Abstract
A hallmark of prion diseases is the conversion of the host-encoded prion protein (PrPC where C is cellular) into an alternatively folded, disease-related isoform (PrPSc, where Sc is scrapie), the accumulation of which is associated with synapse degeneration and ultimately neuronal death. The formation of PrPSc is dependent upon the presence of PrPC in specific, cholesterol-sensitive membrane microdomains, commonly called lipid rafts. PrPC is targeted to these lipid rafts because it is attached to membranes via a glycosylphosphatidylinositol anchor. Here, we show that treatment of prion-infected neuronal cell lines (ScN2a, ScGT1, or SMB cells) with synthetic glycosylphosphatidylinositol analogues, glucosamine-phosphatidylinositol (glucosamine-PI) or glucosamine 2-O-methyl inositol octadecyl phosphate, reduced the PrPSc content of these cells in a dose-dependent manner. In addition, ScGT1 cells treated with glucosamine-PI did not transmit infection following intracerebral injection to mice. Treatment with glucosamine-PI increased the cholesterol content of ScGT1 cell membranes and reduced activation of cytoplasmic phospholipase A2 (PLA2), consistent with the hypothesis that the composition of cell membranes affects key PLA2-dependent signaling pathways involved in PrPSc formation. The effect of glucosamine-PI on PrPSc formation was also reversed by the addition of platelet-activating factor. Glucosamine-PI caused the displacement of PrPC from lipid rafts and reduced expression of PrPC at the cell surface, putative sites for PrPSc formation. We propose that treatment with glucosamine-PI modifies local micro-environments that control PrPC expression and activation of PLA2 and subsequently inhibits PrPSc formation.
Keywords: Glycosylphosphatidylinositol Anchors, Lipid Raft, Neuron, Phospholipase A, Prions, Platelet-activating Factor
Introduction
The transmissible spongiform encephalopathies, otherwise known as prion diseases, are neurodegenerative disorders that include scrapie in sheep and goats, bovine spongiform encephalopathy in cattle, and Creutzfeldt-Jakob disease in humans. A key event in prion disease is the conversion of a normal host protein (PrPC)3 (1) into a disease isoform (PrPSc), via a process whereby a portion of the α-helix and random coil structure in PrPC is refolded into a β-pleated sheet (2). PrPSc represents the major component of infectious scrapie prions. The conversion of PrPC to PrPSc is accompanied by changes in biological and biochemical properties, including reduced solubility and an increased resistance to proteases (3). Aggregates of PrPSc accumulate around neurons in affected brain areas (4), a process that is thought to lead to neuronal degeneration and subsequently the clinical symptoms of infection.
The production of PrPSc is dependent on the presence of PrPC (5–7). More specifically, the formation of PrPSc in neuronal cell lines was dependent on the specific intracellular trafficking pathways of PrPC (8, 9). One of the factors that determines the intracellular trafficking of PrPC is the localization of PrPC at the cell surface within detergent-resistant membrane microdomains, commonly called lipid rafts (10). Cholesterol synthesis inhibitors affect the formation of lipid rafts required for PrPSc formation (11–13). However, many neuronal processes are sensitive to changes in membrane cholesterol, and cholesterol synthesis inhibitors are regarded as crude pharmacological tools. Because lipid rafts exist as heterogeneous subsets (10), we examined the potential of compounds to alter specific lipid raft subsets involved in PrPSc formation.
The majority of PrPC molecules are linked to membranes via a glycosylphosphatidylinositol (GPI) anchor (14), which targets proteins to lipid rafts (15). Replacing the GPI anchor attached to PrPC with the transmembrane and cytoplasmic domains of the CD4 molecule reduced PrPSc formation in vitro (11) suggesting that the GPI anchor attached to PrPC might affect PrPSc formation. In other studies, transgenic mice producing PrPC without a GPI anchor produced high amounts of infectious PrPSc in the absence of clinical scrapie (16). However, the loss of the GPI anchor affected both the glycosylation of PrPC and its expression at the cell surface (16). Because these factors can affect PrPSc production (17), it is not clear whether the effects of removing the entire GPI anchor in these mice was a direct effect of the loss of the GPI anchor or an indirect effect resulting from altered glycosylation. Such observations indicate that the full role of the GPI anchor in PrPSc formation is not fully understood.
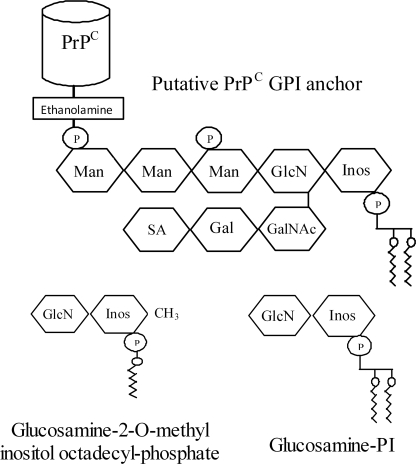
The basic structure of GPI anchors contains a conserved core consisting of ethanolamine phosphate in an amide linkage to the carboxyl terminus of the protein, three mannose residues, and glucosamine linked to phosphatidylinositol (PI) (15, 18). Many variations on this core structure have been found, and the GPI attached to PrPC has been reported to contain high amounts of sialic acid, galactose, and mannose (19) as illustrated in Fig. 1. Some GPI anchors are more than a simple mechanism of attaching proteins to the cell membranes and have cell signaling functions. For example, GPIs from the protozoan Plasmodium falciparum stimulate macrophages (20), whereas other GPIs stimulate lipogenesis in adipocytes (21), and GPIs isolated from PrPC stimulate phospholipase A2 (PLA2) (22). Because PLA2 affected the formation of prions (23), we used synthetic GPI analogues to examine the relationship between GPI anchors, lipid rafts, cell activation, and prion formation. We report that two synthetic GPI anchor analogues altered the composition of cell membranes in three prion-infected neuronal cell lines, reduced cell signaling and the formation of PrPSc, and greatly diminished infectivity of ScGT1 cells.
FIGURE 1.
Structures of the PrPC GPI anchor and glucosamine-PI. Schematic showing the structures of the GPI anchor that is attached to PrPC and the GPI anchor analogues, glucosamine-2-O-methyl inositol octadecyl phosphate and glucosamine-PI. Glycan residues shown include mannose (Man), sialic acid (SA), galactose (Gal), N-acetylgalactosamine (GalNAc), glucosamine (GlcN), phosphate (P), and inositol (Inos).
EXPERIMENTAL PROCEDURES
Cell Lines
Prion-infected ScN2a, ScGT1, and SMB cells and their uninfected controls (N2a, GT1, or SMB-PS cells) were grown in Ham's F-12 medium containing 2 mm glutamine, 2% fetal calf serum, and standard antibiotics (100 units/ml penicillin and 100 μg/ml streptomycin). To determine the effect of compounds on PrPSc formation, ScN2a, SMB, or ScGT1 cells were plated in 6-well plates (105 cells/well) and cultured in the presence or absence of test compounds. Cells were grown with daily changes of media, and the amount of cell-associated PrPSc was evaluated after 7 days. Cells were washed twice in PBS before cell extracts were obtained. Spent medium was collected to see if PrPSc was released into the culture supernatant. These were concentrated by centrifugation with a 10-kDa filter and diluted to an equivalent of 106 cells/ml.
Neuronal Cultures
Primary cortical neurons were prepared from the brains of mouse embryos (day 15.5) after mechanical dissociation and cell sieving. Neuronal precursors were plated (500,000 cells/well in 24-well plates coated with 5 μg/ml poly-l-lysine) in Ham's F-12 medium containing 5% fetal calf serum for 2 h. Cultures were shaken (600 rpm for 5 min), and nonadherent cells were removed by two washes in PBS. Neurons were grown in neurobasal medium containing B27 components (PAA) for 7 days and subsequently incubated with test compounds. Immunolabeling studies showed that after 7 days cultures contained less than 5% glial cells (about 3% glial fibrillary acidic protein-positive and less than 1% MAC-1-positive cells).
Cell Treatments
To determine the short term effects of GPI anchor analogues, cells were plated in 6-well plates (106 cells/well) and allowed to adhere overnight. The following day, cells were washed and incubated for 24 h in the presence or absence of different concentrations of test compounds as shown. Cells were subsequently washed five times with warm PBS before further use.
