Abstract
Qualitative impairments in communication such as delayed language and poor interactive communication skills are fundamental to the diagnosis of autism. Investigations into social communication in adult BTBR T+tf/J (BTBR) mice are needed to determine whether this inbred strain incorporates phenotypes relevant to the second diagnostic symptom of autism, communication deficits, along with its strong behavioral phenotypes relevant to the first and third diagnostic symptoms, impairments in social interactions and high levels of repetitive behavior. The aim of the present study was to simultaneously measure female urine-elicited scent marking and ultrasonic vocalizations in adult male BTBR mice, in comparison to a standard control strain with high sociability, C57BL/6J (B6), for the assessment of a potential communication deficit in BTBR. Adult male BTBR mice displayed lower scent marking and minimal ultrasonic vocalization responses to female urine obtained from both B6 and BTBR females. Lower scent marking and ultrasonic vocalizations in a social setting by BTBR as compared to B6 are consistent with the well-replicated social deficits in this inbred mouse strain. Our findings support the interpretation that BTBR incorporate communication deficits, and suggest that scent marking and ultrasonic vocalizations offer promising measures of interest in social cues that may be widely applicable to investigations of mouse model of autism.
Keywords: autism, mice, communication, social behavior, olfaction, scent marking, ultrasonic vocalizations, USV
Introduction
Recent studies demonstrated that the BTBR T+tf/J (BTBR) inbred strain of mice displays behavioral phenotypes with analogies to all three diagnostic symptoms of autism, including abnormal reciprocal social interactions as juveniles, lack of sociability as adults, and repetitive behavior (Bolivar et al., 2007; McFarlane et al., 2008; Moy et al., 2007; 2008; Yang et al., 2007a; 2007b; 2009). Scattoni et al. (2008) reported that BTBR pups isolated from their mother and littermates displayed an unusual pattern of call categories, including high levels of harmonics, two-syllable, and composite calls, as compared to pups of three other inbred strains, and vocalized more than C57BL/6J (B6) pups. Qualitative impairments in communication such as delayed language and poor communication skills are fundamental to the diagnosis of autism (DSM-IV, 2000; Frith & Happé, 1994; Lord et al., 2001; 2006; Losh & Piven, 2007; Volkmar et al., 2004). Further investigations into communication in adult BTBR are needed to determine the extent to which this inbred strain incorporates phenotypes relevant to the second diagnostic symptom of autism, communication deficits, along with its strong behavioral phenotypes relevant to the first and third diagnostic symptoms, impairments in social behavior and high levels of repetitive behavior.
Mice communicate primarily by using olfactory (Arakawa et al., 2008b; Roberts 2007; Wyatt, 2003) and acoustic signals (Costantini & D’Amato, 2006; Nyby & Whitney, 1978; Portfors, 2007; Whitney & Nyby, 1979). Scent marking, the deposition of urinary pheromone traces in strategic environmental locations, is used by mice to demarcate territories, attract mates, recognize individuals, and maintain family organization. In a Y-maze olfactory discrimination task, subject mice were able to discriminate between mouse species, strains, genders, and individuals (Bowers & Alexander, 1967). Mice use scent marks to recognize the reproductive state of potential partners (Hurst, 1990b). Moreover, scent marks appears to be a key signal through which male mice announce their identity as a negative advertisement, to exclude other adult males from their territory and prevent potential competition (Desjardins et al., 1973; Hurst, 1990a). Scent marks by male mice may also serve as a positive advertisement directed towards females for mate selection. Male mice actively scent mark to adult females (Davies & Bellamy, 1972; Hurst 1989; Maruniak et al., 1974; Reynolds, 1971), more than to adult or juvenile males, and more than to juvenile females (Arakawa et al., 2007).
Adult male mice produce ultrasonic vocalizations when courting and copulating with females (Sales, 1972). Call production is most prominent during initial investigatory behavior, and temporally correlates with male behaviors that indicate a high level of sexual arousal. The emission of ultrasonic vocalizations is considered to convey an important communicative function, namely to attract females, as described in playback experiments (Hammerschmidt et al., 2009; Pomerantz et al., 1973) and devocalization studies (Pomerantz et al., 1973). Female urine alone, in the absence of a female mouse, is sufficient for the induction of ultrasonic vocalizations by male mice (Whitney et al., 1974). Exposure to female urine may offer an ideal paradigm to investigate ultrasonic vocalizations in social settings in mouse models of autism. Several practical advantages are apparent. Recorded ultrasonic vocalizations can be easily attributed to the male, since the presence of other mice is not required. The male ultrasonic vocalization response is specific to female urine, since male urine is ineffective for eliciting calls by male subject mice (Dizinno et al., 1978; Guo & Holy, 2007; Nyby et al., 1977; 1979; 1983; Nyby & Zakeski, 1980; Wang et al., 2008; Whitney et al., 1974). Male ultrasonic vocalizations in response to both females and female urine are highly sensitive to critical social factors such as previous social experience (Dizinno et al., 1978; Guo & Holy, 2007; Maggio et al., 1983; Nyby et al., 1977; 1983; Sipos et al., 1992) and current social status (D’Amato, 1991; Nyby et al., 1976). Ultrasonic vocalization emissions are dependent on the integrity of olfactory structures, as disruption of the vomeronasal system by bulbectomy or by transection of the vomeronasal tract reduced ultrasonic vocalization emission in male mice in response to female urine (Bean et al., 1981).
The aim of the present study was therefore to use female urine-elicited ultrasonic vocalizations and scent marking in adult male mice for the assessment of a potential communication deficit in BTBR, in comparison to a standard control strain with high sociability, B6. In accordance with their previously reported social deficits and repetitive behaviors (Bolivar et al., 2007; McFarlane et al., 2008; Moy et al., 2007; 2008; Scattoni et al., 2008; Yang et al., 2007a; 2007b; 2009), we predicted that BTBR would display less scent marking and ultrasonic vocalizations in response to female urine as compared to B6.
Materials and methods
Animals and housing
Subject mice were adult male C57BL/6J (B6, N = 30) and BTBR T+tf/J (BTBR, N = 30) mice. Breeding pairs were purchased from The Jackson Laboratory (Bar Harbor, ME, USA) and bred at the National Institute of Mental Health in Bethesda, MD, USA. About 2 weeks after pairing for breeding, the females were individually housed and subsequently inspected daily for pregnancy and delivery. After weaning on postnatal day 21, mice were socially housed in groups of 2 – 4 with same-sex partners. All mice were housed in polycarbonate Makrolon cages (369 × 156 × 132 mm, 435 cm2; 1145T; Tecniplast, Milan, Italy). Bedding, paper strips, a nestlet square and a cardboard tube were provided in each cage. Standard rodent chow and water were available ad libitum. The colony room was maintained on a 12:12 light/dark cycle with lights on at 06:00 h, at 20°C temperature and 55 % humidity.
