies-activities of daily living scale; CDR, Clinical Dementia Rating; CFB, change from baseline; MMSE, Mini-Mental State Examination; N/A, not available; T/P, time period.
P Values were based on the significance of the time by treatment interaction term in a repeated measures linear mixed model including random intercepts and slopes, baseline score and time as covariates, and factors for treatment group, clinical site, and current acetylcholinesterase inhibitor/memantine use.
Ad hoc slope analysis not done due to small number of time points.
Figure 2.
Alzheimer Disease Assessment Cognitive Subscale and Alzheimer Disease Cooperative Studies–Activities of Daily Living Scale Scores by Visit
Values represent means using imputed last observation carried forward. Error bars represent 95% CIs.
Secondary Outcomes Analysis
Results of secondary outcome measures are also shown in Table 2 and Figure 2. Change from baseline differed only for the CDR-sb, which favored placebo at months 9 (P=.04) and 18 (P=.004). The slopes for CDR-sb, but not the 70-point ADAS-Cog scale or the Neuropsychiatric Inventory, significantly favored placebo (P=.046).
Additional prespecified change-from-baseline analyses within the followingsubgroups did not show any significant differences: concomitant treatment, sex, age (2 analyses, split at 65 years and at 75 years, respectively). The percentages of improvers did not differ at any visit for the co-primary end points (eTable 2, available at http://www.jama.com).
Plasma concentrations of S-flurbiprofen were determined from blood samples. Of 858 patients receiving tarenflurbil with PK measurements, 417 (48.6%) had at least 1 sample with a measurable concentration of S-flurbiprofen. The median concentration of S-flurbiprofen was 0.67 μm/L with a range of 0.5–9.21 μm/L. A 1-compartment population PK model was developed using the plasma concentration data and the tarenflurbil PK parameters AUC(0–12) and Cmax for each patient were estimated. A correlational analysis exploring the relationship between primary outcome measures and estimates of tarenflurbil exposure (AUC(0–12) and Cmax) derived from population PK analysis did not reveal any significant association. Using the individual parameter measurements, the mean AUC(0–12) and Cmax were calculated to be 669.39-hour × μg/mL (90% CI, 655.85–682.93)and68.59 μg/mL (90% CI, 67.48–69.69), respectively, which were similar to those observed in the previous study of tarenflurbil.7 Post hoc subgroup analyses included severity of disease using median split on each outcome in separate analyses, whether or not patients had 1 or 2 copies of the APOE ε4 allele, body mass index (split ≤26 vs >26), and genotypic variants in the cytochrome P450 2C9 (CYP2C9) enzyme which is largely responsible for tarenflurbil metabolism.17 None of these analyses suggested a subpopulation wherein tarenflurbil demonstrated efficacy.
Adverse Events
Rates of adverse events are listed in Table 3. Overall, 87.2% of participants in the tarenflurbil group and 85.0% of participants in the placebo group experienced at least 1 adverse event (P=.19). More participants taking tarenflurbil than those taking placebo experienced dizziness (P=.03), upper respiratory tract infection (P=.25), and constipation (P=.18).
Table 3.
Adverse Events Occurring in at Least 5% of Participants
| No. (%) of Participants |
|||
|---|---|---|---|
| Placebo (n = 821)a | Tarenflurbil (n = 860)a | P Valueb | |
| Adverse events | |||
| ≥1 Event | 698 (85.0) | 750 (87.2) | .19 |
|
| |||
| Discontinued | 95 (11.6) | 158 (18.4) | <.001 |
|
| |||
| ≥1 Serious | 163 (19.9) | 195 (22.7) | .16 |
|
| |||
| Deaths | 18 (2.2) | 24 (2.8) | .43 |
|
| |||
| MedDRA preferred term | |||
| Urinary tract infection | 109 (13.3) | 111 (12.9) | .82 |
|
| |||
| Fall | 83 (10.1) | 77 (9.0) | .42 |
|
| |||
| Dizziness | 47 (5.7) | 73 (8.5) | .03 |
|
| |||
| Diarrhea | 60 (7.3) | 71 (8.3) | .47 |
|
| |||
| Upper respiratory tract infection | 46 (5.6) | 60 (7.0) | .25 |
|
| |||
| Depression | 70 (8.5) | 58 (6.7) | .17 |
|
| |||
| Agitation | 57 (6.9) | 52 (6.0) | .46 |
|
| |||
| Constipation | 35 (4.3) | 49 (5.7) | .18 |
|
| |||
| Nasopharyngitis | 57 (6.9) | 44 (5.1) | .12 |
|
| |||
| Nausea | 48 (5.8) | 40 (4.7) | .27 |
|
| |||
| Headache | 45 (5.5) | 40 (4.7) | .44 |
|
| |||
| Back pain | 41 (5.0) | 30 (3.5) | .13 |
Abbreviation: MedDRA, Medical Dictionary for Regulatory Activities.
