Abstract
Steroid receptor coactivator-3 (SRC-3) has been demonstrated to regulate lipid metabolism by inhibiting adipocyte differentiation. In the present study, the potential role of SRC-3 in experimental autoimmune encephalomyelitis (EAE), which characterized by inflammatory demyelination in CNS, was examined by analyzing disease progression in SRC-3 deficient (SRC-3−/−) mice. We found that SRC-3 deficiency significantly attenuated the disease severity of EAE along with decreased inflammatory infiltration and demyelination. However, these effects are not caused by inhibition of peripheral T cell response, but by up-regulated expression of peroxisome proliferator-activated receptor (PPAR)-β in CNS, which induced an alternative activation state of microglia in SRC-3−/− mice. These alternatively activated microglia inhibited CNS inflammation through inhibition of pro-inflammatory cytokines and chemokines, such as TNF-α, IFN-γ, CCL2, CCL3, CCL5 and CXCL10, as well as up-regulation of anti-inflammatory cytokine IL-10 and opsonins such as C1qa and C1qb. Moreover, microglia alternative activation promoted myelin regeneration through increased accumulation of oligodendrocyte precursors (OPCs) in white matter and elevated expression of myelin genes in the spinal cords of SRC-3−/− mice. Our results build up a link between lipid metabolic regulation and immune functions, and the modulation of the expression of SRC-3 or PPAR-β may hopefully has therapeutic modality in MS and possibly other neurodegenerative diseases.
Keywords: SRC-3, PPAR-β, alternative activation, microglia, EAE
INTRODUCTION
Lipid has a high concentration in CNS. Accumulating evidence indicates that lipid metabolism is a key event in tissue physiology and cell signaling in many neurological disorders (Adibhatla and Hatcher, 2007). Even subtle perturbations in lipid content of CNS can disrupt their functions and lead to myelin and axon degradation (Adibhatla and Hatcher, 2007; Sobrado et al., 2009; White et al., 2009), while it still remains open how lipid metabolism functions to induce or inhibit various disease progresses. Multiple sclerosis (MS), an inflammatory, demyelinating disease of CNS, affects more than one million people worldwide. Many MS patients have altered plasma lipid profile (Omoúlu et al., 2004); as a result, premises of some diagnostic tests are based on lipid metabolism. With the consequence of change in lipid metabolism even in normal appearing brain tissues, a mild alter in lipid composition could affect the biophysical properties of myelin structure (Wheeler et al., 2008), which may explain the disruption of myelin under imbalanced lipid metabolism. Experimental autoimmune encephalomyelitis (EAE), the commonly used animal model for MS, was reported to be linked with lipid metabolism during disease progression. Genetic deletion of fatty acid amide hydrolase resulted in improved long-term outcome in chronic autoimmune encephalitis (Webb et al., 2008). Besides, it was announced that epidermal fatty acid-binding protein has the ability in potentiating inflammatory responses, and mice deficient for this lipid chaperone have reduced inflammatory reaction, which resulted in impaired Th1 as well as Th17 generation and protection from development of EAE (Li et al., 2009). However, direct evidences of lipid metabolism in the pathogenesis of EAE and the related mechanisms still remain a lot unknown and need to be further elucidated.
The steroid receptor coactivator (SRC)-3 is a member of the p160 family of coactivators that interact with nuclear receptors to enhance their transactivation in a ligand-dependent manner (Xu et al., 2000). Targeted disruption of SRC-3 gene in mice revealed many developmental and physiological functions, such as retardation in growth and development (Coste et al., 2008; Wang et al., 2000), increased insulin sensitivity and adipocyte differentiation (Louet et al., 2006). In addition, SRC-3 has been reported to control the expression of peroxisome proliferator-activated receptors (PPARs), play critical roles in lipid metabolism, controls lipid transportation, usage, and storage (Michalik and Wahli, 2006; Wu et al., 2004; Yu et al., 2007). Recently, a serial of PPARs agonist have been discovered to exhibit therapeutic effects in EAE (Diab et al., 2002; Gocke et al., 2009; Polak et al., 2005). For example, PPAR-β agonist, GW0742, was found to ameliorate EAE through inhibition of microglial inflammatory activation and up-regulation of myelin genes in CNS (Polak et al., 2005).
Due to the importance of PPARs in SRC-3-mediated lipid metabolic regulation and the beneficial activity of specific synthetic agonists of PPARs in inflammatory diseases, we hypothesized that SRC-3 may participate in disease progress of MS through PPARs-mediated functions. Based on this, EAE was induced to investigate the potential role of SRC-3 in the pathogenesis of EAE. We found that SRC-3 deficiency significantly attenuated the disease severity of EAE, which was attributed to the up-regulation of PPAR-β in CNS. Moreover, PPAR-β up-regulation in SRC-3−/− mice induced an alternative activation state of CNS microglia, which in turn modulated CNS inflammation and help promote myelin regeneration. We hope this work will provide new insight into the role of lipid metabolism in neurological diseases.
MATERIALS AND METHODS
Mice
C57BL/6 × 129Sv SRC-3-deficient (SRC-3−/−) mice were generated as described previously (Xu et al., 2000; Yu et al., 2007) and were kept under pathogen-free conditions in the animal center of the Shanghai Jiao Tong University School of Medicine (Shanghai, China). Where indicated, mice were fed with a high-fat diet (45% kJ from fat) for 2 months. Wild-type (SRC-3+/+) littermate control mice were used for comparison in all experiments. All animal experiments complied with the animal protocols approved by the institutional review board of the Institute of Health Sciences (Shanghai, China).
Induction of EAE
Mice (6–8 wk) were immunized s.c. with a synthetic peptide (100 μg) of myelin oligodendrocyte glycoprotein (MOG35–55) (MEVGWYRSPFSRVVHLYRNGK) (GL Biochem). Immunization was performed by mixing MOG peptide in CFA (Sigma-Aldrich) containing 5 mg/ml Mycobacterium tuberculosis H37Ra (Difco). Pertussis toxin (200 ng; List Biological Laboratories) in PBS was administered i.v. on days 0 and 2. Mice were examined daily and scored for disease severity using the standard scale: 0, no clinical signs; 1, limp tail; 2, paraparesis (weakness, incomplete paralysis of one or two hind limbs); 3, paraplegia (complete paralysis of two hind limbs); 4, paraplegia with forelimb weakness or paralysis; 5, moribund or death. After the onset of EAE, food and water were provided on the cage floor. To eliminate any diagnostic bias, scores were assigned by researchers blinded to mouse identity.
Histology
Frozen sections of livers and hearts from mice were stained with Oil Red-O to show triglyceride accumulation. For H&E and Luxol Fast Blue staining, paraffin-embedded sections of spinal cords were stained with H&E or Luxol Fast Blue and examined by light microscopy. Semiquantitative analysis of inflammation and demyelination was performed in a blinded manner as previously described (Bright et al., 1998).
For immunofluorescence staining, sections of spinal cords were incubated with rat anti-mouse CD11b or rabbit anti-mouse NG2 Ab (Chemicon), which were then labeled with FITC-conjugated goat anti-rat IgG (Santa Cruz) or Cy3-conjugated goat anti-rabbit IgG (Jackson Laboratories), counterstained with DAPI, and examined by confocal microscopy (Leica). Oligodendrocyte precursors (OPCs) were quantified as described (Aharoni et al., 2008; Lin et al., 2006). Briefly, immunopositive NG2-expression cells were counted in a field of 0.04 mm2, and only those with nuclei observable by DAPI staining were counted.
