Abstract
LKB1 is a tumor suppressor protein whose loss leads to HIF1α-mediated activation of a proangiogenic program in intestinal polyps. LKB1 is also protein kinase regulator of AMP-activated protein kinase (AMPK) signaling, which is essential for endothelial cell responses to tissue ischemia. To discern whether LKB1 signaling is either pro- or antiangiogenic, we investigated ischemia-induced revascularization in mice that were deficient for LKB1 in Tie2-Cre-expressing cells. Whereas homozygous deletion of LKB1 led to embryonic lethality, heterozygous LKB1-knock-out (KO) (Lkb1flox/+;Tie2Tg/+) mice were viable. Unchallenged heterozygous LKB1-KO mice displayed normal capillary density, but the revascularization of hind limb following ischemic surgery was significantly impaired as evaluated by laser Doppler flow and capillary density measurements. Reduction of LKB1 in cultured endothelial cells, using either small interfering RNA or an adenovirus expressing nonfunctional kinase-dead LKB1 protein, attenuated endothelial proliferation, migration, and differentiation into network structures on Matrigel that was accompanied by diminished AMPK phosphorylation at Thr-172. Conversely, adenovirus-mediated LKB1 overexpression (Ad-LKB1) augmented network structure formation, and this was associated with elevated AMPK phosphorylation. The augmented differentiation of endothelial cells into network structures induced by Ad-LKB1 was abrogated by the co-transduction of a dominant negative mutant of AMPK. These observations suggest that the LKB1-AMPK signaling axis in endothelial cells is a positive regulator of the revascularization response to tissue ischemia.
Keywords: AMP-activated Kinase (AMPK), Cell Migration, Endothelium, Ischemia, Signal Transduction, LKB1, eNOS, Endothelial Cells, Revascularization
Introduction
LKB1, a serine-threonine protein kinase that is ubiquitously expressed in many organs, including liver, heart, and skeletal muscle (1, 2). Clinically, LKB1 deficiency leads to Peutz-Jeghers syndrome, a disease associated with gastrointestinal polyps and an increased risk of cancers (1, 3). Heterozygous ablation of LKB1 in mice leads to increased gastrointestinal polyps consistent with the human syndrome (4, 5). These LKB1-deficient polyps up-regulate the HIF1α transcription factor and its downstream targets (6). Homozygous ablation of LKB1 results in lethality at embryonic day 11, which is associated with disorganized capillary network development and an up-regulation of vascular endothelial growth factor signaling (7). Collectively, these data indicate that LKB1 has an antiangiogenic function. Consistent with this hypothesis, Xie et al. (8) recently reported that overexpression of LKB1 in endothelial cells inhibits network formation on a Matrigel surface in vitro, a surrogate angiogenesis assay.
On the other hand, it is conceivable that LKB1 has a proangiogenic activity within endothelial cells based upon its ability to phosphorylate and activate AMP-activated protein kinase (AMPK).4 In this regard, AMPK signaling promotes endothelial cell function and angiogenesis in response to hypoxic stress (9–11), and tissue ischemia is a powerful activating stimulus for AMPK signaling (12). AMPK phosphorylates eNOS at residue Ser-1177 leading to the production of nitric oxide, a key determinant of endothelial cell function (9, 13–15). Thus, it is plausible that LKB1 has a proangiogenic function via its ability to stimulate AMPK/eNOS signaling in endothelial cells under ischemic conditions. However, one cannot assume a priori that LKB1 is functionally similar to AMPK because it is also likely to have AMPK-independent functions. For example, LKB1 also targets downstream regulators that are distinct from AMPK, including 12 protein kinases (BRSK1, BRSK2, NUAK1, NUAK2, QIK, QSK, SIK, MARK1, MARK2, MARK3, MARK4, and SNRK) (16). Furthermore, in addition to LKB1, AMPK is regulated by other upstream protein kinases, including Ca2+/calmodulin-dependent protein kinase kinase-β (CaMKKβ) and transforming growth factor-β-activated kinase 1 (TAK1) (17). In particular, studies in cultured endothelial cells have provided evidence for a functionally significant CaMKKβ-AMPK signaling interaction (18). Thus, the quantitative significance of the LKB1-AMPK signaling axis in endothelial cells is not clear, nor is it known whether LKB1 has a pro- or antiangiogenic function in this cell type.
Recently, it has been reported that homozygous ablation of LKB1 in cells expressing Cre from the Tie1 promoter also results in embryonic lethality (19). Analysis of Lkb1flox/flox;Tie1Tg/+ embryos revealed bloodless yolk sacs and pericardial swelling, and blood vessels within these embryos appear dilated and congested with blood. Although these phenotypes show that LKB1 is an important regulator of proper vascular development, nothing is known about the function of LKB1 in the adult vascular cells nor whether LKB1 has a pro- or antiangiogenic function in this context. To address these issues, we generated a mouse model of partial LKB1 reduction by mating Lkb1flox/flox mice with Tie2-Cre transgenic mice to produce heterozygous LKB1-KO mice. Analyses of these mice revealed a partial reduction in LKB1 expression in vascular endothelial cells, and this was associated with impaired ischemia-induced angiogenic responses. A series of in vitro studies further suggest that endothelial cell LKB1 exerts proangiogenic activities via an AMPK-dependent pathway.
EXPERIMENTAL PROCEDURES
Materials
Mouse CD31 antibody was purchased from BD Pharmingen. Antibodies LKB1 and eNOS were purchased from Santa Cruz Biotechnology. Antibodies phospho-AMPK (Thr-172) and phospho-eNOS (Ser-1177) were purchased from Cell Signaling Technology (Beverly, MA). Phospho-ACC (Ser-79) antibody was purchased from Upstate Biotechnology. Tubulin antibody was purchased from Oncogene (Cambridge, MA). Antibody CD45 was purchased from R&D. Adenovirus vectors containing the gene for β-galactosidase (Ad-β-gal), the α subunit of LKB1 (Ad-LKB1), kinase-dead LKB1, which expresses the Asp to Ala mutation at position 194 in the kinase domain of human LKB1 (Ad-kd-LKB1), constitutive active AMPK and dominant negative AMPKα2 (Ad-dn-AMPK) were described previously (9, 20–22).
