Abstract
Group A rotaviruses (RV), members of the Reoviridae family, are a major cause of infantile acute gastroenteritis. The RV genome consists of 11 double-stranded RNA segments. In some cases, an RNA segment is replaced by a rearranged RNA segment, which is derived from its standard counterpart by partial sequence duplication. We report here a reverse genetics system for RV based on the preferential packaging of rearranged RNA segments. Using this system, wild-type or in vitro-engineered forms of rearranged segment 7 from a human rotavirus (encoding the NSP3 protein), derived from cloned cDNAs and transcribed in the cytoplasm of COS-7 cells with the help of T7 RNA polymerase, replaced the wild-type segment 7 of a bovine helper virus (strain RF). Recombinant RF viruses (i.e., engineered monoreassortant RF viruses) containing an exogenous rearranged RNA were recovered by propagating the viral progeny in MA-104 cells, with no need for additional selective pressure. Our findings offer the possibility to extend RV reverse genetics to segments encoding nonstructural or structural proteins for which no potent selective tools, such as neutralizing antibodies, are available. In addition, the system described here is the first to enable the introduction of a mutated gene expressing a modified nonstructural protein into an infectious RV. This reverse genetics system offers new perspectives for investigating RV protein functions and developing recombinant live RV vaccines containing specific changes targeted for attenuation.
Group A rotaviruses (RV), members of the Reoviridae family, are a major cause of infantile viral gastroenteritis and are responsible for up to 700,000 deaths each year (6, 24). The RV genome consists of 11 segments of double-stranded RNA (dsRNA) which can be separated by polyacrylamide gel electrophoresis (PAGE), resulting in typical dsRNA profiles exhibiting four well-defined size classes of segments. However, some RV show unusual dsRNA profiles in which standard-sized segments are replaced by larger, rearranged forms (for a review, see reference 5). Gene rearrangements were first reported for RV isolated from immunodeficient children with chronic infection (11, 26) and can be obtained experimentally by serial passages at a high multiplicity of infection (MOI) in cell culture (10, 14, 21). We recently showed that rearrangements also occur during acute RV infection (36). Gene rearrangement usually consists of a partial head-to-tail duplication of a segment sequence. In most cases, sequence duplication occurs after the stop codon, leaving the open reading frame (ORF) untouched and the encoded protein unchanged (5, 6). Less frequently, the duplication occurs within the ORF, which thus encodes a modified protein (8, 38).
Manipulation at the cDNA level of most positive- and negative-strand RNA viral genomes, followed by rescue of infectious virus, is now well established and has provided a better understanding of RNA virus replication and pathogenesis. In the case of Reoviridae, the development of reverse genetics systems has been hampered by the nature of the genome, which carries 10 to 12 dsRNA segments that are densely packed within the viral particle and are transcribed and replicated within a subviral structure. Recent major advances were obtained in the development of reverse genetics systems for some Reoviridae. For mammalian orthoreo- and orbiviruses, reverse genetics systems based on the transfection of plasmid cDNAs (13) or cDNA-derived mRNAs (2) corresponding to a complete set of 10 RNA segments have been established, allowing the rescue of infectious viral progeny. However, for RV, there is no report indicating that transfection of a complete set of viral mRNAs can result in the rescue of infectious viruses, and it is still unknown whether the RV genome can be infectious. In 2006, Komoto et al. (16) described the first reverse genetics system for RV, based on a model originally developed for influenza viruses by Palese and colleagues (17). In this system, an exogenous RV mRNA synthesized in the cell cytoplasm by the T7 RNA polymerase (T7pol), expressed by a recombinant vaccinia virus, is packaged in place of its homologous counterpart into a helper RV. This reverse genetics system has allowed the recovery of engineered monoreassortant infectious RV (designated recombinant RV) with an incorporated exogenous segment 4 encoding the spike protein VP4. An in vitro-modified cDNA-derived segment 4 was also successfully introduced into a recombinant RV to obtain a virus carrying a VP4 antigenic chimera (15). However, this system is restricted to segments encoding antigenically distinct viral surface proteins (like VP4) because the selection of recombinant viruses requires the use of specific and potent neutralizing antibodies to eliminate wild-type (WT) helper viruses.
Results obtained from a preliminary study suggested that rearranged RNA segments might overcome this limitation. Indeed, we first characterized human RV clones containing rearranged segment 7, 11, or both (8) and then compared the fitness levels of rearranged versus WT viruses. We found that viruses with rearranged segment 7 or 11 replicated less well than or equally to WT viruses, as judged by viral growth curve experiments. Surprisingly, in competition growth experiments, rearranged segment 7 or 11 was always selected into the viral progenies, even when mixed infections were performed with a ratio of 1 rearranged to 1,000 WT viruses (4). The absence of a growth advantage conferred on the virus by rearranged segments, combined with their preferential segregation into the viral progenies, suggested that rearranged RNA segments might be packaged preferentially. These observations are in agreement with results of earlier studies showing that rearranged segment 5 or 11 segregated preferentially into viral progenies issued from mixed infections with WT virus (10, 19).
We developed a reverse genetics system for RV on the basis of the preferential packaging of rearranged RNAs. We report here the rescue of recombinant viruses carrying cDNA-derived rearranged segment 7 (either unmodified or containing silent mutations introduced by site-directed mutagenesis to generate restriction enzyme sites as markers), with no selection pressure other than serial passage in cell culture. We also report the rescue of a recombinant virus expressing a double-sized recombinant NSP3 protein encoded by an in vitro-modified cDNA-derived rearranged segment 7, showing for the first time that an in vitro-engineered gene encoding a modified nonstructural protein can be introduced into an infectious RV.
MATERIALS AND METHODS
Viruses and cells.
Human RV M1 and M3 are two previously described cell culture-adapted viruses containing a rearranged segment 7 (8). Virus M1 has a rearranged segment 7R (GenBank accession no. AF338247), and virus M3 has a rearranged segment 7RΔ (GenBank accession no. AF338248), encoding a modified NSP3 protein (mNSP3). The same clonal stock of bovine RV strain RF (5 × 108 PFU/ml) was used in all experiments. RV propagation on confluent monolayers of MA-104 cells and plaque assay for RV titration were performed as previously described (7, 8). Recombinant vaccinia virus rDIs-T7pol, kindly provided by K. Ishii (National Institute of Infectious Diseases, Tokyo, Japan) and expressing T7pol (12), was produced in the DF1 chicken embryonic fibroblast cell line, kindly provided by N. Naffakh (Pasteur Institute, Paris, France), and the resulting vaccinia virus stock (1 × 107 focus-forming units [FFU]/ml) was titrated on BHK-21 cells by a fluorescent-focus assay. The MA-104, COS-7, and DF1 cell lines were cultured in Dulbecco's minimum essential medium (DMEM) supplemented with 10% fetal calf serum (FCS), 2 mM glutamine, 100 U/ml penicillin, and 1 μg/ml streptomycin. The BHK-21 cell line was cultured in Glasgow's minimum essential medium supplemented with 10% FCS, 5% tryptose phosphate broth, 100 U/ml penicillin, and 1 μg/ml streptomycin.