Cell proliferation and cytokine determination
Splenocytes isolated from MOG35–55-immunized mice were cultured in triplicate in complete RPMI 1640 medium at a density of 5 × 105 per well in 96-well plates in the presence or absence of MOG peptide at indicated concentration, and were maintained at 37°C in 5% CO2 for 72 h. To measure T cell proliferation, cells were pulsed with 1 μCi [3H] thymidine during the last 16–18 h of culture before harvest and were measured as cpm as detected by a MicroBeta β counter (PerkinElmer). Splenocyte cell culture supernatants collected at 48 h and spinal cord extracts from from SRC-3+/+ and SRC-3−/− EAE mice 15 days post immunization were collected as described previously (Lin et al., 2006), the concentrations of IFN-γ, TNF-α, IL-4 and IL-5 were measured by ELISA (BioSource) according to the manufacturer’s instructions.
Preparation of CNS mononuclear cells and flow cytometry
For the preparation of CNS mononuclear cells, brains and spinal cords from MOG35–55-immunized mice were excised and dissociated for 45 min at 37 °C by digestion with collagenase IV (2 mg/ml, Sigmal-Aldrich) and DNase I (100 μg/ml, Sigmal-Aldrich) in DMEM medium. Dispersed cells were passed through a 70 μm nylon mesh and collected by centrifugation. CNS mononuclear cells were isolated through a Percoll density gradient and collected on the interface fraction between 37% and 70% Percoll. After intensive washing, suspensions of cells were stained with FITC-labeled anti-CD4, PE-conjugated anti-CD11b, APC-conjugated anti-CD45 or PE-Cy7-conjugated anti-CD8 (all from BD PharMingen, San Diego, CA). Isotype controls were used for determination of negative cells. The stained cells were analyzed on a FACSAria instrument (BD Bioscience).
Microglial culture and stimulation
Microglial cell cultures were prepared as previously described (Ponomarev et al., 2005). The purity of microglia were >90% through staining of CD11b and detected by flow cytometry. For stimulation assay, microglia were incubated in DMEM and stimulated with 50 ng/ml IFN-γ (R&D) at 37 °C in a humidified incubator with 5% CO2.
Quantitative real-time PCR
Total RNA was isolated using TRIzol (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions, and reverse transcribed. The mRNA expression was determined by real-time PCR using SYBR Green master mix (ABI, Foster City, CA). Primer sequences are detailed in Table 1. Data were collected and quantitatively analyzed on an ABI Prism 7900 sequence detection system (ABI). Mouse β-actin gene was used as an endogenous control for sample normalization.
Table I.
Specific primers used in real-time PCR analysis
| Gene | Primer | Sequence (5′→3′) |
|---|---|---|
| β-actin | FWa RV |
ATGGAGGGGAATACAGCCC TTCTTTGCAGCTCCTTCGTT |
| SCD1 | FW RV |
GCCGAGCCTTGTAAGTTCTG CCTCCTGCAAGCTCTACACC |
| FAS | FW RV |
GTTGGCCCAGAACTCCTGTA GTCGTCTGCCTCCAGAGC |
| ACC1 | FW RV |
GAAGCCACAGTGAAATCTCG GATGGTTTGGCCTTTCACAT |
| Cpt1 | FW RV |
AGTGGCCTCACAGACTCCAG GCCCATGTTGTACAGCTTCC |
| Acad | FW RV |
TCTTGCGATCAGCTCTTTCA GGTACATGTGGGAGTACCCG |
| PPAR-β | FW RV |
CTGTGGCTGTTCCATGACTG AGATGAAGACAAACCCACGG |
| CCL2 | FW RV |
ATTGGGATCATCTTGCTGGT CCTGCTGTTCACAGTTGCC |
| CCL3 | FW RV |
ACCATGACACTCTGCAACCA GTGGAATCTTCCGGCTGTAG |
| CCL5 | FW RV |
CCACTTCTTCTCTGGGTTGG GTGCCCACGTCAAGGAGTAT |
| CXCL10 | FW RV |
CCTATGGCCCTCATTCTCAC CTCATCCTGCTGGGTCTGAG |
| Ym1/2 | FW RV |
GGGCATACCTTTATCCTGAG CCACTGAAGTCATCCATGTC |
| FIZZ1 | FW RV |
TCCCAGTGAATACTGATGAGA CCACTCTGGATCTCCCAAGA |
| MRC1 | FW RV |
CAGGTGTGGGCTCAGGTAGT TGTGGTGAGCTGAAAGGTGA |
| Arg1 | FW RV |
TTTTTCCAGCAGACCAGCTT CATGAGCTCCAAGCCAAAGT |
| IL-10 | FW RV |
GGTTGCCAAGCCTTATCGGA ACCTGCTCCACTGCCTTGCT |
| IGF-I | FW RV |
GCAACACTCATCCACAATGC TGGATGCTCTTCAGTTCGTG |
| BDNF | FW RV |
GCCTTCATGCAACCGAAGTA TGAGTCTCCAGGACAGCAAA |
| MBP | FW RV |
GCTCCCTGCCCCAGAAGT TGTCACAATGTTCTTGAAGAAATGG |
| PLP | FW RV |
GCCCCTACCAGACATCTAGC AGTCAGCCGCAAAACAGACT |
FW, Forward; RV, Reverse.
Statistical analysis
GraphPad Prism (version 4.0, GraphPad) software was used for statistical analysis. One-way ANOVA, where applicable, was performed to determine whether an overall statistically significant change existed before Student’s t test to analyze the difference between any two groups. Data are shown as means ± SD. A P-value of <0.05 was considered statistical significance.
Results
SRC-3−/− mice exhibited increased level of lipid metabolism
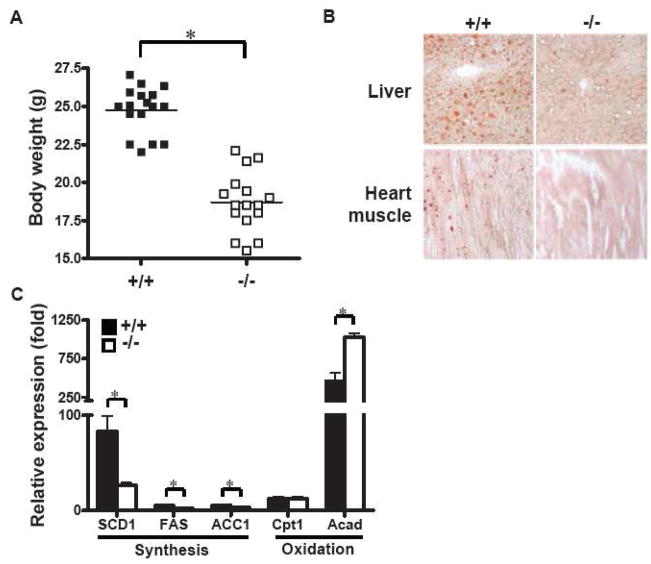
To characterize the relationship between SRC-3 and lipid metabolism, we investigated the differences of body weight and levels of lipid metabolism between C57BL/6 × 129Sv SRC-3−/− mice and their wild-type littermate mice. As illustrated in Fig. 1A, SRC-3−/− mice were lean and had lower body weight compared to SRC-3+/+ mice (18.47 ± 0.46 vs. 24.60 ± 0.40, p < 0.05), which were consistent with the results in C57BL/6 mice (Coste et al., 2008). In addition, after maintaining on a high-fat diet for 2 months, SRC-3+/+ mice developed a massive accumulation of triglyceride, whereas SRC-3−/− mice did not accumulate much triglyceride as indicated by Oil Red-O staining in livers and heart muscles (Fig. 1B). We then analyzed the expression of key enzymes that regulate lipid metabolism in the mice livers, and found that compared with SRC-3+/+ mice, genes responsible for lipid oxidation such as stearoyl-CoA desaturase 1 (SCD1), fatty acid synthase (FAS) and acetyl-CoA carboxylase (ACC1) were significantly down-regulated, whereas genes responsible for lipid synthesis such as Acyl CoA dehydrogenase (Acad) were markedly up-regulated in SRC-3−/− mice. These findings suggest that SRC-3−/− mice exhibit increased level of lipid metabolism compared to their wild-type littermate.