Membrane Extracts
At the end of treatment, cells were homogenized in a buffer containing 10 mm Tris-HCl, pH 7.4, 150 mm NaCl, 10 mm EDTA, 0.5% Nonidet P-40, 0.5% sodium deoxycholate, 0.2% SDS at 106 cells/ml, and nuclei and large fragments were removed by centrifugation (300 × g for 5 min). Mixed protease inhibitors (4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride, aprotinin, leupeptin, bestatin, pepstatin A, and E-46 (Sigma)) and a phosphatase inhibitor mixture, including PP1, PP2A, microcystin LR, cantharidin, and p-bromotetramisole (Sigma), were added to some cell extracts. To determine the amount of PrPSc in cell extracts, they were digested with 1 μg/ml proteinase K (1 h at 37 °C) to remove PrPC. Digestion of noninfected GT1 cell extracts with proteinase K (as above) completely reduced the PrP signal, i.e. complete digestion of PrPC. The soluble material split into two samples, one of which was heated to 95 °C for 5 min and tested in a PrP-specific ELISA (see below). The other sample was mixed 1:1 with Laemmli buffer containing β-mercaptoethanol and boiled for 5 min. This fraction was run on a 12% polyacrylamide gel. Proteins were transferred onto a Hybond-ECL nitrocellulose membrane (Amersham Biosciences) by semi-dry blotting. Membranes were blocked using 10% milk powder, and PrP was detected by incubation with a mouse monoclonal antibody (mAb) ICSM18 (d-Gen), followed by biotinylated rabbit anti-mouse IgG (Dako) and ExtrAvidin-peroxidase (Sigma). Detection of bound antibody was by the enhanced chemiluminescence kit (Amersham Biosciences). Nondigested samples were boiled in Laemmli buffer containing β-mercaptoethanol for 5 min and run on a 12% polyacrylamide gel. Proteins were transferred onto a Hybond-P polyvinylidene difluoride membrane by semi-dry blotting. Membranes were blocked using 10% milk powder, and β-actin was detected by incubation with a mouse mAb (clone AC-74, Sigma), followed by biotinylated rabbit anti-mouse IgG and ExtrAvidin-peroxidase. Detection of bound antibody was by the enhanced chemiluminescence kit.
Isolation of Detergent-resistant Membranes (DRMs)
To differentiate between the normal membrane and lipid rafts, cells were homogenized in an ice-cold buffer containing 1% Triton X-100, 10 mm Tris-HCl, pH 7.4, 150 mm NaCl, 10 mm EDTA, and mixed protease inhibitors at 106 cells/ml (as above), and nuclei and large fragments were removed by centrifugation (300 × g for 5 min at 4 °C). The subsequent postnuclear supernatant was incubated on ice (4 °C) for 1 h and centrifuged (16,000 × g for 30 min at 4 °C). The supernatant was reserved as the normal cell membrane, whereas the insoluble pellet was homogenized in 10 mm Tris-HCl, pH 7.4, 150 mm NaCl, 10 mm EDTA, 0.5% Nonidet P-40, 0.5% sodium deoxycholate, 0.2% SDS, and mixed protease inhibitors at an equivalent of 106 cells/ml and centrifuged (10 min at 16,000 × g), and the soluble material was reserved as DRMs/lipid rafts.
Sucrose Density Gradients
Cells were harvested with a Teflon scraper and homogenized at 1 × 106 cells/ml in a buffer containing 250 mm sucrose, 10 mm Tris-HCl, pH 7.4, 1 mm EGTA, 1 mm dithiothreitol, and mixed protease inhibitors. Particulate membrane fragments and nuclei were removed by centrifugation (1000 × g for 5 min). Membranes were washed by centrifugation at 16,000 × g for 20 min at 4 °C and resuspended in an ice-cold buffer containing 1% Triton X-100, 10 mm Tris-HCl, pH 7.4, 150 mm NaCl, 10 mm EDTA. 5–40% sucrose solutions were prepared and layered to produce a gradient. Solubilized membranes were layered on top and centrifuged at 50,000 × g for 18 h at 4 °C. Serial 1-ml aliquots were collected from the bottom of gradients.
The amount of GM-1 ganglioside (GM-1) in whole cell extracts or detergent-resistant membranes cells was determined by incubating cells with 100 ng/ml fluorescein isothiocyanate-cholera toxin subunit B (Sigma), 1 × 106 cells for 1 h. Cells were washed and cell extracts collected as above. Extracts were transferred into Sterlin 96-well black microplates, and fluorescence was measured using excitation at 495 nm and measuring emission at 521 nm. Values were expressed as a percentage of the amount of fluorescein isothiocyanate-cholera toxin subunit B added.
PrP ELISA
The amount of PrP present in cell extracts was determined by ELISA using commercially available mAbs as described previously (24). Nunc Maxisorb immunoplates were coated with 0.5 μg/ml mAb ICSM18, which recognizes amino acids 146–159 of PrP (25). Samples were applied and detected with biotinylated mAb ICSM35 (d-Gen) (which recognizes an epitope between amino acids 91 and 110) (26). Biotinylated mAb was detected using ExtrAvidin-alkaline phosphatase and 1 mg/ml 4-nitrophenyl phosphate (Sigma). Absorbance was measured on a microplate reader at 405 nm, and the amount of PrP in cell extracts was calculated by reference to a standard curve of recombinant murine PrP (Prionics); its limit of detection was 0.05 ng/ml.
Activated cPLA2 ELISA
The activation of cPLA2 is accompanied by phosphorylation of the 505 serine residues and measured by phospho-specific antibodies. The amount of activated cPLA2 in cell extracts was measured by ELISA as described previously (27). Nunc Maxisorb immunoplates were coated with 0.5 μg/ml mAb anti-cPLA2, clone CH-7 (Upstate), for 1 h and blocked with 10% fetal calf serum. Samples were incubated for 1 h, and the amount of activated cPLA2 was detected using a rabbit polyclonal anti-phospho-cPLA2 (Cell Signaling Technology). Bound antibodies were detected with biotinylated anti-rabbit IgG, ExtrAvidin-alkaline phosphatase, and 1 mg/ml 4-nitrophenyl phosphate. Absorbance was measured at 405 nm, and the amounts of activated cPLA2 were calculated from a standard curve using nonlinear regression. Samples were expressed as “units cPLA2,” where 100 units was defined as the amount of activated cPLA2 in 106 untreated cells. A standard curve was generated from this sample using sequential log 2 dilutions (range 100 to 1.56 units/well).
Quantification of Cell Surface PrPC
The amounts of PrPC expressed at the cell surface were determined by treating cells with 0.2 unit of phosphatidylinositol-phospholipase C for 1 h at 37 °C (106 cells/ml). PI-phospholipase C is cell-impermeable and acts on the GPI anchors that tether PrPC to the cell surface. The amount of PrPC released into culture supernatants following PI-phospholipase C digestion was measured by PrP ELISA.
Isolation of GPI Anchors
GPIs were isolated from GT1 neuronal cells solubilized in a buffer containing 10 mm Tris-HCl, pH 7.4, 100 mm NaCl, 10 mm EDTA, 0.5% Nonidet P-40, 0.5% sodium deoxycholate. Nuclei and cell debris were removed by centrifugation and the postnuclear supernatant incubated with antibodies to PrPC (ICSM18, d-Gen), Thy-1, or CD55 (Serotec). mAb-protein complexes were precipitated following the addition of protein G-agarose (Sigma). Precipitates were washed five times with PBS and digested with proteinase K (100 μg/ml at 37 °C for 24 h) resulting in GPI anchors attached to the terminal amino acid. The released GPIs were extracted with water-saturated butan-1-ol, washed with water five times, and lyophilized, and stock solutions were dissolved in ethanol at 2 μm. A preparation in which ICSM18 was incubated with recombinant PrP (lacking a GPI anchor) and treated as above was used as a control. Extracted GPIs were applied to Silica Gel 60 high performance TLC plates (Whatman) and developed using a mixture of chloroform/methanol/water (4:4:1, v/v). GPI anchors were detected by immunoblotting as described previously (22). Plates were soaked in 0.1% poly(isobutyl methacrylate) in hexane, dried, and blocked with PBS containing 5% milk powder. They were probed with a mAb that binds to phosphatidylinositol, washed with PBS/Tween, and incubated with goat anti-mouse IgG conjugated to peroxidase (Sigma) for 1 h. The bound antibody was washed and visualized using an enhanced chemiluminescence kit.