One participant randomized to placebo and 2 participants randomized to the treatment group discontinued prior to receiving medication and are not included in this table. All percentages and P values are based on the indicated sample sizes.
P values were based on the Pearson χ2 test.
Participants receiving tarenflurbil also experienced the following adverse events more frequently than those taking placebo: all anemias, 70 (8.1%) vs 31 (3.8%; P<.001); pneumonia, 26 (3.0%) vs 14 (1.7%; P=.08); herpes zoster, 17 (2.0%) vs 6 (0.7%; P=.03); gastrointestinal ulcers, 12 (1.4%) vs 3 (0.4%; P=.02); and eosinophilia, 10 (1.2%) vs 3 (0.4%; P=.06). Seven participants (0.8%) in the tarenflurbil group vs 19 (2.3%) in the placebo group experienced a stroke or transient ischemic attack (P=.01). One hundred fifty-eight participants in the tarenflurbil group discontinued because of an adverse event vs 95 in the placebo group (P<.001). Of the 42 deaths during the study, 24 occurred in the tarenflurbil group and 18 in the placebo group (P=.43).
Mean eosinophil count in the tarenflurbil group increased 33% from baseline to month 1 with a subsequent slow decline to baseline levels by month 15 and were unaccompanied by any clinical features. The mean increase in low-density lipoprotein cholesterol over the course of the study was significantly greater (P<.001) for the tarenflurbil group than for the placebo group (14.5% vs 3.1% increase over baseline mean).
COMMENT
Tarenflurbil did not slow cognitive decline or loss of ADLs in patients with mild AD nor did any secondary outcome measure or post hoc analysis favor tarenflurbil. Tarenflurbil was reasonably well tolerated, although more participants dropped out due to adverse events in the active treatment group than in the placebo group.
Several possibilities may explain the lack of efficacy. Plasma concentrations of tarenflurbil indicated that the compound was absorbed as expected; however, data from earlier studies in healthy older individuals had indicated a dose-dependent penetration of drug from plasma to cerebrospinal fluid of only 0.5% to 1%.18 Low brain penetration in that study did not deter plans for the phase 2 or 3 trials because preclinical studies had shown that administration of γ-secretase modulators could reduce amyloid levels and deposits in amyloid precursor protein transgenic mice despite low levels of brain penetration (tarenflurbil plasma to brain tissue concentration ratio of 1.8% to 2.4%).4,19–23
The same 21-day study in healthy older individuals failed to demonstrate sustained reduction in Aβ42 in either plasma or cerebrospinal fluid at 200, 400, or 800 mg twice daily of tarenflurbil vs placebo. Although at peak drug concentration, higher plasma levels were related to lower Aβ42 plasma concentration.18 It is possible that the 800-mg dose may have been too low for a therapeutically relevant γ-secretase modulatory effect in humans. However, no trends in the present results suggest that higher plasma concentrations of tarenflurbil had different clinical outcomes.
Tarenflurbil is the R-enantiomer in R-S-flurbiprofen marketed in the United States as an analgesic and anti-inflammatory. The S-enantiomer acts as a cyclooxygenase-inhibiting NSAID. Tarenflurbil by comparison has very little such activity.24 Due to the low concentrations observed, the presence of S-flurbiprofen was not likely to have any significant effect on efficacy or safety outcomes.