Plasmid construction.
Primers used for reverse transcription-PCR (RT-PCR) amplification or site-directed mutagenesis are given in Table 1, and schematics of the pRiboz plasmids are shown in Fig. 1. pRiboz is a pBluescript II SK-derived plasmid containing the sequence of the hepatitis delta virus (HDV) ribozyme downstream of a unique XmaI site, followed by the T7 terminator (TT7). Full-length cDNAs for the 7R and 7RΔ segments were obtained from dsRNAs extracted from the M1 and M3 viruses, respectively. The dsRNAs were reverse transcribed as previously described (36), and PCR amplifications were performed using 5 μl of cDNA in a 50-μl reaction mixture containing 1× Pfx buffer, 1× Pfx enhancer, 1 mM MgSO4, a 0.3 mM concentration of each deoxynucleoside triphosphate (dNTP), a 0.3 μM concentration of each primer, and 2.5 U of Pfx DNA polymerase (Invitrogen). Amplification was achieved with a model 9700 Perkin Elmer thermocycler. PCR conditions for amplification of full-length cDNA were 35 cycles of 94°C for 15 s, 52°C for 30 s, and 68°C for 3 min. Forward primers used for PCR had a 5′ extension containing a NotI site and the T7 promoter (PT7.) Amplified cDNAs were digested with NotI and AgeI and then ligated to the NotI and XmaI sites of pRiboz. The generated plasmids, pRiboz7R and pRiboz7RΔ, contained the authentic full-length cDNAs of the 7R and 7RΔ segments flanked by PT7 and the HDV ribozyme, followed by TT7.
TABLE 1.
Primers used in this study
| Primer | Nucleotide sequence (5′-3′) (restriction enzyme)a | Use |
|---|---|---|
| For cDNA cloning | ||
| A2 | GGTCACATAACGCCCCTATAGCCAT | RT of segment 7R or 7RΔ |
| 7ribozS | ACGGTGGCGGCCGCTAATACGACTCACTATAGGCTTTTAA (NotI) | Full-length PCR of segment 7R or 7RΔ |
| 7ribozR | GACGTCACCGGTCACATAACGCCCCTATAGCCATTTAGGT (AgeI) | Full-length PCR of segment 7R or 7RΔ |
| For site-directed mutagenesis | ||
| 7-mut-Bst-S | AGCTCTATTATTAATACTTCTTTCGAATCTGCAGTCGTTGCTGC (BstBI) | Creation of BstBI site in segment 7R (change T to C) |
| 7-mut-Eco-S | GAATGATATTGAACAGCAGTTGAATTCAATTGATTTAATTAATCCC (EcoRI) | Creation of EcoRI site in segment 7RΔ (change A to G) |
| 7-del-stop-S | AGCAATGCAACTATGAATATGCATATGATTATGCTTTTCAGTGGTTG | Deletion of stop codon in segment 7R |
| For specific detection of rearranged segments 7 | ||
| RPZJ3 | CTAAGTTTATTCACGTCTTCATC | RT and PCR |
| DPZJ3 | GAGTGGTATCTAAGGTCTATGG | PCR |
Restriction enzyme sites are underlined. Letters in italics indicate the T7 RNA polymerase promoter sequence, and letters in bold indicate the nucleotides substituted in the gene 7 sequence.
FIG. 1.
pRiboz plasmids constructs containing 7R (A) and 7RΔ (B) derivatives. All pRiboz constructs contain a full-length rearranged segment 7 cDNA flanked by the T7pol promoter (PT7) and by the HDV ribozyme (Rib), followed by the T7pol terminator (TT7). Numbers indicate the nucleotide position in the rearranged segment, open boxes indicate the 5′- and 3′-UTRs, and gray boxes indicate the NSP3 coding sequence. Arrows and arrowheads indicate the transcription initiation site and the ribozyme self-cleavage site, respectively. The nucleotide changes used to create BstBI and EcoRI sites are indicated in bold. An asterisk indicates the A967 deletion that abrogates the stop codon and AseI site in 7R-∂Stop.
A QuikChange Multi site-directed mutagenesis kit (Stratagene) was used according to the manufacturer's instructions to introduce nucleotide changes in the pRiboz7R and pRiboz7RΔ sequences, creating sites for the restriction enzyme BstBI in 7R (pRiboz7R-BstBI) and for EcoRI in 7RΔ (pRiboz7RΔ-EcoRI), or to delete a single nucleotide to destroy the NSP3 stop codon in 7R (pRiboz7R-∂Stop). All plasmid constructs were checked by DNA sequencing as described below.
Plasmid pRibozGFP, containing the green fluorescent protein (GFP) gene flanked by the 5′- and 3′-untranslated terminal regions (UTRs) of RV segment 5, cloned between PT7 and the HDV ribozyme, and plasmid pEGFP-C1 (Clontech), containing the GFP gene downstream of the cytomegalovirus immediate-early promoter, were used as controls.
Reverse genetics system.
Confluent monolayers of COS-7 cells in six-well plates (1.5 × 106 cells/well) were transfected with 2.7 μg of pRiboz7R, pRiboz7RΔ, pRiboz7R-BstBI, pRiboz7R-∂Stop, or pRiboz7RΔ-EcoRI, using 5 μl of TransIT-LT1 transfection reagent (Mirus) per microgram of plasmid DNA according to the manufacturer's instructions. Under the same conditions, the percentage of COS-7 cells transfected with the control plasmid pEGFP-C1 was repeatedly approximately 90%. Sixteen hours after transfection, COS-7 cells were infected by recombinant vaccinia virus rDIs-T7pol at an MOI of 3. After a 2-h culture in DMEM supplemented with 2.5% FCS, cells were washed three times with DMEM containing 0.25 μg/ml of porcine pancreatic trypsin type IX-S (Sigma) and were infected by the helper virus bovine rotavirus RF at an MOI of 30. After 24 h of culture, COS-7 cells were harvested, freeze-thawed three times, and centrifuged at low speed to remove cell debris. To rescue recombinant RF viruses, supernatants containing the viral progenies produced in COS-7 cells were propagated in MA-104 cells. At the first passage, confluent monolayers of MA-104 cells in 25-cm2 flasks were inoculated with the supernatant corresponding to one well of COS-7 cells. For further serial passage in MA-104 cell culture, 1/10 of the undiluted MA-104 cell lysate was used to infect MA-104 cells in 25-cm2 flasks for 48 h, and at each passage, aliquots were kept frozen at −80°C for further analysis. Recombinant RF viruses carrying the exogenous rearranged segment 7 were rescued from the 18th passage in MA-104 cells by three plaque-to-plaque cloning steps in MA-104 cells, as previously described (7). All reverse genetics experiments were run in duplicate, by separately testing, in MA-104 cells, the viral progeny issued from two independent wells of COS-7 cells.