Cholesterol and Protein Content
Cholesterol and protein content were determined in cell extracts (106 cells/ml). Protein concentrations were measured using a micro-BCA protein assay kit (Pierce). The amount of cholesterol was measured using the Amplex Red cholesterol assay kit (Invitrogen), according to the manufacturer's instructions. Cholesterol was oxidized by cholesterol oxidase to yield hydrogen peroxide and ketones. The hydrogen peroxide reacts with 10-acetyl-3,7-dihydroxyphenoxazine (Amplex Red reagent) to produce highly fluorescent resorufin, which is measured by excitation at 550 nm and emission detection at 590 nm. By performing the assay with and without cholesterol esterase, the assay can also determine the amount of esterified cholesterol within samples.
Evaluation of Infectivity
Treated ScGT1 cells were detached and counted, washed twice with PBS, and then put through one rapid freeze-thaw cycle. The homogenate was precipitated by centrifugation (16,000 × g for 30 min), washed twice with PBS, and finally homogenized in sterile 0.9% (w/v) saline at 2.5 × 106 cell eq/ml. C57/Bl mice under halothane anesthesia were injected intracerebrally with 30 μl (7.5 × 104 cell eq) of this homogenate. Mice were monitored for clinical signs of scrapie until reaching a pre-defined clinical end point. All animal work was conducted according to local and national guidelines.
Reagents
The GPI analogues glucosamine 2-O-methyl inositol octadecyl phosphate and glucosamine-PI were supplied by Dr. A. Crossman, Dundee, Scotland, UK. PI, galactosamine, glucosamine, mannose, arachidonic acid, platelet-activating factor (PAF), carbamyl-PAF (C-PAF), lyso-PAF, lysophosphatidic acid, lyso-phosphatidylcholine, and lyso-phosphatidylethanolamine were obtained from Sigma.
Statistical Analysis
Comparison of treatment effects was carried out using one- and two-way analysis of variance techniques as appropriate. For all statistical tests, significance was set at the 1% level.
RESULTS
GPI Analogues Reduced the PrPSc Content of Prion-infected Cells
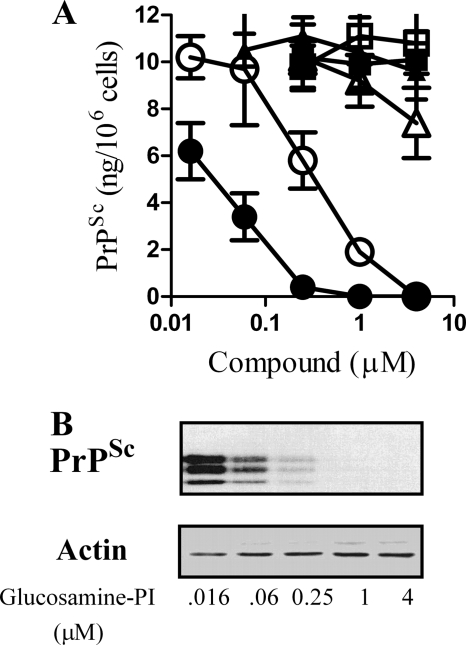
The effect of GPI analogues on PrPSc formation was determined by daily treatment of ScGT1 cells with GPI-related compounds. After 7 days, the amount of PrPSc in proteinase K-digested cell extracts was determined by ELISA. Glucosamine-PI, glucosamine 2-O-methyl inositol octadecyl phosphate, and PI all caused a dose-dependent reduction in the amount of PrPSc these cells contained. The concentration of glucosamine-PI required to reduce PrPSc levels by 50% was 40 nm, and the same effect required 800 nm glucosamine 2-O-methyl inositol octadecyl phosphate (Fig. 2A). Treatment with 5 μm glucosamine-PI or glucosamine 2-O-methyl inositol octadecyl phosphate did not affect the survival of ScGT1 cells as measured by thiazyl blue tetrazolium (data not shown). Immunoblots were used to verify ELISA data; these showed that although the amount of PrPSc in glucosamine-PI treated cells was reduced, there were no significant differences in the amount of β-actin (Fig. 2B). ScGT1 cells treated for 7 days with 1 μm glucosamine-PI did not contain detectable levels of PrPSc, and these cells remained clear of PrPSc for a further 2 months after the cessation of treatment (data not shown). The PrPSc content of ScGT1 cells was not affected by glucosamine, galactose, or mannose. Moreover, the effect of 1 μm glucosamine-PI was not replicated by the combination of 1 μm glucosamine and 1 μm PI; PrPSc levels in these cells were not significantly different from that of vehicle-treated ScGT1 cells (10.6 ng/106 cells ± 1.2 (mean average ± S.D.) compared with 10.2 ± 0.8, n = 9, p = 0.7). The amounts of PrPSc in two other prion-infected cell lines (ScN2a and SMB cells) were also reduced following treatment with 1 μm glucosamine-PI or glucosamine 2-O-methyl inositol octadecyl phosphate, but not by treatment with 50 μm glucosamine, galactosamine, galactose, or mannose (Table 1). At the concentrations used, none of the compounds affected the viability or the growth rate of cell lines.
FIGURE 2.
GPI analogues reduced PrPSc formation in ScGT1 cells. A, amount of PrPSc in ScGT1 cells treated for 7 days with varying concentrations of glucosamine-PI (●), glucosamine 2-O-methyl inositol octadecyl phosphate (○), PI (Δ), glucosamine (■), galactose (□), or mannose (▴). Values shown are the mean average amount of PrPSc (ng/106 cells) ± S.D., n = 15. B, immunoblots showing the amount of PrPSc or β-actin in cell extracts from ScGT1 cells treated for 7 days with varying concentrations of glucosamine-PI.
TABLE 1.
GPI anchor analogues reduced the PrPSc content of ScN2a and SMB cells
ScN2a or SMB cells were treated daily with GPI anchor analogues as shown for 7 days, and the amount of cell-associated PrPSc was determined using a PrP ELISA. Values given are the mean average amount of PrPSc (pg/106 cells) ± S.D., n = 12.
| Treatment | PrPSc (pg/106 cells) |
|
|---|---|---|
| ScN2a | SMB | |
| None | 2128 ± 95 | 6057 ± 639 |
| Vehicle control | 2096 ± 140 | 5988 ± 458 |
| 1 μm glucosamine-PI | <50a | <50a |
| 1 μm glucosamine-O-methyl inositol octadecyl phosphate | 186 ± 120a | 748 ± 161a |
| 50 μm glucosamine | 2318 ± 92 | 5789 ± 634 |
| 50 μm galactosamine | 1988 ± 221 | 6255 ± 589 |
| 50 μm galactose | 2090 ± 320 | 6425 ± 663 |
| 50 μm mannose | 2150 ± 337 | 6271 ± 713 |
a Amount of PrPSc is significantly less than that of vehicle-treated cells (p < 0.01).
Reports that infectious PrPSc is released from cells as exosomes (28–30) raised the possibility that treatment with GPI analogues reduced cellular PrPSc by promoting the release of PrPSc from cells. To examine this possibility, the amount of PrPSc in the supernatants of treated ScGT1 cells was also measured. There were no significant differences in the amount of PrPSc in supernatants from cells treated with a vehicle control, galactosamine, glucosamine, galactose, or mannose. Treatment with glucosamine-PI or glucosamine 2-O-methyl inositol octadecyl phosphate significantly reduced the PrPSc content of supernatants (Table 2).
TABLE 2.
GPI analogues reduced extracellular PrPSc
The amount of PrPSc (pg/106 cells) in culture supernatants collected from ScGT1 cells treated with GPI anchor analogues is shown. Values shown are the mean average amount of PrPSc (pg/106 cells) ± S.D., n = 12.
| Treatment | PrPSc (pg/106 cells) |
|---|---|
| None | 984 ± 164 |
| Vehicle control | 945 ± 140 |
| 1 μm glucosamine-PI | <50a |
| 1 μm glucosamine 2-O-methyl inositol octadecyl phosphate | 348 ± 122a |
| 50 μm glucosamine | 889 ± 202 |
| 50 μm galactosamine | 946 ± 221 |
| 50 μm galactose | 955 ± 98 |
| 50 μm mannose | 1014 ± 158 |
a Amount of PrPSc is significantly less than that of vehicle-treated cells (p < 0.01).