This was the largest randomized, placebo-controlled trial completed for AD to date. The participants had mild AD with MMSE scores from 20 to 26, and as a group they had the expected APOE genotype distribution.25 The placebo group declined at about the same rates as did those in the placebo groups in other 18-month trials that included patients who were both mildly and more moderately impaired.26–29 Thus, the trial showed external validity and consistency with other trials, establishing that the lack of significant efficacy for tarenflurbil was not due to trial design. Nevertheless, as with the other 18-month trials, there was considerable variance around the mean (SD) change scores, eg, ADAS-Cog 70-point version, 6.68 (9.85) for tarenflurbil and 6.44 (8.69) for placebo, indicating that a substantial proportion of patients did not worsen on the ADAS-Cog outcomes over 18 months. The proportion of study participants taking concomitant medications for AD was striking. This trial was limited by the high discontinuation rate, which was similar to that of other recent 18-month trials.26–28,30
The large sample size of this trial would have allowed it to detect a difference as small as 1.4 points on the ADAS-Cog 80-item version if such an effect had been present. The large sample size also provides insight into the overall rate of decline in patients with mild AD as well as the variability in decline among the patients selected for AD clinical trials and provides stable estimates of changes on the outcome measures commonly used in AD trials. Thus, these results provide important information for estimating power in future trials of mild AD. Our results are also a reminder that interventions affecting amyloid have not yet been shown to alter the course of AD.
Acknowledgments
Financial Disclosures: Dr Green reports having received grant support (but no consulting fees) for this clinical trial from Myriad Pharmaceuticals Inc. He reports receiving grant support from Elan and Eli Lilly. Dr Schneider reports having been a paid consultant to and having received grant support for a clinical trial from Myriad Pharmaceuticals Inc, being an editor on the Cochrane Collaboration's Dementia and Cognitive Improvement Group, which oversees systematic reviews of drugs for cognitive impairment and dementia; receiving a grant from the Alzheimer's Association for developing a registry for dementia and cognitive impairment trials; receiving grant or research support from AstraZeneca, Baxter, Elan Pharmaceuticals, Forest Laboratories, Johnson & Johnson, Eli Lilly, Myriad, Novartis, Pfizer, Takeda, and Wyeth; and having served as a consultant for or receiving consulting fees from Abbott Laboratories, Allergan, Allon, Alzheimer Drug Discovery Foundation, AstraZeneca, Bristol-Myers Squibb, Elan, Eli Lilly, Forest, GlaxoSmithKline, Institute IPSEN, Johnson & Johnson, Lundbeck, Myriad, Medivation, Merck, Novartis, Roche, Sanofi-Aventis, Servier, Schering-Plough, Schwabe, Teva, Toyama, Transition Therapeutics, Voyager, and Wyeth. Dr Wilcock reports having been a paid consultant to and having received grant support from Myriad and having served as a consultant for or receiving consulting fees from Eisai, Evotec, Lund-beck, Marix Drug Development, Myriad Pharmaceuticals, Neuropharm, Novartis, Pfizer, Roche, and Shire Pharmaceuticals. Drs Amato, Beelen, Swabb, and Zavitz, as employees of Myriad Pharmaceuticals Inc, reported having an equity ownership and holding stock options in Myriad Pharmaceuticals Inc.
Funding/Support: This study was primarily supported by Myriad Pharmaceuticals. Dr Green reported that he is supported by National Institutes of Health grants RO1-HG02213, K24-AG027841, P30-AG13846, and M01-RR00533. Dr Wilcock was partly supported by the Oxford Comprehensive Biomedical Research Centre with funding from the UK Department of Health's National Institute for Health Research Biomedical Research Centres funding scheme. The statistical analysis was conducted under the direction of Dr Amato, with assistance from Dr Alfred Balch and Mr Brent Evans, MS, both employees of Myriad Pharmaceuticals.
Role of the Sponsor: Myriad Pharmaceuticals Inc provided all financial and material support for the research, consulted with the authors and the members of the Tarenflurbil Phase 3 Study Group on the study design, monitored the conduct of the study and collection of the data, and assisted the authors in the analysis and interpretation of the data and the preparation and review of the manuscript.