Mouse Model of Angiogenesis
Lkb1flox/+;Tie2Tg/+ mice and littermate control mice (Lkb1flox/+;Tie2+/+) in the FVB/N background were used for this study. The floxed LKB1 strain was described previously (23). Mice expressing Cre recombinase under control of the Tie2 gene promoter (Tie2-Cre) were purchased from Jackson Laboratory. Lkb1flox/+;Tie2Tg/+ mice were interbred with Lkb1flox/flox;Tie2+/+ littermates to generate endothelial cell-specific LKB1 diminished mice (Lkb1flox/+;Tie2Tg/+) and littermate control mice (Lkb1flox/+;Tie2+/+). Study protocols were approved by the Institutional Animal Care and Use Committee at Boston University. Mice at the ages of 8–11 weeks were subjected to unilateral hind limb surgery to remove the left femoral artery and vein under anesthesia (10, 24, 25). Prior to surgery, body weight and systolic blood pressure were determined using a tail cuff pressure analysis system on conscious mice.
Laser Doppler Blood Flow Analysis
Hind limb blood flow was measured on postoperative days 0, 3, 7, 14, and 28 using a laser Doppler blood flow (LDBF) analyzer (Moor LDI; Moor Instruments). LDBF was assessed quantitatively as changes in the laser image using different color pixels. To avoid mouse to mouse and experiment to experiment variations, hind limb blood flow was expressed as the ratio of left (ischemic) to right (nonischemic) LDBF signal.
Capillary Density Analysis
Capillary density within the thigh adductor muscle was quantified in histological sections. Muscle samples were imbedded in OCT compound (Miles), snap frozen in liquid nitrogen, and cut into 5-μm slices. Capillary endothelial cells were identified by immunohistostaining for CD31 (PECAM-1; BD Biosciences). Fifteen randomly chosen microscopic fields from three different sections in each tissue block were examined for the presence of capillary endothelial cells for each mouse specimen. Capillary density was expressed as the number of CD31-positive cells/muscle fiber.
Mouse Lung Endothelial Cell Isolation
Mouse lung endothelial cells were isolated from 8-week-old Lkb1flox/+;Tie2Tg/+ heterozygous LKB1-KO and Lkb1flox/+;Tie2+/+ control mice as described previously (25). Mice were killed by CO2 inhalation. The lungs were excised, minced, and digested with 0.1% collagenase in phosphate-buffered saline for 45 min. The digest was homogenized by multiple passages through a 14-gauge needle and then filtered through a 100-μm tissue sieve. The cell suspension was isolated by immunoselection with CD31-conjugated (BD Pharmingen) magnetic beads (Invitrogen). When plated cells reached confluence, a second immunoisolation was performed by ICAM-2-conjugated (BD Pharmingen) magnetic beads (Invitrogen).
RNA Interference
The siRNAs targeting LKB1 were purchased from Dharmacon (ON-TARGET plus SMART Pool Human STK11, L-005035-00). Control cultures were transfected with unrelated siRNAs (Dharmacon, ON-TARGET plus Control Pool Nontargeting pool). HUVECs were transfected for 48 h with siRNAs by using Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer's instructions.
Assessment of Endothelial Cell Responses
Migration activity was measured under basal conditions in the absence of angiogenic-stimulatory growth factors using a modified Boyden chamber assay as described previously (15). Serum-deprived HUVECs were resuspended in endothelial cell growth medium-2 with 0.5% fetal bovine serum. Cell suspension (250 μl, 2.0 × 104 cells/well) were placed to the Transwell fibronectin-coated insert (6.4-mm diameter, 3.0-μm pore size, BD Biosciences). Migrated cells on the lower surface of the membrane were fixed and stained with Giemsa stain solution, and eight random microscopic fields/well were quantified. The formation of network structures by HUVECs on growth factor-reduced Matrigel (BD Biosciences) was performed as described previously (26). Twenty four-well culture plates were coated with Matrigel according to the manufacturer's instructions. Serum-deprived HUVECs were seeded on coated plates at 5 × 104 cells/well in endothelial cell basement medium-2 with 0.5% fetal bovine serum and incubated at 37 °C for 18 h. The degree of cell network formation was quantified by measuring the network area in three randomly chosen fields from each well using ImageJ software. Each experiment was repeated three times.
Cell Proliferation
Cell proliferation was assessed by direct cell counting using a hemocytometer and the CellTiter 96 AQueous kit (Promega) according to the manufacturer's instructions with MTS reagent. Briefly, in direct cell counting, HUVECs were plated at 10,000 cells/cm2 on 24-well plates and incubated in endothelial cell basal medium-2 with 2% fetal bovine serum. HUVECs were transfected with siRNAs according to the manufacturer's instructions. After 48 h, cell number was assessed by a hemocytometer. In the assay using the CellTiter 96 AQueous kit, HUVECs were plated at a density of 0.5 × 104 cells/well in a 96-well plate and incubated in endothelial cell basal medium-2 with 2% fetal bovine serum for 48 h. The 490-nm absorbance was measured after 1-h incubations with MTS.
Assessment of Endothelial Cell Apoptosis
Apoptosis was assayed by a terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) staining method with a commercial kit (Roche) (27). The mean number of apoptotic (TUNEL-positive) cells from three random fields (×100) in each well was calculated.
Western Blot Analysis
Tissue samples were homogenized in lysis buffer containing 20 mm Tris-HCl (pH 8.0), 1% Nonidet P-40, 150 mm NaCl, 0.5% deoxycholic acid, 1 mm sodium orthovanadate, and a protease inhibitor mixture (Sigma). Immunoblot analysis was performed with antibodies at a 1:1000 dilution, followed by incubation with a secondary antibody conjugated with horseradish peroxidase at a 1:5000 dilution. An ECL Plus Western blotting detection kit (Amersham Biosciences) was used.