To assess the efficiency of transfection and/or viral coinfection during the first step of the reverse genetics system, COS-7 cells were fixed at 24 h postinfection and tested by fluorescence assays using two different dyes. The percentage of COS-7 cells coinfected by vaccinia virus and RV was evaluated using immunofluorescence to detect vaccinia virus green staining, using vaccine-specific rabbit polyclonal antiserum (U.S. Biological) and fluorescein isothiocyanate (FITC)-conjugated donkey anti-rabbit IgG (Jackson Immunoresearch), together with RV red staining, using an RV VP6-specific mouse monoclonal antibody (clone RV133; kindly provided by Jean Cohen) and Cy3-conjugated donkey anti-mouse IgG (Jackson Immunoresearch). The percentage of COS-7 cells transfected by plasmid pRibozGFP and coinfected by vaccinia virus and RV was evaluated by detecting direct fluorescence of GFP (the expression of GFP was driven by T7pol of vaccinia virus) together with red staining of RV, obtained by indirect immunofluorescence as described above.
Nucleic acid analysis and sequencing.
For PAGE and RT-PCR analysis, RV genomic dsRNA was extracted from cell culture lysates by use of Tri-Reagent LS (Euromedex) according to the manufacturer's recommendations. RNA genomic profiles were determined by PAGE of 14% polyacrylamide gels for 16 h at 200 V at room temperature, followed by ethidium bromide staining. The RT-PCR assay for specific detection of rearranged segments 7 in the viral progeny was based on a strategy that we previously described for specific detection of segment 11 rearrangements (36). Primers used for RT-PCR detection of rearranged segments 7 are given in Table 1. The RT conditions were the same as those described for the cloning procedures. PCRs were performed in 50-μl reaction mixtures containing 10 mM Tris-HCl (pH 8.3), 2 mM MgCl2, a 0.2 mM concentration of each dNTP, a 0.25 μM concentration of each primer, and 1.5 U of AmpliTaq Gold DNA polymerase (Applied Biosystems). PCR conditions were 45 cycles of 95°C for 30 s, 58°C for 30 s, and 72°C for 1 min, using a model 9700 Perkin Elmer thermocycler. PCR products, left undigested or digested with EcoRI, BstBI, or AseI when appropriate, were separated in 1.5% agarose gels stained with ethidium bromide.
DNA sequencing of plasmid constructs, cDNAs, and RT-PCR products was performed according to the dideoxynucleotide chain termination method, using an ABI Prism Big Dye Terminator cycle sequencing ready reaction kit and an ABI Prism 3130 XL automatic sequencer (Applied Biosystems) according to the manufacturer's instructions.
Protein analysis.
To detect NSP3 viral protein, MA-104 cell cultures were harvested at 18 h postinfection in a lysis buffer containing 50 mM Tris-HCl (pH 6.8), 2% sodium dodecyl sulfate (SDS), and 2% β-mercaptoethanol. SDS-PAGE and Western blotting were performed as previously described (8), using the ID3 anti-NSP3 mouse monoclonal antibody (1).
Figure processing.
Figures were assembled using Adobe Photoshop (version 8.0 for Mac OS X.5). For clarity, positive white-on-black pictures of ethidium bromide-stained gels were converted to negative ones, with the invert function applied to the whole picture.
Nucleotide sequence accession number.
The 7R-∂Stop sequence is available under GenBank accession number GU144588.
RESULTS
Efficiency of reverse genetics system.
To generate infectious recombinant RV carrying an exogenous RV mRNA, we used a modified version of Komoto et al.'s reverse genetics system (16). The exogenous viral mRNA was produced from a full-length cDNA copy of a viral RNA segment cloned into a pRiboz backbone plasmid, downstream of the T7pol promoter (PT7) and upstream of the HDV ribozyme, followed by the T7pol terminator (TT7). This plasmid was transfected into COS-7 cells, where T7pol expressed by a recombinant vaccinia virus drives the transcription of the viral mRNA from the first nucleotide (G) of the 5′ end to the last nucleotide (C) of the 3′ end, after ribozyme cleavage. A key point for rescue of recombinant viruses is to obtain a significant percentage of COS-7 cells not only transfected by the plasmid but also coinfected by vaccinia virus and a helper RV.
The efficiency of the system was assessed and optimized by performing successive experiments combining (i) a reporter plasmid, pRibozGFP, which contained the GFP gene flanked by the 5′- and 3′-UTRs of RV segment 5, cloned between PT7 and the HDV ribozyme; (ii) the recombinant vaccinia virus strain rDIs-T7pol, selected for its low pathogenicity in COS-7 cells (12); and (iii) the bovine RV strain RF, used as a helper virus. The percentages of transfected and/or infected COS-7 cells were evaluated by fluorescence imaging. Infection of COS-7 cells by vaccinia virus or RV alone resulted in >90% infected cells, whereas coinfection of COS-7 cells by vaccinia virus and RV was never higher than 60%, even with an RV MOI as high as 30. When COS-7 cells were transfected by the pRibozGFP reporter plasmid after vaccinia virus infection, the percentage of transfected cells actually infected by RV dramatically dropped, to almost zero. In contrast, when the plasmid was transfected 16 h before the viral infections, the percentage of transfected and coinfected cells reached up to 10%.
These results indicated that despite the optimization of system efficiency, the proportion of recombinant RV carrying the exogenous RNA segment would be in a minority within the viral progeny produced in COS-7 cells. Consequently, the rescue of recombinant viruses would require a strong selective advantage for the recombinant gene to replace the WT gene of the helper virus.
Rescue of recombinant RF viruses containing cDNA-derived rearranged segments 7.
We first tested the possibility of obtaining recombinant viruses containing a rearranged segment 7 derived from two previously described human RV: virus M1, with a rearranged segment, termed 7R, encoding WT NSP3; and virus M3, with a rearranged segment, 7RΔ, encoding a modified NSP3 protein (mNSP3) (8). Our hypothesis was that preferential packaging of rearranged RNAs might favor the rescue of recombinant viruses with no selection pressure other than serial passage in culture itself, since during serial passage in cell culture, recombinant viruses carrying the rearranged segment will be self-selected and might overgrow the WT helper virus.