GPI Analogues Reduced the Infectivity of ScGT1 Cells
Groups of mice were injected intracerebrally with homogenates from ScGT1 cells treated for 7 days with a vehicle control, 1 μm glucosamine-PI or 1 μm glucosamine 2-O-methyl inositol octadecyl phosphate. The mean incubation period of mice given the control homogenate was 164 days ± 4 (incubation period ± S.D., n = 13). After 600 days, four of eight mice given homogenate from cells treated with glucosamine 2-O-methyl inositol octadecyl phosphate remained alive; those that died did so only after a significantly greater incubation period (mean = 232 days ± 13 (p < 0.01)). None of the eight mice inoculated with homogenates from cells treated with glucosamine-PI had died by day 600 (Fig. 3). Such observations indicate that treatment with these GPI anchor analogues reduced infectivity as well as reducing PrPSc.
FIGURE 3.
Treatment with GPI analogues reduced the infectivity of ScGT1 cells. Survival times of mice following intracerebral inoculation with cell homogenates from vehicle-treated ScGT1 cells (–) or from ScGT1 cells treated for 7 days with either 1 μm glucosamine-PI (●) or 1 μm glucosamine 2-O-methyl inositol octadecyl phosphate (○).
Glucosamine-PI Increased Cholesterol
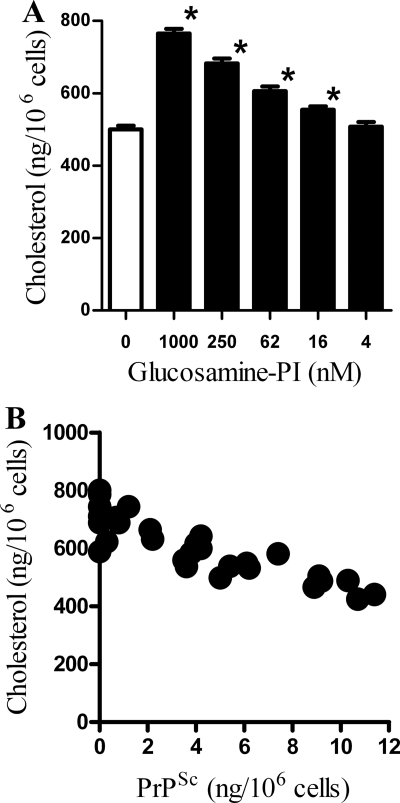
Because several studies show that the cholesterol content of cell membranes is a major factor regulating PrPSc formation, the effect of GPI analogues on the composition of ScGT1 cell membranes was examined. Treatment with glucosamine-PI for 24 h increased the amount of cholesterol in cell membranes (Fig. 4A). After 24 h, glucosamine-PI treatment had not affected the amount of PrPSc in ScGT1 cells (data not shown) indicating that the effect of glucosamine-PI on cholesterol levels was independent of its effect on PrPSc. More detailed analysis indicated that in ScGT1 cells incubated with different amounts of glucosamine-PI, there was a significant inverse correlation between the amount of cholesterol, measured after 24 h, and the amount of PrPSc after 7 days, Pearson's coefficient = −0.872, p < 0.01 (Fig. 4B). The addition of 1 μm glucosamine-PI significantly increased the amount of cholesterol in ScN2a cells (562 ng/106 cells ± 60 compared with 390 ± 48, n = 9, p < 0.01) and SMB cells (704 ng/106 cells ± 68 compared with 519 ± 42, n = 9, p < 0.01). This effect of glucosamine-PI was not selective for prion-infected cell lines; the addition of 1 μm glucosamine-PI also increased the cholesterol content of GT1 cells (534 ng/106 cells ± 62 compared with 382 ± 38, n = 9, p < 0.01), N2a cells (558 ng/106 cells ± 51 compared with 395 ± 40, n = 9, p < 0.01), and cortical neurons (741 ng/106 cells ± 63 compared with 528 ± 45, n = 9, p < 0.01).
FIGURE 4.
Glucosamine-PI altered the cholesterol distribution in ScGT1 cells. A, amount of cholesterol in cell membranes extracted from ScGT1 cells incubated with different concentrations of glucosamine-PI for 24 h. Values shown are the mean average amount of cholesterol (ng/106 cells) ± S.D., n = 9. * = amount of cholesterol significantly higher than in vehicle-treated cells (p < 0.01). B, correlation between the amounts of cholesterol in cell membranes extracted from ScGT1 cells incubated with different concentrations of glucosamine-PI for 24 h and the amount of PrPSc in glucosamine-PI-treated cells after 7 days.
The amount of cholesterol within cell membranes is controlled via a mixture of biosynthesis, uptake/efflux, and by esterification of cholesterol in the endoplasmic reticulum (31). Excess cholesterol entering the endoplasmic reticulum is esterified by acyl-coenzyme A:cholesterol acyltransferase, which keeps the level of cholesterol within cell membranes under tight control (32). Consistent with this hypothesis, we found that the addition of mevalonate, a precursor that is rapidly converted to cholesterol, increased the amount of cholesterol esters in ScGT1 cells without affecting the amount of cholesterol, indicating that any cholesterol formed was rapidly esterified (Table 3). Here, we showed that glucosamine-PI had the novel ability of increasing the amount of cholesterol found within cell membranes. Glucosamine-PI also reduced the amount of cholesterol esters in ScGT1 cells (Table 3) suggesting that the increase in membrane cholesterol was partly derived from the hydrolysis of cholesterol esters. To explore this possibility, ScGT1 cells were treated with a combination of 1 μm glucosamine-PI and 100 μm diethylumbelliferyl phosphate (DEUP), which inhibited the hydrolysis of cholesterol esters (33). We report that the DEUP reduced the glucosamine-PI-induced rise in membrane cholesterol.
TABLE 3.
Glucosamine-PI altered the cholesterol distribution in ScGT1 cells
The amount of cholesterol and cholesterol esters (measured after 24 h) and PrPSc (measured after 7 days) in 106 ScGT1 cells treated with 200 μm mevalonate, 1 μm glucosamine-PI, 100 μm DEUP, or a combination of 1 μm glucosamine-PI and 100 μm DEUP is shown. Cholesterol, cholesterol ester, and PrPSc values are the mean average ± S.D., n = 12.
| Treatment | Cholesterol | Cholesterol esters | PrPSc |
|---|---|---|---|
| ng/106 cells | ng/106 cells | ng/106 cells | |
| Vehicle | 528 ± 42 | 185 ± 29 | 9.8 ± 1.2 |
| Mevalonate | 543 ± 38 | 296 ± 35 | 10.1 ± 1.4 |
| Glucosamine-PI | 754 ± 60 | 18 ± 10 | <0.05 |
| DEUP | 554 ± 41 | 169 ± 35 | 9.5 ± 0.9 |
| Glucosamine-PI + DEUP | 566 ± 51 | 164 ± 36 | <0.05 |
Glucosamine-PI Reduced PLA2 Activity in ScGT1 Cells
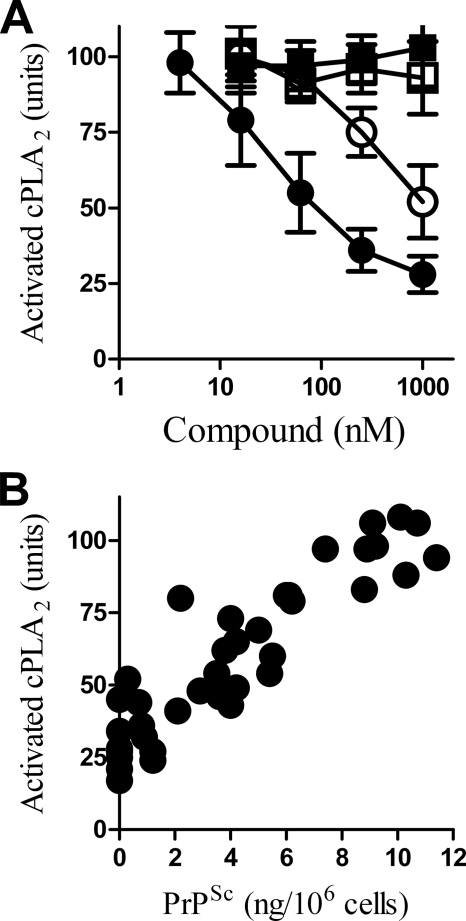
Because the activation PLA2 is necessary for PrPSc formation (23), the effect of glucosamine-PI on the amount of activated cPLA2 in prion-infected cells was measured. Treatment with glucosamine-PI for 1 h reduced the amount of activated cPLA2 in ScGT1 cells (Fig. 5A). There was a significant correlation between the amount of activated cPLA2 in ScGT1 cells incubated with different amounts of glucosamine-PI for 1 h and the amount of PrPSc in ScGT1 cells treated with glucosamine-PI for 7 days, Pearson's coefficient = −0.899, p < 0.01 (Fig. 5B). The addition of 1 μm glucosamine-PI also reduced the amount of activated cPLA2 in ScN2a cells (48 ± 8 units compared with 100 ± 21, n = 6, p < 0.01) and SMB cells (62 ± 12 compared with 100 ± 8, n = 9, p < 0.01).