Statistical Methods
Data are presented as mean ± S.E. Differences between groups were evaluated by the Student's t test or analysis of variance with Fisher's protected least significant difference test. A p value <0.05 denoted the presence of a statistically significant difference. All calculations were performed by using StatView for Windows, version 5.0.
RESULTS
Decreased LKB1 Expression in Endothelial Cells Isolated from Lkb1flox/+;Tie2Tg/+ Mice
To investigate the role of LKB1 in angiogenesis in vivo, we produced conditional LKB1 knock-out mice using Cre-loxP system. Lkb1flox/flox mice were crossed with Tie2-Cre transgenic (Tie2Tg/+) mice. An examination of 226 F2 offspring revealed that Lkb1flox/flox;Tie2Tg/+ (homozygous-KO) mice were not generated by this cross (supplemental Table 1). This result indicates that homozygous Lkb1 ablation with Tie2-Cre leads to embryonic death, and this is consistent with the findings of Londesborough et al. (19), who utilized a Tie1-Cre mouse system to ablate this gene.
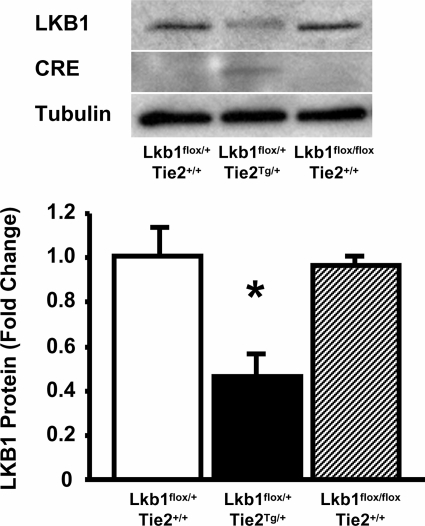
LKB1 expression levels were examined in mouse lung endothelial cells isolated from Lkb1flox/+;Tie2Tg/+ (heterozygous LKB1-KO), Lkb1flox/+;Tie2+/+ (control), and Lkb1flox/flox;Tie2+/+ mice. LKB1 expression was decreased in endothelial cells isolated from Lkb1flox/+;Tie2Tg/+ mice by ∼50% compared with those from Lkb1flox/+;Tie2+/+ and Lkb1flox/flox;Tie2+/+ mice (Fig. 1). Correspondingly, Cre expression was detected only in mouse lung endothelial cells from heterozygous LKB1-KO but not control mice (Fig. 1). As expected from the expression of Tie2-Cre in hematopoietic cells (28), LKB1 expression was also reduced in spleen, but expression was largely maintained in liver and kidney (supplemental Fig. 1).
FIGURE 1.
Reduced LKB1 expression in endothelial cells isolated from Lkb1flox/+;Tie2Tg/+ (heterozygous LKB1-KO) mice. Upper, Western blot analysis for LKB1, Cre, and α-tubulin in endothelial cells isolated from lung of Lkb1flox/+;Tie2+/+, Lkb1flox/+;Tie2Tg/+, and Lkb1flox/flox;Tie2+/+ mice. Lower, histogram indicating LKB1 expression reproducible in isolated lung endothelial cells from Lkb1flox/+;Tie2Tg/+ mice (n = 6 in each group).
Impaired Ischemia-induced Angiogenesis in Heterozygous LKB1-KO Mice
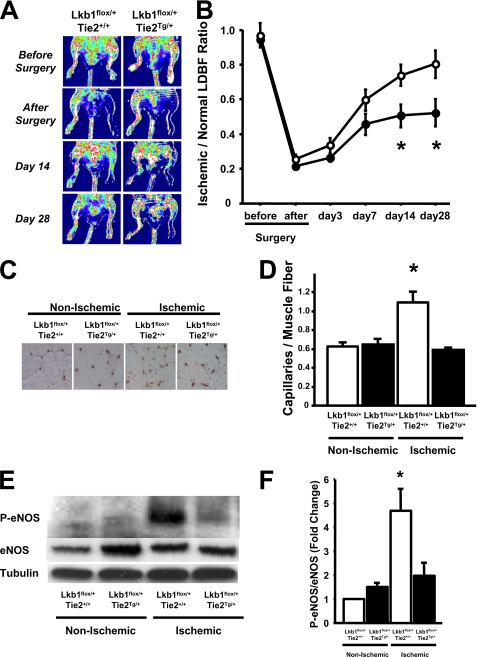
To evaluate in vivo effects of LKB1 deficiency in Tie2-Cre-expressing cells, hind limb ischemia surgery was performed on Lkb1flox/+;Tie2+/+ (control) and heterozygous LKB1-KO mice. Prior to the operation, there were no significant differences in body weight, systolic blood pressure, heart rate, and fasting plasma glucose levels between these mice at 8 weeks of age (supplemental Table 2). Fig. 2A shows representative laser Doppler images of hind limb blood flow before surgery and at different time points after surgery. Consistent with prior reports (10, 24, 25), ischemic hind limb blood flow perfusion increased to 80% of the nonischemic limb by day 28 in control mice (Fig. 2B). In contrast, blood flow in heterozygous LKB1-KO mice was significantly less than that in control mice, particularly at 14 and 28 days after surgery.
FIGURE 2.