For that purpose, we cloned the full-length rearranged segment 7R of virus M1 and 7RΔ of virus M3 into the pRiboz backbone plasmid, to obtain pRiboz7R and pRiboz7RΔ plasmids, respectively (Fig. 1). COS-7 cells were transfected with pRiboz7R or pRiboz7RΔ. At 16 hours posttransfection, cells were infected by the vaccinia virus rDIs-T7pol. Two hours after vaccinia virus infection, cells were superinfected by the helper virus RF, and at 24 h post-RV infection, viral progenies were serially propagated in MA-104 cells. All experiments were performed in duplicate, and at each passage, viral RNAs were analyzed by RT-PCR and PAGE. The RT-PCR strategy we used was designed to specifically detect low-copy-number rearranged segments among a vast majority of their WT counterparts (36). With this assay, rearranged segments 7 containing sequence duplication were exponentially amplified, while WT segment 7 of virus RF was not (Fig. 2A and 3A, lanes RF, M1, and M3). Among viral progenies derived from transfection of the pRiboz7R and pRiboz7RΔ plasmids, RT-PCR detection of a rearranged segment 7 was negative for the first two MA-104 cell passages and became positive at cell passage 3 (Fig. 2A and 3A). As expected, the corresponding RT-PCR products were the same size as those obtained from the rearranged segment 7R of virus M1 (537 bp) and 7RΔ of virus M3 (466 bp). The intensity of RT-PCR signals increased with the number of passages, indicating an increase of the recombinant viral population carrying the exogenous rearranged segment. By PAGE, segments 7R and 7RΔ were detected from passage 18 and passage 15 onwards, respectively, and they displayed the same mobility as the corresponding segments, 7R of virus M1 and 7RΔ of virus M3 (Fig. 2B and 3B). Recombinant viruses r-RF(7R) and r-RF(7RΔ), carrying the corresponding exogenous segments 7, were obtained after three plaque-to-plaque cloning steps with the viral progenies from passage 18 (Fig. 2B and 3B). At the first cloning step, 6 of 15 clones had a 7R segment and 7 of 10 clones had a 7RΔ segment, indicating that viral progenies from passage 18 were still a mixture of recombinant and WT RF helper viruses. The sequences of segments 7R and 7RΔ obtained from two independent viral clones were identical to those cloned into the plasmids used for transfection. Thus, our reverse genetics system allowed the rescue of recombinant RV containing an exogenous segment 7, with no selection pressure other than serial cell culture passage itself.
FIG. 2.
Rescue of recombinant RF viruses containing cDNA-derived rearranged segment 7R. Viral progenies produced in COS-7 cells transfected with pRiboz7R plasmid were serially propagated in MA-104 cells, and viral dsRNA was analyzed at each passage, by RT-PCR using primers DPZJ3 and RPZJ3 (A) and by PAGE (B). Pn indicates the passage number. Recombinant virus r-RF(7R) is a representative clone rescued from MA-104 cell passage 18. dsRNAs prepared from M1 and RF viruses were used as controls. Arrows indicate the location of segment 7R in M1 and recombinant RF viruses. Mw, 100-bp-ladder molecular size marker.
FIG. 3.
Rescue of recombinant RF viruses containing cDNA-derived rearranged segment 7RΔ. Viral progenies produced in COS-7 cells transfected by pRiboz7RΔ plasmid were serially propagated in MA-104 cells, and viral dsRNA was analyzed at each passage, by RT-PCR using primers DPZJ3 and RPZJ3 (A) and by PAGE (B). Pn indicates the passage number. Recombinant virus r-RF(7RΔ) is a representative clone rescued from MA-104 cell passage 18. dsRNAs prepared from M3 and RF viruses were used as controls. Arrows indicate the location of segment 7RΔ in M3 and recombinant RF viruses. Mw, 100-bp-ladder molecular size marker.
Rescue of recombinant RF viruses containing in vitro-modified, cDNA-derived rearranged segment 7R or 7RΔ.
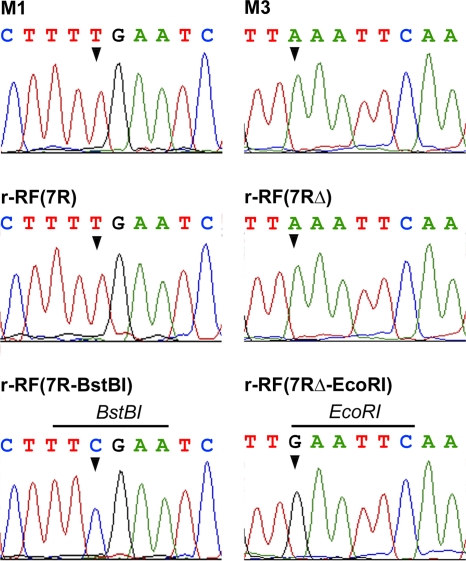
The possibility that recombinant RF viruses could in fact result from natural reassortment events involving RF and M1 or M3 viruses was ruled out by performing the same experiments with pRiboz plasmids containing mutated 7R or 7RΔ sequences. Silent mutations were introduced by site-directed mutagenesis into the 7R and 7RΔ sequences in order to create BstBI and EcoRI restriction sites, respectively, in the sequence targeted by our RT-PCR assay. These sites were chosen because they were absent in full-length sequences of WT or rearranged segments 7 from RF, M1, and M3 viruses. The BstBI and EcoRI sites were created by changing T to C at position 1040 in the 7R sequence and A to G at position 823 in the 7RΔ sequence, to obtain the 7R-BstBI and 7RΔ-EcoRI plasmids, respectively (Fig. 1). When the pRiboz plasmids containing the mutated or unmutated 7R or 7RΔ sequence were used in reverse genetics experiments, the exogenous rearranged segments 7 in the viral progeny produced in MA-104 cell culture were detected between passages 3 and 6 by RT-PCR and at passage 15 by PAGE. Recombinant RF viral clones were then isolated from passage 18 by three plaque-to-plaque cloning steps and were analyzed by PAGE and RT-PCR (Fig. 4). The exogenous 7R-BstBI segment present in r-RF(7R-BstBI) had the same mobility by PAGE as its homologous counterpart in the r-RF(7R) or M1 virus (Fig. 4A) but was the only one to yield an RT-PCR product containing a BstBI site (Fig. 4B). Similarly, r-RF(7RΔ-EcoRI) had an exogenous segment 7 of the same size as segment 7RΔ of r-RF(7RΔ) or M3 virus (Fig. 4A) but was the only one to contain an EcoRI site (Fig. 4B). Lastly, the presence of the BstBI or EcoRI restriction site sequence in the mutated 7R or 7RΔ segment incorporated into the recombinant RF viruses was confirmed by DNA sequencing of RT-PCR products obtained from reverse-transcribed genomic RNAs (Fig. 5).
FIG. 4.
Rescue of recombinant RF viruses containing in vitro-modified, cDNA-derived rearranged segment 7R or 7RΔ. Recombinant viruses r-RF(7R), r-RF(7R-BstBI), r-RF(7RΔ), and r-RF(7RΔ-EcoRI) are representative clones rescued from MA-104 cell culture. (A) dsRNA profiles of recombinant and control (M1 [7R], M3 [7RΔ], and RF) viruses. Segments 7R and 7RΔ are indicated by arrows. (B) BstBI and EcoRI restriction profiles of RT-PCR products obtained from segments 7 of recombinant and control viruses. +, digested; −, not digested. Numbers indicate the expected sizes of fragments. Mw, 100-bp-ladder molecular size marker.