FIGURE 5.
Glucosamine-PI reduced the activation of cPLA2 in ScGT1 cells. A, amount of activated cPLA2 in cell membrane extracts from ScGT1 cells incubated for 24 h with different concentrations of glucosamine-PI (●), glucosamine 2-O-methyl inositol octadecyl phosphate (○), glucosamine (■), or mannose (□). Values shown are the mean average amount of activated cPLA2 (units) ± S.D., n = 9. B, correlation between the amount of activated cPLA2 in cell extracts from ScGT1 cells incubated with different concentrations of glucosamine-PI for 24 h and the amount of PrPSc in glucosamine-PI treated cells after 7 days.
PAF Reversed the Effect of Glucosamine-PI on PrPSc Formation
Activation of PLA2 results in the production of lyso-phospholipids that affect membrane structure and the generation of prostaglandins, leucotrienes, and PAF. To test the hypothesis that the effect of glucosamine-PI on PrPSc formation resulted from inhibition of PLA2, ScGT1 cells were treated with a combination of 1 μm glucosamine-PI and some of the second messengers generated following PLA2 activation. Addition of PAF or C-PAF (a PAF receptor agonist) reversed the effect of glucosamine-PI on PrPSc formation in ScGT1 cells, whereas lyso-PAF, an inactive precursor of PAF (34), had no effect (Fig. 6). The addition of some of the other compounds generated following PLA2 activation, including lyso-phospholipids and arachidonic acid, did not reverse glucosamine-PI-induced inhibition of PrPSc formation (Table 4). The addition of 1 μm PAF also increased the PrPSc content of glucosamine-PI-treated ScN2a cells (2.15 ng of PrPSc/106 cells ± 0.34 compared with <0.05 ng, n = 9, p < 0.01) and SMB cells (6.6 ng of PrPSc/106 cells ± 1.1 compared with <0.05 ng, n = 9, p < 0.01).
FIGURE 6.
Glucosamine-PI-induced inhibition of PrPSc formation is reversed by PAF. The amount of PrPSc in ScGT1 cells treated for 7 days with a combination of 1 μm glucosamine-PI and varying concentrations of PAF (●), C-PAF (○), or lyso-PAF (▴) as shown. Values shown are the mean average amount of PrPSc (ng/106 cells) ± S.D., n = 12.
TABLE 4.
PAF restored PrPSc formation to glucosamine-PI-treated ScGT1 cells
Amount of PrPSc in ScGT1 cells treated for 7 days with glucosamine-PI combined with products released after PLA2 activation is shown. The inhibition of PrPSc formation induced by glucosamine-PI was reversed by the addition of PAF receptor agonists (PAF and C-PAF) but not by lyso-PAF, arachidonic acid, or lyso-phospholipids as shown. Values shown are the mean average PrPSc (pg/106 cells) ± S.D., n = 12.
| Treatment 1 | Treatment 2 | PrPSc (pg/106 cells) |
|---|---|---|
| None | None | 9772 ± 314 |
| 1 μm glucosamine-PI | None | <50a |
| 1 μm glucosamine-PI | 1 μm PAF | 11420 ± 658 |
| 1 μm glucosamine-PI | 1 μm C-PAF | 9412 ± 466 |
| 1 μm glucosamine-PI | 1 μm lyso-PAF | <50a |
| 1 μm glucosamine-PI | 10 μm arachidonic acid | <50a |
| 1 μm glucosamine-PI | 10 μm lyso-phosphatidic acid | <50a |
| 1 μm glucosamine-PI | 10 μm lyso-phosphatidylcholine | <50a |
| 1 μm glucosamine-PI | 10 μm lyso-phosphatidylethanolamine | <50a |
a Amount of PrPSc is significantly less than that of vehicle-treated cells (p < 0.01).
Glucosamine-PI Reduced the Expression of PrPC at the Cell Surface
Because PrPC is necessary for prion formation (5–7), the effect of glucosamine-PI on the amount and distribution of PrPC within cells was examined. To avoid confusion between PrPC and PrPSc, the following studies were conducted on noninfected GT1 cells. There was no significant difference between the amount of PrPC in cell extracts from untreated cells and cells treated for 24 h with 1 μm glucosamine-PI (31.2 ng/106 cells ± 3.8 compared with 29.8 ± 5, n = 9, p = 0.7). Although the amount of PrPC within cells was not affected by treatment with 1 μm glucosamine-PI, it changed the cellular distribution of PrPC. In vehicle-treated cells, greater than 90% of PrPC was found within DRMs, consistent with its localization to lipid rafts (35). However, treatment with 1 μm glucosamine-PI for 24 h reduced the amount of PrPC in DRMs (Table 5). Treatment with glucosamine-PI did not affect the distribution of all GPI anchored proteins equally. For example, immunoblots showed that the GPI anchored proteins Thy-1 and CD55 remained in DRMs in treated cells (data not shown). The addition of 1 μm glucosamine-PI affected other lipid raft constituents, although the amount of GM-1 was reduced (69 ± 7% compared with 100 ± 9%, n = 12, p < 0.01), it remained predominantly within DRMs (data not shown).
TABLE 5.
PAF reversed the effect of glucosamine-PI on PrPC distribution
The amount of PrPC in DRMs (lipid rafts) or non-raft (detergent-soluble fractions) of membranes isolated from GT1 cells treated for 24 h with combinations of 1 μm glucosamine-PI and 1 μm PAF, 1 μm C-PAF, or 1 μm lyso-PAF is as shown. Values are the mean average amount of PrPC (ng/106 cells) ± S.D., n = 12. Also shown is the amount of PrPC expressed at the surface of GT1 cells incubated for 24 h with combinations of 1 μm glucosamine-PI and 1 μm PAF, 1 μm C-PAF, or 1 μm lyso-PAF as shown. Values are the mean average amount of cell surface PrPC (ng/106 cells) ± S.D., n = 12.
| Treatment | PrPC ng/106 cells |
||
|---|---|---|---|
| Lipid raft | Non-raft | Cell surface | |
| Vehicle-treated | 26.7 ± 2.1 | 3.6 ± 1.4 | 5.9 ± 0.8 |
| 1 μm glucosamine-PI | 4.8 ± 0.9a | 23.9 ± 3.8 | 0.5 ± 0.4a |
| 1 μm glucosamine-PI + 1 μm PAF | 28.4 ± 3.7 | 4.5 ± 1.1 | 5.4 ± 1.1 |
| 1 μm glucosamine-PI + 1 μm C-PAF | 30 ± 3.6 | 4.2 ± 1.6 | 6.3 ± 1.8 |
| 1 μm glucosamine-PI ± 1 μm lyso-PAF | 3.5 ± 1.7a | 22.2 ± 5.4 | 1.2 ± 0.6a |
a Amount of PrPC is significantly less than that of vehicle-treated cells.
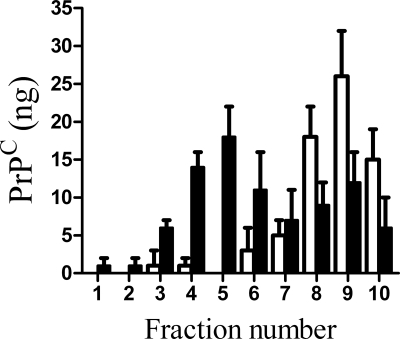
Because the detergent solubility assay is a crude test of membrane targeting, DRM constituents were also separated by flotation on sucrose density gradients. When membrane extracts from GT1 cells were separated by sucrose density flotation, most of the PrPC was found in low density fractions. However, in GT1 cells treated with 1 μm glucosamine-PI for 24 h, a significant amount of PrPC was found in higher density fractions (Fig. 7).
FIGURE 7.
Glucosamine-PI altered the targeting of PrPC in GT1 cell membranes. GT1 cells were treated with a vehicle control (□) or 1 μm glucosamine-PI (■) for 24 h. Cell extracts were subsequently separated by ultracentrifugation on a sucrose density gradient and the amount of PrPC detected in each fraction determined by ELISA. Values shown are the mean average amount of PrPC (ng/106 cells) ± S.D., n = 9.