Impaired ischemia-induced revascularization in Lkb1flox/+;Tie2Tg/ + (heterozygous LKB1-KO) mice. A, representative LDBF images showing decreased perfusion in ischemic limbs of Lkb1flox/+;Tie2Tg/+ mice. A low perfusion signal (blue) was observed in the ischemic hind limbs of Lkb1flox/+;Tie2Tg/+ mice, whereas a high perfusion signal (white to red) was detected in Lkb1flox/+;Tie2+/+ on postoperative day 28. B, quantitative analysis of the ischemic/nonischemic LDBF ratio of Lkb1flox/+;Tie2+/+ (open circles) and Lkb1flox/+;Tie2Tg/+ (filled circles) mice on postoperative days 0, 3, 7, 14, and 28 (n = 6). *, p < 0.05 for Lkb1flox/+;Tie2+/+ mice. C, representative immunostaining of nonischemic and ischemic muscle tissues with anti-CD31 antibody (brown) on postoperative day 28. D, quantitative analyses of capillary density in nonischemic and ischemic muscles of Lkb1flox/+;Tie2+/+ and Lkb1flox/+;Tie2Tg/+ mice on postoperative day 28 (n = 6 in each group). Capillary density was expressed as the number of capillaries/muscle fiber. E, Western blot analysis of phosphorylated eNOS at Ser-1177 (P-eNOS), total eNOS (eNOS), and α-tubulin (Tubulin) in nonischemic and ischemic muscles of Lkb1flox/+;Tie2+/+ and Lkb1flox/+;Tie2Tg/+ mice on postoperative day 7. F, quantitative analysis of Western blots. *, p < 0.05 versus Lkb1flox/+;Tie2+/+ (n = 4 in each group).
To examine revascularization at the microcirculatory level, capillary density was measured in nonischemic and ischemic muscle by immunohistological staining of CD31. Fig. 2C shows representative photos of adductor muscle tissue immunostained with CD31. Quantitative analysis revealed that the frequency of CD31-positive cells was greater in ischemic muscles than in nonischemic muscles in control mice at day 28 after ischemic surgery, but the proportion of CD31-positive cells in ischemic limbs was significantly less in heterozygous LKB1-KO mice compared with control mice (Fig. 2D). Staining with the leukocyte marker CD45 revealed ∼100-fold fewer positive cells (supplemental Fig. 2). The capillary density of nonischemic muscle did not differ statistically between the two groups. These data show that a reduction of LKB1 expression results in impaired revascularization in response to ischemia.
To evaluate the regulation of candidate effectors downstream of LKB1 in the revascularization response, the phosphorylation level of eNOS at Ser-1177 was examined in ischemic skeletal muscles of heterozygous LKB1-KO and control mice. Quantitative Western blot analysis revealed that ischemic surgery significantly increased the phosphorylation levels of eNOS in ischemic muscles of control mice, but this up-regulation was significantly abrogated in the muscle of heterozygous LKB1-KO mice on postoperative day 7 (Fig. 2, E and F).
LKB1 Regulates Angiogenic Cell Responses in HUVECs
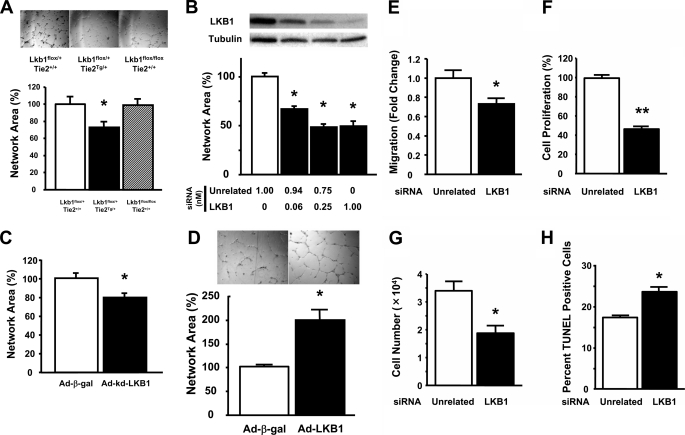
Endothelial cell differentiation into network structures on Matrigel was employed to examine LKB1 signaling at a mechanistic level. As shown in Fig. 3A, endothelial cells isolated from heterozygous LKB1-KO mice exhibited lower levels of network formation than those from Lkb1flox/+;Tie2+/+ and Lkb1flox/flox;Tie2+/+. To corroborate these observations, LKB1 knockdown experiments were performed with HUVECs. Transduction of HUVECs with siRNA targeting LKB1 led to a dose-dependent reduction in LKB1 protein expression (Fig. 3B). Accordingly, increasing levels of LKB1-siRNA led to a dose-dependent reduction in the ability of HUVECs to differentiate into network structures on Matrigel (Fig. 3B). We also examined the consequences of LKB1 loss of function using an adenoviral vector expressing kinase-dead LKB1 (Ad-kd-LKB1). Treatment of HUVECs with Ad-kd-LKB1 at a m.o.i. of 50 also abrogated polyphenol-induced phosphorylation of AMPK by 80% (data not shown). Under these transduction conditions, Ad-kd-LKB1 significantly diminished the ability of HUVECs to form cell networks relative to HUVECs treated with a control vector (Fig. 3C). Conversely, the gain of LKB1 function, via transduction of an adenoviral vector expressing the α subunit of wild-type LKB1 (Ad-LKB1), augmented the morphogenesis of HUVECs on Matrigel (Fig. 3D).
FIGURE 3.
Diminished LKB1 expression impairs endothelial cell functions. A, network formation assay in isolated mice lung endothelial cells from Lkb1flox/+;Tie2+/+, Lkb1flox/+;Tie2Tg/+, and Lkb1flox/flox;Tie2+/+ mice is shown. *, p < 0.05 versus Lkb1flox/+;Tie2+/+ (n = 6 in each group). B, treatment with siRNA targeting LKB1 dose dependently reduced LKB1 protein levels and abrogated network structures in HUVECs. Forty-eight hours after siRNA transfection, differentiation assays were performed (n = 3 in each group). C, network structures under 24-h treatment with adenovirus kinase-dead LKB1 (Ad-kd-LKB1) or adenovirus producing β-galactosidase (Ad-β-gal) in HUVECs (50 m.o.i. each, n = 4 in each group). D, transduction with an adenovirus expressing the α subunit of LKB1 promotes endothelial cell morphogenesis relative to control HUVEC cultures transduced with Ad-β-gal. E–H, treatment with siRNA targeting LKB1 decreased migration (E), proliferation (F and G), and increased apoptosis (H) in HUVECs. Forty-eight hours after siRNA transfection, migration and proliferation assays were performed using a modified Boyden chamber method and MTS-based assay, respectively (n = 4 in each group at migration assay, n = 8 in each group at proliferation assay). In direct cell counting, HUVECs were plated at 10,000 cells/cm2 on 24-well plates and incubated in endothelial cell basal medium-2 with 2% fetal bovine serum for 48 h after siRNA transfection. Cell number was assessed by a hemocytometer (n = 4 in each group). Apoptosis was assayed by TUNEL staining method. The mean number of apoptotic (TUNEL-positive) cells from three random fields (×100) in each well was calculated (n = 4 in each group).