FIGURE 1.
SRC-3−/− mice are lean with increased lipid metabolism rate compared to SRC-3+/+ mice. (A) Body weight of SRC-3+/+ (n=17) and SRC-3−/− mice (n=16). * P < 0.05. (B) Mice were maintained on a high-fat diet for 2 months, representative triglyceride accumulation in livers (×200) and heart muscles (×100) from SRC-3+/+ and SRC-3−/− mice were indicated by Oil-Red O staining. (C) Quantification of mRNA expression of SCD1, FAS, ACC1, Cpt1 and Acad in livers from high-fat diet-fed SRC-3+/+ (solid bars) and SRC-3−/− (open bars).
SRC-3 deficiency attenuated disease severity of EAE
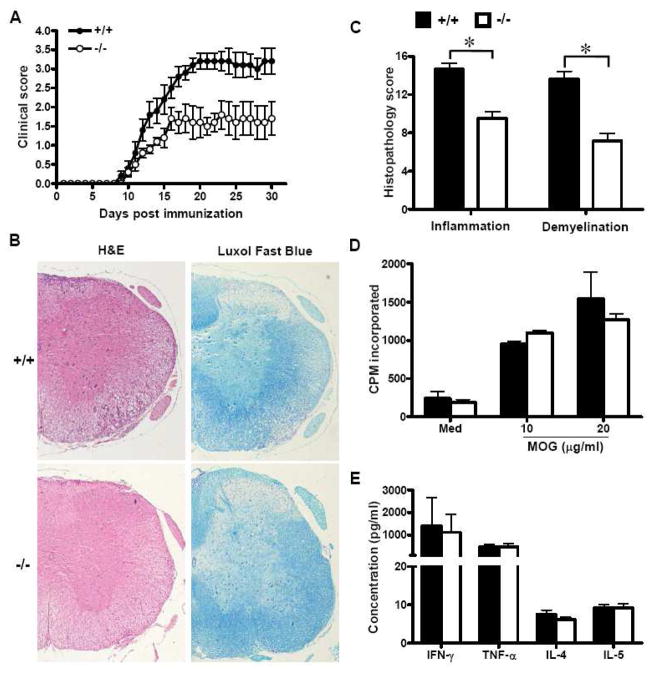
To study the role of SRC-3 in the pathogenesis of EAE, age-matched SRC-3−/− and SRC-3+/+ mice were immunized with MOG35–55 and disease progressions were monitored by clinical assessments. All mice developed typical neurological signs of EAE such as loss of tail tonicity and paralysis starting at about day 9 post immunization (Fig. 2A and Table 2), indicating that the priming phase of the disease was unaltered. SRC-3+/+ mice followed a typical disease course and developed signs of severe paralysis, reaching a mean maximum clinical score of 3.5 (Table 2). In contrast, SRC-3−/− mice developed considerably less severe disease, showing only mild paralysis, and reached a mean maximum clinical score of 2.3. These results indicate that SRC-3 has a pathogenic function in EAE.
FIGURE 2.
SRC-3 deficiency attenuates MOG35-55-induced EAE without impairment of peripheral T cell response. (A) Clinical course and severity of EAE were assessed after immunization with MOG35–55 in SRC-3+/+ (n=10, closed circle) and SRC-3−/− mice (n=10, open circle). Mice were monitored and scored daily as described in Materials and Methods. Data are representative of three independent experiments. (B) Histopathology of spinal cord tissue sections from SRC-3+/+ and SRC-3−/− EAE mice by H&E and Luxol Fast Blue staining (×40). (C) Semiquantitative analysis of inflammation and demyelination in spinal cords from SRC-3+/+ (solid bars) and SRC-3−/− (open bars) EAE mice. *P < 0.05. (D) Splenocytes were isolated from SRC-3+/+ (solid bars) and SRC-3−/− (open bars) EAE mice 15 days post immunization and examined ex vivo for proliferation in the absence (Med) or indicated concentration of the MOG35-55 peptide. Data are presented as mean ± SD in triplicates. (E) Splenocytes from SRC-3+/+ (solid bars) and SRC-3−/− (open bars) EAE mice 15 days post immunization were challenged with the MOG35-55 peptide (20 μg/ml), and culture supernatants were collected at 48 h for cytokine measurement by ELISA. Data are presented as mean mean ± SD of triplicate samples.
Table II.
EAE in SRC-3−/− and wild-type micea
| SRC-3+/+ | SRC-3−/− | |
|---|---|---|
| Incidence | 10/10 (100%) | 4/10 (20%)* |
| Day of onset | 9.8 ± 0.4 | 10.6 ± 0.4 |
| Maximal score | 3.5 ± 0.2 | 2.3 ± 0.3* |
SRC-3−/− and SRC-3+/+ mice in which EAE was induced by s.c. immunization with MOG35–55.
P < 0.05
To analyze pathological differences between SRC-3−/− and wild-type mice in EAE, histologic staining was performed to examine CNS inflammatory infiltration and demyelination. The results revealed spinal cord sections from SRC-3−/− mice exhibited significantly less inflammatory infiltration as well as demyelinated lesions, compared to SRC-3+/+ mice. Therefore, mice absent of SRC-3 appear to be more resistant to EAE, leading to reduced inflammatory infiltration and myelin damage in CNS.
Less pro-inflammatory expression in SRC-3-deficient CNS
The efficient activation of T lymphocytes is a critical requirement for the induction of CNS inflammation and pathology in EAE. To determine whether the deficiency of SRC-3 affects peripheral T cell proliferation and activation, splenocytes were isolated from SRC-3−/− and wild-type EAE mice, and characterized for T cell reactivity and cytokine profile in response to in vitro challenge by the disease-eliciting MOG peptide. Results revealed that the activation and proliferation status of encephalitogenic T cells between SRC-3−/− and wild-type mice were comparable with each other, as indicted by FACS analysis of activation markers in T cells and [3H] thymidine incorporation (Supp. Info. Fig. 1 and Fig. 2D). In addition, there was no significant difference in the amounts of cytokines (IFN-γ, TNF-α, IL-4 and IL-5) released by T cells obtained from SRC-3+/+ and SRC-3−/− mice (Fig. 2E). Therefore, SRC-3 deficiency appeared unable to inhibit peripheral encephalitogenic T cell response, implying that the observed protection from EAE in SRC-3−/− mice is not caused by directly inhibition of T cell response in the peripheral.
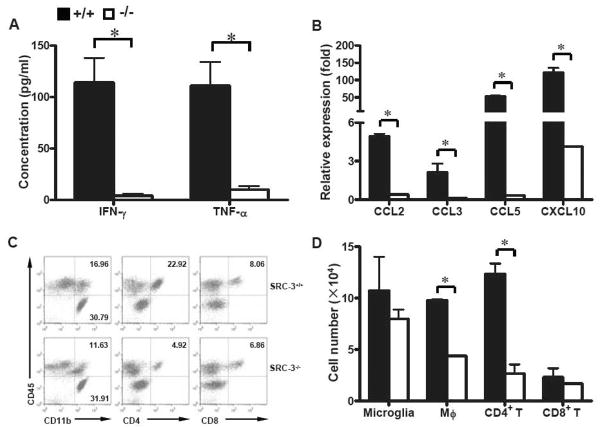
As SRC-3−/− mice had normal peripheral immune responses, we hypothesized that the resistance of these mice to EAE pathology might be due to the impairment of pro-inflammatory activity in CNS. It is clear that pro-inflammatory cytokines and chemokines, such as IFN-γ, TNF-α, CCL2, CCL3, CCL5 and CXCL10, play pivotal roles in establishment and maintenance of EAE (Carter et al., 2007; Gerard and Rollins, 2001; Kassiotis et al., 2001; Lees et al., 2008). Therefore, we examined the expression of these pro-inflammatory cytokines and chemokines in spinal cords of EAE mice. We found that in SRC-3−/− mice, as compared to SRC-3+/+ mice, the production of IFN-γ and TNF-α decreased significantly in the spinal cords (Fig. 3A), suggestive of decreased infiltration of inflammatory cells in CNS. Expectedly, real-time PCR analysis revealed significantly down-regulated expression of CCL2, CCL3, CCL5 and CXCL10 in SRC-3−/− mice as compared to SRC-3+/+ mice (Fig. 3B). Accordingly, the infiltration of macrophages (CD11b+CD45hi) and CD4+ T cells (CD4+CD45hi) in CNS of MOG35–55-immunized SRC-3−/− mice were significantly decreased as compared with that in SRC-3+/+ mice. Meanwhile, the number of microglia (CD11b+CD45lo) in CNS and CD8+ T cell (CD8+CD45hi) infiltration displayed no significant difference in both types of EAE mice (Fig. 3C, D). These findings collectively suggested that the deficiency of SRC-3 decreased the expression of key mediators involved in CNS inflammation, leading to reduced inflammatory infiltration in CNS during the pathogenesis of EAE.