FIG. 5.
Site-specific mutations in segments 7 of recombinant RF viruses. Sequence electropherograms are shown for RT-PCR products obtained from recombinant viruses r-RF(7R), r-RF(7R-BstBI), r-RF(7RΔ), and r-RF(7RΔ-EcoRI) and from control viruses M1 (7R) and M3 (7RΔ). Arrowheads indicate nucleotides changes that created BstBI and EcoRI sites in r-RF(7R-BstBI) and r-RF(7RΔ-EcoRI), respectively.
These results confirmed the reproducibility of the system and indicated the possibility of obtaining infectious RV containing site-specific mutations in a gene coding for a nonstructural protein.
Rescue of a recombinant RF virus expressing a fully duplicated NSP3 protein derived from an in vitro-modified segment 7R cDNA.
We next evaluated the possibility of rescuing recombinant RV expressing a modified NSP3 protein resulting from in vitro site-directed mutagenesis of segment 7R cDNA. For that purpose, we constructed a rearranged segment 7 encoding a fully duplicated NSP3 protein sequence. We chose this strategy because the partially duplicated NSP3 protein encoded by segment 7RΔ, termed mNSP3 (8), could replace the WT NSP3 protein in viable viruses, as it actually did, not only in virus M3 but also in recombinant viruses r-RF(7RΔ) and r-RF(7RΔ-EcoRI). Viruses carrying segment 7R express a WT NSP3 protein. However, the NSP3 ORF of segment 7R is followed by a long 3′-noncoding region containing a fully duplicated copy of the NSP3 ORF sequence. Site-directed mutagenesis was performed on the pRiboz7R plasmid to delete A967 in the stop codon of the NSP3 coding sequence. This deletion resulted in the in-frame fusion of the NSP3 ORF to the duplicated NSP3 coding sequence and in the loss of a restriction site for AseI endonuclease (Fig. 1A).
The resulting mutated plasmid, pRiboz7R-∂Stop, was used in reverse genetics experiments. At passage 18, r-RF(7R-∂Stop) viruses were isolated by three plaque-to-plaque cloning steps, and their dsRNA segments 7 were analyzed (Fig. 6). Segments 7R-∂Stop and 7R, from recombinant RF and M1 viruses, respectively, had the same mobility by PAGE (Fig. 6A) and gave RT-PCR products of the same size, but with different AseI restriction patterns (Fig. 6B). AseI digestion of RT-PCR products obtained from 7R or 7R-∂Stop was expected to give six (222, 115, 78, 63, 50, and 9 bp) or five (222, 177, 78, 50, and 9 bp) DNA fragments, respectively. The presence of a 177-bp fragment for 7R-∂Stop indicated the loss of the AseI site due to the A967 deletion, whereas for 7R this fragment was resolved into two fragments, of 115 bp and 78 bp (DNA fragments of ≤100 bp are not visible in Fig. 6B). Sequencing of the r-RF(7R-∂Stop) segment 7 confirmed the A967 deletion, the loss of the AseI site, and especially the suppression of the NSP3 ORF stop codon (Fig. 6C). Thus, the 7R-∂Stop segment of r-RF(7R-∂Stop) encoded a recombinant NSP3 protein of 629 amino acids (aa) (r-NSP3), which consisted of the complete NSP3 sequence (aa 1 to 310) fused to a 9-aa linker sequence (YAFQWLLLK), corresponding to the translation of 27 nucleotides (nt) of the duplicated 5′-UTR, and followed by a complete duplication of the NSP3 sequence (aa 320 to 629). Western blot analysis showed that the r-RF(7R-∂Stop) virus did express the r-NSP3 protein, with an apparent molecular mass of approximately 70 kDa, which is double the molecular mass of NSP3 expressed by the WT RF helper virus (Fig. 6D). Interestingly, recombinant viruses expressing r-NSP3 grew to titers similar to those obtained for r-RF(7R) virus expressing the WT NSP3 protein (4.31 × 107 ± 2.38 × 107 and 7.73 × 107 ± 2.44 × 107 PFU/ml, respectively) but had a small-plaque phenotype (mean plaque size of 0.61 ± 0.16 mm versus 1.46 ± 0.56 mm; P ≤ 0.0001 by the Student t test). This might indicate a change in r-NSP3 function.
FIG. 6.
Rescue of a recombinant RF virus containing an in vitro-modified, cDNA-derived rearranged segment 7R encoding a duplicated NSP3 protein. (A) dsRNA profiles of a representative r-RF(7R-∂Stop) viral clone rescued from MA-104 cell passage 18 and of control viruses RF and M1 (7R). An arrow indicates the location of segment 7R. (B) Electrophoretic analysis of RT-PCR products obtained from r-RF(7R-∂Stop) and M1 viruses, digested (+) or not (−) by AseI (numbers indicate the expected sizes of fragments). Mw, 100-bp-ladder molecular size marker. (C) Sequence electropherograms for RT-PCR products obtained from M1 (7R) and r-RF(7R-∂Stop) viruses. The arrowhead indicates the A967 residue deleted in the AseI site and the NSP3 stop codon. The predicted aa translation is indicated above the nucleotide sequences. For 7R-∂Stop (encoding r-NSP3), the 9 aa linking the last aa (YD) of NSP3 to the first aa (ME) of the duplicated NSP3 sequence are boxed. (D) Western blot analysis of r-RF(7R-∂Stop) and RF virus-infected cell lysates, using the ID3 monoclonal antibody specific for NSP3. Arrows indicate the NSP3 and r-NSP3 proteins, expressed by the RF and r-RF(7R-∂Stop) viruses, respectively. Numbers indicate molecular size, in kilodaltons.
These results clearly illustrate the ability of our reverse genetics system to obtain infectious RV expressing a specifically designed viral nonstructural protein.