Endothelial cell migration and proliferation contribute to the angiogenic response. Thus, the effects of decreased LKB1 signaling on these parameters were also investigated. Using a modified Boyden chamber method, we found that siRNA targeting LKB1 led to a reduction in endothelial cell migration (Fig. 3E). Similarly, treatment with siRNA targeting LKB1 significantly diminished endothelial cell proliferation compared with unrelated siRNA treatment using an MTS-based assay and direct cell counting (Fig. 3, F and G).
To test the prosurvival effect of LKB1, HUVECs were deprived of serum in the presence of siRNA directed against LKB1 or unrelated sequence. After 48 h, TUNEL staining was performed. Quantitative analysis revealed that treatment with LKB1 siRNA significantly increased the fraction of TUNEL-positive cells compared with that of unrelated siRNA (Fig. 3H).
LKB1 Regulates Angiogenic Cell Responses via an AMPK-dependent Pathway
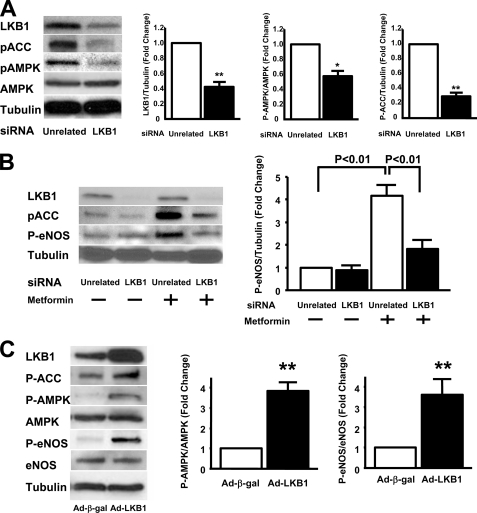
To investigate the signaling pathways downstream of LKB1 in endothelial cells, Western blot analyses were performed on cells treated with siRNA targeting LKB1 or an unrelated siRNA. Knockdown of LKB1 by siRNA decreased phosphorylation levels of AMPK at Thr-172 and ACC at Ser-79 under basal conditions (Fig. 4A). Whereas the ablation of LKB1 by siRNA had little or no effect on eNOS phosphorylation at Ser-1179 under basal conditions, the knockdown of LKB1 diminished metformin-induced phosphorylation of eNOS in HUVECs (Fig. 4B). To examine the consequences of LKB1 gain of function, HUVECs were transduced with Ad-LKB1 leading to a 2-fold overexpression of the α-subunit of LKB1 (Fig. 4C). Overexpression of LKB1 led to an increase in the phosphorylation levels of ACC, AMPK, and eNOS (Fig. 4C).
FIGURE 4.
Transduction with siRNA targeting LKB1 diminished an AMPK signaling in HUVECs. A, LKB1 is required for full AMPK signaling. Western blot analysis for LKB1, phosphorylated ACC at Ser-79 (pACC), phosphorylated AMPK at Thr-172 (pAMPK), total AMPK (AMPK), and α-tubulin (Tubulin) is shown. Right panel shows quantification of LKB1, pAMPK, and pACC. *, p < 0.05 versus unrelated siRNA (n = 3 in each group). HUVECs were treated with siRNA targeting LKB1 for 48 h. B, LKB1 is required for metformin-stimulated eNOS phosphorylation. Western blot analysis for LKB1, phosphorylated ACC, phosphorylated eNOS (P-eNOS), and α-tubulin is shown. HUVECs were treated with siRNA targeting LKB1 for 48 h followed by treatment with metformin (2 mm) or vehicle for 3 h. A representative Western immunoblot is shown. C, LKB1 overexpression activates AMPK signaling and leads to an increase in eNOS phosphorylation. Western blot analysis for LKB1, phosphorylated-ACC at Ser-79, phosphorylated-AMPK at Thr-172, total AMPK, phosphorylated-eNOS at Ser-1177, total eNOS, and α-tubulin is shown. HUVECs were infected with Ad-β-gal or Ad-LKB1 for 24 h. Right panels show quantitative Western blot analyses for P-AMPK and P-eNOS. **, p < 0.01 versus Ad-β-gal treatment (n = 3 in each group).
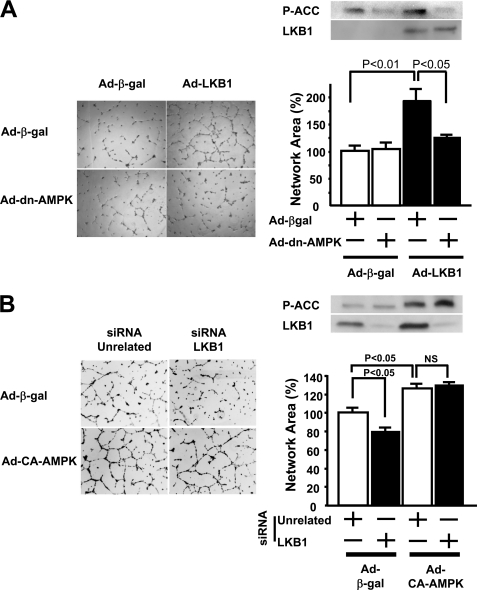
To investigate whether LKB1 overexpression promotes an in vitro angiogenic response through the activation of AMPK, HUVECs were co-transduced with an adenovirus producing dominant negative AMPK (Ad-dn-AMPK) or a control vector expressing β-galactosidase (Ad-β-gal). Treatment of HUVECs with Ad-LKB1 resulted in enhanced LKB1 expression and phosphorylation of ACC, a downstream kinase of AMPK (Fig. 5A). Transduction with Ad-dn-AMPK abolished Ad-LKB1-stimulated phosphorylation of ACC (Fig. 5A). The increase in network formation induced by Ad-LKB1 was significantly abrogated by co-transduction with Ad-dn-AMPK (Fig. 5A). Furthermore, the increase in endothelial cell proliferation induced by Ad-LKB1 was significantly reduced by co-transduction with Ad-dn-AMPK (supplemental Fig. 3).