FIGURE 3.
Ablation of SRC-3 inhibits the expression of pro-inflammatory mediators and reduces inflammatory infiltration in CNS. (A) ELISA analysis of IFN-γ and TNF-α in spinal cords from SRC-3+/+ (solid bars) and SRC-3−/− (open bars) EAE mice 15 days post immunization. Data are presented as mean ± SD in triplicates. * P < 0.05. (B) Quantification of mRNA expression of CCL2, CCL3, CCL5 and CXCL10 in spinal cords from SRC-3+/+ (solid bars) and SRC-3−/− (open bars) EAE mice 15 days post immunization. The data were analyzed by real-time PCR using β-actin as a reference. The values represent mean ± SD in triplicates. * P < 0.05. (C) CNS mononuclear cells from SRC-3+/+ and SRC-3−/− EAE mice 15 days post immunization were isolated by Percoll gradient and assessed by flow cytometry. (D) Quantification of absolute numbers of CNS-infiltrating cells in SRC-3+/+ (solid bars) and SRC-3−/− (open bars) EAE mice 15 days post immunization. Results are presented as mean ± SD of three independent experiments.
SRC-3 deficiency promoted PPAR-β-mediated alternative activation of microglia
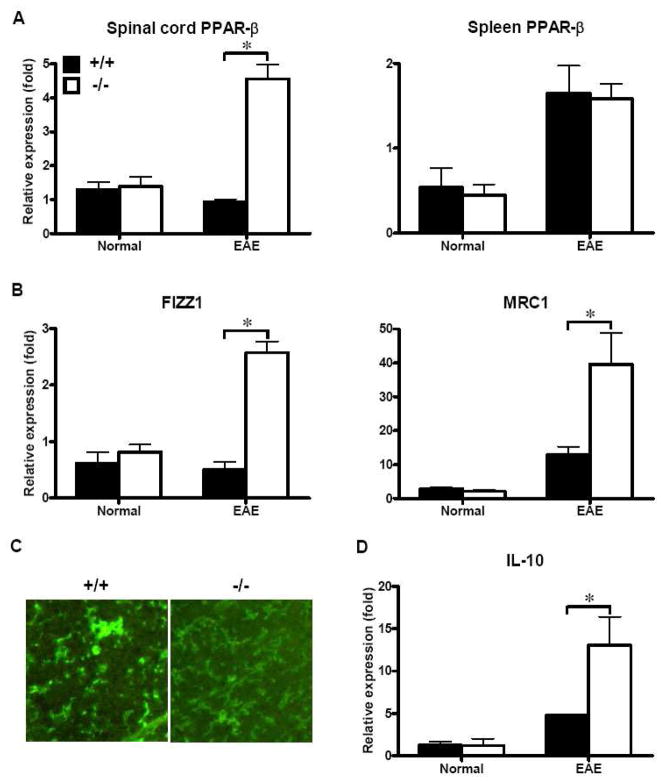
PPARs were found to play critical roles in the regulation of lipid metabolism and inflammatory activities (Bensinger and Tontonoz, 2008), and elevated PPARs activity up-regulates expression of genes involved in cholesterol trafficking while down-regulates expression of genes encoding pro-inflammatory proteins (Makowski et al., 2005). Previous reports have indicted that SRC-3 deficiency promote the activation of PGC-1α, which play important roles in the up-regulation of PPARs (Coste et al., 2008; Evans et al., 2004; Pan et al., 2009). As SRC-3−/− mice exhibit decreased expression of pro-inflammatory cytokines and chemokines in CNS during the process of EAE, we hypothesized that the suppressive effect of EAE in SRC-3−/− mice might be mediated by up-regulation of PPARs. Because SRC-3−/− mice are observed to be lean, and PPAR-β is mainly responsible for fat-burning through modulation of lipid metabolic genes (Evans et al., 2004). We then analyzed the expression of PPAR-β in spinal cords from EAE mice. As shown in Fig. 4A, the mRNA expression of PPAR-β was comparable between SRC-3−/− and wide-type mice in spinal cords and spleens under normal conditions, but was specifically up-regulated in spinal cords of SRC-3−/− mice as compared with those of wild-type mice during EAE disease process. However, this up-regulation of PPAR-β was not detected in spleens in SRC-3−/− mice. These results confirmed our hypothesis that SRC-3 deficiency lead to up-regulation of CNS PPAR-β, through which inhibit CNS inflammation in EAE.
FIGURE 4.
Up-regulation of alternative activation genes through PPAR-β in CNS of SRC-3−/− EAE mice. (A) Quantification of mRNA expression of PPAR-β in spinal cords and spleen from SRC-3+/+ (solid bars) and SRC-3−/− (open bars) normal mice and EAE mice 25 days post immunization. (B, D) Quantification of mRNA expression of FIZZ1, MRC1 and IL-10 in spinal cords from SRC-3+/+ (solid bars) and SRC-3−/− (open bars) normal mice and EAE mice 25 days post immunization. The data were analyzed by real-time PCR using β-actin as a reference. The values represent mean ± SD in triplicates. * P < 0.05. (C) Representative images of CD11b expression showing microglial activation in the lesion site of spinal cords from SRC-3+/+ and SRC-3−/− mice (×200).
Recently, it has been demonstrated that PPAR-β promoted alternative activation of resident macrophages in adipose tissue and liver, respectively (Kang et al., 2008; Odegaard et al., 2008). Thus, we examined the expression of some phenotypic markers, such as Ym1/2, FIZZ1 and MRC1, for microglia alternative activation under PPAR-β up-regulation in CNS. We found that there was no significant difference of the expression of these markers in spinal cords from SRC-3−/− and wide-type mice under normal conditions. However, when mice were induced with EAE, the expression of these markers in spinal cords from SRC-3−/− mice was significantly increased as compared to SRC-3+/+ mice (Fig. 4B). In addition, immunofluorescence analysis revealed that microglia in SRC-3−/− EAE mice showed more ramified morphology distinguished from that in SRC-3+/+ EAE mice, which exhibited more round soma (Fig. 4C), a typical hallmark of reactive microglia (Ling and Wong, 1993; Thomas, 1992). Moreover, we found that IL-10, which was reported to be produced by alternatively activated macrophages to suppress inflammation (Gordon, 2003), was significantly up-regulated in SRC-3−/− mice as compared with that SRC-3+/+ mice under EAE conditions (Fig. 4D). These together with impaired inflammatory profile in CNS shown in Fig. 3 implying that microglia might be induced to an alternative activation state in SRC-3−/− mice during the process of EAE.