Additional Contributions: We thank Mary Jackson, RN, for overseeing the clinical operations of this study and Gary Mather, DVM, PhD, DABT, for the pharmacokinetic analyses; both are employees of Myriad Pharmaceuticals. We thank the members of the data and safety monitoring committee: Chris Clark, MD, and Jason Karlawish, MD (University of Pennsylvania), Gerald Faich, MD, MPH (United BioSource), Peter Kowey, MD (Main Line Health Heart Center), and Weichung Shih, PhD (University of Medicine and Dentistry of New Jersey, School of Public Health).
Footnotes
Independent Statistical Analysis: Independent statistical analysis was conducted by Adrienne Cupples, PhD, Department of Biostatistics, Boston University, Boston, Massachusetts. The analysis, for which she received no commercial compensation, is presented in this article.
Additional Information: Tarenflurbil Phase 3 Study Group affiliations and eTables are available at http://www.jama.com.
Trial Registration clinicaltrials.gov Identifier: NCT00105547
REFERENCES
- 1.Hardy JA, Higgins GA. Alzheimer's disease: the amyloid cascade hypothesis. Science. 1992;256(5054):184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- 2.Selkoe DJ. Alzheimer's disease: genes, proteins, and therapy. Physiol Rev. 2001;81(2):741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 3.McGowan E, Pickford F, Kim J, et al. Abeta42 is essential for parenchymal and vascular amyloid deposition in mice. Neuron. 2005;47(2):191–199. doi: 10.1016/j.neuron.2005.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eriksen JL, Sagi SA, Smith TE, et al. NSAIDs and enantiomers of flurbiprofen target gamma-secretase and lower Abeta 42 in vivo. J Clin Invest. 2003;112(3):440–449. doi: 10.1172/JCI18162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kukar TL, Ladd TB, Bann MA, et al. Substrate-targeting gamma-secretase modulators. Nature. 2008;453(7197):925–929. doi: 10.1038/nature07055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kukar T, Prescott S, Eriksen JL, et al. Chronic administration of R-flurbiprofen attenuates learning impairments in transgenic amyloid precursor protein mice. BMC Neurosci. 2007;8:54–66. doi: 10.1186/1471-2202-8-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilcock GK, Black SE, Hendrix SB, Zavitz KH, Swabb EA, Laughlin MA. Tarenflurbil Phase II Study investigators. Efficacy and safety of tarenflurbil in mild to moderate Alzheimer's disease: a randomised phase II trial. Lancet Neurol. 2008;7(6):483–493. doi: 10.1016/S1474-4422(08)70090-5. [DOI] [PubMed] [Google Scholar]
- 8.McKhann G, Drachman D, Folstein MF, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 9.Rosen WG, Terry RD, Fuld PA, Katzman R, Peck A. Pathologic verification of ischemic score in differentiation of dementias. Ann Neurol. 1980;7(5):486–488. doi: 10.1002/ana.410070516. [DOI] [PubMed] [Google Scholar]
- 10.Rosen W, Mohs RC, Davis KL. A new rating scale for Alzheimer's disease. Am J Psychiatry. 1984;141(11):1356–1364. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- 11.Galasko D, Bennett D, Sano M, et al. An inventory to assess activities of daily living for clinical trials in Alzheimer's disease. Alzheimer Dis Assoc Disord. 1997;11(suppl 2):S33–S39. [PubMed] [Google Scholar]
- 12.Morris JC. The Clinical Dementia Rating (CDR) Neurology. 1993;43(11):2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 13.Folstein MF, Folstein SE, McHugh PR. Mini-Mental State. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 14.Cummings JL. The neuropsychiatric inventory. Neurology. 1997;48(5)(suppl 6):S10–S16. doi: 10.1212/wnl.48.5_suppl_6.10s. [DOI] [PubMed] [Google Scholar]