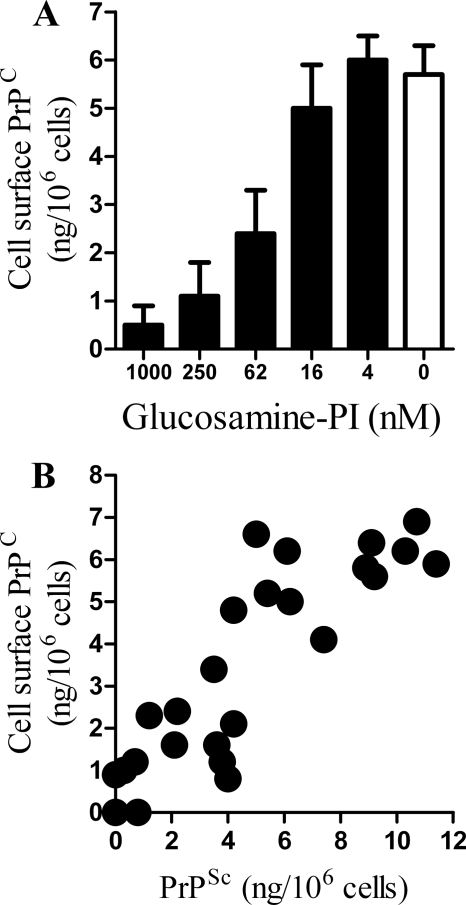
Antibody studies suggest that the conversion of PrPC to PrPSc may occur at the cell surface (25, 36). The amount of cell surface PrPC was measured by digestion with PI-phospholipase C, which released PrPC but not PrPSc (37, 38). Treatment of GT1 cells with glucosamine-PI for 24 h reduced the amount of cell surface PrPC (Fig. 8A). Treatment with glucosamine-PI for 24 h also reduced the amount of PrPC expressed at the surface of the prion-infected ScGT1 cells. In these cells there was a significant correlation between the amount of PrPC expressed at the surface 24 h after treatment with different amounts of glucosamine-PI, and the amount of PrPSc in these cells after 7 days, Pearson's coefficient = 0.895, p < 0.01 (Fig. 8B).
FIGURE 8.
Treatment with glucosamine-PI altered PrPC distribution. A, amount of PrPC at the surface of vehicle-treated GT1 cells (□) or cells treated for 24 h with different concentrations of glucosamine-PI as shown (■). Values shown are the mean average amount of PrPC (ng/106 cells) ± S.D., n = 12. B, correlation between the amount of PrPC expressed at the surface of ScGT1 cells incubated with different concentrations of glucosamine-PI for 24 h and the amount of PrPSc in glucosamine-PI treated ScGT1 cells after 7 days.
PAF Reversed the Effect of Glucosamine-PI on PrPC Expression
The effect of glucosamine-PI on PrPC was similar to those of PLA2 inhibitors (23). Here, we show that the addition of PAF or C-PAF reversed the effect of 1 μm glucosamine-PI on the distribution of PrPC between raft and non-raft membranes in GT1 cells. As shown in Table 5, the addition of 1 μm PAF or 1 μm C-PAF, but not 1 μm lyso-PAF, to glucosamine-PI-treated cells increased the amount of PrPC in lipid rafts. PAF also affected the amount of PrPC at the cell surface; the addition of 1 μm PAF or C-PAF increased the amount of PrPC at the surface of GT1 cells treated with 1 μm glucosamine-PI.
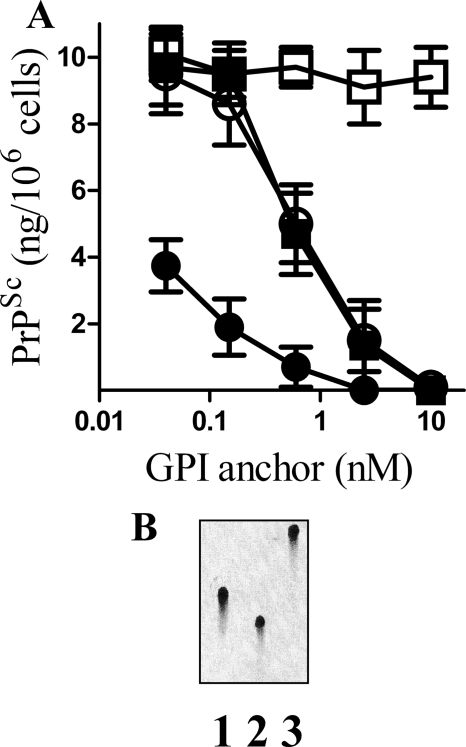
Specific GPI Anchors Reduced PrPSc Formation
Next, we sought to determine whether specific GPI anchors also reduced PrPSc formation. Because the production of synthetic GPI anchors is not a trivial task, GPI anchors were isolated from three different proteins (PrPC, CD55, and Thy-1) as described previously (22). ScGT1 cells were treated daily with these GPI anchors, and the amount of PrPSc in cell extracts was determined after 7 days. Although pretreatment with GPI anchors isolated from PrPC, CD55, or Thy-1 all reduced PrPSc formation, a control preparation had no effect (Fig. 9A). The concentration of GPI anchor required to reduce PrPSc formation to 50% of control cultures (ED50) was lower for the GPI anchor isolated from PrPC than for the GPI anchors isolated from Thy-1 or CD55. High performance TLC analysis showed that the GPI anchors isolated from PrPC, CD55, and Thy-1 had different migration patterns consistent with reports that their glycan composition differed (Fig. 9B).
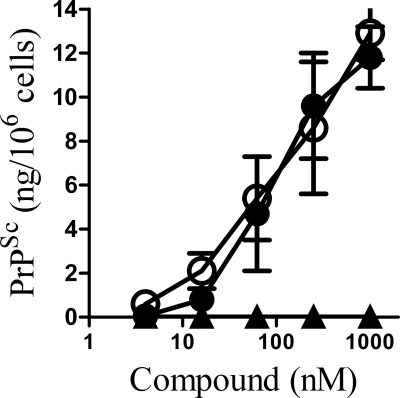
FIGURE 9.
Specific GPI anchors reduced PrPSc formation in ScGT1 cells. A, amount of PrPSc in ScGT1 cells treated for 7 days with varying concentrations of GPI anchors isolated from PrPC (●), Thy-1 (○), CD55 (■), or an anchorless control (□). Values shown are the mean average amount of PrPSc (ng/106 cells) ± S.D., n = 15. B, HPTLC analysis of GPI anchors isolated from PrPC (lane 1), Thy-1 (lane 2), or CD55 (lane 3).
DISCUSSION
Treatment with the GPI analogues, glucosamine-PI or glucosamine-2-O-methyl inositol octadecyl phosphate, reduced PrPSc formation in ScGT1, ScN2a, and SMB cells, whereas treatment with other GPI components galactose, galactosamine, glucosamine, or mannose had no effect. Treatment with glucosamine-PI had a far greater effect than that of PI alone; about 250-fold less was required to halve the amount of PrPSc (40 nm compared with 10 μm). Furthermore, the addition of 1 μm glucosamine to ScGT1 cells treated with 1 μm PI did not alter PrPSc formation indicating that glucosamine had to be covalently bound to PI to be effective. Treatment with glucosamine-PI and glucosamine 2-O-methyl inositol octadecyl phosphate also reduced the amount of PrPSc released into cell supernatants indicating that the loss of cell-associated PrPSc was not simply due to these compounds promoting the exocytosis of PrPSc. The loss of PrPSc from ScGT1 cells that had been treated with glucosamine-PI was accompanied by a loss of infectivity. When ScGT1 cells were treated with glucosamine 2-O-methyl inositol octadecyl phosphate, their infectivity was greatly reduced; four of eight mice survived for longer than 600 days, and a highly significant increase in the incubation period was observed in the four mice that died.