FIGURE 5.
LKB1 overexpression enhances endothelial cell morphogenesis in an AMPK-dependent manner in HUVECs. Twenty-four hours after infection with adenoviral constructs encoding β-galactosidase (Ad-β-gal), LKB1 (Ad-LKB1) (100 m.o.i.), network formation assays were performed in HUVECs. A, left panels show representative photographs of endothelial cell differentiation into network structures. Right upper panels show representative Western blots for phosphorylated-ACC (Ser-79) and LKB1. Right lower panel shows quantitative analysis of network area under treatment with adenovirus producing dominant negative AMPK (Ad-dn-AMPK) (10 m.o.i.) for 24 h. n = 4 in each group. B, left panels show representative photographs of endothelial cell differentiation into network structures. Right upper panels show representative Western blots for phosphorylated-ACC (Ser-79) and LKB1. Right lower panel shows quantitative analysis of network area with 24-h stimulation by adenovirus producing constitutive active AMPK (Ad-ca-AMPK) (50 m.o.i.) under 48-h treatment with siRNA targeting LKB1 (1 nm) (n = 4 in each group).
To explore the LKB1-AMPK signaling axis in endothelial cells further, we tested whether the impairment in network formation in HUVECs treated with siRNA targeting LKB1 could be rescued by treatment with an adenovirus expressing constitutively active AMPK (Ad-ca-AMPK). Ad-ca-AMPK treatment increased phosphorylation of ACC both in the presence and absence of LKB1 (Fig. 5B). Whereas LKB1 knockdown diminished network formation on Matrigel, transduction with Ad-ca-AMPK reversed the effect of LKB1 ablation (Fig. 5B). Transduction of Ad-ca-AMPK alone also increased network formation in this assay.
DISCUSSION
LKB1 is a tumor suppressor (29–31), and enhanced expression of LKB1 in cancer cells results in attenuated tumor growth, invasion, and metastasis, at least in part, through its ability to inhibit tumor angiogenesis (32). Consistent with these observations, whole body, homozygous LKB1 ablation results in an embryonic lethal phenotype that is associated with increased vascular endothelial growth factor expression and abnormal capillary development (7). Based on these observations, LKB1 has been viewed as an antiangiogenic factor. However, AMPK, a downstream effector of LKB1, functions in endothelial cells to promote angiogenic responses under hypoxic conditions (9). Thus, it is conceivable that the angiogenic-regulatory properties of LKB1 are cell type-specific, resulting in different regulatory responses in vascular versus parenchymal cells.
Because nothing is known about the function of endothelial cell LKB1 in the postnatal revascularization response to tissue ischemia, we analyzed the consequences of acute hind limb ischemia in adult mice that were deficient for LKB1 expression in Tie2-Cre-expressing cells. In the present study, no viable pups were produced from matings intended to produce the homozygous ablation of the LKB1 allele with Tie2-Cre. These findings are consistent with the recent report of embryonic lethality when a different transgenic Cre-expressing line (Tie1-Cre) was used to ablate the floxed LKB1 allele (19). Consequently, we utilized heterozygous LKB1-KO mice for the hind limb ischemia study. Although Lkb1flox/+;Tie2Tg/+ mice exhibit approximately half the level of LKB1 protein in lung endothelial cells, their vascular network is indistinguishable from control mice under nonstress conditions. However, this reduction in LKB1 expression in Tie2-Cre-expressing cells led to an impaired revascularization response to tissue ischemia that was associated with reductions in ischemia-induced eNOS phosphorylation in the tissue. The diminished tissue reperfusion in heterozygous LKB1-KO mice was consistent with anatomic evidence of a reduction in capillary density in the limbs that were recovering from ischemia. Collectively, these results show that a partial reduction of LKB1 signaling in vascular endothelial cells impairs the revascularization response to ischemic conditions in adult mice.
Although many studies have used the Tie2-Cre model for gene ablation in vascular endothelial cells, it is documented that these Tie2-Cre transgenic mice also express Cre in hematopoietic cells (28). Because hematopoietic cells are important for a revascularization response (33, 34), we analyzed LKB1 expression levels in spleen, which contains many hematopoietic cells. LKB1 expression in spleen was reduced in the heterozygous LKB1-KO mice compared with control mice. Therefore, LKB1 signaling in hematopoietic derived cells, such as macrophages or vascular progenitor cells, may also contribute to this impaired vascularization phenotype.
The in vivo findings in the murine ischemic hind limb model were corroborated by a series of in vitro experiments that examined the behavior of cultured endothelial cells. Reductions in LKB1 expression in HUVECs using siRNA ablation led to reductions in the ability of these cells to proliferate, migrate, and differentiate into network structures on Matrigel. LKB1 ablation also exacerbated endothelial cell apoptosis under conditions of serum deprivation. Similarly, LKB1 inactivation using an adenoviral vector expressing kinase-dead LKB1 (Ad-kd-LKB1) diminished the ability of HUVECs to form cell networks, whereas adenovirus-mediated overexpression of the α subunit of LKB1 promoted this morphogenic response. Endothelial cells isolated from lungs of heterozygous LKB1-KO mice also displayed impaired network formation in the morphogenesis.