DISCUSSION
Mammalian orthoreoviruses are the first members of the Reoviridae family for which RNA was reported to be infectious (35). In the infectious reovirus RNA system, viral ssRNA, viral dsRNA, and in vitro-translated viral ssRNA products are transfected (by use of Lipofectamine) together into cells, which are then infected by a helper reovirus of a distinct serotype. Although complex, the system allowed the rescue of temperature-sensitive reovirus mutants, opening the way to Reoviridae reverse genetics (31). Indeed, recombinant reoviruses expressing the chloramphenicol acetyltransferase (CAT) protein were obtained by use of this system and a cell line constitutively expressing the product of the engineered gene (30). In this system, the helper reovirus does not act as an acceptor for exogenous segments to generate engineered reassortants, and its role is not completely understood. More recently, new helper virus-independent reverse genetics systems have been established for mammalian orthoreoviruses and bluetongue virus (BTV), two members of the Reoviridae family. The strategies are based on the transfection of a complete set of plasmid cDNAs or plasmid cDNA-derived mRNAs that allow for the rescue of recombinant infectious viruses (2, 13). It is essential for such systems that the complete set of segmented viral RNAs can be infectious when transfected into cells permissive for viral replication. There is no report that infectious RV have been recovered in any cellular system from RNAs produced by viral cores, plasmid cDNAs, or plasmid cDNA-derived mRNAs, and the use of a helper RV to generate engineered reassortants (i.e., recombinant for the exogenous segments) is still mandatory, as described here and by Komoto et al. (15, 16). Our results confirm the feasibility of integrating an exogenous segment into an infectious RV, as reported by Komoto et al. for segment 4. Additionally, our findings indicate that although initial recombination events probably occur at a low frequency, the capacity of rearranged segments to be packaged preferentially over WT segments is efficient enough to support the growth of recombinant over WT viruses.
Results obtained during optimization experiments showed that vaccine infection and timing of plasmid transfection can drastically affect the permissiveness of COS-7 cells for RV infection, possibly related to some changes in cell membrane properties. Consequently, only 10% of COS-7 cells could actually be both transfected by a pRiboz plasmid and coinfected by vaccinia virus and RV, and the proportion of recombinant RV carrying an exogenous RNA segment should be infinitesimally low among the viral progenies produced in COS-7 cells. We previously reported that in a mixture of viruses with WT and rearranged segments 11, the rearranged segments could be detected by RT-PCR if they were present at a threshold ratio as low as 1 rearranged segment to 104 WT segments and by PAGE for a ratio as high as 1 to 1 (36). Similarly, the RT-PCR assay we used here was able to detect a rearranged segment 7 at a threshold ratio of 1 rearranged to 105 WT segments 7. Considering these detection thresholds, we can estimate that the ratio of recombinant to WT viruses was approximately 1:105 at passage 3 (first positive RT-PCR detection) and increased 10-fold every 2 or 3 passages, to reach a ratio of 1:1 at passage 15 to 18 (first positive PAGE detection). We can thus estimate that in the viral progeny produced by COS-7 cells, the ratio of recombinant to WT viruses was ≤1:106. In addition to this low ratio, it may be important to consider the impact of the MOI used for further serial passage in MA-104 cell culture on favoring the recovery of recombinant viruses. Indeed, it has been reported that in mixed infections of WT bovine RV and bovine RV with a rearranged segment 5, viruses with a rearranged genome overgrew during passage at a high MOI (undiluted inoculum), whereas WT virus overgrew during passage at a low MOI (1:100-diluted inoculum) (10). In this study, undiluted inoculum was used for serial passage, and recombinant viruses were recovered after a period of 5 to 6 weeks (15 to 18 cell passages, with 3 passages per week). This time lag could be shortened with the help of RT-PCR detection, and only 1 week (3 passages) was required to ascertain that recombinant viruses had actually been generated, improving the feasibility of the system. We are currently working on further improving the efficiency by RT-PCR testing for recombinant viruses in plaques or pools of plaques obtained from earlier cell culture passages.
To rescue recombinant RV expressing genetically engineered VP4 proteins (15, 16), Komoto et al. relied on the use of potent neutralizing antibodies, thus limiting the system to genes encoding surface capsid proteins. Here we show that distinct forms of exogenous rearranged segment 7 can be rescued by propagating the viral progeny in cell culture without any additional selective pressure. We set up our reverse genetics system by using rearranged segments 7 with a bovine RV as a helper virus, but whether this system can be extended to other rearranged segments or to different strains of helper viruses remains to be determined. In particular, this system might not apply to helper viruses that grow poorly in cell culture, like most human RV, or to segments for which rearrangements have not yet been identified in viable RV. Infectious RV carrying one or more rearranged segments have been described for 7 of the 11 genomic RNA segments (segments 5 to 11) (5), and it has been shown that rearranged segment 5 or 11 segregated preferentially to the homologous WT counterpart in viral progenies issued from mixed infections (10, 19). It is thus reasonable to hypothesize that our system should allow the production of viable recombinant viruses for at least these seven segments and should extend the possibilities of RV reverse genetics to gene segments encoding viral proteins (such as NSP3) for which potent selection tools (such as neutralizing antibodies) are not and will not be available. Because of steric constraints due to the huge concentration of viral dsRNA inside the viral particle, the length or number of rearranged segments could be a limiting factor for packaging. We previously described an RV carrying two rearranged segments, segments 7 and 11, corresponding to 1,531 additional bp packaged into the virus (8). RV can package as many as 1,800 additional bp, i.e., approximately 10% of the standard genome, and RV containing three rearranged segments with partially duplicated sequences have been reported (5, 11, 20). Similarly, it has been shown that the capacity of reovirus to package additional genetic material is probably limited to 2 kb, which is also about 10% of the whole genome (33). The lengths of RNA segments may also interfere with RNA replication. Skehel and Joklik reported that the relative number of RNA transcripts obtained from reovirus cores was inversely proportional to their molecular weight (37). In a study using a cell-free RV replication system, Patton et al. showed that the size of the RNA template is an important factor affecting the synthesis of dsRNA by open cores, with an inverse relationship between RNA length and replication efficiency (25). Interestingly, this was not the case for a rearranged RNA, which was 1.5-fold longer than the WT RNA but was replicated with a similar efficiency. Thus, rearrangement of RNA does not seem to affect RNA replication. This is important to consider, since one limitation of our reverse genetics system is that all generated recombinant viruses will contain a rearranged segment. However, to study protein function by mutagenesis of the ORF carried by a rearranged segment, it will be necessary to compare the mutated to the unmutated ORF in the context of the same long 3′-UTR generated by the rearrangement for assignment of changes in virus phenotype to ORF modification.
We show here for the first time that an in vitro-engineered gene 7 encoding a modified NSP3 protein can be introduced into an infectious RV. Three functional and structural domains have been established for NSP3 (3, 27, 28): the N-terminal domain (aa 1 to 150), which specifically binds to the 3′ end of viral mRNAs; the central domain (aa 150 to 241), which contains a coiled-coil domain required for dimerization; and the C-terminal domain (aa 206 to 313), responsible for interaction with the eIF4G translation initiation factors. It has been established that during RV infection, NSP3 evicts PABP from eIF4GI, leading to a concomitant shutoff of cellular mRNA translation (28, 39, 40). However, enhancement of RV mRNA translation by NSP3 was recently disputed, on the basis of RNA interference experiments showing that NSP3 knockdown has limited effects on viral protein synthesis and viral production in cell culture (22). Our reverse genetics system makes it possible to introduce specific mutations into the NSP3 gene that will be useful for specifying NSP3 functions during the viral replication cycle. Note that recombinant viruses expressing the modified r-NSP3 protein have a small-plaque phenotype. Since it has been suggested that plaque size directly correlates with the degree of suppression of host cell mRNA translation (10, 11), this small-plaque phenotype may indicate a change in the NSP3 function related to the shutoff of cellular protein synthesis. One hypothesis could be that duplication of binding domains of the r-NSP3 protein may interfere with some host factor.