All procedures were conducted in strict compliance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and approved by the National Institute of Mental Health Animal Care and Use Committee.
Experimental Design
Adult male B6 and BTBR mice with previous female experience (subject strain) were exposed to mouse urine obtained from either B6 or BTBR female mice (source of urine). N = 15 adult male mice of each strain were compared in both condition. Mice were approximately 10 – 12 weeks old at the start of behavioral testing.
Previous female experience
In order to provide a standardized prior history of social experience, adult male subject mice were exposed to adult females of their own strain. Each adult male mouse was placed with a female of the same strain (B6 male with B6 female and BTBR male with BTBR female) for 5 min in a clean polycarbonate Makrolon cage (369 × 156 × 132 mm, 435 cm2; 1145T; Tecniplast) with fresh bedding, approximately 1 week before testing.
Urine collection
Subject mice were exposed to fresh urine obtained from B6 or BTBR females. Urine was collected from adult females in estrous by using a method adopted from Nyby et al. (1979). Female donor mice were socially housed in groups of 2 – 4 and were approximately 10 – 12 weeks of age at the time of urine collection. The donor female was gently taken out of its home cage by the tail and held by the base of the tail on the home cage lid. Gentle pressure was applied to lift the back and expose the genital area, to determine the phase of the estrus cycle. The female was scored as in estrus when the vaginal area appeared wide, opened, and red. The act of handling the female in this manner was usually sufficient to cause it to urinate. If the animal did not urinate from this handling, its ventral area was gently stroked in an anterior to posterior direction. Urine was collected in a 1.0 ml Eppendorf tube. 15 μl of fresh female urine was immediately pipetted into the center of an open field.
Test setting
Adult male mice were exposed to female urine samples in an open field (40 × 40 × 30 cm), equipped with a Versamax animal activity monitor (AccuScan Instruments, Inc., Columbus, OH, USA). One sheet of specialized paper (Strathmore Drawing Paper Premium, recycled, microperforated, 400 series; Strathmore Artist Papers, Neenah, WI, USA) that effectively absorbed drops of mouse urine was placed on the floor of the open field. A transparent Plexiglas lid containing 24 holes (diameter: 1.3 cm/hole) was placed on top of the open field to reduce background noise. The test setting was illuminated by red light. Four open fields were used simultaneously in the same testing room.
Test procedure
The adult male mouse was habituated for 60 min to the clean open field, lined with the Strathmore paper and containing some of his own home cage bedding in one corner of the arena. At the end of the habituation period, the mouse was placed back in a clean polycarbonate Makrolon cage (369 × 156 × 132 mm, 435 cm2; 1145T; Tecniplast) with fresh bedding. The home cage bedding and any feces deposited by the mouse were removed from the open field. Scent marks deposited on the paper during habituation were visualized under ultraviolet (UV) light using a UV lamp (Sleeklook Super 18” Black Light-eParty unlimited; Can You Imagine, Chatsworth, CA, USA). Visualized scent marks were outlined using a pencil. 15 μl of female urine was then pipetted onto the center of the Strathmore paper, and the mouse was placed back into the open field for 5 min. The second set of scent marks deposited on the paper during the 5 min exposure to the female urine was visualized under the UV lamp and outlined with a blue colored pen. Prior to each session with a new subject mouse, the open field was cleaned with a 70 % ethanol solution, followed by water and drying with paper towels. Behavioral testing was conducted between 09:00 and 17:00 h during the light phase of the circadian cycle.
Open field activity
Locomotor activity was automatically recorded by the Accuscan software (AccuScan Instruments, Inc., Columbus, OH) during the 60 min habituation session of free exploration in the open field and during the 5 min test session with exposure to the female urine spot. Time spent within an area of 10 cm2 surrounding the female mouse urine spot was automatically recorded using the Versamap option of the Accuscan software (AccuScan Instruments, Inc.). N = 11 during habituation and N = 8 during testing for B6 subjects exposed to B6 female urine, due to data loss.
Scent marking
Urinary scent marks by the subject male mice were scored along with ultrasonic vocalizations within the same test sessions. At the end of each female urine exposure, the marked sheets of Strathmore paper were treated with Ninhydrin spray (LC-NIN-16; TritechForensics Inc., Southport, NC, USA) then left to dry for about 12 h, which allowed the visualization of the urine traces as purple spots. Scent marks appeared as irregular purple spots on the paper, circled either in pencil (subject male’s scent marks during 60 min in the empty open field during habituation) or blue pen (subject male’s scent marks during 5 min in the open field in the presence of female urine). Scent marks were counted as previously described (Arakawa et al., 2008a, b). Briefly, a transparent plastic grid (40 cm2) divided into squares, each 1 cm2, was placed on the top of the sheet of Strathmore paper. The total number of scent marks and the number of scent marks within an area of 10 cm2 around the female urine spot were counted.
Ultrasonic vocalizations
Ultrasonic vocalizations were recorded during the 5 min female urine exposure. Vocal emissions were monitored by an UltraSoundGate Condenser Microphone (CM 16; Avisoft Bioacoustics, Berlin, Germany) placed in the lid of each of the four open fields, 23 cm above the floor. This microphone is sensitive to frequencies of 15 – 180 kHz with a flat frequency response (± 6 dB) between 25 – 140 kHz. Microphones were connected via an Avisoft UltraSoundGate 416 USB Audio device (Avisoft Bioacoustics) to a personal computer, where acoustic data were displayed in real time by Avisoft RECORDER (version 2.97; Avisoft Bioacoustics), and were recorded with a sampling rate of 300,000 Hz in16 bit format.
For acoustical analysis, recordings were transferred to SASLab Pro (version 4.50; Avisoft Bioacoustics) and a fast Fourier transform was conducted (512 FFT length, 100 % frame, Hamming window and 75 % time window overlap). Correspondingly, the spectrograms were produced at 586 Hz of frequency resolution and 0.427 ms of time resolution. An experienced observer counted the total number of ultrasonic vocalizations as well as their numbers in 10 s time bins, to visualize the time course of the ultrasonic vocalization response.
Statistical analysis
Comparisons of subject strains, i.e. BTBR versus B6 male mice, for scent marking and open field activity in the absence of female urine were statistically analyzed using unpaired Student t-tests. To test whether BTBR and B6 differ on scent marking and open field activity in the presence of female urine, a Two-Way ANOVA was performed with the between subject factors of subject strain and source of urine. To compare ultrasonic vocalizations between B6 versus BTBR, a Three-Way ANOVA for Repeated Measures was calculated, using a within subject factor of session time and the between subject factors of subject strain and source of urine. When a significant ANOVA was detected, Bonferroni post-hoc tests were conducted to compare individual means. Finally, Pearson’s product moment statistics were used to run correlation analyses between numbers of scent marks and numbers of ultrasonic vocalizations. A p-value of < 0.050 was considered statistically significant.