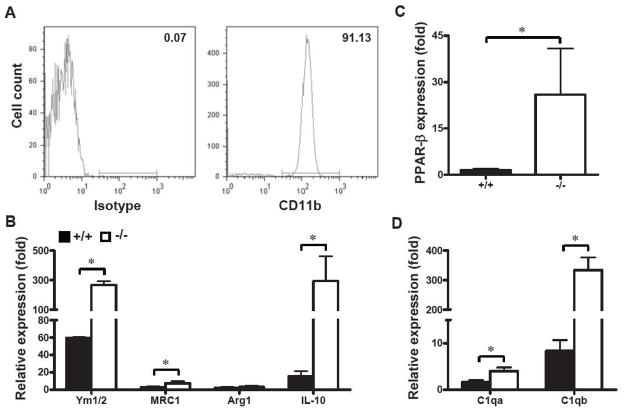
To further validate the concept that SRC-3 deficiency promoted PPAR-β-mediated alternative activation of microglia during the pathogenesis of EAE, we isolated CNS microglia from unimmunized adult SRC-3+/+ and SRC-3−/− mice and stimulated by IFN-γ in vitro. The results revealed that there is no significant difference of the expression of alternative activation markers between unstimulated SRC-3+/+ and SRC-3−/− microglia (data not shown). However, IFN-γ significantly elevated the expression of Ym1/2 and MRC1 (FIZZ1 not detected), most prominently the anti-inflammatory cytokine IL-10 (>10 times) in SRC-3−/− microglia as compared with that in SRC-3+/+ microglia (Fig. 5B). Accordingly, SRC-3−/− microglia exhibited higher PPAR-β expression upon IFN-γ stimulation than that in SRC-3+/+ microglia (Fig. 5C). In addition, we fount that SRC-3 deficiency significantly up-regulated the expression of opsonins such as complement component-1qa (C1qa) and C1qb in microglia (Fig. 5D), which is in accordance with the most recently published data that PPAR-β promote opsonins-mediated phagocytosis of apoptotic cells and production of anti-inflammatory cytokine IL-10 in macrophages to counteract autoimmune disease (Mukundan et al., 2009). These results suggested that up-regulation of opsonins could be one of the mechanisms by which alternatively activated microglia promote tolerance of EAE in SRC-3−/− mice.
FIGURE 5.
SCR-3 deficiency promotes PPAR-β-mediated alternative activation of microglia. (A) The purity of isolated microglia from naïve mice CNS were assessed by staining CD11b through flow cytometry. (B, C, D) Microglia were stimulated by 50 ng/ml IFN-γ for 24 h, and the mRNA expression of Ym1/2, MRC1, Arg1, IL-10, PPAR-β, C1qa and C1qb in SRC-3+/+ (solid bars) and SRC-3−/− (open bars) microglia were quantified through real-time PCR using β-actin as a reference. The values represent mean ± SD in triplicates. * P < 0.05.
Th2 cytokines, such as IL-4 and IL-13, have been reported to up-regulate PPAR-β expression, and then promote alternative activation of adipose tissue resident macrophages and Kupffer cells (Kang et al., 2008; Odegaard et al., 2008). However, we found here that the expression of IL-4 as well as IL-13, was not increased in spinal cords of SRC-3−/− EAE mice (Supp. Info. Fig. 2), indicating that PPAR-β-mediated alternative activation of microglia in SRC-3−/− mice was independent of Th2 cytokines, but the deficiency of SRC-3. These results collectively suggest that SRC-3 deficiency specifically up-regulate CNS PPAR-β, which induce alternatively activated microglia to counteract CNS inflammation during the process of EAE.
Promoted neuroprotective effect in SCR-3−/− mice through PPAR-β-mediated alternative activation of microglia
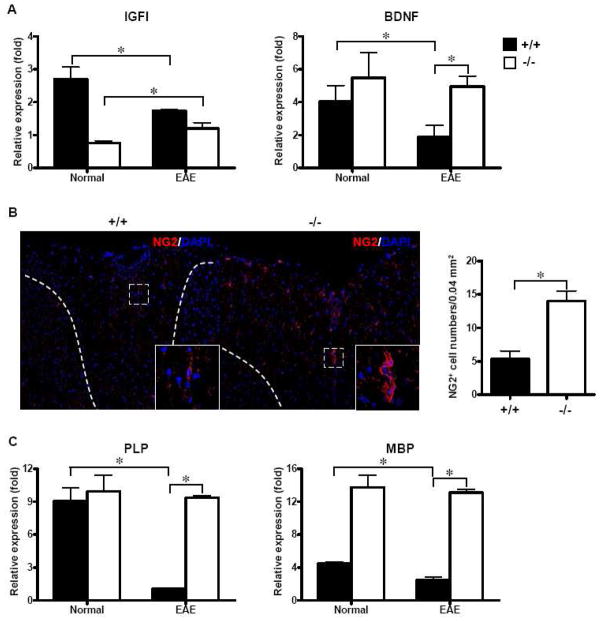
The induction of alternatively activated microglia/macrophage has been demonstrated to limit inflammation through reduced pro-inflammatory cytokine secretion and synthesis of trophic factors, which promote tissue regeneration (Gordon, 2003; Ponomarev et al., 2007). Thus, we examined the expression of neurotropic factors, such as insulin-like growth factor-I (IGF-I) and brain-derived neurotrophic factor (BDNF) in spinal cords. As shown in Fig. 6A, both IGF-I and BDNF decreased significantly in SRC-3+/+ EAE mice, while IGF-I expression in SRC-3−/− mice increased significantly upon EAE induction. Although in SRC-3−/− EAE mice, BDNF expression was not up-regulated, its level was significantly higher than that in SRC-3+/+ mice under EAE condition.
FIGURE 6.
SCR-3 deficiency exhibits promoted neuroprotective effect during the pathogenesis of EAE. (A) Quantification of mRNA expression of IGF-I and BDNF in spinal cords from SRC-3+/+ (solid bars) and SRC-3−/− (open bars) normal mice and EAE mice 25 days post immunization. The data were analyzed by real-time PCR using β-actin as a reference. The values represent mean ± SD in triplicates. * P < 0.05. (B) Immunofluorescence staining of NG2+ OPCs in the white matter of lumbar spinal cords of SRC-3+/+ and SRC-3−/− EAE mice 25 days post immunization (×200). NG2 positive cells in the spinal cords of SRC-3+/+ and SRC-3−/− EAE mice were quantified by counting in a field of 0.04 mm2. (C) Quantification of mRNA expression of MBP and PLP in spinal cords from SRC-3+/+ (solid bars) and SRC-3−/− (open bars) normal mice and EAE mice 25 days post immunization. The data were analyzed by real-time PCR using β-actin as a reference. The values represent mean ± SD in triplicates. * P < 0.05.
The evidence that OPCs are major source of remyelinating oligodendrocyte is compelling, and NG2-expressing OPCs seem to be responsible for producing new myelin sheaths (Franklin and Ffrench-Constant, 2008). As IGF-I and BDNF both were demonstrated to exhibit neuroprotective effects through promotion of NG2+ OPCs differentiation (Butovsky et al., 2006; Girard et al., 2005; Van’t Veer et al., 2009), the status of OPCs in SRC-3−/− and wide-type EAE mice were examined. Results revealed that the number of NG2+ OPCs significantly increased in white matter of spinal cords from SRC-3−/− EAE mice, while fewer were observed in demyelinated lesions of SRC-3+/+ EAE mice (Fig. 6B). These were in accordance with the tendency of IGF-I/BDNF expression in SRC-3−/− and wide-type EAE mice, indicating that the increased accumulation of NG2+ OPCs in demyelinated lesions in spinal cords of SRC-3−/− EAE mice may be result from the up-regulated expression of neurotrophic factors.