The reasons that RV rearranged segments are preferentially packaged into viruses remain to be determined. One could hypothesize that duplication of some packaging signals in rearranged segments may double their probability of being encapsidated. Packaging signals have not yet been identified for RV. In this respect, comparison between homologous rearranged segments from different RV strains may tentatively identify conserved duplicated sequences and/or conserved duplicated stem or stem-loop secondary structures possibly involved in packaging. For reovirus, the existence of packaging signals was first suggested by the ability to package subgenomic S1 segments into defective interfering particles (23). More recently, Roner et al. identified, with the help of reverse genetics, the packaging signals contained in the 5′- and 3′-terminal sequences of the L1, M1, and S2 reovirus genes. The minimal 5′ and 3′ sequences required for packaging range from 96 to 129 nt and from 98 to 173 nt, respectively, extending across the coding sequences. Additionally, the nucleotides used to identify the ssRNAs are localized to the 5′, not the 3′, termini (29, 32-34). The cis-acting sequences required for packaging of the BTV VP6 gene have also been shown to overlap coding sequences (18). For RV, it has also been suggested that a cis-acting structure essential for packaging might exist outside the 5′ and 3′ ends (9). It is interesting that in the RV rearranged segment 7 we used, the 5′ part of the coding sequence is fully or almost fully duplicated, while the 3′-untranslated terminus is unique. This might suggest that, as for reovirus, RV packaging sequences are not in the 3′ terminus and reach further inside the 5′ part of the gene. Similarly to the reovirus model, our reverse genetics system should allow testing of mutants deleted for distinct parts of the duplicated sequence in order to specify (at least for gene 7) the minimal sequence essential for preferential packaging. As opposed to the reovirus or BTV system, which requires a cell line constitutively expressing the product of the gene that is tested for packaging by deletion analysis (18), a system using RV rearranged segments deleted for noncoding duplicated sequences does not require complementation. Characterization of the minimal sequence essential for preferential packaging could allow us to shorten the sequence duplication in engineered segment cDNAs and to optimize vector size for reverse genetics experiments. This will be most useful for the manipulation of the large genomic RNA segments 1 to 4 or for incorporating a set of several exogenous segments together. Thus, by engineering partial gene duplication at the cDNA level, reverse genetics could be possible for virtually every RV RNA segment.
In conclusion, the reverse genetics system for RV described here opens new possibilities for investigating RV protein functions, particularly those linked to virulence and pathogenesis. A better understanding of RV attenuation mechanisms would be helpful for the development of recombinant live RV vaccines containing targeted changes for attenuation.
Acknowledgments
Cécile Troupin was supported by a Ph.D. fellowship funded by an MRT grant. This work was supported in part by UPMC and INRA funds and by grants from the ACI Microbiologie Fondamentale.
We thank Claire Deback for sharing experimental results and Sophie Abelanet and Annie Charpilienne for excellent technical assistance. We thank Yasushi Uematsu (Novartis Vaccines, Siena, Italy) for providing us with the rDls-T7pol vaccinia virus.
Footnotes
Published ahead of print on 28 April 2010.
REFERENCES
- 1.Aponte, C., N. M. Mattion, M. K. Estes, A. Charpilienne, and J. Cohen. 1993. Expression of two bovine rotavirus non-structural proteins (NSP2, NSP3) in the baculovirus system and production of monoclonal antibodies directed against the expressed proteins. Arch. Virol. 133:85-95. [DOI] [PubMed] [Google Scholar]
- 2.Boyce, M., C. C. Celma, and P. Roy. 2008. Development of reverse genetics systems for bluetongue virus: recovery of infectious virus from synthetic RNA transcripts. J. Virol. 82:8339-8348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chizhikov, V., and J. T. Patton. 2000. A four-nucleotide translation enhancer in the 3′-terminal consensus sequence of the nonpolyadenylated mRNAs of rotavirus. RNA 6:814-825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dehee, A., N. Schnepf, C. Deback, A. L. Poeury, and A. Garbarg-Chenon. 2006. Preferential segregation of rearranged segments in rotaviral progeny after mixed infection, abstr. PW4.1, p. 81. Abstr. 9th Int. Symp. ds-RNA Viruses.
- 5.Desselberger, U. 1996. Genome rearrangements of rotaviruses. Adv. Virus Res. 46:69-95. [DOI] [PubMed] [Google Scholar]
- 6.Estes, M. K., and A. Kapikian. 2007. Rotaviruses, p. 1917-1974. In D. M. Knipe et al. (ed.), Fields virology, 5th ed., vol. 2. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 7.Garbarg-Chenon, A., F. Bricout, and J. C. Nicolas. 1984. Study of genetic reassortment between two human rotaviruses. Virology 139:358-365. [DOI] [PubMed] [Google Scholar]
- 8.Gault, E., N. Schnepf, D. Poncet, A. Servant, S. Teran, and A. Garbarg-Chenon. 2001. A human rotavirus with rearranged genes 7 and 11 encodes a modified NSP3 protein and suggests an additional mechanism for gene rearrangement. J. Virol. 75:7305-7314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gorziglia, M. I., and P. L. Collins. 1992. Intracellular amplification and expression of a synthetic analog of rotavirus genomic RNA bearing a foreign marker gene: mapping cis-acting nucleotides in the 3′-noncoding region. Proc. Natl. Acad. Sci. U. S. A. 89:5784-5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hundley, F., B. Biryahwaho, M. Gow, and U. Desselberger. 1985. Genome rearrangements of bovine rotavirus after serial passage at high multiplicity of infection. Virology 143:88-103. [DOI] [PubMed] [Google Scholar]