Results
Scent marking and open field activity in the absence of female urine
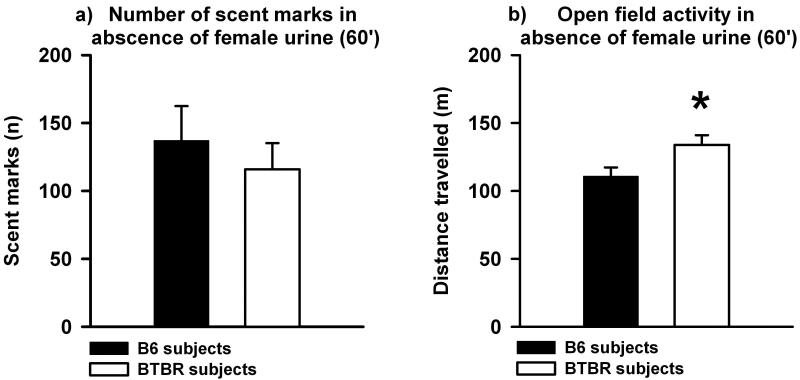
During the 60 min habituation session, the two strains did not differ in the number of scent marks deposited in the clean open field (t58 = 0.656, p = 0.515, Figure 1a) but BTBR had higher locomotor activity as compared to B6 (t54 = −2.364, p = 0.022, Figure 1b), consistent with previous reports (McFarlane et al., 2008; Yang et al., 2009).
Fig. 1. Scent marking and open field activity in adult male C57BL/6J (B6) and BTBR T+tf/J (BTBR) mice in the absence of female urine.
a) BTBR subjects (white bar) and B6 subjects (black bar) did not differ in the number of scent marks deposited in the clean open field during the prior 60 min habituation session in the absence of the female urine spot. b) BTBR had higher exploratory locomotion as compared to B6 during the 60 min habituation session in the clean open field. Data are presented as means ± standard errors of the mean. (* p < 0.050, *** p < 0.001 for B6 versus BTBR).
Scent marking and open field activity in the presence of female urine
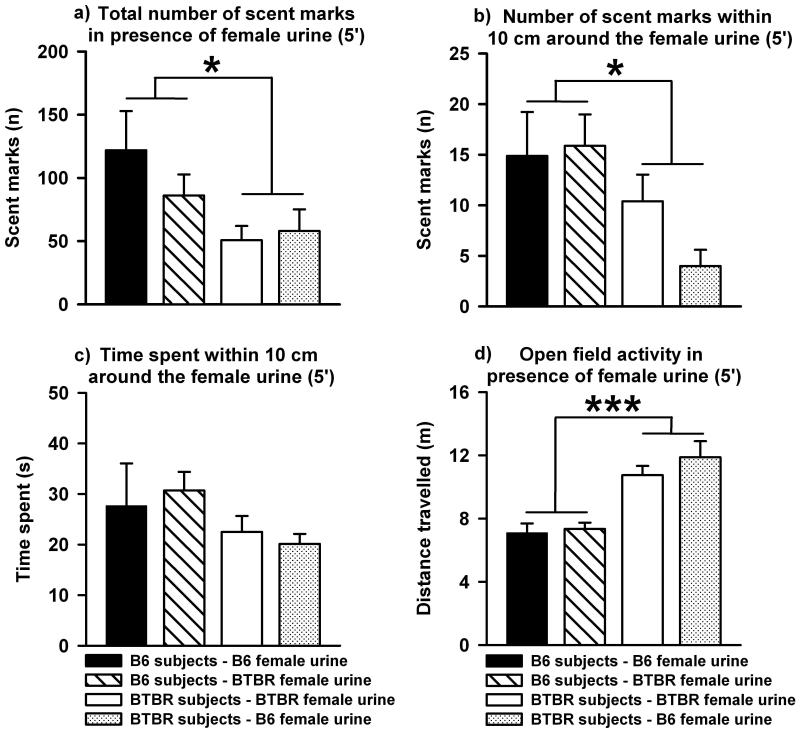
As shown in Figure 2a, BTBR deposited fewer scent marks than B6 during the 5 min test session in the open field in which 15 μl fresh urine from an estrus female was placed in the center (F1,56 = 5.965, p = 0.018, Figure 2a). No main effect of source of urine was detected (F1,56 = 1.129, p = 0.293) and no evidence for an interaction between subject strain and source of urine was found (F1,56 = 0.492, p = 0.486). This shows that the strain difference in female urine-elicited scent marking is independent of source of urine. When tallied for only the grid squares within an area of 10 cm2 surrounding the female mouse urine spot this strain difference was confirmed (F1,56 = 7.016, p = 0.010, Figure 2b). Again, this strain difference was seen in response to both B6 and BTBR female urine as no main effect of source of urine was detected (F1,56 = 1.440, p = 0.235) and no evidence for an interaction between subject strain and source of urine was found (F1,56 = 0.767, p = 0.385). Time spent within this proximal area did not differ between BTBR and B6, although a trend was indicated (F1,53 = 3.824, p = 0.056, Figure 2c). Also, the time spent proximal to the female urine spot was not dependent on source of urine (F1,53 = 0.006, p = 0.940) and no evidence for an interaction between subject strain and source of urine was detected (F1,53 = 3.824, p = 0.056). This indicates that B6 and BTBR female urine evoked similar levels of interest in male B6 and BTBR mice. BTBR had higher open field activity as compared to B6 during the 5 min test session (F1,53 = 29.550, p < 0.001, Figure 2d). Open field activity was not dependent on source of urine (F1,53 = 0.347, p = 0.558) and no evidence for an interaction between subject strain and source of urine was detected (F1,53 = 0.832, p = 0.366).
Fig. 2. Scent marking and open field activity in adult male C57BL/6J (B6) and BTBR T+tf/J (BTBR) mice in the presence of female urine.
a) Fewer scent marks were deposited by BTBR subjects (white bar: in the presence of BTBR female urine; dotted bar: in the presence of B6 female urine) than by B6 subjects (black bar: in the presence of B6 female urine; stripped bar: in the presence of BTBR female urine) throughout the entire open field when the female urine spot was present. b) Fewer scent marks were deposited by BTBR than by B6 within an area of 10 cm2 surrounding the female urine spot. c) BTBR and B6 did not differ in their time spent within this proximal area. d) BTBR had higher exploratory locomotion as compared to B6 in presence of the female urine spot. Data are presented as means ± standard errors of the mean. (* p < 0.050, *** p < 0.001 for B6 versus BTBR).