Myelin genes, such as myelin basic protein (MBP) and proteolipid protein (PLP), are late markers during oligodendroglial maturation cascade (Hemmer et al., 2002). Previous studies have shown the expression of myelin genes was up-regulated during remyelination, and this early alteration in gene expression is believed to reflect an important step in the generation of new mature oligodendrocytes during remyelination. (Jurevics et al., 2002; Lin et al., 2006). In this study, we found that the expression of these myelin genes decreased significantly in SRC-3+/+ mice upon EAE induction, exhibit similar trends as neurotrophic factors. Moreover, the expression levels of these myelin genes in spinal cords from SRC-3−/− mice were significantly higher than that from SRC-3+/+ mice under EAE condition (Fig. 6C). Therefore, SRC-3 deficiency exhibited neuroprotective effect in EAE disease process through up-regulation of neurotrophic factors, which may be produced by PPAR-β-induced alternatively activated microglia.
Discussion
Lipid metabolic regulation has long been found to be associated with the pathogenesis of MS (Omoúlu et al., 2004; Wheeler et al., 2008). During periods of disease inactivity, axons may partially normalize CNS lipid profile, but the inability to normalization may contribute to the onset and progressive damage in MS. However, the mechanisms by which lipid metabolism functions in MS or EAE, the commonly used animal model for MS, still need to be elucidated.
SRC-3 has been reported to play important roles in many developmental and physiological processes especially lipid metabolism (Coste et al., 2008; Louet et al., 2006; Wang et al., 2000; Xu et al., 2000). In this study, we found that genetic ablation of SRC-3 in mice exhibited up-regulated lipid metabolism. Additionally, SRC-3 deficiency significantly attenuated disease severity of EAE with inhibited inflammatory infiltration, and reduced demyelination in the CNS. However, the activation and proliferation of peripheral encephalitogenic T cells was not impaired in SRC-3−/− EAE mice as compared with that in SRC-3+/+ EAE mice. But the expression of pro-inflammatory cytokines and chemokines in CNS were significantly decreased in SRC-3−/− mice, suggesting that the attenuation of EAE in SRC-3−/− mice was not caused by an impairment of periphery T cell response, but by modulating the inflammatory environment in CNS.
Recently, it has been reported that SRC-3 promoted the expression of the prime PGC-1α acetyltransferase GCN5, which facilitates the acetylation and the consecutive inactivation of PGC-1α (Coste et al., 2008; Lerin et al., 2006). Genetic deletion of SRC-3 was found to reduce GCN5 acetylation of PGC-1α and lead to its activation, which improve insulin sensitivity and protect against obesity (Coste et al., 2008). As a transcriptional co-activatior, PGC-1α could positively regulate the expression of PPAR-β and PPAR-γ (Evans et al., 2004; Pan et al., 2009). In addition, PPARs are major modulators that regulate lipid metabolism, among which PPAR-β is mainly responsible for burning the fat through modulation of lipid metabolic genes (Evans et al., 2004; Lee et al., 2006). Moreover, up-regulation of PPAR-β was found to promote tolerance in autoimmune diseases (Mukundan et al., 2009; Polak et al., 2005). Expectedly, we found that the expression of PPAR-β was specifically up-regulated in spinal cord of SRC-3−/− mice, but not in SRC-3+/+ mice under EAE condition. PPAR-β has diverse functions, not only regulate lipid metabolism, but also exhibit anti-inflammatory effect and promoting tissue repair and regeneration activities (Michalik and Wahli, 2006). It was reported that PPAR-β was stimulated upon the pro-inflammatory cytokine produced by injured cells after tissue damage, and PPAR-β-null mice exhibit impaired function of tissue repair (Michalik and Wahli, 2006; Tan et al., 2001). Moreover, PPAR-β agonist was found to ameliorate EAE not by inhibition of peripheral T cell response, but by modulation of local environment in CNS (Polak et al., 2005), which was consistent with our results. These findings together suggest that PPAR-β may act as a stress-induced molecule that counteracts tissue damage where inflammation occurs. However, SRC-3 could negatively regulate the expression of PPAR-β through GCN5-mediated acetylation of PGC-1α, which was confirmed by limited expression of PPAR-β in CNS from SRC-3+/+ EAE mice, in which leading to persisted and uncontrolled inflammation and aggravated CNS damage. In contrast, the expression of PPAR-β was significantly up-regulated in SRC-3−/− EAE mice, in which CNS inflammation and damage was attenuated.
Although PPAR-β agonist exhibit protective effect in EAE, the mechanism of which is far from uncovered. Recently, it has been demonstrated that PPAR-β played a prime role in inducing alternative activation of adipose tissue resident macrophages and liver Kupffer cells (Kang et al., 2008; Odegaard et al., 2008). It is known that alternatively activated macrophages limit tissue damage and promote tissue repair under inflammation (Gordon, 2003). In autoimmune diseases as EAE, CNS resident macrophages, microglia, are over-activated in response to injury signals, together with infiltrated macrophages, produce large amount of neurotoxic pro-inflammatory substances, which increased myelin destruction and uncontrolled inflammation in CNS. (Becher et al., 2000; Carson, 2002). In addition, modulation of microglial activities could effectively suppress the disease severity of EAE (Butovsky et al., 2006; Heppner et al., 2005). Here we demonstrat that microglia in SRC-3−/− EAE mice are induced to an alternative activation state upon PPAR-β up-regulation, with phenotypic and functional changes including decreased pro-inflammatory cytokines and chemokines production, and promoted anti-inflammatory cytokines and alternatively activated markers expression. Moreover, we found SRC-3−/− microglia showed higher expression of oposinins than SRC-3+/+ microglia upon IFN-γ stimulation. Oposinins, such as C1qa and C1qb, are major modulators responsible for complement-mediated phagocytosis and clearance of apoptotic cells by macrophages. Most recently, macrophage PPAR-β has been found to sense the apoptotic signals and up-regulate the expression of oposinins to promote the phagocytosis and clearance of apoptotic cells, thus induce the tolerance in autoimmune disease (Mukundan et al., 2009). Thus, elevated expression of oposinins in SRC-3−/− microglia and oposinins-mediated clearance function of apoptotic cell could be another mechanism that synergistically alleviated EAE pathology, or another property of PPAR-β-induced alternatively activated microglia or maybe other type macrophages.
In EAE, inflammatory damage to myelin and oligodendrocytes is the major cause of demyelination. To minimize the damaging potential of over-activated microglia, maintaining their ability to promote CNS repair may have therapeutic potential in EAE disease process (Popovich and Longbrake, 2008). In this study, we found SRC-3 deficiency promoted the expression of IGF-I and BDNF, which may be due to PPAR-β-induced alternatively activated microglia. It is known that neurotrophic factors, such as IGF-I and BDNF, play important roles in promoting OPCs activation, recruitment, and differentiate into remyelinating oligodendrocytes, which are critical for remyelination (Franklin and Ffrench-Constant, 2008). In response to up-regulation of IGF-I and BDNF, NG2+ OPCs accumulation in CNS white matter and the expression of myelin genes were significantly increased in SRC-3−/− EAE mice. These results could explain the neuroprotective effect of PPAR-β-induced alternatively activated microglia, which up-regulated neurotrophic factors, together with more energy that produced by PPAR-β-induced up-regulation of lipid metabolism, to promote CNS repair in SRC-3−/− EAE mice.
We conclude from our findings that SRC-3 deficiency attenuated the disease severity of EAE through PPAR-β up-regulation, which induce an alternative activation state of microglia. These alternatively activated microglia inhibited the production of pro-inflammatory cytokines and chemokines, and increased the expression of anti-inflammatory cytokine, oposinins and neurotrophic factors, which help promote myelin regeneration through increased OPCs accumulation in demyelinated regions and elevated myelin gene expression. More importantly, the present study uncovered the possible mechanism by which PPAR-β promotes tissue repair in response to injury, and build up a link between lipid metabolic regulation and immune function. The modulations of the expression of SRC-3 and/or PPAR-β may have therapeutic modality in MS and possibly other neurodegenerative diseases.