- 15.Logsdon RG, Gibbons LE, McCurry SM, et al. Quality of life in Alzheimer's disease. J Ment Health Aging. 1999;5:21–32. [Google Scholar]
- 16.Novak M, Guest C. Application of a multidimensional caregiver burden inventory. Gerontologist. 1989;29(6):798–803. doi: 10.1093/geront/29.6.798. [DOI] [PubMed] [Google Scholar]
- 17.Tracy TS, Rosenbluth BW, Wrighton SA, Gonzalez FJ, Korzekwa KR. Role of cytochrome P450 2C9 and an allelic variant of the 4'-hydroxylation of (R)- and (S)-flurbiprofen. Bioch Pharm. 2005;49(9):1269–1127. doi: 10.1016/0006-2952(95)00048-5. [DOI] [PubMed] [Google Scholar]
- 18.Galasko DR, Graff-Radford N, May S, et al. Safety, tolerability, pharmacokinetics, and Abeta levels after short-term administration of R-flurbiprofen in healthy elderly individuals. Alzheimer Dis Assoc Disord. 2007;21(4):292–299. doi: 10.1097/WAD.0b013e31815d1048. [DOI] [PubMed] [Google Scholar]
- 19.Weggen S, Eriksen JL, Das P, et al. A subset of NSAIDs lower amyloidgenic Abeta42 independently of cyclooxygenase activity. Nature. 2001;414(6860):212–216. doi: 10.1038/35102591. [DOI] [PubMed] [Google Scholar]
- 20.Lim G, Yang F, Chu T, et al. Ibuprofen suppresses plaque pathology and inflammation in a mouse model for Alzheimer's disease. J Neurosci. 2000;20(15):5709–5714. doi: 10.1523/JNEUROSCI.20-15-05709.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yan Q, Zhang J, Liu H, et al. Anti-inflammatory drug therapy alters beta-amyloid processing and deposition in an animal model of Alzheimer's disease. J Neurosci. 2003;23(20):7504–7509. doi: 10.1523/JNEUROSCI.23-20-07504.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jantzen PI, Connor K, DiCarlo G, et al. Microglial activation and beta-amyloid deposit reduction caused by a nitric oxide-releasing nonsteroidal anti-inflammatory drug in amyloid precurser protein plus presenilin-1 transgenic mice. J Neurosci. 2002;22(6):2246–2254. doi: 10.1523/JNEUROSCI.22-06-02246.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yao Y, Chinnici C, Tang H. Brain inflammation and oxidative stress in a transgenic mouse model of Alzheimer-like brain amyloidosis. J Neuroinflammation. 2004;1:1–21. doi: 10.1186/1742-2094-1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brune K, Geisslinger G, Menzel-Soglowek S. Pure enantiomers of 2-arylproprionic acids. J Clin Pharmacol. 1992;32(10):944–952. doi: 10.1002/j.1552-4604.1992.tb04643.x. [DOI] [PubMed] [Google Scholar]
- 25.Farrer LA, Cupples LA, Haines JL, et al. Effects of age, sex and ethnicity on the association between apolipoprotein E genotype and Alzheimer's Disease: A meta-analysis. JAMA. 1997;278(16):1349–1356. [PubMed] [Google Scholar]
- 26.Aisen PS, Schneider LS, Sano M, et al. High-dose B vitamin supplementation and cognitive decline in Alzheimer disease. JAMA. 2008;300(15):1774–1783. doi: 10.1001/jama.300.15.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gauthier S, Douillet P, Doody R, Fox NC, Orgogozo JM. Effect of xaliproden, a compound with neurotrophic properties, on the progression of Alzheimer's disease. Clinical Trials in Alzheimer Disease; Montpellier, France. September 17–19, 2008;. [Google Scholar]
- 28.Sano M. Multi-center, randomized, double-blind, placebo controlled trial of Simvastatin to slow the progression of Alzheimer's disease. Alzheimers Dement. 2008;4:T200. doi: 10.1016/j.trci.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. [Accessed April 15, 2009];PhRMA Web Synopsis: protocol A2581078 01 June 2008 Final. http://www.clinicalstudyresults.org/documents/company-study_4374_0.pdf.
- 30.Schneider LS, Sano M. Current Alzheimer's disease clinical trials. Alzheimers Dement. 2009;5(5):388–397. doi: 10.1016/j.jalz.2009.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]