Ultrasonic vocalizations in the presence of female urine
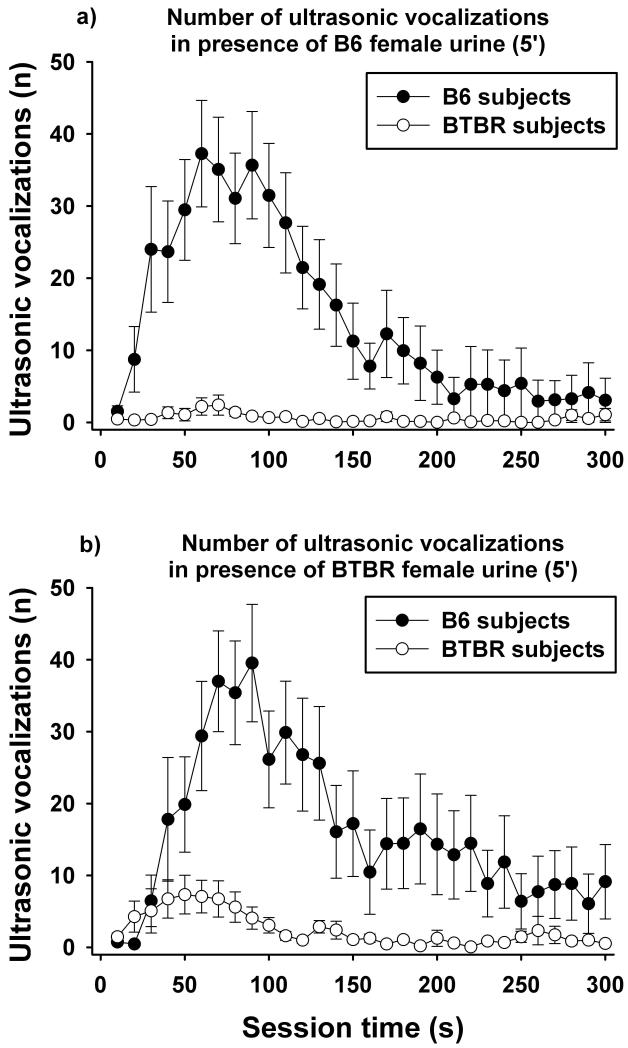
As shown in Figure 3, call emission in adult male mice exposed to fresh female mouse urine was dependent on the subject strain. Significantly more ultrasonic vocalizations were emitted by adult male B6 than BTBR mice (F1,56 = 28.310, p < 0.001). There was no effect of source of female urine (F1,56 = 0.502, p = 0.481). This means that both B6 and BTBR female urine were similarly potent for eliciting ultrasonic vocalizations in male mice. Since no evidence for an interaction between subject strain and source of urine was found (F1,56 = 0.001, p = 0.993), the possibility that BTBR emitted fewer ultrasonic vocalizations to female B6 urine because the urine was taken from an unfamiliar mouse strain can be ruled out. In addition to subject strain, call emission was affected by session time (F29,1624 = 12.209, p < 0.001) and a significant interaction between strain and session time was found (F29,1624 = 8.628, p < 0.001). This interaction was due to the fact that B6 started to emit calls almost immediately after exposure to female urine, emitted a maximum of approximately 40 calls per 10 s between 1 and 2 min after the start of the session, and slowly declined in number of calls throughout the remaining 3 min of testing. In contrast, BTBR displayed minimal vocalization responses throughout the entire 5 min of testing. In the case of B6 female urine (Figure 3a), Bonferroni post-hoc tests detected significant strain differences in the number of calls emitted at the time points of 20 to 180 s, with the exception of time points 160 to 170 s. In the case of BTBR female urine (Figure 3b), Bonferroni post-hoc tests detected significant strain differences in the number of calls emitted at the time points of 50 to 220 s, with the exception of time points 150 to 160 s. In both cases, the difference in call emission between subject strains was not due to a difference in number of mice vocalizing. In response to B6 female urine 11 out of 15 B6 and 12 out of 15 BTBR emitted more than 5 calls. Similar numbers were obtained in response to BTBR female urine. Here, 10 out of 15 B6 and 11 out of 15 BTBR emitted more than 5 calls.
Fig. 3. Ultrasonic vocalizations emitted by adult male C57BL/6J (B6) and BTBR T+tf/J (BTBR) mice in the presence of female urine.
a) Time course for the number of ultrasonic vocalizations emitted by B6 subjects (black circles) and BTBR subjects (white circles) in the presence of a B6 female urine spot. b) Time course for the number of ultrasonic vocalizations emitted by B6 and BTBR in the presence of a BTBR female urine spot. Data are presented as means ± standard errors of the mean. The time course panels illustrate the test sessions in 10 s time blocks.
Correlation between scent marking and ultrasonic vocalizations
As shown in the first row of Table 1, call emission by B6 was positively correlated with female urine-elicited scent marking within an area of 10 cm2 surrounding the female B6 urine spot (r = 0.571, p = 0.026). Call emission by BTBR was not significantly correlated with scent marking within 10 cm2 of the female B6 urine spot (r = 0.369, p = 0.176). For the entire open field arena area, total call emission and total number of scent marks in the presence of female B6 urine were correlated in both B6 (r = 0.552, p = 0.033) and BTBR (r = 0.526, p = 0.044). As shown in the second row of Table 1, call emission and female urine-elicited scent marking within an area of 10 cm2 surrounding the female BTBR urine spot did not correlate in B6 (r = 0.053, p = 0.852) nor in BTBR (r = 0.270, p = 0.331). For the entire open field arena area, total call emission and total number of scent marks in the presence of female BTBR urine were not correlated in B6 (r = 0.050, p = 0.859) but were significantly correlated in BTBR (r = 0.719, p = 0.002).
Table 1.
Correlations (r value and * significance) between number of scent marks and ultrasonic vocalizations elicited by female urine
| B6 male subject mouse | BTBR male subject mouse | |||
|---|---|---|---|---|
| Correlation between ultrasonic vocalizations and total number of scent marks |
Correlation between ultrasonic vocalizations and number of scent marks within 10 cm of the female urine spot |
Correlation between ultrasonic vocalizations and total number of scent marks |
Correlation between ultrasonic vocalizations and number of scent marks within 10 cm of the female urine spot |
|
| B6 female urine |
0.552* | 0.571* | 0.526* | 0.369 |
| BTBR female urine |
0.050 | 0.053 | 0.719** | 0.270 |
p < 0.050
p < 0.010
Discussion
Scent marking (Brennan & Kendrick, 2006; Roberts, 2007; Wyatt, 2003) and the production of ultrasonic vocalizations (Costantini & D’Amato, 2006; Nyby & Whitney, 1978; Portfors, 2007; Whitney & Nyby, 1979) appear to be the two major modes of communication in mice. We addressed the possibility that scent marking and ultrasonic vocalizations by male mice can be used as simultaneous assays to detect communication deficits. Our findings with BTBR, an inbred strain used as a mouse model of autism because of its deficits on multiple social tasks including reciprocal social interactions, social approach, and social transmission of food preference (Bolivar et al., 2007; McFarlane et al., 2008; Moy et al., 2007; Yang et al., 2007a, b; 2009), revealed evidence for scent marking and ultrasonic vocalization deficits in BTBR in relevant components of these two assays.