Supplementary Material
Acknowledgments
We express our gratitude to Dr. Sheri M. Skinner (University of Texas Medical School at Houston, Houston, TX) for critical review of the manuscript.
This work was supported by grants from the Ministry of Science and Technology of China (2010CB945600), Innovation Fund of New Drug 2009ZX09503-024, National Natural Science Foundation of China (30670911, 30873045, 30901317 and 30971385), Science and Technology Commission of Shanghai Municipality Programs (074119634, 08JC1413300, 07JC14070 and 09ZR1416100), and Leading Academic Discipline Project of Shanghai Municipal Education Commission (J50207).
Abbreviations
- MS
multiple sclerosis
- EAE
experimental autoimmune encephalomyelitis
- SRC
steroid receptor coactivator
- PPAR
peroxisome proliferator-activated receptor
- MOG
myelin oligodendrocyte glycoprotein
- OPCs
oligodendrocyte precursors
- IGF-I
Insulin-like growth factor-I
- BDNF
brain-derived neurotropic factor
- MBP
myelin basic protein
- PLP
proteolipid protein
Footnotes
Financial disclosures: The authors have no financial conflict of interest.
References
- Adibhatla RM, Hatcher JF. Role of lipids in brain injury and diseases. Future Lipidol. 2007;2:403–422. doi: 10.2217/17460875.2.4.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharoni R, Herschkovitz A, Eilam R, Blumberg-Hazan M, Sela M, Bruck W, Arnon R. Demyelination arrest and remyelination induced by glatiramer acetate treatment of experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA. 2008;105:11358–11363. doi: 10.1073/pnas.0804632105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becher B, Prat A, Antel JP. Brain-immune connection: immuno-regulatory properties of CNS-resident cells. Glia. 2000;29:293–304. [PubMed] [Google Scholar]
- Bensinger SJ, Tontonoz P. Integration of metabolism and inflammation by lipid-activated nuclear receptors. Nature. 2008;454:470–477. doi: 10.1038/nature07202. [DOI] [PubMed] [Google Scholar]
- Bright JJ, Du C, Coon M, Sriram S, Klaus SJ. Prevention of experimental allergic encephalomyelitis via inhibition of IL-12 signaling and IL-12-mediated Th1 differentiation: an effect of the novel anti-inflammatory drug lisofylline. J Immunol. 1998;161:7015–7022. [PubMed] [Google Scholar]
- Bruck W, Kuhlmann T, Stadelmann C. Remyelination in multiple sclerosis. J Neurol Sci. 2003;206:181–185. doi: 10.1016/s0022-510x(02)00191-0. [DOI] [PubMed] [Google Scholar]
- Butovsky O, Landa G, Kunis G, Ziv Y, Avidan H, Greenberg N, Schwartz A, Smirnov I, Pollack A, Jung S, Schwartz M. Induction and blockage of oligodendrogenesis by differently activated microglia in an animal model of multiple sclerosis. J Clin Invest. 2006;116:905–915. doi: 10.1172/JCI26836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson MJ. Microglia as liaisons between the immune and central nervous systems: functional implications for multiple sclerosis. Glia. 2002;40:218–231. doi: 10.1002/glia.10145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter SL, Müller M, Manders PM, Campbell IL. Induction of the genes for Cxcl9 and Cxcl10 is dependent on IFN-γ but shows differential cellular expression in experimental autoimmune encephalomyelitis and by astrocytes and microglia in vitro. Glia. 2007;55:1728–1739. doi: 10.1002/glia.20587. [DOI] [PubMed] [Google Scholar]
- Coste A, Louet JF, Lagouge M, Lerin C, Antal MC, Meziane H, Schoonjans K, Puigserver P, O’Malley BW, Auwerx J. The genetic ablation of SRC-3 protects against obesity and improves insulin sensitivity by reducing the acetylation of PGC-1α. Proc Natl Acad Sci USA. 2008;105:17187–17192. doi: 10.1073/pnas.0808207105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diab A, Deng C, Smith JD, Hussain RZ, Phanavanh B, Lovett-Racke AE, Drew PD, Racke MK. Peroxisome proliferator-activated receptor-γ agonist 15-deoxy-Δ12,14-prostaglandin J2 ameliorates experimental autoimmune encephalomyelitis. J Immunol. 2002;168:2508–2515. doi: 10.4049/jimmunol.168.5.2508. [DOI] [PubMed] [Google Scholar]
- Evans RM, Barish GD, Wang YX. PPARs and the complex journey to obesity. Nat Med. 2004;10:1–7. doi: 10.1038/nm1025. [DOI] [PubMed] [Google Scholar]
- Franklin RJ, Ffrench-Constant C. Remyelination in the CNS: from biology to therapy. Nat Rev Neurosci. 2008;9:839–855. doi: 10.1038/nrn2480. [DOI] [PubMed] [Google Scholar]
- Gerard C, Rollins BJ. Chemokines and disease. Nat Immunol. 2001;2:108–115. doi: 10.1038/84209. [DOI] [PubMed] [Google Scholar]
- Girard C, Bemelmans AP, Dufour N, Mallet J, Bachelin C, Nait Oumesmar B, Baron Van Evercooren A, Lachapelle F. Grafts of brain-derived neurotrophic factor and neurotrophin 3-transduced primate schwann cells lead to functional recovery of the demyelinated mouse spinal cord. J Neurosci. 2005;25:7924–7933. doi: 10.1523/JNEUROSCI.4890-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gocke AR, Hussain RZ, Yang Y, Peng H, Weiner J, Ben LH, Drew PD, Stuve O, Lovett-Racke AE, Racke MK. Transcriptional modulation of the immune response by peroxisome proliferator-activated receptor-α agonists in autoimmune disease. J Immunol. 2009;182:4479–4487. doi: 10.4049/jimmunol.0713927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- Hemmer B, Archelos JJ, Hartung HP. New concepts in the immunopathogenesis of multiple sclerosis. Nat Rev Neurosci. 2002;3:291–301. doi: 10.1038/nrn784. [DOI] [PubMed] [Google Scholar]
- Heppner FL, Greter M, Marino D, Falsig J, Raivich G, Hövelmeyer N, Waisman A, Rülicke T, Prinz M, Priller J, Becher B, Aguzzi A. Experimental autoimmune encephalomyelitis repressed by microglial paralysis. Nat Med. 2005;11:146–152. doi: 10.1038/nm1177. [DOI] [PubMed] [Google Scholar]
- Jurevics H, Largent C, Hostettler J, Sammond DW, Matsushima GK, Kleindienst A, Toews AD, Morell P. Alterations in metabolism and gene expression in brain regions during cuprizone-induced demyelination and remyelination. J Neurochem. 2002;82:126–36. doi: 10.1046/j.1471-4159.2002.00954.x. [DOI] [PubMed] [Google Scholar]
- Kang K, Reilly SM, Karabacak V, Gangl MR, Fitzgerald K, Hatano B, Lee CH. Adipocyte-derived Th2 cytokines and myeloid PPARδ regulate macrophage polarization and insulin sensitivity. Cell Metab. 2008;7:485–495. doi: 10.1016/j.cmet.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassiotis G, Kranidioti K, Kollias G. Defective CD4T cell priming and resistance to experimental autoimmune encephalomyelitis in TNF-deficient mice due to innate immune hypo-responsiveness. J Neuroimmunol. 2001;119:239–247. doi: 10.1016/s0165-5728(01)00403-9. [DOI] [PubMed] [Google Scholar]