The cell culture experiments performed in this study indicate that the proangiogenic activity of LKB1 is mediated by its ability to regulate AMPK through the activating phosphorylation of Thr-172. These findings are consistent with other studies in cultured vascular endothelial cells showing that LKB1 functions to regulate AMPK. For example, AICAR, but not thrombin, activates AMPK in an LKB1-dependent pathway in HUVECs (18). Other studies have shown that reactive nitrogen species activate AMPK in endothelial cells through an LKB1-dependent manner (35). It has also been shown that LKB1 is a regulator of AMPK signaling in nonvascular tissues. Liver-specific LKB1 deficiency causes hyperglycemia associated with reduced hepatic AMPK activity and an increase in hepatic gluconeogenesis (23). Liver-specific LKB1 deficiency results in impaired responses to metformin, an antidiabetic drug that activates AMPK (23). Skeletal muscle-specific LKB1 deficiency also leads to reduced AMPK phosphorylation at basal and AICAR- or phenformin-stimulated conditions (36, 37). Finally, ablation of LKB1 in the heart leads to hypertrophy that is associated with a reduction in AMPK signaling (38).
It is well established that Akt signaling functions downstream of growth factors in endothelial cells to promote angiogenesis (39). Whereas hypoxia will activate AMPK signaling in endothelial cells (40), these conditions will lead to a reduction in Akt signaling (9, 41). Thus, we speculate that hypoxia-induced AMPK activation serves to promote a vascularization response under conditions of stress and nutritional deprivation when Akt signaling is down-regulated. This proposed revascularization function of AMPK is consistent with the widely recognized cytoprotective role of this kinase in fuel preservation while the tissue recovers from the ischemic injury. The data reported here support the notion that the LKB1-AMPK signaling axis promotes proangiogenic responses in cultured endothelial cells, including proliferation, migration, survival, eNOS activation and differentiation into network structures. Furthermore, its expression in Tie2-Cre-expressing cells is essential for a robust revascularization response to ischemia in vivo. In contrast to these findings, another group recently reported that adenovirus-mediated overexpression of LKB1 inhibits the formation of networks by HUVECs on Matrigel (8). The reasons for these disparate findings are not clear, but they indicate the need for further in vitro and in vivo investigations to appreciate more fully the role of endothelial cell LKB1 in angiogenesis.
In summary, we demonstrated for the first time that the heterozygous deficiency of LKB1 expression in Tie2-Cre-expressing cells leads to an impaired revascularization response to tissue ischemia in vivo. These results were corroborated by a series of in vitro studies showing that reductions in LKB1 impair endothelial cell morphogenesis, whereas the overexpression of LKB1 stimulates this process. These data indicate that LKB1 plays a role in stress-induced tissue revascularization through its ability to regulate AMPK signaling in postnatal vascular endothelial cells.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grants AG15052, HL91949, and HL81587 (to K. W.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Tables 1 and 2 and Figs. 1–3.
- AMPK
- AMP-activated protein kinase
- eNOS
- endothelial nitric-oxide synthase
- KO
- knock-out
- Ad
- adenovirus
- kd
- kinase-dead
- dn
- dominant negative
- LDBF
- laser Doppler blood flow
- siRNA
- small interfering RNA
- HUVEC
- human umbilical vein endothelial cell
- TUNEL
- terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling
- ca
- constitutively active
- β-gal
- β-galactosidase
- m.o.i.
- multiplicity of infection
- ACC
- acetyl-CoA carboxylase
- HIF-1α
- hypoxia-inducible factor-1α
- MTS
- 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium.
REFERENCES
- 1.Hemminki A., Markie D., Tomlinson I., Avizienyte E., Roth S., Loukola A., Bignell G., Warren W., Aminoff M., Höglund P., Järvinen H., Kristo P., Pelin K., Ridanpää M., Salovaara R., Toro T., Bodmer W., Olschwang S., Olsen A. S., Stratton M. R., de la Chapelle A., Aaltonen L. A. (1998) Nature 391, 184–187 [DOI] [PubMed] [Google Scholar]
- 2.Jenne D. E., Reimann H., Nezu J., Friedel W., Loff S., Jeschke R., Müller O., Back W., Zimmer M. (1998) Nat. Genet. 18, 38–43 [DOI] [PubMed] [Google Scholar]
- 3.Hemminki A., Tomlinson I., Markie D., Järvinen H., Sistonen P., Björkqvist A. M., Knuutila S., Salovaara R., Bodmer W., Shibata D., de la Chapelle A., Aaltonen L. A. (1997) Nat. Genet. 15, 87–90 [DOI] [PubMed] [Google Scholar]
- 4.Bardeesy N., Sinha M., Hezel A. F., Signoretti S., Hathaway N. A., Sharpless N. E., Loda M., Carrasco D. R., DePinho R. A. (2002) Nature 419, 162–167 [DOI] [PubMed] [Google Scholar]
- 5.Miyoshi H., Nakau M., Ishikawa T. O., Seldin M. F., Oshima M., Taketo M. M. (2002) Cancer Res. 62, 2261–2266 [PubMed] [Google Scholar]
- 6.Shackelford D. B., Vasquez D. S., Corbeil J., Wu S., Leblanc M., Wu C. L., Vera D. R., Shaw R. J. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 11137–11142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ylikorkala A., Rossi D. J., Korsisaari N., Luukko K., Alitalo K., Henkemeyer M., Mäkelä T. P. (2001) Science 293, 1323–1326 [DOI] [PubMed] [Google Scholar]