- 11.Hundley, F., M. McIntyre, B. Clark, G. Beards, D. Wood, I. Chrystie, and U. Desselberger. 1987. Heterogeneity of genome rearrangements in rotaviruses isolated from a chronically infected immunodeficient child. J. Virol. 61:3365-3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishii, K., Y. Ueda, K. Matsuo, Y. Matsuura, T. Kitamura, K. Kato, Y. Izumi, K. Someya, T. Ohsu, M. Honda, and T. Miyamura. 2002. Structural analysis of vaccinia virus DIs strain: application as a new replication-deficient viral vector. Virology 302:433-444. [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi, T., A. A. Antar, K. W. Boehme, P. Danthi, E. A. Eby, K. M. Guglielmi, G. H. Holm, E. M. Johnson, M. S. Maginnis, S. Naik, W. B. Skelton, J. D. Wetzel, G. J. Wilson, J. D. Chappell, and T. S. Dermody. 2007. A plasmid-based reverse genetics system for animal double-stranded RNA viruses. Cell Host Microbe 1:147-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kojima, K., K. Taniguchi, M. Kawagishi-Kobayashi, S. Matsuno, and S. Urasawa. 2000. Rearrangement generated in double genes, NSP1 and NSP3, of viable progenies from a human rotavirus strain. Virus Res. 67:163-171. [DOI] [PubMed] [Google Scholar]
- 15.Komoto, S., M. Kugita, J. Sasaki, and K. Taniguchi. 2008. Generation of recombinant rotavirus with an antigenic mosaic of cross-reactive neutralization epitopes on VP4. J. Virol. 82:6753-6757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Komoto, S., J. Sasaki, and K. Taniguchi. 2006. Reverse genetics system for introduction of site-specific mutations into the double-stranded RNA genome of infectious rotavirus. Proc. Natl. Acad. Sci. U. S. A. 103:4646-4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luytjes, W., M. Krystal, M. Enami, J. D. Parvin, and P. Palese. 1989. Amplification, expression, and packaging of foreign gene by influenza virus. Cell 59:1107-1113. [DOI] [PubMed] [Google Scholar]
- 18.Matsuo, E., and P. Roy. 2009. Bluetongue virus VP6 acts early in the replication cycle and can form the basis of chimeric virus formation. J. Virol. 83:8842-8848.19553329 [Google Scholar]
- 19.Mattion, N. M., R. C. Bellinzoni, J. O. Blackhall, M. K. Estes, S. Gonzalez, J. L. La Torre, and E. A. Scodeller. 1990. Genome rearrangements in porcine rotaviruses: biochemical and biological comparisons between a supershort strain and its standard counterpart. J. Gen. Virol. 71:355-362. [DOI] [PubMed] [Google Scholar]
- 20.McIntyre, M., V. Rosenbaum, W. Rappold, M. Desselberger, D. Wood, and U. Desselberger. 1987. Biophysical characterization of rotavirus particles containing rearranged genomes. J. Gen. Virol. 68:2961-2966. [DOI] [PubMed] [Google Scholar]
- 21.Mendez, E., C. F. Arias, and S. Lopez. 1992. Genomic rearrangements in human rotavirus strain Wa; analysis of rearranged RNA segment 7. Arch. Virol. 125:331-338. [DOI] [PubMed] [Google Scholar]
- 22.Montero, H., C. F. Arias, and S. Lopez. 2006. Rotavirus nonstructural protein NSP3 is not required for viral protein synthesis. J. Virol. 80:9031-9038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ni, Y., and M. C. Kemp. 1994. Subgenomic S1 segments are packaged by avian reovirus defective interfering particles having an S1 segment deletion. Virus Res. 32:329-342. [DOI] [PubMed] [Google Scholar]
- 24.Parashar, U. D., C. J. Gibson, J. S. Bresse, and R. I. Glass. 2006. Rotavirus and severe childhood diarrhea. Emerg. Infect. Dis. 12:304-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patton, J. T., J. Chnaiderman, and E. Spencer. 1999. Open reading frame in rotavirus mRNA specifically promotes synthesis of double-stranded RNA: template size also affects replication efficiency. Virology 264:167-180. [DOI] [PubMed] [Google Scholar]
- 26.Pedley, S., F. Hundley, I. Chrystie, M. A. McCrae, and U. Desselberger. 1984. The genomes of rotaviruses isolated from chronically infected immunodeficient children. J. Gen. Virol. 65:1141-1150. [DOI] [PubMed] [Google Scholar]
- 27.Piron, M., T. Delaunay, J. Grosclaude, and D. Poncet. 1999. Identification of the RNA-binding, dimerization, and eIF4GI-binding domains of rotavirus nonstructural protein NSP3. J. Virol. 73:5411-5421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Piron, M., P. Vende, J. Cohen, and D. Poncet. 1998. Rotavirus RNA-binding protein NSP3 interacts with eIF4GI and evicts the poly(A) binding protein from eIF4F. EMBO J. 17:5811-5821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roner, M. R., K. Bassett, and J. Roehr. 2004. Identification of the 5′ sequences required for incorporation of an engineered ssRNA into the reovirus genome. Virology 329:348-360. [DOI] [PubMed] [Google Scholar]
- 30.Roner, M. R., and W. K. Joklik. 2001. Reovirus reverse genetics: incorporation of the CAT gene into the reovirus genome. Proc. Natl. Acad. Sci. U. S. A. 98:8036-8041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roner, M. R., I. Nepliouev, B. Sherry, and W. K. Joklik. 1997. Construction and characterization of a reovirus double temperature-sensitive mutant. Proc. Natl. Acad. Sci. U. S. A. 94:6826-6830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roner, M. R., and J. Roehr. 2006. The 3′ sequences required for incorporation of an engineered ssRNA into the reovirus genome. Virol. J. 3:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roner, M. R., and B. G. Steele. 2007. Features of the mammalian orthoreovirus 3 Dearing l1 single-stranded RNA that direct packaging and serotype restriction. J. Gen. Virol. 88:3401-3412. [DOI] [PubMed] [Google Scholar]
- 34.Roner, M. R., and B. G. Steele. 2007. Localizing the reovirus packaging signals using an engineered m1 and s2 ssRNA. Virology 358:89-97. [DOI] [PubMed] [Google Scholar]
- 35.Roner, M. R., L. A. Sutphin, and W. K. Joklik. 1990. Reovirus RNA is infectious. Virology 179:845-852. [DOI] [PubMed] [Google Scholar]
- 36.Schnepf, N., C. Deback, A. Dehee, E. Gault, N. Parez, and A. Garbarg-Chenon. 2008. Rearrangements of rotavirus genomic segment 11 are generated during acute infection of immunocompetent children and do not occur at random. J. Virol. 82:3689-3696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Skehel, J. J., and W. K. Joklik. 1969. Studies on the in vitro transcription of reovirus RNA catalyzed by reovirus cores. Virology 39:822-831. [DOI] [PubMed] [Google Scholar]
- 38.Tian, Y., O. Tarlow, A. Ballard, U. Desselberger, and M. A. McCrae. 1993. Genomic concatemerization/deletion in rotaviruses: a new mechanism for generating rapid genetic change of potential epidemiological importance. J. Virol. 67:6625-6632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Varani, G., and F. H. Allain. 2002. How a rotavirus hijacks the human protein synthesis machinery. Nat. Struct. Biol. 9:158-160. [DOI] [PubMed] [Google Scholar]
- 40.Vende, P., M. Piron, N. Castagne, and D. Poncet. 2000. Efficient translation of rotavirus mRNA requires simultaneous interaction of NSP3 with the eukaryotic translation initiation factor eIF4G and the mRNA 3′ end. J. Virol. 74:7064-7071. [DOI] [PMC free article] [PubMed] [Google Scholar]