- Lee CH, Olson P, Hevener A, Mehl I, Chong LW, Olefsky JM, Gonzalez FJ, Ham J, Kang H, Peters JM, Evans RM. PPARδ regulates glucose metabolism and insulin sensitivity. Proc Natl Acad Sci USA. 2006;103:3444–3449. doi: 10.1073/pnas.0511253103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees JR, Golumbek PT, Sim J, Dorsey D, Russell JH. Regional CNS responses to IFN-γ determine lesion localization patterns during EAE pathogenesis. J Exp Med. 2008;205:2633–2642. doi: 10.1084/jem.20080155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerin C, Rodgers JT, Kalume DE, Kim SH, Pandey A, Puigserver P. GCN5 acetyltransferase complex controls glucose metabolism through transcriptional repression of PGC-1alpha. Cell Metab. 2006;3:429–438. doi: 10.1016/j.cmet.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Li B, Reynolds JM, Stout RD, Bernlohr DA, Suttles J. Regulation of Th17 differentiation by epidermal fatty acid-binding protein. J Immunol. 2009;182:7625–7633. doi: 10.4049/jimmunol.0804192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W, Kemper A, Dupree JL, Harding HP, Ron D, Popko B. Interferon-γ inhibits central nervous system remyelination through a process modulated by endoplasmic reticulum stress. Brain. 2006;129:1306–1318. doi: 10.1093/brain/awl044. [DOI] [PubMed] [Google Scholar]
- Ling EA, Wong WC. The origin and nature of ramified and amoeboid microglia: a historical review and current concepts. Glia. 1993;7:9–18. doi: 10.1002/glia.440070105. [DOI] [PubMed] [Google Scholar]
- Louet J, Coste A, Amazit L, Tannour-Louet M, Wu R, Tsai SY, Tsai M, Auwerx J, O’Malley BW. Oncogenic steroid receptor coactivator-3 is a key regulator of the white adipogenic program. Proc Natl Acad Sci USA. 2006;103:17868–17873. doi: 10.1073/pnas.0608711103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makowski L, Brittingham KC, Reynolds JM, Suttles J, Hotamisligil GS. The fatty acid-binding protein, aP2, coordinates macrophage cholesterol trafficking and inflammatory activity: macrophage expression of aP2 impacts peroxisome proliferator-activated receptor γ and IκB kinase activities. J Biol Chem. 2005;280:12888–12895. doi: 10.1074/jbc.M413788200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalik L, Wahli W. Involvement of PPAR nuclear receptors in tissue injury and wound repair. J Clin Invest. 2006;116:598–606. doi: 10.1172/JCI27958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukundan L, Odegaard JI, Morel CR, Heredia JE, Mwangi JW, Ricardo-Gonzalez RR, Goh YP, Eagle AR, Dunn SE, Awakuni JU, Nguyen KD, Steinman L, Michie SA, Chawla A. PPAR-delta senses and orchestrates clearance of apoptotic cells to promote tolerance. Nat Med. 2009;15:1266–1272. doi: 10.1038/nm.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odegaard JI, Ricardo-Gonzalez RR, Red Eagle A, Vats D, Morel CR, Goforth MH, Subramanian V, Mukundan L, Ferrante AW, Chawla A. Alternative M2 activation of Kupffer cells by PPARδ ameliorates obesity-induced insulin resistance. Cell Metab. 2008;7:496–507. doi: 10.1016/j.cmet.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omoúlu S, Yardimci S, Oku Z. Body fat distribution and plasma lipid profiles of patients with multiple sclerosis. Turk J Med Sci. 2004;34:43–48. [Google Scholar]
- Pan D, Fujimoto M, Lopes A, Wang YX. Twist-1 is a PPARδ-inducible, negative-feedback regulator of PGC-1α in brown fat metabolism. Cell. 2009;137:73–86. doi: 10.1016/j.cell.2009.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponomarev ED, Maresz K, Tan Y, Dittel BN. CNS-derived interleukin-4 is essential for the regulation of autoimmune inflammation and induces a state of alternative activation in microglial cells. J Neurosci. 2007;27:10714–10721. doi: 10.1523/JNEUROSCI.1922-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponomarev ED, Novikova M, Maresz K, Shriver L, Dittel BN. J Immunol Methods. 2005;300:32–46. doi: 10.1016/j.jim.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Popovich PG, Longbrake EE. Can the immune system be harnessed to repair the CNS? Nat Rev Neurosci. 2008;9:481–493. doi: 10.1038/nrn2398. [DOI] [PubMed] [Google Scholar]
- Polak PE, Kalinin S, Dello Russo C, Gavrilyuk V, Sharp A, Peters JM, Richardson J, Willson TM, Weinberg G, Feinstein DL. Protective effects of a peroxisome proliferator-activated receptor-β/δ agonist in experimental autoimmune encephalomyelitis. J Neuroimmunol. 2005;168:65–75. doi: 10.1016/j.jneuroim.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Sobrado M, Pereira MP, Ballesteros I, Hurtado O, Fernández-López D, Pradillo JM, Caso JR, Vivancos J, Nombela F, Serena J, Lizasoain I, Moro MA. Synthesis of lipoxin A4 by 5-lipoxygenase mediates PPARγ-dependent, neuroprotective effects of rosiglitazone in experimental stroke. J Neurosci. 2009;29:3875–3884. doi: 10.1523/JNEUROSCI.5529-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan NS, Michalik L, Noy N, Yasmin R, Pacot C, Heim M, Flühmann B, Desvergne B, Wahli W. Critical roles of PPAR β/δ in keratinocyte response to inflammation. Genes Dev. 2001;15:3263–3277. doi: 10.1101/gad.207501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van’t Veer A, Du Y, Fischer TZ, Boetig DR, Wood MR, Dreyfus CF. Brain-derived neurotrophic factor effects on oligodendrocyte progenitors of the basal forebrain are mediated through trkB and the MAP kinase pathway. J Neurosci Res. 2009;87:69–78. doi: 10.1002/jnr.21841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Rose DW, Hermanson O, Liu F, Herman T, Wu W, Szeto D, Gleiberman A, Krones A, Pratt K, Rosenfeld R, Glass CK, Rosenfeld MG. Regulation of somatic growth by the p160 coactivator p/CIP. Proc Natl Acad Sci USA. 2000;97:13549–13554. doi: 10.1073/pnas.260463097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb M, Luo L, Ma JY, Tham CS. Genetic deletion of fatty acid amide hydrolase results in improved long-term outcome in chronic autoimmune encephalitis. Neurosci Lett. 2008;439:106–110. doi: 10.1016/j.neulet.2008.04.090. [DOI] [PubMed] [Google Scholar]
- Wheeler D, Bandaru VV, Calabresi PA, Nath A, Haughey NJ. A defect of sphingolipid metabolism modifies the properties of normal appearing white matter in multiple sclerosis. Brain. 2008;131:3092–3102. doi: 10.1093/brain/awn190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AB, Givogri MI, Lopez-Rosas A, Cao H, van Breemen R, Thinakaran G, Bongarzone ER. Psychosine accumulates in membrane microdomains in the brain of krabbe patients, disrupting the raft architecture. J Neurosci. 2009;29:6068–6077. doi: 10.1523/JNEUROSCI.5597-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu RC, Qin J, Yi P, Wong J, Tsai SY, Tsai MJ, O’Malley BW. Selective phosphorylations of the SRC-3/AIB1 coactivator integrate genomic reponses to multiple cellular signaling pathways. Mol Cell. 2004;15:937–949. doi: 10.1016/j.molcel.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Xu J, Liao L, Ning G, Yoshida-Komiya H, Deng C, O’Malley BW. The steroid receptor coactivator SRC-3 (p/CIP/RAC3/AIB1/ACTR/TRAM-1) is required for normal growth, puberty, female reproductive function, and mammary gland development. Proc Natl Acad Sci USA. 2000;97:6379–6384. doi: 10.1073/pnas.120166297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C, York B, Wang S, Feng Q, Xu J, O’Malley BW. An essential function of the SRC-3 coactivator in suppression of cytokine mRNA translation and inflammatory response. Mol Cell. 2007;25:765–778. doi: 10.1016/j.molcel.2007.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.