Evidence for a major communication deficit was discovered in adult male BTBR mice. Firstly, the BTBR mouse model of autism displayed reduced scent marking to salient olfactory social cues, indicating a deficit in social olfactory responses in male BTBR mice. BTBR male mice deposited fewer scent marks than B6 in response to female urine. This strain difference in female urine-elicited scent marking was independent of source of urine, i.e. seen in response to B6 female urine as well as in response to BTBR female urine. Secondly, male BTBR mice displayed a major deficit in ultrasonic vocalizations to social cues. Only minimal ultrasonic vocalization responses to female urinary pheromones were found in male BTBR mice. Male B6 mice emitted significantly more vocalizations than BTBR when exposed to B6 female urine. As for scent marking, this strain difference was not dependent on the source of urine. This means that BTBR emitted significantly less ultrasonic vocalizations than B6 in response to either the BTBR or the B6 female urine spot.
At least four possible mechanisms for the low level of scent marking and the minimal ultrasonic vocalization response in BTBR can be considered. Firstly, the low numbers of scent marks and ultrasonic vocalizations seen in BTBR could be due to olfactory deficits. However, BTBR displays normal non-social olfactory abilities in a buried food task (Moy et al., 2007) and normal social olfactory abilities in the preference for social novelty task (Moy et al., 2007; McFarlane et al., 2008). Further, the present results show that BTBR and B6 spent a similar amount of time in proximity to the female urine spot in the center of the open field, indicating that B6 and BTBR female urine evoked similar levels of interest in male B6 and BTBR mice. This supports the interpretation that BTBR is able to process social olfactory cues.
Secondly, the low numbers of scent marks and ultrasonic vocalizations seen in BTBR could be due to a physical impairment in the production of scent marks and ultrasonic vocalizations. With regard to scent marks, this possibility can be ruled out, since during the habituation phase the number of scent marks in a novel empty open field devoid of female urine was similar in B6 and BTBR. With regard to ultrasonic vocalizations, however, it has indeed been shown that some mice cannot produce ultrasonic vocalizations due to motor impairment, for example in Foxp2 deficient mice (Fujita et al., 2008; Groszer, 2008; Shu et al., 2005). BTBR mouse pups, however, emit a substantial amount of isolation-induced ultrasonic vocalizations when isolated from their mother and littermates, and emit highly complex calls of long durations as shown in a detailed comparison of ultrasonic vocalization behavior in four mouse strains (Scattoni et al., 2008). Thus, it appears unlikely that a physical dysfunction in vocal abilities is the cause of the low number of calls in adult BTBR in response to female urine, although a physical abnormality later in development remains possible.
Thirdly, the low numbers of scent marks and ultrasonic vocalizations seen in BTBR could be due to sedation or anxiety-related inhibition. However, exploratory activity in the open field was generally higher in BTBR than in B6, as previously reported (Moy et al., 2007; McFarlane et al., 2008; Yang et al., 2009). Also, time spent within the 10 cm2 area surrounding the female urine spot did not differ between strains. This finding indicates a dissociation between interest in the novel open field and interest in the female urinary pheromones. It is possible that interest in non-social cues competes with interest in social cues in BTBR. However, a direct inverse relationship between these two types of exploration was not seen in the present open field activity data. The higher general exploratory activity by BTBR rules out sedation or anxiety-related inhibition of exploratory activity as the cause of lower scent marks and ultrasonic vocalizations by BTBR.
A more likely interpretation is that the low number of scent marks and ultrasonic vocalizations seen in adult male BTBR mice are due to a lack of social motivation. It is widely accepted that scent marks serve important communicative functions such as mate attraction. Male mice use female scent marks to recognize the reproductive state of potential partners (Hurst, 1990b) and deposit their own scent marks as a positive advertisement directed towards females for mate selection. Thus, male mice actively scent mark to adult females (Davies & Bellamy, 1972; Hurst 1989; Maruniak et al., 1974; Reynolds, 1971), more than to adult or juvenile males and more than to juvenile females (Arakawa et al., 2007). Moreover, scent marks appears to be a key signal through which male mice announce their identity as a negative advertisement, to exclude other adult males from their territory and prevent potential competition (Desjardins et al., 1973; Hurst, 1990a).
Similarly, there is compelling evidence that ultrasonic vocalizations serve to establish or to maintain social contact. By means of playback experiments it was shown that isolation-induced ultrasonic vocalizations emitted by pups induce maternal retrieval behavior (Ehret & Haack, 1982, Sewell, 1970; Wöhr et al., 2008; Wöhr et al., in press), and that female-induced ultrasonic vocalizations emitted by adult males in response to females or female urine attract females (Hammerschmidt et al., in press; Pomerantz et al., 1973). Moreover, ultrasonic vocalizations in juvenile and adult mice are positively correlated with social investigation, such as anogenital sniffing, in the sexual (Sales, 1972; Nyby, 1983) as well as in the non-sexual context (Panksepp et al., 2007). Importantly, female-induced ultrasonic vocalizations have successfully been used as a measure of responses to social cues in mouse models of autism such as neuroligin-3- and neuroligin-4-deficient mice (Jamain et al., 2008; Radyushkin et al., 2009). Low social interactions in BTBR are a well replicated finding in multiple social tasks and across three laboratories. Compared to the commonly used B6 strain, BTBR showed lower levels of social interaction as juveniles and adults (Bolivar et al., 2007; McFarlane et al., 2008; Yang et al., 2007b; 2009), as well as lower levels of social approach as adults (McFarlane et al., 2008; Moy et al., 2007; Yang et al., 2007a; 2007b; 2009). Thus, it appears likely that low social motivation could explain low scent marks and ultrasonic vocalizations to female urine in BTBR. To test this hypothesis further, it will be interesting to conduct a systematic study on strain differences in scent marking behavior and ultrasonic vocalizations in response to female urine.
Motivational issues may also explain why BTBR show exaggerated ultrasonic vocalization responses when isolated from mother and littermates as pups (Scattoni et al., 2008) but minimal vocalization responses to female urine in adulthood. While female-urine elicited ultrasonic vocalizations are likely to be linked to social motivation, isolation-induced ultrasonic vocalizations have been associated with anxiety-like behaviors in behavioral and pharmacological studies (Miczek et al., 1995; Wöhr & Schwarting, 2008). Anxiolytic benzodiazepines and other positive modulators of GABA-receptors inhibited isolation-induced calls (Benton & Nastiti, 1988; Cirulli et al., 1994; Fish et al., 2000; Insel et al., 1986; Nastiti et al., 1991; Takahashi et al., 2009). High levels of ultrasonic vocalization responses to isolation seen in BTBR mice could reflect higher responses to stress or higher levels of anxiety-like traits in the pups. Benno et al. (2009) observed exaggerated responses to stress in adult BTBR mice. However, adult BTBR have not shown an anxiety-like phenotype in standard tasks, including the elevated plus-maze, zero maze, and light↔dark exploration (Benno et al., 2009; McFarlane et al., 2008; Moy et al., 2007; Yang et al., 2009). It will be interesting to explore the developmental trajectory of anxiety-related behaviors in BTBR in greater detail in future studies.