To understand the mechanisms by which glucosamine-PI reduced PrPSc formation, we first examined its effects on cholesterol. Cholesterol synthesis inhibitors reduced prion formation in vitro (11–13) and delayed the progression of experimental scrapie infections (39, 40). The effects of these drugs are thought to be due to cholesterol depletion affecting the formation of lipid rafts (10), which have a critical role in prion formation (41). The GPI anchor targets proteins to lipid rafts (42). Isolated GPIs also target lipid rafts (43) suggesting that the GPI anchors may precipitate the formation of lipid rafts. The high incidence of saturated fatty acids attached to GPI anchors increased the solubilization of cholesterol and precipitated lipid raft formation (44, 45). In addition, the glycan component of GPI anchors has an extended conformation along the plane of the membrane that protects cholesterol from water and stabilizes lipid rafts (46). The addition of glucosamine-PI containing saturated fatty acids had the novel ability of increasing the amount of cholesterol in cell membranes. This observation was surprising as the amount of cholesterol within cell membranes is tightly controlled (31, 32), and as shown in Table 3, cholesterol formed following the addition of mevalonate to ScGT1 cells was rapidly esterified. The increase in membrane cholesterol in glucosamine-PI-treated cells was accompanied by a reduction in cholesterol esters. This was blocked by inhibition of cholesterol ester hydrolase with DEUP showing that the increased cholesterol in glucosamine-PI treated cells was partly derived from cytoplasmic cholesterol ester stores. Notably, the addition of DEUP did not affect PrPSc formation in glucosamine-PI-treated cells indicating that this response to glucosamine-PI was not involved in PrPSc formation. We propose that it is the sequestration of cholesterol by glucosamine-PI that depletes cholesterol from other cellular pools that are necessary for PrPSc formation.
Lipid rafts are enriched in signaling molecules suggesting that they form a platform in which GPIs can interact with cell signaling pathways (47). High concentrations of GPI anchors isolated from PrPC activate PLA2 (22), which had a critical role in PrPSc formation (23). We propose that the aggregation of PrPSc causes the clustering of specific GPI anchors that activate PLA2 and facilitates the production of PrPSc. The addition of glucosamine-PI altered the composition of lipid rafts and reduced the activation of PLA2 that was required for further PrPSc formation. Activation of PLA2 results in the production of bioactive lipids, including eicosanoids, lyso-phospholipids, and PAF. The addition of arachidonic acid or lyso-phospholipids did not reverse the effect of glucosamine-PI on PrPSc formation. In contrast, PAF reversed the effect of glucosamine-PI on PrPSc formation in all three cell lines indicating that the inhibitory effect of glucosamine-PI is through inhibition of PLA2 and a reduction in PAF formation. The hypothesis that PAF regulates the composition, and possibly the function, of a sub-set of lipid rafts that contains PrPC is compatible with reports that PAF increased cholesterol (48) possibly through inhibition of cholesterol esterification (49).
Raft residents proteins, especially GPI-anchored proteins, often display distinctive trafficking pathways (50). Although PrPC is commonly found within lipid rafts at the cell surface (35), it is also found outside lipid rafts following inhibition of cholesterol synthesis (11, 12). We report that treatment with glucosamine-PI did not affect the total amount of PrPC in cells; rather it caused a redistribution of PrPC into the normal cell membrane. The sequestration of cholesterol by glucosamine-PI might reduce the amount of cholesterol available to stabilize PrPC within lipid rafts. We noted that PrPSc remained within lipid rafts in glucosamine-PI-treated cells (data not shown) suggesting that in these cells there are limited interactions between raft-associated PrPSc and non-raft PrPC.
Cholesterol concentrations are critical determinants of the intracellular trafficking of many GPI-anchored proteins. GPI-anchored proteins associate with lipid rafts during their passage through the Golgi, and cholesterol depletion results in impaired trafficking to the plasma membrane (51). More specifically, treatment with the cholesterol synthesis inhibitor lovastatin reduced surface expression of PrPC (52), and here we report that treatment with glucosamine-PI also reduced the expression of PrPC at the cell surface. In addition, many GPI anchored proteins are delivered to a common recycling compartment (53) and recycle back to the cell membrane. This pathway is cholesterol-sensitive, and cholesterol depletion reduced the recycling of GPI-anchored proteins back to the cell membrane (54). Thus, sequestration of cholesterol by glucosamine-PI may affect the recycling of PrPC and reroute PrPC away from sites conducive to the conversion of PrPC to PrPSc. This hypothesis is consistent with the observation that glucosamine-PI inhibited PLA2, that PLA2 inhibitors also reduced PrPC expression at the cell surface (23), and that the addition of PAF reversed the effects of glucosamine-PI on both the distribution of PrPC to lipid rafts and on the expression of PrPC at the cell surface. Although the exact role of PLA2 and PAF in the trafficking of PrPC is not known, PLA2 activation is essential for the maintenance of the Golgi network (55), which is involved in the trafficking of a green fluorescent protein-tagged PrPC (56). We propose that the specific GPIs attached to PrPC activate PLA2 resulting in PAF formation, which directs PrPC to specific sites conducive to prion formation.
The localization of GPI-anchored proteins to specific membrane microdomains depends upon the chemical composition of the GPI anchor (57). A recent paper demonstrated that the composition of the GPI anchor directed antigens to specific membrane microdomains in the absence of noninteractive external domains (58). This observation suggested that complex GPI anchors might inhibit PrPSc formation with greater efficacy than glucosamine-PI. The GPI anchor isolated from PrPC reduced PrPSc formation at concentrations significantly lower than those of GPI anchors isolated from Thy-1 or CD55 suggesting that specific GPI anchors can be used to either displace specific GPI-anchored proteins or inhibit the function of specific lipid raft subsets that are involved in the formation of PrPSc.
Reports that transgenic mice expressing PrPC lacking the GPI anchor did not suffer from clinical scrapie increased interest in the role of the GPI anchor in the pathogenesis of prion diseases (16). Experiments described here indicate that treatment with the GPI anchor analogue glucosamine-PI altered the composition of cell membranes, reduced activation of cPLA2, and reduced PrPSc formation. A causative role of PLA2 activation in PrPSc formation was strengthened by the observation that the effects of glucosamine-PI were reversed by the addition of PAF, which is formed in neurons following PLA2 activation. Treatment with glucosamine-PI also affected the distribution of PrPC; it displaced PrPC from within lipid rafts and reduced expression of PrPC at the cell surface, putative sites for PrPSc formation. Altered trafficking of PrPC away from sites conducive to conversion of PrPC to PrPSc may explain the inhibitory effect of GPI analogues on PrPSc formation. We conclude that GPI anchor analogues may provide a novel means of disturbing lipid raft-dependent processes involved in prion formation.
This work was supported by the European Commission FP6-NeuroPrion-Network of Excellence.
- PrPC
- cellular prion protein
- PrPSc
- scrapie prion protein
- GPI
- glycosylphosphatidylinositol
- PI
- phosphatidylinositol
- PLA2
- A2
- cPLA2
- cytoplasmic PLA2
- PBS
- phosphate-buffered saline
- mAb
- monoclonal antibody
- DRM
- detergent-resistant membrane
- ELISA
- enzyme-linked immunosorbent assay
- PAF
- platelet-activating factor
- DEUP
- diethylumbelliferyl phosphate.