- 8.Xie Z., Dong Y., Zhang J., Scholz R., Neumann D., Zou M. H. (2009) Mol. Cell. Biol. 29, 3582–3596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagata D., Mogi M., Walsh K. (2003) J. Biol. Chem. 278, 31000–31006 [DOI] [PubMed] [Google Scholar]
- 10.Shibata R., Ouchi N., Kihara S., Sato K., Funahashi T., Walsh K. (2004) J. Biol. Chem. 279, 28670–28674 [DOI] [PubMed] [Google Scholar]
- 11.Ouchi N., Shibata R., Walsh K. (2005) Circ. Res. 96, 838–846 [DOI] [PubMed] [Google Scholar]
- 12.Kudo N., Barr A. J., Barr R. L., Desai S., Lopaschuk G. D. (1995) J. Biol. Chem. 270, 17513–17520 [DOI] [PubMed] [Google Scholar]
- 13.Chen Z. P., Mitchelhill K. I., Michell B. J., Stapleton D., Rodriguez-Crespo I., Witters L. A., Power D. A., Ortiz de Montellano P. R., Kemp B. E. (1999) FEBS Lett. 443, 285–289 [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y., Lee T. S., Kolb E. M., Sun K., Lu X., Sladek F. M., Kassab G. S., Garland T., Jr., Shyy J. Y. (2006) Arterioscler. Thromb. Vasc. Biol. 26, 1281–1287 [DOI] [PubMed] [Google Scholar]
- 15.Ouchi N., Kobayashi H., Kihara S., Kumada M., Sato K., Inoue T., Funahashi T., Walsh K. (2004) J. Biol. Chem. 279, 1304–1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lizcano J. M., Göransson O., Toth R., Deak M., Morrice N. A., Boudeau J., Hawley S. A., Udd L., Mäkelä T. P., Hardie D. G., Alessi D. R. (2004) EMBO J. 23, 833–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Witczak C. A., Sharoff C. G., Goodyear L. J. (2008) Cell Mol. Life Sci. 65, 3737–3755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stahmann N., Woods A., Carling D., Heller R. (2006) Mol. Cell. Biol. 26, 5933–5945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Londesborough A., Vaahtomeri K., Tiainen M., Katajisto P., Ekman N., Vallenius T., Mäkelä T. P. (2008) Development 135, 2331–2338 [DOI] [PubMed] [Google Scholar]
- 20.Fujio Y., Walsh K. (1999) J. Biol. Chem. 274, 16349–16354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Noga A. A., Soltys C. L., Barr A. J., Kovacic S., Lopaschuk G. D., Dyck J. R. (2007) Am. J. Physiol. Heart Circ. Physiol. 292, H1460–H1469 [DOI] [PubMed] [Google Scholar]
- 22.Zhou J., Huang W., Tao R., Ibaragi S., Lan F., Ido Y., Wu X., Alekseyev Y. O., Lenburg M. E., Hu G. F., Luo Z. (2009) Oncogene 28, 1993–2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shaw R. J., Lamia K. A., Vasquez D., Koo S. H., Bardeesy N., Depinho R. A., Montminy M., Cantley L. C. (2005) Science 310, 1642–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galasso G., Schiekofer S., Sato K., Shibata R., Handy D. E., Ouchi N., Leopold J. A., Loscalzo J., Walsh K. (2006) Circ. Res. 98, 254–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohashi K., Ouchi N., Sato K., Higuchi A., Ishikawa T. O., Herschman H. R., Kihara S., Walsh K. (2009) Mol. Cell. Biol. 29, 3487–3499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skurk C., Maatz H., Rocnik E., Bialik A., Force T., Walsh K. (2005) Circ. Res. 96, 308–318 [DOI] [PubMed] [Google Scholar]
- 27.Shibata R., Sato K., Pimentel D. R., Takemura Y., Kihara S., Ohashi K., Funahashi T., Ouchi N., Walsh K. (2005) Nat. Med. 11, 1096–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alva J. A., Zovein A. C., Monvoisin A., Murphy T., Salazar A., Harvey N. L., Carmeliet P., Iruela-Arispe M. L. (2006) Dev. Dyn. 235, 759–767 [DOI] [PubMed] [Google Scholar]
- 29.Tiainen M., Vaahtomeri K., Ylikorkala A., Mäkelä T. P. (2002) Hum. Mol. Genet. 11, 1497–1504 [DOI] [PubMed] [Google Scholar]
- 30.Tiainen M., Ylikorkala A., Mäkelä T. P. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 9248–9251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karuman P., Gozani O., Odze R. D., Zhou X. C., Zhu H., Shaw R., Brien T. P., Bozzuto C. D., Ooi D., Cantley L. C., Yuan J. (2001) Mol. Cell 7, 1307–1319 [DOI] [PubMed] [Google Scholar]
- 32.Zhuang Z. G., Di G. H., Shen Z. Z., Ding J., Shao Z. M. (2006) Mol. Cancer Res. 4, 843–849 [DOI] [PubMed] [Google Scholar]
- 33.Arras M., Ito W. D., Scholz D., Winkler B., Schaper J., Schaper W. (1998) J. Clin. Invest. 101, 40–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stabile E., Kinnaird T., la Sala A., Hanson S. K., Watkins C., Campia U., Shou M., Zbinden S., Fuchs S., Kornfeld H., Epstein S. E., Burnett M. S. (2006) Circulation 113, 118–124 [DOI] [PubMed] [Google Scholar]
- 35.Xie Z., Dong Y., Zhang M., Cui M. Z., Cohen R. A., Riek U., Neumann D., Schlattner U., Zou M. H. (2006) J. Biol. Chem. 281, 6366–6375 [DOI] [PubMed] [Google Scholar]
- 36.Sakamoto K., McCarthy A., Smith D., Green K. A., Grahame Hardie D., Ashworth A., Alessi D. R. (2005) EMBO J. 24, 1810–1820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koh H. J., Arnolds D. E., Fujii N., Tran T. T., Rogers M. J., Jessen N., Li Y., Liew C. W., Ho R. C., Hirshman M. F., Kulkarni R. N., Kahn C. R., Goodyear L. J. (2006) Mol. Cell. Biol. 26, 8217–8227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ikeda Y., Sato K., Pimentel D. R., Sam F., Shaw R. J., Dyck J. R., Walsh K. (2009) J. Biol. Chem. 284, 35839–35849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shiojima I., Walsh K. (2002) Circ. Res. 90, 1243–1250 [DOI] [PubMed] [Google Scholar]
- 40.Zou M. H., Hou X. Y., Shi C. M., Kirkpatick S., Liu F., Goldman M. H., Cohen R. A. (2003) J. Biol. Chem. 278, 34003–34010 [DOI] [PubMed] [Google Scholar]
- 41.Zou M. H., Hou X. Y., Shi C. M., Nagata D., Walsh K., Cohen R. A. (2002) J. Biol. Chem. 277, 32552–32557 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.