The present findings explore the correlation between call emission and female urine-elicited scent marking. A significant positive correlation between call emission and female urine-elicited scent marking within an area of 10 cm2 surrounding the female urine spot was detected in B6 exposed to female B6 urine. Such a correlation was not found in BTBR. Correlations between the total number of scent marks in the presence of female urine and the number of ultrasonic vocalizations to the female urine spot were detected for both B6 and BTBR presented with B6 female urine as well as for BTBR presented with BTBR female urine. Focusing on the region proximal to the female urine spot distinguished the high sociability B6 strain from the low sociability BTBR strain on both number of vocalizations and scent marking. Overall these results suggest that the number of scent marks within an area of 10 cm2 surrounding the female urine spot offers a better representation of motivation and communication, as compared to the total number of scent marks deposited throughout the entire open field. Correlations detected in B6 indicate that olfactory and acoustic communication may be positively associated, at least in a social context with high biological significance, i.e. when a male is exposed to fresh urine from its own strain that had previously acquired signal value by a brief prior exposure to a female.
The present findings show that adult male BTBR mice displayed lower scent marking and minimal ultrasonic vocalization responses to female urine obtained from both B6 and BTBR females. These results are indicative of a major deficit in social motivation and communication in male BTBR mice. Low scent marking and emission of ultrasonic vocalizations by BTBR in a social setting is consistent with the robust social deficits in this inbred mouse strain (Bolivar et al., 2007; McFarlane et al., 2008; Moy et al., 2007; Yang et al., 2007a; 2007b; 2009), suggesting that low social responsiveness is likely the primary cause for the observed low scent marking and vocalization responses to social olfactory cues. The present findings in BTBR add to its phenotypes relevant to the diagnostic symptoms of autism. Our results suggest that scent marking and ultrasonic vocalizations offer promising measures of interest in social cues, which may be broadly useful for investigations of many mouse model of autism.
Acknowledgement
This work was supported by the National Institute of Mental Health Intramural Research Program.
References
- Arakawa H, Arakawa K, Blanchard DC, Blanchard RJ. Scent marking behavior in male C57BL/6J mice: sexual and developmental determination. Behav Brain Res. 2007;182:73–79. doi: 10.1016/j.bbr.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakawa H, Arakawa K, Blanchard DC, Blanchard RJ. A new test paradigm for social recognition evidenced by urinary scent marking behavior in C57BL/6J mice. Behav Brain Res. 2008a;190:97–104. doi: 10.1016/j.bbr.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakawa H, Blanchard DC, Arakawa K, Dunlap C, Blanchard RJ. Scent marking behavior as an odorant communication in mice. Neurosci Biobehav Rev. 2008b;32:1236–1248. doi: 10.1016/j.neubiorev.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean NY, Nunez AA, Conner R. Effects of medial preoptic lesions on male mouse ultrasonic vocalizations and copulatory behavior. Brain Res Bull. 1981;6:109–112. doi: 10.1016/s0361-9230(81)80033-0. [DOI] [PubMed] [Google Scholar]
- Benno R, Smirnova Y, Vera S, Liggett A, Schanz N. Exaggerated response to stress in the BTBR T+tf/J mouse: An unusual behavioral phenotype. Behav Brain Res. 2004;197:462–465. doi: 10.1016/j.bbr.2008.09.041. [DOI] [PubMed] [Google Scholar]
- Benton D, Nastiti K. The influence of psychotropic drugs on the ultrasonic calling of mouse pups. Psychopharmacology (Berl) 1988;95:99–102. doi: 10.1007/BF00212775. [DOI] [PubMed] [Google Scholar]
- Bowers JM, Alexander BK. Mice: individual recognition by olfactory cues. Science. 1967;158:1208–1210. doi: 10.1126/science.158.3805.1208. [DOI] [PubMed] [Google Scholar]
- Bolivar VJ, Walters SR, Phoenix JL. Assessing autism-like behaviour in mice: Variations in social interactions among inbred strains. Behav Brain Res. 2007;176:21–26. doi: 10.1016/j.bbr.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan PA, Kendrick KM. Mammalian social odours: attraction and individual recognition. Phil Trans Roy Soc London. 2006;361:2061–2078. doi: 10.1098/rstb.2006.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirulli F, Santucci D, Laviola G, Alleva E, Levine S. Behavioral and hormonal responses to stress in the newborn mouse: effects of maternal deprivation and chlordiazepoxide. Dev Psychobiol. 1994;27:301–316. doi: 10.1002/dev.420270505. [DOI] [PubMed] [Google Scholar]
- Costantini F, D’Amato FR. Ultrasonic vocalizations in mice and rats: social contexts and functions. Acta Zool Sin. 2006;52:619–633. [Google Scholar]
- D’Amato FR. Courtship ultrasonic vocalizations and social status in mice. Anim Behav. 1991;41:875–885. [Google Scholar]
- Davies VJ, Bellamy D. The olfactory response of mice to urine and effects of gonadectomy. J Endocrinol. 1972;55:11–20. doi: 10.1677/joe.0.0550011. [DOI] [PubMed] [Google Scholar]
- Diagnostic and Statistical Manual of Mental Disorders. 4th edn. American Psychiatric Publishing; Arlington, VA, USA: 2000. [Google Scholar]
- Desjardins C, Maruniak JA, Bronson FH. Social rank in house mice: differentiation revealed by ultraviolet visualization of urinary marking patterns. Science. 1973;182:939–941. doi: 10.1126/science.182.4115.939. [DOI] [PubMed] [Google Scholar]
- Dizinno G, Whitney G, Nyby J. Ultrasonic vocalizations by male mice (Mus musculus) to female sex pheromone: experimental determinants. Behav Biol. 1978;22:104–113. [Google Scholar]
- Ehret G, Haack B. Ultrasound recognition in house mice: key-stimulus configuration and recognition mechanisms. J Comp Physiol. 1982;148:245–251. [Google Scholar]