REFERENCES
- 1.Prusiner S. B. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 13363–13383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pan K. M., Baldwin M., Nguyen J., Gasset M., Serban A., Groth D., Mehlhorn I., Huang Z., Fletterick R. J., Cohen F. E. (1993) Proc. Natl. Acad. Sci. U.S.A. 90, 10962–10966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prusiner S. B. (1982) Science 216, 136–144 [DOI] [PubMed] [Google Scholar]
- 4.Jeffrey M., Halliday W. G., Bell J., Johnston A. R., MacLeod N. K., Ingham C., Sayers A. R., Brown D. A., Fraser J. R. (2000) Neuropath. Appl. Neurobiol. 26, 41–54 [DOI] [PubMed] [Google Scholar]
- 5.Brandner S., Isenmann S., Raeber A., Fischer M., Sailer A., Kobayashi Y., Marino S., Weissmann C., Aguzzi A. (1996) Nature 379, 339–343 [DOI] [PubMed] [Google Scholar]
- 6.Büeler H., Aguzzi A., Sailer A., Greiner R. A., Autenried P., Aguet M., Weissmann C. (1993) Cell 73, 1339–1347 [DOI] [PubMed] [Google Scholar]
- 7.Mallucci G., Dickinson A., Linehan J., Klöhn P. C., Brandner S., Collinge J. (2003) Science 302, 871–874 [DOI] [PubMed] [Google Scholar]
- 8.Gilch S., Winklhofer K. F., Groschup M. H., Nunziante M., Lucassen R., Spielhaupter C., Muranyi W., Riesner D., Tatzelt J., Schätzl H. M. (2001) EMBO J. 20, 3957–3966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Béranger F., Mangé A., Goud B., Lehmann S. (2002) J. Biol. Chem. 277, 38972–38977 [DOI] [PubMed] [Google Scholar]
- 10.Pike L. J. (2004) Biochem. J. 378, 281–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taraboulos A., Scott M., Semenov A., Avrahami D., Laszlo L., Prusiner S. B., Avraham D. (1995) J. Cell Biol. 129, 121–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bate C., Salmona M., Diomede L., Williams A. (2004) J. Biol. Chem. 279, 14983–14990 [DOI] [PubMed] [Google Scholar]
- 13.Hagiwara K., Nakamura Y., Nishijima M., Yamakawa Y. (2007) Biol. Pharm. Bull. 30, 835–838 [DOI] [PubMed] [Google Scholar]
- 14.Stahl N., Borchelt D. R., Hsiao K., Prusiner S. B. (1987) Cell 51, 229–240 [DOI] [PubMed] [Google Scholar]
- 15.Major S., Reizman H. (2004) Nat. Rev. Mol. Cell Biol. 5, 110–120 [DOI] [PubMed] [Google Scholar]
- 16.Chesebro B., Trifilo M., Race R., Meade-White K., Teng C., LaCasse R., Raymond L., Favara C., Baron G., Priola S., Caughey B., Masliah E., Oldstone M. (2005) Science 308, 1435–1439 [DOI] [PubMed] [Google Scholar]
- 17.Tuzi N. L., Cancellotti E., Baybutt H., Blackford L., Bradford B., Plinston C., Coghill A., Hart P., Piccardo P., Barron R. M., Manson J. C. (2008) PLoS Biol 6, e100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ikezawa H. (2002) Biol. Pharm. Bull. 25, 409–417 [DOI] [PubMed] [Google Scholar]
- 19.Stahl N., Baldwin M. A., Hecker R., Pan K. M., Burlingame A. L., Prusiner S. B. (1992) Biochemistry 31, 5043–5053 [DOI] [PubMed] [Google Scholar]
- 20.Vijaykumar M., Naik R. S., Gowda D. C. (2001) J. Biol. Chem. 276, 6909–6912 [DOI] [PubMed] [Google Scholar]
- 21.Frick W., Bauer A., Bauer J., Wied S., Müller G. (1998) Biochemistry 37, 13421–13436 [DOI] [PubMed] [Google Scholar]
- 22.Bate C., Williams A. (2004) J. Gen. Virol. 85, 3797–3804 [DOI] [PubMed] [Google Scholar]
- 23.Bate C., Reid S., Williams A. (2004) J. Biol. Chem. 279, 36405–36411 [DOI] [PubMed] [Google Scholar]
- 24.Wadsworth J. D., Joiner S., Linehan J. M., Cooper S., Powell C., Mallinson G., Buckell J., Gowland I., Asante E. A., Budka H., Brandner S., Collinge J. (2006) Brain 129, 1557–1569 [DOI] [PubMed] [Google Scholar]
- 25.White A. R., Enever P., Tayebi M., Mushens R., Linehan J., Brandner S., Anstee D., Collinge J., Hawke S. (2003) Nature 422, 80–83 [DOI] [PubMed] [Google Scholar]
- 26.Beringue V., Mallinson G., Kaisar M., Tayebi M., Sattar Z., Jackson G., Anstee D., Collinge J., Hawke S. (2003) Brain 126, 2065–2073 [DOI] [PubMed] [Google Scholar]
- 27.Bate C., Tayebi M., Williams A. (2008) BMC Biol. 6, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fevrier B., Vilette D., Archer F., Loew D., Faigle W., Vidal M., Laude H., Raposo G. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 9683–9688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leblanc P., Alais S., Porto-Carreiro I., Lehmann S., Grassi J., Raposo G., Darlix J. L. (2006) EMBO J. 25, 2674–2685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vella L. J., Sharples R. A., Lawson V. A., Masters C. L., Cappai R., Hill A. F. (2007) J. Pathol. 211, 582–590 [DOI] [PubMed] [Google Scholar]
- 31.Simons K., Ikonen E. (2000) Science 290, 1721–1726 [DOI] [PubMed] [Google Scholar]
- 32.Chang T. Y., Chang C. C., Cheng D. (1997) Annu. Rev. Biochem. 66, 613–638 [DOI] [PubMed] [Google Scholar]
- 33.Gocze P. M., Freeman D. A. (1992) Endocrinology 131, 2972–2978 [DOI] [PubMed] [Google Scholar]
- 34.Shukla S. D. (1992) FASEB J. 6, 2296–2301 [DOI] [PubMed] [Google Scholar]
- 35.Vey M., Pilkuhn S., Wille H., Nixon R., DeArmond S. J., Smart E. J., Anderson R. G., Taraboulos A., Prusiner S. B. (1996) Proc. Natl. Acad. Sci. U.S.A. 93, 14945–14949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Enari M., Flechsig E., Weissmann C. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 9295–9299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Caughey B., Neary K., Buller R., Ernst D., Perry L. L., Chesebro B., Race R. E. (1990) J. Virol. 64, 1093–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stahl N., Borchelt D. R., Prusiner S. B. (1990) Biochemistry 29, 5405–5412 [DOI] [PubMed] [Google Scholar]
- 39.Mok S. W., Thelen K. M., Riemer C., Bamme T., Gültner S., Lütjohann D., Baier M. (2006) Biochem. Biophys. Res. Commun. 348, 697–702 [DOI] [PubMed] [Google Scholar]
- 40.Kempster S., Bate C., Williams A. (2007) Neuroreport 18, 479–482 [DOI] [PubMed] [Google Scholar]
- 41.Taylor D. R., Hooper N. M. (2006) Mol. Membr. Biol. 23, 89–99 [DOI] [PubMed] [Google Scholar]
- 42.Sotgia F., Razani B., Bonuccelli G., Schubert W., Battista M., Lee H., Capozza F., Schubert A. L., Minetti C., Buckley J. T., Lisanti M. P. (2002) Mol. Cell. Biol. 22, 3905–3926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Legler D. F., Doucey M. A., Schneider P., Chapatte L., Bender F. C., Bron C. (2005) FASEB J. 19, 73–75 [DOI] [PubMed] [Google Scholar]
- 44.Schroeder F., Gallegos A. M., Atshaves B. P., Storey S. M., McIntosh A. L., Petrescu A. D., Huang H., Starodub O., Chao H., Yang H., Frolov A., Kier A. B. (2001) Exp. Biol. Med. 226, 873–890 [DOI] [PubMed] [Google Scholar]
- 45.Brown D. A., London E. (1998) Annu. Rev. Cell Dev. Biol. 14, 111–136 [DOI] [PubMed] [Google Scholar]
- 46.Huang J., Feigenson G. W. (1999) Biophys. J. 76, 2142–2157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Simons K., Toomre D. (2000) Nat. Rev. Mol. Cell Biol. 1, 31–39 [DOI] [PubMed] [Google Scholar]
- 48.Bergelson L. D., Kulikov V. I., Muzia G. I. (1985) FEBS Lett. 190, 305–306 [DOI] [PubMed] [Google Scholar]
- 49.Feliste R., Perret B., Braquet P., Chap H. (1989) Atherosclerosis 78, 151–158 [DOI] [PubMed] [Google Scholar]
- 50.Rajendran L., Simons K. (2005) J. Cell Sci. 118, 1099–1102 [DOI] [PubMed] [Google Scholar]
- 51.Helms J. B., Zurzolo C. (2004) Traffic 5, 247–254 [DOI] [PubMed] [Google Scholar]
- 52.Gilch S., Kehler C., Schätzl H. M. (2006) Mol. Cell. Neurosci. 31, 346–353 [DOI] [PubMed] [Google Scholar]
- 53.Chatterjee S., Smith E. R., Hanada K., Stevens V. L., Mayor S. (2001) EMBO J. 20, 1583–1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mayor S., Sabharanjak S., Maxfield F. R. (1998) EMBO J. 17, 4626–4638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.de Figueiredo P., Drecktrah D., Katzenellenbogen J. A., Strang M., Brown W. J. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 8642–8647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Magalhães A. C., Silva J. A., Lee K. S., Martins V. R., Prado V. F., Ferguson S. S., Gomez M. V., Brentani R. R., Prado M. A. (2002) J. Biol. Chem. 277, 33311–33318 [DOI] [PubMed] [Google Scholar]
- 57.Anderson R. G., Jacobson K. (2002) Science 296, 1821–1825 [DOI] [PubMed] [Google Scholar]
- 58.Nicholson T. B., Stanners C. P. (2006) J. Cell Biol. 175, 647–659 [DOI] [PMC free article] [PubMed] [Google Scholar]