- Fish EW, Sekinda M, Ferrari PF, Dirks A, Miczek KA. Distress vocalizations in maternally separated mouse pups: modulation via 5-HT(1A), 5-HT(1B) and GABA(A) receptors. Psychopharmacology. 2000;149:277–285. doi: 10.1007/s002130000370. [DOI] [PubMed] [Google Scholar]
- Frith U, Happé F. Language and communication in autistic disorders. Phil Trans R Soc Lond B. 1994;346:97–104. doi: 10.1098/rstb.1994.0133. [DOI] [PubMed] [Google Scholar]
- Fujita E, Tanabe Y, Shiota A, Ueda M, Suwa K, Momoi MY, Momoi T. Ultrasonic vocalization impairment of Foxp2 (R552H) knockin mice related to speech-language disorder and abnormality of Purkinje cells. Proc Natl Acad Sci U S A. 2008;105:3117–3122. doi: 10.1073/pnas.0712298105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groszer M, Keays DA, Deacon RM, de Bono JP, Prasad-Mulcare S, Gaub S, Baum MG, French CA, Nicod J, Coventry JA, Enard W, Fray M, Brown SD, Nolan PM, Paabo S, Channon KM, Costa RM, Eilers J, Ehret G, Rawlins JN, Fisher SE. Impaired synaptic plasticity and motor learning in mice with a point mutation implicated in human speech deficits. Curr Biol. 2008;18:354–362. doi: 10.1016/j.cub.2008.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, Holy TE. Sex selectivity of mouse ultrasonic songs. Chem Senses. 2007;23:463–473. doi: 10.1093/chemse/bjm015. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt K, Radyushkin K, Ehrenreich H, Fischer J. Female mice respond to male ultrasonic ‘songs’ with approach behaviour. Biol Lett. 2009;5:589–592. doi: 10.1098/rsbl.2009.0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst JL. The complex network of olfactory communication in populations of wild house mice Mus domesticus Rutty: urine marking and investigation within family groups. Anim Behav. 1989;37:705–725. [Google Scholar]
- Hurst JL. Urine marking in populations of wild house mice (Mus domesticus Rutty). I. Communication between males. Anim Behav. 1990a;40:209–222. [Google Scholar]
- Hurst JL. Urine marking in populations of wild house mice (Mus domesticus Rutty). III. Communication between the sexes. Anim Behav. 1990b;40:233–243. [Google Scholar]
- Insel TR, Hill JL, Mayor RB. Rat pup ultrasonic isolation calls: possible mediation by the benzodiazepine receptor complex. Pharmacol Biochem Behav. 1986;24:1263–1267. doi: 10.1016/0091-3057(86)90182-6. [DOI] [PubMed] [Google Scholar]
- Jamain S, Radyushkin K, Hammerschmidt K, Granon S, Boretius S, Varoqueaux F, Ramanantsoa N, Gallego J, Ronnenberg A, Winter D, Frahm J, Fischer J, Bourgeron T, Ehrenreich H, Brose N. Reduced social interaction and ultrasonic communication in a mouse model of monogenic heritable autism. Proc Natl Acad Sci USA. 2008;105:1710–1715. doi: 10.1073/pnas.0711555105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Leventhal BL, Cook EH. Quantifying the phenotype in autism spectrum disorders. Am J Med Genet. 2001;105:36–38. [PubMed] [Google Scholar]
- Lord C, Risi S, Dilavore PS, Shulman C, Thurm A, Pickles A. Autism from 2 to 9 years of age. Arch Gen Psychiatry. 2006;63:694–701. doi: 10.1001/archpsyc.63.6.694. [DOI] [PubMed] [Google Scholar]
- Losh M, Piven J. Social-cognition and the broad autism phenotype: identifying genetically meaningful phenotypes. J Child Psychol Psychiatry. 2007;48:105–112. doi: 10.1111/j.1469-7610.2006.01594.x. [DOI] [PubMed] [Google Scholar]
- Maggio JC, Maggio JH, Whitney G. Experience-based vocalization of male mice to female chemosignals. Physiol Behav. 1983;31:269–272. doi: 10.1016/0031-9384(83)90186-5. [DOI] [PubMed] [Google Scholar]
- Maruniak JA, Owen K, Bronson FH, Desjardins C. Urinary marking in male house mice: responses to novel environmental and social stimuli. Physiol Behav. 1974;12:1035–1039. doi: 10.1016/0031-9384(74)90151-6. [DOI] [PubMed] [Google Scholar]
- McFarlane HG, Kusek GK, Yang M, Phoenix JL, Bolivar VJ, Crawley JN. Autism-like behavioral phenotypes in BTBR T+tf/J mice. Genes Brain Behav. 2008;7:152–163. doi: 10.1111/j.1601-183X.2007.00330.x. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Weerts EM, Vivian JA, Barros HM. Aggression, anxiety and vocalizations in animals: GABAA and 5-HT anxiolytics. Psychopharmacology (Berl) 1995;121:38–56. doi: 10.1007/BF02245590. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler, Poe MD, Nonneman RJ, Young NB, Koller BH, Crawley JN, Duncan GE, Bodfish JW. Development of a mouse test for repetitive, restricted behaviours: Relevance to autism. Behav Brain Res. 2008;188:178–194. doi: 10.1016/j.bbr.2007.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Young NB, Perez AP, Holloway LP, Barbaro RP, Barbaro JR, Wilson LM, Threadgill DW, Lauder JM, Magnuson TR, Crawley JN. Mouse behavioral tasks relevant to autism: Phenotypes of 10 inbred strains. Behav Brain Res. 2007;176:4–20. doi: 10.1016/j.bbr.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nastiti K, Benton D, Brain PF. The effects of compounds acting at the benzodiazepine receptor complex on the ultrasonic calling of mouse pups. Behav Pharmacol. 1991;2:121–128. [PubMed] [Google Scholar]
- Nyby J. Ultrasonic vocalizations during sex behavior of male house mice (Mus musculus): a description. Behav Neural Biol. 1983;39:128–134. doi: 10.1016/s0163-1047(83)90722-7. [DOI] [PubMed] [Google Scholar]
- Nyby J, Bigelow J, Kerchner M, Barbehenn F. Male mouse (Mus musculus) ultrasonic vocalizations to female urine: why is heterosexual experience necessary? Behav Neural Biol. 1983;38:32–46. doi: 10.1016/s0163-1047(83)90354-0. [DOI] [PubMed] [Google Scholar]
- Nyby J, Dizinno GA, Whitney G. Social status and ultrasonic vocalizations of male mice. Behav Biol. 1976;18:285–289. doi: 10.1016/s0091-6773(76)92198-2. [DOI] [PubMed] [Google Scholar]
- Nyby J, Whitney G. Ultrasonic communication of adult myomorph rodents. Neurosci Biobehav Rev. 1978;2:1–14. [Google Scholar]
- Nyby J, Wysocki CJ, Whitney G, Dizinno G. Pheromonal regulation of male mouse ultrasonic courtship (Mus musculus) Anim Behav. 1977;25:333–341. doi: 10.1016/0003-3472(77)90009-4. [DOI] [PubMed] [Google Scholar]
- Nyby J, Wysocki CJ, Whitney G, Dizinno G, Schneider J. Elicitation of male mouse (Mus musculus) ultrasonic vocalizations: I. Urinary cues. J Comp Physiol Psychol. 1979;93:957–975. [Google Scholar]
- Nyby J, Zakeski D. Elicitation of male mouse ultrasounds: bladder urine and aged urine from females. Physiol Behav. 1980;24:737–740. doi: 10.1016/0031-9384(80)90405-9. [DOI] [PubMed] [Google Scholar]
- Panksepp JB, Jochman KA, Kim JU, Koy JJ, Wilson ED, Chen Q, Wilson CR, Lahvis GP. Affiliative behavior, ultrasonic communication and social reward are influenced by genetic variation in adolescent mice. PLoS ONE. 2007;2:e351. doi: 10.1371/journal.pone.0000351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerantz SM, Nunez AA, Bean NJ. Female behavior is affected by male ultrasonic vocalizations in house mice. Physiol Behav. 1973;31:91–96. doi: 10.1016/0031-9384(83)90101-4. [DOI] [PubMed] [Google Scholar]
- Portfors CV. Types and functions of ultrasonic vocalizations in laboratory rats and mice. J Am Assoc Lab Anim Sci. 2007;46:28–34. [PubMed] [Google Scholar]
- Radyushkin K, Hammerschmidt K, Boretius S, Varoqueaux F, El-Kordi A, Ronnenberg A, Winter D, Frahm J, Fischer J, Brose N, Ehrenreich H. Neuroligin-3-deficient mice: model of a monogenic heritable form of autism with an olfactory deficit. Genes Brain Behav. 2009;8:416–425. doi: 10.1111/j.1601-183X.2009.00487.x. [DOI] [PubMed] [Google Scholar]
- Reynolds E. Urination as a social response in mice. Nature. 1971;234:481–483. doi: 10.1038/234481a0. [DOI] [PubMed] [Google Scholar]
- Roberts . Scent Marking. 255-266. In: Wolf JO, Sherman PW, editors. Rodent societies. An Ecological and Evolutionary Perspective. The University of Chicago Press; Chicago: 2007. [Google Scholar]
- Sales GD. Ultrasound and mating behaviour in rodents with some observations on other behavioural situations. J Zool. 1972;168:149–164. [Google Scholar]
- Scattoni ML, Gandhy SU, Ricceri L, Crawely JN. Unusual repertoire of vocalizations in the BTBR T+tf/J mouse model of autism. PLoS ONE. 2008;3:e3067. doi: 10.1371/journal.pone.0003067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sewell GD. Ultrasonic communication in rodents. Nature. 1970;227:410. doi: 10.1038/227410a0. [DOI] [PubMed] [Google Scholar]
- Shu W, Cho JY, Jiang Y, Zhang M, Weisz D, Elder GA, Schmeidler J, De Gasperi R, Sosa MA, Rabidou D, Santucci AC, Perl D, Morrisey E, Buxbaum JD. Altered ultrasonic vocalization in mice with a disruption in the Foxp2 gene. Proc Natl Acad Sci USA. 2005;102:9643–9648. doi: 10.1073/pnas.0503739102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipos ML, Kerchner M, Nyby JG. An ephemeral sex pheromone in the urine of female house mice (Mus domesticus) Behav Neural Biol. 1992;58:138–143. doi: 10.1016/0163-1047(92)90375-e. [DOI] [PubMed] [Google Scholar]
- Takahashi A, Yap JJ, Bohager DZ, Faccidomo S, Clayton T, Cook JM, Miczek KA. Glutamatergic and GABAergic modulations of ultrasonic vocalizations during maternal separation distress in mouse pups. Psychopharmacology (Berl) 2009;204:61–71. doi: 10.1007/s00213-008-1437-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkmar FR, Lord C, Bailey A, Schultz RT, Klin AJ. Autism and pervasive developmental disorders. J Child Psychol Psychiatry. 2004;45:135–170. doi: 10.1046/j.0021-9630.2003.00317.x. [DOI] [PubMed] [Google Scholar]
- Wang H, Liang S, Burgdorf J, Wess J, Yeomans J. Ultrasonic vocalizations induced by sex and amphetamine in M2, M4, M5 muscarinic and D2 dopamine receptor knockout mice. PLoS ONE. 2008;3:e1893. doi: 10.1371/journal.pone.0001893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney G, Nyby J. Cues that elicit ultrasounds from adult male mice. Amer Zool. 1979;19:457–463. [Google Scholar]
- Whitney G, Alpern M, Dizinno G, Horowitz G. Female odors evoke ultrasounds from male mice. Anim Learn Behav. 1974;2:13–18. doi: 10.3758/bf03199109. [DOI] [PubMed] [Google Scholar]
- Wöhr M, Dahlhoff M, Wolf E, Holsboer F, Schwarting RK, Wotjak CT. Effects of genetic background, gender, and early environmental factors on isolation-induced ultrasonic calling in mouse pups: an embryo-transfer study. Behav Genet. 2008;38:579–595. doi: 10.1007/s10519-008-9221-4. [DOI] [PubMed] [Google Scholar]
- Wöhr M, Oddi D, D’Amato FR. Effect of altricial pup ultrasonic vocalization on maternal behavior. In: Brudzynski SM, editor. Handbook of Mammalian Vocalization. An Integrative Neuroscience Approach. Elsevier; Amsterdam: pp. 159–166. in press. Chapter 5.2. [Google Scholar]
- Wöhr M, Schwarting RKW. Maternal care, isolation-induced infant ultrasonic calling, and their relations to adult anxiety-related behavior in the rat. Behav Neurosci. 2008;122:310–330. doi: 10.1037/0735-7044.122.2.310. [DOI] [PubMed] [Google Scholar]
- Wyatt TD. Pheromones and animal behaviour. Communication by taste and smell. Cambridge University Press; Cambridge: 2003. [Google Scholar]
- Yang M, Clarke AM, Crawley JN. Postnatal lesion evidence against a primary role for the corpus callosum in mouse sociability. Eur J Neurosci. 2009;29:1663–1677. doi: 10.1111/j.1460-9568.2009.06714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Scattoni ML, Zhodzishsky V, Chen T, Caldwell H, Young WS, McFarlance HG, Crawley JN. Social approach behaviors are similar on conventional versus reverse lighting cycles, and in replications across cohorts, in BTBR T+tf/J, C57BL/6J, and vasopressin receptor 1B mutant mice. Front Behav Neurosci. 2007a;1:1. doi: 10.3389/neuro.08/001.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Zhodzishsky V, Crawley JN. Social deficits in BTBR T+tf/J mice are unchanged by cross-fostering with C57BL/6J mothers. Int J Dev Neurosci. 2007b;25:515–521. doi: 10.1016/j.ijdevneu.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]